Abstract
Primary ciliary dyskinesia (PCD) is a rare genetic disease that affects the motility of cilia, leading to impaired mucociliary clearance. It is estimated that the vast majority of patients with PCD have not been diagnosed as such, providing a major obstacle to delivering appropriate care. Challenges in diagnosing PCD include lack of disease-specific symptoms and absence of a single, “gold standard”, diagnostic test. Management of patients is currently not based on high-level evidence because research findings are mostly derived from small observational studies with limited follow-up period. In this review, we provide a critical overview of the available literature on clinical care for PCD patients, including recent advances. We identify barriers to PCD research and make suggestions for overcoming challenges.
Short abstract
Challenges in PCD must be overcome through international collaboration; networks must build on recent advances http://ow.ly/4d4I30dXzWg
Introduction
Primary ciliary dyskinesia (PCD) is a heterogeneous genetic disease characterised by abnormal ciliary function. Impaired mucociliary clearance due to abnormal function of epithelial cilia in lungs, paranasal sinuses and ears leads to recurrent and chronic infections of the upper and lower airways. Clinical presentation of PCD is heterogeneous, and patients present different combinations of symptoms, some of which vary over time [1, 2]. Moreover, the symptoms are nonspecific, contributing to the challenge for general clinicians to identify patients to refer to specialist units for diagnostic testing. However, the pattern and combination of symptoms can be strongly suggestive [3, 4].
Symptoms usually occur within the first few hours after birth, with over 70% of PCD patients suffering from unexplained neonatal respiratory symptoms (NRS) [3, 5, 6]. Persistent wet cough is one of the most frequently reported symptoms [1, 3]. Chronic suppurative pulmonary infections lead to irreversible lung damage, with bronchiectasis seen in both children and adults [1]. Recurrent otitis media is often present at an early age [7] and can lead to significant hearing loss and speech impairment [8].
Approximately half of PCD patients have complete mirror image of major organs situs (situs inversus) [9, 10]. This occurs due to disruption of embryonic nodal flow, which is responsible for determining left–right symmetry [11]. Patients with heterotaxy (or situs ambiguous) often present with congenital heart disease [12]. The sperm flagella and the fimbriae of fallopian tubes exhibit a similar structure to respiratory cilia, with studies reporting fertility problems in men and women [1, 13, 14].
Prevalence is estimated to be around 1:10 000, and higher in consanguineous populations [15, 16], although true prevalence of the disease is unknown and many patients remain undiagnosed [17].
There are a number of recent reviews on the diagnosis and management of PCD [18–27]. This review aims to provide a critical evaluation of the literature, identifying obstacles to patient care and suggesting future directions for PCD research and clinical care.
Referral for testing
A major challenge is identifying patients to refer for diagnostic testing. PCD centres are highly specialised, making geographical access a problem. Referral of appropriate patients for testing is therefore a crucial step on the diagnostic pathway.
There is a lack of awareness of PCD by general practitioners and paediatricians. An international survey of 271 PCD patients reported 37% had over 40 visits to medical professionals due to PCD-related symptoms before being referred for testing [28]. The most prevalent symptoms in PCD are not disease specific; perhaps not surprisingly, patients with situs inversus, a rare condition in the general population [29], are diagnosed at an earlier age [17].
The development of screening tests and predictive tools is paramount to improve accurate identification of patients for diagnostic testing. The recently published European Respiratory Society (ERS) guidelines for the diagnosis of PCD provide recommendations on patient referrals [30]. Simple and easy-to-use predictive tools that provide disease probability scores, such as PICADAR (PrImary CiliARy DyskinesiA Rule) [3], and expert-defined clinical symptoms scores [10] will probably improve referrals from secondary care but require validation in different settings [26].
Despite the majority of patients presenting with NRS [5], studies have shown that neonates are rarely diagnosed with PCD [2, 17], nor are they referred early for testing [28]. Mullowney et al. [6] demonstrated that the combination of lobar collapse, situs inversus and/or persistent oxygen therapy for over 2 days in term newborns with respiratory distress can accurately predict PCD. The diagnosis should also be considered in term neonates with normal situs.
A recently published meta-analysis highlighted variations on the reporting of clinical manifestations in PCD in different studies [1]. Despite reports of over 70% PCD patients suffering from NRS, the review found only 33% of the studies described NRS. It is unclear if these findings reflect limitations in data collection by the authors, issues in recording and reporting these symptoms by clinicians, or a combination of both. Symptoms described in the majority of studies lacked standardisation, limiting comparisons between them.
A well-defined referral pathway is essential to guarantee patient access to diagnostic testing. A Europe-wide survey found that differences in referral patterns have an impact upon disease prevalence [17]. For example, the active search for potential PCD cases in Cyprus led to a higher than average disease prevalence.
PCD centres can centralise the diagnosis and management of PCD patients, with the majority of patients willing to travel longer distances to have access to specialised tests and care [28]. In rare disorders, it is essential to have a high throughput of cases for appropriate training of healthcare professionals and diagnostic scientists. Research in PCD has shown that centres managing over 20 patients are associated with diagnosis at an earlier age [17]. It is anticipated that centralised care will provide access for patients to expert centres using standardised protocols with quality assurance, such as the European Reference Network ERN-LUNG (http://ern-lung.eu) [31] and the North American Genetic Disorders of Mucociliary Clearance Consortium.
PCD diagnosis
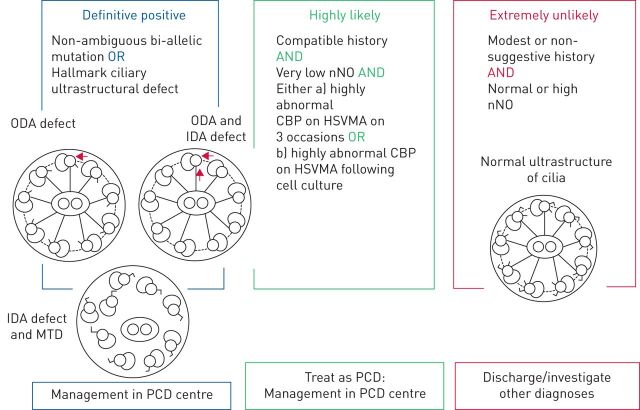
In the absence of a “gold standard” test, diagnosis is reached through a combination of PCD-specific tests. The recently published ERS guidelines provide evidence-based recommendations on PCD diagnosis and offer an algorithm for diagnostic testing, which should be based on clinical symptoms, nasal nitric oxide (nNO) assessment, high-speed video microscopy analysis (HSVMA), transmission electron microscopy (TEM) and genotyping [30]. Importantly, the guidelines provide a hierarchal classification to determine diagnostic certainty based on tests performed and results (figure 1) [30]. Asserting the diagnostic certainty is important to ensure correct enrolment of confirmed cases in prospective cohorts and randomised clinical trials (RCTs).
FIGURE 1.
Primary ciliary dyskinesia (PCD) classification according to the European Respiratory Society guidelines for the diagnosis of PCD [30]. ODA: outer dynein arm; IDA: inner dynein arm; MTD: microtubular disarrangement; nNO: nasal nitric oxide; CBP: ciliary beat pattern; HSVMA: high-speed video microscopy analysis.
The availability of diagnostic tests varies between countries [17], and it might not be feasible to reach a definite diagnosis in centres with few resources due to financial limitations or lack of technical expertise [32]. Over 20% of patients included in the PCD Registry and 10% of studies included in a recent meta-analysis had diagnosis based on suggestive clinical history only, without diagnostic test confirmation [1, 33].
Standardising reporting on diagnostic testing
Although advances have been made to standardise the diagnostic pathway [30], improvements are needed on the conduct and reporting of results from diagnostic tests. Cut-off values vary considerably between centres, as they depend on local expertise, equipment, techniques, and laboratory and sample conditions [34].
For example, nNO has been extensively investigated as a screening test, because levels are extremely low in patients with PCD [35, 36]. A technically acceptable plateau of nNO levels is obtained using a stationary chemiluminescense analyser, preferably during a velum closure manoeuvre. However, a recent meta-analysis found high levels of heterogeneity between studies, reflecting differences in study design, age, control group and breathing techniques [36].
Equally important is the selection of appropriate cut-off values that can be applied to different settings, analysers and populations. Leigh et al. [37] have shown that 77 nL·min−1 is a disease-specific discriminatory cut-off value reproducible in multiple sites when using a standardised protocol. However, there is still no consensus on appropriate thresholds, particularly for children <6 years of age. The ERS guidelines argue that nNO should not be used in isolation or as a general screening test, as it would perform poorly in populations with low prevalence of PCD (e.g. primary and secondary care settings) [36], but instead should contribute to the final diagnostic decision [30].
HSVMA is the only diagnostic test in common use to directly assess ciliary function. Ciliary beat frequency and pattern are analysed by experts using video images recorded by a camera attached to a microscope. Researchers have questioned the validity of HSVMA as ciliary beat pattern analysis is observer dependent [38], but recent studies have shown associations between specific changes in ciliary beat pattern, hallmark ultrastructural defects and genotype [39, 40]. For example, immotile cilia are seen in patients with combined absence of outer dynein arms (ODA) and inner dynein arms (IDA) (e.g. ZMYND10), while isolated ODA absence (e.g. DNAH5) is associated with residual movement and vast areas of static cilia [39, 40]. Circular beating from a top view is present in those with transposition defects [40]. While “typical” cases (e.g. immotile or circular cilia) are relatively easy to detect, subtle changes are difficult to differentiate from secondary defects by HSVMA and are only likely to be picked up by specialised scientists in high-throughput centres and even then risk being missed. There are significant challenges in standardising HSVMA testing and in using appropriate terminology for different beat patterns [41], so it is important to address these in order to generate comparable data.
TEM is used to visualise respiratory cilia ultrastructure in transverse sections of chemically fixed epithelium. TEM was once the “gold standard” for PCD diagnosis. However, it is now known that 15–20% of PCD cases have disease-causative mutations but normal ultrastructure by TEM [30, 39, 41–43]. A quantitative approach to cilia evaluation is recommended, but there is no agreement on the minimal number of cilia scored or on the terminology used for ultrastructural defects.
Pathogenic bi-allelic mutations in over 30 genes have been published to date [30]. These are estimated to be responsible for 60–70% of PCD cases [25, 38, 44] and, as new genes are discovered, this is likely to increase. Candidate gene analysis is still the main tool for gene identification, but next-generation sequencing technologies might offer a greater insight once technical challenges are overcome [16]. Yet, the role of genetic testing in the diagnostic pathway is still unclear. Most studies to date have focused on discovering novel genes and therefore were conducted in small cohorts with PCD already confirmed by TEM. Genetic testing can undoubtedly provide a diagnosis in cases where HSVMA and TEM are normal or subtly abnormal, e.g. DNAH11 and CCDC103 [42, 45]. It is imperative that future studies are designed to assess the use of genetic screening as a diagnostic tool.
Immunofluorescence is based on the visualisation of fluorescence-labelled antibodies specific for cilia proteins in epithelial cells. Antibodies targeting ODA, IDA, radial spoke head and dynein regulatory complex proteins are available but do not cover all known ultrastructural defects. Shoemark et al. [46] recently published the first diagnostic study assessing immunofluorescence accuracy, which showed that immunofluorescence correctly identified 22 out of 25 PCD patients and all negative controls. These findings strongly suggest that immunofluorescence can play an important role in the diagnostic pathway but there is need for a wider range and better quality of antibodies, and standardisation of immunofluorescence conduct and reporting.
Electron microscopy tomography is a computer-assisted TEM technique that allows for the generation of three-dimensional images, providing high-resolution projections of cilia ultrastructure. There have been no diagnostic studies to date, but Shoemark and Hogg [43] have shown that patients with mutations in HYDIN or DNAH11, which are difficult to diagnose using standard TEM [42, 47], can be identified using electron microscopy tomography.
PCD management
Similar to other rare diseases, the evidence base for treating patients with PCD is poor. Therefore, the choice of management strategy is based on expert consensus and the clinician's personal experience [48].
There are two expert consensus statements on PCD management: the 2009 ERS Task Force Consensus in Children [48] and the 2016 North American PCD Foundation Consensus [12], neither of which are evidence based. Both recommend regular follow-up visits at least every 6 months, conducted in specialist centres with multidisciplinary teams experienced in managing chronic suppurative lung disease.
Lower airway management
Respiratory management is largely extrapolated from cystic fibrosis (CF) and non-CF bronchiectasis, despite differences of PCD pathophysiology, prognosis and response to treatment [49, 50]. The cornerstones of lower airway management are regular monitoring of lung function, pulmonary physiotherapy coupled with exercise, and active treatment of infections with antibiotics.
Pulmonary monitoring usually combines lung function tests, sputum microbiology cultures and chest imaging. Spirometry-derived forced expiratory volume in 1 s (FEV1) is used to monitor disease progression in most respiratory diseases, with chest radiography reserved for acute episodes or occasional monitoring.
Studies have questioned the sensitivity of FEV1 to monitor disease progression and to detect early lung damage. Marthin et al. [51] found a high degree of variation in FEV1 decline, unrelated to age at diagnosis or baseline FEV1. A recent study on adults with PCD found a steeper annual decline of FEV1 in women and in patients chronically infected with Pseudomonas aeruginosa [52]. The combination of physiotherapy and treatment of pulmonary exacerbations with antibiotics might lead to recovery of baseline FEV1 and prevent progression of lung damage, but the results are contradictory [53–55]. These findings suggest that pulmonary function decline is heterogeneous and might be associated with different genetic mutations, gene modifiers or environmental influences. However, conclusions are based on small studies with different designs, populations, outcome measures, diagnostic methods and definitions of pulmonary exacerbation. It is therefore imperative to apply standardised definitions where available (e.g. diagnosis based on ERS guidelines) and develop clear definitions where necessary (e.g. pulmonary exacerbation in PCD patients).
High-resolution computed tomography (HRCT) can detect a variety of abnormalities in PCD patients early in life. Radiation risk restricts the use of HRCT for regular monitoring of PCD where patients may have normal life expectancy; however, HRCT can detect bronchiectasis at an early age before changes in lung function or chest radiography occur. Radiation risks need to be balanced against the benefits of identifying early airway changes, but the data on which to base the assessments are limited. Correlations between bronchiectasis severity scores derived from HRCT and FEV1 have produced conflicting results [56–58]. Future studies must avoid selection bias, as patients who have routine imaging tests tend to have more severe disease. For example, Kennedy et al. [56] found that all adults and over half of children had bronchiectasis, and 27% had undergone lobectomy. Lung clearance index (LCI) derived from multiple breath washout is a radiation-free candidate for disease monitoring and seems to be particularly sensitive for detecting early lung disease compared to FEV1 [59]. However, correlations between LCI and HRCT have also shown opposing results, and longitudinal data are lacking [58, 60].
There is no consensus on a definition for pulmonary exacerbation in PCD. Most experts recommend prescribing oral antibiotics if there is worsening of respiratory symptoms or deterioration of lung function [44]. Both European and North American consensus statements recommend the following at every patient visit: 1) collection of sputum culture or oropharyngeal cough swabs for infection surveillance and 2) evaluation of lung function to monitor disease progression [12, 48]. Choice of antibiotic should be directed by culture results, when available. A recent study found age-dependent changes in bacterial pathogens in the lower respiratory tract, with Haemophilus influenzae and Moraxella catarrhalis being more frequent in children and P. aeruginosa in teenagers and adults [61]. Antibiotic prophylaxis should be considered if repeated courses of antibiotics are required [12, 48], but prospective studies on the use of prophylaxis [62] and on different prophylaxis regimes are needed.
Experts recommend airway clearance through physiotherapy and exercise, but there is no evidence or consensus on the most effective physiotherapy technique, with practice varying between centres [48]. Routine use of inhaled β-agonists is not recommended in PCD, as there is no evidence to suggest benefits [63]. Dornase alfa promotes clearance of secretions by reducing mucus viscosity in the airways in CF management [64]. However, mucus fluidity is usually conserved in PCD and there is only low-level evidence for its use, derived solely from case studies with short follow-up [65]. Equally, there is no evidence for the use of hypertonic saline in PCD. A recently published double-blind crossover RCT did not show any clinically significant difference in quality of life (QoL) between patients on 7% hypertonic saline and isotonic saline [66]. However, it was underpowered, the primary outcome was not disease specific, and the control group received nasal rinsing with saline, which might have some therapeutic benefit in PCD [67].
Upper airway management
Literature on the management of hearing loss and chronic rhinosinusitis in PCD is scarce. Outcome measures are rarely defined and mostly derived from a limited number of old retrospective studies with few patients [8, 68–70].
Management of otitis media aims to improve hearing levels and delays to language and academic development. Current options include antibiotic therapy, hearing aids and myringotomy with or without insertion of ventilation tubes (VTs), with the main debate revolving around the latter [8]. The European consensus guidelines advised against VT use in children, based on low-level evidence derived from small studies reporting offensive otorrhoea after surgery [8, 48]. The recent North American consensus guidelines, conversely, highlighted the poor evidence available on VT insertion and did not advocate against their use in PCD. Instead, they recommend counselling on the likelihood of repeated VT insertions after initial placement and post-operative otorrhoea [12].
Two recent retrospective studies assessed hearing outcomes in children with PCD. Wolter et al. [71] reported that hearing levels were maintained in a third of those treated conservatively (i.e. antibiotics or hearing aids) and were restored in 80% of those in whom VTs were inserted, with the groups achieving similar hearing thresholds. However, a direct comparison between management strategies was not possible as patients with worse baseline hearing thresholds systematically received surgical intervention. Prulière-Escabasse et al. [72] observed a decrease in acute otitis media and an increase in chronic otitis media with age. Persistent otitis media with effusion was prevalent (over 80%) throughout all age groups. VTs were inserted in half of the children, of which a further half required repeated replacements afterwards. Conductive deafness, defined as mean air conduction threshold ≥25 dB, decreased with age but was still considerably high (46%) for those aged 12–17 years.
There is no clear consensus on the best treatment option for chronic rhinosinusitis in PCD [12, 48]. Sinus irrigation is a safe, cheap and normally well tolerated treatment option, but studies are needed to assess its effectiveness in PCD. Similar to CF, the sinuses might act as a bacterial reservoir in PCD [73] and therefore bacterial eradication through endoscopic sinus surgery might prove beneficial. Alanin et al. [74] reported a significant improvement in health-related QoL SNOT-22 score after endoscopic sinus surgery and adjuvant treatment (i.e. saline nasal rinsing, topical steroids and 2 weeks of antibiotic treatment).
Cardiac management
Patients with heterotaxy experience increased post-operative mortality [75] and risk for respiratory complications [76], associated with ciliary dysfunction in 42% of cases [77]. The inference is that at least some of these patients had undiagnosed PCD, suggesting that they might benefit from routine pre-surgical screening for PCD and intense peri-surgical respiratory management [77].
Studies have used different definitions and classification systems for heterotaxy [12, 78–83], despite standardisation efforts [84]. While some authors group situs inversus and situs ambiguous under the umbrella of heterotaxy [82], others believe it should be restricted to situs ambiguous only [2, 81, 83, 84]. Using the latter definition, Kennedy et al. [83] reported that over 6% of PCD patients presented with heterotaxy. This is believed to be an underestimation, as abdominal imaging and echocardiograms are not carried out routinely in PCD patients.
Fertility
Prevalence of infertility and subfertility in PCD is still unknown. A systematic review suggested that 58% of women and 100% of men were infertile, but the included studies were highly heterogeneous [1]. Munro et al. [85] reported azoospermia and oligospermia in a small sample size, with normal motility seen in only one patient. Raidt et al. [86] demonstrated that the motor protein composition of fallopian tube cilia is similar to that of respiratory cilia.
Management strategies
A European survey reported wide variations between management strategies for PCD, highlighting differences between countries and within European regions [87]. The number of patients treated per centre was the most important predictor of choice of management strategy. Centres with more PCD patients favoured well-established treatments such as airway clearance, while smaller centres were more likely to prescribe inhaled corticosteroids, a therapy that is not routinely recommended in PCD [48].
Centralised delivery of care has improved patient outcomes in CF, including mitigating the severity of pulmonary disease [88], and a similar strategy has been adopted for PCD in many countries. In the UK, for example, four PCD management services have been commissioned since 2013 [89]. Expert multidisciplinary reference centres working together through international networks are starting to optimise diagnostic and clinical management [31]. The optimal number of centres per country is not known, but estimates suggest that, in a region of 10 million people, there should be 10–12 new PCD cases every year [26].
Outcome measures
There is a lack of validated outcome measures for PCD. Health-related QoL, measured through the age-specific questionnaire QOL-PCD, is the only validated PCD-specific outcome so far [90–92]. The US Food and Drug Administration and the European Medicines Agency promote the use of patient-reported outcome measures such as QOL-PCD in clinical trials, assessing the impact of the disease on the patient's daily symptoms and functioning [93–95].
Furthermore, definitions of important outcomes can vary considerably, hampering comparisons [34]. Pulmonary exacerbation, for example, has been defined in one study as any change in respiratory status that requires intravenous antibiotic treatment [53], and in another as the need for systemic antibiotics and/or a decline of FEV1 % predicted of ≥10% [62]. Treatment efficacy and prognosis are largely derived from a handful of small longitudinal studies [53], while most observational studies use a cross-sectional design. The first adequately powered RCT in PCD is investigating the efficacy and safety of azithromycin prophylaxis, and the results should be available soon [62]. A phase 2 study is evaluating the safety and efficacy of a sodium channel inhibitor, VX-371, in subjects with PCD (ClinicalTrials.gov identifier NCT02871778).
The paucity of research studies for PCD reflects some of the challenges in undertaking good-quality clinical research in rare diseases. There is, by definition, a small number of patients suffering from the disease, and they are spread over a large geographical area. This is particularly concerning for RCTs, as conditions in different centres vary considerably [96]. Additionally, there is a lack of investment in the development of novel disease-specific therapies due to a reduced “potential market”, as the drug development process requires large initial investments.
Potential future therapies
In CF, early results from studies investigating the use of small-molecule compounds that target the underlying defect have been encouraging [97–99]. However, RCTs in gene therapy for CF have so far failed to demonstrate clinical effectiveness [100]. Artificially engineered nucleases for DNA editing and antisense oligonucleotides for RNA editing might offer a better option for future therapy, but studies are at the pre-clinical stage.
The discovery and development of therapies that can potentially rectify the basic disease defect or the resulting defective proteins in CF might serve as a model for other genetic disorders. However, the challenge is considerably higher in PCD, as it is caused by mutations in more than 30 genes, many still unidentified, and it is genetically more heterogeneous than CF.
Pushing the pipeline for new therapies requires a coordinated approach from scientists, clinicians and industry. BEAT-PCD (Better Experimental Approaches to Treat Primary Ciliary Dyskinesia) (www.beatpcd.org) is an example of an international network including experts from diverse clinical specialties (e.g. paediatric and adult pulmonology, ear, nose and throat, and physiotherapy) and multidisciplinary scientists (e.g. genetics, imaging, cell biology, microbiology, and bioinformatics), working with industrial partners. BEAT-PCD is supported by the European Union Framework Programme Horizon 2020 (COST Action BM1407), with the aim of identifying gaps in knowledge and then facilitating PCD-related research to identify mechanisms, study disease patterns and progression, define outcome measures, improve clinical management and identify high-priority therapies [101]. In the USA, the PCD Foundation (www.pcdfoundation.org) is promoting the advance of PCD through annual conferences, community education, patient advocacy and research funding.
A summary of current knowledge, recent advances and remaining barriers in PCD, with suggestions for future research and implementation of findings, is shown in table 1.
TABLE 1.
Summary of current knowledge, recent advances and remaining barriers in primary ciliary dyskinesia (PCD), with suggestions for future research and implementation of findings
| Current knowledge | Recent advances | Barriers | Possible action points | |
| Access to service | Numerous visits to medical professionals before appropriate referral [28] | Development of screening through clinical predictive tools [3, 10]; ERS guidelines with clear patient referral criteria [30] |
Clinical predictive tools developed in specialist setting; adoption of referral criteria by GPs and general paediatricians |
Validation of screening tools in appropriate settings; dissemination to nonspecialists through support groups, conferences and media |
| Early diagnosis | Majority of PCD patients present with unexplained NRD syndrome | Studies investigating combination of neonatal symptoms to predict PCD [6] | Limited literature on neonatal symptoms in PCD | Increase involvement of neonatologists in collaborative PCD studies |
| Clinical manifestations | Limited knowledge on prevalence of symptoms | Meta-analysis reporting prevalence of clinical manifestations [1] | Lack of standardised definitions for symptoms; lack of reporting of symptoms |
Develop definition of PCD symptoms; improve reporting of symptoms in future studies |
| Diagnostic testing | Recommendations on diagnostic testing | Classification system derived from the ERS guidelines on diagnostic testing for PCD [30] | Lack of standardisation on test reporting; no diagnostic studies on genotyping |
Develop protocols for diagnostic test reporting; clarify role of genetics, IF and EMT on diagnostic pathway |
| PCD management | Mainly extrapolated from CF and non-CF bronchiectasis [12, 48] | Limited number of prospective cohorts [34, 54, 55, 74] and RCTs [62, 63, 66] | Lack of standardised and validated outcome measures; no definition for commonly used terms |
Develop clear definition of terms and outcome measures |
| Future therapies | New advancements in CF on molecular and gene therapy [97, 100] | Approval of two new therapies targeting the underlying defect in CF [98, 99] | Complexity of PCD genetics | Improve gene identification; genotype–phenotype correlations |
ERS: European Respiratory Society; GP: general practitioner; NRD: neonatal respiratory distress; IF: immunofluorescence; EMT: electron microscopy tomography; CF: cystic fibrosis; RCTs: randomised controlled trials.
Conclusion
Significant advances have been made in PCD diagnosis in the last decade, culminating with the publication of the first evidence-based diagnostic guidelines. In PCD management, however, there is still no evidence for the different strategies currently employed. The shortage of validated standardised outcome measures hampers comparisons between studies, limiting conclusions based on the currently limited literature available.
Future PCD studies in diagnosis and, particularly, management will benefit immensely from collaborative partnerships between specialised multidisciplinary healthcare professionals from different countries. The Europe-led BEAT-PCD and the North American Genetic Disorders of Mucociliary Clearance Consortium provide the ideal platform to enable collaborative international studies, allowing for pooling of PCD populations and the generation of shareable and meaningful results. It is therefore imperative that PCD experts, facilitated by these networks, develop consensus definitions for commonly used terms in PCD and validate outcomes for large prospective longitudinal studies and RCTs.
Disclosures
J. Lucas ERR-0023-2017_Lucas (1.2MB, pdf)
Footnotes
Support statement: The National PCD Centre in Southampton is commissioned and funded by NHS England. Research in Southampton is supported by NIHR Southampton Biomedical Research Centre, NIHR Wellcome Trust Clinical Research Facility and The AAIR Charity (Reg. No. 1129698). J.S. Lucas and B. Rubbo are members of EU-funded COST Action BEAT-PCD (BM1407).
Conflict of interest: Disclosures can be found alongside this article at err.ersjournals.com
Provenance: Publication of this peer-reviewed article was sponsored by Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, Germany (principal sponsor, European Respiratory Review issue 145).
References
- 1.Goutaki M, Meier AB, Halbeisen FS, et al. Clinical manifestations in primary ciliary dyskinesia: systematic review and meta-analysis. Eur J Respir 2016; 48: 1081–1095. [DOI] [PubMed] [Google Scholar]
- 2.Boon M, Jorissen M, Proesmans M, et al. Primary ciliary dyskinesia, an orphan disease. Eur J Pediatr 2013; 172: 151–162. [DOI] [PubMed] [Google Scholar]
- 3.Behan L, Dimitrov BD, Kuehni CE, et al. PICADAR: a diagnostic predictive tool for primary ciliary dyskinesia. Eur J Respir 2016; 47: 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leigh MW, Ferkol TW, Davis SD, et al. Clinical features and associated likelihood of primary ciliary dyskinesia in children and adolescents. Ann Am Thorac Soc 2016; 13: 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noone PG, Leigh MW, Sannuti A, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 2004; 169: 459–467. [DOI] [PubMed] [Google Scholar]
- 6.Mullowney T, Manson D, Kim R, et al. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics 2014; 134: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer JU, Schafer K, Omran H, et al. ENT manifestations in patients with primary ciliary dyskinesia: prevalence and significance of otorhinolaryngologic co-morbidities. Eur Arch Otorhinolaryngol 2011; 268: 383–388. [DOI] [PubMed] [Google Scholar]
- 8.Campbell RG, Birman CS, Morgan L. Management of otitis media with effusion in children with primary ciliary dyskinesia: a literature review. Int J Pediatr Otorhinolaryngol 2009; 73: 1630–1638. [DOI] [PubMed] [Google Scholar]
- 9.Aylsworth AS. Clinical aspects of defects in the determination of laterality. Am J Med Genet 2001; 101: 345–355. [PubMed] [Google Scholar]
- 10.Leigh MW, Pittman JE, Carson JL, et al. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med 2009; 11: 473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirokawa N, Tanaka Y, Okada Y. Left–right determination: involvement of molecular motor KIF3, cilia, and nodal flow. Cold Spring Harb Perspect Biol 2009; 1: a000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro AJ, Zariwala MA, Ferkol T, et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol 2016; 51: 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliasson R, Mossberg B, Camner P, et al. The immotile-cilia syndrome. N Engl J Med 1977; 297: 1–6. [DOI] [PubMed] [Google Scholar]
- 14.Afzelius BA, Eliasson R. Male and female infertility problems in the immotile-cilia syndrome. Eur J Repir Dis Suppl 1983; 127: 144–147. [PubMed] [Google Scholar]
- 15.O'Callaghan C, Chetcuti P, Moya E. High prevalence of primary ciliary dyskinesia in a British Asian population. Arch Dis Child 2010; 95: 51–52. [DOI] [PubMed] [Google Scholar]
- 16.Zariwala MA, Omran H, Ferkol TW. The emerging genetics of primary ciliary dyskinesia. Proc Am Thorac Soc 2011; 8: 430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuehni CE, Frischer T, Strippoli MP, et al. Factors influencing age at diagnosis of primary ciliary dyskinesia in European children. Eur Respir J 2010; 36: 1248–1258. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald DA, Shapiro AJ. When to suspect primary ciliary dyskinesia in children. Paediatr Respir Rev 2016; 18: 3–7. [DOI] [PubMed] [Google Scholar]
- 19.Lucas JS, Paff T, Goggin P, et al. Diagnostic methods in primary ciliary dyskinesia. Paediatr Respir Rev 2016; 18: 8–17. [DOI] [PubMed] [Google Scholar]
- 20.Horani A, Ferkol TW, Dutcher SK, et al. Genetics and biology of primary ciliary dyskinesia. Paediatr Respir Rev 2016; 18: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison MJ, Shapiro AJ, Kennedy MP. Congenital heart disease and primary ciliary dyskinesia. Paediatr Respir Rev 2016; 18: 25–32. [DOI] [PubMed] [Google Scholar]
- 22.Morgan LC, Birman CS. The impact of primary ciliary dyskinesia on the upper respiratory tract. Paediatr Respir Rev 2016; 18: 33–38. [DOI] [PubMed] [Google Scholar]
- 23.Polineni D, Davis SD, Dell SD. Treatment recommendations in primary ciliary dyskinesia. Paediatr Respir Rev 2016; 18: 39–45. [DOI] [PubMed] [Google Scholar]
- 24.Daniels ML, Noone PG. Genetics, diagnosis, and future treatment strategies for primary ciliary dyskinesia. Expert Opin Orphan Drugs 2015; 3: 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowles MR, Daniels LA, Davis SD, et al. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med 2013; 188: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuehni CE, Lucas JS. Toward an earlier diagnosis of primary ciliary dyskinesia. Which patients should undergo detailed diagnostic testing? Ann Am Thorac Soc 2016; 13: 1239–1243. [DOI] [PubMed] [Google Scholar]
- 27.Honoré I, Burgel PR. Primary ciliary dyskinesia in adults. Rev Mal Respir 2016; 33: 165–189. [DOI] [PubMed] [Google Scholar]
- 28.Behan L, Dunn Galvin A, Rubbo B, et al. Diagnosing primary ciliary dyskinesia: an international patient perspective. Eur Respir J 2016; 48: 1096–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin AE, Krikov S, Riehle-Colarusso T, et al. Laterality defects in the National Birth Defects Prevention Study (1998–2007): birth prevalence and descriptive epidemiology. Am J Med Genet A 2014; 164A: 2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas JS, Barbato A, Collins SA, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J 2017; 49: 1601090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humbert M, Wagner TO. Rare respiratory diseases are ready for primetime: from Rare Disease Day to the European Reference Networks. Eur Respir J 2017; 49: 1700085. [DOI] [PubMed] [Google Scholar]
- 32.Rumman N, Jackson C, Collins S, et al. Diagnosis of primary ciliary dyskinesia: potential options for resource-limited countries. Eur Respir Rev 2017; 26: 160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werner C, Lablans M, Ataian M, et al. An international registry for primary ciliary dyskinesia. Eur Respir J 2016; 47: 849–859. [DOI] [PubMed] [Google Scholar]
- 34.Goutaki M, Maurer E, Halbeisen FS, et al. The international primary ciliary dyskinesia cohort (iPCD Cohort): methods and first results. Eur Respir J 2017; 49: 1601181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker WT, Jackson CL, Lackie PM, et al. Nitric oxide in primary ciliary dyskinesia. Eur Respir J 2012; 40: 1024–1032. [DOI] [PubMed] [Google Scholar]
- 36.Collins SA, Gove K, Walker W, et al. Nasal nitric oxide screening for primary ciliary dyskinesia: systematic review and meta-analysis. Eur Respir J 2014; 44: 1589–1599. [DOI] [PubMed] [Google Scholar]
- 37.Leigh MW, Hazucha MJ, Chawla KK, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc 2013; 10: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horani A, Brody SL, Ferkol TW. Picking up speed: advances in the genetics of primary ciliary dyskinesia. Pediatr Res 2014; 75: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raidt J, Wallmeier J, Hjeij R, et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respir J 2014; 44: 1579–1588. [DOI] [PubMed] [Google Scholar]
- 40.Chilvers MA, Rutman A, O'Callaghan C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J Allergy Clin Immunol 2003; 112: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson CL, Behan L, Collins SA, et al. Accuracy of diagnostic testing in primary ciliary dyskinesia. Eur Respir J 2016; 47: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowles MR, Leigh MW, Carson JL, et al. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax 2012; 67: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoemark A, Hogg C. Electron tomography of respiratory cilia. Thorax 2013; 68: 190–191. [DOI] [PubMed] [Google Scholar]
- 44.Davis SD, Ferkol TW, Rosenfeld M, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med 2015; 191: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shoemark A, Moya E, Hirst RA, et al. High prevalence of CCDC103 p.His154Pro mutation causing primary ciliary dyskinesia disrupts protein oligomerisation and is associated with normal diagnostic investigations. Thorax 2017; in press [ 10.1136/thoraxjnl-2017-209999]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shoemark A, Frost E, Dixon M, et al. Accuracy of immunofluorescence in the diagnosis of primary ciliary dyskinesia. Am J Respir Crit Care Med 2017; 196: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olbrich H, Schmidts M, Werner C, et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am J Hum Genet 2012; 91: 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbato A, Frischer T, Kuehni CE, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J 2009; 34: 1264–1276. [DOI] [PubMed] [Google Scholar]
- 49.Lucas JS, Carroll M. Primary ciliary dyskinesia and cystic fibrosis: different diseases require different treatment. Chest 2014; 145: 674–676. [DOI] [PubMed] [Google Scholar]
- 50.Cohen-Cymberknoh M, Simanovsky N, Hiller N, et al. Differences in disease expression between primary ciliary dyskinesia and cystic fibrosis with and without pancreatic insufficiency. Chest 2014; 145: 738–744. [DOI] [PubMed] [Google Scholar]
- 51.Marthin JK, Petersen N, Skovgaard LT, et al. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med 2010; 181: 1262–1268. [DOI] [PubMed] [Google Scholar]
- 52.Frija-Masson J, Bassinet L, Honoré I, et al. Clinical characteristics, functional respiratory decline and follow-up in adult patients with primary ciliary dyskinesia. Thorax 2017; 72: 154–160. [DOI] [PubMed] [Google Scholar]
- 53.Sunther M, Bush A, Hogg C, et al. Recovery of baseline lung function after pulmonary exacerbation in children with primary ciliary dyskinesia. Pediatr Pulmonol 2016; 51: 1362–1366. [DOI] [PubMed] [Google Scholar]
- 54.Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Respir J 1997; 10: 2376–2379. [DOI] [PubMed] [Google Scholar]
- 55.Ratjen F, Waters V, Klingel M, et al. Changes in airway inflammation during pulmonary exacerbations in patients with cystic fibrosis and primary ciliary dyskinesia. Eur Respir J 2016; 47: 829–836. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy MP, Noone PG, Leigh MW, et al. High-resolution CT of patients with primary ciliary dyskinesia. AJR Am J Roentgenol 2007; 188: 1232–1238. [DOI] [PubMed] [Google Scholar]
- 57.Maglione M, Bush A, Montella S, et al. Progression of lung disease in primary ciliary dyskinesia: is spirometry less accurate than CT? Pediatr Pulmonol 2012; 47: 498–504. [DOI] [PubMed] [Google Scholar]
- 58.Boon M, Vermeulen FL, Gysemans W, et al. Lung structure–function correlation in patients with primary ciliary dyskinesia. Thorax 2015; 70: 339–345. [DOI] [PubMed] [Google Scholar]
- 59.Green K, Buchvald FF, Marthin JK, et al. Ventilation inhomogeneity in children with primary ciliary dyskinesia. Thorax 2012; 67: 49–53. [DOI] [PubMed] [Google Scholar]
- 60.Irving SJ, Ives A, Davies G, et al. Lung clearance index and high-resolution computed tomography scores in primary ciliary dyskinesia. Am J Respir Crit Care Med 2013; 188: 545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alanin MC, Nielsen KG, von Buchwald C, et al. A longitudinal study of lung bacterial pathogens in patients with primary ciliary dyskinesia. Clin Microbiol Infect 2015; 21: 1093.e1–1093.e7. [DOI] [PubMed] [Google Scholar]
- 62.Kobbernagel HE, Buchvald FF, Haarman EG, et al. Study protocol, rationale and recruitment in a European multi-centre randomized controlled trial to determine the efficacy and safety of azithromycin maintenance therapy for 6 months in primary ciliary dyskinesia. BMC Pulm Med 2016; 16: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koh YY, Park Y, Jeong JH, et al. The effect of regular salbutamol on lung function and bronchial responsiveness in patients with primary ciliary dyskinesia. Chest 2000; 117: 427–433. [DOI] [PubMed] [Google Scholar]
- 64.Bryson HM, Sorkin EM. Dornase alfa. A review of its pharmacological properties and therapeutic potential in cystic fibrosis. Drugs 1994; 48: 894–906. [DOI] [PubMed] [Google Scholar]
- 65.NHS England Specialised Services Clinical Reference Group for Paediatric Medicine . Clinical Commissioning Policy: Dornase Alfa Inhaled Therapy for Primary Ciliary Dyskinesia (All Ages). Reference: NHS England 16029/P. NHS England, 2016. www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2016/07/16029_FINAL.pdf [Google Scholar]
- 66.Paff T, Daniels JM, Weersink EJ, et al. A randomised controlled trial on the effect of inhaled hypertonic saline on quality of life in primary ciliary dyskinesia. Eur Respir J 2017; 49: 1601770. [DOI] [PubMed] [Google Scholar]
- 67.Kuehni CE, Goutaki M, Kobbernagel HE. Hypertonic saline in patients with primary ciliary dyskinesia: on the road to evidence-based treatment for a rare lung disease. Eur Respir J 2017; 49: 1602514. [DOI] [PubMed] [Google Scholar]
- 68.Mener DJ, Lin SY, Ishman SL, et al. Treatment and outcomes of chronic rhinosinusitis in children with primary ciliary dyskinesia: where is the evidence? A qualitative systematic review. Int Forum Allergy Rhinol 2013; 3: 986–991. [DOI] [PubMed] [Google Scholar]
- 69.Parsons DS, Greene BA. A treatment for primary ciliary dyskinesia: efficacy of functional endoscopic sinus surgery. Laryngoscope 1993; 103: 1269–1272. [DOI] [PubMed] [Google Scholar]
- 70.Hadfield PJ, Rowe-Jones JM, Bush A, et al. Treatment of otitis media with effusion in children with primary ciliary dyskinesia. Clin Otolaryngol Allied Sci 1997; 22: 302–306. [DOI] [PubMed] [Google Scholar]
- 71.Wolter NE, Dell SD, James AL, et al. Middle ear ventilation in children with primary ciliary dyskinesia. Int J Pediatr Otorhinolaryngol 2012; 76: 1565–1568. [DOI] [PubMed] [Google Scholar]
- 72.Prulière-Escabasse V, Coste A, Chauvin P, et al. Otologic features in children with primary ciliary dyskinesia. Arch Otolaryngol Head Neck Surg 2010; 136: 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alanin MC, Johansen HK, Aanaes K, et al. Simultaneous sinus and lung infections in patients with primary ciliary dyskinesia. Acta Otolaryngol 2015; 135: 58–63. [DOI] [PubMed] [Google Scholar]
- 74.Alanin MC, Aanaes K, Hoiby N, et al. Sinus surgery can improve quality of life, lung infections, and lung function in patients with primary ciliary dyskinesia. Int Forum Allergy Rhinol 2017; 7: 240–247. [DOI] [PubMed] [Google Scholar]
- 75.Kim SJ, Kim WH, Lim HG, et al. Outcome of 200 patients after an extracardiac Fontan procedure. J Thorac Cardiovasc Surg 2008; 136: 108–116. [DOI] [PubMed] [Google Scholar]
- 76.Swisher M, Jonas R, Tian X, et al. Increased postoperative and respiratory complications in patients with congenital heart disease associated with heterotaxy. J Thorac Cardiovasc Surg 2011; 141: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakhleh N, Francis R, Giese RA, et al. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation 2012; 125: 2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anderson RH, Devine WA, Uemura H. Diagnosis of heterotaxy syndrome. Circulation 1995; 91: 906–908. [PubMed] [Google Scholar]
- 79.Hashmi A, Abu-Sulaiman R, McCrindle BW, et al. Management and outcomes of right atrial isomerism: a 26-year experience. J Am Coll Cardiol 1998; 31: 1120–1126. [DOI] [PubMed] [Google Scholar]
- 80.Gilljam T, McCrindle BW, Smallhorn JF, et al. Outcomes of left atrial isomerism over a 28-year period at a single institution. J Am Coll Cardiol 2000; 36: 908–916. [DOI] [PubMed] [Google Scholar]
- 81.Van Praagh R. Terminology of congenital heart disease. Glossary and commentary. Circulation 1977; 56: 139–143. [DOI] [PubMed] [Google Scholar]
- 82.Peeters H, Devriendt K. Human laterality disorders. Eur J Med Genet 2006; 49: 349–362. [DOI] [PubMed] [Google Scholar]
- 83.Kennedy MP, Omran H, Leigh MW, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 2007; 115: 2814–2821. [DOI] [PubMed] [Google Scholar]
- 84.Jacobs JP, Anderson RH, Weinberg PM, et al. The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiol Young 2007; 17: 1–28. [DOI] [PubMed] [Google Scholar]
- 85.Munro NC, Currie DC, Lindsay KS, et al. Fertility in men with primary ciliary dyskinesia presenting with respiratory infection. Thorax 1994; 49: 684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raidt J, Werner C, Menchen T, et al. Ciliary function and motor protein composition of human fallopian tubes. Hum Reprod 2015; 30: 2871–2880. [DOI] [PubMed] [Google Scholar]
- 87.Strippoli MP, Frischer T, Barbato A, et al. Management of primary ciliary dyskinesia in European children: recommendations and clinical practice. Eur Respir J 2012; 39: 1482–1491. [DOI] [PubMed] [Google Scholar]
- 88.Mahadeva R, Webb K, Westerbeek RC, et al. Clinical outcome in relation to care in centres specialising in cystic fibrosis: cross sectional study. BMJ 1998; 316: 1771–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucas JS, Chetcuti P, Copeland F, et al. Overcoming challenges in the management of primary ciliary dyskinesia: the UK model. Paediatr Respir Rev 2014; 15: 142–145. [DOI] [PubMed] [Google Scholar]
- 90.Lucas JS, Botting NJ, Dunn Galvin A, et al. Development of a health related quality of life questionnaire for adult patients with primary ciliary dyskinesia. Cilia 2012; 1: Suppl. 1, P6. [Google Scholar]
- 91.Dell SD, Leigh MW, Lucas JS, et al. Primary ciliary dyskinesia: first health-related quality-of-life measures for pediatric patients. Ann Am Thorac Soc 2016; 13: 1726–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Behan L, Leigh MW, Dell SD, et al. Validation of a health-related quality of life instrument for primary ciliary dyskinesia (QOL-PCD). Thorax 2017; in press [ 10.1136/thoraxjnl-2016-209356]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Apolone G, De Carli G, Brunetti M, et al. Health-related quality of life (HR-QOL) and regulatory issues. An assessment of the European Agency for the Evaluation of Medicinal Products (EMEA) recommendations on the use of HR-QOL measures in drug approval. Pharmacoeconomics 2001; 19: 187–195. [DOI] [PubMed] [Google Scholar]
- 94.European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) . Reflection Paper on the Regulatory Guidance for the Use of Health-Related Quality of Life (HRQL) Measures in the Evaluation of Medicinal Products. London, European Medicines Agency, 2005. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003637.pdf [Google Scholar]
- 95.US Dept of Health and Human Services, Food and Drug Administration (FDA) . Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. FDA, 2009. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf [Google Scholar]
- 96.Spagnolo P, du Bois RM. The challenges of clinical research in orphan diseases. In: Cottin V, Cordier JF, Richeldi L, eds. Orphan Lung Diseases: A Clinical Guide to Rare Lung Diseases. 1st Edn. London, Springer, 2015; pp. 5–15. [Google Scholar]
- 97.Quon BS, Rowe SM. New and emerging targeted therapies for cystic fibrosis. BMJ 2016; 352: i859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davies JC, Wainwright CE, Canny GJ, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 2013; 187: 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alton EW, Armstrong DK, Ashby D, et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 2015; 3: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rubbo B, Behan L, Dehlink E, et al. Proceedings of the COST Action BM1407 inaugural conference BEAT-PCD: translational research in primary ciliary dyskinesia – bench, bedside, and population perspectives. BMC Proc 2016; 10: Suppl. 9, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
J. Lucas ERR-0023-2017_Lucas (1.2MB, pdf)