Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a type of pulmonary hypertension, resulting from fibrotic transformation of pulmonary artery clots causing chronic obstruction in macroscopic pulmonary arteries and associated vascular remodelling in the microvasculature.
Pulmonary endarterectomy (PEA) offers the best chance of symptomatic and prognostic improvement in eligible patients; in expert centres, it has excellent results. Current in-hospital mortality rates are <5% and survival is >90% at 1 year and >70% at 10 years. However, PEA, is a complex procedure and relies on a multidisciplinary CTEPH team led by an experienced surgeon to decide on an individual's operability, which is determined primarily by lesion location and the haemodynamic parameters. Therefore, treatment of patients with CTEPH depends largely on subjective judgements of eligibility for surgery by the CTEPH team.
Other controversies discussed in this article include eligibility for PEA versus balloon pulmonary angioplasty, the new treatment algorithm in the European Society of Cardiology/European Respiratory Society guidelines and the definition of an “expert centre” for the management of this condition.
Short abstract
Pulmonary endarterectomy in an expert centre is the treatment of choice for eligible patients with CTEPH http://ow.ly/T0kv308BGeY
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a type of pulmonary hypertension resulting from fibrotic transformation of pulmonary artery clots causing chronic obstruction of pulmonary arteries and associated vascular remodelling in the microvasculature [1–3]. Consequently, pressure and vascular resistance in the pulmonary vasculature are increased, leading eventually to right heart failure and premature mortality [4]. Not all patients with CTEPH have a history of acute pulmonary embolism [5].
Evidence suggests that the incidence of CTEPH after pulmonary embolism is ∼1.5% [6]. Registry data indicate a prevalence of CTEPH of 3–30 per million in the general population [3].
Diagnosis of CTEPH requires ≥3 months of effective anticoagulation and a mean pulmonary arterial pressure (mPAP) >25 mmHg with a pulmonary capillary wedge pressure ≤15 mmHg, and at least one (segmental) perfusion defect [1, 3]. Some patients suffer from symptomatic chronic thromboembolic pulmonary disease without pulmonary hypertension at rest, and they may also benefit from pulmonary endarterectomy (PEA) [7].
PEA surgery offers the best chance of symptomatic and prognostic improvement in eligible patients, and long-term results are excellent in expert centres [8, 9]. The recent European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines [1] recommend PEA as the treatment of choice for patients with CTEPH. The guidelines recognise that operability is determined by multiple factors that cannot easily be standardised [1]; previous guidelines [10] stated which factors should be considered when assessing a patient's eligibility. Not all patients will be eligible for PEA, and factors such as advanced age, comorbidities, an imbalance between increased pulmonary vascular resistance (PVR) and the number of accessible occlusions, and a poor general condition need to be considered to determine operability [11–13]. Registry data suggest that more than a third of patients diagnosed with CTEPH did not proceed to PEA surgery in the past [5], although this still means that PEA can be performed in the majority of patients.
In this article, we review the current status of PEA in the management of CTEPH, and potential future developments.
Patient selection for PEA
PEA is the recommended treatment for patients with CTEPH if they are considered operable by an experienced multidisciplinary CTEPH team, including at least one experienced surgeon [1, 2]. To be considered operable, a patient must have sufficient surgically accessible thromboembolic material, with a proportional PVR indicating the absence of extensive secondary vasculopathy [5]. An experienced surgeon is defined as one who has performed >20 PEAs in the year they started to assess study cases, and/or >20 in the year before they started to assess study cases, and/or >40 in the 3 years before they started to assess study cases [13].
Thromboembolic disease located proximally in the main, lobar or segmental arteries is amenable to PEA in most surgical centres; distal disease from mid-segmental and subsegmental branches is more challenging for the surgeon [12, 13]. However, with experience, CTEPH surgical teams are operating successfully on more distal disease with good haemodynamic and functional results. Hence, experienced surgeons have proposed that PEA should be considered in all patients who have evidence of thromboembolic disease, including those with more distal disease [12, 14–16].
Without an objective scoring system for evaluation of operative risk or eligibility for PEA, a second opinion may be useful to ensure that no patient is overlooked for a potentially highly beneficial treatment. The task force on chronic thromboembolic pulmonary hypertension at the 5th world symposium recommended referring patients who have been deemed inoperable by one CTEPH centre to a second, more experienced centre for reconsideration [2]. A second opinion may be particularly relevant for patients managed in less experienced centres [15], if the patient is operable in terms of disease distribution but the surgical risk is high or if a patient deemed inoperable some years ago is now reconsidered for surgery. The International CTEPH Association has established the online CTEPH Image Consultation Community (www.cteph-association.org/educational-platform/), where registered users can upload images and documents such as computed tomography (CT) or magnetic resonance imaging (MRI) scans, ventilation/perfusion (V/Q) scintigrams and pulmonary angiograms for review by an expert panel of CTEPH specialists. However, there are challenges to obtaining a second opinion: having two rounds of subjective opinion does not make it an objective approach; a second opinion from a surgeon with little expertise in PEA may be incorrect; there may be technical, regulatory or ethical difficulties with the sharing of diagnostic images between hospitals; a clinician who views diagnostic images digitally without examining the patient does not obtain a full view of the case and may give incorrect advice; and some surgeons may not be open to accepting a second opinion. However, as the key decision-making is based on a subjective evaluation of the imaging, a second opinion on operability in CTEPH can be valuable.
As perioperative mortality rates have declined and experience has grown, CTEPH surgical teams have become willing to operate on more challenging cases [15, 17, 18]. As well as a growth in confidence to operate on patients with more severe pulmonary hypertension and more distal obstruction, it is also realised that patients with less severe haemodynamic derangement may also benefit from PEA. Patients with significant chronic vascular occlusions but near-normal pulmonary haemodynamics at rest are considered to have CTEPH and are therefore eligible for PEA [1]. Some of these individuals have mPAP that is higher than that seen in the healthy population, but below the CTEPH definition threshold [7, 19]. Currently, suitable terminology is lacking to describe this condition as in the absence of pulmonary hypertension the term “CTEPH” is inappropriate, and the Cambridge group have therefore used the term chronic thromboembolic disease (CTED); others may use the term “chronic thromboembolic pulmonary vascular disease” [4]. Completely unilateral CTED is rare, although it can occur [4]; however, most patients have bilateral disease [7], but it may be major (operable) on one side and minor (inoperable) on the other. Patients with CTED are being considered for surgery for symptomatic benefit and for improvement of the significant V/Q mismatch, or conservative treatment with regular follow-up. However, it is important that the risks of surgery are weighed up against its benefit and that this is discussed with the patient [7]. Even if a patient has no symptoms, restoration of perfusion to a lung with complete occlusion can be beneficial (e.g. by removing risks to the patient from sudden loss of function of the remaining lung). In some centres, PEA is offered to selected patients with CTED to improve symptoms and prevent potential chronic parenchymal changes, scarring and secondary vasculopathy. However, it is not known whether this strategy prevents the development of potential CTEPH, that is, is CTED a precursor of CTEPH with a variable time course or a separate entity?
PEA: the surgical technique
PEA requires specialist training and sophisticated intensive care postoperatively. A median sternotomy is performed, with cardiopulmonary bypass (CPB) enabling gradual cooling to 20°C (facilitated by use of a cooling blanket and a head jacket) and safe arrest of the circulation [20, 21]. Deep hypothermic circulatory arrest (DHCA) is initiated when blood obscures the surgical field and provides a clear operating field [20, 21]. DHCA is limited to 20-min intervals, and usually one period is enough for the dissection to be completed on each side. Identification of the correct plane is crucial to prevent perforation of the pulmonary artery while permitting adequate removal of thromboembolic material [20, 21]. The ideal layer leaves a pearly white, smooth residual vessel wall and the easiest dissection plane [20, 21]. The procedure is bilateral, and on completion of the right PEA, bypass is resumed and the patient reperfused while the arteriotomy is closed so that the procedure can be repeated on the left side, with circulatory arrest being initiated as necessary [20, 21].
The PEACOG (PEA and COGnition) trial [22] was conducted to investigate the impact of DHCA on cognitive function at 3 months and 1 year, compared with cerebral perfusion during PEA. The study showed no difference in cognitive function between the two techniques, and improvement postoperatively, indicating that PEA with DHCA at 20°C provides reproducible, excellent results for the lungs and the brain, and, with careful anaesthetic and CPB management, is safe and well tolerated. Registry results suggest that longer circulatory arrest times may be associated with neurological complications [9].
Outcomes of PEA
Haemodynamic and functional outcomes
Postoperative haemodynamics become normal or near normal in most patients after PEA. From the University of California, San Diego (UCSD; CA, USA) and international CTEPH databases, improvements from PVR 700–800 dyn·s·cm–5 to 250 dyn·s·cm–5 have been experienced [15, 23] following surgery, a fall of ∼65%. Other parameters that have been shown to improve markedly following PEA include mPAP (from 46 to 26 mmHg) [15] and median 6-min walking distance (from 362 to 459 m) [23]. Many patients may experience a shift towards improved New York Heart Association (NYHA) functional class after PEA [23], as well as improvements in other measures of exercise capacity, such as the Bruce protocol [24], and quality of life [7]. Reverse remodelling of the right ventricle, with improvement of structure and function, also occurs [25]. The haemodynamic improvements are rapid, whereas, depending on their mechanisms, structural and functional improvements can take longer [26].
Acute mortality and its predictors
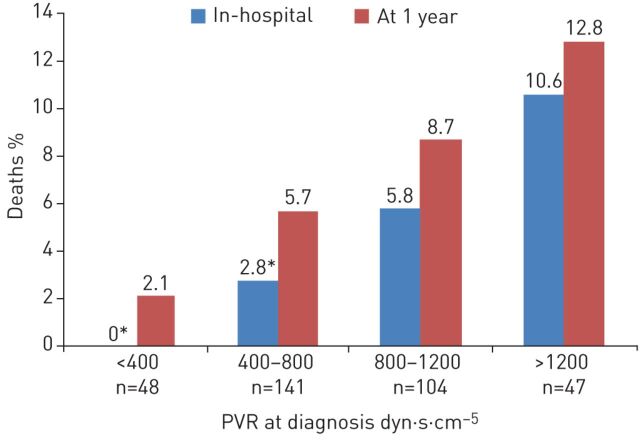
Outcomes of PEA may vary depending on several factors, including chronicity of disease, CTEPH team experience, pre-operative PVR and exercise capacity and the patient's NYHA functional class, comorbidities and distribution of disease [9, 14, 15, 23]. In high-volume centres, the in-hospital mortality is now <5% [27], having improved over time [15]. Higher mortality rates have been reported, although certain contributory factors have been identified; for example, higher pre-operative PVR may increase mortality [15, 23, 28]. At the UCSD centre, 4.1% of patients with a pre-operative PVR >1000 dyn·s·cm–5 died, whereas only 1.6% who had a PVR <1000 dyn·s·cm–5 died [15]. Data from the international CTEPH registry showed in-hospital mortality approximately three times greater in those with PVR >1200 dyn·s·cm–5 at diagnosis compared with PVR 400–800 dyn·s·cm–5 [23] (figure 1). However, it is important to stress that those with high PVR also have the most to gain from PEA surgery as they have the greatest relative improvement and the most potential prognostic benefit. At particularly higher risk are patients with high PVR >1200 dyn·s·cm–5 with poor right ventricular function and more distal disease on imaging. These patients are operable at experienced centres, but it is vital to ensure an effective clearance. Reduced exercise capacity at diagnosis was more apparent in those who died than in those who survived following surgery [23] and was also associated with the risk of residual pulmonary hypertension after PEA [29].
FIGURE 1.
Effects of pulmonary vascular resistance (PVR) at diagnosis on in-hospital and 1-year mortality in patients with chronic thromboembolic pulmonary hypertension (CTEPH) undergoing pulmonary endarterectomy. Data from the international CTEPH registry [23]. *: p<0.05 compared with group with PVR >1200 dyn·s·cm-5.
Persistent pulmonary hypertension after PEA
Up to a third of patients may have persistent (or residual) pulmonary hypertension despite apparently successful PEA surgery [30–32]. Persistent pulmonary hypertension can result from incomplete removal of more distal thrombi by inexperienced surgeons, or from concomitant small-vessel disease in patients with operable proximal disease [12, 33]. Its precise incidence is unclear because many centres do not routinely perform right heart catheterisation in all patients after PEA and there is no agreed definition of persistent pulmonary hypertension [12].
Persistent pulmonary hypertension after surgery remains the most important cause of early postoperative morbidity and mortality. In the international CTEPH registry, persistent pulmonary hypertension affected 16.7% of patients and was associated with a higher early mortality [23]. Similarly, in the UCSD centre, overall death rates (based on retrospective Social Security Death Index search) were 10.3% and 0.9% in those with (>500 dyn·s·cm–5) and without (<500 dyn·s·cm–5) persistent pulmonary hypertension, respectively [15]. Data from the UK national cohort showed that higher mPAP, right atrial pressure and PVR, and lower cardiac index were negatively correlated with long-term survival in multivariate analyses [8]. An mPAP ≥36 mmHg and a PVR ≥416 dyn·s·cm–5 (as time-varying measures) were the optimal thresholds correlated with a higher risk of death from any cause, whereas an mPAP ≥38 mmHg and a PVR ≥425 dyn·s·cm–5 identified those patients at higher risk of death because of CTEPH.
Recurrent pulmonary hypertension after PEA
Generally, the early haemodynamic benefits of PEA remain unchanged over the medium term [34]. However, occasionally, patients can re-present with CTEPH or pulmonary hypertension, which can be proximal or distal, many years after successful PEA. This condition is much less common than persistent pulmonary hypertension after PEA and has distinct pathology. It is caused by a further thrombotic episode after successful PEA [12] and is often associated with poorly controlled anticoagulation (D. Jenkins, Papworth Hospital, Cambridge, UK; unpublished data). Importantly, as with persistent pulmonary hypertension, there is no consensus on the definition of recurrent pulmonary hypertension after PEA and no clear guidance on the follow-up of patients who undergo PEA to detect recurrent pulmonary hypertension [12].
Longer-term outcomes of PEA
In a recent study from a centre in Pavia, Italy, PEA outcomes were compared between patients with proximal disease (type 1 and type 2 lesions, n=221) and distal disease (type 3 lesions, n=110) [24]. There was no significant difference in overall in-hospital mortality between groups, and immediate, 3-month and 1-year haemodynamic and functional improvements were comparable (table 1). These results illustrate that technically difficult patients have good results and could be good candidates for PEA in expert centres.
TABLE 1.
Outcomes of pulmonary endarterectomy in patients with proximal (type 1 and type 2) or distal (type 3) chronic thromboembolic pulmonary hypertension (CTEPH) disease distribution
| Proximal | Distal | |
| Subjects n | 221 | 110 |
| mPAP mmHg | ||
| Pre-operative | 44±10 | 46±11 |
| At discharge | 22±7 | 24±6 |
| 3-month follow-up | 24±9 | 25±7 |
| 12-month follow-up | 23±7 | 24±8 |
| p-value# | <0.001 | <0.001 |
| PVR dyn·s·cm–5 | ||
| Pre-operative | 876±392 | 926±337 |
| At discharge | 251±146 | 295±161 |
| 3-month follow-up | 270±175 | 300±139 |
| 12-month follow-up | 243±115 | 300±224 |
| p-value# | <0.001 | <0.001 |
| PaO2 mmHg | ||
| Pre-operative | 65±12 | 66±11 |
| 3-month follow-up | 82±13 | 80±11 |
| 12-month follow-up | 80±11 | 80±11 |
| p-value# | <0.001 | <0.001 |
| Modified Bruce exercise test m | ||
| Pre-operative | 51 (0−143) | 52 (0−102) |
| 3-month follow-up | 495 (182−658) | 435 (143−586) |
| 12-month follow-up | 520 (261−709) | 474 (225−620) |
| p-value# | <0.001 | <0.001 |
| 6-min walking distance m | ||
| Pre-operative | 277±118 | 289±112 |
| 3-month follow-up | 391±118 | 398±107 |
| 12-month follow-up | 389±118 | 396±112 |
| p-value# | <0.001 | <0.001 |
Data are presented as mean±sd or median (interquartile range), unless otherwise stated. mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; PaO2: arterial oxygen tension. Tests of interaction are as follows. mPAP: p=0.975; PVR: p=0.777; PaO2: p=0.317; modified Bruce exercise test: p=0.205; 6-min walking distance: p=0.962. #: versus pre-operative. Reproduced and modified from [24] with permission from the publisher.
Survival rates in the medium to long term of >90% at 1 year, >80% at 5 years and >70% at 6–10 years have been reported [8, 27, 35]. In a UK follow-up programme, the 5-year survival rate was 92.5%, conditional from 3-month follow-up, indicating that for patients who survive the perioperative period, excellent medium-term outcomes are achievable [36]. Recent data from the international CTEPH registry report estimated survival rates of 93% at 1 year, 91% at 2 years and 89% at 3 years after PEA; significantly better than for those who did not have PEA [9]. The importance of PEA is emphasised in an analysis of operated and nonoperated patients, whereby PEA was the strongest independent predictor of survival, reducing the relative risk of death by 63% (hazard ratio 0.37, 95% CI 0.24–0.58; p<0.0001) [9].
In patients who had PEA, mortality was associated with NYHA functional class, right atrial pressure, a history of cancer, bridging therapy with pulmonary arterial hypertension-targeted drugs, surgical complications and additional cardiac procedures [9]. Consideration of identified prognostic factors when assessing operability has the potential to bring mortality rates down further. A recent UK study has managed to track survival in a national series of 880 consecutive patients following PEA with complete follow-up. This confirmed the excellent medium- and long-term survival and also institutional learning with improved survival in the most recent cohort compared with the initial cohort. It also demonstrated that 49% of late deaths were unrelated to CTEPH [8].
Postoperative pulmonary hypertension has been shown to have no association with medium-term survival; prospectively collected data from the Papworth Hospital (Cambridge, UK) revealed 5-year survival rates of 90.3% and 89.9% (nonsignificant) in discharged patients with a postoperative mPAP <30 mmHg compared with those with mPAP ≥30 mmHg, respectively [29]. This threshold was chosen as it was the level that was associated with worse survival in the original series describing the natural history of untreated patients with CTEPH [37]. However, data from the international CTEPH registry have indicated that patients with postoperative pulmonary hypertension have 3.66-fold greater mortality risk than those without postoperative pulmonary hypertension (p<0.001) [9]. The largest and most comprehensive follow-up to date has clarified the influence of residual pulmonary hypertension following PEA [8]. The degree of residual pulmonary hypertension necessary to influence late survival is much higher than the 25 mmHg needed for diagnosis, at mPAP >38 mmHg or PVR >450 dyn·s·cm–5. These findings explain the conflicting results found in earlier smaller series and set the definition of postoperative residual pulmonary hypertension and the benchmark threshold for good results needed to improve prognosis.
Data on the longer term outcome for patients with CTED are limited, although the UK CTEPH centre published data from 42 patients with symptomatic thromboembolic disease and baseline mPAP of <25 mmHg who underwent PEA [7]. Following surgery, there was no in-hospital mortality, and patients had significant improvement in functional status and quality of life, with 95% of patients returning to NYHA class I or II within 1 year; however, complications occurred in 40% of patients [7]. At present there is no evidence that PEA improves prognosis or prevents the development of CTEPH in this patient group and it should therefore be offered only to symptomatic patients.
Identification of expert centres for PEA
Every patient diagnosed with CTEPH should be referred to an expert centre so that they can be assessed by a specialist and experienced CTEPH team to determine their eligibility for PEA [2, 14]. This approach is at the heart of the ESC/ERS treatment algorithm, which begins by advising confirmation of the diagnosis at a CTEPH expert centre [1]. Missing from the guidelines is an explicit definition of what constitutes a CTEPH expert centre, although a pulmonary hypertension centre has been defined. Historically, it has been suggested that to be considered “expert” a centre should perform ≥20 PEA operations per year with a <10% mortality rate [10]. The definition of a CTEPH expert centre becomes more important as new treatment modalities are developed and more drugs become licensed. There is a potential concern that centres with limited expertise may offer unlicensed drug treatment or balloon pulmonary angioplasty (BPA) to patients who are eligible for, and would benefit more from, PEA. As described later, combined PEA and BPA may be indicated in selected patients: a CTEPH expert centre should therefore also have experienced BPA interventionists available.
Prerequisites for a CTEPH expert centre are shown in table 2. The centre and team's experience in managing CTEPH contributes toward a successful outcome [15]. In the international CTEPH registry in which PEA was performed on 386 patients, in-hospital mortality rates for hospitals that performed >50, 11–50 and 1–10 PEAs per year were 3.4%, 4.5% and 8.8%, respectively [9]. A retrospective case series from the UCSD reported an in-hospital mortality of 2.2% in the last 500 consecutive cases, compared with 5.2% for the preceding 1000 cases, highlighting the importance of increasing institutional experience [15]. Data from the international CTEPH registry published in 2011 suggested a trend towards lower mortality in centres that performed >50 PEA procedures per year [23]. However, a more recent publication from this registry indicated that the number of PEAs performed per centre did not predict improved long-term survival on multivariable analysis [9]. Despite this, the consensus is that PEA surgery is best conducted at an experienced CTEPH centre, with a suggestion that there should be one centre per 40–50 million population performing no fewer than 50 cases per year and with ≥5 years' experience [38]. Importantly, mortality rates in such centres are consistently <5% and this could also be included in the definition of an expert centre.
TABLE 2.
Characteristics of an expert centre
| Extensive experience with cardiothoracic surgery, including procedures requiring DHCA |
| Excellent pulmonary and cardiac services |
| Emphasis on pulmonary hypertension |
| Expert diagnostic imaging |
| Experienced multidisciplinary team comprising surgeons, radiologists, anaesthetists, intensivists, nurses, perfusionists, respiratory therapists and interventionalists, including specialists experienced in BPA |
DHCA: deep hypothermic circulatory arrest; BPA: balloon pulmonary angioplasty. Data from [12].
In addition to centre experience, the clinical status (World Health Organization class) of the patient before PEA is a critical determinant of outcome, with mortality being significantly higher in patients with worse clinical status before surgery, independently of other considerations [9]. This illustrates the importance of early referral of patients with suspected CTEPH to an expert centre for evaluation and management.
International networks could be important for communication and sharing of information to improve the standards of all CTEPH centres. International registries with prospective data on risk factors and early and late outcome after PEA or other treatments will help expand our knowledge. Registries provide a wealth of data on real-world clinical experience, supplement data from randomised clinical trials and may be useful in validating the criteria for identifying centres of excellence. Some centres may be unable to share their data because of local or national regulations. This could hamper categorisation. Another potential barrier is that there is no current method for standardising operability or surgical risk, which to a large extent is a subjective decision of surgeons based on their experience [12, 39]. The relatively small number of patients with CTEPH and difficulty of objectively measuring disease distribution and surgeon expertise hinder the development of a statistically reliable scoring system along the lines of the EuroSCORE system for cardiac surgery, which was developed and validated from tens of thousands of patients. An attempt to develop a simple ranking system based on operative mortality, number of interventions performed and ability to operate on distal/segmental disease is proposed in table 3. This definition is subjective and is not based on evidence. To give a more accurate reflection of the expertise of a centre, this definition should also include validated long-term outcomes and the risk profile of the patients operated. A recent analysis of data from the international CTEPH registry suggested that mortality rates for intermediate and higher volume CTEPH centres were similar because the latter took on more challenging patients [9]. Introduction of a ranking system for centres of excellence and restricting PEA to only the highest quality centres would require cooperation and consensus among centres, and may fail to influence patient care because of geographical boundaries and differences between healthcare systems.
TABLE 3.
Proposed identification criteria of expert or high-quality centres
| Level of expertise | Criteria | ||
| I | 30-day or in-hospital mortality <5% | ||
| II | 30-day or in-hospital mortality <5% | plus ≥50 procedures·year−1 | |
| III | 30-day or in-hospital mortality <5% | plus ≥50 procedures·year−1 |
plus ability to perform segmental endarterectomy/operate on distal disease plus ability to provide PEA, BPA and medical therapy |
PEA: pulmonary endarterectomy; BPA: balloon pulmonary angioplasty.
Challenges of the ESC/ERS guidelines for the management of CTEPH
The ESC/ERS guidelines for the diagnosis and treatment of CTEPH [1] suggest that patients move only “one way” between evaluation of operability, decision on PEA and medical or interventional therapy. In practice, patients may be reconsidered for various options during the course of their disease, and some patients require all three modalities.
The algorithm includes patients who are “technically operable”, but have a “nonacceptable risk:benefit ratio”. It is not practicable to give a single definition of this population because the risk:benefit ratio depends on so many variables. This consideration illustrates the importance of evaluation of all patients with CTEPH at an expert centre to identify those who could benefit from PEA and to evaluate the risk:benefit ratio of the procedure. For this population (technically operable but with nonacceptable risk:benefit ratio) the guideline recommends targeted medical therapy and suggests that BPA may be considered, but technically operable patients with CTEPH were excluded from the clinical trials of pharmacological treatments, and data to support the use of BPA in this setting are sparse. BPA should not currently be offered as a substitute for PEA.
For technically inoperable patients, the algorithm suggests BPA or targeted medical therapy. However, this does not fully reflect the written recommendations. BPA is less invasive than PEA and it has been reported to improve haemodynamics with low procedural risk [40], but more data are required before it becomes established, and it has a IIb/C recommendation [1]. Targeted medical therapy with riociguat has a IB recommendation for the treatment of patients with inoperable CTEPH and those with persistent or recurrent pulmonary hypertension after PEA [1]. Use of endothelin antagonists, prostacyclin analogues or phosphodiesterase type-5 inhibitors is off-label, has a IIb/B recommendation [1] and may lead to worse outcomes [9].
Another controversial aspect of these guidelines is the removal of a recommendation for a second surgical opinion that was present in the guideline from the 5th world symposium on pulmonary hypertension [2].
Summary and future directions
PEA is the gold standard of care for operable patients with CTEPH, with a complete bilateral PEA (with DHCA) at an experienced centre remaining the best treatment option to provide excellent long-term outcomes with low mortality rates. Residual pulmonary hypertension is a risk factor for postoperative morbidity and mortality. It is thought that a substantial component of persistent postoperative pulmonary hypertension is related to distal pulmonary vasculopathy in small precapillary vessels. Patients with residual pulmonary hypertension could potentially benefit from medical treatment once the mechanical obstruction has been removed, as suggested by the recent clinical trials with riociguat in this setting [41–43]. Currently there is no clear guidance on optimal follow-up of patients who undergo PEA to detect persistent or recurrent pulmonary hypertension.
Developments in PEA may also demand more refined definitions of CTEPH. More distal segmental and subsegmental disease may be considered inoperable CTEPH at less experienced centres, but an experienced surgeon may consider this patient group operable. It is our opinion that all patients who have evidence of thromboembolic disease, including those with distal disease, should be considered for PEA. The specific role of BPA in CTEPH remains uncertain; we await longer-term follow-up data and the current CTEPH registry should provide this. The role of drug treatment in the algorithm will need to be established further. Currently, riociguat is approved for inoperable CTEPH or the treatment of persistent/recurrent CTEPH after PEA, but other pulmonary arterial hypertension vasodilator drugs have not been proven to benefit patients with CTEPH in randomised controlled trials. We do not have good evidence that drug pre-treatment is beneficial prior to PEA.
With the advent of BPA, the classification of patients as “operable” or “inoperable” may become less relevant: patients may be eligible for surgical (PEA), interventional/percutaneous (BPA) or noninvasive (medical) therapy and may receive two of these strategies, or all three, in the course of their disease [44]. The definition of “operability” must clarify the borderline between surgical (PEA) and interventional (BPA) cases and consider the CTED group who benefit from PEA despite not meeting the haemodynamic definition of CTEPH [45]. However, currently only patients deemed inoperable should be treated with BPA.
Another interesting development is combined PEA and BPA and the early experience in highly selected cases has been reported. This can occur as intraoperative planned BPA during PEA, acute rescue BPA after failure of PEA or BPA for residual or recurrent pulmonary hypertension months or years after PEA. The intraoperative approach was reported in three patients who underwent PEA of the right pulmonary artery, while the left pulmonary artery territory, inoperable because of distal location of disease, was treated using BPA. Pulmonary haemodynamics improved dramatically in all patients and exercise capacity was significantly improved at 6−10 months' follow-up [44]. Rescue BPA after PEA has been described in an acute setting in three patients in France [46]: the results suggest that this strategy can improve haemodynamics (although two of these severely ill patients died of septic complications). In nine Japanese patients who had gradually deteriorated after an initially favourable response to PEA, BPA performed a mean 4.1 years after surgery improved haemodynamics and NYHA functional class [47]. Although the place of combination interventional therapy in the CTEPH management algorithm has not been determined and it has been performed in only a few patients to date, it seems to be promising. However, it should be attempted only in carefully selected patients managed by expert PEA centres.
Surgical classification of CTEPH is being refined (led by M. Madani's group at UCSD). The new classification was developed specifically to address distal disease, and to improve understanding of long-term outcomes using different therapies for these patients. The ability to distinguish segmental and subsegmental disease, and the efficacy of different treatment modalities in such patients, is the basis behind this new classification of thromboembolic disease. Since 2013, the UCSD group have been using and evaluating a new surgical classification to address the current advances in surgical techniques. Although the final outcomes are still under investigation and soon to be published, the preliminary results are quite promising (M. Madani, Division of Cardiovascular and Thoracic Surgery, University of California, San Diego, La Jolla, CA, USA; unpublished data). Under this new classification, named the “UCSD classification”, thromboembolic disease is characterised into “levels”, based on the location of the disease (table 4). Levels also indicate the degree of difficulty in surgical resection, with higher levels indicating more challenging and advanced resection.
TABLE 4.
The University of California, San Diego (UCSD) classification; a proposed new surgical classification of chronic thromboembolic pulmonary hypertension
| Surgical level | Location of thromboembolic disease |
| 0 | No evidence of thromboembolic disease |
| I | Starts in the main pulmonary arteries (patients with complete occlusion of one lung are classified as level IC) |
| II | Starts at the level of the lobar or intermediate pulmonary arteries |
| III | Starts at the level of segmental arteries only |
| IV | Starts at the subsegmental branches only |
Preliminary results (M. Madani, Division of Cardiovascular and Thoracic Surgery, University of California, San Diego, La Jolla, CA, USA; unpublished data).
Several aspects of the management of CTEPH continue to evolve as three effective treatment options are now available and some areas are in need of refinement and clarification. However, for patients with CTEPH who are considered operable, the message is clear: there is no better treatment than a complete bilateral PEA carried out by an experienced CTEPH team and so this remains the treatment of choice for operable patients with CTEPH.
Disclosures
A.M. D'Armini ERR-0111-2016_DArmini (1.2MB, pdf)
D. Jenkins ERR-0111-2016_Jenkins (1.2MB, pdf)
M. Madani ERR-0111-2016_Madani (1.2MB, pdf)
E. Mayer ERR-0111-2016_Mayer (1.2MB, pdf)
Acknowledgements
Editorial assistance was provided by Adelphi Communications Ltd (Bollington, UK), supported by Bayer AG (Berlin, Germany).
Footnotes
Conflict of interest: Disclosures can be found alongside this article at err.ersjournals.com
Provenance: Publication of this peer-reviewed article was sponsored by Bayer AG, Berlin, Germany (principal sponsor, European Respiratory Review issue 143).
References
- 1.Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 2.Kim NH, Delcroix M, Jenkins DP, et al. . Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62: Suppl. 25, D92–D99. [DOI] [PubMed] [Google Scholar]
- 3.Lang IM, Pesavento R, Bonderman D, et al. . Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 2013; 41: 462–468. [DOI] [PubMed] [Google Scholar]
- 4.Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014; 130: 508–518. [DOI] [PubMed] [Google Scholar]
- 5.Pepke-Zaba J, Delcroix M, Lang I, et al. . Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 6.Guérin L, Couturaud F, Parent F, et al. . Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014; 112: 598–605. [DOI] [PubMed] [Google Scholar]
- 7.Taboada D, Pepke-Zaba J, Jenkins DP, et al. . Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J 2014; 44: 1635–1645. [DOI] [PubMed] [Google Scholar]
- 8.Cannon JE, Su L, Kiely DG, et al. . Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom national cohort. Circulation 2016; 133: 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delcroix M, Lang I, Pepke-Zaba J, et al. . Long-term outcome of patients with chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2016; 133: 859–871. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Hoeper MM, Humbert M, et al. . Guidelines for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 11.Auger WR, Kim NH, Trow TK. Chronic thromboembolic pulmonary hypertension. Clin Chest Med 2010; 31: 741–758. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins D. Pulmonary endarterectomy: the potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2015; 24: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins DP, Biederman A, D'Armini AM, et al. . Operability assessment in CTEPH: lessons from the CHEST-1 study. J Thorac Cardiovasc Surg 2016; 152: 669–674. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins D, Mayer E, Screaton N, et al. . State-of-the-art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur Respir Rev 2012; 21: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madani MM, Auger WR, Pretorius V, et al. . Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012; 94: 97–103. [DOI] [PubMed] [Google Scholar]
- 16.Ng C, Jenkins DP. Surgical management of chronic thromboembolic pulmonary hypertension. Br J Hosp Med 2013; 74: 31–35. [DOI] [PubMed] [Google Scholar]
- 17.de Perrot M, McRae K, Shargall Y, et al. . Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: the Toronto experience. Can J Cardiol 2011; 27: 692–697. [DOI] [PubMed] [Google Scholar]
- 18.Poch DS, Auger WR. Chronic thromboembolic pulmonary hypertension: detection, medical and surgical treatment approach, and current outcomes. Heart Fail Rev 2016; 21: 309–322. [DOI] [PubMed] [Google Scholar]
- 19.Hadinnapola C, Gopalan D, Jenkins DP, et al. . Diagnosing chronic thromboembolic pulmonary hypertension: current perspectives. J Vasc Diagnostics 2014; 2: 75–83. [Google Scholar]
- 20.Jamieson SW, Kapelanski DP. Pulmonary endarterectomy. Curr Probl Surg 2000; 37: 165–252. [DOI] [PubMed] [Google Scholar]
- 21.Madani MM, Jamieson SW. Pulmonary endarterectomy for chronic thromboembolic disease. Oper Tech Thorac Cardiovasc Surg 2006; 11: 264–274. [DOI] [PubMed] [Google Scholar]
- 22.Vuylsteke A, Sharples L, Charman G, et al. . Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOG): a randomised controlled trial. Lancet 2011; 378: 1379–1387. [DOI] [PubMed] [Google Scholar]
- 23.Mayer E, Jenkins D, Lindner J, et al. . Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. [DOI] [PubMed] [Google Scholar]
- 24.D'Armini AM, Morsolini M, Mattiucci G, et al. . Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2014; 148: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 25.D'Armini AM, Zanotti G, Ghio S, et al. . Reverse right ventricular remodeling after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2007; 133: 162–168. [DOI] [PubMed] [Google Scholar]
- 26.Morsolini M, Boffini M, Paciocco G, et al. . Pulmonary endarterectomy: the lancet first, tears for pills. Minerva Med 2014; 105: 7–13. [PubMed] [Google Scholar]
- 27.Hoeper MM, Madani MM, Nakanishi N, et al. . Chronic thromboembolic pulmonary hypertension. Lancet Respir Med 2014; 2: 573–582. [DOI] [PubMed] [Google Scholar]
- 28.Saouti N, de Man F, Westerhof N, et al. . Predictors of mortality in inoperable chronic thromboembolic pulmonary hypertension. Respir Med 2009; 103: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 29.Freed DH, Thomson BM, Berman M, et al. . Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg 2011; 141: 383–387. [DOI] [PubMed] [Google Scholar]
- 30.Bonderman D, Skoro-Sajer N, Jakowitsch J, et al. . Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation 2007; 115: 2153–2158. [DOI] [PubMed] [Google Scholar]
- 31.Jamieson SW, Kapelanski DP, Sakakibara N, et al. . Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg 2003; 76: 1457–1462. [DOI] [PubMed] [Google Scholar]
- 32.Thistlethwaite PA, Madani MM, Kemp AD, et al. . Venovenous extracorporeal life support after pulmonary endarterectomy: indications, techniques, and outcomes. Ann Thorac Surg 2006; 82: 2139–2145. [DOI] [PubMed] [Google Scholar]
- 33.Kim NH, Fesler P, Channick RN, et al. . Preoperative partitioning of pulmonary vascular resistance correlates with early outcome after thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Circulation 2004; 109: 18–22. [DOI] [PubMed] [Google Scholar]
- 34.Corsico AG, D'Armini AM, Cerveri I, et al. . Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med 2008; 178: 419–424. [DOI] [PubMed] [Google Scholar]
- 35.Archibald CJ, Auger WR, Fedullo PF, et al. . Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med 1999; 160: 523–528. [DOI] [PubMed] [Google Scholar]
- 36.Freed DH, Thomson BM, Tsui SS, et al. . Functional and haemodynamic outcome 1 year after pulmonary thromboendarterectomy. Eur J Cardiothorac Surg 2008; 34: 525–529. [DOI] [PubMed] [Google Scholar]
- 37.Riedel M, Stanek V, Widimsky J, et al. . Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982; 81: 151–158. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins DP, Madani M, Mayer E, et al. . Surgical treatment of chronic thromboembolic pulmonary hypertension. Eur Respir J 2013; 41: 735–742. [DOI] [PubMed] [Google Scholar]
- 39.Auger WR, Kerr KM, Kim NH, et al. . Evaluation of patients with chronic thromboembolic pulmonary hypertension for pulmonary endarterectomy. Pulm Circ 2012; 2: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang I, Meyer BC, Ogo T, et al. . Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghofrani HA, D'Armini AM, Grimminger F, et al. . Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 42.Simonneau G, D'Armini AM, Ghofrani HA, et al. . Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J 2015; 45: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 43.Simonneau G, D'Armini AM, Ghofrani HA, et al. . Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 372–380. [DOI] [PubMed] [Google Scholar]
- 44.Wiedenroth CB, Liebetrau C, Breithecker A, et al. . Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2016; 35: 591–596. [DOI] [PubMed] [Google Scholar]
- 45.de Perrot M, Mayer E. Chronic thromboembolic pulmonary hypertension: do we need a new definition? Eur Respir J 2014; 44: 1401–1403. [DOI] [PubMed] [Google Scholar]
- 46.Collaud S, Brenot P, Mercier O, et al. . Rescue balloon pulmonary angioplasty for early failure of pulmonary endarterectomy: the earlier the better? Int J Cardiol 2016; 222: 39–40. [DOI] [PubMed] [Google Scholar]
- 47.Shimura N, Kataoka M, Inami T, et al. . Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2015; 183: 138–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A.M. D'Armini ERR-0111-2016_DArmini (1.2MB, pdf)
D. Jenkins ERR-0111-2016_Jenkins (1.2MB, pdf)
M. Madani ERR-0111-2016_Madani (1.2MB, pdf)
E. Mayer ERR-0111-2016_Mayer (1.2MB, pdf)