Abstract
Platelet-derived growth factors (PDGFs) and their receptors (PDGFRs) play a fundamental role in the embryonic development of the lung. Aberrant PDGF signalling has been documented convincingly in a large variety of pulmonary diseases, including idiopathic pulmonary arterial hypertension, lung cancer and lung fibrosis. Targeting PDGF signalling has been proven to be effective in these diseases. In clinical practice, the most effective way to block PDGF signalling is to inhibit the activity of the intracellular PDGFR kinases. Although the mechanism of action of such drugs is not specific for PDGF signalling, the medications have a broad therapeutic index that allows clinical use. The safety profile and therapeutic opportunities of these and future medications that target PDGFs and PDGFRs are reviewed.
Short abstract
An increasing role for PDGF signalling inhibitors in clinical trials for the treatment of various pulmonary diseases http://ow.ly/buaI30f9HcN
Introduction
Platelet-derived growth factors (PDGFs) and their receptors (PDGFRs) represent one of the most intensively studied families of signalling factors over the past four decades. PDGFs constitute a family of four gene products (PDGF-A–D) acting by means of two receptor tyrosine kinases, PDGFR-α and -β. The PDGF signalling pathway is found in most cell types.
In vivo studies have documented an important role of PDGF signalling in the normal development of several organs, such as the kidney, eye and lung. PDGF signalling plays an essential role in cell proliferation, differentiation, migration and survival [1–3]. In adults, PDGF signalling is involved in formation of de novo connective tissue during wound healing. Aberrant expression and signalling of PDGF ligands and receptors is associated with several connective tissue disorders, and lung diseases such as pulmonary arterial hypertension (PAH), lung cancer and idiopathic pulmonary fibrosis (IPF) [4]. This review first focuses on the PDGF signalling pathways that involve specific ligands and their receptors. This is followed by a description of the role of this important class of molecules in lung disease.
PDGF signalling pathways
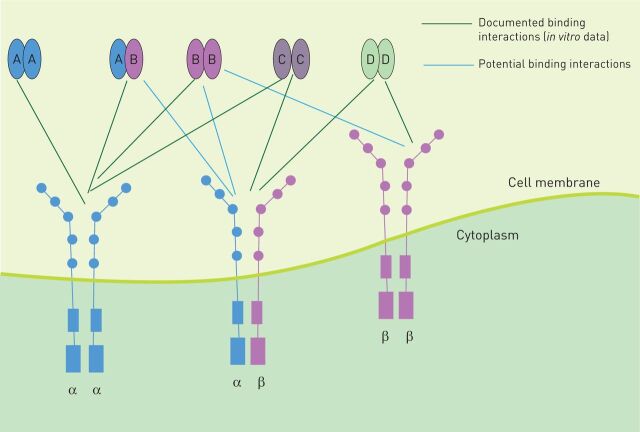
The PDGFRs are transmembrane proteins belonging to the receptor tyrosine kinase (RTK) class. PDGF signalling is initiated by the binding of distinct dimeric PDGF ligands to the extracellular domain of two monomeric receptors at the same time, thereby inducing dimerisation of PDGFR and autophosphorylation of the tyrosine residues within its intracellular domain. Five different ligand isoforms (PDGF-AA, PDGF-BB, PDGF-CC, PDGF-DD and PDGF-AB) and two PDGFR isotypes (PDGFR-α and PDGFR-β) with three different PDGFR dimers (PDGFR-α/α, PDGFR-α/β and PDGFR-β/β) have been described (figure 1). The ligands bind to these receptor pairs with different affinities. PDGF-AA and PDGF-CC bind with high affinity to PDGFR-α/α in vitro and in vivo. PDGF-BB shows binding affinity to all three PDGFR dimers in vitro and signals through PDGFR-β/β in vivo. PDGF-AB binds to PDGFR-α/α and PDGFR-α/β in vitro. PDGF-DD shows affinity for PDGFR-β/β in vitro. PDGF-CC and -DD activate the heterodimer PDGFR-α/β in vitro [5–7]. In vivo binding affinity toward the different PDGFRs is largely unknown for PDGF-DD and -AB.
FIGURE 1.
Platelet-derived growth factor receptors (PDGFRs) and ligand patterns. PDGFRs are transmembrane proteins. The extracellular domain consists of five immunoglobulin-like domains; binding occurs at domains 2 and 3. The intracellular domain is a tyrosine kinase. There are three dimeric forms of PDGFRs (-αα, -ββ and -αβ). Five different ligand isoforms are known to bind to PDGFRs (AA, AB, BB, CC and DD).
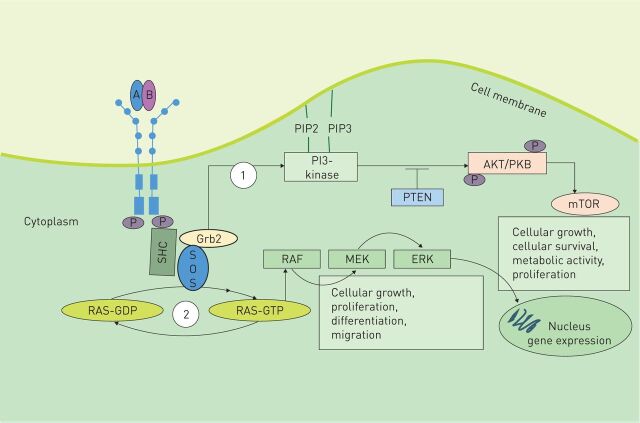
Phosphorylation of the tyrosine residues within the intracellular domain results in transduction of signals via recruitment of surrounding proteins containing Src homology region 2 domains (figure 2). The two main intracellular signalling pathways activated by PDGF signalling are the phosphatidylinositol 3′-kinase/Akt (also known as protein kinase B)/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway and the mitogen-activated protein kinase (MAPK) cascade pathway [8–10]. mTOR suppresses the synthesis of PDGFR. Although the mechanism has not been fully elucidated, data suggest that suppression occurs by reduced PDGFR transcription. Aberrant activation of this pathway is known to appear in several types of human cancer [11–13].
FIGURE 2.
Platelet-derived growth factor (PDGF) signalling pathway. Binding of distinct dimeric PDGF ligands to the respective receptor dimer leads to autophosphorylation of the tyrosine residues within their intracellular domains. Autophosphorylation then leads to transduction of signals via recruitment of surrounding proteins containing Src homology region 2. These include domains such as Grb2, Grb7, SHC, PI3K and GTPase-activating protein for Ras. Signal transduction occurs via two main pathways: 1) the PI3K pathway, which mediates Akt signalling for the promotion of cell survival and 2) the mitogen-activated protein kinase (MAPK) cascade. Hydrolytic conversion of RAS-guanosine diphosphate (GDP) to RAS-guanosine triphosphate (GTP) leads to activation of MAPK cascade members, such as extracellular signal-regulated kinase (ERK) or MAPK kinase (MEK) via RAF1, resulting in gene target transcription. This pathway is important for cell growth, proliferation, differentiation and migration. Son of sevenless (SOS) is a nucleotide exchange factor for Ras.
The MAPK cascade signalling pathway is important in stimulating cell migration, differentiation and proliferation. Conversion of RAS-guanosine diphosphate to RAS-guanosine triphosphate leads to activation of the MAPK cascade members via RAF 1. These proteins such as extracellular signal-regulated kinase or MAPK kinase result in gene target transcription.
Differences in signalling result from PDGFR-α and PDGFR-β activation. In vivo, embryos of transgenic mice lacking PDGFR-α showed deficiencies in a large number of mesenchymal cells, such as smooth muscle cell progenitors, whereas embryos lacking PDGFR-β are deficient in vascular smooth muscle cells and pericytes [14]. At a transcriptome level, PDGFR-α/α, PDGFR-α/β and PDGFR-ββ activated a set of 33, 25 and 15 genes, respectively [15].
The role of PDGF signalling in lung development
PDGF signalling plays a crucial role in the embryonic development of the lung. In vivo studies have documented an essential role of mesenchymal cells expressing PDGFR-α mRNA in postnatal alveolar septation. PDGF-A-null mice died during embryogenesis or shortly after birth, while surviving PDGF-A-deficient mice develop lung emphysema secondary to failure of alveolar septation caused by defective alveolar myofibroblast differentiation [3]. In addition, overexpression of PDGF-A in the lung epithelium resulted in perinatal death caused by fetal lung enlargement, failure of airspace development and mesenchymal cell hyperplasia [16].
PDGF signalling in lung disease
Aberrant PDGF signalling has been documented convincingly in a large variety of pulmonary diseases. In addition, the role of PDGF signalling inhibitors as a therapeutic option has been investigated in multiple clinical trials. PDGF inhibitors currently in use include DNA aptamers or soluble extracellular parts of the receptors that bind PDGF isoforms and thus prevent their binding to signalling receptors [17, 18], neutralising antibodies [18] or decoy receptors that sequester PDGFs and thus prevent their binding to and activation of PDGFRs [19]. Alternatively, the activity of PDGFRs can be inhibited by neutralising antibodies [20–22]. These types of antagonists have the advantage of being reasonably specific; however, they are expensive. One of the most effective ways to block PDGF signalling is to inhibit the activity of the intracellular PDGFR kinases. Several potent inhibitors of PDGFR kinases have been tested, including imatinib, linifanib, nintedanib and sorafenib [20, 23]. Table 1 provides a summary of completed clinical trials. Table 2 provides a summary of targets for each drug and the corresponding median inhibitory concentration (IC50). The mechanism of action of these drugs is not specific for PDGF signalling.
TABLE 1.
Summary of completed clinical trials
| Lung disease/documented PDGF-PDGFR involved in pathogenesis | Drug | Completed clinical study, study phase, study design and number of patients | Patient population | Intervention | Primary outcome | Results |
|
IPAH PDGF-BB PDGFR-β |
Imatinib mesylate | IMPRES [24] Phase 3, 24 weeks, multicentre, randomised, double blind, placebo controlled (n=202) |
PVR ≥800 dyn·s·cm−5 symptomatic on ≥2 PAH therapies | Randomised to receive imatinib 200 mg once daily or placebo | Change in 6MWD | Mean treatment effect on 6MWD 32 m; p=0.002 PVR decreased by 379 dyn·s·cm−5 p<0.001 Serious AEs were more frequent with imatinib compared to placebo (44% versus 30%) |
| Sorafenib | Gomberg-Maitland
et al. [25] Phase 1b, 16 weeks, single centre, open label (n=22) |
Advanced but stable PAH on parenteral prostanoids (with or without oral sildenafil) | Received sorafenib started at 200 mg daily then escalated to 400 mg twice daily | Safety and tolerability | Sorafenib was well tolerated at 200 mg twice daily AEs: moderate skin reactions on the hands and feet and alopecia |
|
|
NSCLC PDGF-A/B PDGFR-α/β |
Imatinib mesylate (in combination with paclitaxel) | Bauman
et al. [26] Phase 2, multicentre, single stage, open label (n=34) |
Age ≥70 years with untreated, stage IIIB/IV NSCLC and ECOG performance status 0–2 | Received up to six 28-day cycles of imatinib and paclitaxel | RR | Met primary end-point; however, no improvement in overall survival and PFS. Regimen not recommended for further study |
| Linifanib (in combination with carboplatin and paclitaxel) | Ramalingam
et al. [27] Phase 2, multinational, open label (n=139) |
Stage IIIB/IV nonsquamous NSCLC | Received carboplatin and paclitaxel plus placebo, linifanib 7.5 mg or linifanib 12.5 mg | PFS | Improved PFS. Higher incidence of AEs known to be associated with VEGF/PDGF inhibition | |
| Nintedanib (BIBF 1120) with pemetrexed |
Ellis
et al. [28] Phase 1, multicentre, open label (n=26) |
Metastatic, unresectable, or locally advanced NSCLC and had to have relapsed during or following one prior platinum-based chemotherapy regimen | Received BIBF 1120 starting dose of 100 mg twice daily (days 2–21) with pemetrexed 500 mg·m−2 (day 1) over a 21-day cycle BIBF 1120 dose was escalated until the MTD was determined |
Safety, tolerability and MTD | The MTD of BIBF 1120 in combination with standard-dose pemetrexed was 200 mg twice daily BIBF 1120 in this combination was tolerable, with promising signs of efficacy |
|
| Nintedanib (in combination with docetaxel) |
LUME-Lung 1 [29] Phase 3, multicentre, double blind, randomised, placebo controlled (n=1314) |
Stage IIIb/IV recurrent NSCLC progressing after first-line chemotherapy | Randomised to docetaxel 75 mg·m−2 by i.v. infusion on day 1 plus nintedanib 200 mg twice daily orally or placebo on days 2–21, every 3 weeks Treatment was continued until unacceptable AEs or disease progression |
PFS | PFS was significantly improved after a median follow-up of 7.1 months Improved overall survival in all patients with adenocarcinoma (12.6 versus 10.3 months); more pronounced in the subgroup of patients who progressed within 9 months after start of first-line therapy (10.9 versus 7.9 months) AEs: diarrhoea, reversible increase in ALT and AST, gastrointestinal side-effects |
|
| Nintedanib (in combination with premetrexed) |
LUME-Lung 2 [30] Phase 3, multicentre, double blind, randomised, placebo controlled (n=713) |
Stage IIIb/IV or recurrent NSCLC previously treated with chemotherapy. ECOG performance score of 0–1 | Randomised to pemetrexed 500 mg·m−2 i.v. on day 1, combined with either oral nintedanib 200 mg twice daily or placebo, given on days 2–21, every 3 weeks | PFS | Stopped early. ITT analysis of PFS favoured the treatment arm (median 4.4 months versus 3.6 months; HR 0.83, 95% CI 0.70–0.99; p=0.0435) | |
|
IPF PDGF-AA, -BB and -CC |
Imatinib mesylate (Gleevec) | Daniels
et al. [31] Phase 2, 96 weeks, multicentre, randomised, double blind, placebo controlled (n=119) |
Mild to moderate IPF diagnosed within 3–36 months of screening with clinical worsening in the past year (10% decline in FVC % pred or worsening chest radiograph or worsening dyspnoea) | Randomised to receive imatinib mesylate 600 mg (six tablets) orally once daily or placebo | Combined measure of disease progression (defined as 10% decline from baseline FVC) or death |
No effect on survival or lung function |
| Nintedanib (BIBF 1120) |
TOMORROW [32] Phase 2, 12 months, multicentre, randomised, double blind, placebo controlled (n=432) |
Age ≥40 years with <5 years diagnosis of IPF, FVC ≥50% pred, DLCO 30–79% pred, PaO2 ≥55 mmHg at <1500 m altitude or ≥50 mmHg at >1500 m altitude | Randomisation to receive one of four doses of BIBF 1120 (50 mg once a day, 50 mg twice a day, 100 mg twice a day or 150 mg twice a day) or placebo | Annual rate of decline in FVC | Compared to placebo, BIBF 1120 at a dose of 150 mg twice daily was associated with: a trend toward a reduction in the decline in lung function (0.06 versus 0.19 L·year-1); fewer acute exacerbations (2.4 versus 15.7 per 100 patient-years, p=0.02); preserved quality of life (−0.66 versus 5.46, p=0.007); gastrointestinal AEs, which led to more discontinuation in the 150 mg BIBF 1120 twice a day group; increase in liver aminotransferase levels |
|
| INPULSIS-1 and 2 [33] Phase 3, 52 weeks, multicentre, randomised double blind, placebo controlled (n=1061) |
Age ≥40 years with <5 years diagnosis of IPF, FVC ≥50% pred, DLCO 30–79% pred, | Randomised to receive 150 mg nintedanib twice daily or placebo | Annual rate of decline in FVC | INPULSIS-1 No difference in time to first acute exacerbation (HR 1.15, 95% CI 0.54–2.42) Comparable proportion of patients with at least one investigator-reported acute exacerbation (6.1% versus 5.4%, respectively, in the nintedanib and placebo groups) INPULSIS-2 Significant increase in time to first acute exacerbation (HR 0.38, 95% CI 0.19–0.77) Lower proportion of patients with at least one investigator-reported acute exacerbation in the nintedanib group (3.6%) compared with placebo (9.6%) Most common AEs: diarrhoea followed by nausea |
||
| Pirfenidone | ASCEND [34] Phase 3, 52 weeks, multicentre, double blind, placebo-controlled (n=555) |
Age 40–80 years, centrally confirmed diagnosis of IPF | Randomised to receive pirfenidone 2403 mg per day or placebo | Change in FVC or death at week 52 | 47.9% relative reduction in the proportion of patients who had an absolute decline of ≥10% of FVC % pred or who died No significant difference in dyspnoea scores (p=0.16) No significant difference in rates of death from any cause (p=0.10) or from IPF (p=0.23) |
|
| CAPACITY 004 [35] Phase 3, 72 weeks, multicentre, double blind, placebo randomised controlled trial (n=435) |
Age 40–80 years, diagnosis of IPF in the previous 48 months, FVC ≥50% and ≤90% pred, DLCO ≥35% and ≤90% pred 6MWT≥150 m |
Randomised to receive pirfenidone 2403 mg·day−1, or pirfenidone 1197 mg·day−1 or placebo | Change in percentage of FVC % pred from baseline to week 72 | Significant reduction in decline of FVC (p=0.001). Mean FVC change at week 72 was –8·0±16.5% in the pirfenidone 2403 mg·day−1 group and –12.4±18.5% in the placebo group (difference 4.4%, 95% CI 0.7–9.1). Outcomes in the pirfenidone 1197 mg·day−1 group were intermediate to the pirfenidone 2403 mg·day−1 and placebo groups AEs: patients in the pirfenidone 2403 mg·day−1 group had higher incidences of nausea, dyspepsia, vomiting, anorexia, photosensitivity and dizziness |
||
| CAPACITY 006 [35] Phase 3, 72 weeks, multicentre, double blind, randomised, placebo controlled (n=344) |
Age 40–80 years, diagnosis of IPF in the previous 48 months FVC ≥50% pred and ≤90% pred, DLCO ≥35% pred and ≤90% pred 6MWT ≥150 m |
Randomised to receive pirfenidone 2403 mg·day−1 or placebo |
Change in FVC % pred from baseline to week 72 | Significant reduction in decline of FVC. Mean change at week 72 was –9.0±19.6% in patients in the pirfenidone 2403 mg·day−1 group and –9.6±19.1% in patients in the placebo group AEs: same as CAPACITY 004 |
||
| HPS | Pirfenidone | Gahl
et al. [36] 44 months, single centre, double blind, randomised controlled (n=21) |
Confirmed diagnosis of HPS and FVC 40–75% pred All 21 subjects were Puerto Rican; 20 were homozygous for the 16-bp duplication in exon 15 of HPS1; one was homozygous for a 3904-bp deletion in HPS3 |
Randomised to receive pirfenidone 800 mg three times daily or placebo | Average rate of decline of FVC, FEV1, TLC and DLCO, measured as % pred, in the two treatment groups | 5% difference in the yearly rate of FVC decline (p=0.001) In patients with an initial FVC >50% pred, pirfenidone slowed lost pulmonary function (FVC, FEV1, TLC and DLCO) at a rate of 8% per year, compared to placebo |
| O'Brien
et al. [37] Single centre, double blind, randomised, placebo controlled (n=35) |
HPS-1 or -4 confirmed by molecular analysis and FVC 51–85% pred, regardless of radiographic evidence of fibrosis on HRCT | Randomised to receive pirfenidone or placebo. A dosage escalation schedule was employed to reach 801 mg pirfenidone three times daily | Rate of decline in FVC | Stopped early (after 12 months) due to futility No significant safety concerns |
||
| Diffuse cutaneous systemic sclerosis | Nintedanib | Spiera
et al. [38] Phase 2a, 12 months, single centre, single arm, open label (n=30) |
Fulfilled the American College of Rheumatology classification criteria for systemic sclerosis and had the diffuse subtype. Stable MRSS of ≥16 points in the month between screening and baseline visits, and had disease duration of <10 years DLCO ≥30% pred |
Imatinib 400 mg daily | Change in the MRSS after 12 months of treatment | Improved MRSS 6.6 points or 22.4% (p=0.001) FVC improved by 6.4% pred (p=0.008) |
PDGF: platelet-derived growth factor; PDGFR: PDGF receptor; IPAH: idiopathic pulmonary arterial hypertension; NSCLC: nonsmall cell lung cancer; IPF: idiopathic pulmonary fibrosis; HPS: Hermansky–Pudlak syndrome; PVR: pulmonary vascular resistance; PAH: pulmonary arterial hypertension; 6MWD: 6-min walking distance; AEs: adverse events; ECOG: Eastern Cooperative Oncology Group; RR: response rate; PFS: progression-free survival; VEGF: vascular endothelial growth factor; MTD: maximum tolerated dose; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ITT: intention to treat; FVC: forced vital capacity; % pred: % predicted; DLCO: diffusing capacity of the lung for carbon monoxide; PaO2: arterial oxygen tension; 6MWT: 6-min walk test; FEV1: forced expiratory volume in 1 s; TLC: total lung capacity; HRCT: high-resolution computed tomography; MRSS: modified Rodnan skin score.
TABLE 2.
Summary of drug targets and median inhibitory concentration (IC50)
| Drug target | IC50 nM | |
| Imatinib [39, 40] | PDGFR V-Abl C-Kit |
100 600 100 |
| Linifanib [41] | PDGFR-β KDR CSF-1R Flt-1/3 |
66 4 3 3/4 |
| Nintedanib [42, 43] | PDGFR-α/β VEGFR1/2/3 FGFR1/2/3 |
59/65 34/13/13 69/37/108 |
| PK10453 [44] | PDGFR-α/β | 10.1/35 |
| Sorafenib [43, 45] | PDGFR-β Raf-1 B-Raf VEGFR-2 |
57 6 22 90 |
| Sunitinib [46] | PDGFR-β VEGFR2 (Flk-1) |
2 80 |
| Pirfenidone | TGF-β PDGF-A and -B |
NA NA |
PDGFR: platelet-derived growth factor receptor; VEGFR: vascular endothelial growth factor receptor; FGFR: fibroblast growth factor receptor; TGF: transforming growth factor; NA: not available.
Idiopathic PAH
Idiopathic PAH is a disease that involves vascular remodelling characterised by enhanced proliferation of pulmonary artery smooth muscle cells (PASMCs) and suppressed apoptosis. Previous studies have shown that PDGF and its receptors, particularly PDGF-BB and PDGFR-β are upregulated in lung tissues and PASMCs isolated from patients and animals with PAH [47–49]. PDGF-BB induces the proliferation and migration of PASMCs and has been proposed to be a key mediator in the progression of PAH [47]. Animal models have suggested that activated PDGFR-β is a key contributor to pulmonary vascular remodelling and idiopathic PAH [50]. Patients with PAH exhibit enhanced expression and phosphorylation of the PDGF-β receptor in remodelled pulmonary arterioles, particularly at the binding sites for phosphatidyl-inositol-3-kinase (PI3K) and phospholipase C (PLC)γ at tyrosine residues 751 and 1021, respectively. Selective disruption of PDGF-dependent PI3K and PLCγ activity is sufficient to abolish these pathogenic responses in vivo, identifying these signalling events as valuable targets for antiremodelling strategies in PAH [50].
Currently available PAH specific therapies, such as phosphodiesterase type 5 inhibitors, endothelin receptor antagonists or prostacyclin, inhibit vasoconstriction and improve endothelial dysfunction. However, their impact on vascular remodelling is not strong. Such compounds are not able to significantly inhibit the hyperproliferation of PASMCs or the fibrogenesis in the vascular wall [51].
The inhibition of PDGF signalling for the treatment of PAH has been investigated in several studies after the publication of a promising case report [52]. Imatinib is a selective inhibitor of the tyrosine kinase receptors, including PDGFR-β and c-Kit and their relevant ligands PDGF-BB and stem cell factor [53]. IMPRES (Imatinib in Pulmonary Arterial Hypertension, a Randomised, Efficacy Study) investigated the safety and efficacy of the tyrosine kinase inhibitor, imatinib mesylate, in advanced PAH over 24 weeks. Improvements of exercise capacity and haemodynamics were observed, but drug discontinuations and adverse effects including nausea, oedema, diarrhoea, and subdural haematoma in this anticoagulated population were common [24]. Whether this beneficial effect of imatinib on PAH is mediated through PDGF signalling is unclear. This therapy is unlikely to be advanced, because of the associated side-effects in this population.
Sorafenib is a multikinase/angiogenesis inhibitor that inhibits Raf1 kinase, a regulator of endothelial apoptosis, and inhibits the angiogenesis growth factor receptors vascular endothelial growth factor receptor (VEGFR)-2, VEGFR-3, PDGFR-β and c-Kit [54]. Gomberg-Maitland et al. [25] investigated the safety and tolerability of sorafenib in patients with advanced yet stable PAH on parenteral prostanoids (with or without oral sildenafil). The results at 200 mg twice daily demonstrated that the treatment was safe, with the most common adverse reactions being skin reactions occurring on the hands and feet, and alopecia. This small trial did not meet efficacy goals.
Medarametla et al. [44] tested the hypothesis that a novel, nonselective inhaled PDGF receptor inhibitor, PK10453, would decrease pulmonary hypertension both in the rat monocrotaline (MCT) model and the rat MCT plus pneumonectomy (MCT+PN) model of PAH. Results showed decreased progression of PAH in the rat MCT and MCT+PN models. The authors concluded that nonselective inhibition of both PDGFR-α and PDGFR-β may have a therapeutic advantage over inhaled imatinib in PAH. In this study imatinib was 8.5-fold more selective for PDGFR-α than for PDGFR-β (IC50 71 nM versus 607 nM, respectively). Most PAH-related cell-based studies interrogating the PDGFR pathway have used high doses of imatinib (5–10 μM), which would not allow a differentiation between inhibition of the PDGFR-α and PDGFR-β isoforms.
Overall, the PDGF signalling pathway seems to be a promising therapeutic target in PAH. Future studies will be required to balance efficacy and safety in this anticoagulated population.
Lung cancer
PDGF signalling is important for tumour growth, lymphangiogenesis and angiogenesis in vivo [53–55]. Aberrant PDGF signalling has been detected in several tumours, such as chronic myelomonocytic leukaemia; glial brain tumours; prostate, breast and lung adenocarcinoma; hepatocellular carcinoma; and nonsmall cell lung cancer (NSCLC) [20, 55–58].
Tumour expression of PDGFR-α/β and PDGF-A/B in NSCLC is associated with poor prognosis [59–61]. Inhibition of PDGFR-α and-β by therapeutic antibodies in NSCLC reduces tumour mass, highlighting the importance of the PDGF/PDGFR axis for tumour growth [62]. However, PDGFR expression patterns vary significantly in different NSCLC subtypes [61]. Preclinical models have demonstrated that inhibition of PDGF signalling with imatinib improved the drug delivery and efficacy of other chemotherapeutic agents in NSCLC, emphasising the potential role of imatinib as an adjunct to small-molecule or liposomal chemotherapy [63]. However, clinical trials with imatinib, showed little or no efficacy, and no improvement in progression-free survival (PFS) [26, 59, 64].
Linifanib (ABT-869) is an orally active, selective receptor tyrosine kinase inhibitor with IC50 values in the low nanomolar range for VEGF (FLT1, KDR and FLT4) and PDGF (PDGFR-α and-β, CSF-1R, c-KIT and FLT3) receptors. A phase II study evaluated the safety and efficacy of linifanib in patients with stage IIIB/IV nonsquamous NSCLC. The study arms included carboplatin and paclitaxel with either linifanib 7.4 mg (arm A) or 12.5 mg (arm B) or placebo. Addition of linifanib to chemotherapy significantly improved PFS (arm A 5.4 months versus arm B 8.3 months; hazard ratio (HR) 0.51, p=0.02). However, there was increased toxicity for both doses reflective of known VEGF/PDGF inhibitory effects [27].
Inhibition of mTOR, a downstream molecule of the PI3K/Akt/mTOR pathway, with rapamycin, had been shown to induce growth arrest in the G1 phase of the cell cycle, and in some cases induced apoptosis in several tumour cell lines [65–68]. Jiang et al. [69] revealed the synergistic effect between mTOR complex 1/2 and glycolysis inhibitors, suggesting that the combined application of mTORC1/2 and glycolysis inhibitors may be a new promising approach to treat NSCLC. The safety and efficacy of newly developed inhibitors of mTOR, such as CCI-779, RAD001 and AP23573 in NSCLC are under clinical investigation.
Nintedanib, formerly called BIBF 1120, is a potent triple angiokinase inhibitor that targets VEGFR1/2/3, basic fibroblast growth factor receptor-1/2/3 and PDGFR-α/β signalling. Nintedanib has an interesting safety profile, as it does not lead to the typical side-effects of antiangiogenic drugs such as hypertension or hand–foot syndrome, probably because of its innovative triple-blocking mechanism of action [70]. In vitro and in vivo studies showed a marked inhibition of tumour growth in xenograft models of human NSCLC with nintedanib [28, 42]. The efficacy and safety of nintedanib in NSCLC has been evaluated in two phase III double blind, randomised, placebo-controlled clinical trials: LUME-Lung 1 and 2.
LUME-Lung 1 compared docetaxel plus placebo against docetaxel plus nintedanib. 1314 patients with stage IIIb/IV recurrent NSCLC progressing after first-line chemotherapy were included. After 7.1 months, the first preplanned primary analysis showed a higher PFS in the nintedanib arm (3.4 versus 2.7 months; HR 0.79, p=0.002). A subsequent analysis was performed at 31.7 months in order to evaluate the overall survival. These results were significant in all patients with adenocarcinoma (12.6 versus 10.3 months; HR 0.83, p=0.04). An even more significant result was observed in a subgroup of patients with adenocarcinoma who had progressed within 9 months after start of first-line therapy, achieving an improvement in overall survival of 4 months (10.9 versus 7.9 months; HR 0.75, p=0.007). The more common side-effects reported for the nintedanib plus docetaxel arm included diarrhoea (6.6% versus 2.6%), reversible increase in alanine aminotransferase (7.8% versus 0.9%), reversible increases in aspartate aminotransferase (3.4% versus 0.5%) and gastrointestinal side-effects, mostly attributed to nintedanib [29].
LUME-Lung 2 compared pemetrexed plus placebo against pemetrexed plus nintedanib in patients with stage IIIb/IV or recurrent NSCLC previously treated with chemotherapy. After randomising 713 of the 1300 planned patients, the trial was stopped due to the results of the preplanned data monitoring committee futility analysis, which suggested that the primary end-point would not be met for PFS. Ongoing patients were unblended, and follow-up continued per protocol. Subsequent intention-to-treat analysis of the primary end-point (PFS) favoured the treatment arm (median 4.4 versus 3.6 months; HR 0.83, 95% CI 0.7–0.99; p=0.04) [30].
In November 2014, the combination of nintedanib and docetaxel obtained European Medicines Agency approval as a second-line option for NSCLC patients with adenocarcinoma histology.
Lung fibrosis
Fibrotic diseases are characterised by active tissue remodelling involving proliferation of mesenchymal cell types such as myofibroblasts and accumulation of extracellular matrix components such as collagen. These lead to progressive scaring and loss of organ function. Upregulation of PDGF signalling has been linked with fibrotic diseases affecting the lung, kidney, liver, skin and heart [23, 71, 72]. Several environmental factors, such as asbestos and air pollutants, stimulate the expression of PDGFR-α in small-animal models [73–75]. An important role of PDGF signalling in the development of pulmonary fibrosis is suggested by studies of mouse models of silica, radiation and bleomycin-induced lung fibrosis [76–79]. PDGF-C is involved in the progression of pulmonary and cardiac fibrosis [79, 80].
IPF
IPF is a progressive fibrosing interstitial pneumonia of unknown cause, primarily occurring in older adults, and associated with the histopathological and/or radiological pattern of usual interstitial pneumonia. In IPF, transforming growth factor-β signal transduction promotes the expression of PDGF-B by regulatory T-cells (Tregs), which stimulate PDGF-B-mediated fibroblast proliferation [77]. Imatinib mesylate abolished fibroblast proliferation induced by Tregs in a mouse model of lung fibrosis induced by silica [77]. However, a multicentre randomised controlled trial showed that imatinib mesylate did not affect lung function or survival in patients with mild-to-moderate IPF [31]. Whether resistance to imatinib mesylate mediated by α1-acid glycoprotein, which is upregulated in patients with IPF, was responsible for this outcome is not known [81].
The most effective dose of nintedanib (BIBF 1120) in IPF was determined in a clinical phase 2 study, TOMORROW (To Improve Pulmonary Fibrosis with BIBF 1120). 432 patients underwent randomisation to receive one of four doses of BIBF 1120 (50 mg once a day, 50 mg twice a day, 100 mg twice a day or 150 mg twice a day) or placebo. The group taking the highest dose of BIBF 1120, 150 mg twice daily, showed a trend towards a slower decline in lung function and fewer exacerbations compared with placebo [32]. Based on these results, two double-blind, phase-3 clinical trials (INPULSIS-1 and INPULSIS-2) were conducted [33].
INPULSIS-1 and -2, which involved a total of 1061 patients with IPF randomly assigned to nintedanib 150 mg or placebo twice daily, met the primary end-point of reduction in the annual rate of decline in forced vital capacity (FVC) over 52 weeks. In these trials, nintedanib reduced the annual rate of FVC decline compared to placebo by 48% in INPULSIS-1 (−115 versus −240 mL·year−1, respectively; 95% CI 78–173 mL·year−1) and by 55% in INPULSIS-2 (−114 versus −207 mL·year−1, respectively; 95% CI 45–143 mL·year−1) [33].
In INPULSIS-1, there was no difference between the nintedanib and placebo groups in time to first acute exacerbation (HR 1.15, 95% CI 0.54–2.42), and the proportion of patients with at least one investigator-reported acute exacerbation was comparable between the nintedanib and placebo groups (61% versus 54%, respectively).
In INPULSIS-2, there was a significant increase in time to first acute exacerbation in the nintedanib group compared with placebo (HR 0.38, 95% CI 0.19–0.77). In addition, the proportion of patients with at least one investigator-reported acute exacerbation was lower in the nintedanib group (36%) compared with placebo (96%).
Diarrhoea was the most frequent adverse event in the nintedanib groups compared to the placebo groups, and was reported in 61.5% versus 18.6% of participants, respectively, in INPULSIS-1 and 63.2% versus 18.3% of participants, respectively, in INPULSIS-2. Nausea was the second most common adverse event among patients treated with nintedanib versus placebo, occurring in 22.7% versus 5.9% of participants, respectively, in INPULSIS-1 and 26.1% versus 7.3% of participants, respectively, in INPULSIS-2.
Pirfenidone is an antifibrotic agent that inhibits TGF-β-stimulated collagen synthesis, decreases extracellular matrix production and blocks fibroblast proliferation in vitro. It was demonstrated that pirfenidone inhibited synthesis of both PDGF-A and -B isoforms by lung macrophages [82], reduced inflammation and suppressed the bleomycin-induced increase in the levels of TGF-β [83]. How much of the antifibrotic effects are mediated through a PDGF pathway remains unclear.
Pirfenidone has been investigated for IPF since 1999 [84, 85]. ASCEND (Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis) was a phase-3 trial in 555 patients with IPF randomly assigned to receive oral pirfenidone (2403 mg per day) or placebo for 52 weeks [34]. Compared to the placebo group, pirfenidone resulted in a significant reduction in the 1-year rate of decline in FVC, but did not reduce dyspnoea.
Two concurrent, multicentre clinical trials (CAPACITY 004 and 006) studied pirfenidone in IPF; the primary end-point was change in FVC % predicted at week 72 [35]. 779 patients with mild-to-moderate IPF (i.e. FVC ≥50% pred and diffusing capacity of the lung for carbon monoxide (DLCO) ≥35% pred) were randomly assigned in a 2:1:2 ratio to oral pirfenidone 2403 mg·day−1, 1197 mg·day−1 or placebo in the 004 trial and oral pirfenidone 2403 mg·day−1 or placebo in the 006 trial. The higher dose of pirfenidone significantly decreased the percentage fall in FVC in the 004 trial (difference between groups 4.4%; p=0.001), but not in the 006 trial (difference between groups 0.6%; p=0.51). The higher dose of pirfenidone significantly reduced the decline in the 6-min walk test distance, a secondary end-point, in the 006 trial (absolute difference 32 m; p=0.0009), but not in the 004 trial.
In a prespecified analysis that pooled results of the ASCEND trial and CAPACITY 004 and 006 (n=1247 patients), pirfenidone decreased death from any cause relative to placebo (22 (3.5%) deaths in the pirfenidone group compared with 42 (6.7%) deaths in the placebo group; HR 0.52, 95% CI 0.31–0.87). As the ASCEND trial was 52 weeks in duration, the pooled survival analysis only considered data from the first 52 weeks of the CAPACITY trials (which were 72 weeks in duration). A separate pooled analysis considering all available data on all-cause mortality showed a trend favouring pirfenidone, but was not statistically significant (Kaplan–Meier estimate 0.75, 95% CI 0.51–1.11) [34].
The US Food and Drug Administration approved pirfenidone (Esbriet) and nintedanib (Ofev) for the treatment of IPF in October 2014.
Hermansky–Pudlak syndrome
Hermansky–Pudlak syndrome (HPS) is a rare autosomal recessive disorder characterised by tyrosinase-positive oculocutaneous albinism, a bleeding diathesis from platelet dysfunction and systemic complications associated with lysosomal dysfunction, including pulmonary fibrosis.
In a randomised placebo-controlled trial, treatment with pirfenidone (800 mg three times daily) for ≤44 months led to a 5% difference in the yearly rate of FVC decline in 11 pirfenidone-treated patients compared to 10 placebo-treated patients (p=0.001). Using data restricted to patients with an initial FVC of >50% pred, patients in the pirfenidone group lost pulmonary function (FVC, forced expiratory volume in 1 s, total lung capacity and DLCO) at a rate of 8% per year slower than the placebo group. The authors concluded that pirfenidone slowed the progression of pulmonary fibrosis in patients with HPS who have significant residual lung function [36].
Following these initial promising results, the National Institutes of Health conducted a second randomised controlled study which enrolled 35 subjects with HPS-1 pulmonary fibrosis; 23 subjects received pirfenidone and 12 received placebo. The study was stopped due to futility. This was after an interim analysis, performed 12 months after 30 patients were enrolled showed no statistical difference between the placebo and prifenidone groups for the rate of decline in FVC (0.7% per year less for the pirfenidone group compared with the placebo group) [37].
Connective tissue disease
PDGF signalling plays an important role during embryonic development and contributes to the maintenance of many elements of the connective tissue matrix in adults. For example, elevated expression of PDGF and PDGFR has been found in scleroderma skin and lung tissues [86].
Divekar et al. [87] demonstrated that imatinib reduced interleukin (IL)-4-producing T-cells, but increased CD41 T-cells in the lungs of patients with systemic sclerosis (SSc). Therefore, targeting PDGF signalling to control immune cell populations in lung diseases might also be important.
In earlier experiences in clinical use of imatinib, several case reports showed the favourable activity of imatinib in patients with connective tissue diseases including SSc and mixed connective tissue disease (MCTD) [88–90]. Chung et al. [88] reported two cases of SSc treated with imatinib. Both patients showed improvement of skin tightening after the use of 200 mg·day−1 of imatinib for 3 months. Sfikakis et al. [89] reported a severe case of SSc treated with 400 mg·day−1 imatinib for 6 months. In this patient FVC improved from 68.0% pred to 88.3% pred. In addition, Distler et al. [90] reported the improvement of pulmonary function and high-resolution computed tomography findings in patients with MCTD with imatinib therapy. In a phase-IIa, single-arm, open-label clinical trial, 30 patients with diffuse cutaneous SSc were treated with 400 mg of imatinib daily. Improved modified Rodnan skin scores and FVC were seen [38]. Adverse events were common, but were mild to moderate. Some trials were terminated prematurely for safety reasons [91]. The most common adverse events reported included fluid retention, weakness and nausea or vomiting. Imatinib use in severe SSc requires further study.
Lymphangioleiomyomatosis
Lymphangioleiomyomatosis (LAM) is a rare and unusual cancer that involves the lungs either as part of tuberous sclerosis complex or in a sporadic form. Growth factors such as PDGF and epidermal growth factor have been shown to enhance LAM and renal angiomyolipoma (AML) cell proliferation in vitro [92, 93]. Shiomi et al. [94] demonstrated that LAM are cells of mesenchymal origin that stain positive for PDGFR-β. Imatinib mesylate, which targets PDGF, could completely block the growth of the LAM/AML cells resulting in cell death. Cells treated with rapamycin did not undergo cell death, although growth was inhibited. These findings suggest that imatinib could be a potential therapy in the treatment of LAM. Clinical trials have not been performed.
Acute respiratory distress syndrome-associated lung fibrosis
Acute respiratory distress syndrome (ARDS) is characterised by damage to the alveolar capillary membrane, oedema formation and repair of the alveolar–capillary membrane with a varying degree of fibrosis.
A recent study on human lung fibroblasts showed that PDGF and TGF-β1 regulate ARDS-associated lung fibrosis through distinct signalling pathway-mediated activation of fibrosis-related proteins [95]. The authors suggested that treatments with both PDGF and TGF-β1 antagonists may result in better antifibrotic outcomes for lung fibrosis induced by acute lung injury. Clinical trials have not been performed.
Summary
PDGF is a growth factor involved in many different lung diseases. Although the use of multitarget tyrosine kinase inhibitors to attenuate PDGF signalling is approved for lung cancer and pulmonary fibrosis, expanded uses of newer and more specific agents will enter clinical trials and find a place in the treatment of other pulmonary diseases.
Disclosures
C. Strange ERR-0061-2017_Strange (1.2MB, pdf)
Footnotes
Support statement: C. Strange has received research funding to the Medical University of South Carolina from Novartis.
Conflict of interest: Disclosures can be found alongside this article at err.ersjournals.com
Provenance: Submitted article, peer reviewed.
References
- 1.Boström H, Gritli-Linde A, Betsholtz C. PDGF-A/PDGF alpha-receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Dev Dyn 2002; 223: 155–162. [DOI] [PubMed] [Google Scholar]
- 2.Kimani PW, Holmes AJ, Grossmann RE, et al. PDGF-Rα gene expression predicts proliferation, but PDGF-A suppresses transdifferentiation of neonatal mouse lung myofibroblasts. Respir Res 2009; 10: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boström H, Willetts K, Pekny M, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 1996; 85: 863–873. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc 2006; 81: 1241–1257. [DOI] [PubMed] [Google Scholar]
- 5.Gilbertson DG, Duff ME, West JW, et al. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF α and β receptor. J Biol Chem 2001; 276: 27406–27414. [DOI] [PubMed] [Google Scholar]
- 6.Cao R, Bråkenhielm E, Li X, et al. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-αα and -αβ receptors. FASEB J 2002; 16: 1575–1583. [DOI] [PubMed] [Google Scholar]
- 7.LaRochelle WJ, Jeffers M, McDonald WF, et al. PDGF-D, a new protease-activated growth factor. Nat Cell Biol 2001; 3: 517–521. [DOI] [PubMed] [Google Scholar]
- 8.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1997; 275: 661–665. [DOI] [PubMed] [Google Scholar]
- 9.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 1997; 385: 544–548. [DOI] [PubMed] [Google Scholar]
- 10.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501. [DOI] [PubMed] [Google Scholar]
- 11.Cai SL, Tee AR, Short, JD, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol 2006; 173: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Bajraszewski N, Wu, E. et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest 2007; 117: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005; 8: 179–183. [DOI] [PubMed] [Google Scholar]
- 14.Wu E, Palmer N, Tian Z, et al. Comprehensive dissection of PDGF-PDGFR signaling pathways in PDGFR genetically defined cells. PLoS One 2008; 3: e3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lokker NA, Sullivan CM, Hollenbach SJ, et al. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res 2002; 62: 3729–3735. [PubMed] [Google Scholar]
- 16.Li J, Hoyle GW. Overexpression of PDGF-A in the lung epithelium of transgenic mice produces a lethal phenotype associated with hyperplasia of mesenchymal cells. Dev Biol 2001; 239: 338–349. [DOI] [PubMed] [Google Scholar]
- 17.Green LS, Jellinek D, Jenison R, et al. Inhibitory DNA ligands to platelet-derived growth factor B-chain. Biochemistry 1996; 35: 14413–14424. [DOI] [PubMed] [Google Scholar]
- 18.Hawthorne T, Giot L, Blake L, et al. A phase I study of CR002, a fully-human monoclonal antibody against platelet-derived growth factor-D. Int J Clin Pharmacol Ther 2008; 46: 236–244. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki H, Yoshimatsu Y, Akatsu Y, et al. Expression of platelet-derived growth factor receptor β is maintained by Prox1 in lymphatic endothelial cells and is required for tumor lymphangiogenesis. Cancer Sci 2014; 105: 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal 2013; 11: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayson GC, Parker GJ, Mullamitha S, et al. Blockade of platelet-derived growth factor receptor-beta by CDP860, a humanized, PEGylated di-Fab′, leads to fluid accumulation and is associated with increased tumor vascularized volume. J Clin Oncol 2005; 23: 973–981. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, Vil MD, Zhang H, et al. An antibody directed against PDGF receptor β enhances the antitumor and the anti-angiogenic activities of an anti-VEGF receptor 2 antibody. Biochem Biophys Res Commun 2007; 357: 1142–1147. [DOI] [PubMed] [Google Scholar]
- 23.Heldin CH. Targeting the PDGF signaling pathway in the treatment of non-malignant diseases. J Neuroimmune Pharmacol 2014; 9: 69–79. [DOI] [PubMed] [Google Scholar]
- 24.Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation 2013; 127: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 25.Gomberg-Maitland M, Maitland ML, Barst RJ, et al. A dosing/cross-development study of the multikinase inhibitor sorafenib in patients with pulmonary arterial hypertension. Clin Pharmacol Ther 2010; 87: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauman JE, Eaton KD, Wallace SG, et al. A phase II study of pulse dose imatinib mesylate and weekly paclitaxel in patients aged 70 and over with advanced non-small cell lung cancer. BMC Cancer 2012; 12: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramalingam SS, Shtivelband M, Soo RA, et al. Randomized phase II study of carboplatin and paclitaxel with either linifanib or placebo for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2015; 33: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis PM, Kaiser R, Zhao Y, et al. Phase I open-label study of continuous treatment with BIBF 1120, a triple angiokinase inhibitor, and pemetrexed in pretreated non-small cell lung cancer patients. Clin Cancer Res 2010; 16: 2881–2889. [DOI] [PubMed] [Google Scholar]
- 29.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014; 15: 143–155. [DOI] [PubMed] [Google Scholar]
- 30.Hanna NH, Kaiser R, Sullivan RN, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-Lung 2): a randomized, double-blind, phase III trial. Lung Cancer 2016; 102: 65–73. [DOI] [PubMed] [Google Scholar]
- 31.Daniels CE, Lasky JA, Limper AH, et al. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med 2010; 181: 604–610. [DOI] [PubMed] [Google Scholar]
- 32.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011; 365: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 33.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 34.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 35.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 36.Gahl WA, Brantly M, Troendle J, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky–Pudlak syndrome. Mol Genet Metab 2002; 76: 234–242. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien K, Troendle J, Gochuico BR, et al. Pirfenidone for the treatment of Hermansky–Pudlak syndrome pulmonary fibrosis. Mol Genet Metab 2011; 103: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiera RF, Gordon JK, Mersten JN, et al. Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 1-year, phase IIa, single-arm, open-label clinical trial. Ann Rheum Dis 2011; 70: 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchdunger E, Zimmermann J, Mett H, et al. Selective inhibition of the platelet-derived growth factor signal transduction pathway by a protein-tyrosine kinase inhibitor of the 2-phenylaminopyrimidine class. Proc Natl Acad Sci USA 1995; 92: 2558–2562. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Heinrich MC, Griffith DJ, Druker BJ, et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 2000; 96: 925–932. [PubMed] [Google Scholar]
- 41.Albert DH, Tapang P, Magoc TJ, et al. Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther 2006; 5: 995–1006. [DOI] [PubMed] [Google Scholar]
- 42.Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008; 68: 4774–4782. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006; 66: 11851–11858. [DOI] [PubMed] [Google Scholar]
- 44.Medarametla V, Festin S, Sugarragchaa C, et al. PK10453, a nonselective platelet-derived growth factor receptor inhibitor, prevents the progression of pulmonary arterial hypertension. Pulm Circ 2014; 4: 82–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004; 64: 7099–7109. [DOI] [PubMed] [Google Scholar]
- 46.Sun L, Liang C, Shirazian S, et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J Med Chem 2003; 46: 1116–1119. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Lv W, Piao H, et al. Role of platelet-derived growth factor-BB (PDGF-BB) in human pulmonary artery smooth muscle cell proliferation. J Recept Signal Transduct Res 2014; 34: 254–260. [DOI] [PubMed] [Google Scholar]
- 48.Balasubramaniam V, Le Cras TD, Ivy DD, et al. Role of platelet-derived growth factor in vascular remodeling during pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 2003; 284: L826–L833. [DOI] [PubMed] [Google Scholar]
- 49.Perros F, Montani D, Dorfmüller P, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 178: 81–88. [DOI] [PubMed] [Google Scholar]
- 50.Ten Freyhaus H, Berghausen EM, Janssen W, et al. Genetic ablation of PDGF-dependent signaling pathways abolishes vascular remodeling and experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol 2015; 35: 1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antoniu SA. Targeting PDGF pathway in pulmonary arterial hypertension. Expert Opin Ther Targets 2012; 16: 1055–1063. [DOI] [PubMed] [Google Scholar]
- 52.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med 2005; 353: 1412–1413. [DOI] [PubMed] [Google Scholar]
- 53.Kadivar A, Kamalidehghan B, Akbari Javar H, et al. Antiproliferation effect of imatinib mesylate on MCF7, T-47D tumorigenic and MCF 10A nontumorigenic breast cell lines via PDGFR-β, PDGF-BB, c-Kit and SCF genes. Drug Des Devel Ther 2017; 11: 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adnane L, Trail PA, Taylor I, et al. Sorafenib (BAY 43–9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol 2006; 407: 597–612. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Liu C, Qiu L, et al. Overexpression of both platelet-derived growth factor-BB and vascular endothelial growth factor-C and its association with lymphangiogenesis in primary human non-small cell lung cancer. Diagn Pathol 2014; 9: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donnem T, Al-Saad S, Al-Shibli K, et al. Prognostic impact of platelet-derived growth factors in non-small cell lung cancer tumor and stromal cells. J Thorac Oncol 2008; 3: 963–970. [DOI] [PubMed] [Google Scholar]
- 57.Fleming TP, Saxena A, Clark WC, et al. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res 1992; 52: 4550–4553. [PubMed] [Google Scholar]
- 58.Puputti M, Tynninen O, Sihto H, et al. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res 2006; 4: 927–934. [DOI] [PubMed] [Google Scholar]
- 59.Tsao AS, Liu S, Fujimoto J, et al. Phase II trials of imatinib mesylate and docetaxel in patients with metastatic non-small cell lung cancer and head and neck squamous cell carcinoma. J Thorac Oncol 2011; 6: 2104–2111. [DOI] [PubMed] [Google Scholar]
- 60.Pietras K, Sjöblom T, Rubin K, et al. PDGF receptors as cancer drug targets. Cancer Cell 2003; 3: 439–443. [DOI] [PubMed] [Google Scholar]
- 61.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008; 455: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDermott U, Ames RY, Iafrate AJ, et al. Ligand-dependent platelet-derived growth factor receptor (PDGFR)-alpha activation sensitizes rare lung cancer and sarcoma cells to PDGFR kinase inhibitors. Cancer Res 2009; 69: 3937–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlahovic G, Ponce AM, Rabbani Z, et al. Treatment with imatinib improves drug delivery and efficacy in NSCLC xenografts. Br J Cancer 2007; 97: 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang CH, Williamson SK, Van Veldhuizen PJ, et al. Potential role of platelet-derived growth factor receptor inhibition using imatinib in combination with docetaxel in the treatment of recurrent non-small cell lung cancer. J Thorac Oncol 2011; 6: 372–377. [DOI] [PubMed] [Google Scholar]
- 65.Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 1994; 369: 756–758. [DOI] [PubMed] [Google Scholar]
- 66.Eng CP, Sehgal SN, Vézina C. Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot 1984; 37: 1231–1237. [DOI] [PubMed] [Google Scholar]
- 67.Seufferlein T, Rozengurt E. Rapamycin inhibits constitutive p70s6k phosphorylation, cell proliferation, and colony formation in small cell lung cancer cells. Cancer Res 1996; 56: 3895–3897. [PubMed] [Google Scholar]
- 68.Wiederrecht GJ, Sabers CJ, Brunn GJ, et al. Mechanism of action of rapamycin: new insights into the regulation of G1-phase progression in eukaryotic cells. Prog Cell Cycle Res 1995; 1: 53–71. [DOI] [PubMed] [Google Scholar]
- 69.Jiang S, Zou Z, Nie P, et al. Synergistic effects between mTOR complex 1/2 and glycolysis inhibitors in non-small-cell lung carcinoma cells. PLoS One 2015; 10: e0132880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caglevic C, Grassi M, Raez L, et al. Nintedanib in non-small cell lung cancer: from preclinical to approval. Ther Adv Respir Dis 2015; 9: 164–172. [DOI] [PubMed] [Google Scholar]
- 71.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 2004; 15: 255–273. [DOI] [PubMed] [Google Scholar]
- 72.Antoniades HN, Bravo MA, Avila RE, et al. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest 1990; 86: 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonner JC, Goodell AL, Coin PG, et al. Chrysotile asbestos upregulates gene expression and production of alpha-receptors for platelet-derived growth factor (PDGF-AA) on rat lung fibroblasts. J Clin Invest 1993; 92: 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lasky JA, Tonthat B, Liu JY, et al. Upregulation of the PDGF-alpha receptor precedes asbestos-induced lung fibrosis in rats. Am J Respir Crit Care Med 1998; 157: 1652–1657. [DOI] [PubMed] [Google Scholar]
- 75.Lindroos PM, Coin PG, Badgett A, et al. Alveolar macrophages stimulated with titanium dioxide, chrysotile asbestos, and residual oil fly ash upregulate the PDGF receptor-alpha on lung fibroblasts through an IL-1beta-dependent mechanism. Am J Respir Cell Mol Biol 1997; 16: 283–292. [DOI] [PubMed] [Google Scholar]
- 76.Aono Y, Kishi M, Yokota Y, et al. Role of platelet-derived growth factor/platelet-derived growth factor receptor axis in the trafficking of circulating fibrocytes in pulmonary fibrosis. Am J Respir Cell Mol Biol 2014; 51: 793–801. [DOI] [PubMed] [Google Scholar]
- 77.Lo Re S, Lecocq M, Uwambayinema F, et al. Platelet-derived growth factor-producing CD4+ Foxp3+ regulatory T lymphocytes promote lung fibrosis. Am J Respir Crit Care Med 2011; 184: 1270–1281. [DOI] [PubMed] [Google Scholar]
- 78.Abdollahi A, Li M, Ping G, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 2005; 201: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhuo Y, Zhang J, Laboy M, et al. Modulation of PDGF-C and PDGF-D expression during bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2004; 286: L182–L188. [DOI] [PubMed] [Google Scholar]
- 80.Pontén A, Li X, Thorén P, et al. Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am J Pathol 2003; 163: 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nishioka Y, Azuma M, Kishi M, et al. Targeting platelet-derived growth factor as a therapeutic approach in pulmonary fibrosis. J Med Invest 2013; 60: 175–183. [DOI] [PubMed] [Google Scholar]
- 82.Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol 1999; 276: L311–L318. [DOI] [PubMed] [Google Scholar]
- 83.Corbel M, Lanchou J, Germain N, et al. Modulation of airway remodeling-associated mediators by the antifibrotic compound, pirfenidone, and the matrix metalloproteinase inhibitor, batimastat, during acute lung injury in mice. Eur J Pharmacol 2001; 426: 113–121. [DOI] [PubMed] [Google Scholar]
- 84.Raghu G, Johnson WC, Lockhart D, et al. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label phase II study. Am J Respir Crit Care Med 1999; 159: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 85.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005; 171: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 86.Trojanowska M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology 2008; 47: Suppl. 5, v2–v4. [DOI] [PubMed] [Google Scholar]
- 87.Divekar AA, Khanna D, Abtin F, et al. Treatment with imatinib results in reduced IL-4-producing T cells, but increased CD4+ T cells in the broncho-alveolar lavage of patients with systemic sclerosis. Clin Immunol 2011; 141: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chung L, Fiorentino DF, Benbarak MJ, et al. Molecular framework for response to imatinib mesylate in systemic sclerosis. Arthritis Rheum 2009; 60: 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sfikakis PP, Gorgoulis VG, Katsiari CG, et al. Imatinib for the treatment of refractory, diffuse systemic sclerosis. Rheumatology 2008; 47: 735–737. [DOI] [PubMed] [Google Scholar]
- 90.Distler JH, Manger B, Spriewald BM, et al. Treatment of pulmonary fibrosis for twenty weeks with imatinib mesylate in a patient with mixed connective tissue disease. Arthritis Rheum 2008; 58: 2538–2542. [DOI] [PubMed] [Google Scholar]
- 91.Bournia VK, Evangelou K, Sfikakis PP. Therapeutic inhibition of tyrosine kinases in systemic sclerosis: a review of published experience on the first 108 patients treated with imatinib. Semin Arthritis Rheum 2013; 42: 377–390. [DOI] [PubMed] [Google Scholar]
- 92.Goncharova EA, Goncharov DA, Spaits M, et al. Abnormal growth of smooth muscle-like cells in lymphangioleiomyomatosis: role for tumor suppressor TSC2. Am J Respir Cell Mol Biol 2006; 34: 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lesma E, Grande V, Carelli S, et al. Isolation and growth of smooth muscle-like cells derived from tuberous sclerosis complex-2 human renal angiomyolipoma: epidermal growth factor is the required growth factor. Am J Pathol 2005; 167: 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shiomi T, Anguiano V, Chada KK, et al. Lymphangiomyomatosis: a disease of mesenchymal progenitor cells. Am J Respir Crit Care Med 2014; 189: A2127. [Google Scholar]
- 95.Deng X, Jin K, Li Y, et al. Platelet-derived growth factor and transforming growth factor β1 regulate ARDS-associated lung fibrosis through distinct signaling pathways. Cell Physiol Biochem 2015; 36: 937–946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
C. Strange ERR-0061-2017_Strange (1.2MB, pdf)