Abstract
The clinical expression of idiopathic pulmonary fibrosis (IPF) is directly related to multiple alterations in lung function. These alterations derive from a complex disease process affecting all compartments of the lower respiratory system, from the conducting airways to the lung vasculature. In this article we review the profound alterations in lung mechanics (reduced lung compliance and lung volumes), pulmonary gas exchange (reduced diffusing capacity, increased dead space ventilation, chronic arterial hypoxaemia) and airway physiology (increased cough reflex and increased airway volume), as well as pulmonary haemodynamics related to IPF. The relative contribution of these alterations to exertional limitation and dyspnoea in IPF is discussed.
Short abstract
Physiological impairment in IPF is complex and involves all compartments of the respiratory system http://ow.ly/gyao30hdHUb
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common idiopathic interstitial pneumonia and one of the most frequently diagnosed interstitial lung diseases (ILDs). Although the novel antifibrotic agents pirfenidone and nintedanib attenuate the progressive decline in lung function characteristic of IPF [1], reduce the risk of hospitalisation or exacerbation [2, 3], and reduce the risk of death [3–5], IPF is a very severe disease where clinical decline is common. IPF is of particular interest to the lung physiologist because its clinical expression, which ranges from exertional dyspnoea occurring early in the disease to end-stage respiratory failure, is directly related to alterations in lung physiology. The aim of the present article is to review the multiple alterations in lung function in IPF. The diagnosis of IPF relies on high-resolution computed tomography (HRCT) scanning and pathological studies [6, 7], and is not discussed here. The role of lung function studies for prognostication and clinical decision making has been the focus of recent reviews [8–10]. Few data are available regarding the additional physiological derangements occurring during IPF exacerbations, so the present review focuses on stable or progressive IPF.
Pathobiology and pathology of IPF
The factors driving the course of IPF are not definitively identified. Current concepts implicate chronic and/or repetitive microinjuries of the alveolar epithelium as triggers of the disease. Such microinjuries may include environmental pollutants such as cigarette smoke, acid aspiration due to gastro-oesophageal reflux, and viral infections. Epithelial damage is followed by injury and/or activation of cells lining the vascular (capillary endothelial cells, pericytes) and interstitial (resident mesenchymal cells including alveolar fibroblasts) compartments of the lung, as well as the epithelium of distal airways and resident macrophages. Eventually, lung mesenchymal cells accumulate, undergo glycolytic reprogramming [11], and differentiate to myofibroblasts, which are considered the effector cells of fibrogenesis. Whether the epithelial-to-mesenchymal transition or the recruitment of circulating fibrocytes participates in the increase in myofibroblast numbers in IPF is subject to debate. Myofibroblasts synthetise an abnormally stiff extracellular matrix [12] that further drives mesenchymal cell activation through mechano-transduced signals [13].
The contribution of genetic factors to IPF is suggested by the occurrence of IPF-like disease in patients with rare genetic disorders [14] and by cases of familial idiopathic interstitial pneumonia [15]. Analysis of genetic factors provides valuable insight into IPF pathogenesis. The development of acute and chronic fibrotic lung disease in patients with mutations in the pulmonary surfactant apoproteins SFTPA2 and SFTPC [15], or lipid transport genes such as ABCA3 [16], suggests that alterations in surfactant composition or metabolism play important roles in IPF. The occurrence of IPF-like disease in patients with mutations in components of the telomerase complex (TERT, TERC) or other telomere-associated proteins (DKC1, TINF2, RTEL1) suggests the contribution of genomic instability, defective cell homeostasis and/or cell senescence to IPF [14, 17]. A gain-of-function mutant allele in the promoter regions of the gene coding the secreted mucin MUC5B is found in ∼40% of patients with sporadic idiopathic interstitial pneumonia, including patients with IPF, versus 9–10% of control subjects [18, 19], although the mechanisms by which increased mucin production relates to alveolar fibrosis are not known.
IPF is associated with multiple pathological alterations involving most compartments of the lower respiratory system (figure 1). Lung fibrosis is defined by the replacement of the normal, compliant lung extracellular matrix, which is rich in elastin, with an abnormal matrix that is rich in fibrillar collagen [20]. Alveolar lesions conform to the usual interstitial pneumonia (UIP) pattern. The UIP pattern is defined by: 1) spatial heterogeneity, as lesions alternate with areas of normal lung; 2) temporal heterogeneity, with the concomitant presence of discrete lesions in lung tissue that appears otherwise normal (called fibroblastic foci) and fibrotic areas composed mainly of dense acellular collagen; and 3) the presence of honeycomb lesions, which are abnormal dilated airspaces with walls composed of fibrotic tissue, lined by an epithelium that shares characteristics with the airway epithelium [21, 22]. Tissular alterations are also present in other parts of the respiratory system in IPF. Expression of Ki67, a marker of cell proliferation, is detected in conducting airway epithelial cells [23, 24].
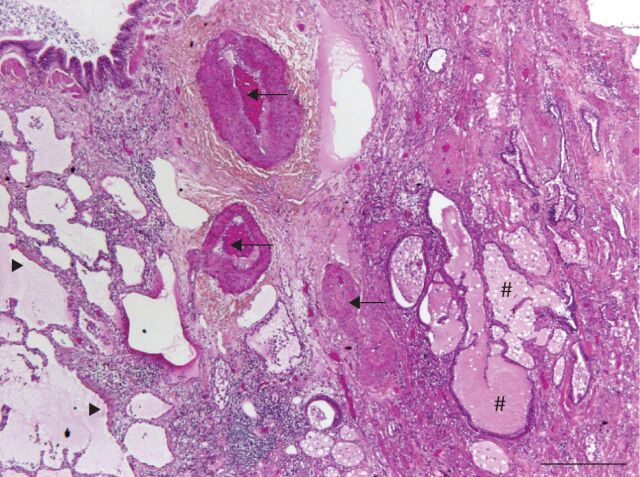
FIGURE 1.
Pathological alterations in idiopathic pulmonary fibrosis (IPF). Lung biopsy of a patient with IPF showing the usual interstitial pneumonia pattern (haematoxylin–eosin–saffron stain; ×10 magnification; scale bar=500 µm). Fibrotic lung with microscopic honeycomb change (#) and remodelled arteries (arrows) is visible on the right, adjacent to preserved alveolar parenchyma on the left. Fibroblastic foci (arrowheads) are visible in between. A bronchiole is visible in the upper left corner of the micrograph.
Distinct vascular alterations are observed in the fibrotic regions and the more preserved regions of IPF lungs. In areas of dense fibrosis, sharp decreases in vessel density are observed [25, 26]. The walls of pulmonary arteries show intimal fibrosis and medial hypertrophy, and are thickened [27, 28]. Pulmonary veins and venules present loose and intimal fibrosis with reduced calibre [28]. In the histologically preserved areas, occlusion of pulmonary venules is frequent (65% of patients) and is associated with alveolar capillary multiplication [26] and/or muscularisation of arterioles, whereas arterial lesions are rare [28]. Capillary density is increased in regions bordering fibroblastic foci and honeycomb cysts [25].
Alterations in the mechanical properties of the lung
Reduction in lung compliance
Lung compliance describes the ability of the lung to expand and is key to describing the lung from a mechanical point of view. It is defined as the change in lung volumes divided by the change in transpulmonary pressure. IPF results in profound reductions in lung compliance. This reduction in lung compliance is driven both by reductions in the compliance of the lung extracellular matrix and by alterations in the pulmonary surfactant. In patients with IPF, lung surfactant shows alterations in its lipid profile (reduced phosphatidyl glycerol, increased phosphatidylinositol and increased sphingomyelin levels, alterations in fatty acid composition) [29, 30], leading to severely impaired surface activity compared to controls [29]. The association of IPF with mutations in genes associated with surfactant metabolism [15, 16] or with mutations in the telomerase complex [14, 17] driving accelerated epithelial cell senescence suggests that surfactant alterations may contribute to the progress of IPF.
Lung compliance is typically measured under quasi-static conditions during lung deflation, when flow is interrupted at several points during slow exhalation from total lung capacity (TLC). Transpulmonary pressure is defined as the difference between mouth pressure and oesophageal pressure in the absence of flow, under the assumption that mouth pressure approximates alveolar pressure and that oesophageal pressure approximates pleural pressure. Lung compliance measurements thus require placement of an oesophageal pressure probe. This invasive measurement is not routinely done in the clinic. Normal static lung compliance is 328±102 mL·cmH2O−1 and normal dynamic compliance is 285±105 mL·cmH2O−1 in Swedish men, with little impact of age [31].
Reductions in lung compliance occur early in IPF. In one series, static lung compliance was reduced in all but one out of 25 patients with IPF [32]. Among 31 IPF patients with a mean vital capacity (VC) of 79±17% predicted values, static lung compliance was constantly and strikingly reduced (44±6% pred) [33]. In another series of 14 IPF patients, none had normal static lung compliance [34]. Anecdotally, lung compliance was markedly reduced in a patient with biopsy-proven IPF but a normal chest HRCT scan [35]. Altogether, these data suggest that measurements of lung compliance may be helpful for the early diagnosis of IPF.
Reductions in lung compliance may be tightly correlated with the degree of lung fibrosis. Among 23 patients with biopsy-proven IPF, static lung compliance correlated with VC and TLC, but not with the diffusing capacity of the lung for carbon monoxide (DLCO) [36]. Importantly, although no correlation was observed between standard physiological studies (VC, TLC, DLCO) and pathological severity, static lung compliance was strongly correlated with the degree of fibrosis assessed by scoring of lung biopsies. Such an association between lung compliance and the extent of fibrosis was not replicated in another study [34]. Reduction in lung compliance appears to progress with disease. In seven patients with end-stage IPF requiring mechanical ventilation, dynamic lung compliance was considerably reduced (19±2.4 mL·cmH2O−1) [37]. Reductions in dynamic compliance occur to the same extent as reductions in static compliance in subjects with ILD [38]. The forced oscillation technique allows noninvasive approximation of the dynamic compliance of the respiratory system in the absence of airway obstruction and may be of interest in IPF [39]. However, in an earlier study [40], no correlation was observed in five patients between lung compliance and either respiratory system resistance or reactance.
It remains to be defined how reductions in lung compliance relate to clinical features such as dyspnoea. Such an association is highly likely, considering that lung compliance is a major determinant of the load of the respiratory muscles and thus of the work of breathing [41]. The distribution of lesions is heterogeneous in IPF. It is therefore expected that the compliance of the lung is uneven among lung regions, as was shown in a sheep model of lung fibrosis [42], and consequently that convective ventilation predominantly occurs in the less affected regions of the lungs. In support of this hypothesis, the distribution of radiolabelled aerosols predominates in the upper regions of the lungs in IPF, whereas lesions predominate in the basal regions [43]. The distribution of ventilation to the less affected regions is an obstacle to the development of inhaled therapeutics for IPF.
Reduction of lung volumes
A restrictive ventilatory defect, defined by a reduction in static (TLC) and/or operating (VC) lung volumes, is typical in patients with IPF as in other ILDs [9]. Reduction of lung compliance is key to restriction because both chest wall compliance [37] and respiratory muscle strength, as assessed by measurements of transdiaphragmatic pressure [44] and maximal inspiratory pressure at the mouth [45], are mostly preserved.
Restriction is often absent at the time of diagnosis. In 96 patients with biopsy-confirmed IPF, forced vital capacity (FVC) ranged from 26% to 112% pred, while TLC ranged from 42% to 125% pred [46]. In recent clinical trials, mean FVC was close to 80% pred, consistent with half of patients having normal operating volumes [47]. These elements indicate poor sensitivity of lung volume measurements for the diagnosis of IPF. Although restriction of operating lung volumes is consistently associated with an increased risk of death [9], it correlates weakly with dyspnoea or an altered quality of life in IPF [48], consistent with other physiological alterations also playing key roles in clinical expression of the disease.
It is not known whether the lack of restriction in some patients reflects the natural history of IPF, or illustrates a limitation of population-based reference values. For instance, IPF subjects who had better than average lung function when healthy may present with apparently normal lung volumes before disease reaches a severe stage. The confounding effect of smoking could explain the preservation of static lung volumes in a fraction of patients, due to the effects of comorbid pulmonary emphysema on lung compliance [49]. Patients with the combined pulmonary fibrosis and emphysema (CPFE) syndrome have higher residual volume and TLC than patients with IPF [50].
Alterations in pulmonary gas exchange
IPF is associated with multiple alterations in the lung vasculature. In concert with alterations of the alveolar–capillary membrane, these lesions impair both gas diffusion and ventilation/perfusion (V′/Q′) relationships in the lung, leading to reduced diffusing capacity of the lung, increased dead space ventilation, and increases in the alveolar–arterial oxygen tension difference (PA–aO2) and chronic arterial hypoxaemia.
Reduced diffusing capacity of the lung
Lung diffusing capacity is almost always reduced in patients with IPF. The DLCO was reduced in 98% of IPF patients at the time of initial evaluation, although 27% of these patients had normal TLC and 56% had normal FVC [51]. Reduction of DLCO results from parenchymal and vascular lesions, as described by the Roughton–Forster model where gas diffusion across the alveolar barrier depends on membrane conductance (DmCO) and vascular conductance, the latter being mostly dependent on the pulmonary capillary volume [41].
DLCO is usually measured by a single-breath test where the subject inhales a gas mix comprising an insoluble gas such as helium (He) or methane (CH4) along with carbon monoxide. The volume where gas exchange occurs (alveolar volume (VA)) and the transfer constant of carbon monoxide (KCO) are calculated based on the reduction of He/CH4 and carbon monoxide concentrations in exhaled breath. DLCO is calculated by multiplying VA and KCO [52]. KCO can also be referred to as DLCO/VA, although this term is misleading as it implies that DLCO is the primary measurement from which DLCO/VA is then calculated, when the opposite is actually correct. Overall, DLCO reflects the general gas-exchanging function of the whole lungs, while KCO reflects gas exchange per unit of lung volume. Reference values for DLCO, VA and KCO were obtained in healthy subjects at full lung inflation [53]. When the lungs are inflated below TLC (i.e. low VA), DLCO slightly decreases while KCO increases [54]. The increase in KCO at low lung volume in normal individuals is due to the incomplete expansion (unfolding) of alveolar walls resulting in increased mass of gas-exchanging tissue per unit of volume.
Both VA and KCO are reduced to varying degrees in IPF. Of note, KCO is in the normal range in up to 30% of patients with IPF [55], particularly in patients with moderately altered DLCO [56]. It is important not to misinterpret this finding as being indicative of a preservation of gas exchange units, as it can be surmised that full lung inflation may not be attainable in IPF where subpleural fibrosis impairs lung inflation. In normal subjects, KCO increases at low lung volumes, so predicted values are inadequate to interpret KCO in patients with restrictive disease [57]. In addition, the spatial heterogeneity of lesions in IPF may influence KCO as relatively preserved areas of the lung are preferentially ventilated [43]. Our opinion is that a normal KCO value in IPF patients does not indicate that pulmonary gas exchange is normal. DLCO correlates more strongly than KCO with exertional increases in PA–aO2 in IPF [58]. DLCO and KCO both strongly correlate with the extent of disease as determined by scoring of computed tomography scans [59]. In support of the importance of DLCO measurements to the clinical appraisal of IPF, DLCO is highly correlated both with dyspnoea [60] and survival [61].
It is unclear whether alterations in the alveolar–capillary membrane or the lung vasculature are the predominant mechanism of DLCO reductions in IPF. KCO is inversely correlated both with oxygen diffusion limitation and with alveolar ventilation (V′A)/Q′ mismatch, as shown by the multiple inert gas elimination technique (MIGET) [62]. Simultaneous carbon monoxide and nitric oxide diffusion measurements can help to dissect to what extent alterations in the alveolar–capillary membrane or the lung vasculature contribute to reductions in DLCO. Nitric oxide reacts almost immediately with haemoglobin, so the diffusing capacity of the lung for nitric oxide (DLNO) is mostly independent of vascular conductance and is equal to the conductance of the alveolar–capillary membrane to nitric oxide (DmNO). Because DmNO/DmCO is fixed, both DmCO and vascular conductance can be calculated with the DLCO/DLNO technique [63]. Such studies show severe and similar decreases in both membrane conductance and lung capillary volume in IPF patients [64], indicating that alterations in the alveolar membrane and the lung vasculature both contribute to the impairment of gas diffusion in IPF [65]. However, it is worth noting that in one study, the capillary volume was in the normal range for half of 30 patients with IPF [66]. It is unclear whether discrepancies between these studies reflect differences in patient selection or are due to differences in data acquisition, as different equipment and procedures were used for carbon monoxide and nitric oxide measurements. Interestingly, in one study, the DLNO correlated better than DLCO with the extent of fibrotic lesions as assessed by HRCT [66]. Recent approaches based on hyperpolarised 129Xe magnetic resonance imaging suggest that both impaired membrane conductance and transfer to red blood cells participate in the reduced pulmonary gas exchange in IPF [67].
Dead space ventilation
Patients with IPF have increased physiological dead space ventilation (increased ratio of dead space volume to tidal volume (VD/VT)) at rest and at exercise [62, 68]. This feature results from both increased anatomical dead space due to the increased volume of conducting airways [69], and from regional increases in V′/Q′ ratios, i.e. alveolar dead space. V′/Q′ lung scans demonstrate that fibrotic lesions, and honeycomb lesions in particular, are very poorly perfused although they still receive some ventilation [70].
Interestingly, severe dead space ventilation may be a peculiar feature of IPF in comparison with other ILDs, as patients with IPF fail to reduce VD/VT at exercise [62], in contrast with patients with asbestosis [71]. VD/VT at exercise is strongly correlated with DLCO in IPF [55]. It is not known whether direct or indirect measures of VD/VT at rest and exercise provide additional information in comparison with resting measurements of gas diffusion in the lung, although experience acquired in the context of pulmonary hypertension (PH) [72] or heart failure [73] suggests this may be so.
Chronic arterial hypoxaemia
Alterations in the mechanical properties of the lungs, abnormalities of the lung vasculature and diffusion impairment lead to early-onset exertional chronic arterial hypoxaemia and later-onset resting chronic arterial hypoxaemia in IPF. Alveolar hypoventilation (hypercapnia) while awake is not common in IPF and is considered a feature of end-stage disease [74], when respiratory muscles fail in the face of a highly increased mechanical load (strongly reduced lung compliance). Alveolar hypoventilation is frequent during sleep in IPF [75]. An increase in PA–aO2 is the main mechanism driving hypoxaemia in IPF [62]. PA–aO2, which is calculated from arterial oxygen tension (PaO2) and arterial carbon dioxide tension (PaCO2) using the ideal alveolar gas equation [41], can be increased because of reduced V′/Q′ ratios, right-to-left shunting, or impairment of oxygen diffusion per se (referred to in the past as “alveolar–capillary block”). In a series of 15 patients, MIGET demonstrated that V′/Q′ mismatch and diffusion impairment contributed to chronic arterial hypoxaemia in IPF [62]. In that study, 2% and 4% of cardiac output perfused areas with absent (shunting) or altered (low V′/Q′) ventilation, respectively, while breathing room air at rest, suggesting that right-to-left shunting was in the physiological range in these patients. MIGET allows the calculation of a predicted PaO2 value based on the observed V′A/Q′ mismatch, under the assumption that diffusion limitation does not occur. In IPF, the observed PaO2 was lower than the predicted value, allowing the attribution of 19% of PA–aO2 to diffusion limitation [62] at rest. At exercise, V′/Q′ and shunt accounted for 60% of PA–aO2 and diffusion limitation for 40% [62]. These data are consistent with a more recent MIGET study [76]. It is not known whether exaggerated decreases in central venous oxygen tension contribute to hypoxaemia in IPF at rest. At submaximal exercise, the oxygen tension of mixed venous blood was 29 mmHg in IPF patients [62], similar to healthy subjects [77].
Although V′/Q′ mismatch and diffusion limitation are the main contributors to increased PA–aO2 in IPF, anatomical right-to-left shunting may contribute in a fraction of patients. In a study of 15 IPF patients breathing 100% oxygen, mean PaO2 and PaCO2 were 481 mmHg and 38 mmHg [62], which translate to a shunt fraction of 12% according to the shunt equation. At variance with earlier reports [62, 78], brain imaging following intravenous injection of 99mTc-labelled albumin aggregates demonstrated right-to-left shunting in two out of 22 patients with IPF [79]. It was not reported whether contrast echocardiography confirmed the existence of anatomical shunting, and it is unclear whether shunting resulted from the IPF disease process or was incidental in these patients. Identifying the few patients with anatomical shunting may be clinically important. It is reported that closure of the abnormal communication partially corrected chronic arterial hypoxaemia in a patient with IPF [80]. However, the benefit of shunt closure should be precisely evaluated because shunt could be life-preserving if the patient had concomitant PH.
Alterations in the structure and function of the conducting airways
IPF is understood to primarily involve the alveolar regions. Several lines of evidence, however, suggest that IPF also affects the airways. IPF lungs show evidence of airway epithelial cell proliferation [23] and differentiation [69], along with increased numbers of bronchioles in the distal regions [24]. In line with these observations, alterations in the function of conducting airways have been observed.
Elevation of nerve growth factor levels in induced sputum, which preferentially samples proximal airways, raises the hypothesis that the proximal airways may be involved in IPF [81]. Patients with IPF have an increased cough reflex to inhaled capsaicin. The inhalation of substance P induces cough in some IPF patients, which does not occur in normal subjects [59]. These data suggest functional upregulation of airway sensory neurons in IPF. Cough, however, may not be related to alterations in conducting airways only, as direct stimulation of the chest wall suffices to induce cough in IPF patients [82].
Multiple data suggest reduced resistance of the conducting airways in IPF. Among 55 IPF patients with a mean age of 71 years, the mean ratio of the forced expiratory volume in 1 s (FEV1) to FVC (FEV1/FVC) was 0.83 [56], which is higher than expected (0.74 for males, 0.75 for females according to European Respiratory Society reference equations) [83]. The ratio of the forced expiratory flow at 25–75% of FVC (FEF25–75%) to FVC (FEF25–75%/FVC) correlates positively with HRCT indices of IPF [39], suggesting that airway dilation occurs as part of the disease process. The increase in FEV1/FVC and FEF25–75%/FVC is consistent with data obtained with aerosol-derived morphometry, which show increased airway dimensions at all lung depths in IPF [84]. Recently, we measured the volume of conducting airways by volumetric capnography in patients with IPF, other ILDs and healthy controls. Interestingly, airway volume was higher in IPF than in controls and non-IPF ILDs, suggesting that increased airway volume may be somewhat IPF-specific [69]. Anecdotal evidence indicates reduced distensibility of the proximal airways in IPF, although it is not clear whether this is related to either reduced compliance of the airway wall or to changes in airway transmural pressure due to increased lung recoil [85].
Alterations in pulmonary haemodynamics
Vascular lesions lead to disproportionate increases in pulmonary vascular resistance (PVR) and PH in a subset of patients with IPF. Right heart catheterisation is the gold standard for the diagnosis of PH, which is defined as a mean pulmonary artery pressure (mPAP) ≥25 mmHg at rest [86].
PH associated with IPF may represent a specific phenotype of IPF. In a series of 488 IPF patients with minimal honeycomb changes (<5% of the lung) and mild-to-moderate restriction (mean FVC between 67% and 69% pred), right-heart catheterisation showed PH without elevated capillary wedge pressure in 14% of patients, PH with elevated wedge pressure in 5%, and isolated elevated wedge pressure in 4% [87]. Patients with PH and normal capillary wedge pressure had lower DLCO and increased haemoglobin desaturation at exercise. Interestingly, PH does not seem to progress rapidly in these patients, as repeat catheterisation at 48 weeks yielded levels quite similar to baseline [87].
The prevalence of PH is 46.1% in patients with severe IPF listed for transplantation [88]. Severe PH correlates with elevated capillary wedge pressure, suggesting the participation of left-sided ventricular dysfunction to PH in IPF patients [88]. The prevalence of PH appears to be lower in IPF compared to connective tissue disease-associated ILD of similar severity [89]. PH is associated with increased mortality in IPF. In a series of 135 patients, there was a 2.4 increase in the hazard ratio (HR) for death per 10 mmHg increase in mPAP, while an increase in PVR of 1 Wood unit was associated with a 1.4 increase in the HR for mortality [90].
The precise nature of lesions driving PH in IPF is not well understood. There is no correlation between mPAP and operating lung volumes [87, 91]. Anatomical–functional correlation analysis in 26 explanted lungs showed that mPAP is significantly correlated with the extent of fibrosis in damaged regions, but no correlation exists between mPAP and the extent of venous lesions in less-damaged areas [28]. It is probable that the rarefaction of vessels in fibrotic areas contributes to PH in IPF, as suggested by the presence of major defects on V′/Q′ lung scans [92]. It is possible that the discrepancy between global vascular rarefaction [93] and the higher density of capillary vessels in alveolar septa of IPF lungs [94] pertains to variation in the sites of biopsy.
Impairment of pulmonary haemodynamics at rest is detected late in IPF, although early alterations may be detected at exercise. Increases in mPAP are observed in IPF patients during exercise at 60% of the maximal workload, while PVR does not decrease as observed in normal subjects [62]. In favour of the early onset of vascular damage in IPF, mPAP increased from 20 mmHg to 40 mmHg in seven patients with mild-to-moderate IPF [76]. The increase in oxygen diffusion impairment at exercise, documented by MIGET, is consistent with decrease of the alveolar–capillary contact time subsequent to the increase in cardiac output, most probably indicating reduced recruitment of the lung vasculature in IPF [62].
Measurements of systolic pulmonary artery pressure by echocardiography lack sensitivity and specificity for the diagnosis of PH in IPF [75]. Other echocardiographic indices may be useful for the identification of right ventricle dysfunction and PH. A right ventricle to left ventricle diameter ratio >1 is associated with a 5.4 increase in the HR for mortality in IPF [90], while moderate-to-severe right ventricle dysfunction, identified by tricuspid annular plane systolic excursion <1.6 cm, is associated with a 7.5 increase, suggesting that echocardiography may indeed have a role for the identification of IPF patients with clinically significant pulmonary vascular impairment [90]. HRCT measurements of the diameter of the pulmonary artery do not accurately indicate PH in IPF, possibly due to the confounding effect of reduced pleural pressure causing the dilation of cavitary intrathoracic organs [76]. Noninvasive measurements of pulmonary blood flow using rebreathing of sulfur hexafluoride may be an interesting tool for the exploration of haemodynamic limitation in IPF [77].
Central control of ventilation
Alterations in lung mechanics and gas exchange drive persistent activation of the central command of ventilation in IPF. An elevated ventilatory drive, detected by a rise in the 100 ms occlusion pressure (P0.1), was reported in patients with ILD, at rest and under carbon dioxide rebreathing [44, 95]. Correlations between P0.1 and both lung compliance and VC were observed in IPF, suggesting that the increased ventilatory drive reflects the mechanical load imposed by fibrosis [96, 97]. An association was also observed between P0.1 and KCO in patients with ILD, suggesting that impaired gas exchange contributes to ventilatory drive [98]. Diaphragm activation is increased in IPF in comparison with healthy subjects, both during carbon dioxide rebreathing [99] and at exercise [38], consistent with the preservation of the command of ventilation in this disease.
Impact of comorbidities on lung function in IPF
Because IPF typically affects older patients and smokers, multiple comorbidities can affect patients with IPF [100, 101]. In particular, chronic obstructive pulmonary disease (COPD) and pulmonary emphysema are common. The prevalence of emphysema in patients with IPF, which defines the CPFE syndrome, ranges from 6% to 67% [100]. Emphysema is associated with higher FVC and TLC at diagnosis in patients with IPF [102]. CPFE is associated with markedly lower DLCO, especially when emphysema is present in lung areas not affected by fibrosis [103]. Of note, the pattern of lung function impairment in CPFE is quite distinct from COPD. Some, but not all, patients with CPFE present with airway obstruction and hyperinflation [104]. Impulse oscillometry showed that the expiratory increase in the reactance of the respiratory system at low frequency (5 Hz), which indicates expiratory collapse of the distal airways, was much lower in CPFE than in COPD [104], consistent with the lack of dynamic hyperinflation in CPFE [104]. It is not known whether lung compliance is affected in CPFE to the same extent as in IPF without emphysema. 9.1% of IPF patients show reversible airflow obstruction as indicated by a 200 mL and 12% increase in either FVC or FEV1 after inhalation of a bronchodilator, although it is not clear whether this reflects comorbid asthma or COPD, or an intrinsic feature of IPF [105].
Heart disease is a common occurrence in patients with IPF. The prevalence of coronary heart disease has been reported to range from 3.2% to 68% [100]. 9% of patients with mild to moderate IPF and left ventricle ejection fraction ≥40% have increased pulmonary artery wedge pressure, indicating occult heart failure [87]. In terms of lung function, heart failure can be associated with restriction, obstruction, reduced DLCO [106, 107] and PH [108]. Heart failure with preserved ejection fraction is associated with reduced DLCO [109]. Venous thromboembolism may also contribute to low DLCO in a fraction of IPF patients. Venous thrombosis is twice as frequent in IPF patients as in the control population [110], while the incidence of pulmonary embolism is 6.4-fold higher in IPF than in the general population.
Lung function indices as indicators of prognosis and outcomes in clinical trials
The severity of lung function impairment at the time of diagnosis and the decline of lung function over time are both tightly associated with survival in IPF. Impairment of operating lung volumes, static lung volumes and carbon monoxide transfer are associated with worse prognosis in IPF, with the strongest associations observed with FVC, TLC and DLCO, respectively [111]. In a large cohort (n=1156), FVC <80% pred and DLCO ≤45% pred were associated with increased mortality [112]. Since a >5% absolute decline in FVC (% pred) and a ≥15% decline in DLCO (% pred) over 6 months are also highly associated with mortality [112], follow-up investigations are critical to determine prognosis in patients with IPF. The prognostic impact of lung function may be assessed using composite indices such as the Composite Physiological Index, which combines FVC, FEV1 and DLCO [113], or the Gender Age Physiology score, which combines FVC and DLCO with gender and age [114].
The rate of decline in FVC was the most common primary outcome end-point in recent clinical trials in IPF, expressed either in millilitres per year [47] or as percentage of the predicted value [115–117]. It is currently debated whether change in FVC over time is the optimal outcome for clinical trials in IPF. The presence of emphysema affects the rate of FVC decline in IPF. Patients with more emphysema show less decrease in FVC, while DLCO does decline, raising the hypothesis that FVC may not be the best end-point in patients with emphysema [118]. In a series of 32 patients with IPF and moderate to severe emphysema, a 10% decline in FVC also failed to predict mortality [119]. In addition, although decline in both FVC (10%) and DLCO (15%) do predict mortality in the subsequent year, neither predicts change in lung function in the subsequent year [120]. These limitations to the use of FVC decline have led to the use of progression-free survival, defined as time to all-cause death or a categorical decrease from baseline in FVC % pred, in a recent trial of LOXL2 antibodies [121].
How do physiological alterations integrate in IPF? Exercise limitation and dyspnoea
The multiple alterations of lung physiology in IPF translate into profound alterations in exercise capacity and dyspnoea. Oxygen uptake, power, tidal volume and PaO2 are reduced at exercise in IPF patients compared with healthy controls [38, 68], while PA–aO2 and VD/VT are increased.
It is not clear whether reduced lung compliance, haemodynamic dysfunction, hypoxaemia or increased VD/VT are the prime determinants of dyspnoea and exertional limitation in IPF. Short-term therapeutic intervention studies addressed this question. Alleviation of the load of the respiratory muscles by noninvasive ventilation during submaximal exercise leads to increases in endurance time and arterial haemoglobin saturation, and reductions in breathlessness [122]. These data clearly indicate that alterations in the mechanical properties of the respiratory system play an important role in exercise limitation in IPF patients, and are consistent with both the reduction in operating lung volumes and increased diaphragmatic activity at exercise [38].
PH may play key roles in a subset of patients. When PH is present, it is associated with reduced oxygen pulse at exercise, consistent with haemodynamic limitation [123], with more severe arterial haemoglobin desaturation at exercise [87] and with increased VD/VT, as suggested by an increase in the ratio of minute ventilation (V′E) to carbon dioxide elimination (V′CO2) at the ventilatory threshold [124]. Likewise, correlations exist between the 6-min walk distance and both mPAP and PVR [125]. It is unclear whether these associations reflect a causative relationship between the alteration of pulmonary haemodynamics and exertional limitation in IPF patients without severe PH. In seven patients with IPF (mean FVC 60% pred, DLCO 52% pred), two of whom had mPAP slightly over 25 mmHg at rest, the inhalation of nitric oxide during submaximal exercise reduced the increase in mPAP and reduced PVR, but did not increase cardiac output or PaO2 and did not reduce VD/VT [76]. However, a 12-week course of oral sildenafil, which potentiates the effect of endogenous nitric oxide, yielded clinically significant benefits in terms of dyspnoea and quality of life in patients with advanced IPF [126].
Gas exchange abnormalities during exercise are highly prevalent in IPF. Patients with mild-to-moderate IPF and normal or near-normal resting PaO2 have a significant decline in arterial haemoglobin saturation after a 6-min walk [127]. Multiple factors contribute to exertional arterial hypoxaemia in IPF, with alterations of both V′/Q′ ratios [128] and diffusion [129] playing key roles. Uncontrolled retrospective [130, 131] and prospective [132] studies show increased walk performance, better quality of life and reduced dyspnoea in IPF patients treated with supplemental oxygen. This effect was observed both in patients with resting arterial hypoxaemia and in patients without resting arterial hypoxaemia [131, 132]. Whether the effect of oxygen therapy is related to a placebo effect remains subject to debate. In a controlled study, supplemental oxygen given at exercise in IPF patients with exertional arterial hypoxaemia but without resting arterial hypoxaemia failed to improve the 6-min walk distance and dyspnoea in comparison with placebo (air), despite improvements in arterial haemoglobin saturation [133], while another study reported beneficial effects with regard to endurance time and dyspnoea [134]. Indirect evidence suggests key roles of dead space ventilation in the genesis of dyspnoea, although the lack of a possibility to experimentally amend the VD/VT ratio precludes definitive demonstration. In a series of 25 IPF patients, the V′E/V′CO2 slope, which is tightly correlated with VD/VT, was the physiological parameter most strongly correlated with patient-reported exertional dyspnoea [135]. In addition to alterations in pulmonary anatomy and physiology, anaemia may occasionally contribute to gas exchange abnormalities in patients with IPF. This condition may be more prevalent in patients bearing mutations of the telomerase complex [136].
Dyspnoea is the main complaint in patients with IPF. Exertional dyspnoea correlates with markers of both reduced lung compliance and altered pulmonary gas exchange [98, 137]. It is conceivable that the increased V′E required to maintain PaCO2 at normal levels in the face of high VD/VT, in combination with the reduced lung compliance requiring higher effort to increase ventilation and a small-volume/high-respiratory-rate breathing pattern [38], are responsible for increased ventilatory drive and dyspnoea in IPF. Strong correlations exist between increases in diaphragm activation and dyspnoea at exercise [38], as well as between P0.1 at rest and patient-reported exertional dyspnoea [98]. Increased respiratory drive, secondary both to the increased elastic load and the higher ventilation levels required by abnormal pulmonary gas exchange, is a key contributor to dyspnoea in patients with IPF [138].
The role of physiological tests in the diagnostic workup of IPF patients presenting with an increase in dyspnoea remains to be defined. A key aim in this context is to determine whether clinical worsening relates to the natural history of IPF, or to other conditions. Exacerbations of IPF and comorbidities such as infection or heart failure would probably result in worsening of restriction, diffusion impairment and hypoxaemia. Because physiological alterations are nonspecific, it is unclear whether such tests would bring additional diagnostic information to clinical, biological and imaging studies in the context of acute or subacute dyspnoea, although pulmonary embolism may be associated with an isolated decrease in DLCO [139]. Occasionally, specific physiological tests may help to recognise differential diagnoses of IPF. For example, respiratory muscle studies may help with recognising polymyositis-associated ILD [140].
Conclusion
In IPF, pathological processes affect not only alveoli but also other regions of the respiratory system, such as the lung vasculature and conducting airways. As a result, patients with IPF show multiple alterations in lung physiology (summarised in table 1 and figure 2) that combine to a different degree in each individual patient, along with infrequent or rare alterations such as severe PH or right-to-left shunting. We advocate that, although spirometry and DLCO measurements provide critical information and remain the backbone of the functional evaluation of IPF patients, physiological testing may not be limited to these tests when questions remain as to the explanation of the clinical picture in a given patient. In such a context, measurements of lung compliance, blood gas analysis while breathing 100% oxygen, assessment of pulmonary haemodynamics and exploration of the ventilatory drive, as well as exercise testing with quantification of dead space ventilation, may provide useful information when relevant comorbidities such as heart failure or anaemia have been ruled out. This aspect may be of particular importance for the definition of clinical–physiological disease phenotypes, analogous with other fields such as severe asthma [141]. Future studies will inform us about the respective roles of select physiological tests in the general IPF care strategy.
TABLE 1.
Alterations of lung function tests in idiopathic pulmonary fibrosis (IPF)
| Mild IPF | Moderate to severe IPF | |
| Spirometry | ||
| FVC | Normal | Decreased |
| FEV1/FVC | Normal or increased | Normal or increased |
| Static lung volumes | ||
| TLC | Normal | Decreased |
| FRC | Normal | Decreased |
| Blood gases at rest | ||
| PaO2 | Normal | Decreased |
| PaCO2 | Normal | Decreased |
| Carbon monoxide transfer | ||
| DLCO | Decreased | Decreased |
| VA | May be normal | Decreased |
| KCO | May be normal | Decreased |
| Airways | ||
| Cough reflex | Increased | Increased |
| Airway resistance | Decreased | Decreased |
| Pulmonary haemodynamics at rest | ||
| PAP | May be increased | Frequently increased |
| PCWP | Normal | May be increased |
| Ventilatory drive | ||
| P0.1 | May be normal | Increased |
| Ventilatory response to CO2 rebreathing | Normal | Normal |
| Exercise physiology | ||
| Peak V′O2 | May be normal | Decreased |
| VD/VT | Increased | Increased |
| V′E/V′CO2 | Increased | Increased |
| PAP at exercise | Increased | Increased |
| PA–aO2 at exercise | Increased | Increased |
FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; TLC: total lung capacity; FRC: functional residual capacity; PaO2: arterial oxygen tension; PaCO2: arterial carbon dioxide tension; DLCO: diffusing capacity of the lung for carbon monoxide; VA: alveolar volume; KCO: transfer constant of carbon monoxide; PAP: pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; P0.1: 100 ms occlusion pressure; V′O2: oxygen uptake; VD/VT: ratio of dead space volume to tidal volume; V′E/V′CO2: ratio of minute ventilation to carbon dioxide elimination; PA–aO2: alveolar–arterial oxygen tension difference.
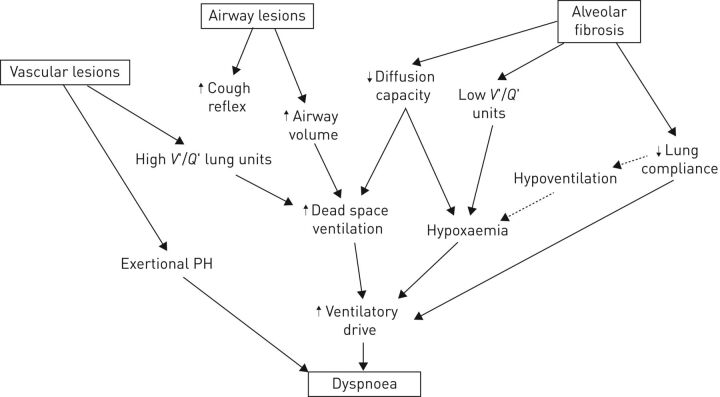
FIGURE 2.
Model for the association between pathological features, physiological alterations and their association with pathological and clinical features. Filled arrows represent strong and/or demonstrated associations; dotted arrows represent associations seen in end-stage disease. V′/Q′: ventilation/perfusion ratio; PH: pulmonary hypertension.
Acknowledgements
The authors thank Benoit Wallaert (University of Lille, Lille, France) for advice.
Authors contributions were as follows. L. Plantier prepared the first draft of the manuscript. C. Bancal, A. Cazes, A-T. Dinh-Xuan, S. Marchand-Adam and B. Crestani made important intellectual input and revised the manuscript. All authors have agreed to the final content.
Footnotes
Conflict of interest: None declared.
Provenance: Submitted article, peer reviewed.
References
- 1.Richeldi L, Cottin V, du Bois RM, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS trials. Respir Med 2016; 113: 74–79. [DOI] [PubMed] [Google Scholar]
- 2.Ley B, Swigris J, Day B-M, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2017; 196: 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogliani P, Calzetta L, Cavalli F, et al. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Pulm Pharmacol Ther 2016; 40: 95–103. [DOI] [PubMed] [Google Scholar]
- 4.Nathan SD, Albera C, Bradford WZ, et al. Effect of continued treatment with pirfenidone following clinically meaningful declines in forced vital capacity: analysis of data from three phase 3 trials in patients with idiopathic pulmonary fibrosis. Thorax 2016; 71: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher M, Nathan SD, Hill C, et al. Predicting life expectancy for pirfenidone in idiopathic pulmonary fibrosis. J Manag Care Spec Pharm 2017; 23: Suppl. 3b, S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cottin V, Crestani B, Valeyre D, et al. Diagnosis and management of idiopathic pulmonary fibrosis: French practical guidelines. Eur Respir Rev 2014; 23: 193–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan JJ, Martinez FJ, Wells AU, et al. Lung function estimates in idiopathic pulmonary fibrosis: the potential for a simple classification. Thorax 2005; 60: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FJ, Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006; 3: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan SD, Shlobin OA, Weir N, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011; 140: 221–229. [DOI] [PubMed] [Google Scholar]
- 11.Kottmann RM, Kulkarni AA, Smolnycki KA, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med 2012; 186: 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev 2015; 24: 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Huang X, Hecker L, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 2013; 123: 1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spagnolo P, Cottin V. Genetics of idiopathic pulmonary fibrosis: from mechanistic pathways to personalised medicine. J Med Genet 2017; 54: 93–99. [DOI] [PubMed] [Google Scholar]
- 15.Borie R, Kannengiesser C, Crestani B. Familial forms of nonspecific interstitial pneumonia/idiopathic pulmonary fibrosis: clinical course and genetic background. Curr Opin Pulm Med 2012; 18: 455–461. [DOI] [PubMed] [Google Scholar]
- 16.Campo I, Zorzetto M, Mariani F, et al. A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant. Respir Res 2014; 15: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J 2016; 48: 1721–1731. [DOI] [PubMed] [Google Scholar]
- 18.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 2011; 364: 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borie R, Crestani B, Dieude P, et al. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. PLoS One 2013; 8: e70621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thannickal VJ, Henke CA, Horowitz JC, et al. Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute. Am J Pathol 2014; 184: 1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plantier L, Crestani B, Wert SE, et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax 2011; 66: 651–657. [DOI] [PubMed] [Google Scholar]
- 22.Seibold MA, Smith RW, Urbanek C, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One 2013; 8: e58658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vuorinen K, Ohlmeier S, Leppäranta O, et al. Peroxiredoxin II expression and its association with oxidative stress and cell proliferation in human idiopathic pulmonary fibrosis. J Histochem Cytochem 2008; 56: 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chilosi M, Poletti V, Murer B, et al. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN-p63. Lab Invest 2002; 82: 1335–1345. [DOI] [PubMed] [Google Scholar]
- 25.Hanumegowda C, Farkas L, Kolb M. Angiogenesis in pulmonary fibrosis: too much or not enough? Chest 2012; 142: 200–207. [DOI] [PubMed] [Google Scholar]
- 26.Ebina M, Shimizukawa M, Shibata N, et al. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2004; 169: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 27.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med 2007; 175: 875–880. [DOI] [PubMed] [Google Scholar]
- 28.Colombat M, Mal H, Groussard O, et al. Pulmonary vascular lesions in end-stage idiopathic pulmonary fibrosis: histopathologic study on lung explant specimens and correlations with pulmonary hemodynamics. Hum Pathol 2007; 38: 60–65. [DOI] [PubMed] [Google Scholar]
- 29.Günther A, Schmidt R, Nix F, et al. Surfactant abnormalities in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and sarcoidosis. Eur Respir J 1999; 14: 565–573. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt R, Meier U, Markart P, et al. Altered fatty acid composition of lung surfactant phospholipids in interstitial lung disease. Am J Physiol Lung Cell Mol Physiol 2002; 283: L1079–L1085. [DOI] [PubMed] [Google Scholar]
- 31.Galetke W, Feier C, Muth T, et al. Reference values for dynamic and static pulmonary compliance in men. Respir Med 2007; 101: 1783–1789. [DOI] [PubMed] [Google Scholar]
- 32.Radwan L, Zielonka TM, Maszczyk Z, et al. Zaburzenia czynnosciowe u chorych na srodmiazszowe choroby pluc bez cech restrykcji [Functional disturbances in patients with interstitial lung diseases without signs of restriction]. Pneumonol Alergol Pol 1999; 67: 180–188. [PubMed] [Google Scholar]
- 33.Zielonka TM, Demkow U, Radzikowska E, et al. Angiogenic activity of sera from interstitial lung disease patients in relation to pulmonary function. Eur J Med Res 2010; 15: Suppl. 2, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sansores RH, Ramirez-Venegas A, Pérez-Padilla R, et al. Correlation between pulmonary fibrosis and the lung pressure–volume curve. Lung 1996; 174: 315–323. [DOI] [PubMed] [Google Scholar]
- 35.Orens JB, Kazerooni EA, Martinez FJ, et al. The sensitivity of high-resolution CT in detecting idiopathic pulmonary fibrosis proved by open lung biopsy. A prospective study. Chest 1995; 108: 109–115. [DOI] [PubMed] [Google Scholar]
- 36.Fulmer JD, Roberts WC, von Gal ER, et al. Morphologic-physiologic correlates of the severity of fibrosis and degree of cellularity in idiopathic pulmonary fibrosis. J Clin Invest 1979; 63: 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nava S, Rubini F. Lung and chest wall mechanics in ventilated patients with end stage idiopathic pulmonary fibrosis. Thorax 1999; 54: 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faisal A, Alghamdi BJ, Ciavaglia CE, et al. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am J Respir Crit Care Med 2016; 193: 299–309. [DOI] [PubMed] [Google Scholar]
- 39.Lopes AJ, Capone D, Mogami R, et al. Correlacao dos achados tomograficos com parametros de funcao pulmonar na fibrose pulmonar idiopatica em nao fumantes [Correlation of tomographic findings with pulmonary function parameters in nonsmoking patients with idiopathic pulmonary fibrosis]. J Bras Pneumol 2007; 33: 671–678. [DOI] [PubMed] [Google Scholar]
- 40.van Noord JA, Clément J, Cauberghs M, et al. Total respiratory resistance and reactance in patients with diffuse interstitial lung disease. Eur Respir J 1989; 2: 846–852. [PubMed] [Google Scholar]
- 41.Crystal R, West J, Weibel E. The Lung: Scientific Foundations. 2nd Edn. Philadelphia, Lippincott Williams & Wilkins, 1997. [Google Scholar]
- 42.Organ L, Bacci B, Koumoundouros E, et al. Structural and functional correlations in a large animal model of bleomycin-induced pulmonary fibrosis. BMC Pulm Med 2015; 15: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanazawa M, Suzuki Y, Ishizaka A, et al. [Assessment of pulmonary aerosol deposition and epithelial permeability in 99mTc-DTPA inhalation scintigram]. Nihon Kyōbu Shikkan Gakkai Zasshi 1993; 31: 593–600. [PubMed] [Google Scholar]
- 44.DiMarco AF, Kelsen SG, Cherniack NS, et al. Occlusion pressure and breathing pattern in patients with interstitial lung disease. Am Rev Respir Dis 1983; 127: 425–430. [DOI] [PubMed] [Google Scholar]
- 45.Jastrzebski D, Kozielski J, Zebrowska A. Rehabilitacja oddechowa chorych z idiopatycznym srodmiazszowym wloknieniem pluc za pomoca programu z cwiczeniami miesni wdechowych [Pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis with inspiratory muscle training]. Pneumonol Alergol Pol 2008; 76: 131–141. [PubMed] [Google Scholar]
- 46.Cherniack RM, Colby TV, Flint A, et al. Correlation of structure and function in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1995; 151: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 47.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 48.du Bois RM, Weycker D, Albera C, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 184: 459–466. [DOI] [PubMed] [Google Scholar]
- 49.Doherty MJ, Pearson MG, O'Grady EA, et al. Cryptogenic fibrosing alveolitis with preserved lung volumes. Thorax 1997; 52: 998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mura M, Zompatori M, Pacilli AMG, et al. The presence of emphysema further impairs physiologic function in patients with idiopathic pulmonary fibrosis. Respir Care 2006; 51: 257–265. [PubMed] [Google Scholar]
- 51.Cortes-Telles A, Forkert L, O'Donnell DE, et al. Idiopathic pulmonary fibrosis: new insights on functional characteristics at diagnosis. Can Respir J 2014; 21: e55–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg E. The 1995 update of recommendations for a standard technique for measuring the single-breath carbon monoxide diffusing capacity (transfer factor). Am J Respir Crit Care Med 1996; 154: 827–828. [DOI] [PubMed] [Google Scholar]
- 53.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26: 720–735. [DOI] [PubMed] [Google Scholar]
- 54.Johnson DC. Importance of adjusting carbon monoxide diffusing capacity (DLCO) and carbon monoxide transfer coefficient (KCO) for alveolar volume. Respir Med 2000; 94: 28–37. [DOI] [PubMed] [Google Scholar]
- 55.Wallaert B, Wemeau-Stervinou L, Salleron J, et al. Do we need exercise tests to detect gas exchange impairment in fibrotic idiopathic interstitial pneumonias? Pulm Med 2012; 2012: 657180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pastre J, Plantier L, Planes C, et al. Different KCO and VA combinations exist for the same DLCO value in patients with diffuse parenchymal lung diseases. BMC Pulm Med 2015; 15: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frans A, Nemery B, Veriter C, et al. Effect of alveolar volume on the interpretation of single breath DLCO. Respir Med 1997; 91: 263–273. [DOI] [PubMed] [Google Scholar]
- 58.Agustí C, Xaubet A, Agustí AG, et al. Clinical and functional assessment of patients with idiopathic pulmonary fibrosis: results of a 3 year follow-up. Eur Respir J 1994; 7: 643–650. [DOI] [PubMed] [Google Scholar]
- 59.Wells AU, King AD, Rubens MB, et al. Lone cryptogenic fibrosing alveolitis: a functional-morphologic correlation based on extent of disease on thin-section computed tomography. Am J Respir Crit Care Med 1997; 155: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 60.Swigris JJ, Han M, Vij R, et al. The UCSD shortness of breath questionnaire has longitudinal construct validity in idiopathic pulmonary fibrosis. Respir Med 2012; 106: 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamada K, Nagai S, Tanaka S, et al. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 2007; 131: 650–656. [DOI] [PubMed] [Google Scholar]
- 62.Agustí AG, Roca J, Gea J, et al. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis 1991; 143: 219–225. [DOI] [PubMed] [Google Scholar]
- 63.Guenard H, Varene N, Vaida P. Determination of lung capillary blood volume and membrane diffusing capacity in man by the measurements of NO and CO transfer. Respir Physiol 1987; 70: 113–120. [DOI] [PubMed] [Google Scholar]
- 64.Martinot JB, Guénard H, Dinh-Xuan AT, et al. Nitrogen monoxide and carbon monoxide transfer interpretation: state of the art. Clin Physiol Funct Imaging 2017; 37: 357–365. [DOI] [PubMed] [Google Scholar]
- 65.Wémeau-Stervinou L, Perez T, Murphy C, et al. Lung capillary blood volume and membrane diffusion in idiopathic interstitial pneumonia. Respir Med 2012; 106: 564–570. [DOI] [PubMed] [Google Scholar]
- 66.Barisione G, Brusasco C, Garlaschi A, et al. Lung diffusing capacity for nitric oxide as a marker of fibrotic changes in idiopathic interstitial pneumonias. J Appl Physiol 2016; 120: 1029–1038. [DOI] [PubMed] [Google Scholar]
- 67.Wang JM, Robertson SH, Wang Z, et al. Using hyperpolarized 129Xe MRI to quantify regional gas transfer in idiopathic pulmonary fibrosis. Thorax 2018; 73: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miki K, Maekura R, Hiraga T, et al. Acidosis and raised norepinephrine levels are associated with exercise dyspnoea in idiopathic pulmonary fibrosis. Respirology 2009; 14: 1020–1026. [DOI] [PubMed] [Google Scholar]
- 69.Plantier L, Debray MP, Estellat C, et al. Increased volume of conducting airways in idiopathic pulmonary fibrosis is independent of disease severity: a volumetric capnography study. J Breath Res 2016; 10: 016005. [DOI] [PubMed] [Google Scholar]
- 70.Strickland NH, Hughes JM, Hart DA, et al. Cause of regional ventilation–perfusion mismatching in patients with idiopathic pulmonary fibrosis: a combined CT and scintigraphic study. AJR Am J Roentgenol 1993; 161: 719–725. [DOI] [PubMed] [Google Scholar]
- 71.Agusti AG, Roca J, Rodriguez-Roisin R, et al. Different patterns of gas exchange response to exercise in asbestosis and idiopathic pulmonary fibrosis. Eur Respir J 1988; 1: 510–516. [PubMed] [Google Scholar]
- 72.Paolillo S, Farina S, Bussotti M, et al. Exercise testing in the clinical management of patients affected by pulmonary arterial hypertension. Eur J Prev Cardiol 2012; 19: 960–971. [DOI] [PubMed] [Google Scholar]
- 73.Poggio R, Arazi HC, Giorgi M, et al. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: a meta-analysis of the published literature. Am Heart J 2010; 160: 1004–1014. [DOI] [PubMed] [Google Scholar]
- 74.Bennett D, Fossi A, Bargagli E, et al. Mortality on the waiting list for lung transplantation in patients with idiopathic pulmonary fibrosis: a single-centre experience. Lung 2015; 193: 677–681. [DOI] [PubMed] [Google Scholar]
- 75.Milioli G, Bosi M, Poletti V, et al. Sleep and respiratory sleep disorders in idiopathic pulmonary fibrosis. Sleep Med Rev 2016; 26: 57–63. [DOI] [PubMed] [Google Scholar]
- 76.Blanco I, Ribas J, Xaubet A, et al. Effects of inhaled nitric oxide at rest and during exercise in idiopathic pulmonary fibrosis. J Appl Physiol 2011; 110: 638–645. [DOI] [PubMed] [Google Scholar]
- 77.Stringer WW, Hansen JE, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol 1997; 82: 908–912. [DOI] [PubMed] [Google Scholar]
- 78.Miller WC, Heard JG, Unger KM, et al. Anatomical lung shunting in pulmonary fibrosis. Thorax 1986; 41: 208–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Graves MW, Kiratli PO, Mozley D, et al. Scintigraphic diagnosis of a right to left shunt in end-stage lung disease. Respir Med 2003; 97: 549–554. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen S, Leroy S, Bautin N, et al. Fibrose pulmonaire idiopathique et shunt droit-gauche par foramen ovale permeable: amelioration clinique et gazometrique apres fermeture percutanee [Idiopathic pulmonary fibrosis and right-to left shunt by patent foramen ovale]. Rev Mal Respir 2007; 24: 631–634. [DOI] [PubMed] [Google Scholar]
- 81.Hope-Gill BDM, Hilldrup S, Davies C, et al. A study of the cough reflex in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003; 168: 995–1002. [DOI] [PubMed] [Google Scholar]
- 82.Jones RM, Hilldrup S, Hope-Gill BD, et al. Mechanical induction of cough in idiopathic pulmonary fibrosis. Cough 2011; 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party. Standardization of Lung Function Tests. European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J 1993; 6: Suppl. 16, 5–40. [PubMed] [Google Scholar]
- 84.Brand P, Kohlhäufl M, Meyer T, et al. Aerosol-derived airway morphometry and aerosol bolus dispersion in patients with lung fibrosis and lung emphysema. Chest 1999; 116: 543–548. [DOI] [PubMed] [Google Scholar]
- 85.Baier H, Zarzecki S, Wanner A. Influence of lung inflation on the cross-sectional area of central airways in normals and in patients with lung disease. Respiration 1981; 41: 145–154. [DOI] [PubMed] [Google Scholar]
- 86.Caminati A, Cassandro R, Harari S. Pulmonary hypertension in chronic interstitial lung diseases. Eur Respir Rev 2013; 22: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raghu G, Nathan SD, Behr J, et al. Pulmonary hypertension in idiopathic pulmonary fibrosis with mild-to-moderate restriction. Eur Respir J 2015; 46: 1370–1377. [DOI] [PubMed] [Google Scholar]
- 88.Shorr AF, Wainright JL, Cors CS, et al. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J 2007; 30: 715–721. [DOI] [PubMed] [Google Scholar]
- 89.Todd NW, Lavania S, Park MH, et al. Variable prevalence of pulmonary hypertension in patients with advanced interstitial pneumonia. J Heart Lung Transplant 2010; 29: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rivera-Lebron BN, Forfia PR, Kreider M, et al. Echocardiographic and hemodynamic predictors of mortality in idiopathic pulmonary fibrosis. Chest 2013; 144: 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nathan SD, Shlobin OA, Ahmad S, et al. Pulmonary hypertension and pulmonary function testing in idiopathic pulmonary fibrosis. Chest 2007; 131: 657–663. [DOI] [PubMed] [Google Scholar]
- 92.Bourke SJ, Hawkins T, Keavey PM, et al. Ventilation perfusion radionuclide imaging in cryptogenic fibrosing alveolitis. Nucl Med Commun 1993; 14: 454–464. [DOI] [PubMed] [Google Scholar]
- 93.Renzoni EA, Walsh DA, Salmon M, et al. Interstitial vascularity in fibrosing alveolitis. Am J Respir Crit Care Med 2003; 167: 438–443. [DOI] [PubMed] [Google Scholar]
- 94.Kim KH, Maldonado F, Ryu JH, et al. Iron deposition and increased alveolar septal capillary density in nonfibrotic lung tissue are associated with pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Res 2010; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Meerhaeghe A, Scano G, Sergysels R, et al. Respiratory drive and ventilatory pattern during exercise in interstitial lung disease. Bull Eur Physiopathol Respir 1981; 17: 15–26. [PubMed] [Google Scholar]
- 96.Launois S, Clergue F, Medrano G, et al. Controle de la respiration dans les fibroses pulmonaires. Effets de l'O2 et du CO2 [The control of respiration in pulmonary fibrosis. The effect of O2 and CO2]. Rev Mal Respir 1991; 8: 67–73. [PubMed] [Google Scholar]
- 97.Renzi G, Milic-Emili J, Grassino AE. The pattern of breathing in diffuse lung fibrosis. Bull Eur Physiopathol Respir 1982; 18: 461–472. [PubMed] [Google Scholar]
- 98.Londner C, Al Dandachi G, Plantier L, et al. Cross-sectional assessment of the relationships between dyspnea domains and lung function in diffuse parenchymal lung disease. Respiraton 2014; 87: 105–112. [DOI] [PubMed] [Google Scholar]
- 99.Gorini M, Spinelli A, Ginanni R, et al. Neural respiratory drive and neuromuscular coupling during CO2 rebreathing in patients with chronic interstitial lung disease. Chest 1989; 96: 824–830. [DOI] [PubMed] [Google Scholar]
- 100.Raghu G, Amatto VC, Behr J, et al. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J 2015; 46: 1113–1130. [DOI] [PubMed] [Google Scholar]
- 101.Oldham JM, Collard HR. Comorbid conditions in idiopathic pulmonary fibrosis: recognition and management. Front Med 2017; 4: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bodlet A, Maury G, Jamart J, et al. Influence of radiological emphysema on lung function test in idiopathic pulmonary fibrosis. Respir Med 2013; 107: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 103.Jacob J, Bartholmai BJ, Rajagopalan S, et al. Functional and prognostic effects when emphysema complicates idiopathic pulmonary fibrosis. Eur Respir J 2017; 50: 1700379. [DOI] [PubMed] [Google Scholar]
- 104.Kitaguchi Y, Fujimoto K, Hanaoka M, et al. Pulmonary function impairment in patients with combined pulmonary fibrosis and emphysema with and without airflow obstruction. Int J Chron Obstruct Pulmon Dis 2014; 9: 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Assayag D, Vittinghoff E, Ryerson CJ, et al. The effect of bronchodilators on forced vital capacity measurement in patients with idiopathic pulmonary fibrosis. Respir Med 2015; 109: 1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agostoni P, Bussotti M, Cattadori G, et al. Gas diffusion and alveolar-capillary unit in chronic heart failure. Eur Heart J 2006; 27: 2538–2543. [DOI] [PubMed] [Google Scholar]
- 107.Melenovsky V, Andersen MJ, Andress K, et al. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail 2015; 17: 1161–1171. [DOI] [PubMed] [Google Scholar]
- 108.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 109.Olson TP, Johnson BD, Borlaug BA. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail 2016; 4: 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hubbard RB, Smith C, Le Jeune I, et al. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med 2008; 178: 1257–1261. [DOI] [PubMed] [Google Scholar]
- 111.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 183: 431–440. [DOI] [PubMed] [Google Scholar]
- 112.du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011; 184: 1382–1389. [DOI] [PubMed] [Google Scholar]
- 113.Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003; 167: 962–969. [DOI] [PubMed] [Google Scholar]
- 114.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012; 156: 684–691. [DOI] [PubMed] [Google Scholar]
- 115.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 116.Parker JM, Glaspole IN, Lancaster LH, et al. A phase 2 randomized controlled study of tralokinumab in subjects with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2018; 197: 94–103. [DOI] [PubMed] [Google Scholar]
- 117.Raghu G, Martinez FJ, Brown KK, et al. CC-chemokine ligand 2 inhibition in idiopathic pulmonary fibrosis: a phase 2 trial of carlumab. Eur Respir J 2015; 46: 1740–1750. [DOI] [PubMed] [Google Scholar]
- 118.Cottin V, Hansell DM, Sverzellati N, et al. Effect of emphysema extent on serial lung function in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2017; 196: 1162–1171. [DOI] [PubMed] [Google Scholar]
- 119.Schmidt SL, Nambiar AM, Tayob N, et al. Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema. Eur Respir J 2011; 38: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmidt SL, Tayob N, Han MK, et al. Predicting pulmonary fibrosis disease course from past trends in pulmonary function. Chest 2014; 145: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raghu G, Brown KK, Collard HR, et al. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med 2017; 5: 22–32. [DOI] [PubMed] [Google Scholar]
- 122.Moderno EV, Yamaguti WPS, Schettino GPP, et al. Effects of proportional assisted ventilation on exercise performance in idiopathic pulmonary fibrosis patients. Respir Med 2010; 104: 134–141. [DOI] [PubMed] [Google Scholar]
- 123.Boutou AK, Pitsiou GG, Trigonis I, et al. Exercise capacity in idiopathic pulmonary fibrosis: the effect of pulmonary hypertension. Respirology 2011; 16: 451–458. [DOI] [PubMed] [Google Scholar]
- 124.van der Plas MN, van Kan C, Blumenthal J, et al. Pulmonary vascular limitation to exercise and survival in idiopathic pulmonary fibrosis. Respirology 2014; 19: 269–275. [DOI] [PubMed] [Google Scholar]
- 125.Minai OA, Santacruz JF, Alster JM, et al. Impact of pulmonary hemodynamics on 6-min walk test in idiopathic pulmonary fibrosis. Respir Med 2012; 106: 1613–1621. [DOI] [PubMed] [Google Scholar]
- 126.Idiopathic Pulmonary Fibrosis Clinical Research Network , Zisman DA, Schwarz M, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med 2010; 363: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nishiyama O, Taniguchi H, Kondoh Y, et al. Dyspnoea at 6-min walk test in idiopathic pulmonary fibrosis: comparison with COPD. Respir Med 2007; 101: 833–838. [DOI] [PubMed] [Google Scholar]
- 128.Hempleman SC, Hughes JM. Estimating exercise DLO2 and diffusion limitation in patients with interstitial fibrosis. Respir Physiol 1991; 83: 167–178. [DOI] [PubMed] [Google Scholar]
- 129.Hughes JM, Lockwood DN, Jones HA, et al. DLCO/Q and diffusion limitation at rest and on exercise in patients with interstitial fibrosis. Respir Physiol 1991; 83: 155–166. [DOI] [PubMed] [Google Scholar]
- 130.Visca D, Montgomery A, de Lauretis A, et al. Ambulatory oxygen in interstitial lung disease. Eur Respir J 2011; 38: 987–990. [DOI] [PubMed] [Google Scholar]
- 131.Frank RC, Hicks S, Duck AM, et al. Ambulatory oxygen in idiopathic pulmonary fibrosis: of what benefit? Eur Respir J 2012; 40: 269–270. [DOI] [PubMed] [Google Scholar]
- 132.Visca D, Mori L, Tsipouri V, et al. AmbOx: a randomised controlled, crossover trial evaluating the effect of ambulatory oxygen on health status in patients with fibrotic interstitial lung disease. Am J Respir Crit Care Med 2017; 195: A7603. [Google Scholar]
- 133.Nishiyama O, Miyajima H, Fukai Y, et al. Effect of ambulatory oxygen on exertional dyspnea in IPF patients without resting hypoxemia. Respir Med 2013; 107: 1241–1246. [DOI] [PubMed] [Google Scholar]
- 134.Dowman LM, McDonald CF, Bozinovski S, et al. Greater endurance capacity and improved dyspnoea with acute oxygen supplementation in idiopathic pulmonary fibrosis patients without resting hypoxaemia. Respirology 2017; 22: 957–964. [DOI] [PubMed] [Google Scholar]
- 135.Manali ED, Lyberopoulos P, Triantafillidou C, et al. MRC Chronic Dyspnea Scale: relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study. BMC Pulm Med 2010; 10: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007; 356: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 137.Swigris JJ, Streiner DL, Brown KK, et al. Assessing exertional dyspnea in patients with idiopathic pulmonary fibrosis. Respir Med 2014; 108: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Triantafillidou C, Manali E, Lyberopoulos P, et al. The role of cardiopulmonary exercise test in IPF prognosis. Pulm Med 2013; 2013: 514817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Prost JF, Desfonds P, Genevray B, et al. Evaluation de la gravite des embolies pulmonaires. Interet de la mesure de la capacite de transfert du monoxyde de carbone a l'etat stable [Evaluation of the severity of pulmonary embolism. Value of the measurement of stable carbon monoxide transfer capacity]. Presse Med 1984; 13: 1193–1197. [PubMed] [Google Scholar]
- 140.Schiavi EA, Roncoroni AJ, Puy RJ. Isolated bilateral diaphragmatic paresis with interstitial lung disease. An unusual presentation of dermatomyositis. Am Rev Respir Dis 1984; 129: 337–339. [PubMed] [Google Scholar]
- 141.Sears MR. Predicting asthma outcomes. J Allergy Clin Immunol 2015; 136: 829–836. [DOI] [PubMed] [Google Scholar]