Abstract
Although these conditions are rare, a proportion of patients with interstitial lung diseases (ILDs) may develop a progressive-fibrosing phenotype. Progressive fibrosis is associated with worsening respiratory symptoms, lung function decline, limited response to immunomodulatory therapies, decreased quality of life and, potentially, early death. Idiopathic pulmonary fibrosis may be regarded as a model for other progressive-fibrosing ILDs. Here we focus on other ILDs that may present a progressive-fibrosing phenotype, namely idiopathic nonspecific interstitial pneumonia, unclassifiable idiopathic interstitial pneumonia, connective tissue disease-associated ILDs (e.g. rheumatoid arthritis-related ILD), fibrotic chronic hypersensitivity pneumonitis, fibrotic chronic sarcoidosis and ILDs related to other occupational exposures. Differential diagnosis of these ILDs can be challenging, and requires detailed consideration of clinical, radiological and histopathological features. Accurate and early diagnosis is crucial to ensure that patients are treated optimally.
Short abstract
Other chronic ILDs with a progressive-fibrosing phenotype may have a clinical course similar to IPF. Although challenging, identification of these patients is crucial, and requires a multidisciplinary approach, to ensure optimal diagnosis and management. http://ow.ly/8q8M30mGDsQ
Introduction
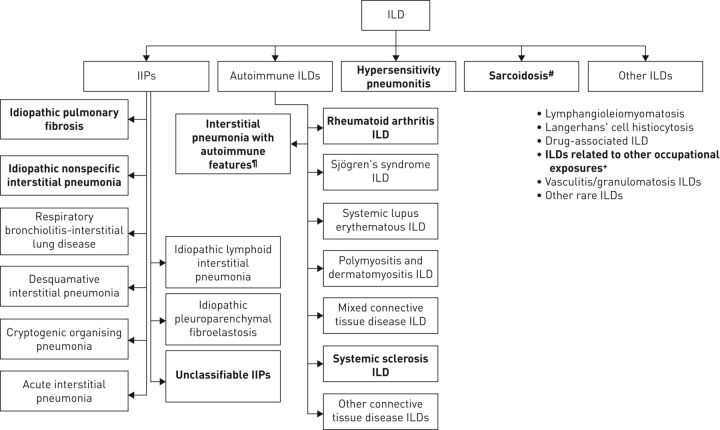
The term interstitial lung disease (ILD) encompasses a large group of > 200 parenchymal pulmonary disorders, of which the majority are classified as rare [1, 2]. Early and accurate diagnosis can be challenging, and it is difficult to predict disease progression. Most patients with ILDs are cared for by a pulmonologist, but specialists from other disciplines may also be involved (e.g. rheumatologists) [3]. Idiopathic pulmonary fibrosis (IPF) and several other ILDs (such as hypersensitivity pneumonitis (HP), rheumatoid arthritis-associated ILD (RA-ILD) and unclassifiable disease) may present a progressive-fibrosing phenotype (figure 1).
FIGURE 1.
Types of interstitial lung disease (ILD) most likely to have a progressive-fibrosing phenotype (indicated in bold). IIPs: idiopathic interstitial pneumonias. #: stage IV sarcoidosis only; ¶: not an established clinical diagnosis; +: e.g. asbestosis, silicosis.
IPF is the most widely studied and most common ILD. It is characterised by progressive fibrosis, lung scarring and a radiological pattern known as usual interstitial pneumonia (UIP) [4–6]. There are a number of clinical and mechanistic parallels between IPF and other fibrosing ILDs that may present a progressive phenotype [7–9]. Given their overlapping clinical, radiological and pathological presentations, the terminology recently used to describe patients with fibrosing ILDs that may present a progressive phenotype, despite currently available treatment, is “progressive-fibrosing ILD (PF-ILD)” [1, 2, 9]. The commonalities of ILDs that may present a progressive-fibrosing phenotype suggest the potential for a common treatment pathway. Most patients will have a combination of inflammation and fibrosis, and the effectiveness of immunomodulatory therapy varies between conditions (it is most likely to stabilise and/or to slow disease progression in nonspecific interstitial pneumonia (NSIP), connective tissue disease-associated ILD (CTD-ILD) and subsets of chronic fibrosis HP) [1].
Progressive fibrosis of the lung parenchyma is self-sustaining and causes progressive deterioration in lung function, respiratory symptoms and quality of life [10, 11]. It therefore increases the risk of early death. Pulmonary fibrosis is sometimes regarded as an indicator of disease progression, and the prognosis associated with an ILD is generally worsened by its presence [1]. However, aside from IPF, ILDs with progressive fibrosis may be clinically stable, especially if the disease is mild. In addition, a progressive phenotype can exist in the absence of pulmonary fibrosis. One question in this regard is how the term “progressive” should be defined. There are no uniformly accepted criteria, but we suggest patients meeting any of the following criteria within a 24-month period have experienced disease progression: a relative decline of ≥10% in forced vital capacity (FVC); a relative decline of ≥15% in diffusing capacity of the lung for carbon monoxide (DLCO); or worsening symptoms or a worsening radiological appearance accompanied by a ≥5–<10% relative decrease in FVC [1, 12].
In this review, we explore the current understanding of fibrosing ILDs (other than IPF) that may present a progressive phenotype and consider how they are diagnosed, highlighting similarities and differences between their presentation and clinical course. Fibrosis is a common characteristic in some patients with interstitial pneumonia with autoimmune features (IPAF). However, IPAF is not an established diagnostic entity: IPAF criteria were constructed by the European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force in order to standardise research but have yet to be validated in clinical practice [13, 14]. Idiopathic pleuroparenchymal fibroelastosis has been excluded because this disease is not limited to the lung parenchyma and there is no conclusive evidence to enable definitive consideration of its histology and natural course. Several further ILDs with an intermediate frequency of progressive fibrosis have also been excluded, such as inflammatory myopathy, mixed connective tissue disease (CTD) or desquamative interstitial pneumonia. Although these diseases are considered outside the scope of this review, they may be considered as possibilities during diagnostic workup.
Diagnostic methods
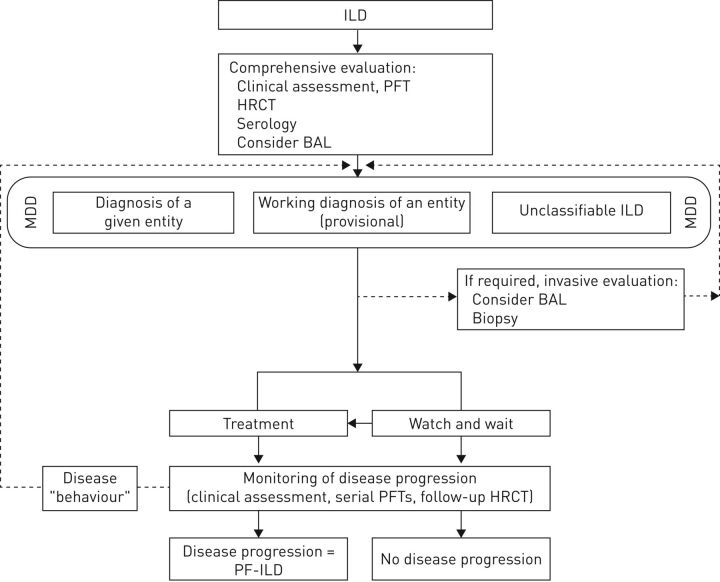
The differential diagnosis of ILDs requires a multidisciplinary approach, usually involving pulmonologists, radiologists and pathologists [15]. Evaluations include clinical presentation, specific history assessment, smoking status, lung function evolution, serological test results, imaging and, if required, lung biopsy [4, 5, 16]. In nearly all cases, high-resolution computed tomography (HRCT) is the primary tool for diagnosis [5]. HRCT (in particular, the fibrosis score) can also help determine the prognosis in ILDs, and quantitative computed tomography (CT) analysis may be useful for evaluating disease progression [17–19] (the article by Walsh et al. [20] in this issue of the European Respiratory Review provides more detail on imaging in fibrosing ILDs that may present a progressive phenotype). ILD diagnoses should be categorised as confident, provisional or unclassifiable depending on the level of diagnostic confidence. Based on current knowledge, we would propose the (previously unpublished) algorithm shown in figure 2 for diagnosing ILDs that may present a progressive-fibrosing phenotype; clinical data are needed to refine and validate this approach.
FIGURE 2.
Diagnosis of fibrosing interstitial lung diseases (ILD) that may present a progressive phenotype. PFTs: pulmonary function test; HCRT: high-resolution computed tomography; BAL: bronchoalveolar lavage; MDD: multidisciplinary diagnosis; PF-ILD: progressive-fibrosing ILD.
With due consideration of the clinical context, serological testing can determine whether there is an underlying autoimmune disease or autoreactive component [4]. Lung biopsy and bronchoscopy tend to be reserved for the minority of cases where other assessments are inconclusive. The risk of complications (including acute exacerbations of underlying disease) and mortality must be considered before a biopsy is undertaken, although novel methods such as transbronchial cryobiopsy may reduce the risks. Bronchoalveolar lavage is helpful for the diagnosis of some ILDs, although it may not be required in typical IPF (or UIP) [4].
Lung function tests and blood gas analyses usually have limited diagnostic value, because restrictive lung capacity and abnormal gas exchange are common to all ILDs with fibrosis [21]. However, they can help determine the severity of disease and the prognosis, and occasionally refine a working diagnosis based on disease behaviour [22–28]. Through serial measurements, lung function tests (particularly FVC) provide the primary means of monitoring disease progression [21].
Presentation and clinical course of fibrosing ILDs that may present a progressive phenotype, other than IPF
Idiopathic interstitial pneumonias
Idiopathic interstitial pneumonia (IIP) is a term used to describe a wide range of ILDs characterised by unique clinical, radiological and pathological features [29]. IPF is the most common type of IIP, accounting for ∼50% of all IIPs [30]. Diagnosis of patients with IIP can be challenging due to the mixed patterns of lung injury that can be observed [16, 31]. Within this classification, there are at least two entities that can develop a progressive-fibrosing phenotype and present similar clinical features to those seen in IPF: idiopathic NSIP (iNSIP) and unclassifiable IIP.
iNSIP
iNSIP accounts for about one-quarter of all IIPs [30]. Although the aetiology of iNSIP is unknown, the disease is more common in women, never-smokers and in individuals in their sixth decade of life [32, 33].
The diagnosis of iNSIP is predominantly a process of exclusion, where the possibility of a CTD or an ILD with autoimmune features should be ruled out. On HRCT, iNSIP exhibits peripheral, basal, symmetric, predominantly lower-lung reticular ground-glass opacities with irregular lines, traction bronchiectasis, consolidation and lower lobe volume loss that is usually diffuse or subpleural in the axial dimension but which sometimes spares the subpleural lung [32–34]. Honeycombing is generally absent. iNSIP is distinguished radiographically from IPF by its spatial and temporal homogeneity, and (in some cases) by the fact that it is subpleural sparing. The histology of iNSIP involves uniform alveolar and interstitial mononuclear cell inflammation with preserved lung architecture that is almost always accompanied by fibrosis [33].
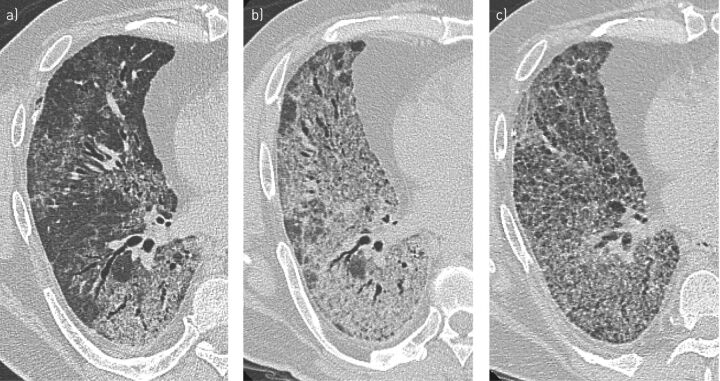
Patients with fibrosing iNSIP have a restrictive ventilatory defect on lung function tests and a low DLCO [32, 33]. The clinical presentation of iNSIP is nonspecific, with dyspnoea, cough and constitutional symptoms frequently reported [33]. Progression is highly heterogeneous: some cases can remain stable or improve, but others can progress to end-stage fibrosis [16, 17, 30]. A disease-behaviour classification is therefore useful to manage patients with iNSIP [35]. Serial HRCT images from a patient with NSIP are shown in figure 3.
FIGURE 3.
Nonspecific interstitial pneumonia: high-resolution computed tomography images from a 46-year-old male patient who underwent lung transplantation. a) The initial scan, taken at first admission, shows ground-glass attenuation and consolidation with reticulation and traction bronchiectasis along with a bronchovascular bundle, sparing the subpleural lung. b) A follow-up scan 3 years later showed increased ground-glass opacity and consolidation despite corticosteroids and immunosuppressive therapy. c) After another 3 years, a decrease in ground-glass opacity and consolidation was evident, together with increased traction bronchiectasis and cysts.
Neovascularisation within intra-alveolar fibrotic lesions can be more extensive in lung tissues from patients with fibrotic NSIP than patients with UIP [36]. The survival of patients with iNSIP may be poorer than patients with NSIP associated with CTDs [37]. Furthermore, histopathological findings of organising diffuse alveolar damage overlap or UIP overlap, or a clinical diagnosis of chronic HP overlap have been associated with increased risk of death [38]. However, survival rates in iNSIP are usually higher than in IPF [25, 39]. The 5-year survival rate has been reported to be 82% in iNSIP, whereas in IPF the median survival is <5 years [5, 32]. The outcome of iNSIP can be influenced by immunosuppressive therapy [40], and survival appears to be related to both lung function and histology [25, 41].
Unclassifiable IIP
IIPs may remain unclassifiable due to inadequate, nonspecific or conflicting clinical, radiological or histopathological findings, or because patients are unwilling or unable to undergo lung biopsy [16, 17, 42, 43]. Unclassifiable IIP was first defined in international guidelines in 2013, meaning the disease has been largely unrecognised and further investigation is needed for improved understanding [16, 44]. It has been reported that ∼10% of all IIPs are unclassifiable [17, 30]. The risk factors for the prognosis of unclassifiable IIP are not clearly defined.
Some patients with unclassifiable IIP have one or more of the defined pathological entities (UIP or NSIP) that characterise ILDs that may present a progressive-fibrosing phenotype [17, 44, 45]. Therefore, unclassifiable IIPs often have a progressive fibrotic component and can present similarly to define ILDs that may present a progressive fibrosing phenotype. Clinical features include dyspnoea, dry cough and auscultatory crackles with no evidence of exposures or features of autoimmune disease [46]. HRCT findings vary markedly, with mixed patterns of lung injury common. The prognosis for unclassifiable IIPs may be slightly better than that of IPF, but the mortality rate is still high (estimated in one study to be 31% at 5 years) [17]. A low DLCO, the presence of a UIP-like fibrotic pattern on HRCT and the extent of fibrosis on HRCT predict mortality in unclassifiable ILD [1, 17]. Patients with unclassifiable IIP and HRCT features suggesting fibrosis (a high fibrosis score, the presence of honeycombing or the presence of UIP or a possible UIP pattern) may have a poor prognosis, similar to patients with IPF [17]. The extent of fibrosis, which can be measured by quantitative CT analysis, may help to predict outcomes in patients with unclassifiable IIP [19].
CTD-ILD
CTDs, also known as collagen vascular diseases, encompass a spectrum of systemic immune diseases in which self-reactive T- and B-cells produce circulating autoantibodies leading to inflammation and organ damage [47, 48]. While the causes of these diseases are unknown, both genetic and environmental factors appear to contribute [47, 49]. ILDs occur in ∼15% of all patients with CTDs, with higher rates in certain conditions such as systemic sclerosis (SSc) and rheumatoid arthritis (RA), and they are associated with varying rates of mortality [50, 51]. CTD-ILDs are the second most common diagnosis in tertiary ILD referral centres [52, 53]. The risk of CTD-ILDs appears to be higher in women and in patients <50 years of age [48].
CTD-ILDs can present with fibrosing lung injury, and HRCT assessments have shown that NSIP is the most common pattern [48]. CTD-ILD may alternatively present with a pattern of UIP, and in this case it is important to differentiate the disease from IPF. Expertise is needed to understand the radiological or pathological features that enable differential diagnosis, and the frequent presence of comorbidities may complicate the process [54, 55].
CTDs associated with fibrosing ILD include RA, SSc, idiopathic inflammatory myopathy (polymyositis and dermatomyositis), Sjögren's syndrome, systemic lupus erythematosus and mixed CTDs [48]. Of these conditions, SSc and RA are most commonly associated with ILD, and these are discussed in more detail below.
RA-ILD
RA is characterised by swelling, tenderness and destruction of synovial joints, along with extra-articular manifestations that can include pulmonary involvement [56, 57]. The mean age at diagnosis of RA is ∼60 years [58]. Under the age of 75 years, incidence rates of RA are up to four times higher in women than men. Fibrosing ILD, the most common pulmonary manifestation of disease (occurring in ∼10% of patients), is a major cause of morbidity and mortality in patients with RA and in some cases can be the first symptom of RA [49, 59–61]. RA-ILD is believed to be caused by chronic immune activation and inflammation, which stimulates fibroproliferation in the lung parenchyma of genetically susceptible individuals. There are strong associations between RA-ILD and older age, male sex, cigarette smoking, high rheumatoid factor titres and increased anti-citrullinated protein antibody levels [62].
When diagnosing RA-ILD, it is important first to rule out infection and drug-induced lung injury caused by common RA medications, such as disease-modifying anti-rheumatic drugs and tumour necrosis factor-ɑ inhibitors, which are known to cause pulmonary toxicity [49]. The histological lung injury in RA-ILD can be any of the interstitial pneumonia patterns, including overlaps. Most studies report UIP and NSIP as being the most common patterns, and coexisting UIP and NSIP are common in RA-ILD [63]. In addition, lymphoid hyperplasia with germinal centres, and pleural and peribronchiolar lesions are more common in RA-ILD than in IIPs, while fibroblastic foci are observed less frequently [64].
RA-ILD typically progresses faster in patients with UIP than other subtypes, although mortality rates are variable [49]. Most RA-ILD patients with UIP have a similar slightly improved prognosis compared with IPF, with acute exacerbations having a major impact on survival [57, 65–68]. Conversely, recently published data suggest the existence of a subset of RA-ILD patients with UIP in whom the disease stabilises in the long term, differentiating these patients from those with IPF [69].
SSc-associated ILD
SSc, also known as scleroderma, is an autoreactive immune CTD characterised by inflammation, immune dysregulation, microvascular damage and progressive fibrosis of the skin and internal organs [70, 71]. The peak onset of SSc is 30–70 years of age, with women being four times more likely to develop the disease than men [72]. Fibrosing ILD is a very common manifestation of SSc and accounts for about one-third of all disease-related deaths [73]. The pathogenesis of fibrosing SSc-associated ILD (SSc-ILD) is not clearly understood but is believed to involve repetitive epithelial injury, activation of innate and adaptive immune mechanisms, and fibroblast recruitment and activation leading to extracellular matrix accumulation and fibrosis [74]. SSc is characterised by small vessel vasculopathy, autoantibody production and fibroblast dysfunction subsequently leading to extracellular matrix deposition [75].
Key features of SSc-ILD include pulmonary fibrosis, most pronounced in the basilar portions of the lungs, and “velcro” crackles on auscultation that cannot be attributed to another cause [71, 74]. Most patients have a fibrotic NSIP pattern on HRCT, with a high proportion of ground-glass opacities (peripheral distribution with subpleural and basilar predominance) and a low proportion of course reticulation [74, 76, 77]. However, a UIP pattern may sometimes be seen [74, 77].
Symptoms of SSc-ILD include exertional dyspnoea, non-productive cough and fatigue [74, 77]. Lung function tests generally show a restrictive ventilatory deficit with a low DLCO. Nearly all SSc-ILD patients test positive for antinuclear antibodies, and SSc-specific antibodies (e.g. anti-topoisomerase I) are commonly found [74]. Median survival among patients with SSc-ILD appears to be better than among IPF patients, probably because of the higher prevalence of the NSIP pattern [77–79]. The extent of disease on HRCT has been shown to be an independent predictor of mortality [80].
HP
HP (also known as extrinsic alveolar alveolitis) is a complex, diffuse ILD caused by repeated exposure and sensitisation to an inhaled antigen, most commonly avian, microbial (especially moulds) or chemical [81, 82]. HP is the third most prevalent ILD diagnosis after IPF- and CTD-ILDs [53]. It is believed that antigen inhalation results in type III/IV hypersensitivity reactions, which cause alveolar epithelial injury [83]. Abnormal repair mechanisms may then lead to fibroblast expansion/activation, excessive collagen deposition (through accumulation of the extracellular matrix) and destruction of the lung architecture, similar to that seen in IPF [83, 84]. The principal forms of HP are acute/subacute and chronic [82, 85]. Acute/subacute HP is predominantly characterised by influenza-like symptoms (e.g. fever, cough and dyspnoea) that emerge 2–9 h after exposure to an antigen, and may last or increase over a period of hours or days [82]. Patients with chronic HP have slowly progressive disease, with dyspnoea, cough, fatigue and weight loss [81, 82]. The chronic form of HP arises from repetitive, low-level exposure to the causative antigen, but the identity of the causative antigen may remain unknown in more than half of all cases [82, 86]. The search for a cause of chronic HP may include consideration of specific circulating immunoglobulin G antibodies [16].
Fibrosis develops in many patients with chronic HP. Because of its similarity to IPF, chronic fibrotic HP is difficult to diagnose [87–92]. On HRCT, a “possible UIP” pattern of fibrosis is often seen in fibrosing HP [93]. Potential radiological features that distinguish chronic HP from UIP include: a patchy pattern of ground-glass opacities with upper lung predominance; a combination of ground-glass, reticular and centrilobular nodular opacities with fibrosis; and lobular areas with mosaic attenuation and air trapping [16, 81, 84, 90, 91]. The diagnosis can be supported by lymphocyte predominance on bronchoalveolar lavage, although the absence of this finding should not exclude the diagnosis of fibrosing HP [84]. Potential histological findings include fibrosis in a bronchiolocentric pattern, infrequent or inconsistently distributed fibroblast foci, a variable number of poorly formed granulomas, giant cells, inflammation, organising pneumonia or chronic bronchiolitis [16, 94]. An international group of experts recently attached highest importance to the following diagnostic criteria for chronic HP: identification of a causative antigen, time relation between exposure and disease, mosaic attenuation on chest imaging, and poorly formed non-necrotising granulomas on pathology [95].
The natural history of fibrosing HP with a UIP pattern is similar to IPF, whereby progression occurs despite treatment with an agent considered appropriate for the ILD. However, in other subsets of patients with HP, fibrosis that initially appears progressive may stabilise in the longer term in response to therapy. Chronic fibrosing HP develops over a period of months to years, and is usually consequential to continuous low-level exposure to the causal antigen, although in a significant number of cases an aetiological agent cannot be found. Patients with chronic HP have significantly worse health-related quality of life than those with IPF [96]. The median survival from diagnosis of chronic HP is ∼5 years [86]. HP with either a UIP-like or an NSIP pattern may be associated with similar survival rates to IPF [81]. Potentially fatal acute exacerbations of HP have been reported [97]. The extent of fibrosis on HRCT in patients with chronic HP is predictive of mortality [86, 92, 98–101].
Sarcoidosis
Sarcoidosis is a rare systemic, inflammatory disease of unknown aetiology [102]. It may result from a chronic immunological response to an unknown antigen(s) in genetically susceptible individuals. Approximately 90% of patients with sarcoidosis have pulmonary involvement [103]. Signs and symptoms of pulmonary sarcoidosis include dry cough, chronic dyspnoea on exertion, chest tightness, wheezing, hypoxaemia and lung function decline [102, 104].
Approximately 20% of patients with pulmonary sarcoidosis develop pulmonary fibrosis (stage IV sarcoidosis), which is associated with significant morbidity and mortality [102]. Pulmonary fibrosis predominantly affects the upper lobes (posterior segments) and is airway-centred with no air trapping; the fibrosis tends to follow the bronchovascular bundles [105]. The extent and type of fibrosis are highly variable. Imaging tends to reveal faint reticulations to dense linear bands, cystic lung disease, traction bronchiectasis and airway distortion [104]. Extensive parenchymal destruction and mycetoma are also occasionally observed. A UIP pattern of lung injury is uncommonly seen in patients with fibrosing pulmonary sarcoidosis, although honeycombing may be seen in the upper zones of the lungs [105].
The natural history of lung function decline in patients with sarcoidosis has not been well established [102]. A 10-year mortality rate of 16% has been reported in patients with lung fibrosis [106], which, allowing for comorbidities and other causes of death, may suggest that a small subset of patients have the progressive-fibrosing phenotype despite appropriate therapy. Acute exacerbations have not been reported in stage IV pulmonary sarcoidosis [105].
ILDs related to other occupational exposures
ILDs related to other occupational exposures are caused by inhalation and retention in the lungs of various dusts. The most common of these conditions are: asbestosis, which is caused by asbestos fibres; and silicosis, which is related to free crystalline silicon dioxide or silica [107–109]. Exposure to farming, livestock, metal dust, coal, sand and dust arising from the preparation of dental prosthetics have also been linked to the development of ILDs [110]. ILDs related to other occupational exposures are diagnosed principally from exposure history and imaging. In simple silicosis, multiple, small rounded nodules of hyalanised collagen are visualised using HRCT as bilateral nodules in the upper lobes [110]. In more advanced, complicated silicosis, convergence of the nodules may result in larger opacities, which can develop into a progressive massive fibrosis pattern in the upper lobes, comparable to pulmonary fibrosis in sarcoidosis. However, some patients with silicosis present with a UIP pattern [111, 112]. In fibrosing asbestosis, HRCT findings resemble those seen in IPF [108]; some patients may have pleural thickening and/or plaques, which are suggestive of asbestos exposure. Additionally, histology may reveal iron-coated asbestos fibres (“asbestos bodies”) embedded in the peribronchiolar interstitium and alveolar spaces, which are the hallmark of asbestosis [113].
Progression of silicosis from simple to complicated disease is generally slow and can take >10 years [109]. For fibrosing asbestosis, the prognosis is usually better than for IPF because mild disease progresses slowly, although deterioration is quicker in patients retaining larger quantities of asbestos in the lungs [114]. According to a US study of individuals dying from asbestosis, the condition shortens life by a median of 8 years [115]. As there are no known treatments for ILDs related to other occupational exposures, avoidance of exposure is the only means of reducing the burden of disease.
Conclusion
Progressive fibrosis is an important characteristic of several ILDs that is strongly linked with morbidity and mortality. Although individual ILDs that may present a progressive-fibrosing phenotype have distinguishing features in terms of their clinical, radiological and histopathological presentations, they also have many overlapping characteristics that are similar to the classical fibrosing ILD with a progressive phenotype, IPF. Antifibrotic therapy is currently being investigated in these diseases. Accurate and early diagnosis is challenging but crucial to ensure that each patient receives treatment that is appropriate for the rate of progression seen with their particular disease.
Acknowledgements
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Writing assistance was provided by Ken Sutor of GeoMed, an Ashfield company, part of UDG Healthcare plc, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Footnotes
Provenance: Publication of this peer-reviewed article was sponsored by Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA (principal sponsor, European Respiratory Review issue 150).
Conflict of interest: V. Cottin reports receiving the following, outside the submitted work: personal fees from Actelion for consultancy, lectures and travel to medical meetings; personal fees from Boehringer Ingelheim for the development of educational presentations, consultancy, lectures and travel to medical meetings; personal fees from Bayer for consultancy; personal fees from Gilead for acting as a member of an adjudication committee; personal fees from GSK for consultancy; personal fees from MSD for consultancy and travel to medical meetings; personal fees from Novartis for consultancy and lectures; personal fees from Roche for consultancy, lecture fees and travel to medical meetings; personal fees from Sanofi for consultancy and lectures; a grant to his institution from Boehringer Ingelheim; a grant to his institution from Roche; personal fees from Promedior for acting as Chair of the DSMB; personal fees from Celgene for the DSMB; and personal fees from Galapagos for consultancy and for acting as Chair of the DSMB.
Conflict of interest: N.A. Hirani has nothing to disclose.
Conflict of interest: D.L. Hotchkin has nothing to disclose.
Conflict of interest: A.M. Nambiar reports receiving the following, outside the submitted work: grants, personal fees, non-financial support and other support from Boehringer Ingelheim; and grants from Genentech-Roche.
Conflict of interest: T. Ogura reports receiving the following, outside the submitted work: grants and personal fees from Boehringer Ingelheim, Japan; grants from the Ministry of Health, Labour and Welfare, Japan; personal fees from Astellas Pharma Inc., Shionogi & Co. Ltd, Toray Industries Inc., AstraZeneca K.K. and Kyorin Inc.
Conflict of interest: M. Otaola has nothing to disclose.
Conflict of interest: D. Skowasch reports receiving the following, outside the submitted work: personal fees/honoraria for consulting and speaking from Boehringer Ingelheim and Roche.
Conflict of interest: J.S. Park has nothing to disclose.
Conflict of interest: H.K. Poonyagariyagorn has nothing to disclose.
Conflict of interest: W. Wuyts reports receiving the following, outside the submitted work: grants paid to his university from Boehringer Ingelheim and Hoffmann La Roche; and travel fees from Galapagos.
Conflict of interest: A.U. Wells reports receiving the following, outside the submitted work: personal fees for speaking and for acting on advisory boards from Boehringer Ingelheim, Roche and Bayer.
Support statement: The authors received no direct compensation related to the development of the manuscript. Writing assistance was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI).
References
- 1.Flaherty KR, Brown KK, Wells AU, et al. . Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir Res 2017; 4: e000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikolasch TA, Porter JC. Transbronchial cryobiopsy in the diagnosis of interstitial lung disease: a cool new approach. Respirology 2014; 19: 623–624. [DOI] [PubMed] [Google Scholar]
- 3.Schoenheit G, Becattelli I, Cohen AH. Living with idiopathic pulmonary fibrosis: an in-depth qualitative survey of European patients. Chron Respir Dis 2011; 8: 225–231. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Collard HR, Egan JJ, et al. . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez FJ, Collard HR, Pardo A, et al. . Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 2017; 3: 17074. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017; 389: 1941–1952. [DOI] [PubMed] [Google Scholar]
- 7.Kreuter M, Walscher J, Behr J. Antifibrotic drugs as treatment of nonidiopathic pulmonary fibrosis interstitial pneumonias: the time is now (?). Curr Opin Pulm Med 2017; 23: 418–425. [DOI] [PubMed] [Google Scholar]
- 8.Solomon JJ, Olson AL, Fischer A, et al. . Scleroderma lung disease. Eur Respir Rev 2013; 22: 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells AU, Brown KK, Flaherty KR, et al. . What's in a name? That which we call IPF, by any other name would act the same. Eur Respir J 2018; 51: 1800692. [DOI] [PubMed] [Google Scholar]
- 10.Olson AL, Brown KK, Swigris JJ. Understanding and optimizing health-related quality of life and physical functional capacity in idiopathic pulmonary fibrosis. Patient Relat Outcome Meas 2016; 7: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sgalla G, Iovene B, Calvello M, et al. . Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res 2018; 19: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley B, Branley HM, Egan JJ, et al. . Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008; 63: Suppl. 5, v1–v58. [DOI] [PubMed] [Google Scholar]
- 13.Fischer A, Antoniou KM, Brown KK, et al. . An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015; 46: 976–987. [DOI] [PubMed] [Google Scholar]
- 14.Strek ME, Costabel U. Interstitial pneumonia with autoimmune features: a critical appraisal of the new definition. Curr Opin Pulm Med 2016; 22: 442–449. [DOI] [PubMed] [Google Scholar]
- 15.De Sadeleer LJ, Meert C, Yserbyt J, et al. . Diagnostic ability of a dynamic multidisciplinary discussion in interstitial lung diseases: a retrospective observational study of 938 cases. Chest 2018; 153: 1416–1423. [DOI] [PubMed] [Google Scholar]
- 16.Travis WD, Costabel U, Hansell DM, et al. . An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryerson CJ, Urbania TH, Richeldi L, et al. . Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J 2013; 42: 750–757. [DOI] [PubMed] [Google Scholar]
- 18.Humphries SM, Yagihashi K, Huckleberry J, et al. . Idiopathic pulmonary fibrosis: data-driven textural analysis of extent of fibrosis at baseline and 15-month follow-up. Radiology 2017; 285: 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob J, Bartholmai BJ, Rajagopalan S, et al. . Unclassifiable-interstitial lung disease: outcome prediction using CT and functional indices. Respir Med 2017; 130: 43–51. [DOI] [PubMed] [Google Scholar]
- 20.Walsh SLF, Devaraj A, Enghelmayer JI, et al. . Role of imaging in progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018; 27: 180073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buzan MT, Pop CM. State of the art in the diagnosis and management of interstitial lung disease. Clujul Med 2015; 88: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.du Bois RM, Weycker D, Albera C, et al. . Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011; 184: 1382–1389. [DOI] [PubMed] [Google Scholar]
- 23.Flaherty KR, Mumford JA, Murray S, et al. . Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003; 168: 543–548. [DOI] [PubMed] [Google Scholar]
- 24.Gimenez A, Storrer K, Kuranishi L, et al. . Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax 2018; 73: 391–392. [DOI] [PubMed] [Google Scholar]
- 25.Jegal Y, Kim DS, Shim TS, et al. . Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005; 171: 639–644. [DOI] [PubMed] [Google Scholar]
- 26.Park IN, Jegal Y, Kim DS, et al. . Clinical course and lung function change of idiopathic nonspecific interstitial pneumonia. Eur Respir J 2009; 33: 68–76. [DOI] [PubMed] [Google Scholar]
- 27.Zappala CJ, Latsi PI, Nicholson AG, et al. . Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35: 830–836. [DOI] [PubMed] [Google Scholar]
- 28.Latsi PI, du Bois RM, Nicholson AG, et al. . Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003; 168: 531–537. [DOI] [PubMed] [Google Scholar]
- 29.Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev 2015; 24: 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neurohr C, Behr J. Changes in the current classification of IIP: a critical review. Respirology 2015; 20: 699–704. [DOI] [PubMed] [Google Scholar]
- 31.Jankowich MD, Rounds SIS. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest 2012; 141: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis WD, Hunninghake G, King TE Jr, et al. . Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med 2008; 177: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 33.Belloli EA, Beckford R, Hadley R, et al. . Idiopathic non-specific interstitial pneumonia. Respirology 2016; 21: 259–268. [DOI] [PubMed] [Google Scholar]
- 34.Sverzellati N, Lynch DA, Hansell DM, et al. . American Thoracic Society-European Respiratory Society classification of the idiopathic interstitial pneumonias: advances in knowledge since 2002. Radiographics 2015; 35: 1849–1871. [DOI] [PubMed] [Google Scholar]
- 35.Yamakawa H, Kitamura H, Takemura T, et al. . Prognostic factors and disease behaviour of pathologically proven fibrotic non-specific interstitial pneumonia. Respirology 2018; 23: 1032–1040. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi M, Kunugi S, Terasaki Y, et al. . The difference of neovascularization in early intra-alveolar fibrosis between nonspecific interstitial pneumonia and usual interstitial pneumonia. Pathol Int 2013; 63: 237–244. [DOI] [PubMed] [Google Scholar]
- 37.Nunes H, Schubel K, Piver D, et al. . Nonspecific interstitial pneumonia: survival is influenced by the underlying cause. Eur Respir J 2015; 45: 746–755. [DOI] [PubMed] [Google Scholar]
- 38.Kambouchner M, Levy P, Nicholson AG, et al. . Prognostic relevance of histological variants in nonspecific interstitial pneumonia. Histopathology 2014; 65: 549–560. [DOI] [PubMed] [Google Scholar]
- 39.Bjoraker JA, Ryu JH, Edwin MK, et al. . Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998; 157: 199–203. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Park MS, Kim SY, et al. . Factors affecting treatment outcome in patients with idiopathic nonspecific interstitial pneumonia: a nationwide cohort study. Respir Res 2017; 18: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Travis WD, Matsui K, Moss J, et al. . Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000; 24: 19–33. [DOI] [PubMed] [Google Scholar]
- 42.American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002; 165: 277–304. [DOI] [PubMed] [Google Scholar]
- 43.Hyldgaard C, Bendstrup E, Wells AU, et al. . Unclassifiable interstitial lung diseases: clinical characteristics and survival. Respirology 2017; 22: 494–500. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura Y, Sugino K, Kitani M, et al. . Clinico-radio-pathological characteristics of unclassifiable idiopathic interstitial pneumonias. Respir Investig 2018; 56: 40–47. [DOI] [PubMed] [Google Scholar]
- 45.Traila D, Oancea C, Tudorache E, et al. . Clinical profile of unclassifiable interstitial lung disease: comparison with chronic fibrosing idiopathic interstitial pneumonias. J Int Med Res 2018; 46: 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skolnik K, Ryerson CJ. Unclassifiable interstitial lung disease: a review. Respirology 2016; 21: 51–56. [DOI] [PubMed] [Google Scholar]
- 47.Wells AU, Denton CP. Interstitial lung disease in connective tissue disease – mechanisms and management. Nat Rev Rheumatol 2014; 10: 728–739. [DOI] [PubMed] [Google Scholar]
- 48.Cottin V. Idiopathic interstitial pneumonias with connective tissue diseases features: a review. Respirology 2016; 21: 245–258. [DOI] [PubMed] [Google Scholar]
- 49.Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am 2015; 41: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antoniou KM, Margaritopoulos G, Economidou F, et al. . Pivotal clinical dilemmas in collagen vascular diseases associated with interstitial lung involvement. Eur Respir J 2009; 33: 882–896. [DOI] [PubMed] [Google Scholar]
- 51.Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ 2016; 352: h6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmad K, Barba T, Gamondes D, et al. . Interstitial pneumonia with autoimmune features: clinical, radiologic, and histological characteristics and outcome in a series of 57 patients. Respir Med 2017; 123: 56–62. [DOI] [PubMed] [Google Scholar]
- 53.Hyldgaard C, Hilberg O, Muller A, et al. . A cohort study of interstitial lung diseases in central Denmark. Respir Med 2014; 108: 793–799. [DOI] [PubMed] [Google Scholar]
- 54.Spagnolo P, Cordier JF, Cottin V. Connective tissue diseases, multimorbidity and the ageing lung. Eur Respir J 2016; 47: 1535–1558. [DOI] [PubMed] [Google Scholar]
- 55.Wuyts WA, Cavazza A, Rossi G, et al. . Differential diagnosis of usual interstitial pneumonia: when is it truly idiopathic? Eur Respir Rev 2014; 23: 308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aletaha D, Neogi T, Silman AJ, et al. . 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010; 69: 1580–1588. [DOI] [PubMed] [Google Scholar]
- 57.Kim EJ, Collard HR, King TE Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest 2009; 136: 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doran MF, Pond GR, Crowson CS, et al. . Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum 2002; 46: 625–631. [DOI] [PubMed] [Google Scholar]
- 59.Bongartz T, Nannini C, Medina-Velasquez YF, et al. . Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010; 62: 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olson AL, Swigris JJ, Sprunger DB, et al. . Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011; 183: 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyldgaard C, Hilberg O, Pedersen AB, et al. . A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017; 76: 1700–1706. [DOI] [PubMed] [Google Scholar]
- 62.Johnson C. Recent advances in the pathogenesis, prediction, and management of rheumatoid arthritis-associated interstitial lung disease. Curr Opin Rheumatol 2017; 29: 254–259. [DOI] [PubMed] [Google Scholar]
- 63.Smith M, Dalurzo M, Panse P, et al. . Usual interstitial pneumonia-pattern fibrosis in surgical lung biopsies. Clinical, radiological and histopathological clues to aetiology. J Clin Pathol 2013; 66: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee HK, Kim DS, Yoo B, et al. . Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 2005; 127: 2019–2027. [DOI] [PubMed] [Google Scholar]
- 65.Hozumi H, Nakamura Y, Johkoh T, et al. . Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open 2013; 3: e003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim EJ, Elicker BM, Maldonado F, et al. . Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010; 35: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 67.Park JH, Kim DS, Park IN, et al. . Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007; 175: 705–711. [DOI] [PubMed] [Google Scholar]
- 68.Moua T, Zamora Martinez AC, Baqir M, et al. . Predictors of diagnosis and survival in idiopathic pulmonary fibrosis and connective tissue disease-related usual interstitial pneumonia. Respir Res 2014; 15: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacob J, Hirani N, van Moorsel CHM, et al. . Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J 2018; in press [ 10.1183/13993003.00869-2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cutolo M, Sulli A, Pizzorni C, et al. . Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 2000; 27: 155–160. [PubMed] [Google Scholar]
- 71.van den Hoogen F, Khanna D, Fransen J, et al. . 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013; 65: 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayes MD, Lacey JV Jr, Beebe-Dimmer J, et al. . Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 2003; 48: 2246–2255. [DOI] [PubMed] [Google Scholar]
- 73.Tyndall AJ, Bannert B, Vonk M, et al. . Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69: 1809–1815. [DOI] [PubMed] [Google Scholar]
- 74.Cappelli S, Bellando Randone S, Camiciottoli G, et al. . Interstitial lung disease in systemic sclerosis: where do we stand? Eur Respir Rev 2015; 24: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pattanaik D, Brown M, Postlethwaite BC, et al. . Pathogenesis of systemic sclerosis. Front Immunol 2015; 6: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Desai SR, Veeraraghavan S, Hansell DM, et al. . CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004; 232: 560–567. [DOI] [PubMed] [Google Scholar]
- 77.Schoenfeld SR, Castelino FV. Interstitial lung disease in scleroderma. Rheum Dis Clin North Am 2015; 41: 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouros D, Wells AU, Nicholson AG, et al. . Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 2002; 165: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 79.Fischer A, Swigris JJ, Groshong SD, et al. . Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest 2008; 134: 601–605. [DOI] [PubMed] [Google Scholar]
- 80.Winstone TA, Assayag D, Wilcox PG, et al. . Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest 2014; 146: 422–436. [DOI] [PubMed] [Google Scholar]
- 81.Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med 2012; 186: 314–324. [DOI] [PubMed] [Google Scholar]
- 82.Sforza GG R, Marinou A. Hypersensitivity pneumonitis: a complex lung disease. Clin Mol Allergy 2017; 15: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takemura T, Akashi T, Ohtani Y, et al. . Pathology of hypersensitivity pneumonitis. Curr Opin Pulm Med 2008; 14: 440–454. [DOI] [PubMed] [Google Scholar]
- 84.Kouranos V, Jacob J, Nicholson A, et al. . Fibrotic hypersensitivity pneumonitis: key issues in diagnosis and management. J Clin Med 2017; 6: pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vasakova M, Morell F, Walsh S, et al. . Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am J Respir Crit Care Med 2017; 196: 680–689. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez Perez ER, Swigris JJ, Forssen AV, et al. . Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest 2013; 144: 1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacob J, Bartholmai BJ, Brun AL, et al. . Evaluation of visual and computer-based CT analysis for the identification of functional patterns of obstruction and restriction in hypersensitivity pneumonitis. Respirology 2017; 22: 1585–1591. [DOI] [PubMed] [Google Scholar]
- 88.Jeong YJ, Lee KS, Chung MP, et al. . Chronic hypersensitivity pneumonitis and pulmonary sarcoidosis: differentiation from usual interstitial pneumonia using high-resolution computed tomography. Semin Ultrasound CT MR 2014; 35: 47–58. [DOI] [PubMed] [Google Scholar]
- 89.Morell F, Villar A, Montero MA, et al. . Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir Med 2013; 1: 685–694. [DOI] [PubMed] [Google Scholar]
- 90.Salisbury ML, Myers JL, Belloli EA, et al. . Diagnosis and treatment of fibrotic hypersensitivity pneumonia. where we stand and where we need to go. Am J Respir Crit Care Med 2017; 196: 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Silva CI, Muller NL, Lynch DA, et al. . Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology 2008; 246: 288–297. [DOI] [PubMed] [Google Scholar]
- 92.Walsh SL, Sverzellati N, Devaraj A, et al. . Chronic hypersensitivity pneumonitis: high resolution computed tomography patterns and pulmonary function indices as prognostic determinants. Eur Radiol 2012; 22: 1672–1679. [DOI] [PubMed] [Google Scholar]
- 93.Sahin H, Brown KK, Curran-Everett D, et al. . Chronic hypersensitivity pneumonitis: CT features comparison with pathologic evidence of fibrosis and survival. Radiology 2007; 244: 591–598. [DOI] [PubMed] [Google Scholar]
- 94.Glazer CS. Chronic hypersensitivity pneumonitis: important considerations in the work-up of this fibrotic lung disease. Curr Opin Pulm Med 2015; 21: 171–177. [DOI] [PubMed] [Google Scholar]
- 95.Morisset J, Johannson KA, Jones KD, et al. . Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: an international modified Delphi survey. Am J Respir Crit Care Med 2017; in press 10.1164/rccm.201710-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lubin M, Chen H, Elicker B, et al. . A comparison of health-related quality of life in idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. Chest 2014; 145: 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olson AL, Huie TJ, Groshong SD, et al. . Acute exacerbations of fibrotic hypersensitivity pneumonitis: a case series. Chest 2008; 134: 844–850. [DOI] [PubMed] [Google Scholar]
- 98.Mooney JJ, Elicker BM, Urbania TH, et al. . Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest 2013; 144: 586–592. [DOI] [PubMed] [Google Scholar]
- 99.Vourlekis JS, Schwarz MI, Cherniack RM, et al. . The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med 2004; 116: 662–668. [DOI] [PubMed] [Google Scholar]
- 100.Hanak V, Golbin JM, Hartman TE, et al. . High-resolution CT findings of parenchymal fibrosis correlate with prognosis in hypersensitivity pneumonitis. Chest 2008; 134: 133–138. [DOI] [PubMed] [Google Scholar]
- 101.Jacob J, Bartholmai BJ, Rajagopalan S, et al. . Automated computer-based CT stratification as a predictor of outcome in hypersensitivity pneumonitis. Eur Radiol 2017; 27: 3635–3646. [DOI] [PubMed] [Google Scholar]
- 102.Patterson KC, Strek ME. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann Am Thorac Soc 2013; 10: 362–370. [DOI] [PubMed] [Google Scholar]
- 103.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med 2011; 183: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patterson KC, Chen ES. The pathogenesis of pulmonary sarcoidosis and implications for treatment. Chest 2017; 153: 1432–1442. [DOI] [PubMed] [Google Scholar]
- 105.Salvatore M, Ishikawa G, Padilla M. Is it idiopathic pulmonary fibrosis or not? J Am Board Fam Med 2018; 31: 151–162. [DOI] [PubMed] [Google Scholar]
- 106.Nardi A, Brillet PY, Letoumelin P, et al. . Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J 2011; 38: 1368–1373. [DOI] [PubMed] [Google Scholar]
- 107.American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 2004; 170: 691–715. [DOI] [PubMed] [Google Scholar]
- 108.Gulati M, Redlich CA. Asbestosis and environmental causes of usual interstitial pneumonia. Curr Opin Pulm Med 2015; 21: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leung CC, Yu IT, Chen W. Silicosis. Lancet 2012; 379: 2008–2018. [DOI] [PubMed] [Google Scholar]
- 110.Castranova V, Vallyathan V. Silicosis and coal workers’ pneumoconiosis. Environ Health Perspect 2000; 108: Suppl. 4, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arakawa H, Johkoh T, Honma K, et al. . Chronic interstitial pneumonia in silicosis and mix-dust pneumoconiosis: its prevalence and comparison of CT findings with idiopathic pulmonary fibrosis. Chest 2007; 131: 1870–1876. [DOI] [PubMed] [Google Scholar]
- 112.Arakawa H, Fujimoto K, Honma K, et al. . Progression from near-normal to end-stage lungs in chronic interstitial pneumonia related to silica exposure: long-term CT observations. AJR Am J Roentgenol 2008; 191: 1040–1045. [DOI] [PubMed] [Google Scholar]
- 113.Larsen BT, Smith ML, Elicker BM, et al. . Diagnostic approach to advanced fibrotic interstitial lung disease: bringing together clinical, radiologic, and histologic clues. Arch Pathol Lab Med 2017; 141: 901–915. [DOI] [PubMed] [Google Scholar]
- 114.Cullinan P, Reid P. Pneumoconiosis. Prim Care Respir J 2013; 22: 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bang KM, Mazurek JM, Wood JM, et al. . Diseases attributable to asbestos exposure: years of potential life lost, United States, 1999–2010. Am J Ind Med 2014; 57: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]