Abstract
Despite the availability of effective inhaled therapies, many patients with asthma have poor asthma control. Uncontrolled asthma presents a significant burden on the patient and society, and, for many, remains largely preventable. There are numerous reasons why a patient may remain uncontrolled despite access to therapies, including incorrect inhaler technique, poor adherence to treatment, oversight of triggers and suboptimal medical care. Shared decision-making, good patient–clinician communication, supported self-management, multidisciplinary patient education, new technology and risk stratification may all provide solutions to this major unmet need in asthma. Novel treatments such as biologics could benefit patients’ lives, while the investigations into biomarkers, non-Type 2 asthma, treatable traits and disease modification give an exciting glimpse into the future of asthma care.
Short abstract
Despite effective therapies, many patients with asthma have poor asthma control, which is preventable. The benefits of shared decision-making, supported self-management, risk stratification and novel treatments in transforming patient care are reviewed. https://bit.ly/3A386Nm
Introduction
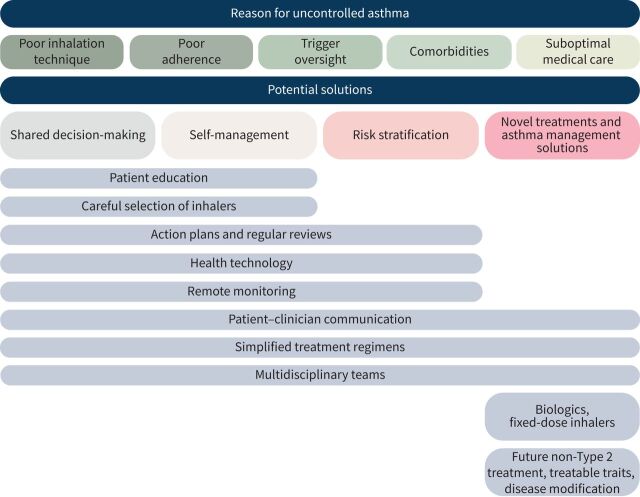
Globally, 339.4 million people are affected by asthma [1]. The cornerstone of asthma treatment is inhaled corticosteroids (ICS), with the addition of a second controller (e.g. a long-acting β2-agonist (LABA) for those with uncontrolled symptoms) [2]. However, despite the availability of these effective inhaled therapies, approximately 39% of patients with asthma remain uncontrolled [3]. Uncontrolled disease can have potential negative consequences, including risk of exacerbations [4], side-effects from oral corticosteroid (OCS) use [5–7] and reduced quality of life (QoL) [8]. A United Kingdom (UK) report of asthma deaths between 2012 and 2013 revealed that two-thirds of deaths due to asthma could be prevented if care were improved [4]. There are numerous complex reasons why a patient may remain uncontrolled despite access to therapies, including: poor management [9–12], poor adherence to treatment [11, 13–15], incorrect inhaler technique [16, 17] and asthma that is treatment-resistant. This narrative review explores the current unmet needs and the potential future of asthma management (figure 1).
FIGURE 1.
Interventions for uncontrolled asthma.
What is uncontrolled asthma?
There is a general consensus that uncontrolled asthma is defined by poor symptom control and/or frequent exacerbations that require OCS treatment or hospitalisation (table 1) [2, 18–28]. However, there is currently no universal definition of uncontrolled asthma. Global guidelines include the Global Initiative for Asthma (GINA) report [2] and the European Respiratory Society/American Thoracic Society (ERS/ATS) taskforce, and many countries have national guidelines [29]. Guidelines have varying, although similar, management recommendations (table 1). The GINA report stresses the importance of distinguishing between uncontrolled and severe asthma, as the former may be more easily improved than the latter. The definition of severe asthma from the ERS/ATS taskforce is similar to the GINA report (table 1), but stresses severe asthma only includes patients with refractory asthma and those in whom treatment of comorbidities (e.g. severe sinus disease, obesity) remains incomplete [18]. It should be noted that, since biologics are now in use, GINA advises against use of the term “refractory” to describe asthma unresponsive to high-dose ICS. GINA offers guidance on identifying common causes of uncontrolled asthma which need to be excluded before a diagnosis of severe asthma can be made, such as poor inhaler technique, poor medication adherence, comorbidities, exposure to triggers and incorrect diagnosis of asthma, all of which are discussed in this review [2]. The ERS/ATS taskforce also recommend patients who present with “difficult asthma” should have their asthma diagnosis confirmed, evaluated and managed by an asthma specialist for >3 months to determine whether asthma is severe or uncontrolled [18]. There are various terms to describe uncontrolled asthma used in guidelines and literature, such as “difficult-to-control” and “poorly controlled” [2, 20]. In this review, we use the term “uncontrolled” asthma throughout. Individual articles should be consulted for exact terms and definitions.
TABLE 1.
Definitions of uncontrolled and severe asthma
| Report [ref.] | Uncontrolled asthma | Severe asthma |
| GINA report [2] | At least one of the following: 1) Poor symptom control (frequent symptoms or reliever use, activity limited by asthma, night waking due to asthma) 2) Frequent exacerbations (≥2/year) requiring OCS treatment, or serious exacerbations (≥1/year) requiring hospitalisation |
Asthma that is difficult to treat (not controlled despite GINA Step 4 or 5 treatment,# or that requires such treatment to maintain good symptom control) and despite good adherence and inhaler technique, or that worsens when high-dose treatment is decreased |
| International ERS/ATS guidelines [18] | At least one of the following: 1) ACQ consistently ≥1.5 or ACT <20 2) Frequent severe exacerbations (≥2 courses of SCS in the previous year) 3) Serious exacerbations (≥1 hospitalisation in the previous year) Predicted post-bronchodilator FEV1 of <80% (in the face of reduced FEV1/FVC defined as less than the lower limit of normal) |
Asthma requiring treatment with high-dose ICS and ≥1 maintenance treatment (LABA, LAMA, leukotriene modifier or theophylline) or SCS for ≥50% of the previous year to prevent it from becoming uncontrolled, or remains uncontrolled despite this therapy |
| NHLBI and NEAPP Guidelines for the Diagnosis and Management of Asthma (EPR-3) [27, 28] | 1) Symptoms >2 days·week−1 2) Night-time awakening ≥1/week 3) Limitations with normal activity 4) SABA use >2 days·week−1 5) FEV1 or peak flow ≤80% predicted/personal best 6) ATAQ ≥1 7) ACQ ≥1.5 8) ACT ≤19 Exacerbations ≥2/year |
Asthma with EPR-3 Step 5 or 6# as the lowest level of treatment required to maintain control |
| Canadian Thoracic Society position statement [19] | At least one of the following: 1) Poor symptom control: as per CTS asthma control criteria or other standardised questionnaires: ACQ consistently >1.5, ACT <20, or cACT <20 2) Frequent severe exacerbations: ≥2 courses of SCS (3 days each) in the previous year 3) Serious exacerbations: ≥1 hospitalisation, ICU stay or mechanical ventilation in the previous year 4) Airflow limitation: after appropriate bronchodilator withhold FEV1 <80% of personal best (or <the LLN, in the face of reduced FEV1/FVC defined as less than the LLN) |
Asthma requiring treatment with high-dose ICS and a second controller for the previous year, or SCS for 50% of the previous year to prevent it from becoming uncontrolled, or remains uncontrolled despite this therapy |
| BTS/SIGN British Guideline on the Management of Asthma [20] | “Difficult asthma” is defined as at least one of the following: 1) Persistent symptoms 2) Frequent asthma exacerbations despite treatment with: a) High-dose ICS (adults) or medium-dose ICS (children) plus a LABA (age ≥5 years) or an LTRA b) Medium-dose ICS (adults) or low-dose ICS (children) plus LABA (age ≥5 years) or LTRA and an appropriate additional therapy c) Continuous or frequent use of OCS |
ND |
| NICE guidelines [26] | At least one of the following: 1) ≥3 days a week with symptoms 2) ≥3 days a week with required use of a SABA for symptomatic relief 3) ≥1 nights a week with awakening due to asthma |
ND |
| Japanese Guidelines for Adult Asthma [21] | ND | Asthma controlled by, or uncontrolled despite administration of high doses of ICS, LABA, LTRA, theophylline, anti-IgE antibody or OCS. Also known as intractable asthma |
| Brazilian Thoracic Association [22] | Three to four of the following: 1) Diurnal symptoms ≥2 times a week 2) Nocturnal awakenings due to asthma 3) Rescue medication ≥2 times a week 4) Limitation of activities due to asthma or ACQ-7 >1.5 or ACT ≤15 |
Asthma that remains uncontrolled on high-dose ICS and a second controller medication within the previous year or the use of OCS on ≥50% of the days in the last year, or that needs this treatment to prevent the disease from becoming uncontrolled (in an attempt to reduce the dose of ICS or OCS), despite the suppression or minimisation of factors that impair asthma control |
| The Saudi Initiative for Asthma [24] | 1) Symptoms and/or SABA use required throughout the day 2) ≥2 exacerbations a week 3) Extremely limited daily activities 4) FEV1 or peak flow <60% of predicted/personal best 5) ACT <16 >2 exacerbations per year requiring OCS or hospitalisation |
Asthma that requires treatment SINA Step 4 or 5# |
| Australian Asthma Handbook [23] | “Poor control” defined as three or more, and “partial control” as two or more of: 1) Daytime symptoms >2 days per week 2) Need for SABA reliever >2 days per week 3) Any limitation of activities 4) Any symptoms during night or on waking |
Asthma that is uncontrolled despite treatment with high-dose ICS/LABA (with correct inhaler technique and good adherence) or maintenance OCS, or that requires such treatment to prevent it becoming uncontrolled |
| NZ Adolescent and Adult Asthma Guidelines [25] | “Poor control” defined as three or more, and “partial control” as two or more of: 1) Daytime symptoms >2 days per week 2) Need for SABA reliever >2 days per week 3) Any limitation of activities 4) Any symptoms during night or on waking |
ND |
GINA: Global Initiative for Asthma; OCS: oral corticosteroid; ERS/ATS: European Respiratory Society/American Thoracic Society; ACQ: asthma control questionnaire; ACT: asthma control test; SCS: systemic corticosteroid; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; ICS: inhaled corticosteroids; LABA: long-acting β2 agonist; LAMA: long-acting muscarinic antagonist; NHLBI: National Heart, Lung, and Blood Institute; NEAPP: National Asthma Education and Prevention Program; EPR 3: expert panel report 3; SABA: short-acting β-agonist; ATAQ: Asthma Therapy Assessment Questionnaire; CTS: Canadian Thoracic Society; cACT: child asthma control test; ICU: Intensive Care Unit; LLN: lower limits of normal; BTS: British Thoracic Society; SIGN: Scottish Intercollegiate Guidelines Network; LTRA: leukotriene receptor antagonist; ND: no definition; NICE: National Institute for Health and Care Excellence; SINA: Saudi Initiative for Asthma; NZ: New Zealand. #: medium- or high-dose ICS/LABA ± add-on therapy (LAMA, LTRA, OCS, biologics).
What are the consequences of uncontrolled asthma?
Impact on the life of the patient
The greater risk of symptoms, exacerbations, comorbidities and side-effects that patients with uncontrolled asthma experience can each have a major impact on their lives. Uncontrolled asthma can negatively impact patients’ health-related QoL (limitations due to physical health problems, vitality, social functioning and emotional wellbeing) [8, 30], and patients are more likely to have anxiety and depression versus patients with better asthma control [31]. Sleep quality is also affected, with uncontrolled asthma contributing to insomnia [32]. Children with uncontrolled asthma may have later bedtimes, increased frequency of night-time awakenings and poorer sleep quality than children with controlled asthma [33]. Absenteeism from work/school, and decreased productivity are also associated with uncontrolled asthma [34, 35], and when children are affected, this can negatively impact absenteeism and work productivity for caregivers.
Patients with uncontrolled asthma are also at risk of side-effects from corticosteroids, largely through frequent OCS use to control symptoms [5–7]. Add-on low-dose OCS (≤7.5 mg·day−1 prednisone equivalent) can be prescribed for patients requiring GINA Step 5 treatment to help control severe asthma [2]. OCS are widely used in asthma management due to their anti-inflammatory, immunosuppressive and vasoconstrictive properties [36]. Frequent OCS use is associated with serious short- and long-term adverse effects including weight gain [5–7], diabetes [5–7], osteoporosis [5–7], cataracts [6, 7], anxiety [6], depression [6], hypertension [5–7] and adrenal insufficiency [37]. Adrenal insufficiency may persist following corticosteroid discontinuation, potentially causing serious clinical consequences such as shock, seizure, coma and sometimes death [37, 38]. Even short-term OCS use can increase rates of sepsis, venous thromboembolism and fracture [39]. High OCS exposure is also associated with increased emergency department (ED) and inpatient visits compared with low OCS exposure in patients with asthma [40]. ICS, the gold-standard first-line therapy for asthma, can also cause adverse events with long-term use, including oropharyngeal candidiasis, adrenal suppression, bone loss and cataracts [41].
Impact on healthcare and the economy
Patients with uncontrolled asthma are at greater risk of symptoms [42], exacerbations [43], increased ED visits and hospitalisations [34]. Importantly, poor adherence to maintenance ICS has a negative impact on asthma outcomes. In 115 patients referred to a specialist clinic for difficult-to-control asthma, 65% of those prescribed ICS or ICS/LABA had poor adherence, and poor adherence was associated with reduced lung function (lower forced expiratory volume in 1 s (FEV1)) and inflammation (higher percentage sputum eosinophil count) [44]. Furthermore, greater OCS use and more frequent exacerbation are associated with poor adherence to ICS in patients prescribed ICS with add-on anti-interleukin (IL)-5 monoclonal antibody therapy, highlighting the importance of ICS maintenance with add-on therapy [45].
Exacerbations and uncontrolled symptoms can also be fatal. Since the 1980s, worldwide asthma mortality rates have declined, in part, due to improved diagnosis and treatment, particularly with the availability of ICS [46]. However, this trend may have stalled. The World Health Organization mortality database reported no improvement in asthma mortality from 2006–2012 (estimated global asthma mortality rate: 0.19 deaths/100 000 people (90% CI 0.16–0.21)) [46]. Patients with severe, uncontrolled asthma are at greater risk of mortality than those with severe asthma, and it has been found that this is largely preventable [4].
The economic impact of uncontrolled asthma can be difficult to measure, as productivity-loss, side-effects of OCS, burden of comorbidities, etc. may be underestimated. However, it is evident that uncontrolled asthma is associated with major direct and indirect costs. In the UK, the direct and indirect costs of asthma are estimated at GBP 6.2 billion/year [34], and double in patients with uncontrolled versus well-controlled asthma [34]. Over-reliance on OCS can place additional strain on the economy: in a UK study, OCS-dependent asthma patients had an average 43% greater associated direct treatment costs than patients not receiving maintenance OCS [47].
Why is asthma uncontrolled in many patients?
Misdiagnosis of asthma
Asthma is characterised by variable respiratory symptoms and variable airflow limitation; this heterogeneity, coupled with a wide variety of conditions and comorbidities that can present with symptoms similar to asthma, can make asthma diagnosis challenging [48]. Studies suggest 20‒70% of people with asthma remain undiagnosed [49], and this can be caused by patient underreporting of symptoms, poor communication and poor diagnostics [49, 50]. However, studies suggest that around one-third of patients diagnosed with asthma do not have current asthma [51]. This may be due to failure to use objective lung function tests at the time of diagnosis, or unrecognition of sustained remission. Patients with overdiagnosed asthma may experience adverse effects related to unnecessary treatment, and a delay in alternative diagnosis of their respiratory symptoms.
Poor inhalation technique
Different devices (pressurised metered-dose inhalers (pMDIs), dry powder inhalers (DPIs), soft mist inhalers and nebulisers) offer various benefits and drawbacks, such as ease of use and portability [52]. If a patient does not use their inhaler correctly, it is likely to diminish medication delivery. According to a relevant systematic review, the most frequent pMDI errors were coordination, speed and/or depth of inspiration and no post-inhalation breath-hold. Frequent DPI errors were incorrect preparation, no full expiration before inhalation and no post-inhalation breath-hold. The overall prevalence of correct technique was just 31%. Interestingly, errors in technique did not differ over the 20-year period observed [16]. In an analysis of 3981 asthma patient reviews in the UK, significantly more patients who had ≥1 exacerbation failed their first inhaler technique check than those without exacerbations, and 67% of patients who required OCS for exacerbations were unable to use their pMDI [17]. Inhaler misuse may be driven by lack of instruction, and/or physical/cognitive factors: in a study of 1664 adults in Italy with COPD or asthma, the incorrect use of inhalers was common, with a strong association between inhaler misuse and older age, lower education and lack of instruction for inhaler use [53]. Moreover, inhaler mishandling increased risk of hospitalisation, ED visits, OCS use, antimicrobial use and poor disease control [53].
Poor adherence to medication
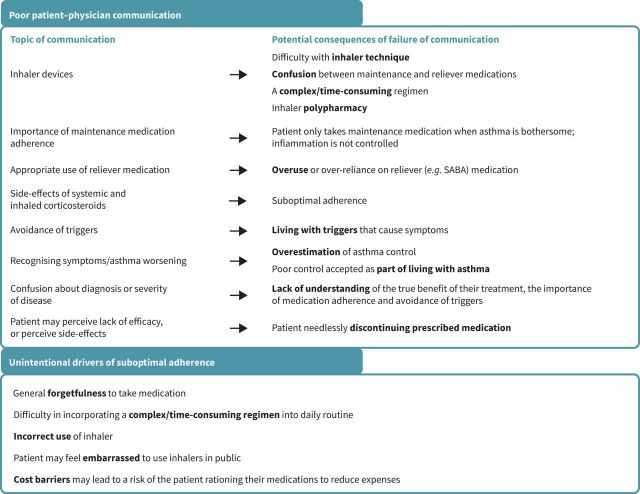
Suboptimal adherence to medication use is the most common reason for treatment failure in asthma and has several known drivers (figure 2) [2, 15, 54–56]. Firstly, patients overestimate their asthma control, and accept poor control as part of living with asthma. In a UK qualitative analysis of 42 adults with asthma, patients often did not think daily treatments preventing inflammation were needed if their asthma was not bothersome [13]. In a study of the perception of control among 329 adults in Trinidad, uncontrolled asthma correlated with routine work limitation, night-time disturbances, work absenteeism, exacerbations and rescue inhalation; however, 65% believed that they had to live with their symptoms [11]. In an online survey of 2467 adults with asthma from eight Asian countries/regions (REALISE Asia), 50% of patients had diagnosed uncontrolled asthma [10]; however, approximately 90% of respondents felt their asthma was under control. Patients consistently overestimated their asthma control and considered control as the management of exacerbations [10].
FIGURE 2.
Key reasons for poor adherence to asthma medication. SABA: short-acting beta-agonist.
Smart inhalers, which feature a sensor and link to an app on a phone/tablet to track inhaler usage, are becoming increasingly widely available and enable reliable, longitudinal adherence data to be shared with physicians [57]. Data suggest electronic inhaler-monitoring devices could have a beneficial effect on medication adherence. In a randomised, open-label trial of an inhaler-monitoring device in severe uncontrolled asthma, improvement in adherence after 3 months was greater in patients given biofeedback on inhaler usage and technique versus counterparts blinded to biofeedback [58].
While the onus of adherence to medication is considered to be mainly on the patient, the patient–clinician relationship exerts a large influence on whether a patient will take medication as directed (figure 2) [2, 15, 54–56]. Health care professionals (HCPs) may not give patients an asthma action plan: in an asthma call-back survey in the USA, only 35% of adults with asthma reported receiving a written asthma action plan [42]. For patients, suboptimal adherence could be unintentional: as they may have a lack of understanding of asthma as a chronic illness, or not know the importance of taking their medication [54].
Lack of awareness about trigger avoidance
Exposure to triggers can worsen asthma symptoms and cause exacerbations. Triggers include house dust mites, moulds, pets, pollens, air pollution, weather/temperature changes, viral infections and smoking (including inhaled cannabis) [59, 60]. E-cigarettes may also worsen lung function and airway inflammation [61]. In a survey of 1202 adults with asthma in five European countries, uncontrolled asthma was more common in patients with a high trigger burden (>16 triggers) than those with a low trigger burden (1–5 triggers). High versus low trigger burden was also associated with an increased likelihood of previous severe asthma, exacerbations during a lifetime, more hospitalisations and more missed days at work or study [62]. Aeroallergen exposure may contribute to the chronicity of disease and difficulty to maintain disease control. Allergen avoidance has proven difficult, but experience in selected patients with immunotherapy has proven beneficial [63]. Furthermore, immunotherapy may have ongoing benefit following discontinuation of treatment, suggesting its effects may represent disease modification [64].
Impact of comorbidities
Conditions such as obesity, chronic sinusitis with nasal polyps (CRSwNP), gastroesophageal reflux disease (GERD), anxiety/depression, obstructive sleep apnoea, rhinitis and COPD are often observed in patients with asthma and may affect asthma control [65–67]. These are potential confounding factors for diagnosis/assessment of asthma control, and/or may alter responses to therapy [65, 66]. For example, patients with asthma and comorbid obesity experience more symptoms, more frequent and severe exacerbations, greater impairment of QoL, and have a reduced response to several asthma medications versus people with asthma and healthy weight [68]. Patients with obesity and asthma have been demonstrated to have higher asthma medication use than asthma patients with healthy weight, despite lower lung function [69]. Weight loss has also been demonstrated to be effective in improving asthma outcomes [70, 71]. It is therefore debatable if conventional asthma therapy or weight loss-based approaches offer the greater likelihood of improving symptom control. Low-grade systemic inflammation of obesity may contribute to airway inflammation [71], and other comorbidities such as GERD can lead to airway inflammation and contribute to asthma obesity association [72]. There is a need for research to better understand the interaction between metabolic and inflammatory dysregulation [71]. Asthma with comorbid CRSwNP is another difficult-to-treat phenotype that remains poorly characterised [73]. CRSwNP shares inflammatory traits with asthma, and there is a need for research that characterises biomarkers in people with CRSwNP and asthma to predict response to intervention [73]. Furthermore, some comorbidities such as vocal cord dysfunction and dysfunctional breathing may mimic the symptoms of asthma and be confused for poor asthma control [74].
Suboptimal medical and professional care
There are many factors – such as poor patient–clinician communication, overestimation of asthma control and little importance given to symptoms that do not cause exacerbations – that may contribute to suboptimal medical and professional care.
For patients diagnosed with asthma, physicians may overestimate their patients’ asthma control: in a survey conducted in patients undergoing routine asthma reviews, physicians perceived 80% of patients as well-controlled, whereas only 52% had well-controlled asthma using asthma control questionnaire scores [9]. HCPs may also not offer an action plan or regular asthma reviews [12], and lack of consensus of global and national asthma guidelines with respect to definitions, terminology and treatment could cause uncertainty among primary care HCPs for the management of asthma (table 1). Consequently, patients may not be stepped up to optimal treatment, or patients with severe disease not referred to a specialist [75]. However, even among patients who are stepped up in their treatment, some remain uncontrolled. In a 1-year follow-up of patients with asthma in the UK, of the 1046 patients uncontrolled on medium-dose ICS/LABA and subsequently stepped up to high-dose ICS/LABA, 35% remained uncontrolled [3]. Physicians may also overprescribe short-acting β-agonists (SABAs), leading to reliance on a rescue medication that does not control inflammation. In the SABINA study, prescription and/or dispensing data for >1 000 000 patients with asthma (2006–2017) revealed the prevalence of SABA overuse was: 9% in Italy, 16% in Germany, 29% in Spain, 30% in Sweden, and 38% in the UK. SABA overuse was not correlated with asthma severity, except in the UK – where overuse was greater in patients with moderate-to-severe asthma (58%) versus mild asthma (27%) [76]. Overuse of SABAs is associated with increased risk of exacerbation and death [4, 56].
For patients uncontrolled despite adhering to high-dose ICS/LABA, GINA suggests phenotypic assessment to guide the selection of add-on treatment, such as biologics [2]. Biologics are currently approved for severe asthma target type 2 cytokines such as IL-4, IL-5 and IL-13, and immunoglobulin E (IgE) and therefore should only be prescribed to patients with typical biomarkers of type 2 airway inflammation (e.g. high eosinophils or IgE) [2]. These patients with underlying type 2 inflammation mediated by a T-helper (Th) type 2 pathway are generally known as having Type 2, or eosinophilic asthma. However, physicians may have difficulty identifying the optimal biologic for their patient, especially when agents target similar pathways. Also, they may have trouble identifying responders or recognising when a switch should be considered because of suboptimal control [77]. Biomarkers (e.g. IgE, fractional exhaled nitric oxide (FENO), sputum or blood eosinophils), when available, can help inform biologic selection but may not be specific enough to clearly identify an asthma phenotype and the biologic best suited to a patient [78]. The National Asthma Education and Prevention Program (NEAPP) guidelines conditionally recommend frequent assessment of FENO levels [27]. Identification of further biomarkers is ongoing. The high cost of biologics may also be prohibitive for patients, practitioners and insurers [79]. For patients with non-Type 2 asthma, there are currently no approved therapies with specific clinical benefits [80].
Potential solutions to the challenge of uncontrolled asthma
Shared decision-making
Firstly, verifying that patients are diagnosed accurately with asthma ensures they are on the first step to managing their condition; data indicate that up to one-third of presumed patients with asthma may not have asthma [49]. Once diagnosed, talking with patients openly and sharing treatment decisions with them can empower them to take control of their asthma [55]. Good patient–clinician communication can improve adherence, QoL and asthma control [81]. It is therefore important for HCPs to arrange regular asthma reviews or reassessments with their patients. The National Review of Asthma Deaths (NRAD) – a UK enquiry report – recommends asthma reviews to be carried out by a primary care practitioner at least annually, but more frequently in patients with poor control or high risk of exacerbation [4]. Sufficient time should be given to explore concerns about medication and potential nonadherence. Clinicians should help patients understand that asthma is a chronic illness that requires adherence to maintenance medication, including during periods of reduced symptoms. For difficult and severe asthma, systematic assessment (identifying and managing poor adherence, poor inhaler technique, uncontrolled comorbidities, misdiagnosis, and/or undertreated severe asthma biology) has been shown to significantly improve asthma outcomes [82]. Clinical guidelines in asthma advocate use of an asthma action plan, including information on how and when to take medicines, how to avoid triggers and when to seek medical help. These are particularly important for patients following emergency treatment for asthma [4].
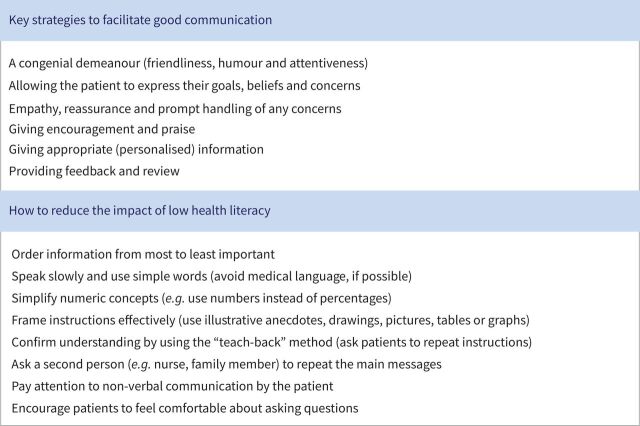
HCPs should also consider a patient's health literacy – whether a patient has the capacity to understand basic health information (figure 3) [2]. Encouraging patients to lose weight and stop smoking can also help with managing asthma [83, 84]. As well as physicians and nurses, pharmacists can play an important part in disease management: community pharmacists see patients regularly and may therefore be able to address common issues by screening for asthma, providing training and monitoring treatment [85].
FIGURE 3.
Communication strategies for healthcare professionals. Reproduced from [2] with permission.
Self-management
Asthma management should not lie solely with the HCP. It is vital patients are given personalised advice to develop their knowledge and skills to enable them to optimise how they self-manage their asthma. Regularly supported self-management – face-to-face, or remote via telehealth/digital technology – may improve QoL and reduce healthcare resource use across all levels of asthma severity [86]. Education and training are key for patient self-management. HCPs ensuring patients understand how to use their inhalers correctly can improve asthma control and reduce SABA use [87]. Analysis of data from a community pharmacy dataset in Australia found that 24% of patients with asthma had correct inhaler technique. Following training in inhalation technique, this rose to 50% after 1 month [88]. This highlights the need for patients to practice their inhaler technique, and for HCPs to check their technique regularly. This is particularly important before switching medications/devices or increasing dosages. Devices that monitor inhaler technique and usage-frequency may be beneficial; data can be shared with HCPs, allowing adherence monitoring [89]. Training that includes visual aids could reinforce correct inhaler technique [2, 90]. Inhaled devices that are intuitive and minimise critical errors, as well as simplifying treatment regimens to once-daily fixed-dose combination inhalers may also facilitate successful dosing, adherence and asthma control [91, 92].
Educating patients about asthma is essential. Patients can be taught to recognise asthma worsening, how to avoid triggers and to understand the importance of taking their medications correctly to control inflammation. Training from nurses [93], school nurses/teachers [94, 95], pharmacists [85], community health workers [96] and physiotherapists [97] can benefit asthma control, lung function and QoL [85, 94, 95, 97]. Even coaching performed remotely via telephone, text and e-mail can improve patient-reported outcomes [98]. Learning about asthma is also accessible via pamphlets, websites and smartphone applications (apps) [99, 100]. For children, innovative ways to make learning fun and engaging have been utilised, such as activity books, music, videos and smartphone apps that include games and quizzes [101].
With the ubiquity of smartphones and tablets, there has been a drive for digital health solutions for asthma management. Smartphone or mHealth apps present an opportunity to engage with patients on an everyday basis using a familiar format. Apps allow users to create reminders, identify triggers and manually record symptoms and exacerbations that can be shared with HCPs [89]. Apps can have a positive impact on asthma control [100]; some apps can be used in conjunction with smart spirometers and peak flow meters so lung function results can be shared with HCPs [89, 102]. Using a smart inhaler and certain apps, patients can enter details of asthma control and passively/manually record medication use – which can be shared with their HCP – and also create reminders and access asthma management tips [57, 89]. Smart inhalers can improve asthma control and reduce SABA use [103].
Risk stratification
Close monitoring of the patients most at risk of uncontrolled asthma can facilitate timely identification and treatment interventions to avoid exacerbations. Risk factors include: poor adherence and inhaler technique, prior exacerbations, continued exposure to triggers, obesity, female sex, smoking, low educational level, low income, eosinophilia and comorbidities (e.g. COPD, depression, allergic rhinitis) [34, 65, 104–110]. Prior ED visits and hospitalisations, excessive OCS use, and poor lung function predict the risk of future exacerbations [43, 111, 112], so the close monitoring of patients who experience these concerns is paramount. The NRAD report made several recommendations to prevent life-threatening exacerbations, including: regular asthma reviews, in which excessive SABA use, inhaler technique and regimens should be discussed; encouragement of patient self-management; and outlining systems that should be implemented within healthcare organisations (such as the referral of patients with severe asthma to specialist care, documenting asthma reviews in medical records, electronic alerts for excessive SABA use/preventer inhaler underuse, and follow-up arrangements after every ED visit) [4]. Electronic alerts that notify HCPs of their patients’ ED visits could be useful to prevent exacerbations [4, 89]. Remote monitoring, such as smart inhalers and compliance devices, gives HCPs further opportunities to regularly monitor patients and provide tailored feedback [89].
Although the risk of exacerbations increases with asthma severity, even patients with mild asthma are at risk of potentially life-threatening exacerbations. The NRAD report showed that, of 155 asthma deaths where severity could be estimated, 9% were in patients with mild asthma [4]. Overuse of SABAs is associated with increased risk of asthma exacerbation and mortality [56], and use of a SABA alone can mask inflammation until a severe exacerbation occurs [49]. Because of these detrimental effects, GINA recently made significant updates to their recommendations in mild asthma management to no longer support SABA-only therapy [2, 113].
Advances in treatment: biologics
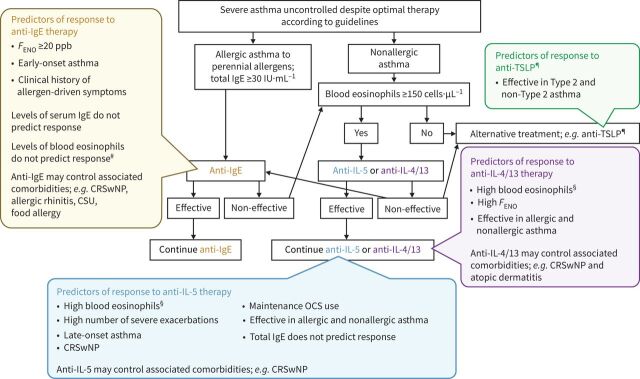
The breakthrough in the treatment of severe asthma in recent years is the introduction of biologics. Currently, five monoclonal antibody treatments are approved for asthma in the US and EU (omalizumab (anti-IgE); mepolizumab, benralizumab, reslizumab (anti-IL-5/IL-5R); dupilumab (anti-IL-4/13)), and all target patients with Type 2 asthma. Figure 4 gives a suggested care pathway and predictors of response for biologics in asthma [2, 77, 114–127].
FIGURE 4.
Care pathways and predictors of response for biologics in asthma. CRSwNP: chronic rhinosinusitis with nasal polyposis; CSU: chronic spontaneous urticaria; FENO: fractional exhaled nitric oxide; IgE: immunoglobulin E; IL: interleukin; OCS: oral corticosteroid; TSLP: thymic stromal lymphopoietin. #In randomised control trials, there was a greater decrease in exacerbations in patients with blood eosinophil levels of ≥260 cells·μL−1 (omalizumab versus placebo) [115, 127]. ¶The higher the eosinophil level, the higher the expected impact. §Anti-TSLP not approved in patients with asthma at time of publication. Reproduced and modified from [77].
As discussed earlier, physicians may have difficulty in identifying which biologic should be chosen for their patient, especially as current biologics target similar pathways [128]. Guidance for the consideration of specific approved biologics appear in the GINA 2021 report [2] and EACCI guidelines [117]. Patients can undergo aeroallergen skin prick testing and IgE measurement to assess for the allergic asthma phenotype, and a FENO test to detect type 2 inflammation (FENO >50 ppb). Blood count and white cell differential can also detect the presence of eosinophilia as a marker of type 2 inflammation [2, 129]. However, critical research into biomarkers that can easily and cheaply identify patients likely to benefit from specific biologics is ongoing, and biomarkers to assess non-Type 2 asthma represent a particular unmet need [129]. Integrated care pathways – structured multidisciplinary care plans detailing essential patient management steps – could help physicians identify which biologics suit specific patients [77]. Biologics can be administered subcutaneously or intravenously, in the clinic or at home, and with different dose regimens (every 2, 4 or 8 weeks) [130]. If patients have the option of ≥1 biologic, patient preference should be taken into consideration.
Advances in treatment: fixed-dose combination inhaled therapies
The GINA 2021 report now recommends long-acting muscarinic antagonists (LAMA) as an add-on therapy for patients at Step 4 or 5, uncontrolled on ICS/LABA [2]. This can be in the form of tiotropium, or a triple combination inhaler – ICS/LABA/LAMA (fluticasone furoate/vilanterol/umeclidinium (FF/VI/UMEC), mometasone/indacaterol/glycopyrronium (MF/IND/GLY), or beclometasone/formoterol/glycopyrronium (BDP/FF/GLY)). This decision was based on positive results from phase 3 trials which demonstrated improvements in lung function and exacerbations for ICS/LABA/LAMA versus ICS/LABA alone [131–133]. In the ARGON phase 3 trial, high-dose MF/IND/GLY also improved QoL and lung function versus the loose triple combination of twice-daily fluticasone propionate/salmeterol plus tiotropium [134]. Simplifying treatment regimens to a single, once-daily inhaler may also improve adherence and reduce healthcare resource use [135].
Adherence to maintenance therapy can be low if symptoms are not bothersome [13], leading to reliance on SABAs that do not control inflammation. As evidence suggests significant adverse effects with SABA overuse, GINA no longer supports SABA-only therapy, and now recommends as-needed low-dose ICS/formoterol as the preferred reliever [2]. The 2020 updates to the NEAPP guidelines also recommend ICS/formoterol at Steps 3 and 4 [27].
Advances in treatment: therapies for severe non-Type 2 asthma
While effective treatments for Type 2 asthma exist, there are currently no specific therapies for non-Type 2 asthma [80]. This phenotype is less well characterised and is generally described as noneosinophilic asthma without the presence of Type 2 inflammatory markers. Potential mechanisms include neutrophilic-, IFN- and IL-17-mediated airway inflammation [68]. Recognising these patients is important, as they may not benefit from increased doses of maintenance ICS [136]. In a recent study of 301 patients with severe asthma, that categorised patients using a composite biomarker strategy, 23% were deemed “T2-low” on the basis of FENO, blood eosinophils and serum periostin at study entry; however, only 5% were consistently T2-low in serial assessments every 8 weeks for 48 weeks, highlighting the difficulty of identifying biomarkers for noneosinophilic/nontype 2 asthma [137].
Data show that patients with asthma and obesity are more likely to have non-Type 2 asthma, and neutrophilia is associated with pollution, infections, cigarette smoke and some occupational exposures [138]. Nonpharmacological interventions such as cessation of smoking and avoidance of exposure to pollutants may reduce neutrophilic inflammation in asthma [139, 140]. Pharmacological interventions include off-label medications such as macrolides and PDE (phosphodiesterase)-4 inhibitors [2, 141]. However, these therapies currently lack the clinical data to support wider use in non-Type 2 asthma. Novel therapies that target neutrophils and cytokines associated with non-Type 2 asthma are under development; however, they have so far been unsuccessful. Clinical trials with brodalumab (an anti-IL-17 receptor mAb) and AZD5069 (a CXCR2 antagonist) failed to meet their primary objectives [142, 143].
One therapy under development shows promise in patients with non-Type 2 asthma. Tezepelumab is a mAb that blocks thymic stromal lymphopoietin (TSLP), an upstream cytokine mediator that initiates multiple Type 2 signalling pathways. In the phase 3 NAVIGATOR trial, tezepelumab reduced asthma exacerbation rates in patients with both Type 2 and non-Type 2 severe uncontrolled asthma (defined as IgE level ≤100 IU·mL−1 or blood eosinophil count <140 cells·µL−1) [144]. However, the recent 48-week tezepelumab SOURCE trial failed to meet its primary end-point in reduction of daily OCS use. Other end-points were consistent with results from NAVIGATOR [145]. The efficacy of tezepelumab may be due to the activity of TSLP early in the inflammatory cascade [123]. While tezepelumab is administered by subcutaneous injection, another anti-TSLP, CSJ117, is a once-daily inhaled formulation of a mAb fragment. In a 12-week phase 1 trial in patients with mild asthma, the primary end-point was met: CSJ117 reduced late-asthmatic response versus placebo [146]. A phase 2 trial is also in progress (NCT04410523) [147].
Future directions
Treatable traits
Although patients with asthma are broadly split into Type 2 and non-Type 2 asthma, this approach is likely to be over-simplified, given the growing evidence of asthma populations that do not neatly fit into either phenotype. A precision medicine strategy based on “treatable traits” that are specific to distinct phenotypes and endotypes of patients with asthma may represent the next step in the search for new therapeutic approaches. Treatable traits can be put into three domains: pulmonary (e.g. eosinophilia, neutrophilia, airflow variability, exacerbation-prone asthma); extrapulmonary (e.g. obesity, osteoporosis, depression, cardiovascular disease); and behavioural/risk factors (e.g. smoking, aspergillus sensitisation, multiple inhaler use) [110]. A trial in 55 patients with severe asthma found that individualised treatment that targeted predefined treatable traits led to significantly improved health-related QoL (p<0.001) and asthma control (p=0.01) [148]. Ongoing research into the underlying pathobiological mechanisms of patients with asthma is leading to the discovery of multiple subpopulations of patients displaying distinct phenotypes and endotypes.
Disease modification: journey towards remission
At present, there is no cure for asthma and treatment typically involves therapies that prevent or reduce asthma symptoms, without modifying the underlying disease [149]. If a treatment can address the pathogenesis of a disease, preventing progression or leading to a long-term reduction in symptoms, this can be classed as disease modifying. Such therapies have been investigated and approved in other indications, such as rheumatoid arthritis [150]. The heterogeneous nature of asthma has made the discovery of similar therapies in asthma more difficult, although novel therapies such as biologics may have the potential to exhibit disease-modifying properties. For example, omalizumab can reduce reticular basement membrane thickness [151], mepolizumab can reduce expression of markers of airway remodelling [152] and tezepelumab can decrease matrix remodelling [153]. Clinical trials investigating the disease-modifying properties of biologics are ongoing but incomplete [154, 155]. Other therapies such as prostaglandin D2 (PGD2) receptor 2 antagonists have also shown promise [156]. Halting or reversing the progression of asthma with disease-modifying therapies could potentially lead to the “holy grail” of full disease remission or prevention; therefore, further investigation into these therapies is imperative, with careful consideration of end-points, trial length and study population.
Conclusions
Despite the availability of effective treatments, asthma still causes significant disease burden and mortality. Exacerbations may be largely preventable: we know that action plans, regular asthma reviews, patient self-management and risk stratification can improve asthma outcomes, yet there is evidence that these strategies are not always put into practice. An individualised and effective patient–clinician partnership that involves good communication, patient education and supported self-management is particularly vital for maintaining asthma control. Translating this into routine care from physicians and multidisciplinary teams should be a focus in asthma management. Research into existing and new molecular targets is vital, and novel therapies for patients whose asthma does not respond to available treatments need to be developed and applied effectively in clinical practice. As these therapies become available, physicians should have the guidelines and tools available to them to optimally implement treatment. Encouraging patient–clinician communication and embracing present and future technology and therapies may offer the best potential for further advances in the management of this chronic disease.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: W.W. Busse reports consultant fees from AstraZeneca, Genentech, GSK, Novartis, Regeneron Pharmaceuticals Inc., and Sanofi, outside the submitted work.
Conflict of interest: M. Kraft reports grants from NIH, Sanofi, ALA, Chiesi and AstraZeneca, consultant fees from Sanofi and AstraZeneca, and Chief Medical Officer fees from RaeSedo LLC, outside the submitted work.
Support statement: This publication was funded by Novartis Pharma, Basel, Switzerland. Novartis did not have any input into the content. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Ellen Maxwell, PhD, of Ashfield MedComms, an Ashfield Health company and funded by Novartis. This publication was written in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017: 390: 1211–1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2021. Available from: https://ginasthma.org/
- 3.Buhl R, Heaney LG, Loefroth E, et al. One-year follow up of asthmatic patients newly initiated on treatment with medium- or high-dose inhaled corticosteroid-long-acting beta2-agonist in UK primary care settings. Respir Med 2020; 162: 105859. doi: 10.1016/j.rmed.2019.105859 [DOI] [PubMed] [Google Scholar]
- 4.Royal College of Physicians UK . Why asthma still kills. National Review of Asthma Deaths. www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills. Date last updated: August 11, 2015. Date last accessed: January 7, 2021.
- 5.Sullivan PW, Ghushchyan VH, Globe G, et al. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol 2018; 141: 110–116.e117. doi: 10.1016/j.jaci.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 6.Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016; 71: 339–346. doi: 10.1136/thoraxjnl-2015-207630 [DOI] [PubMed] [Google Scholar]
- 7.Al Efraij K, Johnson KM, Wiebe D, et al. A systematic review of the adverse events and economic impact associated with oral corticosteroids in asthma. J Asthma 2019; 56: 1334–1346. doi: 10.1080/02770903.2018.1539100 [DOI] [PubMed] [Google Scholar]
- 8.Foster JM, McDonald VM, Guo M, et al. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J 2017; 50: 1700765. doi: 10.1183/13993003.00765-2017 [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga K, Hamada K, Oishi K, et al. Factors associated with physician-patient discordance in the perception of asthma control. J Allergy Clin Immunol Pract 2019; 7: 2634–2641. doi: 10.1016/j.jaip.2019.04.046 [DOI] [PubMed] [Google Scholar]
- 10.Price D, David-Wang A, Cho SH, et al. Time for a new language for asthma control: results from REALISE Asia. J Asthma Allergy 2015; 8: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beharry S, Gidla D, Maharaj A, et al. Reality and understanding of asthma control. Chron Respir Dis 2015; 12: 340–346. doi: 10.1177/1479972315598692 [DOI] [PubMed] [Google Scholar]
- 12.Chapman KR, Hinds D, Piazza P, et al. Physician perspectives on the burden and management of asthma in six countries: the Global Asthma Physician Survey (GAPS). BMC Pulm Med 2017; 17: 153. doi: 10.1186/s12890-017-0492-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bidad N, Barnes N, Griffiths C, et al. Understanding patients’ perceptions of asthma control: a qualitative study. Eur Respir J 2018; 51: 1701346. doi: 10.1183/13993003.01346-2017 [DOI] [PubMed] [Google Scholar]
- 14.Axelsson M. Personality and reasons for not using asthma medication in young adults. Heart Lung 2013; 42: 241–246. doi: 10.1016/j.hrtlng.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 15.Pelaez S, Lamontagne AJ, Collin J, et al. Patients’ perspective of barriers and facilitators to taking long-term controller medication for asthma: a novel taxonomy. BMC Pulm Med 2015; 15: 42. doi: 10.1186/s12890-015-0044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchis J, Gich I, Pedersen S, Aerosol drug management improvement team. systematic review of errors in inhaler use: has patient technique improved over time? Chest 2016; 150: 394–406. doi: 10.1016/j.chest.2016.03.041 [DOI] [PubMed] [Google Scholar]
- 17.Levy ML, Hardwell A, McKnight E, et al. Asthma patients’ inability to use a pressurised metered-dose inhaler (pMDI) correctly correlates with poor asthma control as defined by the Global Initiative for Asthma (GINA) strategy: a retrospective analysis. Prim Care Respir J 2013; 22: 406–411. doi: 10.4104/pcrj.2013.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 19.FitzGerald JM, Lemiere C, Lougheed MD, et al. Recognition and management of severe asthma: a Canadian Thoracic Society position statement. Can J Respir Crit Care Sleep Med 2017; 1: 199–221. [Google Scholar]
- 20.BTS/SIGN . British Guideline on the Management of Asthma. A National Clinical Guideline. Edinburgh, Healthcare Improvement Scotland, 2019. www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma. Date last updated: July 2019. Date last accessed: January 7, 2021. [Google Scholar]
- 21.Ichinose M, Sugiura H, Nagase H, et al. Japanese guidelines for adult asthma 2017. Allergol Int 2017; 66: 163–189. doi: 10.1016/j.alit.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 22.Pizzichini MMM, Carvalho-Pinto RM, Cancado JED, et al. 2020. Brazilian Thoracic Association recommendations for the management of asthma. J Bras Pneumol 2020; 46: e20190307. doi: 10.1590/1806-3713/e20190307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Asthma Council Australia . Australian Asthma Handbook, Version 2.1. Melbourne, National Asthma Council Australia; 2020. www.asthmahandbook.org.au/. Date last updated: September 2020. Date last accessed: January 7, 2021. [Google Scholar]
- 24.Al-Moamary MS, Alhaider SA, Alangari AA, et al. The Saudi Initiative for Asthma – 2019 update: guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med 2019; 14: 3–48. doi: 10.4103/atm.ATM_327_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beasley R, Beckert L, Fingleton J, et al. Asthma and Respiratory Foundation NZ Adolescent and Adult Asthma Guidelines 2020: a quick reference guide. NZMJ 2020; 133: 73–99. [PubMed] [Google Scholar]
- 26.National Institute for Health and Care Excellence (NICE) . Asthma: diagnosis, monitoring and chronic asthma management. NICE guideline [NG80]. London, NICE; 2017. www.nice.org.uk/guidance/ng80. Date last updated: February 12, 2020. Date last accessed: January 7, 2021. [Google Scholar]
- 27.National Heart Lung and Blood Institute . 2020 focused updates to the Asthma Management Guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. Bethesda, MD, National Heart Lung and Blood Institute; 2020. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines. Date last accessed: January 7, 2021. [Google Scholar]
- 28.National Heart Lung and Blood Institute, National Asthma Education and Prevention Program . Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD, National Heart Lung and Blood Institute; 2007. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf. Date last accessed: January 7, 2021. [Google Scholar]
- 29.Global Asthma Network . Asthma Management Guidelines. http://globalasthmanetwork.org/management/country.php. Date last updated: 2020. Date last accessed: November 1, 2020.
- 30.Ilmarinen P, Juboori H, Tuomisto LE, et al. Effect of asthma control on general health-related quality of life in patients diagnosed with adult-onset asthma. Sci Rep 2019; 9: 16107. doi: 10.1038/s41598-019-52361-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lomper K, Chudiak A, Uchmanowicz I, et al. Effects of depression and anxiety on asthma-related quality of life. Pneumonol Alergol Pol 2016; 84: 212–221. doi: 10.5603/PiAP.2016.0026 [DOI] [PubMed] [Google Scholar]
- 32.Luyster FS, Strollo PJ, Jr, Holguin F, et al. Association between insomnia and asthma burden in the Severe Asthma Research Program (SARP) III. Chest 2016; 150: 1242–1250. doi: 10.1016/j.chest.2016.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meltzer LJ, Pugliese CE. Sleep in young children with asthma and their parents. J Child Health Care 2017; 21: 301–311. doi: 10.1177/1367493517712064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavord ID, Mathieson N, Scowcroft A, et al. The impact of poor asthma control among asthma patients treated with inhaled corticosteroids plus long-acting beta2-agonists in the United Kingdom: a cross-sectional analysis. NPJ Prim Care Respir Med 2017; 27: 17. doi: 10.1038/s41533-017-0014-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu J, Qin X, Beavers SF, et al. Asthma-related school absenteeism, morbidity, and modifiable factors. Am J Prev Med 2016; 51: 23–32. doi: 10.1016/j.amepre.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol 2013; 9: 30. doi: 10.1186/1710-1492-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broersen LHA, Pereira AM, Jørgensen JOL, et al. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100: 2171–2180. doi: 10.1210/jc.2015-1218 [DOI] [PubMed] [Google Scholar]
- 38.Puar TH, Stikkelbroeck NM, Smans LC, et al. Adrenal crisis: still a deadly event in the 21st century. Am J Med 2016; 129: 339.e331. [DOI] [PubMed] [Google Scholar]
- 39.Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017; 357: j1415. doi: 10.1136/bmj.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol 2015; 136: 1488–1495. doi: 10.1016/j.jaci.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 41.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med 2006; 100: 1307–1317. doi: 10.1016/j.rmed.2005.11.020 [DOI] [PubMed] [Google Scholar]
- 42.Christensen GM, Tomasallo CD, Meiman JG. Adult asthma control and self-management, Wisconsin 2012-2016. WMJ 2019; 118: 187–190. [PubMed] [Google Scholar]
- 43.Quezada W, Kwak ES, Reibman J, et al. Predictors of asthma exacerbation among patients with poorly controlled asthma despite inhaled corticosteroid treatment. Ann Allergy Asthma Immunol 2016; 116: 112–117. doi: 10.1016/j.anai.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy AC, Proeschal A, Brightling CE, et al. The relationship between clinical outcomes and medication adherence in difficult-to-control asthma. Thorax 2012; 67: 751–753. doi: 10.1136/thoraxjnl-2011-201096 [DOI] [PubMed] [Google Scholar]
- 45.d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J 2020; 55: 1902259. doi: 10.1183/13993003.02259-2019 [DOI] [PubMed] [Google Scholar]
- 46.Ebmeier S, Thayabaran D, Braithwaite I, et al. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet 2017; 390: 935–945. doi: 10.1016/S0140-6736(17)31448-4 [DOI] [PubMed] [Google Scholar]
- 47.O'Neill S, Sweeney J, Patterson CC, et al. The cost of treating severe refractory asthma in the UK: an economic analysis from the British Thoracic Society Difficult Asthma Registry. Thorax 2015; 70: 376–378. doi: 10.1136/thoraxjnl-2013-204114 [DOI] [PubMed] [Google Scholar]
- 48.Papi A, Brightling C, Pedersen SE, et al. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med 2018; 198: 1012–1020. doi: 10.1164/rccm.201804-0682CI [DOI] [PubMed] [Google Scholar]
- 49.Aaron SD, Boulet LP, Reddel HK, et al. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med 2018; 198: 1012–1020. [DOI] [PubMed]
- 50.Haughney J, Price D, Kaplan A, et al. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med 2008; 102: 1681–1693. doi: 10.1016/j.rmed.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 51.Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA 2017; 317: 269–279. doi: 10.1001/jama.2016.19627 [DOI] [PubMed] [Google Scholar]
- 52.Usmani OS. Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manag 2019; 15: 461–472. doi: 10.2147/TCRM.S160365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011; 105: 930–938. doi: 10.1016/j.rmed.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 54.Amin S, Soliman M, McIvor A, et al. Understanding patient perspectives on medication adherence in asthma: a targeted review of qualitative studies. Patient Prefer Adherence 2020; 14: 541–551. doi: 10.2147/PPA.S234651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gruffydd-Jones K, Hansen K. Working for better asthma control: how can we improve the dialogue between patients and healthcare professionals? Adv Ther 2020; 37: 1–9. doi: 10.1007/s12325-019-01131-0 [DOI] [PubMed] [Google Scholar]
- 56.Nwaru BI, Ekstrom M, Hasvold P, et al. Overuse of short-acting beta2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J 2020; 55: 1901872. doi: 10.1183/13993003.01872-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Himes BE, Leszinsky L, Walsh R, et al. Mobile health and inhaler-based monitoring devices for asthma management. J Allergy Clin Immunol Pract 2019; 7: 2535–2543. doi: 10.1016/j.jaip.2019.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sulaiman I, Greene G, MacHale E, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J 2018; 51: 1701126. doi: 10.1183/13993003.01126-2017 [DOI] [PubMed] [Google Scholar]
- 59.Gautier C, Charpin D. Environmental triggers and avoidance in the management of asthma. J Asthma Allergy 2017; 10: 47–56. doi: 10.2147/JAA.S121276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med 2019; 19: 52. doi: 10.1186/s12890-019-0814-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotoulas SC, Pataka A, Domvri K, et al. Acute effects of e-cigarette vaping on pulmonary function and airway inflammation in healthy individuals and in patients with asthma. Respirology 2020; 25: 1037–1045. doi: 10.1111/resp.13806 [DOI] [PubMed] [Google Scholar]
- 62.Price D, Dale P, Elder E, et al. Types, frequency and impact of asthma triggers on patients’ lives: a quantitative study in five European countries. J Asthma 2014; 51: 127–135. doi: 10.3109/02770903.2013.846369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA 2016; 315: 1715–1725. doi: 10.1001/jama.2016.3964 [DOI] [PubMed] [Google Scholar]
- 64.Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol 2012; 129: 717–725 e715. doi: 10.1016/j.jaci.2011.12.973 [DOI] [PubMed] [Google Scholar]
- 65.Hekking PP, Amelink M, Wener RR, et al. Comorbidities in difficult-to-control asthma. J Allergy Clin Immunol Pract 2018; 6: 108–113. doi: 10.1016/j.jaip.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 66.Mahdavian M, Power BH, Asghari S, et al. Effects of comorbidities on asthma hospitalization and mortality rates: a systematic review. Can Respir J 2018; 2018: 6460379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008; 63: Suppl. 86, 8–160. doi: 10.1111/j.1398-9995.2007.01620.x [DOI] [PubMed] [Google Scholar]
- 68.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 2018; 141: 1169–1179. doi: 10.1016/j.jaci.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson CA, Eslick SR, Berthon BS, et al. Asthma medication use in obese and healthy weight asthma: systematic review/meta-analysis. Eur Respir J 2021; 57: 2000612. doi: 10.1183/13993003.00612-2020 [DOI] [PubMed] [Google Scholar]
- 70.Okoniewski W, Lu KD, Forno E. weight loss for children and adults with obesity and asthma. A systematic review of randomized controlled trials. Ann Am Thorac Soc 2019; 16: 613–625. doi: 10.1513/AnnalsATS.201810-651SR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miethe S, Karsonova A, Karaulov A, et al. Obesity and asthma. J Allergy Clin Immunol 2020; 146: 685–693. doi: 10.1016/j.jaci.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 72.Gupta S, Lodha R, Kabra SK. Asthma, GERD and obesity: triangle of inflammation. Indian J Pediatr 2018; 85: 887–892. doi: 10.1007/s12098-017-2484-0 [DOI] [PubMed] [Google Scholar]
- 73.Langdon C, Mullol J. Nasal polyps in patients with asthma: prevalence, impact, and management challenges. J Asthma Allergy 2016; 9: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fretzayas A, Moustaki M, Loukou I, et al. Differentiating vocal cord dysfunction from asthma. J Asthma Allergy 2017; 10: 277–283. doi: 10.2147/JAA.S146007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asthma UK . Living in limbo: the scale of unmet need in difficult and severe asthma. London, Asthma UK; 2019. www.asthma.org.uk/69841483/globalassets/get-involved/external-affairs-campaigns/.publications/living-in-limbo/living-in-limbo---the-scale-of-unmet-need-in-difficult-and-severe-asthma.pdf. Date last updated: 2019. Date last accessed: January 7, 2021. [Google Scholar]
- 76.Janson C, Menzies-Gow A, Nan C, et al. SABINA: an overview of short-acting beta2–agonist use in asthma in European countries. Adv Ther 2020; 37: 1124–1135. doi: 10.1007/s12325-020-01233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bousquet J, Brusselle G, Buhl R, et al. Care pathways for the selection of a biologic in severe asthma. Eur Respir J 2017; 50: 1701782. doi: 10.1183/13993003.01782-2017 [DOI] [PubMed] [Google Scholar]
- 78.Carr TF, Kraft M. Use of biomarkers to identify phenotypes and endotypes of severe asthma. Ann Allergy Asthma Immunol 2018; 121: 414–420. doi: 10.1016/j.anai.2018.07.029 [DOI] [PubMed] [Google Scholar]
- 79.Anderson WC, III, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: To biologic or not to biologic? Ann Allergy Asthma Immunol 2019; 122: 367–372. doi: 10.1016/j.anai.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 80.Fitzpatrick AM, Chipps BE, Holguin F, et al. T2-“low” asthma: overview and management strategies. J Allergy Clin Immunol Pract 2020; 8: 452–463. doi: 10.1016/j.jaip.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 81.Kew KM, Malik P, Aniruddhan K, et al. Shared decision-making for people with asthma. Cochrane Database Syst Rev 2017; 10: CD012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denton E, Lee J, Tay T, et al. Systematic assessment for difficult and severe asthma improves outcomes and halves oral corticosteroid burden independent of monoclonal biologic use. J Allergy Clin Immunol Pract 2020; 8: 1616–1624. doi: 10.1016/j.jaip.2019.12.037 [DOI] [PubMed] [Google Scholar]
- 83.Dias-Junior SA, Reis M, de Carvalho-Pinto RM, et al. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J 2014; 43: 1368–1377. doi: 10.1183/09031936.00053413 [DOI] [PubMed] [Google Scholar]
- 84.Polosa R, Morjaria JB, Caponnetto P, et al. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov Med 2016; 21: 99–108. [PubMed] [Google Scholar]
- 85.Kovacevic M, Culafic M, Jovanovic M, et al. Impact of community pharmacists’ interventions on asthma self-management care. Res Social Adm Pharm 2018; 14: 603–611. doi: 10.1016/j.sapharm.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 86.Hodkinson A, Bower P, Grigoroglou C, et al. Self-management interventions to reduce healthcare use and improve quality of life among patients with asthma: systematic review and network meta-analysis. BMJ 2020; 370: m2521. doi: 10.1136/bmj.m2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Janson SL, McGrath KW, Covington JK, et al. Individualized asthma self-management improves medication adherence and markers of asthma control. J Allergy Clin Immunol 2009; 123: 840–846. doi: 10.1016/j.jaci.2009.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Azzi E, Srour P, Armour C, et al. Practice makes perfect: self-reported adherence a positive marker of inhaler technique maintenance. NPJ Prim Care Respir Med 2017; 27: 29. doi: 10.1038/s41533-017-0031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asthma UK . Connected asthma: how technology will transform care. London, Asthma UK; 2016. www.asthma.org.uk/f29019fc/globalassets/get-involved/external-affairs-campaigns/publications/connected-asthma/connected-asthma---aug-2016.pdf. Date last updated: 2016. Date last accessed: January 7, 2021. [Google Scholar]
- 90.Normansell R, Kew KM, Mathioudakis AG. Interventions to improve inhaler technique for people with asthma. Cochrane Database Syst Rev 2017; 3: CD012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lokke A, Ahlbeck L, Bjermer L, et al. Expert Nordic perspectives on the potential of novel inhalers to overcome unmet needs in the management of obstructive lung disease. Eur Clin Respir J 2015; 2: 29445. doi: 10.3402/ecrj.v2.29445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cazzola M, Matera MG. Fixed-dose combination inhalers. Handb Exp Pharmacol 2017; 237: 117–129. doi: 10.1007/164_2016_66 [DOI] [PubMed] [Google Scholar]
- 93.Chung LP, Johnson P, Summers Q. Models of care for severe asthma: the role of primary care. Med J Aust 2018; 209: S34–S40. doi: 10.5694/mja18.00119 [DOI] [PubMed] [Google Scholar]
- 94.Francisco B, Rood T, Nevel R, et al. Teaming up for asthma control: EPR-3 compliant school program in missouri is effective and cost-efficient. Prev Chronic Dis 2017; 14: E40. doi: 10.5888/pcd14.170003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suwannakeeree P, Deerojanawong J, Prapphal N. School-based educational interventions can significantly improve health outcomes in children with asthma. J Med Assoc Thai 2016; 99: 166–174. [PubMed] [Google Scholar]
- 96.Bryant-Stephens T, Kenyon C, Apter AJ, et al. Creating a community-based comprehensive intervention to improve asthma control in a low-income, low-resourced community. J Asthma 2020; 57: 820–828. doi: 10.1080/02770903.2019.1619083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bruton A, Lee A, Yardley L, et al. Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med 2018; 6: 19–28. doi: 10.1016/S2213-2600(17)30474-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rasulnia M, Burton BS, Ginter RP, et al. Assessing the impact of a remote digital coaching engagement program on patient-reported outcomes in asthma. J Asthma 2018; 55: 795–800. doi: 10.1080/02770903.2017.1362430 [DOI] [PubMed] [Google Scholar]
- 99.Asthma UK . Resources. www.asthma.org.uk/advice/resources/. Date last updated: March 2021. Date last accessed: January 7, 2021.
- 100.Hui CY, Walton R, McKinstry B, et al. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc 2017; 24: 619–632. doi: 10.1093/jamia/ocw143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iio M, Miyaji Y, Yamamoto-Hanada K, et al. Beneficial features of a mHealth asthma app for children and caregivers: qualitative study. JMIR Mhealth Uhealth 2020; 8: e18506. doi: 10.2196/18506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Antalffy T, De Simoni A, Griffiths CJ. Promising peak flow diary compliance with an electronic peak flow meter and linked smartphone app. NPJ Prim Care Respir Med 2020; 30: 19. doi: 10.1038/s41533-020-0178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol Pract 2016; 4: 455–463. doi: 10.1016/j.jaip.2015.11.022 [DOI] [PubMed] [Google Scholar]
- 104.Braido F, Brusselle G, Guastalla D, et al. Determinants and impact of suboptimal asthma control in Europe: the International Cross-Sectional and Longitudinal Assessment on Asthma Control (LIAISON) study. Respir Res 2016; 17: 51. doi: 10.1186/s12931-016-0374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yawn BP, Rank MA, Bertram SL, et al. Obesity, low levels of physical activity and smoking present opportunities for primary care asthma interventions: an analysis of baseline data from The Asthma Tools Study. NPJ Prim Care Respir Med 2015; 25: 15058. doi: 10.1038/npjpcrm.2015.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zahran HS, Bailey CM, Qin X, et al. Assessing asthma control and associated risk factors among persons with current asthma – findings from the child and adult Asthma Call-back Survey. J Asthma 2015; 52: 318–326. doi: 10.3109/02770903.2014.956894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kampe M, Lisspers K, Stallberg B, et al. Determinants of uncontrolled asthma in a Swedish asthma population: cross-sectional observational study. Eur Clin Respir J 2014; 1. eCollection 22014. [https://doi:10.3402/ecrj.v3401.24109] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shah R, Newcomb DC. Sex bias in asthma prevalence and pathogenesis. Front Immunol 2018; 9: 2997. doi: 10.3389/fimmu.2018.02997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med 2015; 3: 849–858. doi: 10.1016/S2213-2600(15)00367-7 [DOI] [PubMed] [Google Scholar]
- 110.McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: Treatable Traits Down Under International Workshop report. Eur Respir J 2019; 53: 1802058. doi: 10.1183/13993003.02058-2018 [DOI] [PubMed] [Google Scholar]
- 111.Wu DJ, Hipolito E, Bilderback A, et al. Predicting future emergency department visits and hospitalizations for asthma using the Pediatric Asthma Control and Communication Instrument – Emergency Department version (PACCI-ED). J Asthma 2016; 53: 387–391. doi: 10.3109/02770903.2015.1115520 [DOI] [PubMed] [Google Scholar]
- 112.Blakey JD, Price DB, Pizzichini E, et al. Identifying risk of future asthma attacks using UK medical record data: a respiratory effectiveness group initiative. J Allergy Clin Immunol Pract 2017; 5: 1015–1024.e1018. doi: 10.1016/j.jaip.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 113.Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J 2019; 53: 1901046. [DOI] [PubMed] [Google Scholar]
- 114.Brusselle G, Michils A, Louis R, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med 2009; 103: 1633–1642. doi: 10.1016/j.rmed.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 115.Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013; 187: 804–811. doi: 10.1164/rccm.201208-1414OC [DOI] [PubMed] [Google Scholar]
- 116.Humbert M, Taille C, Mala L, et al. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J 2018; 51: 1702523. doi: 10.1183/13993003.02523-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agache I, Akdis CA, Akdis M, et al. EAACI biologicals guidelines-recommendations for severe asthma. Allergy 2021; 76: 14–44. doi: 10.1111/all.14425 [DOI] [PubMed] [Google Scholar]
- 118.Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med 2016; 4: 549–556. doi: 10.1016/S2213-2600(16)30031-5 [DOI] [PubMed] [Google Scholar]
- 119.FitzGerald JM, Bleecker ER, Menzies-Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med 2018; 6: 51–64. doi: 10.1016/S2213-2600(17)30344-2 [DOI] [PubMed] [Google Scholar]
- 120.Brusselle G, Germinaro M, Weiss S, et al. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm Pharmacol Ther 2017; 43: 39–45. doi: 10.1016/j.pupt.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 121.Chipps BE, Newbold P, Hirsch I, et al. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol 2018; 120: 504–511.e504. doi: 10.1016/j.anai.2018.01.030 [DOI] [PubMed] [Google Scholar]
- 122.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 123.Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med 2017; 377: 936–946. doi: 10.1056/NEJMoa1704064 [DOI] [PubMed] [Google Scholar]
- 124.Patel GB, Peters AT. The role of biologics in chronic rhinosinusitis with nasal polyps. Ear Nose Throat J 2021; 100: 44–47. doi: 10.1177/0145561320964653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Busse W, Muñoz X, Casale T, et al. S31: Dupilumab reduces severe exacerbations across baseline disease characteristics in patients with elevated baseline type 2 biomarkers: the LIBERTY ASTHMA QUEST study. Thorax 2019; 74: A21–A22. [Google Scholar]
- 126.European Medicines Agency (EMA) . Dupixent. Summary of Product Characteristics (SmPC). www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf. Date last updated: September 26, 2017. Date last accessed: January 7, 2021.
- 127.Casale TB, Chipps BE, Rosen K, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy 2018; 73: 490–497. doi: 10.1111/all.13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Viswanathan RK, Busse WW. How to compare the efficacy of biologic agents in asthma. Ann Allergy Asthma Immunol 2020; 125: 137–149. doi: 10.1016/j.anai.2020.04.031 [DOI] [PubMed] [Google Scholar]
- 129.Diamant Z, Vijverberg S, Alving K, et al. Toward clinically applicable biomarkers for asthma: an EAACI position paper. Allergy 2019; 74: 1835–1851. doi: 10.1111/all.13806 [DOI] [PubMed] [Google Scholar]
- 130.Tan L, Chupp G, Castro M, et al. Going beyond “bio-markers,” think “life-markers”. Chest 2020; 157: 503–505. doi: 10.1016/j.chest.2019.08.2210 [DOI] [PubMed] [Google Scholar]
- 131.Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med 2021; 9: 69–84. doi: 10.1016/S2213-2600(20)30389-1 [DOI] [PubMed] [Google Scholar]
- 132.Kerstjens HAM, Maspero J, Chapman KR, et al. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med 2020; 8: 1000–1012. doi: 10.1016/S2213-2600(20)30190-9 [DOI] [PubMed] [Google Scholar]
- 133.Virchow JC, Kuna P, Paggiaro P, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet 2019; 394: 1737–1749. doi: 10.1016/S0140-6736(19)32215-9 [DOI] [PubMed] [Google Scholar]
- 134.Gessner C, Kornmann O, Maspero J, et al. Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: a randomised, Phase IIIb, non-inferiority study (ARGON). Respir Med 2020; 170: 106021. doi: 10.1016/j.rmed.2020.106021 [DOI] [PubMed] [Google Scholar]
- 135.Zhang S, King D, Rosen VM, et al. Impact of single combination inhaler versus multiple inhalers to deliver the same medications for patients with asthma or COPD: a systematic literature review. Int J Chron Obstruct Pulmon Dis 2020; 15: 417–438. doi: 10.2147/COPD.S234823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Berry M, Morgan A, Shaw DE, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007; 62: 1043–1049. doi: 10.1136/thx.2006.073429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heaney LG, Busby J, Hanratty CE, et al. Composite type-2 biomarker strategy versus a symptom–risk-based algorithm to adjust corticosteroid dose in patients with severe asthma: a multicentre, single-blind, parallel group, randomised controlled trial. Lancet Respir Med 2021; 9: 57–68. doi: 10.1016/S2213-2600(20)30397-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chang HS, Lee TH, Jun JA, et al. Neutrophilic inflammation in asthma: mechanisms and therapeutic considerations. Expert Rev Respir Med 2017; 11: 29–40. doi: 10.1080/17476348.2017.1268919 [DOI] [PubMed] [Google Scholar]
- 139.Chaudhuri R, Livingston E, McMahon AD, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med 2006; 174: 127–133. doi: 10.1164/rccm.200510-1589OC [DOI] [PubMed] [Google Scholar]
- 140.Maghni K, Lemiere C, Ghezzo H, et al. Airway inflammation after cessation of exposure to agents causing occupational asthma. Am J Respir Crit Care Med 2004; 169: 367–372. doi: 10.1164/rccm.200309-1238OC [DOI] [PubMed] [Google Scholar]
- 141.Luo J, Yang L, Yang J, et al. Efficacy and safety of phosphodiesterase 4 inhibitors in patients with asthma: a systematic review and meta-analysis. Respirology 2018; 23: 467–477. doi: 10.1111/resp.13276 [DOI] [PubMed] [Google Scholar]
- 142.Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 2013; 188: 1294–1302. doi: 10.1164/rccm.201212-2318OC [DOI] [PubMed] [Google Scholar]
- 143.O'Byrne PM, Metev H, Puu M, et al. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2016; 4: 797–806. doi: 10.1016/S2213-2600(16)30227-2 [DOI] [PubMed] [Google Scholar]
- 144.Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med 2021; 384: 1800–1809. doi: 10.1056/NEJMoa2034975 [DOI] [PubMed] [Google Scholar]
- 145.PR Newswire , Amgen . Update on SOURCE phase 3 trial for tezepelumab in patients with severe, oral corticosteroid-dependent asthma. www.prnewswire.com/news-releases/update-on-source-phase-3-trial-for-tezepelumab-in-patients-with-severe-oral-corticosteroid-dependent-asthma-301197136.html. Date last updated: December 22, 2020. Date last accessed: January 7, 2021.
- 146.Gauvreau GM, Hohlfeld JM, Grant S, et al. Efficacy and safety of an inhaled anti-TSLP antibody fragment in adults with mild atopic asthma. Am J Respir Crit Care Med 2020; 201: A4207. [Google Scholar]
- 147.ClinicalTrials.gov . NCT04410523. Study of efficacy and safety of CSJ117 in patients with severe uncontrolled asthma. www.clinicaltrials.gov/ct2/show/NCT04410523?term=04410523&draw=2&rank=1. Date last updated: March 9, 2021. Date last accessed: March 11, 2021.
- 148.McDonald VM, Clark VL, Cordova-Rivera L, et al. Targeting treatable traits in severe asthma: a randomised controlled trial. Eur Respir J 2020; 55: 1901509. doi: 10.1183/13993003.01509-2019 [DOI] [PubMed] [Google Scholar]
- 149.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006; 354: 1985–1997. doi: 10.1056/NEJMoa051378 [DOI] [PubMed] [Google Scholar]
- 150.Benjamin O, Bansal P, Goyal A, et al. Disease Modifying Anti-Rheumatic Drugs (DMARD). Treasure Island/Florida, StatPearls Publishing LLC, 2020. [Google Scholar]
- 151.Riccio AM, Mauri P, De Ferrari L, et al. Galectin-3: an early predictive biomarker of modulation of airway remodeling in patients with severe asthma treated with omalizumab for 36 months. Clin Transl Allergy 2017; 7: 6. doi: 10.1186/s13601-017-0143-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest 2003; 112: 1029–1036. doi: 10.1172/JCI17974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sridhar S, Zhao W, Pham T-H, et al. Tezepelumab decreases matrix remodelling and inflammatory pathways in patients with asthma. Eur Respir J 2019; 54: RCT3785. [Google Scholar]
- 154.ClinicalTrials.gov . NCT03797404: Airway Remodeling During Mepolizumab Treatment (REMOMEPO). www.clinicaltrials.gov/ct2/show/NCT03797404?term=03797404&draw=1&rank=1. Date last updated: May 20, 2020. Date last accessed: January 7, 2021.
- 155.ClinicalTrials.gov . NCT03953300: Benralizumab Airway Remodeling Study in Severe Eosinophilic Asthmatics (CHINOOK). www.clinicaltrials.gov/ct2/show/NCT03953300?term=03953300&draw=2&rank=1. Date last updated: December 2, 2020. Date last accessed: January 7, 2021.
- 156.Saunders R, Kaul H, Berair R, et al. DP2 antagonism reduces airway smooth muscle mass in asthma by decreasing eosinophilia and myofibroblast recruitment. Sci Transl Med 2019; 11: eaao6451. doi: 10.1126/scitranslmed.aao6451 [DOI] [PubMed] [Google Scholar]