Abstract
In Gamma Knife (GK) radiosurgery, dose rate decreases during the life cycle of its radiation source, extending treatment times. Prolonged treatments influence the amount of sublethal radiation injury that is repaired during exposure, and is associated with decreased biologically-equivalent dose (BED). We assessed the impact of treatment times on clinical outcomes following GK of the trigeminal nerve – a rare clinical model to isolate the effects of treatment times.
This is a retrospective analysis of 192 patients with facial pain treated across three source exchanges. All patients were treated to 80 Gy with a single isocenter. Treatment time was analyzed in terms of patient anatomy-specific dose rate, as well as BED calculated from individual patient beam-on times. An outcome tool measuring pain in three distinct domains (pain intensity, interference with general and oro-facial activities of daily living), was administered before and after intervention. Multivariate linear regression was performed with dose rate/BED, brainstem dose, sex, age, diagnosis, and prior intervention as predictors. BED was an independent predictor of the degree of improvement in all three dimensions of pain severity. A decrease in dose rate by 1.5 Gy/min corresponded to 31.8% less improvement in the overall severity of pain. Post-radiosurgery incidence of facial numbness was increased for BEDs in the highest quartile.
Treatment time is an independent predictor of pain outcomes, suggesting that prescription dose should be customized to ensure iso-effective treatments, while accounting for the possible increase in adverse effects at the highest BEDs.
Keywords: Trigeminal neuralgia, facial pain, Gamma Knife, stereotactic radiosurgery, dose rate, biologically equivalent dose
INTRODUCTION
Gamma Knife (GK) radiosurgery of the trigeminal nerve is widely used to treat refractory facial pain, most commonly trigeminal neuralgia (TN). Complete pain relief is achieved in 70% of patients, which is maintained in 40-55% at three years, and in 25% at 10 years1–5. GK also demonstrates a favorable adverse event profile among therapeutic modalities, most commonly sensory changes associated with trigeminal dysfunction, such as facial numbness/hypesthesia. The impact of key treatment parameters on pain outcomes, e.g., prescription/integral dose, as well as isocenter numerosity and location have been reported on6–11. However, in addition to dose, the biological effect of radiation is dependent on the time over which the dose is delivered12–16. In contrast to linear accelerators, GK utilizes a fixed radiation source (Cobalt-60; half-life 5.26 years). Hence, even with source replacement every 5-10 years, dose rates, and hence treatment times can vary significantly during its life cycle.
The concept that the radiobiological response of tissue, or the biologically-equivalent dose (BED) of a treatment, decreases with prolonged exposure times has been supported by both preclinical and clinical studies in not only fractionated radiotherapy but also single-fraction radiosurgery12–14,16. Given the high doses used in the treatment of functional disorders, and the relatively simple and uniform treatment plans across patients, GK of the trigeminal nerve represents a rare clinical model to study the effects of treatment times. However, two early clinical studies, as well as a more recent study, did not find any robust evidence for the relevance of treatment time to pain outcomes17–19.
What explains these negative results? Our hypothesis was that a more granular metric of pain, as well as its measurement both before and after treatment would be essential to understand the impact of treatment time. Previously, our group reported that fixed-geometry dose rate, reflecting only source decay, correlated with short-term pain relief and its long-term durability20. In the current study, we present additional evidence for the clinical relevance of treatment time in an extended cohort of patients spanning the life cycles of three consecutive radiation sources. Furthermore, we expand upon our previous analyses by assessing patient anatomy-specific dose rate, as well as BED computed from individual treatment times with biologically-plausible parameters. Finally, we also assessed the impact of treatment time on facial numbness, which has recently been associated with higher BEDs19.
METHODS
Population characteristics and radiosurgical procedure
This is a retrospective analysis of patients undergoing GK for facial pain at a single institution (2006-2018). Patients were clinically diagnosed by the senior author following Burchiel’s classification: TN type 1 (TN1; >50% episodic pain), TN type 2 (TN2; >50% constant pain), symptomatic TN secondary to multiple sclerosis (TN-MS), post-herpetic TN, and trigeminal neuropathic pain secondary to unintentional injury21. Inclusion criteria were: (1) treatment to a maximum dose of 80 Gy with a single 4 mm isocenter without use of any plugging/sector blocking; (2) completion of the Penn Facial Pain Scale-Revised22,23 (University of Pennsylvania, PA; PFPS-R) questionnaire, both before and after GK. Isocenter location targeted the mid-posterior portion of the trigeminal nerve. Although isocenter location was standardized, anatomic variability in the length of the trigeminal nerve can lead to differences in brainstem dose across patients. We accounted for this by including the dose at the surface of the brainstem in our analyses (see Statistics).
Of the 283 patients who underwent GK of the trigeminal nerve for facial pain during the study period, a total of 192 patients met inclusion criteria (Table 1), including 104 patients treated on the Leksell 4C unit (Elekta, Sweden) with the first Cobalt-60 source, and 88 patients treated on the Leksell Perfexion with the latter two sources. Of note, there is excellent agreement between dosimetric parameters of these two systems24. The majority of patients were diagnosed with TN1 (68.2%) or TN2 (15.1%). Patients with a history of prior radiosurgery on the ipsilateral trigeminal nerve were all treated with doses <80 Gy at our institution, and hence were excluded. Overall, 19.8% of patients had a history of other neurosurgical interventions for their facial pain. Data from a subset of patients treated with the first source have previously been reported on20. Patient consent was obtained, and the study protocol was approved by our local Institutional Review Board.
Table 1.
Demographics and Treatment Parameters
| Number of patients | 192 |
|---|---|
| First Cobalt source (11/9/2005; %) | 54.2 |
| Second Cobalt source (06/29/2012; %) | 39.0 |
| Third Cobalt source (12/13/2016; %) | 6.8 |
| Dose rate (Gy/min) | 2.13 ± 0.03 [1.19-2.86] |
| Treatment time (min) | 40 ± 0.8 [28-69] |
| Biologically-equivalent dose | 1962 ± 0.008 [1703-2104] |
| Brainstem dose (Gy) | 18.9 ± 0.7 [12-14] |
| Female (%) | 60.4 |
| Age (years) | 68 ± 1.0 |
| Right-sided pain (%) | 59.4 |
| Diagnosis | |
| TN1 (%) | 68.2 |
| TN2 (%) | 15.1 |
| TN-MS (%) | 15.1 |
| Other (%) | 2.5 |
| Prior Procedures (%) | 19.8 |
| Microvascular decompression (%) | 12.5 |
| Glycerol rhizotomy (%) | 5.2 |
| Radiofrequency thermocoagulation (%) | 2.1 |
| Balloon compression (%) | 0.5 |
| Gamma Knife (%) | 0 |
| Central/peripheral neuro-stimulation (%) | 1.0 |
TN1: trigeminal neuralgia type 1, TN2: TN type 2,
TN-MS: TN secondary to multiple sclerosis.
Dose rate and biologically equivalent dose
The dose rate at focus and beam-on time were obtained from individual patient treatment plans (Leksell GammaPlan). In addition to the three factors that determine fixed-geometry dose rate (dose rate at calibration time, source decay half-life, and collimator output factor), the dose rate at focus also reflects attenuation through tissue. For each isocenter, the planning software calculates the attenuation based on the coordinates of the target and the inputted measurements of the patient’s head size/shape. Hence, dose rate at focus represents the patient anatomy-specific dose rate.
The concept of BED derives from increased repair of sublethal radiation-induced injury during extended periods of exposure (during both inter-fraction intervals and intra-fraction durations), ultimately leading to a decline in the biological effectiveness of a prescribed dose. BED is calculated based on the linear-quadratic (LQ) model as follows:
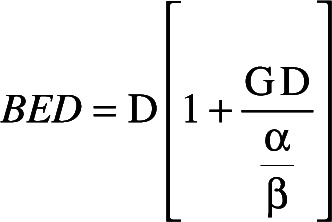
where D is the prescribed dose (80 Gy), and α/β reflects the radio-sensitivity of cells in target tissue. For a single continuous exposure with GK (i.e. one isocenter), the protraction factor G is
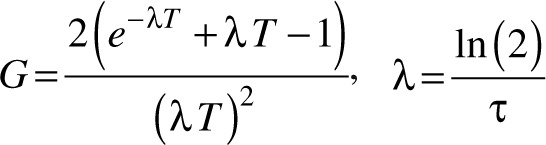
where T is treatment time (for treatments with one isocenter, T is equal to beam-on time), λ is recovery constant, and τ is repair half-time of sublethal radiation damage. Repair rates are thought to have two components defined by a fast (τ1) and a slow repair time (τ2)12. Values for α/β (2.47 Gy), τ 1 (0.19 hours), and τ 2 (2.16 hours), as well as a 50:50 weighted average of G across the two time scales were based on data from rodent spinal cord models of normal CNS white matter25, following previous studies19,26,27.
Although the LQ model is the most commonly-employed estimate of BED, its accuracy for large fraction sizes (>18 Gy) is controversial28. Nonetheless, several studies have validated the LQ model in the context of tumor radiosurgery at high doses of up to 50 Gy15,16. Moreover, the LQ model has been widely applied in clinical studies of GK for functional disorders, in which the target is non-tumorous neural tissue17,19,29.
Clinical outcomes
The PFPS-R (originally Brief Pain Inventory-Facial) provides outcome measures for facial pain in three dimensions, which have been shown to capture distinct components of pain severity22,23: interference with general activities of daily living (ADL; seven items), interference with oro-facial specific ADLs (seven items), and pain intensity (four items). PFPS-R depicts pain severity in the preceding week, with each item rated on an ordinal scale (0 to 10). For general ADL interference, patients described how pain has interfered with general activity, mood, walking, household/workplace work, interpersonal relations, sleep, and enjoyment of life; for odor-facial ADL, patients reported on interference with eating, facial touching/grooming, brushing/flossing teeth, smiling/laughing, talking, and opening mouth widely; for pain intensity, patients rated their pain at its worst, at its least, on average, and right now.
All included patients had baseline PFPS-R ratings, as well as a repeat at the first post-radiosurgery follow-up. Questionnaires were provided on paper forms, which were completed without any direct supervision from the clinical team. Longer-term outcomes were obtained with telephone questionnaires, which included the PFPS-R, as well as questions on development of any new or worsening facial numbness. Those reporting numbness were asked whether the symptoms were “distressful” and/or had “negatively impacted your quality of life.” Telephone questionnaires were attempted in all included patients, and data were included only from those patients who had not undergone any subsequent surgical interventions. At both post-radiosurgery time points, patients were not allowed to reference previous PFPS-R ratings.
Statistics
Pain outcomes were quantified with four variables: the mean change across all PFPS-R items (referred to as overall pain severity), as well as mean change across items comprising each of the three PFPS-R components. We calculated relative changes between pre- and post-radiosurgery scores, which were treated with winsorization to replace extreme outliers <-100%. Multivariate linear regression was conducted with the following independent variables: dose rate or BED, in addition to dose at brainstem surface as continuous variables; sex, prior intervention, and diagnosis (TN1 vs. TN2, TN1/2 vs. other diagnoses) as categorical/binary variables; age (in deciles) as ordinal variable. Variables with p<0.3 on univariate regression were entered into the multivariate model. For multi-isocenter GK, BED is a complex function of the interplay between dose and treatment time (i.e., dose contribution and beam-on time of each isocenter, time between isocenters)30. However, in this study, as all patients were treated to 80 Gy with a single isocenter, BED varied only with respect to treatment time, and hence patient anatomy specific dose rate. We therefore evaluated these two metrics independent of each other in the multivariate analysis. All analysis was conducted with Matlab (Mathworks, Natick, MA), and p<0.05 was considered significant. Data reported as mean ± s.e.m. [min, max], unless otherwise noted.
RESULTS
Dose rate accounting for source age and patient anatomy varied significantly within our cohort (N=192), ranging between 1.19 and 2.86 Gy/min. As a result, treatment times to deliver the same physical dose of 80 Gy varied between 28 and 69 minutes, corresponding to BEDs of 2104 and 1703, respectively. For the three Cobalt-60 sources used during the study period, BED declined annually by 3.0, 2.6, and 4.4%, respectively. Overall pain severity (mean across all PFPS-R items) was improved at short-term follow-up (1.3 ± 0.1 months) from an absolute score of 5.4 (out of 10) to 2.5, representing a 44% improvement (Table 2). In the subset of patients in whom longer-term follow up was available (8.2 ± 0.5 years; N=41), overall pain severity further improved to 1.4 (72% improvement). Similarly, patients improved in each of the three dimensions of pain severity (intensity, general ADL and oro-facial ADL interference) captured by PFPS-R.
Table 2.
Pre- and post-Gamma Knife Penn Facial Pain Scale-Revised (PFPS-R) scores
| Baseline | Short-term** | Long-term* (N=41) | |
|---|---|---|---|
| Pain intensity items | 5.4 ± 0.2 | 2.5 ± 0.2 (44 ± 4.9 %) | 1.4 ± 0.4 (72 ± 7.2 %) |
| General ADL interference items | 5.5 ± 0.2 | 2.3 ± 0.2 (49 ± 5.0 %) | 1.3 ± 0.4 (78 ± 6.4 %) |
| Facial ADL interference items | 6.8 ± 0.2 | 2.9 ± 0.2 (50 ± 4.5 %) | 1.3 ± 0.4 (77 ± 7.1 %) |
| All items | 6.0 ± 0.2 | 2.5 ± 0.2 (49 ± 4.1 %) | 1.3 ± 0.4 (77 ± 6.4 %) |
Table values represent group-average scores, shown separately for each of the three dimensions of the PFPS-R, as well as for the entire questionnaire. For each subject, we calculated the average absolute score across the items comprising each of the three dimensions of the PFPS-R, as well as across all 18 items of the questionnaire. We also show the percentage change at short- and long-term follow up in parenthesis. The pre- vs. post-GK absolute scores were compared using a paired-sample Wilcoxon signed rank test (*p < 10−6, **>p < 10−20).
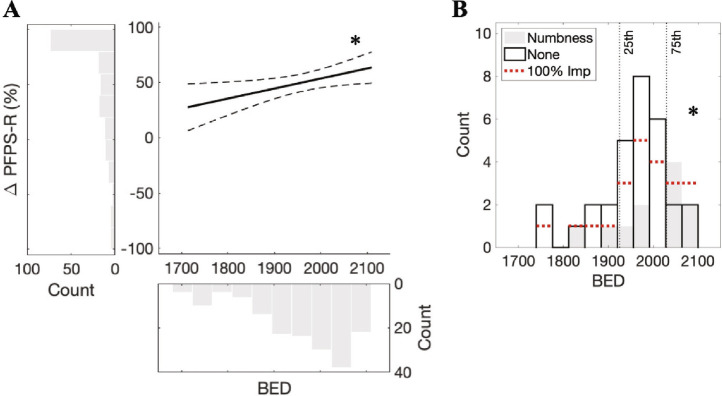
Multivariate linear regression was performed to determine predictors of short-term pain relief (Table 3). BED was a significant predictor of all three dimensions of pain, as well as overall pain severity (Figure 1A). Inclusion of dose rate in place of BED led to congruent results. A decrease in dose rate by 1.5 Gy/min corresponded to 31.8% less improvement in overall pain severity. For the demographic predictors that were included in the multivariate models, we did not observe consistent results (female sex was a positive predictor of overall pain severity). Furthermore, interaction terms were not included in the final models, as none of them were significant predictors, and their inclusion did not increase the explanatory power of models (adjusted R2). Together, these results indicate that treatment time is a robust, independent predictor of short-term pain outcomes.
Table 3.
Predictors of short-term pain relief based on percentage change in Penn Facial Pain Scale-Revised (PFPS-R) items
| Pain intensity items | General ADL interference items | Facial ADL interference items | All PFPS-R items | |||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |
| Dose rate (Gy/min) | 0.034 | 0.048 | 0.002 | 0.003 | 0.034 | 0.054 | 0.011 | 0.023 |
| BED | 0.035 | 0.049 | 0.001 | 0.003 | 0.048 | 0.047 | 0.012 | 0.025 |
| Age | 0.969 | 0.352 | 0.408 | 0.209 | 0.166 | |||
| Brainstem dose (Gy) | 0.897 | 0.832 | 0.786 | 0.741 | ||||
| Dx: TN1 vs. TN2 | 0.940 | 0.750 | 0.578 | 0.736 | ||||
| Dx: TN1/2 vs. other | 0.174 | 0.157 | 0.551 | 0.105 | 0.101 | 0.342 | ||
| Prior intervention | 0.857 | 0.300 | 0.403 | 0.862 | 0.366 | |||
| Sex | 0.142 | 0.189 | 0.130 | 0.271 | 0.876 | 0.022 | 0.030 | |
Table values are p-values of corresponding t-statistics, shown separately for univariate and multivariate regression models, where statistically-significant predictors on multivariate analysis are marked in bold. ADL: activities of daily living; TN 1/2: trigeminal neuralgia type 1/type 2, dx: diagnosis.
Figure 1.
A) Multivariate linear model of short-term percentage change in the overall severity of pain (ΔPFPS-R) was constructed with predictors significant on univariate analysis (model F-test, p = 0.006): BED, sex, and age. Histograms shows pain outcome and BED across individual patients (pain outcome outliers treated with winsorization). Fitted line (solid) was adjusted to isolate the effect of BED (predictor t-test, *p = 0.02, coefficient/slope = 0.09). Dotted lines indicate 95% confidence bounds. B) Distribution of BEDs in subjects in whom long-term data was available, divided into subsets based on post-radiosurgery development of facial numbness. The incidence of numbness was increased in patients treated with BEDs in the highest quartile (*p = 0.01). Patients reporting a 100% improvement in overall pain severity indicated with horizontal dotted lines. Vertical lines indicate 25th and 75th percentiles of BED.
For long-term pain outcomes, a similar multivariate analysis was precluded by the small number of subjects, and the fact that 53.7% of patients reported 100% improvement in overall pain severity (vs. 25.0% during short-term follow-up). BEDs in patients who achieved 100% improvement trended towards being greater compared to the other patients (two-sample Wilcoxon rank sum test, U = 509, p = 0.22). In terms of adverse effects, 26.8% of patients had developed new facial numbness at long-term follow-up, although symptoms were reported to be distressing in only a minority of these patients (2 of 11). We similarly noted a trend towards BED being greater in patients with numbness (U = 281, p = 0.15). In fact, patients treated with BEDs in the top 25th percentile had a significantly higher incidence of numbness vs. patients treated with BEDs lower than this threshold (83.9% vs. 40.0%, Fisher’s exact test, p = 0.01; Figure 1B).
DISCUSSION
While a number of key treatment parameters have been proposed to influence pain outcomes following GK (dose, isocenter number and location6–10), there has been controversy regarding the clinical relevance of the time over which radiation is delivered. Our data revealed that biologically-equivalent dose (BED), which conceptualizes the radiobiological effect of both treatment time as well as total dose, was a robust independent predictor of pain outcomes. Concurrently, the risk of post-radiosurgery facial numbness was dramatically increased in those treated with BEDs in the highest quartile.
Our results build upon an extensive body of pre-clinical and clinical work demonstrating that treatment time (accounting for both inter-fraction interval and intra-fraction duration) determines the amount of sublethal radiation damage that is repaired during the period of exposure12–14,16. Consequently, shorter overall treatment times have enhanced radiation biological effectiveness on both pathologic and normal tissue. Importantly, the intra-fraction duration can be clinically relevant when it exceeds 30 minutes, even at the relatively high dose rates used in radiosurgery12,13. Intra-fraction repair of sublethal damage is particularly relevant in functional GK procedures, which require much higher doses compared to those used for tumors, leading to treatment times that can vary substantially during source lifespan. For example, time to deliver 80 Gy to the trigeminal nerve with a single isocenter ranged from 28 to 69 minutes in our cohort.
Given the relative simplicity of treatment plans, the clinical significance of treatment time has been investigated in the setting of GK for facial pain. However, two early studies reported no difference in pain outcomes17,18. In the first study, Balamucki et al. included patients treated with variable doses (80-90 Gy) and with either one or two isocenters. The authors reported that neither treatment time nor dose rate was associated with pain outcomes, but how their analysis adequately controlled for the variability in treatment parameters was unclear17. In the second study, Arai et al. only included patients treated to 80 Gy with a single isocenter, but also did not find a difference in outcomes between those treated with high vs. low dose rates18. Importantly, both studies quantified pain on a one-dimensional 4-6 point scale, and pain was only measured once during the post-operative period. In a more recent study, Tuleasca et al. evaluated pain outcomes in 408 patients treated with one isocenter to a dose of 76.1-97.9 Gy19. Notably, a variable degree of blocking was used in the majority of patients. Although there was no statistical difference in pain outcomes at <30 days or at 1-2 years, on stratification of patients into eight groups with respect to BED, the lowest group of patients appeared to have worse acute pain outcomes. As in the initial two studies, clinical outcomes were poorly captured, with pain measured in a binary fashion (presence of pain freedom), and only in the post-operative period.
In contrast, our group utilized measurement of pain both before and after intervention to previously demonstrate that fixed-geometry dose rate is a significant predictor of improvement in the intensity of pain at its worst20. In the current study, we expand upon these findings in the following aspects: (1) dose rate accounting for individual patient anatomy in addition to source age was a significant predictor of outcomes, as well as the BEDs calculated from individual patient treatment times; (2) these variables were significant predictors of all three distinct components of pain measured by the PFPS-R (pain intensity, interference with general and oro-facial ADLs); (3) the correlation held true for an extended cohort of patients treated across three consecutive Cobalt-60 sources; (4) while a high BED was favorable for pain outcomes, it was also associated with increased rates of facial numbness at long-term follow-up, specifically for the highest BED quartile.
Our long-term follow-up data were not sufficient to definitively assess the impact of treatment times on the durability of pain control. Nonetheless, we would like to note that the onset latency to maximal pain relief following GK is remarkably quick, generally reported to be within a month in the majority of reported series, with 40 and 80% of patients responding within one week and one month, respectively1,6,31–35. In contrast, adverse effects related to trigeminal dysfunction are more delayed in onset, and requires a follow-up of at least four months to ascertain2,5,31,34,35.
Our results suggest that to deliver iso-effective treatments, prescription dose should be tailored to compensate for the decrease in dose rates throughout source lifespan19,36. For example, the BED of treatments delivering 80 Gy decreases by 5.9% with protraction of treatment times from 30 to 40 minutes. Using the LQ model with biologically-plausible parameters, the prescription dose should be increased to approximately 82.5 Gy to match BEDs. Importantly, consistent with our data, higher doses have been shown to increase the risk of facial numbness, although this association has been observed primarily for doses exceeding 90 Gy2,8,37,38. Therefore, any clinical benefit obtained from dose escalation must be weighed against any potential increases in complications. It is also worth noting that, in our cohort, symptoms was not perceived as distressing in the majority of patients developing numbness.
Our results also suggest that the clinical effect of dose escalation in GK for facial pain may be better elucidated by stratifying patients based on BED, which would account for differences in treatment times in addition to dose. In fact, there is considerable variability across institutions in prescription dose, ranging between 60 and 95 Gy in the literature1,5,8. Kondziolka et al. showed that the minimum dose should be 70 Gy, and over time efforts have been made to increase the dose to 80-90 Gy to enhance pain outcomes. However, Pollack et al. found no improvement in outcomes with dose escalation from 70 to 90 Gy38. On the other hand, in a more recent study based on 870 patients, increasing the dose from 82 to 90 Gy was associated with improved rates of pain relief8.
The current study is subject to the limitations of a retrospective study. Notably, however, pain was measured in a prospective manner. Patients also directly reported their pain on the questionnaire both before and after intervention, without reference to their previous ratings, and without any influence from the clinical team.
CONCLUSIONS
Our data provide convincing evidence that treatment times in GK for facial pain are an independent predictor of improved pain outcomes, suggesting that prescription dose should be systematically increased to deliver iso-effective treatments throughout source lifespan. However, the incidence of facial numbness was increased for the highest BED quartile. Further work is warranted to determine the optimal range of BEDs that achieves a balance between pain control and avoidance of adverse effects following GK of the trigeminal nerve.
ACKNOWLEDGMENTS
We would like to acknowledge Keren Somers, Arjun Patel, Megan Daly, Marie Kerr, Eileen Maloney, as well as other members of the Clinical Research Division for research support.
Footnotes
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception and design: Andrew I. Yang, John Y.K. Lee
Data collection: Andrew I. Yang, Emily F. Shekhtman, Svetlana Kvint, Connor A. Wathen, Kobina G. Mensah-Brown, Frederick L. Hitti
Data analysis and interpretation: Andrew I. Yang
Manuscript writing: Andrew I. Yang
Final approval of manuscript: Andrew I. Yang, Michelle Alonso-Basanta, Stephen M. Avery, Jay F. Dorsey, John Y.K. Lee
REFERENCES
- 1.Lopez BC, Hamlyn PJ, Zakrzewska JM. Stereotactic radiosurgery for primary trigeminal neuralgia: state of the evidence and recommendations for future reports J Neurol Neurosurg Psychiatry 2004. Jul;75(7):1019-1024. PMCID: PMC1739098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollock BE, Phuong LK, Gorman DA, Foote RL, Stafford SL. Stereotactic radiosurgery for idiopathic trigeminal neuralgia J Neurosurg 2002. Aug;97(2):347-353. PMID: 12186463 [DOI] [PubMed] [Google Scholar]
- 3.Kondziolka D, Zorro O, Lobato-Polo J, Kano H, Flannery TJ, Flickinger JC, Lunsford LD. Gamma Knife stereotactic radiosurgery for idiopathic trigeminal neuralgia: Clinical article Journal of Neurosurgery 2010. Apr 1;112(4):758-765. [DOI] [PubMed] [Google Scholar]
- 4.Lopez BC, Hamlyn PJ, Zakrzewska JM. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia Neurosurgery 2004. Apr;54(4):973-982; discussion 982-983. PMID: 15046666 [DOI] [PubMed] [Google Scholar]
- 5.Tuleasca C, Régis J, Sahgal A, De Salles A, Hayashi M, Ma L, Martínez-Álvarez R, Paddick I, Ryu S, Slotman BJ, Levivier M. Stereotactic radiosurgery for trigeminal neuralgia: a systematic review J Neurosurg 2018. Apr 27;130(3):733-757. PMID: 29701555 [DOI] [PubMed] [Google Scholar]
- 6.Shrivastava A, Mohammed N, Hung Y-C, Xu Z, Schlesinger D, Heinrichs T, Kearns K, Li CE, Lavezzo K, Narayan A, Sheehan JP. Impact of Integral Dose on the Maintenance of Pain Relief in Patients with Idiopathic Trigeminal Neuralgia Treated with Upfront Gamma Knife Radiosurgery World Neurosurg 2019. Sep;129:e375-e380. PMID: 31132503 [DOI] [PubMed] [Google Scholar]
- 7.Mousavi SH, Niranjan A, Akpinar B, Monaco EA, Cohen J, Bhatnagar J, Chang Y-F, Kano H, Huq S, Flickinger JC, Dade Lunsford L. A proposed plan for personalized radiosurgery in patients with trigeminal neuralgia J Neurosurg 2018. Feb;128(2):452-459. PMID: 28298016 [DOI] [PubMed] [Google Scholar]
- 8.Kotecha R, Kotecha R, Modugula S, Murphy ES, Jones M, Kotecha R, Reddy CA, Suh JH, Barnett GH, Neyman G, Machado A, Nagel S, Chao ST. Trigeminal Neuralgia Treated With Stereotactic Radiosurgery: The Effect of Dose Escalation on Pain Control and Treatment Outcomes Int J Radiat Oncol Biol Phys 2016. January;96(1):142-148. PMID: 27325473 [DOI] [PubMed] [Google Scholar]
- 9.Xu Z, Schlesinger D, Moldovan K, Przybylowski C, Sun X, Lee C-C, Yen C-P, Sheehan J. Impact of target location on the response of trigeminal neuralgia to stereotactic radiosurgery J Neurosurg 2014. Mar;120(3):716-724. PMID: 24313616 [DOI] [PubMed] [Google Scholar]
- 10.Boling W, Song M, Shih W, Karlsson B. Gamma Knife Radiosurgery for Trigeminal Neuralgia: A Comparison of Dose Protocols Brain Sciences 2019. Jun;9(6):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flickinger JC, Pollock BE, Kondziolka D, Phuong LK, Foote RL, Stafford SL, Lunsford LD. Does increased nerve length within the treatment volume improve trigeminal neuralgia radiosurgery? A prospective double-blind, randomized study Int J Radiat Oncol Biol Phys 2001. Oct 1;51(2):449-454. PMID: 11567820 [DOI] [PubMed] [Google Scholar]
- 12.Fowler JF, Welsh JS, Howard SP. Loss of biological effect in prolonged fraction delivery Int J Radiat Oncol Biol Phys 2004. May 1;59(1):242-249. PMID: 15093921 [DOI] [PubMed] [Google Scholar]
- 13.Hallgren S, Hill MA, Thompson JM, Elliott A, Paddick I, Jones B, Hopewell JW. Effects of variations in overall treatment time on the clonogenic survival of V79-4 cells: Implications for radiosurgery J Radiosurg SBRT 2019;6(1):1-9. PMCID: PMC6355450 [PMC free article] [PubMed] [Google Scholar]
- 14.Fu KK, Phillips TL, Kane LJ, Smith V. Tumor and normal tissue response to irradiation in vivo: variation with decreasing dose rates Radiology 1975. Mar;114(3):709-716. PMID: 1118577 [DOI] [PubMed] [Google Scholar]
- 15.Matsuo T, Shibata S, Yasunaga A, Iwanaga M, Mori K, Shimizu T, Hayashi N, Ochi M, Hayashi K. Dose optimization and indication of Linac radiosurgery for brain metastases Int J Radiat Oncol Biol Phys 1999. Nov 1;45(4):931-939. PMID: 10571200 [DOI] [PubMed] [Google Scholar]
- 16.Shuryak I, Carlson DJ, Brown JM, Brenner DJ. High-dose and fractionation effects in stereotactic radiation therapy: Analysis of tumor control data from 2965 patients Radiother Oncol 2015. Jun;115(3):327-334. PMID: 26058991 [DOI] [PubMed] [Google Scholar]
- 17.Balamucki CJ, Stieber VW, Ellis TL, Tatter SB, Deguzman AF, McMullen KP, Lovato J, Shaw EG, Ekstrand KE, Bourland JD, Munley MT, Robbins M, Branch C. Does dose rate affect efficacy? The outcomes of 256 gamma knife surgery procedures for trigeminal neuralgia and other types of facial pain as they relate to the half-life of cobalt J Neurosurg 2006. Nov;105(5):730-735. PMID: 17121135 [DOI] [PubMed] [Google Scholar]
- 18.Arai Y, Kano H, Lunsford LD, Novotny J, Niranjan A, Flickinger JC, Kondziolka D. Does the Gamma Knife dose rate affect outcomes in radiosurgery for trigeminal neuralgia? J Neurosurg 2010. Dec;113 Suppl:168-171. PMID: 21121798 [DOI] [PubMed] [Google Scholar]
- 19.Tuleasca C, Paddick I, Hopewell JW, Jones B, Millar WT, Hamdi H, Porcheron D, Levivier M, Régis J. Establishment of a Therapeutic Ratio for Gamma Knife Radiosurgery of Trigeminal Neuralgia: The Critical Importance of Biologically Effective Dose Versus Physical Dose World Neurosurg 2020. Feb;134:e204-e213. PMID: 31606504 [DOI] [PubMed] [Google Scholar]
- 20.Lee JYK, Sandhu S, Miller D, Solberg T, Dorsey JF, Alonso-Basanta M. Higher dose rate Gamma Knife radiosurgery may provide earlier and longer-lasting pain relief for patients with trigeminal neuralgia J Neurosurg 2015. Oct;123(4):961-968. PMID: 26252452 [DOI] [PubMed] [Google Scholar]
- 21.Burchiel KJ. A new classification for facial pain Neurosurgery 2003. Nov;53(5):1164-1166; discussion 1166-1167. PMID: 14580284 [DOI] [PubMed] [Google Scholar]
- 22.Lee JYK, Chen HI, Urban C, Hojat A, Church E, Xie SX, Farrar JT. Development of and psychometric testing for the Brief Pain Inventory-Facial in patients with facial pain syndromes J Neurosurg 2010. Sep;113(3):516-523. PMID: 20151778 [DOI] [PubMed] [Google Scholar]
- 23.Symonds T, Randall JA, Hoffman DL, Zakrzewska JM, Gehringer W, Lee JY. Measuring the impact of trigeminal neuralgia pain: the Penn Facial Pain Scale-Revised J Pain Res 2018;11:1067-1073. PMCID: PMC5993035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novotny J, Bhatnagar JP, Niranjan A, Quader MA, Huq MS, Bednarz G, Flickinger JC, Kondziolka D, Lunsford LD. Dosimetric comparison of the Leksell Gamma Knife Perfexion and 4C J Neurosurg 2008. Dec;109 Suppl:8-14. PMID: 19123882 [DOI] [PubMed] [Google Scholar]
- 25.Pop LA, Millar WT, van der Plas M, van der Kogel AJ. Radiation tolerance of rat spinal cord to pulsed dose rate (PDR-) brachytherapy: the impact of differences in temporal dose distribution Radiother Oncol 2000. Jun;55(3):301-315. PMID: 10869745 [DOI] [PubMed] [Google Scholar]
- 26.Jones B, Klinge T, Hopewell JW. The influence of the α/β ratio on treatment time iso-effect relationships in the central nervous system Int J Radiat Biol 2020. Jul;96(7):903-909. PMID: 32243225 [DOI] [PubMed] [Google Scholar]
- 27.Graffeo CS, Donegan D, Erickson D, Brown PD, Perry A, Link MJ, Young WF, Pollock BE. The Impact of Insulin-Like Growth Factor Index and Biologically Effective Dose on Outcomes After Stereotactic Radiosurgery for Acromegaly: Cohort Study Neurosurgery 2020. Sep 1;87(3):538-546. PMCID: PMC7426191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction Semin Radiat Oncol 2008. Oct;18(4):234-239. PMCID: PMC2750078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kann BH, Yu JB, Stahl JM, Bond JE, Loiselle C, Chiang VL, Bindra RS, Gerrard JL, Carlson DJ. The impact of cobalt-60 source age on biologically effective dose in high-dose functional Gamma Knife radiosurgery J Neurosurg 2016;125(Suppl 1):154-159. PMID: 27903196 [DOI] [PubMed] [Google Scholar]
- 30.Millar WT, Hopewell JW, Paddick I, Lindquist C, Nordströn H, Lidberg P, Gårding J. The role of the concept of biologically effective dose (BED) in treatment planning in radiosurgery Phys Med 2015. Sep;31(6):627-633. PMID: 25982304 [DOI] [PubMed] [Google Scholar]
- 31.Tuleasca C, Carron R, Resseguier N, Donnet A, Roussel P, Gaudart J, Levivier M, Régis J. Patterns of pain-free response in 497 cases of classic trigeminal neuralgia treated with Gamma Knife surgery and followed up for least 1 year J Neurosurg 2012. Dec;117 Suppl:181-188. PMID: 23205808 [DOI] [PubMed] [Google Scholar]
- 32.Dhople AA, Adams JR, Maggio WW, Naqvi SA, Regine WF, Kwok Y. Long-term outcomes of Gamma Knife radiosurgery for classic trigeminal neuralgia: implications of treatment and critical review of the literature. Clinical article J Neurosurg 2009. Aug;111(2):351-358. PMID: 19326987 [DOI] [PubMed] [Google Scholar]
- 33.Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of The Barrow Neurological Institute Int J Radiat Oncol Biol Phys 2000. Jul 1;47(4):1013-1019. PMID: 10863073 [DOI] [PubMed] [Google Scholar]
- 34.Linskey ME, Ratanatharathorn V, Peñagaricano J. A prospective cohort study of microvascular decompression and Gamma Knife surgery in patients with trigeminal neuralgia J Neurosurg 2008. Dec;109 Suppl:160-172. PMID: 19123904 [DOI] [PubMed] [Google Scholar]
- 35.Young B, Shivazad A, Kryscio RJ, St Clair W, Bush HM. Long-term outcome of high-dose γ knife surgery in treatment of trigeminal neuralgia J Neurosurg 2013. Nov;119(5):1166-1175. PMID: 23600932 [DOI] [PubMed] [Google Scholar]
- 36.Jones B, Hopewell JW. Modelling the influence of treatment time on the biological effectiveness of single radiosurgery treatments: derivation of “protective” dose modification factors BJR 2018. Aug 21;92(1093):20180111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall K, Chan MD, McCoy TP, Aubuchon AC, Bourland JD, McMullen KP, deGuzman AF, Munley MT, Shaw EG, Tatter SB, Ellis TL. Predictive Variables for the Successful Treatment of Trigeminal Neuralgia With Gamma Knife Radiosurgery Neurosurgery 2012. Mar 1;70(3):566-573. [DOI] [PubMed] [Google Scholar]
- 38.Pollock BE, Phuong LK, Foote RL, Stafford SL, Gorman DA. High-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction Neurosurgery 2001. Jul;49(1):58-62; discussion 62-64. PMID: 11440460 [DOI] [PubMed] [Google Scholar]