Abstract
COPD and obstructive sleep apnoea (OSA) are highly prevalent and different clinical COPD phenotypes that influence the likelihood of comorbid OSA. The increased lung volumes and low body mass index (BMI) associated with the predominant emphysema phenotype protects against OSA whereas the peripheral oedema and higher BMI often associated with the predominant chronic bronchitis phenotype promote OSA. The diagnosis of OSA in COPD patients requires clinical awareness and screening questionnaires which may help identify patients for overnight study. Management of OSA-COPD overlap patients differs from COPD alone and the survival of overlap patients treated with nocturnal positive airway pressure is superior to those untreated. Sleep-related hypoventilation is common in neuromuscular disease and skeletal disorders because of the effects of normal sleep on ventilation and additional challenges imposed by the underlying disorders. Hypoventilation is first seen during rapid eye movement (REM) sleep before progressing to involve non-REM sleep and wakefulness. Clinical presentation is nonspecific and daytime respiratory function measures poorly predict nocturnal hypoventilation. Monitoring of respiration and carbon dioxide levels during sleep should be incorporated in the evaluation of high-risk patient populations and treatment with noninvasive ventilation improves outcomes.
Short abstract
Sleep disordered breathing disorders, such as obstructive sleep apnoea, are common in patients with chronic respiratory diseases. Diagnosis is often challenging, but treatment options seem to improve outcomes. http://bit.ly/2LaJMlf
Introduction
Sleep occupies up to one-third of every adult's life, yet insufficient attention is given to the influence of sleep on medical disorders. This lack of awareness is unfortunate as sleep may have deleterious effects on many physiological and organ functions, which are frequently exacerbated in disease states. In the respiratory system, sleep has important effects on breathing and gas exchange that may exacerbate the dysfunction seen while awake in respiratory disorders such as COPD and asthma, among others [1]. Furthermore, there are specific respiratory disorders associated with sleep such as obstructive sleep apnoea (OSA), which may co-exist with other chronic respiratory diseases and exacerbate sleep-related breathing disturbances [2]. The present review is based on a session at the 8th Sleep and Breathing Conference held in Marseilles, France, on 11–13 April, 2019, and focusses on the relationships of sleep with two specific chronic respiratory disorders, namely COPD and hypoventilation due to neuromuscular and thoracic cage disorders.
Sleep quality is impaired in patients with chronic respiratory disease [3] and diminished sleep efficiency with a reduction in REM sleep has been reported in patients with COPD, which correlates with awake arterial oxygen tension (PaO2) but not with the degree of airflow obstruction [4], although lung hyperinflation has been associated with poor sleep quality in COPD patients [5]. Sleep disturbance in COPD is associated with reduced quality of life measures such as the Short Form-12 and the St George's Respiratory Questionnaire [6].
Physiological changes in ventilation during sleep
Sleep has adverse effects on breathing that include disturbances in respiratory control, respiratory muscle function and lung mechanics. Respiratory control effects include diminished cortical inputs to the respiratory centre with reduced respiratory motor neurone output, and diminished chemoreceptor sensitivity affecting ventilatory responses to hypoxia and hypercapnia, in addition to increased upper airway resistance [7, 8]. Respiratory muscle function is also adversely affected, especially the accessory muscles of respiration during rapid eye movement (REM) sleep, whereas diaphragmatic contraction is not greatly affected during sleep [9]. In normal REM sleep, there is active inhibition of skeletal muscles, including the accessory muscles of respiration, whereas diaphragm contraction is relatively preserved [10]. Adverse effects on lung mechanics are also reported that include changes in functional residual capacity and disturbances in ventilation–perfusion relationships [11]. Overall, these physiological effects result in hypoventilation with associated hypoxaemia and hypercapnia, which can be recognised in normal subjects to a very mild and clinically insignificant degree [12]. Minute ventilation decreases from wakefulness to non-REM (NREM) sleep by about 15% in healthy subjects with further decrease during REM sleep [12].
Pathophysiology of sleep disordered breathing in COPD patients
In patients with chronic respiratory disease such as COPD, the physiological changes during sleep may be enough to result in clinically significant disturbances in gas exchange, especially during REM sleep [13]. The loss of accessory muscle contraction in REM sleep is especially important in COPD, where lung hyperinflation may diminish the efficacy of diaphragmatic contraction, and such patients become more reliant on accessory muscle contraction to maintain ventilation [14]. Furthermore, disturbed ventilation–perfusion relationships contribute to hypoxaemia, which increases the degree of nocturnal oxygen desaturation resulting from physiological hypoventilation during sleep [11, 13].
Sleep and the supine position contribute to worsening airflow obstruction [15], which may exacerbate hyperinflation and hypoventilation in COPD. Hyperinflation increases the work of breathing, which contributes to increased arousability and sleep disturbance. Furthermore, decreased skeletal muscle contraction, especially during REM sleep, contributes to upper airway obstruction by diminished ability to withstand upper airway collapsing forces during inspiration [16].
Factors influencing the association of COPD with OSA
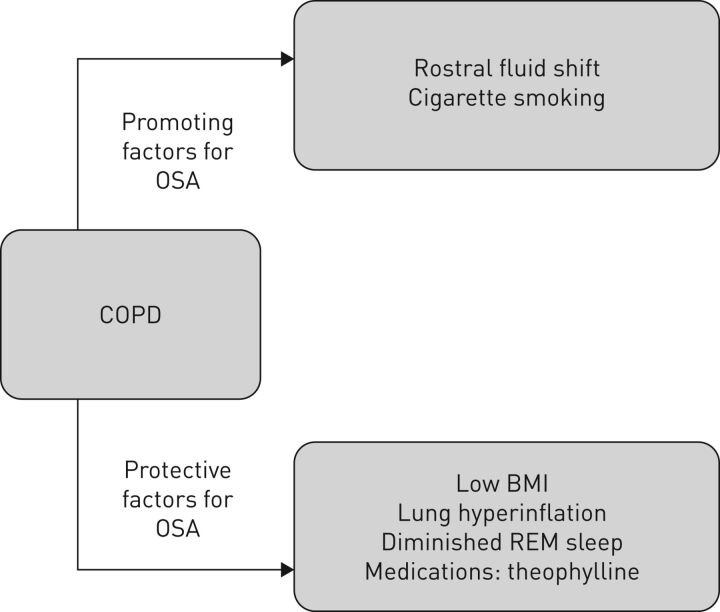
While sleep has many effects on breathing, some factors relating to COPD promote the development of OSA whereas others are protective (figure 1). COPD-related factors that may promote OSA include rostral fluid shift in the supine position [17], which is especially relevant in COPD complicated by right heart failure. Other promoting factors include cigarette smoking, which contributes to upper airway inflammation [18], and medications, especially corticosteroids [19, 20]. COPD-related factors that protect against OSA include low body mass index (BMI), which is common in patients with COPD, lung hyperinflation, older age, diminished REM sleep, and medications such as theophylline. Thus, the likelihood of OSA being present in COPD patients (the overlap syndrome) reflects the balance of promoting and protective factors [21]. Previous reports have provided differing findings on the likelihood of COPD and OSA co-existing, which likely reflects differences in anthropometric and pathophysiological variables in the respective patient populations [22–24].
FIGURE 1.
Interactions between COPD and obstructive sleep apnoea (OSA) that may influence the prevalence of the overlap syndrome. BMI: body mass index; REM: rapid eye movement.
Lung hyperinflation has been demonstrated to protect against OSA, likely as a result of caudal traction effects on the upper airway, and computed tomography measures of hyperinflation and gas trapping are reported to be negatively associated with the apnoea–hypopnoea index (AHI) frequency per hour [25]. COPD patients with lung hyperinflation have a more negative critical value of positive end-expiratory pressure (Pcrit) of the upper airway than control subjects [26], which is likely the most important protective measure against OSA. Furthermore, hypoventilation during NREM sleep in COPD is reported to be a consequence of decreased neural respiratory drive, whereas in the overlap syndrome increased upper airway resistance appears to be more important [27]. Sleep disordered breathing (SDB) in COPD may not follow the accepted definitions of apnoea and hypopnoea and may present as periods of non-apnoeic hypoventilation with associated oxygen desaturation [28].
Steveling et al. [29] evaluated the association of COPD and OSA in a cohort of 177 patients with stable COPD and found in multivariate analysis that BMI and pack year smoking were positively associated with AHI. Lambert et al. [30] reported that obesity is a contributing factor for a range of adverse COPD outcomes including lower quality of life and higher exacerbation rate. However, the possibility of co-existing OSA was not evaluated, which could have represented a contributing factor to these adverse outcomes.
Rostral fluid shift in the supine position during sleep is a well-established contributing factor to the development of OSA and has been studied extensively in a range of disorders including heart failure and end-stage renal disease [31, 32]. Pathophysiological mechanisms include the accumulation of fluid in the neck resulting in upper airway narrowing, increased pharyngeal wall compliance, and possible reduced pharyngeal dilator muscle contraction [17]. COPD patients with right heart failure are prone to OSA by this mechanism, especially where peripheral oedema is present.
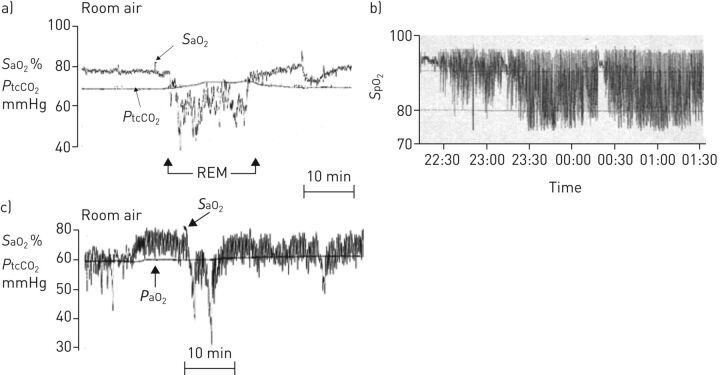
The pattern of oxygen desaturation differs between COPD, OSA and overlap syndrome. In COPD alone, periods of sustained desaturation may develop that is most pronounced in REM [2]. The degree of nocturnal desaturation typically reflects the awake PaO2 and nocturnal oxygen desaturation in COPD usually occurs in patients with low PaO2 levels while awake [33]. This association reflects the impact of the oxyhaemoglobin dissociation curve, where greater oxygen desaturation will occur for a similar degree of hypoventilation when the starting PaO2 level is diminished. In OSA, episodic desaturation develops that usually reverts to normal saturation levels between apnoea. In patients with COPD-OSA overlap, episodic desaturation also develops, typically from a low saturation baseline, and desaturation may be more pronounced as a result of this low starting baseline. Typical oxygen saturation profiles in these three clinical scenarios are given in figure 2.
FIGURE 2.
Different patterns of oxygen desaturation during sleep in patients with a) COPD, b) obstructive sleep apnoea and c) overlap syndrome. SaO2: arterial oxygen saturation; SpO2: arterial oxygen saturation measured by pulse oximetry; PtcCO2: transcutaneous carbon dioxide tension; REM: rapid eye movement.
Airway and systemic inflammation are reported in COPD and OSA, which may contribute to the development of cardiovascular comorbidity. OSA has been reported to increase lower airway inflammation in COPD, which may aggravate clinical features and predispose to exacerbations [34]. Conversely, COPD may increase nasal and pharyngeal inflammation, especially in COPD patients who continue to smoke [18]. OSA may also contribute to other clinical manifestations such as gastro-oesophageal reflux [35], which may be a factor in airway inflammation.
Medications used in the treatment of COPD may also influence the development of OSA. Inhaled corticosteroids have been reported to adversely affect pharyngeal muscle function with reduced Pcrit, thus predisposing to upper airway collapse [19]. Theophyllines, however, have been reported to reduce AHI in patients with OSA [36], which does not appear to be a consequence of improved lung mechanics as theophylline diminishes lung hyperinflation in patients with COPD [37].
Assessment of SDB in COPD
The diagnosis of SDB in patients with COPD can be challenging. Three separate important components need to be considered; a detailed clinical interview, a physical examination and an objective overnight sleep recording.
Clinical interview
Sleep complaints reported by COPD patients include difficulties initiating and maintaining sleep as well as short sleep time. However, these symptoms often overlap with symptoms of SDB, such as non-restorative sleep, waking up gasping and choking, morning headaches and increased daytime sleepiness [38]. Daytime fatigue is a frequent complaint of COPD patients which may be aggravated by coexisting OSA. Features such as nocturnal dyspnoea or disturbed sleep may lack sensitivity for SDB and often overlap with symptoms of COPD [39]. Finally, nocturnal hypoxaemia, while commonly observed in COPD patients with borderline daytime oxygen levels of 90–95%, does not produce specific symptoms and often remains undetected [40].
Questionnaires to screen and determine the probability of OSA, such as the Epworth Sleepiness Scale, the STOP BANG or the Berlin questionnaire, have not been specifically validated for patients with coexisting COPD and should therefore be used with some caution [41]. Specific sleep-related questionnaires for COPD patients have been developed but clinical experience is limited [42, 43].
Physical examination
Clinical findings found to correlate with SDB among COPD patients include obesity, anatomical upper airway obstruction such as nasal congestion, signs of right ventricular failure including peripheral oedema, and deranged blood gases. Hence, blood-gas testing is important to detect persistent daytime hypoxaemia and hypercapnia. The clinical features identified as indications for evaluation of possible sleep-related breathing disorders in COPD are summarised in table 1 [44].
TABLE 1.
Indications for performing a sleep diagnostic test in COPD patients
| Symptoms or findings indicative for sleep disordered breathing in patients with COPD |
| Sleep-related symptoms such as snoring, gasping and choking, as well as nocturia or morning headache |
| Increased daytime sleepiness |
| Signs of obesity including BMI >30 kg·m−2 in men and >35 kg·m−2 in women, neck circumference >43 cm in men and >41 cm in women |
| Reduced daytime pulse oxygen saturation (<93%) at rest or during exercise |
| Daytime hypercapnia |
| Signs of pulmonary hypertension or right heart failure, such as peripheral oedema |
| Polycythaemia |
| Patients who use opioids and/or hypnotic medications |
| Comorbidities such as atrial fibrillation, end-stage renal disease, type 2 diabetes, heart failure, difficult to treat hypertension and stroke |
BMI: body mass index.
When to perform a sleep diagnostic test?
The threshold to carry out a sleep diagnostic test should be low as questionnaires and clinical assessment are blunt tools. This is unfortunate as the prevalence of SDB in COPD is likely to be high, if only because of the high general population prevalence of SDB, and treatment opportunities are abundant. Some reports indicate a higher prevalence of SDB in COPD compared to the general population [29, 42], whereas other reports indicate no increase [24, 45]. These contrasting findings may reflect differences in promoting and protective factors for SDB between study populations [21].
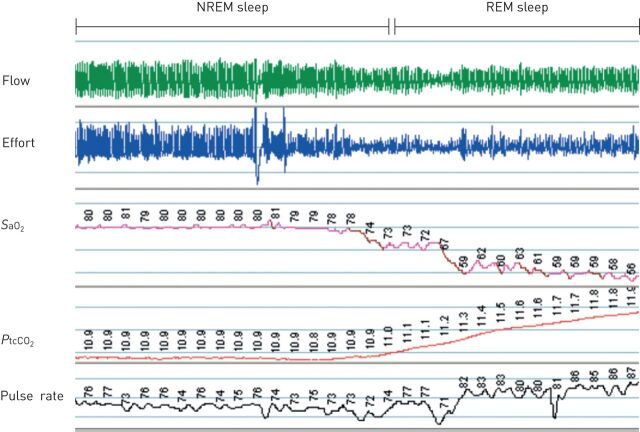
The sleep diagnostic test aims to determine the degree of hypoxaemia and hypercapnia, the amount and type of breathing disorder (central/obstructive apnoea/hypoventilation) and the degree of sleep disturbance (such as REM sleep-associated hypoventilation demonstrated in figure 3). The choice of sleep study depends on local resources and specific diagnostic guidelines for COPD patients with suspected overlap or hypoventilation syndrome do not exist. Stable COPD patients with preserved sleep quality may be investigated with a home sleep study whereas patients with more complex sleep-related symptoms or an unstable COPD condition may need testing in a sleep laboratory.
FIGURE 3.
Sleep-related hypoventilation in COPD. Image shows ∼30 min of respiration during non-rapid and rapid eye movement (NREM and REM) sleep in a female patient with stable, advanced COPD. The level of arterial oxygen saturation (SaO2) and transcutaneous carbon dioxide tension (PtcCO2) is already reduced during NREM stage 2 sleep compared to values during wakefulness (SaO2 90%). No repetitive apnoea/hypopnea occurred. In the transition to REM sleep a physiological reduction in respiratory efforts (reduced amplitude in the thoracic effort signal) with a corresponding decrease in airflow amplitude occurred. A further reduction in SaO2 and a significant increase in PtcCO2 (a qualitative, non-calibrated signal) is the consequence of the REM sleep hypoventilation. Heart rate increases as an indirect sign of increased sympathetic activity.
Diagnostic tools
Overnight pulse oximetry may be used as a screening device. The method is particularly useful for the detection of transient or sustained nocturnal hypoxaemia during the sleep period [40]. However, the method does not allow for any differentiation of central versus obstructive sleep apnoea or any sleep wake classification.
The home sleep apnoea test (HSAT) is a frequently used user-friendly and reliable method to detect OSA [46]. Recorded signals, in addition to pulse-oximetry, include respiratory effort, oro-nasal flow, actigraphy pattern and/or sleep position. Improvements in HSAT technology have led to a better acceptance and tolerability of HSAT among patients with suspected overlap syndrome [47]. However, high rates of recording failures with HSAT, as well as variable correlation with in-laboratory polysomnography (PSG), has limited the use in COPD patients [48]. In fact, this variability may, in part, be caused by the generally poor sleep quality in COPD patients [49].
In-laboratory PSG is the gold standard method used to diagnose the full range of COPD-specific SDB such as hypoventilation, central sleep apnoea and periods of inspiratory and expiratory airflow limitation leading to frequent arousal [44]. Recorded signals on top of HSAT devices include sleep-EEG and possible continuous assessment of transcutaneous and/or end-expiratory CO2 level. In addition, arterial blood gases may be monitored prior to lights out and immediately upon waking in the morning. More advanced signals include quantitative flow assessment with a pneumotachograph and diaphragmatic EMG activity in more expanded recording settings to quantify repetitive dynamic hyperinflation frequently seen in COPD patients. Major limitations of PSG include the lack of availability and high cost.
Carbon dioxide levels can be measured using invasive and noninvasive techniques, by monitoring end-tidal carbon dioxide tension (PetCO2) and transcutaneous carbon dioxide tension (PtcCO2). PetCO2 measures breath-to-breath changes in exhaled carbon dioxide whereas PtcCO2 assesses arterialised capillary carbon dioxide at the skin surface. PetCO2 is known to be influenced by nasal congestion and secretion, oxygen insufflations, noninvasive ventilation (NIV) and mask leaks [50]. PtcCO2 measurement techniques have undergone recent improvements and is now considered reliable as a diagnostic tool to detect hypoventilation in the guidelines of the American Academy of Sleep Medicine (AASM) [51], although the method may overestimate the absolute arterial carbon dioxide tension (PaCO2) [52, 53].
Treatment of sleep disordered treatment in COPD
There are no specific guidelines on how to treat patients with the overlap syndrome. Both diseases should be treated according to current disease specific guidelines [54, 55]. The goal of therapy is to alleviate hypoxaemia and hypercapnia during sleep, improve sleep quality and improve health-related quality of life. There is increasing evidence that treatment of SDB may contribute to a significant reduction of COPD-related morbidity, exacerbation frequency and mortality [39, 56, 57].
Positive airway pressure treatment in OSA
Continuous positive airway pressure (CPAP) is the first-line therapy in moderate-to-severe OSA [55]. Ventilation, sleep quality and cardiovascular function improves as soon as the therapeutic pressure level has been reached. Many patients with mild-to-moderate COPD and OSA may have positive airway pressure (PAP) treatment initiated at home, but in patients with severe COPD and/or complex comorbid conditions, the supervised titration in a specialist sleep centre setting is recommended [55]. Coaching at treatment initiation and close follow-up of efficacy and potential side-effects improve treatment acceptance and adherence [55].
Importantly, PAP is reported to improve daytime blood gases and reduce mortality, morbidity and exacerbation rates in patients with overlap syndrome [57, 58]. Clinical cohort studies indicate that exacerbation rate and mortality is reduced in subjects compliant with PAP treatment [57]. However, as randomised CPAP trials have not been performed in the OSA–COPD overlap syndrome, the level of evidence is still limited.
The application of PAP in patients with severe emphysema can worsen dynamic hyperinflation and increase work of breathing, particularly at pressures >10 cmH2O. In these patients, newer PAP devices that allow reduction in expiratory pressures or even the application of bilevel PAP may help to get patients adjusted to PAP, reducing the number of side-effects and increasing overall compliance with treatment [59].
The effect of oral appliances or positional treatment in mild-moderate OSA has not been systematically evaluated in COPD patients. Beneficial effects on the AHI comparable to that reported in other patient groups may be expected, but benefits to COPD-related outcomes such as daytime blood gases or exacerbation frequency are unknown.
PAP treatment of central sleep apnoea
Central sleep apnoea in COPD may be caused by atrial fibrillation, heart failure with and without preserved ejection fraction or opiate use [60]. Specific data regarding the prevalence and consequences of central sleep apnoea events in patients with COPD are sparse. Treatment should be considered to target the underlying cause, i.e. by intensifying heart failure treatment or opiate withdrawal [38]. In symptomatic CSA, PAP treatment including CPAP, bilevel PAP or adaptive servo ventilator may be considered [60].
Treatment of sleep-related hypoventilation by NIV
Patients with stable hypercapnia outside the acute exacerbation of COPD may benefit from NIV with bilevel treatment [60–62]. The treatment goal is to reverse daytime hypercapnia, which is associated with improvements in health-related quality of life. In order to achieve improved ventilation and reduction of elevated carbon dioxide tension levels, high levels of pressure support may be necessary. Recent studies in patients with severe COPD demonstrated an improvement in survival, and one study reported a prolonged time to hospital readmission [62]. Treatment compliance needs to be good in order to achieve the treatment goal.
Oxygen supplementation during sleep
Long-term oxygen therapy (LTOT) has been offered to patients with nocturnal hypoxaemia [63]. However, nocturnal oxygen supplementation for patients with nocturnal, but not daytime, hypoxaemia appears not to provide important benefits to the patients [64]. LTOT has been shown to improve survival in COPD patients with severe daytime hypoxaemia, but oxygen therapy has not improved survival in patients with exercise-induced mild-to-moderate daytime hypoxaemia [65]. While oxygen therapy at night may worsen hypoventilation during sleep, a sleep-related increase in carbon dioxide rarely worsens daytime hypercapnia and acidosis [66, 67]. Nonetheless, it is recommended that overnight oxygen is used in COPD patients with comorbid SDB only in combination with CPAP or other forms of NIV [39].
Additional treatment strategies to improve sleep in COPD patients
Drug treatment of COPD
Only a few studies have evaluated the effect of COPD-specific treatment on sleep and breathing during sleep. The long-acting β-receptor agonist (LABA) salmeterol induced a significant improvement in overnight oxygenation (arterial oxygen saturation (SaO2) 91.0% on placebo, 92.9% on LABA) and a reduction of time in profound hypoxaemia during sleep (SaO2 <90% from 25.6% to 1.8%). However, sleep quality remained poor during active treatment (total sleep time 5.1 h, slow wave sleep 8.3%) [68]. Similar improvements in nocturnal SaO2 levels have been reported with the long-acting muscarinic antagonist tiotropium without improvements in objective sleep quality [69], and another, aclidinium, resulted in a significant reduction of sleep-related COPD specific complaints like breathlessness, wheezing or cough [70]. However, objective sleep data were not provided in this latter report. There is evidence that targeting COPD specific complaints during sleep with available treatment is clinically relevant in this patient group.
Insomnia treatment
Concomitant insomnia complaints are quite frequent in COPD patients and may reduce acceptance and compliance with any PAP treatment. Cognitive behavioural treatment is regarded as the first-line treatment of insomnia [71] and existing limited evidence suggests that cognitive behavioural treatment is feasible for insomnia management in COPD patients [72]. Benzodiazepines and benzodiazepine receptor agonists like zolpidem and zopiclone should be used with caution in patients with severe COPD due to a potential harmful influence on breathing during sleep [73].
Research agenda for the treatment of SDB in COPD
An agenda on research priorities has recently been proposed addressing the question whether nocturnal oxygen therapy in combination with PAP treatment modes is beneficial in overlap syndrome, whether REM-related hypoxaemia is a treatment target in COPD patients, and whether more comfortable and effective interfaces and modes for treating SDB in patients with COPD will improve sleep quality and adherence [28].
Novel therapies for the treatment of SDB in COPD have been suggested including nasal high flow therapy which may reduce dead space ventilation and work of breathing [74, 75]. In COPD patients with ongoing LTOT therapy due to hypoxic respiratory failure, nocturnal nasal high flow therapy reduced both the exacerbation frequency and the number of hospital admissions over 12 months in a recent randomised trial [76].
Sleep-related hypoventilation in neuromuscular and skeletal disorders
Hypoventilation conventionally exists when PaCO2 exceeds the upper limit of normal, which is estimated to be ∼45 mmHg [77]. Under physiological conditions, the respiratory control system effectively regulates ventilation, so that PaCO2 is maintained within a range of 39–41 mmHg. Failure of mechanisms regulating ventilation results in hypoventilation, excess accumulation of carbon dioxide and destabilisation of the acid-base balance which leads to a lowering of the pH; however, as the clinical problems are generally not acute, the pH level returns closer to normal as a result of renal compensation with retention of bicarbonate in chronic alveolar hypoventilation syndromes [78].
The decrease of minute ventilation may be generated from all parts of the respiratory system. Regardless of the cause, hypoventilation usually manifests during sleep prior to wakefulness. While obesity is the most prevalent cause of hypoventilation, the present review addresses sleep-related hypoventilation (SRH) in disorders primarily affecting the respiratory pump including NMDs and skeletal disorders affecting the chest wall.
Definitions
The definition of sleep hypoventilation has evolved over time and the most recent criteria proposed by an AASM Task Force in 2012 revised scoring criteria for sleep hypoventilation [79], which are defined as: 1) arterial carbon dioxide (or a surrogate such as transcutaneous or PETCO2) >55 mmHg for ≥10 min; or 2) an increase in carbon dioxide tension >10 mmHg from awake supine value to a value >50 mmHg for ≥10 min.
A recent study showed significant differences in the prevalence of nocturnal hypoventilation, depending on the definition used, reaching up to one-third of the patients with daytime normocapnia. This could result in practical consequences, since the indication to start therapy relies on the detection of hypoventilation [80].
Causes of SRH
SRH disorders are classified according to the third edition of the International Classification of Sleep Disorders published by the AASM, as follows [81]: obesity hypoventilation syndrome; congenital central alveolar hypoventilation syndrome; late-onset central hypoventilation with hypothalamic dysfunction; idiopathic central alveolar hypoventilation; SRH due to a medication or substance; and SRH due to a medical disorder.
Obesity hypoventilation syndrome and chronic hypoventilation due to medical disorders or medication/substance abuse represent the huge majority of chronic and SRH.
SRH in neuromuscular disorders
Neuromuscular diseases (NMDs) comprise a diverse group of disorders involving various components of the nervous system, such as muscles, neuromuscular junction, peripheral nerves and the spinal cord. All NMDS are characterised by a reduction in muscle strength; however, they vary markedly according to underlying cause, clinical presentation and rate of progression, pattern of respiratory involvement, prognosis and therapy. The major NMDs that can affect the muscles of respiration are listed in table 2.
TABLE 2.
Major neuromuscular and skeletal disorders that can provoke sleep hypoventilation
| Neuromuscular diseases | Skeletal chest wall diseases |
| Guillain-Barré syndrome | Kyphoscoliosis |
| Myasthenia gravis | Ankylosing spondylitis |
| Poliomyelitis | |
| Post-polio syndrome | |
| Amyotrophic lateral sclerosis | |
| Cervical or thoracic spinal cord injury | |
| Polymyositis | |
| Muscular dystrophies |
Several NMDs may unfavourably affect respiratory stimulant signal transmission from the brainstem respiratory centre to the respiratory muscles resulting in a reduced capacity of the respiratory system to withstand a normal respiratory load, insufficient muscle contraction or dysfunction, and hypoventilation [82]. Hypoventilation is most notable during sleep and there may also be associated sleep apnoea [83].
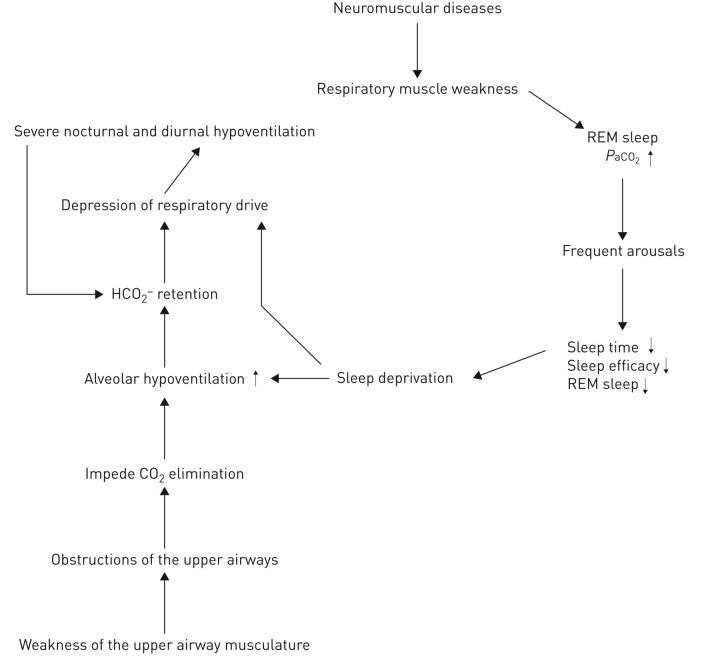
SRH is common in NMDs and may be present months to years before daytime respiratory failure ensues [84]. Patients with diaphragmatic weakness who depend on recruitment of accessory inspiratory muscles and expiratory abdominal muscles are particularly susceptible to significant hypoventilation during REM sleep. As a consequence, SRH first appears in REM sleep before progressing to NREM sleep, and then to wakefulness [85]. The rate of progression from mild REM-related hypoventilation to diurnal hypoventilation could be slow in disorders such as Duchenne muscular dystrophy or more rapid in amyotrophic lateral sclerosis [86]. In NMDs, sleep fragmentation with shorter total sleep time, frequent arousals and a significant reduction of REM sleep have been reported [87, 88]. This sleep fragmentation and sleep deficit may further suppress the arousal response and worsen hypoventilation. Furthermore, weakness of the upper airway musculature can cause obstructive events, which may be further exacerbated by an imbalance between the inspiratory force generated by the inspiratory muscles and activity of the muscles responsible for stabilising the upper airway [89]. Therefore, both SRH and OSA may coexist, such as in Duchenne muscular dystrophy, which may begin with OSA followed by SRH when diaphragmatic weakness becomes critical and end with chronic hypoventilation [90]. Upper airway obstruction further stresses the ventilatory system and may impede carbon dioxide elimination, especially during sleep (figure 4). Based on computer models, carbon dioxide may accumulate over the long term when apnoea episodes become more than three times longer than the hyperventilation period between them [91].
FIGURE 4.
Pathophysiology of sleep-related hypoventilation in neuromuscular diseases. PaO2: arterial carbon dioxide tension.
SRH in skeletal disorders
Skeletal chest wall deformities (table 2), especially scoliosis, often accompany neuromuscular disorders and may also result in SRH. The chest wall is a major component of the respiratory pump and consists of the rib cage and abdomen. Disorders affecting the skeletal structures of the chest wall, such as the thoracic spine, ribs, costovertebral joints and sternum, may lead to respiratory dysfunction. In some disorders, such as kyphoscoliosis, the load on the respiratory muscles is chronic and progressive. By contrast, in other disorders, such as ankylosing spondylitis and pectus excavatum, the impact on respiratory function is minimal.
Patients with scoliosis have reduced chest wall and lung compliance, increased work of breathing, respiratory muscle weakness, ventilation–perfusion mismatching and reduced respiratory drive. The degree of respiratory impairment is associated with the severity of spinal deformity [92]. Studies have shown decreased sleep efficiency with increased stage 1 and reduced slow-wave sleep [93, 94]. As with neuromuscular disorders, gas exchange is most disturbed during REM sleep [94–97]. SDB is highly prevalent in kyphoscoliosis and hypoventilation is the predominant form [93, 94, 97–104].
Diagnosis of SRH
As with other forms of SDB, a detailed sleep history is an essential component for the clinical evaluation. SRH during sleep may be associated with dyspnoea, orthopnea, poor sleep quality, excessive daytime sleepiness, fatigue and morning headaches. However, there are inter-individual differences depending on the underlying disease and there are also patients who report minor or no complaints [105]. Paradoxical movement of the thorax and abdomen, use of accessory muscles of inspiration, and shallow breathing are typically late signs of disease.
Monitoring of respiration and carbon dioxide levels during sleep is required to establish the diagnosis of SRH. Thus, early in-laboratory PSG with continuous carbon dioxide monitoring is indicated in this patient population. PSG is the preferred diagnostic method for assessing sleep quality and respiration in adults with neuromuscular disease and/or known or suspected hypoventilation. Respiratory polygraphy may also be used but has several limitations in this patient group, in particular to rule out relevant SRH [47]. Moreover, although continuous nocturnal pulse oximetry monitoring is readily available and may be used as a screening tool, this method is not recommended as a main diagnostic test [106].
Identification of patients at risk for SRH
A high index of clinical suspicion for nocturnal hypoventilation is necessary, especially in patients with NMDs and skeletal disorders. As hypoventilation is usually associated with long-term oxygen desaturations, awake arterial oxygen saturation measured by pulse oximetry of <95% should trigger further diagnostic evaluation for SRH [107]. Furthermore, elevated levels of bicarbonate (HCO3−) after awakening could also indicate sleep hypoventilation even if the PaCO2 is within the normal range during wakefulness [108]. Daytime measures of respiratory function have limited ability to accurately detect nocturnal hypoventilation; however, assessments of respiratory muscle strength and pulmonary functions could be important indicators of nocturnal hypoventilation and are best used in combination [109]. Vital capacity and maximal inspiratory muscle pressure have been shown to predict hypoventilation at thresholds that may vary according to the underlying NMD. Measurement of supine vital capacity is a sensitive marker of diaphragmatic dysfunction [110, 111].
Treatment of SRH
Individualised treatment is desirable due to the large heterogeneity of underlying diseases. However, treatment of causative factors is ineffective in most patients with NMDs and skeletal disorders presenting with chronic hypoventilation or SRH. Thus, symptomatic therapy with NIV has become the therapy of choice, although the NIV mode and settings must be adjusted according to the underlying pathophysiological mechanisms and the specific patient's needs [86, 91, 109, 112].
The current criteria to start ventilation in NMDs were defined in a consensus conference in 1999, and are based on daytime hypercapnia or nocturnal desaturations as an indirect sign of nocturnal hypoventilation [113]. Uncertainty remains about the most appropriate timing to start NIV, although there are data suggesting that it should be initiated early enough at the stage of nocturnal hypoventilation to prevent acute respiratory events prior to the presence of daytime hypercapnia [114, 115].
Although the optimal criteria for initiation of NIV for SRH in NMDs remain uncertain, NIV improves nocturnal oxygen saturation, diurnal and nocturnal PaCO2, subjective sleep quality, quality of life and mortality in various NMDs [61, 116–120]. There are still conflicting data regarding the effect of NIV on objective sleep quality [121–123]. Importantly, in amyotrophic lateral sclerosis, NIV appears to be the only treatment option to improve survival [87, 118, 120, 124–127]. Furthermore, NIV extends the median age of death in Duchenne's muscle dystrophy [128, 129].
There are no randomised controlled trials of outcomes when NIV is initiated early based solely on SRH. Ideally, the diagnostic criterion to start NIV should differ in rapidly progressive and less rapidly progressive NMDs. In one of the few randomised trials available, patients with nocturnal hypoventilation, defined as a peak PtcCO2 ≥49 mmHg, demonstrated benefit from home NIV even in the absence of daytime hypercapnia [115]. Thus, peak nocturnal PtcCO2 ≥49 mmHg should be considered as one of the criteria to start NIV in patients with NMDs [130].
In-laboratory titration is a preferable setting to achieve optimal NIV settings during sleep and to minimise patient–NIV asynchrony, which may decrease comfort and compliance in patients with NMDs. [116]. However, multiple training sessions in an outpatient setting may be very helpful to get NIV treatment adapted to the patient. The 2010 AASM best clinical practices guideline for NIV in stable chronic alveolar hypoventilation syndromes recommend close follow-up after initiation of NIV to establish effective utilisation patterns, assessment of respiratory function on a regular basis or if signs of clinical deterioration are present [116].
There are no large-scale studies examining the effects of SRH treatment in skeletal disorders. In small studies, nocturnal NIV has been shown to improve gas change despite mixed results on sleep architecture and sleep quality [93, 95, 98, 99, 131–133]. Daytime symptoms related to sleep hypoventilation and hospitalisation rate have been reported to be improved [60]. Additionally, survival was better when NIV was used with or without LTOT than LTOT alone [98, 99, 104, 132, 60, 134, 135], supporting the recommendation to employ NIV with or without LTOT as the first treatment option in these patients [60].
Footnotes
Provenance: Commissioned article, peer reviewed
Conflict of interest: W.T. McNicholas has nothing to disclose.
Conflict of interest: D. Hansson reports non-financial support from Itamar, during the conduct of the study.
Conflict of interest: S. Schiza has nothing to disclose.
Conflict of interest: L. Grote reports non-financial support from Itamar, personal fees from AstraZeneca, during the conduct of the study; and personal fees from Resmed, Philips, Fisher and Paykel, Itamar outside the submitted work. In addition, Dr. Grote has a patent on drug treatment in sleep apnea licensed.
References
- 1.Newton K, Malik V, Lee-Chiong T. Sleep and breathing. Clin Chest Med 2014; 35: 451–456. [DOI] [PubMed] [Google Scholar]
- 2.McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnoea – the overlap syndrome. J Thorac Dis 2016; 8: 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valipour A, Lavie P, Lothaller H, et al. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med 2011; 12: 367–372. [DOI] [PubMed] [Google Scholar]
- 4.McSharry DG, Ryan S, Calverley P, et al. Sleep quality in chronic obstructive pulmonary disease. Respirology 2012; 17: 1119–1124. [DOI] [PubMed] [Google Scholar]
- 5.Tsai SC, Lee-Chiong T. Lung hyperinflation and sleep quality in the overlap syndrome. COPD 2009; 6: 419–420. [DOI] [PubMed] [Google Scholar]
- 6.Zeidler MR, Martin JL, Kleerup EC, et al. Sleep disruption as a predictor of quality of life among patients in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS). Sleep 2018; 41: 10.1093/sleep/zsy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev 2010; 90: 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillipson EA. Control of breathing during Sleep. Am Rev Respir Dis 1978; 118: 909–939. [DOI] [PubMed] [Google Scholar]
- 9.Johnson M, Remmers J. Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol Respir Environ Exerc Physiol 1984; 57: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 10.Tabachnik E, Muller NL, Bryan AC, et al. Changes in ventilation and chest wall mechanics during sleep in normal adolescents. J Appl Physiol Respir Environ Exerc Physiol 1981; 51: 557–564. [DOI] [PubMed] [Google Scholar]
- 11.Hudgel DW, Devadatta P. Decrease in functional residual capacity during sleep in normal humans. J Appl Physiol Respir Environ Exerc Physiol 1984; 57: 1319–1322. [DOI] [PubMed] [Google Scholar]
- 12.Douglas NJ, White DP, Pickett CK, et al. Respiration during sleep in normal man. Thorax 1982; 37: 840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNicholas WT. Impact of sleep in COPD. Chest 2000; 117: Suppl. 2, 48S–53S. [DOI] [PubMed] [Google Scholar]
- 14.White JE, Drinnan MJ, Smithson AJ, et al. Respiratory muscle activity during rapid eye movement (REM) sleep in patients with chronic obstructive pulmonary disease. Thorax 1995; 50: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badr C, Elkins MR, Ellis ER. The effect of body position on maximal expiratory pressure and flow. Aust J Physiother 2002; 48: 95–102. [DOI] [PubMed] [Google Scholar]
- 16.Deegan PC, McNicholas WT. Pathophysiology of obstructive sleep apnoea. Eur Respir J 1995; 8: 1161–1178. [DOI] [PubMed] [Google Scholar]
- 17.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol (Lond) 2013; 591: 1179–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renner B, Mueller CA, Shephard A. Environmental and non-infectious factors in the aetiology of pharyngitis (sore throat). Inflamm Res 2012; 61: 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teodorescu M, Xie A, Sorkness CA, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med 2014; 10: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens RL, Macrea MM, Teodorescu M. The overlaps of asthma or COPD with OSA: a focused review. Respirology 2017; 22: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 21.McNicholas WT. COPD-OSA overlap syndrome: evolving evidence regarding epidemiology, clinical consequences, and management. Chest 2017; 152: 1318–1326. [DOI] [PubMed] [Google Scholar]
- 22.Zhao YY, Blackwell T, Ensrud KE, et al. Sleep apnea and obstructive airway disease in older men: Outcomes of Sleep Disorders in Older Men Study. Sleep 2016; 39: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg-Dotan S, Reuveni H, Tal A, et al. Increased prevalence of obstructive lung disease in patients with obstructive sleep apnea. Sleep Breath 2014; 18: 69–75. [DOI] [PubMed] [Google Scholar]
- 24.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med 2003; 167: 7–14. [DOI] [PubMed] [Google Scholar]
- 25.Krachman SL, Tiwari R, Vega ME, et al. Effect of emphysema severity on the apnea–hypopnea index in smokers with obstructive sleep apnea. Ann Am Thorac Soc 2016; 13: 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biselli P, Grossman PR, Kirkness JP, et al. The effect of increased lung volume in chronic obstructive pulmonary disease on upper airway obstruction during sleep. J Appl Physiol 2015; 119: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He B-T, Lu G, Xiao S-C, et al. Coexistence of OSA may compensate for sleep related reduction in neural respiratory drive in patients with COPD. Thorax 2017; 72: 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malhotra A, Schwartz AR, Schneider H, et al. Research priorities in pathophysiology for sleep-disordered breathing in patients with chronic obstructive pulmonary disease. An official American Thoracic Society research statement. Am J Respir Crit Care Med 2018; 197: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steveling EH, Clarenbach CF, Miedinger D, et al. Predictors of the overlap syndrome and its association with comorbidities in patients with chronic obstructive pulmonary disease. Respiration 2014; 88: 451–457. [DOI] [PubMed] [Google Scholar]
- 30.Lambert AA, Putcha N, Drummond MB, et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest 2017; 151: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons OD, Inami T, Perger E, et al. The effect of fluid overload on sleep apnoea severity in haemodialysis patients. Eur Respir J 2017; 49: 1601789. [DOI] [PubMed] [Google Scholar]
- 32.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57: 119–127. [DOI] [PubMed] [Google Scholar]
- 33.Catterall JR, Douglas NJ, Calverley PMA, et al. Transient hypoxemia during sleep in chronic obstructive pulmonary disease is not a sleep apnea syndrome. Am Rev Respir Dis 1983; 128: 24–29. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med 2015; 16: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 35.Kiely JL, Murphy M, McNicholas WT. Subjective efficacy of nasal CPAP therapy in obstructive sleep apnoea syndrome: a prospective controlled study. Eur Respir J 1999; 13: 1086–1090. [DOI] [PubMed] [Google Scholar]
- 36.Mulloy E, McNicholas W T. Theophylline in obstructive sleep apnea. A double-blind evaluation. Chest 1992; 101: 753–757. [DOI] [PubMed] [Google Scholar]
- 37.Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1993; 148: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 38.Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med 2015; 11: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNicholas WT, Verbraecken J, Marin JM. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev 2013; 22: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis CA, Fergusson W, Eaton T, et al. Isolated nocturnal desaturation in COPD: prevalence and impact on quality of life and sleep. Thorax 2009; 64: 133–138. [DOI] [PubMed] [Google Scholar]
- 41.Faria AC, da Costa CH, Rufino R. Sleep Apnea Clinical Score, Berlin Questionnaire, or Epworth Sleepiness Scale: which is the best obstructive sleep apnea predictor in patients with COPD? Int J Gen Med 2015; 8: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soler X, Liao SY, Marin JM, et al. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): the challenge to predict OSA in advanced COPD. PLoS One 2017; 12: e0177289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan N, Vestbo J, Garrow A, et al. The Manchester Respiratory-related Sleep Symptoms scale for patients with COPD: development and validation. Int J Chron Obstruct Pulmon Dis 2018; 13: 3885–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946. [DOI] [PubMed] [Google Scholar]
- 45.Bednarek M, Plywaczewski R, Jonczak L, et al. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration 2005; 72: 142–149. [DOI] [PubMed] [Google Scholar]
- 46.Rosen IM, Kirsch DB, Carden KA, et al. Clinical use of a home sleep apnea test: an updated American Academy of Sleep Medicine Position Statement. J Clin Sleep Med 2018; 14: 2075–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017; 13: 479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira MG, Nery LE, Santos-Silva R, et al. Is portable monitoring accurate in the diagnosis of obstructive sleep apnea syndrome in chronic pulmonary obstructive disease? Sleep Med 2012; 13: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 49.Vukoja M, Kopitovic I, Milicic D, et al. Sleep quality and daytime sleepiness in patients with COPD and asthma. Clin Respir J 2018; 12: 398–403. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz BD, Shapiro BA. Capnography. Respir Care Clin N Am 1995; 1: 107–117. [PubMed] [Google Scholar]
- 51.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8: 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Randerath WJ, Stieglitz S, Galetke W, et al. Evaluation of a system for transcutaneous long-term capnometry. Respiration 2010; 80: 139–145. [DOI] [PubMed] [Google Scholar]
- 53.Storre JH, Magnet FS, Dreher M, et al. Transcutaneous monitoring as a replacement for arterial PCO(2) monitoring during nocturnal non-invasive ventilation. Respir Med 2011; 105: 143–150. [DOI] [PubMed] [Google Scholar]
- 54.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2019 report. https://goldcopd.org/gold-reports
- 55.Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2019; 15: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee R, McNicholas WT. Obstructive sleep apnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2011; 17: 79–83. [DOI] [PubMed] [Google Scholar]
- 57.Marin JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010; 182: 325–331. [DOI] [PubMed] [Google Scholar]
- 58.Schreiber A, Surbone S, Malovini A, et al. The effect of continuous positive airway pressure on pulmonary function may depend on the basal level of forced expiratory volume in 1 second. J Thorac Dis 2018; 10: 6819–6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Donoghue FJ, Catcheside PG, Ellis EE, et al. Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factors. Eur Respir J 2003; 21: 977–984. [DOI] [PubMed] [Google Scholar]
- 60.Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J 2017; 49: 1600959. [DOI] [PubMed] [Google Scholar]
- 61.Kohnlein T, Windisch W, Kohler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014; 2: 698–705. [DOI] [PubMed] [Google Scholar]
- 62.Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA 2017; 317: 2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandberg D. Home oxygen program review: regionalization in Vancouver coastal health and British Columbia. Can J Respir Ther 2015; 51: 19–23. [PMC free article] [PubMed] [Google Scholar]
- 64.Lacasse Y, Bernard S, Series F, et al. Multi-center, randomized, placebo-controlled trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease: a study protocol for the INOX trial. BMC Pulm Med 2017; 17: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albert RK, Au DH, Blackford AL, et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016; 375: 1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dunn WF, Nelson SB, Hubmayr RD. Oxygen-induced hypercarbia in obstructive pulmonary disease. Am Rev Respir Dis 1991; 144: 526–530. [DOI] [PubMed] [Google Scholar]
- 67.Moloney ED, Kiely JL, McNicholas WT. Controlled oxygen therapy and carbon dioxide retention during exacerbations of chronic obstructive pulmonary disease. Lancet 2001; 357: 526–528. [DOI] [PubMed] [Google Scholar]
- 68.Ryan S, Doherty LS, Rock C, et al. Effects of salmeterol on sleeping oxygen saturation in chronic obstructive pulmonary disease. Respiration 2010; 79: 475–481. [DOI] [PubMed] [Google Scholar]
- 69.McNicholas WT, Calverley PMA, Lee A, et al. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J 2004; 23: 825–831. [DOI] [PubMed] [Google Scholar]
- 70.Kerwin EM, D'Urzo AD, Gelb AF, et al. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012; 9: 90–101. [DOI] [PubMed] [Google Scholar]
- 71.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017; 26: 675–700. [DOI] [PubMed] [Google Scholar]
- 72.Kapella MC, Herdegen JJ, Perlis ML, et al. Cognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. Int J Chron Obstruct Pulmon Dis 2011; 6: 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grote L. Drug-induced sleep-disordered breathing and ventilatory impairment. Sleep Med Clin 2018; 13: 161–168. [DOI] [PubMed] [Google Scholar]
- 74.Biselli P, Fricke K, Grote L, et al. Reductions in dead space ventilation with nasal high flow depend on physiological dead space volume: metabolic hood measurements during sleep in patients with COPD and controls. Eur Respir J 2018; 51: 1702251. [DOI] [PubMed] [Google Scholar]
- 75.Biselli PJ, Kirkness JP, Grote L, et al. Nasal high-flow therapy reduces work of breathing compared with oxygen during sleep in COPD and smoking controls: a prospective observational study. J Appl Physiol 2017; 122: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Storgaard LH, Hockey HU, Laursen BS, et al. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis 2018; 13: 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown LK. Hypoventilation syndromes. Clin Chest Med 2010; 31: 249–270. [DOI] [PubMed] [Google Scholar]
- 78.Piper AJ, Yee BJ. Hypoventilation syndromes. Compr Physiol 2014; 4: 1639–1676. [DOI] [PubMed] [Google Scholar]
- 79.Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, version 2.0. Darien, IL, American Academy of Sleep Medicine, 2012. [Google Scholar]
- 80.Ogna A, Quera Salva MA, Prigent H, et al. Nocturnal hypoventilation in neuromuscular disease: prevalence according to different definitions issued from the literature. Sleep Breath 2016; 20: 575–581. [DOI] [PubMed] [Google Scholar]
- 81.The international classification of sleep disorders: diagnostic and coding manual. Westchester, IL, American Academy of Sleep Medicine, 2005. [Google Scholar]
- 82.Benditt JO, Boitano LJ. Pulmonary issues in patients with chronic neuromuscular disease. Am J Respir Crit Care Med 2013; 187: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 83.McNicholas WT. Impact of sleep in respiratory failure. Eur Respir J 1997; 10: 920–933. [PubMed] [Google Scholar]
- 84.Piper A. Sleep abnormalities associated with neuromuscular disease: pathophysiology and evaluation. Semin Respir Crit Care Med 2002; 23: 211–219. [DOI] [PubMed] [Google Scholar]
- 85.Ragette R, Mellies U, Schwake C, et al. Patterns and predictors of sleep disordered breathing in primary myopathies. Thorax 2002; 57: 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hilbert J. Sleep-disordered breathing in neuromuscular and chest wall diseases. Clin Chest Med 2018; 39: 309–324. [DOI] [PubMed] [Google Scholar]
- 87.Bourke SC, Gibson GJ. Sleep and breathing in neuromuscular disease. Eur Respir J 2002; 19: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 88.Douglas NJ, White DP, Weil JV, et al. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis 1982; 126: 758–762. [DOI] [PubMed] [Google Scholar]
- 89.Piper AJ. Nocturnal hypoventilation – identifying & treating syndromes. Indian J Med Res 2010; 131: 350–365. [PubMed] [Google Scholar]
- 90.Khan Y, Heckmatt JZ. Obstructive apnoeas in Duchenne muscular dystrophy. Thorax 1994; 49: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simonds AK. Chronic hypoventilation and its management. Eur Respir Rev 2013; 22: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kinnear WJM. Scoliosis. In: Elliott M, Nava S, Schonhofer B, eds. Non-invasive ventilation and weaning: principles and practice. 2010. London, Hodder Arnold. pp: 379–388. [Google Scholar]
- 93.Ferris G, Servera-Pieras E, Vergara P, et al. Kyphoscoliosis ventilatory insufficiency: noninvasive management outcomes. Am J Phys Med Rehabil 2000; 79: 24–29. [DOI] [PubMed] [Google Scholar]
- 94.Mezon BL, West P, Israels J, et al. Sleep breathing abnormalities in kyphoscoliosis. Am Rev Respir Dis 1980; 122: 617–621. [DOI] [PubMed] [Google Scholar]
- 95.Masa JF, Celli BR, Riesco JA, et al. Noninvasive positive pressure ventilation and not oxygen may prevent overt ventilatory failure in patients with chest wall diseases. Chest 1997; 112: 207–213. [DOI] [PubMed] [Google Scholar]
- 96.Masa Jimenez JF, Sanchez de Cos Escuin J, Disdier Vicente C, et al. Nasal intermittent positive pressure ventilation. Analysis of its withdrawal. Chest 1995; 107: 382–388. [DOI] [PubMed] [Google Scholar]
- 97.Sawicka EH, Branthwaite MA. Respiration during sleep in kyphoscoliosis. Thorax 1987; 42: 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bach JR, Robert D, Leger P, et al. Sleep fragmentation in kyphoscoliotic individuals with alveolar hypoventilation treated by NIPPV. Chest 1995; 107: 1552–1558. [DOI] [PubMed] [Google Scholar]
- 99.Brooks D, De Rosie J, Mousseau M, et al. Long term follow-up of ventilated patients with thoracic restrictive or neuromuscular disease. Can Respir J 2002; 9: 99–106. [DOI] [PubMed] [Google Scholar]
- 100.Cirignotta F, Gerardi R, Mondini S, et al. Breathing disorders during sleep in chest wall diseases. Monaldi Arch Chest Dis 1993; 48: 315–317. [PubMed] [Google Scholar]
- 101.Ellis ER, Grunstein RR, Chan S, et al. Noninvasive ventilatory support during sleep improves respiratory failure in kyphoscoliosis. Chest 1988; 94: 811–815. [DOI] [PubMed] [Google Scholar]
- 102.Guilleminault C, Kurland G, Winkle R, et al. Severe kyphoscoliosis, breathing, and sleep: the “Quasimodo” syndrome during sleep. Chest 1981; 79: 626–630. [DOI] [PubMed] [Google Scholar]
- 103.Nauffal D, Domenech R, Martinez Garcia MA, et al. Noninvasive positive pressure home ventilation in restrictive disorders: outcome and impact on health-related quality of life. Respir Med 2002; 96: 777–783. [DOI] [PubMed] [Google Scholar]
- 104.Piper AJ, Sullivan CE. Effects of long-term nocturnal nasal ventilation on spontaneous breathing during sleep in neuromuscular and chest wall disorders. Eur Respir J 1996; 9: 1515–1522. [DOI] [PubMed] [Google Scholar]
- 105.Donic V, Tomori Z. Hypoventilation syndromes/chronic respiratory insufficiency in sleep. In: Simonds AK, De Backer W, eds. ERS Handbook Respiratory Sleep Medicine. Sheffield, European Respiratory Society, 2012; pp. 48–51. [Google Scholar]
- 106.Paiva R, Krivec U, Aubertin G, et al. Carbon dioxide monitoring during long-term noninvasive respiratory support in children. Intensive Care Med 2009; 35: 1068–1074. [DOI] [PubMed] [Google Scholar]
- 107.Piper AJ, Gonzalez-Bermejo J, Janssens J. Sleep hypoventilation: diagnostic considerations and technological limitations. Sleep Med Clin; 9: 301–313. [Google Scholar]
- 108.Boing S, Randerath WJ. Chronic hypoventilation syndromes and sleep-related hypoventilation. J Thorac Dis 2015; 7: 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Augelli DM, Krieger AC. Social and economic impacts of managing sleep hypoventilation syndromes. Sleep Med Clin 2017; 12: 87–98. [DOI] [PubMed] [Google Scholar]
- 110.American Thoracic Society, European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002; 166: 518–624. [DOI] [PubMed] [Google Scholar]
- 111.Allen SM, Hunt B, Green M. Fall in vital capacity with posture. Br J Dis Chest 1985; 79: 267–271. [PubMed] [Google Scholar]
- 112.Hess DR. Noninvasive ventilation for neuromuscular disease. Clin Chest Med 2018; 39: 437–447. [DOI] [PubMed] [Google Scholar]
- 113.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation – a consensus conference report. Chest 1999; 116: 521–534. [DOI] [PubMed] [Google Scholar]
- 114.Bach JR, Goncalves MR, Hon A, et al. Changing trends in the management of end-stage neuromuscular respiratory muscle failure: recommendations of an international consensus. Am J Phys Med Rehabil 2013; 92: 267–277. [DOI] [PubMed] [Google Scholar]
- 115.Ward S, Chatwin M, Heather S, et al. Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime normocapnia. Thorax 2005; 60: 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berry RB, Chediak A, Brown LK, et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med 2010; 6: 491–509. [PMC free article] [PubMed] [Google Scholar]
- 117.Bourke SC, Tomlinson M, Williams TL, et al. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006; 5: 140–147. [DOI] [PubMed] [Google Scholar]
- 118.Carratu P, Spicuzza L, Cassano A, et al. Early treatment with noninvasive positive pressure ventilation prolongs survival in amyotrophic lateral sclerosis patients with nocturnal respiratory insufficiency. Orphanet J Rare Dis 2009; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dorst J, Behrendt G, Ludolph AC. Non-invasive ventilation and hypercapnia-associated symptoms in amyotrophic lateral sclerosis. Acta Neurol Scand 2019; 139: 128–134. [DOI] [PubMed] [Google Scholar]
- 120.Lechtzin N, Scott Y, Busse AM, et al. Early use of non-invasive ventilation prolongs survival in subjects with ALS. Amyotroph Lateral Scler 2007; 8: 185–188. [DOI] [PubMed] [Google Scholar]
- 121.Boentert M, Brenscheidt I, Glatz C, et al. Effects of non-invasive ventilation on objective sleep and nocturnal respiration in patients with amyotrophic lateral sclerosis. J Neurol 2015; 262: 2073–2082. [DOI] [PubMed] [Google Scholar]
- 122.de Carvalho M, Costa J, Pinto S, et al. Percutaneous nocturnal oximetry in amyotrophic lateral sclerosis: periodic desaturation. Amyotroph Lateral Scler 2009; 10: 154–161. [DOI] [PubMed] [Google Scholar]
- 123.Vrijsen B, Buyse B, Belge C, et al. Noninvasive ventilation improves sleep in amyotrophic lateral sclerosis: a prospective polysomnographic study. J Clin Sleep Med 2015; 11: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dreyer P, Lorenzen CK, Schou L, et al. Survival in ALS with home mechanical ventilation non-invasively and invasively: a 15-year cohort study in west Denmark. Amyotroph Lateral Scler Frontotemporal Degener 2014; 15: 62–67. [DOI] [PubMed] [Google Scholar]
- 125.Jacobs TL, Brown DL, Baek J, et al. Trial of early noninvasive ventilation for ALS: a pilot placebo-controlled study. Neurology 2016; 87: 1878–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kleopa KA, Sherman M, Neal B, et al. BiPAP improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci 1999; 164: 82–88. [DOI] [PubMed] [Google Scholar]
- 127.Pinto AC, Evangelista T, Carvalho M, et al. Respiratory assistance with a non-invasive ventilator (BiPAP) in MND/ALS patients: survival rates in a controlled trial. J Neurol Sci 1995; 129: Suppl, 19–26. [DOI] [PubMed] [Google Scholar]
- 128.Bach JR, Martinez D. Duchenne muscular dystrophy: continuous noninvasive ventilatory support prolongs survival. Respir Care 2011; 56: 744–750. [DOI] [PubMed] [Google Scholar]
- 129.Simonds AK, Muntoni F, Heather S, et al. Impact of nasal ventilation on survival in hypercapnic Duchenne muscular dystrophy. Thorax 1998; 53: 949–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Orlikowski D, Prigent H, Quera Salva MA, et al. Prognostic value of nocturnal hypoventilation in neuromuscular patients. Neuromuscul Disord 2017; 27: 326–330. [DOI] [PubMed] [Google Scholar]
- 131.Buyse B, Meersseman W, Demedts M. Treatment of chronic respiratory failure in kyphoscoliosis: oxygen or ventilation? Eur Respir J 2003; 22: 525–528. [DOI] [PubMed] [Google Scholar]
- 132.Gonzalez C, Ferris G, Diaz J, et al. Kyphoscoliotic ventilatory insufficiency: effects of long-term intermittent positive-pressure ventilation. Chest 2003; 124: 857–862. [DOI] [PubMed] [Google Scholar]
- 133.Jager L, Franklin KA, Midgren B, et al. Increased survival with mechanical ventilation in posttuberculosis patients with the combination of respiratory failure and chest wall deformity. Chest 2008; 133: 156–160. [DOI] [PubMed] [Google Scholar]
- 134.Duiverman ML, Bladder G, Meinesz AF, et al. Home mechanical ventilatory support in patients with restrictive ventilatory disorders: a 48-year experience. Respir Med 2006; 100: 56–65. [DOI] [PubMed] [Google Scholar]
- 135.Simonds AK, Elliott MW. Outcome of domiciliary nasal intermittent positive pressure ventilation in restrictive and obstructive disorders. Thorax 1995; 50: 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]