Abstract
This review presents and discusses a new frontier for fast, risk-free and potentially inexpensive diagnostics of respiratory diseases by detecting volatile organic compounds (VOCs) present in exhaled breath. One part of the review is a didactic presentation of the overlaying concept and the chemistry of exhaled breath. The other part discusses diverse sensors that have been developed and used for the detection of respiratory diseases (e.g. chronic obstructive pulmonary disease, asthma, lung cancer, pulmonary arterial hypertension, tuberculosis, cystic fibrosis, obstructive sleep apnoea syndrome and pneumoconiosis) by analysis of VOCs in exhaled breath. The strengths and pitfalls are discussed and criticised, particularly in the perspective in disseminating information regarding these advances. Ideas regarding the improvement of sensors, sensor arrays, sensing devices and the further planning of workflow are also discussed.
Short abstract
Detection of volatile organic compounds from exhaled breath by nanomaterial-based sensors is a new diagnostics frontier in the screening of pulmonary diseases. http://bit.ly/2JoBKXn
Introduction
Respiratory diseases are often diagnosed in later stages, reducing the chance of effective treatment [1]. Diagnosis currently includes physical examination followed by a series of tests that include chemical, imaging, endoscopic, immunological and genomic procedures among others [2, 3]. Extensive screening and detection of respiratory diseases at an early stage can dramatically decrease morbidity and mortality [4], because this enables prompt intervention/treatment, with the prospect of achieving the best possible therapeutic outcome for the patient. Moreover, these programmes may enable diagnosis of high-risk conditions for the development of a particular disease [1]. By identifying and managing these high-risk conditions, it may be possible to act preventatively, thereby reducing the incidence of disease occurrence [5]. Monitoring of individuals identified as being high-risk cases is important in terms of determining the point at which the disease begins to progress, notably in subjects where transformation from a benign to a malignant state occurs (as in lung cancer), and planning interventions for such individuals [6]. The accuracy and/or accessibility of tests available in currently existing programmes fails to reach the desired levels, whereas new developments (e.g. sputum tests, radiography and computed tomography (CT) scans) are costly [7]. In other instances, the tests can be more invasive (endoscopy, pulmonary catheterisation, biopsy and bone marrow tests) and, therefore, run the risk of complications to the patients screened and/or require special facilities (such as CT) with healthcare professionals operating the instruments [8]. The ideal test for respiratory diseases should have high-accuracy, low cost, be noninvasive, easily repeatable at specific intervals and nontechnical. A distinct advantage is for the procedure(s) to have minimal impact on the daily activities of the person being screened [9].
Volatile organic compounds as biomarkers for respiratory diseases
A new frontier for fast, risk-free and potentially inexpensive diagnosis of respiratory diseases is based on volatile organic compounds (VOCs), i.e. organic compounds that have high vapor pressure at ambient conditions. The rationale rests on the fact that VOCs show distinct and immediate changes when pathological conditions arise, altering the body's biochemistry by one or a combination of the following processes: oxidative stress, cytochrome p450, liver enzymes, carbohydrate metabolism and lipid metabolism [2, 10]. Part of these VOCs, which appear both in normal and abnormal cells, are mixtures of distinctly different compositions [11]. The remainder come from exclusively abnormal cells. The particularly significant feature that can be exploited in this approach is that each disease has its own unique VOC pattern, and therefore the presence of one disease would not screen out others [12]. These VOCs can therefore be detected: 1) directly from the headspace (i.e. the mixture of VOCs trapped above the abnormal cells in a sealed vessel); or 2) in exhaled breath, blood or other body fluids, something highly dependent on their tissue–blood and blood–air partition coefficients [13]. Therefore, isolation and detection of VOCs in these body fluids can serve as a pathway for the early detection of respiratory and other diseases. The monitoring of respiratory diseases by breath analysis is noninvasive and breath sampling can be carried out without the need for specialist settings and specialist technical expertise.
Several spectrometry and spectroscopy techniques have been used to collect, detect and analyse exhaled VOCs of respiratory diseases [14–18]. Frequently used techniques include proton transfer reaction-mass spectrometry (MS), selected ion flow tube (SIFT)-MS, ion mobility spectrometry, laser spectroscopy and gas chromatography (GC), which is considered the most utilised method [11]. In GC techniques, the exhaled breath is collected and usually stored in inert bags or sorption tubes. After desorption, VOCs are assessed and analysed by GC which is usually followed by MS or flame ionisation detection [19]. VOCs are separated based on their chemical properties being consecutively ionised and separated by their mass-to-charge (m/z) ratio [2]. While GC-MS-based techniques are powerful in detecting disease-related VOCs, they unfortunately require expensive equipment, high levels of expertise to operate the instruments, considerable time and effort for sampling and analysis, and a need for preconcentration techniques [10].
These approaches have been used to identify the VOCs distinctive of several respiratory diseases, including chronic obstructive pulmonary disease (COPD), asthma, lung cancer, pulmonary arterial hypertension (PAH), obstructive sleep apnoea syndrome (OSAS), tuberculosis (TB), cystic fibrosis (CF) and pneumoconiosis. An updated list compiling the characteristic VOCs for these respiratory diseases is shown in table S1.
Sensor arrays for disease detection in exhaled breath
To overcome the challenges associated with spectroscopic and/or MS techniques for breath analysis of respiratory diseases, chemical sensors have been adopted. Detection of the disease-related VOCs from exhaled breath can be achieved using two main chemical sensing strategies. The first is based on a selective mechanism in which a chemical sensor is designed to interact and detect the presence of a single compound in exhaled breath [2]. This approach, though quite sensitive, is cumbersome due to the complex synthesis of separate and highly selective nanomaterials for the detection of each VOC, mainly with nonpolar targets. Moreover, there are currently no individual unique VOCs that are specific to any particular disease [20]. The second approach involves an array of broadly cross-reactive sensors along with methods of pattern recognition (figure 1a) [11, 21]. In contrast to the selective sensing method, the sensors array approach, bioinspired by the sense of smell, is capable of detecting a compendium of VOCs. Each sensor in this approach responds to a range of VOCs which allows the sensing and analysis of individual components from a mixture of compounds [22]. The underlying mechanism of this approach depends on the nature of the sensors (figure 1b); for example, chemiresistors change their electrical resistivity due to sorption of VOCs on the organic film, or by steric changes within the sensing layer affecting the charge transfer from/to the inorganic nanomaterial (figure 1b) [20]. Acoustic sensors detect changes in the propagation (velocity and amplitude) of acoustic waves through or on the surface of the sensor's coating material due to sorption of VOCs [23]. Colorimetric sensors are based on indicators, specifically chemoresponsive dyes, which chemically react and change colour on exposure to VOCs, thereby identifying the exposed species [24].
FIGURE 1.
a) Overview of the working principal of nanomaterial-based sensors array. b) Different nanomaterial-based sensors. 1) Chemiresistor based on monolayer-capped nanoparticles; 2) chemiresistors based on single-wall carbon nanotubes; 3) chemiresistor based on conducting polymers; 4) chemiresistor based on metal oxide film; 5) quartz microbalance with selective coating; 6) colorimetric sensor; and 7) surface acoustic wave sensor. Reproduced from [11] with permission from the publisher.
The most widely used approach for sensors array is possibly based on conductive polymers, which operate on electrical resistance changes from steady state induced by the attachment of VOCs to the sensor [25]. While polymer-based chemiresistors offer several advantages (e.g. low power consumption, small size, low operating temperature and low cost), their sensitivity depends on the type of coating; they also show a drift in baseline due to polymer instability [25, 26]. Nevertheless, selection of a sensors array type depends on the physical characteristics that are optimal for clinical purposes; sensor arrays with low recovery time are not optimal for screening purposes [25]. Table 1 lists representative diseases and related types of sensor arrays used for their detection. In the following sections, this table will be extended to discuss the use of sensor arrays conjugated with pattern recognition methods as diagnostic tools for different respiratory diseases.
TABLE 1.
Sensor arrays in the diagnosis of respiratory diseases
| Disease | Sensor type | References |
| Lung cancer | CBPC, MO, SWNTs, SiNW FET, MCMNPs, QMB, colourimetric | [27, 28, 29, 30, 31, 32, 33, 34, 35, 36] |
| COPD | CBPC, QMB, MO | [37, 38, 39, 40, 41, 42] |
| Asthma | CBPC, MO | [37, 43, 44, 45, 46, 47, 48] |
| PAH | MCMNPs, colourimetric | [49] |
| OSAS | CBPC | [50, 51, 52] |
| CF | CBPC | [53, 54, 55] |
| TB | MO, SWNTs, MCMNPs, | [56, 57, 58, 59, 60, 61] |
| Pneumoconiosis | CBPC | [62] |
COPD: chronic obstructive pulmonary disease; PAH: pulmonary arterial hypertension; OSAS: obstructive sleep apnoea syndrome; CF: cystic fibrosis; TB: tuberculosis. CBPC: carbon black polymer composite; MO: metal oxide; SWNTs: single-walled carbon nantotubes; SiNW FET: silicon nanowire field effect transistors; MCMNPs: monolayer-capped metal-coated nanoparticles; QMB: quartz microbalance.
Chronic obstructive pulmonary disease
COPD is characterised by oxidative stress and production of VOCs secreted by the lungs [38]. Diagnosis is based on identifying characteristic symptoms and lung functioning parameters [23, 63]. Current diagnostic tools poorly reflect the severity and other distinctive features of the disease [63].
Nuclear magnetic resonance and GC-MS analysis of exhaled breath condensates (aerosolised nonvolatile particles contained in the fluid lining of the airway) and noncondensated exhaled breath concentrations of lactate, acetate, propionate, serine, proline and tyrosine are raised, but valine and lysine are lower than non-COPD controls [37, 45, 64–66]. Relying on these breath-print signatures, other research groups have developed sensors to detect COPD-related VOCs in exhaled breath. In a cross-sectional study of 100 patients with asthma and COPD, breath VOCs were analysed by a sensors array based on 32 derivatives of polymer/carbon black sensors [37]. Principle component analysis (PCA) of the sensing signals had 88% accuracy for distinguishing fixed asthma from COPD, and 83% for classic asthma, and the detection accuracy was not confounded by smoking status [37]. Since both COPD and asthma patients have chronic airway inflammation, the results might include overlapping features, in the sense that COPD patients could be misdiagnosed as asthmatics and vice versa. Hence, it is essential to clearly discriminate COPD from asthma, especially in elderly people who have a higher probability of adverse reactions to different classes of inhaled agents or systemic corticosteroid [67, 68]. Thus, a combined system consisting of an array of quartz crystal microbalance (QMB) sensors coated with six derivatives of metal-based metalloporphyrins linked with a GC system found nine VOCs that were significantly correlated with COPD, of which two positively correlated with COPD and seven negatively correlated with COPD [38]. These results showed that the following were significantly increased in healthy subjects: limonene; butylated hydroxytoluene (BHT); 2-propanol; benzene, 1,3,5-tri-tert-butyl-; hexane, 3-ethyl-4-methyl-; hexyl ethylphosphono-fluoridate; and 1-pentene, 2,4,4-trimethyl. The alkanes decane and 6-ethyl-2-methyl decane were raised in COPD patients [38]. Parallel to the GC-MS analysis, the cross-validated model provided the correct classification of 26 out of 27 COPD patients, and five out of seven control subjects, with an accuracy of 91% [38].
Asthma
Symptoms of atopic asthma often begin in early childhood and mostly improve, or even disappear, at puberty, but can relapse later in life [68]. Analysis of exhaled breath may, therefore, be used to assess inflammation and oxidative stress in the respiratory tract, thereby providing a diagnostic approach to the condition [69, 70]. The most common breath analysis approach is based on the detection and monitoring of exhaled nitric oxide fraction (FeNO) [70]. Indeed, increased exhalation of nitric oxide (NO) as a result of interleukin (IL)-13-induced induction of NO synthase in the airway epithelium have been widely documented in asthmatic patients. As a result, asthmatic patients exhale >30 ppbv of NO, whereas a healthy population exhales lower concentrations [71].
Portable NO-selective sensors are already routinely used to detect asthma, generally being sensitive to NO levels of <1 ppb, with a relatively rapid response time [45]. Nevertheless, raised NO concentrations have been reported in other diseases, including hypertension, arthritis, lung diseases, bronchiectasis and CF, in addition to inflammatory bowel disease of the colon and small intestine [72]. Consequently, a pattern recognition approach of exhaled breath is more likely to be fruitful for predicting asthma than a single biomarker. Indeed, sensors array have given a higher degree of diagnostic accuracy for asthma than exhaled NO or lung function [14, 44, 73]. For example, a cross-sectional study with polymer/carbon black sensors array could distinguish between 30 patients with COPD, 20 patients with mild-to-severe asthma, 20 healthy smokers and 20 healthy nonsmokers [45]. The breath-prints of patients with COPD and asthmatics have been compared, and the data analysed by PCA and canonical linear discriminant analysis. Moreover, cross-validation with the leave-one-out method has been used to estimate the accuracy, showing that the polymer-based sensors could successfully discriminate patients with mild-to-severe asthma from those with COPD, healthy smokers and healthy nonsmokers with accuracies of 96%, 93% and 95%, respectively [45]. Similarly, polymer/carbon black sensors array successfully separated mild asthma from young controls; however, it failed to distinguish severe from mild asthmatics [66]. Paredi et al. [14] have shown that the VOC profile can assess asthma severity and control. In addition to the collective analysis of breath-prints, raised ethane levels have been recorded in the breath of steroid-naïve asthmatics compared to subjects treated with steroids. Moreover, ethane was found to be higher in patients with severe asthma compared with patients with mild asthma, suggesting that ethane might be a preselected marker for the detection of asthma.
Lung cancer
Lung cancer is typically asymptomatic in its early stages and, as such, most of the cases are diagnosed in later stages when treatment is no longer effective [74]. The 5-year survival rate increases dramatically from 10% to 80% if the disease is detected at the early stages [75].
Numerous GC-MS and proton transfer reaction-MS studies have examined the profile of VOCs in lung cancer; >1000 trace VOCs have been found in the exhaled breath of lung cancer patients at concentrations ranging from parts per million by volume to parts per trillion by volume [6, 9, 15, 76–81]. Typical examples are isoprene, methanol, acetone and 2-propanol (appearing in all human breath samples), acetonitrile, furan, 2-methyl furan (primarily found in smokers) and many others [82]. Hence, efforts have been invested in analysing exhaled breath as a simple noninvasive method of the early detection of lung cancer [83, 84]. Relying on these findings, Di Natale et al. [27] reported 100% classification of patients with lung cancer versus healthy subjects by using eight QMB sensors coated with different metalloporphyrins. Similarly, the same sensor array was used to detect lung cancer in a pool of 36 healthy controls, 28 patients with lung cancer and 28 patients with diverse lung diseases [34]. The sensors response was analysed using PCA and discriminate analysis, combined with partial least square ; it could classify between the groups with 85%, 92.8% and 89.3% sensitivity, respectively.
Surface acoustic wave (SAW) sensors were used as a detector for the breath analysis of 42 volunteers, including 15 healthy subjects, 20 patients with lung cancer and seven patients with chronic bronchitis. This array was a SAW coated with a film of isobutylene regarded as the detector, with the other sensor being used as a reference. After calibration of the sensors, breath samples were collected in Tedlar bags and absorbed on solid-phase microextraction fibres for preconcentration. The VOCs extracted from the thermal desorber column were absorbed on the SAW sensors. Frequency response (Hz) and the corresponding retention times were recorded. Using artificial neural network analysis, the sensors could correctly diagnose patients with lung cancer from exhaled breath with 80% sensitivity and specificity [28].
Despite their sensitivity and good response time, SAW sensors are temperature sensitive, such that certain analyte compounds are affected by the different sensor coatings [10]. Therefore, others have tried to verify the potential of these different types of nanoarray sensors to detect lung cancer. For example, a colorimetric sensors array was designed for noninvasive lung cancer detection of exhaled breath of 49 patients with nonsmall cell lung cancer, 21 healthy volunteers and 73 patients with different pulmonary diseases, including COPD [29]. Each colorimetric sensors array was composed of 36 chemically sensitive spots with different sensitivities to volatile compounds. The data gave 73.3% sensitivity and 72.4% specificity for the diagnosis of lung cancer. In a following study, breath samples were taken from 229 volunteers divided into a control group of individuals at high risk of developing lung cancer, subjects with intermediate lung nodules, and untreated patients with lung cancer validated by biopsy. All groups were examined by a colorimetric sensors array and compared with breath signatures of eight binary groups for the identification and characterisation of lung cancer with high sensitivities and specificities. The resulting model could discriminate between different lung cancer histologies with 90% sensitivity; however, prediction and differentiation between healthy volunteers and patients with lung cancer was less accurate (70%) [35]. Arguably, the disposable features of the colorimetric sensors might be a limitation in real-world applications.
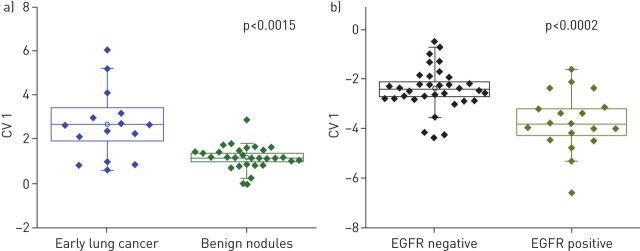
With these challenges in mind, chemiresistors based on monolayer-coated metal nanoparticles have been used to detect and monitor lung cancer as having advantages over other sensing techniques, such as: a larger surface-to-volume ratio of the sensors, operation at room temperature, lower detection limits for the VOCs of interest (sub-ppb), lower operating voltage, a wider dynamic range, faster response and recovery times, higher tolerance to humidity and compatibility with standard microelectronic industry [2, 30]. Using this approach, the group of Haick and co-workers. [31–33, 36] successfully discriminated early and late stages of lung cancer with 88% accuracy. Moreover, this sensors array could distinguish between small cell lung carcinoma and nonsmall cell carcinoma, as well as in differentiating between subhistologies of adenocarcinoma and squamous cell carcinoma with 93% and 88% accuracy, respectively. Furthermore, in a study that included 144 breath samples from 39 patients with advanced lung cancer, one gold nanoparticle (GNP) sensor could differentiate between patients with lung cancer after surgery, as well as monitoring the response of patients to therapy with an accuracy of 59%. Discriminant factor analysis of the collective responses from the nanoarray could monitor changes in tumour response during therapy and also indicate lack of any further response to therapy with a success rate of 85% [85]. Using the same sensors array, these authors managed to detect lung cancer at early onset and monitor breath volatolomics after lung cancer resection. Moreover, patients with lung cancer and volunteers with benign nodules before and after surgery could clearly be differentiated by DFA maps [32]. Ultimately, the nanomaterial-based sensors array distinguished between pre-surgery and post-surgery lung cancer states yielding an 80% classification accuracy [32]. In another study by the same group, exhaled breath was analysed in the diagnosis of epidermal growth factor receptor (EGFR) mutation in patients with lung cancer [36]. A nanomaterial-based sensors array composed of 40 cross-reactive, chemically diverse chemiresistors based on organically stabilised spherical GNPs, and single-walled carbon nanotubes, were used to discriminate patients with lung cancer who harboured the EGFR mutation from those with wild-type with an accuracy of 83%. Nanoarray sensors also showed that patients with early lung cancer could be discriminated from patients with benign pulmonary nodules, with a sensitivity, specificity and accuracy of 75%, 93% and 87%, respectively (figure 2).
FIGURE 2.
Discriminant factor analysis plots calculated from the responses of the nanoarray sensors for a) early lung cancer and benign pulmonary nodules and b) patients with lung cancer with and without the epidermal growth factor receptor (EGFR) mutation. Each point represents one patient. The positions of the mean values are marked with an unfilled square; the boxes correspond to the first and third quartiles, and the error bars correspond to the sd. CV1: first canonical variable. Reproduced from [36] with permission from the publisher.
Pulmonary arterial hypertension
PAH is a progressive cardiopulmonary disease characterised by the extensive occlusion of small to mid-sized pulmonary arterioles, as well as structural alterations in the vascular wall that eventually lead to right heart failure [12, 13, 86]. Although a wide range of therapeutic agents have been established for the management of PAH, it remains incurable, with lung transplantation continuing to be the main treatment in severe cases [87]. Most diagnostic methods involve assessment of the cardiac structure or estimation of pulmonary artery pressure; however, PAH does not manifest until pulmonary vascular disease is advanced [86]. Accordingly, screening for PAH in a high-risk population would aid early diagnosis and intervention, thereby improving patient outcomes [88, 89].
In PAH, changes in the exhaled volatolome could result from the pathophysiological process of remodelling in arterioles, as well as the compensatory mechanisms accompanying the development of PAH [13]. Moreover, the physiological changes in the lungs and heart, and fluctuations in the pulmonary circulation, could be other sources of volatolomic alterations [90]. Consequently, many groups are trying to use changes in the exhaled breath pattern to detect PAH in its earlier stages. Exhaled breath of PAH patients has been examined by SIFT-MS in predetermined training and validation sets [90]. A total of 31 PAH patients and 34 controls were enrolled in the study. The breath of patients with PAH had raised concentrations of 2-nonene, 2-propanol, acetaldehyde, ammonia, ethanol and pentane compared with control subjects, whereas 1-decene and 1-octene were significantly lower. The model also gave 86.1% accuracy in the training phase and 79.3% in the independent validation set. In contrast, there was no association between levels of ammonia in the breath and plasma (plasma levels of ammonia were similar in both groups). Ammonia is generated by the breakdown of nucleic acids, polyamines and amino acids, mainly glutamine. Moreover, PAH patients showed higher glutaminolysis, thereby generating more ammonia in the exhaled breath seen in this study, although not enough to affect whole blood concentration [90].
While SIFT-MS offers real-time VOC detection and quantification of exhaled breath, its clinical applications suffer from high cost, with high levels of expertise being required to operate the instrument, and a long time needed to complete the analysis of breath samples. Thus, efforts continue to be made to develop nanoscale sensors for the rapid detection of VOCs in exhaled breath. In fact, Cohen-Kaminsky et al. [49] have established a proof of concept that GNP-based sensors can successfully detect and classify PAH cases in a pool of 22 patients and 23 healthy controls with an accuracy of 92%. Similarly, a colorimetric sensors array was used as a diagnostic method for the discrimination of patients with lung cancer from other common lung diseases, wherein 20 patients with PAH were enrolled as a control group [29]. The results showed that the breath signatures of patients with lung cancer differed from PAH, idiopathic pulmonary fibrosis, COPD and healthy controls. In the validation set of the nanoarray, the sensors could discriminate between the diseases with a sensitivity of 73.3% and a specificity of 72.4%. The results were not influenced by sex, age, histology or smoking history.
Tuberculosis
Diagnosis of TB remains a major global public health challenge, with its social burden increasing because many patients may also be infected with HIV. The rates of multidrug-resistant TB are increasing [58, 91]. In 2010, there was an estimated incident case count of 8.8 million active TB infections, resulting in 1.5 million deaths [57]. Current diagnostic methods rely on either insensitive smear microscopy or sensitive, but lengthy, microbiologic culture, unlikely to be used in poorly resourced centres [56, 59]. More recently, the Xpert MTB/RIF assay, a fully automated sample-to-answer nucleic acid amplification test, has significantly improved sensitivity compared with smear microscopy [58]. However, this assay requires a sputum sample or an invasive sample from patients that cannot expectorate. Its high cost also limits it use in poor and resource-limited countries where TB is rampant [61]. Hence, development of a rapid, affordable and noninvasive assay is needed for TB screening, especially in developing countries.
Phillips et al. [60] analysed the breath of 226 symptomatic high-risk patients using GC-MS, pointing out several biomarkers of active pulmonary TB. They suggested biomarkers in oxidative stress products, such as alkanes and alkane derivatives, and volatile metabolites of Mycobacterium tuberculosis, such as cyclohexane and benzene derivatives. Their results differentiated between positive and negative TB with 85% overall accuracy, 84% sensitivity and 64.7% specificity, using C-statistic values [60]. In an attempt to use the distinctive breath-prints of TB patients in a point-of-care setting, a metal-oxide-based sensors array was tested in a pilot study for the detection of TB in Bangladesh on 30 participants and 194 in the validation set [57]. The response was analysed and validated using traditional sputum smear microscopy and culture on Lowenstein–Jensen media. The results showed a sensitivity of 93.5% and a specificity of 98.5% in the validation set. Moreover, the sensors array had a sensitivity of 93.5% and a specificity of 85.3% in discriminating healthy controls from patients with TB, and a sensitivity of 76.5% and specificity of 87.2% in identifying patients with TB within the entire test population [57].
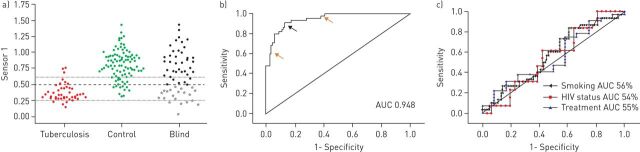
Nakhleh et al. [56] used arrays of molecularly modified GNP and molecularly modified single-walled carbon nanotubes for the detection of active TB. Their study included 64 individuals in whom M. tuberculosis was proven by culture. The control group consisted of two main subcategories, 67 healthy volunteers and a group of 67 subjects in whom TB was suspected but had had negative smears, culture and GeneXpert MTB/RIF. The two groups were age- and sex-matched, but differed in their smoking habits, HIV infection rates and treatment for TB [56]. The classification ability of the sensors was evaluated using receiver operating characteristic (ROC)-derived Youden's index as a cut-off point (best sensitivity+specificity−1) as a binary classifier threshold for the training set. Three single-walled carbon nanotubes sensors modified with a layer of polyaromatic hydrocarbon derivative showed >80% accuracy in the training set, whereas the other nine showed either <80% accuracy or a random classification. Alternatively, the chemiresistor based on dodecanethiol-capped GNPs correctly classified 121 out of the 138 in the training set, with an accuracy of 88%, a sensitivity of 85% and a specificity of 89%. In the validation set, the same sensor scored a sensitivity of 90%, a specificity of 93% and an accuracy of 92%. Moreover, the sensor's function was unaffected by possible confounding factors, including smoking habits, HIV infection and antibiotic treatment (figure 3) [56].
FIGURE 3.
a) Dot plots response of a single molecularly modified gold nanoparticle. Each symbol represents a single sample. The dashed line represents Youden's cut-off point, and the dotted lines represent the cut-off points to rule in and rule out tuberculosis. Samples from the validation set with responses lower than the threshold were classified as tuberculosis positive (open stars) or nontuberculosis positive (closed stars) according to Youden's cut-off point. b) Receiver operating characteristic curve of the sensor. c) Receiver operating characteristic curves of the sensor when comparing smoking, HIV and treatment status. AUC: area under curve. Reproduced from [56] with permission from the publisher.
Another group used a sensors array composed of eight metalloporphyrin-coated QMB sensors to assess the exhaled breath of patients with TB during treatment. Breath samples of 51 patients with TB and 20 controls were analysed before and 2, 7, 14 and 30 days after therapy. The sensors response was validated and correlated with clinical and microbiological measurements on sputum samples [58]. The sensors scored 93% accuracy in distinguishing TB cases from controls; additionally, serial measurements of VOCs also showed signal changes during TB treatment among patients.
In addition to TB, nanomaterial sensor arrays have been successfully employed for the detection of other respiratory infections including sinusitis, ventilator-associated pneumonia and invasive pulmonary aspergillosis in prolonged chemotherapy-induced neutropenia [92–98].
Pneumoconiosis
Approximately 15% of lung diseases are attributed to pneumoconiosis, a lung condition that is associated with occupational exposure leading to inhalation of dust, silica, asbestos or smoke [62]. The high-risk population includes shipyard workers, construction workers, asbestos textile workers and asbestos miners [23]. Although asbestos has been gradually banned in many countries, mortality due to asbestos exposure continues to increase in many developed countries. However, diagnosis of early-stage pneumoconiosis remains difficult in clinical practice [19, 23]. The inhaled dust particles are phagocytosed by alveolar macrophages, resulting in reactive oxygen species being produced. Consequently, lipid peroxidation occurs and hence screening for lipid peroxidation-related VOCs should be useful in diagnosing pneumoconiosis [62].
Yang et al. [62] analysed the exhaled breath of 34 subjects with pneumoconiosis and 64 healthy patients using a polymer/carbon black sensors array. The prediction model based on linear discriminant analysis indicated that the discriminations of the sample yielded a specificity of 88.0%, a sensitivity of 67.9% and an accuracy of 80.8% in the training set. In the test set, sensitivity was 66.7%, specificity was 71.4%, and accuracy was 70.0% by linear discriminant analysis, suggesting that nanoarray sensors based on polymer/carbon are potential valuable in screening for pneumoconiosis.
Obstructive sleep apnoea syndrome
OSAS is a common disease associated with an increased risk for cardiovascular disorders [50]. Despite the introduction of several screening tools, the diagnosis of OSAS still needs to be confirmed by polysomnography, and hence the use of expensive instruments by trained personnel is required limiting its large-scale application [51].
In an attempt to utilise sensors array technology for the detection of OSAS, the polymer composite-based sensors were utilised in the discrimination of OSAS between healthy controls showing adequate sensitivity and specificity (93% and 70%, respectively) [50]. In another study, the same sensors array was employed to test the breath-prints of 18 children with OSAS and 10 non-OSAS subjects with habitual snoring (aged 6–11 years). The exhaled biomarker pattern of patients with OSAS was discriminated from that of control subjects (p=0.03, cross-validation accuracy: 64%), ROC curve analysis (area: 0.83) showed 78% sensitivity and 70% specificity [99]. In a more recent study, 136 subjects (20 obese non-OSAS, 20 hypoxic OSAS, 20 nonhypoxic OSAS, and 20 nonhypoxic COPD versus 56 healthy controls) were tested using seven quartz crystals. Their collective breath samples were analysed, and controls were distinguished from others scoring 100% correct classification. Moreover, the sensors array identified 60% of hypoxic, and 35% of nonhypoxic patients with OSAS [100]. Similarly, a 100% correct classification was obtained when control and COPD groups were compared. Finally, Incalzi et al. [52] used the same quartz-based sensors array to show that breath-prints of patients with OSAS significantly change after a single night of continuous positive airways pressure and it largely depends upon studied comorbidities like diabetes mellitus, metabolic syndrome and chronic heart failure.
Cystic fibrosis
CF is characterised by inflammation and oxidative stress; thus, monitoring of airway inflammation and oxidative stress can be helpful in the diagnosis and monitoring of CF, especially since inflammation arises before clinical symptoms appear [101].
The currently available techniques for measuring inflammation and oxidative stress in the airways are bronchoscopy, bronchoalveolar lavage and biopsy; however, these techniques are too invasive for repeated routine use, especially in children [19]. The oxidative stress that accompanies inflammation in CF and other respiratory diseases leads to the formation of distinctive volatile substances in the breath, which has led to increasing interest in exhaled breath analysis for the detection of CF. FeNO is the most extensively studied marker in exhaled breath, and has been proved to be helpful clinically in some pulmonary diseases, including CF [6, 53]. Nevertheless, monitoring NO has several limitations, most noted in that FeNO is largely a marker of allergic inflammation, thereby limiting its use in nonallergic patients. Next to monitoring preselected unique markers, it is possible to assess the profiles of VOCs in exhaled air [101]. Indeed, the exhaled breath of 64 patients with CF and 21 with primary ciliary dyskinesia were analysed using the polymer composite-based sensors array along with PCA analysis [55]. A cross-validated ROC curve was constructed; breath profiles of patients with CF showed a significant difference from controls (p=0.001) and from patients with primary ciliary dyskinesia (p=0.005). The sensors' response could also differentiate between CF and primary ciliary dyskinesia with or without a number of well-characterised chronic pulmonary infections [55]. Furthermore, the sensors array had the ability to discriminate between patients with CF suffering from chronic pulmonary Pseudomonas aeruginosa infection from those without a chronic pulmonary infection. However, the results indicated that it was impossible to detect any overall difference between chronically and nonchronically infected patients [55]. It was also impossible to differentiate nonchronically infected patients with CF from patients with CF having other chronic pulmonary infections with other pathogens, such as Achromobacter xylosoxidans, Stenotrophomonas maltophilia or a species of the Burkholderia cepacia complex. The authors explained these findings regarding possible bacteria-specific VOCs that patients with CF with chronic pulmonary infection emit in their exhaled breath [55].
McGrath et al. [53] found that patients with CF with an acute exacerbation had lower levels of exhaled isoprene compared with controls [53]. Moreover, when these patients were treated with antibiotics, their isoprene levels increased to normal levels. Ethane levels were also raised in steroid-naïve patients with CF compared with steroid-treated patients [53]. Overall, the data indicate that VOC profiling could be useful in assessing and following up exacerbations, and for rapid detection of P. aeruginosa in patients with CF.
Future perspectives and concluding remarks
Sensor arrays are potentially becoming convenient devices for physicians in the detection and monitoring of therapy of patients with respiratory diseases. Improvement in sensor technologies, machine-learning methods, disease-specific reference libraries and databases, in addition to the identification of respiratory disease biomarkers, have all contributed to the advance in diagnostic methods based on exhaled breath [6, 102]. Nevertheless, before VOC profiling can become a potent clinical tool, considerably more work is needed to allow it to be applied in clinical practice. Several different stages have to be addressed in every part of the development of chemical sensing systems for disease diagnostics. These include clinical and engineering aspects, as well as commercial and ethical issues, which could take years [2]. An important step is for the extensive validation of the currently available VOC profiles [20]. This should be done by worldwide population studies that can statistically confirm many of the suggested patterns. Another approach ought to rely on basic research studies that can connect breath profiles to biological pathways of a particular disease [30, 103, 104]. Other validation aspects to consider, for instance, are the substantial differences in methodology, such as breath collection and sampling techniques across different studies. A standardised methodology is required to take advantage of the different datasets [105]. For example, the relative humidity of exhaled breath may vary and influence measurements; water absorption reduces the sensitivity of metal oxide sensors by preventing electron donation to the surface charge layer. Alternatively, gold or platinum metal monolayer-capped nanoparticle chemiresistors have low sensitivity to water [104]. Another example might be related to the breath manoeuvres; a recent study on chronic rhinosinusitis showed that patients that had underlying asthma presented some confounding influence [20]. This could also relate to differences in sampling procedure due to asthmatic individuals having more difficulties in breathing (e.g. change in exhalation rate, shallow breaths, etc.) [20].
Environmental influences and the effects of ambient and background VOCs also have to be taken into account. For example, during the offline procedure of exhaled VOC collection, Tedlar bags could release VOCs into the collected breath, and storage in Tenax tubes might disturb the composition of the breath sample [106]. Concerning reproducibility, instrumental and classification repeatability between different sensor models must also be considered to allow identical profile and output [107]. In regard to control group selection, an appropriate group of individuals should be selected, taking into account sex, smoking habits, fasting and comorbidities, among other confounding factors. Although age and sex are known to modify individual VOCs, this does not seem to affect the overall profile as analysed by the sensors array [5]. However, the presence of comorbidities, such as renal failure, heart diseases and several forms of cancer, along with smoking habits, hamper the composition of exhaled VOC and therefore the resulting breath-print [108]. Such confounding factors may prevent the diagnosis of interest and should always be taken into consideration [5, 107, 109]. Lastly, one of the most crucial aspects of nanomaterial-based sensor technology is data analysis; the digital outputs generated by the sensors have to be analysed and interpreted in order to provide useful information. The choice of method depends on the type of available input data acquired from the sensors and the type of information that is sought [110].
Sensor response to VOCs can be analysed by pattern recognition algorithms to classify different cases individually, in which the principal component reduction and subsequent pattern recognition by discriminant analysis are the most frequently used types of raw-data analysis for their responses [5]. Other techniques are also used for data analysis, such as machine-learning algorithms and neural networks [111]. These techniques mimic the cognitive process of the human brain, containing interconnected data processing algorithms that work in parallel [110]. The results of the artificial neural network data analysis are usually in the form of a percentage match of identification elements in a given breath sample with those of VOC patterns seen in a training set-up. The diversity of analytical techniques that are available may hinder the standardisation of sensors array technologies, and consequently special care must be given to avoid overfitting the training data and validation sets.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
ERR-0011-2019_Supplementary tables ERR-0011-2019_Supplementary_tables (357.2KB, pdf)
Acknowledgements
The authors acknowledge Yoav Broza (Dept of Chemical Engineering, Technion, Haifa, Israel) for his constructive criticism and proof-reading of this manuscript. D. Hashoul acknowledges the Neubauer Doctoral Fellowship Fund for a PhD scholarship.
Footnotes
This article has supplementary material available from err.ersjournals.com
Provenance: Submitted article, peer reviewed.
Conflict of interest: D. Hashoul has nothing to disclose.
Conflict of interest: H. Haick has nothing to disclose.
References
- 1.Gasparri R, Sedda G, Spaggiari L. The electronic nose's emerging role in respiratory medicine. Sensors (Basel) 2018; 18: E3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broza YY, Vishinkin R, Barash O, et al. . Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem Soc Rev 2018; 47: 4781–4859. [DOI] [PubMed] [Google Scholar]
- 3.Karnon J, Goyder E, Tappenden P, et al. . A review and critique of modelling in prioritising and designing screening programmes. Health Technol Assess 2007; 11. [DOI] [PubMed] [Google Scholar]
- 4.Wilson AD, Baietto M. Advances in electronic-nose technologies developed for biomedical applications. Sensors 2011; 11: 1105–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dragonieri S, Pennazza G, Carratu P, et al. . Electronic nose technology in respiratory diseases. Lung 2017; 195: 157–165. [DOI] [PubMed] [Google Scholar]
- 6.Miekisch W, Schubert JK, Noeldge-Schomburg GF. Diagnostic potential of breath analysis – focus on volatile organic compounds. Clin Chim Acta 2004; 347: 25–39. [DOI] [PubMed] [Google Scholar]
- 7.Azar FE, Azami-Aghdash S, Pournaghi-Azar F, et al. . Cost-effectiveness of lung cancer screening and treatment methods: a systematic review of systematic reviews. BMC Health Serv Res 2017; 17: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nardi-Agmon I, Peled N. Exhaled breath analysis for the early detection of lung cancer: recent developments and future prospects. Lung Cancer 2017; 8: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buszewski B, Kesy M, Ligor T, et al. . Human exhaled air analytics: biomarkers of diseases. Biomed Chromatogr 2007; 21: 553–566. [DOI] [PubMed] [Google Scholar]
- 10.Haick H, Broza YY, Mochalski P, et al. . Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev 2014; 43: 1423–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broza YY, Haick H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine 2013; 8: 785–806. [DOI] [PubMed] [Google Scholar]
- 12.Nakhleh MK, Amal H, Jeries R, et al. . Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017; 11: 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakhleh MK, Haick H, Humbert M, et al. . Volatolomics of breath as an emerging frontier in pulmonary arterial hypertension. Eur Respir J 2017; 49 1601897. [DOI] [PubMed] [Google Scholar]
- 14.Paredi P, Kharitonov SA, Barnes PJ. Elevation of exhaled ethane concentration in asthma. Am J Respir Crit Care Med 2000; 162: 1450–1454. [DOI] [PubMed] [Google Scholar]
- 15.Kischkel S, Miekisch W, Sawacki A, et al. . Breath biomarkers for lung cancer detection and assessment of smoking related effects – confounding variables, influence of normalization and statistical algorithms. Clin Chim Acta 2010; 411: 1637–1644. [DOI] [PubMed] [Google Scholar]
- 16.de Gennaro G, Dragonieri S, Longobardi F, et al. . Chemical characterization of exhaled breath to differentiate between patients with malignant pleural mesothelioma from subjects with similar professional asbestos exposure. Anal Bioanal Chem 2010; 398: 3043–3050. [DOI] [PubMed] [Google Scholar]
- 17.Barker M, Hengst M, Schmid J, et al. . Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur Respir J 2006; 27: 929–936. [DOI] [PubMed] [Google Scholar]
- 18.Alkhouri N, Cikach F, Eng K, et al. . Analysis of breath volatile organic compounds as a noninvasive tool to diagnose nonalcoholic fatty liver disease in children. Eur J Gastroenterol Hepatol 2014; 26: 82–87. [DOI] [PubMed] [Google Scholar]
- 19.van de Kant KD, van der Sande LJ, Jobsis Q, et al. . Clinical use of exhaled volatile organic compounds in pulmonary diseases: a systematic review. Respir Res 2012; 13: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vishinkin R, Haick H. Nanoscale sensor technologies for disease detection via volatolomics. Small 2015; 11: 6142–6164. [DOI] [PubMed] [Google Scholar]
- 21.Rock F, Barsan N, Weimar U. Electronic nose: current status and future trends. Chem Rev 2008; 108: 705–725. [DOI] [PubMed] [Google Scholar]
- 22.Thaler ER, Kennedy DW, Hanson CW. Medical applications of electronic nose technology: review of current status. Am J Rhinol 2001; 15: 291–295. [PubMed] [Google Scholar]
- 23.Montuschi P, Mores N, Trove A, et al. . The electronic nose in respiratory medicine. Respiration 2013; 85: 72–84. [DOI] [PubMed] [Google Scholar]
- 24.Tang Z, Yang J, Yu J, et al. . A colorimetric sensor for qualitative discrimination and quantitative detection of volatile amines. Sensors 2010; 10: 6463–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikov A, Lazar Z, Horvath I. Established methodological issues in electronic nose research: how far are we from using these instruments in clinical settings of breath analysis? J Breath Res 2015; 9: 034001. [DOI] [PubMed] [Google Scholar]
- 26.Szulczyński B, Gębicki J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments 2017; 4: 21. [Google Scholar]
- 27.Di Natale C, Macagnano A, Martinelli E, et al. . Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens Bioelectron 2003; 18: 1209–1218. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Cao M, Li Y, et al. . A study of an electronic nose for detection of lung cancer based on a virtual SAW gas sensors array and imaging recognition method. Meas Sci Technol 2005; 16: 1535. [Google Scholar]
- 29.Mazzone PJ, Hammel J, Dweik R, et al. . Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 2007; 62: 565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakim M, Broza YY, Barash O, et al. . Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev 2012; 112: 5949–5966. [DOI] [PubMed] [Google Scholar]
- 31.Peled N, Hakim M, Bunn PA, et al. . Non-invasive breath analysis of pulmonary nodules. J Thorac Oncol 2012; 7: 1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broza YY, Kremer R, Tisch U, et al. . A nanomaterial-based breath test for short-term follow-up after lung tumor resection. Nanomedicine 2013; 9: 15–21. [DOI] [PubMed] [Google Scholar]
- 33.Barash O, Peled N, Hirsch FR, et al. . Sniffing the unique “odor print” of non-small-cell lung cancer with gold nanoparticles. Small 2009; 5: 2618–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Amico A, Pennazza G, Santonico M, et al. . An investigation on electronic nose diagnosis of lung cancer. Lung Cancer 2010; 68: 170–176. [DOI] [PubMed] [Google Scholar]
- 35.Mazzone PJ, Wang XF, Xu Y, et al. . Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer. J Thorac Oncol 2012; 7: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlomi D, Abud M, Liran O, et al. . Detection of lung cancer and EGFR mutation by electronic nose system. J Thorac Oncol 2017; 12: 1544–1551. [DOI] [PubMed] [Google Scholar]
- 37.Fens N, Roldaan AC, van der Schee MP, et al. . External validation of exhaled breath profiling using an electronic nose in the discrimination of asthma with fixed airways obstruction and chronic obstructive pulmonary disease. Clin Exp Allergy 2011; 41: 1371–1378. [DOI] [PubMed] [Google Scholar]
- 38.Cazzola M, Segreti A, Capuano R, et al. . Analysis of exhaled breath fingerprints and volatile organic compounds in COPD. COPD Res Pract 2015; 1: 7. [Google Scholar]
- 39.Sibila O, Garcia-Bellmunt L, Giner J, et al. . Identification of airway bacterial colonization by an electronic nose in chronic obstructive pulmonary disease. Respir Med 2014; 108: 1608–1614. [DOI] [PubMed] [Google Scholar]
- 40.Hattesohl AD, Jorres RA, Dressel H, et al. . Discrimination between COPD patients with and without alpha 1-antitrypsin deficiency using an electronic nose. Respirology 2011; 16: 1258–1264. [DOI] [PubMed] [Google Scholar]
- 41.Dragonieri S, Annema JT, Schot R, et al. . An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 2009; 64: 166–170. [DOI] [PubMed] [Google Scholar]
- 42.van Geffen WH, Bruins M, Kerstjens HA. Diagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronic nose: a pilot study. J Breath Res 2016; 10: 036001. [DOI] [PubMed] [Google Scholar]
- 43.Finnegan LP. Treatment issues for opioid-dependent women during the perinatal period. J Psychoactive Drugs 1991; 23: 191–201. [DOI] [PubMed] [Google Scholar]
- 44.Montuschi P, Santonico M, Mondino C, et al. . Diagnostic performance of an electronic nose, fractional exhaled nitric oxide, and lung function testing in asthma. Chest 2010; 137: 790–796. [DOI] [PubMed] [Google Scholar]
- 45.Fens N, Zwinderman AH, van der Schee MP, et al. . Exhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med 2009; 180: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 46.Hora MS, Rana RK, Wilcox CL, et al. . Development of a lyophilized formulation of interleukin-2. Dev Biol Stand 1992; 74: 295–303. [PubMed] [Google Scholar]
- 47.Plaza V, Crespo A, Giner J, et al. . Inflammatory asthma phenotype discrimination using an electronic nose breath analyzer. J Investig Allergol Clin Immunol 2015; 25: 431–437. [PubMed] [Google Scholar]
- 48.de Vries R, Brinkman P, van der Schee MP, et al. . Integration of electronic nose technology with spirometry: validation of a new approach for exhaled breath analysis. J Breath Res 2015; 9: 046001. [DOI] [PubMed] [Google Scholar]
- 49.Cohen-Kaminsky S, Nakhleh M, Perros F, et al. . A proof of concept for the detection and classification of pulmonary arterial hypertension through breath analysis with a sensor array. Am J Respir Crit Care Med 2013; 188: 756–759. [DOI] [PubMed] [Google Scholar]
- 50.Greulich T, Hattesohl A, Grabisch A, et al. . Detection of obstructive sleep apnoea by an electronic nose. Eur Respir J 2013; 42: 145–155. [DOI] [PubMed] [Google Scholar]
- 51.Dragonieri S, Porcelli F, Longobardi F, et al. . An electronic nose in the discrimination of obese patients with and without obstructive sleep apnoea. J Breath Res 2015; 9: 026005. [DOI] [PubMed] [Google Scholar]
- 52.Antonelli Incalzi R, Pennazza G, Scarlata S, et al. . Comorbidity modulates non-invasive ventilation-induced changes in breath print of obstructive sleep apnea syndrome patients. Sleep Breath 2015; 19: 623–630. [DOI] [PubMed] [Google Scholar]
- 53.McGrath LT, Patrick R, Mallon P, et al. . Breath isoprene during acute respiratory exacerbation in cystic fibrosis. Eur Respir J 2000; 16: 1065–1069. [DOI] [PubMed] [Google Scholar]
- 54.Paff T, van der Schee MP, Daniels JM, et al. . Exhaled molecular profiles in the assessment of cystic fibrosis and primary ciliary dyskinesia. J Cyst Fibros 2013; 12: 454–460. [DOI] [PubMed] [Google Scholar]
- 55.Joensen O, Paff T, Haarman EG, et al. . Exhaled breath analysis using electronic nose in cystic fibrosis and primary ciliary dyskinesia patients with chronic pulmonary infections. PLoS One 2014; 9: e115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakhleh MK, Jeries R, Gharra A, et al. . Detecting active pulmonary tuberculosis with a breath test using nanomaterial-based sensors. Eur Respir J 2014; 43: 1522–1525. [DOI] [PubMed] [Google Scholar]
- 57.Bruins M, Rahim Z, Bos A, et al. . Diagnosis of active tuberculosis by e-nose analysis of exhaled air. Tuberculosis 2013; 93: 232–238. [DOI] [PubMed] [Google Scholar]
- 58.Zetola NM, Modongo C, Matsiri O, et al. . Diagnosis of pulmonary tuberculosis and assessment of treatment response through analyses of volatile compound patterns in exhaled breath samples. J Infect 2017; 74: 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips M, Cataneo RN, Condos R, et al. . Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis 2007; 87: 44–52. [DOI] [PubMed] [Google Scholar]
- 60.Phillips M, Basa-Dalay V, Bothamley G, et al. . Breath biomarkers of active pulmonary tuberculosis. Tuberculosis 2010; 90: 145–151. [DOI] [PubMed] [Google Scholar]
- 61.Sandlund J, Lim S, Queralto N, et al. . Development of colorimetric sensor array for diagnosis of tuberculosis through detection of urinary volatile organic compounds. Diagn Microbiol Infect Dis 2018; 92: 299–304. [DOI] [PubMed] [Google Scholar]
- 62.Yang HY, Peng HY, Chang CJ, et al. . Diagnostic accuracy of breath tests for pneumoconiosis using an electronic nose. J Breath Res 2017; 12: 016001. [DOI] [PubMed] [Google Scholar]
- 63.Ho WE, Xu YJ, Xu F, et al. . Metabolomics reveals altered metabolic pathways in experimental asthma. Am J Respir Cell Mol Biol 2013; 48: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basanta M, Jarvis RM, Xu Y, et al. . Non-invasive metabolomic analysis of breath using differential mobility spectrometry in patients with chronic obstructive pulmonary disease and healthy smokers. Analyst 2010; 135: 315–320. [DOI] [PubMed] [Google Scholar]
- 65.Fens N, de Nijs SB, Peters S, et al. . Exhaled air molecular profiling in relation to inflammatory subtype and activity in COPD. Eur Respir J 2011; 38: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 66.Fens N, van Rossum AG, Zanen P, et al. . Subphenotypes of mild-to-moderate COPD by factor and cluster analysis of pulmonary function, CT imaging and breathomics in a population-based survey. COPD 2013; 10: 277–285. [DOI] [PubMed] [Google Scholar]
- 67.Zeki AA, Schivo M, Chan A, et al. . The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy 2011; 2011: 861926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pite H, Morais-Almeida M, Rocha SM. Metabolomics in asthma: where do we stand? Curr Opin Pulm Med 2018; 24: 94–103. [DOI] [PubMed] [Google Scholar]
- 69.Kharitonov SA, Barnes PJ. Clinical aspects of exhaled nitric oxide. Eur Respir J 2000; 16: 781–792. [DOI] [PubMed] [Google Scholar]
- 70.Barnes PJ, Dweik RA, Gelb AF, et al. . Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 2010; 138: 682–692. [DOI] [PubMed] [Google Scholar]
- 71.Ashutosh K. Nitric oxide and asthma: a review. Curr Opin Pulm Med 2000; 6: 21–25. [DOI] [PubMed] [Google Scholar]
- 72.Wilson AD. Application of electronic-nose technologies and VOC-biomarkers for the noninvasive early diagnosis of gastrointestinal diseases. Sensors (Basel) 2018; 18: E2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nurmatov UB, Tagiyeva N, Semple S, et al. . Volatile organic compounds and risk of asthma and allergy: a systematic review. Eur Respir Rev 2015; 24: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jia Z, Zhang H, Ong CN, et al. . Detection of lung cancer: concomitant volatile organic compounds and metabolomic profiling of six cancer cell lines of different histological origins. ACS Omega 2018; 3: 5131–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cutler DM. Are we finally winning the war on cancer? J Econ Perspect 2008; 22: 3–26. [DOI] [PubMed] [Google Scholar]
- 76.Peng G, Hakim M, Broza YY, et al. . Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer 2010; 103: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bajtarevic A, Ager C, Pienz M, et al. . Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009; 9: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amann A, Corradi M, Mazzone P, et al. . Lung cancer biomarkers in exhaled breath. Expert Rev Mol Diagn 2011; 11: 207–217. [DOI] [PubMed] [Google Scholar]
- 79.Phillips M, Herrera J, Krishnan S, et al. . Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl 1999; 729: 75–88. [DOI] [PubMed] [Google Scholar]
- 80.Mendis S, Sobotka PA, Euler DE. Pentane and isoprene in expired air from humans: gas-chromatographic analysis of single breath. Clin Chem 1994; 40: 1485–1488. [PubMed] [Google Scholar]
- 81.Smith D, Wang T, Sule-Suso J, et al. . Quantification of acetaldehyde released by lung cancer cells in vitro using selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom 2003; 17: 845–850. [DOI] [PubMed] [Google Scholar]
- 82.Phillips M, Altorki N, Austin JH, et al. . Detection of lung cancer using weighted digital analysis of breath biomarkers. Clin Chim Acta 2008; 393: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Phillips M, Cataneo RN, Cummin AR, et al. . Detection of lung cancer with volatile markers in the breath. Chest 2003; 123: 2115–2123. [DOI] [PubMed] [Google Scholar]
- 84.Devillier P, Salvator H, Naline E, et al. . Metabolomics in the diagnosis and pharmacotherapy of lung diseases. Curr Pharm Des 2017; 23: 2050–2059. [DOI] [PubMed] [Google Scholar]
- 85.Nardi-Agmon I, Abud-Hawa M, Liran O, et al. . Exhaled breath analysis for monitoring response to treatment in advanced lung cancer. J Thorac Oncol 2016; 11: 827–837. [DOI] [PubMed] [Google Scholar]
- 86.Lai YC, Potoka KC, Champion HC, et al. . Pulmonary arterial hypertension: the clinical syndrome. Circ Res 2014; 115: 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galie N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force f. or the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 88.Humbert M, Morrell NW, Archer SL, et al. . Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43: 12 Suppl S, 13S–24S. [DOI] [PubMed] [Google Scholar]
- 89.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004; 351: 1425–1436. [DOI] [PubMed] [Google Scholar]
- 90.Cikach FS Jr, Tonelli AR, Barnes J, et al. . Breath analysis in pulmonary arterial hypertension. Chest 2014; 145: 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Samad A, Sultana Y, Akhter MS, et al. . Treatment of tuberculosis: use of active pharmaceuticals. Recent Pat Antiinfect Drug Discov 2008; 3: 34–44. [DOI] [PubMed] [Google Scholar]
- 92.de Heer K, van der Schee MP, Zwinderman K, et al. . Electronic nose technology for detection of invasive pulmonary aspergillosis in prolonged chemotherapy-induced neutropenia: a proof-of-principle study. J Clin Microbiol 2013; 51: 1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Heer K, Kok MG, Fens N, et al. . Detection of airway colonization by Aspergillus fumigatus by use of electronic nose technology in patients with cystic fibrosis. J Clin Microbiol 2016; 54: 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bos LD, Martin-Loeches I, Kastelijn JB, et al. . The volatile metabolic fingerprint of ventilator-associated pneumonia. Intensive Care Med 2014; 40: 761–762. [DOI] [PubMed] [Google Scholar]
- 95.Hockstein NG, Thaler ER, Torigian D, et al. . Diagnosis of pneumonia with an electronic nose: correlation of vapor signature with chest computed tomography scan findings. Laryngoscope 2004; 114: 1701–1705. [DOI] [PubMed] [Google Scholar]
- 96.Hanson CW 3rd, Thaler ER. Electronic nose prediction of a clinical pneumonia score: biosensors and microbes. Anesthesiology 2005; 102: 63–68. [DOI] [PubMed] [Google Scholar]
- 97.Dutta R, Hines EL, Gardner JW, et al. . Bacteria classification using Cyranose 320 electronic nose. Biomed Eng Online 2002; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shafiek H, Fiorentino F, Merino JL, et al. . Using the electronic nose to identify airway infection during COPD exacerbations. PLoS One 2015; 10: e0135199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benedek P, Lazar Z, Bikov A, et al. . Exhaled biomarker pattern is altered in children with obstructive sleep apnoea syndrome. Int J Pediatr Otorhinolaryngol 2013; 77: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 100.Scarlata S, Pennazza G, Santonico M, et al. . Screening of obstructive sleep apnea syndrome by electronic-nose analysis of volatile organic compounds. Sci Rep 2017; 7: 11938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith D, Sovova K, Dryahina K, et al. . Breath concentration of acetic acid vapour is elevated in patients with cystic fibrosis. J Breath Res 2016; 10: 021002. [DOI] [PubMed] [Google Scholar]
- 102.Zhang M, Sun JJ, Khatib M, et al. . Time-space-resolved origami hierarchical electronics for ultrasensitive detection of physical and chemical stimuli. Nat Commun 2019; 10: 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martinez-Lozano Sinues P, Zenobi R, Kohler M. Analysis of the exhalome: a diagnostic tool of the future. Chest 2013; 144: 746–749. [DOI] [PubMed] [Google Scholar]
- 104.Konvalina G, Haick H. Effect of humidity on nanoparticle-based chemiresistors: a comparison between synthetic and real-world samples. ACS Appl Mater Interfaces 2012; 4: 317–325. [DOI] [PubMed] [Google Scholar]
- 105.Broza YY, Braverman I, Haick H. Breath volatolomics for diagnosing chronic rhinosinusitis. Int J Nanomedicine 2018; 13: 4661–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pereira J, Porto-Figueira P, Cavaco C, et al. . Breath analysis as a potential and non-invasive frontier in disease diagnosis: an overview. Metabolites 2015; 5: 3–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Konvalina G, Haick H. Sensors for breath testing: from nanomaterials to comprehensive disease detection. Acc Chem Res 2013; 47: 66–76. [DOI] [PubMed] [Google Scholar]
- 108.Bikov A, Paschalaki K, Logan-Sinclair R, et al. . Standardised exhaled breath collection for the measurement of exhaled volatile organic compounds by proton transfer reaction mass spectrometry. BMC Pulm Med 2013; 13: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McWilliams A, Beigi P, Srinidhi A, et al. . Sex and smoking status effects on the early detection of early lung cancer in high-risk smokers using an electronic nose. IEEE Trans Biomed Eng 2015; 62: 2044–2054. [DOI] [PubMed] [Google Scholar]
- 110.Wilson A, Baietto M. Applications and advances in electronic-nose technologies. Sensors 2009; 9: 5099–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanna GB, Boshier PR, Markar SR, et al. . Accuracy and methodologic challenges of volatile organic compound-based exhaled breath tests for cancer diagnosis: a systematic review and meta-analysis. JAMA Oncol 2018: e182815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
ERR-0011-2019_Supplementary tables ERR-0011-2019_Supplementary_tables (357.2KB, pdf)