Abstract
Nontuberculous mycobacterial pulmonary disease (NTM-PD) remains a challenging condition to diagnose and treat effectively. Treatment of NTM-PD is prolonged, frequently associated with adverse effects and has variable success. In this review, we consider the factors influencing clinicians when treating NTM-PD and discuss outcomes from key studies on the pharmacological management of Mycobacterium avium complex pulmonary disease and M. abscessus pulmonary disease. We highlight issues relating to treatment-related toxicity and provide an overview of repurposed and emerging therapies for NTM-PD.
Short abstract
Despite advances in treatment regimens for nontuberculous mycobacterial pulmonary disease, significant challenges remain. Repurposed and emerging therapies may hold promise. https://bit.ly/3IBPh9q
Background
Nontuberculous mycobacteria (NTM) are ubiquitous environmental organisms, most commonly found in water and soil, comprising all mycobacterial species other than those in the Mycobacterium tuberculosis complex and M. leprae. In humans, the commonest site of infection is the lungs; other sites include the lymph nodes, sinuses, central nervous system, skin, bones and joints [1]. NTM species are subdivided according to their speed of growth. Rapid-growing NTM can be identified within 7 days on subculture, while slow-growing NTM can take up to 12 weeks [1]. Close to 200 NTM species have been identified to date, although only a proportion of these cause clinically significant disease [2]. NTM may cause substantial pathology in the lungs, giving rise to NTM pulmonary disease (NTM-PD) characterised by nonspecific symptoms including cough, sputum production, haemoptysis, dyspnoea, weight loss, night sweats, pyrexia and fatigue [1].
The global incidence of NTM infections and NTM-PD is rising, particularly among women and older individuals. In the USA, between 2008 and 2015, incidence rose from 4.16 per 100 000 to 6.69 per 100 000 among women and from 12.70 per 100 000 to 18.37 per 100 000 among those aged ≥65 years [3]. In England, Wales and Northern Ireland, NTM isolate incidence rose from 5.6 per 100 000 to 7.6 per 100 000 between 2007 and 2012 [4]. There is no evidence for human-to-human transmission of NTM infection. However, M. abscessus subsp. massiliense clones are widely dispersed globally in patients with cystic fibrosis (CF) and there have been reports of transmission within a hospital or clinic setting; whether transmission occurred directly or indirectly between patients has not been established unequivocally [5–7]. Clustering of M. abscessus subsp. abscessus and Mycobacterium avium complex (MAC) isolates among patients with CF has also been reported [8]. Compared to M. avium, mortality risk is higher for M. intracellulare (adjusted hazard ratio (aHR) 1.40, 95% CI 1.03–1.91) and M. abscessus subsp. abscessus (aHR 2.19, 95% CI 1.36–3.51) [9].
Risk factors for developing NTM-PD can broadly be divided into environmental and host factors. Environmental factors include exposure to NTM reservoirs, such as in contaminated water supplies and soil, and high levels of environmental humidity [10–12]. Host factors include underlying structural lung diseases such as bronchiectasis, COPD and idiopathic pulmonary fibrosis [13–15]; genetic disorders such as CF, primary ciliary dyskinesia and α1-antitrypsin deficiency [16–18]; and conditions such as gastro-oesophageal reflux disease and rheumatoid arthritis [19, 20]. Decrease in body mass index (BMI) has been associated with increased NTM-PD incidence [21]. Irrespective of the aetiology, from a pathophysiological perspective it is bronchiectasis that plays a central role in the development of NTM-PD. Increased risk is also associated with impaired immunity secondary to immunosuppressive agents such as high-dose inhaled corticosteroids and anti-tumour necrosis factor (TNF)-α therapies [22, 23]. Interleukin-12–interferon (IFN)-γ–STAT1 pathway mutations and AIDS are usually associated with disseminated NTM infection [24, 25].
In this review, we consider the factors influencing clinicians when treating NTM-PD and discuss outcomes from key studies on the pharmacological management of MAC pulmonary disease (MAC-PD) and M. abscessus (MAB) pulmonary disease (MAB-PD). We highlight issues relating to treatment-related toxicity and provide an overview of repurposed and emerging therapies for NTM-PD.
Diagnosis and treatment considerations
Diagnosis of NTM-PD is contingent upon meeting clinical, radiographic and microbiological criteria [26]. Clinical criteria require the presence of pulmonary or systemic symptoms compatible with NTM-PD and exclusion of other potential causes. Radiological criteria require a chest radiograph showing nodular or cavitary opacities, or a computed tomography (CT) chest scan showing multifocal bronchiectasis with multiple small nodules. To meet microbiological criteria, patients must have at least two sputum culture results positive for the same species of NTM (or subspecies in the setting of M. abscessus); or one bronchial wash or bronchial lavage culture result positive for NTM; or one lung biopsy with histology consistent with NTM plus one biopsy or sputum culture result positive for NTM. Diagnostic criteria are pertinent to the small number of NTM species known to be pathogenic. For commonly isolated NTM environmental contaminants and for rarely isolated NTM species, clinicians must be cautious and are encouraged to obtain expert consultation.
International clinical guidelines and consensus recommendations on managing NTM-PD have been published [26–28]. NTM-PD treatment is prolonged, associated with frequent adverse effects and has variable success. In an observational study of 170 patients with NTM-PD caused mainly by MAC, M. kansasii or M. xenopi, NTM therapy failed in 4.1%, disease recurred in 11.2% and treatment was halted in 13.5%, mainly due to treatment intolerance [29]. A survey of >3500 physicians in France, Germany, Italy, Spain, the UK and Japan revealed poor physician adherence to treatment guidelines for MAC-PD: only 9.2% of patients from the European countries and 41.9% of patients from Japan received >6 months of treatment [30]. In another survey of physicians treating NTM-PD, clinicians reported that factors influencing their decision to start treatment included symptoms such as breathlessness, persisting fever or chronic cough; NTM species isolation in multiple sputum or bronchoalveolar lavage (BAL) samples; isolation of MAC, M. abscessus subsp. abscessus or M. kansasii; and concomitant bronchiectasis, CF or HIV infection [31]. In addition, clinicians are more inclined to start treatment when there is a clinical history of night sweats or weight loss; and high-resolution CT chest imaging consistent with cavitary disease [32].
Compared to stable MAC-PD, progressive MAC-PD has been more associated with positive sputum smear and radiographic changes consistent with fibrocavitary and more extensive disease [33]. Patients with stable MAC-PD are more likely than patients with progressive disease to have a higher BMI and fewer systemic symptoms at the time of diagnosis [33]. A multivariate analysis has shown that treatment initiation for noncavitary nodular-bronchiectatic MAC-PD is predicted by patient age ≤60 years, positive sputum smear, systemic symptoms, BMI >18.5 kg·m−2 and having at least four lobes affected by disease [34]. However, it must be acknowledged that initiating treatment remains a clinical decision that takes account of multiple factors. Consideration should be given to the presence of comorbidities that increase mortality risk in NTM-PD, including concurrent chronic pulmonary aspergillosis, malignancy and chronic cardiac or hepatic disease; in addition to old age and low BMI [9]. It is important to note that factors that increase the likelihood of poor outcomes are understandably more likely to lead to the decision to initiate therapy, but the majority of these studies have documented poor outcomes despite therapy. Therefore, it is important that in addition to clinical factors a shared decision-making process that also takes account of patients’ preferences and treatment tolerance is followed. Rates of success, relapse or reinfection need to be considered before determining whether to proceed with treatment [35] and determining the goal of treatment (which may not always be culture conversion and may in some patients be stability or symptom relief) is critical before embarking on therapy.
Equally complex decision-making processes are required for those patients with poor clinical trajectories despite treatment, again with reference to the overall realistic benefit and harm of any therapy. Correct identification of the NTM species and drug susceptibility testing of isolates is important to guide appropriate antibiotic use in selected circumstances. NTM-PD guidelines recommend using in vitro susceptibility testing for selecting antibiotics, but notably, aside from macrolides and amikacin, there are currently no other antibiotics for which an association exists between in vitro susceptibility and clinical response for either MAC or MAB [36]. Furthermore, the use of sustained culture conversion to define treatment end-points is limited by the fact that it relies on the quality of sputum provided by patients; decisions regarding management have to be made before sputum results are available as they can take up to 8 weeks to culture; and culture conversion on its own may not be linked to symptomatic improvement [37]. Surgical intervention, such as resection of diseased lung, may need to be considered in patients who either fail to respond to medical therapy or in whom there are complicating factors such as significant cavitary disease, bronchiectasis or haemoptysis [26].
MAC-PD treatment
Treatment of MAC-PD typically comprises a macrolide, a rifamycin and ethambutol (table 1) [26]. In nodular-bronchiectatic MAC-PD, clinical guidelines give a conditional recommendation for these drugs to be taken three times weekly, whereas a daily regimen is recommended for advanced or severe nodular-bronchiectatic MAC-PD. In cavitary NTM-PD, intravenous amikacin or intramuscular/intravenous streptomycin may be given in addition. The regimen for refractory MAC-PD entails at least four drugs: a macrolide, a rifamycin, ethambutol and amikacin liposome inhalation suspension. A daily dosing frequency is recommended for both cavitary NTM-PD and refractory NTM-PD, although aminoglycosides may be given three times weekly. Treatment should continue until 12 months post-culture conversion [26].
TABLE 1.
Treatment regimens for Mycobacterium avium complex pulmonary disease and M. abscessus pulmonary disease
| Treatment regimen | Frequency | |
| M. avium complex | ||
| Nodular-bronchiectatic | Macrolide (azithromycin or clarithromycin) Rifamycin (rifampicin or rifabutin) |
3 times per week (daily if advanced or severe disease) |
| Ethambutol | ||
| Cavitary | At least 3 of: Macrolide (azithromycin or clarithromycin) Rifamycin (rifampicin or rifabutin) Ethambutol |
Daily (or 3 times per week for aminoglycosides) |
| Systemic aminoglycoside (amikacin (intravenous) or streptomycin) | ||
| Refractory | At least 4 of: Macrolide (azithromycin or clarithromycin) Rifamycin (rifampicin or rifabutin) Ethambutol |
Daily (or 3 times per week for parenteral aminoglycosides) |
| Amikacin liposome inhalation suspension or systemic aminoglycoside (amikacin (intravenous) or streptomycin) | ||
| M. abscessus | ||
| Macrolide susceptible | Initial phase At least 3 of: 1–2 parenteral: amikacin, imipenem (or cefoxitin), tigecycline |
Daily (or 3 times per week for parenteral aminoglycosides) |
| 2 oral: macrolide (azithromycin or clarithromycin), clofazimine, linezolid | ||
| Continuation phase | ||
| At least 2 of: | ||
| 2–3 oral or inhaled: macrolide (azithromycin or clarithromycin), clofazimine, linezolid, inhaled amikacin | ||
| Inducible macrolide resistance | Initial phase At least 4 of | Daily (or 3 times per week for parenteral aminoglycosides) |
| 2–3 parenteral: amikacin, imipenem (or cefoxitin), tigecycline | ||
| 2–3 oral: macrolide (azithromycin or clarithromycin), clofazimine, linezolid | ||
| Continuation phase | ||
| At least 2 of: | ||
| 2–3 oral or inhaled: macrolide (azithromycin or clarithromycin), clofazimine, linezolid, inhaled amikacin | ||
| Constitutive macrolide resistance (with or without inducible macrolide resistance) | Initial phase At least 4 of: | Daily (or 3 times per week for parenteral aminoglycosides) |
| 2–3 parenteral: amikacin, imipenem (or cefoxitin), tigecycline | ||
| 2–3 oral: macrolide (azithromycin or clarithromycin), clofazimine, linezolid | ||
| Continuation phase | ||
| At least 2 of: | ||
| 2–3 oral or inhaled: macrolide (azithromycin or clarithromycin), clofazimine, linezolid, inhaled amikacin |
Information from [26].
Macrolide-containing regimens
Macrolide-containing regimens for MAC-PD have long been established as having better outcomes than those without macrolides [38]. A key determinant as to whether MAC-PD treatment will be successful with the standard combination of rifampicin, ethambutol and a macrolide is whether the isolate exhibits macrolide susceptibility. Mutations in the peptidyltransferase region of the 23S rRNA gene confer MAC isolates with clarithromycin resistance [39, 40]. In a recent meta-analysis, 1-year all-cause mortality in patients with macrolide-resistant MAC-PD was 10% [41]. Survival outcomes for patients with macrolide-resistant MAC-PD are not significantly different to those seen in patients with multidrug-resistant tuberculosis (MDR-TB) [42].
A study in which patients with macrolide-susceptible MAC-PD were treated with macrolide-containing regimens demonstrated sputum culture conversion in 86% of patients; no cases of macrolide resistance arose [43]. Despite 48% of patients having microbiological recurrences, overall treatment success, defined as sputum culture conversion with no subsequent microbiological relapse, occurred in 84% [43]. A comparison of clarithromycin-containing regimens for MAC-PD found that after 18 months of treatment, regimens containing a clarithromycin dose of 400 mg·day−1 had a significantly lower rate of sputum culture conversion than regimens containing 800 mg·day−1 [44]. A meta-analysis comprising 1462 patients treated for MAC-PD with macrolide-containing regimens found that the treatment success rate was 60.0% (95% CI 55.1–64.8%) and that 6.4% of patients required either macrolide dose modification or cessation due to toxicity [45].
Number of drugs
Miwa et al. [46] demonstrated noninferiority between a two-drug regimen that comprises clarithromycin and ethambutol, for which the sputum culture conversion rate was 55.0%, and a three-drug regimen consisting of clarithromycin, ethambutol and rifampicin, for which the conversion rate was 40.6%. Notably, this study used just clarithromycin as the macrolide. Levels of the latter may have been reduced in the three-drug regimen due to an interaction with rifampicin and the doses of clarithromycin used were below the standard (200 mg three times daily). In addition, this study was not blinded and dropout numbers were significant. In a follow-up study, the two-drug regimen did not cause a higher incidence of clarithromycin-resistance than the three-drug regimen, although it was underpowered to detect this [47]. A recent study compared outcomes among MAC-PD patients treated with a standard regimen of rifampicin, ethambutol and a macrolide with outcomes for patients treated with ethambutol and clarithromycin either with or without a fluoroquinolone; or with rifampicin, clarithromycin and a fluoroquinolone. The alternative regimens were associated with sputum nonconversion, treatment failure and the development of clarithromycin resistance [48]. Current guidelines suggest that there is not enough evidence at present for a two-drug regimen and three drugs are recommended [26]. It is possible that two-drug regimens may be suitable in those with milder disease and there are clinical trials ongoing to assess this [49].
Amikacin liposome inhalation suspension
Amikacin liposome inhalation suspension (ALIS) refers to amikacin that has been encapsulated in liposomes and can be administered by aerosol delivery. By delivering amikacin directly into the respiratory tract, it reduces adverse effects such as nephrotoxicity and ototoxicity associated with systemic administration, and allows a higher drug concentration to be achieved in the lungs [50–52]. In addition, liposomal formulations are able to penetrate biofilms and macrophages, although there are currently limited data demonstrating that this can be achieved in vivo [50, 52, 53]. Pharmacokinetic studies have demonstrated that delivery of amikacin by this inhalation route once daily results in lower systemic exposure to amikacin compared to parenteral administration [54]. Negative sputum culture results obtained from MAC-PD patients during treatment with ALIS have been shown to reflect the true microbiological status of patients’ lungs rather than being confounded by the presence of residual amikacin in the sputum sample [55].
In the CONVERT study on patients with treatment-refractory MAC-PD, addition of ALIS to guideline-based therapy (GBT) resulted in a significantly higher rate of culture conversion compared to GBT alone (29.0% versus 8.9%, OR 4.22, 95% CI 2.08–8.57; p<0.001), while rates of adverse events were similar in both groups [56]. In an extension open-label study, patients with refractory MAC-PD who did not achieve culture conversion by month 6 in the aforementioned study were invited to enrol for once-daily ALIS in addition to GBT for 12 months. Among the cohort that was previously ALIS-naïve, sputum culture conversion occurred in 26.7% by month 6 and 33.3% by month 12, while among those who had received ALIS previously, it occurred in 46.6% by month 6 and 13.7% by month 12 [57]. Serious treatment-emergent adverse events were reported in both cohorts (35.6% and 27.4% in ALIS-naïve and prior-ALIS, respectively) [57].
A study evaluating the sustainability and durability of culture conversion found a higher rate of sustained culture conversion both at the end of 12 months post-conversion treatment and following 3 months off all treatment in patients treated using ALIS plus GBT versus GBT alone (16.1% versus 0%, p<0.0001) [58]. Among patients who received ALIS plus GBT and whose sputum converted, 63.1% remained culture-negative at 3 months and 53.8% at 12 months off treatment, whereas no patients who received GBT alone and converted their sputum remained culture negative at 3 months off treatment [58].
MAB-PD treatment
Clinical guidelines recommend that treatment regimens for MAB-PD comprise an induction phase that includes intravenous therapy for several weeks to months, followed by a continuation phase with oral or inhaled antibiotics for ⩾12 months following sputum culture conversion (table 1) [26]. However, it must be recognised that this phase approach to MAB-PD therapy has arisen not due to clinical evidence favouring its use over continuous intravenous treatment, but rather due to a pragmatic need to avoid the toxicity of prolonged parenteral therapy and the lack of alternative therapeutic options [36]. While phase therapy is effective in treating macrolide-susceptible MAB-PD, it is less effective at achieving and sustaining an effective response in macrolide-resistant MAB-PD [36].
Macrolide-containing regimens
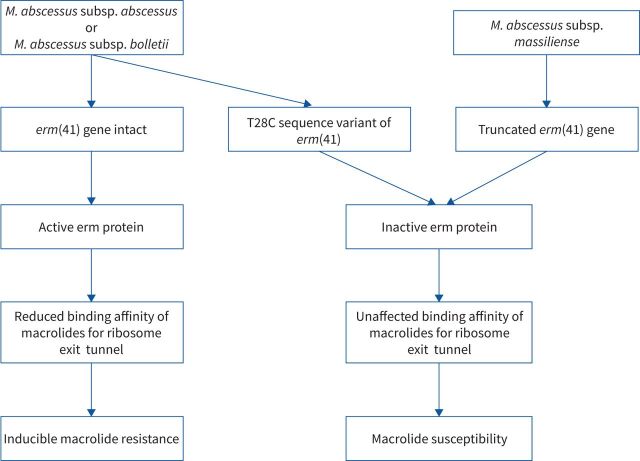
MAB-PD treatment should be tailored to the subspecies that is cultured and guided by the macrolide susceptibility of the isolate. M. abscessus subsp. abscessus and M. abscessus subsp. bolletii exhibit inducible macrolide resistance as they possess an intact erythromycin resistance methylase (erm) gene called erm(41) (figure 1) [59, 60]. Notably, a T28C sequence variant of the erm(41) gene has been shown to confer isolates with macrolide susceptibility [61]. M. abscessus subsp. massiliense possesses a truncated erm(41) gene due to a frameshift mutation, deletion and truncated C-terminal region, conferring this subspecies with macrolide susceptibility [62]. Mutations in the 23S rRNA (rrl) gene confer MAB isolates with constitutive macrolide resistance [63].
FIGURE 1.
Inducible macrolide resistance in Mycobacterium abscessus (MAB) isolates: flowchart demonstrating the mechanisms underlying inducible macrolide resistance and macrolide susceptibility in MAB isolates. erm: erythromycin resistance methylase.
Macrolides exhibit immunomodulatory properties [64]. Guidelines recommend that macrolides may be used for treating macrolide-resistant MAB-PD for their immunomodulatory effects, but should not be considered an active drug in the treatment regimen in this context [26]. A meta-analysis has evaluated the success rate of macrolide-containing combination therapy in MAB-PD: 34% of patients with new M. abscessus subsp. abscessus lung disease and 54% of patients with M. abscessus subsp. massiliense lung disease achieved sustained sputum culture conversion; this reduced to 20% in patients with refractory disease [65]. In a study of 14 patients with macrolide-susceptible MAB-PD in which the MAB isolates all had a nonfunctional erm(41) gene, 93% of patients achieved sputum culture conversion following macrolide-based treatment [66]. Macrolide resistance is associated with poor outcomes, with one study reporting a 5-year mortality rate of 33% among patients with macrolide-resistant M. abscessus subsp. massiliense lung disease [67]. It is as important to guard against the acquisition of macrolide resistance-conferring mutations in MAB isolates as it is to achieve MAB eradication [36].
Number of drugs
A study in patients with MAB-PD (not further subspeciated) found that an initial regimen of intravenous amikacin and cefoxitin for 4 weeks combined with clarithromycin, ciprofloxacin and doxycycline resulted in symptomatic improvement in 83% of patients, radiographic improvement in 74% and sputum culture conversion and maintenance of negative cultures for 12 months in 58%, but success rates were lower among those with clarithromycin-resistant isolates [68]. In another study in which 41 patients with MAB-PD were treated using either a macrolide with amikacin or a macrolide with two parenteral agents (amikacin and cefoxitin or imipenem) for a total antibiotic treatment median duration of 511 days, treatment success occurred in 80.5%, failure in 12.2% and default in 7.3% [69]. There was no significant difference in treatment success or relapse rates between the two treatment groups [69]. In a study of 69 MAB-PD patients who were followed up for a mean 34 months and among whom 97% received a macrolide while 74% received a macrolide and intravenous amikacin with or without an additional antibiotic, 29% remained culture positive, 23% converted but subsequently relapsed, 48% converted and did not relapse and 16% died [70]. In the absence of studies directly comparing outcomes between different regimens and taking account of both the severity of MAB-PD and the potential rate of progression, guidelines recommend including at least three active drugs when treating MAB-PD [26]. Notably there still remains a need for validated in vitro and animal models capable of predicting patients’ responses to antibiotics [36].
Treatment-related toxicity
Drug toxicity associated with NTM-PD treatment must be closely monitored in accordance with guidelines (table 2) [26]. An observational study of 170 patients with NTM-PD, in whom the majority of infections were caused by MAC, M. kansasii and M. xenopi, found that 37.6% of patients experienced adverse effects over a median 31 months, mainly due to drug intolerance, attributed but not limited to abdominal pain, pruritus and deteriorating performance status [29]. While treatment was halted at a higher frequency among non-MAC than MAC patients (22.4% versus 9.9%, p=0.030), unsuccessful outcomes were similar in both groups (34.7% versus 35.5%) [29].
TABLE 2.
Adverse effects of selected commonly used drugs in the treatment of Mycobacterium avium complex pulmonary disease and M. abscessus pulmonary disease
| Adverse effects | |
| Amikacin (intravenous) | Electrolyte disturbance, nephrotoxicity, ototoxicity, vestibular toxicity |
| Amikacin (liposome inhalation suspension) | Cough, dysphonia, dyspnoea, nephrotoxicity, ototoxicity, vestibular toxicity |
| Azithromycin | Gastrointestinal, hearing loss/tinnitus, hepatotoxicity, prolonged QTc |
| Cefoxitin | Cytopenias, hypersensitivity |
| Clarithromycin | Gastrointestinal, hearing loss/tinnitus, hepatotoxicity, prolonged QTc |
| Clofazimine | Dermatological (skin tanning and dryness), hepatotoxicity, prolonged QTc |
| Ethambutol | Neuropathy, ocular toxicity |
| Imipenem | Cytopenias, nephrotoxicity, rashes |
| Linezolid | Cytopenias, neuropathy, optic neuritis |
| Rifabutin | Cytopenias, hepatoxicity, hypersensitivity, red-orange discolouration of secretions, uveitis |
| Rifampicin | Cytopenias, hepatoxicity, hypersensitivity, red-orange discolouration of secretions |
| Streptomycin | Electrolyte disturbance, nephrotoxicity, ototoxicity, vestibular toxicity |
| Tigecycline | Nausea, hepatitis, pancreatitis, vomiting |
Reproduced and modified from [26].
In a study of 364 patients receiving long-term treatment for MAC-PD, the most common adverse effects included thrombocytopenia (28.6%) at median 61.5 days following treatment commencement, leukocytopenia (20.0%) at 41 days, hepatotoxicity (19.5%) at 55 days, raised serum creatinine (12.4%) at 430.5 days, dermatological reactions (9.3%) at 30 days and ocular toxicity (7.7%) at 278 days [71]. Ototoxicity with low-dose amikacin in the treatment of NTM-PD has been particularly associated with female sex (OR 4.96, 95% CI 1.24–19.87) and total dose of amikacin per bodyweight (OR 1.62, 95% CI 1.08–2.43) [72]. In the CONVERT study, 17.5% of patients receiving ALIS with GBT experienced treatment-emergent adverse events that resulted in ALIS discontinuation [56]. Treatment with ALIS plus GBT was associated with higher rates of tinnitus, dizziness and hearing loss than when using GBT alone [56]. Other notable treatment-emergent adverse events included dysphonia, which occurred in 45.7% of patients in the ALIS plus GBT arm compared to 0.9% in the GBT-only arm, and cough, which occurred in 37.2% of the former arm compared to 15.2% of the latter [56]. Fluoroquinolone use in mycobacterial disease treatment may be associated with various side-effects [73]. In the treatment of MAC-PD, fluoroquinolone-containing regimens have a similar rate of adverse effects to standard MAC-PD regimens [74].
There are limited data regarding the appropriate course of action when NTM drug-related adverse events occur. A survey of NTM experts resulted in a general consensus, albeit with caveats, to guide decision making when managing drug toxicity. This included preferences for watchful waiting in cases of mild rifampicin-induced hepatotoxicity, halting rifampicin in severe hepatotoxicity before cautious reintroduction when appropriate, decreasing the dose of azithromycin when it is associated with tinnitus or diarrhoea and stopping ethambutol if associated with ocular toxicity [75].
Repurposed and emerging treatments
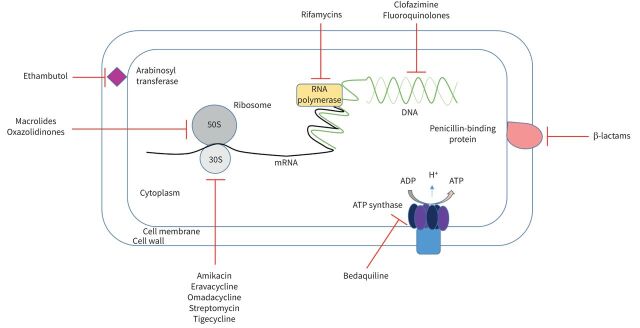
While there has been considerable focus on new drug pipelines for drug-resistant TB, the number of active trials for new agents for NTM-PD is more limited [76]. Various drugs repurposed for treating NTM-PD have been under investigation and synergy between existing antimicrobials to potentiate anti-NTM activity is a promising avenue. For example, rifabutin synergises with clarithromycin or tigecycline both in dual and triple therapy combinations in the treatment of M. abscessus cultures [77]. The sites of action of current and emerging therapies for NTM-PD are summarised in figure 2.
FIGURE 2.
Site of action of current and emerging treatments for nontuberculous mycobacterial (NTM) pulmonary disease: schematic showing the site of action of antimicrobials against NTM.
Oral agents
Bedaquiline, a diarylquinoline that inhibits mycobacterial ATP synthase, is approved for use in MDR-TB [78]. A case series found that among six patients with MAC-PD and four with MAB-PD, of whom eight patients had isolates exhibiting macrolide resistance, 50% of the group had one or more negative cultures following 6 months of bedaquiline treatment [79]. Development of bedaquiline resistance, as evidenced by whole genome sequencing and a rise in minimum inhibitory concentration (MIC) following bedaquiline treatment, has been reported in MAC-PD [80]. A phase II/III clinical trial evaluating the efficacy and safety of bedaquiline-containing regimens for the treatment of MAC-PD was suspended recently [81]. Adding clofazimine to bedaquiline improves the bacteriostatic effect of the latter against M. abscessus (although this appears to promote bedaquiline resistance), and results in a modest effect on the bactericidal effect of bedaquiline against M. avium, while slowing emergence of bedaquiline resistance [82]. In vitro studies have demonstrated that co-administering bedaquiline with imipenem and cefoxitin results in inhibition of the bactericidal ATP burst normally induced by the latter two drugs, diminishing their bactericidal activity against MAB [83].
Tedizolid, an oxazolidinone, is a potential alternative to linezolid in cases of linezolid-associated myelotoxicity, nephrotoxicity or gastrointestinal intolerance when treating NTM-PD [84]. An in vitro study has shown that tedizolid exhibits concentration-dependent activity against M. avium, which is enhanced when combined with ethambutol, and it has weak bacteriostatic activity against M. abscessus, which is enhanced when combined with amikacin [85]. A retrospective cohort study in which solid-organ transplant recipients were treated for NTM infection with regimens containing either tedizolid or linezolid found no significant difference in the safety profiles of the two drugs [86]. Long-term tedizolid administration was well-tolerated and efficacious in a patient with pulmonary TB and a liver transplant due to hepatic failure secondary to anti-TB medications [87]. However, a case series reported that tedizolid probably contributed to anaemia developing in a patient with pulmonary mycobacterial infection and thrombocytopenia in a patient with disseminated mycobacterial infection [88].
Novel tetracycline analogues have been investigated. Omadacycline, an aminomethylcycline available in both oral and intravenous formulations, is approved by the United States Food and Drug Administration (FDA) for treating community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections. In vitro studies have shown that omadacycline has potent activity against several rapid-growing NTM and its activity against MAB, M. chelonae and M. fortuitum is similar to tigecycline [89–91]. A case report has described how the use of a 4-week course of omadacycline with amikacin and aztreonam for treating MAB-PD resulted in symptomatic improvement and radiographic stability at 1-month follow-up in a patient who had previously failed NTM-PD treatment [92]. In a case series of four patients who were treated for MAB disease at different sites, omadacycline was tolerated for >7 months in a patient with MAB-PD, although they required a lobectomy 5 months into omadacycline treatment [93]. In another case series in which seven out of 12 patients had MAB-PD, omadacycline therapy was associated with treatment success in five out of seven MAB-PD patients, while treatment failure in MAB-PD occurred in one patient with fibrocavitary disease and one patient with nodular-bronchiectatic disease with dissemination [94]. Gastrointestinal, renal and hepatic adverse effects were reported in one patient each [94]. A phase II clinical trial evaluating the use of omadacycline in treating MAB-PD is currently in progress [95]. Eravacycline, a fluorocycline, is approved by the FDA for treating complicated intra-abdominal infections in an intravenous formulation. It exhibits in vitro activity against MAB, although the MIC required to inhibit the growth of 50% (MIC50) and 90% (MIC90) of MAB is two-fold lower than that of tigecycline and omadacycline [96].
Intravenous agents
The use of dual β-lactam agents has shown potential. In vitro studies have shown that ceftazidime synergises with imipenem or ceftaroline, resulting in significantly lower MICs in the treatment of MAB isolates [97]. Other in vitro studies have shown that amoxicillin synergises with imipenem-relebactam against MAB, as does cefoxitin with imipenem [98, 99]. A ceftaroline–imipenem combination has been shown to reduce the MIC50 and MIC90 of MAB isolates [100]. The addition of β-lactamase inhibitors such as relebactam or vaborbactam to cephalosporins or carbapenems also results in enhanced anti-MAB activity in vitro [101], although combining either of these β-lactamase inhibitors with ceftaroline and imipenem has been shown to have a modest impact on isolate susceptibility compared to using the β-lactams alone [100]. Notably the addition of relebactam to ceftaroline–imipenem does not appear to enhance the activity of the latter combination [102]. Avibactam has been shown to inhibit β-lactamase produced by MAB in vitro and in vivo in a zebrafish model [103]. However, the relevance of these in vitro studies of synergy does remain theoretical and their utility in clinical practice remains to be seen.
Inhaled agents
Recent studies have investigated the utility of inhaled antibiotics other than amikacin for treating MAB-PD. In a case series of two paediatric MAB-PD patients with poor response to various inhaled regimens, inhaled imipenem–cilastatin was well-tolerated and resulted in lung function stability [104]. In a granulocyte–macrophage colony-stimulating factor (GM-CSF) knockout mouse model of MAB infection, inhaled high-dose tigecycline was found to be effective in a dose-dependent manner at reducing pulmonary bacterial load and did not cause toxicity [105]. In another mouse study, clofazimine inhalation suspension demonstrated MICs of 0.125–2 μg·mL−1 for M. abscessus and MAC, achieving a four-fold higher concentration in the lungs than oral clofazimine and remaining well-tolerated at high doses [106].
The potential of nonantibiotic inhaled therapies has also been the focus of much investigation. A case report has described how inhaled IFN-γ improved NTM clearance in patients with IFN-γ deficiency [107]. However, a large study in MAC-PD patients found that inhaled IFN-γ was poor at promoting sputum culture conversion among those with cavitary disease and at improving symptoms among those with a history of treatment for MAC-PD, bronchiectasis and COPD [108]. In another study in MAC-PD patients, administering adjuvant intramuscular IFN-γ was associated with higher treatment response rates relative to placebo (72% versus 36%, p=0.037) and fewer disease-related deaths (11.1% versus 35.7%) [109].
GM-CSF is a cytokine that activates and mediates differentiation of macrophages [110]. In a GM-CSF knockout mouse model, exposure to aerosolised M. abscessus has been shown to result in chronic pulmonary NTM infection becoming established [111], highlighting a role of GM-CSF in protection against NTM infections. Building on this, a novel case report described how administration of adjuvant inhaled GM-CSF was associated with improved lung function and culture conversion in two patients with CF and MAB-PD who were previously on a deteriorating clinical trajectory [112]. A phase II trial on the use of nebulised recombinant human GM-CSF for the treatment of chronic NTM infection in patients with CF was terminated in 2021 due to limitations related to coronavirus disease 2019 and the primary end-point being confounded by changes to the standard of care for CF during the study period [113].
Case reports on the use of inhaled nitric oxide (NO) in patients with CF and MAB-PD have described variable outcomes. In one case, administration of 160 ppm inhaled NO for 21 days followed by 240 ppm for 8 days resulted in improvement in quality of life, lung function and 6-min walk distance (6MWD), but not MAB eradication; the higher dose NO was stopped due to intolerance [114]. In a case series comprising two patients, intermittent inhalations of 160 ppm NO resulted in significant reductions in MAB load [115]. In a pilot study including nine patients with CF and MAB-PD, intermittent inhaled NO treatment resulted in improvements in forced expiratory volume in 1 s and 6MWD, but culture conversion was not achieved [116]. The results of a proof-of-concept study on using inhaled NO in NTM-PD are awaited [117]. A pilot study assessing the safety of intermittent inhaled NO in treating MAC-PD and MAB-PD in patients with and without CF is underway [118]. Another phase II clinical trial investigating inhaled NO administration in patients with CF and MAB-PD is due to commence in 2021 [119].
Novel therapeutics
Benzimidazoles such as SPR719 and EJMCh-6 have exhibited potency in vitro against various NTM species [120, 121]. A phase IIa study on using the benzimidazole SPR720 in MAC-PD had started recruiting in the USA, but was terminated due to a FDA hold [122]. Fragment-based screening approaches have been used to identify potential therapeutic target sites in M. abscessus [123]. In vitro inhibitors have been developed against M. abscessus tRNA-(N(1)G37) methyltransferase (TrmD), an enzyme that prevents translational frameshift errors [124]. This may hold therapeutic potential, as such compounds have been found to be effective against M. leprae and M. tuberculosis [125]. Therapy with engineered bacteriophages was associated with clinical improvement, including in lung function, in a patient with CF with disseminated drug-resistant MAB infection [126]. Advances in understanding the structural proteome of MAB will be valuable in the search for new drug targets [127]. A summary of repurposed and emerging treatments for NTM-PD is provided in table 3.
TABLE 3.
Summary of repurposed and emerging treatments for nontuberculous mycobacterial pulmonary disease (NTM-PD)
| Evidence | References | |
| Bedaquiline | 6 months of bedaquiline treatment associated with one or more negative cultures among 50% of a cohort of 10 patients with NTM-PD | [79] |
| Adding clofazimine to bedaquiline in vitro improves bacteriostatic effect of bedaquiline against M. abscessus (but promotes bedaquiline resistance); has modest effect on bactericidal effect of bedaquiline against M. avium (and slows emergence of bedaquiline resistance) | [82] | |
| Tedizolid | Concentration-dependent activity in vitro against M. avium; enhanced when combined with ethambutol Weak bacteriostatic activity in vitro against MAB; enhanced when combined with amikacin |
[85] |
| Well-tolerated and efficacious in patient with pulmonary TB and liver transplant due to hepatic failure secondary to anti-TB medications | [87] | |
| No significant difference in safety profile between tedizolid and linezolid when used to treat NTM infections in solid-organ transplant recipients | [86] | |
| Probably contributed to anaemia in patient with pulmonary mycobacterial infection, and thrombocytopenia in patient with disseminated mycobacterial infection | [88] | |
| Omadacycline | Potent activity against rapid-growing NTM in vitro; similar activity to tigecycline against MAB, M. chelonae and M. fortuitum | [89–91] |
| Symptomatic improvement and radiographic stability at 1-month follow-up in a patient who had previously failed NTM-PD treatment following treatment with 4-week course of omadacycline plus amikacin and aztreonam | [92] | |
| Tolerated for >7 months in a patient with MAB-PD; lobectomy required 5 months into omadacycline treatment | [93] | |
| Associated with treatment success in five out of seven patients with MAB-PD; treatment failure in MAB-PD in one patient with fibrocavitary disease and one patient with nodular-bronchiectatic disease with dissemination | [94] | |
| Eravacycline | In vitro activity against MAB; MIC50 and MIC90 two-fold lower than tigecycline and omadacycline | [96] |
| Dual β-lactams | Ceftazidime synergises with imipenem or ceftaroline against MAB in vitro | [97] |
| Amoxicillin synergises with imipenem–relebactam against MAB in vitro | [98] | |
| Cefoxitin synergises with imipenem against MAB in vitro | [99] | |
| Ceftaroline synergises with imipenem against MAB in vitro | [100] | |
| Inhaled antibiotics (other than ALIS) | Inhaled imipenem–cilastatin well-tolerated and resulted in lung function stability in two paediatric MAB-PD patients | [104] |
| Inhaled high-dose tigecycline effective in a dose-dependent manner at reducing pulmonary bacterial load in a GM-CSF knockout model of MAB infection | [105] | |
| Clofazimine inhalation suspension achieved four-fold higher concentration in the lungs than oral clofazimine and was well-tolerated in a mouse model of NTM lung disease | [106] | |
| IFN-γ | Inhaled IFN-γ improves NTM clearance in patients with IFN-γ deficiency | [107] |
| Inhaled IFN-γ poor at promoting sputum culture conversion among those with cavitary disease | [108] | |
| Adjuvant intramuscular IFN-γ associated with higher treatment response rates relative to placebo and fewer disease-related deaths in MAC-PD | [109] | |
| GM-CSF | Adjuvant inhaled GM-CSF associated with improved lung function and culture conversion in two patients with CF and MAB-PD | [112] |
| Inhaled NO | 160 ppm inhaled NO for 21 days followed by 240 ppm for 8 days resulted in improvement in quality of life, lung function and 6MWD, but not MAB eradication | [114] |
| Intermittent inhalations of 160 ppm NO associated with reductions in pulmonary MAB loads in two patients | [115] | |
| Intermittent inhaled NO treatment improved FEV1 and 6MWD among nine patients with CF and MAB-PD, but culture conversion not achieved | [116] | |
| Benzimidazoles | SPR719 and EJMCh-6 potent against various NTM species in vitro | [120, 121] |
| Engineered bacteriophage therapy | Clinical improvement in lung function, liver function and skin lesions in a patient with CF with disseminated drug-resistant MAB infection | [126] |
ALIS: amikacin liposome inhalation suspension; IFN: interferon; GM-CSF: granulocyte–macrophage colony-stimulating factor; NO: nitric oxide; TB: tuberculosis; MAB: Mycobacterium abscessus; MIC50/90: minimum inhibitory concentration required to inhibit the growth of 50%/90%; MAC: Mycobacterium avium complex; CF: cystic fibrosis; 6MWD: 6-min walk distance; FEV1: forced expiratory volume in 1 s.
Outlook
The management of mycobacterial lung disease is fraught with challenges. Deciding when to commence treatment, selecting the appropriate combination of drugs and monitoring therapeutic response remains inherently complex. However, just as there has been progress in the management of TB [128], so too have advances been made in NTM-PD treatment.
From both a patient and a clinician perspective, evaluating patient-reported outcome measures (PROMs) is vital when assessing response to NTM therapy. Indeed, there have been calls for increased reporting of patient-reported experiences from NTM centres to inform cost–benefit assessments [129]. A survey of patients with NTM-PD revealed that their top concerns included fatigue, cough, exacerbations and treatment side-effects, and they highlighted the need for increased awareness of NTM-PD management among primary care clinicians [130]. However, there are currently no clinically validated and widely used PROMs for patients with NTM-PD. The NTM module was developed recently as a PROM specifically for NTM patients, reflecting NTM symptoms, body image, digestive symptoms and eating problems to provide a score between 0 and 100, where a higher score reflects better functioning [131]. It provides consistent and reproducible results, but limitations include that it does not account for respiratory symptoms, requires concomitant use of other validated PROM tools and patient perspective has yet to be evaluated [37]. Optimising such tools will be useful but will require additional validation. Furthermore, while there has been an emergence in digital health technologies to promote TB treatment adherence, the potential role of such technologies in supporting adherence in NTM-PD is yet to be determined.
Therapeutic regimens for NTM-PD that have greater efficacy and better safety profiles are still needed. Achieving this will require further clinical trials of existing and novel therapies, as well as identification of new drug targets. Bureaucratic barriers that impede the timely approval of NTM studies should be addressed to facilitate rapid set-up of much needed trials [132]. Enhancing collaboration between clinicians, patients and other key stakeholders will be crucial for setting future research strategies and addressing the unmet need that persists in the management of NTM-PD [133].
Acknowledgements
K. Kumar is an Imperial 4i Clinician Scientist at Imperial College London.
Provenance: submitted article, peer reviewed.
Author contributions: K. Kumar and M.R. Loebinger planned the content of the article. K. Kumar prepared the original draft. C.L. Daley, D.E. Griffith and M.R. Loebinger critically revised the manuscript for content. All authors approved the version of the article that was submitted for publication.
Conflict of interest: K. Kumar reports support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC) and the Lee Family endowment to the Faculty of Medicine at Imperial College London.
Conflict of interest: C.L. Daley reports receiving grants or contracts from Insmed and BugWorks. Consulting fees received from Insmed, Spero, Paratek, AN2, Matinas, Cipla, Johnson and Johnson, outside the submitted work.
Conflict of interest: D.E. Griffith reports support from Insmed Inc. and AN2 Pharmaceuticals. Research grant received from Insmed Inc. Consulting fees received from Insmed Inc., and AN2 Pharmaceuticals. Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from Insmed Inc. Support received from Insmed Inc. for attending meetings and/or travel. Participation on an Advisory Board for Insmed Inc., outside the submitted work.
Conflict of interest: M.R. Loebinger reports receiving consulting fees from Insmed, Savara and Meiji. Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from Insmed. Participation on a Data Safety Monitoring Board or Advisory Board for Redhill, outside the submitted work.
Support statement: K. Kumar is supported by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. K. Kumar is also supported by the Lee Family endowment to the Faculty of Medicine at Imperial College London.
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367– 416. doi: 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 2.Fedrizzi T, Meehan CJ, Grottola A, et al. Genomic characterization of nontuberculous mycobacteria. Sci Rep 2017; 7: 45258. doi: 10.1038/srep45258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winthrop KL, Marras TK, Adjemian J, et al. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008–2015. Ann Am Thorac Soc 2020; 17: 178– 185. doi: 10.1513/AnnalsATS.201804-236OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah NM, Davidson JA, Anderson LF, et al. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infect Dis 2016; 16: 195. doi: 10.1186/s12879-016-1521-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitken ML, Limaye A, Pottinger P, et al. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 2012; 185: 231– 232. doi: 10.1164/ajrccm.185.2.231 [DOI] [PubMed] [Google Scholar]
- 6.Bryant JM, Grogono DM, Greaves D, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 2013; 381: 1551– 1560. doi: 10.1016/S0140-6736(13)60632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant JM, Grogono DM, Rodriguez-Rincon D, et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 2016; 354: 751– 757. doi: 10.1126/science.aaf8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson RM, Hasan NA, Epperson LE, et al. Population genomics of Mycobacterium abscessus from U.S. cystic fibrosis care centers. Ann Am Thorac Soc 2021; 18: 1960– 1969. doi: 10.1513/AnnalsATS.202009-1214OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jhun BW, Moon SM, Jeon K, et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. Eur Respir J 2020; 55: 1900798. doi: 10.1183/13993003.00798-2019 [DOI] [PubMed] [Google Scholar]
- 10.Falkinham JO. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis 2011; 17: 419– 424. doi: 10.3201/eid1703.101510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda JR, Virdi R, Chan ED. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front Microbiol 2018; 9: 2029. doi: 10.3389/fmicb.2018.02029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adjemian J, Olivier KN, Seitz AE, et al. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med 2012; 186: 553– 558. doi: 10.1164/rccm.201205-0913OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler SJ, French J, Screaton NJ, et al. Nontuberculous mycobacteria in bronchiectasis: prevalence and patient characteristics. Eur Respir J 2006; 28: 1204– 1210. doi: 10.1183/09031936.06.00149805 [DOI] [PubMed] [Google Scholar]
- 14.Pyarali FF, Schweitzer M, Bagley V, et al. Increasing non-tuberculous mycobacteria infections in veterans with COPD and association with increased risk of mortality. Front Med 2018; 5: 311. doi: 10.3389/fmed.2018.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odashima K, Kagiyama N, Kanauchi T, et al. Incidence and etiology of chronic pulmonary infections in patients with idiopathic pulmonary fibrosis. PLoS One 2020; 15: e0230746. doi: 10.1371/journal.pone.0230746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park IK, Olivier KN. Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med 2015; 36: 217– 224. doi: 10.1055/s-0035-1546751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roden L, Görlich D, Omran H, et al. A retrospective analysis of the pathogens in the airways of patients with primary ciliary dyskinesia. Respir Med 2019; 156: 69– 77. doi: 10.1016/j.rmed.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Chan ED, Kaminska AM, Gill W, et al. Alpha-1-antitrypsin (AAT) anomalies are associated with lung disease due to rapidly growing mycobacteria and AAT inhibits Mycobacterium abscessus infection of macrophages. Scand J Infect Dis 2007; 39: 690– 696. doi: 10.1080/00365540701225744 [DOI] [PubMed] [Google Scholar]
- 19.Koh W, Lee JH, Kwon YS, et al. Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest 2007; 131: 1825– 1830. doi: 10.1378/chest.06-2280 [DOI] [PubMed] [Google Scholar]
- 20.Mori S, Koga Y, Nakamura K, et al. Mortality in rheumatoid arthritis patients with pulmonary nontuberculous mycobacterial disease: a retrospective cohort study. PLoS One 2020; 15: e0243110. doi: 10.1371/journal.pone.0243110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song JH, Kim BS, Kwak N, et al. Impact of body mass index on development of nontuberculous mycobacterial pulmonary disease. Eur Respir J 2021; 57: 2000454. doi: 10.1183/13993003.00454-2020 [DOI] [PubMed] [Google Scholar]
- 22.Liu VX, Winthrop KL, Lu Y, et al. Association between inhaled corticosteroid use and pulmonary nontuberculous mycobacterial infection. Ann Am Thorac Soc 2018; 15: 1169– 1176. doi: 10.1513/AnnalsATS.201804-245OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winthrop KL, Chang E, Yamashita S, et al. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-α therapy. Emerg Infect Dis 2009; 15: 1556– 1561. doi: 10.3201/eid1510.090310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosain J, Kong XF, Martinez-Barricarte R, et al. Mendelian susceptibility to mycobacterial disease: 2014–2018 update. Immunol Cell Biol 2019; 97: 360– 367. doi: 10.1111/imcb.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horsburgh CR, Selik RM. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis 1989; 139: 4– 7. doi: 10.1164/ajrccm/139.1.4 [DOI] [PubMed] [Google Scholar]
- 26.Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020; 56: 2000535. doi: 10.1183/13993003.00535-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haworth CS, Banks J, Capstick T, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017; 72: Suppl. 2, ii1– ii64. doi: 10.1136/thoraxjnl-2017-210927 [DOI] [PubMed] [Google Scholar]
- 28.Floto RA, Olivier KN, Saiman L, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016; 71: Suppl. 1, i1– i22. doi: 10.1136/thoraxjnl-2015-207360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliberti S, Sotgiu G, Castellotti P, et al. Real-life evaluation of clinical outcomes in patients undergoing treatment for non-tuberculous mycobacteria lung disease: a ten-year cohort study. Respir Med 2020; 164: 105899. doi: 10.1016/j.rmed.2020.105899 [DOI] [PubMed] [Google Scholar]
- 30.van Ingen J, Wagner D, Gallagher J, et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J 2017; 49: 1601855. doi: 10.1183/13993003.01855-2016 [DOI] [PubMed] [Google Scholar]
- 31.Lombardi A, Colaneri M, Sambo M, et al. Predictors of starting antimicrobial treatment in patients with nontuberculous mycobacterial lung disease in the Italian scenario: a SITA GIOVANI-IRENE promoted web-survey. Respir Med 2021; 179: 106341. doi: 10.1016/j.rmed.2021.106341 [DOI] [PubMed] [Google Scholar]
- 32.Rawson TM, Abbara A, Kranzer K, et al. Factors which influence treatment initiation for pulmonary non-tuberculous mycobacterium infection in HIV negative patients; a multicentre observational study. Respir Med 2016; 120 101– 108. doi: 10.1016/j.rmed.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 33.Hwang JA, Kim S, Jo K, et al. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J 2017; 49: 1600537. doi: 10.1183/13993003.00537-2016 [DOI] [PubMed] [Google Scholar]
- 34.Kwon BS, Lee JH, Koh Y, et al. The natural history of non-cavitary nodular bronchiectatic Mycobacterium avium complex lung disease. Respir Med 2019; 150: 45– 50. doi: 10.1016/j.rmed.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 35.Loebinger MR. Mycobacterium avium complex infection: phenotypes and outcomes. Eur Respir J 2017; 50: 1701380. doi: 10.1183/13993003.01380-2017 [DOI] [PubMed] [Google Scholar]
- 36.Griffith DE, Daley CL. Treatment of Mycobacterium abscessus pulmonary disease. Chest 2022; 161: 64– 75. [DOI] [PubMed] [Google Scholar]
- 37.Loebinger MR, Birring SS. Patient reported outcomes for non-tuberculous mycobacterial disease. Eur Respir J 2020; 55: 1902204. doi: 10.1183/13993003.02204-2019 [DOI] [PubMed] [Google Scholar]
- 38.Wallace RJ, Brown BA, Griffith DE, et al. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med 1996; 153: 1766– 1772. doi: 10.1164/ajrccm.153.6.8665032 [DOI] [PubMed] [Google Scholar]
- 39.Meier A, Heifets L, Wallace RJ, et al. Molecular mechanisms of clarithromycin resistance in Mycobacterium avium: observation of multiple 23S rDNA mutations in a clonal population. J Infect Dis 1996; 174: 354– 360. doi: 10.1093/infdis/174.2.354 [DOI] [PubMed] [Google Scholar]
- 40.Meier A, Kirschner P, Springer B, et al. Identification of mutations in 23S rRNA gene of clarithromycin-resistant Mycobacterium intracellulare. Antimicrob Agents Chemother 1994; 38: 381– 384. doi: 10.1128/AAC.38.2.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park Y, Lee EH, Jung I, et al. Clinical characteristics and treatment outcomes of patients with macrolide-resistant Mycobacterium avium complex pulmonary disease: a systematic review and meta-analysis. Respir Res 2019; 20: 286. doi: 10.1186/s12931-019-1258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morimoto K, Namkoong H, Hasegawa N, et al. Macrolide-resistant Mycobacterium avium complex lung disease: analysis of 102 consecutive cases. Ann Am Thorac Soc 2016; 13: 1904– 1911. doi: 10.1513/AnnalsATS.201604-246OC [DOI] [PubMed] [Google Scholar]
- 43.Wallace RJ, Brown-Elliott BA, McNulty S, et al. Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest 2014; 146: 276– 282. doi: 10.1378/chest.13-2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasegawa N, Nishimura T, Ohtani S, et al. Therapeutic effects of various initial combinations of chemotherapy including clarithromycin against Mycobacterium avium complex pulmonary disease. Chest 2009; 136: 1569– 1575. doi: 10.1378/chest.08-2567 [DOI] [PubMed] [Google Scholar]
- 45.Kwak N, Park J, Kim E, et al. Treatment outcomes of Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis 2017; 65: 1077– 1084. doi: 10.1093/cid/cix517 [DOI] [PubMed] [Google Scholar]
- 46.Miwa S, Shirai M, Toyoshima M, et al. Efficacy of clarithromycin and ethambutol for Mycobacterium avium complex pulmonary disease. A preliminary study. Ann Am Thorac Soc 2014; 11: 23– 29. doi: 10.1513/AnnalsATS.201308-266OC [DOI] [PubMed] [Google Scholar]
- 47.Ito Y, Miwa S, Shirai M, et al. Macrolide resistant Mycobacterium avium complex pulmonary disease following clarithromycin and ethambutol combination therapy. Respir Med 2020; 169: 106025. doi: 10.1016/j.rmed.2020.106025 [DOI] [PubMed] [Google Scholar]
- 48.Fukushima K, Kitada S, Komukai S, et al. First line treatment selection modifies disease course and long-term clinical outcomes in Mycobacterium avium complex pulmonary disease. Sci Rep 2021; 11: 1178. doi: 10.1038/s41598-021-81025-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Comparison of Two- Versus Three-antibiotic Therapy for Pulmonary Mycobacterium avium Complex Disease. https://clinicaltrials.gov/ct2/show/NCT03672630 Date last accessed: 26 November 2021. Date last updated: 13 October 2021.
- 50.Meers P, Neville M, Malinin V, et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother 2008; 61: 859– 868. doi: 10.1093/jac/dkn059 [DOI] [PubMed] [Google Scholar]
- 51.Malinin V, Neville M, Eagle G, et al. Pulmonary deposition and elimination of liposomal amikacin for inhalation and effect on macrophage function after administration in rats. Antimicrob Agents Chemother 2016; 60: 6540– 6549. doi: 10.1128/AAC.00700-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rose SJ, Neville ME, Gupta R, et al. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS One 2014; 9: e108703. doi: 10.1371/journal.pone.0108703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J, Leifer F, Rose S, et al. Amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol 2018; 9: 915. doi: 10.3389/fmicb.2018.00915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubino CM, Onufrak NJ, van Ingen J, et al. Population pharmacokinetic evaluation of amikacin liposome inhalation suspension in patients with treatment-refractory nontuberculous mycobacterial lung disease. Eur J Drug Metab Pharmacokinet 2021; 46: 277– 287. doi: 10.1007/s13318-020-00669-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eagle G, Brown K, Floto RA. Examining the effect of residual amikacin on sputum culture for nontuberculous mycobacteria. Am J Respir Crit Care Med 2018; 197: 267– 269. doi: 10.1164/rccm.201704-0792LE [DOI] [PubMed] [Google Scholar]
- 56.Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med 2018; 198: 1559– 1569. doi: 10.1164/rccm.201807-1318OC [DOI] [PubMed] [Google Scholar]
- 57.Winthrop KL, Flume PA, Thomson R, et al. Amikacin liposome inhalation suspension for Mycobacterium avium complex lung disease: a 12-month open-label extension clinical trial. Ann Am Thorac Soc 2021; 18: 1147– 1157. doi: 10.1513/AnnalsATS.202008-925OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Griffith DE, Thomson R, Flume PA, et al. Amikacin liposome inhalation suspension for refractory Mycobacterium avium complex lung disease: sustainability and durability of culture conversion and safety of long-term exposure. Chest 2021; 160: 831– 842. doi: 10.1016/j.chest.2021.03.070 [DOI] [PubMed] [Google Scholar]
- 59.Nash KA, Brown-Elliott BA, Wallace RJ. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 2009; 53: 1367– 1376. doi: 10.1128/AAC.01275-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi G, Shin SJ, Won C, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med 2012; 186: 917– 925. doi: 10.1164/rccm.201111-2005OC [DOI] [PubMed] [Google Scholar]
- 61.Bastian S, Veziris N, Roux A, et al. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 2011; 55: 775– 781. doi: 10.1128/AAC.00861-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim H, Kim BJ, Kook Y, et al. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol Immunol 2010; 54: 347– 353. doi: 10.1111/j.1348-0421.2010.00221.x [DOI] [PubMed] [Google Scholar]
- 63.Maurer FP, Rüegger V, Ritter C, et al. Acquisition of clarithromycin resistance mutations in the 23S rRNA gene of Mycobacterium abscessus in the presence of inducible erm(41). J Antimicrob Chemother 2012; 67: 2606– 2611. doi: 10.1093/jac/dks279 [DOI] [PubMed] [Google Scholar]
- 64.Zarogoulidis P, Papanas N, Kioumis I, et al. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol 2012; 68: 479– 503. doi: 10.1007/s00228-011-1161-x [DOI] [PubMed] [Google Scholar]
- 65.Pasipanodya JG, Ogbonna D, Ferro BE, et al. Systematic review and meta-analyses of the effect of chemotherapy on pulmonary Mycobacterium abscessus outcomes and disease recurrence. Antimicrob Agents Chemother 2017; 61: e01206-17. doi: 10.1128/AAC.01206-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi H, Jhun BW, Kim S, et al. Treatment outcomes of macrolide-susceptible Mycobacterium abscessus lung disease. Diagn Microbiol Infect Dis 2018; 90: 293– 295. doi: 10.1016/j.diagmicrobio.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 67.Choi H, Kim S, Lee H, et al. Clinical characteristics and treatment outcomes of patients with macrolide-resistant Mycobacterium massiliense lung disease. Antimicrob Agents Chemother 2017; 61: e02189-16. doi: 10.1128/AAC.02189-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeon K, Kwon OJ, Lee NY, et al. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 2009; 180: 896– 902. doi: 10.1164/rccm.200905-0704OC [DOI] [PubMed] [Google Scholar]
- 69.Lyu J, Jang HJ, Song JW, et al. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respir Med 2011; 105: 781– 787. doi: 10.1016/j.rmed.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 70.Jarand J, Levin A, Zhang L, et al. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 2011; 52: 565– 571. doi: 10.1093/cid/ciq237 [DOI] [PubMed] [Google Scholar]
- 71.Kamii Y, Nagai H, Kawashima M, et al. Adverse reactions associated with long-term drug administration in Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis 2018; 22: 1505– 1510. doi: 10.5588/ijtld.18.0171 [DOI] [PubMed] [Google Scholar]
- 72.Aznar ML, Marras TK, Elshal AS, et al. Safety and effectiveness of low-dose amikacin in nontuberculous mycobacterial pulmonary disease treated in Toronto, Canada. BMC Pharmacol Toxicol 2019; 20: 37. doi: 10.1186/s40360-019-0302-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar K, McHugh TD, Lipman M. Fluoroquinolones for treating tuberculosis. Clinical Pharmacist 2017; 9: 142– 149. doi: 10.1211/PJ.2017.20202555 [DOI] [Google Scholar]
- 74.Shuto H, Komiya K, Goto A, et al. Efficacy and safety of fluoroquinolone-containing regimens in treating pulmonary Mycobacterium avium complex disease: a propensity score analysis. PLoS One 2020; 15: e0235797. doi: 10.1371/journal.pone.0235797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Ingen J, Aliberti S, Andrejak C, et al. Management of drug toxicity in Mycobacterium avium complex pulmonary disease: an expert panel survey. Clin Infect Dis 2021; 73: e256- e259. doi: 10.1093/cid/ciaa1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee SFK, Laughon BE, McHugh TD, et al. New drugs to treat difficult tuberculous and nontuberculous mycobacterial pulmonary disease. Curr Opin Pulm Med 2019; 25: 271– 280. doi: 10.1097/MCP.0000000000000570 [DOI] [PubMed] [Google Scholar]
- 77.Pryjma M, Burian J, Thompson CJ. Rifabutin acts in synergy and is bactericidal with frontline Mycobacterium abscessus antibiotics clarithromycin and tigecycline, suggesting a potent treatment combination. Antimicrob Agents Chemother 2018; 62: e00283-18. doi: 10.1128/AAC.00283-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005; 307: 223– 227. doi: 10.1126/science.1106753 [DOI] [PubMed] [Google Scholar]
- 79.Philley JV, Wallace RJ, Benwill JL, et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 2015; 148: 499– 506. doi: 10.1378/chest.14-2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zweijpfenning SMH, Schildkraut JA, Coolen JPM, et al. Failure with acquired resistance of an optimised bedaquiline-based treatment regimen for pulmonary Mycobacterium avium complex disease. Eur Respir J 2019; 54: 1900118. doi: 10.1183/13993003.00118-2019 [DOI] [PubMed] [Google Scholar]
- 81.A Phase 2/3, Multicenter, Randomized, Open-label, Active-controlled Study to Evaluate the Efficacy and Safety of Bedaquiline Administered as Part of a Treatment Regimen With Clarithromycin and Ethambutol in Adult Patients With Treatment-refractory Mycobacterium avium Complex-lung Disease (MAC-LD). https://clinicaltrials.gov/ct2/show/NCT04630145 Date last accessed: 5 August 2021. Date last updated: 24 November 2021.
- 82.Ruth MM, Sangen JJN, Remmers K, et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother 2019; 74: 935– 943. doi: 10.1093/jac/dky526 [DOI] [PubMed] [Google Scholar]
- 83.Lindman M, Dick T. Bedaquiline eliminates bactericidal activity of β-lactams against Mycobacterium abscessus. Antimicrob Agents Chemother 2019; 63: e00827-19. doi: 10.1128/AAC.00827-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuste JR, Bertó J, Del Pozo JL, et al. Prolonged use of tedizolid in a pulmonary non-tuberculous mycobacterial infection after linezolid-induced toxicity. J Antimicrob Chemother 2017; 72: 625– 628. doi: 10.1093/jac/dkw484 [DOI] [PubMed] [Google Scholar]
- 85.Ruth MM, Koeken VACM, Pennings LJ, et al. Is there a role for tedizolid in the treatment of non-tuberculous mycobacterial disease? J Antimicrob Chemother 2020; 75: 609– 617. doi: 10.1093/jac/dkz511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poon YK, La Hoz RM, Hynan LS, et al. Tedizolid vs linezolid for the treatment of nontuberculous mycobacteria infections in solid organ transplant recipients. Open Forum Infect Dis 2021; 8: ofab093. doi: 10.1093/ofid/ofab093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuste JR, Serrano-Alonso M, Carmona-Torre F, et al. Efficacy and safety of long-term use of tedizolid after liver transplantation in an adolescent with pulmonary tuberculosis. J Antimicrob Chemother 2019; 74: 2817– 2819. doi: 10.1093/jac/dkz216 [DOI] [PubMed] [Google Scholar]
- 88.Mensa Vendrell M, Tasias Pitarch M, Salavert Lletí M, et al. Safety and tolerability of more than six days of tedizolid treatment. Antimicrob Agents Chemother 2020; 64: e00356-20. doi: 10.1128/AAC.00356-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bax HI, de Vogel CP, Mouton JW, et al. Omadacycline as a promising new agent for the treatment of infections with Mycobacterium abscessus. J Antimicrob Chemother 2019; 74: 2930– 2933. doi: 10.1093/jac/dkz267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown-Elliott BA, Wallace RJ. In vitro susceptibility testing of omadacycline against nontuberculous mycobacteria. Antimicrob Agents Chemother 2021; 65: e001947-20. doi: 10.1128/AAC.01947-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shoen C, Benaroch D, Sklaney M, et al. In vitro activities of omadacycline against rapidly growing mycobacteria. Antimicrob Agents Chemother 2019; 63: e002522-18. doi: 10.1128/AAC.02522-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Minhas R, Sharma S, Kundu S. Utilizing the promise of omadacycline in a resistant, non-tubercular mycobacterial pulmonary infection. Cureus 2019; 11: e5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pearson JC, Dionne B, Richterman A, et al. Omadacycline for the treatment of Mycobacterium abscessus disease: a case series. Open Forum Infect Dis 2020; 7: ofaa415. doi: 10.1093/ofid/ofaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morrisette T, Alosaimy S, Philley JV, et al. Preliminary, real-world, multicenter experience with omadacycline for Mycobacterium abscessus infections. Open Forum Infect Dis 2021; 8: ofab002. doi: 10.1093/ofid/ofab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oral Omadacycline vs. Placebo in Adults With NTM Pulmonary Disease Caused by Mycobacterium Abscessus Complex (MABc). https://clinicaltrials.gov/ct2/show/NCT04922554. Date last accessed: 5 August 2021. Date last updated: 23 November 2021.
- 96.Kaushik A, Ammerman NC, Martins O, et al. In vitro activity of new tetracycline analogs omadacycline and eravacycline against drug-resistant clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother 2019; 63: e00470-19. doi: 10.1128/AAC.00470-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pandey R, Chen L, Manca C, et al. Dual β-lactam combinations highly active against Mycobacterium abscessus complex in vitro. mBio 2019; 10: 2895. doi: 10.1128/mBio.02895-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopeman RC, Harrison J, Rathbone DL, et al. Effect of amoxicillin in combination with imipenem-relebactam against Mycobacterium abscessus. Sci Rep 2020; 10: 928. doi: 10.1038/s41598-020-57844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Story-Roller E, Galanis C, Lamichhane G. β-Lactam combinations that exhibit synergy against Mycobacteroides abscessus clinical isolates. Antimicrob Agents Chemother 2021; 65: e02545-20. doi: 10.1128/AAC.02545-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dousa KM, Kurz SG, Taracila MA, et al. Insights into the l,d-transpeptidases and d,d-carboxypeptidase of Mycobacterium abscessus: ceftaroline, imipenem, and novel diazabicyclooctane inhibitors. Antimicrob Agents Chemother 2020; 64: e00098-20. doi: 10.1128/AAC.00098-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaushik A, Ammerman NC, Lee J, et al. In vitro activity of the new β-lactamase inhibitors relebactam and vaborbactam in combination with β-lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob Agents Chemother 2019; 63: e02623-18. doi: 10.1128/AAC.02623-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen DC, Dousa KM, Kurz SG, et al. ‘One-two punch’: synergistic β-lactam combinations for Mycobacterium abscessus and target redundancy in the inhibition of peptidoglycan synthesis enzymes. Clin Infect Dis 2021; 73: 1532– 1536. doi: 10.1093/cid/ciab535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dubée V, Bernut A, Cortes M, et al. β-Lactamase inhibition by avibactam in Mycobacterium abscessus . J Antimicrob Chemother 2015; 70: 1051– 1058. [DOI] [PubMed] [Google Scholar]
- 104.Jones LA, Doucette L, Dellon EP, et al. Use of inhaled imipenem/cilastatin in pediatric patients with cystic fibrosis: a case series. J Cyst Fibros 2019; 18: e42– e44. doi: 10.1016/j.jcf.2019.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pearce C, Ruth MM, Pennings LJ, et al. Inhaled tigecycline is effective against Mycobacterium abscessus in vitro and in vivo. J Antimicrob Chemother 2020; 75: 1889– 1894. doi: 10.1093/jac/dkaa110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Banaschewski B, Verma D, Pennings LJ, et al. Clofazimine inhalation suspension for the aerosol treatment of pulmonary nontuberculous mycobacterial infections. J Cyst Fibros 2019; 18: 714– 720. doi: 10.1016/j.jcf.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 107.Hallstrand TS, Ochs HD, Zhu Q, et al. Inhaled IFN-γ for persistent nontuberculous mycobacterial pulmonary disease due to functional IFN-γ deficiency. Eur Respir J 2004; 24: 367– 370. doi: 10.1183/09031936.04.00036704 [DOI] [PubMed] [Google Scholar]
- 108.Lam PK, Griffith DE, Aksamit TR, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2006; 173: 1283– 1289. doi: 10.1164/rccm.200509-1531OC [DOI] [PubMed] [Google Scholar]
- 109.Milanés-Virelles MT, García-García I, Santos-Herrera Y, et al. Adjuvant interferon gamma in patients with pulmonary atypical Mycobacteriosis: a randomized, double-blind, placebo-controlled study. BMC Infect Dis 2008; 8: 17. doi: 10.1186/1471-2334-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol 2016; 100: 481– 489. doi: 10.1189/jlb.3RU0316-144R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Groote MA, Johnson L, Podell B, et al. GM-CSF knockout mice for preclinical testing of agents with antimicrobial activity against Mycobacterium abscessus. J Antimicrob Chemother 2014; 69: 1057– 1064. doi: 10.1093/jac/dkt451 [DOI] [PubMed] [Google Scholar]
- 112.Scott JP, Ji Y, Kannan M, et al. Inhaled granulocyte-macrophage colony-stimulating factor for Mycobacterium abscessus in cystic fibrosis. Eur Respir J 2018; 51: 1702127. doi: 10.1183/13993003.02127-2017 [DOI] [PubMed] [Google Scholar]
- 113.Trial of Inhaled Molgramostim in Cystic Fibrosis Subjects With Nontuberculous Mycobacterial Infection (ENCORE). https://clinicaltrials.gov/ct2/show/NCT03597347. Date last accessed: 14 November 2021. Date last updated: 27 October 2021.
- 114.Bogdanovski K, Chau T, Robinson CJ, et al. Antibacterial activity of high-dose nitric oxide against pulmonary Mycobacterium abscessus disease. Access Microbiol 2020; 2: acmi000154. doi: 10.1099/acmi.0.000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yaacoby-Bianu K, Gur M, Toukan Y, et al. Compassionate nitric oxide adjuvant treatment of persistent mycobacterium infection in cystic fibrosis patients. Pediatr Infect Dis J 2018; 37: 336– 338. doi: 10.1097/INF.0000000000001780 [DOI] [PubMed] [Google Scholar]
- 116.Bentur L, Gur M, Ashkenazi M, et al. Pilot study to test inhaled nitric oxide in cystic fibrosis patients with refractory Mycobacterium abscessus lung infection. J Cyst Fibros 2020; 19: 225– 231. doi: 10.1016/j.jcf.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 117.A Proof of Concept Study of Inhaled Nitric Oxide for Adults With Pulmonary Non-Tuberculous Mycobacterial Infection. https://clinicaltrials.gov/ct2/show/NCT03748992 Date last accessed: 26 November 2021. Date last updated: 18 May 2021. [DOI] [PubMed]
- 118.A Pilot Study to Assess the Effect of Intermittent Inhaled Nitric Oxide on the Treatment of Nontuberculous Mycobacteria (NTM) Lung Infection in Cystic Fibrosis and Non-Cystic Fibrosis Patients. https://clinicaltrials.gov/ct2/show/NCT04685720 Date last accessed: 16 August 2021. Date last updated: 28 December 2020.
- 119.30 Technology . 30 Technology Launches a Critical Clinical Trial of RESP301 in Cystic Fibrosis Patients with Serious Lung Infections. https://30.technology/30-technology-cystic-fibrosis-lung-infections/ Date last accessed: 5 August 2021. Date last updated: 19 February 2021. [Google Scholar]
- 120.Raynaud C, Daher W, Johansen MD, et al. Active benzimidazole derivatives targeting the MmpL3 transporter in Mycobacterium abscessus. ACS Infect Dis 2020; 6: 324– 337. doi: 10.1021/acsinfecdis.9b00389 [DOI] [PubMed] [Google Scholar]
- 121.Brown-Elliott BA, Rubio A, Wallace RJ. In vitro susceptibility testing of a novel benzimidazole, SPR719, against nontuberculous mycobacteria. Antimicrob Agents Chemother 2018; 62: e01503-18. doi: 10.1128/AAC.01503-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Phase 2a Safety, Tolerability, Pharmacokinetics and Efficacy of SPR720 for the Treatment of Patients With Mycobacterium Avium Complex (MAC) Pulmonary Disease. https://clinicaltrials.gov/ct2/show/NCT04553406 Date last accessed: 26 November 2021. Date last updated: 23 March 2021.
- 123.Thomas SE, Collins P, James RH, et al. Structure-guided fragment-based drug discovery at the synchrotron: screening binding sites and correlations with hotspot mapping. Philos Trans A Math Phys Eng Sci 2019; 377: 20180422. doi: 10.1098/rsta.2018.0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas SE, Whitehouse AJ, Brown K, et al. Fragment-based discovery of a new class of inhibitors targeting mycobacterial tRNA modification. Nucleic Acids Res 2020; 48: 8099– 8112. doi: 10.1093/nar/gkaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Whitehouse AJ, Thomas SE, Brown KP, et al. Development of inhibitors against Mycobacterium abscessus tRNA (m1G37) methyltransferase (TrmD) using fragment-based approaches. J Med Chem 2019; 62: 7210– 7232. doi: 10.1021/acs.jmedchem.9b00809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 2019; 25: 730– 733. doi: 10.1038/s41591-019-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Skwark MJ, Torres PHM, Copoiu L, et al. Mabellini: a genome-wide database for understanding the structural proteome and evaluating prospective antimicrobial targets of the emerging pathogen Mycobacterium abscessus. Database 2019; 2019: baz113. doi: 10.1093/database/baz113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kumar K, Kon OM. Diagnosis and treatment of tuberculosis: latest developments and future priorities. Ann Res Hosp 2017; 1: 37. doi: 10.21037/arh.2017.08.08 [DOI] [Google Scholar]
- 129.Satta G, McHugh TD, Mountford J, et al. Managing pulmonary nontuberculous mycobacterial infection. Time for a patient-centered approach. Ann Am Thorac Soc 2014; 11: 117– 121. doi: 10.1513/AnnalsATS.201308-278OT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shteinberg M, Boyd J, Aliberti S, et al. What is important for people with nontuberculous mycobacterial disease? An EMBARC-ELF patient survey. ERJ Open Res 2021; 7: 00807-2020. doi: 10.1183/23120541.00807-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Henkle E, Winthrop KL, Ranches GP, et al. Preliminary validation of the NTM module: a patient-reported outcome measure for patients with pulmonary nontuberculous mycobacterial disease. Eur Respir J 2020; 55: 1901300. doi: 10.1183/13993003.01300-2019 [DOI] [PubMed] [Google Scholar]
- 132.Duplancic C, Crough T, Bell SC. Multi-centre ethics and research governance review can impede non-interventional clinical research. Intern Med J 2019; 49: 722– 728. doi: 10.1111/imj.14158 [DOI] [PubMed] [Google Scholar]
- 133.Lipman M, Kunst H, Loebinger MR, et al. Non tuberculous mycobacteria pulmonary disease: patients and clinicians working together to improve the evidence base for care. Int J Infect Dis 2021; 113: S73– S77. doi: 10.1016/j.ijid.2021.03.064 [DOI] [PubMed] [Google Scholar]