Abstract
Nasal high flow is a promising novel oxygen delivery device, whose mechanisms of action offer some beneficial effects over conventional oxygen systems. The administration of a high flow of heated and humidified gas mixture promotes higher and more stable inspiratory oxygen fraction values, decreases anatomical dead space and generates a positive airway pressure that can reduce the work of breathing and enhance patient comfort and tolerance. Nasal high flow has been used as a prophylactic tool or as a treatment device mostly in patients with acute hypoxaemic respiratory failure, with the majority of studies showing positive results. Recently, its clinical indications have been expanded to post-extubated patients in intensive care or following surgery, for pre- and peri-oxygenation during intubation, during bronchoscopy, in immunocompromised patients and in patients with “do not intubate” status. In the present review, we differentiate studies that suggest an advantage (benefit) from other studies that do not suggest an advantage (no benefit) compared to conventional oxygen devices or noninvasive ventilation, and propose an algorithm in cases of nasal high flow application in patients with acute hypoxaemic respiratory failure of almost any cause.
Short abstract
Most studies favour NHF over conventional oxygen therapy, but it is unknown which patients will benefit the most http://ow.ly/Dmk830cBTKY
Introduction
Over the past decade, nasal high flow (NHF) has been introduced for oxygen therapy in adults, as a natural extension of its use in neonates and children. The device consists of an air/oxygen blender connected via an active heated humidifier to a nasal cannula, through a single-limb heated inspiratory circuit. It delivers a fraction of inspired oxygen (FiO2) from 21% to 100%, with a flow rate up to 60 L·min−1. FiO2 adjustments are independent of the setting flow rate, so that the patient is administered heated humidified high-flow oxygen, with a flow that can be set above the patient's maximum inspiratory flow rate, thus increasing confidence about the FiO2 being delivered to the patient [1]. These device characteristics make it more promising in comparison with conventional low- and high-flow oxygen devices (e.g. nasal cannula, non-rebreathing masks and Venturi masks), especially in patients with high inspiratory flow rates, such as patients with acute respiratory failure (ARF) [1, 2].
Recently, indications for the NHF system have been expanded, although there are no established guidelines or algorithms to guide its use. In the present review we discuss the physiological effects of NHF, its current clinical implications in adults and differentiate studies that suggest an advantage (benefit) from other studies that do not suggest an advantage (no benefit) compared to conventional oxygen devices or noninvasive ventilation (NIV). Moreover, we propose an algorithm for the rational clinical application of NHF in patients with acute hypoxaemic respiratory failure of almost any cause.
Search criteria
We searched for trials and reviews of NHF therapy in adult patients on PubMed and the Cochrane Database. We limited our search to English-language publications using the terms “high flow” OR “heated” OR “humidified” AND “oxygen” OR “nasal oxygen” OR “nasal cannulae” in the text or title. The last search was performed on April 1, 2017.
Mechanisms of action
NHF can adequately supplement oxygen, support the respiratory system and hydrate the airways effectively. FiO2 values administered via NHF are more stable and higher than those of standard oxygen delivery systems [1], because NHF can generate high flow rates up to 60 L·min−1 that can match or even exceed the patient's inspiratory flow demand, thus reducing entrainment of room air and dilution of administered oxygen [3, 4]. In addition, these high flow rates create a positive airway pressure which linearly correlates with the administered flow rate [3–8]. For every 10 L·min−1 increase in gas flow, the mean airway pressure increases by ∼0.69 cmH2O in the mouth-closed position and by 0.35 cmH2O in the mouth-open position [8]. Analysis of the pressure generated during different parts of the respiratory cycle demonstrated that higher pressures are obtained during expiration than inspiration, which are flow dependent [9]. This mechanism resembles the application of positive end-expiratory pressure (PEEP), and it results in recruitment of the atelectatic areas and thus increase of end-expiratory lung volume (EELV) by ∼25% [5]. This effect is more pronounced in subjects with higher body mass index (BMI), regardless of body position [5, 10]. Furthermore, lung compliance increases and the patient breathes more slowly, with almost 10% higher tidal volumes [5]. The imposed expiratory resistance to the patient's exhalation against the continuous high flow of incoming oxygen gas and the pressurisation of the upper airway above atmospheric pressure are the possible explanations for this mechanism [7, 8].
Reduced dead space ventilation and thus reduced rebreathing of expired air in a flow-dependent manner is another benefit that is attributed to high gas flow that is delivered by this device, as demonstrated in studies using upper airway models as well as on healthy volunteers [11, 12]. The mechanism differs between sleep and awake states, because of different ventilatory responses to NHF in each state. Indeed, during wakefulness, respiratory rate is slowed and tidal volume is increased in response to NHF, whereas during sleep there is no change in respiratory rate, but a reduction in tidal volume, which leads to ∼20% reduction of minute ventilation without any increase in dead space. Therefore, it seems that the decrease in dead space by caused by NHF during wakefulness is attributed to increased tidal volume, whereas the stable dead space during sleep is attributed to washout of nasopharyngeal dead space volume by the high flow of incoming gas [13].
NHF has a significant role in heating to the core temperature level (37°C) and humidifying the respiratory system to saturation, especially in high flow rates, where conventional oxygen delivery systems fail [14]. By this mechanism, mucociliary function and secretion clearance is improved [15], as shown in patients with idiopathic bronchiectasis from the reduced percentage of retention of an inhaled tracer before and after receiving warm and humidified air through NHF [16]. Furthermore, the required metabolic cost of warming and increasing the relative humidity of inspired gas is reduced, especially in patients with increased minute ventilation [17].
All these NHF mechanisms of actions exert various effects in the respiratory system, including improved gas exchange, lower respiratory rate and effort, improved lung volume, dynamic compliance, transpulmonary pressures and homogeneity of ventilation, as shown in a study of ARF patients in whom an oesophageal balloon catheter along with electrical impedance tomography allowed advanced physiological measurements [18]. Therefore, patients breathe more comfortably with less subjective dyspnoea as a result of the reduced work of breathing [19, 20].
Clinical implications
Acute hypoxaemic respiratory failure
Benefit
Conventional low- and high-flow oxygen systems are traditionally used as first-line supportive treatment of hypoxaemic ARF. Unfortunately, in both systems, at high inspiratory flow rates there is a limitation of heating and humidifying of inspired oxygen as well as increased entrainment of room air, which leads to dilution of oxygen and lowering of FiO2 [21]. The principles and mechanisms of action of NHF make it an attractive and promising oxygen delivery device for adult patients with hypoxaemic ARF. The beneficial effects of NHF in both objective parameters (respiratory rate and arterial oxygen saturation measured by pulse oximetry (SpO2)) and subjective ones, such as the degree of sensed dyspnoea, were confirmed in two studies on patients with ARF receiving oxygen through NHF or face mask. NHF was associated with less dyspnoea, a lower respiratory rate and greater overall oxygenation and comfort [19, 22]. The short (30 min) implementation period of NHF [22] and the late initiation of its use [19] did not allow any further conclusions regarding the effectiveness of NHF on ARF.
Sztrymf et al. [23] studied the efficacy, safety and outcome of the immediate application of NHF in intensive care unit (ICU) patients with hypoxaemic ARF without any time limitation of its application. Use of NHF was associated with a significant reduction in respiratory rate, heart rate, dyspnoea score, supraclavicular retraction and thoraco-abdominal asynchrony and a significant improvement in SpO2. These improvements were evident within the first 15–30 min after applying NHF and lasted throughout the study period without any unexpected side-effects. >75% of patients avoided intubation, and thus the authors concluded that NHF has a favourable effect on clinical signs and oxygenation in critically ill patients with ARF [23]. These encouraging results were further confirmed by the same group of authors 1 year later in a prospective observational study on patients with ARF caused by community-acquired pneumonia and sepsis [24]. Moreover, NHF may be more effective in treating mild to moderate hypoxaemic ARF compared to oxygen delivered through a face mask, with fewer desaturations and less need for NIV, as reported in a prospective randomised study by Parke et al. [25].
Although the aforementioned studies included patients with hypoxaemic ARF and various level of hypoxaemia, none of them assessed the effect of NHF on other important clinical outcomes, such as intubation rate and mortality. In a recent randomised controlled trial involving patients admitted to the ICU with hypoxaemic ARF, NHF, NIV and standard oxygen therapy (SOT) were evaluated for their effect on endotracheal intubation and outcome [26]. Neither NIV nor NHF decreased the rate of intubation among the overall studied population. However, the intubation rate was significantly lower with NHF in the subgroup of patients with more severe respiratory insufficiency (arterial oxygen tension (PaO2)/FiO2 ≤200). Moreover, therapy with NHF resulted in a significantly lower ICU and 90-day mortality, as compared with SOT or NIV, which was attributed to lower intubation rates among patients with severe hypoxaemia. Thereafter, it seems that NHF can be used in every stage of hypoxaemic ARF, even in patients with acute respiratory distress syndrome [27].
No benefit
As with NIV [28], there is a great concern about the optimum duration of NHF use. The potential masking of any respiratory deterioration may increase mortality due to delayed intubation [29]. In fact, findings from a retrospective observational study in ICU patients suggested that early intubation (i.e. within 48 h of NHF initiation) was associated with lower overall ICU mortality, better extubation success and more ventilator-free days than late intubation when patients had received NHF therapy that failed [29]. The 48 h time frame is in accordance with that of Moretti et al. [28], who recorded particularly high mortality (67.7%) in patients with NIV failure who were intubated after the aforementioned time. Some significant factors could have influenced the results of this study [29]. Flow settings of NHF were not provided, which is crucial since the benefits arising from its application are all flow dependent. In addition, more patients in the late group were intubated because of acute-on-chronic respiratory failure, in whom only NIV is recommended to date [30].
In the HOT-ER study [31] performed in patients admitted to the emergency department with ARF, no differences were detected between NHF and SOT regarding the rate of initiation of mechanical ventilation (invasive or noninvasive) in the emergency department, the length of emergency department or hospital stay and 90-day mortality. However, during the study, it became apparent that some participants received NIV soon after leaving the emergency department, and a post hoc analysis showed that fewer participants in the NHF group required mechanical ventilation either in the emergency department or within 24 h of leaving the emergency department. It is possible that NHF exerts its benefit beyond the first few hours because the developed airway pressures are low and thus a longer time period is needed to recruit any atelectatic lung areas. A significant message that can be drawn from these two studies [29, 31] could be that although NHF application in patients with ARF seems to be very effective, we need more randomised trials to define predictors of success or failure in order to avoid early discontinuation of the therapy, or, in contrast, for timely diagnosis of treatment failure and intubation, thus avoiding any undesired evolution. A summary of these studies is shown in table 1.
TABLE 1.
Relevant studies for nasal high flow (NHF) in patients with acute respiratory failure
| Design | Patients | Results | |
| Benefit | |||
| Lenglet et al. [19] | Prospective, observational study | 17 ARF patients on oxygen with a non-rebreathing face mask who switched to NHF | NHF was associated with a significant decrease in dyspnoea, respiratory rate and a significant increase in SpO2 within the first 15 min of its application |
| Roca et al. [22] | Prospective, comparative study of sequential interventions | 20 patients with hypoxaemic ARF received oxygen via face mask and NHF for 30 min each | NHF was associated with less dyspnoea and mouth dryness and more comfort than face mask; it was also associated with higher PaO2 and lower respiratory rate, with no differences in PaCO2 |
| Sztrymf et al. [23] | Prospective observational study | 38 ICU patients with ARF who received oxygen through NHF | NHF was associated with a significant reduction in respiratory rate, heart rate, dyspnoea score, supraclavicular retraction and thoraco-abdominal asynchrony and a significant improvement in SpO2 |
| Sztrymf et al. [24] | Prospective observational study | 20 patients with persistent ARF who received oxygen through NHF | Use of NHF enabled a significant reduction of respiratory rate and a significant increase in oxygen saturation and PaO2 |
| Frat et al. [26] | Multicentre, open-label RCT | 310 ARF patients admitted to ICU, randomly assigned to NHF, SOT or NIV | ICU and 90-day mortality were significantly lower with NHF compared with SOT or NIV, although no significant difference in intubation rate was found between the three study groups. In the subgroup of patients with PaO2/FiO2 ≤200 the intubation rate was significantly lower with NHF |
| Messika et al. [27] | Prospective observational study | Effectiveness and frequency of NHF use in patients with ARDS | 29% of patients requiring noninvasive ventilatory support were treated via NHF as first-line treatment. Among them, the intubation rate was 40%. Haemodynamic failure was associated with NHF failure and intubation |
| No benefit | |||
| Jones et al. [31] | Pragmatic open randomised controlled trial | 303 ARF patients at emergency departments randomised to NHF or SOT | There was no difference between the groups in NIV or intubation rate during the emergency department stay in mortality, on emergency department and hospital length of stay |
ARF: acute respiratory failure; SpO2: arterial oxygen saturation measured by pulse oximetry; PaO2: arterial oxygen tension; PaCO2: arterial carbon dioxide tension; ICU: intensive care unit; SOT: standard oxygen treatment; NIV: noninvasive ventilation; FiO2: inspiratory oxygen fraction; ARDS: acute respiratory distress syndrome.
Post-extubation in the ICU
Benefit
The incidence of extubation failure in ICU varies between 6% and 47%, with respiratory failure being its most common cause, and is associated with a significant morbidity and mortality more than double that of successful extubation [32]. SOT devices are used to treat this complication, but they may be inadequate in patients with high inspiratory flow, since they can provide oxygen at flow rates <15 L·min−1 [21, 32, 33]. Rittayamai et al. [34], in a crossover study, compared 30-min interventions with NHF and a non-rebreathing mask in recently extubated patients. They reported significant reductions in dyspnoea, respiratory rate and heart rate with NHF [34]. These results were further confirmed and strengthened by another study comparing NHF with the Venturi mask in critically ill patients requiring oxygen therapy after extubation (PaO2/FiO2 <300) [35]. It was found that use of NHF was associated with a lower respiratory rate, less discomfort and better oxygenation for the same set FiO2, until 48 h after extubation, with fewer episodes of oxygen desaturation. Moreover, the use of NHF in the post-extubation period was associated with less need for NIV and endotracheal intubation than the Venturi mask (7.5% versus 34.6%) [35]. Both these studies [34, 35] support the protective role of NHF after extubation, attributing this effect to higher flow of the administered gas with the NHF system, which could meet the ventilator demand of the patient after extubation. Given the fact that the patients included in these studies [34, 35] had residual respiratory impairment, these results could not be generalised to the overall population. Indeed, even in patients at low risk of re-intubation, NHF successfully reduced re-intubation rates from 12.2% [36, 37] to 4.9%, compared to SOT devices, without any reported complications, which was attributed to NHF-favourable mechanisms on the work of breathing and the conditioning of the inspired gas, even at high flow rates [38].
No benefit
Although the consistent benefit in comfort and tolerance is confirmed in almost all studies applying NHF after extubation [34, 35, 38, 39], there are conflicting data about its effect on gas exchange and physiological parameters. In a crossover study [39], patients were randomised after extubation to either NHF followed by high-flow face mask or high-flow face mask followed by NHF. No differences in gas exchange, heart rate, blood pressure or respiratory rate were found between the two groups [39]. However, the fact that the face mask was applied with similar flow rates as NHF, i.e. 30 L·min−1, could explain the results. In another study of patients at high risk of re-intubation [40], NHF was not inferior to NIV in re-intubation rates and time to re-intubation, although NHF group had lower post-extubation respiratory failure rates. It is possible that the 24 h limit of using NHF as a preventive measure for re-intubation might have influenced the results, since the application of NHF for 48 h in the study of Maggiore et al. [35] showed persistent improvement in oxygenation and comfort parameters and achieved a lower re-intubation rate than the former study [40]. Conversely, delaying intubation is associated with increased morbidity and mortality, as previously reported using NHF or NIV to treat ARF [29, 41]. Perhaps more studies are needed to clarify whether specific clinical parameters should be used instead of fixed periods of time in order to achieve the greatest benefit from NHF use while avoiding any delay in intubation. A summary of these studies is shown in table 2.
TABLE 2.
Relevant studies for nasal high flow (NHF) use in intensive care unit (ICU) post-extubated patients
| Design | Patients | Results | |
| Benefit | |||
| Rittayamai et al. [34] | Randomised, non-blinded, crossover study | 17 successfully weaned patients received NHF for 30 min followed by SOT for another 30 min and vice versa | Use of NHF was associated with significant reductions in dyspnoea, heart rate and breathing frequency compared with a non-rebreathing mask |
| Maggiore et al. [35] | Randomised, controlled, open-label trial | 105 extubated patients with PaO2/FiO2 ≤300 randomised to either NHF or Venturi mask | NHF in the post-extubation period resulted in better oxygenation for the same set FiO2, decreased respiratory rate, improved patient comfort, reduced episodes of oxygen desaturations and frequency of need for ventilator support of any kind |
| Hernández et al. [38] | Multicentre, randomised clinical trial | 527 patients at low risk of post-extubation respiratory failure randomised to NHF or SOT after extubation | NHF in comparison with SOT resulted in less post-extubation respiratory failure (8.3% versus 14.4%) and lower re-intubation rate (4.9% versus 12.2%) |
| No benefit | |||
| Tiruvoipati et al. [39] | Randomised crossover study | 50 extubated patients received either NHF followed by high-flow face mask for 30 min each or vice versa | No significant difference in gas exchange, respiratory rate or haemodynamics was observed between groups. Tolerance was significantly better with NHF |
| Hernández et al. [40] | Multicentre, prospective cohort study | 604 patients at high risk of post-extubation respiratory failure randomised to NHF or NIV after extubation | NHF had similar re-intubation rates and median time to re-intubation with NIV. ICU length of stay was lower in the NHF group |
SOT: standard oxygen treatment; PaO2: arterial oxygen tension; FiO2: inspiratory oxygen fraction; NIV: noninvasive ventilation.
Post-extubation following surgery
Benefit
Respiratory complications play a significant role after major surgery, and can determine morbidity and mortality and substantially increase healthcare cost [42, 43]. Traditionally, low- and high-flow oxygen systems have been used to reverse post-surgical respiratory complications. NHF might be superior in post-cardiac and thoracic surgery patients, since it can provide a high flow of heated and hydrated oxygen while the positive airway pressure created by the high gas flow can recruit alveoli and increase the EELV [5, 6, 25, 44, 45]. In two studies conducted by the same authors [25, 45], NHF was compared with SOT after extubation in patients undergoing cardiovascular surgery. Patients on NHF had significantly fewer desaturations and succeeded more in their allocated therapy, with less need for NIV or intubation, although respiratory (SpO2, respiratory rate and forced expiratory volume in 1 s) and cardiovascular (systolic and diastolic arterial pressure and heart rate) variables did not differ between groups. Positive airway pressure, enabling the recruitment of atelectatic lung areas, along with facilitation of mucociliary clearance by the heated and humidified gas administered by NHF, were the possible key features that improved overall treatment effectiveness in both studies [25, 45], given that atelectasis is the more common respiratory complication after surgery, presenting in 90% of patients undergoing general anaesthesia [42].
The specific mechanisms of benefits of NHF on post-surgical respiratory complications were studied further by Corley et al. [5] and it was found that NHF compared to low-flow oxygen on post-cardiac surgery patients significantly increased mean airway pressure by 3.0 cmH2O, tidal volume by 10.5% and EELV by 25.6%, regardless of whether they breathed with the mouth open or closed. There was a linear correlation between the increase in gas flow and EELV [5]. These findings were more apparent in patients with higher BMI, although they were not confirmed in a subsequent study by the same group of authors [46], probably because of the difference in the study protocols and the limited exposure to NHF (only 8 h) in the latter study.
No benefit
Recent randomised controlled trials have demonstrated that continuous positive airway pressure (CPAP) or bi-level positive airway pressure are better than standard oxygen devices in the postoperative period [47, 48] and are increasingly used in patients undergoing cardiothoracic surgery in order to prevent or treat postoperative ARF [49–51]. In an attempt to compare NHF with these techniques, a current randomised noninferiority trial [52] in post-cardiothoracic surgery patients compared the use of NHF to NIV; similar rates of treatment failure, discomfort and ICU mortality were detected in both study groups. Although NIV was associated with a higher PaO2/FiO2 ratio, probably because of the higher applied PEEP, NHF was associated with lower values of PaCO2 and respiratory rate, with possible explanations being higher tidal volumes, improved inspiratory flow dynamics and a carbon dioxide washout effect [52]. Given the noninferiority of NHF compared to NIV in this group of patients, NHF could be used as a first option, since it provides the advantages of ease of application and lower nurse workload without hampering a patient's prognosis.
Few studies have investigated the role of NHF application in other types of surgery. There is only one study [53] in patients with lung resection surgery where immediate application of NHF versus low-flow oxygen did not improve early functional recovery as assessed by 6-min walk test and standard spirometry, although it resulted in reduced hospital length of stay and improved patient satisfaction. However, most patients included in the study had a good 6-min walk test before surgery. In another study, the preventive application of NHF directly after extubation was compared to SOT in patients undergoing major abdominal surgeries. NHF was ineffective at reducing the incidence of hypoxaemia as well as other postoperative outcomes such as pulmonary complications and length of hospital stay [54]. The small sample size, along with the lower than expected rate of hypoxaemia after surgery might have influenced the results, and thus more studies are needed to clarify the role of NHF after abdominal surgery. A summary of these studies is shown in table 3.
TABLE 3.
Relevant studies for nasal high flow (NHF) use in post-extubated patients following surgery
| Design | Patients | Results | |
| Benefit | |||
| Corley et al. [5] | Prospective interventional study | 20 patients post-cardiac surgery with ARF under SOT and then NHF | Compared with SOT, NHF increased mean Paw by 3.0 cmH2O, expiratory lung volume by 25.6% and tidal volume by 10.5%. Patients with higher BMI had larger increases in end-expiratory lung volume |
| Parke et al. [25] | Prospective randomised comparative study | 56 postoperative patients with ARF, randomised to NHF or to high-flow face mask | Significantly more NHF patients succeeded with their allocated therapy. Patients in the NHF group tended to need NIV less frequently than the high-flow face mask group and had significantly fewer desaturations |
| Parke et al. [45] | Pragmatic, open-label randomised controlled trial | 340 post-cardiac surgery patients randomised to NHF or SOT after extubation | No differences in SpO2/FiO2 ratio at day 3 or in-hospital and ICU length of stay and mortality at day 28 were observed between the two study groups. NHF reduced the requirement for escalation of respiratory support |
| No benefit | |||
| Corley et al. [46] | Randomised controlled trial | 155 patients post-cardiac surgery with BMI ≥30 kg·m−2 randomised to NHF or SOT | No difference was seen between groups in atelectasis scores on day 1 or 5, in mean PaO2/FiO2 ratio or respiratory rate in the first 24 h post-extubation and the length of ICU stay |
| Stéphan et al. [52] | Multicentre, randomised, unblinded noninferiority trial | BiPAP or NHF in 830 cardiothoracic patients who developed ARF after extubation or with pre-existing risk factors for post-extubation ARF | NHF was non-inferior to BiPAP in treatment failure, defined as re-intubation for mechanical ventilation, switch to the other study treatment or premature study treatment discontinuation. No significant differences were found in ICU mortality |
| Ansari et al. [53] | Randomised, controlled, blinded study | 59 post-elective lung resection surgery patients randomised to NHF or SOT | Similar results were observed in the difference between pre-operative and postoperative 6-min walk test and spirometry between the two study groups. Length of hospital stay was significantly lower in the NHF group |
| Futier et al. [54] | Multicentre, randomised controlled trial | 220 post-abdominal surgery patients randomised to NHF or SOT | No differences for postoperative hypoxaemia, pulmonary complications or length of hospital stay were found between the two groups studied |
ARF: acute respiratory failure; SOT: standard oxygen treatment; Paw: airway pressure; BMI: body mass index; NIV: noninvasive ventilation; SpO2: arterial oxygen saturation measured by pulse oximetry; FiO2: inspiratory oxygen fraction; ICU: intensive care unit; BiPAP: bi-level positive airway pressure.
Pre- and peri-oxygenation during intubation
Benefit
Endotracheal intubation in the ICU is one of the highest risk procedures, resulting in complications up to 40% or cases [55]. Pre-existing hypoxaemia and haemodynamic instability of ICU patients are the main reasons for insufficient pre-oxygenation and severe desaturations during laryngoscopy, throughout which pre-oxygenation is interrupted, even for a short period [55–58]. NHF (60 L·min−1) has been evaluated as an alternative technique for pre- and peri-oxygenation (i.e. oxygenation during laryngoscopy) during the intubation procedure in comparison with non-rebreathing bag reservoir face mask (15 L·min−1) in a nonrandomised prospective study [59]. Oxygen with 6 L·min−1 flow was administered to patients through a nasopharyngeal catheter during intubation in the face mask group, while in the NHF group the initial flow rate and FiO2 were maintained throughout the intubation procedure. The median SpO2 was significantly lower and the prevalence of severe hypoxaemia (SpO2 <80%) was present exclusively in the face mask group. Similar results were observed in a recent study, comparing the combination of NHF and NIV versus NIV alone, during the intubation procedure in patients with severe hypoxaemic respiratory failure [60]. Even without lung expansion, oxygen diffusion from the alveoli to the capillaries decreases alveolar pressure, and by increasing pharyngeal oxygen content a flow of air is generated from the pharynx to the distal airway, which is called apnoeic oxygenation [61]. High flow rates with NHF create a great oxygen reservoir in nasopharyngeal area, which together with carbon dioxide elimination by flushing of the dead space [11, 12] and the generated CPAP [3–8] make NHF a promising oxygenation device in the peri-intubation period, which maintains oxygenation without a rapid and dangerous rise in carbon dioxide concentration [62].
No benefit
In contrast to the study by Miguel-Montanes et al. [59], in which patients with severe hypoxaemia were excluded, and given that severe hypoxaemia before endotracheal intubation is an independent risk factor for severe desaturation during intubation [55], a multicentre controlled randomised study was conducted, comparing NHF versus a non-rebreathing bag reservoir face mask in the pre-oxygenation of severely hypoxaemic patients [63]. In the NHF group, the initials settings were FiO2 100% with a flow rate of 60 L·min−1, and they were maintained throughout the procedure in order to achieve apnoeic oxygenation. In the face mask group, the flow rate was set at 15 L·min−1 and the mask was removed after pre-oxygenation to enable laryngoscopy. There was no difference between the two oxygenation devices in preventing desaturation, not even during the apnoeic period, regardless of the severity of respiratory distress. The main difference between the studies by Miguel-Montanes et al. [59] and Vourc'h et al. [63], which could explain the discordant results, was the level of hypoxia of the patients included, which was more severe in the latter study. In addition, apnoeic oxygenation has been the subject of debate in recent literature, with conflicting results regarding the prevention of desaturation [64, 65]. However, in both studies [64, 65] lower flow rates were used (≤15 L·min−1) for apnoeic oxygenation; thus, it is evident that further studies are needed to define the effectiveness of different levels of flow rate for apnoeic oxygenation in distinct groups of patients.
Acute hypoxaemic respiratory failure in immunocompromised patients
Benefit
Choosing the optimal device for delivering oxygen in immunocompromised patients with ARF is extremely important, since invasive mechanical ventilation is associated with high mortality rates up to 40–60%. In order to avoid intubation and invasive mechanical ventilation, NIV is traditionally used as first-line treatment in this group of patients, although NIV failure has also been associated with increased mortality [66–68].
In two studies, the effects of NHF and NIV were assessed in immunocompromised patients admitted to the ICU for ARF [69, 70]. In both studies, the rates of intubation and mortality were lower in patients treated with NHF than in those randomly assigned to NIV. Moreover, NIV remained independently associated with increased rate of intubation and mortality [69, 70]. The results could be attributed to the better tolerance of NHF and the potential harmful effects of NIV through increasing tidal volumes, thus leading to high transpulmonary pressure and ventilator-induced lung injury [70].
In another study including patients with cancer and ARF, NHF in combination with NIV was associated with a lower 28-day mortality rate compared to either NHF alone or to a combination of SOT with NIV [71]. These results contrast with the results of the FLORALI (Clinical Effect of the Association of Non-invasive Ventilation and High Flow Nasal Oxygen Therapy in Resuscitation of Patients with Acute Lung Injury) study [26], in which the combination of NHF–NIV in an unselected population with hypoxaemic ARF was associated with high mortality. It is possible that the differences in the NIV application protocol (time and number of sessions) as well as the different study populations could explain the dissimilar results between studies [26, 71]. The beneficial effects of NHF have been highlighted in two further studies in patients with solid tumours [72] and haematologic malignancies [73], where more than one-third of the patients improved while on NHF and another large percentage (44%) remained stable [72], often obviating the need for ICU admission or invasive mechanical ventilation. Tolerance of the device was good with few complaints (e.g. nasal discomfort) [72].
Lung transplant patients comprise another group of immunocompromised patients who often require admission to the ICU and intubation because of ARF, with high mortality rates. NHF has been shown to reduce this risk by ∼29.8% compared to conventional oxygen therapy; the number of patients needed to treat with NHF in order to prevent one intubation was three [74]. Failure of NHF treatment and further need for mechanical ventilation was not associated with higher mortality [74].
No benefit
In contrast to the aforementioned studies, when NHF was compared to Venturi mask in immunocompromised patients admitted to the ICU with clinical signs of respiratory distress or hypoxaemia, NHF application neither decreased the need for invasive mechanical ventilation or NIV nor improved the patient's comfort, dyspnoea, respiratory rate or heart rate [75]. These results could be explained by the fact that the study was underpowered to demonstrate the potential inferiority of NHF compared to Venturi mask. In addition, sources of discomfort were not assessed, since the underlying disease associated with immunodeficiency may have been a significant source of patient discomfort, not influenced by the mode of oxygen delivery.
In another retrospective study in patients with haematologic diseases complicated by ARF, it was found that although NHF therapy was safe and well tolerated, only 20% of the patients showed a good response to NHF therapy, while the remaining 80% required a second-line intervention including endotracheal intubation and mechanical ventilation, NIV or narcotic palliation alone [76]. A significant factor that could have led to underestimation of NHF efficacy in this study was that a lot of the patients underwent palliation therapy or died while they were on NHF, and they were then classified as nonresponders to NHF regardless of the response.
A question that is raised in cases of NHF failure in immunocompromised patients is the delay of optimal treatment initiation, which could further increase the mortality rates in this group of patients at very high risk of death, as shown with NIV failure in the general population [28]. To date, only one study has shown that NHF failure was not associated with higher mortality rates [74], but undoubtedly, further studies are needed to clarify the safe time frame of NHF application as well as the specific indications of NHF oxygen delivery in immunocompromised patients. A summary of these studies is shown in table 4.
TABLE 4.
Relevant studies for nasal high flow (NHF) use in immunocompromised patients with acute respiratory failure (ARF)
| Design | Patients | Results | |
| Benefit | |||
| Coudroy et al. [69] | Retrospective observational study | 115 immunocompromised patients with ARF treated with NIV or NHF | The NHF group had significantly lower rates of intubation and mortality in the ICU and at day 28 than the NIV group |
| Frat et al. [70] | Post hoc analysis | 82 immunocompromised patients with ARF treated with NIV, NHF or SOT | The NHF group had significantly lower rates of intubation and mortality than the NIV group. No significant difference in intubation and mortality rates was noted between SOT and NHF |
| Mokart et al. [71] | Retrospective propensity-score analysis | 178 cancer patients with severe ARF received oxygen through SOT, NHF or through the combinations NHF–NIV or SOT–NIV | Compared to the other patients, patients who received NHF–NIV combination presented a lower day-28 mortality rate, a longer time from ICU admission to intubation and a higher, but not significant number of ventilator-free days. NHF–NIV was independently associated with improved survival |
| Epstein et al. [72] | Retrospective | 183 cancer patients with hypoxia treated with NHF | 41% improved while on the device, 44% remained stable and 15% declined. The device was well tolerated with few complaints |
| Lee et al. [73] | Retrospective | 45 patients with haematological malignancies who developed ARF | 20% of the patients showed a good response to NHF therapy while the remaining 80% of patients failed to respond to the initial NHF therapy requiring intubation with mechanical ventilation, NIV or narcotic palliation alone |
| Roca et al. [74] | Retrospective | 37 lung transplant recipients with ARF who received NHF or SOT | Absolute risk reduction for mechanical ventilation with NHF therapy was 29.8% and the NNT to prevent one intubation with NHF was 3. NHF therapy was associated with a decreased risk of mechanical ventilation |
| No benefit | |||
| Lemiale et al. [75] | Prospective, multicentre, parallel-group RCT | 100 immunocompromised patients with ARF, randomised to NHF or SOT | No significant difference regarding the need for invasive ventilation/NIV during the 2-h study period. Additionally, no significant difference reported regarding dyspnoea score, respiratory rate and heart rate |
| Harada et al. [76] | Retrospective | 56 patients with haematological malignancies with ARF under NHF treatment | 20% responded well to NHF therapy, while 80% failed, and they underwent a second-line therapy with invasive mechanical ventilation, NIV or narcotic palliation |
NIV: noninvasive ventilation; ICU: intensive care unit; SOT: standard oxygen treatment; NNT: number needed to treat; RCT: randomised controlled trial.
NHF in other clinical conditions
NHF could be used during bronchoscopy in patients with different levels of respiratory dysfunction. High flow rates (50–60 L·min−1) protect patients from hypoxaemia during bronchoscopy with remarkable tolerance and minimal variations in SpO2 [77–80]. NIV and CPAP are usually used in more severe hypoxaemic patients during bronchoscopy and studies have shown that NIV is superior both to SOT [81–83] and to NHF [84]. Moreover, it seems that NHF benefits are more remarkable when bronchoscopy is performed through the nares than through the mouth; nonetheless, even if bronchoscopy is performed through the mouth, maximum flow rate (60 L·min−1) should be used, since a PEEP value of 3.6 cmH2O has been measured at this flow rate in healthy volunteers with a partially obstructed mouth [77].
Stable chronic obstructive pulmonary disease (COPD) patients with chronic respiratory failure could benefit from NHF application through a reduction of anatomical dead space [85, 86], improvement in mucociliary clearance in conducting airways [16, 87] and passive elevation in end-expiratory pressure [6, 7, 9, 85]. These mechanisms lead to improved exercise performance with fewer symptoms and better gas exchange [85, 88, 89], improved lung mechanics [90] with less respiratory muscle load [91] and less work of breathing [86]. Since NIV is used currently as a treatment option in patients with COPD and chronic hypercapnia [92], it seems that NHF could be used instead of NIV in the least tolerant and compliant patients, or in association with NIV, to reduce mask-related side-effects [89]. Conversely, whether NHF might have an impact on hypercapnic ARF is questionable, since two case report studies [93, 94] have presented two patients with exacerbation of COPD in whom NIV was poorly tolerated and NHF was used instead. Progressively, respiratory rate was reduced and arterial blood gases were improved. Until more studies clarify this issue, NIV or invasive mechanical ventilation should be used based on the specific indications.
NHF application in patients with decompensated heart failure improved the intensity of dyspnoea, respiratory rate and oxygenation indices [95]. High gas flow with NHF has been shown to significantly decrease (>20%) the inspiratory collapse of the inferior vena cava and thus decrease the right ventricular preload [96]. However, more randomised trials are needed in order to compare NHF versus NIV in the intubation rates and mortality of patients with decompensated heart failure.
Finally, NHF seems a promising oxygen device for patients with a “do not intubate” status presenting with ARF, since it can provide optimum symptom relief, improve oxygenation [97] and thus avoid transportation to the ICU for a possible application of NIV [98].
Algorithm for clinical use
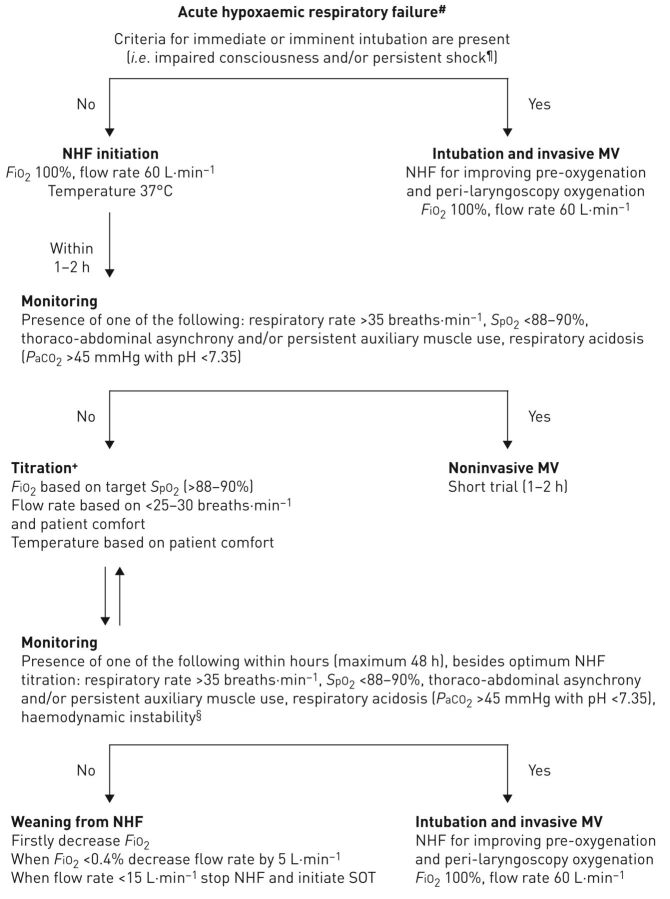
Summarising the existing literature led us to propose the following algorithm in case NHF is available and has been chosen as the initial oxygen delivery device in patients with ARF (figure 1). If a patient is admitted with clinical signs of acute respiratory distress and blood gas analysis demonstrates hypoxaemia (PaO2/FiO2 <300) of almost any cause without hypercapnia (PaCO2 >45 mmHg with pH <7.35), check primarily if the criteria for imminent intubation and invasive mechanical ventilation are met. If the answer is yes, intubation should be performed and NHF could be used for pre-oxygenation and apnoeic oxygenation during laryngoscopy with maximum settings (flow rate 60 L·min−1, 100% FiO2) [59, 60, 62]. If the answer is no, NHF should be applied as soon as possible with 100% FiO2, flow rate 40–60 L·min−1 and temperature 37°C [2, 4, 26, 35]. The proposed initial flow rate differs between the studies, with some authors suggesting initial lower values (35–40 L·min−1) that will be better tolerated by patients [2, 4], while others suggest initial maximum flow rate values (60 L·min−1) to rapidly relieve dyspnoea and avoid the occurrence of muscle fatigue [26, 35]. We believe that maximum flow rate values are more beneficial, and we thus recommend the application of the latter option in the algorithm.
FIGURE 1.
Recommended algorithm for high-flow nasal cannula use in acute hypoxaemic respiratory failure in immunocompetent or immunocompromised patients. #: arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FiO2) <300 (patients with arterial carbon dioxide tension (PaCO2) >45 mmHg and pH <7.35 are excluded); ¶: systolic arterial blood pressure <90 mmHg despite adequate fluid administration; +: the rationale for change in nasal high flow (NHF) settings are as follows. 1) Flow rate could be adjusted downwards by 5–10 L·min−1 per 1–2 h if none of the negative prognostic factors are present. However, if targets of arterial oxygen saturation measured by pulse oximetry (SpO2) and respiratory rate are not achieved, while the flow rate is <60 L·min−1, increase of flow rate by 5–10 L·min−1 is preferred to raising FiO2; 2) increase in FiO2 causes increases in PaO2 and SpO2; 3) temperature can be set at 37°C or lower (31–34°C), based on the patient's comfort; §: haemodynamic instability is defined by heart rate >140 beats·min−1 or change >20% from baseline and/or systolic arterial blood pressure >180 mmHg, <90 mmHg or decrease >40 mmHg from baseline. MV: mechanical ventilation; SOT: standard oxygen treatment.
Within 1–2 h of NHF initiation the presence of respiratory parameters that have negative prognostic significance should be checked, such as SpO2 <88–90%, respiratory rate >35 breaths·min-1, thoraco-abdominal asynchrony and auxiliary respiratory muscle use [23]. The presence of one of these identifies patients who do not respond to NHF very early; a short NIV trial could be considered before intubating the patient. If none of the parameters is present, NHF could be continued and its initial settings should be titrated based on the patient's respiratory rate (<25–30 breaths·min−1), SpO2 (>88–90%) and the patient's comfort and tolerance. In addition, NHF settings should be checked and adjusted accordingly during the monitoring of the patient, as follows. Flow rate could be adjusted downwards by 5–10 L·min−1 per 1–2 h if none of the aforementioned negative prognostic factors are present. However, if targets of SpO2 and respiratory rate are not achieved, while the flow rate is <60 L·min−1, increase of flow rate by 5–10 L·min−1 is rather preferred to raising FiO2, because higher flow rates reduce entrainment of room air during inspiration and increase the airway pressure linearly, thus recruiting more alveolar units. If SpO2 remains low, then an increase of FiO2 is required [2].
It is necessary to monitor closely the patient under NHF to avoid undesired respiratory and cardiac complications with a maximum time frame of 48 h [29]. The parameters that need regular monitoring during this time frame and which have prognostic significance are the respiratory parameters listed earlier, as well as haemodynamic instability [23, 27]. If the patient does not improve within 48 h, NHF treatment should be considered to have failed and we should proceed to intubation and invasive mechanical ventilation as soon as possible. Maintaining a failed NHF treatment could mask any further respiratory deterioration and increase the mortality rate. If the patient's clinical and gasometric parameters gradually improve, then FiO2 should first be lowered to 40–50%, proceeding with a stepped decrease in flow rate of 5–10 L·min−1. The intervals of these decrements could be shorter or longer based on the patient's clinical and physiological parameters. When the patient is stable for 1–2 h with FiO2 40% and flow rate <15 L·min−1, NHF should be stopped and a Venturi mask or nasal oxygen could be started [2, 4, 99].
Future research
Although the available clinical data for adult application of NHF are increasing over time, there are still some unanswered questions regarding practical aspects of its use. In cases of hypoxaemic ARF, most of the published trials include study population with different causes of ARF (i.e. pneumonia, pulmonary oedema, asthma exacerbation, etc.) of varying levels of severity, which could confuse the results. Undoubtedly, specific randomised clinical trials are needed to make recommendations for each separate cause. Moreover, different NHF initial settings, titration and weaning approaches could potentially be required in each separate disease, given the different pathophysiological underlying mechanisms. In case of hypercapnic ARF, controlled trials are needed to clarify the role of NHF and whether it could be used instead of and/or during breaks in NIV. Additionally, the haemodynamic effects of NHF should be studied in detail, such as NHF effects on cardiac output, heart pressures and pulmonary vascular resistance.
Conclusion
The beneficial effects of NHF over SOT are reported in most of the studies. The more stable FiO2, carbon dioxide washout effect, positive airway pressure generation and effective hydration of the administrated gas are the main mechanisms behind the greater perceived comfort and tolerance by the patient, more effective oxygenation and the improved breathing pattern with less dyspnoea. However, there is a great need for further research with physiological and randomised controlled studies in specific diseases and types of respiratory failure, in order to find out which patient will benefit the most from NHF therapy. Special attention should be given to the settings of FiO2 and flow rate per disease, and the maximum safe duration of NHF application before the initiation of NIV or invasive mechanical ventilation. Until then, the choice of oxygen supplemental treatment should be personalised and based on the patient's clinical status, underlying disease, severity of hypoxaemia, coexistence of hypercapnia and patient tolerance and comfort.
Footnotes
Conflict of interest: None declared.
Provenance: Submitted article, peer reviewed.
References
- 1.Chanques G, Riboulet F, Molinari N, et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol 2013; 79: 1344–1355. [PubMed] [Google Scholar]
- 2.Spoletini G, Alotaibi M, Blasi F, et al. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest 2015; 148: 253–261. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie JE, Williams AB, Gerard C, et al. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care 2011; 39: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 4.Gotera C, Díaz Lobato S, Pinto T, et al. Clinical evidence on high flow oxygen therapy and active humidification in adults. Rev Port Pneumol 2013; 19: 217–227. [DOI] [PubMed] [Google Scholar]
- 5.Corley A, Caruana LR, Barnett AG, et al. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 2011; 107: 998–1004. [DOI] [PubMed] [Google Scholar]
- 6.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth 2009; 103: 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 2007; 20: 126–131. [DOI] [PubMed] [Google Scholar]
- 8.Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care 2011; 56: 1151–1155. [DOI] [PubMed] [Google Scholar]
- 9.Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care 2013; 58: 1621–1624. [DOI] [PubMed] [Google Scholar]
- 10.Riera J, Pérez P, Cortés J, et al. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care 2013; 58: 589–596. [DOI] [PubMed] [Google Scholar]
- 11.Möller W, Celik G, Feng S, et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol 2015; 118: 1525–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Möller W, Feng S, Domanski U, et al. Nasal high flow reduces dead space. J Appl Physiol 2017; 122: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mündel T, Feng S, Tatkov S, et al. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol 2013; 114: 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chikata Y, Izawa M, Okuda N, et al. Humidification performance of two high-flow nasal cannula devices: a bench study. Respir Care 2014; 59: 1186–1190. [DOI] [PubMed] [Google Scholar]
- 15.Williams R, Rankin N, Smith T, et al. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med 1996; 24: 1920–1929. [DOI] [PubMed] [Google Scholar]
- 16.Hasani A, Chapman TH, McCool D, et al. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis 2008; 5: 81–86. [DOI] [PubMed] [Google Scholar]
- 17.Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: mechanisms of action. Respir Med 2009; 103: 1400–1405. [DOI] [PubMed] [Google Scholar]
- 18.Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2017; 195: 1207–1215. [DOI] [PubMed] [Google Scholar]
- 19.Lenglet H, Sztrymf B, Leroy C, et al. Humidified high flow nasal oxygen during respiratory failure in the emergency department: feasibility and efficacy. Respir Care 2012; 57: 1873–1878. [DOI] [PubMed] [Google Scholar]
- 20.Frizzola M, Miller TL, Rodriguez ME, et al. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol 2011; 46: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Driscoll BR, Howard LS, Davison AG, et al. BTS guideline for emergency oxygen use in adult patients. Thorax 2008; 63: Suppl. 6, vi1–vi68. [DOI] [PubMed] [Google Scholar]
- 22.Roca O, Riera J, Torres F, et al. High-flow oxygen therapy in acute respiratory failure. Respir Care 2010; 55: 408–413. [PubMed] [Google Scholar]
- 23.Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med 2011; 37: 1780–1786. [DOI] [PubMed] [Google Scholar]
- 24.Sztrymf B, Messika J, Mayot T, et al. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care 2012; 27: 324. [DOI] [PubMed] [Google Scholar]
- 25.Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care 2011; 56: 265–270. [DOI] [PubMed] [Google Scholar]
- 26.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372: 2185–2196. [DOI] [PubMed] [Google Scholar]
- 27.Messika J, Ben Ahmed K, Gaudry S, et al. Use of high-flow nasal cannula oxygen therapy in subjects with ARDS: a 1-year observational study. Respir Care 2015; 60: 162–169. [DOI] [PubMed] [Google Scholar]
- 28.Moretti M, Cilione C, Tampieri A, et al. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax 2000; 55: 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med 2015; 41: 623–632. [DOI] [PubMed] [Google Scholar]
- 30.Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2017; 49: 1600791. [DOI] [PubMed] [Google Scholar]
- 31.Jones PG, Kamona S, Doran O, et al. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER Study. Respir Care 2016; 61: 291–299. [DOI] [PubMed] [Google Scholar]
- 32.Krinsley JS, Reddy PK, Iqbal A. What is the optimal rate of failed extubation? Crit Care 2012; 16: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarni A, Agarwal V. Extubation failure in intensive care unit: predictors and management. Indian J Crit Care Med 2008; 12: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rittayamai N, Tscheikuna J, Rujiwit P. High-flow nasal oxygen versus conventional oxygen therapy after endotracheal extubation: a randomized crossover physiologic study. Respir Care 2014; 59: 485–490. [DOI] [PubMed] [Google Scholar]
- 35.Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort and clinical outcome. Am J Respir Crit Care Med 2014; 190: 282–288. [DOI] [PubMed] [Google Scholar]
- 36.Thille AW, Harrois A, Schortgen F, et al. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med 2011; 39: 2612–2618. [DOI] [PubMed] [Google Scholar]
- 37.Funk GC, Anders S, Breyer MK, et al. Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur Respir J 2010; 35: 88–94. [DOI] [PubMed] [Google Scholar]
- 38.Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA 2016; 315: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 39.Tiruvoipati R, Lewis D, Haji K, et al. High-flow nasal oxygen vs high-flow face mask: a randomized crossover trial in extubated patients. J Crit Care 2010; 25: 463–468. [DOI] [PubMed] [Google Scholar]
- 40.Hernández G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA 2016; 316: 1565–1574. [DOI] [PubMed] [Google Scholar]
- 41.Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350: 2452–2460. [DOI] [PubMed] [Google Scholar]
- 42.Ferreyra G, Long Y, Ranieri VM. Respiratory complications after major surgery. Curr Opin Crit Care 2009; 15: 342–348. [DOI] [PubMed] [Google Scholar]
- 43.Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199: 531–537. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care 2016; 61: 529–541. [DOI] [PubMed] [Google Scholar]
- 45.Parke R, McGuinness S, Dixon R, et al. Open-label, phase II study of routine high-flow nasal oxygen therapy in cardiac surgical patients. Br J Anaesth 2013; 111: 925–931. [DOI] [PubMed] [Google Scholar]
- 46.Corley A, Bull T, Spooner AJ, et al. Direct extubation onto high-flow nasal cannulae post-cardiac surgery versus standard treatment in patients with a BMI ≥30: a randomised controlled trial. Intensive Care Med 2015; 41: 887–894. [DOI] [PubMed] [Google Scholar]
- 47.Jaber S, Lescot T, Futier E, et al. Effect of noninvasive ventilation on tracheal reintubation among patients with hypoxemic respiratory failure following abdominal surgery: a randomized clinical trial. JAMA 2016; 315: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 48.Squadrone V, Coha M, Cerutti E, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA 2005; 293: 589–595. [DOI] [PubMed] [Google Scholar]
- 49.Zarbock A, Mueller E, Netzer S, et al. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications: a prospective, randomized, controlled trial in 500 patients. Chest 2009; 135: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 50.Auriant I, Jallot A, Hervé P, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med 2001; 164: 1231–1235. [DOI] [PubMed] [Google Scholar]
- 51.Zhu G-F, Wang DJ, Liu S, et al. Efficacy and safety of noninvasive positive pressure ventilation in the treatment of acute respiratory failure after cardiac surgery. Chin Med J 2013; 126: 4463–4469. [PubMed] [Google Scholar]
- 52.Stéphan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA 2015; 313: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 53.Ansari BM, Hogan MP, Collier TJ, et al. A randomized controlled trial of high-flow nasal oxygen (Optiflow) as part of an enhanced recovery program after lung resection surgery. Ann Thorac Surg 2016; 101: 459–464. [DOI] [PubMed] [Google Scholar]
- 54.Futier E, Paugam-Burtz C, Godet T, et al. Effect of early postextubation high-flow nasal cannula vs conventional oxygen therapy on hypoxaemia in patients after major abdominal surgery: a French multicentre randomized controlled trial (OPERA). Intensive Care Med 2016; 42: 1888–1898. [DOI] [PubMed] [Google Scholar]
- 55.Jaber S, Amraoui J, Lefrant JY, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective multiple-center study. Crit Care Med 2006; 34: 2355–2361. [DOI] [PubMed] [Google Scholar]
- 56.Cook TM, Woodall N, Harper J, et al. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth 2011; 106: 632–642. [DOI] [PubMed] [Google Scholar]
- 57.Mort TC. Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med 2005; 33: 2672–2675. [DOI] [PubMed] [Google Scholar]
- 58.Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 2006; 174: 171–177. [DOI] [PubMed] [Google Scholar]
- 59.Miguel-Montanes R, Hajage D, Messika J, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med 2015; 43: 574–583. [DOI] [PubMed] [Google Scholar]
- 60.Jaber S, Monnin M, Girard M, et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med 2016; 42: 1877–1887. [DOI] [PubMed] [Google Scholar]
- 61.Teller LE, Alexander CM, Frumin MJ, et al. Pharyngeal insufflation of oxygen prevents arterial desaturation during apnea. Anesthesiology 1988; 69: 980–982. [DOI] [PubMed] [Google Scholar]
- 62.Patel A, Nouraei SAR. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015; 70: 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vourc'h M, Asfar P, Volteau C, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med 2015; 41: 1538–1548. [DOI] [PubMed] [Google Scholar]
- 64.Wimalasena Y, Burns B, Reid C, et al. Apneic oxygenation was associated with decreased desaturation rates during rapid sequence intubation by an Australian helicopter emergency medicine service. Ann Emerg Med 2015; 65: 371–376. [DOI] [PubMed] [Google Scholar]
- 65.Semler MW, Janz DR, Lentz RJ, et al. Randomized trial of apneic oxygenation during endotracheal intubation of the critically ill. Am J Respir Crit Care Med 2016; 193: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azoulay E, Lemiale V, Mokart D, et al. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med 2014; 40: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 67.Azevedo LC, Caruso P, Silva UV, et al. Outcomes for patients with cancer admitted to the ICU requiring ventilatory support: results from a prospective multicenter study. Chest 2014; 146: 257–266. [DOI] [PubMed] [Google Scholar]
- 68.Bello G, De Pascale G, Antonelli M. Noninvasive ventilation for the immunocompromised patient: always appropriate? Curr Opin Crit Care 2012; 18: 54–60. [DOI] [PubMed] [Google Scholar]
- 69.Coudroy R, Jamet A, Petua P, et al. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: an observational cohort study. Ann Intensive Care 2016; 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frat JP, Ragot S, Girault C, et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. Lancet Respir Med 2016; 4: 646–652. [DOI] [PubMed] [Google Scholar]
- 71.Mokart D, Geay C, Chow-Chine L, et al. High-flow oxygen therapy in cancer patients with acute respiratory failure. Intensive Care Med 2015; 41: 2008–2010. [DOI] [PubMed] [Google Scholar]
- 72.Epstein AS, Hartridge-Lambert SK, Ramaker JS, et al. Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center. J Palliat Med 2011; 14: 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee HY, Rhe CK, Lee JW. Feasibility of high-flow nasal cannula oxygen therapy for acute respiratory failure in patients with hematologic malignancies: a retrospective single-center study. J Crit Care 2015; 30: 773–777. [DOI] [PubMed] [Google Scholar]
- 74.Roca O, de Acilu MG, Caralt B, et al. Humidified high flow nasal cannula supportive therapy improves outcomes in lung transplant recipients readmitted to the intensive care unit because of acute respiratory failure. Transplantation 2015; 99: 1092–1098. [DOI] [PubMed] [Google Scholar]
- 75.Lemiale V, Mokart D, Mayaux J, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care 2015; 19: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harada K, Kurosawa S, Hino Y, et al. Clinical utility of high-flow nasal cannula oxygen therapy for acute respiratory failure in patients with hematological disease. Springerplus 2016; 5: 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lucangelo U, Vassallo FG, Marras E, et al. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit Care Res Pract 2012; 2012: 506382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.La Combe B, Messika J, Labbé V, et al. High-flow nasal oxygen for bronchoalveolar lavage in acute respiratory failure patients. Eur Respir J 2016; 47: 1283–1286. [DOI] [PubMed] [Google Scholar]
- 79.Diab S, Fraser JF. Maintaining oxygenation successfully with high flow nasal cannula during diagnostic bronchoscopy on a postoperative lung transplant patient in the intensive care. Case Rep Crit Care 2014; 2014: 198262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyagi K, Haranaga S, Higa F, et al. Implementation of bronchoalveolar lavage using a high-flow nasal cannula in five cases of acute respiratory failure. Respir Investig 2014; 52: 310–314. [DOI] [PubMed] [Google Scholar]
- 81.Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. A randomized double-blind study using a new device. Am J Respir Crit Care Med 2000; 162: 1063–1067. [DOI] [PubMed] [Google Scholar]
- 82.Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation vs. conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 2002; 121: 1149–1154. [DOI] [PubMed] [Google Scholar]
- 83.Antonelli M, Conti G, Riccioni L, et al. Noninvasive positive-pressure ventilation via face mask during bronchoscopy with BAL in high-risk hypoxemic patients. Chest 1996; 110: 724–728. [DOI] [PubMed] [Google Scholar]
- 84.Simon M, Braune S, Frings D, et al. High-flow nasal cannula oxygen versus non-invasive ventilation in patients with acute hypoxaemic respiratory failure undergoing flexible bronchoscopy – a prospective randomised trial. Crit Care 2014; 18: 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fricke K, Tatkov S, Domanski U, et al. Nasal high flow reduces hypercapnia by clearance of anatomical dead space in a COPD patient. Respir Med Case Rep 2016; 19: 115–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biselli PJ, Kirkness JP, Grote L, et al. Nasal high-flow therapy reduces work of breathing compared with oxygen during sleep in COPD and smoking controls: a prospective observational study. J Appl Physiol 2017; 122: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rea H, McAuley S, Jayaram L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med 2010; 104: 525–533. [DOI] [PubMed] [Google Scholar]
- 88.Cirio S, Piran M, Vitacca M, et al. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir Med 2016; 118: 128–132. [DOI] [PubMed] [Google Scholar]
- 89.Bräunlich J, Seyfarth HJ, Wirtz H. Nasal high-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med 2015; 10: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fraser JF, Spooner AJ, Dunster KR, et al. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomized crossover trial. Thorax 2016; 71: 759–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pisani L, Fasano L, Corcione N, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax 2017; 72: 373–375. [DOI] [PubMed] [Google Scholar]
- 92.Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014; 2: 698–705. [DOI] [PubMed] [Google Scholar]
- 93.Millar J, Lutton S, O'Connor P. The use of high-flow nasal oxygen therapy in the management of hypercarbic respiratory failure. Ther Adv Respir Dis 2014; 8: 63–64. [DOI] [PubMed] [Google Scholar]
- 94.Lepere V, Messika J, La Combe B, et al. High-flow nasal cannula oxygen supply as treatment in hypercapnic respiratory failure. Am J Emerg Med 2016; 34: 1914. [DOI] [PubMed] [Google Scholar]
- 95.Carratalá Perales JM, Llorens P, Brouzet B, et al. High-flow therapy via nasal cannula in acute heart failure. Rev Esp Cardiol 2011; 64: 723–725. [DOI] [PubMed] [Google Scholar]
- 96.Roca O, Pérez-Terán P, Masclans JR, et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care 2013; 28: 741–746. [DOI] [PubMed] [Google Scholar]
- 97.Peters SG, Holets SR, Gay PC. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care 2013; 58: 597–600. [DOI] [PubMed] [Google Scholar]
- 98.Azoulay E, Kouatchet A, Jaber S, et al. Noninvasive mechanical ventilation in patients having declined tracheal intubation. Intensive Care Med 2013; 39: 292–301. [DOI] [PubMed] [Google Scholar]
- 99.Roca O, Hernández G, Díaz-Lobato S, et al. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care 2016; 20: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]