Abstract
Background
The risk for thromboembolisms in nonsmall cell lung cancer (NSCLC) patients is increased and often requires treatment or prophylaxis with direct oral anticoagulants (DOACs). Small-molecule inhibitors (SMIs) to treat NSCLC may cause relevant drug–drug interactions (DDIs) with DOACs. Guidance on how to combine these drugs is lacking, leaving patients at risk of clotting or bleeding. Here, we give practical recommendations to manage these DDIs.
Methods
For all DOACs and SMIs approved in Europe and the USA up to December 2021, a literature review was executed and reviews by the US Food and Drug Administration and European Medicines Agency were analysed for information on DDIs. A DDI potency classification for DOACs was composed and brought together with DDI characteristics of each SMI, resulting in recommendations for each combination.
Results
Half of the combinations result in relevant DDIs, requiring an intervention to prevent ineffective or toxic treatment with DOACs. These actions include dose adjustments, separation of administration or switching between anticoagulant therapies. Combinations of SMIs with edoxaban never cause relevant DDIs, compared to more than half of combinations with other DOACs and even increasing to almost all combinations with rivaroxaban.
Conclusions
Combinations of SMIs and DOACs often result in relevant DDIs that can be prevented by adjusting the DOAC dosage, separation of administration or switching between anticoagulants.
Short abstract
Lung cancer patients treated with targeted therapy often develop thromboembolic events requiring direct oral anticoagulants (DOACs). This article provides practical recommendations on how to safely combine DOACs and targeted therapies. https://bit.ly/382NBZ1
Introduction
Lung cancer patients frequently have a vital indication for the use of anticoagulant therapy. They have an increased risk of venous thromboembolisms (VTEs) and other thromboembolic complications, with an incidence of one in six amongst all lung cancer patients [1, 2]. In ROS1 gene-rearranged nonsmall cell lung cancer (NSCLC), this even increases to one in three [3]. Cancer-related VTEs are associated with higher mortality compared to noncancer-related VTEs [4]. The incidence of stroke and atrial fibrillation is also increased in the lung cancer population compared to the general population [5]. Therefore, prevention (both primary and secondary) and treatment of thromboembolisms is a highly relevant issue for lung cancer patients.
To date, direct oral anticoagulants (DOACs) are the standard of care in the treatment of VTEs and in the prevention of thromboembolic complications of atrial fibrillation because they are at least as effective, safe and more convenient than low molecular weight heparins (LMWHs) or vitamin K antagonists (VKAs). DOACs, formerly referred to as “novel or new oral anticoagulants”, are direct inhibitors of activated factor Xa (apixaban, edoxaban and rivaroxaban) or prevent activation of factor II reversibly (dabigatran). DOACs can be taken orally and do not require frequent monitoring, in contrast to LMWHs and VKAs, which either require daily subcutaneous injection or frequent monitoring of the internal normalised ratio. Double-blind randomised clinical studies support the use of the factor Xa inhibitors edoxaban, apixaban and rivaroxaban to treat cancer-associated VTEs [6–9]. Due to the proven efficacy, convenience and patient preference of DOACs compared to LMWHs and VKAs [10–12], recommendations for the use of DOACs in cancer patients have also been included in guidelines. DOACs are recommended in cancer patients without renal and/or hepatic impairment, with low risk of bleeding and without genitourinary or gastro–intestinal tumours [13, 14]. However, guidelines also acknowledge that the use of DOACs is not without caveats when in combination with anticancer drugs as there are still concerns about the risk of bleeding and drug–drug interactions (DDIs) may occur [13, 14].
Small-molecule inhibitors (SMIs) are increasingly used as targeted therapy for NSCLC. At present, over 20 SMIs are approved by the US Food and Drug Administration (FDA), the majority of which as first line treatment, whilst by 2011 only three were available in the USA [15]. Given the inhibiting and/or inducing effects that the majority of SMIs have on cytochrome P450 isoenzyme 3A4 (CYP3A4) or drug efflux pumps P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), SMIs may cause DDIs with DOACs [16], which result in either increased or decreased exposure to DOACs. These DDIs can be clinically relevant [17, 18] as a clear relationship between systemic exposure to DOACS and the risk of bleeding events or VTEs has been well established [19–22]. Of note, DOACs are not expected to influence the efficacy and/or toxicity of the SMIs, so when it comes to DDI terminology, the SMIs are the perpetrators and the DOACs the victims of a potential DDI.
Despite the need to combine DOACs with SMIs, practical guidance on how to combine these drugs with the current SMI armamentarium to treat NSCLC is lacking, leaving the individual patient often deprived of optimal treatment. Here, we provide practical recommendations on how to combine DOACs with SMIs, when the combination of these drugs is indicated.
Methods
All SMIs with registration for the treatment of NSCLC in the USA or Europe and all DOACs approved in the USA or Europe were selected on 1 November 2021. First, all documents on DOACs and SMIs available from the FDA and the European Medicines Agency were reviewed for DDIs. Second, PubMed and Embase were searched for articles published until 1 November 2021, using the terms “drug interactions”, “P-glycoprotein”, “cytochrome p-450 CYP3A”, “breast-cancer resistance protein”, “factor Xa inhibitors”, “dabigatran” and all SMIs currently approved for NSCLC in the European Union and the USA. Retrieved publications were snowballed to find other relevant articles. For CYP3A4 (not P-gp or BCRP) DDIs, SMIs were reported as weak, moderate or strong inhibitors or inducers, consistent with regulatory grading [23, 24]. Currently, standardised in vivo methods or classifications for P-gp and BCRP interactions do not exist. If such a DDI is assessed in vitro, the DDI is considered relevant if the in vivo plasma concentrations exceed the concentrations needed to inhibit the P-gp or BCRP (Ki ≤ maximum expected concentration at site of action). If a DDI is considered irrelevant, it is not reported. DDI potency classifications were defined for each DOAC to achieve transparent and structured grading of DDIs (table 1). Based on these DDI potency classifications for DOACs, specific recommendations for each combination of SMI and DOAC were made by expert opinion in a panel consisting of pharmacists, lung oncologists and haematologists. Grading for these specific recommendations is also mentioned in parentheses in the separate SMI sections.
TABLE 1.
Drug–drug interaction (DDI) potency classification, reflecting the approach used for recommendations in figure 1
| Colour | DDI potency classification |
| Green | No relevant interaction, combination is expected to be safe. |
| Yellow | Interaction of weak magnitude, relevance unclear or unlikely. No a priori dose adjustment of the DOAC required. |
| Amber | Interaction of moderate-potent magnitude, requiring an a priori dose or administration time adjustment of the DOAC. |
| Red | Interaction of potent magnitude which cannot be managed by a dose adjustment. Co-administration should be avoided as it can have deleterious consequences. |
Choosing the DDI with the lowest classification is recommended (see “General recommendations” section). DOAC: direct oral anticoagulant. Adapted from [25].
Results
In total, five DOACs and 21 SMIs were selected. An overview of our recommendations for each combination is provided in figure 1. First, general recommendations are given. Second, the potential role of laboratory monitoring to individualise DOAC treatment is discussed. Third, considerations regarding practical recommendations to combine each SMI with DOACs are specified. The DDI potency classification for each DOAC is described in the supplementary material.
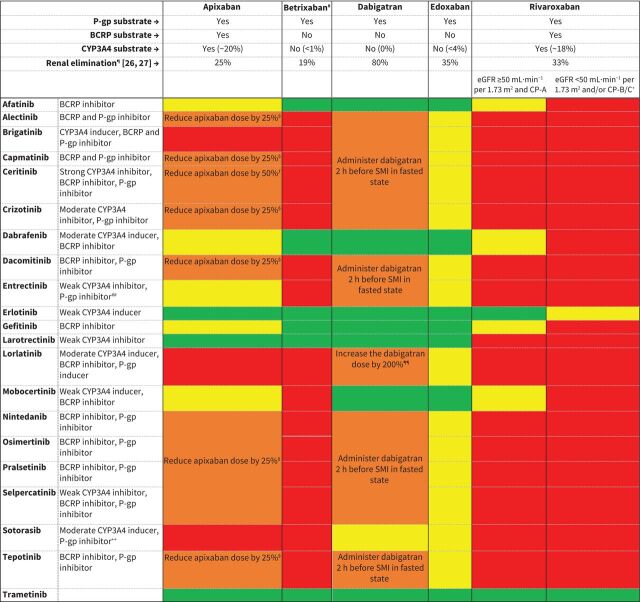
FIGURE 1.
Recommendations for combining direct oral anticoagulants (DOACs) and small-molecule inhibitors (SMIs) and dose adjustments of DOACs (classification based on table 1). BCRP: breast cancer resistance protein; CP-A/B/C: Child–Pugh A/B/C; CYP3A4: cytochrome P450 isoenzyme 3A4; dabigatran: dabigatran-etexilate; eGFR: estimated glomerular filtration rate; P-gp: P-glycoprotein. #: only available in the USA. ¶: percentage of dose excreted in urine as unchanged drug. +: in patients with renal or hepatic impairment (eGFR <50 mL·min−1 per 1.73 m2 and/or CP-B/C), concomitant use of inhibitors of P-gp, BCRP or CYP3A4 could result in an extensive increase in exposure of rivaroxaban and hence, bleeding risk [28]; thus, extra caution is warranted. §: reduce apixaban dose by 25% with P-gp inhibitors: 5 mg twice daily → 5 mg morning and 2.5 mg afternoon/10 mg twice daily → 7.5 mg twice daily. ƒ: reduce apixaban dose by 50% with strong CYP3A4 inhibitors, e.g. ceritinib: 5 mg twice daily → 2.5 mg twice daily/10 mg twice daily → 5 mg twice daily. ##: entrectinib increases exposure to digoxin, a sensitive P-gp substrate, by only 18%; therefore, no dose reduction of apixaban with entrectinib is warranted (yellow). ¶¶: increase dabigatran dose by 200%: 150 mg twice daily → 450 mg twice daily/110 mg twice daily → 330 mg twice daily. ++: sotorasib increases exposure to digoxin, a sensitive P-gp substrate, by only 21%; therefore, separation of administration of dabigatran with sotorasib is not needed (yellow).
General recommendations
Figure 1 summarises the recommendations for combination of all SMIs and DOACs. These are further explained below in detail for each SMI. For each SMI, we advise choosing the DOAC where the classification is green. In the absence of a green classification, we advise choosing the yellow option and in the absence of a yellow option we advise choosing the amber option. As betrixaban is only available in the USA, this DOAC is not an option for other countries. For dabigatran, the physician should also take into consideration that currently no double-blind randomised clinical studies have been performed to assess its efficacy and safety in the oncology population. Furthermore, DOAC dose individualisation based on laboratory monitoring may be of added value, which is further explained below.
The potential role of laboratory monitoring to individualise DOAC treatment
Although laboratory monitoring of DOAC treatment cannot be considered routine care, there is accumulating evidence that laboratory monitoring to individualise the dose of DOACs may be useful in certain situations, such as in the presence of a DDI [29–31]. As mentioned earlier, a clear relationship between plasma concentrations and treatment outcomes exists for all DOACs [19–22]. Furthermore, due to dose-exposure linearity, an adjustment of DOAC dosage will result in a proportional change in exposure, facilitating straightforward dose adjustments to correct for loss or increase in exposure [28, 32–34]. Since the magnitude of the effect of the DDIs between SMIs and DOACs remains largely uninvestigated, we postulate that laboratory monitoring of DOAC treatment may have the potential to overcome relevant (e.g. amber category) DDIs.
There are yet still hurdles to overcome to individualise DOAC dosing in case of DDIs. Although various routine coagulation assays, such as prothrombin time, thrombin time, activated partial thromboplastin time and chromogenic anti-Xa, may be able to detect differences in DOAC exposure and efficacy, the current gold standard to monitor DOAC treatment is considered quantification of DOAC plasma concentrations by means of liquid chromatography with mass spectrometry detection [35]. Although this technique is slowly becoming commonplace, it is not yet widely and routinely available in all clinics, hampering its routine implementation. Another hurdle may be that it is unknown what DOAC exposure should be targeted. Although normal ranges for peak and trough levels, associated with safe and effective treatment, are known for all DOACs in the absence of a DDI, reference values for the presence of a DDI may be different since peak and trough concentrations may not change equally during a DDI [35]. Lastly, there are no prospective studies showing the superiority of pharmacokinetically guided dose individualisation of DOAC treatment on the risk of bleeding and VTEs in the presence of DDIs. It should be noted, however, that this evidence is hard to gather, considering the many different DOACs, further complicated by the ever-increasing number of SMIs that are developed for small populations with rare mutations.
Although a clear recommendation cannot be made regarding the use of pharmacokinetically guided dosing of DOACs, we advise measuring DOAC plasma concentrations when a relevant DDI is expected (e.g. amber category) and switching to another anticoagulant is not an option. The measured concentration could then be interpreted based on known normal values, as proposed earlier, or based on a measured DOAC trough concentration in the absence of the interaction, if possible [35].
Practical recommendations to combine an SMI with DOACs
Afatinib
Afatinib inhibits BCRP in vitro [36–38]. Apixaban and rivaroxaban are transported by BCRP [39–42]. Thus, exposure to these DOACs may increase when combined with afatinib, which is expected to result in a DDI of weak magnitude (yellow). Afatinib also inhibits P-gp in vitro, but afatinib plasma concentrations in clinical use are lower than concentrations needed to inhibit P-gp. Therefore, no relevant DDI via P-gp is expected between afatinib and P-gp substrates. This rationale was confirmed by clinical data [43]. Since dabigatran, betrixaban and edoxaban are only transported by P-gp, no DDI between afatinib and exposure to these DOACs is expected (green) [44–48]. Therefore, the use of dabigatran, edoxaban or betrixaban is recommended when the combination of afatinib and a DOAC is indicated.
Alectinib
The potency of alectinib to inhibit BCRP and P-gp was assessed in vitro for M4 (active metabolite) and alectinib itself. The inhibitory effect of alectinib and M4 on these transporters was potent [49]. Co-administration of alectinib with all DOACs may increase their exposure. The effect of P-gp inhibition (without simultaneous CYP3A4 inhibition) on rivaroxaban exposure is unknown and, as an increase in exposure may result in an unacceptable increase in toxicity, co-administration is contraindicated (red). Betrixaban is contraindicated in combination with P-gp inhibitors as well (red). Exposure to edoxaban can also be increased when co-administered with alectinib, but this effect is expected to be of weak magnitude (yellow). For dabigatran, an increase in exposure via P-gp inhibition by alectinib can be prevented by administering dabigatran 2 h before alectinib in fasted state (amber). The increase in apixaban exposure can be mitigated by reducing the dose by 25% (amber). Alternatively, LMWHs or VKAs can be used to avoid any DDI. If treatment with these drugs is unwanted, edoxaban may be combined with alectinib.
Brigatinib
Brigatinib is reported to induce CYP3A4 (classification unknown) and to inhibit P-gp and BCRP in vitro [50, 51]. The net effect of these opposite mechanisms on exposure of apixaban and rivaroxaban, which are both CYP3A4 and P-gp/BCRP substrates, is unknown. Therefore, co-administration of brigatinib with apixaban or rivaroxaban is contraindicated (red). As all DOACs are P-gp substrates, exposure to all DOACs may alter and the use of an LMWH or VKA is recommended. Co-administration of betrixaban with a P-gp inhibitor is associated with bleeding events and thus, co-administration is contraindicated (red). The effect of P-gp inhibition on exposure to edoxaban is expected to be weak (yellow). For dabigatran, increase of exposure may be limited by administering dabigatran 2 h before brigatinib in fasted state (amber). If a treatment with a DOAC is preferred, then edoxaban may be the most appropriate option.
Capmatinib
Capmatinib inhibits both P-gp and BCRP in vivo [52]. Co-administration of capmatinib with digoxin or rosuvastatin, sensitive P-gp and BCRP substrates, increased exposure to these drugs by 47% and 108%, respectively [53]. Similar effects could occur with all DOACs, as they are all substrates of P-gp and/or BCRP. As the use of metabolism/transporter inhibitors, i.e. capmatinib, may increase bleeding risk of betrixaban and rivaroxaban, co-administration is contraindicated (red). If needed, apixaban may be combined with capmatinib whilst reducing the dose of apixaban by 25% or with dabigatran whilst administrating dabigatran 2 h before capmatinib in fasted state (amber). To avoid any DDI, the use of an LMWH or VKA is recommended. If combined use with a DOAC is indicated, the use of edoxaban could be considered, as the expected increase and consequent increase in bleeding risk is likely to be the least (yellow) compared to other DOACs.
Ceritinib
Ceritinib inhibits P-gp and BCRP in vitro and is a strong inhibitor of CYP3A4 in vivo [54]. Exposure to midazolam, a sensitive CYP3A4 substrate, was increased 5.4-fold after co-administration with ceritinib in vivo [54, 55]. Thus, combining DOACs with ceritinib is expected to increase exposure to all DOACs, which will lead to an unacceptable increase in bleeding risk with betrixaban and rivaroxaban. These DOACs are therefore contraindicated in combination with ceritinib (red). The effect of ceritinib to exposure of edoxaban is expected to be weak (yellow), as possible increases in edoxaban exposure are not expected to result in an unfavourable risk–benefit ratio. Thus, treatment with edoxaban is recommended when VKAs or LMWHs are considered undesirable treatment options. If co-administration of ceritinib with apixaban or dabigatran is deemed necessary, a dose reduction of apixaban by 50% should be applied or dabigatran should be administered 2 h before ceritinib in fasted state (amber).
Crizotinib
Crizotinib is classified as a moderate CYP3A4 inhibitor and has been shown to increase exposure to midazolam 3.7-fold in a single-dose in vivo study [56–58]. This moderate classification is currently under discussion as crizotinib inhibits its own metabolism time-dependently, which may result in fluctuating CYP3A4 inhibition. No CYP3A4 inhibition was found in mice treated with crizotinib for 14 days as measured by erythromycin N-demethylation in liver microsomes [59]. However, a study using a physiologically based pharmacokinetic model investigated the effects of multiple doses of crizotinib on midazolam exposure and found comparable results to the initial clinical single-dose study [60]. Therefore, crizotinib is classified as a moderate CYP3A4 inhibitor in this article. Crizotinib also inhibits P-gp in vitro [56, 57] and, consequently, increases exposure to all DOACs. As the use of P-gp inhibitors, e.g. crizotinib, increases bleeding risk of betrixaban and rivaroxaban, co-administration is contraindicated (red). Apixaban may be combined with crizotinib if apixaban dosage is reduced (amber). Dabigatran may be combined if administered 2 h before crizotinib in fasted state (amber). The effect of P-gp inhibition is expected to be of weak magnitude with edoxaban (yellow) and, therefore, treatment with edoxaban is recommended if VKAs or LMWHs are not an option.
Dabrafenib
Dabrafenib decreased exposure to midazolam, a sensitive CYP3A4 substrate, by 65% in vivo and is, therefore, classified as a moderate CYP3A4 inducer [61, 62]. A similar decrease of exposure could occur with apixaban and rivaroxaban as these DOACs are substrates of CYP3A4. The European label also states that dabrafenib may induce P-gp, but this is not mentioned in the FDA label and further information is lacking in the European Public Assessment Report and FDA Clinical Pharmacology and Biopharmaceutics Review [61–64]. Dabrafenib is therefore not classified as a P-gp inducer in this article. Co-administration of dabrafenib with rosuvastatin, a BCRP substate, increased its peak concentration 2.6-fold in vivo but did not significantly alter its area under the concentration–time curve [65]. Dabrafenib is therefore classified a BCRP inhibitor. Apixaban and rivaroxaban are both BCRP substrates but the net effect of CYP3A4 induction and BCRP inhibition on exposure to apixaban and rivaroxaban is unknown. DDIs of weak magnitude (yellow) are expected when combining apixaban or rivaroxaban with dabrafenib in patients with normal renal and hepatic function (estimated glomerular filtration rate ≥50 mL·min−1 per 1.73 m2 and Child–Pugh A). Betrixaban, dabigatran or edoxaban can be safely applied with dabrafenib (green).
Dacomitinib
In vitro studies indicate that dacomitinib inhibits P-gp and BCRP [66, 67]. Exposure may increase to all DOACs and may lead to an unacceptable increase in bleeding risk with betrixaban and rivaroxaban. These DOACs are therefore contraindicated when combined with dacomitinib (red). If needed, apixaban may be used concomitantly with dacomitinib if the apixaban dosage is reduced by 25% (amber). Dabigatran may be combined with dacomitinib if dabigatran is administered 2 h before dacomitinib in fasted state (amber). The effect of dacomitinib on exposure to edoxaban is expected to be least of all DOACs (yellow). To avoid any DDIs, LMWHs or VKAs may be applied concomitantly with dacomitinib.
Entrectinib
Co-administration of entrectinib with midazolam, a CYP3A4 substrate, increased exposure to midazolam by 50% in vivo [68, 69]. Accordingly, entrectinib is classified as a weak CYP3A4 inhibitor and may increase exposure to apixaban and rivaroxaban. The in vivo inhibitory potency of entrectinib on P-gp was assessed by co-administration with digoxin, a P-gp substrate, and exposure to digoxin was increased by 18% [68, 69]. A similar effect may occur with all DOACs, as they are all P-gp substrates. As this is unlikely to exceed the limits of increase in exposure of apixaban and edoxaban, only a weak interaction is expected, and no dose adjustments are advised (yellow). For rivaroxaban, every increase of exposure will lead to an increased risk of side effects and, thus, co-administration is contraindicated (red). Betrixaban is contraindicated with P-gp inhibitors, e.g. entrectinib, as well (red). If needed, co-administration of entrectinib with dabigatran may be applied while administrating dabigatran 2 h before entrectinib in fasted state (amber). If co-administration of a DOAC with entrectinib is indicated, apixaban or edoxaban could be applied as DDIs with entrectinib affect these DOACs the least.
Erlotinib
Erlotinib induces CYP3A4. When combining erlotinib with midazolam, a sensitive CYP3A4 substrate, in vivo, erlotinib only decreased exposure to midazolam by 24% [70]. According to official grading, erlotinib is therefore classified as a weak CYP3A4 inducer (this contradicts with erlotinib's summary of product characteristics, where it is classified as a moderate CYP3A4 inducer [70]) and is not expected to affect exposure to the CYP3A4 substrates apixaban and rivaroxaban significantly. Erlotinib did not alter exposure to edoxaban, a P-gp substrate, in vivo either [71]. All DOACs can be co-administered with erlotinib safely (green) in patients with normal renal and hepatic function (estimated glomerular filtration rate ≥50 mL·min−1 per 1.73 m2 and Child–Pugh A).
Gefitinib
Gefitinib inhibits BCRP in vitro, which may increase exposure to DOACs that are BCRP substrates in vivo [72]. Thus, combined use of apixaban and rivaroxaban with gefitinib should be approached with caution (yellow). Gefitinib did not increase exposure to edoxaban, not a BCRP substrate, in vivo [71]. Therefore, the use of dabigatran, edoxaban and betrixaban, which are not BCRP substrates, is recommended (green).
Larotrectinib
Co-administration of larotrectinib with midazolam, a sensitive CYP3A4 substrate, increased exposure to midazolam by 70% in vivo [73]. Therefore, larotrectinib can be classified as a weak CYP3A4 inhibitor. Inhibition of metabolism of apixaban and rivaroxaban is expected, as they are substrates of CYP3A4. Any increase in exposure of rivaroxaban will lead to increased risk of bleeding complications and, hence, co-administration of rivaroxaban with larotrectinib should be avoided (red). However, co-administration of apixaban with diltiazem, a moderate CYP3A4 inhibitor, increased exposure to apixaban by 40%. The effect of larotrectinib, a weak CYP3A4 inhibitor, will be of smaller magnitude and unlikely to be clinically relevant (green). However, to avoid any possible DDI, the use of DOACs that are not substrates of CYP3A4 (betrixaban, dabigatran or edoxaban) is recommended (green).
Lorlatinib
Lorlatinib decreased exposure to midazolam, a sensitive CYP3A4 substrate, by 60% in vivo and is therefore classified as a moderate CYP3A4 inducer. Because lorlatinib also decreased exposure to fexofenadine by 67% in vivo, which is a sensitive P-gp substrate, lorlatinib is also a P-gp inducer. It is unknown what the effect is of a decrease in exposure of betrixaban through P-gp induction and, therefore, co-administration of betrixaban with lorlatinib is contraindicated (red). Furthermore, in vitro studies indicate that lorlatinib inhibits BCRP [74, 75]. It is unknown what the effect of moderate CYP3A4 induction and P-gp induction is on the exposure to apixaban and rivaroxaban, which are both CYP3A4 and P-gp substrates. The effect of lorlatinib on apixaban and rivaroxaban may be estimated when compared with the effect of rifampicin, a strong CYP3A4 and P-gp inducer. Rifampicin reduced exposure to apixaban and rivaroxaban by approximately 50% [39, 40, 42]. For apixaban, co-administration with lorlatinib may exceed the limit of 25% reduction of exposure. Therefore, co-administration of lorlatinib with apixaban is contraindicated (red). For rivaroxaban, a dose increment to overcome decreased exposure cannot be recommended, as the precise effect of lorlatinib on rivaroxaban exposure cannot be predicted [20]. Therefore, co-administration of lorlatinib with rivaroxaban is contraindicated as well (red). P-gp induction by lorlatinib also reduces exposure to dabigatran, which is a sensitive P-gp substrate. This loss of exposure can be corrected by increasing the dose by 200% (amber). This is an off-label dose increase which may lead to higher exposure compared to dabigatran monotherapy, but increases of dabigatran exposure up to 150% are considered irrelevant (see supplementary material “DDI potency classification of DOACs, dabigatran”). However, lorlatinib will only affect exposure to edoxaban for a minor fraction as it induces P-gp but inhibits organic anion transporting polypeptide 1B1 just as rifampicin (see supplementary material “DDI potency classification of DOACs, edoxaban”) and therefore, edoxaban may be the most appropriate option if co-administration of lorlatinib with a DOAC is indicated (yellow).
Mobocertinib
Mobocertinib decreased exposure to midazolam, a sensitive CYP3A4 substrate, by 32% in vivo and can therefore be classified as a weak CYP3A4 inducer [76]. Mobocertinib is also reported to be an inhibitor of P-gp and BCRP, but no relevant changes in pharmacokinetic characteristics of sensitive P-gp substrates (e.g. dabigatran) are expected as no clinically relevant effect was predicted in modelling techniques [76, 77]. The impact of co-administration of mobocertinib on BCRP substrates, e.g. apixaban and rivaroxaban, in vivo remains unclear, as the analysis to clarify this potential DDI is considered inadequate by the FDA [77]. For safety, we considered mobocertinib as a BCRP inhibitor. The net effect of weak CYP3A4 induction and BCRP inhibition on exposure to rivaroxaban and apixaban, both BCRP and CYP3A4 substrates, is unknown but expected to result in a DDI of weak magnitude (yellow). Although no relevant changes in exposure to P-gp substrates are predicted, co-administration of mobocertinib with betrixaban remains contraindicated as the adverse outcomes of combining betrixaban with P-gp inhibitors cannot be explained by increased exposure of betrixaban but may be related to underlying patient factor(s) (red). Co-administration of mobocertinib with dabigatran or edoxaban, which are solely P-gp substrates, is not expected to result in a clinically relevant DDI and is therefore considered safe (green).
Nintedanib
Nintedanib inhibits P-gp and BCRP in vitro [78, 79]. Therefore, nintedanib may increase exposure to all DOACs and co-administration with rivaroxaban and betrixaban is contraindicated (red). The increase in exposure of apixaban should be corrected by reducing the dose by 25% (amber). For dabigatran, an increase in exposure can be avoided by administrating dabigatran 2 h before nintedanib in fasted state (amber). As the effect of nintedanib on exposure of edoxaban is expected to be of weak magnitude (yellow), edoxaban may be the most appropriate option when co-administration of nintedanib with DOACs is indicated.
Osimertinib
Co-administration of osimertinib and simvastatin, a sensitive CYP3A4 substrate, increased simvastatin exposure by only 9% in vivo [80, 81]. As this does not exceed 20%, osimertinib cannot be classified a weak CYP3A4 inhibitor and the inhibitory effect is considered irrelevant. Osimertinib inhibits P-gp and BCRP. Co-administration of osimertinib with fexofenadine and rosuvastatin, a P-gp and BCRP substrate, increased exposure of fexofenadine and rosuvastatin by 56% and 35% in vivo, respectively [80]. A similar effect may occur with DOACs that are substrates of P-gp and/or BCRP. Thus, co-administration of osimertinib with betrixaban and rivaroxaban is contraindicated (red). If needed, osimertinib may be combined with apixaban by reducing the apixaban dose by 25% (amber). Dabigatran and edoxaban are only P-gp and not BCRP substrates. For dabigatran, a DDI via P-gp inhibition can be circumvented if dabigatran is administered 2 h before osimertinib in fasted state (amber). For edoxaban, co-administration with osimertinib does not require dose adjustments (yellow). Therefore, edoxaban is considered the best alternative for LMWHs or VKAs.
Pralsetinib
Pralsetinib is reported to be an inhibitor of P-gp and BCRP in vitro [81]. Dabigatran and edoxaban are only substrates of P-gp and, thus, minimal impact of a DDI via P-gp on exposure and bleeding risk of edoxaban is expected (yellow). Dabigatran may be applied while administrating it 2 h before pralsetinib in fasted state (amber). Rivaroxaban and betrixaban are contraindicated with P-gp inhibitors, e.g. pralsetinib (red). Apixaban may be applied while reducing its dose by 25% (amber). Thus, edoxaban is expected to be safest to use of all DOACs when co-administered with pralsetinib.
Selpercatinib
After co-administration of selpercatinib with the CYP3A4 substrate midazolam, exposure to midazolam increased by 54% in vivo and is therefore classified a weak CYP3A4 inhibitor [82, 83]. As apixaban and rivaroxaban are CYP3A4 substrates, exposure to these DOACs may increase. Selpercatinib also inhibits P-gp and BCRP in vitro and, thus, increases exposure to all DOACs as all are P-gp and/or BCRP substrates [82, 83]. Co-administration of betrixaban with P-gp inhibitors, e.g. selpercatinib, is contraindicated due to the observed increased bleeding risk (red). The P-gp and BCRP inhibitory effect of selpercatinib on exposure to apixaban may be diminished by reducing the apixaban dose (amber). For dabigatran, the P-gp inhibitory effect of selpercatinib may be circumvented by administrating dabigatran 2 h before selpercatinib in fasted state (amber). As every increase in rivaroxaban exposure leads to an increase in bleeding risk and no dose reduction can be applied (due to available tablet doses), co-administration of selpercatinib with rivaroxaban is contraindicated (red). If co-administration with a DOAC is indicated, edoxaban is considered the safest to use of all DOACs.
Sotorasib
Sotorasib has the potential to cause DDIs due to moderate induction of CYP3A4. Co-administration of sotorasib with midazolam, a CYP3A4 substrate, decreased exposure to midazolam by 53% in vivo [84]. A similar effect could occur with apixaban and rivaroxaban, which are CYP3A4 substrates. Furthermore, sotorasib may increase exposure to all DOACs as it inhibits P-gp. Combined use of sotorasib and digoxin increased exposure to digoxin by 21% in vivo [84]. The net effect of CYP3A4 induction and P-gp inhibition on DOACs which are both CYP3A4 and P-gp substrates (apixaban and rivaroxaban) is unknown and, accordingly, co-administration is contraindicated (red). Co-administration of sotorasib and betrixaban is contraindicated (red), as the combination of P-gp inhibitors, e.g. sotorasib with betrixaban, is associated with an increased bleeding risk. The effect of sotorasib on the P-gp substrates dabigatran and edoxaban is expected to be of weak magnitude as an approximately 20% increase of exposure is not likely to result in adverse effects (yellow). Therefore, these DOACs are considered safest of all DOACs to use with sotorasib.
Tepotinib
Co-administration of tepotinib with dabigatran, a sensitive P-gp substrate, has been investigated as part of tepotinib's registration studies. Exposure to dabigatran increased by 50% in vivo after multiple doses of tepotinib, an increase which is considered of weak magnitude for edoxaban (yellow). Co-administration of tepotinib with apixaban may exceed the limit of 50% [85], and thus, apixaban's dose should be reduced by 25% when combined with tepotinib (amber). To avoid increases in dabigatran exposure via P-gp inhibition by tepotinib, dabigatran should be administered 2 h before tepotinib in fasted state (amber). As co-administration of betrixaban with P-gp inhibitors is associated with an unacceptable increase in bleeding risk, combined use of tepotinib with betrixaban is contraindicated (red). Tepotinib inhibits CYP3A4, but did not significantly change exposure to midazolam (a sensitive CYP3A4 substrate) in vivo [85]. The CYP3A4 inhibitory effect of tepotinib is therefore considered irrelevant. Tepotinib also inhibits BCRP in vitro and may increase exposure to apixaban and rivaroxaban, which are BCRP substrates [86]. As any increase in rivaroxaban exposure results in an unacceptable increase in bleeding risk, co-administration of tepotinib with rivaroxaban is contraindicated (red). Thus, co-administration of tepotinib with edoxaban is considered the most appropriate option when co-administration of tepotinib with a DOAC is indicated.
Trametinib
Trametinib induces CYP3A4 in vitro, but in vivo no relevant effect on exposure to everolimus (a sensitive CYP3A4 substrate) was demonstrated [87]. Therefore, no DDIs via CYP3A4 inhibition are expected. Moreover, trametinib does not inhibit P-gp or BCRP at relevant concentrations [87]. Thus, all DOACs may be used safely with trametinib (green).
Discussion
In approximately 50% of all combinations, co-administration of DOACs and SMIs results in moderately potent or potent DDIs. Thus, many relevant DDIs may occur, and this requires interference by the physician to prevent ineffective treatment or increased risk of bleeding caused by DOACs. Edoxaban never causes relevant DDIs that require intervention of physicians, as DDIs are categorised as yellow at most. Moreover, the SMI trametinib is the only SMI that does not cause any DDI with DOACs. In this article, we provide practical recommendations on how to manage DDIs between SMIs and DOACs.
When interpreting our recommendations, it is important to take in account other factors besides the DDIs between DOACs and SMIs. First, polypharmacy occurs in approximately 60% of NSCLC patients [88–90]. The use of multiple drugs may introduce DDIs in addition to DDIs between DOACs and SMIs. We would therefore urge pulmonologists and pharmacologists to carefully check the entire list of medications used by the patient. Second, patients’ characteristics such as body weight or age may also influence exposure to a DOAC. For example, dosage of apixaban should be reduced by 50% in patients 80 years or older and weighing 60 kg or less [39, 40]. In summary, such aspects should be taken into consideration when considering treatment modifications. Next to polypharmacy and patient characteristics, it is also important to take into account that increases of exposure and consequent increases of bleeding risk are gradient. Thus, it should be noted that yellow categories do not indicate that no increase of bleeding risk occurs, as seen in some retrospective studies [91, 92]. However, before introducing a drug, one should always balance the efficacy and toxicity and, based on those considerations, comparative assessments were made to categorise DDIs either yellow or amber.
Besides the established efficacy and toxicity of DOACs, e.g. prevention/treatment of thrombosis and risk of bleeding/thrombosis, there are also signs that DOACs may influence cancer disease behaviour. A systematic review by Najidh et al. [93] describes that DOACs affected cancer growth and metastasis in animal cancer models. Another retrospective observational database study in patients with nonvalvular atrial fibrillation found no association between the use of DOACs with the incidence of cancer [94]. Thus, further research should be conducted to elucidate the possible effect(s) of DOACs on cancer.
It is apparent that multiple DDIs have not been assessed in vivo or have not been assessed at all, e.g. P-gp inhibition and induction by SMIs. Consequently, it is unknown what the effect of these DDIs is on DOAC exposure. However, as all DOACs are P-gp substrates, and given the large number of patients using SMIs and DOACs concurrently, there is a high probability of DDI occurrence in clinical practice. In future, prospective in vivo DDI studies with probe P-gp substrates or DOACs should be mandatory as part of the official registration studies for SMIs.
Next to making in vivo DDI studies with DOACs mandatory, DOAC treatment could be further enhanced by individualising DOAC dosages. As mentioned in the results section under “The potential role of laboratory monitoring to individualise DOAC treatment”, monitoring of DOAC concentrations could assist in reducing the bleeding and thromboembolic risk. Dose regimens are already adjusted on expected increases or decreases of exposure to DOACs to correct for increases of bleeding risk or treatment failure in groups, e.g. reduced dosages of DOACs in impaired renal function and/or reduced body weight and/or older ages [39, 42, 45, 46, 48]. These group recommendations cause difficulty in treatment decisions for the individual patient. Individual patient characteristics often deviate from those of groups or the general population and certain patient characteristics may concomitantly increase and decrease exposure to DOACs, which creates uncertainty in the prediction of the net effect of these patient characteristics on exposure to DOACs. The pharmaceutical industry should therefore enable physicians and pharmacists to personalise DOAC treatment. In order to assess the multifactorial effects on exposure to DOACs in vivo, it should be made possible to measure exposure to DOACs and have practical recommendations how to deal with the measured exposures.
Thus, to improve anticoagulant treatment in patients treated with SMIs for lung cancer, we think the several stakeholders, such as clinicians, pharmaceutical industry and regulatory agencies, may actively contribute. First, for existing SMIs it should be investigated whether DOAC pharmacokinetics are relevantly altered in vivo. Second, for existing DOACs, pharmacokinetic therapeutic ranges should be established, to allow dose individualisation by means of therapeutic drug monitoring to counter relevant DDIs and other factors that affect DOAC exposure [31]. Lastly, regulatory agencies should encourage DDI studies between DOACs and drugs that are prescribed in populations where VTEs are frequent, such as cancer patients.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material: DDI potency of DOACs ERR-0004-2022.SUPPLEMENT (355.9KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: L.S. Otten has nothing to disclose.
Conflict of interest: B. Piet declares research funding paid to their institution by Amgen, AstraZeneca, Bristol-Myers Squibb, Janssen, Mirati and Novartis; honoraria for presentations, paid to their institution by AstraZeneca, Janssen and Pfizer; a personal travel grant from Roche; and advisory board fees paid to their institution by Bristol-Myers Squibb, Janssen, Pfizer, Takeda and Merck, all in the 36 months prior to manuscript submission; and that they hold the following roles: Dutch Society for Medical Oncology (NVMO): member of the committee Offlabel-indications Oncological Medications (Cie-OOM); Dutch Association of Physicians in Chest Medicine and Tuberculosis (NVALT): member of the committee for Professional Interests and the Guideline Committee on patient counselling about genetical Tumour diagnostics.
Conflict of interest: M.M. van den Heuvel declares research funding paid to their institution by AstraZeneca, Bristol-Myers Squibb, Janssen, Merck, Novartis, Stichting Treatmeds, Merck Sharp & Dohme, Pamgene, Pfizer, Roche and Roche Diagnostics; advisory board fees paid to their institution by Bristol-Myers Squibb, Pfizer, Merck, Merck Sharp & Dohme, AstraZeneca, Novartis and Roche-Genentech, all in the 36 months prior to manuscript submission; and that they are a member of the Dutch Association of Physicians in Chest Medicine and Tuberculosis (NVALT) and the Dutch Society for Medical Oncology (NVMO).
Conflict of interest: C. Marzolini declares research funding paid to their institution by Gilead; and honoraria for lectures, paid to their institution by Merck Sharp & Dohme, ViiV Healthcare and AbbVie, all in the 36 months prior to manuscript submission.
Conflict of interest: R.M.J.M. van Geel has nothing to disclose.
Conflict of interest: J.L. Gulikers has nothing to disclose.
Conflict of interest: D.M. Burger has nothing to disclose.
Conflict of interest: J. Leentjens has nothing to disclose.
Conflict of interest: R. ter Heine has nothing to disclose.
References
- 1.Tagalakis V, Levi D, Agulnik JS, et al. . High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. J Thorac Oncol 2007; 2: 729–734. doi: 10.1097/JTO.0b013e31811ea275 [DOI] [PubMed] [Google Scholar]
- 2.Numico G, Garrone O, Dongiovanni V, et al. . Prospective evaluation of major vascular events in patients with nonsmall cell lung carcinoma treated with cisplatin and gemcitabine. Cancer 2005; 103: 994–999. doi: 10.1002/cncr.20893 [DOI] [PubMed] [Google Scholar]
- 3.Ng TL, Smith DE, Mushtaq R, et al. . ROS1 gene rearrangements are associated with an elevated risk of peridiagnosis thromboembolic events. J Thorac Oncol 2019; 14: 596–605. doi: 10.1016/j.jtho.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 4.Puurunen MK, Gona PN, Larson MG, et al. . Epidemiology of venous thromboembolism in the Framingham Heart Study. Thromb Res 2016; 145: 27–33. doi: 10.1016/j.thromres.2016.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorigue M, Miljkovic MD. Atrial fibrillation and stroke risk in patients with cancer: a primer for oncologists. J Oncol Pract 2019; 15: 641–650. doi: 10.1200/JOP.18.00592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raskob GE, van Es N, Verhamme P, et al. . Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2017; 378: 615–624. doi: 10.1056/NEJMoa1711948 [DOI] [PubMed] [Google Scholar]
- 7.Agnelli G, Becattini C, Meyer G, et al. . Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med 2020; 382: 1599–1607. doi: 10.1056/NEJMoa1915103 [DOI] [PubMed] [Google Scholar]
- 8.McBane RD 2nd, Wysokinski WE, Le-Rademacher JG, et al. . Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost 2020; 18: 411–421. doi: 10.1111/jth.14662 [DOI] [PubMed] [Google Scholar]
- 9.Young AM, Marshall A, Thirlwall J, et al. . Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018; 36: 2017–2023. doi: 10.1200/JCO.2018.78.8034 [DOI] [PubMed] [Google Scholar]
- 10.Rostamnjad L, Dagogo-Jack I. Evaluation of direct oral anticoagulant use for cancer-associated venous thromboembolism (VTE) in lung cancer. J Clin Oncol 2021; 39: Suppl. 28, 243. doi: 10.1200/JCO.2020.39.28_suppl.243 [DOI] [Google Scholar]
- 11.O'Connell C, Escalante CP, Goldhaber SZ, et al. . Treatment of cancer-associated venous thromboembolism with low-molecular-weight heparin or direct oral anticoagulants: patient selection, controversies, and caveats. Oncologist 2021; 26: e8–e16. doi: 10.1002/onco.13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojtukiewicz MZ, Skalij P, Tokajuk P, et al. . Direct oral anticoagulants in cancer patients. Time for a change in paradigm. Cancers 2020; 12: 1144. doi: 10.3390/cancers12051144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Streiff MB, Abutalib SA, Farge D, et al. . Update on guidelines for the management of cancer-associated thrombosis. Oncologist 2021; 26: e24–e40. doi: 10.1002/onco.13596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsubaie NS, Al Rammah SM, Alshouimi RA, et al. . The use of direct oral anticoagulants for thromboprophylaxis or treatment of cancer-associated venous thromboembolism: a meta-analysis and review of the guidelines. Thromb J 2021; 19: 76. doi: 10.1186/s12959-021-00326-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roskoski R. Properties of FDA-approved small molecule protein kinase inhibitors: a 2021 update. Pharmacol Res 2021; 165: 105463. doi: 10.1016/j.phrs.2021.105463 [DOI] [PubMed] [Google Scholar]
- 16.van Leeuwen RWF, van Gelder T, Mathijssen RHJ, et al. . Drug–drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol 2014; 15: e315–e326. doi: 10.1016/S1470-2045(13)70579-5 [DOI] [PubMed] [Google Scholar]
- 17.Rashdan S, Yang H, Le T, et al. . Prevalence and significance of potential pharmacokinetic drug–drug interactions among patients with lung cancer: implications for clinical trials. Clin Drug Investig 2021; 41: 161–167. doi: 10.1007/s40261-020-00994-4 [DOI] [PubMed] [Google Scholar]
- 18.van Leeuwen RWF, Brundel DHS, Neef C, et al. . Prevalence of potential drug–drug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer 2013; 108: 1071–1078. doi: 10.1038/bjc.2013.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruff CT, Giugliano RP, Braunwald E, et al. . Association between edoxaban dose, concentration, anti-factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 2015; 385: 2288–2295. doi: 10.1016/S0140-6736(14)61943-7 [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Yan X, Nandy P, et al. . Influence of model-predicted rivaroxaban exposure and patient characteristics on efficacy and safety outcomes in patients with acute coronary syndrome. Ther Adv Cardiovasc Dis 2019; 13: 1753944719863641. doi: 10.1177/1753944719863641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly PA, Lehr T, Haertter S, et al. . The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 2014; 63: 321–328. doi: 10.1016/j.jacc.2013.07.104 [DOI] [PubMed] [Google Scholar]
- 22.Byon W, Garonzik S, Boyd RA, et al. . Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet 2019; 58: 1265–1279. doi: 10.1007/s40262-019-00775-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration . Drug Development and Drug Interactions. Table of Substrates, Inhibitors and Inducers. Date last updated: 10 March 2020. www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers
- 24.European Medicines Agency . Guideline on the investigation of drug interactions. Date last updated: 21 June 2012. www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf
- 25.Seden K, Gibbons S, Marzolini C, et al. . Development of an evidence evaluation and synthesis system for drug-drug interactions, and its application to a systematic review of HIV and malaria co-infection. PLoS One 2017; 12: e0173509. doi: 10.1371/journal.pone.0173509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber J, Olyaei A, Shatzel J. The efficacy and safety of direct oral anticoagulants in patients with chronic renal insufficiency: a review of the literature. Eur J Haematol 2019; 102: 312–318. doi: 10.1111/ejh.13208 [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration . BevyxXa (betrixaban) Clinical Pharmacology and Biopharmaceutics Review. 2016. www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208383Orig1s000ClinPharmR.pdf [Google Scholar]
- 28.European Medicines Agency . CHMP assessment report for Xarelto. Date last updated: 10 November 2008. www.ema.europa.eu/en/documents/assessment-report/xarelto-epar-public-assessment-report_en.pdf
- 29.Powell JR. Are new oral anticoagulant dosing recommendations optimal for all patients? JAMA 2015; 313: 1013–1014. doi: 10.1001/jama.2015.59 [DOI] [PubMed] [Google Scholar]
- 30.Patel JP, Byrne RA, Patel RK, et al. . Progress in the monitoring of direct oral anticoagulant therapy. Br J Haematol 2019; 184: 912–924. doi: 10.1111/bjh.15756 [DOI] [PubMed] [Google Scholar]
- 31.Bernier M, Lancrerot SL, Parassol N, et al. . Therapeutic drug monitoring of direct oral anticoagulants may increase their benefit-risk ratio. J Cardiovasc Pharmacol 2020; 76: 472–477. doi: 10.1097/FJC.0000000000000870 [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration . Eliquis (apixaban) Clinical Pharmacology and Biopharmaceutics Review. 2012. www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202155Orig1s000ClinPharmR.pdf
- 33.European Medicines Agency . CHMP assessment report for Pradaxa. Date last updated: 23 April 2008. www.ema.europa.eu/en/documents/assessment-report/pradaxa-epar-public-assessment-report_en.pdf
- 34.European Medicines Agency . Lixiana (edoxaban) Public Assessment Report. Date last updated: 3 July 2015. www.ema.europa.eu/en/documents/assessment-report/lixiana-epar-public-assessment-report_en.pdf
- 35.Dunois C. Laboratory monitoring of direct oral anticoagulants (DOACs). Biomedicines 2021; 9: 445. doi: 10.3390/biomedicines9050445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration . Gilotrif (afatinib) US Prescribing Information. Date last updated: January 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2018/201292s014lbl.pdf
- 37.European Medicines Agency . Giotrif (afatinib) Summary of Product Characteristics. Date last updated: 21 April 2021. www.ema.europa.eu/en/documents/product-information/giotrif-epar-product-information_en.pdf
- 38.Wind S, Schnell D, Ebner T, et al. . Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin Pharmacokinet 2017; 56: 235–250. doi: 10.1007/s40262-016-0440-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.European Medicines Agency . Eliquis (apixaban) Summary of Product Characteristics. Date last updated: 4 April 2022. www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf
- 40.US Food and Drug Administration . Eliquis (apixaban) US Prescribing Information. Date last updated: July 2016. www.accessdata.fda.gov/drugsatfda_docs/label/2016/202155s012lbl.pdf
- 41.European Medicines Agency . Xarelto (rivaroxaban) Summary of Product Characteristics. Date last updated: 13 December 2021. www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_en.pdf
- 42.US Food and Drug Administration . Xarelto (rivaroxaban) US Prescribing Information. Date last updated: December 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/215859s000lbl.pdf
- 43.US Food and Drug Administration . Gilotrif (afatinib) Clinical Pharmacology and Biopharmaceutics Review. 2012. www.accessdata.fda.gov/drugsatfda_docs/nda/2013/201292Orig1s000ClinPharmR.pdf
- 44.US Food and Drug Administration . Pradaxa (dabigatran-etexilate) US Prescribing Information. Date last updated: June 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/214358s000lbl.pdf
- 45.European Medicines Agency . Pradaxa (dabigatran-etexilate) Summary of Product Characteristics. Date last updated: 4 February 2022. www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_en.pdf
- 46.US Food and Drug Administration . Bevyxxa (betrixaban) US Prescribing information. Date last updated: August 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/208383s007lbl.pdf
- 47.US Food and Drug Administration . Savaysa (edoxaban) US Prescribing Information. Date last updated: August 2019. www.accessdata.fda.gov/drugsatfda_docs/label/2019/206316s015lbl.pdf
- 48.European Medicines Agency . Lixiana (edoxaban) Summary of Product Characteristics. Date last updated: 23 April 2021. www.ema.europa.eu/en/documents/product-information/lixiana-epar-product-information_en.pdf
- 49.US Food and Drug Administration . Alecensa (alectinib) Clinical Pharmacology and Biopharmaceutics Review. 2016. www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208434Orig1s000ClinPharmR.pdf
- 50.European Medicines Agency . Alunbrig (brigatinib) Summary of Product Characteristics. Date last updated: 29 March 2022. www.ema.europa.eu/en/documents/product-information/alunbrig-epar-product-information_en.pdf
- 51.US Food and Drug Administration . Alunbrig (brigatinib) US Prescribing Information. Date last updated: May 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/208772s008lbl.pdf
- 52.US Food and Drug Administration . Tabrecta (capmatinib) US Prescribing Information. Date last updated: May 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/213591s000lbl.pdf
- 53.US Food and Drug Administration . Tabrecta (capmatinib) Multi-Discipline Review. 2020. www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213591Orig1s000MultidisciplineR.pdf
- 54.European Medicines Agency . Zykadia (ceritinib) Summary of Product Characteristics. Date last updated: 25 February 2022. www.ema.europa.eu/en/documents/product-information/zykadia-epar-product-information_en.pdf
- 55.Hurtado FK, de Braud F, De Castro Carpeño J, et al. . Effect of ceritinib on the pharmacokinetics of coadministered CYP3A and 2C9 substrates: a phase I, multicenter, drug–drug interaction study in patients with ALK + advanced tumors. Cancer Chemother Pharmacol 2021; 87: 475–486. doi: 10.1007/s00280-020-04180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.European Medicines Agency . Xalkori (crizotinib) Summary of Product Characteristics. Date last updated: 21 February 2022. www.ema.europa.eu/en/documents/product-information/xalkori-epar-product-information_en.pdf
- 57.US Food and Drug Administration . Xalkori (crizotinib) US Prescribing Information. Date last updated: January 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/202570s030lbl.pdf
- 58.Tan W, Wilner KD, Bang Y, et al. . Pharmacokinetics (PK) of PF-02341066, a dual ALK/MET inhibitor after multiple oral doses to advanced cancer patients. J Clin Oncol 2010; 28: Suppl. 15, 2596. doi: 10.1200/jco.2010.28.15_suppl.2596 [DOI] [Google Scholar]
- 59.Bland AR, Shrestha N, Rosengren RJ, et al. . Does crizotinib auto-inhibit CYP3A in vivo? Pharmacology 2020; 105: 715–718. doi: 10.1159/000506996 [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki S, Johnson TR, Smith BJ. Prediction of drug–drug interactions with crizotinib as the CYP3A substrate using a physiologically based pharmacokinetic model. Drug Metab Dispos 2015; 43: 1417. doi: 10.1124/dmd.115.064618 [DOI] [PubMed] [Google Scholar]
- 61.European Medicines Agency . Tafinlar (dabrafenib) Summary of Product Characteristics. Date last updated: 9 December 2021. www.ema.europa.eu/en/documents/product-information/tafinlar-epar-product-information_en.pdf
- 62.US Food and Drug Administration . Tafinlar (dabrafenib) US Prescribing Information. Date last updated: May 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2018/202806s010lbl.pdf
- 63.European Medicines Agency . Tafinlar (dabrafenib) Public Assessment Report. Date last updated: 18 September 2013. www.ema.europa.eu/en/documents/assessment-report/tafinlar-epar-public-assessment-report_en.pdf
- 64.US Food and Drug Administration . Tafinlar (dabrafenib) Clinical Pharmacology and Biopharmaceutics Review. 2013. www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202806orig1s000clinpharmr.pdf
- 65.Nebot N, Won CS, Moreno V, et al. . Evaluation of the effects of repeat-dose dabrafenib on the single-dose pharmacokinetics of rosuvastatin (OATP1B1/1B3 substrate) and midazolam (CYP3A4 substrate). Clin Pharmacol Drug Dev 2021; 10: 1054–1063. doi: 10.1002/cpdd.937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.US Food and Drug Administration . Vizimpro (dacomitinib) US Prescribing Information. Date last updated: December 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/211288s003lbl.pdf
- 67.European Medicines Agency . Vizimpro (dacomitinib) Summary of Product Characteristics. Date last updated: 21 July 2021. www.ema.europa.eu/en/documents/product-information/vizimpro-epar-product-information_en.pdf
- 68.European Medicines Agency . Rozlytrek (entrectinib) CHMP assessment report. Date last updated: 11 September 2020. www.ema.europa.eu/en/documents/assessment-report/rozlytrek-epar-public-assessment-report_en.pdf
- 69.US Food and Drug Administration . Rozlytrek (entrectinib) US Prescribing Information. Date last updated: August 2019. www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf
- 70.European Medicines Agency . Tarceva (erlotinib) Summary of Product Characteristics. Date last updated: 18 August 2021. www.ema.europa.eu/en/documents/product-information/tarceva-epar-product-information_en.pdf
- 71.Hotta T, Tsubata Y, Hamai K, et al. . Pharmacokinetics of edoxaban in EGFR-mutated non-small cell lung cancer patients with venous thromboembolism. Respir Investig 2021; 59: 327–334. doi: 10.1016/j.resinv.2020.11.007 [DOI] [PubMed] [Google Scholar]
- 72.European Medicines Agency . Iressa (gefitinib) Summary of Product Characteristics. Date last updated: 5 March 2021. www.ema.europa.eu/en/documents/product-information/iressa-epar-product-information_en.pdf
- 73.European Medicines Agency . Vitrakvi (larotrectinib) Summary of Product Characteristics. Date last updated: 9 February 2022. www.ema.europa.eu/en/documents/product-information/vitrakvi-epar-product-information_en.pdf
- 74.US Food and Drug Administration . Lorbrena (lorlatinib) US Prescribing Information. Date last updated: March 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/210868s004lbl.pdf
- 75.European Medicines Agency . Lorviqua (lorlatinib) Summary of Product Characteristics. Date last updated: 7 April 2022. www.ema.europa.eu/en/documents/product-information/lorviqua-epar-product-information_en.pdf
- 76.US Food and Drug Administration . Exkivity (mobocertinib) US Prescribing Information. Date last updated: September 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/215310s000lbl.pdf
- 77.US Food and Drug Administration . Exkivity (mobocertinib) Multi-Discipline Review. 2021. www.accessdata.fda.gov/drugsatfda_docs/nda/2021/215310Orig1s000MultidisciplineR.pdf
- 78.European Medicines Agency . Ofev (nintedanib) Summary of Product Characteristics. Date last updated: 9 December 2021. www.ema.europa.eu/en/documents/product-information/ofev-epar-product-information_en.pdf
- 79.US Food and Drug Administration . Ofev (nintedanib) US Prescribing Information. Date last updated: March 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/205832s013lbl.pdf
- 80.European Medicines Agency . Tagrisso (osimertinib) Summary of Product Characteristics. Date last updated: 7 April 2022. www.ema.europa.eu/en/documents/product-information/tagrisso-epar-product-information_en.pdf
- 81.US Food and Drug Administration . Gavreto (pralsetinib) Multi-Discipline Review. 2020. www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213721Orig1s000MultidisciplineR.pdf
- 82.US Food and Drug Administration . Retevmo (selpercatinib) US Prescribing Information. Date last updated: May 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/213246s000lbl.pdf
- 83.European Medicines Agency . Retsevmo (selpercatinib) Summary of Product Characteristics. Date last updated: 14 March 2022. www.ema.europa.eu/en/documents/product-information/retsevmo-epar-product-information_en.pdf
- 84.US Food and Drug Administration . Lumakras (sotorasib) US Prescribing Information. Date last updated: May 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/214665s000lbl.pdf
- 85.Yalkinoglu Ö, Heuer J, Becker A, et al. . 480P – Drug–drug interaction profile of tepotinib with CYP3A and P-gp substrates. Ann Oncol 2019; 30: Suppl. 5, v182. doi: 10.1093/annonc/mdz244.042 [DOI] [Google Scholar]
- 86.US Food and Drug Administration . Tepmetko (tepotinib) US Prescribing Information. Date last updated: February 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/214096s000lbl.pdf
- 87.US Food and Drug Administration . Mekinist (trametinib) Clinical Pharmacology and Biopharmaceutics Review. 2013. www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204114Orig1s000ClinPharmR.pdf
- 88.Young EH, Pan S, Yap AG, et al. . Polypharmacy prevalence in older adults seen in United States physician offices from 2009 to 2016. PLoS One 2021; 16: e0255642. doi: 10.1371/journal.pone.0255642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morin L, Johnell K, Laroche ML, et al. . The epidemiology of polypharmacy in older adults: register-based prospective cohort study. Clin Epidemiol 2018; 10: 289–298. doi: 10.2147/CLEP.S153458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hakozaki T, Hosomi Y, Shimizu A, et al. . Polypharmacy as a prognostic factor in older patients with advanced non-small-cell lung cancer treated with anti-PD-1/PD-L1 antibody-based immunotherapy. J Cancer Res Clin Oncol 2020; 146: 2659–2668. doi: 10.1007/s00432-020-03252-4 [DOI] [PubMed] [Google Scholar]
- 91.Pham P, Schmidt S, Lesko L, et al. . Association of oral anticoagulants and verapamil or diltiazem with adverse bleeding events in patients with nonvalvular atrial fibrillation and normal kidney function. JAMA Netw Open 2020; 3: e203593. doi: 10.1001/jamanetworkopen.2020.3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanigan S, Das J, Pogue K, et al. . The real world use of combined p-glycoprotein and moderate CYP3A4 inhibitors with rivaroxaban or apixaban increases bleeding. J Thromb Thrombolysis 2020; 49: 636–643. doi: 10.1007/s11239-020-02037-3 [DOI] [PubMed] [Google Scholar]
- 93.Najidh S, Versteeg HH, Buijs JT. A systematic review on the effects of direct oral anticoagulants on cancer growth and metastasis in animal models. Thromb Res 2020; 187: 18–27. doi: 10.1016/j.thromres.2019.12.022 [DOI] [PubMed] [Google Scholar]
- 94.Abrahami D, Renoux C, Yin H, et al. . The association between oral anticoagulants and cancer incidence among individuals with nonvalvular atrial fibrillation. Thromb Haemost 2020; 120: 1384–1394. doi: 10.1055/s-0040-1714213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material: DDI potency of DOACs ERR-0004-2022.SUPPLEMENT (355.9KB, pdf)