Abstract
Primary ciliary dyskinesia is a genetic disease of ciliary function leading to chronic upper and lower respiratory tract symptoms. The diagnosis is frequently overlooked because the symptoms are nonspecific and the knowledge about the disease in the primary care setting is poor. Additionally, none of the available tests is accurate enough to be used in isolation. These tests are expensive, and need sophisticated equipment and expertise to analyse and interpret results; diagnosis is therefore only available at highly specialised centres. The diagnosis is particularly challenging in countries with limited resources due to the lack of such costly equipment and expertise.
In this review, we discuss the importance of early and accurate diagnosis especially for countries where the disease is clinically prevalent but diagnostic tests are lacking. We review the diagnostic tests available in specialised centres (nasal nitric oxide, high-speed video microscopy, transmission electron microscopy, immunofluorescence and genetics). We then consider modifications that might be considered in less well-resourced countries whilst maintaining acceptable accuracy.
Short abstract
PCD diagnosis with limited resources http://ow.ly/eYN7302Y2wg
Introduction
Primary ciliary dyskinesia (PCD) is a genetic disease that causes abnormalities in ciliary function leading to impaired mucociliary clearance. The clinical features usually start with neonatal respiratory distress despite term gestation, with early-onset persistent rhinosinusitis, serous otitis media with hearing impairment and persistent daily wet cough [1, 2]. Laterality disorders (e.g. situs inversus) occur in about 50% of cases [3]. Approximately 50% of males with PCD are believed to be infertile due to dyskinetic sperm [4]. However, there is little evidence of the rates of female infertility or ectopic pregnancy risk.
PCD is rare, with an estimated prevalence of one in 10–40 000 [5]. This is likely to be an underestimate since PCD is often underdiagnosed due to poor knowledge of the disease, the lack of diagnostic facilities and the fact that symptoms are commonly mistaken for common respiratory infections in otherwise healthy children. Indeed, diagnosed cases of PCD have previously been reported to cluster around specialist centres [6]. In a European survey, the median age at diagnosis was 5.3 years and late diagnosis strongly correlated with general government expenditures on health [5]. The predominant mode of inheritance is autosomal recessive, which makes it more common in populations with high rates of consanguineous marriages [7, 8]. For example, a significantly higher prevalence (one in 2265) has been reported in a British Asian population where consanguineous marriages are common [8]. It is likely that PCD is even more underdiagnosed and diagnosed later in countries with limited resources.
In addition to the factors above, the social stigma that accompanies the diagnosis of a chronic illness or a genetic disease in some communities is a barrier to engagement with health professionals. Chronic productive cough maybe considered socially unacceptable and from the authors' experience, some patients, especially young females, attempt to hide their symptoms and suppress their cough. Associations with infertility add to the stigma.
The diagnosis of PCD remains challenging since none of the available tests can be used as a stand-alone test, and all require expensive equipment and highly trained technical staff. Although an international consensus statement [9] provides guidance on which tests to conduct, there is no standardised algorithm for what constitutes a diagnosis, and no international standards for conduct and report of the tests. Most specialised centres follow “local” diagnostic algorithms, which differ in the combination of five tests (nasal nitric oxide (nNO), high-speed video microscopy (HSVM), transmission electron microscopy (TEM), immunofluorescence (IF) and genetics) [10]. For example, in the UK, diagnosis is based on consistent clinical history plus at least two abnormal tests (TEM, HSVM and low nNO; repeating nNO and HSVM if TEM is normal) [11] whilst in North America, genetic testing is more prominent [12, 13] and in some centres, HSVM is only suggested as a research tool [14].
The aim of this review is to briefly discuss the diagnostic tests available in specialised centres. We then consider modifications that might be utilised in less resourced countries at a cheaper cost whilst maintaining acceptable accuracy for PCD diagnosis.
Why is an accurate diagnosis necessary?
Early and accurate diagnosis of PCD is crucial because manifestations start early [15]. Impaired lung function [16] and radiological evidence of bronchiectasis can already be evident in infancy [17]. Although there has been a perception that PCD has a good prognosis with a normal life expectancy [18, 19], this may not always be the case because PCD is progressive and can lead to respiratory failure, and need for lobectomy [20, 21] or lung transplantation [22, 23]. Progressive decline in lung function occurs [20, 24, 25] but appropriate treatments, if instituted early, may help to stabilise lung function [26]. Appropriate early management may reduce recurrent infections, lung damage and progression of bronchiectasis, which is almost inevitable by adulthood [20].
PCD can also cause significant growth impairment during childhood, and it is likely that careful monitoring and intervention will improve growth and general health [27, 28]. Failure to correctly diagnose patients may lead to unnecessary ear, nose and throat (ENT) surgeries, since management of serous otitis media and rhinosinusitis differs from other causes. In PCD, conservative management is often advocated, since hearing impairment resolves spontaneously over time [29, 30]. Early use of hearing aids is therefore the preferred management in many countries, although there is no high-quality evidence for this.
There is evidence in cystic fibrosis (CF) that early diagnosis decreases extensive, costly and unnecessary diagnostic and therapeutic interventions [31], and this is likely to be true for PCD. In addition, accurate diagnosis is a prerequisite to participating in international registries, research and clinical trials. PCD is clinically prevalent in countries where diagnostic testing is not readily available, e.g. Palestine. Inclusion of patients from these countries in research would have positive consequences for patients and other stakeholders not only from these regions but globally. It is important for consanguineous populations to receive appropriate genetic counselling based on a correct diagnosis and screening of siblings of a confirmed patient.
Who should be referred for diagnostic testing?
Neonatologists are usually the first to face the problems associated with PCD, or even earlier if situs inversus is noted on antenatal ultrasound. The diagnosis of PCD should be considered in all children with unexplained neonatal respiratory distress, especially if accompanied by atelectasis or rhinitis [15]. Not all children with situs inversus will have PCD but it is recommended to test them if nasal or respiratory symptoms are present [11, 32]. Diagnostic testing should be considered in all children with bronchiectasis if no other cause is found. In fact, it is suggested that if you are considering testing for CF also consider PCD [10]. Paediatricians need a high index of suspicion, should consider referring all infants and older children with chronic wet cough that “does not seem to go away”, and should investigate if this is associated with persistent rhinitis or serous otitis media; all these symptoms could be easily overlooked especially in a busy general paediatric or ENT clinic. Siblings of known cases should all be referred particularly if there are daily upper or lower respiratory tract symptoms.
PCD should be considered in any patient with laterality defects accompanied by respiratory symptoms. It is important for clinicians treating patients with heterotaxy and complex heart disease (e.g. atrial isomerism or transposition of the great arteries) to avoid blaming the cardiac anomaly for a “wet chest” until PCD has been excluded. Males with immotile sperm should be considered for testing if there are other features [9].
Symptoms of PCD are nonspecific and the above indications remain general. PICADAR is a recent validated predictive tool based on clinical characteristics that can help identify appropriate patients to refer for further testing [1]. The score is based on seven simple questions relating to clinical history providing the probability of a positive diagnosis. The score was developed and then externally validated in specialist PCD referral centres in UK, demonstrating good sensitivity and specificity. The score now needs validating and perhaps modifying for use in different populations and settings; in particular, we need to establish the negative and positive predictive values in different settings [33]. It could potentially be very effective for identifying and excluding patients for full testing, particularly if combined with nNO analysis. In resource-limited countries, PICADAR could potentially guide physicians to determine the probability of having PCD, in order not to overwhelm diagnostic resources, especially if they have to be sent to other specialised centres [1].
What are the tests for PCD?
Screening tests
Saccharine test
The saccharine test was historically used as a screening test [18]. However, it is technically difficult to perform and is impossible in young children. It is therefore no longer used as a screening test [9].
Nasal nitric oxide
nNO is the screening test of choice [9, 34, 35]. NO is produced throughout the airways but is more abundant in the paranasal sinuses [36, 37]. It is low in patients with PCD (10–15% of normal values) [38] but this is not diagnostic since low levels have been reported in other conditions [9, 34, 39]. Rarely, normal or high nNO has been reported in patients with PCD [40, 41]. Therefore, nNO is only appropriate as an adjunct to definitive tests. nNO should not be used for general population screening and should only be used in patients with relevant symptoms to avoid excessive false-positive results [42].
American Thoracic Society/European Respiratory Society (ERS) guidelines [43] recommend sampling by aspiration of air from one nostril through a catheter connected to a stationary chemiluminescence NO analyser as the “gold standard”. To limit contamination with lower airway gases, velum closure should be achieved, for example, by oral exhalation against resistance. The nNO level is considered technically accurate when a real-time display of NO confirms a plateau of at least 3 s [43].
Many details to ensure standardisation of nNO testing and reporting are lacking. There is limited evidence to guide measurement in infants and preschool children, and no age-related cut-off values, although ideally, this is the targeted age for early diagnosis. In a study from North America, the procedure of nNO measurement was standardised and applied in seven centres. They defined a cut-off of 77 nL·min−1, which had a sensitivity of 98% and specificity of 99.9% [44]. An ERS task force recommended that centres should generate their own reference values due to the variable analysers and methods used at different centres [9].
Countries with limited resources face many challenges. Firstly, the high cost of the gold standard chemiluminescence analysers and consumables, and the need for trained physiologists to take measurements make this test unattainable. Chemiluminescence nNO analysers are not usually available in nonspecialist centres and are not easily transportable. In some countries, alternative and cheaper portable analysers using electrochemical detection are in use [45–48] with small studies reporting 100% sensitivity for nNO detection of PCD [45, 47]. Limitations of these analysers include the inability to show the data in real time so that the operator cannot ensure a plateau has been achieved. Additionally, these analysers usually require a longer breath-hold time than chemiluminescence devices [45]. However, considering the cost, they provide an alternative option for resource-limited centres. In addition, being portable makes electrochemical devices easier to use in “outreach clinics”, enabling physicians to screen patients who are geographically distant and have difficult access to big centres (table 1).
TABLE 1.
Comparison between stationary and portable nasal nitric oxide analysers
| Stationary analyser | Portable analyser |
| Gold standard | Not gold standard |
| Chemiluminescence detection | Electrochemical detection |
| Real-time analysis with visual display | End-point analysis with no visual display |
| Measurement taken from plateau of 3 s, enabling quality assurance | No visual display; potential for measurements from technically unacceptable manoeuvres |
| Calibration required | Internal standards apply, no calibration needed |
| Approximate costs | Approximate costs |
| €45 000–50 000 for the device | €3000–4000 for the device |
| €4200 for annual maintenance | Annual maintenance is not required |
| €15 per test (including nasal olive, catheter and filters) | €15 per test (including nasal olive) |
Diagnostic tests
High-speed video microscopy
Ciliary function assessed by ciliary beat pattern (CBP) and ciliary beat frequency (CBF) has the potential to identify almost all cases of PCD. High-resolution HSVM analysis can quantify CBF and allow assessment of CBP [49, 50].
HSVM [51] requires expensive equipment and microscopists with extensive experience of normal and abnormal ciliary function, including more subtle beat pattern abnormalities [52]. There is no global consensus for standardisation of equipment, sampling, processing, or analysis conditions such as temperature, medium, resolution (optical and digital magnification) or video recording and analysis rates in frames per second (fps) [53, 54]. The three English PCD diagnostic centres use shared protocols, discuss difficult cases and perform regular cross-auditing of data [10], and similar approaches could be adopted between countries. CBP analysis by experienced microscopists remains a subjective qualitative method with potential for observer bias [11]. In an attempt to overcome the subjectivity, several groups have developed software to automate analysis [55–57]. There are cases where HSVM is not able to differentiate genetic causes from secondary dyskinesia; for example, patients with MCIDAS and CCNO mutations [58, 59] have reduced generation of motile cilia, which is difficult to distinguish from the deciliation commonly associated with bacterial or viral infections.
Obtaining ciliated nasal epithelial cells using a brush or curette is quick and simple, causing only minor discomfort [11]. Live cilia are digitally imaged using high-magnification objectives coupled to a high-resolution, high-speed digital video camera. Images are recorded at a high frame rate (e.g. 500 fps) to provide enough frames per ciliary stroke for accurate CBF analysis and CBP assessment. The high-speed videos are replayed in slow motion (e.g. 30 fps) to review the ciliary movement [50, 51]. CBF is dependent on temperature. For example, at 37°C, normal cilia beat at 15.4 Hz (sd 2.3, n=554) [51]. If cilia beating at 15 Hz are recorded at 500 fps, 33 frames will capture each ciliary stroke, allowing appropriately detailed analysis (CBF=recording frame rate/average number of frames for 10 ciliary beats×10) [60]. In comparison, a “standard” video camera capable of low light sensitivity and high pixel resolution with a hypothetical recording rate of 30 fps would only provide two frames per ciliary stroke (15 Hz), which would be inappropriate for analysis.
In resource-limited countries, purchasing expensive specialist equipment may be difficult. In addition, the test requires experience to identify not only the immotile/static cilia but also subtle changes that might be difficult to differentiate from secondary dyskinesia. Abnormalities in CBP and CBF can be secondary to infections, inflammation, allergies and pollutants. Damaged or disrupted epithelium during sample collection can cause slower and dyskinetic cilia [61]. Training and support from an established specialist centre is essential before implementing HSVM.
Options in resource-limited countries
The CBF is physiologically reduced at lower temperatures, reportedly with little or no effect on CBP in healthy samples, providing a potential approach for analysis of CBP without the need for expensive HSVM [62]. Smith et al. [62] described a method of a Peltier cooling system by flow of cooled air into the sample chamber. They assessed healthy ciliary function after sample cooling, and demonstrated a sigmoidal relationship between CBF and temperature (CBF decreased between 37°C and 2°C), and no significant difference in the dyskinesia score, suggesting that normal ciliary function was maintained. However, Jackson et al. [63] reported improvement in the beat pattern of a single PCD patient when cooled from 37°C to room temperature and suggested that ciliary function analysis below 37°C may miss occasional cases.
Santamaria et al. [64] made direct observation of ciliary motility using standard light microscopy (LM) and results were compared to TEM. They found this method was not reliable for PCD diagnosis, with only 83% agreement between LM and TEM, but given the somewhat reasonable sensitivity of 92% and specificity of 80%, it could be considered in less-resourced centres where cases would otherwise be undiagnosed.
Centres that use any of the above methods should be aware of their limitations. Visualisation of immotile cilia or obviously abnormal pattern by these methods might be indicative of PCD when combined with a typical clinical picture, but it may not be possible to discern the more subtle ciliary function abnormalities in PCD reported in the literature [52].
Transmission electron microscopy
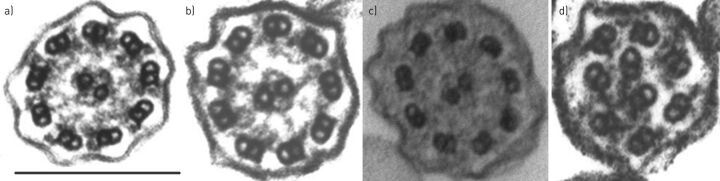
TEM showing “hallmark” defects in ciliary ultrastructure was previously considered the gold-standard test but a number of publications have demonstrated that ciliary ultrastructure is normal (figure 1a) in approximately 20% of patients with PCD [51, 65–67].
FIGURE 1.
Transmission electron micrographs of human airway cilia in transverse section of a) normal subjects, and examples of hallmark defects depicting b) the absence of outer dynein arms, c) the combined absence of inner and outer dynein arms, and d) inner arm absence combined with microtubular disarrangement. Scale bar=200 nm.
The process of preparing ciliated epithelial cells for testing by TEM is lengthy. Samples are immersed immediately after collection in 3% glutaraldehyde fixative and processed to resin blocks. Ultrathin sections are cut, placed on copper grids and stained with heavy metals to enhance contrast. Cilia are observed at high magnification (>60 000×) in transverse and longitudinal section, and the number of defects in microtubular arrangement and the dynein arms are counted. There is no global consensus to standardise the analysis of the cilia. In the UK, quantitative analysis of 100–300 cilia is usual [51, 67], while others analyse at least 30 ciliary cross-sections containing >60 high-quality cilia images [12].
The hallmark ultrastructural defects in PCD include the absence of outer dynein arms (ODAs) (figure 1b), combined absence of inner dynein arms (IDAs) and ODAs (figure 1c), and IDA absence combined with microtubular disarrangement (figure 1d). Other abnormalities, such as isolated IDA defects and central microtubule defects, are controversial since they are also associated with secondary damage to the ciliary ultrastructure [67]. Defects in radial spoke head proteins (RSPH1, RSPH3, RSPH4A and RSPH9), nexin proteins (GAS8) and the central apparatus protein hydin are frequently reported with normal ultrastructure [68], although subtle abnormalities might be detected. Moreover, it is sometimes difficult to determine the presence or absence of dynein arms in transverse sections since they are intermittent structures repeated at intervals along the length of ciliary axoneme. IDAs are particularly difficult to visualise because they occur less frequently along the axoneme and defects can be secondary; some IDA defects seen in unhealthy samples have not persisted on repeat testing [69].
Electron microscopic tomography [70] and cryo-electron tomography [68] may identify some of the defects not resolved by conventional TEM but are extremely specialised and not widely available.
Many challenges make TEM difficult for resource-limited countries. The electron microscope is very expensive, the process is time consuming and the reagents may be difficult to obtain or import. TEM requires appropriate sample fixation and is technically capricious, requiring staff with experience of abnormalities associated with primary and secondary defects [71]. Even for developed countries, TEM is limited if samples are inadequate due to poor health, yield or fixation, demonstrating the need for appropriate patient referral, good sampling technique and knowledge of processing.
Options in resource-limited countries
TEM should only be done by highly specialised PCD diagnostic centres with expertise to distinguish between primary and secondary defects. Centres where TEM is not available should consider collaborating with a PCD service with electron microscopy capacity. An advantage of TEM is that samples in fixative or resin blocks may be sent by land or air mail to specialist centres.
Immunofluorescence
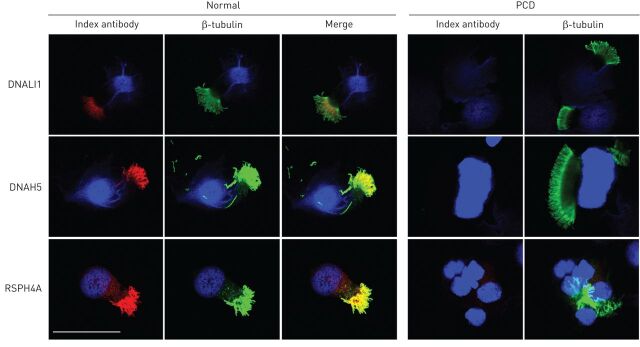
Labelling of ciliary proteins using IF-linked antibodies was developed to understand the downstream effects of genetic mutations in patients with PCD. The potential to use IF diagnostically is attractive and a number of centres are exploring its role as a clinical investigation. Absence or mislocalisation of protein labelling can be indicative of PCD (e.g. ODA protein DNAH5 is mislocalised in PCD-positive patients with ODA defects by TEM) [72, 73]. Radial spoke head protein mutations are associated with subtle abnormalities or normal ultrastructure when observed by TEM, whilst absence of protein can be confirmed by IF [52]. IF may hold further advantages: IDAs that are difficult to observe by TEM are clearly identifiable by IF; and turnaround time for processing and analysis is more rapid than TEM.
IF is used to localise proteins along the ciliary axoneme. Ciliated cells in suspension are dropped onto glass slides and air-dried before incubation with antibodies to the proteins of interest (e.g. DNAH5 to identify ODAs) [72]. The slides are then viewed under confocal or fluorescent microscopes to determine the presence or absence of the proteins (figure 2).
FIGURE 2.
Fluorescence confocal micrograph images of human ciliated nasal epithelial cells showing presence (normal) or absence (examples here from different primary ciliary dyskinesia (PCD) patients) of ciliary proteins by labelling with antibodies against DNALI1 (inner dynein arm), DNAH5 (outer dynein arm) and RSPH4A (radial spoke head) in red. β-tubulin was used as a consistent cilium marker (green) and cell nuclei were counterstained with DAPI (blue). Scale bar=40 μm.
Options in resource-limited countries
IF is promising for countries with limited resources because, once validated as a diagnostic test, it could provide a less expensive method for determining ciliary protein defects when TEM is not available or limited.
Genetics
The challenge for using genetics as a diagnostic test is that PCD is genetically heterogeneous, with >30 genes able to identify approximately 60% of cases [12]. Furthermore, most of the mutations are isolated. Gene discovery projects are underway to increase the numbers of genes and mutations able to contribute to genetic testing, which will improve sensitivity.
Genetic mutations are usually associated with specific ultrastructural defects; for example, DNAH5 mutations [73] and DNAI1 mutations [74] are causes of ODA defects; CCDC40 and CCDC39 mutations cause axonemal disorganisation and absent inner arms [75]; while HYDIN mutants appear to have normal ultrastructure by standard TEM though defects of the central apparatus may be evident by detailed tomography [76]. Newer genetic techniques (e.g. next-generation sequencing) hold promise for identification of new genes [10].
Genetic testing may, in the future, be a way to screen newborns suspected of PCD to improve diagnosis, or to inform PCD patients or relatives with PCD carrier status to provide informed counselling for family planning.
Despite PCD genetics, it is believed that functional tests will remain necessary in the diagnostic pathway, similar to the need for functional sweat chloride testing in CF, which has remained the primary gold-standard diagnostic test despite the well characterised CF genetics [77].
Options in resource-limited countries
Centres with limited resources could consider participating in international collaborations for genetic research. This will increase the chances of gene discovery since it will include DNA from more diverse populations.
In the future, if population-specific genes are found, centres could potentially develop their own diagnostic kits based on the most common mutations in their population.
Summary
Countries with limited resources are challenged in their diagnosis of PCD due to a lack of confirmatory tests. In such cases, cost-effective alternatives should be considered. Neonatologists, paediatricians and ENT specialists should keep a high index of suspicion for patients. Features that might increase suspicion of PCD include offspring from consanguineous families, with recurrent upper and lower respiratory symptoms since birth, with dextrocardia or situs inversus, or with a positive family history of PCD. However, many patients have normal situs, no family history and are from nonconsanguineous backgrounds. PICADAR is a score based on clinical characteristics, which might help identify appropriate patients for further testing [1]. In countries with no diagnostic testing, PICADAR could potentially be used to estimate the diagnostic likelihood of patients, but the score needs validation in different settings and populations. Portable nNO analysers can be used as an alternative to expensive chemiluminescent analysers, which are usually available only in specialist centres. Evaluation of CBP using basic equipment might be informative if cilia are immotile or have an obviously abnormal pattern in the context of a classical history, but subtle pattern abnormalities can be easily missed. Therefore, in difficult cases, a PCD diagnosis should not be dismissed, and repeat testing and consultation with specialist centres should be performed. In the absence of TEM, diagnostic IF may be helpful in excluding PCD when normal protein labelling is detected. However, this is dependent on the inclusion of a panel of antibodies; otherwise, some cases may be missed. There are currently several international networks and collaborations that aim to improve our understanding of this disease, and develop standardised protocols for diagnosis and management, and initiatives to widen the accessibility of diagnostic tests to include countries with limited resources.
Disclosures
J.S. Lucas ERR-0058-2016_Lucas (1.2MB, pdf)
N. Rumman ERR-0058-2016_Rumman (1.2MB, pdf)
Footnotes
Support statement: N. Rumman is the recipient of an European Respiratory Society Fellowship (STRTF 2014-6816). The National PCD Diagnostic Service at University Hospital Southampton (UHS) is commissioned and funded by NHS England. Research at UHS is supported by AAIR Charity, NIHR Southampton Respiratory Biomedical Research Unit and NIHR Wellcome Trust Clinical Research Facility, Southampton, UK. All authors are participants of COST Action BEAT-PCD: Better Evidence to Advance Therapeutic options for PCD (BM 1407). J.S. Lucas chairs the European Respiratory Society Task Force for the development of a practice guideline for diagnosis of PCD (ERS TF-2014-04) and receives research funding from the European Union's Seventh Framework Programme under EC-GA No. 305404 BESTCILIA: Better Experimental Screening and Treatment for Primary Ciliary Dyskinesia. Funding information for this article has been deposited with the Open Funder Registry.
Conflict of Interest: Disclosures can be found alongside this article at err.ersjournals.com
Provenance: Submitted article, peer reviewed.
References
- 1.Behan L, Dimitrov BD, Kuehni CE, et al. PICADAR: a diagnostic predictive tool for primary ciliary dyskinesia. Eur Respir J 2016; 47: 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis SD, Ferkol TW, Rosenfeld M, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med 2015; 191: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro AJ, Davis SD, Ferkol T, et al. Laterality defects other than situs inversus totalis in primary ciliary dyskinesia: insights into situs ambiguus and heterotaxy. Chest 2014; 146: 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munro NC, Currie DC, Lindsay KS, et al. Fertility in men with primary ciliary dyskinesia presenting with respiratory infection. Thorax 1994; 49: 684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuehni CE, Frischer T, Strippoli MP, et al. Factors influencing age at diagnosis of primary ciliary dyskinesia in European children. Eur Respir J 2010; 36: 1248–1258. [DOI] [PubMed] [Google Scholar]
- 6.Meeks M, Bush A. Primary ciliary dyskinesia (PCD). Pediatr Pulmonol 2000; 29: 307–316. [DOI] [PubMed] [Google Scholar]
- 7.Lie H, Zariwala MA, Helms C, et al. Primary ciliary dyskinesia in Amish communities. J Pediatr 2010; 156: 1023–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Callaghan C, Chetcuti P, Moya E. High prevalence of primary ciliary dyskinesia in a British Asian population. Arch Dis Child 2010; 95: 51–52. [DOI] [PubMed] [Google Scholar]
- 9.Barbato A, Frischer T, Kuehni CE, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J 2009; 34: 1264–1276. [DOI] [PubMed] [Google Scholar]
- 10.Lucas JS, Burgess A, Mitchison HM, et al. Diagnosis and management of primary ciliary dyskinesia. Arch Dis Child 2014; 99: 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas JS, Paff T, Goggin P, et al. Diagnostic methods in primary ciliary dyskinesia. Paediatr Respir Rev 2016; 18: 8–17. [DOI] [PubMed] [Google Scholar]
- 12.Knowles MR, Daniels LA, Davis SD, et al. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med 2013; 188: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leigh MW, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: improving the diagnostic approach. Curr Opin Pediatr 2009; 21: 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horani A, Brody SL, Ferkol TW. Picking up speed: advances in the genetics of primary ciliary dyskinesia. Pediatr Res 2014; 75: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullowney T, Manson D, Kim R, et al. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics 2014; 134: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DE, Pittman JE, Leigh MW, et al. Early lung disease in young children with primary ciliary dyskinesia. Pediatr Pulmonol 2008; 43: 514–516. [DOI] [PubMed] [Google Scholar]
- 17.Jain K, Padley SP, Goldstraw EJ, et al. Primary ciliary dyskinesia in the paediatric population: range and severity of radiological findings in a cohort of patients receiving tertiary care. Clin Radiol 2007; 62: 986–993. [DOI] [PubMed] [Google Scholar]
- 18.Bush A, Cole P, Hariri M, et al. Primary ciliary dyskinesia: diagnosis and standards of care. Eur Respir J 1998; 12: 982–988. [DOI] [PubMed] [Google Scholar]
- 19.Perraudeau M, Scott J, Walport M, et al. Late presentation of Kartagener's syndrome. Consequences of ciliary dysfunction. BMJ 1994; 308: 519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noone PG, Leigh MW, Sannuti A, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 2004; 169: 459–467. [DOI] [PubMed] [Google Scholar]
- 21.Yiallouros PK, Kouis P, Middleton N, et al. Clinical features of primary ciliary dyskinesia in Cyprus with emphasis on lobectomized patients. Respir Med 2015; 109: 347–356. [DOI] [PubMed] [Google Scholar]
- 22.Graeter T, Schäfers HJ, Wahlers T, et al. Lung transplantation in Kartagener's syndrome. J Heart Lung Transplant 1994; 13: 724–726. [PubMed] [Google Scholar]
- 23.Schertler T, Lardinois D, Boehm T, et al. Lung transplantation in Kartagener syndrome and situs inversus: potential of multidetector row computed tomography and three-dimensional postprocessing. J Thorac Cardiovasc Surg 2007; 134: 814–815. [DOI] [PubMed] [Google Scholar]
- 24.Magnin ML, Cros P, Beydon N, et al. Longitudinal lung function and structural changes in children with primary ciliary dyskinesia. Pediatr Pulmonol 2012; 47: 816–825. [DOI] [PubMed] [Google Scholar]
- 25.Marthin JK, Petersen N, Skovgaard LT, et al. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med 2010; 181: 1262–1268. [DOI] [PubMed] [Google Scholar]
- 26.Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Respir J 1997; 10: 2376–2379. [DOI] [PubMed] [Google Scholar]
- 27.Cohen-Cymberknoh M, Simanovsky N, Hiller N, et al. Differences in disease expression between primary ciliary dyskinesia and cystic fibrosis with and without pancreatic insufficiency. Chest 2014; 145: 738–744. [DOI] [PubMed] [Google Scholar]
- 28.Svobodova T, Djakow J, Zemková D, et al. Impaired growth during childhood in patients with primary ciliary dyskinesia. Int J Endocrinol 2013; 731423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadfield PJ, Rowe-Jones JM, Bush A, et al. Treatment of otitis media with effusion in children with primary ciliary dyskinesia. Clin Otolaryngol Allied Sci 1997; 22: 302–306. [DOI] [PubMed] [Google Scholar]
- 30.Majithia A, Fong J, Hariri M, et al. Hearing outcomes in children with primary ciliary dyskinesia – a longitudinal study. Int J Pediatr Otorhinolaryngol 2005; 69: 1061–1064. [DOI] [PubMed] [Google Scholar]
- 31.Sims EJ, Mugford M, Clark A, et al. Economic implications of newborn screening for cystic fibrosis: a cost of illness retrospective cohort study. Lancet 2007; 369: 1187–1195. [DOI] [PubMed] [Google Scholar]
- 32.Coren ME, Meeks M, Morrison I, et al. Primary ciliary dyskinesia: age at diagnosis and symptom history. Acta Paediatr 2002; 91: 667–669. [DOI] [PubMed] [Google Scholar]
- 33.Kuehni CE, Lucas JS. Towards an earlier diagnosis of primary ciliary dyskinesia: which patients should undergo detailed diagnostic testing? Ann Am Thorac Soc 2016; 13: 1239–1243. [DOI] [PubMed] [Google Scholar]
- 34.Collins SA, Gove K, Walker W, et al. Nasal nitric oxide screening for primary ciliary dyskinesia: systematic review and meta-analysis. Eur Respir J 2014; 44: 1589–1599. [DOI] [PubMed] [Google Scholar]
- 35.O'Callaghan C, Chilvers M, Hogg C, et al. Diagnosing primary ciliary dyskinesia. Thorax 2007; 62: 656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundberg JO, Rinder J, Weitzberg E, et al. Nasally exhaled nitric oxide in humans originates mainly in the paranasal sinuses. Acta Physiol Scand 1994; 152: 431–432. [DOI] [PubMed] [Google Scholar]
- 37.Walker WT, Jackson CL, Lackie PM, et al. Nitric oxide in primary ciliary dyskinesia. Eur Respir J 2012; 40: 1024–1032. [DOI] [PubMed] [Google Scholar]
- 38.Wodehouse T, Kharitonov SA, Mackay IS, et al. Nasal nitric oxide measurements for the screening of primary ciliary dyskinesia. Eur Respir J 2003; 21: 43–47. [DOI] [PubMed] [Google Scholar]
- 39.Piacentini GL, Bodini A, Peroni D, et al. Nasal nitric oxide for early diagnosis of primary ciliary dyskinesia: practical issues in children. Respir Med 2008; 102: 541–547. [DOI] [PubMed] [Google Scholar]
- 40.Marthin JK, Nielsen KG. Choice of nasal nitric oxide technique as first-line test for primary ciliary dyskinesia. Eur Respir J 2011; 37: 559–565. [DOI] [PubMed] [Google Scholar]
- 41.Pifferi M, Bush A, Caramella D, et al. Agenesis of paranasal sinuses and nasal nitric oxide in primary ciliary dyskinesia. Eur Respir J 2011; 37: 566–571. [DOI] [PubMed] [Google Scholar]
- 42.Collins SA, Behan L, Harris A, et al. The dangers of widespread nitric oxide screening for primary ciliary dyskinesia. Thorax 2016; 71: 560–561. [DOI] [PubMed] [Google Scholar]
- 43.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 2005; 171: 912–930. [DOI] [PubMed] [Google Scholar]
- 44.Leigh MW, Hazucha MJ, Chawla KK, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc 2013; 10: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris A, Bhullar E, Gove K, et al. Validation of a portable nitric oxide analyzer for screening in primary ciliary dyskinesias. BMC Pulm Med 2014; 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maniscalco M, de Laurentiis G, Weitzberg E, et al. Validation study of nasal nitric oxide measurements using a hand-held electrochemical analyser. Eur J Clin Invest. 2008; 38: 197–200. [DOI] [PubMed] [Google Scholar]
- 47.Marthin JK, Nielsen KG. Hand-held tidal breathing nasal nitric oxide measurement - a promising targeted case-finding tool for the diagnosis of primary ciliary dyskinesia. PLoS One 2013; 8: e57262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montella S, Alving K, Maniscalco M, et al. Measurement of nasal nitric oxide by hand-held and stationary devices. Eur J Clin Invest 2011; 41: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 49.Chilvers MA, Rutman A, O'Callaghan C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J Allergy Clin Immunol 2003; 112: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stannard WA, Chilvers MA, Rutman AR, et al. Diagnostic testing of patients suspected of primary ciliary dyskinesia. Am J Respir Crit Care Med 2010; 181: 307–314. [DOI] [PubMed] [Google Scholar]
- 51.Jackson CL, Behan L, Collins SA, et al. Accuracy of diagnostic testing in primary ciliary dyskinesia. Eur Respir J 2016; 47: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frommer A, Hjeij R, Loges NT, et al. Immunofluorescence analysis and diagnosis of primary ciliary dyskinesia with radial spoke defects. Am J Respir Cell Mol Biol 2015; 53: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raidt J, Wallmeier J, Hjeij R, et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respir J 2014; 44: 1579–1588. [DOI] [PubMed] [Google Scholar]
- 54.Strippoli MP, Frischer T, Barbato A, et al. Management of primary ciliary dyskinesia in European children: recommendations and clinical practice. Eur Respir J 2012; 39: 1482–1491. [DOI] [PubMed] [Google Scholar]
- 55.Papon JF, Bassinet L, Cariou-Patron G, et al. Quantitative analysis of ciliary beating in primary ciliary dyskinesia: a pilot study. Orphanet J Rare Dis 2012; 7: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sears PR, Yin WN, Ostrowski LE. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 2015; 309: L99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith CM, Djakow J, Free RC, et al. ciliaFA: a research tool for automated, high-throughput measurement of ciliary beat frequency using freely available software. Cilia 2012; 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boon M, Wallmeier J, Ma L, et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Commun 2014; 5: 4418. [DOI] [PubMed] [Google Scholar]
- 59.Wallmeier J, Al-Mutairi DA, Chen CT, et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet 2014; 46: 646–651. [DOI] [PubMed] [Google Scholar]
- 60.Chilvers MA, O'Callaghan C. Analysis of ciliary beat pattern and beat frequency using digital high speed imaging: comparison with the photomultiplier and photodiode methods. Thorax 2000; 55: 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas B, Rutman A, O'Callaghan C. Disrupted ciliated epithelium shows slower ciliary beat frequency and increased dyskinesia. Eur Respir J 2009; 34: 401–404. [DOI] [PubMed] [Google Scholar]
- 62.Smith CM, Hirst RA, Bankart MJ, et al. Cooling of cilia allows functional analysis of the beat pattern for diagnostic testing. Chest 2011; 140: 186–190. [DOI] [PubMed] [Google Scholar]
- 63.Jackson CL, Goggin PM, Lucas JS. Ciliary beat pattern analysis below 37 degrees C may increase risk of primary ciliary dyskinesia misdiagnosis. Chest 2012; 142: 543–544. [DOI] [PubMed] [Google Scholar]
- 64.Santamaria F, de Santi MM, Grillo G, et al. Ciliary motility at light microscopy: a screening technique for ciliary defects. Acta Paediatr 1999; 88: 853–857. [DOI] [PubMed] [Google Scholar]
- 65.Boon M, Smits A, Cuppens H, et al. Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J Rare Dis 2014; 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwabe GC, Hoffmann K, Loges NT, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat 2008; 29: 289–298. [DOI] [PubMed] [Google Scholar]
- 67.Shoemark A, Dixon M, Corrin B, et al. Twenty-year review of quantitative transmission electron microscopy for the diagnosis of primary ciliary dyskinesia. J Clin Pathol 2012; 65: 267–271. [DOI] [PubMed] [Google Scholar]
- 68.Lin J, Yin W, Smith MC, et al. Cryo-electron tomography reveals ciliary defects underlying human RSPH1 primary ciliary dyskinesia. Nat Commun 2014; 5: 5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Callaghan C, Rutman A, Williams GM, et al. Inner dynein arm defects causing primary ciliary dyskinesia: repeat testing required. Eur Respir J 2011; 38: 603–607. [DOI] [PubMed] [Google Scholar]
- 70.Shoemark A, Hogg C. Electron tomography of respiratory cilia. Thorax 2013; 68: 190–191. [DOI] [PubMed] [Google Scholar]
- 71.Leigh MW, O'Callaghan C, Knowles MR. The challenges of diagnosing primary ciliary dyskinesia. Proc Am Thorac Soc 2011; 8: 434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fliegauf M, Olbrich H, Horvath J, et al. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am J Respir Crit Care Med 2005; 171: 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hornef N, Olbrich H, Horvath J, et al. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med 2006; 174: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zariwala MA, Leigh MW, Ceppa F, et al. Mutations of DNAI1 in primary ciliary dyskinesia: evidence of founder effect in a common mutation. Am J Respir Crit Care Med 2006; 174: 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antony D, Becker-Heck A, Zariwala MA, et al. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum Mutat 2013; 34: 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olbrich H, Schmidts M, Werner C, et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am J Hum Genet 2012; 91: 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hogg C, Bush A. Genotyping in primary ciliary dyskinesia: ready for prime time, or a fringe benefit? Thorax 2012; 67: 377–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
J.S. Lucas ERR-0058-2016_Lucas (1.2MB, pdf)
N. Rumman ERR-0058-2016_Rumman (1.2MB, pdf)