Abstract
Objectives
Cystic fibrosis (CF) lung disease is characterised by mucus stasis, chronic infection and inflammation, causing progressive structural lung disease and eventual respiratory failure. CF airways are inhabited by an ecologically diverse polymicrobial environment with vast potential for interspecies interactions, which may be a contributing factor to disease progression. Pseudomonas aeruginosa and Aspergillus fumigatus are the most common bacterial and fungal species present in CF airways respectively and coinfection results in a worse disease phenotype.
Methods
In this review we examine existing expert knowledge of chronic co-infection with P. aeruginosa and A. fumigatus in CF patients. We summarise the mechanisms of interaction and evaluate the clinical and inflammatory impacts of this co-infection.
Results
P. aeruginosa inhibits A. fumigatus through multiple mechanisms: phenazine secretion, iron competition, quorum sensing and through diffusible small molecules. A. fumigatus reciprocates inhibition through gliotoxin release and phenotypic adaptations enabling evasion of P. aeruginosa inhibition. Volatile organic compounds secreted by P. aeruginosa stimulate A. fumigatus growth, while A. fumigatus stimulates P. aeruginosa production of cytotoxic elastase.
Conclusion
A complex bi-directional relationship exists between P. aeruginosa and A. fumigatus, exhibiting both mutually antagonistic and cooperative facets. Cross-sectional data indicate a worsened disease state in coinfected patients; however, robust longitudinal studies are required to derive causality and to determine whether interspecies interaction contributes to disease progression.
Short abstract
A complex relationship exists between P. aeruginosa and A. fumigatus and is associated with worsened clinical disease in cystic fibrosis. Further study of organism interactions, longitudinal outcomes and therapeutic strategies is warranted. https://bit.ly/2A161bh
Introduction
Cystic fibrosis (CF) is the most common inherited lung disease worldwide, caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Resultant dysfunctional, or absent CFTR protein on the apical airway epithelial membrane, leads to anion depletion and reduction in the airway surface liquid, causing a triad of mucus stasis, chronic infection and inflammation in the CF airways. Over the past decade there has been much focus on the development of small molecule therapies that are capable of restoring CFTR function. While these therapies may be transformational for some people with CF, individual responses may be variable and improved CFTR function does not halt inflammation, eradicate microbial pathogens residing in the airways or reverse existing lung damage [1–4].
In addition to the traditional CF respiratory pathogens such as Pseudomonas aeruginosa, Staphlococcus aureus, Haemophilus influenzae and Burkholderia cepacia complex, recent metagenomic microbiome studies have identified a much more diverse airway microbial environment than was previously thought [5]. Rich communities of aerobic and anaerobic bacteria, viruses and fungi coinhabit the airways, with a dynamic composition and waning diversity as CF lung disease progresses [6, 7]. These new insights have led to the identification of novel pathogens such as Ralstonia mannitolilytica, Prevotella spp. and Veillonella spp. [5, 8, 9] and raised questions of how previously overlooked organisms and interspecies interactions may influence disease progression.
The Gram-negative bacterium P. aeruginosa contributes significantly to respiratory morbidity and mortality in CF lung disease [10]. The presence of several organisms coinhabiting the CF airways have been shown to influence the virulence of P. aeruginosa, producing both gainful or inhibitory effects [11–17]. However, the potential for interspecies interactions is vast and we are at an early stage in our understanding of this novel aspect of pathogenesis in CF lung disease. Despite the demonstration of multiple direct and indirect organism interactions in vitro, it remains unclear whether these interactions are clinically significant and how they contribute to disease progression. Here we review the interaction of the most common bacterial and fungal species in the CF respiratory tract, P. aeruginosa and A. fumigatus [18–20], consider the clinical implications and future directions for management of polymicrobial infections in CF.
Epidemiology
Because of the physiological basis of the disease, acquisition of microbes occurs from the immediate environment via the upper gastrointestinal tract or the upper respiratory tract, through ingestion or inhalation, respectively. Aspiration of microbes may also occur from the gastrointestinal tract to the upper respiratory tract. Combinations of these routes of entry manifest in the presence of a diverse variety of bacteria and fungi in the sputum of CF patients. A comprehensive review on the microbiology of CF has been reported previously [21, 22].
P. aeruginosa and A. fumigatus represent the most dominant bacterial and fungal species, respectively, within the CF respiratory tract. Presently, there are 255 species described within the genus Pseudomonas, of which. P. aeruginosa is the most prevalent in patients with CF. Despite the aggressive eradication protocols widely used in CF care, 60–70% of CF patients are intermittent or chronically colonised with P. aeruginosa by the age of 20 years [23, 24].
There are approximately 180 species of Aspergillus spp., of which A. fumigatus is the most common and clinically significant in patients with CF. The prevalence of A. fumigatus colonisation in CF patients is between 16% and 58% [25–28], with rising rates of isolation over the past decade [29]. A range of factors including P. aeruginosa eradication treatment, frequent courses of antibiotics, prolonged use of inhaled antibiotics, inhaled corticosteroids and the widespread use of azithromycin are related to the early acquisition and rising prevalence of A. fumigatus [29–32]. However, variation in diagnostic techniques and surveillance practices between centres is undoubtedly a contributory factor to both the increased and variable prevalence [33]. The use of nonculture-based diagnostic techniques, such as nucleic acid amplification technologies and matrix-assisted laser desorption/ionisation, have been shown to significantly improve detection of fungal organisms over conventional culture techniques [34, 35]; however, access to these technologies may be limited to specialist mycological laboratories [35]
Epidemiological studies indicate there is a wide variation in the prevalence of chronic co-infection with P. aeruginosa and A. fumigatus of 16–35% reported in the Irish CF population [36] and a recent meta-analysis showed a pooled prevalence of 15.8% with significant variation, ranging between 2.3% and 44.8% among CF patients [37]. Accurate estimation of the prevalence of P. aeruginosa and A. fumigatus co-infection poses several challenges due to inconsistent definitions relating to fungal disease, nonstandardised diagnostic techniques, sampling frequency and clinical interpretation of culture results between centres.
Clinical significance of P. aeruginosa and A. fumigatus in CF
It is well established that P. aeruginosa plays a central role in the progression of CF lung disease [24] and it is considered to be the primary pathogen leading to deterioration of lung function, hospitalisation and death in CF [24]. However, the role of A. fumigatus is less clearly defined. The ubiquitous environmental filamentous fungi pose a serious threat causing invasive infection in immunocompromised patients; however, it is effectively cleared in the immunocompetent host through robust multifaceted immune responses. In CF and other chronic respiratory conditions, impaired mucociliary clearance and structural lung damage favours A. fumigatus persistence in the airways, furthermore, ineffective clearance of bacterial and fungal pathogens by phagocytes expressing defective CFTR places people with CF at particular risk of A. fumigatus infection [38–41].
The findings of several studies have indicated that persistent isolation of A. fumigatus is uncommon in children aged <10 years [42–44], as such it has been considered to be a late occurring infection, likely to be acquired as a result of changes in the microbial milieu or disease state over time. However, recent findings from the AREST-CF study group, using early bronchoalveolar lavage (BAL) sample, indicate that A. fumigatus is one of the most prevalent organisms isolated from the lower airways of young children [45, 46]. They have also shown that early Aspergillus infections are an independent risk factor for the progression of lung disease in children with CF [47]. These findings indicate that the presence of A. fumigatus is not dependant on advanced disease states, age of the patient or on the presence of other pathogens as was previously thought.
Several observational studies have demonstrated a worsened clinical condition associated with co-colonisation with P. aeruginosa and A. fumigatus [25, 36, 48–50]. These include two large cross-sectional registry data studies, which included both paediatric and adult patients. The Irish registry data showed that co-colonisation was associated with reduced forced expiratory volume in 1 s (FEV1), more frequent hospitalisation, more frequent pulmonary exacerbations and increased antimicrobial usage, when compared to those not co-colonised [36], while the UK CF registry showed co-colonised patients had an increased use of intravenous antibiotics without correlating with a lower FEV1 [49]. A further single-centre UK study of adult patients showed they had lower FEV1, more i.v. antibiotics and a lower body mass index [50].
A. fumigatus-related lung disease in CF
The hypersensitivity lung disease allergic bronchopulmonary aspergillosis (ABPA) affects up to 10% of people with CF [51]. Diagnostic criteria for ABPA were established in 2003 [27], and are based on a range of clinical, serologic and radiological parameters that continue to be in widespread use today. While there is some variation in management practices, there is consensus amongst clinicians that a diagnosis of ABPA has significant clinical implications that warrants treatment with systemic corticosteroids, with or without antifungal therapy [51, 52]. In addition to ABPA, Aspergillus-related lung disease may have other manifestations in people with CF. A classification system proposed by Baxter et al. [53] based on a cluster analysis in adults identified four distinct classes of Aspergillus-related lung disease in CF: ABPA, Aspergillus sensitisation, Aspergillus colonisation and Aspergillus bronchitis [53].
Aspergillus bronchitis refers to fungal infection which is confined to the bronchial tree, causing superficial mucosal invasion and symptoms of cough and increased mucus production and is distinguished from the other entities by qPCR and Aspergillus immunoglobulin-G alongside negative serological markers of allergic disease [53]. Its first description was based on a small group of CF patients who were nonresponsive to antimicrobial therapy, who chronically isolated A. fumigatus without ABPA and all of whom had a clinical response to antifungal treatment [48]. A proportion of patients exposed to A. fumigatus will become immunologically sensitised; these patients more commonly develop ABPA and the immunological response to the fungi may represent a spectrum of hypersensitivity disease. Several studies have shown that Aspergillus sensitisation without ABPA has been associated with poorer lung function in its own right [54–56].
While the above classification provides a useful clinical and research framework for A. fumigatus lung disease, the proposed biomarkers to differentiate between these clusters have yet to be prospectively validated. Further study of these subgroups, particularly A. fumigatus-colonised and bronchitis patients would be beneficial to understand which patients have inconsequential colonisation and which have active fungal infection. Furthermore, while the proposed classification separates hypersensitivity from nonhypersensitivity Aspergillus disease, it does not incorporate other manifestations including pulmonary aspergilloma [57] or invasive aspergillosis, which may rarely complicate CF lung disease [58].
Uncertainty around the significance of the chronic isolation of A. fumigatus is fuelled by conflicting evidence around its direct effect on progression of lung disease. Several studies show that it does not directly affect absolute FEV1 [42, 43, 59]; however, a number of other studies indicate that it is independently associated with a lower and more accelerated decline in FEV1 [25, 29, 60–62]. Additionally, it has been shown to be associated with more frequent pulmonary exacerbations [25], more advanced bronchiectasis on high-resolution computed tomography (HRCT) [63], elevated BAL neutrophil count [64] and persistent inflammation in a CF murine model [65, 66].
Survival of P. aeruginosa and A. fumigatus in CF airways
Over the course of chronic infections, P. aeruginosa displays a range of mechanisms, phenotypic and genetic adaptations which enable it to persist in the CF lung. Early in infection, P. aeruginosa releases virulence factors which enable it to overcome host defences and establish infection, they include toxic phenazines and rhamnolipids, which promote ciliary stasis [67], and proteases, including LepA, which activate nuclear factor (NF)-kB to increase inflammation [68]. Over time, P. aeruginosa maintains infection through release of immunosuppressing factors (exoproteins) such as the elastases LasA and LasB, which cleave the connective tissue protein elastin and the immune modulator surfactant protein D [69]. It also mutates into small-colony variants (SCVs), mucoid strains and forms impenetrable biofilms, which create a physical and chemical barrier against antimicrobial agents and the host immune system [70].
On exposure to A. fumigatus, alveolar macrophages recognise fungal surface antigens (galactomannan, β-d-glucan) through alveolar macrophage surface receptors such as Dectin-1 and Toll-like receptors. Recognition of A. fumigatus leads to production of proinflammatory cytokines through activation of the NF-kB and inflammasome pathways, triggering an influx of neutrophils, natural killer and T-cells to the site of infection. T-helper (Th) cells are differentiated into a predominant Th1 response with generation of tumour necrosis factor-α and interferon-γ .
A. fumigatus has a range of immune-evasion strategies to avoid clearance by the host protective responses, primarily through the release of secondary metabolites, including mycotoxins and, like P. aeruginosa it forms biofilms. Gliotoxin is the most abundant mycotoxin released by A. fumigatus, its action is through suppressing immune responses including NF-kB [71], macrophage phagocytosis [72], T-cell function [73] and neutrophil activation [74]. Furthermore, gliotoxin may impair the integrity of the epithelial cell wall and has been shown to kill lung epithelial cells in vitro [75].
Interactions between P. aeruginosa and A. fumigatus
Inhibition by P. aeruginosa
In CF, P. aeruginosa inhibits A. fumigatus through a range of different mechanisms and to a greater extent than in non-CF isolates [76]. The primary inhibitory mechanism is through the release of the virulence factors, phenazines. These include pyocyanin (PYO), phenazine-1-carboxamide, 1-hydroxyphenazine (1-HP) and phenazine-1-carboxylic acid (PCA), which promote P. aeruginosa growth and are toxic to surrounding bacteria, fungi and mammalian cells [77]. Phenazines inhibit the growth of A. fumigatus through the generation of reactive oxygen species (ROS) and reactive nitrogen species, which damage the mitochondrial ultrastructure of A. fumigatus hyphae [78]. In addition to causing oxidative stress to the lung, PYO is directly toxic to cilia, upregulates interleukin (IL)-8 activity, causes cellular senescence [78–82] and inactivates α1-antitrypsin, an important component of the endogenous antiprotease shield, contributing to protease/antiprotease imbalance within the lung. PYO is regulated by the P. aeruginosa quorum sensing (QS) system [77] and levels have been directly correlated with prognosis [83] and frequency of pulmonary exacerbations [84].
The QS system allows bacteria to sense each other and to regulate physiological activities such as virulence, motility and biofilm formation through small diffusible signalling molecules, which modulate the pathogenicity of microorganisms found in the CF respiratory tract. The role of the QS in A. fumigatus inhibition was demonstrated recently by Sass et al. [85] in P. aeruginosa QS knockout strains, showing that A. fumigatus growth was significantly higher than when in direct co-culture with wild-type P. aeruginosa, confirming inhibition of A. fumigatus by P. aeruginosa via QS. Furthermore, the viability of conidia and A. fumigatus biofilm mass was reduced by diffusible and heat-soluble molecules released by P. aeruginosa, which are structurally similar to QS molecules[86], though the effect was less pronounced in established mixed-species biofilms [80, 85, 86].
The QS system also controls the production of the P. aeruginosa virulence molecules, rhamnolipids. These induce A. fumigatus production of an extracellular matrix that inhibits A. fumigatus growth by altering cell wall architecture [87]. P. aeruginosa also secrete the interkingdom signalling molecules alkylhydroxyquinolones [88], produced in response to increasing density of bacterial cells, which influence gene expression, phenazine secretion [80] and have been shown to disrupt A. fumigatus biofilm integrity [88].
In addition to intermicrobial signals and the release of redox-active toxins, nutrient competition is a further mechanism of P. aeruginosa inhibition of A. fumigatus growth. Iron is a central micronutrient for the survival of both P. aeruginosa and A. fumigatus, with a particular role in biofilm formation [89]. P. aeruginosa produces the siderophore, pyoverdine [90], which captures iron from the environment and stores it. Through iron deprivation, pyoverdine has a substantial antifungal activity [78, 85, 86]. Sass et al. [90] have shown that P. aeruginosa mutants lacking pyoverdine have less inhibitory capacity for A. fumigatus growth, indicating the importance of pyoverdine as a means of A. fumigatus inhibition. Similarly, the production of siderophores has also been shown to be increased in the presence of other competing bacterial organisms, including S. aureus [91] and Burkholderia spp. [92].
Denial of iron to A. fumigatus is also the mechanism of inhibition by the P. aeruginosa-produced bacteriophage Pf4 [93]. This endogenous phage inhibits the metabolic activity of A. fumigatus biofilms and was more pronounced against preformed A. fumigatus biofilm rather than biofilm formation, while conidial growth was unaffected. The authors also demonstrated that the inhibition of A. fumigatus metabolism by Pf4 could be overcome with supplemental ferric iron, again demonstrating the central role this micronutrient in bacterial–fungal competition.
Reciprocal antagonism by A. fumigatus
Despite a range of antagonistic mechanisms and fungicidal properties of P. aeruginosa, A. fumigatus manages to survive in CF airways in close proximity to P. aeruginosa within the shared ecosystem of the CF airways. Investigation of the antifungal properties of P. aeruginosa has shown that P. aeruginosa clinical isolates fail to completely inhibit A. fumigatus [85, 94]. These findings illustrate that A. fumigatus has the ability to counteract antagonistic actions of P. aeruginosa and that the relationship may shift between antagonism and cooperation.
Variation in the inhibitory capacity of P. aeruginosa has been demonstrated though several studies. Mowat et al. [86] showed that once filamentous A. fumigatus biofilms have been produced, the inhibitory capacity of P. aeruginosa is significantly restricted through small diffusible and heat stable molecules. The antifungal capacity of P. aeruginosa also diminishes as A. fumigatus condita transition into hyphae as their walls become impermeable to P. aeruginosa metabolites and the antibacterial mycotoxin, gliotoxin, is released [72, 95, 96]. This was demonstrated in the Galleria mellonella infection model, indicating that P. aeruginosa and A. fumigatus exert mutual antagonism within shared biofilms [96].
As a further line of defence against the antifungal effects of P. aeruginosa, A. fumigatus produces its own siderophores, allowing it to preserve iron, a vital capability for survival in iron-scarce conditions, such as during pulmonary exacerbations or advanced lung disease. The central role of these siderophores was confirmed using A. fumigatus mutant strains lacking the SidA gene, which exhibited less capacity to preserve A. fumigatus biofilms than A. fumigatus wild type when exposed to the toxic phenazine pyoverdine [90].
Toxic phenazines produced by P. aeruginosa, inhibit A. fumigatus in high concentrations through production of reactive oxygen and nitrogen species; however, concentrations of PYO and PCA occur in the range of 1–100 µM in CF sputum samples, these concentrations have been demonstrated to be to be subinhibitory to A. fumigatus [78]. Furthermore, in the presence of low concentrations of phenazines in CF airways, iron bioavailability is enhanced, thereby sustaining A. fumigatus biofilms. A. fumigatus has also been shown to have the ability to bio-transform phenazines into alternative forms with more favourable properties, including PCA conversion to 1-HP, which induces A. fumigatus siderophore production [80]. These findings demonstrate the complex interplay between these organisms and how A. fumigatus has mechanisms to evade antagonism by P. aeruginosa.
Cooperation
The antagonistic and counter-antagonistic mechanisms enable both organisms to coexist; however, beyond tolerance of each other, cooperative, virulence-enhancing effects have also been demonstrated. As A. fumigatus infection is found in many CF patients following P. aeruginosa infection [97], it is likely that P. aeruginosa facilitates the establishment and growth of A. fumigatus. As described, one mechanism facilitating this is the sub-bacteriostatic airway concentrations of phenazines, which induce A. fumigatus growth through increasing iron bioavailability [78].
Volatile organic compounds released by P. aeruginosa can communicate at a distance with A. fumigatus, without direct contact with the effect of promoting fungal growth [98]. The compound dimethyl sulphide mediates this effect through communication in the gas phase, thus P. aeruginosa may create an environment which is conducive to inhabitation by A. fumigatus and precipitate fungal growth once infection is established. It has been shown that the phenazine 1-HP is able to chelate iron [78] thereby contributing to iron starvation in A. fumigatus. However, it has also been shown that the iron chelating activity of 1-HP induces the transcription of genes for adaptation to iron starvation in A. fumigatus, demonstrating the adaptive capability of A. fumigatus in the presence of P. aeruginosa [78, 99].
P. aeruginosa also gains from the presence of A. fumigatus. In the Galleria mellonella insect model, Reece et al. [96] showed that P. aeruginosa had enhanced killing capacity when pre-exposed to A. fumigatus larvae. Within in vitro-mixed P. aeruginosa and A. fumigatus biofilms, P. aeruginosa displayed increased antimicrobial resistance, when compared to P. aeruginosa in monomicrobial biofilms, likely due to altered permeability of the biofilm extracellular matrix, however the same was not observed of A. fumigatus antifungal susceptibility, which showed no difference between mixed and monomicrobial biofilms [100]. This work indicates that co-presence of A. fumigatus may accelerate phenotypic adaptations and genetic mutations in P. aeruginosa, thereby enhancing virulence of the organism.
Relationship dynamics over time and disease course
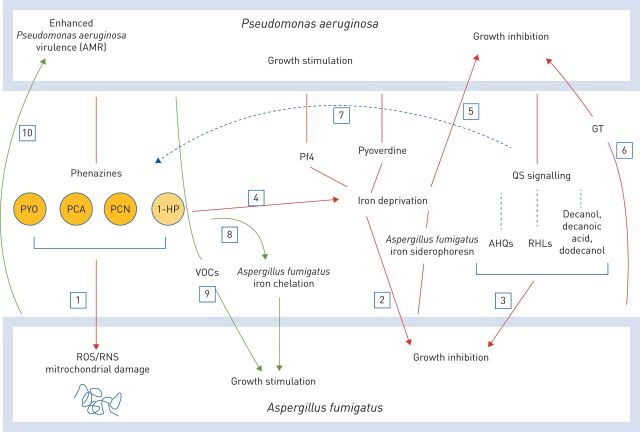
As we have described, the relationship between P. aeruginosa and A. fumigatus is complex with potential for both inhibitory and cooperative interactions (figure 1). Mutual antagonism allows each organism to coexist despite the hostile conditions of the CF airways, theoretically maintaining balance by preventing proliferation of either organism. However, in a cooperative state, enhanced virulence of these organisms may contribute to disease progression.
FIGURE 1.
Interactions between Pseudomonas aeruginosa and Aspergillus fumigatus. Inhibition is shown by red arrows. 1) Phenazines inhibit A. fumigatus growth through generation of reactive oxygen and nitrogen species (ROS and RNS, respectively) which damage A. fumigatus mitochondrial ultrastructure. 2) Pyoverdine and bacteriophage Pf4. 3) P. aeruginosa quorum sensing system (QS). 4) Phenazine 1-hydroxyphenazine (1-HP) chelates iron, contributing to A. fumigatus iron deprivation. 5) A. fumigatus siderophores compete with P. aeruginosa siderophores for iron. 6) Gliotoxin (GT), a primary mycotoxin released by A. fumigatus. Regulation is shown by black arrow. 7) QS signalling controls phenazine release. Stimulation is shown by green arrows. 8) Subinhibitory phenazine levels promote A. fumigatus iron availability. 9) Volatile organic compounds (VOCs) released by P. aeruginosa stimulate A. fumigatus growth. 10) P. aeruginosa phenotypic adaptations influenced by A. fumigatus, including antimicrobial resistance (AMR). PYO: pyocyanin; PCA: phenazine-1-carboxylic acid; PCN: phenazine-1-carboxamide; AHQ: alkylhydroxyquinolones; RHL: rhamnolipids.
The factors influencing shifts between antagonism and cooperation in the co-infection state are not clear. Severity of CF lung disease may be one of the factors determining the nature of species interaction. In hypoxic, anaerobic conditions, the inhibitory effect of phenazines on both planktonic and biofilm forms of A. fumigatus is diminished [101]. Regional ventilation inhomogeneity exists in the CF lung where mucus impaction and bronchiectasis occur. These findings indicate that advancing lung disease or pulmonary exacerbation may favour A. fumigatus growth. A further example of the variation of inhibition in different infection stage was demonstrated in P. aeruginosa SCVs, which showed variation in inhibitory capacity towards A. fumigatus which is directly related to levels of pyoverdine production [102].
The nature of the interspecies relationship may also vary between planktonic and biofilm forms of infection. This was demonstrated through the finding that pyoverdine production by P. aeruginosa in biofilms is higher than in the planktonic state [76, 103]. However, several studies have demonstrated that while P. aeruginosa can inhibit A. fumigatus biofilm formation, its inhibition is rather ineffective on established biofilms [76, 86]. In monomicrobial A. fumigatus biofilms, metabolic activity of the fungus progressively wanes as germinating conidia transition to hyphae and form mature filamentous biofilms [86]. This lower metabolic state desensitises A. fumigatus to P. aeruginosa phenazines, whose action is concentrated in metabolically active sites. Other conditions including host immunity, comorbidity and exogenous factors, such as the use of antibiotics or corticosteroids, may influence interspecies interaction; however, these factors have yet to be evaluated.
Host immunity and inflammation
It is known that co-infection with P. aeruginosa and S. aureus has an additive effect on endobronchial inflammation. Furthermore, an intensifying degree of inflammation has been observed with rising number of species in polymicrobial infections [104]. However, effects of cross-kingdom polymicrobial infection on airway inflammation have not been examined. Although ineffective clearance of bacterial pathogens by CFTR defective phagocytes and other immune mechanisms have been well demonstrated [39, 40, 105], only a few studies have examined the immune responses of CFTR defective immune cells to A. fumigatus and other fungal pathogens [38, 106, 107].
Individually, both P. aeruginosa and A. fumigatus, are capable of inducing proinflammatory responses in the lung epithelium. Inhaled conidia can be cleared without evoking any immune response; however, in the transition into hyphae, proinflammatory cell surface constituents promote phagocyte activity [108]. This was demonstrated in a CF mouse model where A. fumigatus elicited hyperinflammatory responses in airway epithelial cells [107], with elevated percentages of macrophages, neutrophils and neutrophilic chemokines in BAL fluid within 24 h of exposure of CFTR−/− mutants to conidia. Furthermore, in that study, CFTR−/− mice were unable to effectively clear conidia by 6 h, in contrast to the complete clearance by wild-type mice. Further murine models have demonstrated release of IL-1b, exaggerated neutrophil response [66], and profound Th2 hyperinflammatory response to A. fumigatus antigens [109]. These findings illustrate the proinflammatory effects and dysregulated immune responses in response to A. fumigatus, and the effects of impaired innate antifungal immunity in Aspergillus infections in CF.
There is an intense, neutrophilic inflammation present in CF airways with abundant degranulating neutrophils overwhelming endogenous antiprotease mechanisms, thus causing a protease–antiprotease imbalance [110]. It has been shown that A. fumigatus enhances the production of P. aeruginosa cytotoxic elastase, adding to this imbalance and causing direct epithelial damage, mucociliary and CFTR dysfunction [111, 112]. However, Reece et al. [96] did not find elevation of IL-6 and IL-8 levels in co-cultures, compared to infection with P. aeruginosa alone, possibly related to the effect of mutual antagonism, suppression of immune responses by organisms or saturated pathways for cytokine production.
It has recently been shown that the fungal recognition receptor Dectin-1 is cleaved by elastase in BAL fluid, leading to under-detection of the arrival of fungal pathogens in the lung [113]. This work indicates for the first time that neutrophil derived proteases lead to A. fumigatus persistence and infection through inactivation of fungal receptors; the effects of other proteases has not been examined.
With clear inflammatory implications and likely contribution to protease–antiprotease imbalance, examination of inflammatory effects and a better understanding of the innate immune responses is needed to better understand how these pathogens and their interaction influence disease progression.
Should we treat A. fumigatus in coinfected patients?
Combined antimicrobial and antifungal treatment in patients chronically coinfected with P. aeruginosa and A. fumigatus has not been evaluated in clinical trials. Baxter et al. [114] showed that the use of short-term i.v. antibiotics, administered during pulmonary exacerbation to target P. aeruginosa, reduced the quantities of both P. aeruginosa and A. fumigatus isolated in sputum at the end of treatment by PCR. The reason for this is unclear; however, the authors postulate that P. aeruginosa biofilms may protect and sustain A. fumigatus from host immunity and in provision of nutrients.
The benefits of use of antifungal therapies in A. fumigatus disease is unclear due to insufficient existing research. There is a single randomised controlled trial that evaluates the effect of eradication of A. fumigatus in 35 chronically infected patients with 24 weeks of itraconazole or placebo. The study found no improvement in frequency of pulmonary exacerbations or FEV1 [115], which supports a conservative clinical management strategy. However, a major flaw of this study was failure to achieve therapeutic antifungal levels in the majority of patients.
Three further studies, all case–series or cohort studies without randomisation, have evaluated the effect of different azoles in CF patients chronically colonised with A. fumigatus. The first included a small number of people with CF with chronic A. fumigatus in their sputum (without serological evidence of ABPA) treated with voriconazole for a median of 22 weeks. There was no change in FEV1 following antifungal treatment and serum drug levels were not tested in any of the patients [116]. In contrast to the above findings, two small studies using posaconazole [117] and itraconazole [118] showed that eradication of A. fumigatus in chronically colonised patients resulted in fewer pulmonary exacerbations, improved respiratory symptoms, lung function and in one study improvements were seen on HRCT [118].
The effect of treatment in Aspergillus-sensitised and Aspergillus bronchitis patients has received even less attention. Two small studies have evaluated the effect of antifungal therapy in Aspergillus bronchitis patients [48, 119]; both showed improved respiratory symptoms and improvement in lung function. In Aspergillus-sensitised patients, Kanthan et al. [120] retrospectively compared two cohorts of sensitised children; the second cohort, who received more antifungal treatment had higher FEV1 than the second cohort. However, this was a low-quality study comparing groups of patients from different time-points with variable rates of ABPA between the two cohorts . Even within ABPA, the role of antifungal therapy is unclear with variations in clinical practice amongst clinicians [52]. A systematic review of antifungal treatment in ABPA including four randomised controlled trials, showed that symptoms, frequency of pulmonary exacerbations and lung function all improved with antifungal treatment. [121]. However, adverse effects were common, therefore recommendations for use of antifungals in ABPA is classed as “weak” by the British Society for Allergy and Clinical Immunology.
In the absence of clear data on the effectiveness, safety and tolerability of antifungal therapies in chronically infected patients and across the different Aspergillus phenotypes, clinicians have to weigh up the potential treatment gains against the significant and well-documented adverse effects of long-term treatment, including toxicity, drug interactions and the emerging issue of azole resistance. Furthermore, to warrant such treatment, clinicians need to be certain about the clinical implications of A. fumigatus colonisation. Those coinfected with A. fumigatus and P. aeruginosa represent a particular subgroup of patients in whom complex mechanistic interactions appear to confer a worse disease state, in whom targeted antifungal treatment is likely to be beneficial.
Going forward, there is a need for robust longitudinal data and identification of biomarkers to separate “harmless” colonisation from those which have active Aspergillus infection. This needs to be followed by robust therapeutic antifungal trials to determine the optimal antifungal treatment across different subsets of patients (i.e. those with Aspergillus colonisation, bronchitis and sensitisation). Novel treatment strategies must also be evaluated, which may obviate the need for prolonged toxic antifungal treatment, including anti-inflammatory treatments and immunotherapeutic approaches. Although evaluation of a range of different anti-inflammatory therapies is ongoing in CF, in-depth characterisation of the inflammatory implications of this co-infection may identify potential pathways for targeted anti-inflammatory treatments. Recent preclinical evaluation of anakinra, the IL-1-receptor antagonist has shown potential in reducing inflammation through inhibition of IL-1b and correction of dysregulated inflammasome responses [66]. Finally, the effects of CFTR modulator treatments on polymicrobial infections warrants close evaluation, as a reduction in fungal colonisation has been observed following ivacaftor treatment in patients with G551D [122], likely due to complete or partial restoration of CFTR function in innate immune cells.
Conclusion
It is clear that P. aeruginosa and A. fumigatus interact through a range of mechanisms, producing a variably competitive and cooperative relationship, enabling organisms to coexist and thrive in a shared habitat. However, there are many gaps in our understanding of how this relationship evolves over time and disease state and crucially, the clinical implications of this interaction. There is an urgent need for standardisation of terminology, definitions and culture techniques in relation to fungal infection in CF, to enable robust longitudinal studies to be performed and to explore novel therapeutic strategies. Furthermore, clinically accessible biomarkers are needed to identify those most significantly affected by direct targeted treatment in co-colonised patients.
Footnotes
Submitted article, peer reviewed
Conflict of interest: K. Keown has nothing to disclose.
Conflict of interest: A. Reid has nothing to disclose.
Conflict of interest: J.E. Moore has nothing to disclose.
Conflict of interest: C.C. Taggart has nothing to disclose.
Conflict of interest: D.G. Downey has nothing to disclose.
References
- 1.Rowe SM, Heltshe SL, Gonska T, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014; 190: 175–184. doi: 10.1164/rccm.201404-0703OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernarde C, Keravec M, Mounier J, et al. Impact of the CFTR-potentiator ivacaftor on airway microbiota in cystic fibrosis patients carrying a G551D mutation. PLoS One 2015; 10: e0124124. doi: 10.1371/journal.pone.0124124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brody A, Nagle S, Hug C, et al. S93 Effect of lumacaftor/ivacaftor on total, bronchiectasis, and air trapping computed tomography (CT) scores in children homozygous for f508del-cftr: exploratory imaging substudy. Thorax 2017; 72: A57. [Google Scholar]
- 4.Sawicki GS, McKone EF, Pasta DJ, et al. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med 2015; 192: 836–842. doi: 10.1164/rccm.201503-0578OC [DOI] [PubMed] [Google Scholar]
- 5.Fodor AA, Klem ER, Gilpin DF, et al. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One 2012; 7: e45001. doi: 10.1371/journal.pone.0045001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox MJ, Allgaier M, Taylor B, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One 2010; 5: e11044. doi: 10.1371/journal.pone.0011044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim YW, Evangelista JS 3rd, Schmieder R, et al. Clinical insights from metagenomic analysis of sputum samples from patients with cystic fibrosis. J Clin Microbiol 2014; 52: 425–437. doi: 10.1128/JCM.02204-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coman I, Bilodeau L, Lavoie A, et al. Ralstonia mannitolilytica in cystic fibrosis: a new predictor of worse outcomes. Respir Med Case Rep 2016; 20: 48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutin S, Graeber SY, Weitnauer M, et al. Comparison of microbiomes from different niches of upper and lower airways in children and adolescents with cystic fibrosis. PLoS One 2015; 10: e0116029. doi: 10.1371/journal.pone.0116029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez MI, Prince A. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr Opin Pharmacol 2007; 7: 244–251. doi: 10.1016/j.coph.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 11.DeVault JD, Kimbara K, Chakrabarty AM. Pulmonary dehydration and infection in cystic fibrosis: evidence that ethanol activates alginate gene expression and induction of mucoidy in Pseudomonas aeruginosa. Mol Microbiol 1990; 4: 737–745. doi: 10.1111/j.1365-2958.1990.tb00644.x [DOI] [PubMed] [Google Scholar]
- 12.Armbruster CR, Wolter DJ, Mishra M, et al. Staphylococcus aureus protein a mediates interspecies interactions at the cell surface of Pseudomonas aeruginosa. mBio 2016; 7: e00538. doi: 10.1128/mBio.00538-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan K, Dammel C, Stein J, et al. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol 2003; 50: 1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x [DOI] [PubMed] [Google Scholar]
- 14.Korgaonkar A, Trivedi U, Rumbaugh KP, et al. Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc Natl Acad Sci USA 2013; 110: 1059–1064. doi: 10.1073/pnas.1214550110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costello A, Reen FJ, O'Gara F, et al. Inhibition of co-colonizing cystic fibrosis-associated pathogens by Pseudomonas aeruginosa and Burkholderia multivorans. Microbiology (Reading, Engl) 2014; 160: 1474–1487. doi: 10.1099/mic.0.074203-0 [DOI] [PubMed] [Google Scholar]
- 16.Brand A, Barnes JD, Mackenzie KS, et al. Cell wall glycans and soluble factors determine the interactions between the hyphae of Candida albicans and Pseudomonas aeruginosa. FEMS Microbiol Lett 2008; 287: 48–55. doi: 10.1111/j.1574-6968.2008.01301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur J, Pethani BP, Kumar S, et al. Pseudomonas aeruginosa inhibits the growth of Scedosporium aurantiacum, an opportunistic fungal pathogen isolated from the lungs of cystic fibrosis patients. Front Microbiol 2015; 6: 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chotirmall SH, Mirkovic B, Lavelle GM, et al. Immunoevasive Aspergillus virulence factors. Mycopathologia 2014; 178: 363–370. doi: 10.1007/s11046-014-9768-y [DOI] [PubMed] [Google Scholar]
- 19.Nagano Y, Millar BC, Johnson E, et al. Fungal infections in patients with cystic fibrosis. Rev Med Microbiol 2007; 18: 11–16. [Google Scholar]
- 20.Pihet M, Carrere J, Cimon B, et al. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis--a review. Med Mycol 2009; 47: 387–397. doi: 10.1080/13693780802609604 [DOI] [PubMed] [Google Scholar]
- 21.Hauser AR, Jain M, Bar-Meir M, et al. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin Microbiol Rev 2011; 24: 29–70. doi: 10.1128/CMR.00036-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 2002; 15: 194–222. doi: 10.1128/CMR.15.2.194-222.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart CA, Winstanley C. Persistent and aggressive bacteria in the lungs of cystic fibrosis children. Br Med Bull 2002; 61: 81–96. doi: 10.1093/bmb/61.1.81 [DOI] [PubMed] [Google Scholar]
- 24.Emerson J, Rosenfeld M, McNamara S, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34: 91–100. doi: 10.1002/ppul.10127 [DOI] [PubMed] [Google Scholar]
- 25.Amin R, Dupuis A, Aaron SD, et al. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 2010; 137: 171–176. doi: 10.1378/chest.09-1103 [DOI] [PubMed] [Google Scholar]
- 26.Skov M, McKay K, Koch C, et al. Prevalence of allergic bronchopulmonary aspergillosis in cystic fibrosis in an area with a high frequency of atopy. Respir Med 2005; 99: 887–893. doi: 10.1016/j.rmed.2004.11.018 [DOI] [PubMed] [Google Scholar]
- 27.Stevens DA, Moss RB, Kurup VP, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis – state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003; 37: Suppl. 3, S225–S264. doi: 10.1086/376525 [DOI] [PubMed] [Google Scholar]
- 28.Becker JW, Burke W, McDonald G, et al. Prevalence of allergic bronchopulmonary aspergillosis and atopy in adult patients with cystic fibrosis. Chest 1996; 109: 1536–1540. doi: 10.1378/chest.109.6.1536 [DOI] [PubMed] [Google Scholar]
- 29.Fillaux J, Bremont F, Murris M, et al. Aspergillus sensitization or carriage in cystic fibrosis patients. Pediatr Infect Dis J 2014; 33: 680–686. doi: 10.1097/INF.0000000000000231 [DOI] [PubMed] [Google Scholar]
- 30.Burns JL, Van Dalfsen JM, Shawar RM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis 1999; 179: 1190–1196. doi: 10.1086/314727 [DOI] [PubMed] [Google Scholar]
- 31.Hodson ME, Gallagher CG, Govan JRW. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J 2002; 20: 658–664. doi: 10.1183/09031936.02.00248102 [DOI] [PubMed] [Google Scholar]
- 32.Harun SN, Holford NHG, Grimwood K, et al. Pseudomonas aeruginosa eradication therapy and risk of acquiring Aspergillus in young children with cystic fibrosis. Thorax 2019; 74: 740–748. doi: 10.1136/thoraxjnl-2018-211548 [DOI] [PubMed] [Google Scholar]
- 33.Borman AM, Palmer MD, Delhaes L, et al. Lack of standardization in the procedures for mycological examination of sputum samples from CF patients: a possible cause for variations in the prevalence of filamentous fungi. Med Mycol 2010; 48: Suppl. 1, S88–S97. doi: 10.3109/13693786.2010.511287 [DOI] [PubMed] [Google Scholar]
- 34.Nagano Y, Elborn JS, Millar BC, et al. Comparison of techniques to examine the diversity of fungi in adult patients with cystic fibrosis. Med Mycol 2010; 48: 166–176. doi: 10.3109/13693780903127506 [DOI] [PubMed] [Google Scholar]
- 35.Schelenz S, Owens K, Guy R, et al. National mycology laboratory diagnostic capacity for invasive fungal diseases in 2017: evidence of sub-optimal practice. J Infect 2019; 79: 167–173. doi: 10.1016/j.jinf.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 36.Reece E, Segurado R, Jackson A, et al. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: an Irish registry analysis. BMC Pulm Med 2017; 17: 70. doi: 10.1186/s12890-017-0416-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Cheng W, He X, et al. The co-colonization prevalence of Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis: a systematic review and meta-analysis. Microb Pathog 2018; 125: 122–128. doi: 10.1016/j.micpath.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 38.Brunel SF, Willment JA, Brown GD, et al. Aspergillus-induced superoxide production by cystic fibrosis phagocytes is associated with disease severity. ERJ Open Res 2018; 4: 00068-2017. doi: 10.1183/23120541.00068-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Painter RG, Bonvillain RW, Valentine VG, et al. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J Leukoc Biol 2008; 83: 1345–1353. doi: 10.1189/jlb.0907658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Painter RG, Valentine VG, Lanson NA Jr, et al. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 2006; 45: 10260–10269. doi: 10.1021/bi060490t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porto PD, Cifani N, Guarnieri S, et al. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One 2011; 6: 1–8. doi: 10.1371/journal.pone.0019970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milla CE, Wielinski CL, Regelmann WE. Clinical significance of the recovery of Aspergillus species from the respiratory secretions of cystic fibrosis patients. Pediatr Pulmonol 1996; 21: 6–10. doi: [DOI] [PubMed] [Google Scholar]
- 43.de Vrankrijker AM, van der Ent CK, van Berkhout FT, et al. Aspergillus fumigatus colonization in cystic fibrosis: implications for lung function? Clin Microbiol Infect 2011; 17: 1381–1386. doi: 10.1111/j.1469-0691.2010.03429.x [DOI] [PubMed] [Google Scholar]
- 44.Saunders RV, Modha DE, Claydon A, et al. Chronic Aspergillus fumigatus colonization of the pediatric cystic fibrosis airway is common and may be associated with a more rapid decline in lung function. Med Mycol 2016; 54: 537–543. doi: 10.1093/mmy/myv119 [DOI] [PubMed] [Google Scholar]
- 45.Breuer O, Schultz A, Turkovic L, et al. Changing prevalence of lower airway infections in young children with cystic fibrosis. Am J Respir Crit Care Med 2019; 200: 590–599. doi: 10.1164/rccm.201810-1919OC [DOI] [PubMed] [Google Scholar]
- 46.Harun SN, Wainwright CE, Grimwood K, et al. Aspergillus and progression of lung disease in children with cystic fibrosis. Thorax 2019; 74: 125–131. doi: 10.1136/thoraxjnl-2018-211550 [DOI] [PubMed] [Google Scholar]
- 47.Breuer O, Schultz A, Garratt LW, et al. Aspergillus infections and progression of structural lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 2019; 200: 11–21. doi: 10.1164/rccm.201908-1585OC [DOI] [PubMed] [Google Scholar]
- 48.Shoseyov D, Brownlee KG, Conway SP, et al. Aspergillus bronchitis in cystic fibrosis. Chest 2006; 130: 222–226. doi: 10.1378/chest.130.1.222 [DOI] [PubMed] [Google Scholar]
- 49.Hughes D, Archangelidi O, Armstrong-James D, et al. Clinical characteristics of Pseudomonas aeruginosa and Aspergillus species co-infected cystic fibrosis patients in the UK. Pediatr Pulmonol 2018; 53: Suppl. 2, 346. doi: 10.1093/med/9780198813170.003.0017 [DOI] [Google Scholar]
- 50.Ferguson C, Moore JE, McCaughan J, et al. Bacterial and fungal co-colonisation leads to poorer clinical outcomes in an adult cystic fibrosis population. J Cyst Fibros 2019; 18: Suppl. 1, P170. [Google Scholar]
- 51.Maturu VN, Agarwal R. Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: systematic review and meta-analysis. Clin Exp Allergy 2015; 45: 1765–1778. doi: 10.1111/cea.12595 [DOI] [PubMed] [Google Scholar]
- 52.Boyle M, Moore JE, Whitehouse JL, et al. The diagnosis and management of respiratory tract fungal infection in cystic fibrosis: a UK survey of current practice. Med Mycol 2019; 57: 155–160. doi: 10.1093/mmy/myy014 [DOI] [PubMed] [Google Scholar]
- 53.Baxter CG, Dunn G, Jones AM, et al. Novel immunologic classification of aspergillosis in adult cystic fibrosis. J Allergy Clin Immunol 2013; 132: 560–566. doi: 10.1016/j.jaci.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 54.Wojnarowski C, Eichler I, Gartner C, et al. Sensitization to Aspergillus fumigatus and lung function in children with cystic fibrosis. Am J Respir Crit Care Med 1997; 155: 1902–1907. doi: 10.1164/ajrccm.155.6.9196093 [DOI] [PubMed] [Google Scholar]
- 55.Nicolai T, Arleth S, Spaeth A, et al. Correlation of IgE antibody titer to Aspergillus fumigatus with decreased lung function in cystic fibrosis. Pediatr Pulmonol 1990; 8: 12–15. doi: 10.1002/ppul.1950080106 [DOI] [PubMed] [Google Scholar]
- 56.Kraemer R, Delosea N, Ballinari P, et al. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am J Respir Crit Care Med 2006; 174: 1211–1220. doi: 10.1164/rccm.200603-423OC [DOI] [PubMed] [Google Scholar]
- 57.Maguire CP, Hayes JP, Hayes M, et al. Three cases of pulmonary aspergilloma in adult patients with cystic fibrosis. Thorax 1995; 50: 805–806. doi: 10.1136/thx.50.7.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Padrão E, Amorim A. Subacute invasive aspergillosis in a patient with cystic fibrosis. Pulmonology 2019; 25: 126–127. doi: 10.1016/j.pulmoe.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 59.Hong G, Psoter KJ, Jennings MT, et al. Risk factors for persistent Aspergillus respiratory isolation in cystic fibrosis. J Cyst Fibros 2018; 17: 624–630. doi: 10.1016/j.jcf.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luong ML, Chaparro C, Stephenson A, et al. Pretransplant Aspergillus colonization of cystic fibrosis patients and the incidence of post-lung transplant invasive aspergillosis. Transplantation 2014; 97: 351–357. doi: 10.1097/01.TP.0000437434.42851.d4 [DOI] [PubMed] [Google Scholar]
- 61.Armstead J, Morris J, Denning DW. Multi-country estimate of different manifestations of aspergillosis in cystic fibrosis. PLoS One 2014; 9: e98502. doi: 10.1371/journal.pone.0098502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramsey KA, Ranganathan S, Park J, et al. Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am J Respir Crit Care Med 2014; 190: 1111–1116. doi: 10.1164/rccm.201407-1277OC [DOI] [PubMed] [Google Scholar]
- 63.McMahon MA, Chotirmall SH, McCullagh B, et al. Radiological abnormalities associated with Aspergillus colonization in a cystic fibrosis population. Eur J Radiol 2012; 81: e197–e202. doi: 10.1016/j.ejrad.2011.01.114 [DOI] [PubMed] [Google Scholar]
- 64.Gangell C, Gard S, Douglas T, et al. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin Infect Dis 2011; 53: 425–432. doi: 10.1093/cid/cir399 [DOI] [PubMed] [Google Scholar]
- 65.Iannitti RG, Carvalho A, Cunha C, et al. Th17/Treg imbalance in murine cystic fibrosis is linked to indoleamine 2,3-dioxygenase deficiency but corrected by kynurenines. Am J Respir Crit Care Med 2013; 187: 609–620. doi: 10.1164/rccm.201207-1346OC [DOI] [PubMed] [Google Scholar]
- 66.Iannitti RG, Napolioni V, Oikonomou V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Commun 2016; 7: 10791. doi: 10.1038/ncomms10791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Read RC, Roberts P, Munro N, et al. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J Appl Physiol 1992; 72: 2271–2277. doi: 10.1152/jappl.1992.72.6.2271 [DOI] [PubMed] [Google Scholar]
- 68.Kida Y, Higashimoto Y, Inoue H, et al. A novel secreted protease from Pseudomonas aeruginosa activates NF-κB through protease-activated receptors. Cell Microbiol 2008; 10: 1491–1504. doi: 10.1111/j.1462-5822.2008.01142.x [DOI] [PubMed] [Google Scholar]
- 69.Alcorn JF, Wright JR. Degradation of pulmonary surfactant protein D by Pseudomonas aeruginosa elastase abrogates innate immune function. J Biol Chem 2004; 279: 30871–30879. doi: 10.1074/jbc.M400796200 [DOI] [PubMed] [Google Scholar]
- 70.Hoiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 2010; 5: 1663–1674. doi: 10.2217/fmb.10.125 [DOI] [PubMed] [Google Scholar]
- 71.Fitzpatrick LR, Wang J, Le T. In vitro and in vivo effects of gliotoxin, a fungal metabolite: efficacy against dextran sodium sulfate-induced colitis in rats. Dig Dis Sci 2000; 45: 2327–2336. doi: 10.1023/A:1005630723111 [DOI] [PubMed] [Google Scholar]
- 72.Schlam D, Canton J, Carreno M, et al. Gliotoxin suppresses macrophage immune function by subverting phosphatidylinositol 3,4,5-trisphosphate homeostasis. mBio 2016; 7: e02242. doi: 10.1128/mBio.02242-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stanzani M, Orciuolo E, Lewis R, et al. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 2005; 105: 2258–2265. doi: 10.1182/blood-2004-09-3421 [DOI] [PubMed] [Google Scholar]
- 74.Eichner RD, Al Salami M, Wood PR, et al. The effect of gliotoxin upon macrophage function. Int J Immunopharmacol 1986; 8: 789–797. doi: 10.1016/0192-0561(86)90016-0 [DOI] [PubMed] [Google Scholar]
- 75.Geissler A, Haun F, Frank DO, et al. Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ 2013; 20: 1317–1329. doi: 10.1038/cdd.2013.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferreira JA, Penner JC, Moss RB, et al. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa is dependent on the source, phenotype and growth conditions of the bacterium. PLoS One 2015; 10: e0134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dietrich LE, Price-Whelan A, Petersen A, et al. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol 2006; 61: 1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x [DOI] [PubMed] [Google Scholar]
- 78.Briard B, Bomme P, Lechner BE, et al. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci Rep 2015; 5: 8220. doi: 10.1038/srep08220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kerr JR, Taylor GW, Rutman A, et al. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J Clin Pathol 1999; 52: 385–387. doi: 10.1136/jcp.52.5.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moree WJ, Phelan VV, Wu C-H, et al. Interkingdom metabolic transformations captured by microbial imaging mass spectrometry. Proc Natl Acad Sci USA 2012; 109: 13811. doi: 10.1073/pnas.1206855109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Malley YQ, Abdalla MY, McCormick ML, et al. Subcellular localization of Pseudomonas pyocyanin cytotoxicity in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2003; 284: L420–L430. doi: 10.1152/ajplung.00316.2002 [DOI] [PubMed] [Google Scholar]
- 82.Muller M. Premature cellular senescence induced by pyocyanin, a redox-active Pseudomonas aeruginosa toxin. Free Radic Biol Med 2006; 41: 1670–1677. doi: 10.1016/j.freeradbiomed.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 83.Hunter RC, Klepac-Ceraj V, Lorenzi MM, et al. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am J Respir Cell Mol Biol 2012; 47: 738–745. doi: 10.1165/rcmb.2012-0088OC [DOI] [PubMed] [Google Scholar]
- 84.Fothergill JL, Mowat E, Ledson MJ, et al. Fluctuations in phenotypes and genotypes within populations of Pseudomonas aeruginosa in the cystic fibrosis lung during pulmonary exacerbations. J Med Microbiol 2010; 59: 472–481. doi: 10.1099/jmm.0.015875-0 [DOI] [PubMed] [Google Scholar]
- 85.Sass G, Nazik H, Penner J, et al. Aspergillus-Pseudomonas interaction, relevant to competition in airways. Med Mycol 2019; 57: S228–S232. doi: 10.1093/mmy/myy087 [DOI] [PubMed] [Google Scholar]
- 86.Mowat E, Rajendran R, Williams C, et al. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol Lett 2010; 313: 96–102. doi: 10.1111/j.1574-6968.2010.02130.x [DOI] [PubMed] [Google Scholar]
- 87.Briard B, Rasoldier V, Bomme P, et al. Dirhamnolipids secreted from Pseudomonas aeruginosa modify anjpegungal susceptibility of Aspergillus fumigatus by inhibiting beta1,3 glucan synthase activity. ISME J 2017; 11: 1578–1591. doi: 10.1038/ismej.2017.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reen FJ, Phelan JP, Woods DF, et al. Harnessing bacterial signals for suppression of biofilm formation in the nosocomial fungal pathogen Aspergillus fumigatus. Front Microbiol 2016; 7: 2074–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim SH, Clark ST, Surendra A, et al. Global analysis of the fungal microbiome in cystic fibrosis patients reveals loss of function of the transcriptional repressor Nrg1 as a mechanism of pathogen adaptation. PLoS Pathog 2015; 11: e1005308. doi: 10.1371/journal.ppat.1005308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sass G, Ansari SR, Dietl AM, et al. Intermicrobial interaction: Aspergillus fumigatus siderophores protect against competition by Pseudomonas aeruginosa. PLoS One 2019; 14: e0216085. doi: 10.1371/journal.pone.0216085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harrison F, Paul J, Massey RC, et al. Interspecific competition and siderophore-mediated cooperation in Pseudomonas aeruginosa. ISME J 2008; 2: 49–55. doi: 10.1038/ismej.2007.96 [DOI] [PubMed] [Google Scholar]
- 92.Weaver VB, Kolter R. Burkholderia spp. alter Pseudomonas aeruginosa physiology through iron sequestration. J Bacteriol 2004; 186: 2376–2384. doi: 10.1128/JB.186.8.2376-2384.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Penner JC, Ferreira JA, Secor PR, et al. Pf4 bacteriophage produced by Pseudomonas aeruginosa inhibits Aspergillus fumigatus metabolism via iron sequestration. Microbiology (Reading) 2016; 162: 1583–1594. doi: 10.1099/mic.0.000344 [DOI] [PubMed] [Google Scholar]
- 94.Kerr J. Inhibition of fungal growth by Pseudomonas aeruginosa and Pseudomonas cepacia isolated from patients with cystic fibrosis. J Infect 1994; 28: 305–310. doi: 10.1016/S0163-4453(94)91943-7 [DOI] [PubMed] [Google Scholar]
- 95.Sutton P, Newcombe NR, Waring P, et al. In vivo immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infect Immun 1994; 62: 1192–1198. doi: 10.1128/IAI.62.4.1192-1198.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reece E, Doyle S, Greally P, et al. Aspergillus fumigatus inhibits Pseudomonas aeruginosa in co-culture: implications of a mutually antagonistic relationship on virulence and inflammation in the CF airway. Front Microbiol 2018; 9: 1–14. doi: 10.3389/fmicb.2018.01205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paugam A, Baixench M-T, Demazes-Dufeu N, et al. Characteristics and consequences of airway colonization by filamentous fungi in 201 adult patients with cystic fibrosis in France. Med Mycol 2010; 48: S32–S36. doi: 10.3109/13693786.2010.503665 [DOI] [PubMed] [Google Scholar]
- 98.Briard B, Heddergott C, Latge JP. Volatile compounds emitted by Pseudomonas aeruginosa stimulate growth of the fungal pathogen Aspergillus fumigatus. mBio 2016; 7: e00219-16. doi: 10.1128/mBio.00219-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phelan VV, Moree WJ, Aguilar J, et al. Impact of a transposon insertion in phzF2 on the specialized metabolite production and interkingdom interactions of Pseudomonas aeruginosa. J Bacteriol 2014; 196: 1683–1693. doi: 10.1128/JB.01258-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manavathu EK, Vager DL, Vazquez JA. Development and antimicrobial susceptibility studies of in vitro monomicrobial and polymicrobial biofilm models with Aspergillus fumigatus and Pseudomonas aeruginosa. BMC Microbiol 2014; 14: 53–53. doi: 10.1186/1471-2180-14-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anand R, Clemons KV, Stevens DA. Effect of anaerobiasis or hypoxia on Pseudomonas aeruginosa inhibition of Aspergillus fumigatus biofilm. Arch Microbiol 2017; 199: 881–890. doi: 10.1007/s00203-017-1362-5 [DOI] [PubMed] [Google Scholar]
- 102.Anand R, Moss RB, Sass G, et al. Small colony variants of Pseudomonas aeruginosa display heterogeneity in inhibiting Aspergillus fumigatus biofilm. Mycopathologia 2018; 183: 263–272. doi: 10.1007/s11046-017-0186-9 [DOI] [PubMed] [Google Scholar]
- 103.Visaggio D, Pasqua M, Bonchi C, et al. Cell aggregation promotes pyoverdine-dependent iron uptake and virulence in Pseudomonas aeruginosa. Front Microbiol 2015; 6: 902–902. doi: 10.3389/fmicb.2015.00902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sagel SD, Gibson RL, Emerson J, et al. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J Pediatr 2009; 154: 183–188. doi: 10.1016/j.jpeds.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bruscia EM, Zhang PX, Satoh A, et al. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J Immunol 2011; 186: 6990–6998. doi: 10.4049/jimmunol.1100396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Warris A, Bercusson A, Armstrong-James D. Aspergillus colonization and antifungal immunity in cystic fibrosis patients. Med Mycol 2019; 57: S118–s126. doi: 10.1093/mmy/myy074 [DOI] [PubMed] [Google Scholar]
- 107.Chaudhary N, Datta K, Askin FB, et al. Cystic fibrosis transmembrane conductance regulator regulates epithelial cell response to Aspergillus and resultant pulmonary inflammation. Am J Respir Crit Care Med 2012; 185: 301–310. doi: 10.1164/rccm.201106-1027OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev 2009; 230: 38–50. doi: 10.1111/j.1600-065X.2009.00793.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allard JB, Poynter ME, Marr KA, et al. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol 2006; 177: 5186–5194. doi: 10.4049/jimmunol.177.8.5186 [DOI] [PubMed] [Google Scholar]
- 110.Twigg MS, Brockbank S, Lowry P, et al. The role of serine proteases and antiproteases in the cystic fibrosis lung. Mediators Inflamm 2015; 2015: 1–10. doi: 10.1155/2015/293053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amitani R, Wilson R, Rutman A, et al. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol 1991; 4: 26–32. doi: 10.1165/ajrcmb/4.1.26 [DOI] [PubMed] [Google Scholar]
- 112.Smith K, Rajendran R, Kerr S, et al. Aspergillus fumigatus enhances elastase production in Pseudomonas aeruginosa co-cultures. Med Mycol 2015; 53: 645–655. doi: 10.1093/mmy/myv048 [DOI] [PubMed] [Google Scholar]
- 113.Griffiths JS, Thompson A, Stott M, et al. Differential susceptibility of Dectin-1 isoforms to functional inactivation by neutrophil and fungal proteases. FASEB J 2018; 32: 3385–3397. doi: 10.1096/fj.201701145R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baxter CG, Rautemaa R, Jones AM, et al. Intravenous antibiotics reduce the presence of Aspergillus in adult cystic fibrosis sputum. Thorax 2013; 68: 652–657. doi: 10.1136/thoraxjnl-2012-202412 [DOI] [PubMed] [Google Scholar]
- 115.Aaron SD, Vandemheen KL, Freitag A, et al. Treatment of Aspergillus fumigatus in patients with cystic fibrosis: a randomized, placebo-controlled pilot study. PLoS One 2012; 7: 1–7. doi: 10.1371/journal.pone.0036077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hilliard T, Edwards S, Buchdahl R, et al. Voriconazole therapy in children with cystic fibrosis. J Cyst Fibros 2005; 4: 215–220. doi: 10.1016/j.jcf.2005.05.019 [DOI] [PubMed] [Google Scholar]
- 117.Patel D, Popple S, Claydon A, et al. Posaconazole therapy in children with cystic fibrosis and Aspergillus-related lung disease. Med Mycol 2019; 58: 11–21. doi: 10.1093/mmy/myz015 [DOI] [PubMed] [Google Scholar]
- 118.Coughlan CA, Chotirmall SH, Renwick J, et al. The effect of Aspergillus fumigatus infection on vitamin D receptor expression in cystic fibrosis. Am J Respir Crit Care Med 2012; 186: 999–1007. doi: 10.1164/rccm.201203-0478OC [DOI] [PubMed] [Google Scholar]
- 119.Brandt C, Roehmel J, Rickerts V, et al. Aspergillus bronchitis in patients with cystic fibrosis. Mycopathologia 2018; 183: 61–69. doi: 10.1007/s11046-017-0190-0 [DOI] [PubMed] [Google Scholar]
- 120.Kanthan SK, Bush A, Kemp M, et al. Factors affecting impact of Aspergillus fumigatus sensitization in cystic fibrosis. Pediatr Pulmonol 2007; 42: 785–793. doi: 10.1002/ppul.20656 [DOI] [PubMed] [Google Scholar]
- 121.Moreira AS, Silva D, Ferreira AR, et al. Antifungal treatment in allergic bronchopulmonary aspergillosis with and without cystic fibrosis: a systematic review. Clin Exp Allergy 2014; 44: 1210–1227. doi: 10.1111/cea.12333 [DOI] [PubMed] [Google Scholar]
- 122.Heltshe SL, Mayer-Hamblett N, Burns JL, et al. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis 2015; 60: 703–712. doi: 10.1093/cid/ciu944 [DOI] [PMC free article] [PubMed] [Google Scholar]