Abstract
Cadmium in tobacco smoke may contribute to the development of pulmonary emphysema. However, there is poor understanding of the mechanisms behind the pathogenic role of cadmium in this and other smoking-related lung diseases. The traditional focus on the total body burden of cadmium, estimated through analysis of urine, may not fully reflect the local burden of cadmium, since it is inhaled by smokers. Thus, assessing the local accumulation of cadmium in the lungs appears more relevant, given that there is tissue-specific retention of cadmium.
In this review, we outline the principal sources of cadmium exposure and the clinical effects of occupational exposure. In addition, we review evidence on local cadmium and its association with alterations in innate immunity in tobacco smokers. Moreover, we scrutinise the data on cadmium as a cause of lung disease in translational models.
We conclude that cadmium may contribute to smoking-related lung diseases, possibly via an altered redox balance and by making macrophages dysfunctional. However, there is a need for new studies on local cadmium levels and their relation to pathology in long-term tobacco smokers, as well as for more in-depth studies on cellular and molecular mechanisms, to elucidate the importance of cadmium in smoking-related lung diseases.
Short abstract
Cadmium in tobacco smoke emerges as a potentially important pathogenic factor in smoking-related lung disease http://ow.ly/msOm30irmg7
Introduction
Tobacco smoking is the single most common identified cause of chronic obstructive pulmonary disease (COPD) in the Western world and its medical and economic impact at the global level is huge [1]. Tobacco smoking is also an important cause of comorbidities to COPD, such as chronic bronchitis, pulmonary emphysema and repeated bacterial infections in the lungs [1]. Currently, the World Health Organization (WHO) predicts that COPD will become the third leading cause of disease-related death globally by 2030 [2]. Although tobacco smoking is decreasing in several European countries, it is increasing at the global level. Given that the only truly effective therapy against tobacco-related lung disease currently is smoking cessation, it seems imperative to improve the understanding of the underlying pathogenic mechanisms and their relationship to various compounds in tobacco smoke. One such compound of interest is cadmium, a toxic heavy metal which was associated with lung disease as early as 1950 [3–5].
Sources of cadmium exposure
Tobacco
According to the WHO, cadmium is a major concern for public health [5, 6], and this metal is naturally accumulated in the tobacco plant (Nicotiana tabacum) [7]. This “hyper-accumulation” leads to very high cadmium concentrations in the tobacco leaf, relatively independent of its soil content [7]. In general, the cadmium content in tobacco leaves ranges between 1 and 2 μg·g−1 dry weight, resulting in 0.5–1 μg cadmium per cigarette. Cadmium oxide, generated during tobacco smoking, is likely to either be deposited locally in lung tissue or absorbed into the systemic blood circulation, or both [5]. The absorption after inhalation in the lungs is thought to be much higher than that from food, via the intestine, and as a result, cadmium concentrations in blood can be up to four or five times higher and kidney concentrations up to two or three times higher in tobacco smokers, compared to nonsmokers [5, 7–9]. However, very little is known about its local deposition in the lungs.
Food
Agricultural and other industrial activities result in the contamination of soil with cadmium, making diet the primary source of cadmium exposure in nonsmokers. A comparatively high accumulation of cadmium in crops results in plant food generally being the primary dietary source (>80%). Cereals, especially wholegrains, leafy vegetables such as spinach, potatoes and other root vegetables, and certain seeds contain high concentrations of cadmium. Moreover, contaminated aquatic environments display enhanced accumulation of cadmium in crustaceans and molluscs [10]. In red meat products and fish, the cadmium content is relatively lower [8, 10–13].
Occupational exposure
Occupational exposure and, to some extent, house dust in contaminated areas constitute important sources of cadmium [14]. Occupational exposure to cadmium primarily takes place in industrial factories such as zinc smelters, battery manufacturing and metal-recovering factories, cadmium-refining companies, production units for paint and pigment and via other anthropogenic factors [8]. As a consequence of regulatory activities, currently, occupational exposure to cadmium is relatively less common among workers in developed countries [15].
Cadmium accumulation in tobacco smokers
Systemic accumulation
The analysis of cross-sectional data from the US National Health and Nutrition Examination Survey (NHANES) 2007–2010 revealed an association of progressive increase of cadmium concentrations in blood with impaired forced expiratory volume in 1 s (FEV1) % predicted among smokers. Moreover, the study claimed that cadmium partially mediates the association between smoking and obstructive lung disease (OLD), as defined by FEV1/forced vital capacity (FVC) ratio <0.7, thus possibly including patients with asthma as well as those with true COPD [16]. The study involved 1164 OLD patients and 8411 non-OLD subjects with the mean serum cadmium concentrations of 0.51 µg·L−1 (males 0.48 µg·L−1, females 0.58 µg·L−1) and 0.33 µg·L−1 (males 0.31 µg·L−1, females 0.36 µg·L−1), respectively. In addition, the mean cadmium concentration was significantly higher in the OLD group compared to the non-OLD group in each stratum of former and current smokers. However, there was no difference in the mean cadmium concentration among never-smokers with or without OLD, implying that smoking-related COPD accounts for the referred statistical association. Among active smokers, the mean cadmium concentration was almost 50% elevated in patients with OLD (1.04 µg·L−1) compared to those without OLD (0.70 µg·L−1). Whether these differences reflect more intense or longer periods of smoking in those who developed OLD remains unknown. The study further reported an association of increased cadmium concentration with higher risk for mild-moderate-severe OLD.
Local accumulation in the lungs
It was reported that there was a higher accumulation of cadmium in human lung tissue obtained from the Lung Tissue Research Consortium (National Institutes of Health) among patients with very severe (Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage IV) compared to patients with very mild (GOLD stage 0) COPD [17]. In fact, the cadmium content was directly proportional to the total tobacco consumption (“tobacco load”) among patients with GOLD stage IV (58±10.8 pack-years) and among those with GOLD stage 0 (22.5±12.1 pack-years). The fraction of accumulated cadmium in the lung tissue of GOLD stage IV patients was 0.0015–0.0032 µg·mg−1 dry weight tissue, whereas in the case of GOLD stage 0 patients, it was below the detection limit [17]. However, the investigation was limited to a relatively small number of subjects per study group (n=9–11).
Sundblad et al. [18] have published evidence for a link between local cadmium and alterations in innate immunity in the lungs. The study demonstrated increased extracellular cadmium concentrations in bronchoalveolar lavage (BAL) fluid from long-term smokers (n=29), with or without COPD (0.07–0.10 µg·L−1), compared to that from healthy nonsmokers (n=19). Among the latter, the extracellular cadmium concentrations were so low that they were not detectable. The observation of similar BAL cadmium concentrations for smokers with and without COPD is consistent with the well-matched tobacco load (35–40 pack-years) among the two study groups, thus demonstrating that long-term smoking per se rather than the COPD diagnosis is the critical determinant factor. Notably, the study demonstrated higher extracellular cadmium concentration in BAL fluid samples from females than in those from males (males 0.028 µg·L−1, females 0.085 µg·L−1), although this difference failed to prove statistically significant. However, the increased cadmium concentrations did display statistically significant positive correlations with markers of activity in innate immunity, at the local as well as the systemic level. These markers included the transcription of tumour necrosis factor (TNF)-α in alveolar macrophages, the concentrations of neutrophils and CD8+ cells in blood samples and the concentrations of critical cytokines such as interleukin (IL)-6 and -8, as well as concentrations of the neutrophil activity marker matrix metalloproteinase (MMP)-9 in sputum samples. This detection of locally increased cadmium concentrations and their correlation with markers of activity in innate immunity support the idea that a lung cell/tissue–cadmium interaction may contribute to the pathogenic mechanisms leading to alterations in host defence that resemble those observed in COPD. Eventually, those alterations contribute to the development of smoking-related lung diseases. The positive correlations between concentrations of cadmium with transcript levels or proteins concentrations of TNF-α, IL-6, IL-8 and MMP-9 in BAL samples, with trends towards stronger correlations among smokers with COPD, further attest the idea that this heavy metal plays a role in the development of smoking-related lung diseases.
Clinical effects on lung health caused by exposure to cadmium
Short-term exposure
Most of the studies on the immediate effects of short-term inhalation of cadmium in human lungs originate from the 1950s [5]. In essence, these studies show that inhalation exposure to high concentrations of cadmium oxide in fumes is intensely irritating to the respiratory system, and symptoms often appear after a certain time lag. For example, after an acute exposure to cadmium (8.63 mg·m−3) for 5 h [5, 19], subjects reported few symptoms, limited to coughing and slight irritation of the throat and mucosa during the first two subsequent hours. However, during the following 2–8 h, influenza-like symptoms including cough, tight chest, pain in chest on coughing and dyspnoea emerged. Beyond 8 h after cadmium exposure, the exposed subjects reported serious respiratory symptoms, including severe dyspnoea and wheezing, chest pain and precordial constriction, as well as persistent cough. Other studies have shown that acute inhalation of high doses of cadmium can be fatal and survivors of these high-dose exposures may display a more or less chronic impairment of lung function [20, 21].
Long-term exposure
Several studies from the 1950s and 1960s reported emphysema and dyspnoea among subjects exposed to lower doses of cadmium [3, 5, 22–24]. Kjellström [25] reported increased mortality due to respiratory diseases among battery factory workers exposed to cadmium for >5 years. A significant, dose-dependent increase in the ratio for reported and expected deaths from bronchitis was observed among 6995 men with occupational exposure to cadmium during an average of 11 years [26]. However, this was not the case for the corresponding ratio for emphysema. Moreover, a rapid decline in lung function, with a faster onset of emphysema, was reported for male long-term smokers (defined as 1 pack·day-1 and no history of respiratory disease) who were exposed to cadmium during 4 years at work as furnace operators, compared to other smokers [27]. This implies that tobacco smoking may accelerate the detrimental effects on the respiratory system caused by occupational cadmium exposure. It seems plausible that the additional cadmium deposition in the lungs caused by tobacco smoking is responsible for this effect among workers who suffer from occupational exposure to cadmium.
Chronic exposure
An association of chronic cadmium exposure with impaired lung function has been indicated in a study by Lampe et al. [28]. This study reported an inverse association of increased urinary cadmium to FEV1 (% pred), FVC (% pred) and FEV1/FVC ratio. In addition, the study indicated that the reduced FEV1/FVC ratio in smokers is associated with enhanced cadmium concentrations in urine, thereby suggesting a relationship between systemic cadmium and ventilatory lung function. Moreover, the findings of this study suggested that exposure to cadmium from sources other than tobacco may predispose individuals to COPD. However, the modest sample size (n=96) sets statistical limits on the conclusions from this study [28]. Along these lines, the third NHANES survey on 16 024 adult subjects in the United States presents evidence that elevated concentrations of cadmium in urine, a perceived indicator of the total burden of cadmium, are associated with a decreased ventilatory lung function. As expected, a higher mean value for urinary cadmium/creatinine ratio was detected among current smokers (0.46±0.01 µg·g−1) than among former smokers (0.32±0.01 µg·g−1) and never-smokers (0.23±0.01 µg·g−1). Increased concentrations of cadmium in urine were associated with lower FEV1, FVC and FEV1/FVC ratio among both current and former smokers, which is compatible with long-lasting effects of cadmium [29, 30].
Given the publications reviewed on various kinds of cadmium exposure, it seems that studies of both short- and long-term exposure, as well as chronic exposure to cadmium, indicate an association of the exposure to this heavy metal with the loss of lung function and development of lung diseases. Further support for this type of association has been provided by the fact that the pulmonary effects among long-term smokers are more prominent than among those with lesser tobacco load. The predisposition of tobacco smokers to the respiratory effects of occupational cadmium exposure underlines the possibility that local uptake of cadmium through inhalation may play an important role in the pathogenesis of smoking-related lung diseases.
Effects of experimental cadmium inhalation
Short-term exposure
A solid body of evidence from animal studies supports the causal link between acute inhalation exposure to cadmium and lung disease. Single acute inhalation, in the form of cadmium oxide dust or cadmium oxide fume (5–10 mg cadmium·m−3 for 1–5 h) results in multifocal interstitial pneumonitis, diffuse alveolitis, inhibition of macrophages, focal interstitial thickening, oedema and type 2 cell hyperplasia in rats in vivo [5, 31–37]. Repeated short-term inhalation, in the form of cadmium oxide (0.088 mg·m−3) for 2 weeks results in alveolar macrophage infiltration, focal inflammation surrounding alveolar ducts and hyperplasia in the tracheobronchial lymph nodes in rats and mice in vivo [36]. Thus, the effects of short-term acute cadmium inhalation is consistent with the pathology observed in smoking-related lung diseases, including chronic bronchitis and emphysema.
Long-term exposure
There are very few truly long-term studies on the effects of cadmium administered through inhalation in animal models. However, there is a fair number of studies on what can be referred to as “intermediate-term” exposure. These studies indicate that intermediate-term exposure to cadmium (0.004–0.07 mg cadmium·m−3) results in respiratory effects that are similar to those observed after acute exposure [3, 4, 36, 38–40]. Pathology features such as pulmonary oedema and cell hyperplasia in the bronchoalveolar region have been observed in rats following chronic cadmium exposure [41, 42]. Other studies have shown that repeated low-concentration exposure to cadmium results in the development of an “adaptive survival response” with an increase in metallothionein, glutathione and γ-glutamylcysteine synthetase [33, 43, 44]. Tentatively, the collective evidence from studies of both short- and intermediate-term exposure to cadmium in rodents indicates that cadmium causes alterations locally in the lungs that resemble the pathology in smoking-related lung diseases. These cadmium-induced alterations include biochemical responses, inflammatory reactions and histological changes, whereas the published evidence on induced alterations in the lung physiology in vivo is more limited.
Molecular and cellular targets of cadmium
Oxidative stress
Cadmium binds to metallothioneins, the antioxidant enzyme glutathione (GSH) and other proteins or peptides in a tissue-specific manner. Metallothioneins bind to cadmium, thereby detoxifying and removing it from the cellular environment [8]. Exposure to cadmium chloride causes an increased oxidised GSH pool (glutathione disulphide (GSSG)) in rat lung epithelial cell lines, indicating the generation of reactive oxygen species (ROS). Single-dose inhalation of cadmium chloride (0.1%) in rats results in an acute increase of the GSSG/GSH ratio in cell-free BAL fluid, thereby indicating enhanced oxidative stress. Repeated inhalation of cadmium chloride (1 h per day for 3–5 weeks) also results in a progressive increase of GSH in the cell-free BAL fluid [8, 45–50]. Notably, the enzymatic activity of major antioxidants, including superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase, is altered in response to cadmium exposure [51–53]. Thus, the ability of cadmium to elicit oxidative stress response is proven.
Orotracheal administration of cadmium chloride in a rat model causes centriacinar emphysema defined as reduced mean linear intercept, enhanced collagen content and impaired lung function (FVC) [54]. In this setting, the oral co-administration of the anti-oxidant N-acetylcysteine partially reversed the induced pathology. Moreover, autophagy mediated by haemoxygenase may protect against cell death in pulmonary endothelial cells and the development of emphysema that is caused by intratracheal administration of cadmium chloride in a mouse model [55]. Furthermore, lysyl oxidase (LOX) is a copper-dependent enzyme essential for the cross-linking of the extracellular matrix. The LOX core promoter and redox-sensitive cis-elements have been identified as key targets for cadmium in the downregulation of LOX-mediated mechanisms that signify cadmium-induced emphysema in a rat model [56]. Notably, the increased oxidative stress, persistent inflammation, degradation of extracellular matrix, loss of lung function and increased alveolar size are established phenomena in the pathogenesis of in smoking-related lung diseases including COPD, chronic bronchitis and emphysema.
Alveolar macrophages
Vimentin is a major intracellular intermediate filament protein. It is expressed abundantly by fibroblasts and it is expressed on the cell surface of macrophages and endothelial cells. Interestingly, vimentin-deficient mice are protected from cadmium-induced peribronchiolar fibrosis and remodelling [57]. The uptake of cadmium results in altered immune function in human THP-1-derived macrophages compared to THP-1 monocytes, as suggested by a decrease in the production of TNF-α, IL-6, IL-10 and IL-8, all archetypal pro- and anti-inflammatory cytokines. Moreover, the impaired immune function of macrophages observed in the referred study was attributed to the inhibition of the transcription factor complex nuclear factor (NF)-κB. Indeed, the NF-κB pathway is a well-known critical activator of genes involved in inflammation and immune function [58]. The exposure to cadmium results in elevated protein concentrations of several pro-inflammatory cytokines and their receptors such as chemokine (C-X-C motif) ligand (CXCL)2, CXCL3, IL-8/CXCL8 and chemokine (C-C motif) ligand-26, IL-6 and IL-1 receptor ligand-1 in human fibroblasts in vitro [59]. In addition, in the same study, an increased release of classic pro-inflammatory cytokines such as CXCL2, IL-1β and TNF-α from rat alveolar macrophages was reported [59]. It is interesting to note that alveolar macrophages in cigarette smokers may accumulate significant amounts of cadmium without a concurrent increase in metallothionein content, thereby suggesting a greater saturation of metallothioneins [60]. Notably, these metallothioneins are low molecular weight and cysteine rich proteins that bind both physiological and xenobiotic heavy metals such as cadmium, zinc and copper [60]. The saturation of metallothioneins may impair the ROS-scavenging ability of tissue in mammalian organs. Thus, a redox imbalance, a disruption of extracellular matrix homeostasis and an impaired immune function of alveolar macrophages emerge as potentially important mechanisms in lung diseases caused by cadmium exposure. However, a more detailed understanding of the signalling pathways involved in cadmium-mediated effects in lung disease is needed to make this area of research useful in a clinical context.
Summary and conclusions
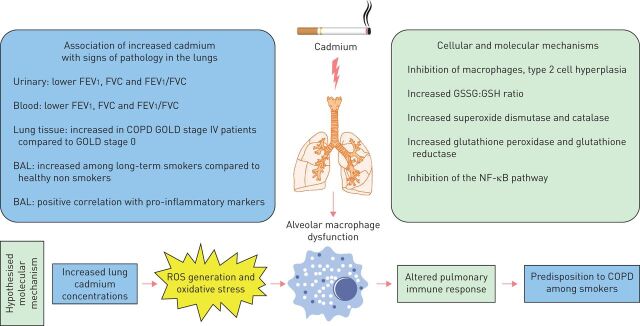
Taken together, the association of increased concentrations of cadmium in urine [29], blood [16] and lungs [17, 18] with smoking, declining ventilatory lung function and the diagnosis of COPD, respectively, has been demonstrated in several studies (summarised in figure 1). However, the current body of evidence is insufficient to conclude whether urinary or blood cadmium concentrations accurately reflect the accumulation of cadmium in the lung. Moreover, the current body of evidence does not allow us to clearly distinguish the cadmium-mediated effects in the lungs from those caused by other potentially pathogenic compounds in tobacco smoke. Local accumulation of cadmium in the lungs appears to be a critical component of predisposition to lung diseases among long-term smokers. This may be particularly important considering that the biological half-life of cadmium in the human body is >25 years, a substantial period of time, suggesting the possibility of significant retention of cadmium in the lungs of long-term smokers [5]. Moreover, it is plausible that retention of cadmium in the lungs and other organs will inevitably influence intracellular signalling. This may in turn result in impaired function of host defence including innate immunity, possibly contributing to the enhanced susceptibility to bacterial colonisation and infections leading to chronic inflammation, fibrosis and emphysema. These events may in turn lead to impaired ventilatory lung function as well as reduced gas exchange, key clinical characteristics in severe COPD among long-term smokers. Given this, we think that studies on large clinical cohorts, simultaneously characterising cadmium concentrations in urine, blood and lung compartments in long-term smokers with and without disease, along with mechanistic animal studies in vivo, are highly justified. Collectively, these types of studies may substantially improve the understanding of the pathogenic significance of cadmium in smoking-related lung diseases and the corresponding potential in targeting cadmium for diagnosis, monitoring or treatment.
FIGURE 1.
Schematic representation of the current evidence for the association of cadmium exposure with smoking-related lung disease including chronic obstructive pulmonary disease (COPD). FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; BAL: bronchoalveolar lavage; GSSG: glutathione disulphide; GSH: glutathione; NF-κB: nuclear factor κ-light-chain-enhancer of activated B cells; ROS: reactive oxygen species.
Acknowledgements
Author contributions: All authors have read and approved the manuscript.
Footnotes
Support statement: Academic funding was obtained from the Swedish Governmental Agency for Innovations Systems to K. Ganguly (VINNOVA, grant number 2016-01951) and the Swedish Heart-Lung Foundation (L. Palmberg and A. Lindén). No funding was obtained from the tobacco industry. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: G. Ganguly reports grants from VINNOVA during the conduct of the study.
Provenance: Submitted manuscript, peer reviewed.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD executive summary. Am J Respir Crit Care Med 2017; 195: 557–582. [DOI] [PubMed] [Google Scholar]
- 2.Adeloye D, Chua S, Lee C, et al. . Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015; 5: 020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friberg L. Health hazards in the manufacture of alkaline accumulators with special reference to chronic cadmium poisoning; a clinical and experimental study. Acta Medica Scand Suppl 1950; 240: 1–124. [PubMed] [Google Scholar]
- 4.Friberg L. Injuries following continued administration of cadmium. preliminary report of a clinical and experimental study. Arch Indust Hyg Occup Med 1950; 1: 458–466. [PubMed] [Google Scholar]
- 5.Agency for Toxic Substances & Disease Registry (ATSDR) . Toxicological Profile for Cadmium. Atlanta, GA, U.S. Department of Health and Human Services, Public Health Service, 2012. [Google Scholar]
- 6.World Health Organization (WHO) . Exposure to Cadmium: a Major Public Health Concern. Geneva, WHO, 2010. [Google Scholar]
- 7.Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect 2004; 112: 1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair AR, Degheselle O, Smeets K, et al. . Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci 2013; 14: 6116–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barregard L, Fabricius-Lagging E, Lundh T, et al. . Cadmium, mercury, and lead in kidney cortex of living kidney donors: impact of different exposure sources. Environ Res 2010; 110: 47–54. [DOI] [PubMed] [Google Scholar]
- 10.Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on cadmium in food. EFSA J 2009; 980: 1–139. [Google Scholar]
- 11.Satarug S, Garrett SH, Sens MA, et al. . Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 2010; 118: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarwar N, Saifullah Malhi SS, et al. . Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 2010; 90: 925–937. [DOI] [PubMed] [Google Scholar]
- 13.Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 2009; 238: 201–208. [DOI] [PubMed] [Google Scholar]
- 14.Hogervorst J, Plusquin M, Vangronsveld J, et al. . House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ Res 2007; 103: 30–37. [DOI] [PubMed] [Google Scholar]
- 15.International Cadmium Association . Cadmium Exposure and Human Health. www.cadmium.org/environment/cadmium-exposure-and-human-health Date last accessed: October 31, 2017.
- 16.Rokadia HK, Agarwal S. Serum heavy metals and obstructive lung disease: results from the National Health and Nutrition Examination Survey. Chest 2013; 143: 388–397. [DOI] [PubMed] [Google Scholar]
- 17.Hassan F, Xu X, Nuovo G, et al. . Accumulation of metals in GOLD4 COPD lungs is associated with decreased CFTR levels. Respir Res 2014; 15: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundblad BM, Ji J, Levänen B, et al. . Extracellular cadmium in the bronchoalveolar space of long-term tobacco smokers with and without COPD and its association with inflammation. Int J Chron Obstruct Pulmon Dis 2016; 11: 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beton DC, Andrews GS, Davies HJ, et al. . Acute cadmium fume poisoning: five cases with one death from renal necrosis. Br J Ind Med 1966; 23: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnhart S, Rosenstock L. Cadmium chemical pneumonitis. Chest 1984; 86: 789–791. [DOI] [PubMed] [Google Scholar]
- 21.Townshend RH. Acute cadmium pneumonitis: a 17-year follow-up. Br J Ind Med 1982; 39: 411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnell JA. Emphysema and proteinuria in men casting copper-cadmium alloys. Br J Ind Med 1955; 12: 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane RE, Campbell AC. Fatal emphysema in two men making a copper cadmium alloy. Br J Ind Med 1954; 11: 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith JP, Smith JC, McCall AJ. Chronic poisoning from cadmium fume. J Pathol Bacteriol 1960; 80: 287–296. [Google Scholar]
- 25.Kjellström T. Exposure and accumulation of cadmium in populations from Japan, the United States, and Sweden. Environ Health Perspect 1979; 28: 169–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong BG, Kazantzis G. The mortality of cadmium workers. Lancet 1983; 1: 1425–1427. [DOI] [PubMed] [Google Scholar]
- 27.Leduc D, de Francquen P, Jacobovitz D, et al. . Association of cadmium exposure with rapidly progressive emphysema in a smoker. Thorax 1993; 48: 570–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampe BJ, Park SK, Robins T, et al. . Association between 24-hour urinary cadmium and pulmonary function among community-exposed men: the VA Normative Aging Study. Environ Health Perspect 2008; 116: 1226–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannino DM, Holguin F, Greves HM, et al. . Urinary cadmium levels predict lower lung function in current and former smokers: data from the Third National Health and Nutrition Examination Survey. Thorax 2004; 59: 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendrick DJ. Smoking, cadmium, and emphysema. Thorax 2004; 59: 184–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boudreau J, Vincent R, Nadeau D, et al. . The response of the pulmonary surfactant-associated alkaline phosphatase following acute cadmium chloride inhalation. Am Ind Hyg Assoc J 1989; 50: 331–335. [DOI] [PubMed] [Google Scholar]
- 32.Buckley BJ, Bassett DJ. Pulmonary cadmium oxide toxicity in the rat. J Toxicol Environ Health 1987; 21: 233–250. [DOI] [PubMed] [Google Scholar]
- 33.Bus JS, Vinegar A, Brooks SM. Biochemical and physiologic changes in lungs of rats exposed to a cadmium chloride aerosol. Am Rev Respir Dis 1978; 118: 573–580. [DOI] [PubMed] [Google Scholar]
- 34.Grose EC, Richards JH, Jaskot RH, et al. . A comparative study of the effects of inhaled cadmium chloride and cadmium oxide: pulmonary response. J Toxicol Environ Health 1987; 21: 219–232. [DOI] [PubMed] [Google Scholar]
- 35.Hart BA, Voss GW, Willean CL. Pulmonary tolerance to cadmium following cadmium aerosol pretreatment. Toxicol Appl Pharmacol 1989; 101: 447–460. [DOI] [PubMed] [Google Scholar]
- 36.NTP toxicity studies of cadmium oxide (CAS No. 1306-19-0) administered by inhalation to F344/N rats and B6C3F1 mice. Toxic Rep Ser 1995; 39: 1–D3. [PubMed] [Google Scholar]
- 37.Palmer KC, Mari F, Malian MS. Cadmium-induced acute lung injury: compromised repair response following thyroidectomy. Environ Res 1986; 41: 568–584. [DOI] [PubMed] [Google Scholar]
- 38.Johansson A, Curstedt T, Robertson B, et al. . Lung morphology and phospholipids after experimental inhalation of soluble cadmium, copper, and cobalt. Environ Res 1984; 34: 295–309. [DOI] [PubMed] [Google Scholar]
- 39.Oberdörster G, Cherian MG, Baggs RB. Importance of species differences in experimental pulmonary carcinogenicity of inhaled cadmium for extrapolation to humans. Toxicol Lett 1994; 72: 339–343. [DOI] [PubMed] [Google Scholar]
- 40.Prigge E. Early signs of oral and inhalative cadmium uptake in rats. Arch Toxicol 1978; 40: 231–247. [DOI] [PubMed] [Google Scholar]
- 41.Oldiges H, Glaser U. The inhalative toxicity of different cadmium compounds in rats. Trace Elem Med 1986; 3: 72–75. [Google Scholar]
- 42.Takenaka S, Oldiges H, König H, et al. . Carcinogenicity of cadmium chloride aerosols in W rats. J Natl Cancer Inst 1983; 70: 367–373. [PubMed] [Google Scholar]
- 43.Hart BA. Cellular and biochemical response of the rat lung to repeated inhalation of cadmium. Toxicol Appl Pharmacol 1986; 82: 281–291. [DOI] [PubMed] [Google Scholar]
- 44.Hart BA, Potts RJ, Watkin RD. Cadmium adaptation in the lung – a double-edged sword? Toxicology 2001; 160: 65–70. [DOI] [PubMed] [Google Scholar]
- 45.Moulis JM. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 2010; 23: 877–896. [DOI] [PubMed] [Google Scholar]
- 46.Vesey DA. Transport pathways for cadmium in the intestine and kidney proximal tubule: focus on the interaction with essential metals. Toxicol Lett 2010; 198: 13–19. [DOI] [PubMed] [Google Scholar]
- 47.Dalton TP, He L, Wang B, et al. . Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci USA 2005; 102: 3401–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 2010; 23: 783–792. [DOI] [PubMed] [Google Scholar]
- 49.Abouhamed M, Wolff NA, Lee WK, et al. . Knockdown of endosomal/lysosomal divalent metal transporter 1 by RNA interference prevents cadmium-metallothionein-1 cytotoxicity in renal proximal tubule cells. Am J Physiol Renal Physiol 2007; 293: F705–F712. [DOI] [PubMed] [Google Scholar]
- 50.Thévenod F. Cadmium and cellular signaling cascades: to be or not to be? Toxicol Appl Pharmacol 2009; 238: 221–239. [DOI] [PubMed] [Google Scholar]
- 51.Casalino E, Sblano C, Landriscina C. Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys 1997; 346: 171–179. [DOI] [PubMed] [Google Scholar]
- 52.Ikediobi CO, Badisa VL, Ayuk-Takem LT, et al. . Response of antioxidant enzymes and redox metabolites to cadmium-induced oxidative stress in CRL-1439 normal rat liver cells. Int J Mol Med 2004; 14: 87–92. [PubMed] [Google Scholar]
- 53.Waisberg M, Joseph P, Hale B, et al. . Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003; 192: 95–117. [DOI] [PubMed] [Google Scholar]
- 54.Heili Frades S, Del Puerto-Nevado L, Pérez-Rial S, et al. . Improving the cadmium-induced centriacinar emphysema model in rats by concomitant anti-oxidant treatment. Clin Exp Pharmacol Physiol 2008; 35: 1337–1342. [DOI] [PubMed] [Google Scholar]
- 55.Surolia R, Karki S, Kim H, et al. . Heme oxygenase-1-mediated autophagy protects against pulmonary endothelial cell death and development of emphysema in cadmium-treated mice. Am J Physiol Lung Cell Mol Physiol 2015; 309: L280–L292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Cheng G, Zheng M, et al. . The core promoter and redox-sensitive cis-elements as key targets for inactivation of the lysyl oxidase gene by cadmium. J Nat Sci 2015; 1: e38. [PMC free article] [PubMed] [Google Scholar]
- 57.Li FJ, Surolia R, Li H, et al. . Low-dose cadmium exposure induces peribronchiolar fibrosis through site-specific phosphorylation of vimentin. Am J Physiol Lung Cell Mol Physiol 2017; 313: L80–L91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cox JN, Rahman MA, Bao S, et al. . Cadmium attenuates the macrophage response to LPS through inhibition of the NF-κB pathway. Am J Physiol Lung Cell Mol Physiol 2016; 311: L754–L765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Låg M, Rodionov D, Ovrevik J, et al. . Cadmium-induced inflammatory responses in cells relevant for lung toxicity: expression and release of cytokines in fibroblasts, epithelial cells and macrophages. Toxicol Lett 2010; 193: 252–260. [DOI] [PubMed] [Google Scholar]
- 60.Grasseschi RM, Ramaswamy RB, Levine DJ, et al. . Cadmium accumulation and detoxification by alveolar macrophages of cigarette smokers. Chest 2003; 124: 1924–1928. [DOI] [PubMed] [Google Scholar]