Abstract
Sarcoidosis-associated pulmonary hypertension (SAPH) is an important complication of advanced sarcoidosis. Over the past few years, there have been several studies dealing with screening, diagnosis and treatment of SAPH. This includes the results of two large SAPH-specific registries. A task force was established by the World Association of Sarcoidosis and Other Granulomatous disease (WASOG) to summarise the current level of knowledge in the area and provide guidance for the management of patients. A group of sarcoidosis and pulmonary hypertension experts participated in this task force. The committee developed a consensus regarding initial screening including who should undergo more specific testing with echocardiogram. Based on the results, the committee agreed upon who should undergo right-heart catheterisation and how to interpret the results. The committee felt there was no specific phenotype of a SAPH patient in whom pulmonary hypertension-specific therapy could be definitively recommended. They recommended that treatment decisions be made jointly with a sarcoidosis and pulmonary hypertension expert. The committee recognised that there were significant defects in the current knowledge regarding SAPH, but felt the statement would be useful in directing future studies.
Short abstract
Sarcoidosis-associated pulmonary hypertension is a significant cause of morbidity and mortality. A guide to screening, diagnosis and treatment has been developed by a team of experts. https://bit.ly/3FzOcgb
Introduction
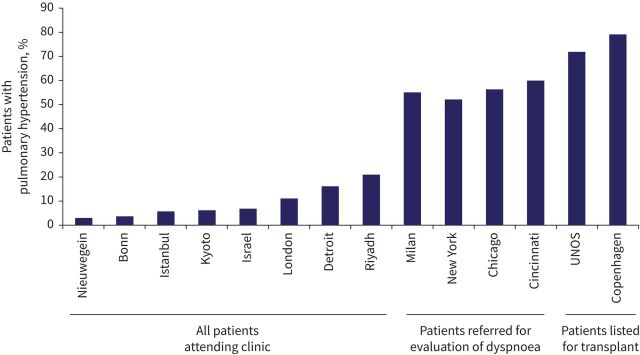
Pulmonary hypertension (PH) is haemodynamically defined by right-heart catheterisation (RHC) by a mean pulmonary artery pressure (mPAP) above 20 mmHg. Post-capillary PH is characterised by a pulmonary artery wedge pressure (PAWP) above 15 mmHg while pre-capillary PH is defined by PAWP ≤15 mmHg and pulmonary vascular resistance (PVR) >3 Wood's units (WU). Right ventricle (RV) remodelling and dysfunction induced by increased afterload results in exercise limitation and death. The purpose of the recently updated PH 6th World Symposium clinical classification was to categorise clinical conditions associated with PH based on similar pathophysiological mechanisms, clinical presentation, haemodynamic characteristics and therapeutic management. Sarcoidosis-associated pulmonary hypertension (SAPH) can be caused by different and sometimes overlapping mechanisms and it has remained in Group 5 of the clinical classification of PH. SAPH is a significant cause of morbidity and mortality in patients with advanced sarcoidosis [1, 2]. The reported incidence of SAPH has been around 5% in studies from across the world. Higher incidences were reported in patients who had persistent dyspnoea or who were being evaluated for lung transplantation (figure 1) [3–16]. Over the past few years, there has been increasing recognition of the prevalence of this major complication of sarcoidosis, as well as studies regarding screening/early detection, diagnosis and treatment.
FIGURE 1.
The reported incidence of SAPH from various centres across the world. The city or country of origin of the study is indicated. A higher incidence of SAPH was found for patients being evaluated for persistent dyspnoea or lung transplantation [3–16]. SAPH: sarcoidosis-associated pulmonary hypertension; UNOS: United Network Organ Sharing.
Based on the increasing evidence and the need for better awareness of SAPH, a task force was established by the World Association of Sarcoidosis and Other Granulomatous diseases (WASOG) and the Foundation for Sarcoidosis Research (FSR). This global task force was chaired by three international experts: two of sarcoidosis (RP Baughman, AU Wells) and one of PH (M Humbert). The committee met physically and virtually over 3 years (2019–2021). The committee acknowledges that the present recommendations were for the most part based on limited information available for SAPH. Many organisations such as the European Respiratory Society use the GRADE method and PICO questions (patient, intervention, comparison, outcomes) [17] for clinical practice guidelines. We decided not to use this approach because there were too few well-designed large multicentre SAPH registries and randomised clinical trials. The committee therefore focused on specific areas regarding screening, diagnosis and treatment and made specific comments (table 1). The comments were based on a literature review and a series of polls of the committee members to provide answers where there was consensus among the committee members. Consensus was defined as >70% of voting members agreeing on an individual statement. This Statement is narrative and pragmatic rather than systematic and is further complemented by a description of usual clinical practice and the experience of the panel members [18]. This Statement summarises the current state of knowledge and it does not make formal recommendations for clinical practice.
TABLE 1.
Summary of Task Force comments.
|
|
|
|
|
|
|
|
|
SAPH: sarcoidosis-associated pulmonary hypertension; WHO FC: World Health Organization Functional Class; 6MWD: 6-min walk distance; RHC: right-heart catheterisation; PH: pulmonary hypertension; PAH: pulmonary arterial hypertension.
SAPH clinical outcomes
Is SAPH associated with increased morbidity?
The presence of PH in sarcoidosis is associated with greater supplemental oxygen requirements [10, 12, 15, 19, 20] as exemplified by an analysis of 363 patients with sarcoidosis listed for lung transplantation, in which subjects with SAPH required more supplemental oxygen (2.7±1.8 versus 1.6±1.4 L·min−1) compared with those without PH [15]. In the ReSAPH registry analysis of 176 patients, most desaturated during the 6-min walk test (6MWT), with a median degree of desaturation of 5% [20]. Similarly, in the French Registry cohort of 126 patients with moderate-to-severe SAPH followed over a 10-year period, 54% were on long-term oxygen [21]. Presence of SAPH also increases the burden on functional capacity [10, 22–24], employment status and need for caregiver assistance [19]. In the French Registry, 83% of the patients with SAPH reported World Health Organization (WHO) functional class (FC) III–IV symptoms [21]. In the Duke cohort of 95 patients followed for 11 years, almost all patients (99%) were symptomatic with activity (WHO FC II–IV symptoms) and 77% of the cohort reported WHO FC III/IV symptoms [22]. In a small British cohort of 24 patients, 48% had WHO FC III symptoms, while 42% had WHO FC IV symptoms [23]. Moreover, objective assessment of functional capacity supports subjectively reported decreased exercise tolerance in patients with SAPH. The 6-min walk distance (6MWD) was consistently found to be reduced in several studies, with mean values ranging between 305 and 320 metres [20, 21, 24]. The French Registry analysis which captured dyspnoea assessed by the Borg scale found it to be elevated at 4.0±2.3 [21] during the performance of the 6MWT.
Summary
Presence of SAPH is associated with significant symptomatology and morbidity, as evidenced by increased WHO FC, decreased 6MWD or desaturation and increased oxygen use.
Is SAPH an independent predictor of mortality?
Several studies have assessed the presence of RV dysfunction in patients with SAPH. In the Duke cohort, the median N-terminal pro-brain natriuretic peptide (NT-proBNP) level at the time of initial evaluation was 910 pg·mL−1 (Q1–Q3: 225–2807). On transthoracic echocardiogram, the majority (59%) of patients had either moderate or severe RV enlargement and 55% had moderate or severe RV dysfunction [22]. Interestingly, in this cohort, neither baseline echocardiographic nor haemodynamic characterisation of RV function were associated with outcomes, but follow-up NT-proBNP levels were higher in those who died or were hospitalised (1258.0 versus 262.0 pg·mL−1, p=0.007).
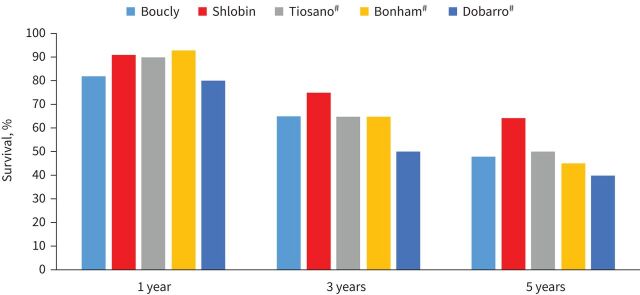
The mortality implications of any PH, both pre- and post-capillary, in the context of sarcoidosis are also profound, with a 10-fold increase in mortality and an estimated 5-year survival of only 59% [1, 14, 25]. Figure 2 shows the 1-, 3-, and 5-year survival reported from several centres across the world [7, 13, 21, 23, 24]. In the Duke cohort, the median time from diagnosis of SAPH to either death or hospitalisation was 6 months (Q1–Q3: 3.0–12.0 months) [22]. Patients with SAPH are more likely to be listed for lung transplant and also have a greater likelihood of succumbing while on the waiting list [15, 26]. In the British cohort, the rate of mortality or transplantation was 41.2% with the median survival without transplantation being 5.3 years. In that study, more patients who died or underwent transplantation during follow-up had baseline RV dysfunction (80%) [23]. The ReSAPH registry analysis of 159 patients with pre-capillary SAPH demonstrated the 1-, 3- and 5-year transplant-free survival to be 83.7%, 70.6% and 58.5%, respectively. Severe gas transfer impairment (diffusing lung capacity for carbon monoxide (DLCO) <35% predicted) and 6MWD <300 m were strong predictors of decreased survival (p=0.0151 and p <0.0001, respectively) [24]. These results were similar to the French Registry, which demonstrated 3- and 5-year survival rates of 74% and 55%, respectively [21].
FIGURE 2.
The 1-, 3- and 5-year survival from sarcoidosis-associated pulmonary hypertension [7, 13, 21, 23, 24]. #: For three of the studies [7, 13, 23], the survival was estimated based on the Kaplan–Meier survival curves presented in the papers.
In patients with SAPH from the French Registry, univariate analysis showed that WHO FC IV, 6MWD and reduced forced vital capacity (FVC) or DLCO were associated with a poor survival [21]. In multivariate analysis, only 6MWD remained independently associated with mortality [21]. It is still not clear if patients with SAPH are succumbing because of PH or in the presence of PH. In one study, which demonstrated that SAPH was associated with mortality, the only haemodynamic factor that remained predictive of mortality after multivariable analysis was the right arterial pressure [26]. In another study of a small cohort of 24 patients, presence of right ventricular dysfunction on echocardiography was the most powerful predictor of death or transplantation (OR 83.1, 95% CI 2.2–31.02, p=0.017) [23]. This evidence of right-sided heart failure implies that patients with SAPH are indeed dying from their PH, rather than the PH being an epiphenomenon.
In two multi-regression analyses PH has been found to be an independent risk factor for mortality [1, 2]. In evaluating patients awaiting lung transplant the final prediction model for mortality included mPAP [19]. In an analysis of fibrotic sarcoidosis patients, presence of PH by echocardiography had a hazard ratio for mortality of 3.42. In the study by Kirkil et al. [1], haemodynamically confirmed pre-capillary SAPH had a hazard ratio of 8.96 and, along with fibrosis >20% by high-resolution computed tomography (HRCT) and age, remained an independent predictor of mortality by Cox modelling.
Summary
PH is an independent predictor of mortality in patients with sarcoidosis.
Classification/cause of SAPH
Should SAPH be divided into various subclasses
SAPH can be caused by a variety of mechanisms (table 2). Due to the predilection of granulomas for the lymphatics, which are found in the bronchovascular bundles and the interlobular septae, pulmonary vascular granulomatous involvement typically occurs on both the arterial and venous sides of the circulation. In one study of organ explants from patients with sarcoidosis undergoing lung transplantation, features of venous involvement were identified in addition to arterial disease [25]. An increased incidence of pulmonary emboli is frequently encountered in sarcoidosis patients [27–29], and chronic thromboembolism can potentially lead to Group 4 PH [30]. Interstitial changes are also highly associated with the development of PH [20, 21]. Although more than half of patients with SAPH have clinical fibrotic lung disease, most studies confirm that up to 20% of patients with SAPH have no radiographic evidence of parenchymal lung disease [12, 20, 31]. Alveolar hypoxia, most frequently in the setting of parenchymal involvement, may also contribute to the development of PH. Hilar adenopathy compressing the pulmonary arteries and veins can also lead to PH. However, compression usually has to be of multiple vessels to lead to significant PH. In a study of 156 patients with SAPH, significant compressive hilar adenopathy leading to PH was identified in only two patients [21]. Fibrosing mediastinitis due to sarcoidosis can also contribute to PH [32–34]. In the French Registry, 3 of 156 patients with SAPH had fibrosing mediastinitis as the identified cause [21]. In a prospective study of 72 patients with SAPH from a single institution, eight patients were found to have vascular compression/distortion. In all eight patients, stenting was successful in reducing pulmonary artery pressure [35].
TABLE 2.
Causes of pulmonary hypertension in sarcoidosis patients
| Condition | Potential treatments |
| Vascular disease | |
| Vasculitis | Glucocorticoids and other anti-inflammatory treatments Pulmonary vasodilators |
| Granulomatous vascular involvement | Glucocorticoids and other anti-inflammatory treatments Pulmonary vasodilators |
| Veno-occlusive disease | Glucocorticoids and other anti-inflammatory treatments Careful use of pulmonary vasodilators |
| Pulmonary embolism (CTEPH) | Anticoagulation Balloon pulmonary angioplasty Pulmonary endarterectomy Pulmonary vasodilators |
| Interstitial lung disease | |
| Parenchymal lung disease due to granulomas | Glucocorticoids and other anti-inflammatory treatments |
| Parenchymal lung disease due to fibrosis | Anti-fibrotic agents |
| Hilar and mediastinal distortion | |
| Pulmonary artery/vein extrinsic compression | Glucocorticoids and other anti-inflammatory treatments Dilation and/or stenting of compressed vessels |
| Fibrosing mediastinitis | Dilation and/or stenting of compressed vessels |
| Extrapulmonary disease | |
| Left ventricular systolic dysfunction | Glucocorticoids and other anti-inflammatory treatments Diuretics, afterload reduction |
| Left ventricular diastolic dysfunction | Diuretics |
| Sleep apnoea | CPAP, oxygen and other measures |
| Liver disease | Glucocorticoids and other anti-inflammatory treatments |
CTEPH: chronic thromboembolic pulmonary hypertension; CPAP: continuous positive airway pressure.
Left ventricular disease can lead to development of post-capillary PH. In one study, approximately 20% of patients with SAPH demonstrated elevated PAWP, consistent with left ventricular failure [14]. Sarcoidosis patients may have comorbid coronary artery disease. This can be a cause of significant morbidity in sarcoidosis [36]. Evaluation of patients with elevated pulmonary artery pressure should exclude left ventricular disease as the basis of either ischaemic or nonischaemic heart disease. Granulomatous inflammation can also cause an infiltrative cardiomyopathy often leading to left heart failure [37]. However, in some cases, the left ventricular ejection fraction may be normal or only mildly impaired but still have a less compliant left ventricle [38, 39].
Sleep-related breathing disorders can contribute to PH [40, 41], and patients with sarcoidosis have an increased incidence of obstructive sleep apnoea syndrome (OSAS) [42–44]. Of note, chronic use of corticosteroids increases the risk for OSAS in sarcoidosis patients, regardless of gender of patient [44]. If OSAS is diagnosed, treatment with continuous positive airway pressure (CPAP) should be initiated. Although the liver is one of the most commonly affected organs in sarcoidosis [45, 46], severe liver disease is relatively uncommon [47, 48] and portopulmonary hypertension is rarely seen.
Summary
Since the cause of SAPH is multifactorial, the committee commented that the current recommendation to keep SAPH in Group 5 was reasonable. The committee considered that patients with left ventricular disease either due to sarcoidosis or other conditions should be treated as WHO Group 2. The committee felt it was important to identify a dominant cause for SAPH on an individual basis, as it is likely to have treatment implications.
Screening and diagnosis of SAPH
Who should get a transthoracic echocardiogram?
Screening is defined as the systematic use of a test, or tests, in at-risk individuals to identify disease before symptom onset. Previous studies have shown that screening allows earlier management and better clinical outcome in patients with systemic sclerosis-associated pulmonary arterial hypertension (PAH) [49]. While the consequences of SAPH screening remains unknown, the task force felt that it is desirable to improve awareness of this severe complication of sarcoidosis in an attempt to diagnose it earlier when functional impairment and haemodynamics are less severely compromised. Therefore, screening (in asymptomatic patients) and early diagnosis (when symptoms and functional impairment are present) have the potential to identify SAPH at an early stage. It has been previously recommended using GRADE methodology that a transthoracic echocardiogram should be performed in those patients with sarcoidosis in whom PH is suspected (conditional recommendation) [50].
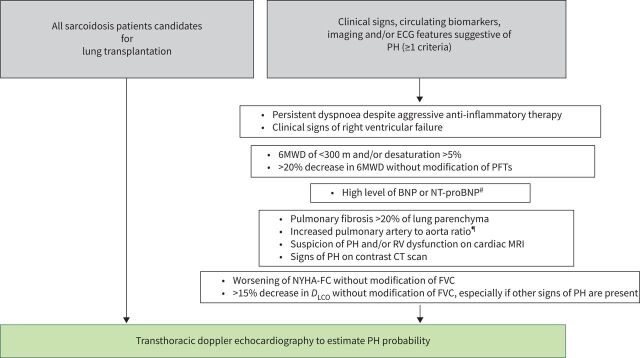
The task force evaluated what factors would lead to the decision to perform a transthoracic echocardiogram in patients with sarcoidosis and identified several features which were likely to be associated with SAPH (figure 3). The task force felt the presence of one or more of these features should lead to echocardiography. RHC should be performed when echocardiography gives a high or intermediate probability of PH (according to the ESC/ERS PH guidelines) (figure 4) [51] and for all patients being considered for lung transplant. For those patients, the RHC should be followed by left catheterisation and coronarography because of the potential implication of the results for the patient's management and on future lung transplantation in eligible cases (timing of transplantation, lung or heart-lung transplantation, need of extracorporeal circulation). Over 70% of sarcoidosis patients undergoing lung transplant will have SAPH and presence of SAPH increases the indication for transplant [16, 19]. However, SAPH was not associated with higher mortality after transplant [52]. While a recent study did not find that SAPH was associated with increased mortality for patients on the lung transplant list [53], patients with SAPH still had significant morbidity.
FIGURE 3.
Algorithm for who should undergo transthoracic echocardiogram to evaluate for SAPH. 6MWD: 6-min walk distance; BNP: B-type natriuretic peptide; BSA: body surface area; CT: computed tomography; DLCO: diffusing capacity of the lung for carbon monoxide; ECG: electrocardiogram; FVC: forced vital capacity; MRI: magnetic resonance imaging; NT-proBNP: N-terminal (NT)-pro hormone BNP; NYHA-FC: New York Heart Association functional class; PFT: pulmonary function test; PH: pulmonary hypertension; RV: right ventricle; SAPH: sarcoidosis-associated pulmonary hypertension. #: Transthoracic echocardiogram recommended to assess for SAPH and left ventricular dysfunction. ¶: May be more accurate if corrected for BSA.
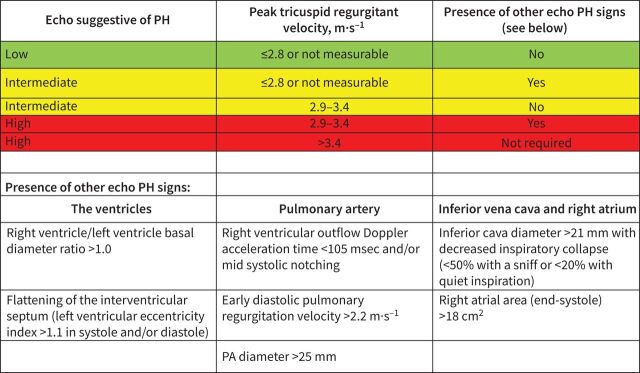
FIGURE 4.
Probability of pulmonary hypertension from echocardiography findings of direct estimate of right ventricular systolic pressure and indirect evidence of right ventricular strain. Reproduced from Galie et al. [51]. PA: pulmonary artery; PH: pulmonary hypertension.
Other features, ranging from chest imaging to serum biomarkers, have been associated with the presence of SAPH and therefore could be considered as part of the screening process. In three large studies, approximately half of sarcoidosis patients with persistent dyspnoea despite anti-inflammatory therapy were noted to have SAPH [11, 12, 14]. These studies did not identify which specific complaint or level of dyspnoea led to further evaluation. However, all three studies found that the rate of SAPH was up to ten times higher than the general sarcoidosis population. Patients with SAPH generally have a median 6MWD of <350 m [9, 20, 21, 54]. Most patients also desaturate >5% during the test [9]. It has also been noted that some patients with SAPH have elevated serum B-type natriuretic peptide (BNP) and NT-proBNP levels [8, 22, 55]. Chest imaging has also been shown to be abnormal in SAPH patients. Patients with SAPH are more likely to have pulmonary fibrosis than a comparable symptomatic group [11, 12]. This has led to the recommendation that all sarcoidosis patients with fibrosis be screened for SAPH [56]. However, there was the concern among the committee about the variable interpretation of presence of pulmonary fibrosis on chest radiograph [57]. The presence of greater than 20% fibrosis on HRCT has proved a more reproducible assessment of fibrosis with good agreement between radiologists and clinicians [58, 59]. The presence of >20% fibrosis on HRCT has been found to be associated with increased mortality in pulmonary sarcoidosis [1, 2, 58]. The presence of an increased pulmonary artery diameter, corrected for by either aorta diameter or body surface area [2, 60, 61], is associated with SAPH. The group also reached consensus regarding an enlarged right ventricle on a contrast computed tomography (CT) scan as an indicator for the patient to have a transthoracic echocardiogram. Cardiac MRI findings of RV dysfunction or PH have been reported in SAPH [62, 63]. Cardiac MRI is useful in identifying cardiac sarcoidosis and as a result any potential treatment implications. Several studies in patients with SAPH have shown that patients with SAPH have a degree of pulmonary restriction (FVC <60% pred) and at least moderate gas transfer impairment (DLCO <50% pred) [11, 12, 24]. Patients with sarcoidosis such lung function parameters would be considered for SAPH screening. Using the paradigm of systemic sclerosis-associated PH, a KCO (transfer coefficient of the lung for carbon monoxide) <60% pred or an FVC/ DLCO ratio >1.6 may be parameters predicting SAPH [49].
There was consensus that the presence of one or more of these factors should lead to echocardiography to determine the probability of PH. In addition, other features were identified by the group and consensus for screening echocardiography was achieved if there is a worsening of New York Heart Association (NYHA) functional class, a decrease >20% in the 6MWD, or a decrease >15% of the DLCO when there was no significant change in lung volumes.
Summary
The suggestions of who should undergo transthoracic echocardiogram are summarised in figure 3.
Who should get right-heart catheterisation?
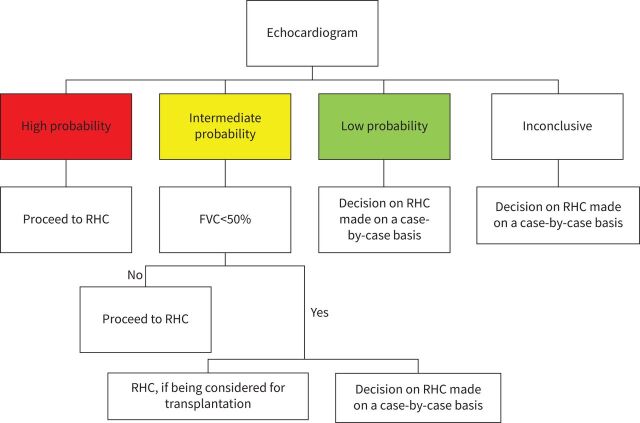
RHC is the gold standard for diagnosing PH. It has been recognised for several years that echocardiography may over or underestimate pulmonary arterial pressure (PAP), especially in those with interstitial lung disease [64, 65]. In sarcoidosis, RHC can be particularly helpful in separating pre- and post-capillary PH [14]. A transthoracic echocardiogram can estimate the probability of PH and raise suspicion, but it cannot define SAPH. The echocardiogram can provide information regarding RV function and evaluate left ventricular function may suggest post-capillary PH. However, a RHC remains the definitive test to distinguish between pre- and post-capillary PH [14]. Therefore, the committee felt the results of echocardiography should be used to determine who should undergo RHC.
The estimated RV systolic pressure based on tricuspid regurgitation velocity (TRV) is the most validated echocardiographic parameter to screen for PH. However, the echo may not be a well visualised tricuspid regurgitation (TR) jet seen on transthoracic echocardiogram. Also, other signs suggestive of PH may be seen on transthoracic echocardiogram when analysing the ventricles, the pulmonary arteries, the inferior vena cava and right atrium. The ESC/ERS PH guidelines [51] have proposed a simple algorithm which allows one to incorporate both the TRV and other indirect measures (figure 4) leading to a scoring of patients as having high, intermediate or low probability for PH. For SAPH, for those with low probability, the decision to proceed with a right catheterisation should be made on a case-by-case basis. For those in whom the results were inconclusive, the patient should be considered on a case-by-case basis (figure 5) with a joint decision between a PH and a sarcoidosis expert. Factors which may influence the decision to perform a RHC include echocardiographic evidence for RV dysfunction (figure 4), pulmonary function tests, 6MWT, BNP or NT-proBNP and imaging results. For members of the committee, there was not agreement regarding systematically performing a RHC in those with an intermediate probability of PH associated with severe interstitial lung disease. The presence of right ventricular dysfunction on echocardiogram adds further support to the decision to proceed with RHC, but there was insufficient evidence in sarcoidosis to make this a formal recommendation. Some felt this was not needed unless in the context of a lung transplant evaluation. Others felt that the decision for RHC should be made on a case-by-case basis. This ambivalence is predicated by the current literature that suggests this group may not be responsive to PAH therapies.
FIGURE 5.
Algorithm for who should undergo RHC based on echocardiography. FVC: forced vital capacity; RHC: right-heart catheterisation.
Summary
The committee's suggestions regarding who should undergo RHC are summarised in figure 5.
How should one interpret RHC in SAPH?
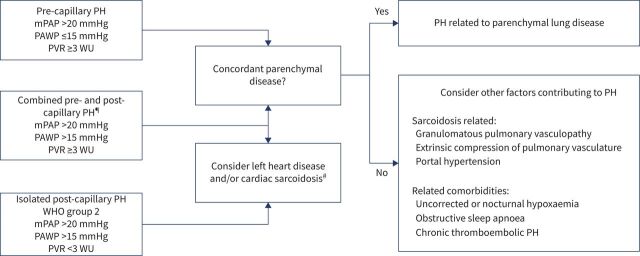
The committee felt that the RHC should be performed in a PH expert centre and its results (and consequences) should be interpreted by a multidisciplinary team with expertise in PH, sarcoidosis, imaging and transplantation, if available and appropriate. In some cases, the sarcoidosis and PH expert may be the same person. A cardiologist's input is needed for those with post-capillary PH to distinguish sarcoidosis-related causes from left-sided heart failure. Review of the RHC tracings may be useful in order to precisely define the pre- or post-capillary mechanisms of PH.
Figure 6 summarises the suggestions of the committee on assessing the RHC. For patients with an elevated mPAP (>20 mm Hg), the results of the PAWP measurement will determine whether this is pre- or post-capillary PH (WHO Group 2). As noted in the figure, a PAWP >15 mm Hg confirms a post-capillary PH. If there is any uncertainty about the validity of the PAWP measurement, then direct measurement of the left ventricular end-diastolic pressure measurement should be considered. The distinction is important for both treatment decisions as well as prognosis [14]. The committee recognised that patients might have combined pre- and post-capillary PH which can be either due to pre-capillary vascular changes due to left heart disease or due to sarcoidosis-related factors. Therefore, careful follow-up evaluation after the initial treatment decision should be undertaken for these patients in particular to ensure that the chosen treatment course is accompanied by a salutary and not a deleterious response. Pre-capillary PH due to pulmonary vascular disease is robustly defined when the PVR is ≥3 WU but remains likely when the PVR is between 2 and 3.
FIGURE 6.
Interpretation of right-heart catheterisation and subsequent treatment recommendations are made in the figure. mPAP: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; WHO: World Health Organization; WU: Wood's unit. #: Cardiac sarcoidosis may be evident in pre-capillary PH without changing haemodynamics. ¶: Careful evaluation should be performed by a cardiologist/PH specialist to distinguish PH due to left heart disease with subsequent pre-capillary vascular changes and sarcoidosis-associated PH.
For patients with a mean PAP of <20 mm Hg, the clinician should look elsewhere for the cause of symptoms and offer regular follow-up.
In patients with a PAWP 13–15 mmHg, left-sided cardiac involvement can still be contributory to PH. However, a RHC occasionally with the need for provocative manoeuvres, such as fluid challenge or exercise, remains the definitive test to distinguish between pre and post-capillary PH.
The cardiac MRI may provide additional information in these cases. It may indicate cardiac involvement as the cause of increased pressures, especially for those WHO Group 2 patients. These patients should still be considered for a multidisciplinary discussion [37]. In addition, the presence of right ventricular abnormalities were prognostic factors in a population of sarcoidosis patients with suspected cardiac sarcoidosis [66]. No study has been conducted to the correlation between right ventricular sarcoidosis and the presence of PH.
Summary
The committee indicated that the RHC results should be interpreted by a multidisciplinary team with at least a sarcoidosis and PH expert and decisions should be made as summarised in figure 6.
Treatment
Should pulmonary vasculature be visualised to rule in/out narrowing
SAPH may be caused by direct compression of the pulmonary artery by either mediastinal adenopathy or fibrosis. For patients with adenopathy compressing the pulmonary arteries, anti-inflammatory therapy may lead to reduction of the size of the nodes and relief of the compression. Boucly et al. [21] identified this situation in five of their 126 (4%) patients with severe SAPH. The authors performed positron emission tomography (PET) scanning to detect this situation. Distortion of the vasculature due to mediastinal fibrosis can also lead to SAPH. In some cases, stenting of the vasculature may reduce PAP [35, 67]. In a prospective study, all patients with SAPH underwent computer tomographic pulmonary angiography. Eight of 72 (11%) were found to have significant pulmonary artery stenosis and underwent successful stenting to relieve pressure [35]. A recent meta-analysis of the literature found that pulmonary artery angioplasty with or without stenting was successful in improving 6MWD [68].
Summary
Evaluate for pulmonary artery stenosis and mediastinal compression chest imaging.
Should pre-capillary SAPH be treated?
The committee focused on treatment of pre-capillary PH. The World Symposium on Pulmonary Hypertension concluded in 2019 that there was insufficient information to make routine recommendations for therapy in SAPH [69]. The results of treatment of SAPH for individual PAH drugs are summarised in table 3 [70–82], with results from the studies with the highest level of evidence for each treatment presented. To date, there have been only two double-blind, placebo controlled (DBPC) trials for SAPH [70, 71]. Three studies reported the results of prospective open label trials [72–74]. The remaining studies were retrospective case series. Initial or sequential combination therapy has been shown to be effective in treating PAH [75, 76]. Three series have reported the outcome of treatment with various treatment regimens in SAPH [8, 21, 77]. In those studies, measuring haemodynamics before and after therapy, the majority of patients showed improvement [8, 16, 21, 71, 72, 77–79]. There was only one study evaluating haemodynamics for placebo-treated patients demonstrating no significant change in pressures after 16 weeks [71]. Other end-points, including 6MWD and quality of life measures, had more mixed results. As summarised in table 3, not all studies found a positive response to treatment in SAPH. To date, only one study has evaluated time to clinical worsening (TCW) with therapy [70]. That study has been published online and did find a significant improvement in TCW for riociguat compared with placebo.
TABLE 3.
Treatment of pre-capillary SAPH
| Highest level of evidence study in patients with SAPH | Total number of patients with SAPH treated | Results in sarcoidosis | |
| Prostenoids | |||
| Epoprostenol | Retrospective OL positive [78, 79] | 12 | Haemodynamics improved [78, 79] |
| Iloprost | Prospective, OL [72] | 15 of 22 enrolled completed 16 weeks’ therapy | In sarcoidosis, haemodynamics and QoL improved [72] |
| Endothelin receptor antagonists | |||
| Bosentan | DBPC [71] | 23 | Haemodynamics improved, no change in 6MWD [71] |
| Ambrisentan | Prospective OL [73] | 21 | Nonsignificant improved QoL, no change 6MWD [73] |
| Macitentan | Retrospective OL [82] | 6 | WHO FC improved in 4/6 treated patients [82] |
| Phosphodiesterase 5 inhibitors | |||
| Sildenafil | Retrospective OL [16] | 12 | Haemodynamics improved, 6MWD no changes |
| Tadalafil | Prospective OL [74] | 12 | No significant changes in 6MWD and QoL |
| Others | |||
| Riociguat | DBPC [70] | 16 | TCW and 6MWD significantly better compared with placebo |
| Combination therapy | Retrospective OL positive [8, 21, 77] | 29 | Haemodynamics and 6MWD improved in some |
SAPH: sarcoidosis-associated pulmonary hypertension; OL: open-label; QoL: quality of life; DBPC: double-blind, placebo-controlled; 6MWD: 6-min walk distance; WHO FC: World Health Organization functional class; TCW: time to clinical worsening.
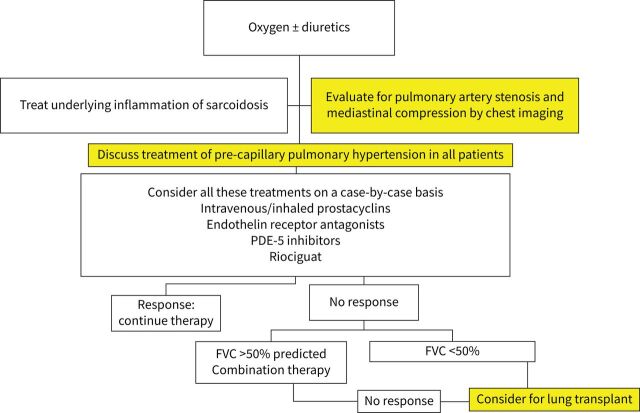
Because of the limited number of randomised trials, the committee did not feel any specific recommendation could be made regarding one or other agent to treat pre-capillary PH, The committee felt that off-label use of PAH drugs may be considered on a case-by-case basis [21, 22]. While not specifically studied in any of the trials, the committee felt that specific PAH therapies should be considered after taking into account the mechanisms involved in the development of PH, the severity of PH and the severity of the underlying parenchymal lung disease. Table 2 points out the various factors which can lead to SAPH. Therapy for pre-capillary hypertension is directed to vascular disease. Care must be taken when using these treatments in patients with veno-occlusive disease. For those with moderate-to-severe parenchymal lung disease (FVC <50% pred), treatment of SAPH may not be as effective. However, if there is evidence for RV dysfunction, treatment of SAPH may still be indicated. For patients with milder SAPH, other factors may be the major cause of patient's symptoms. A recently published study did demonstrate that treatment of mild PH in idiopathic interstitial lung disease was associated with a positive response [80]. However, that study did not include sarcoidosis patients. Worsening of ventilation/perfusion mismatch may occur with pulmonary vasodilator therapy, but in one 16 week study the frequency was similar to that seen with placebo-treated patients [71]. Another potential limitation is uncovering pulmonary veno-occlusive disease (PVOD) [79]. Treatment decision and follow-up should be made by a multidisciplinary team with a sarcoidosis and a PH expert.
Summary
In SAPH, treatment decision and follow-up should be made by a multidisciplinary team with a sarcoidosis and a PH expert. Off-label use of PAH therapy may be considered for symptomatic patients on a case-by-case basis (figure 7). As noted above, nearly three-quarters of sarcoidosis patients listed for lung transplant have SAPH [16, 19]. It has been observed that post-transplant survival in patients with pulmonary sarcoidosis was similar to that in patients with other indications for lung transplantation [52, 81].
FIGURE 7.
Proposed algorithm for treating pre-capillary sarcoidosis-associated pulmonary hypertension. FVC: forced vital capacity; PDE-5: phosphodiesterase 5.
Summary
Patients who have failed to respond to treatment for PH should be referred for lung transplant evaluation, if they are deemed otherwise to be appropriate candidates.
Future research and next steps
Screening
The development of noninvasive screening tools, including serum biomarkers and MRI, need to be further evaluated in SAPH. In addition, the indications for when to repeat screening for SAPH need to be better defined.
Treatment trials
The first priority is the need to develop therapy for SAPH based on well-designed placebo controlled trials. The data summarised in this statement provide ample support for the likelihood that highly efficacious therapies will emerge and the existence of a large multinational SAPH registry indicates that the sarcoidosis community is now geared for definitive studies. The recent treprostinil data in nonsarcoid interstitial lung disease (ILD)-PH [80], suggesting major efficacy in the short-term, is an additional spur: based on past data, there has been more basis for optimism in SAPH than in nonsarcoid ILD-PH. However, SAPH poses unique challenges in two respects.
Treatment trial phenotypes
The definition of optimal patient phenotypes for treatment trials must take into account the multiple mechanisms driving SAPH. Ideally, based on research into haemodynamic profiles, individual phenotypes will be integrated into combined phenotype trials or selectively excluded. Core phenotypes include pre-capillary vasculopathy (whether due to direct granulomatous involvement or to pathways associated with pulmonary fibrosis), vascular compression, and post-capillary pulmonary pathways (veno-occlusive processes). When SAPH coexists with cardiac disease (including cardiac sarcoidosis and comorbidities), robust algorithms will need to be developed to optimise inclusion and exclusion criteria. The distinction between SAPH resulting from end-stage lung disease and other forms of SAPH needs to be considered further, based on the definition of realistic treatment goals, short-term haemodynamic data in these patient subgroups and accumulating data on the specific effects of individual candidate therapies. Imaging assessment of the extent and distribution of parenchymal lung involvement in relation to the haemodynamic profile will also be integral to any phenotyping.
Trial end-points
Because sarcoidosis is multidimensional in its nature, with variably severe co-existent pulmonary and systemic disease, end-point selection is likely to be heavily influenced by the distinction between: 1) short-term trials designed to establish safety and proof of concept; and 2) phase 3 studies evaluating major clinical benefit. Pivotal end-point selection is not confined to the selection of the primary end-point but includes the choice of key secondary end-points. There is a need to cover the spectrum of haemodynamic effects, major clinical end-points (mortality, TCW), multidimensional functionality (6MWT data and actigraphy) and measures of quality of life, crucially including patient-reported outcomes. In short-term trials, haemodynamic effects may continue to be the primary focus but pivotal secondary end-points should cover the domains listed above. In phase 3 trials, with clinical end-points likely to have primacy, possible strategies include the use of co-primary end-points (e.g. TCW, 6MWT data) to capture both major adverse outcomes and average cohort treatment effects. Composite end-points should be constituted by relevant clinical outcomes including but not necessarily limited to cardiopulmonary hospitalisation, mortality and categorical changes in 6MWT. In summary, whether the end-point focus is haemodynamic or primarily clinical, depending upon trial duration, all domains should be represented by pivotal secondary end-points, including the individual components of composite indices. Ongoing clinical research, defining the optimal end-point and its performance characteristics, is pivotal. Continued efforts are ongoing to identify biomarkers especially those that might identify longer-term efficacy based on short-term change.
Optimal prognostic evaluation
Clinicians now have easy access to markers of PH, in SAPH and in ILD-PH alike. Noninvasive PH markers in various forms of PH are listed in table 4. More clinical research is required in SAPH to validate the prognostic significance of individual variables, with comparisons between variables of prognostic values in large well-phenotyped cohorts. These should ideally include patients with SAPH and those with less advanced disease, in order to identify earlier markers of pulmonary vasculopathy.
TABLE 4.
Noninvasive PH markers
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PH: pulmonary hypertension; BNP: B-type natriuretic peptide; PA: pulmonary artery; CT: computed tomography; 6MWD: 6-min walk distance; DLCO: diffusing capacity of the lung for carbon monoxide; KCO: transfer coefficient of the lung for carbon monoxide; FVC: forced vital capacity.
Composite noninvasive indices
Whether combinations of noninvasive indices, covering the multiple vasculopathic domains listed in the previous section, may enhance prognostic evaluation is ripe for further investigation. Studies are ongoing to determine optimal weighting of composite indices, defined by multivariable evaluation against key clinical outcomes and by the presence of PH at RHC. In this way, the utility of noninvasive composite indices in the selection of patients for RHC and their inclusion as end-points in pivotal trials can be established.
Harnessing of technological advances
Recent advances in PH and in ILD have included deep phenotyping data (CT vascular morphometry, SPECT/CT, PET metabolomics, four-dimensional MRI, machine learning) and in ILD, automated quantification of pulmonary vascular volume using CALIPER, with major added value in prognostic evaluation. In particular, it appears increasing likely that machine learning, applied both to individual modalities and to combinations of techniques, will enhance prognostic evaluation. This is likely to be particularly relevant to powering trials by selecting patients more likely to have adverse outcomes.
Summary and conclusion
SAPH is a significant cause of morbidity and mortality in advanced pulmonary disease. An approach to detection of SAPH was developed by the committee. This approach relies on information from patient history, physical examination, pulmonary function testing, chest imaging and serum biomarkers. The echocardiogram remains the most commonly used next step in the patient with risk factors for SAPH. However, RHC is needed to confirm the diagnosis and properly categorise the type of PH. Potential treatments for SAPH are available. The committee felt that the interpretation of the RHC and the use of specific treatments should be made by a multidisciplinary team including both a specialist in sarcoidosis and PH.
Footnotes
Provenance: Commissioned article, peer reviewed.
Endorsed by the World Association of Sarcoidosis and Other Granulomatous diseases (WASOG).
Conflict of interest: L. Savale reports support for the present manuscript from Janssen and Janssen, and MSD. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events received from Janssen and Janssen, and MSD, outside the submitted work. Support for attending meetings and/or travel received from Janssen and Janssen, and MSD, outside the submitted work.
Conflict of interest: O. Shlobin reports participation on a Data Safety Monitoring Board or Advisory Board for Bayer, United Therapeutics, Johnson and Johnson, and Altavant, outside the submitted work.
Conflict of interest: V. Kouranos reports receiving payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, and Roche, outside the submitted work.
Conflict of interest: S.D. Nathan reports receiving consulting fees from United Therapeutics, Bellerophon, Merck, Bayer, Roche, and Boehringer Ingelheim, outside the submitted work. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from United Therapeutics, Bayer, Roche, and Boehringer Ingelheim, outside the submitted work. Payment for expert testimony received from Roche.
Conflict of interest: H. Nunes reports receiving consulting fees from Actelion (now Janssen), outside the submitted work.
Conflict of interest: R. Gupta reports receiving grants of contracts from Bayer, outside the submitted work.
Conflict of interest: J.C. Grutters reports receiving grants or contracts from SPHINX trial (Actelion) outside the submitted work. J.C. Grutters also reports to be member of the SPHINX trial steering committee.
Conflict of interest: D.A. Culver reports participation on a Data Safety Monitoring Board or Advisory Board for Actelion (Janssen), and United Therapeutics, outside the submitted work.
Conflict of interest: D. Ouellette reports receiving grants or contracts from US Federal Government/PICORI, and Sanofi, outside the submitted work. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sunrise 2019, India, outside the submitted work. Payment for expert testimony received from Adam & McGrevey Law Firm, and Spangenberg, Shibley & Liber, outside the submitted work. Unpaid Incoming chair Critical Care Network; CHEST.
Conflict of interest: E.E. Lower reports receiving grants or contracts from Bayer, Bellephron, Actelion, Genentech, Mallinckrodt, aTyr, Novartis, and Gilead, outside the submitted work.
Conflict of interest: T. Al-Hakim reports support for the present manuscript from Foundation for Sarcoidosis Research, and Bayer Pharmaceuticals.
Conflict of interest: A.U. Wells reports participation on a Data Safety Monitoring Board or Advisory Board for Roche, outside the submitted work. A.U. Wells also reports to be President Elect of the World Association of Sarcoidosis and Other Granulomatous Diseases.
Conflict of interest: M. Humbert reports receiving grants or contracts from Acceleron, Janssen, and Merck, outside the submitted work. Consulting fees received from Acceleron, Janssen, and Merck, outside the submitted work. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events received from AOP, Janssen, and Merck, outside the submitted work. Participation on a Data Safety Monitoring Board or Advisory Board for Acceleron, Janssen, and Merck.
Conflict of interest: R.P. Baughman reports support for the present manuscript from Foundation for Sarcoidosis Research. Grants or contracts received from Bayer, Bellephron, Actelion, Genentech, Mallinckrodt, aTyr, Novartis, and Gilead, outside the submitted work. Consulting fees received from Mallinckrodt. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events received from Mallinckrodt, Boehringer Ingelheim, and United Therapeutics. Participation on a Data Safety Monitoring Board or Advisory Board for Bellephron, United Therapeutics, Mallinckrodt, and Actelion.
Conflict of interest: M. Huitema and M.C. Post have nothing to disclose.
Support statement: Supported in part by unrestricted grants from Foundation for Sarcoidosis Research and Bayer Pharmaceuticals. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Kirkil G, Lower EE, Baughman RP. Predictors of mortality in pulmonary sarcoidosis. Chest 2018; 153: 105–113. doi: 10.1016/j.chest.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 2.Jeny F, Uzunhan Y, Lacroix M, et al. Predictors of mortality in fibrosing pulmonary sarcoidosis. Respir Med 2020; 169: 105997. doi: 10.1016/j.rmed.2020.105997 [DOI] [PubMed] [Google Scholar]
- 3.Huitema MP, Bakker ALM, Mager JJ, et al. Prevalence of pulmonary hypertension in pulmonary sarcoidosis: the first large European prospective study. Eur Respir J 2019; 54: 1900897. doi: 10.1183/13993003.00897-2019 [DOI] [PubMed] [Google Scholar]
- 4.Pabst S, Hammerstingl C, Grau N, et al. Pulmonary arterial hypertension in patients with sarcoidosis: the Pulsar single center experience. Adv Exp Med Biol 2013; 755: 299–305. doi: 10.1007/978-94-007-4546-9_38.:299-305 [DOI] [PubMed] [Google Scholar]
- 5.Ozen DK, Mutlu B, Kocakaya D, et al. Pulmonary hypertension in patients with sarcoidosis: a single-center experience. Anatol J Cardiol 2021; 25: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest 2006; 129: 1246–1252. doi: 10.1378/chest.129.5.1246 [DOI] [PubMed] [Google Scholar]
- 7.Tiosano S, Versini M, Dar AL, et al. The long-term prognostic significance of sarcoidosis-associated pulmonary hypertension – a cohort study. Clin Immunol 2019; 199: 57–61. doi: 10.1016/j.clim.2018.12.012 [DOI] [PubMed] [Google Scholar]
- 8.Keir GJ, Walsh SL, Gatzoulis MA, et al. Treatment of sarcoidosis-associated pulmonary hypertension: a single centre retrospective experience using targeted therapies. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31: 82–90. [PubMed] [Google Scholar]
- 9.Bourbonnais JM, Samavati L. Clinical predictors of pulmonary hypertension in sarcoidosis. Eur Respir J 2008; 32: 296–302. doi: 10.1183/09031936.00175907 [DOI] [PubMed] [Google Scholar]
- 10.Alhamad EH, Idrees MM, Alanezi MO, et al. Sarcoidosis-associated pulmonary hypertension: clinical features and outcomes in Arab patients. Ann Thorac Med 2010; 5: 86–91. doi: 10.4103/1817-1737.62471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzato G, Pezzano A, Sala G, et al. Right heart impairment in sarcoidosis: haemodynamic and echocardiographic study. Eur J Respir Dis 1983; 64: 121–128. [PubMed] [Google Scholar]
- 12.Sulica R, Teirstein AS, Kakarla S, et al. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest 2005; 128: 1483–1489. doi: 10.1378/chest.128.3.1483 [DOI] [PubMed] [Google Scholar]
- 13.Bonham CA, Oldham JM, Gomberg-Maitland M, et al. Prostacyclin and oral vasodilator therapy in sarcoidosis-associated pulmonary hypertension: a retrospective case series. Chest 2015; 148: 1055–1062. doi: 10.1378/chest.14-2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baughman RP, Engel PJ, Taylor L, et al. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest 2010; 138: 1078–1085. doi: 10.1378/chest.09-2002 [DOI] [PubMed] [Google Scholar]
- 15.Shorr AF, Helman DL, Davies DB, et al. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur Respir J 2005; 25: 783–788. doi: 10.1183/09031936.05.00083404 [DOI] [PubMed] [Google Scholar]
- 16.Milman N, Burton CM, Iversen M, et al. Pulmonary hypertension in end-stage pulmonary sarcoidosis: therapeutic effect of sildenafil? J Heart Lung Transplant 2008; 27: 329–334. doi: 10.1016/j.healun.2007.11.576 [DOI] [PubMed] [Google Scholar]
- 17.Alonso-Coello P, Schunemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016; 353: i2016. doi: 10.1136/bmj.i2016.:i2016 [DOI] [PubMed] [Google Scholar]
- 18.Miravitlles M, Tonia T, Rigau D, et al. New era for European Respiratory Society clinical practice guidelines: joining efficiency and high methodological standards. Eur Respir J 2018; 51: 1800221. doi: 10.1183/13993003.00221-2018 [DOI] [PubMed] [Google Scholar]
- 19.Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest 2003; 124: 922–928. doi: 10.1016/S0012-3692(15)37649-2 [DOI] [PubMed] [Google Scholar]
- 20.Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med 2018; 139: 72–78. doi: 10.1016/j.rmed.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 21.Boucly A, Cottin V, Nunes H, et al. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J 2017; 50: 1700465. doi: 10.1183/13993003.00465-2017 [DOI] [PubMed] [Google Scholar]
- 22.Parikh KS, Dahhan T, Nicholl L, et al. Clinical features and outcomes of patients with sarcoidosis-associated pulmonary hypertension. Sci Rep 2019; 9: 4061. doi: 10.1038/s41598-019-40030-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobarro D, Schreiber BE, Handler C, et al. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. Am J Cardiol 2013; 111: 278–285. doi: 10.1016/j.amjcard.2012.09.031 [DOI] [PubMed] [Google Scholar]
- 24.Shlobin OA, Kouranos V, Barnett SD, et al. Physiological predictors of survival in patients with sarcoidosis-associated pulmonary hypertension: results from an international registry. Eur Respir J 2020; 55: 1901747. doi: 10.1183/13993003.01747-2019 [DOI] [PubMed] [Google Scholar]
- 25.Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax 2006; 61: 68–74. doi: 10.1136/thx.2005.042838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arcasoy SM, Christie JD, Pochettino A, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest 2001; 120: 873–880. doi: 10.1378/chest.120.3.873 [DOI] [PubMed] [Google Scholar]
- 27.Vorselaars AD, Snijder RJ, Grutters JC. Increased number of pulmonary embolisms in sarcoidosis patients. Chest 2012; 141: 826–827. doi: 10.1378/chest.11-2514 [DOI] [PubMed] [Google Scholar]
- 28.Swigris JJ, Olson AL, Huie TJ, et al. Increased risk of pulmonary embolism among US decedents with sarcoidosis from 1988 to 2007. Chest 2011; 140: 1261–1266. doi: 10.1378/chest.11-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawshaw AP, Wotton CJ, Yeates DG, et al. Evidence for association between sarcoidosis and pulmonary embolism from 35-year record linkage study. Thorax 2011; 66: 447–448. doi: 10.1136/thx.2010.134429 [DOI] [PubMed] [Google Scholar]
- 30.Tandon R, Baughman RP, Stanley J, et al. The link between chronic thromboembolic pulmonary hypertension and sarcoidosis: association or visual masquerade? Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 352–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baughman RP, Engel PJ, Meyer CA, et al. Pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006; 23: 108–116. [PubMed] [Google Scholar]
- 32.Seferian A, Steriade A, Jais X, et al. Pulmonary hypertension complicating fibrosing mediastinitis. Medicine (Baltimore) 2015; 94: e1800. doi: 10.1097/MD.0000000000001800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton-Craig CR, Slaughter R, McNeil K, et al. Improvement after angioplasty and stenting of pulmonary arteries due to sarcoid mediastinal fibrosis. Heart Lung Circ 2009; 18: 222–225. doi: 10.1016/j.hlc.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 34.Toonkel RL, Borczuk AC, Pearson GD, et al. Sarcoidosis-associated fibrosing mediastinitis with resultant pulmonary hypertension: a case report and review of the literature. Respiration 2010; 79: 341–345. doi: 10.1159/000243786 [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Xu J, Zhang Y, et al. Interventional therapy in sarcoidosis-associated pulmonary arterial stenosis and pulmonary hypertension. Clin Respir J 2015; 11: 906–914. [DOI] [PubMed] [Google Scholar]
- 36.Ungprasert P, Crowson CS, Matteson EL. Risk of cardiovascular disease among patients with sarcoidosis: a population-based retrospective cohort study, 1976–2013. Eur Respir J 2017; 49: 1601290. doi: 10.1183/13993003.01290-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro Neto ML, Jellis CL, Joyce E, et al. Update in cardiac sarcoidosis. Ann Am Thorac Soc 2019; 16: 1341–1350. doi: 10.1513/AnnalsATS.201902-119CME [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Lower EE, , Li HP, et al. Cardiac sarcoidosis: the impact of age and implanted devices on survival. Chest 2017; 151: 139–148. doi: 10.1016/j.chest.2016.08.1457 [DOI] [PubMed] [Google Scholar]
- 39.Chapelon-Abric C, Sene D, Saadoun D, et al. Cardiac sarcoidosis: diagnosis, therapeutic management and prognostic factors. Arch Cardiovasc Dis 2017; 110: 456–465. doi: 10.1016/j.acvd.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 40.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010; 137: 376–387. doi: 10.1378/chest.09-1140 [DOI] [PubMed] [Google Scholar]
- 41.Boysen PG, Block AJ, Wynne JW, et al. Nocturnal pulmonary hypertension in patients with chronic obstructive pulmonary disease. Chest 1979; 76: 536–542. doi: 10.1378/chest.76.5.536 [DOI] [PubMed] [Google Scholar]
- 42.Patterson KC, Huang F, Oldham JM, et al. Excessive daytime sleepiness and obstructive sleep apnea in patients with sarcoidosis. Chest 2013; 143: 1562–1568. doi: 10.1378/chest.12-1524 [DOI] [PubMed] [Google Scholar]
- 43.Drent M, Verbraecken J, van der GC, et al. Fatigue associated with obstructive sleep apnea in a patient with sarcoidosis. Respiration 2000; 67: 337–340. doi: 10.1159/000029523 [DOI] [PubMed] [Google Scholar]
- 44.Turner GA, Lower EE, Corser BC, et al. Sleep apnea in sarcoidosis. Sarcoidosis 1997; 14: 61–64. [PubMed] [Google Scholar]
- 45.Ungprasert P, Crowson CS, Simonetto DA, et al. Clinical characteristics and outcome of hepatic sarcoidosis: a population-based study 1976–2013. Am J Gastroenterol 2017; 112: 1556–1563. doi: 10.1038/ajg.2017.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baughman RP, Koehler A, Bejarano PA, et al. Role of liver function tests in detecting methotrexate-induced liver damage in sarcoidosis. Arch Intern Med 2003; 163: 615–620. doi: 10.1001/archinte.163.5.615 [DOI] [PubMed] [Google Scholar]
- 47.Sedki M, Fonseca N, Santiago P, et al. Hepatic sarcoidosis: natural history and management implications. Front Med (Lausanne) 2019; 6: 232. doi: 10.3389/fmed.2019.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cremers J, Drent M, Driessen A, et al. Liver-test abnormalities in sarcoidosis. Eur J Gastroenterol Hepatol 2012; 24: 17–24. doi: 10.1097/MEG.0b013e32834c7b71 [DOI] [PubMed] [Google Scholar]
- 49.Young A, Nagaraja V, Basilious M, et al. Update of screening and diagnostic modalities for connective tissue disease-associated pulmonary arterial hypertension. Semin Arthritis Rheum 2019; 48: 1059–1067. doi: 10.1016/j.semarthrit.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2020; 201: e26–e51. doi: 10.1164/rccm.202002-0251ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 52.Le Pavec J, Valeyre D, Gazengel P, et al. Lung transplantation for sarcoidosis: outcome and prognostic factors. Eur Respir J 2021; 58: 2003358. doi: 10.1183/13993003.03358-2020 [DOI] [PubMed] [Google Scholar]
- 53.Gangemi AJ, Myers CN, Zheng M, et al. Mortality for sarcoidosis patients on the transplant wait list in the Lung Allocation Score era: experience from a high volume center. Respir Med 2019; 157: 69–76. doi: 10.1016/j.rmed.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 54.Baughman RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest 2007; 132: 207–213. doi: 10.1378/chest.06-2822 [DOI] [PubMed] [Google Scholar]
- 55.Mirsaeidi M, Omar HR, Baughman R, et al. The association between BNP, 6MWD test, DLCO% and pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2016; 33: 317–320. [PubMed] [Google Scholar]
- 56.Huitema MP, Grutters JC, Rensing BJWM, et al. Pulmonary hypertension complicating pulmonary sarcoidosis. Neth Heart J 2016; 24: 390–399. doi: 10.1007/s12471-016-0847-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baughman RP, Shipley R, Desai S, et al. Changes in chest roentgenogram of sarcoidosis patients during a clinical trial of infliximab therapy: comparison of different methods of evaluation. Chest 2009; 136: 526–535. doi: 10.1378/chest.08-1876 [DOI] [PubMed] [Google Scholar]
- 58.Walsh SL, Wells AU, Sverzellati N, et al. An integrated clinicoradiological staging system for pulmonary sarcoidosis: a case-cohort study. Lancet Respir Med 2014; 2: 123–130. doi: 10.1016/S2213-2600(13)70276-5 [DOI] [PubMed] [Google Scholar]
- 59.Goh NS, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008; 177: 1248–1254. doi: 10.1164/rccm.200706-877OC [DOI] [PubMed] [Google Scholar]
- 60.Huitema MP, Spee M, Vorselaars VM, et al. Pulmonary artery diameter to predict pulmonary hypertension in pulmonary sarcoidosis. Eur Respir J 2016; 47: 673–676. doi: 10.1183/13993003.01319-2015 [DOI] [PubMed] [Google Scholar]
- 61.Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging 1999; 14: 270–278. doi: 10.1097/00005382-199910000-00007 [DOI] [PubMed] [Google Scholar]
- 62.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands. Chest 2005; 128: 30–35. doi: 10.1378/chest.128.1.30 [DOI] [PubMed] [Google Scholar]
- 63.Patel MB, Mor-Avi V, Murtagh G, et al. Right heart involvement in patients with sarcoidosis. Echocardiography 2016; 33: 734–741. doi: 10.1111/echo.13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167: 735–740. doi: 10.1164/rccm.200210-1130OC [DOI] [PubMed] [Google Scholar]
- 65.Nathan SD, Shlobin OA, Barnett SD, et al. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med 2008; 102: 1305–1310. doi: 10.1016/j.rmed.2008.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velangi PS, Chen KA, Kazmirczak F, et al. Right ventricular abnormalities on cardiovascular magnetic resonance imaging in patients with sarcoidosis. JACC Cardiovasc Imaging 2020; 13: 1395–1405. doi: 10.1016/j.jcmg.2019.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Condado JF, Babaliaros V, Henry TS, et al. Pulmonary stenting for the treatment of sarcoid induced pulmonary vascular stenosis. Sarcoidosis Vasc Diffuse Lung Dis 2016; 33: 281–287. [PubMed] [Google Scholar]
- 68.daSilva-deAbreu A, Bracamonte-Baran W, Condado JF, et al. Characteristics and outcomes of pulmonary angioplasty with or without stenting for sarcoidosis-associated pulmonary hypertension: systematic review and individual participant data meta-analysis. Curr Probl Cardiol 2021; 46: 100616. doi: 10.1016/j.cpcardiol.2020.100616 [DOI] [PubMed] [Google Scholar]
- 69.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914. doi: 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baughman RP, Shlobin OA, Gupta RG, et al. Riociguat was effective in increasing time until clinical worsening in sarcoidosis-associated pulmonary hypertension: results of a one year double blind, placebo controlled trial. Am J Resp Crit Care Med 2021; 203: A1828. [Google Scholar]
- 71.Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest 2014; 145: 810–817. doi: 10.1378/chest.13-1766 [DOI] [PubMed] [Google Scholar]
- 72.Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2009; 26: 110–120. [PubMed] [Google Scholar]
- 73.Judson MA, Highland KB, Kwon S, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2011; 28: 139–145. [PubMed] [Google Scholar]
- 74.Ford HJ, Baughman RP, Aris R, et al. Tadalafil therapy for sarcoidosis-associated pulmonary hypertension. Pulm Circ 2016; 6: 557–562. doi: 10.1086/688775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. doi: 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 76.Simonneau G, Rubin LJ, Galie N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008; 149: 521–530. doi: 10.7326/0003-4819-149-8-200810210-00004 [DOI] [PubMed] [Google Scholar]
- 77.Barnett CF, Bonura EJ, Nathan SD, et al. Treatment of sarcoidosis-associated pulmonary hypertension. A two center experience. Chest 2009; 135: 1455–1461. doi: 10.1378/chest.08-1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abston E, Moll M, Hon S, et al. Long-term outcomes of epoprostenol therapy in sarcoid associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2020; 37: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest 2006; 130: 1481–1488. doi: 10.1378/chest.130.5.1481 [DOI] [PubMed] [Google Scholar]
- 80.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med 2021; 384: 325–334. doi: 10.1056/NEJMoa2008470 [DOI] [PubMed] [Google Scholar]
- 81.Taimeh Z, Hertz MI, Shumway S, et al. Lung transplantation for pulmonary sarcoidosis. Twenty-five years of experience in the USA. Thorax 2016; 71: 378–379. doi: 10.1136/thoraxjnl-2015-207497 [DOI] [PubMed] [Google Scholar]
- 82.Mathijssen H, Huitema MP, Bakker ALM, et al. Safety of macitentan in sarcoidosis-associated pulmonary hypertension: a case-series. Sarcoidosis Vasc Diffuse Lung Dis 2020; 37: 74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]