Abstract
COPD is a major cause of morbidity and mortality worldwide. Multimorbidity is common in COPD patients and a key modifiable factor, which requires timely identification and targeted holistic management strategies to improve outcomes and reduce the burden of disease.
We discuss the use of integrative approaches, such as cluster analysis and network-based theory, to understand the common and novel pathobiological mechanisms underlying COPD and comorbid disease, which are likely to be key to informing new management strategies.
Furthermore, we discuss the current understanding of mechanistic drivers to multimorbidity in COPD, including hypotheses such as multimorbidity as a result of shared common exposure to noxious stimuli (e.g. tobacco smoke), or as a consequence of loss of function following the development of pulmonary disease. In addition, we explore the links to pulmonary disease processes such as systemic overspill of pulmonary inflammation, immune cell priming within the inflamed COPD lung and targeted messengers such as extracellular vesicles as a result of local damage as a cause for multimorbidity in COPD.
Finally, we focus on current and new management strategies which may target these underlying mechanisms, with the aim of holistic, patient-centred treatment rather than single disease management.
Short abstract
Multimorbidity is common in COPD and is a global health priority. Using novel approaches to understand the complex mechanisms underlying multimorbidity is key to developing targeted, patient-centred management strategies to improve outcomes. https://bit.ly/3fUUkWB
Overview of multimorbidity in COPD
COPD is a major cause of morbidity and rising mortality worldwide [1, 2]. More than 90% of COPD deaths occur in low- and middle-income countries [3] and COPD mortality is predicted to rise further in these countries despite measures to reduce exposure to risk factors, such as tobacco control policies and declining poverty [4]. Importantly, multimorbidity has been identified as a key modifiable factor, which requires greater recognition, focus and management to improve outcomes and reduce burden of disease in COPD [5].
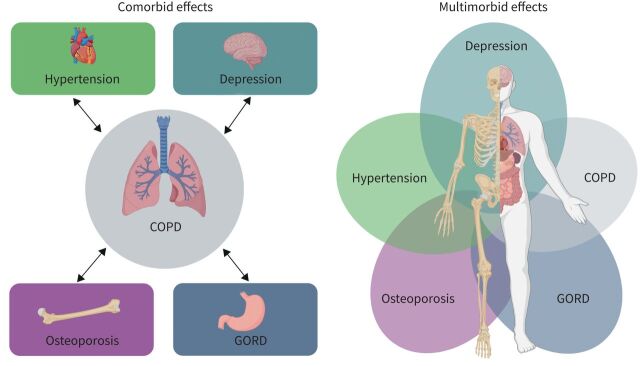
Whereas comorbidity describes the burden of illness coexisting with a particular disease of interest, multimorbidity is defined as the presence of multiple chronic conditions [6, 7] in an individual. It acknowledges that health conditions may overlap, vary in severity and change in importance over time (figure 1). Multimorbidity is a global health priority [8] and its prevalence increases substantially with age and levels of deprivation [9]. COPD is present in the majority of multimorbid patients [9] and multimorbidity in COPD increases the likelihood of hospital admission [10, 11] and healthcare costs [12], reduces quality of life and exercise tolerance [13] and increases mortality [14]. Studies have shown that patients with COPD are more likely to have comorbid disease compared with the general population, with the most prevalent comorbidities being osteoporosis, hypertension and gastro-oesophageal disease (table 1). Importantly, all these studies recognise that concomitant chronic diseases are often misdiagnosed or undiagnosed in patients with COPD and are thus untreated.
FIGURE 1.
Conceptual diagram of comorbidity and multimorbidity in COPD. GORD: gastro-oesophageal reflux disease.
TABLE 1.
Prevalence of comorbidities in England compared to patients with COPD
| Prevalence in control population # | Prevalence in COPD | Reference(s) | |
| Osteoporosis/osteopenia | 18–22 | 50–70 | [16, 17] |
| Hypertension | 34–41 | 40–60 | [10, 18] |
| GORD | 33 | 30–60 | [19] |
| Skeletal muscle dysfunction | 9 | 32 | [20] |
| Depression | 7–12 | 25 | [21] |
| Ischaemic heart disease | 15 | 10–23 | [22] |
| Diabetes | 6–10 | 12–13 | [10, 23] |
| Previous stroke | 3 | 10–14 | [18, 23] |
| Arrhythmia | 5–11 | 11–12 | [18] |
| Congestive heart failure | 4 | 5–19 | [18, 24] |
| Obesity | 17–32 | 18–54 | [25] |
Data are presented as %. GORD: gastro-oesophageal reflux disease. Adapted from [15]. #: control population from the same study(ies) as the COPD comorbidity prevalence reported.
Approaches to understanding complex mechanisms underlying multimorbidity in COPD
Conventionally when studying multimorbidity in COPD, researchers have focused on a single coexistent disease or group of diseases. However, recent evidence supports a more integrative approach, in the hope that this could reveal disease clusters and novel pathobiology mechanisms, leading toward a better understanding and, possibly, integrated co-management of multimorbidity [26–28].
Unsupervised cluster-based analysis uses multivariate techniques to organise information in an unbiased way so that heterogeneous groups of patients can be classified into relatively homogeneous groups or “clusters” [29]. Several studies have used this approach and identified a COPD phenotype with a high prevalence of comorbid disease [30–32] and higher levels of systemic inflammatory markers [31, 32]. Furthermore, Garcia-Aymerich et al. [31] showed measures of systemic inflammation did not correlate with bronchial inflammation suggesting that systemic rather than bronchial inflammation may be driving the origins of both COPD and comorbid disease. To investigate the role of systemic inflammation in multimorbidity further, Vanfleteren et al. [33] used self-organising maps, an alternative neural network-based nonhierarchical clustering approach, to identify five comorbidity clusters (“less comorbidity”, “cardiovascular”, “metabolic”, “psychologic” and “cachectic” clusters) suggesting that different pathophysiological pathways underlie these clusters.
Although clustering approaches have shown promise in identifying COPD subtypes, further work has questioned the reproducibility of these analyses. For example, Castaldi et al. [34] demonstrated only modest reproducibility of COPD clustering results across multiple independent cohorts and suggested that COPD heterogeneity is best characterised by continuous disease traits coexisting within the same individual rather than separate identifiable COPD subtypes. More recently, studies have demonstrated that continuous measures of disease (disease axes) were better at predicting emphysema progression [35] and mortality than subtypes alone [36]. In contrast, Burgel et al. [37] was able to demonstrate reproducible clustering across cohorts in relation to mortality, with enrichment for multimorbidity in subgroup I. Together, these studies suggest there may be multiple distinct sets of subtypes that depend on a specific clinical outcome of interest.
Mathematical-based network theory demonstrates that disease co-occurrence is not the result of simple chance, but as a result of genetic predisposition in concert with cumulative pathobiological processes in response to biological stressors [28]. For example, Park et al. [38] showed that diseases that share genes or involve proteins that interact with each other show elevated comorbidity, demonstrating correlations between the structure of cellular networks and disease patterns in the population. This approach to network analysis maps the molecular and phenotypic interactions that could provide new insights into the pathobiology of multimorbidity in COPD [39]. Using this approach, Grosdidier et al. [40] identified several shared genes, proteins and biological pathways common to the most prevalent COPD multimorbidities (such as ischaemic heart disease, diabetes and obesity) suggesting common biological mechanisms. Furthermore, several of these were targets of the tobacco exposome, suggesting a link for the common exposure theory. Divo et al. [41] used network analysis to show that the prevalence of comorbidities (number of nodes) and number of simultaneous comorbidities is higher in COPD patients compared with controls. In addition, the COPD comorbidity network was found to have a “scale-free” architecture, indicating the existence of a few, highly connected nodes (diseases) in the network, so-called “hubs” that may play a key pathogenic role [42]. Based on this, they proposed that therapies targeted towards these hubs could result in improvement of outcomes in multiple diseases. Finally, they identified a number of “modules” consisting of coexisting diseases, which are interlinked beyond simple coincidence [41]. For example, they described module 1A comprised of 17 nodes connected by 81 edges around the theme of older COPD individuals with cardiovascular comorbidities (such as hypertension and coronary artery disease) and clinical characteristics known to confer worse prognosis in patients with COPD (forced expiratory volume in 1 s (FEV1) <50%, modified Medical Research Council score >2, 6-min walk distance <350 m). Interestingly, these were similar to groups described by the cluster analysis studies [31, 32], thus reinforcing the concept that targeting key modules could be important for achieving meaningful outcomes from treatment across conventional single-morbidity strategies.
Towards an understanding of mechanistic drivers to multimorbidity in COPD
Understanding the mechanisms that could be driving these relationships between COPD and coexisting disease is key to developing novel strategies for treating this complex and heterogeneous disease. In this section, we explore the potential relationships between mechanistic drivers and examine the evidence supporting the competing hypotheses of disease genesis.
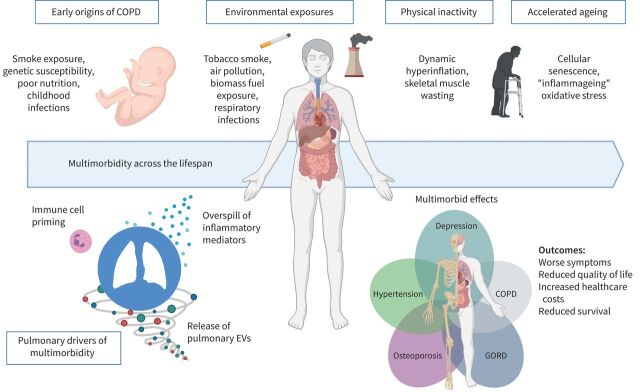
Current knowledge in the field of mechanisms underlying multimorbidity in COPD is summarised in figure 2.
FIGURE 2.
Drivers, mechanisms and outcomes for multimorbidity in COPD. EVs: extracellular vesicles; GORD: gastro-oesophageal reflux disease.
Multimorbidity in COPD over the life course
Early origins of disease
Since the seminal work of Lange et al. [43] on lung function trajectories leading to COPD, there has been increasing interest in lung function development and its early determinants. These analyses and several other studies demonstrated that a low FEV1 in early adulthood is important in the genesis of COPD; however, an accelerated decline in FEV1 is not necessary [44, 45].
A normal lung function trajectory throughout life has three phases: a growth phase from birth to early adulthood, a plateau phase from ∼20 years and a decline phase resulting from physiological ageing. Although smoking is still a major factor, other environmental, genetic and developmental factors with diverse biological mechanisms and effects can lead to an abnormal lung function trajectory, resulting in respiratory disease in adulthood [46]. In addition to COPD, a lower peak lung function in early adulthood is associated with cardiovascular and metabolic disease as well as premature mortality [44]. Therefore, early-life lung health has important implications for multimorbidity as well as single disease entities and further research is needed to better understand the biological mechanisms underlying these different abnormal lung trajectories.
Recently, Kachroo et al. [47] used network approaches to investigate the epigenetic modifications caused by in utero smoke exposure, which may link to risk for COPD in adulthood. They identified several putative disease pathways supportive of exposure-related and age-associated developmental origins of COPD.
COPD and many of its comorbidities are often recognised and diagnosed too late, and therefore investigating these biological mechanisms underlying different abnormal lung function trajectories is key to impacting on early diagnosis and informing new therapeutic and preventative interventions including vaccinations [48, 49].
Environmental exposures and risk factors
Multimorbidity can arise as a consequence of environmental exposures, such as cigarette smoke, biomass fuel and physical inactivity, leading to multiple organ damage and disease.
Smoking
Tobacco smoke exposure is considered the primary risk factor for COPD, and is estimated to account for up to 80–90% of cases in the developed world. Furthermore, smoking is an important risk factor for other comorbid conditions such as lung cancer [50], cardiovascular disease [51] and osteoporosis [52]. Several studies have tried to unpick the complex relationship between common exposure and/or risk factors and presence of comorbid disease in COPD.
Large prospective cohort studies have demonstrated that the association between lung function impairment and cardiovascular disease was largely due to common risk factors such as age, sex, race, smoking, hypertension, diabetes, cholesterol and fibrinogen levels [53]. This was further supported by Van Remoortel et al. [54] who found prevalence of premorbid risk factors (e.g. obesity and hypertension) and comorbid diseases (e.g. heart disease, diabetes, skeletal muscle dysfunction and osteoporosis) was comparable in patients with COPD and in smokers, but was higher than in healthy nonsmokers. Importantly, physical activity and smoking were more strongly associated with multimorbidity than airflow limitation. Furthermore, these findings were in accordance with Thomsen et al. [55], who reported that the risks of cardiovascular comorbidities and all-cause mortality were increased in former and current smokers with COPD, but not in never-smokers with COPD (Copenhagen General Population Study).
Together, these studies provide evidence that exposure to shared risk factors (such as tobacco smoke) may contribute to the multimorbid burden in COPD, even in early and pre-clinical disease. This underlines the importance of treating modifiable risk factors (such as smoking cessation) early to improve multimorbid ill-health.
Although it is clear that there are associations between shared risk factors and multimorbidity in COPD, other studies suggest that airflow limitation itself has a super-added risk on presence and outcome of multimorbidity. A large (>20 000 subjects), population-based study in the United States showed that airflow obstruction was associated with greater comorbidity, independent of shared risk factors such as age, sex, race, smoking status and body mass index (BMI). In addition, those with respiratory impairment and comorbid disease were at significantly higher risk of death and hospitalisation [10]. This was in line with data from Sin et al. [56], which showed a striking relationship between reduced FEV1 and mortality from ischaemic heart disease independent of smoking. Where even a modest decline of FEV1 (from mean 109% predicted to 88% predicted), was associated with a five-fold increase in death from ischaemic heart disease, independent of baseline smoking status and other potential confounding factors such as age, gender, blood pressure, BMI and diabetes. This relationship was not only shown in cross-sectional data, but also prospectively in the Lung Health Study of nearly 6000 subjects, where decline in FEV1 predicted death from cardiovascular disease, independent of smoking (p=0.002) [57].
Based on these studies, one well-described link for this association of cardiovascular disease with COPD, independent of common risk factors, is the role of low-grade chronic systemic inflammation present in both diseases, which could potentially be driving both pathologies. This is further discussed later.
Physical inactivity
Inactivity is prevalent in patients with COPD and is a major risk factor for many systemic manifestations of COPD [54]. Patients with COPD become less active early in the disease course [58] and this becomes more marked during exacerbations [59]. Furthermore, inactivity in COPD is associated with increased exacerbations and early mortality [60, 61]. Inactivity may lead to systemic consequences in COPD by several different mechanisms.
Although the relationship between inactivity and COPD is multifactorial, collective evidence suggests that dynamic hyperinflation plays a major role in exercise limitation [62]. Additionally, hyperinflation is directly associated with worse patient-centred outcomes such as progressive breathlessness, anxiety and lower quality-of-life scores, all of which impact on comorbid disease [63]. However, treatment of hyperinflation with pulmonary rehabilitation, bronchodilators and lung volume reduction interventions does not fully reverse the effects of inactivity, suggesting that there are other factors at play.
Skeletal muscle wasting is an important consequence of inactivity in patients with COPD. Furthermore, skeletal muscle wasting may lead to a “downward disease spiral”, whereby quadriceps myopathy and anaerobic metabolism at low exercise intensity leads to a further reduction in exercise capacity [64]. Inactivity results in protein catabolism within skeletal muscle, leading to muscle atrophy and sarcopenia. Specifically, deconditioning in COPD is associated with reduced oxidative capacity [65], fibre atrophy and reduced skeletal muscle cross-sectional area [66]. Spruit et al. [67] suggest that skeletal muscle weakness during exacerbations may be because of circulating cytokines. In addition, even at low doses, long-term corticosteroid use contributes to myopathy in COPD [68]. Conversely, treatment with antioxidants may improve skeletal muscle endurance, by ameliorating the effects of oxidative stress [69].
In addition to skeletal muscle wasting, inactivity may lead to other systemic sequelae. Studies have shown that inactivity is an independent risk factor for osteoporosis [70], type 2 diabetes, cardiovascular disease [71] and depression [72]. There is some evidence suggesting that low-grade systemic inflammation may be a unifying mechanism. Epidemiological studies have shown that physical inactivity is associated with higher levels of systemic inflammatory markers [73, 74]. Furthermore, the Women's Health Study (>27 000 participants) showed that physical activity could reduce cardiovascular risk, mediated mostly by a reduction in inflammatory markers (C-reactive protein (CRP), fibrinogen and intercellular adhesion molecule (ICAM)-1) [75]. However, most studies investigating the effect of training interventions did not show a reduction in circulating inflammation markers [76, 77].
Accelerated ageing
With a given genetic background, chronic diseases may develop and progress (at different speeds and in various combinations) in response to common risk factors, such as those listed earlier [78]. Accelerated ageing as a result of these environmental factors may be a unifying mechanism for this [79]. In support of this, cellular senescence with telomere shortening, a surrogate marker for accelerated ageing, was reported in circulating leukocytes in several comorbid diseases such as COPD [80], myocardial infarction, stroke and diabetes [81].
Furthermore, ageing is characterised by low-grade chronic inflammation (“inflammageing”) and with this, important age-related perturbations in the gut microbiota have been recognised as a potential source [82]. Interventions directed at the composition of gut microbiota might be important in controlling this imflammageing process and be another key component of multimorbidity management.
Oxidative stress
Increased oxidative stress in the major mechanism that drives accelerated ageing through its damaging effect on DNA, activation of mammalian target of rapamycin (mTOR) signalling and shortening of telomeres. Oxidative stress is implicated in several pathological mechanisms (including NF-κB and phosphoinositide 3-kinase (PI3K)) in COPD, and these have resulted in targets for potential treatments [83]. Furthermore, it is likely that oxidative stress is also a mechanism driving multimorbidity in COPD, with increased levels of serum 8-hydroxy-2′ -deoxyguanosine (a product of DNA damage) in patients with coronary artery disease [84] and diabetes [85].
Pulmonary components of multimorbidity
Systemic inflammation from the COPD lung
Pulmonary inflammation is a hallmark of COPD pathogenesis [86, 87]. The inflammatory profile is characterised by increased numbers of innate and adaptive immune cells and cytotoxic mediators within the airways [88, 89], which have been suggested to “spill over” into the systemic circulation [90]. There is evidence that systemic inflammation is common in patients with clinically stable COPD [91–93] and is implicated in several comorbid conditions [94], including accelerated ageing [95]. However, patterns of inflammation are heterogeneous [96] and quantifying systemic inflammation in COPD is difficult, where many of the studies use cross-sectional data from small sample sizes and it may not be a persistent phenomenon [97]. Agustí et al. [98] showed that although the majority of COPD patients (∼70%) demonstrate a degree of systemic inflammation (defined as elevated levels of one of CRP, interleukin (IL)-6, IL-8, fibrinogen, tumour necrosis factor (TNF)-α or leukocytes), only 16% of COPD patients had persistent inflammation. Importantly, those with persistent inflammation (despite having relatively similar lung function impairment) had significantly increased mortality and exacerbation frequency. Furthermore, this persistence of a systemic inflammatory phenotype is linked to atherosclerosis, ischaemic heart disease, stroke and cardiovascular mortality, independent of COPD [99], and specifically increased systemic TNF-α has been implicated as a mechanism for cachexia and skeletal muscle wasting in COPD [100].
Understanding the drivers of systemic inflammation in COPD is important, as it is likely to be fundamental to the pathogenesis of COPD. Studies have shown that systemic inflammation persists despite smoking cessation [101] and increases during exacerbations [102–104]. In addition, it seems to be an important determinant for patient outcome, with associations with accelerated decline in lung function [105] and exacerbations [106], as well as a two- to four-fold risk of cardiovascular disease, diabetes, lung cancer and pneumonia [107]. Furthermore, systemic inflammation is linked to psychological comorbidity with evidence it is key to the development of depression [108], anxiety and cognitive impairment [109]. The origin of systemic inflammation in COPD is much debated and Sinden and Stockley [90] reviewed the evidence for pulmonary “overspill” in 2010.
A lack of correlation between inflammatory markers in the sputum and blood of COPD patients has provided some evidence against the overspill theory [110–113]. In addition, several other drivers to systemic inflammation are reported including smoking, lung hyperinflation (discussed earlier), tissue hypoxia, skeletal muscle wasting and bone marrow stimulation [114]. Therefore, the origin of systemic inflammation in COPD is complex and likely to be multifactorial.
Immune cell priming in the COPD lung
Despite the lack of direct evidence supporting the overspill theory, other studies suggest that immune cell activation or “priming” occurs within the COPD lung in both stable disease and at exacerbation, leading to systemic inflammation [115, 116]. Leukocyte priming results in enhanced migratory and cytotoxic responses [117, 118], which may contribute to systemic inflammation and comorbid disease. Studies have shown that cytokine priming of neutrophils occurs in response to cigarette smoke exposure [119, 120]. Noguera et al. [116] showed an increase in expression of the endothelial adhesion molecules and production of reactive oxygen species (ROS) in circulating neutrophils in patients with COPD compared with smokers with normal lung function. This demonstrates an important disease effect on neutrophil priming and implicates their role in systemic endothelial dysfunction. Furthermore, monocytes also accumulate in the lungs of smokers in response to cigarette smoke [121] and it is likely that smoke-activated macrophages release monocytic chemokines (e.g. monocyte chemoattractant protein-1) into the peripheral blood [122]. Besides stimulating recruitment of cells to the lungs, in response to smoking these chemokines can also prime circulating monocytes, which increases adhesion to the endothelium and provides a possible mechanism for atherosclerosis [123, 124]. Furthermore, TNF-α is increased in the blood [125, 126] and sputum of COPD patients [127], and increased production of TNF-α in response to cigarette smoke may contribute to the priming of circulating inflammatory cells [119].
Overall, these experimental data suggest that local cytokine release in the periphery, whether in response to acute cigarette smoke exposure, or in the context of COPD, prime immune cells to coordinate a systemic inflammatory response and possible contribute to the burden of multimorbidity.
Although much of the focus has been on the role of macrophage- and neutrophil-driven inflammation in COPD, the importance of eosinophilic inflammation has been highlighted in several studies, showing that eosinophilic COPD patients have more frequent exacerbations [128–130], and are more responsive to corticosteroid treatment [131]. Less is known about their role in comorbid disease, although it has been demonstrated that eosinophils play a pivotal role in metabolic homeostasis [132, 133], and have been found to be associated with coronary artery disease [134, 135] and thrombus formation [136]. More recently, blood eosinophils have been found to correlate with the presence of hypertension in older patients with COPD [137]. It might not be surprising that in COPD patients, different comorbidities may be associated with eosinophil increase, where blood eosinophils could be the result of immune cell priming within the bone marrow.
Pulmonary extracellular vesicles as mediators of local and systemic inflammation
Purposeful, direct intercellular communication is key to inducing and resolving inflammation. Extracellular vesicles (EVs) are intercellular messengers present in all body fluids, transporting proteins, lipids and RNA [138]. They are involved in immunomodulation [138, 139], implicated in inflammatory lung disorders, including COPD [140–142], and may be a universal disseminator of inflammation [143]. EVs can originate from the endosomal compartment (exosomes) or be shed from the cell surface (microvesicles (MVs)). The following section reviews how pulmonary EVs support inflammation in the lungs and how they may exit the lungs and contribute to dissemination of inflammation and therefore multimorbidity.
EVs in the airways
EVs were first identified in the healthy human bronchoalveolar lavage fluid (BALF) in 2003, and found to express CD86, ICAM-1 and major histocompatibility complex class I and II [144]. Pulmonary EVs are likely to originate from different cell sources depending on health and disease; however, bronchial epithelial cells have been suggested as the main source of lung EVs [145]. Epithelial cell injury secondary to cigarette smoke is known to stimulate release of EVs, which are pro-inflammatory (induce IL-8 and vascular endothelial growth factor release) [146] and profibrotic (promote fibroblast differentiation) [147]. Furthermore, epithelial-derived EVs could also have a role in innate defence, whereby when enriched for mucins (glycoproteins vital for maintaining mucus barriers), EVs can bind to and neutralise influenza virus [148]. In addition, EVs are released by alveolar macrophages and are implicated in regulation of several inflammatory processes in response to cigarette smoke and airway pathogens [149–154]. Taken together, these studies suggest a role for pulmonary EVs in regulating pulmonary inflammation in response to noxious stimuli, and further work has suggested these may be driven in part by EV shuttling of microRNA [155, 156].
Circulating EVs
Aberrant release of endothelial MVs occurs in lung disease as a consequence of damage the lung capillary bed [157, 158]. Circulating pulmonary endothelial-derived MVs are increased in smokers with signs of early emphysema [159]. COPD patients have elevated circulating endothelial-derived MVs, which are further elevated during exacerbations. These MVs had markers indicating pulmonary capillary origin, suggesting that their release is associated with pulmonary capillary endothelial damage [160].
Evidence for EVs exiting the lung
Rather than just “overspill” from the lung into the systemic circulation, there is evidence to suggest that pulmonary EVs undergo targeted release into the circulation as a result of increased pulmonary vascular permeability in response to acute and chronic inflammation [161]. This will affect the exchange of EVs between the blood and the epithelial lining, with the possibility of pulmonary EVs reaching systemic circulation and potentially distant organs. For example, EVs from pulmonary endothelial cells carry α1-antitrypsin (A1AT) and may be involved in shuttling A1AT across the alveolar membrane to recipient epithelial cells [162], possibly to prevent excessive pulmonary inflammation. Furthermore, proteomic characterisation of BALF exosomes from sarcoid patients showed proteins associated with inflammation, cellular migration and complement components [163]. In addition, vitamin D-binding protein (a potent chemoattractant for leukocytes) was significantly increased in both BALF and serum EVs, which may be a result of pulmonary exosomes exiting into systemic circulation.
EVs, systemic inflammation and comorbidities
EVs are implicated in the pathogenesis of several other inflammatory disorders. Several studies have demonstrated the presence of pro-inflammatory EVs in rheumatoid arthritis [164], multiple sclerosis (MS) [165] and inflammatory bowel disease [166]. Specifically, pro-inflammatory EVs have been identified both in the synovial fluid of patients with rheumatoid arthritis where active joint inflammation occurs and in the circulation [164, 167, 168], suggesting a role for EVs in mediating systemic inflammation. MS patients have increased levels of circulating platelet MVs, which correlate with disease subtype and increase during active inflammation [169]. In addition, EVs from MS patients have been implicated in disease progression via endothelial barrier disruption [165], degradation of the blood–brain barrier and promotion of neural inflammation [170].
Further to EVs driving systemic inflammation and progression of inflammatory disease, several studies have suggested a role for EVs, particularly endothelial-derived microparticles, in vascular comorbidities [171]. Increased levels of endothelial microparticles have been found in patients with hypertension [172], atrial fibrillation [173], obesity [174] and type 2 diabetes [175]. These microparticles can promote clotting, oxidative stress and endothelial dysfunction, all contributing to the pathogenesis of disease. Therefore, by targeting the production of these microparticles or altering their structure, several risk factors for cardiovascular disease could be modified simultaneously, thereby treating comorbidity in these patients using a unified therapy.
Treatment approaches for multimorbidity in COPD
Management of patients with multimorbidity is now the most important task facing the medical community and presents a fundamental challenge to the single-disease focus that pervades medicine [176]. Self-management approaches to improve knowledge, confidence and skills of patients may prove an important approach in these patients [177, 178]. Recent guidelines suggest that management of multimorbidity should involve personalised assessment and tailored treatment plans. The aim of treatment should be to improve length and quality of life by consolidating management, whereby treatment based on single-disease guidelines is no longer an acceptable approach [179]. Current and future treatment strategies are summarised in table 2 and discussed in detail in the next section.
TABLE 2.
Summary of current and future treatment strategies for multimorbidity in COPD
| Pulmonary effects | Multimorbid effects | |
| Current strategies | ||
| Smoking cessation | Reduction in airway inflammation Improves respiratory symptoms and bronchial hyperresponsiveness Prevents accelerated lung decline |
Reduction of risk of cardiovascular disease and lung cancer [180] |
| Exercise and pulmonary rehabilitation | Delays dynamic hyperinflation Reduces functional breathlessness |
Increases exercise capacity Improves quality of life Reduces anxiety and depression [181] Increases BMI, skeletal muscle mass and improves osteoporosis [182] |
| Inhaled corticosteroids | Limited reduction in airway inflammation: decrease in CD8+ T-lymphocytes in airway biopsies [183] Reduction in exacerbations, especially in eosinophilic COPD [131] Increased risk of pneumonia, especially in severe disease [184] |
Possible reduction in cardiovascular mortality [185] Possible reduction in systemic inflammation (CRP, TNF-α) [86] Improvement in quality of life (in combination with bronchodilator) |
| Theophylline | Possible increased inspiratory muscle strength [186] Reduction in neutrophilic inflammation [187] |
Not known |
| PDE4 inhibitors, e.g. roflumilast | Reduction in exacerbations and improvement in lung function in patients with chronic bronchitis, FEV1 <50% and history of frequent exacerbations | Prevention of bone loss and increase in skeletal muscle mass (in a murine model) [188] |
| Cardiovascular-targeted treatments | ||
| Statins | May reduce exacerbations [189] | Reduction in cardiovascular risk [190] Reduction in oxidative stress and inflammation [191] |
| ACE inhibitors/ARB | Reduction in exacerbations [192] Decrease in hyperinflation [193] |
May improve survival (in those with cardiovascular risk) |
| β-Blockers | Reduction in exacerbations [194] | Reduction in mortality after myocardial infarction [195] and in heart failure [196] Reduction in oxidative stress [197] Improved exercise capacity [198] |
| Future strategies | ||
| Metformin (targeting PI3K-AKT-mTOR pathway) | May improve respiratory symptoms and reduce hospitalisations [199] | Possible reduction in mortality in COPD patients with diabetes [200] |
| Resveratrol (plant-based antioxidant) | Anti-inflammatory in lung epithelial cells [201] | Possible cardioprotective effects [202] |
| Losmapimod (p38 MAPK inhibitor) | No effect on respiratory symptoms or lung function, but a trend towards reduction in exacerbations [203] | Not yet determined; possible effect on arterial inflammation |
| NF-κB inhibitors, e.g. IκB kinase inhibitors | Not yet determined; possible effects on exacerbations given role in activating inflammatory signalling in the COPD lung [204] | Not yet determined; possible effects on skeletal muscle wasting [205], cardiovascular disease, lung cancer, osteoporosis and diabetes [206] |
| Antioxidants, e.g. Nrf2 activators, NOX4 inhibitors | Not yet determined; possible reduction in inflammation and reversal of corticosteroid resistance | Not yet determined; issues with side-effect profile |
| Mesenchymal stem cell EVs | Not yet determined; initial trials failed to show benefit [207] | Not yet determined; possibly neuroprotective [208], cardioprotective [209] and anti-inflammatory [210] |
PDE4: phosphodiesterase-4; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blockers; PI3K: phosphatidylinositol 3-kinase; AKT: also known as protein kinase B; mTOR: mechanistic target of rapamycin; MAPK: p38 mitogen-activated protein kinase; IκB: inhibitor of NF-κB; Nrf2: nuclear factor erythroid 2-related factor 2; NOX: NADPH oxidase; EVs: extracellular vesicles; BMI: body mass index; CRP: C-reactive protein; TNF: tumour necrosis factor; FEV1: forced expiratory volume in 1 s.
Current treatment strategies
Smoking cessation
Smoking is a key risk factor for multimorbidity in COPD [54]. Consequently, smoking cessation may represent a key target for primary and/or secondary prevention of multimorbidity. Effective tobacco control policies (i.e. smoking ban legislation) have been shown to reduce smoking, with rapid improvement in the risks of chronic diseases such as cardiovascular disease and lung cancer [211]. Therefore, although difficult to achieve, smoking cessation should remain an important part of multimorbidity management.
Exercise and pulmonary rehabilitation
Pulmonary rehabilitation has been shown to reduce breathlessness, increase exercise capacity and improve quality of life in COPD patients [212, 213]. Physical activity and regular exercise are both recommended and are beneficial not only for patients with COPD, but also for patients with cardiovascular disease, musculoskeletal disease, obesity, diabetes mellitus and most other chronic medical conditions [182, 214]. Furthermore, a systematic review and meta-analysis including six randomised controlled trials showed that comprehensive pulmonary rehabilitation programmes, (including exercise training, education and psychosocial support) can effectively reduce anxiety and depression [181]. Although traditionally used to bronchospasm, there is evidence to suggest that various β2-agonists increase skeletal muscle mass and strength and prevent fatigue [215]. This suggests that optimal use of inhaled therapy could minimise symptoms and improve compliance with exercise programmes.
Inhaled corticosteroids
Evidence suggests that targeting treatment towards suppressing pulmonary and/or systemic inflammation may benefit multimorbidity in COPD. Although inhaled corticosteroids are a mainstay of COPD therapy, studies show that they may fail to suppress airway inflammation in the COPD [216, 217], and may impair immune cell function in response to common pathogens [218]. Despite this, inhaled corticosteroids have been shown to reduce systemic inflammation [86] and cardiovascular mortality [185]. However, prospective work showed minimal benefit of inhaled corticosteroids on all-cause mortality [219] and no reduction in systemic markers of inflammation [220], suggesting an unlikely beneficial effect on multimorbidity.
Targeting cardiovascular comorbidity in COPD
Observational studies suggest that treatment of cardiovascular disease may have some unexpected benefit on COPD. For example, statins reduce cholesterol and thus cardiovascular risk, but also have pleiotropic effects involving a reduction in oxidative stress and inflammation [221, 222]. Observational studies have shown that statins may reduce exacerbations [189] and cardiovascular mortality in patients with COPD [190]. However, a subsequent systematic review found although statins were associated with a significant reduction in inflammation (CRP and IL-6), this did not translate into improvement in exacerbations, mortality, functional capacity, quality of life, or lung function [191].
Angiotensin-converting enzyme (ACE) inhibitors are widely used to treat hypertension and heart failure, and have been shown to reduce exacerbations and mortality in COPD patients [192]. Both activation of renin–angiotensin system [223, 224] and polymorphisms of the ACE gene have been linked to skeletal muscle wasting in COPD [225]. However, although angiotensin II receptor antagonists reduced hyperinflation in COPD, they had no effect on respiratory or skeletal muscle strength [193]. Furthermore, Curtis et al. [226] showed that ACE inhibitors in combination with pulmonary rehabilitation actually reduced exercise capacity in COPD patients. Therefore, current evidence suggests using this therapy in COPD only in the context of coexisting hypertension or heart failure.
Finally, β-blockers reduce mortality in patients after myocardial infarction [195] and those with heart failure [196]. Despite evidence showing that β-blockers benefit patients with COPD, with reductions in exacerbations and mortality [194], prescribers are often reluctant to use them due to concerns about precipitating bronchospasm [227]. Importantly, similar to statins, cardioselective β-blockers such as carvedilol may exert pleiotropic effects including reducing oxidative stress [197] and skeletal muscle adaptation, resulting in improved exercise capacity [198]. However, the recent BLOCK trial by Dransfield et al. [228] showed that patients with moderate or severe COPD did not benefit from β-blocker (metoprolol) therapy. In fact, hospitalisation for COPD exacerbation was more common in the treatment group. The trial population did not have any overt cardiovascular disease and therefore no therapeutic indication for treatment with a β-blocker. Physicians should continue to use cardioselective β-blockers in patients with COPD who have coexisting cardiovascular disease [229].
Future treatment strategies for multimorbidity in COPD
Anti-ageing molecules
Accelerated ageing is implicated in both multimorbidity and COPD progression and a better understanding of senescence pathways has identified several potential therapeutic targets [230]. The PI3K–protein kinase B (AKT)–mTOR pathway plays a key role in cellular senescence and inhibition of autophagy. Metformin is widely used to treat type 2 diabetes and indirectly inhibits AMP-activated protein kinase, resulting in inhibition of mTOR and extension of lifespan in mice, probably through increasing nuclear factor erythroid 2-related factor 2 (Nrf2)-induced antioxidant gene expression [231, 232]. Observational data have shown reduced mortality in patients with COPD and diabetes taking metformin compared to non-metformin users [200]. However, earlier evidence showed that metformin did not reduce levels of inflammation or improve clinical outcomes in patients admitted with severe COPD exacerbation [233].
PI3K is upregulated in the peripheral lung of COPD and is involved in corticosteroid resistance [234]. Low-dose theophylline may increase sensitivity to inhaled corticosteroids in patients with COPD, by inhibiting oxidant-activated PI3K and restoring histone deacetylase 2 activity (a known anti-ageing molecule) [234]. However, clinical trials of low-dose theophylline have so far had disappointing results [235]. Although these two current therapies have failed to show significant benefits, a newer molecule, resveratrol (a plant-based polyphenol found in grapes, peanuts and red wine), has been shown to extend lifespan by activating Sirtuin-1 [236]. Reports suggest that resveratrol is a powerful antioxidant [237], a PI3K inhibitor [238] and it has been shown to have anti-inflammatory effects in lung epithelial cells [201]. Recent clinical studies in non-COPD patients show improved mitochondrial oxidative metabolism after resveratrol treatment, which could be beneficial for both lung and skeletal muscle impairment in COPD. Moreover, pre-clinical studies suggest that it has cardioprotective effects [202]. Therefore, future treatments for multimorbidity may include novel targets that reduce cellular senescence pathways, thereby reducing accelerated ageing associated with COPD and other comorbid disease.
Targeting inflammation and oxidative stress
Phosphodiesterase (PDE)4 inhibitors are the most developed of the novel anti-inflammatory treatments for COPD. Roflumilast, a selective PDE4 inhibitor, is currently used as an anti-inflammatory treatment in severe COPD to prevent exacerbations in the context of chronic bronchitis. However, the drug's narrow therapeutic window has limited its use [239]. PDE4 inhibition appears to mediate the anti-inflammatory effects of theophylline, and selective PDE4 inhibitors have a wide spectrum of anti-inflammatory effects in the lung and are more effective against neutrophilic inflammation than corticosteroids [240, 241]. Furthermore, in rats a PDE4 inhibitor prevented bone loss and increased skeletal muscle mass, suggesting that PDE4 inhibitors have the potential to prevent osteoporosis and skeletal muscle wasting in COPD [188].
Oxidative stress is present in the lungs of COPD patients and is a major contributor to accelerated ageing and several COPD comorbidities, including atherosclerosis and diabetes [83]. Existing antioxidants, such as N-acetylcysteine and carbocisteine, have proved disappointing in reducing progression of lung function and exacerbations in COPD [242]. The transcription factor Nrf2 regulates many antioxidant genes and is impaired in COPD [243]. However, current Nrf2 activators (e.g. sulforaphane and bardoxolone methyl) are poorly selective, leading to toxicity [244]. ROS may be generated by NADPH oxidases (NOX) in activated inflammatory cells in the airways, and selective NOX4 inhibitors are now in clinical development for various diseases [245]. Furthermore, mitochondria are a major source of ROS in COPD, and selective mitochondria-targeted antioxidants (e.g. mitoquinone) are now in clinical studies [246].
Multiple kinases are involved in driving lung inflammation and remodelling in COPD [247], and kinase inhibitors have trialled in the treatment of COPD [248]. Although promising in animal studies, the oral p38 mitogen-activated protein kinase inhibitor (losmapimod) had no significant effects on symptoms or lung function, but a trend toward reduced exacerbations [203]. Furthermore, a recent phase II trial showed that losmapimod had no effect on arterial inflammation and endothelial function, and so does not support kinase inhibitors as an effective treatment for cardiovascular morbidity COPD [249].
Finally, NF-κB regulates the expression of inflammatory cytokines and chemokines (TNF-α and matrix metalloproteinase-9) and is activated in macrophages and epithelial cells of COPD patients, particularly during exacerbations [204]. NF-κB activation is implicated in mediating systemic inflammation and may be involved in several COPD comorbid diseases including skeletal muscle wasting [205], cardiovascular disease, lung cancer, osteoporosis and diabetes [206]. Inhibitors of NF-κB kinases (IKKs) are essential in NF-κB signalling, and several small-molecule inhibitors of IKK are in development as a promising treatment for COPD [250]. However, there are safety concerns regarding profound immunosuppression and impaired host defences as a consequence of targeting ubiquitously expressed NF-κB. Therefore, there are currently no human trials of these molecules.
Targeted therapy with mesenchymal stem cell derived EVs
Stem cell exhaustion has been implicated in ageing [251] and several chronic diseases including COPD [252]. Mesenchymal stem cells (MSCs) are a proposed regenerative therapy, given their ability to differentiate into a variety of cell types and their ability to migrate and engraft into target tissues [253]. However, more recently their biological effects have been attributed to their secreted EVs which have been shown to be neuroprotective [208], cardioprotective [209] and anti-inflammatory [210]. Although pre-clinical studies have demonstrated immunomodulatory and regenerative potential of MSC-based therapy, clinical studies have failed to demonstrate a proven benefit in COPD [207]. Furthermore, work needs to be done to tackle issues in production, safety, characterisation and delivery.
Conclusions
COPD is a multisystem disease characterised by continued poor outcomes despite current therapeutic strategies targeting the lung. While COPD is associated with both pulmonary and systemic inflammation driven by local responses to toxic stimuli, accelerated ageing, inactivity and other lifestyle factors, the synergistic mechanisms by which multimorbidity is driven remains incompletely described. Understanding this complexity is key to discovering the molecular mechanisms driving inflammation and in doing so will lead to new, holistic therapeutic approaches, treating the patient and not the single disease.
Acknowledgements
The authors would like to acknowledge Alastair Watson (University of Southampton, Southampton, UK) for his help in proof reading and preparation of the manuscript for submission and Laura Reid (University of Southampton) for her help with the figures.
Provenance: Submitted article, peer reviewed.
Conflict of interest: H. Burke has nothing to disclose.
Conflict of interest: T.M.A. Wilkinson reports personal fees and other funding from MMH, grants and personal fees from GSK, AstraZeneca and Synairgen, and personal fees from Boehringer Ingelheim, outside the submitted work.
References
- 1.Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017; 5: 691–706. doi: 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wedzicha JA, Wilkinson T. Impact of chronic obstructive pulmonary disease exacerbations on patients and payers. Proc Am Thorac Soc 2006; 3: 218–221. doi: 10.1513/pats.200510-114SF [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Global Status Report on Noncommunicable Diseases 2010. Description of the Global Burden of NCDs, Their Risk Factors and Determinants. 2011. Available from: www.who.int/nmh/publications/ncd_report2010/en/
- 4.Lortet-Tieulent J, Soerjomataram I, López-Campos JL, et al. International trends in COPD mortality, 1995–2017. Eur Respir J 2019; 54: 1901791. doi: 10.1183/13993003.01791-2019 [DOI] [PubMed] [Google Scholar]
- 5.Hurst JR, Dickhaus J, Maulik PK, et al. Global Alliance for Chronic Disease researchers’ statement on multimorbidity. Lancet Glob Health 2018; 6: e1270–e1271. doi: 10.1016/S2214-109X(18)30391-7 [DOI] [PubMed] [Google Scholar]
- 6.Uijen AA, van de Lisdonk EH. Multimorbidity in primary care: prevalence and trend over the last 20 years. Eur J Gen Pract 2008; 14: Suppl. 1, 28–32. doi: 10.1080/13814780802436093 [DOI] [PubMed] [Google Scholar]
- 7.Fortin M, Stewart M, Poitras ME, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012; 10: 142–151. doi: 10.1370/afm.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) . Multimorbidity. Geneva, WHO, 2016. [Google Scholar]
- 9.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37–43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 10.Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32: 962–969. doi: 10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 11.Williams NP, Coombs NA, Johnson MJ, et al. Seasonality, risk factors and burden of community-acquired pneumonia in COPD patients: a population database study using linked health care records. Int J Chron Obstruct Pulmon Dis 2017; 12: 313–322. doi: 10.2147/COPD.S121389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster TS, Miller JD, Marton JP, et al. Assessment of the economic burden of COPD in the U.S.: a review and synthesis of the literature. COPD 2006; 3: 211–218. doi: 10.1080/15412550601009396 [DOI] [PubMed] [Google Scholar]
- 13.Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013; 107: 1376–1384. doi: 10.1016/j.rmed.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 14.Sin DD, Anthonisen NR, Soriano JB, et al. Mortality in COPD: role of comorbidities. Eur Respir J 2006; 28: 1245–1257. doi: 10.1183/09031936.00133805 [DOI] [PubMed] [Google Scholar]
- 15.Patel AR, Hurst JR. Extrapulmonary comorbidities in chronic obstructive pulmonary disease: state of the art. Expert Rev Respir Med 2011; 5: 647–662. doi: 10.1586/ers.11.62 [DOI] [PubMed] [Google Scholar]
- 16.Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med 2003; 114: 10–14. doi: 10.1016/S0002-9343(02)01297-4 [DOI] [PubMed] [Google Scholar]
- 17.Siris ES, Chen Y-T, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 2004; 164: 1108–1112. doi: 10.1001/archinte.164.10.1108 [DOI] [PubMed] [Google Scholar]
- 18.Curkendall SM, DeLuise C, Jones JK Jr, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol 2006; 16: 63–70. doi: 10.1016/j.annepidem.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 19.García Rodríguez LA, Ruigómez A, Martín-Merino E, et al. Relationship between gastroesophageal reflux disease and COPD in UK primary care. Chest 2008; 134: 1223–1230. doi: 10.1378/chest.08-0902 [DOI] [PubMed] [Google Scholar]
- 20.Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010; 36: 81–88. doi: 10.1183/09031936.00104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanania NA, Müllerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med 2011; 183: 604–611. doi: 10.1164/rccm.201003-0472OC [DOI] [PubMed] [Google Scholar]
- 22.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107: 1514–1519. doi: 10.1161/01.CIR.0000056767.69054.B3 [DOI] [PubMed] [Google Scholar]
- 23.Feary JR, Rodrigues LC, Smith CJ, et al. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax 2010; 65: 956–962. doi: 10.1136/thx.2009.128082 [DOI] [PubMed] [Google Scholar]
- 24.Soriano JB, Visick GT, Muellerova H, et al. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005; 128: 2099–2107. doi: 10.1378/chest.128.4.2099 [DOI] [PubMed] [Google Scholar]
- 25.Hanson C, Rutten EP, Wouters EFM, et al. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis 2014; 9: 723–733. doi: 10.2147/COPD.S50111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabbri LM, Boyd C, Boschetto P, et al. How to integrate multiple comorbidities in guideline development: article 10 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proc Am Thorac Soc 2012; 9: 274–281. doi: 10.1513/pats.201208-063ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hidalgo CA, Blumm N, Barabási A-L, et al. A dynamic network approach for the study of human phenotypes. PLoS Comput Biol 2009; 5: e1000353. doi: 10.1371/journal.pcbi.1000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barabási A-L, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet 2011; 12: 56–68. doi: 10.1038/nrg2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardlaw AJ, Silverman M, Siva R, et al. Multi-dimensional phenotyping: towards a new taxonomy for airway disease. Clin Exp Allergy 2005; 35: 1254–1262. doi: 10.1111/j.1365-2222.2005.02344.x [DOI] [PubMed] [Google Scholar]
- 30.Burgel PR, Paillasseur JL, Caillaud D, et al. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J 2010; 36: 531–539. doi: 10.1183/09031936.00175109 [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Aymerich J, Gómez FP, Benet M, et al. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax 2011; 66: 430–437. doi: 10.1136/thx.2010.154484 [DOI] [PubMed] [Google Scholar]
- 32.Rennard SI, Locantore N, Delafont B, et al. Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc 2015; 12: 303–312. doi: 10.1513/AnnalsATS.201403-125OC [DOI] [PubMed] [Google Scholar]
- 33.Vanfleteren LEGW, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 728–735. doi: 10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 34.Castaldi PJ, Benet M, Petersen H, et al. Do COPD subtypes really exist? COPD heterogeneity and clustering in 10 independent cohorts. Thorax 2017; 72: 998–1006. doi: 10.1136/thoraxjnl-2016-209846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Cho M, Silverman EK, et al. Turning subtypes into disease axes to improve prediction of COPD progression. Thorax 2019; 74: 906–909. doi: 10.1136/thoraxjnl-2018-213005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinney GL, Santorico SA, Young KA, et al. Identification of chronic obstructive pulmonary disease axes that predict all-cause mortality: the COPDGene Study. Am J Epidemiol 2018; 187: 2109–2116. doi: 10.1093/aje/kwy087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgel P-R, Paillasseur J-L, Janssens W, et al. A simple algorithm for the identification of clinical COPD phenotypes. Eur Respir J 2017; 50: 1701034. doi: 10.1183/13993003.01034-2017 [DOI] [PubMed] [Google Scholar]
- 38.Park J, Lee D-S, Christakis NA, et al. The impact of cellular networks on disease comorbidity. Mol Syst Biol 2009; 5: 262. doi: 10.1038/msb.2009.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faner R, Agustí A. Network analysis: a way forward for understanding COPD multimorbidity. Eur Respir J 2015; 46: 591–592. doi: 10.1183/09031936.00054815 [DOI] [PubMed] [Google Scholar]
- 40.Grosdidier S, Ferrer A, Faner R, et al. Network medicine analysis of COPD multimorbidities. Respir Res 2014; 15: 111. doi: 10.1186/s12931-014-0111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Divo MJ, Casanova C, Marin JM, et al. COPD comorbidities network. Eur Respir J 2015; 46: 640–650. doi: 10.1183/09031936.00171614 [DOI] [PubMed] [Google Scholar]
- 42.Agusti A, Sobradillo P, Celli B. Addressing the complexity of chronic obstructive pulmonary disease: from phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am J Respir Crit Care Med 2011; 183: 1129–1137. doi: 10.1164/rccm.201009-1414PP [DOI] [PubMed] [Google Scholar]
- 43.Lange P, Celli B, Agustí A. Lung-function trajectories and chronic obstructive pulmonary disease. N Engl J Med 2015; 373: 1575. doi: 10.1056/NEJMc1508392 [DOI] [PubMed] [Google Scholar]
- 44.Agustí A, Noell G, Brugada J, et al. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med 2017; 5: 935–945. doi: 10.1016/S2213-2600(17)30434-4 [DOI] [PubMed] [Google Scholar]
- 45.Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018; 6: 535–544. doi: 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 46.Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med 2019; 7: 358–364. doi: 10.1016/S2213-2600(18)30529-0 [DOI] [PubMed] [Google Scholar]
- 47.Kachroo P, Morrow JD, Kho AT, et al. Co-methylation analysis in lung tissue identifies pathways for fetal origins of COPD. Eur Respir J 2020; 56: 1902347. doi: 10.1183/13993003.02347-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkinson TMA, Schembri S, Brightling C, et al. Non-typeable Haemophilus influenzae protein vaccine in adults with COPD: a phase 2 clinical trial. Vaccine 2019; 37: 6102–6111. doi: 10.1016/j.vaccine.2019.07.100 [DOI] [PubMed] [Google Scholar]
- 49.Sanei F, Wilkinson T. Influenza vaccination for patients with chronic obstructive pulmonary disease: understanding immunogenicity, efficacy and effectiveness. Ther Adv Respir Dis 2016; 10: 349–367. doi: 10.1177/1753465816646050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004; 328: 1519. doi: 10.1136/bmj.38142.554479.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gakidou E, Afshin A, Abajobir AA, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1345–1422. doi: 10.1016/S0140-6736(17)32366-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int 2001; 68: 259–270. doi: 10.1007/BF02390832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston AK, Mannino DM, Hagan GW, et al. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax 2008; 63: 599–605. doi: 10.1136/thx.2007.088112 [DOI] [PubMed] [Google Scholar]
- 54.Van Remoortel H, Hornikx M, Langer D, et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189: 30–38. [DOI] [PubMed] [Google Scholar]
- 55.Thomsen M, Nordestgaard BG, Vestbo J, et al. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med 2013; 1: 543–550. doi: 10.1016/S2213-2600(13)70137-1 [DOI] [PubMed] [Google Scholar]
- 56.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005; 127: 1952–1959. doi: 10.1378/chest.127.6.1952 [DOI] [PubMed] [Google Scholar]
- 57.Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005; 142: 233–239. doi: 10.7326/0003-4819-142-4-200502150-00005 [DOI] [PubMed] [Google Scholar]
- 58.Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 972–977. doi: 10.1164/rccm.200407-855OC [DOI] [PubMed] [Google Scholar]
- 59.Donaldson GC, Wilkinson TMA, Hurst JR, et al. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 446–452. doi: 10.1164/rccm.200408-1054OC [DOI] [PubMed] [Google Scholar]
- 60.Gimeno-Santos E, Frei A, Steurer-Stey C, et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax 2014; 69: 731–739. doi: 10.1136/thoraxjnl-2013-204763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong CW, Wilkinson TMA. Predicting and preventing hospital readmission for exacerbations of COPD. ERJ Open Res 2020; 6: 00325-2019. doi: 10.1183/23120541.00325-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Donnell DE, Webb KA. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol 2008; 105: 753–755. doi: 10.1152/japplphysiol.90336.2008b [DOI] [PubMed] [Google Scholar]
- 63.Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 2006; 119: Suppl. 1, 21–31. doi: 10.1016/j.amjmed.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 64.Polkey MI, Moxham J. Attacking the disease spiral in chronic obstructive pulmonary disease. Clin Med 2006; 6: 190–196. doi: 10.7861/clinmedicine.6-2-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saey D, Debigaré R, LeBlanc P, et al. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168: 425–430. doi: 10.1164/rccm.200208-856OC [DOI] [PubMed] [Google Scholar]
- 66.Sala E, Roca J, Marrades RM, et al. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 1726–1734. doi: 10.1164/ajrccm.159.6.9804136 [DOI] [PubMed] [Google Scholar]
- 67.Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003; 58: 752–756. doi: 10.1136/thorax.58.9.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med 1994; 150: 11–16. doi: 10.1164/ajrccm.150.1.8025735 [DOI] [PubMed] [Google Scholar]
- 69.Koechlin C, Couillard A, Simar D, et al. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2004; 169: 1022–1027. doi: 10.1164/rccm.200310-1465OC [DOI] [PubMed] [Google Scholar]
- 70.National Institutes of Health. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement 2000 March 27-29; 17: 1–45. https://consensus.nih.gov/2000/2000Osteoporosis111PDF.pdf. [PubMed]
- 71.Sullivan PW, Morrato EH, Ghushchyan V, et al. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000-2002. Diabetes Care 2005; 28: 1599–1603. doi: 10.2337/diacare.28.7.1599 [DOI] [PubMed] [Google Scholar]
- 72.Stephenson J, Bauman A, Armstrong T, et al. The Cost of Illness Attributable to Physical Inactivity in Australia; a Preliminary Study. Canberra, Commonwealth Department of Health and Aged Care, 2000. [Google Scholar]
- 73.Wannamethee SG, Lowe GDO, Whincup PH, et al. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation 2002; 105: 1785–1790. doi: 10.1161/01.CIR.0000016346.14762.71 [DOI] [PubMed] [Google Scholar]
- 74.Mora S, Lee IM, Buring JE, et al. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA 2006; 295: 1412–1419. doi: 10.1001/jama.295.12.1412 [DOI] [PubMed] [Google Scholar]
- 75.Mora S, Cook N, Buring JE, et al. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 2007; 116: 2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol 2005; 78: 819–835. doi: 10.1189/jlb.0505247 [DOI] [PubMed] [Google Scholar]
- 77.Cronin O, Keohane DM, Molloy MG, et al. The effect of exercise interventions on inflammatory biomarkers in healthy, physically inactive subjects: a systematic review. QJM 2017; 110: 629–637. [DOI] [PubMed] [Google Scholar]
- 78.Clini EM, Beghé B, Fabbri LM. Chronic obstructive pulmonary disease is just one component of the complex multimorbidities in patients with COPD. Am J Respir Crit Care Med 2013; 187: 668–671. doi: 10.1164/rccm.201302-0230ED [DOI] [PubMed] [Google Scholar]
- 79.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest 2009; 135: 173–180. doi: 10.1378/chest.08-1419 [DOI] [PubMed] [Google Scholar]
- 80.Savale L, Chaouat A, Marcos E, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 179: A3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D'Mello MJJ, Ross SA, Briel M, et al. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet 2015; 8: 82–90. doi: 10.1161/CIRCGENETICS.113.000485 [DOI] [PubMed] [Google Scholar]
- 82.Shintouo CM, Mets T, Beckwee D, et al. Is inflammageing influenced by the microbiota in the aged gut? A systematic review. Exp Gerontol 2020; 141: 111079. doi: 10.1016/j.exger.2020.111079 [DOI] [PubMed] [Google Scholar]
- 83.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest 2013; 144: 266–273. doi: 10.1378/chest.12-2664 [DOI] [PubMed] [Google Scholar]
- 84.Xiang F, Shuanglun X, Jingfeng W, et al. Association of serum 8-hydroxy-2′-deoxyguanosine levels with the presence and severity of coronary artery disease. Coron Artery Dis 2011; 22: 223–227. doi: 10.1097/MCA.0b013e328344b615 [DOI] [PubMed] [Google Scholar]
- 85.Serdar M, Sertoglu E, Uyanik M, et al. Comparison of 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels using mass spectrometer and urine albumin creatinine ratio as a predictor of development of diabetic nephropathy. Free Radic Res 2012; 46: 1291–1295. doi: 10.3109/10715762.2012.710902 [DOI] [PubMed] [Google Scholar]
- 86.Sin DD, Lacy P, York E, et al. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170: 760–765. doi: 10.1164/rccm.200404-543OC [DOI] [PubMed] [Google Scholar]
- 87.Cosio MG, Guerassimov A. Chronic obstructive pulmonary disease. Inflammation of small airways and lung parenchyma. Am J Respir Crit Care Med 1999; 160: S21–S25. doi: 10.1164/ajrccm.160.supplement_1.7 [DOI] [PubMed] [Google Scholar]
- 88.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 89.McKendry RT, Spalluto CM, Burke H, et al. Dysregulation of antiviral function of CD8+ T cells in the chronic obstructive pulmonary disease lung. Role of the PD-1–PD-L1 axis. Am J Respir Crit Care Med 2016; 193: 642–651. doi: 10.1164/rccm.201504-0782OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax 2010; 65: 930–936. doi: 10.1136/thx.2009.130260 [DOI] [PubMed] [Google Scholar]
- 91.Agustí AGN, Noguera A, Sauleda J, et al. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 347–360. doi: 10.1183/09031936.03.00405703 [DOI] [PubMed] [Google Scholar]
- 92.Gan WQ, Man SFP, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59: 574–580. doi: 10.1136/thx.2003.019588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wouters EFM. Chronic obstructive pulmonary disease. 5: systemic effects of COPD. Thorax 2002; 57: 1067–1070. doi: 10.1136/thorax.57.12.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sevenoaks MJ, Stockley RA. Chronic obstructive pulmonary disease, inflammation and co-morbidity – a common inflammatory phenotype? Respir Res 2006; 7: 70. doi: 10.1186/1465-9921-7-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Martinis M, Franceschi C, Monti D, et al. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett 2005; 579: 2035–2039. doi: 10.1016/j.febslet.2005.02.055 [DOI] [PubMed] [Google Scholar]
- 96.Mayhew D, Devos N, Lambert C, et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax 2018; 73: 422–430. doi: 10.1136/thoraxjnl-2017-210408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Day K, Ostridge K, Conway J, et al. Interrelationships among small airways dysfunction, neutrophilic inflammation, and exacerbation frequency in COPD. Chest 2020; 159: 1391–1399. doi:10.1016/j.chest.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 98.Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 2012; 7: e37483. doi: 10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002; 347: 1557–1565. doi: 10.1056/NEJMoa021993 [DOI] [PubMed] [Google Scholar]
- 100.de Godoy I, Donahoe M, Calhoun WJ, et al. Elevated TNF-alpha production by peripheral blood monocytes of weight-losing COPD patients. Am J Respir Crit Care Med 1996; 153: 633–637. doi: 10.1164/ajrccm.153.2.8564110 [DOI] [PubMed] [Google Scholar]
- 101.Hogg JC. Why does airway inflammation persist after the smoking stops? Thorax 2006; 61: 96–97. doi: 10.1136/thx.2005.049502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hurst JR, Perera WR, Wilkinson TMA, et al. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173: 71–78. doi: 10.1164/rccm.200505-704OC [DOI] [PubMed] [Google Scholar]
- 103.Perera WR, Hurst JR, Wilkinson TMA, et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J 2007; 29: 527–534. doi: 10.1183/09031936.00092506 [DOI] [PubMed] [Google Scholar]
- 104.Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J 2017; 50: 1700853. doi: 10.1183/13993003.00853-2017 [DOI] [PubMed] [Google Scholar]
- 105.Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 2005; 128: 1995–2004. doi: 10.1378/chest.128.4.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 174: 867–874. doi: 10.1164/rccm.200604-506OC [DOI] [PubMed] [Google Scholar]
- 107.Thomsen M, Dahl M, Lange P, et al. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 982–988. doi: 10.1164/rccm.201206-1113OC [DOI] [PubMed] [Google Scholar]
- 108.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016; 16: 22–34. doi: 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allison DJ, Ditor DS. The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflammation 2014; 11: 151. doi: 10.1186/s12974-014-0151-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vernooy JH, Küçükaycan M, Jacobs JA, et al. Local and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors are increased in sputum. Am J Respir Crit Care Med 2002; 166: 1218–1224. doi: 10.1164/rccm.2202023 [DOI] [PubMed] [Google Scholar]
- 111.Sapey E, Ahmad A, Bayley D, et al. Imbalances between interleukin-1 and tumor necrosis factor agonists and antagonists in stable COPD. J Clin Immunol 2009; 29: 508–516. doi: 10.1007/s10875-009-9286-8 [DOI] [PubMed] [Google Scholar]
- 112.Michel O, Dentener M, Corazza F, et al. Healthy subjects express differences in clinical responses to inhaled lipopolysaccharide that are related with inflammation and with atopy. J Allergy Clin Immunol 2001; 107: 797–804. doi: 10.1067/mai.2001.114249 [DOI] [PubMed] [Google Scholar]
- 113.Sapey E, Bayley D, Ahmad A, et al. Inter-relationships between inflammatory markers in patients with stable COPD with bronchitis: intra-patient and inter-patient variability. Thorax 2008; 63: 493–499. doi: 10.1136/thx.2007.086751 [DOI] [PubMed] [Google Scholar]
- 114.Agustí A. Systemic effects of chronic obstructive pulmonary disease: what we know and what we don't know (but should). Proc Am Thorac Soc 2007; 4: 522–525. doi: 10.1513/pats.200701-004FM [DOI] [PubMed] [Google Scholar]
- 115.Koenderman L, Kanters D, Maesen B, et al. Monitoring of neutrophil priming in whole blood by antibodies isolated from a synthetic phage antibody library. J Leukoc Biol 2000; 68: 58–64. [PubMed] [Google Scholar]
- 116.Noguera A, Batle S, Miralles C, et al. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax 2001; 56: 432–437. doi: 10.1136/thorax.56.6.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oudijk EJD, Lammers JWJ, Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Eur Respir J 2003; 46: 5s–13s. doi: 10.1183/09031936.03.00004603a [DOI] [PubMed] [Google Scholar]
- 118.Burnett D, Hill S, Chamba A, et al. Neutrophils from subjects with chronic obstructive lung disease show enhanced chemotaxis and extracellular proteolysis. Lancet 1987; 2: 1043–1046. doi: 10.1016/S0140-6736(87)91476-0 [DOI] [PubMed] [Google Scholar]
- 119.Gustafsson A, Asman B, Bergström K. Cigarette smoking as an aggravating factor in inflammatory tissue-destructive diseases. Increase in tumor necrosis factor-alpha priming of peripheral neutrophils measured as generation of oxygen radicals. Int J Clin Lab Res 2000; 30: 187–190. doi: 10.1007/s005990070005 [DOI] [PubMed] [Google Scholar]
- 120.Koethe SM, Kuhnmuench JR, Becker CG. Neutrophil priming by cigarette smoke condensate and a tobacco anti-idiotypic antibody. Am J Pathol 2000; 157: 1735–1743. doi: 10.1016/S0002-9440(10)64810-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shapiro SD. The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: S29–S32. doi: 10.1164/ajrccm.160.supplement_1.9 [DOI] [PubMed] [Google Scholar]
- 122.Masubuchi T, Koyama S, Sato E, et al. Smoke extract stimulates lung epithelial cells to release neutrophil and monocyte chemotactic activity. Am J Pathol 1998; 153: 1903–1912. doi: 10.1016/S0002-9440(10)65704-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bergmann S, Siekmeier R, Mix C, et al. Even moderate cigarette smoking influences the pattern of circulating monocytes and the concentration of sICAM-1. Respir Physiol 1998; 114: 269–275. doi: 10.1016/S0034-5687(98)00098-X [DOI] [PubMed] [Google Scholar]
- 124.Kalra VK, Ying Y, Deemer K, et al. Mechanism of cigarette smoke condensate induced adhesion of human monocytes to cultured endothelial cells. J Cell Physiol 1994; 160: 154–162. doi: 10.1002/jcp.1041600118 [DOI] [PubMed] [Google Scholar]
- 125.Di Francia M, Barbier D, Mege JL, et al. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994; 150: 1453–1455. doi: 10.1164/ajrccm.150.5.7952575 [DOI] [PubMed] [Google Scholar]
- 126.Takabatake N, Nakamura H, Abe S, et al. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-α system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 1179–1184. doi: 10.1164/ajrccm.161.4.9903022 [DOI] [PubMed] [Google Scholar]
- 127.Keatings VM, Collins PD, Scott DM, et al. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996; 153: 530–534. doi: 10.1164/ajrccm.153.2.8564092 [DOI] [PubMed] [Google Scholar]
- 128.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184: 662–671. doi: 10.1164/rccm.201104-0597OC [DOI] [PubMed] [Google Scholar]
- 129.Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J 2007; 29: 906–913. doi: 10.1183/09031936.00146306 [DOI] [PubMed] [Google Scholar]
- 130.Staples KJ, Nicholas B, McKendry RT, et al. Viral infection of human lung macrophages increases PDL1 expression via IFNβ. PLoS One 2015; 10: e0121527. doi: 10.1371/journal.pone.0121527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012; 186: 48–55. doi: 10.1164/rccm.201108-1553OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 2016; 126: 3279–3295. doi: 10.1172/JCI85664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu D, Molofsky AB, Liang H-E, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011; 332: 243–247. doi: 10.1126/science.1201475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sweetnam PM, Thomas HF, Yarnell JWG, et al. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol 1997; 145: 416–421. doi: 10.1093/oxfordjournals.aje.a009123 [DOI] [PubMed] [Google Scholar]
- 135.Tanaka M, Fukui M, Tomiyasu K-I, et al. Eosinophil count is positively correlated with coronary artery calcification. Hypertens Res 2012; 35: 325–328. doi: 10.1038/hr.2011.191 [DOI] [PubMed] [Google Scholar]
- 136.Mukai HY, Ninomiya H, Ohtani K, et al. Major basic protein binding to thrombomodulin potentially contributes to the thrombosis in patients with eosinophilia. Br J Haematol 1995; 90: 892–899. doi: 10.1111/j.1365-2141.1995.tb05211.x [DOI] [PubMed] [Google Scholar]
- 137.Pignatti P, Visca D, Cherubino F, et al. Do blood eosinophils strictly reflect airway inflammation in COPD? Comparison with asthmatic patients. Respir Res 2019; 20: 145. doi: 10.1186/s12931-019-1111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013; 200: 373–383. doi: 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14: 195–208. doi: 10.1038/nri3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kadota T, Fujita Y, Yoshioka Y, et al. Extracellular vesicles in chronic obstructive pulmonary disease. Int J Mol Sci 2016; 17: 1801. doi: 10.3390/ijms17111801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burke H, Freeman A, Ostridge K, et al. S13 Extracellular vesicle miRNA: a mechanism for chronic airway inflammation and macrophage dysfunction in COPD. Thorax 2018; 73: Suppl. 4, A9–A10. [Google Scholar]
- 142.Bartel S, Deshane J, Wilkinson T, et al. Extracellular vesicles as mediators of cellular cross talk in the lung microenvironment. Front Med 2020; 7: 326. doi: 10.3389/fmed.2020.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wahlund CJE, Eklund A, Grunewald J, et al. Pulmonary extracellular vesicles as mediators of local and systemic inflammation. Front Cell Dev Biol 2017; 5: 39. doi: 10.3389/fcell.2017.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Admyre C, Grunewald J, Thyberg J, et al. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J 2003; 22: 578–583. doi: 10.1183/09031936.03.00041703 [DOI] [PubMed] [Google Scholar]
- 145.Kulshreshtha A, Ahmad T, Agrawal A, et al. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol 2013; 131: 1194–1203. doi: 10.1016/j.jaci.2012.12.1565 [DOI] [PubMed] [Google Scholar]
- 146.Moon HG, Kim SH, Gao J, et al. CCN1 secretion and cleavage regulate the lung epithelial cell functions after cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2014; 307: L326–L337. doi: 10.1152/ajplung.00102.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fujita Y, Araya J, Ito S, et al. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J Extracell Vesicles 2015; 4: 28388. doi: 10.3402/jev.v4.28388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kesimer M, Scull M, Brighton B, et al. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J 2009; 23: 1858–1868. doi: 10.1096/fj.08-119131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cordazzo C, Petrini S, Neri T, et al. Rapid shedding of proinflammatory microparticles by human mononuclear cells exposed to cigarette smoke is dependent on Ca2+ mobilization. Inflamm Res 2014; 63: 539–547. doi: 10.1007/s00011-014-0723-7 [DOI] [PubMed] [Google Scholar]
- 150.Bourdonnay E, Zasłona Z, Penke LRK, et al. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J Exp Med 2015; 212: 729–742. doi: 10.1084/jem.20141675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee H, Zhang D, Laskin DL, et al. Functional evidence of pulmonary extracellular vesicles in infectious and noninfectious lung inflammation. J Immunol 2018; 201: 1500–1509. doi: 10.4049/jimmunol.1800264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soni S, Wilson MR, Dea KP, et al. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax 2016; 71: 1020–1029. doi: 10.1136/thoraxjnl-2015-208032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bhatnagar S, Shinagawa K, Castellino FJ, et al. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007; 110: 3234–3244. doi: 10.1182/blood-2007-03-079152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Giri PK, Schorey JS. Exosomes derived from M. bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One 2008; 3: e2461. doi: 10.1371/journal.pone.0002461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee H, Zhang D, Zhu Z, et al. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep 2016; 6: 35250. doi: 10.1038/srep35250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee SWL, Paoletti C, Campisi M, et al. MicroRNA delivery through nanoparticles. J Control Release 2019; 313: 80–95. doi: 10.1016/j.jconrel.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu H, Ding L, Zhang Y, et al. Circulating endothelial microparticles involved in lung function decline in a rat exposed in cigarette smoke maybe from apoptotic pulmonary capillary endothelial cells. J Thorac Dis 2014; 6: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Letsiou E, Sammani S, Zhang W, et al. Pathologic mechanical stress and endotoxin exposure increases lung endothelial microparticle shedding. Am J Respir Cell Mol Biol 2015; 52: 193–204. doi: 10.1165/rcmb.2013-0347OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gordon C, Gudi K, Krause A, et al. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med 2011; 184: 224–232. doi: 10.1164/rccm.201012-2061OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takahashi T, Kobayashi S, Fujino N, et al. Increased circulating endothelial microparticles in COPD patients: a potential biomarker for COPD exacerbation susceptibility. Thorax 2012; 67: 1067–1074. doi: 10.1136/thoraxjnl-2011-201395 [DOI] [PubMed] [Google Scholar]
- 161.Nagy JA, Benjamin L, Zeng H, et al. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008; 11: 109–119. doi: 10.1007/s10456-008-9099-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lockett AD, Brown MB, Santos-Falcon N, et al. Active trafficking of alpha 1 antitrypsin across the lung endothelium. PLoS One 2014; 9: e93979. doi: 10.1371/journal.pone.0093979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Martinez-Bravo M-J, Wahlund CJE, Qazi KR, et al. Pulmonary sarcoidosis is associated with exosomal vitamin D-binding protein and inflammatory molecules. J Allergy Clin Immunol 2017; 139: 1186–1194. doi: 10.1016/j.jaci.2016.05.051 [DOI] [PubMed] [Google Scholar]
- 164.Berckmans RJ, Nieuwland R, Tak PP, et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum 2002; 46: 2857–2866. doi: 10.1002/art.10587 [DOI] [PubMed] [Google Scholar]
- 165.Marcos-Ramiro B, Oliva Nacarino P, Serrano-Pertierra E, et al. Microparticles in multiple sclerosis and clinically isolated syndrome: effect on endothelial barrier function. BMC Neurosci 2014; 15: 110. doi: 10.1186/1471-2202-15-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mitsuhashi S, Feldbrügge L, Csizmadia E, et al. Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm Bowel Dis 2016; 22: 1587–1595. doi: 10.1097/MIB.0000000000000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sellam J, Proulle V, Jüngel A, et al. Increased levels of circulating microparticles in primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther 2009; 11: R156. doi: 10.1186/ar2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Burbano C, Rojas M, Muñoz-Vahos C, et al. Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci Rep 2018; 8: 17917. doi: 10.1038/s41598-018-36335-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sáenz-Cuesta M, Irizar H, Castillo-Triviño T, et al. Circulating microparticles reflect treatment effects and clinical status in multiple sclerosis. Biomark Med 2014; 8: 653–661. doi: 10.2217/bmm.14.9 [DOI] [PubMed] [Google Scholar]
- 170.Sáenz-Cuesta M, Osorio-Querejeta I, Otaegui D. Extracellular vesicles in multiple sclerosis: what are they telling us? Front Cell Neurosci 2014; 8: 100. doi: 10.3389/fncel.2014.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gohar A, de Kleijn DPV, Hoes AW, et al. Vascular extracellular vesicles in comorbidities of heart failure with preserved ejection fraction in men and women: the hidden players. A mini review. Vascul Pharmacol 2018; 111: 1–6. doi: 10.1016/j.vph.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 172.Amabile N, Cheng S, Renard JM, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J 2014; 35: 2972–2979. doi: 10.1093/eurheartj/ehu153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ederhy S, Di Angelantonio E, Mallat Z, et al. Levels of circulating procoagulant microparticles in nonvalvular atrial fibrillation. Am J Cardiol 2007; 100: 989–994. doi: 10.1016/j.amjcard.2007.04.040 [DOI] [PubMed] [Google Scholar]
- 174.Esposito K, Ciotola M, Schisano B, et al. Endothelial microparticles correlate with endothelial dysfunction in obese women. J Clin Endocrinol Metab 2006; 91: 3676–3679. doi: 10.1210/jc.2006-0851 [DOI] [PubMed] [Google Scholar]
- 175.Burger D, Turner M, Xiao F, et al. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia 2017; 60: 1791–1800. doi: 10.1007/s00125-017-4331-2 [DOI] [PubMed] [Google Scholar]
- 176.Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet 2012; 380: 7–9. [DOI] [PubMed] [Google Scholar]
- 177.Crooks MG, Elkes J, Storrar W, et al. Evidence generation for the clinical impact of myCOPD in patients with mild, moderate and newly diagnosed COPD: a randomised controlled trial. ERJ Open Res 2020; 6: 00460-2020. doi: 10.1183/23120541.00460-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.North M, Bourne S, Green B, et al. A randomised controlled feasibility trial of E-health application supported care vs usual care after exacerbation of COPD: the RESCUE trial. NPJ Digit Med 2020; 3: 145. doi: 10.1038/s41746-019-0211-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.National Institute for Health and Care Excellence (NICE) . Multimorbidity: Clinical Assessment and Management. London, NICE, 2016. Available from: www.nice.org.uk/guidance/ng56/ [Google Scholar]
- 180.Jiménez-Ruiz CA, Andreas S, Lewis KE, et al. Statement on smoking cessation in COPD and other pulmonary diseases and in smokers with comorbidities who find it difficult to quit. Eur Respir J 2015; 46: 61–79. doi: 10.1183/09031936.00092614 [DOI] [PubMed] [Google Scholar]
- 181.Coventry PA, Hind D. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstructive pulmonary disease: systematic review and meta-analysis. J Psychosom Res 2007; 63: 551–565. doi: 10.1016/j.jpsychores.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 182.Franssen FME, Rochester CL. Comorbidities in patients with COPD and pulmonary rehabilitation: do they matter? Eur Respir Rev 2014; 23: 131–141. doi: 10.1183/09059180.00007613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jen R, Rennard SI, Sin DD. Effects of inhaled corticosteroids on airway inflammation in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2012; 7: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013; 68: 1029–1036. doi: 10.1136/thoraxjnl-2012-202872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sin DD, Wu L, Anderson JA, et al. Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax 2005; 60: 992–997. doi: 10.1136/thx.2005.045385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Moxham J. Aminophylline and the respiratory muscles: an alternative view. Clin Chest Med 1988; 9: 325–336. [PubMed] [Google Scholar]
- 187.Culpitt SV, de Matos C, Russell RE, et al. Effect of theophylline on induced sputum inflammatory indices and neutrophil chemotaxis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 165: 1371–1376. doi: 10.1164/rccm.2105106 [DOI] [PubMed] [Google Scholar]
- 188.Yao W, Tian XY, Chen J, et al. Rolipram, a phosphodiesterase 4 inhibitor, prevented cancellous and cortical bone loss by inhibiting endosteal bone resorption and maintaining the elevated periosteal bone formation in adult ovariectomized rats. J Musculoskelet Neuronal Interact 2007; 7: 119–130. [PubMed] [Google Scholar]
- 189.Wang M-T, Lo Y-W, Tsai C-L, et al. Statin use and risk of COPD exacerbation requiring hospitalization. Am J Med 2013; 126: 598–606. doi: 10.1016/j.amjmed.2013.01.036 [DOI] [PubMed] [Google Scholar]
- 190.Young RP, Hopkins RJ. Update on the potential role of statins in chronic obstructive pulmonary disease and its co-morbidities. Expert Rev Respir Med 2013; 7: 533–544. doi: 10.1586/17476348.2013.838018 [DOI] [PubMed] [Google Scholar]
- 191.Walsh A, Perrem L, Khashan AS, et al. Statins versus placebo for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2019; 7: CD011959. doi: 10.1002/14651858.CD011959.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mancini GBJ, Etminan M, Zhang B, et al. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol 2006; 47: 2554–2560. doi: 10.1016/j.jacc.2006.04.039 [DOI] [PubMed] [Google Scholar]
- 193.Andreas S, Herrmann-Lingen C, Raupach T, et al. Angiotensin II blockers in obstructive pulmonary disease: a randomised controlled trial. Eur Respir J 2006; 27: 972–979. doi: 10.1183/09031936.06.00098105 [DOI] [PubMed] [Google Scholar]
- 194.Du Q, Sun Y, Ding N, et al. Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studies. PLoS One 2014; 9: e113048. doi: 10.1371/journal.pone.0113048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 1998; 339: 489–497. doi: 10.1056/NEJM199808203390801 [DOI] [PubMed] [Google Scholar]
- 196.Hjalmarson Å, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). JAMA 2000; 283: 1295–1302. doi: 10.1001/jama.283.10.1295 [DOI] [PubMed] [Google Scholar]
- 197.Wang R, Miura T, Harada N, et al. Pleiotropic effects of the β-adrenoceptor blocker carvedilol on calcium regulation during oxidative stress-induced apoptosis in cardiomyocytes. J Pharmacol Exp Ther 2006; 318: 45–52. doi: 10.1124/jpet.105.099903 [DOI] [PubMed] [Google Scholar]
- 198.Ladage D, Schwinger RHG, Brixius K. Cardio-selective beta-blocker: pharmacological evidence and their influence on exercise capacity. Cardiovasc Ther 2013; 31: 76–83. doi: 10.1111/j.1755-5922.2011.00306.x [DOI] [PubMed] [Google Scholar]
- 199.Zhu A, Teng Y, Ge D, et al. Role of metformin in treatment of patients with chronic obstructive pulmonary disease: a systematic review. J Thorac Dis 2019; 11: 4371–4378. doi: 10.21037/jtd.2019.09.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ho T-W, Huang C-T, Tsai Y-J, et al. Metformin use mitigates the adverse prognostic effect of diabetes mellitus in chronic obstructive pulmonary disease. Respir Res 2019; 20: 69. doi: 10.1186/s12931-019-1035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Donnelly LE, Newton R, Kennedy GE, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol 2004; 287: L774–L783. doi: 10.1152/ajplung.00110.2004 [DOI] [PubMed] [Google Scholar]
- 202.Beijers RJHCG, Gosker HR, Schols AMWJ. Resveratrol for patients with chronic obstructive pulmonary disease: hype or hope? Curr Opin Clin Nutr Metab Care 2018; 21: 138–144. doi: 10.1097/MCO.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Watz H, Barnacle H, Hartley BF, et al. Efficacy and safety of the p38 MAPK inhibitor losmapimod for patients with chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2014; 2: 63–72. doi: 10.1016/S2213-2600(13)70200-5 [DOI] [PubMed] [Google Scholar]
- 204.Caramori G, Romagnoli M, Casolari P, et al. Nuclear localisation of p65 in sputum macrophages but not in sputum neutrophils during COPD exacerbations. Thorax 2003; 58: 348–351. doi: 10.1136/thorax.58.4.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Agustí A, Morlá M, Sauleda J, et al. NF-κB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax 2004; 59: 483–487. doi: 10.1136/thx.2003.017640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Karin M, Yamamoto Y, Wang QM. The IKK NF-κB system: a treasure trove for drug development. Nat Rev Drug Discov 2004; 3: 17–26. doi: 10.1038/nrd1279 [DOI] [PubMed] [Google Scholar]
- 207.Broekman W, Khedoe P, Schepers K, et al. Mesenchymal stromal cells: a novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax 2018; 73: 565–574. doi: 10.1136/thoraxjnl-2017-210672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lee M, Ban J-J, Yang S, et al. The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer's disease. Brain Res 2018; 1691: 87–93. doi: 10.1016/j.brainres.2018.03.034 [DOI] [PubMed] [Google Scholar]
- 209.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010; 4: 214–222. doi: 10.1016/j.scr.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 210.Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles 2015; 4: 30087. doi: 10.3402/jev.v4.30087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Glantz S, Gonzalez M. Effective tobacco control is key to rapid progress in reduction of non-communicable diseases. Lancet 2012; 379: 1269-1271. doi: 10.1016/S0140-6736(11)60615-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Spruit MA, Singh SJ, Garvey C, et al. An Official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 213.Bourne S, DeVos R, North M, et al. Online versus face-to-face pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: randomised controlled trial. BMJ Open 2017; 7: e014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Freeman AT, Hill D, Newell C, et al. Patient perceived barriers to exercise and their clinical associations in difficult asthma. Asthma Res Pract 2020; 6: 5. doi: 10.1186/s40733-020-00058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 2008; 88: 729–767. doi: 10.1152/physrev.00028.2007 [DOI] [PubMed] [Google Scholar]
- 216.Reid DW, Wen Y, Johns DP, et al. Bronchodilator reversibility, airway eosinophilia and anti-inflammatory effects of inhaled fluticasone in COPD are not related. Respirology 2008; 13: 799–809. doi: 10.1111/j.1440-1843.2008.01380.x [DOI] [PubMed] [Google Scholar]
- 217.Verhoeven GT, Hegmans JPJJ, Mulder PGH, et al. Effects of fluticasone propionate in COPD patients with bronchial hyperresponsiveness. Thorax 2002; 57: 694–700. doi: 10.1136/thorax.57.8.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hinks TSC, Wallington JC, Williams AP, et al. Steroid-induced deficiency of mucosal-associated invariant T cells in the chronic obstructive pulmonary disease lung. Implications for nontypeable Haemophilus influenzae infection. Am J Respir Crit Care Med 2016; 194: 1208–1218. doi: 10.1164/rccm.201601-0002OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775–789. doi: 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 220.Sin DD, Man SFP, Marciniuk DD, et al. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177: 1207–1214. doi: 10.1164/rccm.200709-1356OC [DOI] [PubMed] [Google Scholar]
- 221.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005; 45: 89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hothersall E, McSharry C, Thomson NC. Potential therapeutic role for statins in respiratory disease. Thorax 2006; 61: 729–734. doi: 10.1136/thx.2005.057976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jones A, Woods DR. Skeletal muscle RAS and exercise performance. Int J Biochem Cell Biol 2003; 35: 855–866. doi: 10.1016/S1357-2725(02)00342-4 [DOI] [PubMed] [Google Scholar]
- 224.Andreas S, Anker SD, Scanlon PD, et al. Neurohumoral activation as a link to systemic manifestations of chronic lung disease. Chest 2005; 128: 3618–3624. doi: 10.1378/chest.128.5.3618 [DOI] [PubMed] [Google Scholar]
- 225.Hopkinson NS, Nickol AH, Payne J, et al. Angiotensin converting enzyme genotype and strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170: 395–399. doi: 10.1164/rccm.200304-578OC [DOI] [PubMed] [Google Scholar]
- 226.Curtis KJ, Meyrick VM, Mehta B, et al. Angiotensin-converting enzyme inhibition as an adjunct to pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2016; 194: 1349–1357. doi: 10.1164/rccm.201601-0094OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.van der Woude HJ, Zaagsma J, Postma DS, et al. Detrimental effects of β-blockers in COPD: a concern for nonselective β-blockers. Chest 2005; 127: 818–824. doi: 10.1378/chest.127.3.818 [DOI] [PubMed] [Google Scholar]
- 228.Dransfield MT, Voelker H, Bhatt SP, et al. Metoprolol for the prevention of acute exacerbations of COPD. N Engl J Med 2019; 381: 2304–2314. doi: 10.1056/NEJMoa1908142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.MacNee W. Beta-blockers in COPD – a controversy resolved? N Engl J Med 2019; 381: 2367–2368. doi: 10.1056/NEJMe1912664 [DOI] [PubMed] [Google Scholar]
- 230.Ito K, Colley T, Mercado N. Geroprotectors as a novel therapeutic strategy for COPD, an accelerating aging disease. Int J Chron Obstruct Pulmon Dis 2012; 7: 641–652. doi: 10.2147/COPD.S28250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Anisimov VN, Berstein LM, Egormin PA, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 2008; 7: 2769–2773. doi: 10.4161/cc.7.17.6625 [DOI] [PubMed] [Google Scholar]
- 232.Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun 2013; 4: 2192. doi: 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hitchings AW, Lai D, Jones PW, et al. Metformin in severe exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2016; 71: 587–593. doi: 10.1136/thoraxjnl-2015-208035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.To Y, Ito K, Kizawa Y, et al. Targeting phosphoinositide-3-kinase-δ with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 182: 897–904. doi: 10.1164/rccm.200906-0937OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Devereux G, Cotton S, Fielding S, et al. Effect of theophylline as adjunct to inhaled corticosteroids on exacerbations in patients with COPD: a randomized clinical trial. JAMA 2018; 320: 1548–1559. doi: 10.1001/jama.2018.14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of SIRT1: effects on aging and age-related diseases. Nutr Rev 2008; 66: 591–596. doi: 10.1111/j.1753-4887.2008.00109.x [DOI] [PubMed] [Google Scholar]
- 237.de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans 2007; 35: 1156–1160. doi: 10.1042/BST0351156 [DOI] [PubMed] [Google Scholar]
- 238.Fröjdö S, Cozzone D, Vidal H, et al. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J 2007; 406: 511–518. doi: 10.1042/BJ20070236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 81–90. doi: 10.2147/COPD.S89849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rabe KF, Bateman ED, O'Donnell D, et al. Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005; 366: 563–571. doi: 10.1016/S0140-6736(05)67100-0 [DOI] [PubMed] [Google Scholar]
- 241.Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax 2007; 62: 1081–1087. doi: 10.1136/thx.2006.075937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Decramer M, Rutten-van Mölken M, Dekhuijzen PNR, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 2005; 365: 1552–1560. doi: 10.1016/S0140-6736(05)66456-2 [DOI] [PubMed] [Google Scholar]
- 243.Copple IM. The Keap1-Nrf2 cell defense pathway – a promising therapeutic target? Adv Pharmacol 2012; 63: 43–79. doi: 10.1016/B978-0-12-398339-8.00002-1 [DOI] [PubMed] [Google Scholar]
- 244.Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1–Nrf2 pathway. Trends Pharmacol Sci 2013; 34: 340–346. doi: 10.1016/j.tips.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 245.Borbély G, Szabadkai I, Horváth Z, et al. Small-molecule inhibitors of NADPH oxidase 4. J Med Chem 2010; 53: 6758–6762. doi: 10.1021/jm1004368 [DOI] [PubMed] [Google Scholar]
- 246.Murphy MP. Antioxidants as therapies: can we improve on nature? Free Radic Biol Med 2014; 66: 20–23. doi: 10.1016/j.freeradbiomed.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 247.Renda T, Baraldo S, Pelaia G, et al. Increased activation of p38 MAPK in COPD. Eur Respir J 2008; 31: 62–69. doi: 10.1183/09031936.00036707 [DOI] [PubMed] [Google Scholar]
- 248.Norman P. Investigational p38 inhibitors for the treatment of chronic obstructive pulmonary disease. Expert Opin Investig Drugs 2015; 24: 383–392. doi: 10.1517/13543784.2015.1006358 [DOI] [PubMed] [Google Scholar]
- 249.Fisk M, Cheriyan J, Mohan D, et al. The p38 mitogen activated protein kinase inhibitor losmapimod in chronic obstructive pulmonary disease patients with systemic inflammation, stratified by fibrinogen: a randomised double-blind placebo-controlled trial. PLoS One 2018; 13: e0194197. doi: 10.1371/journal.pone.0194197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 2015; 5: 1266–1283. doi: 10.3390/biom5031266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013; 153: 1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax 2015; 70: 482–489. doi: 10.1136/thoraxjnl-2014-206084 [DOI] [PubMed] [Google Scholar]
- 253.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells – current trends and future prospective. Biosci Rep 2015; 35: e00191. doi: 10.1042/BSR20150025 [DOI] [PMC free article] [PubMed] [Google Scholar]