Abstract
Purpose:
Cancer-related fatigue is a prevalent, debilitating condition that can persist for months or years after treatment. In a single-arm clinical trial, the feasibility and safety of a time-restricted eating (TRE) intervention were evaluated among cancer survivors, and initial estimates of within-person change in cancer-related fatigue were obtained.
Methods:
Participants were 4–60 months post-cancer treatment, were experiencing fatigue (≥ 3 on a scale 0–10), and were not following TRE. TRE entailed limiting all food and beverages to a self-selected 10-h window for 14 days. Participants reported their eating window in a daily diary and completed the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Brief Fatigue Inventory (BFI), and symptom inventory pre- and post-intervention. This study was pre-registered at clinicaltrials.gov in January 2020 (NCT04243512).
Results:
Participants (n=39) were 61.5 ± 12.4 years old and 1.8 ± 1.3 years post-treatment; 89.7% had had breast cancer. The intervention was feasible in that 36/39 (92.3%) of participants completed all questionnaires and daily diaries. It was also safe with no severe adverse events or rapid weight loss (average loss of 1.1 ± 2.3 pounds, p=0.008). Most adhered to TRE; 86.1% ate within a 10-h window at least 80% of the days, and the average eating window was 9.33 ± 1.05 h. Fatigue scores improved 5.3 ± 8.1 points on the FACIT-F fatigue subscale (p<0.001, effect size [ES]=0.55), 30.6 ± 35.9 points for the FACIT-F total score (p<0.001, ES=0.50), and −1.0 ± 1.7 points on the BFI (p<0.001, ES=−0.58).
Conclusion:
A 10-h TRE intervention was feasible and safe among survivors, and fatigue improved with a moderate effect size after two weeks.
Limitations:
This was a single-arm study, so it is possible that expectation effects were present for fatigue outcomes, independent of effects of TRE per se. However, this feasibility trial supports evaluation of TRE in randomized controlled trials to address persistent cancer-related fatigue.
Keywords: Oncology, Fatigue, Intermittent fasting, Nutrition, Diet, Supportive care, Behavior
Introduction
Cancer-related fatigue affects at least 30–90% of patients who undergo treatment for cancer treatment, including both chemotherapy and radiation [1]. It is one of the most prevalent and debilitating side effects of these treatments, and can persist for years into survivorship [2,3]. Cancer-related fatigue is tiredness that is not relieved by sleep or rest, and it contributes to substantial adverse physical, psychological, and economic consequences as well as increased mortality [2,4]. Development of effective treatments has been hindered by the lack of knowledge of the etiology and pathophysiology of cancer-related fatigue [5].
“Time-restricted eating” refers to restricting eating to a consistent time window between 6 and 12 hours without an overt attempt to reduce caloric intake. Time-restricted eating as a therapeutic approach has garnered substantial appreciation in the literature and among the public in the last decade for its effectiveness to sustain circadian rhythms to prevent and treat disease [6]. Human and rodent studies have shown that time-restricted eating prevents excess weight gain, improves sleep, and slows age- and diet-induced disease [6]. Until recently, time-restricted eating had been studied only in healthy participants or those who are overweight or with metabolic disorders (e.g., [7–13]). These studies collectively show that a 10-hour time-restricted eating window is feasible, safe, and effective at improving metabolic markers [7–11]. Two studies looked at fatigue as a potential consequence of time-restricted eating—in a study among 11 men who were overweight or obese, five days of time-restricted eating in an 8-hour window did not affect fatigue levels [11]. Also, among eight overweight adults, 16 weeks of time-restricted eating in a 10–12-hour window led to energy levels were significantly greater overall and in the mornings specifically, and improvements in energy were sustained at one year [14]. However, there have not yet been published studies, to our knowledge, in the cancer population [8].
Thus, we hypothesize that time-restricted eating can help alleviate persistent cancer-related fatigue. Herein, we performed a two-week, single-arm pilot study that evaluated the feasibility of recruiting cancer survivors to a 10-hour time-restricted eating study, adherence of the participants to the program, and safety of a 10-hour time-restricted eating program in regard to adverse events with a special attention to weight loss. Further, we assessed within-person changes in patient-reported fatigue from pre- to post-intervention.
Methods
Study design, participants, and procedures
This prospective single-arm pilot clinical trial was conducted at University of Rochester Medical Center (URMC) from June 2020-September 2021. This study was pre-registered at clinicaltrials.gov on 28/01/2020 (NCT04243512). The research protocol was reviewed and approved by the Research Subjects Review Board at URMC (study no. 00004598). All methods were performed in accordance with the Declarations of Helsinki.
Participants were eligible if they had completed adjuvant chemotherapy, surgery, and/or radiation for cancer at least 4 months and not more than 5 years prior to enrolling; had a baseline level of fatigue as determined by reporting a score of 3 or greater for the question, “In the last week, how bad was your worst fatigue on a scale from 0–10?”; spoke English; were at least 18 years old; were not already in the habit of eating all their food within a 10–hour window; had a body mass index (BMI) >20.0 kg/m2; did not have surgery planned in the next month; did not have any contraindications to the proposed nutrition intervention as identified by their medical provider, their designee, or the study team (e.g., high risk for hypoglycemia, medication requirements, recent history of an eating disorder); were not taking insulin; and were not taking enteral or parenteral nutrition.
While all participants were recruited locally, all recruitment and study activities were accomplished remotely during the coronavirus disease 2019 (COVID-19) pandemic. Participants were provided the choice to consent via a paper consent (via mail) or eConsent using REDCap software [15,16]. Participants completed baseline procedures before making any changes to their diet patterns, i.e., questionnaires, 24-hour food record, and body weight. They then were asked to follow time-restricted eating for 14 consecutive days. At the mid-point, a nutritionist on the study team called the participant to check in, inquire about adverse events, and discuss barriers to time-restricted eating. Post-intervention data collection (i.e., questionnaires, body weight, 24-hour food record) occurred on Day 14. Within a week of completing the intervention, study staff conducted a semi-structured interview to get feedback on participants’ experience with the intervention.
Time-restricted eating intervention
The restricted eating window was 10 hours long, during the day, and was selected by the participant based on their normal meal patterns and preferences. We asked that the window be consistent during the study period. Water and medications were allowable any time but, because of the potential of caffeine and artificial sweeteners to affect circadian rhythm [17,18], coffee, tea, chewing gum, and diet beverages were discouraged during the fasting window.
Data collection
Participants completed a paper-based daily diary for each of the 14 days of the intervention, which included the time of their first calorie and time of their last calorie. The daily eating window was calculated as the difference between first and last calorie. The a priori primary outcome was adherence to time-restricted eating, as calculated from the percent of days that each participant adhered to the intervention.
At baseline and Day 14, a battery of three questionnaires was administered to assess fatigue and other symptoms-the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) [19], Brief Fatigue Inventory (BFI) [20], and a Symptom Inventory. The FACIT-F is a validated, common measure of fatigue that is comprised of five subscales: physical well-being, social well-being, emotional well-being, functional well-being, and fatigue [19]. It asks how true 40 statements are over the last 7 days such as “I have lack of energy” and “I have trouble starting things because I am tired” with five response choices ranging from 0, “Not at all,” to 4, “Very much.” The BFI is a 10-item fatigue questionnaire that is also validated and commonly used in the cancer population [20]. It captures fatigue now as well as the usual and worst in the last 24 hours. It also includes six single-item questions regarding how fatigue has interfered with general activity, mood, work, etc. The average of all 10 items yields a global fatigue score. Cronbach alpha reliability ranges from 0.82 to 0.97 [21]. Lastly, the Symptom Inventory consisted of 29-items that captured 21 symptoms and 8 questions regarding how many their symptoms interfered with daily activities, mood, and relationships. We report data on fatigue, sleep problems, drowsiness, and interference of symptoms with quality of life.
A 24-hour food record was collected at baseline and Day 14, in which participants were asked to record all food and beverage intake, including time eaten and portion size. These data were entered into NDSR software (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) and analyzed for total caloric intake.
Adverse events were closely monitored as described by the Common Terminology Criteria for Adverse Events (CTCAE), version 5 [21], with special attention to body weight. Each participant was provided a bathroom scale (Weight Watchers by Conair, Stamford, CT). Participants were instructed to place the scale on a hard, level surface and weigh themselves shortly after rising the mornings of Day 1, Day 8, and Day 15. Body weight was recorded by the participant on their daily diary.
Statistics
Descriptive analyses (count, mean, standard deviation [SD], range, percentage, as appropriate) were used to describe the study sample demographics, feasibility metrics, and outcomes. A paired t-test was used to assess symptom measures at post- vs. pre-intervention for participants with evaluable data at both time points. Effect size (ES) was calculated as the change in the measure from pre- to post-intervention divided by the pooled SD. p<0.05 was deemed statistically significant.
Results
A total of 215 letters were sent to potential participants and we had nine referrals from colleagues. We then discussed our study with 59 potential participants (Figure 1). Of these 59, 39 were eligible and volunteered to participate (66.1% recruitment rate). A total of 36 participants provided data at baseline and Day 14 for a retention rate of 92.3%.
Figure 1.
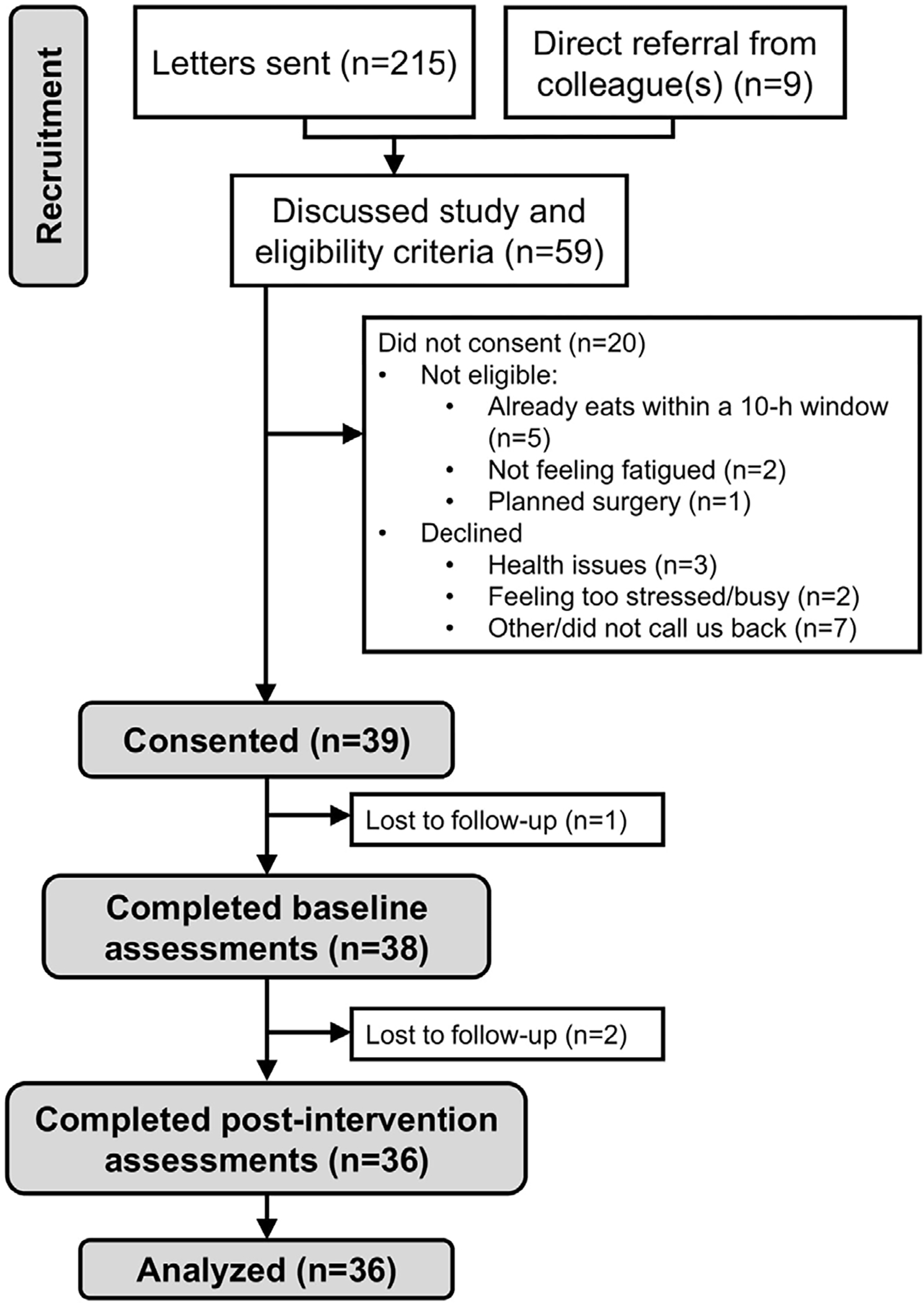
CONSORT flow diagram.
Participants were 61.5 ± 12.4 years old, 92% female, and 84.8% were White, non-Hispanic (Table 1). The majority (89.7%) of participants had been treated for breast cancer, and the majority (71.8%) had had stage 0 or 1 cancer. Among the 36 women, 25 (69.4%) were post-menopausal. Eight (20.5%) participants were normal weight (BMI <25 kg/m2), eight (20.5%) were overweight (25 ≤ BMI <30 kg/m2), and 23 (59.0%) were obese (BMI ≥ 30 kg/m2).
Table 1.
Demographics and clinical characteristics (n=39*).
| Characteristics | Mean ± SD or n (%) |
|---|---|
| Age (years) | 61.5 ± 12.5 |
| Gender | |
| Male | 3 (7.7%) |
| Female | 36 (92.3%) |
| Race and ethnicity | |
| African American/Black | 4 (10.3%) |
| Asian | 1 (2.6%) |
| Hispanic/Latinx | 1 (2.6%) |
| White, non-Hispanic | 33 (84.6%) |
| Marital status | |
| Married or long-term committed significant other | 28 (71.8%) |
| Divorced, separated, single, or widowed | 10 (25.6%) |
| Employment | |
| Employed (including self-employed) | 18 (46.2%) |
| Home Maker | 6 (15.4%) |
| Unemployed | 12 (30.8%) |
| Highest level of education | |
| High school/GED or less | 19 (71.8%) |
| 2 or 4 year degree or some college | 17 (43.6%) |
| Graduate degree | 2 (5.1%) |
| Body mass index (kg/m2) | 32.3±7.1 |
| Type of cancer | |
| Breast | 35 (89.7%) |
| Prostate | 3 (7.7%) |
| Uterine | 1 (2.6%) |
| Cancer stage | |
| 0 | 5 |
| 1 | 23 |
| 2 | 7 |
| 3 or 4 | 3 |
| Previous treatment for cancer | |
| Surgery | 36 (92.3%) |
| Chemotherapy | 14 (35.9%) |
| Radiation | 35 (89.7%) |
| Years since treatment | 1.7±1.2 |
One participant did not complete the On Study form at baseline. n=38 for marital status, employment, and education
Our primary aim was to assess adherence of the participants to the 10-h time-restricted eating intervention. Daily diaries were collected from 36 participants (92.3%), who completed 100% of entries. On average, participants adhered to the 10-h window 90.1% of the days, and the average eating window was 9 hours, 20 minutes (± 1 hour, 3 minutes). A total of 86.1% of participants adhered more than 80% of the days, which was larger than our a priori minimum of 80% to declare the program feasible. In regard to safety, there were two Grade 1 adverse events—one instance of headache, which was possibly related to the intervention, and one instance of insomnia, which was unlikely to be related to the intervention. Both occurred within the first few days of starting time-restricted eating. There were no other adverse events, including severe adverse events. Participants lost 1.1 ± 2.3 pounds over the course of the study (p=0.008). Based on 24-hour food logs, participants (n=35) consumed 1858 ± 554 kcal at baseline and 1663 ± 462 kcal on Day 14, a difference of 202 ± 654 kcal (10.5 ± 35.2%) fewer total calories (p=0.076).
Fatigue was measured at baseline and post-intervention using the multidimensional FACIT-F, the BFI, and the symptom inventory (Table 2). Total FACIT-F total score changed by 9.3 ± 13.3 (mean ± SD) points indicating improvements in fatigue (p<0.001, ES=0.50). For the FACIT-F fatigue subscale, fatigue improved by 5.3 ± 8.1 points (p<0.001, ES=0.55, Figure 2), which is greater than the minimal clinically important difference (MCID) of 3 points [22]. Fatigue also improved 8.4 ± 11.2 points according to the FACIT-F Trial Outcome Index, which is more than the MCID of 5.0 points [22]. Significant improvements were also observed for change in physical well-being (1.4 ± 2.9 points), functional well-being (1.7 ± 3.3 points), and quality of life (as measured from the Functional Assessment of Cancer Therapy-General [FACT-G] subscale, 4.0 ± 7.0 points, p<0.01 for all). The BFI reflected improvements in fatigue, with global scores changing −1.0 ± 1.7 points (p<0.001, ES= −0.58, Table 2). Single-item questions on the symptom inventory regarding fatigue and drowsiness reflected improvements as well as how symptoms interfered with quality of life, but there was not a significant change in reported sleep problems (Table 2).
Table 2.
Fatigue measured at baseline and after 14 days of following a 10-hour time-restricted eating pattern (n=36).
| Fatigue measure | Directionality | Baseline | Day 14 | p-value* | Effect size |
|---|---|---|---|---|---|
| Functional Assessment of Chronic Illness Therapy- Fatigue (FACIT-F) Total score | Higher is better | 107.9 ± 17.3 | 117.2 ± 19.6 | <0.001** | 0.50 |
| FACIT-F: Physical well being | Higher is better | 19.9 ± 4.1 | 21.4 ± 4.7 | 0.006** | 0.32 |
| FACIT-F: Social well being | Higher is better | 22.8 ± 5.7 | 23.1 ± 5.5 | 0.529 | 0.06 |
| FACIT-F: Emotional well being | Higher is better | 18.5 ± 3.7 | 19.1 ± 3.5 | 0.144 | 0.18 |
| FACIT-F: Functional well being | Higher is better | 16.0 ± 4.5 | 17.3 ± 4.9 | 0.005** | 0.35 |
| FACIT-F: Fatigue subscale | Higher is better | 30.6 ± 9.2 | 35.9 ± 9.9 | <0.001** | 0.55 |
| FACIT-F: Trial outcome index (fatigue)† | Higher is better | 66.6 ± 14.8 | 74.9 ± 17.3 | <0.001** | 0.52 |
| FACIT-F: Functional Assessment of Cancer Therapy (FACT)-General‡ | Higher is better | 77.2 ± 12.3 | 81.3 ± 13.0 | 0.001** | 0.32 |
| Brief Fatigue Inventory: Global fatigue score | Lower is better | 3.9 ± 1.6 | 2.9 ± 1.9 | 0.001** | −0.58 |
| Brief Fatigue Inventory: Fatigue at its worst | Lower is better | 6.8 ± 1.9 | 5.2 ± 2.6 | <0.001 | −0.69 |
| Symptom inventory: Fatigue | Lower is better | 5.8 ± 2.4 | 4.4 ± 2.3 | 0.002** | −0.62 |
| Symptom inventory: Sleep problems | Lower is better | 4.7 ± 2.9 | 4.2 ± 3.0 | 0.374 | −0.18 |
| Symptom inventory: Drowsiness | Lower is better | 5.2 ± 2.5 | 3.0 ± 2.3 | <0.001** | −0.89 |
| Symptom inventory: Interference of symptoms with quality of life | Lower is better | 4.0 ± 2.9 | 2.3 ± 2.6 | 0.001** | −0.62 |
p-value derived from a two-sided, paired t-test between baseline and Day 14
p<0.01
Trial outcome index (fatigue) = physical + functional + fatigue subscales
Functional Assessment of Cancer Therapy (FACT)-General = physical + social + emotional + functional subscales; a common measure of quality of life
Figure 2.
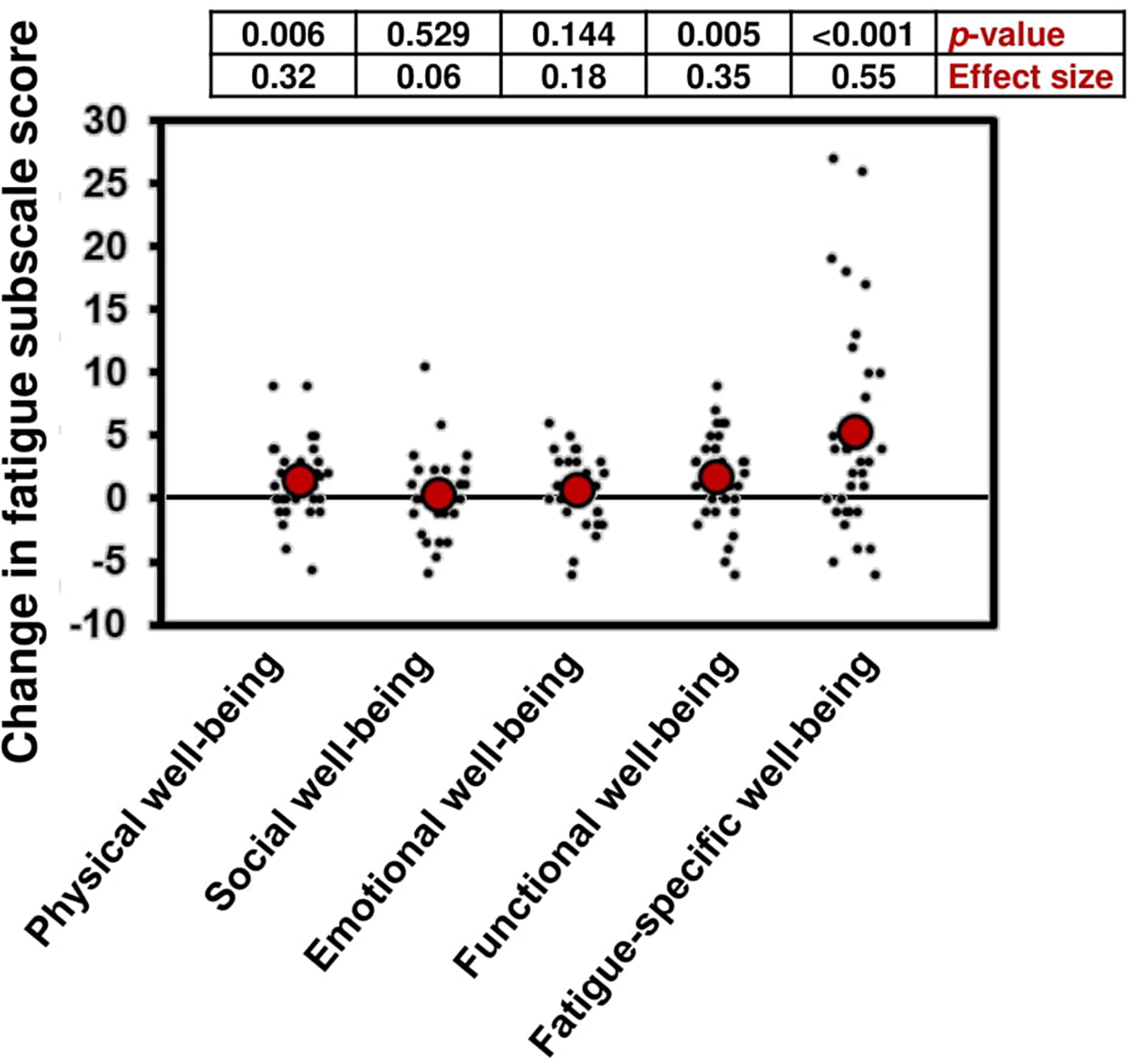
Change in subscales of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) from baseline to Day 14 while participating in a 10-hour time-restricted eating regimen. A greater score indicates greater quality of life and less fatigue (n=36).
Exit interviews were conducted within a week of completing the study (n=37). Overall, participants enjoyed time-restricted eating and many indicated that it facilitated a more regular eating pattern. Several people reported that it took about three days to one week for their bodies to adjust to the new pattern, after which it was easier and more comfortable. The vast majority voiced that they would continue time-restricted eating in some capacity, some with modifications such as drinking coffee earlier. Some did not like the strictness of the time window but said that they benefited from not eating after dinner and will continue to follow that practice. All participants would recommend time-restricted eating to friends or family, often with specific goals including weight management/loss, regulating blood sugar, reducing fatigue, or promoting overall health. The largest barriers to time-restricted eating included coordinating with their work schedule, coordinating meal times with their family/spouse, and not being allowed to drink caffeinated beverages in the morning. Through this study, some participants reflected on other lifestyle behaviors including diet (i.e., composition and quantity of food) and physical activity.
Discussion
Herein, we demonstrated feasibility of a 14-day, 10-hour time-restricted eating intervention among cancer survivors with persistent cancer-related fatigue, as well as initial efficacy. The intervention was very well received, and the vast majority of participants stated that they will continue a time-restricted eating in some fashion. This intervention was safe, and participants reported clinically meaningful improvements in their fatigue using uni- and multidimensional fatigue questionnaires. These results support further testing of a 10-hour time-restricted eating intervention vs. usual care and time- and attention-control interventions to alleviate cancer-related fatigue in cancer survivorship.
Adherence was excellent, with 86% adhering at least 80% of the days, and an average eating window of 9 hours, 20 minutes. This is in line with other studies that have evaluated adherence to 8–10-hour time-restricted eating regimens. For example, healthy participants adhered to a 10–12-hour window (self-selected) 6.5 ± 0.5 days/week during a 12-week-long study (92% adherence, n=19) [7]. Adults with type 2 diabetes adhered to a 9-hour eating window (10:00–17:00) 20 ± 7 out of 28 days of the intervention (average 72% adherence, n=19) [10]. Obese adults adhered to an 8-hour eating window (10:00–18:00) 5.6 ± 0.3 days/week for 12 weeks (80% adherence, n=23) [23]. Further, people with metabolic syndrome adhered at least 5.0 ± 2.2 days/week over a 12-week intervention (72–81% weekly adherence, n=37). While our study was only 14 days long, other studies have demonstrated consistent adherence over time periods up to 12 weeks [23,24].
Our results build upon literature showing that cancer survivors with persistent cancer-related fatigue are willing and able to adhere to behavioral interventions to address their fatigue. Currently, behavioral interventions such as exercise and psychosocial interventions (e.g., cognitive behavioral therapy) are more effective to combat fatigue than the available pharmaceuticals [25], and effective nutritional interventions are emerging [26]. Behavioral interventions are desirable because they have a plethora of health benefits and few side effects. Time-restricted eating, specifically, is appealing because it is free-of-charge and does not require specialized equipment, thereby making it widely accessible [8]. Our adherence rate of 90% was slightly higher than many other studies that evaluate behavioral interventions among cancer survivors. For example, exercise interventions tend to have adherence rates of 54–78% among cancer survivors [27]. Dietary interventions tend to have more variable adherence, with rates ranging from less than 50% to 100%, depending on how adherence is calculated [26]. Notable examples include adherence of 81% at 12 weeks for a Mediterranean diet intervention [28] and 73–94% for food group goals in a 12-week ‘Fatigue Reduction Die’ study [29], both of which were conducted among survivors with persistent cancer-related fatigue.
Time-restricted eating has the potential to improve several pathophysiological mechanisms that underlie cancer-related fatigue. First, time-restricted eating may be able to alleviate fatigue by entraining circadian rhythms. Circadian rhythms are 24-hour biological cycles that work in synchrony to regulate hormone secretion, the sleep/wake cycle, and metabolic processes. Cancer and cancer treatment have been shown to disrupt circadian rhythm, which can lead to sleep disturbances and fatigue [30–33]. Circadian rhythm is affected by external cues—zeitgebers—that include light exposure, sleep, physical activity, and nutrient timing [6]. Consistent animal and human data demonstrate that aberrant eating patterns disrupt circadian rhythm (i.e., disruption of expression of genes that show strong diurnal oscillations) [6]. Only about 10% of people eat within a window less than 12 hours [14], and a consistent, shorter window of eating, for example 10 hours, may help entrain the circadian clock and improve metabolic homeostasis with broad health consequences [6,7]. In addition, bright light therapy, which aims to regulate circadian processes, has shown benefits for cancer-related fatigue [34]. Second, cancer and cancer treatments can interfere with metabolism including glucose, lipid, and redox homeostasis, hormone regulation, and mitochondrial function, possibly causing or exacerbating cancer-related fatigue [5,35,36]. Time-restricted eating can regulate glucose metabolism and metabolic hormones in people with chronic conditions [6,7,24]. It can be combined with other dietary interventions that prescribe the amount and/or composition of the diet (e.g., [9]). Other suggested mechanisms underlying cancer-related fatigue include chronic inflammation, disruption of the hypothalamus-pituitary-adrenal (HPA) axis, neuroendocrine functions, and psychological distress [5,36]. There is preliminary evidence that time-restricted eating can regulate and/or improve some of these functions via circadian or independent processes [8,37]. The effects of time-restricted eating on circadian rhythm have yet to be measured and the relationships between cancer- and treatment-related pathology, persistent psychological distress, circadian rhythm, and cancer-related fatigue are not yet fully elucidated, but this is a rich area for future research.
The results of this study should take into account both its strengths and limitations. It is one of the first studies testing time-restricted eating in the cancer population, and the first, to our knowledge, to apply it to address chronic fatigue and supportive care outcomes. Our study is limited in that it was small and conducted in mostly older breast cancer survivors, so generalizability should occur with prudence. Also, recruitment occurred during 2020–2021; it is unknown how the COVID-19 pandemic impacted the feasibility of recruitment and participants’ ability to adhere to time-restricted eating. However, because our study was conducted completely remotely, our methods may be used for follow-up multisite studies and studies in rural and other hard-to-reach populations. Lastly, our study was only 14 days long and time-restricted eating interventions that report health benefits are usually at least 8 weeks [6,8]; it is unknown how adherence will change over a longer study period.
Conclusion
Cancer survivors with persistent cancer-related fatigue were willing and able to adhere to a 10-hour time-restricted eating intervention for two weeks with eagerness to continue the eating pattern. Time-restricted eating was safe and fatigue improved significantly from pre- to post-intervention with moderate effect sizes. These results support future work investigating the effects of time-restricted eating against usual care and other behavioral control interventions to address cancer-related fatigue.
Acknowledgments
We would like to thank the people who participated in this study and provided valuable feedback on the time-restricted eating program. We would also like to thank the staff in the Physical Exercise, Activity, and Kinesiology (PEAK) Laboratory at University of Rochester for access to NDSR software.
Funding
This work was supported by the National Institutes of Health (NIH) National Cancer Institute (NCI) under grant numbers UG1CA189961 to KMM and Gary Morrow, T32CA102618 to Michelle Janelsins and Gary Morrow, and K07CA221931 to IRK. REDCap access and research support was provided by the NIH National Center for Advancing Translational Sciences (NCATS) under grant number UL1TR002001 to Martin Zand and Nancy Bennett. This publication was also supported by funds through the Maryland Department of Health’s Cigarette Restitution Fund Program. Neither the NIH nor the Maryland Department of Health had any say in the design, conduct, analysis, or publication of the study.
Footnotes
The authors have no relevant financial or non-financial interests to disclose.
References
- 1.Maqbali Al, Mohammed Mohammed Al Sinani, Al Naamani Zakariya, and Al Badi Khalid. “Prevalence of fatigue in patients with cancer: A systematic review and meta-analysis.” J Pain Symptom Manag 61 (2021): 167–189. [DOI] [PubMed] [Google Scholar]
- 2.Bower Julienne E., Wiley Joshua, Petersen Laura, and Irwin Michael R. “Fatigue after breast cancer treatment: Biobehavioral predictors of fatigue trajectories.” Health Psychol 37 (2018): 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esser Peter, Heide Götze Anja Mehnert-Theuerkauf, and Knoop Hans, et al. “Fear of progression and its role in the relationship of cancer-related fatigue with physical functioning and global quality of life–A register-based study among hematological cancer survivors.” J Psychosom Res 127 (2019): 109844. [DOI] [PubMed] [Google Scholar]
- 4.Horneber Markus, Fischer Irene, Dimeo Fernando, and Rüffer Jens Ulrich, et al. “Zertifizierte Fortbildung (cme)-Tumor-assoziierte Fatigue-Epidemiologie, Pathogenese, Diagnostik und Therapie.” Deutsches Arzteblatt-Arztliche Mitteilungen-Ausgabe A 109 (2012): 161. [Google Scholar]
- 5.Saligan LN, Olson K, Filler K, and Larkin DF et al. “Multinational association of supportive care in cancer fatigue study group-biomarker working group. The biology of cancer-related fatigue: A review of the literature.” Support Care Cancer 23 (2015): 2461–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaix Amandine, Manoogian Emily N.C., Melkani Girish C., and Panda Satchidananda. “Time-restricted eating to prevent and manage chronic metabolic diseases.” Annu Rev Nutr 39 (2019): 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson Michael J., Manoogian Emily N.C., Zadourian Adena, and Lo Hannah, et al. “Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome.” Cell metab 31(2020): 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen Rebecca A.G., and Kirkham Amy A. “Time-restricted eating: A novel and simple dietary intervention for primary and secondary prevention of breast cancer and cardiovascular disease.” Nutrients 13 (2021): 3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peeke Pamela M., Greenway Frank L., Billes Sonja K., and Zhang Dachuan, et al. “Effect of time restricted eating on body weight and fasting glucose in participants with obesity: Results of a randomized, controlled, virtual clinical trial.” Nutr Diabetes 11 (2021): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parr Evelyn B., Devlin Brooke L., Lim Karen H.C., and Moresi Laura N.Z., et al. “Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study.” Nutrients 12 (2020): 3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parr Evelyn B., Devlin Brooke L., Radford Bridget E., and Hawley John A. “A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: A randomized controlled trial.” Nutrients 12 (2020): 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow Lisa S., Manoogian Emily N.C., Alvear Alison, and Fleischer Jason G., et al. “Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: A feasibility study.” Obesity 28 (2020): 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchison Amy T., Regmi Prashant, Manoogian Emily N.C., and Fleischer Jason G., et al. “Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial.” Obesity 27 (2019): 724–732. [DOI] [PubMed] [Google Scholar]
- 14.Gill Shubhroz, and Panda Satchidananda. “A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits.” Cell Metab 22 (2015): 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris Paul A., Taylor Robert, Minor Brenda L., and Elliott Veida, et al. “The REDCap consortium: Building an international community of software platform partners.” J Biomed Inform 95 (2019): 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris Paul A., Taylor Robert, Thielke Robert, and Payne Jonathon, et al. “Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support.” J Biomed Inform 42 (2009): 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oishi Katsutaka, Higo-Yamamoto Sayaka, and Yasumoto Yuki. “Moderately high doses of the artificial sweetener saccharin potentially induce sleep disorders in mice.” Nutrition 32 (2016): 1159–1161. [DOI] [PubMed] [Google Scholar]
- 18.Burke Tina M., Markwald Rachel R., McHill Andrew W., and Chinoy Evan D., et al. “Effects of caffeine on the human circadian clock in vivo and in vitro.” Sci Transl Med 7 (2015): 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cella David. “The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue.” In Seminars in Hematology 34 (1997): 13–19. [PubMed] [Google Scholar]
- 20.Mendoza Tito R., Wang X. Shelley, Cleeland Charles S., and Morrissey Marilyn, et al. “The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory.” Cancer 85 (1999): 1186–1196. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. (2017).
- 22.Nordin Åsa, Taft Charles, Lundgren-Nilsson Åsa, and Dencker Anna. “Minimal important differences for fatigue patient reported outcome measures-A systematic review.” BMC Med Res Methodol 16 (2016): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabel Kelsey, Hoddy Kristin K., Haggerty Nicole, and Song Jeehee, et al. “Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study.” Nutr Healthy Aging 4 (2018): 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Przulj Dunja, Ladmore Daniella, Smith Katie Myers, and Phillips-Waller Anna, et al. “Time restricted eating as a weight loss intervention in adults with obesity.” PloS One 16 (2021): e0246186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mustian Karen M., Alfano Catherine M., Heckler Charles, Kleckner Amber S., Kleckner Ian R., et al. “Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis” JAMA Oncol 3 (2017): 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baguley Brenton J., Skinner Tina L., and Wright Olivia R.L. “Nutrition therapy for the management of cancer-related fatigue and quality of life: a systematic review and meta-analysis.” British J Nutr 122 (2019): 527–541. [DOI] [PubMed] [Google Scholar]
- 27.Kampshoff Caroline S., Jansen Femke, Van Mechelen Willem, and May Anne M., et al. “Determinants of exercise adherence and maintenance among cancer survivors: A systematic review.” Int J Behav Nutr Phys Act 11 (2014): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baguley Brenton J., Skinner Tina L., Jenkins David G., and Wright Olivia R.L. “Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: A pilot randomised control trial.” Clin Nutr 40 (2021): 245–254. [DOI] [PubMed] [Google Scholar]
- 29.Zick Suzanna Maria, Colacino Justin, Cornellier Maria, and Khabir Tohfa, et al. “Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial.” Breast Cancer Res Treat 161 (2017): 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roscoe Joseph A., Morrow Gary R., Hickok Jane T., and Bushunow Peter, et al. “Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment.” Supportive Care Cancer 10 (2002): 329–336. [DOI] [PubMed] [Google Scholar]
- 31.Sultan Armiya, Choudhary Vivek, and Parganiha Arti. “Worsening of rest-activity circadian rhythm and quality of life in female breast cancer patients along progression of chemotherapy cycles.” Chronobiol Int 34 (2017): 609–623. [DOI] [PubMed] [Google Scholar]
- 32.Roveda Eliana, Bruno Eleonora, Galasso Letizia, and Mulè Antonino, et al. “Rest-activity circadian rhythm in breast cancer survivors at 5 years after the primary diagnosis.” Chronobiol Int 36 (2019): 1156–1165. [DOI] [PubMed] [Google Scholar]
- 33.Payne Judith K. “Altered circadian rhythms and cancer-related fatigue outcomes.” Integr Cancer Ther 10 (2011): 221–233. [DOI] [PubMed] [Google Scholar]
- 34.Johnson Jillian A., Garland Sheila N., Carlson Linda E., and Savard Josée, et al. “Bright light therapy improves cancer-related fatigue in cancer survivors: A randomized controlled trial.” J Cancer Surviv 12 (2018): 206–215. [DOI] [PubMed] [Google Scholar]
- 35.Dieli-Conwright Christina M., Wong Louise, Waliany Sarah, and Bernstein Leslie, et al. “An observational study to examine changes in metabolic syndrome components in patients with breast cancer receiving neoadjuvant or adjuvant chemotherapy.” Cancer 122 (2016): 2646–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Songwei, Chu Shifeng, Gao Yan and Ai Qidi, et al. “A narrative review of Cancer-related Fatigue (CRF) and its possible pathogenesis.” Cells 8 (2019): 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manoogian Emily N.C., Chow Lisa S., Taub Pam R., and Laferrère Blandine, et al. “Time-restricted eating for the prevention and management of metabolic diseases.” Endocr Rev 43 (2022): 405–436. [DOI] [PMC free article] [PubMed] [Google Scholar]


