Abstract
The mechanism of cardiovascular diseases (CVDs) is complex and threatens human health. Cardiomyocyte death is an important participant in the pathophysiological basis of CVDs. Ferroptosis is a new type of iron-dependent programmed cell death caused by excessive accumulation of iron-dependent lipid peroxides and reactive oxygen species (ROS) and abnormal iron metabolism. Ferroptosis differs from other known cell death pathways, such as apoptosis, necrosis, necroptosis, autophagy and pyroptosis. Several compounds have been shown to induce or inhibit ferroptosis by regulating related key factors or signalling pathways. Recent studies have confirmed that ferroptosis is associated with the development of diverse CVDs and may be a potential therapeutic drug target for CVDs. In this review, we summarize the characteristics and related mechanisms of ferroptosis and focus on its role in CVDs, with the goal of inspiring novel treatment strategies.
Subject terms: Cell death, Cardiovascular diseases
Facts
Ferroptosis is a new type of iron-dependent programmed cell death.
The biological characteristics of ferroptosis include abnormal lipid peroxidation and ROS production.
Iron metabolism disorder is an important factor in inducing ferroptosis.
Open Questions
What are the characteristics of different forms of cell death?
What mechanism is responsible for the production of ferroptosis?
Are there any inducers or inhibitors that can target ferroptosis?
How does ferroptosis participate in diverse cardiovascular diseases?
Introduction
As the power source of blood flow, the heart can transport blood to all parts of the body and provide oxygen and nutrition to other organs and tissues. It is one of the most important organs in the human body. However, with an unhealthy diet structure and lifestyle, as well as the aggravation of ageing, the incidence and mortality of CVDs are increasing each year, especially in developing countries, and CVDs have become the number one killer [1]. CVDs mainly include myocardial infarction (MI), reperfusion injury, atherosclerosis (AS), hypertension, myocardial hypertrophy, heart failure (HF), diabetic cardiomyopathy (DCM) and doxorubicin (DOX)-induced cardiomyopathy (DIC) [2]. Cardiomyocytes make up the largest proportion of mammalian heart tissue, accounting for three-quarters of the total volume of the heart. The state of cardiomyocytes also affects individual heart function to a certain extent. It is worth noting that, in adult mammals, the proliferation ability of cardiomyocytes in vivo becomes limited, and external adverse factors will dominate the fate of cardiomyocytes. Cell death is a stress response to stimulation by external damage factors. Cardiomyocyte death is involved in regulating cardiac development, senescence and homeostasis, which has important physiological significance [3]. Among them, the common forms of cell death mainly include apoptosis, necrosis, necroptosis, autophagy, pyroptosis and ferroptosis, which were discovered in recent years. A sophisticated regulatory network controls most myocardial cell death [4]. Apoptosis is mainly characterized by cell atrophy, an increase in cytoplasmic density, the disappearance of mitochondrial membrane potential (MMP) and a change in permeability [5], leading to a complete apoptotic body. Necrosis is usually an unexpected and unregulated form of cell death after physical or chemical damage [6]. Necroptosis is also regulated by specific signalling networks. The death receptor TNFR1 plays a key role in the process of necroptosis [7]. Autophagy is a prosurvival mechanism that transfers unwanted or damaged cellular components to lysosomes for degradation and plays an important role in maintaining intracellular metabolic homeostasis [8]. Pyroptosis is considered to be an inflammatory and regulated form of cell death that usually occurs in the defence against exogenous pathogens, such as viruses, bacteria and fungi [9].
The human body contains iron as one of the important elements. Iron in the body exists as haemoglobin (approximately 72%), myoglobin (3%), and other compounds (0.2%), and the rest is reserve iron (25%), which is stored as ferritin in the liver, spleen and bone marrow [10]. Iron is involved in metabolic processes and a variety of life activities, including oxygen transport, cell respiration and electron transfer, DNA synthesis, and immune regulation [11]. The abnormal metabolism of iron leads to disorders of many physiological functions. Ferroptosis was first proposed by Brent R. Stockwell et al., and it was considered an iron-dependent form of cell death [12]. Ferroptosis is characterized by the accumulation of lipid peroxides to lethal levels, resulting in oxidative damage to cell membranes [13]. Ferroptosis is distinct from other forms of cell death in terms of morphology and mechanism (Table 1). An increasing number of studies have reported that ferroptosis plays an important role in CVDs [14, 15]. In this review, we introduce the mechanism of ferroptosis and focus on the research progress of ferroptosis in CVDs to provide ideas for novel treatment strategies.
Table 1.
Differences in diverse types of cell death.
| Cell death forms | Morphological characteristics | Biological characteristics | Immunological effects | Key factors | Classification |
|---|---|---|---|---|---|
| Apoptosis | Plasma membrane blistering; Chromatin shrinkage and nuclear fragmentation; Apoptosis bodies | DNA fragments; Activation of the caspase family; Phosphatidylserine exposure | Anti-inflammatory | Caspase family; BCL2 family; Cytc; p53 | Programmed cell death |
| Necrosis | Cell swelling; Plasma membrane rupture; Cell content release | ATP depleted; mPTP on | Promote inflammation | Unknown | Accidental cell death |
| Necroptosis | Cell swelling; Plasma membrane rupture; Cell content release; Moderate chromatin condensation | ATP decreased; Activated levels of RIP1, RIP3 and MLKL phosphorylation; ROS production | Promote inflammation | RIPK1; RIPK3; MLKL | Programmed cell death |
| Autophagy | Accumulation of double-membraned autolysosomes | p62 degradation; LC3-I to LC3-II conversion; Enhanced autophagic flux and lysosomal activity | Anti-inflammatory | AMPK; mTOR; ATG5; ATG7; Beclin 1 | Programmed cell death |
| Pyroptosis | Cell swelling (less than necrosis); Pyrosomes form before rupture of the plasma membrane; Cell content release; Mitochondrial integrity unaffected | Activated caspase-1 and GSDMDN; GSDMDN induces pore formation and IL-1β release | Promote inflammation | Caspase1; Caspase11; GSDMD | Programmed cell death |
| Ferroptosis | Mitochondria are small, and the mitochondrial cristae shrink or disappear, accompanied by rupture of the outer membrane and electron density of the mitochondria. The structure of the plasma membrane and nucleus is intact, and the morphological structure of chromatin remains unchanged. | GPX4 and xCT are inhibited; Deletion of GSH; Iron-dependent lipid peroxidation; ROS production | Promote inflammation | GPX4; Nrf2; TFRC; ACSL4; SLC7A11; NCOA4;Hmox1; p53 | Programmed cell death |
Overview of ferroptosis mechanisms
Brent R. Stockwell et al. previously proposed the term ferroptosis in 2012 [12]. They found that erastin induces cell death in Rasv12 cells, an unknown form of cell death that is distinct from apoptosis. Experiments confirmed that Ras-selective lethal (RSL) compound could also induce the cell death phenotype, and the use of apoptosis, necroptosis, autophagy, and pyroptosis inhibitors could not improve the cell death induced by RSL. However, an iron-chelating agent could inhibit this process. This novel form of cell death is therefore considered iron-dependent [16].
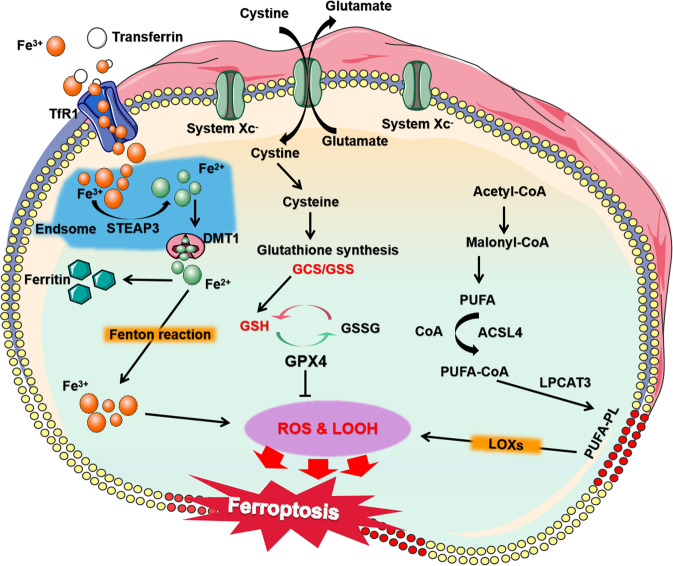
Iron, as an important cofactor in the metabolic process of many enzymes and catalysts for REDOX cycle reactions, participates in diverse key physiological and biochemical processes in vivo [11]. Different physiological conditions and pathological stress may lead to ferroptosis. Among them, abnormal iron metabolism and lipid peroxidation are important factors to induce ferroptosis, and the active state of System Xc− and Glutathione peroxidase 4 (GPX4) is the key mechanism of regulating ferroptosis. Here, we summarize and elaborate on the regulatory mechanism of ferroptosis (Fig. 1).
Fig. 1. The mechanisms of ferroptosis.
Ferroptosis is caused by excessive accumulation of lipid peroxide, ROS production and abnormal iron metabolism. Excessive Fe2+ accumulation will cause ROS production and lipid peroxidation. System Xc− can control the transport of cystine to cells and affect GPX4 activity. GPX4 is a key molecule for endogenous inhibition of lipid peroxidation. The arrows indicate promotion, and the blunt-ended lines indicate inhibition. STEAP3 six-transmembrane epithelial antigen of prostate 3, DMT1 divalent metal transporter 1, TfR1 transferrin receptor 1, GSSG glutathione disulfide, ROS reactive oxygen species, LOOH lipid peroxides.
Iron metabolism
Iron, a basic element in vivo, is indispensable for life activities as it is involved in the synthesis of many important proteins and enzymes [17]. Intracellular iron overload caused by abnormal iron metabolism is one of the important steps of ferroptosis [18]. In in vivo circulation, iron is mainly in the form of ferric ions (Fe3+). Fe3+ binds to transferrin and is specifically recognized and transported intracellularly by membrane transferrin receptor 1 (TfR1). Under the action of the six-transmembrane epithelial antigen of prostate 3 (STEAP3), it is reduced to ferrous ion (Fe2+), and with the help of divalent metal transporter 1 (DMT1), Fe2+ is then released into the cytoplasmic unstable iron pool [19]. In addition to storing Fe2+, the iron pool can also store ferric proteins induced by REDOX reactions, such as heme. Ferroportin mediates intracellular iron output to maintain the dynamic balance of iron, and excess iron will remain intracellular as ferritin.
Ferritin usually exhibits non-REDOX activity to prevent cell damage caused by iron overload. However, excessive iron can lead to the accumulation of ROS and induce ferroportin in cells through Fenton and Haber-Weiss reactions [20, 21]. Erastin treatment of H-RasV12 mutant fibrosarcoma cells induces upregulation of TfR1, resulting in increased iron intake. Intracellular ferritin heavy-chain 1 (Fth1) and ferritin light-chain 1 (Ftl1) downregulation also leads to iron overload. Low expression of nuclear receptor coactivator 4 (NCOA4) or autophagy-related (ATG) genes inhibits ferritin degradation and reduces free iron levels, thus limiting oxidative damage caused by ferroptosis [22, 23]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important transcription factor that regulates the cellular oxidative stress response and is also a central regulator of maintaining intracellular redox homeostasis [24]. When Nrf2 is activated, the storage of iron increases and inhibits oxidative stress, which blocks ferroptosis [4]. In the mouse cardiomyopathy model induced by DOX, the expression of haem oxygenase-1 (Hmox1) was upregulated, which sped up the degradation of haem and the release of free iron, resulting in ferroptosis and myocardial injury [25]. When the transferrins are exhausted, the metal transporter Slc39a14 can act as a nontransferrin bound iron (NTBI) transporter to introduce iron into cells and induce ferroptosis and tissue fibrosis [26]. Sun et al. found that heat shock protein β-1 (HSPb1) plays an important role in iron metabolism [27]. Protein kinase C can mediate the phosphorylation of HSPb1 and reduce iron-mediated ROS production to resist ferroptosis.
Lipid peroxidation
Lipid peroxidation is an important marker of ferroptosis. Excessive production of lipid peroxides can lead to loss of stability of the lipid bilayer and disintegration of the cell membrane. The degree of the unsaturated lipid bilayer affects the sensitivity of cells to ferroptosis [28]. Among them, polyunsaturated fatty acids (PUFAs) are most susceptible to peroxidation. The location and content of PUFAs determine the severity of ferroptosis by affecting the degree of intracellular lipid peroxidation. PUFA is attached to the sn-2 site of phospholipids by acyl-coenzyme A (acyl-CoA)-mediated esterification. Acyl-CoA synthase long-chain family member 4 (ACSL4) catalyses the binding of long-chain-PUFA (LC-PUFA) and adrenergic acid to CoA to form PUFA-CoA, which facilitates the entry of LC-PUFA into lipids and membranes [29]. Then, it is esterified into anionic membrane phospholipids by lysophosphatidylcholine acyltransferase 3 (LPCAT3), which changes the remodelling of membrane phospholipids and affects cell ferroptosis [30]. Tammo et al. found that inhibition of ACSL4 could reduce phospholipid-PUFA and inhibit ferroptosis induced by RSL3 [31]. In addition, PUFAs are easily attacked by lipoxygenase (LOX) in chemical structures, resulting in lipid peroxidation and ROS production. Therefore, inhibiting LOX activity may help us treat the damage caused by ferroptosis.
System Xc−
System Xc−, a cysteine/glutamate reverse transporter on the cell membrane, is mainly composed of substrate-specific subunit SLC7A11 and regulatory subunit SLC3A2. It has been found that System Xc− is an upstream target regulating ferroptosis [32]. System Xc− can exchange extracellular cystine with intracellular glutamate (ratio 1:1). Then, cystine is reduced to cysteine to synthesize the antioxidant GSH. Extracellular high concentrations of glutamate and some compounds, such as erastin, analogues, and sorafenib, can be used as inhibitors of System Xc− to consume intracellular cysteine, reduce GSH concentration, cause oxidative stress, increase ROS production, and lead to ferroptosis. Regulating the stability or activity of the System Xc− subunit may be an effective way to regulate cell ferroptosis in the future. Liu et al. found that the ubiquitin hydrolase OTUB1 could control the stability of SLC7A11 and thus regulate cell ferroptosis [33]. In vascular smooth muscle cells (VSMCs), the expression of SLC3A2 helps to stabilize plaque formation and reduce the risk of atherosclerotic thrombosis [34].
GPX4
GPX4, one of the peroxidase enzymes of GSH, is an important specific marker of ferroptosis. It can maintain the REDOX homeostasis of cells by catalysing the reduction of lipid peroxides or the conversion of free hydrogen peroxide into water, thus protecting cells from oxidative damage [35]. As a specific substrate of GPX4, RSL3 can bind to GPX4 and inactivate it, induce the accumulation of lipid ROS and lead to ferroptosis. Overexpression of GPX4 decreases the sensitivity of RSL3-induced cell ferroptosis [36]. Zhang et al. found that high glucose-induced ferroptosis and cell damage can be regulated by TRIM46 promotion via GPX4 ubiquitin [37]. Recently, Mao et al. revealed that dihydroorotate dehydrogenase (DHODH) could interact with mitochondrial GPX4 to mediate ferroptosis, opening a novel perspective for the mitochondrial pathway of the ferroptosis defence mechanism [38]. Palmitic acid (PA) can reduce the expression levels of heat shock factor 1 (HSF1) and GPX4 in H9c2 cardiomyocytes in a time-dependent and dose-dependent manner. Overexpression of HSF1 can restore intracellular iron homeostasis by regulating iron metabolism-related genes, promoting GPX4 expression and healing the sensitivity of cardiomyocytes to ferroptosis. However, knocking down GPX4 reversed this effect [39].
Inducers and inhibitors
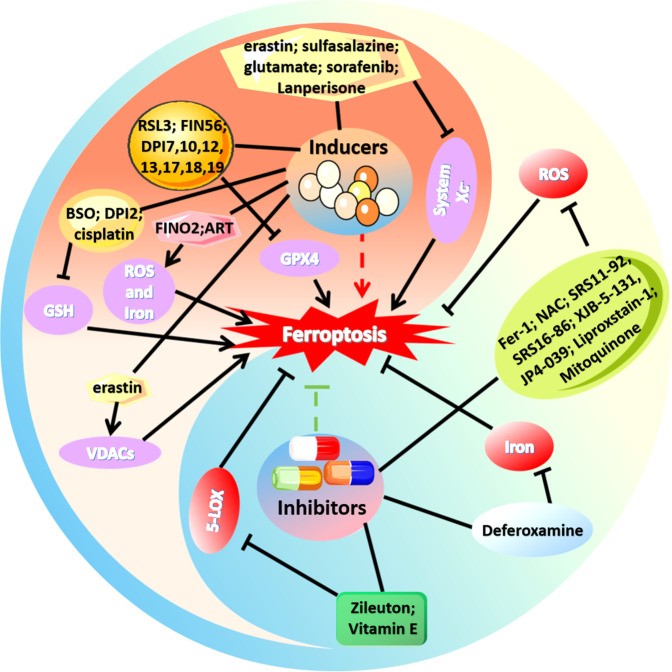
Ferroptosis is an important form of cell death that is different from other types of cell death in morphology and biochemistry. The mechanism of ferroptosis involves many key factors and signalling pathways. Regulating the decomposition and synthesis of some key molecules can change the sensitivity of cells to ferroptosis. Reasonable induction or inhibition of cell ferroptosis is helpful in improving and treating tumours and CVDs. Several drugs or compounds have been found to induce or inhibit ferroptosis (Fig. 2). According to different targets, ferroptosis inducers can be divided into the following categories: (1) Targeting iron ions and ROS (iron ion oxidation, inactivation of GPX4, induction of ferritin autophagy); (2) targeting System Xc− (inhibit System Xc− activity and prevent GSH synthesis); (3) targeting GSH (reduce glutathione synthesis and inactivate GPX4 by binding with GSH); (4) targeting GPX4 (degrade GPX4 and inhibit GPX4 activity); and (5) targeting voltage-dependent anion channels (VDACs) (reduce GPX4). Inhibitors of ferroptosis can be divided into: (1) targeting iron ions (chelating excess iron); (2) targeting ROS (preventing lipid peroxidation from producing ROS, removing intracellular ROS and inhibiting mitochondrial superoxide generation); and (3) targeting LOX (maintaining cell redox homeostasis). However, the targets and potential applications of these inducers or inhibitors still need to be further studied. In addition, for some compounds with multiple targets, further elucidating their mechanisms, exploring the feasibility of drug combination, and developing more specific inducers or inhibitors will provide better application prospects for clinical treatment.
Fig. 2. Inducers and inhibitors of ferroptosis.
Iron accumulation and ROS production are important signs of ferroptosis. Inhibiting the activity of System Xc− can reduce cystine transport into cells and reduce the synthesis of intracellular GSH, resulting in a decreased ability of GPX4 to scavenge peroxide, increased accumulation of lipid peroxide in cells, and ferroptosis. GSH is an important cofactor of GPX4 activity. GPX4 can scavenge peroxide and maintain the dynamic circulation of GSH in cells, which is a central regulator of ferroptosis; VDAC is located in the outer membrane of mitochondria. Its closure can inhibit the function of mitochondria, change the permeability of the mitochondrial membrane and trigger ferroptosis. LOX-overexpressing cells tend to undergo lipid peroxidation and ROS production and are sensitive to ferroptosis. The arrows indicate promotion, and the blunted lines indicate inhibition.
Ferroptosis with CVDs
The pathological mechanism of CVDs is complex, and many cell death types are involved. In recent years, ferroptosis has been proven to play an important role in CVDs in continuous studies [40]. Researchers usually assess the impact of ferroptosis in related CVDs by regulating key factors associated with ferroptosis and intervening in the sensitivity of cells to ferroptosis. Here, we summarize the association between various CVDs and ferroptosis (Table 2), such as MI, reperfusion injury, AS, hypertension, myocardial hypertrophy, HF, DCM and DIC.
Table 2.
The role of ferroptosis in CVDs.
| Disease | Factors | Mechanism | Role | Reference |
|---|---|---|---|---|
| MI | miR-23a-3p | Inhibit DMT1 expression | Inhibit ferroptosis and reduce myocardial injury | [47] |
| BACH1 | Adjust the threshold of iron ion induction | Inhibit ferroptosis | [48] | |
| Reperfusion injury | C3G | Decreased Fe2+, downregulated TfR1 expression, upregulated Fth1 and GPX4 expression | Inhibit ferroptosis and reduce myocardial injury | [57] |
| Res | Decreased Fe2+, downregulated TfR1 expression, upregulated Fth1 and GPX4 expression | Inhibit ferroptosis and reduce myocardial injury | [58] | |
| Lip-1 | Reduce VDAC1 level and increase GPX4 level | Reduce I/R injury | [126] | |
| Eto | Induced Nrf2 nuclear translocation | Inhibit I/R-induced ferroptosis, improve fibrosis | [127] | |
| AS | SIRT1 | Reduce IL-1β and IL-18 levels | Inhibit ferroptosis and limit AS development | [64] |
| PDSS2 | Activate Nrf2, inhibit ROS release and reduce iron levels | Promote the proliferation of HCAECs and limit AS development | [65] | |
| miR-17-92 | Targeting zinc lipoprotein A20 reduces Acsl4 expression and ROS accumulation | Inhibit ferroptosis | [66] | |
| CD98hc | Unknown | Promote VSMCs proliferation and prevent atherosclerotic thrombosis | [34] | |
| Hypertension | Monocrotaline (MCT) | Activate the the HMGB1/TLR4/NLRP3 inflammatory pathway | Promote ferroptosis | [73] |
| Celastrol | Increase HO-1 expression and decrease ROS production | Reduce inflammation and oxidative stress in VSMCs caused by hypertension | [74] | |
| CA | Regulate iron metabolism | Improve PAH | [76] | |
| Elabela | Regulate the IL-6/STAT3/GPX4 signalling pathway | Inhibit AngII-induced ferroptosis in poor myocardial remodelling, fibrosis and cardiac dysfunction | [79] | |
| Myocardial hypertrophy | DHA | Increase IRF3-SLC7A11, decrease ALOX12 and iron levels | Inhibit ferroptosis | [128] |
| miR-351 | Regulate the JNK/p53 signalling pathway | Inhibit ferroptosis and improve fibrosis | [84] | |
| LncRNA AAB | Sponge miR-30b-5p, induced imbalance of MMP9/TIMP1 and enhanced TfR-1 | Promote ferroptosis | [86] | |
| HF | CD147 | Activate TRAF2-TAK1 signalling pathway | Promote cardiac remodelling and dysfunction | [91] |
| TLR4 and NOX4 | Unknown | Inhibit cardiac autophagy and ferroptosis in HF rats | [129] | |
| DCM | TRIM46 | Promote GPX4 ubiquitination | Resist cell damage caused by high glucose | [37] |
| PA | Reduce HSF1 and GPX4 | Promote ferroptosis and enhance endoplasmic reticulum stress | [39] | |
| DIC | EMPA | Participate in NLRP3- and MYD88-related pathways | Inhibit ferroptosis, fibrosis, apoptosis and inflammation | [99] |
| AsIV | Activate Nrf2 signalling pathway and increase GPX4 expression | Inhibit ferroptosis and improve fibrosis | [100] | |
| Sepsis | LPS | Activate NCOA4 and SFXN1, increase free iron | Cause mitochondrial damage and promote ferroptosis | [108] |
| Dexmedetomidine | Reduce HO-1 expression, increase GPX4 expression | Reduce sepsis-induced myocardial cell damage | [107] | |
| Stroke | NCOA4 | USP14 upregulates NCOA4 through deubiquitination | Silencing NCOA4 can eliminate the ferritinophagy induced by I/R injury | [102] |
| CDKN1A | C9orf106/C9orf139-miR-22-3p-CDKN1A axes | Regulate ferroptosis during IS progression | [104] | |
| JUN | GAS5-miR-139-5p/miR-429-JUN axes | |||
| HIF-1α | Inhibits ACSL4 expression in early IS | Against ferroptosis | [105] |
MI
MI refers to injury caused by acute and/or continuous ischaemia and hypoxia of the coronary artery. At present, MI has gradually become one of the main causes of death in patients with CVDs worldwide. Previous reports hold that the adverse consequences of MI mainly include cardiomyocyte apoptosis, necrosis and autophagy [41–43]. However, recent studies have found that the expression of GPX4 is significantly decreased in the early and middle stages of MI [44], suggesting that MI may lead to ferroptosis in myocardial cells. Meanwhile, the downregulation of GPX4 during MI contributes to the ferroptosis of cardiomyocytes under metabolic stress, such as cysteine deprivation [45]. Zhao et al. found that human umbilical cord-derived mesenchymal stem cell exosomes could alleviate acute myocardial infarction (AMI) injury [46]. Song et al. found that the expression of DMT1 was significantly increased in mouse models of AMI and hypoxia-injured myocardial cells [47]. Human umbilical cord blood-derived mesenchymal stem cell exosomes may inhibit DMT1 expression by targeting miR-23a-3p, thereby inhibiting ferroptosis and alleviating MI [47]. The transcription factors BTB and CNC homology 1 (BACH1) are thought to promote ferroptosis at the transcriptional level, and BACH1−/− mice are more resistant to MI than wild-type mice [48]. In addition, ferroptosis often triggers inflammation and leads to the aggravation of cardiac dysfunction and poor myocardial remodelling after MI [49]. Therefore, inhibiting ferroptosis of cardiomyocytes may be a novel avenue for the treatment of MI to improve cardiac function.
Reperfusion injury
As an important risk factor for CVDs, ischaemia/reperfusion (I/R) injury seriously threatens human life and health [49]. Percutaneous coronary intervention is usually used for MI patients to restore blood flow [50]. Unfortunately, reperfusion may cause further damage to the patients’ heart [51]. Intracellular acidification, anaerobic glucose metabolism and ROS accumulation are involved in the pathological process of I/R damage, and this series of oxidative stress reactions further catalyses the process of lipid peroxidation [52]. Tang et al. hold that ferroptosis occurs mainly in the phase of myocardial reperfusion but not ischaemia [53]. Iron overload in coronary artery flow after I/R leads to the attenuation of cardiac function and the aggravation of myocardial oxidative injury [54]. In the simulated I/R model established by Euncheol et al., ferrostatin-1 (Fer-1) significantly reduced cell death, suggesting that reperfusion injury may cause ferroptosis, which is associated with I/R-induced cell death in vivo [55]. Cyanidin-3-glucoside (C3G), a member of the anthocyanin family, is widely distributed in purple or red vegetables and fruits and has anti-inflammatory, antioxidant, and heart-protecting effects [56]. Shan et al. found that C3G could inhibit ferroptosis in cardiomyocytes by decreasing Fe2+ content, downregulating TfR1 expression, and upregulating Fth1 and GPX4 expression, ultimately playing a role in preserving cardiac function [57]. Interestingly, Li et al. found that resveratrol (Res) has a similar function [58]. I/R injury produces oxidized phosphatidylcholines (OxPCs), a bioactive phospholipid intermediate that disrupts mitochondrial bioenergy and calcium transients and triggers cell death through iron overload. Fer-1 or E06 can neutralize OxPCs and prevent cell death during reperfusion [59].
AS
AS is a chronic progressive and inflammatory artery disease with an intricate pathogenesis, in which dyslipidaemia is the main risk factor, and oxidative stress is a key initiating factor [60]. Endothelial cell dysfunction or death is affected by intracellular lipid peroxides and participates in the regulation of AS [61]. Usually, oxidized low-density lipoprotein (ox-LDL) can induce AS in vitro [62]. Macrophages phagocytose a large number of ox-LDL through surface scavenger receptors, forming foam cells, which are the early lesions of AS [63]. SIRT1 can inhibit ferroptosis of foam cells caused by iron overload through autophagy while reducing the levels of IL-1β and IL-18 and limiting the development of AS [64]. By activating the antioxidant Nrf2, propylene diphosphate synthase subunit 2 (PDSS2) can limit ROS release and iron content to inhibit ferroptosis. Meanwhile, it promotes the proliferation of human coronary artery endothelial cells (HCAECs) and ultimately inhibits the progression of AS [65]. High levels of miR-17-92 in human umbilical vein endothelial cells (HUVECs) can inhibit erastin-induced ferroptosis by targeting zinc lipoprotein A20 to reduce the expression of Acsl4 and the accumulation of ROS [66]. Fer-1 can inhibit the excessive accumulation of iron, alleviate lipid peroxidation and increase the activity of mouse aortic endothelial cells (MAECs) by upregulating the levels of SLC7A11 and GPX4 [67].
Hypertension
Hypertension is characterized by increased blood pressure in systemic circulation arteries, accompanied by abnormal functions of the heart, brain and kidney [68, 69]. Hypertension is one of the most common chronic diseases and the main risk factor for CVDs [70]. Inflammation triggers vascular remodelling, pulmonary vascular remodelling and increased pulmonary vascular resistance, leading to pulmonary hypertension (PH) [71, 72]. The HMGB1/TLR4 signalling pathway can activate inflammatory bodies in NLRP3 in PH rats, leading to inflammatory ferroptosis, which can be rescued by Fer-1 [73]. Celastrol alleviates cellular inflammation and oxidative stress caused by hypertension through HO-1 induction [74]. Cinnamaldehyde (CA) can regulate vasodilation and resist hypertension caused by insulin deficiency [75]. Zou et al. speculated that celastrol and CA may contribute to the treatment of idiopathic pulmonary arterial hypertension (IPAH) by targeting the iron metabolic pathway [76]. Hydrostatic pressure is one of the main biomechanical forces of blood vessels and plays a key role in the occurrence and development of hypertension [77]. High HP can downregulate Cythionine γ-lyase/H2S in VSMCs; trigger a decrease in GSH levels; and increase iron accumulation, ROS production and lipid peroxidation, which results in aggravation of VSMC dysfunction caused by ferroptosis [78]. Elabela antagonizes cardiac microvascular endothelial cell (CMVEC) ferroptosis by regulating the IL-6/STAT3/GPX4 signalling pathway and improves adverse myocardial remodelling fibrosis and cardiac dysfunction in hypertensive mice [79]. Zhang et al. suggested that the signalling network between miRNAs and transcription factors may be involved in regulating PAH-related ferroptosis, providing a new view to treat hypertension in the future [80].
Myocardial hypertrophy and HF
HF is one of the leading causes of death worldwide, and its prevalence continues to grow [81, 82]. Adverse cardiac remodelling characterized by pathological myocardial hypertrophy and myocardial fibrosis caused by various extracellular stimuli will eventually develop into HF [83]. The prevention and treatment of pathological myocardial hypertrophy are effective means for the treatment of HF. Mixed lineage kinase 3 (MLK3), a member of the MAP3K family, induces pyroptosis by regulating inflammatory responses mediated by the NF-κB/NLRP3 signalling pathway. Oxidative stress mediated by the JNK/p53 signalling pathway leads to ferroptosis. Pyroptosis and ferroptosis induced by MLK3 lead to the aggravation of myocardial hypertrophy and myocardial fibrosis, contributing to the progression of chronic heart failure (CHF) [84]. Angiotensin II (Ang II) is an important component of the renin angiotensin aldosterone system (RAAS), which can induce cardiomyocyte hypertrophy and reduce xCT expression. Inhibition of xCT exacerbates AngII-induced cardiac hypertrophy and increases the levels of Ptgs2, a biomarker of ferroptosis, malondialdehyde and ROS [85]. LncRNA AAB is upregulated in Ang II-treated CMVECs, which induces MMP9/TIMP1 imbalance, enhances TfR-1 expression and promotes ferroptosis by sponging miR-30B-5p [86]. Patients with HF usually have symptoms of iron deficiency. Systemic iron deficiency leads to reduced iron content in the myocardium, causing insufficient oxygen delivery and erythropoietin resistance, which is one of the risk factors for poor prognosis of patients [87]. Treatment with iron supplements can improve symptoms to some extent, but the exact mechanism is not clear, and it may have potential risks [88]. In Fth-deficient cardiomyocytes, Slc7a11 expression and iron levels were decreased, oxidative stress was increased, and the heart showed mild ageing damage. Because of the low expression of Slc7a11, mice have insufficient ability to regulate iron homeostasis, and a high-iron diet leads to significant ferroptosis characteristics and causes severe heart injury and hypertrophic cardiomyopathy [89]. CD147 is a transmembrane glycoprotein receptor that activates matrix metalloproteinases and promotes inflammation [90]. Zhong et al. demonstrated that CD147 promotes pathological cardiac remodelling and dysfunction in a glycation-dependent manner by binding to the adapter protein TRAF2 and activating the downstream TAK1 signalling pathway, which is accompanied by increased oxidative stress and ferroptosis [91].
DCM
Diabetes affects cardiac structure and function through a variety of mechanisms, such as metabolic disorders, calcium transport defects, microangiopathy, interstitial fibrosis and cardiac autonomic neuropathy [92]. Oxidative stress is the main cause of DCM, and myocardial hypertrophy and fibrosis are some of its main clinical features [93]. Nrf2 is the main regulator of the cellular REDOX state and detoxification. It can maintain metabolic and redox homeostasis by controlling the expression of specific genomes involved in iron and lipid metabolism and redox balance in the heart [24, 94]. Type 1 diabetes (T1D) causes abnormal cardiac autophagy and interrupts the balance of metabolism and REDOX controlled by Nrf2, inducing ferroptosis of cardiomyocytes, which further aggravates the progression of DCM [94]. At the same time, diabetic patients will produce NOX2-related oxidative stress in an AMPK-dependent way, resulting in ferroptosis and aggravating myocardial I/R injury [95]. Li et al. found that inhibiting endoplasmic reticulum oxidative stress can reduce ferroptosis and cell damage. I/R damage in diabetic mice can be reduced by blocking ferroptosis, which provides a novel therapeutic strategy for myocardial injury [96].
DIC
DIC is a life-threatening progressive cardiomyopathy caused by DOX cardiotoxicity [97]. DOX is a chemotherapeutic drug for patients with malignant tumours. Its cardiotoxicity can cause ferroptosis and mitochondrial dysfunction [98]. Vincenzo et al. found that EMPA inhibited DOX-induced ferroptosis, myocardial fibrosis and inflammation by participating in NLRP3 and MyD88-related pathways and significantly improved cardiac function in mice [99]. Luo et al. found that astragaloside IV (AsIV) significantly improved DOX-induced myocardial fibrosis and cardiac dysfunction in rats, which may play a role by activating Nrf2 signalling and increasing GPX4 expression [100]. DOX reduces GPX4 expression and induces excessive lipid peroxidation in mitochondria through the DOX-Fe2+ complex, resulting in mitochondrial-dependent ferroptosis [98]. Fang et al. confirmed that DOX upregulates Hmox1 through Nrf2-mediated regulation and rapid systematic accumulation of nonheme iron-induced DIC in mice [25]. Targeting mitochondrial oxidative damage may be an effective protective strategy to rescue cardiomyocyte ferroptosis in patients with DIC in the future.
Stroke
Stroke is an acute cerebrovascular disease that causes brain tissue damage due to insufficient blood supply following cerebral vascular occlusion [101]. Stroke usually causes hemiplegia and disturbance of consciousness, with high mortality and disability rates. It is a global problem that seriously threatens human health. In a model of neuronal I/R injury, ubiquitin-specific peptidase 14 (USP14) increased the expression of NCOA4 in the cytoplasm through deubiquitination. Silencing NCOA4 can eliminate the ferritinophagy induced by I/R injury, thus inhibiting ferroptosis [102]. In brain microvascular endothelial cells (BMVECs) of diabetic animals, iron can increase ROS levels and ferroptosis, which can be prevented by iron chelated deoxyferriamine (DFX) [103]. Fan et al. found that CDKN1A/JUN may be a promising biomarker for the diagnosis of IS and regulate ferroptosis during IS progression through the C9orf106/C9orf139-miR-22-3p-CDKN1A and GAS5-miR-139-5p/miR-429-JUN axes [104]. At the same time, plasma cells may play an important role in the immune microenvironment of IS, which provides a novel approach for the study of therapeutic targets of IS [104]. Yu et al. found that HIF-1α inhibits ACSL4 expression in early IS, which may be derived from the body’s rescue measures against ferroptosis [105]. These results suggest that understanding the role of ferroptosis in IS is of great significance for the prevention and treatment of this devastating disease.
Others
Studies have shown that sepsis is caused by infection, which can cause cardiac dysfunction, leading to significant morbidity and mortality, and ferroptosis plays an important role in this pathological process [106]. Wang et al. found that the expression of GPX4 decreased and the concentration of iron increased in the hearts of septic mice induced by caecal ligation and puncture (CLP) [107]. In a mouse model of sepsis induced by high-dose lipopolysaccharide (LPS), NCOA4 is activated and releases a large amount of free iron by degrading ferritin. Excessive intracellular Fe2+ activates the mitochondrial membrane protein SFXN1, which transports cytoplasmic Fe2+ in mitochondria, resulting in the production of mitochondrial ROS and ferroptosis [108]. Abdominal aortic aneurysm (AAA) is a life-threatening vascular disease with a fatality rate of up to 80% [109]. Oxidative stress and inflammation caused by iron overload have been confirmed to contribute to the progression of AAA [110]. In addition, the accumulation of oxidized phospholipids (or their decomposition products) in myocardial tissue of COVID-19 patients also shows the important role of ferroptosis in the progression of heart injury [111].
Clinical application in CVDs
Ferroptosis is closely related to the occurrence and development of CVDs. Clinical studies have increasingly shown that targeting ferroptosis may be an effective treatment for CVDs. At present, the anti-inflammatory and antioxidant effects of drugs are mainly used to inhibit ferroptosis in the clinic. Deferiprone is an FDA-approved oral active iron chelator that clears intracardial bleeding to relieve hypertrophic heart disease and has cardioprotective effects in acute myocardial infarction [112]. Jiang et al. found that the combination of L-glutamine and deferoxamine can protect the heart from I/R injury [113]. Dexrazoxane is a cyclic derivative of EDTA that easily penetrates the cell membrane and forms a ring-opening iron chelator. As the only drug approved by the FDA to prevent the toxicity of low cumulative dose DOX, it has been proven to successfully inhibit ferroptosis and protect the heart [25, 114]. N-acetylcysteine (NAC) is the donor of glutathione, and the level of GSH decreases under a high oxidation state. NAC can be used to enhance antioxidant therapy [115]. HO-1 can degrade haem into ferrous iron, and overexpression of HO-1 can alleviate hypertrophy, fibrosis and oxidative stress in a failing heart and promote neovascularization [116]. The mechanistic target of rapamycin (mTOR) can protect cardiomyocytes from iron excess and ferroptosis. mTOR is a serine/threonine protein kinase that acts on a large number of iron transporters and participates in the control of iron metabolism [117]. Statins inhibit the biosynthesis of GPX4 and coenzyme Q10, thereby promoting the ferroptosis of mesenchymal cells. Therefore, statins have also been used as an indirect treatment for cardiovascular disease-related ferroptosis [118, 119]. Some natural products with antioxidant activity, such as vitamins, can effectively inhibit ferroptosis and have been shown to have cardioprotective effects [120]. Vitamin E can effectively prevent atherosclerosis, and its potential mechanism may be to prevent ferroptosis by reducing the oxidation of LDL [121]. Puerarin can inhibit lipid peroxidation and iron overload in H9c2 cells, and baicalein can inhibit erastin-mediated GPX4 degradation and enhance the ability of H9c2 cells to resist ferroptosis [122].
Ferroptosis detection and identification
The morphology of cells can be directly observed by transmission electron microscopy to identify the occurrence of ferroptosis. Iron is an important basic element in the human body and is involved in the maintenance of various physiological functions. During ferroptosis, iron overload occurs in cells, and the detection of significantly increased iron levels can be used as an important indicator to monitor ferroptosis. Recently, an electronic sensing probe compatible with living cells was demonstrated to monitor the dynamics of iron metabolism in real time, which may help us better detect the dynamics of ferroptosis [123]. Iron can produce lipid ROS through the Fenton reaction, causing lipid peroxidation and promoting cell death. Therefore, detecting lipid peroxidation products (such as MDA, LOP, TBARS), ROS levels and cell activity can help us determine ferroptosis. Quantitative polymerase chain reaction (qPCR) or western blot (WB) was used to detect changes in some key factors related to ferroptosis, which can also be used as an important biomarker for determining ferroptosis in cells. In addition, the occurrence of ferroptosis may serve as a biomarker of CVDs and provide important information for the prevention and diagnosis of the disease. The three most important characteristics of biomarkers are specificity, sensitivity and stability. Some indicators of ferroptosis meet these requirements. GPX4 and ACSl4 are two recognized ferroptosis biomarkers [124, 125]. As proteins, ACSl4 and GPX4 are relatively stable in serum; compared with other types of biomarkers, they have the advantage of simple and sensitive determination.
Discussion
CVDs threaten human health and quality of life. Understanding how cardiomyocyte injury participates in the pathological process of heart-related diseases is the key to formulating heart protection strategies. In recent years, the pathogenic role of iron overload in cardiotoxicity has been widely recognized. Compared with the previously discovered types of cell death, such as apoptosis, necrosis, autophagy and pyroptosis, ferroptosis is an iron-dependent programmed cell death with two obvious biochemical characteristics: intracellular iron accumulation and lipid peroxidation.
Iron metabolism, lipid peroxidation, System Xc− and GPX4 play important roles in the regulation of ferroptosis-related pathways. Abnormal iron metabolism is the main cause of intracellular iron overload, and lipid peroxidation is an important marker of ferroptosis. GPX4 is another important marker of ferroptosis and a key central molecule in System Xc−, constituting the metabolic pathway of ferroptosis. As a new type of programmed cell death, the study of ferroptosis involves nervous system diseases, kidney-related diseases, tumours and cardiovascular diseases. In this review, we summarized the regulatory mechanisms of ferroptosis and discussed the role of ferroptosis in CVDs, including MI, reperfusion injury, AS, hypertension, myocardial hypertrophy, HF, DCM and DIC.
Several compounds ameliorate ferroptosis in cardiomyocytes and cardiac dysfunction in CVDs by inhibiting iron accumulation, regulating oxidative stress, and inhibiting lipid peroxidation. However, the specific microscopic reaction targets of these ferroptosis inhibitors are not clear, and whether they have potential toxicity to other organs remains to be confirmed, limiting their clinical application in the treatment of CVDs. At present, studies on ferroptosis are mostly based on animal models and cell levels, and there is still a lack of experimental verification in vivo. Ferroptosis is usually accompanied by an imbalance in ROS signals or an abnormal increase in ROS, which affects cell metabolism and inflammatory signal transduction. However, the specific molecular mechanism by which ROS cause ferroptosis has not been explained in detail. ROS levels, iron concentration, cell viability and some related marker proteins were used to evaluate ferroptosis in experiments. There is still a gap in the accurate detection of ferroptosis progression in vivo. If a specific probe or ferroptosis-related kit can be designed, it will better help in the prevention and treatment of CVDs. In addition, more molecular mechanisms related to ferroptosis remain to be discovered. Gu et al. found that p53 participates in the nonclassical pathway of ferroptosis regulation, which adds more complexity to the research on its mechanism.
In conclusion, ferroptosis is involved in the pathophysiological process of CVDs, suggesting that it can be a potential new drug therapy target. However, further efforts are still required to realize its practical application.
Acknowledgements
We thank Professor Peifeng Li, the head of the Institute for Translation Medicine, who provided substantial scientific support to this work.
Author contributions
All authors provided direction and guidance throughout the preparation of this manuscript. Kai Wang, Xin-Zhe Chen and Yun-Hong Wang drafted the manuscript. Lu-Yu Zhou and Kun Wang reviewed and made significant revisions to the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82070313, 81870236), Chinese Academy of Medical Sciences-Innovation Fund for Medical Sciences (2020-I2M-C&T-B-053), and Guiding Fund of Government’s Science and Technology (YDZX2021004).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally, Kai Wang, Xin-Zhe Chen, Yun-Hong Wang.
Contributor Information
Lu-Yu Zhou, Email: lyzhoucas@163.com.
Kun Wang, Email: wangk696@qdu.edu.cn.
References
- 1.Mendis S, Davis S, Norrving B. Organizational update: The world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46:e121–2. doi: 10.1161/STROKEAHA.115.008097. [DOI] [PubMed] [Google Scholar]
- 2.Arnett D, Blumenthal R, Albert M, Buroker A, Goldberger Z, Hahn E, et al. 2019 acc/aha guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74:1376–414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Re D, Amgalan D, Linkermann A, Liu Q, Kitsis R. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev. 2019;99:1765–817. doi: 10.1152/physrev.00022.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo M, Su J, Gong S, Liang N, Huang W, Chen W, et al. Ferroptosis: New dawn for overcoming the cardio-cerebrovascular diseases. Front Cell Dev Biol. 2021;9:733908. doi: 10.3389/fcell.2021.733908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonora M, Giorgi C, Pinton P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat Rev Mol cell Biol. 2022;23:266–85. doi: 10.1038/s41580-021-00433-y. [DOI] [PubMed] [Google Scholar]
- 6.D’Arcy M. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–92. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Yu S, Ji C, Li J. Structural basis of cell apoptosis and necrosis in tnfr signaling. Apoptosis Int J Program Cell Death. 2015;20:210–5. doi: 10.1007/s10495-014-1061-5. [DOI] [PubMed] [Google Scholar]
- 8.Saleem S. Apoptosis, autophagy, necrosis and their multi galore crosstalk in neurodegeneration. Neuroscience. 2021;469:162–74. doi: 10.1016/j.neuroscience.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Tian P, Wang K, Wang M, Wang K. Pyroptosis: Role and mechanisms in cardiovascular disease. Front Cardiovasc Med. 2022;9:897815. doi: 10.3389/fcvm.2022.897815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravingerová T, Kindernay L, Barteková M, Ferko M, Adameová A, Zohdi V, et al. The molecular mechanisms of iron metabolism and its role in cardiac dysfunction and cardioprotection. Int J Mol Sci. 2020;21:7889.. doi: 10.3390/ijms21217889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoyanovsky D, Tyurina Y, Shrivastava I, Bahar I, Tyurin V, Protchenko O, et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic Biol Med. 2019;133:153–61. doi: 10.1016/j.freeradbiomed.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon S, Lemberg K, Lamprecht M, Skouta R, Zaitsev E, Gleason C, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng H, Stockwell B. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16:e2006203. doi: 10.1371/journal.pbio.2006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillo-Moya J, Rojas-Solé C, Muñoz-Salamanca D, Panieri E, Saso L, Rodrigo R. Targeting ferroptosis against ischemia/reperfusion cardiac injury. Antioxidants (Basel, Switzerland) 2021;10:667.. doi: 10.3390/antiox10050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Disco. 2021;7:193. doi: 10.1038/s41420-021-00579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stockwell B, Friedmann Angeli J, Bayir H, Bush A, Conrad M, Dixon S, et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward D, Cloonan S. Mitochondrial iron in human health and disease. Annu Rev Physiol. 2019;81:453–82. doi: 10.1146/annurev-physiol-020518-114742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11:3052–9. doi: 10.7150/thno.54113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doll S, Conrad M. Iron and ferroptosis: A still ill-defined liaison. IUBMB Life. 2017;69:423–34. doi: 10.1002/iub.1616. [DOI] [PubMed] [Google Scholar]
- 20.He Y, Liu X, Xing L, Wan X, Chang X, Jiang H. Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials. 2020;241:119911. doi: 10.1016/j.biomaterials.2020.119911. [DOI] [PubMed] [Google Scholar]
- 21.Qi X, Zhang Y, Guo H, Hai Y, Luo Y, Yue T. Mechanism and intervention measures of iron side effects on the intestine. Crit Rev Food Sci Nutr. 2020;60:2113–25. doi: 10.1080/10408398.2019.1630599. [DOI] [PubMed] [Google Scholar]
- 22.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–32. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou W, Xie Y, Song X, Sun X, Lotze M, Zeh H, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–8. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Maltagliati A. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50:77–97. doi: 10.1152/physiolgenomics.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672–80. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726–39. doi: 10.1182/blood.2019002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, et al. Hspb1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–25. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chng C, Sadovsky Y, Hsia K, Huang C. Site-specific peroxidation modulates lipid bilayer mechanics. Extreme Mech Lett. 2021;42:101148. doi: 10.1016/j.eml.2020.101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golej D, Askari B, Kramer F, Barnhart S, Vivekanandan-Giri A, Pennathur S, et al. Long-chain acyl-coa synthetase 4 modulates prostaglandin e2 release from human arterial smooth muscle cells. J Lipid Res. 2011;52:782–93. doi: 10.1194/jlr.M013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon S, Winter G, Musavi L, Lee E, Snijder B, Rebsamen M, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10:1604–9. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller T, Dewitz C, Schmitz J, Schröder A, Bräsen J, Stockwell B, et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol life Sci. 2017;74:3631–45. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N, Jiang W, Wang W, Xiong R, Wu X, Geng Q. Ferroptosis and its emerging roles in cardiovascular diseases. Pharm Res. 2021;166:105466. doi: 10.1016/j.phrs.2021.105466. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Jiang L, Tavana O, Gu W. The deubiquitylase otub1 mediates ferroptosis via stabilization of slc7a11. Cancer Res. 2019;79:1913–24. doi: 10.1158/0008-5472.CAN-18-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumer Y, McCurdy S, Alcala M, Mehta N, Lee B, Ginsberg M, et al. Cd98 regulates vascular smooth muscle cell proliferation in atherosclerosis. Atherosclerosis. 2017;256:105–14. doi: 10.1016/j.atherosclerosis.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang K, Dong Y, Liu J, Qian L, Wang T, Gao X, et al. Effects of redox in regulating and treatment of metabolic and inflammatory cardiovascular diseases. Oxid Med Cell Longev. 2020;2020:5860356. doi: 10.1155/2020/5860356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W, SriRamaratnam R, Welsch M, Shimada K, Skouta R, Viswanathan V, et al. Regulation of ferroptotic cancer cell death by gpx4. Cell. 2014;156:317–31. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Qiu Q, Wang H, Chen C, Luo D. Trim46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating gpx4 ubiquitination. Exp Cell Res. 2021;407:112800. doi: 10.1016/j.yexcr.2021.112800. [DOI] [PubMed] [Google Scholar]
- 38.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, et al. Dhodh-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–90. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang N, Ma H, Li J, Meng C, Zou J, Wang H, et al. Hsf1 functions as a key defender against palmitic acid-induced ferroptosis in cardiomyocytes. J Mol Cell Cardiol. 2021;150:65–76. doi: 10.1016/j.yjmcc.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Hu H, Chen Y, Jing L, Zhai C, Shen L. The link between ferroptosis and cardiovascular diseases: A novel target for treatment. Front Cardiovasc Med. 2021;8:710963. doi: 10.3389/fcvm.2021.710963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang K, Zhou L, Liu F, Lin L, Ju J, Tian P, et al. Piwi-interacting rna haapir regulates cardiomyocyte death after myocardial infarction by promoting nat10-mediated ac c acetylation of tfec mrna. Adv Sci (Weinh, Baden-Wurtt, Ger) 2022;9:e2106058. doi: 10.1002/advs.202106058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Tian J, Luo X, Peng J. Targeting the pathways of regulated necrosis: A potential strategy for alleviation of cardio-cerebrovascular injury. Cell Mol Life Sci. 2021;78:63–78. doi: 10.1007/s00018-020-03587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C, Zhang Y, Li R, Zhou L, An T, Zhang R, et al. Lncrna caif inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat Commun. 2018;9:29. doi: 10.1038/s41467-017-02280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Liu C, Zhao Y, Gao G. Mitochondria regulation in ferroptosis. Eur J Cell Biol. 2020;99:151058. doi: 10.1016/j.ejcb.2019.151058. [DOI] [PubMed] [Google Scholar]
- 45.Park T, Park J, Lee G, Lee J, Shin J, Kim M, et al. Quantitative proteomic analyses reveal that gpx4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019;10:835. doi: 10.1038/s41419-019-2061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int. 2015;2015:761643. doi: 10.1155/2015/761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song Y, Wang B, Zhu X, Hu J, Sun J, Xuan J, et al. Human umbilical cord blood-derived mscs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol. 2021;37:51–64. doi: 10.1007/s10565-020-09530-8. [DOI] [PubMed] [Google Scholar]
- 48.Nishizawa H, Matsumoto M, Shindo T, Saigusa D, Kato H, Suzuki K, et al. Ferroptosis is controlled by the coordinated transcriptional regulation of glutathione and labile iron metabolism by the transcription factor bach1. J Biol Chem. 2020;295:69–82. doi: 10.1074/jbc.RA119.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang S, Frangogiannis N. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br J Pharm. 2018;175:1377–1400. doi: 10.1111/bph.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan H, Song Y, Chen J, Zhang N, Wang Q, Li Q, et al. Platelet-like fusogenic liposome-mediated targeting delivery of mir-21 improves myocardial remodeling by reprogramming macrophages post myocardial ischemia-reperfusion injury. Adv Sci (Weinh, Baden-Wurtt, Ger) 2021;8:e2100787. doi: 10.1002/advs.202100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shilo M, Oved H, Wertheim L, Gal I, Noor N, Green O, et al. Injectable nanocomposite implants reduce ros accumulation and improve heart function after infarction. Adv Sci (Weinh, Baden-Wurtt, Ger) 2021;8:e2102919. doi: 10.1002/advs.202102919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hausenloy D, Yellon D. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang L, Luo X, Tu H, Chen H, Xiong X, Li N, et al. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn-Schmiedeberg’s Arch Pharmacol. 2021;394:401–10. doi: 10.1007/s00210-020-01932-z. [DOI] [PubMed] [Google Scholar]
- 54.Chevion M, Jiang Y, Har-El R, Berenshtein E, Uretzky G, Kitrossky N. Copper and iron are mobilized following myocardial ischemia: Possible predictive criteria for tissue injury. Proc Natl Acad Sci USA. 1993;90:1102–6. doi: 10.1073/pnas.90.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Son E, Lee D, Woo C, Kim Y. In vitrothe optimal model of reperfusion injury using h9c2 transformed cardiac myoblasts. Korean J Physiol Pharmacol: Off J Korean Physiological Soc Korean Soc Pharmacol. 2020;24:173–83. doi: 10.4196/kjpp.2020.24.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olivas-Aguirre F, Rodrigo-García J, Martínez-Ruiz N, Cárdenas-Robles A, Mendoza-Díaz S, Álvarez-Parrilla E, et al. Cyanidin-3-o-glucoside: Physical-chemistry, foodomics and health effects. Molecules (Basel, Switzerland) 2016;21:1264.. doi: 10.3390/molecules21091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shan X, Lv Z, Yin M, Chen J, Wang J, Wu Q. The protective effect of cyanidin-3-glucoside on myocardial ischemia-reperfusion injury through ferroptosis. Oxid Med Cell Longev. 2021;2021:8880141. doi: 10.1155/2021/8880141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li T, Tan Y, Ouyang S, He J, Liu L. Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene. 2022;808:145968. doi: 10.1016/j.gene.2021.145968. [DOI] [PubMed] [Google Scholar]
- 59.Stamenkovic A, O’Hara K, Nelson D, Maddaford T, Edel A, Maddaford G, et al. Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury. Am J Physiol Heart Circulatory Physiol. 2021;320:H1170–84. doi: 10.1152/ajpheart.00237.2020. [DOI] [PubMed] [Google Scholar]
- 60.Huang R, Hu Z, Chen X, Cao Y, Li H, Zhang H, et al. The transcription factor sub1 is a master regulator of the macrophage tlr response in atherosclerosis. Adv Sci (Weinh, Baden-Wurtt, Ger) 2021;8:e2004162. doi: 10.1002/advs.202004162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gimbrone M, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–36. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li R, Jiang Q, Zheng Y. Circ_0002984 induces proliferation, migration and inflammation response of vsmcs induced by ox-ldl through mir-326-3p/vamp3 axis in atherosclerosis. J Cell Mol Med. 2021;25:8028–38. doi: 10.1111/jcmm.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tao J, Qiu J, Lu L, Zhang L, Fu Y, Wang M, et al. Zbtb20 positively regulates oxidative stress, mitochondrial fission, and inflammatory responses of ox-ldl-induced macrophages in atherosclerosis. Oxid Med Cell Longev. 2021;2021:5590855. doi: 10.1155/2021/5590855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su G, Yang W, Wang S, Geng C, Guan X. Sirt1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases il-1β and il-18. Biochem Biophys Res Commun. 2021;561:33–9. doi: 10.1016/j.bbrc.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 65.Yang K, Song H, Yin D. Pdss2 inhibits the ferroptosis of vascular endothelial cells in atherosclerosis by activating nrf2. J Cardiovasc Pharm. 2021;77:767–76. doi: 10.1097/FJC.0000000000001030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao F, Zhang D, Wu Y, Jia Q, Zhang L, Li Y, et al. Mirna-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the a20-acsl4 axis. Biochem Biophys Res Commun. 2019;515:448–54. doi: 10.1016/j.bbrc.2019.05.147. [DOI] [PubMed] [Google Scholar]
- 67.Bai T, Li M, Liu Y, Qiao Z, Wang Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. 2020;160:92–102. doi: 10.1016/j.freeradbiomed.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J, Sun H. Extracellular vesicle-mediated vascular cell communications in hypertension: Mechanism insights and therapeutic potential of ncrnas. Cardiovasc Drugs Ther. 2022;36:157–72. doi: 10.1007/s10557-020-07080-z. [DOI] [PubMed] [Google Scholar]
- 69.Ferdinand K, Townsend R. Hypertension in the us black population: Risk factors, complications, and potential impact of central aortic pressure on effective treatment. Cardiovasc Drugs Ther. 2012;26:157–65. doi: 10.1007/s10557-011-6367-8. [DOI] [PubMed] [Google Scholar]
- 70.Shang F, Guo X, Chen Y, Wang C, Gao J, Wen E, et al. Endothelial microrna-483-3p is hypertension-protective. Oxid Med Cell Longev. 2022;2022:3698219. doi: 10.1155/2022/3698219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger J, Nicolls M, et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur Respir J. 2019;53:1801887.. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan R, Li C, Xu C, Wu Q, Gao L, Shi Y, et al. Targeting jp2: A new treatment for pulmonary hypertension. Oxid Med Cell Longev. 2021;2021:2003446. doi: 10.1155/2021/2003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie S, Deng Y, Guo S, Li J, Zhou Y, Liao J, et al. Endothelial cell ferroptosis mediates monocrotaline-induced pulmonary hypertension in rats by modulating nlrp3 inflammasome activation. Sci Rep. 2022;12:3056. doi: 10.1038/s41598-022-06848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu X, Tao W, Jiang F, Li C, Lin J, Liu C. Celastrol attenuates hypertension-induced inflammation and oxidative stress in vascular smooth muscle cells via induction of heme oxygenase-1. Am J Hypertens. 2010;23:895–903. doi: 10.1038/ajh.2010.75. [DOI] [PubMed] [Google Scholar]
- 75.El-Bassossy H, Fahmy A, Badawy D. Cinnamaldehyde protects from the hypertension associated with diabetes. Food Chem Toxicol Int J published Br Ind Biol Res Assoc. 2011;49:3007–12. doi: 10.1016/j.fct.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 76.Zou H, Qiu B, Lai S, Zhou X, Gong C, Wang L, et al. Iron metabolism and idiopathic pulmonary arterial hypertension: New insights from bioinformatic analysis. Biomed Res Int. 2021;2021:5669412. doi: 10.1155/2021/5669412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei W, Rao F, Liu F, Xue Y, Deng C, Wang Z, et al. Involvement of smad3 pathway in atrial fibrosis induced by elevated hydrostatic pressure. J Cell Physiol. 2018;233:4981–9. doi: 10.1002/jcp.26337. [DOI] [PubMed] [Google Scholar]
- 78.Jin R, Yang R, Cui C, Zhang H, Cai J, Geng B, et al. Ferroptosis due to cystathionine γ lyase/hydrogen sulfide downregulation under high hydrostatic pressure exacerbates vsmc dysfunction. Front Cell Dev Biol. 2022;10:829316. doi: 10.3389/fcell.2022.829316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Z, Tang J, Song J, Xie M, Liu Y, Dong Z, et al. Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the il-6/stat3/gpx4 signaling. Free Radic Biol Med. 2022;181:130–42. doi: 10.1016/j.freeradbiomed.2022.01.020. [DOI] [PubMed] [Google Scholar]
- 80.Zhang F, Liu H. Identification of ferroptosis-associated genes exhibiting altered expression in pulmonary arterial hypertension. Math Biosci Eng. 2021;18:7619–30. doi: 10.3934/mbe.2021377. [DOI] [PubMed] [Google Scholar]
- 81.Mozaffarian D, Benjamin E, Go A, Arnett D, Blaha M, Cushman M, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 82.Magadum A, Singh N, Kurian A, Sharkar M, Sultana N, Chepurko E, et al. Therapeutic delivery of pip4k2c-modified mrna attenuates cardiac hypertrophy and fibrosis in the failing heart. Adv Sci (Weinh, Baden-Wurtt, Ger) 2021;8:2004661. doi: 10.1002/advs.202004661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang K, Long B, Liu F, Wang J, Liu C, Zhao B, et al. A circular rna protects the heart from pathological hypertrophy and heart failure by targeting mir-223. Eur Heart J. 2016;37:2602–11. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Deng B, Liu Q, Huang Y, Chen W, Li J, et al. Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (mlk3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis. 2020;11:574. doi: 10.1038/s41419-020-02777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Zheng C, Gao Z, Chen H, Li K, Wang L, et al. Slc7a11/xct prevents cardiac hypertrophy by inhibiting ferroptosis. Cardiovasc Drugs Ther. 2022;36:437–47. doi: 10.1007/s10557-021-07220-z. [DOI] [PubMed] [Google Scholar]
- 86.Shi P, Li M, Song C, Qi H, Ba L, Cao Y, et al. Aabr07017145.1neutrophil-like cell membrane-coated sirna of lncrna therapy for cardiac hypertrophy via inhibiting ferroptosis of cmecs. Mol Ther Nucleic Acids. 2022;27:16–36. doi: 10.1016/j.omtn.2021.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anand I, Gupta P. Anemia and iron deficiency in heart failure: Current concepts and emerging therapies. Circulation. 2018;138:80–98. doi: 10.1161/CIRCULATIONAHA.118.030099. [DOI] [PubMed] [Google Scholar]
- 88.Anker S, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl J Med. 2009;361:2436–48. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 89.Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P, et al. Loss of cardiac ferritin h facilitates cardiomyopathy via slc7a11-mediated ferroptosis. Circ Res. 2020;127:486–501. doi: 10.1161/CIRCRESAHA.120.316509. [DOI] [PubMed] [Google Scholar]
- 90.Kong L, Liao C, Chen L, Yang H, Zhang S, Zhang Z, et al. Promoter hypomethylation up-regulates cd147 expression through increasing sp1 binding and associates with poor prognosis in human hepatocellular carcinoma. J Cell Mol Med. 2011;15:1415–28. doi: 10.1111/j.1582-4934.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhong F, Zhao Y, Zhao C, Gu Z, Lu X, Jiang W, et al. The role of cd147 in pathological cardiac hypertrophy is regulated by glycosylation. Oxid Med Cell Longev. 2022;2022:6603296. doi: 10.1155/2022/6603296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ge Z, Lian Q, Mao X, Xia Z. Current status and challenges of nrf2 as a potential therapeutic target for diabetic cardiomyopathy. Int Heart J. 2019;60:512–20. doi: 10.1536/ihj.18-476. [DOI] [PubMed] [Google Scholar]
- 93.Bugger H, Abel E. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–71. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zang H, Wu W, Qi L, Tan W, Nagarkatti P, Nagarkatti M, et al. Autophagy inhibition enables nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes. 2020;69:2720–34. doi: 10.2337/db19-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang C, Zhu L, Yuan W, Sun L, Xia Z, Zhang Z, et al. Diabetes aggravates myocardial ischaemia reperfusion injury via activating nox2-related programmed cell death in an ampk-dependent manner. J Cell Mol Med. 2020;24:6670–9. doi: 10.1111/jcmm.15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li W, Li W, Leng Y, Xiong Y, Xia Z. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol. 2020;39:210–25. doi: 10.1089/dna.2019.5097. [DOI] [PubMed] [Google Scholar]
- 97.Qin Y, Lv C, Zhang X, Ruan W, Xu X, Chen C, et al. Protective effect of qiliqiangxin against doxorubicin-induced cardiomyopathy by suppressing excessive autophagy and apoptosis. Cardiovasc Ther. 2022;2022:9926635. doi: 10.1155/2022/9926635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5:e132747.. doi: 10.1172/jci.insight.132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quagliariello V, De Laurentiis M, Rea D, Barbieri A, Monti M, Carbone A, et al. The sglt-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20:150. doi: 10.1186/s12933-021-01346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luo L, Guan P, Qin L, Wang J, Wang N, Ji E. Astragaloside iv inhibits adriamycin-induced cardiac ferroptosis by enhancing nrf2 signaling. Mol Cell Biochem. 2021;476:2603–11. doi: 10.1007/s11010-021-04112-6. [DOI] [PubMed] [Google Scholar]
- 101.Edward J, Cornwell W. Impact of exercise on cerebrovascular physiology and risk of stroke. Stroke. 2022;53:2404–10. doi: 10.1161/STROKEAHA.121.037343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C, Sun G, Chen B, Xu L, Ye Y, He J, et al. Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharm Res. 2021;174:105933. doi: 10.1016/j.phrs.2021.105933. [DOI] [PubMed] [Google Scholar]
- 103.Abdul Y, Li W, Ward R, Abdelsaid M, Hafez S, Dong G, et al. Deferoxamine treatment prevents post-stroke vasoregression and neurovascular unit remodeling leading to improved functional outcomes in type 2 male diabetic rats: Role of endothelial ferroptosis. Transl Stroke Res. 2021;12:615–30. doi: 10.1007/s12975-020-00844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fan J, Chen M, Cao S, Yao Q, Zhang X, Du S, et al. Identification of a ferroptosis-related gene pair biomarker with immune infiltration landscapes in ischemic stroke: A bioinformatics-based comprehensive study. BMC Genomics. 2022;23:59. doi: 10.1186/s12864-022-08295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cui Y, Zhang Y, Zhao X, Shao L, Liu G, Sun C, et al. Acsl4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun. 2021;93:312–21. doi: 10.1016/j.bbi.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 106.Zhu H, Santo A, Jia Z, Robert Li Y. Gpx4 in bacterial infection and polymicrobial sepsis: Involvement of ferroptosis and pyroptosis. React Oxyg Species (Apex) 2019;7:154–60. doi: 10.20455/ros.2019.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang C, Yuan W, Hu A, Lin J, Xia Z, Yang C, et al. Dexmedetomidine alleviated sepsis‑induced myocardial ferroptosis and septic heart injury. Mol Med Rep. 2020;22:175–84. doi: 10.3892/mmr.2020.11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C, et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med. 2020;160:303–18. doi: 10.1016/j.freeradbiomed.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 109.Adams L, Brangsch J, Reimann C, Kaufmann J, Buchholz R, Karst U, et al. Simultaneous molecular mri of extracellular matrix collagen and inflammatory activity to predict abdominal aortic aneurysm rupture. Sci Rep. 2020;10:15206. doi: 10.1038/s41598-020-71817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sawada H, Hao H, Naito Y, Oboshi M, Hirotani S, Mitsuno M, et al. Aortic iron overload with oxidative stress and inflammation in human and murine abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:1507–14. doi: 10.1161/ATVBAHA.115.305586. [DOI] [PubMed] [Google Scholar]
- 111.Jacobs W, Lammens M, Kerckhofs A, Voets E, Van San E, Van Coillie S, et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (covid-19): Autopsy reveals a ferroptosis signature. ESC Heart Failure. 2020;7:3772–81. doi: 10.1002/ehf2.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Behrouzi B, Weyers J, Qi X, Barry J, Rabadia V, Manca D, et al. Action of iron chelator on intramyocardial hemorrhage and cardiac remodeling following acute myocardial infarction. Basic Res Cardiol. 2020;115:24. doi: 10.1007/s00395-020-0782-6. [DOI] [PubMed] [Google Scholar]
- 113.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu X, Ruan Y, Shen T, Qiu Q, Yan M, Sun S, et al. Dexrazoxane protects cardiomyocyte from doxorubicin-induced apoptosis by modulating mir-17-5p. BioMed Res Int. 2020;2020:5107193. doi: 10.1155/2020/5107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, et al. N-acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic Res. 2018;52:751–62. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 116.Wang G, Hamid T, Keith R, Zhou G, Partridge C, Xiang X, et al. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–25. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baba Y, Higa J, Shimada B, Horiuchi K, Suhara T, Kobayashi M, et al. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circulatory Physiol. 2018;314:H659–68. doi: 10.1152/ajpheart.00452.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bibli S, Hu J, Leisegang M, Wittig J, Zukunft S, Kapasakalidi A, et al. Shear stress regulates cystathionine γ lyase expression to preserve endothelial redox balance and reduce membrane lipid peroxidation. Redox Biol. 2020;28:101379. doi: 10.1016/j.redox.2019.101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Al-Rasheed N, Al-Rasheed N, Hasan I, Al-Amin M, Al-Ajmi H, Mohamad R, et al. Simvastatin ameliorates diabetic cardiomyopathy by attenuating oxidative stress and inflammation in rats. Oxid Med Cell Longev. 2017;2017:1092015. doi: 10.1155/2017/1092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid peroxidation-dependent cell death regulated by gpx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143–70. doi: 10.1007/82_2016_508. [DOI] [PubMed] [Google Scholar]
- 121.Hinman A, Holst C, Latham J, Bruegger J, Ulas G, McCusker K, et al. Vitamin e hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PLoS One. 2018;13:e0201369. doi: 10.1371/journal.pone.0201369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie Y, Song X, Sun X, Huang J, Zhong M, Lotze M, et al. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem Biophys Res Commun. 2016;473:775–80. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 123.Chen N, Du N, Wang W, Liu T, Yuan Q, Yang Y. Real-time monitoring of dynamic microbial fe(iii) respiration metabolism with a living cell-compatible electron-sensing probe. Angew Chem Int Ed Engl. 2022;61:e202115572.. doi: 10.1002/anie.202115572. [DOI] [PubMed] [Google Scholar]
- 124.Shimada K, Hayano M, Pagano N, Stockwell B. Cell-line selectivity improves the predictive power of pharmacogenomic analyses and helps identify nadph as biomarker for ferroptosis sensitivity. Cell Chem Biol. 2016;23:225–35. doi: 10.1016/j.chembiol.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of acsl4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–43. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 126.Feng Y, Madungwe N, Imam Aliagan A, Tombo N, Bopassa J. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing vdac1 levels and restoring gpx4 levels. Biochem Biophys Res Commun. 2019;520:606–11. doi: 10.1016/j.bbrc.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lv Z, Wang F, Zhang X, Zhang X, Zhang J, Liu R. Etomidate attenuates the ferroptosis in myocardial ischemia/reperfusion rat model via nrf2/ho-1 pathway. Shock (Augusta, Ga) 2021;56:440–9. doi: 10.1097/SHK.0000000000001839. [DOI] [PubMed] [Google Scholar]
- 128.Shi P, Song C, Qi H, Ren J, Ren P, Wu J, et al. Up-regulation of irf3 is required for docosahexaenoic acid suppressing ferroptosis of cardiac microvascular endothelial cells in cardiac hypertrophy rat. J Nutr Biochem. 2022;104:108972.. doi: 10.1016/j.jnutbio.2022.108972. [DOI] [PubMed] [Google Scholar]
- 129.Chen X, Xu S, Zhao C, Liu B. Role of tlr4/nadph oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun. 2019;516:37–43. doi: 10.1016/j.bbrc.2019.06.015. [DOI] [PubMed] [Google Scholar]