Abstract
Objective
Imaging represents an important noninvasive means to assess cystic fibrosis (CF) lung disease, which remains the main cause of morbidity and mortality in CF patients. While the development of new imaging techniques has revolutionised clinical practice, advances have posed diagnostic and monitoring challenges. The authors aim to summarise these challenges and make evidence-based recommendations regarding imaging assessment for both clinicians and radiologists.
Study design
A committee of 21 experts in CF from the 10 largest specialist centres in Italy was convened, including a radiologist and a pulmonologist from each centre, with the overall aim of developing clear and actionable recommendations for lung imaging in CF. An a priori threshold of at least 80% of the votes was required for acceptance of each statement of recommendation.
Results
After a systematic review of the relevant literature, the committee convened to evaluate 167 articles. Following five RAND conferences, consensus statements were developed by an executive subcommittee. The entire consensus committee voted and approved 28 main statements.
Conclusions
There is a need for international guidelines regarding the appropriate timing and selection of imaging modality for patients with CF lung disease; timing and selection depends upon the clinical scenario, the patient's age, lung function and type of treatment. Despite its ubiquity, the use of the chest radiograph remains controversial. Both computed tomography and magnetic resonance imaging should be routinely used to monitor CF lung disease. Future studies should focus on imaging protocol harmonisation both for computed tomography and for magnetic resonance imaging. The introduction of artificial intelligence imaging analysis may further revolutionise clinical practice by providing fast and reliable quantitative outcomes to assess disease status. To date, there is no evidence supporting the use of lung ultrasound to monitor CF lung disease.
Short abstract
There is a need for international guidelines regarding the appropriate timing and selection of imaging modality for patients with cystic fibrosis lung disease. https://bit.ly/3HwjUvG
Introduction
Cystic fibrosis (CF) is the most common fatal congenital disease in the Caucasian population with a frequency of one in 2000 to 3000 live births [1]. Lung involvement in CF is characterised by chronic bacterial infection and inflammation with acute episodes of pulmonary exacerbation resulting in progressive diffuse bronchiectasis and lung function decline [1]. Although lung disease remains the main cause of morbidity and mortality in CF patients, the last decade has seen new drug therapies and lung transplantation lead to a significant improvement in survival [2]. Data from the Cystic Fibrosis Foundation 2019 Patient Registry Annual Data Report shows that the median predicted survival for CF patients in the United States improved from 38 years for those born in 2008 (95% CI, 35–39 years) to 48.4 years (95% CI, 45.9–51.5 years) for those born in 2019 [3]. This significant improvement has been largely achieved by the introduction of prevention and yearly monitoring programmes, which aim to detect disease at an early phase and closely monitor disease progression [2].
The progression of lung disease has been routinely assessed by pulmonary function tests (PFTs) [4], although chest imaging has proved to be more sensitive than PFTs in the detection of structural lung damage [5]. In addition, chest imaging is more feasible when monitoring lung involvement in patients unable to perform PFT manoeuvres, such as neonates and infants [6].
Moving from the routine use of chest radiographs (CRs) to cross-sectional imaging, such as computed tomography (CT) and chest magnetic resonance imaging (MRI), the latter has significantly contributed to the development of patient-tailored therapy [6–13]. However, several issues related to the use of chest imaging modalities in CF remain unaddressed; for example, it is unclear precisely when, and how, to use CR, CT and MRI. For instance, while the most frequent clinical use of CT within the European Cystic Fibrosis Society Clinical Trial Network (ECFS-CTN) is a single biennial scan, other CF centres employ CT imaging annually or every 3 years [14]. This variability translates into significant radiation dose variability between CF centres [15]. Standard operating procedures concerning lung imaging across differing centres are also highly heterogeneous and rely on various factors including local expertise, accessibility to specific imaging modalities, and confidence of the referring physician in the interpretation of imaging findings [15]. To address these variations, a group of experts in CF imaging, including pulmonologists and radiologists, founded the iMAging managEment of cySTic fibROsis (MAESTRO) committee; the overall aim of the group being the development of clear and actionable recommendations for the timing and use of specific lung imaging techniques in the diagnosis and assessment of clinically stable and exacerbated CF lung disease via an evidence-based review of the literature.
Material and methods
A multidisciplinary panel of 13 radiologists and eight pulmonologists, all expert in paediatric and adult CF care, together with one study methodologist, were involved with the identification of clinical questions, expressed in PICOT (patient/population; intervention/indicator; compare/control; outcome; time/type of study) format. The search protocol was set up according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement [16]. Databases used were PubMed and Web of Science; in addition, the reference lists of the included articles were searched for additional articles of interest. The authors included original papers, randomised controlled trials, systematic reviews or meta-analyses, guidelines, and consensus statements; articles not written in English and case reports were excluded. The detailed search strategy is shown in Appendix 1 of the online supplementary material. The search was conducted in November 2019 and updated in June 2021; a second update occurred after first article revision in October 2021.
Relevant papers were ranked, and the level of evidence graded according to the United States Preventative Services Task Force (USPFTF) system for grading the quality of evidence and strength of recommendations. The committee then identified three representative clinical scenarios (table 1) and drafted a list of recommendations based on either evidence or expert opinion relating to the relevant clinical question. Thereafter, areas of agreement and disagreement were identified through online Delphi rounds and voted on using a nine-point Likert scale: strongly disagree (score 1), disagree (score 3), neutral (score 5), agree (score 7), and strongly agree (score 9).
TABLE 1.
The three clinical scenarios
| Clinical scenario | Type of patient | Statements |
| First diagnosis | Asymptomatic | 1.1–1.4 |
| Symptomatic | ||
| Follow-up | Stable | 2.1–2.18 |
| Declining | ||
| Improving (treatment with cystic fibrosis transmembrane conductance regulator modulators) | 2.10 and 2.12 | |
| Pulmonary exacerbation | Diagnosis | 3.1–3.6 |
| Monitoring treatment response |
In addition, the committee classified as “best practice” the recommendations that were felt to be underpinned by a high level of certainty despite limited evidence being available. The term “statement of fact” was used to summarise an important topic explored in the consensus when facts, rather than actions, were discussed and agreed by the committee. Furthermore, five RAND rounds were performed, to vote upon the appropriateness, or otherwise, of each technique in a given clinical scenario. According to the “RAND/UCLA (University of California at Los Angeles) Appropriateness Method’, the appropriateness of timing and selection of a given imaging modality was rated on an ordinal scale of integers from one to nine grouped into three categories [17]. Integers from one to three were in the category “usually not appropriate”, when the harm of undertaking a procedure or treatment outweighed its benefits; integers from four to six represented procedures that “may be appropriate”; and integers from seven to nine were in the category “usually appropriate”, when the benefits of undertaking a procedure or treatment outweighed its harms or risks [17]. The final draft of recommendations and RAND results were discussed during the consensus meeting, held on 20 June 2021, to collect final agreement and vote on the strength of each recommendation.
Clinical scenarios, imaging modalities and definitions
Three clinical scenarios were defined by the committee, each of which included sub-scenarios (table 1). The first scenario (First diagnosis) referred to the initial imaging examination performed in patients with CF. This group usually includes either young asymptomatic infants diagnosed by screening – sweat or genetic testing – or symptomatic patients, which could be child or adult, the latter in the case of late diagnosis (which usually follows clinical manifestation).
The second scenario (Follow-up) included patients with CF undergoing routine (annual or biennial) clinical monitoring. In this group, a distinction was made between patients with stable, declining or improving lung function. Declining lung function was defined as the annual decrease in forced expiratory volume in 1 s (FEV1 % percentage of predicted) larger than 2% and 3% in patients younger and older than 12 years of age, respectively [18–20]. Decline in FEV1, based on recent UK Cystic Fibrosis Registry data, estimates ranges between 0.5 and 2.68%, with an average value of 1.5%, according to age and pancreatic status [20]. Using the lung clearance index (LCI) as a parameter, decline is defined as annual drop of 17% and 15% in CF patients younger and older than 12 years of age, respectively [21, 22]. “Improving lung function” is the sub-scenario in CF patients on treatment with new CF transmembrane conductance regulator (CFTR) modulator therapies [23]. In young children who cannot perform lung function tests, decline was defined by clinically integrated evaluation which included the presence of chronic respiratory symptoms, frequency of respiratory exacerbation and antibiotic therapy [24, 25]. The third scenario (Pulmonary exacerbation) referred to patients experiencing exacerbation defined as rapid decline of lung function combined with increased respiratory symptoms according to Rosenfeld et al. [26]. The category Pulmonary exacerbation involved two sub-scenarios: the initial diagnosis of pulmonary exacerbation and, secondly, follow-up of treatment response.
Recommendations refer to all modalities of thoracic imaging, namely, lung ultrasound (LUS), CR, CT and MRI. During the discussion, the authors discriminate between uncooperative and cooperative patients; an uncooperative patient is one who cannot follow breathing instructions, such as inspiratory and expiratory manoeuvres, during the examination, either because of age (i.e. younger than 6 years old) or level of consciousness (i.e. sedation). A cooperative patient is one who can follow the instructions during the examination. In this article, the term low-dose CT refers to a CT protocol which is based on the recommendations by Kuo et al. [15] according to patient's age. The term PFT refers to standard spirometry and plethysmography assessment performed according to European Respiratory Society/American Thoracic Society Guidelines [27]. When describing MRI techniques, a difference is made between high-resolution ultrashort echo time (UTE) sequences and conventional MRI sequences, which usually have lower spatial resolution [28].
Finally, based on the literature search, the committee provided a series of recommendations related to collaboration between the referring clinician and radiologist, the use of structured reporting in CF, and those specific to each imaging modality.
Results
Study selection
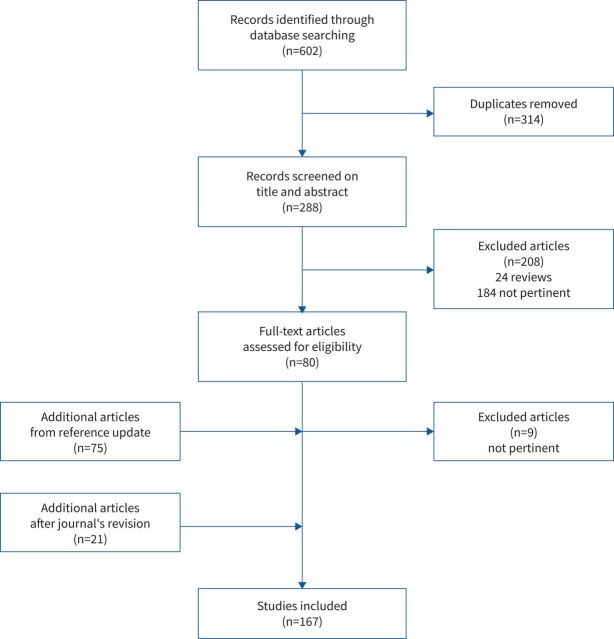
The first search identified 602 articles, of which, after removing duplicates, 288 were screened for title and abstract based on article type. The full text of 80 articles was deemed relevant to the aim of the study. A second search (June 2021) identified 75 articles, of which 66 were included in the study, resulting in a final selection of 146 articles. After the first revision of the publication (October 2021), an additional 21 articles were identified giving a total of 167 articles. The final list of articles is given in the online supplement (1E). The flowchart of the article selection is shown in figure 1.
FIGURE 1.
Flowchart of article selection.
28 recommendations are presented in table 2 (First diagnosis), table 3 (Follow-up) and table 4 (Pulmonary exacerbation) according to the clinical scenarios. Each table includes the USPFTF system of grading, with descriptions of the type of statement, strength of recommendation, quality of evidence, and type of CF patient. The last column of each table refers to the most relevant bibliography supporting the recommendation.
TABLE 2.
First diagnosis
| Statement number | Statement | Type of statement | Strength of recommendation | Quality of evidence | Type of patient | Most relevant supporting articles |
| 1.1 | In infants diagnosed via newborn screening, CT can be used as a sensitive tool to detect early disease, tailor treatment, and monitor disease progression both in symptomatic and asymptomatic patients. | Recommendation | Grade B | Moderate | Asymptomatic and symptomatic | [10, 13, 29, 30] |
| 1.2 | Low-dose CT is feasible both in uncooperative and cooperative patients. | Recommendation | Grade A | High | Asymptomatic and symptomatic | [31–36] |
| 1.3 | Although MRI is more feasible in cooperative patients, it can be performed in uncooperative patients with or without moderate sedation/general anaesthesia according to the patient's age and mental status. | Recommendation | Grade B | Moderate | Asymptomatic and symptomatic | [7–9, 28, 33, 37–39] |
| 1.4 | CT dose should be as low as reasonably achievable without affecting the diagnostic quality of the image. | Recommendation | Grade A | High | Asymptomatic and symptomatic | [14, 40–44] |
CT: computed tomography; MRI: magnetic resonance imaging.
TABLE 3.
Follow-up
| Statement number | Statement | Type of statement | Strength of recommendation | Quality of evidence | Type of patient | Most relevant supporting articles |
| 2.1 | Limited data are available about the use of LUS to monitor lung status or to evaluate pulmonary exacerbation. Preliminary results show good relation between LUS and CT to assess structural changes. | Recommendation | Grade I | Low | Stable and declining | [45, 46] |
| 2.2 | Use of CR scoring systems can improve sensitivity in monitoring CF lung disease. However, their routine use in clinical practice is cumbersome, due to high inter-observer variability. Fully automated CR scoring systems based on artificial intelligence algorithms may overcome this limitation increasing the sensitivity of CR in detecting disease progression. |
Recommendation | Grade C | Moderate | Stable and declining | [47–52] |
| 2.3 | There is little evidence in the literature about the optimal timing of CT monitoring. There is a need for international guidelines to schedule CT surveillance in patients with CF lung disease. | Statement of fact | NA | Low | Stable | [6, 13, 29, 53–56] |
| 2.4 | Current best clinical imaging practice in several CF centres is performing CT biennially (i.e. 1 every 2 years). | Best practice | Grade A | Moderate | Stable | [14, 15] |
| 2.5 | State-of-the-art reviews of risk related to CT radiation exposure highlight a reasonably low risk of cumulative cancer in children using biennial low dose CT. CT protocol harmonisation between CF centres should be promoted to comply with the “as low as reasonably achievable” (ALARA) concept. |
Recommendation | Grade A | High | Stable | [14, 29, 35, 36, 40–44] |
| 2.6 | CT can better detect lung disease progression than standard pulmonary function tests (i.e. FEV1) both in cooperative and uncooperative patients, irrespective of disease severity. | Recommendation | Grade B | High | Stable and declining | [5, 10, 12, 53–66] |
| 2.7 | CT is complementary to lung clearance index in the detection of disease progression or improvement by clinical intervention. | Statement of fact | NA | High | Stable and declining | [67–74] |
| 2.8 | CT provides relevant information possibly capable of modifying disease trajectory, patient management and follow-up, both in uncooperative and cooperative patients. | Statement of fact | NA | Moderate | Stable and declining | [5, 10–12, 48, 75, 76] |
| 2.9 | The use of appropriate scoring systems for CT increases its sensitivity in tracking changes in symptomatic, and asymptomatic, early lung disease. Therefore, their use is recommended to standardise interpretation of CT data according to CF centre expertise and capacity. | Recommendation | Grade A | Moderate | Stable and declining | [60, 65, 77–94] |
| 2.10 | Artificial intelligence-based scoring system and segmentation tools for CF imaging allows a fully automated volumetric quantification of CF-related abnormalities over an entire lung. These novel scoring systems, when further validated, could provide a robust disease outcome in the era of effective CFTR modulator therapy. | Statement of fact | NA | Moderate | Stable, declining and improving | [60, 65, 77–94] |
| 2.11 | CT scans performed in infants and young children with symptoms is a potential clinical trial outcome measure for novel treatments in this age group. | Statement of fact | NA | Moderate | Declining | [34, 48, 53, 60, 63, 92, 95–102] |
| 2.12 | Despite improvement in clinical, lung function, and imaging outcomes in patients undertaking CFTR modulator therapy, no deviation from the usual imaging follow-up scheme should be advised, because there is no evidence in the current literature about long-term benefit of these agents. | Statement of fact | NA | Moderate | Stable, declining and improving | [35, 36, 81, 101, 103–105] |
| 2.13 | Despite conventional MRI sequences having lower sensitivity than CT in the assessment of disease extent, state-of-the-art MRI (e.g. UTE sequence) shows comparable results to CT and provides a convenient, noninvasive, and non-ionising assessment of disease progression in cooperative patients. The beneficial absence of radiation is particularly important with respect to the need for frequent follow-up examinations and the increasing life span of CF patients. | Statement of fact | NA | High | Stable and declining | [8, 9, 106–118] |
| 2.14 | MRI provides information about ventilation, inflammation, perfusion and structure (VIPS-MRI) in a single examination that is difficult to obtain with CT. | Statement of fact | Grade B | Moderate | Stable and declining | [8, 9, 33, 119–133] |
| 2.15 | MRI is a noninvasive, radiation-free endpoint to identify potentially reversible abnormalities (e.g. mucus plugging and lung hypoperfusion) in early phase clinical trials testing novel therapeutics in symptomatic, cooperative, patients with CF. | Statement of fact | Grade B | Moderate | Stable and declining | [118, 131, 134–138] |
| 2.16 | The MRI scoring system is a promising tool to predict the loss of lung function in CF patients and can serve as a clinically relevant outcome predictor for pulmonary manifestations in CF. | Statement of fact | NA | Moderate | Stable and declining | [115, 128, 139–142] |
| 2.17 | Longitudinal studies are needed to compare the sensitivity of CT and MRI in tracking disease progression. | Statement of fact | NA | Moderate | Stable and declining | [8, 116, 143] |
| 2.18 | The use of MRI in clinical practice is hampered by its higher cost than CT; the need for state-of-the-art MR systems; the occasional need for moderate sedation/general anaesthesia in uncooperative children; nonuniformity of MR protocol; as well as substantial image variability/capability between MR brands. | Statement of fact | NA | High | Stable and declining | [7, 8, 34, 37, 144–148] |
CF: cystic fibrosis; CFTR: cystic fibrosis transmembrane conductance regulator; CR: chest radiograph; CT: computed tomography; FEV1: forced expiratory volume in 1 s; LUS: lung ultrasound; MR: MRI: magnetic resonance; MRI: magnetic resonance imaging; NA: not available; UTE: ultrashort echo.
TABLE 4.
Pulmonary exacerbations
| Statement number | Statement | Type of statement | Strength of recommendation | Quality of evidence | Type of patient | Most relevant supporting articles |
| 3.1 | CR may not help with the detection of pulmonary exacerbation, especially in CF patients with more severe disease. | Recommendation | Grade D | Low | Diagnosis | [149] |
| 3.2 | CT can detect acute structural lung abnormalities during and after pulmonary exacerbation (e.g. increase of bronchial wall thickening and mucus plugging) in cooperative, and uncooperative, patients. | Statement of fact | NA | High | Diagnosis | [89, 95, 96, 150–156] |
| 3.3 | CT can be used to define outcome measures of pulmonary damage in clinical trials and test therapeutic interventions in patients with persistent respiratory symptoms and reduced lung function despite appropriate therapy. | Recommendation | Grade C | Low | Diagnosis and monitoring treatment response | [55, 65, 89, 95, 96, 150–154, 157] |
| 3.4 | Routine use of CT for short term follow-up during pulmonary exacerbation is not recommended due to the risk of high cumulative radiation dose. Clinicians should consider the risk/benefit ratio related to dose when prescribing CT in pulmonary exacerbation. | Recommendation | Grade D | Moderate | Monitoring treatment response | [14, 29, 35, 36, 40–44, 158] |
| 3.5 | MRI can be used as a surrogate marker for disease severity and response to treatment during short-term follow-up of symptomatic, or declining, cooperative CF patients. In uncooperative patients, the risk related to moderate sedation or anaesthesia needs to be considered. | Recommendation | Grade A | High | Diagnosis and monitoring treatment response | [118, 119, 130, 136, 159] |
| 3.6 | MRI can be used as a clinically relevant outcome predictor for pulmonary exacerbations in cooperative, declining CF patients. | Statement of fact | NA | High | Diagnosis and monitoring treatment response | [106, 116, 119, 130, 139] |
CF: cystic fibrosis; CR: chest radiograph; CT: computed tomography; MRI: magnetic resonance imaging; NA: not available.
Recommendations relating to collaboration between the pulmonologist and radiologist, structured radiology reporting, and each imaging modality are presented in the supplementary material (Appendix 1, tables E2, E3, E4).
Discussion
The systematic literature search identified 167 relevant publications on CF imaging. Based on this review, the authors report a lack of consensus regarding imaging protocols for patients with CF, including uncertainty surrounding choice of the most appropriate imaging modality according to clinical scenario, patient's age and lung function. To bridge this gap, the authors propose 28 new recommendations by summarising and grading the quality of evidence published in the literature to date.
First diagnosis
Despite many specialist centres using CR as the imaging technique of choice in infants and preschool children, CT shows higher sensitivity in detecting early abnormalities in both symptomatic and asymptomatic CF patients [5, 10, 12, 53, 54, 60–63]. Therefore, using CR in the early post-screening phase seems to be of limited value, with CT being a more efficient way of detecting early disease and monitoring disease progression [10, 13, 29, 50]. A main limitation of CT, however, for routine imaging of preschool children, is the absence of any “CT protocol harmonisation” [15]. There is, indeed, no consensus on what dose level may be considered low and on the optimal timing of the first CT examination. State-of the art CT scanners can provide substantial dose reductions with effective radiation exposure comparable to CR [35, 158, 160]. Further dose reductions are expected by the introduction of the new photon-counting CT scanner, which could provide a dose reduction of up to 70% at no expense to image resolution [161]. Given the speed of operation, CT scanning can be performed without anaesthesia, thus avoiding cumbersome logistics and anaesthetic recovery times [32, 34, 37, 147]. Thus, CT appears to be higher-yielding and easier to perform than MRI for cross-sectional imaging in preschool patients with a new diagnosis of CF, especially when performed in uncooperative patients [32, 146–148, 162] – a group that frequently requires moderate sedation, or anaesthesia, when undergoing MRI [7, 8, 37, 146, 147].
Follow-up
CR versus CT
Despite the absence of strong supporting evidence in the literature, CR remains the most frequently used imaging modality for lung disease monitoring in several specialist centres; this is due to its ready availability and low cost, and the decades-long use of this technique by CF clinicians during routine follow-up. However, the sensitivity of CR is poor, and inter-observer variability between radiologists is high, even when combined with adequate scoring systems [47, 48, 50]. The use of CR is frequently unable to efficiently monitor CF lung disease progression, being less sensitive than CT [48]. The MAESTRO committee believes that the frequent use of CR for follow-up of patients with CF, especially in the early phase of the disease, should be challenged. Furthermore, in view of the progressive increase in life expectancy of the CF population, the risk of radiation exposure could be minimised by an optimised use of CT, increased utilisation of MRI and improved assessment of CR. The latter could be achieved by the introduction of an artificial intelligence (AI) algorithm to increase sensitivity and limit the cumulative radiation dose at low diagnostic cost [49, 52].
Timing of imaging surveillance in the era of CFTRs
Surprisingly, the authors did not find guidelines regarding the optimal timing of imaging follow-up. The scarcity of evidence on this matter contributes to the high degree of heterogeneity within the imaging protocols among CF centres [15]. As observed above, the type and timing of imaging modality are frequently selected based on local experience and accessibility to specific radiological facilities. Current best clinical imaging practice in several CF centres within the ECFS-CTN is to perform CT biennially with a radiation dose as low as reasonably achievable (the “as low as reasonably achievable” (ALARA) concept), while avoiding sedation [163]. Despite the lack of clarity regarding the basis for this follow-up, it seems that the risk related to CT radiation exposure within this scheme is reasonably low [14, 29, 40, 43]. To date, only one study has been conducted to assess the effect of the imaging interval on CF lung disease progression [55]. In future, however, imaging follow-up should be patient-tailored and include stratification for risk factors for disease progression, such as chronic bacterial infection, pulmonary exacerbation rate and access to new CFTR modulators. Other potential risk factors influencing disease progression could include pancreatic insufficiency, nutritional state, age at diagnosis, and therapy adherence [2, 164, 165]. Such a patient-tailored approach could further reduce the risk of radiation exposure by modifying the imaging interval according to disease status, with longer CT scan intervals in more stable CF patients. This is particularly important in the new era of CFTR modulation therapy, which has shown remarkable efficiency in terms of improvement in lung function and reduction in structural abnormalities [36, 81, 101, 166]. Although, in future, patients could benefit from shorter or longer intervals of radiological follow-up according to clinical status, it is not currently possible to make recommendations, because the long-term benefit of CFTR modulators remains unknown. The introduction of a shorter imaging surveillance methodology, either based on CT or MRI, will require in both cases a major effort in imaging protocol standardisation. While for CT this should be focus on the introduction of an ultra-low-dose CT protocol (i.e. radiation dose in the range of a CR), for MRI will need both international agreement on a common set of MRI sequences and development of dedicated post-processing tools for quantification of MRI findings [167]. The authors’ recommendation is, therefore, to maintain routine follow-up of patients who improve under the effects of CFTR modulation; on the other hand, any significant deterioration of lung function, or clinical symptoms, should prompt further imaging investigation either with ultra-low dose CT or chest-MRI according CF centre expertise.
Integrated diagnostics – an opportunity to reduce radiation dose?
CT dose reduction may also be achieved via CT protocol harmonisation. Studies have shown that dose variation between specialist centres strongly contributes to a huge variability of cumulative dose, which has prompted the introduction of uniform dose-management strategies [15, 29]. Recent developments have also shown the potential for further dose reduction, up to 78%, using optimised imaging protocols, with iterative reconstruction techniques via the newest generation CT scanners [35, 36, 44, 158, 160, 167]. Interestingly, these optimised CT protocols have allowed the availability of diagnostic quality CT images at a dose equivalent of a CR, while demonstrating superior sensitivity to the CR and equivalent standard CT in the detection of bronchiectasis [160]. These observations invite future studies that will aim to explore the benefits of replacing CR with low-dose CT in terms of improved diagnostic yield and clinical decision making, and patient outcomes [160]. Within this context, the MAESTRO committee urges the CF research community to define international guidelines to regulate surveillance with CT in patients with CF [14, 29, 160].
Despite the risks associated with frequent imaging, CT has been shown in several studies to offer benefits over the standard PFT (e.g. FEV1), given its improved sensitivity in monitoring pulmonary disease, both in cooperative and uncooperative patients, irrespective of disease severity [5, 10, 12, 60, 63, 64]. On the other hand, chest CT has shown a good correlation with LCI, especially the multiple-breath nitrogen washout technique, in defining the presence, and extent, of CF lung disease in preschool and school-aged children (although it is less sensitive in infants) [67–74, 157, 168, 169]. The LCI reflects overall ventilation inhomogeneity within the lung, even in the presence of normal spirometric volumes, and is correlated with mosaic lung attenuation on chest CT related either to gas trapping or reduced perfusion [170, 171]. These LCI/gas-trapping measurements have been utilised over the past decade in preference to FEV1 as an early, and sensitive, tool in monitoring the progression of CF lung disease, both in terms of radiological change and clinical involvement [67, 169, 171–175]. Further integration of LCI and gas-trapping measurement with chest CT will be pivotal for a better understanding of disease progression, and as a relevant end-point in clinical intervention trials. The use of more sensitive lung function tests, such as LCI, will also help the shift towards the cross-disciplinary implementation of integrated diagnostics [176], when the use of imaging will be adjusted according to lung function and clinical status.
The rise of “machine learning” in CF chest imaging
The introduction of AI algorithms and machine-learning techniques have also found their way into CF imaging. Zucker et al. [52] have recently shown that an AI-based algorithm could be used to perform an automated Brasfield scoring of CRs and it performed similarly to a paediatric radiologist. Improvement of the diagnostic performance of CR is important in limiting the cumulative dose of CF chest imaging and reducing diagnostic uncertainty [49, 52].
AI has been also applied to CT imaging, being shown to modify disease trajectory, patient management and follow-up [10, 13, 48]. By combining the use of CT with appropriate scoring systems, or automatic segmentation tools, its sensitivity further increases, making it possible to assess early CF lung disease and progression both in symptomatic and asymptomatic patients [11, 60, 82, 87–92, 140]. Deployment of quantitative imaging tools based on AI and machine-learning techniques can speed up the development of new CT outcome measures for novel treatments in clinical trials [53, 60, 63, 82]. Recent publications have promoted the clinical use of AI-based segmentation and scoring tools for chest CT, providing reader-independent quantitative outcomes such as: airways–artery ratio, airways tapering, and measurement of trapped air [176 55, 58, 59, 84–88, 177]. Recently, a multi-centre study involving a fully automated AI-based scoring system has proven its high diagnostic efficiency [81]. The upcoming introduction of commercially available software for thoracic imaging and, in particular, for automatic CT scoring might revolutionise clinical practice [178]. Finally, AI applications for MRI in CF are still limited, with chest CT far more advanced in terms of technique validation compared to chest MRI [119, 126, 128, 133, 135, 139, 179].
The use magnetic resonance in CF imaging
Although CT is currently the most commonly used imaging modality for monitoring disease progression, advances in MRI have taken a quantum leap and now offer improved image quality, affording a complementary/alternative imaging tool to CT [7, 8, 33]. Initial studies with conventional MRI sequencing revealed a poorer image quality compared with CT, but more recent MRI technology, such as UTE sequence, shows comparable results [107, 109, 111, 112, 114, 117, 118]. MRI therefore offers a convenient, noninvasive and nonionising means of assessing disease progression in cooperative patients. The beneficial absence of radiation is particularly important with respect to the potential need for frequent follow-up and the increasing life span of CF patients. More importantly, MRI has a unique advantage over CT, which is the ability to provide information about ventilation, inflammation, perfusion and structure in a single examination [178 7, 8, 33, 119, 129, 130, 132, 180]. For example, it has been shown that MRI can capture potentially reversible abnormalities – such as mucus plugging and lung hypoperfusion – which could be used as a new imaging outcome in early phase clinical trials and for testing novel therapeutics in symptomatic, cooperative CF patients [119, 134, 136]. Moreover, a dedicated MRI scoring system has been proven to be a clinically relevant outcome predictor for pulmonary manifestations in CF patients [115, 128, 139–141].
MRI outcomes have also shown strong correlations with lung function parameters in both early and advanced disease. Both MRI and LCI were good predictors of disease progression and response to antibiotic therapy during pulmonary exacerbation in preschool CF children [131, 134, 137]; use of MRI has also been proved to detect early subclinical disease in infants and children [128, 135, 166, 181]. MRI has also shown high sensitivity to the presence of allergic bronchopulmonary aspergillosis and might, therefore, be considered when assessing the effect of treatment on other chronic airway infections (e.g. Pseudomonas aeruginosa) [120, 121].
These results indicate that MRI may become a “one-stop-shop” for CF imaging. To date, what hampers its broader use in clinical practice is its higher cost than CT, the need for state-of-the-art MRI systems, the necessity for sedation/general anaesthesia for uncooperative patients, nonuniformity of MRI protocol, as well as large image variability/capability between MRI brands [180 7, 8, 37, 144–146, 182]. Recent development of a high performance low-field MRI system (0.55 T), capable of high-resolution lung imaging, offers new perspectives on routine MRI application, overcoming the aforementioned limitations with high-strength MRI systems (1.5–3 T) [183, 184]. Future studies should focus on MRI protocol harmonisation to foster the development of comparable image quality between CF centres and MRI vendors. Homogeneous image quality will be important to facilitate the development of dedicated post-processing tools for chest MRI and allow quantitative imaging. Recent single-centre studies have proposed new automatic tools for the detection of trapped air [135] or inflammatory changes quantification [119].
LUS
Although LUS is generally considered to be a safe bedside imaging modality, frequently used in paediatric and adult lung clinics, there is insufficient evidence to propose its use for CF lung disease monitoring [45, 46]. Further multi-centre validation studies will be needed to assess its utility in the assessment of CF lung disease.
Pulmonary exacerbations
Pulmonary exacerbations are major determinants of lung function decline and mortality in the CF population with 25–50% of patients experiencing exacerbations that do not allow them to recover their prior FEV1 value following treatment [180]. The absence of a shared definition compromises the optimal clinical management of pulmonary exacerbation, including the use of imaging for diagnosis and follow-up [180]; this is problematic given the significant role of imaging in the clinical management of exacerbation [79, 95, 119, 130, 136, 151, 152, 154]. The most frequently used imaging modality during exacerbation is CR, although its sensitivity and specificity are debated, especially in patients with severe disease [149]. The latter group is not only at higher risk of exacerbation, but is one where it is difficult to detect new radiographic changes in a lung substrate of diffuse parenchymal abnormality.
Within this context, CT may offer an alternative modality [79, 95, 96, 150–154, 185] where it can be used to detect acute structural lung abnormalities related to pulmonary exacerbation (e.g. increase in bronchial wall thickening and mucus plugging) in uncooperative and cooperative patients [79, 95, 96, 150–154, 183]. CT may also play a role in defining objective outcome measures in clinical trials of new therapies for the treatment of pulmonary exacerbation [79, 95, 96, 150–154, 185]. Despite these advantages, the challenge of cumulative radiation exposure limits its routine use for pulmonary exacerbation diagnosis and short-term follow-up. Clinicians should always consider the risk/benefit ratio related to the annual radiation dose when prescribing CT scans in these instances [14, 29, 40, 43]. A cautious approach would be to use low-dose CT only in patients with persistent respiratory symptoms and reduced lung function despite appropriate therapy.
Conversely, MRI can become a surrogate for disease severity and response to treatment for short-term follow-up of symptomatic, or declining, cooperative CF patients [119, 129, 130, 136]. Several studies have shown that MRI can track functional and morphological changes related to pulmonary exacerbation with adequate sensitivity [119, 131, 136]. The absence of radiation makes MRI an ideal solution for serial imaging during the treatment of pulmonary exacerbation, becoming a clinically relevant outcome predictor in declining cooperative patients [180]. Moreover, some authors have shown that MRI could be used to detect acute inflammatory changes related to pulmonary exacerbation, making MRI an important tool to assess treatment response [119, 129, 130]. Currently, the main limitation to the use of MRI remains the uncooperative patient, where risks related to anaesthesia need to be considered [37, 146–148].
Conclusions
The MAESTRO committee urges the establishment of international guidelines on CF imaging. These guidelines should provide clear indications regarding the timing and selection of the most appropriate imaging modality according to the clinical scenario, patient's age, lung disease severity and type of treatment (i.e. CFTRs). Based on this state-of-the-art review, the committee concludes that, to date, there is no concrete evidence for a role of LUS in monitoring CF lung disease. Although CR remains the most frequently used modality in CF imaging, its ability to monitor CF lung disease is controversial. Regarding CT and MRI, both techniques should be routinely and interchangeably used to monitor CF lung disease according to the patient's age, disease status and type of treatment. However, compared with MRI, CT is at a more advanced stage in terms of validation and quantitative imaging, although there is a need for CT protocol harmonisation and patient-tailored follow-up schemes to further reduce any risk relating to radiation exposure. Full implementation of MRI in CF lung imaging will require a significant effort towards MRI protocol harmonisation to enable multi-centre validation studies and image quantification. Finally, the upcoming introduction of AI-based solutions both for CR and CT might revolutionise clinical practice by providing fast and reliable quantitative outcome measures to assess disease status in patients with CF.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0173-2021.SUPPLEMENT (569.7KB, pdf)
Acknowledgements
We would like to acknowledge S. Maina, from Editamed srl, for her assistance in the literature search, coordination and summary of the committee's meetings.
Provenance: Submitted article, peer reviewed.
This article has supplementary material available from err.ersjournals.com
Conflict of interest: P. Ciet reports personal fees from Editamed, during the conduct of the study. S. Bertolo reports personal fees from Vertex pharmaceuticals, outside the submitted work. M. Ros reports personal fees from Vertex Pharmaceuticals, personal fees from Chiesi Farmaceutici, outside the submitted work. R. Casciaro has nothing to disclose. M. Cipolli has nothing to disclose. S. Colagrande reports that during the last 5 years, he has had and has various projects in place that have involved remuneration, which has always been devolved to the SBSC department for which Prof. Colagrande works. This refers to experimental/conventional activities for which it has received compensation. Nothing has ever been personally perceived by Prof. Colagrande and all the part destined to the university was conferred to the department. The companies involved were Novartis, Sanofi, Lilly, Celther, Pfizer, Janssen, etc. All the fees have been paid following the signing of a contract among the company, the university and the Hospital. S. Costa has nothing to disclose. V. Galici has nothing to disclose. A. Gramegna has nothing to disclose. C. Lanza has nothing to disclose. F. Lucca has nothing to disclose. L. Macconi has nothing to disclose. F. Majo has nothing to disclose. A. Paciaroni has nothing to disclose. G.F. Parisi has nothing to disclose. F. Rizzo has nothing to disclose. I. Salamone has nothing to disclose. T. Santangelo has nothing to disclose. L. Scudeller has nothing to disclose. L. Saba has nothing to disclose. P. Tomà has nothing to disclose. G. Morana has nothing to disclose.
Support statement: This work was supported by an unrestricted educational grant from Chiesi Pharmaceuticals, Parma, Italy and Vertex Pharmaceuticals, Boston, USA.
References
- 1.Egan ME. Genetics of cystic fibrosis: clinical implications. Clin Chest Med 2016; 37: 9–16. doi: 10.1016/j.ccm.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 2.Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med 2020; 8: 65–124. doi: 10.1016/S2213-2600(19)30337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation . Patient Registry Annual Data Report. 2020. Available from: www.cff.org/sites/default/files/2021-10/2019-Patient-Registry-Annual-Data-Report.pdf
- 4.Wagener JS, Elkin EP, Pasta DJ, et al. Pulmonary function outcomes for assessing cystic fibrosis care. J Cyst Fibros 2015; 14: 376–383. doi: 10.1016/j.jcf.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 5.de Jong PA, Nakano Y, Lequin MH, et al. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J 2004; 23: 93–97. doi: 10.1183/09031936.03.00006603 [DOI] [PubMed] [Google Scholar]
- 6.Hota P, Madan R. Cystic fibrosis from childhood to adulthood: what is new in imaging assessment? Radiol Clin North Am 2020; 58: 475–486. doi: 10.1016/j.rcl.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 7.Hirsch FW, Sorge I, Vogel-Claussen J, et al. The current status and further prospects for lung magnetic resonance imaging in pediatric radiology. Pediatr Radiol 2020; 50: 734–749. doi: 10.1007/s00247-019-04594-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods JC, Wild JM, Wielpütz MO, et al. Current state of the art MRI for the longitudinal assessment of cystic fibrosis. J Magn Reson Imaging 2020; 52: 1306–1320. doi: 10.1002/jmri.27030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leutz-Schmidt P, Eichinger M, Stahl M, et al. Ten years of chest MRI for patients with cystic fibrosis: translation from the bench to clinical routine. Radiologe 2019; 59: 10–20. doi: 10.1007/s00117-019-0553-2 [DOI] [PubMed] [Google Scholar]
- 10.Newbegin K, Pilkington K, Shanthikumar S, et al. Clinical utility of surveillance computed tomography scans in infants with cystic fibrosis. Pediatr Pulmonol 2018; 53: 1387–1390. doi: 10.1002/ppul.24132 [DOI] [PubMed] [Google Scholar]
- 11.Szczesniak R, Turkovic L, Andrinopoulou E-RR, et al. Chest imaging in cystic fibrosis studies: what counts, and can be counted? J Cyst Fibros 2017; 16: 175–185. doi: 10.1016/j.jcf.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rybacka A, Karmelita-Katulska K. The role of computed tomography in monitoring patients with cystic fibrosis. Polish J Radiol 2016; 81: 141–145. doi: 10.12659/PJR.896051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiddens HAWM, Rosenow T. What did we learn from two decades of chest computed tomography in cystic fibrosis? Pediatr Radiol 2014; 44: 1490–1495. doi: 10.1007/s00247-014-2964-6 [DOI] [PubMed] [Google Scholar]
- 14.Kuo W, Ciet P, Tiddens HAWM, et al. Monitoring cystic fibrosis lung disease by computed tomography. Radiation risk in perspective. Am J Respir Crit Care Med 2014; 189: 1328–1336. doi: 10.1164/rccm.201311-2099CI [DOI] [PubMed] [Google Scholar]
- 15.Kuo W, Kemner-van de Corput MPC, Perez-Rovira A, et al. Multicentre chest computed tomography standardisation in children and adolescents with cystic fibrosis: the way forward. Eur Respir J 2016; 47: 1706–1717. doi: 10.1183/13993003.01601-2015 [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel MR, Spertus JA, Brindis RG, et al. ACCF Proposed method for evaluating the appropriateness of cardiovascular imaging. J Am Coll Cardiol 2005; 46: 1606–1613. doi: 10.1016/j.jacc.2005.08.030 [DOI] [PubMed] [Google Scholar]
- 18.VandenBranden SL, McMullen A, Schechter MS, et al. Lung function decline from adolescence to young adulthood in cystic fibrosis. Pediatr Pulmonol 2012; 47: 135–143. doi: 10.1002/ppul.21526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konstan MW, Wagener JS, VanDevanter DR, et al. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros 2012; 11: 405–411. doi: 10.1016/j.jcf.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caley L, Smith L, White H, et al. Average rate of lung function decline in adults with cystic fibrosis in the United Kingdom: data from the UK CF registry. J Cyst Fibros 2021; 20: 86–90. doi: 10.1016/j.jcf.2020.04.008 [DOI] [PubMed] [Google Scholar]
- 21.Svedberg M, Gustafsson PM, Robinson PD, et al. Variability of lung clearance index in clinically stable cystic fibrosis lung disease in school age children. J Cyst Fibros 2018; 17: 236–241. doi: 10.1016/j.jcf.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 22.Perrem L, Rayment JH, Ratjen F. The lung clearance index as a monitoring tool in cystic fibrosis: ready for the clinic? Curr Opin Pulm Med 2018; 24: 579–585. doi: 10.1097/MCP.0000000000000515 [DOI] [PubMed] [Google Scholar]
- 23.Goetz DM, Savant AP. Review of CFTR modulators 2020. Pediatr Pulmonol 2021; 56: 3595–3606. doi: 10.1002/ppul.25627 [DOI] [PubMed] [Google Scholar]
- 24.Bayfield KJ, Douglas TA, Rosenow T, et al. Time to get serious about the detection and monitoring of early lung disease in cystic fibrosis. Thorax 2021; 76: 1255–1265. doi: 10.1136/thoraxjnl-2020-216085 [DOI] [PubMed] [Google Scholar]
- 25.Nissenbaum C, Davies G, Horsley A, et al. Monitoring early stage lung disease in cystic fibrosis. Curr Opin Pulm Med 2020; 26: 671–678. doi: 10.1097/MCP.0000000000000732 [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld M, Emerson J, Williams-Warren J, et al. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr 2001; 139: 359–365. doi: 10.1067/mpd.2001.117288 [DOI] [PubMed] [Google Scholar]
- 27.Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax 2006; 61: 744–746. doi: 10.1136/thx.2006.061648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciet P, Tiddens HAWM, Wielopolski PA, et al. Magnetic resonance imaging in children: common problems and possible solutions for lung and airways imaging. Pediatr Radiol 2015; 45: 1901–1915. doi: 10.1007/s00247-015-3420-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilchrist FJ, Buka R, Jones M, et al. Clinical indications and scanning protocols for chest CT in children with cystic fibrosis: a survey of UK tertiary centres. BMJ Paediatr Open 2018; 2: e000367. doi: 10.1136/bmjpo-2018-000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thia LP, Calder A, Stocks J, et al. Is chest CT useful in newborn screened infants with cystic fibrosis at 1 year of age? Thorax 2014; 69: 320–327. doi: 10.1136/thoraxjnl-2013-204176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sly PD, Brennan S, Gangell C, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med 2009; 180: 146–152. doi: 10.1164/rccm.200901-0069OC [DOI] [PubMed] [Google Scholar]
- 32.Oudraad MCJ, Kuo W, Rosenow T, et al. Assessment of early lung disease in young children with CF: a comparison between pressure-controlled and free-breathing chest computed tomography. Pediatr Pulmonol 2020; 55: 1161–1168. doi: 10.1002/ppul.24702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goralski JL, Stewart NJ, Woods JC. Novel imaging techniques for cystic fibrosis lung disease. Pediatr Pulmonol 2021; 56: Suppl 1, S40–S54. doi: 10.1002/ppul.24931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kino A, Zucker EJ, Honkanen A, et al. Ultrafast pediatric chest computed tomography: comparison of free-breathing vs. breath-hold imaging with and without anesthesia in young children. Pediatr Radiol 2019; 49: 301–307. doi: 10.1007/s00247-018-4295-5 [DOI] [PubMed] [Google Scholar]
- 35.Moloney F, Kavanagh RG, Ronan NJ, et al. Ultra-low-dose thoracic CT with model-based iterative reconstruction (MBIR) in cystic fibrosis patients undergoing treatment with cystic fibrosis transmembrane conductance regulators (CFTR). Clin Radiol 2021; 76: 393.e9–393.e17. doi: 10.1016/j.crad.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 36.Ronan NJ, Einarsson GG, Twomey M, et al. CORK study in cystic fibrosis: sustained improvements in ultra-low-dose chest CT scores after CFTR modulation with ivacaftor. Chest 2018; 153: 395–403. doi: 10.1016/j.chest.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 37.Baez JC, Seethamraju RT, Mulkern R, et al. Pediatric chest MR imaging: sedation, techniques, and extracardiac vessels. Magn Reson Imaging Clin N Am 2015; 23: 321–335. doi: 10.1016/j.mric.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 38.Deen J, Vandevivere Y, Van de Putte P. Challenges in the anesthetic management of ambulatory patients in the MRI suites. Curr Opin Anaesthesiol 2017; 30: 670–675. doi: 10.1097/ACO.0000000000000513 [DOI] [PubMed] [Google Scholar]
- 39.Liszewski MC, Ciet P, Lee EY. MR imaging of lungs and airways in children: past and present. Magn Reson Imaging Clin N Am 2019; 27: 201–225. doi: 10.1016/j.mric.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 40.de Jong PA, Mayo JR, Golmohammadi K, et al. Estimation of cancer mortality associated with repetitive computed tomography scanning. Am J Respir Crit Care Med 2006; 173: 199–203. doi: 10.1164/rccm.200505-810OC [DOI] [PubMed] [Google Scholar]
- 41.Brenner DJ, Hall EJ. Computed tomography an increasing source of radiation exposure. N Engl J Med 2007; 357: 2277–2284. doi: 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 42.Huda W. Radiation doses and risks in chest computed tomography examinations. Proc Am Thorac Soc 2007; 4: 316–320. doi: 10.1513/pats.200611-172HT [DOI] [PubMed] [Google Scholar]
- 43.O'Connell OJ, McWilliams S, McGarrigle A, et al. Radiologic imaging in cystic fibrosis: cumulative effective dose and changing trends over 2 decades. Chest 2012; 141: 1575–1583. doi: 10.1378/chest.11-1972 [DOI] [PubMed] [Google Scholar]
- 44.Bayfield K, Weinheimer O, Boyton C, et al. Late Breaking Abstract - Use of ultra-low dose CT does not impact structure-function relationships in early cystic fibrosis lung disease. Eur Respir J 2020; 56: Suppl. 64, 4310. doi: 10.1183/13993003.congress-2020.4310 [DOI] [Google Scholar]
- 45.Peixoto AO, Marson F AL, Dertkigil SS, et al. The use of ultrasound as a tool to evaluate pulmonary disease in cystic fibrosis. Respir Care 2020; 65: 293–303. doi: 10.4187/respcare.07038 [DOI] [PubMed] [Google Scholar]
- 46.Strzelczuk–Judka L, Wojsyk–Banaszak I, Zakrzewska A, et al. Diagnostic value of chest ultrasound in children with cystic fibrosis – Pilot study. PLoS ONE 2019; 14: e0215786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benden C. The Chrispin–Norman score in cystic fibrosis: doing away with the lateral view. Eur Respir J 2005; 26: 894–897. doi: 10.1183/09031936.05.00059105 [DOI] [PubMed] [Google Scholar]
- 48.Bortoluzzi CF, Pontello E, Pintani E, et al. The impact of chest computed tomography and chest radiography on clinical management of cystic fibrosis lung disease. J Cyst Fibros 2020; 19: 641–646. doi: 10.1016/j.jcf.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 49.Lee MZ, Cai W, Song Y, et al. Fully automated scoring of chest radiographs in cystic fibrosis. Annu Int Conf IEEE Eng Med Biol Soc 2013; 2013: 3965–3968. doi: 10.1109/EMBC.2013.6610413 [DOI] [PubMed] [Google Scholar]
- 50.Terheggen-Lagro S, Truijens N, van Poppel N, et al. Correlation of six different cystic fibrosis chest radiograph scoring systems with clinical parameters. Pediatr Pulmonol 2003; 35: 441–445. doi: 10.1002/ppul.10280 [DOI] [PubMed] [Google Scholar]
- 51.de Jong PA, Achterberg JA, Kessels OAM, et al. Modified Chrispin–Norman chest radiography score for cystic fibrosis: observer agreement and correlation with lung function. Eur Radiol 2011; 21: 722–729. doi: 10.1007/s00330-010-1972-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zucker EJ, Barnes ZA, Lungren MP, et al. Deep learning to automate Brasfield chest radiographic scoring for cystic fibrosis. J Cyst Fibros 2020; 19: 131–138. doi: 10.1016/j.jcf.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 53.Brody AS, Molina PL, Klein JS, et al. High-resolution computed tomography of the chest in children with cystic fibrosis: support for use as an outcome surrogate. Pediatr Radiol 1999; 29: 731–735. doi: 10.1007/s002470050684 [DOI] [PubMed] [Google Scholar]
- 54.Cademartiri F, Luccichenti G, Palumbo AA, et al. Predictive value of chest CT in patients with cystic fibrosis: a single-center 10-year experience. Am J Roentgenol 2008; 190: 1475–1480. doi: 10.2214/AJR.07.3000 [DOI] [PubMed] [Google Scholar]
- 55.Robinson TE, Goris ML, Moss RB, et al. Mucus plugging, air trapping, and bronchiectasis are important outcome measures in assessing progressive childhood cystic fibrosis lung disease. Pediatr Pulmonol 2020; 55: 929–938. doi: 10.1002/ppul.24646 [DOI] [PubMed] [Google Scholar]
- 56.Tepper LA, Caudri D, Rovira AP, et al. The development of bronchiectasis on chest computed tomography in children with cystic fibrosis: can pre-stages be identified? Eur Radiol 2016; 26: 4563–4569. doi: 10.1007/s00330-016-4329-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tepper LA, Utens EMWJ, Caudri D, et al. Impact of bronchiectasis and trapped air on quality of life and exacerbations in cystic fibrosis. Eur Respir J 2013; 42: 371–379. doi: 10.1183/09031936.00137612 [DOI] [PubMed] [Google Scholar]
- 58.Kuo W, Andrinopoulou E-R, Perez-Rovira A, et al. Objective airway artery dimensions compared to CT scoring methods assessing structural cystic fibrosis lung disease. J Cyst Fibros 2017; 16: 116–123. doi: 10.1016/j.jcf.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 59.Kuo W, de Bruijne M, Petersen J, et al. Diagnosis of bronchiectasis and airway wall thickening in children with cystic fibrosis: objective airway-artery quantification. Eur Radiol 2017; 27: 4680–4689. doi: 10.1007/s00330-017-4819-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouma NR, Janssens HM, Andrinopoulou E, et al. Airway disease on chest computed tomography of preschool children with cystic fibrosis is associated with schoolage bronchiectasis. Pediatr Pulmonol 2020; 55: 141–148. doi: 10.1002/ppul.24498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brody AS, Klein JS, Molina PL, et al. High-resolution computed tomography in young patients with cystic fibrosis: distribution of abnormalities and correlation with pulmonary function tests. J Pediatr 2004; 145: 32–38. doi: 10.1016/j.jpeds.2004.02.038 [DOI] [PubMed] [Google Scholar]
- 62.Sasihuseyinoglu AS, Altıntaş DU, Soyupak S, et al. Evaluation of high resolution computed tomography findings of cystic fibrosis. Korean J Intern Med 2019; 34: 335–343. doi: 10.3904/kjim.2017.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wijker NE, Vidmar S, Grimwood K, et al. Early markers of cystic fibrosis structural lung disease: follow-up of the ACFBAL cohort. Eur Respir J 2020; 55: 1901694. doi: 10.1183/13993003.01694-2019 [DOI] [PubMed] [Google Scholar]
- 64.de Jong PA, Lindblad A, Rubin L, et al. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax 2006; 61: 80–85. doi: 10.1136/thx.2005.045146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loeve M, van Hal PTW, Robinson P, et al. The spectrum of structural abnormalities on CT scans from patients with CF with severe advanced lung disease. Thorax 2009; 64: 876–882. doi: 10.1136/thx.2008.110908 [DOI] [PubMed] [Google Scholar]
- 66.Mott LS, Park J, Murray CP, et al. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax 2012; 67: 509–516. doi: 10.1136/thoraxjnl-2011-200912 [DOI] [PubMed] [Google Scholar]
- 67.Gustafsson PM, De Jong PA, Tiddens HAWM, et al. Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax 2008; 63: 129–134. doi: 10.1136/thx.2007.077784 [DOI] [PubMed] [Google Scholar]
- 68.Keen C, Gustafsson P, Lindblad A, et al. Low levels of exhaled nitric oxide are associated with impaired lung function in cystic fibrosis. Pediatr Pulmonol 2010; 45: 241–248. doi: 10.1002/ppul.21137 [DOI] [PubMed] [Google Scholar]
- 69.Ellemunter H, Fuchs SI, Unsinn KM, et al. Sensitivity of lung clearance index and chest computed tomography in early CF lung disease. Respir Med 2010; 104: 1834–1842. doi: 10.1016/j.rmed.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 70.Fuchs SI, Gappa M, Eder J, et al. Tracking lung clearance index and chest CT in mild cystic fibrosis lung disease over a period of three years. Respir Med 2014; 108: 865–874. doi: 10.1016/j.rmed.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 71.Owens CM, Aurora P, Stanojevic S, et al. Lung clearance index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax 2011; 66: 481–488. doi: 10.1136/thx.2010.150375 [DOI] [PubMed] [Google Scholar]
- 72.Ramsey KA, Rosenow T, Turkovic L, et al. Lung clearance index and structural lung disease on computed tomography in early cystic fibrosis. Am J Respir Crit Care Med 2016; 193: 60–67. doi: 10.1164/rccm.201507-1409OC [DOI] [PubMed] [Google Scholar]
- 73.Yammine S, Ramsey KA, Skoric B, et al. Single-breath washout and association with structural lung disease in children with cystic fibrosis. Pediatr Pulmonol 2019; 54: 587–594. doi: 10.1002/ppul.24271 [DOI] [PubMed] [Google Scholar]
- 74.Sandvik RM, Kongstad T, Green K, et al. Prospective longitudinal association between repeated multiple breath washout measurements and computed tomography scores in children with cystic fibrosis. J Cyst Fibros 2021; 20: 632–640. doi: 10.1016/j.jcf.2020.09.010 [DOI] [PubMed] [Google Scholar]
- 75.Refait J, Macey J, Bui S, et al. CT evaluation of hyperattenuating mucus to diagnose allergic bronchopulmonary aspergillosis in the special condition of cystic fibrosis. J Cyst Fibros 2019; 18: e31–e36. doi: 10.1016/j.jcf.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 76.Turkovic L, Caudri D, Rosenow T, et al. Structural determinants of long-term functional outcomes in young children with cystic fibrosis. Eur Respir J 2020; 55: 1900748. doi: 10.1183/13993003.00748-2019 [DOI] [PubMed] [Google Scholar]
- 77.Konietzke P, Weinheimer O, Wielpütz MO, et al. Validation of automated lobe segmentation on paired inspiratory-expiratory chest CT in 8–14 year-old children with cystic fibrosis. PLoS One 2018; 13: e0194557. doi: 10.1371/journal.pone.0194557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weinheimer O, Hoff BA, Fortuna AB, et al. Influence of inspiratory/expiratory CT registration on quantitative air trapping. Acad Radiol 2019; 26: 1202–1214. doi: 10.1016/j.acra.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chassagnon G, Martin C, Burgel P-R, et al. An automated computed tomography score for the cystic fibrosis lung. Eur Radiol 2018; 28: 5111–5120. doi: 10.1007/s00330-018-5516-x [DOI] [PubMed] [Google Scholar]
- 80.Ram S, Hoff BA, Bell AJ, et al. Improved detection of air trapping on expiratory computed tomography using deep learning. PLoS One 2021; 16: e0248902. doi: 10.1371/journal.pone.0248902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dournes G, Hall CS, Willmering MM, et al. Artificial intelligence in CT for quantifying lung changes in the era of CFTR modulators. Eur Respir J 2021; in press [ 10.1183/13993003.00844-2021] [DOI] [PubMed] [Google Scholar]
- 82.Rosenow T, Oudraad MCJ, Murray CP, et al. PRAGMA-CF. A quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med 2015; 191: 1158–1165. doi: 10.1164/rccm.201501-0061OC [DOI] [PubMed] [Google Scholar]
- 83.Zorzo C, Caballero P, Diab L, et al. Predictive value of computed tomography scoring systems evolution in adults with cystic fibrosis. Eur Radiol 2020; 30: 3634–3640. doi: 10.1007/s00330-020-06759-z [DOI] [PubMed] [Google Scholar]
- 84.Goris ML, Zhu HJ, Blankenberg F, et al. An automated approach to quantitative air trapping measurements in mild cystic fibrosis. Chest 2003; 123: 1655–1663. doi: 10.1378/chest.123.5.1655 [DOI] [PubMed] [Google Scholar]
- 85.Robinson TE, Goris ML, Zhu HJ, et al. Dornase alfa reduces air trapping in children with mild cystic fibrosis lung disease: a quantitative analysis. Chest 2005; 128: 2327–2335. doi: 10.1378/chest.128.4.2327 [DOI] [PubMed] [Google Scholar]
- 86.Loeve M, Rosenow T, Gorbunova V, et al. Reversibility of trapped air on chest computed tomography in cystic fibrosis patients. Eur J Radiol 2015; 84: 1184–1190. doi: 10.1016/j.ejrad.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 87.Brody AS, Kosorok MR, Li Z, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging 2006; 21: 14–21. doi: 10.1097/01.rti.0000203937.82276.ce [DOI] [PubMed] [Google Scholar]
- 88.Sanders DB, Li Z, Brody AS, et al. Chest computed tomography scores of severity are associated with future lung disease progression in children with cystic fibrosis. Am J Respir Crit Care Med 2011; 184: 816–821. doi: 10.1164/rccm.201105-0816OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brody AS, Sucharew H, Campbell JD, et al. Computed tomography correlates with pulmonary exacerbations in children with cystic fibrosis. Am J Respir Crit Care Med 2005; 172: 1128–1132. doi: 10.1164/rccm.200407-989OC [DOI] [PubMed] [Google Scholar]
- 90.Brody AS. Scoring systems for CT in cystic fibrosis: who cares? Radiology 2004; 231: 296–298. doi: 10.1148/radiol.2312032097 [DOI] [PubMed] [Google Scholar]
- 91.Loeve M, Gerbrands K, Hop WC, et al. Bronchiectasis and pulmonary exacerbations in children and young adults with cystic fibrosis. Chest 2011; 140: 178–185. doi: 10.1378/chest.10-1152 [DOI] [PubMed] [Google Scholar]
- 92.Tiddens HAWM, Andrinopoulou E-R, McIntosh J, et al. Chest computed tomography outcomes in a randomized clinical trial in cystic fibrosis: lessons learned from the first ataluren phase 3 study. PLoS ONE 2020; 15: e0240898. doi: 10.1371/journal.pone.0240898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhalla M, Turcios N, Aponte V, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology 1991; 179: 783–788. doi: 10.1148/radiology.179.3.2027992 [DOI] [PubMed] [Google Scholar]
- 94.Wielpütz MO, Eichinger M, Weinheimer O, et al. Automatic airway analysis on multidetector computed tomography in cystic fibrosis: correlation with pulmonary function testing. J Thorac Imaging 2013; 28: 104–113. doi: 10.1097/RTI.0b013e3182765785 [DOI] [PubMed] [Google Scholar]
- 95.Bortoluzzi C-F, Volpi S, D'Orazio C, et al. Bronchiectases at early chest computed tomography in children with cystic fibrosis are associated with increased risk of subsequent pulmonary exacerbations and chronic Pseudomonas infection. J Cyst Fibros 2014; 13: 564–571. doi: 10.1016/j.jcf.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 96.Judge EP, Dodd JD, Masterson JB, et al. Pulmonary abnormalities on high-resolution CT demonstrate more rapid decline than FEV1 in adults with cystic fibrosis. Chest 2006; 130: 1424–1432. doi: 10.1378/chest.130.5.1424 [DOI] [PubMed] [Google Scholar]
- 97.Loeve M, Lequin MH, de Bruijne M, et al. Cystic fibrosis: are volumetric ultra-low-dose expiratory CT scans sufficient for monitoring related lung disease? Radiology 2009; 253: 223–229. doi: 10.1148/radiol.2532090306 [DOI] [PubMed] [Google Scholar]
- 98.Nasr SZ, Gordon D, Sakmar E, et al. High resolution computerized tomography of the chest and pulmonary function testing in evaluating the effect of tobramycin solution for inhalation in cystic fibrosis patients. Pediatr Pulmonol 2006; 41: 1129–1137. doi: 10.1002/ppul.20447 [DOI] [PubMed] [Google Scholar]
- 99.O'Connor OJ, Vandeleur M, McGarrigle AM, et al. Development of low-dose protocols for thin-section CT assessment of cystic fibrosis in pediatric patients. Radiology 2010; 257: 820–829. doi: 10.1148/radiol.10100278 [DOI] [PubMed] [Google Scholar]
- 100.van Straten M, Brody AS, Ernst C, et al. Guidance for computed tomography (CT) imaging of the lungs for patients with cystic fibrosis (CF) in research studies. J Cyst Fibros 2020; 19: 176–183. doi: 10.1016/j.jcf.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 101.Sheikh SI, Long FR, McCoy KS, et al. Computed tomography correlates with improvement with ivacaftor in cystic fibrosis patients with G551D mutation. J Cyst Fibros 2015; 14: 84–89. doi: 10.1016/j.jcf.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 102.Stick S, Tiddens H, Aurora P, et al. Early intervention studies in infants and preschool children with cystic fibrosis: are we ready? Eur Respir J 2013; 42: 527–538. doi: 10.1183/09031936.00108212 [DOI] [PubMed] [Google Scholar]
- 103.Graeber SY, Boutin S, Wielpütz MO, et al. Effects of lumacaftor–ivacaftor on lung clearance index, magnetic resonance imaging, and airway microbiome in Phe508del homozygous patients with cystic fibrosis. Ann Am Thorac Soc 2021; 18: 971–980. doi: 10.1513/AnnalsATS.202008-1054OC [DOI] [PubMed] [Google Scholar]
- 104.Muston HN, Perrem L, Davis MD, et al. The remaining barriers to normalcy in CF: Advances in assessment of CF lung disease. Pediatr Pulmonol 2021; 56: S90–S96. doi: 10.1002/ppul.24929 [DOI] [PubMed] [Google Scholar]
- 105.Barry PJ, Taylor-Cousar JL. Triple combination cystic fibrosis transmembrane conductance regulator modulator therapy in the real world – opportunities and challenges. Curr Opin Pulm Med 2021; 27: 554–566. doi: 10.1097/MCP.0000000000000819 [DOI] [PubMed] [Google Scholar]
- 106.Roach DJ, Crémillieux Y, Fleck RJ, et al. Ultrashort echo-time magnetic resonance imaging is a sensitive method for the evaluation of early cystic fibrosis lung disease. Ann Am Thorac Soc 2016; 13: 1923–1931. doi: 10.1513/AnnalsATS.201603-203OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dournes G, Menut F, Macey J, et al. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol 2016; 26: 3811–3820. doi: 10.1007/s00330-016-4218-5 [DOI] [PubMed] [Google Scholar]
- 108.Dournes G, Grodzki D, Macey J, et al. Quiet submillimeter MR imaging of the lung is feasible with a PETRA sequence at 1.5 T. Radiology 2015; 276: 258–265. doi: 10.1148/radiol.15141655 [DOI] [PubMed] [Google Scholar]
- 109.Ciet P, Serra G, Bertolo S, et al. Assessment of CF lung disease using motion corrected PROPELLER MRI: a comparison with CT. Eur Radiol 2016; 26: 780–787. doi: 10.1007/s00330-015-3850-9 [DOI] [PubMed] [Google Scholar]
- 110.Puderbach M, Eichinger M, Gahr J, et al. Proton MRI appearance of cystic fibrosis: comparison to CT. Eur Radiol 2007; 17: 716–724. doi: 10.1007/s00330-006-0373-4 [DOI] [PubMed] [Google Scholar]
- 111.Puderbach M, Eichinger M, Haeselbarth J, et al. Assessment of morphological MRI for pulmonary changes in cystic fibrosis (CF) patients: comparison to thinsection CT and chest xray. Invest Radiol 2007; 42: 715–724. doi: 10.1097/RLI.0b013e318074fd81 [DOI] [PubMed] [Google Scholar]
- 112.Eichinger M, Tetzlaff R, Puderbach M, et al. Proton magnetic resonance imaging for assessment of lung function and respiratory dynamics. Eur J Radiol 2007; 64: 329–334. doi: 10.1016/j.ejrad.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 113.Wielpütz MO, Eichinger M, Puderbach M. Magnetic resonance imaging of cystic fibrosis lung disease. J Thorac Imaging 2013; 28: 151–159. doi: 10.1097/RTI.0b013e31828d40d4 [DOI] [PubMed] [Google Scholar]
- 114.Teufel M, Ketelsen D, Fleischer S, et al. Comparison between high-resolution CT and MRI using a very short echo time in patients with cystic fibrosis with extra focus on mosaic attenuation. Respiration 2013; 86: 302–311. doi: 10.1159/000343085 [DOI] [PubMed] [Google Scholar]
- 115.Eichinger M, Optazaite D-E, Kopp-Schneider A, et al. Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol 2012; 81: 1321–1329. doi: 10.1016/j.ejrad.2011.02.045 [DOI] [PubMed] [Google Scholar]
- 116.Tepper LA, Ciet P, Caudri D, et al. Validating chest MRI to detect and monitor cystic fibrosis lung disease in a pediatric cohort. Pediatr Pulmonol 2016; 51: 34–41. doi: 10.1002/ppul.23328 [DOI] [PubMed] [Google Scholar]
- 117.Scholz O, Denecke T, Böttcher J, et al. MRI of cystic fibrosis lung manifestations: sequence evaluation and clinical outcome analysis. Clin Radiol 2017; 72: 754–763. doi: 10.1016/j.crad.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 118.Renz DM, Scholz O, Böttcher J, et al. Comparison between magnetic resonance imaging and computed tomography of the lung in patients with cystic fibrosis with regard to clinical, laboratory, and pulmonary functional parameters. Invest Radiol 2015; 50: 733–742. doi: 10.1097/RLI.0000000000000178 [DOI] [PubMed] [Google Scholar]
- 119.Benlala I, Hocke F, Macey J, et al. Quantification of MRI T2-weighted high signal volume in cystic fibrosis: a pilot study. Radiology 2020; 294: 186–196. doi: 10.1148/radiol.2019190797 [DOI] [PubMed] [Google Scholar]
- 120.Dournes G, Berger P, Refait J, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis: MR imaging of airway mucus contrasts as a tool for diagnosis. Radiology 2017; 285: 261–269. doi: 10.1148/radiol.2017162350 [DOI] [PubMed] [Google Scholar]
- 121.Garg MK, Gupta P, Agarwal R, et al. MRI: a new paradigm in imaging evaluation of allergic bronchopulmonary aspergillosis? Chest 2015; 147: e58–e59. doi: 10.1378/chest.14-2347 [DOI] [PubMed] [Google Scholar]
- 122.Triphan SMF, Stahl M, Jobst BJ, et al. Echo time-dependence of observed lung t1 in patients with cystic fibrosis and correlation with clinical metrics. J Magn Reson Imaging 2020; 52: 1645–1654. doi: 10.1002/jmri.27271 [DOI] [PubMed] [Google Scholar]
- 123.Nyilas S, Bauman G, Sommer G, et al. Novel magnetic resonance technique for functional imaging of cystic fibrosis lung disease. Eur Respir J 2017; 50: 1701464. doi: 10.1183/13993003.01464-2017 [DOI] [PubMed] [Google Scholar]
- 124.Nyilas S, Bauman G, Pusterla O, et al. Ventilation and perfusion assessed by functional MRI in children with CF: reproducibility in comparison to lung function. J Cyst Fibros 2019; 18: 543–550. doi: 10.1016/j.jcf.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 125.Pennati F, Roach DJ, Clancy JP, et al. Assessment of pulmonary structure-function relationships in young children and adolescents with cystic fibrosis by multivolume proton-MRI and CT. J Magn Reson Imaging 2018; 48: 531–542. doi: 10.1002/jmri.25978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pennati F, Salito C, Borzani I, et al. Quantitative multivolume proton-magnetic resonance imaging in patients with cystic fibrosis lung disease: comparison with clinical indicators. Eur Respir J 2019; 53: 1702020. doi: 10.1183/13993003.02020-2017 [DOI] [PubMed] [Google Scholar]
- 127.Leutz-Schmidt P, Stahl M, Sommerburg O, et al. Non-contrast enhanced magnetic resonance imaging detects mosaic signal intensity in early cystic fibrosis lung disease. Eur J Radiol 2018; 101: 178–183. doi: 10.1016/j.ejrad.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 128.Schaefer JF, Hector A, Schmidt K, et al. A semiquantitative MRI-score can predict loss of lung function in patients with cystic fibrosis: preliminary results. Eur Radiol 2018; 28: 74–84. doi: 10.1007/s00330-017-4870-4 [DOI] [PubMed] [Google Scholar]
- 129.Ciet P, Serra G, Andrinopoulou ER, et al. Diffusion weighted imaging in cystic fibrosis disease: beyond morphological imaging. Eur Radiol 2016; 26: 3830–3839. doi: 10.1007/s00330-016-4248-z [DOI] [PubMed] [Google Scholar]
- 130.Ciet P, Bertolo S, Ros M, et al. Detection and monitoring of lung inflammation in cystic fibrosis during respiratory tract exacerbation using diffusion-weighted magnetic resonance imaging. Eur Respir J 2017; 50: 1601437. doi: 10.1183/13993003.01437-2016 [DOI] [PubMed] [Google Scholar]
- 131.Grasemann H, Ciet P, Amin R, et al. Changes in magnetic resonance imaging scores and ventilation inhomogeneity in children with cystic fibrosis pulmonary exacerbations. Eur Respir J 2017; 50: 1700244. doi: 10.1183/13993003.00244-2017 [DOI] [PubMed] [Google Scholar]
- 132.Amaxopoulou C, Gnannt R, Higashigaito K, et al. Structural and perfusion magnetic resonance imaging of the lung in cystic fibrosis. Pediatr Radiol 2018; 48: 165–175. doi: 10.1007/s00247-017-4021-8 [DOI] [PubMed] [Google Scholar]
- 133.Behrendt L, Voskrebenzev A, Klimeš F, et al. Validation of automated perfusion-weighted phase-resolved functional lung (PREFUL)-MRI in patients with pulmonary diseases. J Magn Reson Imaging 2020; 52: 103–114. doi: 10.1002/jmri.27027 [DOI] [PubMed] [Google Scholar]
- 134.Stahl M, Wielpütz MO, Graeber SY, et al. Comparison of lung clearance index and magnetic resonance imaging for assessment of lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 2017; 195: 349–359. [DOI] [PubMed] [Google Scholar]
- 135.Fleischer S, Kraus MS, Gatidis S, et al. New severity assessment in cystic fibrosis: signal intensity and lung volume compared to LCI and FEV1: preliminary results. Eur Radiol 2020; 30: 1350–1358. doi: 10.1007/s00330-019-06462-8 [DOI] [PubMed] [Google Scholar]
- 136.Wielpütz MO, Puderbach M, Kopp-Schneider A, et al. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med 2014; 189: 956–965. doi: 10.1164/rccm.201309-1659OC [DOI] [PubMed] [Google Scholar]
- 137.Stahl M, Joachim C, Wielpütz MO, et al. Comparison of lung clearance index determined by washout of N2 and SF6 in infants and preschool children with cystic fibrosis. J Cyst Fibros 2019; 18: 399–406. doi: 10.1016/j.jcf.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 138.Puderbach M, Eichinger M. The role of advanced imaging techniques in cystic fibrosis follow-up: is there a place for MRI? Pediatr Radiol 2010; 40: 844–849. doi: 10.1007/s00247-010-1589-7 [DOI] [PubMed] [Google Scholar]
- 139.Benlala I, Point S, Leung C, et al. Volumetric quantification of lung MR signal intensities using ultrashort TE as an automated score in cystic fibrosis. Eur Radiol 2020; 30: 5479–5488. doi: 10.1007/s00330-020-06910-w [DOI] [PubMed] [Google Scholar]
- 140.von Steyern KV, Björkman-Burtscher IM, Geijer M. Radiography, tomosynthesis, CT and MRI in the evaluation of pulmonary cystic fibrosis: an untangling review of the multitude of scoring systems. Insights Imaging 2013; 4: 787–798. doi: 10.1007/s13244-013-0288-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sileo C, Corvol H, Boelle P-Y, et al. HRCT and MRI of the lung in children with cystic fibrosis: comparison of different scoring systems. J Cyst Fibros 2014; 13: 198–204. doi: 10.1016/j.jcf.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 142.Verbanck S, Vanderhelst E. The respective roles of lung clearance index and magnetic resonance imaging in the clinical management of patients with cystic fibrosis. Am J Respir Crit Care Med 2018; 197: 409–409. doi: 10.1164/rccm.201706-1137LE [DOI] [PubMed] [Google Scholar]
- 143.Wielpütz MO, Eichinger M, Wege S, et al. Midterm reproducibility of chest magnetic resonance imaging in adults with clinically stable cystic fibrosis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2019; 200: 103–107. doi: 10.1164/rccm.201812-2356LE [DOI] [PubMed] [Google Scholar]
- 144.Wielpütz MO, von Stackelberg O, Stahl M, et al. Multicentre standardisation of chest MRI as radiation-free outcome measure of lung disease in young children with cystic fibrosis. J Cyst Fibros 2018; 17: 518–527. doi: 10.1016/j.jcf.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 145.Triphan SMF, Biederer J, Burmester K, et al. Design and application of an MR reference phantom for multicentre lung imaging trials. PLoS One 2018; 13: e0199148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malviya S, Voepel-Lewis T, Eldevik OP, et al. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth 2000; 84: 743–748. doi: 10.1093/oxfordjournals.bja.a013586 [DOI] [PubMed] [Google Scholar]
- 147.Parad RB. Non-sedation of the neonate for radiologic procedures. Pediatr Radiol 2018; 48: 524–530. doi: 10.1007/s00247-017-4002-y [DOI] [PubMed] [Google Scholar]
- 148.Vinson AE, Houck CS. Neurotoxicity of anesthesia in children: prevention and treatment. Curr Treat Options Neurol 2018; 20: 51. doi: 10.1007/s11940-018-0536-z [DOI] [PubMed] [Google Scholar]
- 149.Greene KE, Takasugi JE, Godwin JD, et al. Radiographic changes in acute exacerbations of cystic fibrosis in adults: a pilot study. AJR Am J Roentgenol 1994; 163: 557–562. doi: 10.2214/ajr.163.3.8079843 [DOI] [PubMed] [Google Scholar]
- 150.Byrnes CA, Vidmar S, Cheney JL, et al. Prospective evaluation of respiratory exacerbations in children with cystic fibrosis from newborn screening to 5 years of age. Thorax 2013; 68: 643–651. doi: 10.1136/thoraxjnl-2012-202342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Davis SD, Fordham LA, Brody AS, et al. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med 2007; 175: 943–950. doi: 10.1164/rccm.200603-343OC [DOI] [PubMed] [Google Scholar]
- 152.Horsley AR, Davies JC, Gray RD, et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax 2013; 68: 532–539. doi: 10.1136/thoraxjnl-2012-202538 [DOI] [PubMed] [Google Scholar]
- 153.Sanders DB, Li Z, Brody AS. Chest computed tomography predicts the frequency of pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc 2015; 12: 64–69. doi: 10.1513/AnnalsATS.201407-338OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shah RM, Sexauer W, Ostrum BJ, et al. High-resolution CT in the acute exacerbation of cystic fibrosis: evaluation of acute findings, reversibility of those findings, and clinical correlation. Am J Roentgenol 1997; 169: 375–380. doi: 10.2214/ajr.169.2.9242738 [DOI] [PubMed] [Google Scholar]
- 155.Rosenow T, Mok LC, Turkovic L, et al. The cumulative effect of inflammation and infection on structural lung disease in early cystic fibrosis. Eur Respir J 2019; 54: 1801771. doi: 10.1183/13993003.01771-2018 [DOI] [PubMed] [Google Scholar]
- 156.Robinson TE, Leung AN, Northway WH, et al. Spirometer-triggered high-resolution computed tomography and pulmonary function measurements during an acute exacerbation in patients with cystic fibrosis. J Pediatr 2001; 138: 553–559. doi: 10.1067/mpd.2001.111820 [DOI] [PubMed] [Google Scholar]
- 157.Hall GL, Logie KM, Parsons F, et al. Air trapping on chest CT is associated with worse ventilation distribution in infants with cystic fibrosis diagnosed following newborn screening. PLoS One 2011; 6: e23932. doi: 10.1371/journal.pone.0023932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ernst CW, Basten IA, Ilsen B, et al. Pulmonary disease in cystic fibrosis: assessment with chest CT at chest radiography dose levels. Radiology 2014; 273: 597–605. doi: 10.1148/radiol.14132201 [DOI] [PubMed] [Google Scholar]
- 159.Martini K, Gygax CM, Benden C, et al. Volumetric dynamic oxygen-enhanced MRI (OE-MRI): comparison with CT Brody score and lung function in cystic fibrosis patients. Eur Radiol 2018; 28: 4037–4047. doi: 10.1007/s00330-018-5383-5 [DOI] [PubMed] [Google Scholar]
- 160.Sheahan KP, Glynn D, Joyce S, et al. Best practices: imaging strategies for reduced-dose chest CT in the management of cystic fibrosis–related lung disease. Am J Roentgenol 2021: 217; 304–313. doi: 10.2214/AJR.19.22694 [DOI] [PubMed] [Google Scholar]
- 161.Willemink MJ, Persson M, Pourmorteza A, et al. Photon-counting CT: technical principles and clinical prospects. Radiology 2018; 289: 293–312. doi: 10.1148/radiol.2018172656 [DOI] [PubMed] [Google Scholar]
- 162.Newman B, Krane EJ, Gawande R, et al. Chest CT in children: anesthesia and atelectasis. Pediatr Radiol 2014; 44: 164–172. doi: 10.1007/s00247-013-2800-4 [DOI] [PubMed] [Google Scholar]
- 163.Conway S, Balfour-Lynn IM, De Rijcke K, et al. European Cystic Fibrosis Society standards of care: framework for the cystic fibrosis centre. J Cyst Fibros 2014; 13: Suppl 1, S3–S22. doi: 10.1016/j.jcf.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kerem E, Reisman J, Corey M, et al. Prediction of mortality in patients with cystic fibrosis. N Engl J Med 1992; 326: 1187–1191. doi: 10.1056/NEJM199204303261804 [DOI] [PubMed] [Google Scholar]
- 165.Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med 2011; 183: 1463–1471. doi: 10.1164/rccm.201009-1478CI [DOI] [PubMed] [Google Scholar]
- 166.Willmering MM, Roach DJ, Kramer EL, et al. Sensitive structural and functional measurements and 1-year pulmonary outcomes in pediatric cystic fibrosis. J Cyst Fibros 2021; 20: 533–539. doi: 10.1016/j.jcf.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Crowley C, Connor OJO, Ciet P, et al. The evolving role of radiological imaging in cystic fibrosis. Curr Opin Pulm Med 2021; 27: 575–585. doi: 10.1097/MCP.0000000000000828 [DOI] [PubMed] [Google Scholar]
- 168.Lin S, Lin M, Lau KK. Image quality comparison between model-based iterative reconstruction and adaptive statistical iterative reconstruction chest computed tomography in cystic fibrosis patients. J Med Imaging Radiat Oncol 2019; 63: 602–609. doi: 10.1111/1754-9485.12895 [DOI] [PubMed] [Google Scholar]
- 169.Ramsey KA, McGirr C, Stick SM, et al. Effect of posture on lung ventilation distribution and associations with structure in children with cystic fibrosis. J Cyst Fibros 2017; 16: 713–718. doi: 10.1016/j.jcf.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 170.Verbanck S, King GG, Zhou W, et al. The quantitative link of lung clearance index to bronchial segments affected by bronchiectasis. Thorax 2018; 73: 82–84. doi: 10.1136/thoraxjnl-2017-210496 [DOI] [PubMed] [Google Scholar]
- 171.Rosenow T, Ramsey K, Turkovic L, et al. Air trapping in early cystic fibrosis lung disease-Does CT tell the full story? Pediatr Pulmonol 2017; 52: 1150–1156. doi: 10.1002/ppul.23754 [DOI] [PubMed] [Google Scholar]
- 172.Cohen-Cymberknoh M, Ben Meir E, Gartner S, et al. How abnormal is the normal? Clinical characteristics of CF patients with normal FEV1. Pediatr Pulmonol 2021; 56: 2007–2013. doi: 10.1002/ppul.25371 [DOI] [PubMed] [Google Scholar]
- 173.Rowan SA, Bradley JM, Bradbury I, et al. Lung clearance index is a repeatable and sensitive indicator of radiological changes in bronchiectasis. Am J Respir Crit Care Med 2014; 189: 586–592. doi: 10.1164/rccm.201310-1747OC [DOI] [PubMed] [Google Scholar]
- 174.Davies G, Thia LP, Stocks J, et al. Minimal change in structural, functional and inflammatory markers of lung disease in newborn screened infants with cystic fibrosis at one year. J Cyst Fibros 2020; 19: 896–901. doi: 10.1016/j.jcf.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 175.Belessis Y, Dixon B, Hawkins G, et al. Early cystic fibrosis lung disease detected by bronchoalveolar lavage and lung clearance index. Am J Respir Crit Care Med 2012; 185: 862–873. doi: 10.1164/rccm.201109-1631OC [DOI] [PubMed] [Google Scholar]
- 176.Lundström CF, Gilmore HL, Ros PR. Integrated diagnostics: the computational revolution catalyzing cross-disciplinary practices in radiology, pathology, and genomics. Radiology 2017; 285: 12–15. doi: 10.1148/radiol.2017170062 [DOI] [PubMed] [Google Scholar]
- 177.Perez-Rovira A, Kuo W, Petersen J, et al. Automatic airway-artery analysis on lung CT to quantify airway wall thickening and bronchiectasis. Med Phys 2016; 43: 5736–5744. doi: 10.1118/1.4963214 [DOI] [PubMed] [Google Scholar]
- 178.Schalekamp S, Klein WM, van Leeuwen KG. Current and emerging artificial intelligence applications in chest imaging: a pediatric perspective. Pediatr Radiol 2022; in press [ 10.1007/s00247-021-05146-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Glandorf J, Klimeš F, Behrendt L, et al. Perfusion quantification using voxel-wise proton density and median signal decay in PREFUL MRI. Magn Reson Med 2021; 86: 1482–1493. doi: 10.1002/mrm.28787 [DOI] [PubMed] [Google Scholar]
- 180.Tiddens HAWM, Stick SM, Wild JM, et al. Respiratory tract exacerbations revisited: ventilation, inflammation, perfusion, and structure (VIPS) monitoring to redefine treatment. Pediatr Pulmonol 2015; 50: Suppl 40, S57–S65. doi: 10.1002/ppul.23266 [DOI] [PubMed] [Google Scholar]
- 181.Marshall H, Horsley A, Taylor CJ, et al. Detection of early subclinical lung disease in children with cystic fibrosis by lung ventilation imaging with hyperpolarised gas MRI. Thorax 2017; 72: 760–762. doi: 10.1136/thoraxjnl-2016-208948 [DOI] [PubMed] [Google Scholar]
- 182.Dong S, Zhu M, Bulas D. Techniques for minimizing sedation in pediatric MRI. J Magn Reson Imaging 2019; 50: 1047–1054. doi: 10.1002/jmri.26703 [DOI] [PubMed] [Google Scholar]
- 183.Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 2019; 293: 384–393. doi: 10.1148/radiol.2019190452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Campbell-Washburn AE, Malayeri AA, Jones EC, et al. T2-weighted lung imaging using a 0.55-T MRI system. Radiol Cardiothorac Imaging 2021; 3: e200611. doi: 10.1148/ryct.2021200611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sanders DB, Bittner RCL, Rosenfeld M, et al. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol 2011; 46: 393–400. doi: 10.1002/ppul.21374 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0173-2021.SUPPLEMENT (569.7KB, pdf)