Abstract
The percentage of α1-antitrypsin protease inhibitor ZZ (PiZZ) genotypes in patients with COPD is controversial, with large differences among various studies. We aimed to estimate the prevalence of PiZZ in COPD patients from 20 European countries with available data, according to the number of PiZZ and COPD individuals in each country.
A systematic review was conducted to select European countries with reliable data on the prevalence of PiZZ and COPD. We created a database with the following data: 1) total population and population aged ≥40 years according to the Eurostat database; 2) number and 95% CI of PiZZ patients aged ≥40 years; 3) application of a conversion factor of genetic penetrance of 60%; 4) number of COPD individuals, with 95% CI, aged ≥40 years; and 5) calculation of the PiZZ/COPD ratio. Finally, results were presented using an Inverse Distance Weighted Interpolation map.
We found 36 298 (95% CI 23 643–56 594) PiZZ individuals at high risk and 30 849 709 (95% CI 21 411 293–40 344 496) COPD patients, with a PiZZ/COPD ratio of 0.12% (range 0.08–0.24%), and a prevalence of 1 out of 408 in Northern, 1 out of 944 in Western, 1 out of 1051 in Central, 1 out of 711 in Southern, and 1 out of 1274 in Eastern Europe.
These data may be useful to plan strategies for future research and diagnosis, and to rationalise the available therapeutic resources.
Short abstract
There is a significant number of PiZZ individuals at high risk of COPD, as well as an impressive number of patients with COPD in Europe. The ratio between PiZZ and COPD ranges between 0.08% and 0.24%, with wide differences among countries. https://bit.ly/2VrOzUv
Introduction
Severe α1-antitrypsin (AAT) deficiency is a rare autosomal codominant genetic condition caused by mutations of the SERPINA1 gene, which mainly affects Caucasian individuals of European heritage [1]. In clinical practice, 96% of severe AAT deficiency index cases are homozygous for the SERPINA1 Z mutation (Glu342Lys) expressing a protease inhibitor ZZ (PiZZ) genotype [2].
SERPINA1 Z mutation results in the synthesis of a structurally malformed Z-AAT glycoprotein, which polymerises and is retained in hepatocytes, predisposing PiZ homozygotes to neonatal hepatitis, liver cirrhosis and hepatocellular carcinoma, while the subsequent secretory defect results in a marked reduction of about 80% of both its inhibitory capacity against neutrophil elastase and the circulating levels of the protein, which may be insufficient to ensure a lifetime of protection of the lungs from the proteolytic damage of proteinases, thus favouring the development of COPD in adults [3]. Less frequently, by mechanisms not yet well clarified, AAT deficiency can be expressed by granulomatosis with polyangiitis (or Wegener disease) and neutrophilic panniculitis [2].
In clinical practice, lung emphysema and chronic bronchitis are the most common clinical phenotypes of COPD associated with PiZZ deficiency [4]. Environmental factors, particularly cigarette smoke, greatly increase the risk of COPD development [2], and while the onset of respiratory disease in smokers occurs in the third or fourth decades of life, in nonsmokers the onset can be delayed to the fifth or sixth decades, and even some nonsmokers may have a normal life span without developing COPD or other diseases associated with AAT deficiency [5–9]. This striking variability of clinical expression suggests that in a number of cases AAT deficiency alone is not enough to induce COPD, and that, in addition to smoking and other environmental pollutants, genetic modifiers not yet definitively identified likely influence this clinical variability [10–12]. Indeed, according to the data provided by registries of patients with AAT deficiency, the genetic penetrance of the SERPINA1 Z homozygotes (i.e. number of individuals with the PiZZ genotype who develop COPD) reaches at most 60% [2, 5, 7]. This is an important fact to consider, as severe AAT deficiency is the only subtype of COPD with a specific augmentative therapy with infusions of purified plasma-derived AAT [13]; hence the importance of knowing the number of patients with PiZZ at high risk of developing COPD in each country, in order to enhance awareness of this association among healthcare providers and the general public, for planning health policies and financial medical resources and their utilisation by the scientific community, governments and pharmaceutical industry [11, 14].
A study from the 1980s found that 1.87% of 965 patients with COPD had PiZZ-AAT deficiency [15]. However, subsequent screening studies provided much lower rates of prevalence, while case-finding programmes that focused on individuals with low serum levels of AAT and clinical or radiological data indicative of AAT deficiency provided higher detection rates [16].
The objective of this study was to make a theoretical estimate, using logical mathematical reasoning, of the prevalence of PiZZ genotypes in the population of individuals with COPD from 20 European countries with available data of both the genetic epidemiology of AAT deficiency and the prevalence of COPD.
Methods
The authors used data from three recent systematic reviews published by their group to select European countries with reliable data on the prevalence of both PiZZ deficiency and COPD [17–19]. The selection criteria and the characteristics of 75 603 PiZZ individuals from 90 European samples are described in detail in the study by Blanco et al. [17], and this publication provides a link to supplementary material that includes all the references analysed. In addition, the COPD point prevalence and characteristics of the samples provided by 62 selected epidemiological studies from European countries are described in detail in another study by Blanco et al. [19]. With this methodology, 20 out of the 50 sovereign European countries were selected. These countries were: Denmark, Estonia, Finland, Latvia, Lithuania, Norway and Sweden from Northern Europe; Belgium, France, Netherlands, Republic of Ireland and UK from Western Europe; Austria, Germany, Poland and Switzerland from Central Europe; Italy, Portugal and Spain from Southern Europe; and Serbia from Eastern Europe.
Then, the following data were consecutively entered into a Microsoft Excel database. 1) Total population and population aged ≥40 years from each of these countries according to the Eurostat database [20]. 2) Total number of PiZZ subjects, with the corresponding 95% CI of each country extracted from the published data [17]. 3) The calculated number and 95% CI of PiZZ individuals aged ≥40 years, assuming a normal allelic distribution of AAT alleles in that age group [21, 22]. 4) The calculated number and 95% CI of the PiZZ individuals aged ≥40 years, resulting from the application of a conversion factor of genetic penetrance of 60% [2]. 5) The estimated number of individuals with COPD, with their corresponding 95% CI, aged ≥40 years, applying the following reported values of COPD prevalence: 11.5% (95% CI 8.8–14.1%), 14.1% (95% CI 10.4–17.9%), 10.8% (95% CI 7.8–13.8%) and 14.2% (95% CI 8.8–19.6%) for Northern, Central, Southern and Western Europe, respectively [18, 19]. 6) The PiZZ/COPD ratio, which was calculated by dividing the total number of PiZZ individuals at high risk (i.e. those aged ≥40 years and a genetic penetrance of 60%) by the total number of COPD subjects aged ≥40 years in each country.
Finally, with the data of the previous calculations, a map of Inverse Distance Weighted (IDW) Interpolation, performed with the same technique used by authors in previous studies [17–19], was drawn to graphically illustrate the results of the study. For this, an ascending chromatic scale (blue-green-yellow-orange-brown-red), in which blue and red expressed the maximum and minimum prevalence values, respectively, was chosen. The areas with a population density of less than two inhabitants per square kilometre were hidden from visualisation, appearing as white. The geographical regions not included in the study were shaded grey.
Results
Numerical results are shown in table 1. In summary, a total of 20 countries (seven from Northern Europe, five from Western Europe, four from Central Europe, three from Southern Europe, and one from Eastern Europe), with a total population of 455 851 098 individuals (51.9% aged ≥40 years), were analysed.
TABLE 1.
Estimated numbers of α1-antitrypsin protease inhibitor ZZ (PiZZ) deficiency and COPD individuals in 20 European countries
| Geographic region and country | Country population/total population ≥40 years n | Total PiZZ (95% CI) | PiZZ ≥40 years (95% CI) | PiZZ ≥40 years and penetrance 60% (95% CI) | Total COPD (95% CI) | PiZZ/COPD ratio % | PiZZ/COPD prevalence (1/x) n |
| Northern Europe | |||||||
| Denmark | 5 627 235/2 835 230 | 4090 (3459–4834) | 2061 (1743–2436) | 1236 (1046–1461) | 326 051 (249 500–399 767) | 0.38 | 264 |
| Estonia | 1 315 819/663 339 | 752 (481–1170) | 379 (242–590) | 227 (145–354) | 76 284 (58 373–93 530) | 0.30 | 335 |
| Finland | 5 451 270/2 811 795 | 1929 (1553–2394) | 995 (801–1235) | 597 (481–741) | 323 356 (247 437–396 463) | 0.18 | 542 |
| Latvia | 2 943 472/1 040 757 | 4005 (1802–8648) | 1416 (637–3058) | 850 (382–1835) | 119 687 (91 586–146 746) | 0.71 | 141 |
| Lithuania | 2 001 468/1 530 171 | 717 (405–1261) | 548 (310–964) | 329 (186–578) | 175 970 (134 655–215 754) | 0.19 | 535 |
| Norway | 5 107 970/2 410 416 | 1798 (1321–2442) | 848 (623–1152) | 509 (374–691) | 277 198 (212 116–339 868) | 0.18 | 545 |
| Sweden | 9 644 864/4 814 821 | 2262 (1674–3052) | 1129 (836–1524) | 678 (501–914) | 553 704 (423 704–678 889) | 0.12 | 817 |
| Subtotal | 32 092 098/16 106 529 | 15 553 (10 695–23 801) | 7377 (5192–10 958) | 4426 (3115–6575) | 1 852 251 (1417–375-) | 0.24 | 418 |
| Western Europe | |||||||
| Belgium | 11 180 840/5 633 352 | 3193 (1745–5787) | 1609 (879–2916) | 965 (528–1749) | 799 936 (495 734–1 104 136) | 0.12 | 829 |
| France | 66 458 153/33 219 475 | 17 191 (13 255–22 270) | 8593 (6626–11 132) | 5156 (3975–6679) | 4 717 165 (2 923 313–6 511 017) | 0.11 | 915 |
| Netherlands | 16 829 289/8 646 510 | 5353 (3057–9298) | 2750 (1571–4777) | 1650 (942–2866) | 1 227 804 (760 892–1 694 715) | 0.13 | 744 |
| Republic of Ireland | 4 637 852/1 938 181 | 2265 (1264–4019) | 947 (528–1,80) | 568 (317–1008) | 275 222 (170 559–379 883) | 0.21 | 485 |
| UK | 64 351 203/31 282 515 | 13 044 (8315–20 421) | 6341 (4042–9927) | 3805 (2425–5956) | 4 442 117 (2 752 861–6 131 372) | 0.09 | 1168 |
| Subtotal | 163 457 337/ 80 720 033 | 41 046 (27 636–61 795) | 20 240 (13 646–30 431) | 13 666 (8187–18 259) | 11 462 245 (7 103 363–15 821 126) | 0.11 | 944 |
| Central Europe | |||||||
| Austria | 8 507 786/4 484 191 | 1529 (646–3536) | 806 (350–1 864) | 484 (210–1 118) | 632 271 (466 355–802 670) | 0.08 | 1308 |
| Germany | 80 767 463/45 778 502 | 20 611 (13 380–31 626) | 11 682 (7584–17 925) | 7009 (4550–10 755) | 6 454 769 (4 760 964–8 194 351) | 0.11 | 921 |
| Poland | 38 017 856/18 015 506 | 6791 (4395–10 454) | 3218 (2083–4954) | 1931 (1250–2972) | 2 540 186 (1 873 612–3 224 775) | 0.08 | 1316 |
| Switzerland | 8 139 631/4 199 289 | 972 (434–2139) | 501 (224–1104) | 301 (134–662) | 592 100 (436 726–751 672) | 0.05 | 1968 |
| Subtotal | 135 432 736/ 72 477 488 | 29 903 (18 873–47 755) | 16 208 (10 240–25 846) | 9725 (6144–15 508) | 10 219 326 (7 537 659–12 973 470) | 0.10 | 1051 |
| Southern Europe | |||||||
| Italy | 60 782 668/33 982 240 | 10 652 (7046–16 049) | 5955 (3939–8973) | 3573 (2364–5384) | 3 670 082 (2 650 614–4 689 549) | 0.10 | 1027 |
| Portugal | 10 427 301/5 546 011 | 4944 (2403–10 004) | 2630 (1278–5321) | 1578 (767–3193) | 598 969 (432 588–765 349) | 0.26 | 380 |
| Spain | 46 512 199/23 903 789 | 14 522 (9405–22 331) | 7479 (4833–11 476) | 4487 (2900–6886) | 2 581 609 (1 864 495–3 298 722) | 0.17 | 575 |
| Subtotal | 117 722 168/63 432 040 | 30 148 (18 854–48 384) | 16 064 (10 051–25 770) | 9638 (6030–15 462) | 6 850 660 (4 947 699–8 753 622) | 0.14 | 711 |
| Eastern Europe | |||||||
| Serbia | 7 146 759/3 751 833 | 1159 (525–2510) | 608 (276–1318) | 365 (165–791) | 465 227 (405 197–525 256) | 0.08 | 1274 |
| Subtotal | 7 146 759/3 751 833 | 1159 (525–2510) | 608 (276–1318) | 365 (165–791) | 465 227 (405 197–525 256) | 0.08 | 1274 |
| TOTAL | 455 851 098/236 487 923 | 117 809 (76 583–184 245) | 60 496 (39 405–94 323) | 36 298 (23 643–56 594) | 30 849 709 (21 411 293–40 344 496) | 0.12 | 850 |
Our tabulation showed a total of 117 809 (95% CI 76 583–184 245) individuals with PiZZ genotypes among the adult populations of these countries. This number fell to 60 496 (95% CI 39 405–94 323; 51%) in the population aged ≥40 years, and decreased to 36 298 (95% CI 23 643–56 594; 30.8%) after applying a conversion factor of genetic penetrance of 60%.
However, according to our calculations there would be a total of 30 849 709 (95% CI 21 411 293–40 344 496) individuals with COPD in the 20 European countries analysed, with the ratio between PiZZ and COPD individuals being 0.12% (0.08–0.24%), and with wide differences among countries.
Finally, the estimated mean prevalence of patients with PiZZ at high risk among the subjects with COPD, expressed as 1/x, was of one PiZZ for every 850 patients with COPD (i.e. 1/850), with wide differences among geographical regions, specifically: 1/408, 1/944, 1/1051, 1/711 and 1/1274 in Northern, Western, Central, Southern and Eastern Europe, respectively.
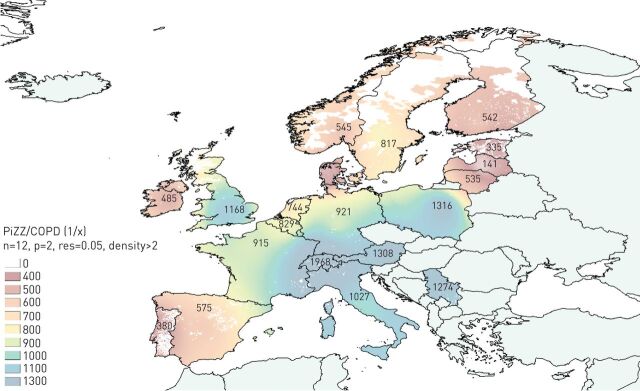
The results are numerically and graphically shown by means of a shaded red-blue scale on the IDW interpolation map in figure 1. With this methodology, virtually all regions of Western Europe, most of Central Europe, and only Serbia in Eastern Europe appear shaded. The highest prevalence (roughly between 1/400 and 1/800), shaded in reddish tones, is in Northern Europe, the Iberian Peninsula and Ireland. The coastal regions of Western Europe and northern Great Britain appear shaded in greenish-yellow tones indicative of an intermediate prevalence of about 1/800–1000. The remaining geographical areas of Eastern and Southern Europe are shaded in blue tones, indicative of a relatively low prevalence (1/1000–1300) (figure 1).
FIGURE 1.
Map of inverse distance weighted interpolation showing the PiZZ/COPD ratio (1/x) in 20 European countries. PiZZ refers to the number of individuals aged ≥40 years, after applying a correction factor of 60% of genetic penetrance. COPD refers to the total number of patients aged ≥40 years from each country. The colour scale corresponds to the prevalence of PiZZ in the total COPD (1/x) of each country. Countries without data are shaded pale green. Unpopulated or sparsely populated regions (with less than two inhabitants per square kilometre) are shaded white. PiZZ: α1-antitrypsin protease inhibitor ZZ. n=total number points with known values in IDW interpolation (12 for the current map); p=power parameter (p=2 for the current map); res=resolution (0.05 for the present map).
Discussion
Our tabulation confirms that there is a significant number of individuals carrying PiZZ genotypes (∼60% of whom are at high risk of developing COPD), as well as an impressive number of patients with COPD in the 20 European countries analysed. As a result of these numbers, the ratio between patients with PiZZ at high risk and COPD individuals ranged between 0.08% and 0.24%, with wide differences among countries. These numbers are based on extrapolations and are, therefore, a reflection of both the prevalence of specific PiZZ and the respective COPD among the population aged >40 years in each country.
Interestingly, several of our theoretical estimates were consistent with those reported by other researchers in different European countries in which real screening studies were conducted in samples constituted by non-selected subjects with COPD, but without clinical evidence or prior knowledge of the AAT levels, and in whom the diagnostic rate ranged from 0.0 to 0.75% (table 2). For example, a screening study in a sample of 1060 German patients with COPD, asthma or bronchiectasis provided a PiZZ diagnostic rate of 0.0% [23]. Similarly, two Spanish screening studies in two samples of 2137 and 596 patients with COPD provided diagnostic rates of 0.37 and 0.34% for PiZZ, respectively [24, 25]. More recently, a screening of non-selected samples of COPD patients, ranging from 1000 to 2000 individuals, from Romania, Hungary, Bulgaria, Lithuania, Latvia and Russia provided modest PiZZ detection rates of between 0.11% and 0.75% [26].
TABLE 2.
Results of screening and targeted detection (case finding) programmes for α1-antitrypsin protease inhibitor ZZ (PiZZ) in several European samples with >1000 individuals
| Country | Type of study | Sample size n | ZZ detection ratio % | First author [ref.] |
| Germany | COPD/asthma/bronchiectasis screening | 1156 | 0.0 | Wencker [23] |
| Spain | COPD screening | 2137 | 0.37 | De la Roza [24] |
| Spain | COPD screening | 596 | 0.34 | Molina [25] |
| Romania | COPD screening | 1858 | 0.11 | Greulich [26] |
| Hungary | COPD screening | 1657 | 0.18 | Greulich [26] |
| Bulgaria | COPD screening | 1474 | 0.20 | Greulich [26] |
| Lithuania | COPD screening | 1263 | 0.48 | Greulich [26] |
| Russia | COPD screening | 1070 | 0.75 | Greulich [26] |
| Latvia | COPD screening | 1052 | 0.38 | Greulich [26] |
| Italy | Case finding | 1841 | 6.4 | Luisetti [27] |
| Germany | Case finding | 2722 | 8.2 | Bals [28] |
| Ireland | Case finding | 13 500 | 1.4 | Carroll [29] |
| Germany | Case finding | 18 638 | 6.9 | Greulich [30] |
Conversely, several targeted case-finding studies focused on individuals with a high risk for AAT deficiency achieved much higher rates of AAT deficiency detection, ranging from 1.4% to 8.2% for PiZZ genotypes, depending on the inclusion criteria, the methodology used, the number of individuals investigated, and the geographic origin of the samples (table 2). Specifically, an Italian programme led by Luisetti et al. [27] tested a sample constituted by individuals with early-onset lung emphysema, familial cluster of COPD, absence of or reduced αl-globulin band on serum protein electrophoresis, AAT plasma levels <80 mg·dL−1, and first degree relatives of subjects with known AAT deficiency (including PiMZ heterozygotes) and found that of 1841 samples screened, 10.6% presented severe AAT deficiency (i.e. 118 PiZZ, 17 PiSZ, eight QO-QO, 4 Z-QO, and four “rare” variants). In addition, in Germany, Bals et al. [28] combined an awareness programme with a diagnostic test free of charge in 2696 subjects, who were preselected based on clinical suspicion or low serum levels of AAT, and identified 12% of individuals with severe AAT deficiency (i.e. 268 ZZ, 13 “rare” and three new deficiency genotypes). Later, Carroll et al. [29] screened 3000 Irish individuals as part of an Irish National Targeted Detection Programme and included subjects with COPD, non-responsive asthma, cryptogenic liver disease, first degree relatives of known AAT deficiency patients (including those with ZZ, SZ and MZ types) and individuals with abnormally low serum AAT levels, finding 42 ZZ genotypes (1.4%). Finally, Greulich et al. [30] analysed 18 638 testing kits and identified 1293 German PiZZ (6.9%) subjects, as well as 271 (1%) “rare” phenotypes. Their diagnostic approach combined an initial measurement of AAT serum levels and an allele-specific PCR for PiS and PiZ mutations, with further testing (phenotyping or gene sequencing) if deemed necessary.
Our calculations were based on epidemiological studies on the prevalence of COPD, in which cases were defined based on spirometric results, and most of the COPD cases identified corresponded to undiagnosed mild COPD. Therefore, our ratio of 0.12% is significantly lower than that observed in the previously mentioned studies, which included clinically diagnosed cases, the majority with moderate-to-severe COPD. One limitation of our study is the estimate of the penetrance of PI*ZZ in COPD. Since there is not a definitive number, we used the 60% suggested as the maximal penetrance in the American Thoracic Society/European Respiratory Society document [2]. In case of a lower penetrance of only 40%, the number of PI*ZZ with COPD in Europe would still be 24 198 with a ratio of 0.08%.
In 1997, the World Health Organization, and thereafter various scientific societies, recommended investigating AAT deficiency using a quantitative test in all patients with COPD and blood relatives of index cases and in other pathologies related to AAT deficiency [2, 11, 14]. Regrettably, despite these recommendations, the vast majority of subjects with AAT deficiency remain undiagnosed and, therefore remain without appropriate care and treatment.
Potential benefits of systematic screening include genetic counselling, lifestyle recommendations (such as smoking prevention or cessation, avoidance of high-risk occupations), augmentative therapy evaluation, and the possibility of taking advantage of future advances that can be provided by the various clinical trials currently under development. Potential limitations of targeted-screening programmes directed at subjects with a high suspicion of having AAT deficiency, such as those with early-onset COPD, basal bilateral panacinar emphysema, family history of α1-antitrypsin deficiency, neonatal cholestasis, cirrhosis of unknown cause, Wegener-type vasculitis, or neutrophilic panniculitis, are that these programmes may be reasonably effective but might result in missing asymptomatic subjects with severe AAT deficiency. In addition, potential harms include psychological effects, social discriminatory effects and costs. All these pros and cons of testing should be taken into account when patients are asked to sign an informed consent form to investigate AAT deficiency [11].
Remarkably, our IDW interpolation map revealed, for the first time, a close relationship between ZZ-AAT deficiency and COPD in Nordic countries, and also a less suspected high relationship between this rare genetic condition and COPD in Ireland and the Iberian Peninsula. In addition, there is also a non-negligible prevalence of ZZ-AAT deficiency in individuals with COPD from many other regions of Western Europe. This ratio is much smaller, albeit not negligible, in Central and Eastern Europe and, therefore, needs to be more extensively studied.
In conclusion, the original approach to the topic of the prevalence of PiZZ and COPD used in this study may provide some useful data to plan future strategies aimed at increasing the diagnosis of severe AAT deficiency in European subjects with COPD.
Footnotes
Author contributions: All the authors have contributed to the elaboration of the manuscript and have approved its final version. I. Blanco, I. Diego and M. Miravitlles are the guarantors of the paper.
Provenance: Submitted article, peer reviewed
Conflict of interest: I. Blanco has nothing to disclose.
Conflict of interest: I. Diego has nothing to disclose.
Conflict of interest: P. Bueno has nothing to disclose.
Conflict of interest: S. Pérez-Holanda has nothing to disclose.
Conflict of interest: F. Casas-Maldonado has nothing to disclose.
Conflict of interest: M. Miravitlles reports speakers fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Zambon, Sandoz, CSL Behring, Grifols and Novartis; consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Kamada, TEVA, Sanofi, pH Pharma, Novartis and Grifols; grants from GlaxoSmithKline and Grifols, outside the submitted work.
References
- 1.Hutchison DCS. Αlpha1-antitrypsin in Europe: geographical distribution of Pi types S and Z. Respir Med 1998; 92: 367–377. doi: 10.1016/S0954-6111(98)90278-5 [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society, European Respiratory Society . American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003; 168: 818–900. doi: 10.1164/rccm.168.7.818 [DOI] [PubMed] [Google Scholar]
- 3.Lomas DA, Evans DL, Finch JT, et al. The mechanism of Z α1 antitrypsin accumulation in the liver. Nature 1992; 357: 605–607. doi: 10.1038/357605a0 [DOI] [PubMed] [Google Scholar]
- 4.Piras B, Ferrarotti I, Lara B, et al. Characteristics of Italian and Spanish patients with alpha-1-antitrypsin deficiency: Identifying clinical phenotypes. Eur Respir J 2013; 42: 54–64. doi: 10.1183/09031936.00104712 [DOI] [PubMed] [Google Scholar]
- 5.McElvaney NG, Stoller JK, Buist AS, et al. Baseline characteristics of enrolees in the National Heart, Lung and Blood Institute Registry of α1-antitrypsin deficiency. Chest 1997; 111: 394–403. doi: 10.1378/chest.111.2.394 [DOI] [PubMed] [Google Scholar]
- 6.Tanash HA, Ekström M, Rönmark E, et al. Survival in individuals with severe alpha 1-antitrypsin deficiency (PiZZ) in comparison to a general population with known smoking habits. Eur Respir J 2017; 50: 1700198. doi: 10.1183/13993003.00198-2017 [DOI] [PubMed] [Google Scholar]
- 7.Stockley RA, Luisetti M, Miravitlles M, et al. Ongoing research in Europe: Alpha One International Registry (AIR) objectives and development. Eur Respir J 2007; 29: 582–586. doi: 10.1183/09031936.00053606 [DOI] [PubMed] [Google Scholar]
- 8.Esquinas C, Serreri S, Barrecheguren M, et al. Long-term evolution of lung function in individuals with alpha-1 antitrypsin deficiency from the Spanish Registry. Int J Chron Obstruct Pulmon Dis 2018; 13: 1001–1007. doi: 10.2147/COPD.S155226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janciauskiene S, DeLuca DS, Barrecheguren M, et al. Serum levels of alpha1-antitrypsin and their relationship with COPD in the general Spanish population. Arch Bronconeumol 2020; 56: 76–83. doi: 10.1016/j.arbres.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 10.Rigobello C, Baraldo S, Tinè M, et al. Exome sequencing reveals immune genes as susceptibility modifiers in individuals with α1-antitrypsin deficiency. Sci Rep 2019; 9: 13088. doi: 10.1038/s41598-019-49409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur Respir J 2017; 50: 1700610. doi: 10.1183/13993003.00610-2017 [DOI] [PubMed] [Google Scholar]
- 12.Esquinas C, Janciauskiene S, Gonzalo R, et al. Gene and miRNA expression profiles in PBMCs from patients with severe and mild emphysema with PiZZ alpha1-antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis 2017; 12: 3381–3390. doi: 10.2147/COPD.S145445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrecheguren M, Miravitlles M. Augmentation therapy for emphysema due to alpha-1 antitrypsin deficiency: Pro. Arch Bronconeumol 2018; 54: 363–364. doi: 10.1016/j.arbres.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 14.Alpha-1-antitrypsin deficiency: memorandum from a WHO meeting. Bull World Health Organ 1997; 75: 397–415. [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberman J, Winter B, Sastre A. Alpha1-antitrypsin Pi-types in 965 COPD patients. Chest 1986; 89: 370–373. doi: 10.1378/chest.89.3.370 [DOI] [PubMed] [Google Scholar]
- 16.Greulich T, Vogelmeier CF. Alpha-1-antitrypsin deficiency: increasing awareness and improving diagnosis. Ther Adv Respir Dis 2016; 10: 72–84. doi: 10.1177/1753465815602162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco I, Bueno P, Diego I, et al. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int J Chron Obstruct Pulmon Dis 2017; 12: 561–569. doi: 10.2147/COPD.S125389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco I, Diego I, Bueno P, et al. Geographical distribution of COPD prevalence in Europe, estimated by an inverse distance weighting interpolation technique. Int J Chron Obstruct Pulmon Dis 2018; 13: 57–67. doi: 10.2147/COPD.S150853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco I, Diego I, Bueno P, et al. Geographic distribution of COPD prevalence in the world displayed by Geographic Information System maps. Eur Respir J 2019; 54: 1900610. doi: 10.1183/13993003.00610-2019 [DOI] [PubMed] [Google Scholar]
- 20.Eurostat . Population (demography, migration and projections). https://ec.europa.eu/eurostat/web/population-demography-migration-projections/data/database Date last accessed:15 January 2020; date last updated: 23 April 2020.
- 21.Blanco I, Fernández E, Rodríguez MC, et al. Allelic frequency of the gene of alpha-1-antitrypsin in the general population in a county in Asturias. Med Clin (Barc) 1999; 113: 366–370. [PubMed] [Google Scholar]
- 22.Blanco I. A well-designed/conducted study on alpha-1 antitrypsin epidemiology not quoted. Eur Respir J 2018; 51: 1702662. doi: 10.1183/13993003.02662-2017 [DOI] [PubMed] [Google Scholar]
- 23.Wencker M, Marx A, Konietzko N, et al. Screening for alpha1-Pi deficiency in patients with lung diseases. Eur Respir J 2002; 20: 319–324. doi: 10.1183/09031936.02.02012001 [DOI] [PubMed] [Google Scholar]
- 24.de la Roza C, Rodríguez-Frías F, Lara B, et al. Results of a case-detection programme for alpha1-antitrypsin deficiency in COPD patients. Eur Respir J 2005; 26: 616–622. doi: 10.1183/09031936.05.00007305 [DOI] [PubMed] [Google Scholar]
- 25.Molina J, Flor X, Garcia R, et al. The IDDEA project: a strategy for the detection of alpha-1 antitrypsin deficiency in COPD patients in the primary care setting. Ther Adv Respir Dis 2011; 5: 237–243. doi: 10.1177/1753465811404919 [DOI] [PubMed] [Google Scholar]
- 26.Greulich T, Averyanov A, Borsa L, et al. European screening for alpha1-antitrypsin deficiency in subjects with lung disease. Clin Respir J 2017; 11: 90–97. doi: 10.1111/crj.12310 [DOI] [PubMed] [Google Scholar]
- 27.Luisetti M, Massi G, Massobrio M, et al. A national program for detection of alpha 1-antitrypsin deficiency in Italy. Gruppo I.D.A. Respir Med 1999; 93: 169–172. doi: 10.1016/S0954-6111(99)90003-3 [DOI] [PubMed] [Google Scholar]
- 28.Bals R, Koczulla R, Kotke V, et al. Identification of individuals with alpha-1-antitrypsin deficiency by a targeted screening program. Respir Med 2007; 101: 1708–1714. doi: 10.1016/j.rmed.2007.02.024 [DOI] [PubMed] [Google Scholar]
- 29.Carroll TP, O'Connor CA, Floyd O, et al. The prevalence of alpha-1 antitrypsin deficiency in Ireland. Respir Res 2011; 12: 91. doi: 10.1186/1465-9921-12-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greulich T, Nell C, Herr C, et al. Results from a large targeted screening program for alpha-1-antitrypsin deficiency: 2003–2015. Orphanet J Rare Dis 2016; 11: 75. doi: 10.1186/s13023-016-0453-8 [DOI] [PMC free article] [PubMed] [Google Scholar]