Abstract
Background
While immunotherapy has been shown to improve survival and decrease neurologic death in patients with brain metastases, it remains unclear whether this improvement is due to prevention of new metastasis to the brain.
Method
We performed a retrospective review of patients presenting with brain metastases simultaneously with the first diagnosis of metastatic disease and were treated with upfront immunotherapy as part of their treatment regimen and stereotactic radiosurgery (SRS) to the brain metastases. We compared this cohort with a historical control population (prior to the immunotherapy era) who were treated with pre-immunotherapy standard of care systemic therapy and with SRS to the brain metastases.
Results
Median overall survival time was improved in the patients receiving upfront immunotherapy compared to the historical cohort (48 months vs 8.4 months, p=0.001). Median time to distant brain failure was statistically equivalent (p=0.3) between the upfront immunotherapy cohort and historical control cohort (10.3 vs 12.6 months). Brain metastasis velocity was lower in the upfront immunotherapy cohort (median 3.72 metastases per year) than in the historical controls (median 9.48 metastases per year, p=0.001). Cumulative incidence of neurologic death at one year was 12% in the upfront immunotherapy cohort and 28% in the historical control cohort (p=0.1).
Conclusions
Upfront immunotherapy appears to improve overall survival and decrease BMV compared to historical controls. While these data remain to be validated, they suggest that brain metastasis patients may benefit from concurrent immunotherapy with SRS.
Keywords: Stereotactic radiosurgery, immunotherapy, brain metastasis, BMV, overall survival
INTRODUCTION
Brain metastasis velocity (BMV) is a recently described metric used to express the rate at which patients experience new brain metastases after receiving upfront stereotactic radiosurgery (SRS).[1] BMV has been shown to be prognostic for survival[2,3] and predictive of which patients actually die of their brain metastases.[1] While BMV has been proposed as a potential tool to help triage patients to the proper salvage treatment after upfront SRS failure, it may also have the potential to assess the efficacy of systemic therapies in their ability to prevent re-seeding of the brain with new brain metastases.
In the past several years, immunotherapy (specifically PDL-1 and PD-1 inhibitors) has improved outcomes for several different cancer histologies including melanoma,[4] lung cancer,[5] and renal cell carcinoma.[6] In patients with brain metastases from these primary cancers, immunotherapy has also been found to improve overall survival.[7] One question that has arisen is to what degree immunotherapy may affect the ability to control brain metastases and prevent the formation of new brain metastases. Several studies have been published demonstrating at least modest activity for immunotherapy agents against brain metastases.[8,9] However, data is sparse to show whether immunotherapy may decrease the likelihood of new brain metastases forming.
Since the original BMV analysis was published, several additional studies have been published looking at how immunotherapy may affect the BMV. One recent competing risk analysis in brain metastasis patients receiving SRS suggested that the improvement in survival seen with patients receiving immunotherapy was driven by a decrease in neurologic death.[7] Another recent analysis demonstrated that even in the immunotherapy era, a high BMV continued to be predictive of the likelihood of dying of brain metastases.[10] In both of these series, the analyses were underpowered to determine whether immunotherapy affected BMV, or if decreased BMV may be a mechanism by which immunotherapy leads to decreased neurologic death. The aforementioned studies were also confounded by heterogeneous populations with patients receiving immunotherapy at various times in the natural history of their cancer.
As immunotherapy has been moved into the upfront setting for treatment of multiple metastatic cancers, the ability to ask the question of how immunotherapy affects BMV is more readily answered. The timing and usage of immunotherapy have become more standardized across multiple histologies and is now commonly given as primary upfront therapy at the time of metastatic cancer diagnosis. In the present study, we sought to analyze whether the use of upfront immunotherapy in brain metastasis patients receiving SRS affects BMV over prior standard therapy. We evaluated patients with newly metastatic cancers and synchronous brain metastases in order to control for the timing of immunotherapy relative to the diagnosis and treatment of brain metastases. Clinical outcomes of patients treated with upfront immunotherapy at the diagnosis of metastatic cancer and synchronous brain metastases were compared to a historical control population treated with standard systemic therapy in the era prior to immunotherapy.
METHODS
Data Acquisition
This study was approved by the Wake Forest School of Medicine Institutional Review Board. The Wake Forest Gamma Knife brain metastasis database was searched to identify patients who were treated with upfront SRS without prior whole brain radiation (WBRT). Patients were included in the present study if they had a synchronous diagnosis of brain metastasis and metastatic cancer and received upfront immunotherapy as part of their systemic treatment regimen within 3 months of their SRS. An equivalent number of patients with new diagnosis of metastatic cancer and synchronous brain metastases were identified in the era prior to 2017 (when upfront chemoimmunotherapy became a standard treatment option for treatment of metastatic disease at our institution) to serve as a historical control group. Clinical outcomes were determined using the electronic medical records. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Upfront Immunotherapy Group (range/ percent) | Historical Control Group | P value | |
|---|---|---|---|
| Total number of patients | 93 | 100 | N/A |
| Age | 64 (26-88) | 65 (31-91) | 0.4 |
| Sex (female) | 51 (55%) | 42 (42%) | 0.07 |
| Disease burden Widespread Oligometastasis No metastasis (except for brain) |
15 (16%) 21 (23%) 57 (61%) |
20 (20%) 31 (31%) 49 (49%) |
0.2 |
| Primary tumor type NSCLC RCC Melanoma SCLC |
76 (81.7%) 7 (7.5%) 8 (8.6%) 2 (2.2%) |
81 (81.0%) 13 (13.0%) 4 (4.0%) 2 (2.0%) |
0.4 |
| Median number intracranial lesions | 2 (1-22) | 2 (1-9) | 0.0015 |
| Lowest SRS margin dose | 18 (12.5-30) | 20 (8-25) | 0.2 |
NSCLC=Non-Small Cell Lung Cancer; RCC=Renal Cell Cancer; SCLC=Small Cell Lung Cancer
p-values are from Chi-square test or Kruskal-Wallis test
Stereotactic Radiosurgery
Patients were treated on the Gamma Knife Perfexion (Elekta AB, Stockholm, Sweden). A same day MRI brain was performed on each patient on a 3T MRI (GE Healthcare, Chicago, USA). High resolution contrast-enhanced T1 axial sequences were acquired for each patient. Treatment planning was performed on the GammaPlan Treatment planning system (Elekta Norcross, GA, USA). Dose prescription was determined based upon the size and volume of each lesion, generally according to the guidelines published by Shaw et al for a single fraction treated with irradiated brain tumors.[11]
Immunotherapy
Immunotherapies were included in the study if they were PDL or PDL-1 inhibitors or anti-CTLA antibodies. PD-1 inhibitors included in this study were pembrolizumab and nivolumab. PDL-1 inhibitors in this study were durvalumab and atezolizumab. Anti-CTLA antibody included in this study was ipilumumab.
Patient Follow-up and Response Assessment
Patients were followed clinically and with an MRI of the brain at 6-8 weeks after SRS and then every 3 months thereafter for the first two years after SRS. Clinical and imaging follow-up was spaced apart thereafter if there was no sign of tumor progression. Distant brain failure was defined as a new brain metastasis outside of the previous SRS target volumes. BMV was defined as published by Farris et al.[1] In short, the BMV was calculated as the total number of new metastases at time of distant brain failure (not including the lesions treated at time of initial SRS) divided by the total amount of time elapsed since initial SRS. Neurologic death was determined based on a definition previously reported by McTyre et al.[12]
Statistics
Median follow-up and time to event outcomes were determined from the time of SRS. Kaplan Meier method was used to calculate overall survival. Patients who had not yet had an event were censored at the date of their last follow-up. Log rank tests were used to evaluate differences in the survival curves by treatment type (upfront immunotherapy vs historical control). Cox proportional hazards models were created using a forward selection process based on a list of predefined baseline covariates. These covariates included age, gender, race, primary histology, lowest marginal dose delivered at SRS, number of brain metastases at time of first SRS. Competing risks analysis was used to analyze the cumulative incidence of distant brain failure and neurologic death. A Kruskal-Wallis test was used to compare the brain metastasis velocity between patients receiving upfront immunotherapy versus the historical controls.
RESULTS
Survival and Neurologic Death
Kaplan Meier analysis was performed to assess overall survival time. Kaplan Meier plots for overall survival in the upfront immunotherapy and historical control cohorts are demonstrated in Figure 1. Overall survival in the upfront immunotherapy cohort was 91% at 3 months, 84% at 6 months and 79% at 12 months. Overall survival in the historical control cohort was 74% at 3 months, 59% at 6 months and 35% at 12 months. Median overall survival time was significantly improved in the patients receiving upfront immunotherapy compared to the historical non-upfront immunotherapy cohort (48 months vs 8.4 months, respectively; log rank p=0.001).
Figure 1.
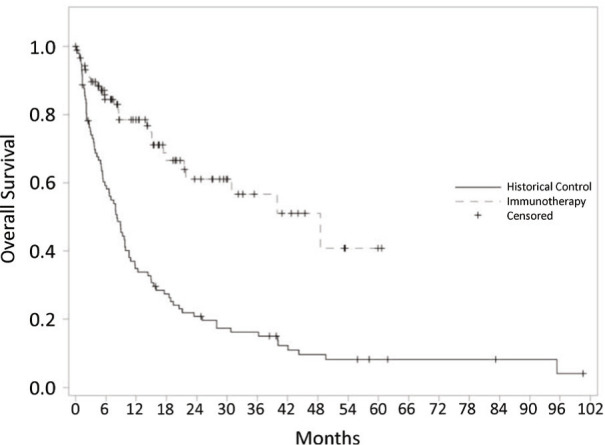
Kaplan Meier plots for overall survival of patients receiving upfront immunotherapy as part of their systemic therapy regimen vs historical controls who did not receive upfront immunotherapy
Cox proportional competing risk model was performed to estimate time to neurologic death. Cumulative incidence plots for neurologic death are depicted in Figure 2. Cumulative incidence of neurologic death at one year was 12% in the upfront immunotherapy cohort and 28% in the historical control cohort. There was a trend in increased time to neurologic death in favor of the patients receiving immunotherapy (p=0.1).
Figure 2.
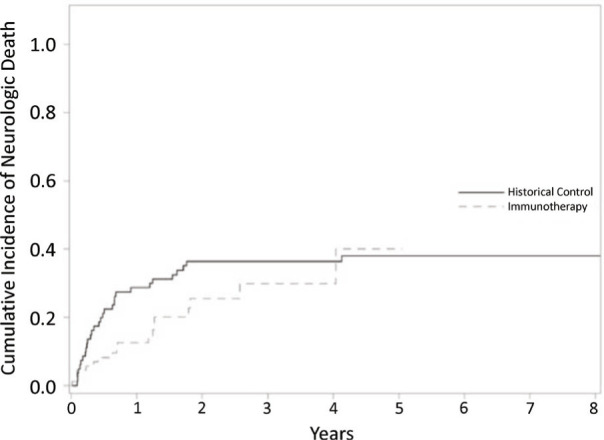
Cumulative incidence plots for time to neurologic death using competing risk analysis comparing patient cohort receiving upfront immunotherapy vs. historical controls.
Patterns of Failure
Kaplan Meier analysis was performed to assess time to distant brain failure. Distant brain failure for patients in the upfront immunotherapy cohort was 9% at 3 months, 28% at 6 months and 57% at 12 months. Distant brain failure for patients in the historical control cohort was 19% at 3 months, 41% at 6 months and 49% at 12 months. Median time to distant brain failure was statistically equivalent between the upfront immunotherapy cohort and historical control cohort (10.3 vs 12.6 months, respectively; p=0.3).
Kruskal-Wallis test was used to compare the brain metastasis velocity between patients receiving upfront immunotherapy versus the historical controls. Brain metastasis velocity was higher in the historical controls (median 9.48 metastases per year) than in the upfront immunotherapy cohort (median 3.72 metastases per year, p=0.001).
Predictive Factors
Cox proportional hazards regression was performed to determine if any factors were predictive of overall survival. Results of the Cox analysis are depicted in Table 2. In summary, a greater number of brain metastases (HR = 1.1, p=0.001) and lack of immunotherapy (HR = 4.4, p<0.001) were the factors that adversely affected overall survival.
Table 2.
Predictive factors for overall survival
| Factors | HR (95% CI) | P-value |
|---|---|---|
| Age | 1.1 (0.9-1.3) | 0.3 |
| Gender (Female VS Male) | 1 (0.7-1.5) | 0.9 |
| Higher number of metastases at first GK | 1.1 (1.0-1.1) | 0.001 |
| Lowest GK dose | 1.0 (0.9-1.1) | 0.9 |
| Primary diagnosis Lung VS Melanoma Renal VS Melanoma |
1.2 (0.5-3.1) 1.9 (0.7-5.5) |
0.6 0.2 |
| Upfront immunotherapy | 0.2 (0.1-0.4) | <0.0001 |
CI: confidence interval; GK: Gamma Knife; HR: hazard ratio
DISCUSSION
BMV has emerged as a potential biomarker to help assign patients to a proper salvage treatment after distant brain failure from upfront SRS. The presently accruing NRG BN009 study is using high and intermediate BMV as an entrance criterion to help determine the proper salvage regimen (WBRT-based or SRS alone). The goal of BN009 is to determine if a WBRT-based approach can mitigate neurologic death associated with a higher risk strata BMV. While previously published series have recognized the potential value of BMV as a prognostic marker in the setting of distant brain failure after SRS, the present series represents the first study to demonstrate that a class of systemic agents can reduce BMV.
Several studies have demonstrated the ability of systemic agents to modulate brain metastasis outcomes. Targeted agents have been shown to decrease the likelihood of local failure of SRS.[13,14] Concurrent cytotoxic chemotherapy has also improved local failure in previous series.[15] Multiple series have shown that brain metastasis patients with cytogenetic variants that are targetable by systemic agents can lead to prolonged survival in these patients and lead to fewer distant brain failures.[16,17,18] No prior series have demonstrated systemic agents’ ability to reduce BMV.
Reduction of BMV may be a clinically significant outcome because patients with BMV of 4 metastases/year or less have been shown to have a decreased likelihood of dying of neurologic death.[1] In the present series, the median BMV in the upfront immunotherapy cohort was less than 4, which is the lowest risk cohort in the Farris analysis. The median BMV in the historical cohort was twice that of the immunotherapy cohort. Patients with BMV less than 4 have been considered good candidates for repeat SRS. As such, patients treated with upfront immunotherapy would be considered to have a greater ability to avoid ultimate WBRT,[19] and instead have new brain metastases treated with serial applications of SRS. As depicted in Figure 2, the present series shows a corresponding strong trend towards decrease of neurologic death (particularly in the first 2 years after diagnosis) in the patients treated with upfront immunotherapy.
Figure 3.
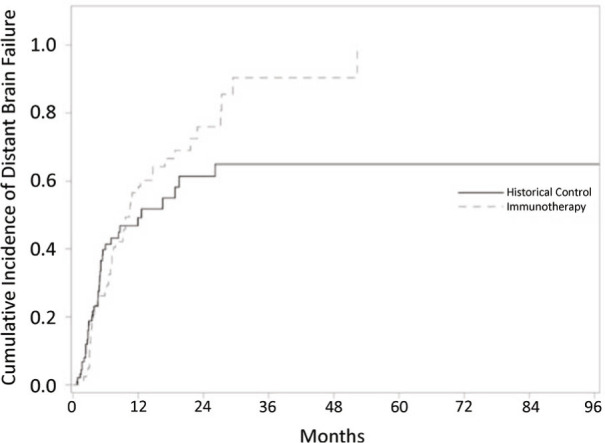
Cumulative incidence plots for time to distant brain failure using competing risk analysis comparing patient cohort receiving upfront immunotherapy vs. historical controls.
A series published by Lanier et al has suggested that the mechanism by which immunotherapy improves survival in patients with brain metastases is by the reduction of neurologic death.[7] In the study by Lanier, non-neurologic death was statistically equivalent between patients who had immunotherapy exposure and those that did not, but neurologic death was significantly decreased by treatment with immunotherapy. The major limitation of the Lanier study was that there was a heterogeneity with regards to when immunotherapy was given (representing multiple different times during the natural history of a patient’s disease process). This limitation led to an inability to detect whether immunotherapy affected BMV as it was not necessarily given concurrently with SRS. The present study was designed specifically to include only patients who had newly diagnosed metastatic cancers synchronous with newly diagnosed brain metastases so that use of upfront immunotherapy could be assessed in a normalized time point in the natural history of patients’ cancers. When controlling for this variable, the effect of immunotherapy on BMV was quite apparent.
It had been assumed that BMV represents a surrogate for the ability to control systemic metastases as better control of extracranial disease leads to lower numbers of metastases re-seeding the brain. However, it may be that some systemic agents actually have activity on microscopic metastatic disease in the brain to decrease the number of brain metastases that ultimately become clinically detectable on MRI. Several previously published series have suggested CNS activity of immunotherapy using either a combination immunotherapy regimen[9] or single agent immunotherapy for small asymptomatic metastases.[8] A recent report from France showed that patients with advanced melanoma with no prior brain metastases experienced lower rates of developing brain metastases when treated with immunotherapy.[20] The present study is unfortunately not designed to be able to distinguish the difference between control of systemic disease versus actively treating microscopic disease. However, several cooperative group studies are presently being designed to evaluate the role of immunotherapy alone for patients with brain metastases. It will be helpful to assess BMV in these studies.
There are several limitations to the present study. First of all, as a retrospective series performed at a single institution, this study is susceptible to patient selection bias. As such, the findings should be considered to be hypothesis generating and will need to be validated by an independent dataset. The decision to use patients with simultaneously diagnosed brain metastases and metastatic cancer was made in order to standardize the timing of upfront immunotherapy to be within 3 months of SRS so that we could best approximate the effect of immunotherapy on the development of new brain metastases. A potential consequence of this is that it has enriched the population studied with patients whose cancers are more likely to metastasize to the brain.
CONCLUSION
Upfront immunotherapy appears to decrease BMV compared to historical controls. While these data remain to be validated, they suggest that brain metastasis patients may benefit from concurrent immunotherapy with SRS when that is an option for the treatment of their metastatic disease.
ACKNOWLEDGMENTS
Authors’ disclosure of potential conflicts of interest
Dr. Michael Chan reports receiving honoraria from Elekta and Monteris. Other authors have nothing to disclose.
Footnotes
Author contributions
Conception or design: Emmanuel Scott, Mohammed Abdulhaleem, Hannah Johnston, Jimmy Ruiz, Michael Chan
Dara acquisition: Emmanuel Scott, Mohammed Abdulhaleem, Hannah Johnston, Claire Lanier, Michael LeCompte, Stephen B. Tatter, Adrian W. Laxton, Jing Su, Michael Chan
Analysis and interpretation of data for the work: Emmanuel Scott, Mohammed Abdulhaleem, Hannah Johnston, Scott Isom, Jimmy Ruiz, Michael Chan
Drafting: Emmanuel Scott, Mohammed Abdulhaleem, Hannah Johnston, Scott Isom, Christina K. Cramer, Jimmy Ruiz, Hui-Wen Lo, Kuonosuke Watabe, Stacey O’neill, Christopher Whitlow, Michael Chan
Critical revision: All authors
Final approval: All authors
REFERENCES
- 1.Farris M, McTyre ER, Cramer CK, Hughes R, Randolph DM, Ayala-Peacock DN, Bourland JD, Ruiz J, Watabe K, Laxton AW, Tatter SB, Zhou X, Chan MD. Brain metastasis velocity: A novel prognostic metric predictive of overall survival and freedom from whole-brain radiation therapy after distant brain failure following upfront radiosurgery alone Int J Radiat Oncol Biol Phys 2017;98(1):131-141. doi: 10.1016/j.ijrobp.2017.01.201 Epub 2017 Jan 26. PMID: 28586952. [DOI] [PubMed] [Google Scholar]
- 2.McTyre ER, Soike MH. Multi-institutional validation of brain metastasis velocity, a recently defined predictor of outcomes following stereotactic radiosurgery Radiother Oncol 2020;142:168-174. doi: 10.1016/j.radonc.2019.08.011 Epub 2019 Sep 13. PMID: 31526671. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M, Aiyama H, Koiso T, Watanabe S, Kawabe T, Sato Y, Higuchi Y, Kasuya H, Barfod BE. Validity of a recently proposed prognostic grading index, brain metastasis velocity, for patients with brain metastasis undergoing multiple radiosurgical procedures Int J Radiat Oncol Biol Phys 2019;103(3):631-637. doi: 10.1016/j.ijrobp.2018.10.036 Epub 2018 Nov 3. PMID: 30395905. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma N Engl J Med 2019. Oct 17;381(16):1535-1546. doi: 10.1056/NEJMoa1910836 Epub 2019 Sep 28. PMID: 31562797. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC. KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer N Engl J Med 2018. May 31;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 Epub 2018 Apr 16. PMID: 29658856. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T. KEYNOTE-426 Investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma N Engl J Med 2019. Mar 21;380(12):1116-1127. doi: 10.1056/NEJMoa1816714 Epub 2019 Feb 16. PMID: 30779529. [DOI] [PubMed] [Google Scholar]
- 7.Lanier CM, Hughes R, Ahmed T, LeCompte M, Masters AH, Petty WJ, Ruiz J, Triozzi P, Su J, O’Neill S, Watabe K, Cramer CK, Laxton AW, Tatter SB, Wang G, Whitlow C, Chan MD. Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries Neurooncol Pract 2019. Sep;6(5):402-409. doi: 10.1093/nop/npz004 Epub 2019 Feb 5. PMID: 31555455; PMCID: PMC6753360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, Yu J, Hegde U, Speaker S, Madura M, Ralabate A, Rivera A, Rowen E, Gerrish H, Yao X, Chiang V, Kluger HM. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial Lancet Oncol 2016. Jul;17(7):976-983. doi: 10.1016/S1470-2045(16)30053-5 Epub 2016 Jun 3. PMID: 27267608; PMCID: PMC5526047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, Atkins MB, Ernstoff MS, Reardon DA, Puzanov I, Kudchadkar RR, Thomas RP, Tarhini A, Pavlick AC, Jiang J, Avila A, Demelo S, Margolin K. Combined nivolumab and ipilimumab in melanoma metastatic to the brain N Engl J Med 2018. Aug 23;379(8):722-730. doi: 10.1056/NEJMoa1805453 PMID: 30134131; PMCID: PMC8011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeCompte MC, Hughes RT, Farris M, Masters A, Soike MH, Lanier C, Glenn C, Cramer CK, Watabe K, Su J, Ruiz J, Whitlow CT, Wang G, Laxton AW, Tatter SB, Chan MD. Impact of brain metastasis velocity on neurologic death for brain metastasis patients experiencing distant brain failure after initial stereotactic radiosurgery J Neurooncol 2020. Jan;146(2):285-292. doi: 10.1007/s11060-019-03368-9 Epub 2020 Jan 1. PMID: 31894518. [DOI] [PubMed] [Google Scholar]
- 11.Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05 Int J Radiat Oncol Biol Phys 2000. May 1;47(2):291-8. doi: 10.1016/s0360-3016(99)00507-6 PMID: 10802351. [DOI] [PubMed] [Google Scholar]
- 12.McTyre ER, Johnson AG, Ruiz J, Isom S, Lucas JT, Hinson WH, Watabe K, Laxton AW, Tatter SB, Chan MD. Predictors of neurologic and nonneurologic death in patients with brain metastasis initially treated with upfront stereotactic radiosurgery without whole-brain radiation therapy. Neuro Oncol 2017. Apr 1;19(4):558-566. doi: 10.1093/neuonc/now184. PMID: 27571883; PMCID: PMC5464318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochran DC, Chan MD, Aklilu M, Lovato JF, Alphonse NK, Bourland JD, Urbanic JJ, McMullen KP, Shaw EG, Tatter SB, Ellis TL. The effect of targeted agents on outcomes in patients with brain metastases from renal cell carcinoma treated with Gamma Knife surgery J Neurosurg 2012. May;116(5):978-83. doi: 10.3171/2012.2.JNS111353 Epub 2012 Mar 2. PMID: 22385005; PMCID: PMC3791504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson AG, Ruiz J, Hughes R, Page BR, Isom S, Lucas JT, McTyre ER, Houseknecht KW, Ayala-Peacock DN, Bourland DJ, Hinson WH, Laxton AW, Tatter SB, Debinski W, Watabe K, Chan MD. Impact of systemic targeted agents on the clinical outcomes of patients with brain metastases Oncotarget 2015. Aug 7;6(22):18945-55. doi: 10.18632/oncotarget.4153 PMID: 26087184; PMCID: PMC4662466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris S, Chan MD, Lovato JF, Ellis TL, Tatter SB, Bourland JD, Munley MT, deGuzman AF, Shaw EG, Urbanic JJ, McMullen KP. Gamma Knife stereotactic radiosurgery as salvage therapy after failure of whole-brain radiotherapy in patients with small-cell lung cancer Int J Radiat Oncol Biol Phys 2012. May 1;83(1):e53-9. doi: 10.1016/j.ijrobp.2011.11.059 Epub 2012 Feb 17. PMID: 22342297; PMCID: PMC3791505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, Beal K, Amini A, Patil T, Kavanagh BD, Camidge DR, Braunstein SE, Boreta LC, Balasubramanian SK, Ahluwalia MS, Rana NG, Attia A, Gettinger SN, Contessa JN, Yu JB, Chiang VL. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: A retrospective multi-institutional analysis. J Clin Oncol 2017. Apr 1;35(10):1070-1077. doi: 10.1200/JCO.2016.69.7144 Epub 2017 Jan 23. PMID: 28113019. [DOI] [PubMed] [Google Scholar]
- 17.Johung KL, Yeh N, Desai NB, Williams TM, Lautenschlaeger T, Arvold ND, Ning MS, Attia A, Lovly CM, Goldberg S, Beal K, Yu JB, Kavanagh BD, Chiang VL, Camidge DR, Contessa JN. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis J Clin Oncol 2016. Jan 10;34(2):123-9. doi: 10.1200/JCO.2015.62.0138 Epub 2015 Oct 5. PMID: 26438117; PMCID: PMC5070549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vern-Gross TZ, Lawrence JA, Case LD, McMullen KP, Bourland JD, Metheny-Barlow LJ, Ellis TL, Tatter SB, Shaw EG, Urbanic JJ, Chan MD. Breast cancer subtype affects patterns of failure of brain metastases after treatment with stereotactic radiosurgery J Neurooncol 2012. Dec;110(3):381-8. doi: 10.1007/s11060-012-0976-3 Epub 2012 Sep 24. PMID: 23001361; PMCID: PMC3852435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McTyre E, Ayala-Peacock D, Contessa J, Corso C, Chiang V, Chung C, Fiveash J, Ahluwalia M, Kotecha R, Chao S, Attia A, Henson A, Hepel J, Braunstein S, Chan M. Multi-institutional competing risks analysis of distant brain failure and salvage patterns after upfront radiosurgery without whole brain radiotherapy for brain metastasis Ann Oncol 2018. Feb 1;29(2):497-503. doi: 10.1093/annonc/mdx740 PMID: 29161348. [DOI] [PubMed] [Google Scholar]
- 20.Marcaillou M, Linder C, Chaltiel L, Sibaud V, Pagès C, Modesto A, Chira C, Dalmasso C, Boulinguez S, Bedane C, Meyer N. PD-1 inhibitors might limit the development of brain metastases in patients with advanced melanoma Melanoma Res 2020. Dec;30(6):580-589. doi: 10.1097/CMR.0000000000000700 PMID: 33156203. [DOI] [PubMed] [Google Scholar]


