Abstract
Objective:
To evaluate the pathologic response, safety, and feasibility of nephrectomy following receipt of immune checkpoint inhibition (ICI) for renal cell carcinoma (RCC).
Methods:
Patients who underwent nephrectomy for RCC after exposure to nivolumab monotherapy or combination ipilimumab/nivolumab were reviewed. Primary surgical outcomes included operative time (OT), estimated blood loss (EBL), length of stay (LOS), readmission rates, and complication rates. Pathologic response in the primary and metastatic sites constituted secondary outcomes.
Results:
Eleven nephrectomies (10 radical, 1 partial) were performed in 10 patients after ICI with median postoperative follow-up 180 days. Six patients received 1 to 4 cycles of ipilimumab/nivolumab, while 5 received 2 to 12 infusions of nivolumab preoperatively. Five surgeries were performed laparoscopically, and 4 patients underwent concomitant thrombectomy. One patient exhibited complete response (pT0) to ICI, and 3/4 patients who underwent metastasectomy for hepatic, pulmonary, or adrenal lesions exhibited no detectable malignancy in any of the metastases resected. No patients experienced any major intraoperative complications, and all surgical margins were negative. Median OT, EBL, and LOS were 180 minutes, 100 ml, and 4 days, respectively. Four patients experienced a complication, including 3 that were addressed with interventional radiology procedures. One patient died of progressive disease >3 months after surgery, and 1 patient succumbed to pulmonary embolism complicated by sepsis. No complications or readmissions were noted in 6 patients.
Conclusion:
Nephrectomy following ICI for RCC is safe and technically feasible with favorable surgical outcomes and pathologic response. Timing of the nephrectomy relative to checkpoint dosing did not seem to impact outcome. Biopsies of lesions responding radiographically to ICI may warrant attention prior to surgical excision.
Keywords: Renal cell carcinoma, Immune checkpoint inhibitors, Nephrectomy, Safety, Complications
1. Introduction
Over 65,000 new cases of kidney cancer, predominantly renal cell carcinoma (RCC) are diagnosed in the United States annually, with nearly 15,000 attributable deaths [1]. At diagnosis, approximately 30% to 40% of patients already harbor metastatic disease [2], and corresponding 5-year survival rates are suboptimal, ranging from 0% to 20% [3–5]. Furthermore, following the surgical management of clinically localized RCC with curative intent, an estimated 20% to 40% of patients recur within 3 years of nephrectomy [6], suggesting aggressive tumor biology or undetected micrometastatic disease in these patients at presentation. Such patients may benefit from early, potentially upfront, systemic therapy. Indeed, while there is a growing role for the integration of multidisciplinary approaches in managing advanced RCC, the utility and optimal timing of surgery versus systemic therapy has yet to be further elucidated.
The introduction of several novel classes of systemic therapies, including targeted therapies and most recently immune checkpoint inhibitors (ICI), has revolutionized the management of metastatic RCC over the last decade. With these new therapies, the role of nephrectomy in the treatment paradigm for advanced RCC has continued to evolve. Contemporary studies assessing the safety of nephrectomy following systemic therapy have primarily involved tyrosine kinase inhibitors with mixed results [7–20], though in the recent phase III EORTC 30073 (SURTIME) trial, cytoreductive nephrectomy after sunitinib was found to be safe [7].
ICIs have been gaining considerable momentum in managing metastatic RCC, initially with the approval of second-line nivolumab monotherapy and subsequently with the approval of combination ipilimumab and nivolumab in the frontline setting, based on the results of the CheckMate 025 and 214 trials, respectively [21,22]. Unlike other therapies for metastatic RCC, ICIs act uniquely by blocking inhibitory signaling to restore tumor-specific T cell-mediated immune responses [23]. In the present immunotherapy era, the role, candidacy, and timing of surgically resecting the primary tumor remains undefined. We recently reported the case of a patient with metastatic RCC who exhibited a remarkable response to nivolumab and underwent an uncomplicated radical nephrectomy with no evidence of viable malignancy in his final pathologic specimen [24]. In another case report of a patient with metastatic RCC, use of nivolumab actually facilitated nephrectomy and partial hepatectomy by reducing the primary tumor size, and the surgery was performed safely in a minimally invasive fashion [25].
Heretofore, the safety of nephrectomy after ICI has not yet been studied systematically. In the present study, we sought to evaluate the safety and feasibility of performing nephrectomy in patients who received prior ICI for RCC at our institution, along with the pathologic response rates of primary and metastatic tumors.
2. Methods
Following institutional review board approval, clinicopathologic data of patients who underwent nephrectomy for RCC between 2016 and 2018 at our institution were reviewed. Patients who had received nivolumab monotherapy or combination ipilimumab and nivolumab prior to surgery were identified. All patients were confirmed to have RCC by biopsy of either the primary or a metastatic lesion prior to initiation of systemic therapy. Decision to pursue ICI was based on failure of other lines of systemic therapies or, following first-line approval of combination ipilimumab and nivolumab for metastatic RCC, was the initial choice of therapy. Indications and timing for proceeding with nephrectomy varied by patient scenario, but was generally pursued in patients who were appropriate surgical candidates (minimal competing comorbidities), with the majority of their tumor burden localized in the kidney and an absence of brain metastases. Rather than receive upfront nephrectomy at metastatic diagnosis, patients included herein were initiated on systemic therapy given the perceived oncologic benefit of ICI. Patients who were demonstrating response to ICI in metastatic sites or in the primary tumor, including improvement in risk classification, were then felt most likely to derive benefit from nephrectomy. All patients were counseled on risks, benefits, and alternatives of pursuing surgical intervention prior to nephrectomy. Surgical approach, including open versus minimally invasive techniques, performance of lymphadenectomy, and performance of metastasectomy were per the treating surgeon’s discretion. If tumors were felt to be safely approachable using robotic or conventional laparoscopic approaches, then these approaches were preferentially used to minimize morbidity. An open approach was pursued in scenarios of local tumor invasion (e.g., to surrounding organs or tumor thrombus extension into the inferior vena cava), extensive resection of metastatic sites, or particularly large tumor size deemed on preoperative imaging.
Patient demographics, tobacco exposure, type and number of ICI cycles received before surgery, immune-related adverse events, tumor laterality, and presence and site(s) of metastatic disease were collected. Patients’ risk categorizations were ascertained by the International Metastatic RCC Database Consortium (IMDC) criteria. Pathologic information including tumor histology, grade, size, stage, multifocality, surgical margins, and presence of necrosis, sarcomatoid features, rhabdoid features, or tumor thrombus were tabulated. Immediate surgical outcomes assessed included intraoperative complications, operative time (OT), estimated blood loss (EBL), need for blood transfusion, admission to the intensive care unit, and hospital length of stay (LOS). Additional information regarding any postoperative complications (graded by the Clavien-Dindo classification scale), 30- and 90-day readmission rates, continuation of ICI postoperatively, and mortality were also collected.
Descriptive statistics were used to analyze clinicopathologic data, treatment response, surgical outcomes, complications, and follow-up. All statistical analyses were conducted using SPSS version 25.0 (IBM, Armonk, NY).
3. Results
3.1. Clinicopathologic characteristics
In total, 11 nephrectomies, including 10 radical and 1 partial nephrectomy, were performed in 10 patients (9 male, 1 female) who had received prior ICI. Clinical data for each patient are summarized in Table 1. Median age at the time of nephrectomy was 64 years. Six patients received 1 to 4 cycles of combination ipilimumab and nivolumab, while 4 received 2 to 12 infusions of nivolumab preoperatively. ICI was not halted for surgical indications in any case, and the median time between ICI dose and surgery was 21 days. One patient with nonmetastatic, synchronous bilateral renal masses underwent staged left radical nephrectomy and right partial nephrectomy after 2 cycles of nivolumab. The remaining patients all harbored metastatic disease. IMDC risk scores for metastatic patients at the time of ICI receipt were either intermediate (7/9, 78%) or poor (2/9, 22%); no patients exhibited favorable IMDC risk. At the time of surgery, 1 of the patients with initially poor risk disease was reclassified as intermediate risk after multiple cycles of nivolumab.
Table 1.
Baseline clinical data of patients who underwent nephrectomy after receipt of ICI
| Patient | Age at nephrectomy (yrs.) | Race | Gender | BMI (kg/m2) | Laterality | Prior tobacco use | IMDC risk score at first ICI | IMDC risk score at nephrectomy | # of metastatic sites | # lines of systemic therapy before ICI | ICI received before nephrectomy (# cycles) | # of days between ICI dose and nephrectomy | Any irAE prior to surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 | Caucasian | Male | 25.0 | Left | No | Intermediate | Intermediate | 4 | 2 | Nivolumab (11) | 2 | No |
| 2 | 64 | Caucasian | Male | 30.9 | Left | No | Poor | Intermediate | 1 | 1 | Nivolumab (11) | 8 | Hypothyroidism, adrenal insufficiencyb |
| 3 | 64 | Hispanic | Male | 26.1 | Right | No | Poor | Poor | 4 | 1 | Nivolumab (12) | 17 | No |
| 4 | 67 | Caucasian | Female | 29.1 | Left | No | Intermediate | Intermediate | 3 | 2 | Ipilimumab/Nivolumab (4) + Nivolumab (1) | 64 | Pneumonitisb |
| 5 | 48 | Caucasian | Male | 27.6 | Left | Yes | Intermediate | Intermediate | 1 | 0 | Ipilimumab/Nivolumab (2) | 264 | Nephritisb |
| 6 | 80 | Caucasian | Male | 24.6 | Right | Yes | Intermediate | Intermediate | 4 | 0 | Ipilimumab/Nivolumab (1) | 36 | Pneumonitisb |
| 7aa | 62 | Caucasian | Male | 45.7 | Left | No | N/A | N/A | 0 | 0 | Nivolumab (2) | 19 | No |
| 7ba | 62 | Caucasian | Male | 45.7 | Right | No | N/A | N/A | 0 | 0 | Nivolumab (2) | 66 | No |
| 8 | 69 | Other | Male | 23.2 | Right | Yes | Intermediate | Intermediate | 3 | 0 | Ipilimumab/Nivolumab (1) | 13 | Dermatitis |
| 9 | 69 | Caucasian | Male | 34.7 | Left | No | Intermediate | Intermediate | 1 | 0 | Ipilimumab/Nivolumab (1) | 71 | Dermatitisb |
| 10 | 41 | Hispanic | Male | 22.0 | Left | No | Intermediate | Intermediate | 4 | 0 | Ipilimumab/Nivolumab (1) | 21 | No |
Abbreviations: BMI = body mass index; ICI = immune checkpoint inhibition; IMDC = International Metastatic RCC Database Consortium; irAE = immune-related adverse event.
Patient 7 underwent staged left radical nephrectomy and right partial nephrectomy for synchronous bilateral renal masses.
Treatment with systemic steroids for irAE.
On final pathologic assessment (Table 2), all but 2 tumors were of clear cell histology; the remainder included 1 papillary type II and 1 translocation RCC. One patient exhibited complete response (pT0) to ICI in the primary tumor. The relative change in size of the index primary tumor from pre-ICI imaging to surgery is summarized in Fig. 1. No patients progressed by iRECIST criteria prior to surgery. Among the 4 patients with tumor thrombi, there was no appreciable shrinkage in the thrombus size in response to ICI. Of the 5 patients who underwent lymphadenectomy, 2 patients exhibited pN1 disease, both of whom had nonclear cell histology. Four patients underwent metastasectomy for hepatic, pulmonary, or adrenal lesions, of whom 3 patients (75%) exhibited no detectable malignancy in any of the metastases resected. The radiographic appearance of a patient’s hepatic lesion that responded completely to ICI is exemplified in Fig. 2 (Patient 5). All surgical margins were negative.
Table 2.
Pathologic findings on surgical specimens exposed to prior ICI
| Patient | Histology | Primary tumor size (cm) | Grade | pT stage | pN stage | pM stage if separate metastasectomy performed | Sarcomatoid features | Rhabdoid features | Necrosis | Tumor thrombus (level per Novick classification) | Surgical margin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Clear cell | 12.5 | 4 | 3a | 0 | x | No | Yes | Yes | No | Negative |
| 2 | Clear cell | 3.6 | N/A | 0 | x | x | No | No | Yes | No | Negative |
| 3 | Clear cell | 8 | 4 | 3a | x | x | Yes | Yes | Yes | No | Negative |
| 4 | Clear cell | 6.3 | 4 | 3a | x | 1 (liver) | No | No | Yes | No | Negative |
| 5 | Clear cell | 7 | 3 | 3b | 0 | 0 (liver) | No | No | Yes | Yes (II) | Negative |
| 6 | Clear cell | 5 | 3 | 3a | x | x | No | No | No | No | Negative |
| 7a | Clear cell | 13.1 | 3 | 3a | 0 | x | No | No | Yes | Yes (Ia) | Negative |
| 7b | Clear cell | 1.7 | 3 | la | x | x | No | No | No | No | Negative |
| 8 | Papillary II | 9.5 | 4 | 3a | 1 | 0 (liver, adrenal) | No | No | Yes | No | Negative |
| 9 | Clear cell | 7.2 | 3 | 3b | x | 0 (lung) | No | No | Yes | Yes (III) | Negative |
| 10 | Translocation | 15 | 3 | 3a | 1 | x | No | No | Yes | Yes (Ia) | Negative |
Thrombus extending to renal vein only.
Fig. 1.
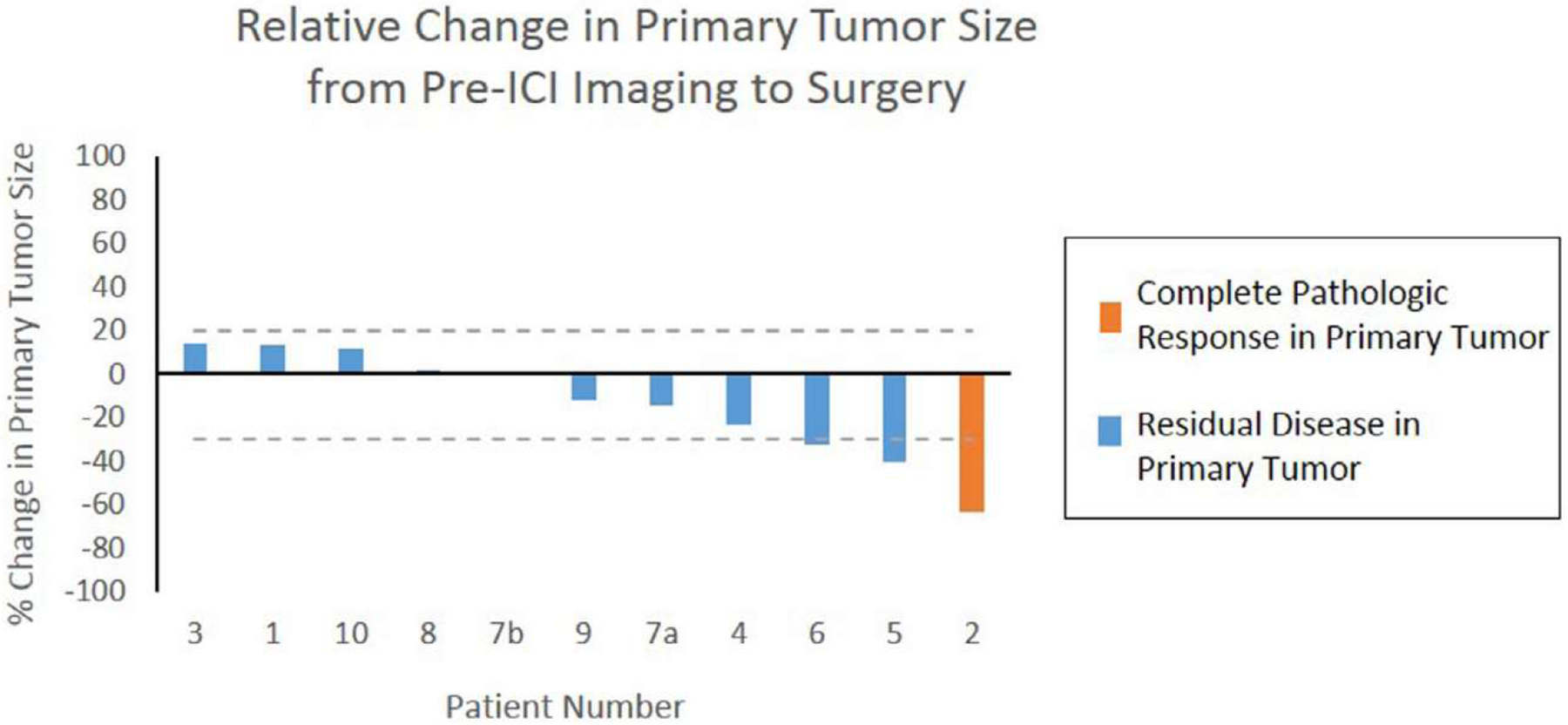
Waterfall plot illustrating relative change in size of the index primary tumor from pre-ICI cross-sectional imaging to surgery. Patient 2 (orange) exhibited complete pathologic response in his primary tumor (pT0). Dotted lines are displayed at 20% and −30% thresholds used for progressive disease and partial response, respectively, by iRECIST criteria. Abbreviations: ICI = immune checkpoint inhibition.
Fig. 2.
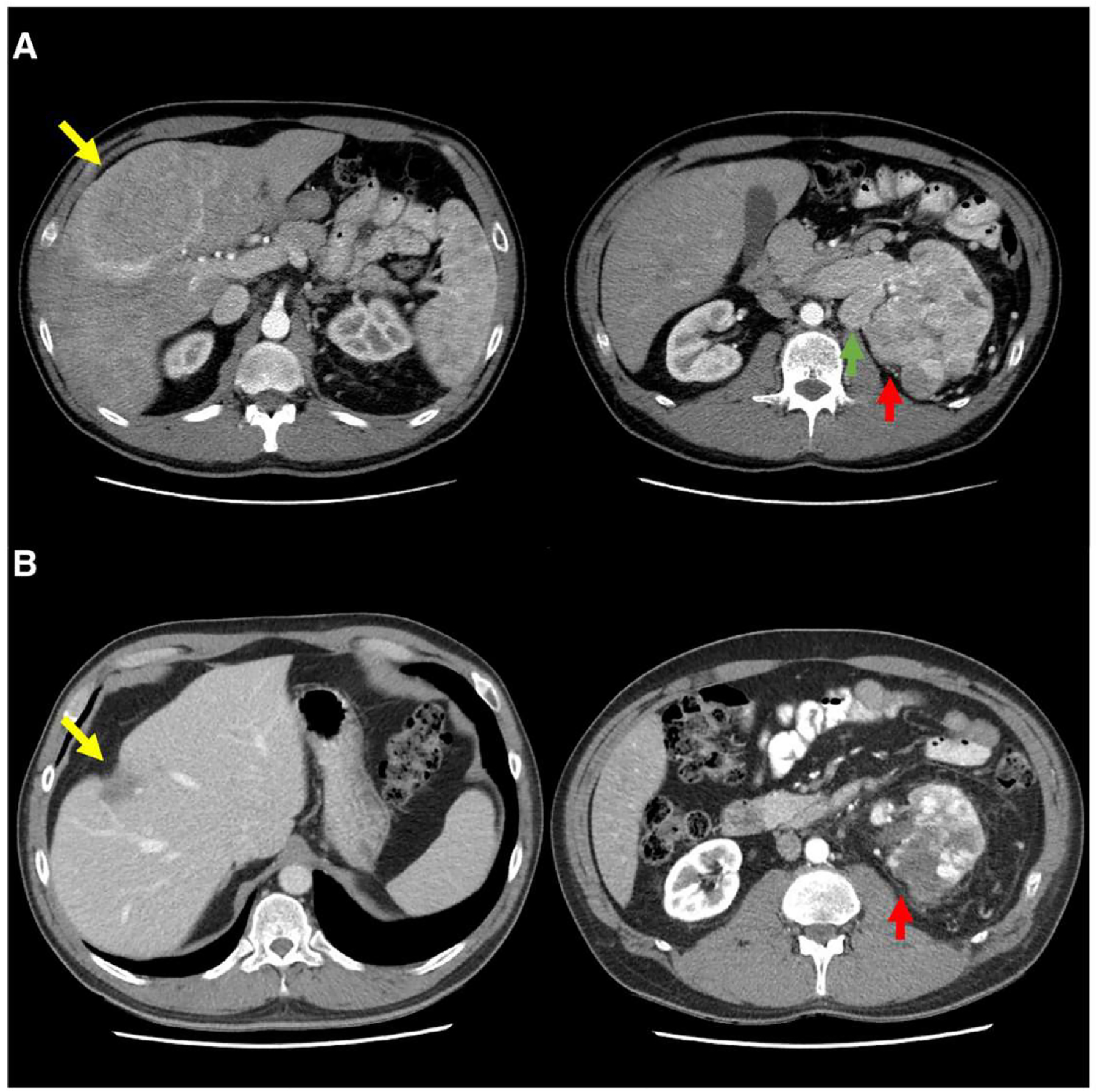
Axial contrast-enhanced abdominal CT images of Patient 5, demonstrating radiographic response to ICI. (A) Baseline images obtained prior to ICI reveal multiple enhancing hepatic metastases, biopsy-confirmed RCC, with the dominant tumor measuring 12.3 × 8.1 cm located primarily in segment 8 (yellow arrow). The large left primary renal tumor (red arrow) measures 11.8 × 9.7 × 9.3 cm and demonstrates heterogeneous enhancement with local infiltration and invasion into the renal vein and inferior vena cava (not shown). Enlarged left para-aortic lymph node measures 4.0 × 2.5 cm (green arrow). (B) Following receipt of 2 cycles of combination ipilimumab and nivolumab, the same dominant hepatic lesion (yellow arrow) shrank considerably to 3.2 × 2.1 cm and demonstrated hypoattenuation with capsular retraction. The primary tumor shrank to 8.2 × 6.6 × 7.0 cm (red arrow), and retroperitoneal lymphadenopathy resolved entirely. Partial hepatectomy at the time of nephrectomy revealed fibrosis, inflammation, edema, and remote hemorrhage, but otherwise no viable malignancy in the liver metastases (final stage pT3bN0M0). Abbreviations: ICI = immune checkpoint inhibition; RCC = renal cell carcinoma.
3.2. Feasibility and safety outcomes
Five surgeries (45%) were successfully performed in a minimally invasive fashion (conventional laparoscopy or robotic-assisted), and 4 patients underwent concomitant tumor thrombectomy, including 2 with thrombus extension into the inferior vena cava. In our experience, there were no untoward intraoperative challenges encountered that we could definitively attribute to ICI exposure. Grossly, we did not find an increased desmoplastic reaction, fibrosis, edema, or adhesions that affected the technical difficulty of the operations. There were no anesthesia challenges encountered either. No patients experienced any major intraoperative complications (Table 3). One patient who underwent left radical nephrectomy and simultaneous resection of a hepatic metastasis experienced a pancreatic laceration that was repaired primarily without any notable postoperative consequence. Median OT and EBL were 180 minutes and 100 ml, respectively. Three patients were admitted to the intensive care unit postoperatively, including 2 who were admitted for routine monitoring after thrombectomy. Median total LOS was 4 days. There were no deaths or complications in the immediate postoperative period, and no patients experienced any wound complications.
Table 3.
Feasibility and safety assessed by perioperative and postoperative outcomes in patients undergoing nephrectomy after receipt of ICI
| Patient | Perioperative outcomes | Postoperative outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Open versus minimally invasive (MIS) approach | OT (mins) | EBL (rnL) | Intraoperative complications | Need for blood transfusion | ICU admission | LOS (days) | 30-day readmission | 90-day readmission | Any complication (highest Clavien-Dindo grade) | Resumption of ICI post-operatively | Dead | Time to last follow-up from surgery (days) | |
| 1 | Open | 180 | 300 | No | No | Yes | 4 | Yes | Yes | Yes (IIIa)a | Yes | Yesb | 105 |
| 2 | Open | 92 | 50 | No | No | No | 5 | No | No | No | No | No | 675 |
| 3 | MIS | 115 | 100 | No | No | No | 2 | No | No | No | Yes | No | 528 |
| 4 | MIS | 262 | 70 | Pancreatic laceration (repaired primarily) | No | No | 4 | No | No | No | No | No | 295 |
| 5 | Open | 312 | 350 | No | No | Yes | 7 | Yes | Yes | Yes (IIIa)a | No | No | 205 |
| 6 | MIS | 139 | 75 | No | No | No | 1 | No | No | No | No | No | 196 |
| 7a | MIS | 192 | 100 | No | No | No | 2 | No | No | No | Yes | No | 164 |
| 7b | MIS | 80 | 100 | No | No | No | 2 | No | No | No | Yes | No | 117 |
| 8 | Open | 223 | 400 | No | No | No | 3 | No | No | No | Yes | No | 141 |
| 9 | Open | 330 | 6000 | No | Yes | Yes | 4 | Yes | Yes | Yes (IIIa)a | No | No | 114 |
| 10 | Open | 130 | 500 | No | Yes | No | 4 | No | Yes | Yes (V)a | Yes | Yes | 81 |
Abbreviations: EBL = estimated blood loss; ICU = intensive care unit; LOS = length of stay; OT = operative time.
Patient 1 developed chylous ascites requiring paracentesis. Patient 5 developed a sterile fluid collection in the hepatic resection bed requiring percutaneous aspiration. Patient 9 developed pleural effusion requiring thoracentesis. Patient 10 developed a pulmonary embolus and sepsis from which he succumbed at 81 days after surgery.
Patient 1 died of progressive disease >3 months after surgery.
Median postoperative follow-up for the 10 patients was 180 days. Within the first 30 days of surgery, there were no deaths and 3 readmissions: 1 patient developed a pericardial effusion and sizable left pleural effusion for which he required thoracentesis, 1 required paracentesis in the setting of chylous ascites, and another required percutaneous aspiration of a sterile fluid collection in the hepatic resection bed. Between 30 and 90 days of surgery, there was 1 additional readmission for a patient who succumbed to pulmonary insufficiency secondary to a pulmonary embolus and sepsis at 81 days postoperatively. One patient died of progressive disease 105 days after surgery. No readmissions or complications of any grade were noted in the remaining 6 patients, and 8 patients are currently alive. ICI was resumed postoperatively in 6 patients based on tolerability and ongoing radiographic benefit from ICI at the time of surgery. Safety outcomes are summarized in Table 3.
4. Discussion
The landmark CheckMate 025 and 214 trials recently revolutionized the paradigm for managing metastatic RCC by introducing ICIs into our therapeutic armamentarium [21,22]. The role and timing of offering nephrectomy in the contemporary immunotherapy era remain largely undefined, and until now, the feasibility and safety of performing nephrectomy after prior receipt of ICI have not been studied systematically. In the present study, we found that nephrectomy following ICI for RCC appears to be both safe and technically feasible in our institutional experience, with favorable surgical outcomes and pathologic response.
Mechanistically, ICIs act uniquely to block inhibitory signaling mediated through the CTLA-4 (ipilimumab) or PD-1 (nivolumab) pathways. In turn, by preventing the binding of CTLA-4 or PD-1 to their respective ligands, ICIs enhance T cell activation, proliferation, and infiltration in tumors to elicit an immune-mediated antitumoral response [23]. Combined blockade of both the CTLA-4 and PD-1 pathways has demonstrated synergy in treating RCC [22], eventually establishing an approved role for ICI in the frontline management of metastatic RCC patients. Despite the immunomodulation induced by these agents, we found that neither the technical difficulty of surgical dissection nor adhesions encountered intraoperatively were increased, and no major intraoperative complications or perioperative deaths occurred. OT, EBL, and LOS in our series were acceptable and comparable to other contemporary nephrectomy series reporting on perioperative outcomes [26–28]. In all 5 cases in which we attempted a minimally invasive approach, surgery was completed successfully without conversion to an open procedure. We were also able to successfully perform tumor thrombectomy in 4 patients, and no patients had positive surgical margins.
Although autoimmune effects could theoretically impact postoperative recovery, such as pancreatitis, pneumonitis, and neuropathy, no such issues arose postoperatively in our cohort, despite that immune-related adverse events occurred in 6 patients prior to surgery. In another recent retrospective study evaluating the feasibility and safety of surgery in patients receiving ICI for various malignancies—including 2 patients with RCC who underwent laparoscopic abdominal wall resection after atezolizumab—Elias et al. reported 1 death and no 30-day grade III-IV complications [29]. They concluded that ICI was safe in the perioperative setting and did not need to be stopped, though notably none of the patients underwent post-ICI nephrectomy, and the 22 surgical procedures evaluated in their study were varied and often less involved than nephrectomies. Furthermore, unlike several prospective studies in which wound-healing concerns were noted after the presurgical use of angiogenic inhibitors for RCC [9,10,13,30]—including 21% such complications after bevacizumab [9]—we found that none of our patients exhibited any wound complications after post-ICI nephrectomy.
Postoperative complication rates overall were acceptable in our cohort. Three of 4 patients who developed any complication over a median postoperative follow-up of 6 months were successfully managed with interventional radiology procedures (paracentesis, percutaneous aspiration, or thoracentesis). Of the 2 patients who died, 1 developed a pulmonary embolus and sepsis >30 days after surgery, which were not definitively attributable to preoperative ICI use, and the other succumbed to disease progression >3 months after surgery. Our complication rates fared favorably to those reported recently in the randomized SURTIME trial, which compared immediate and deferred cytoreductive nephrectomy in patients with metastatic RCC receiving sunitinib [7]. In SURTIME, Bex et al. reported an overall surgical complication rate of 56% across the immediate, per-protocol deferred, and off-protocol deferred nephrectomy groups; this notably included 27% intraoperative complications and 45% postoperative complications.
Our findings also highlight the remarkable pathologic response to ICI in a cohort of patients who only exhibited intermediate or poor IMDC risk. One patient achieved complete pathologic response (pT0) on his nephrectomy specimen, while 3 of 4 (75%) patients who underwent metastasectomy for identified lesions in the liver, lung, or adrenal glands strikingly exhibited no malignancy in any of the metastases resected. Given the favorable pathologic response to ICI, performing biopsies of lesions that respond radiographically can be considered to avoid the morbidity of surgical excision, though false negatives or even tumor scar relapse remain possibilities. Predicting response to ICI remains an area of considerable contemporary interest [31–33], and patient selection, potentially integrating genomic, transcriptomic, or immunohistochemical expression profiles, will likely retain a critical role in understanding which patients would derive oncologic benefit from ICI combined with locoregional control.
Our study is limited by its retrospective nature in a small cohort derived from a single institution. The ICI regimen employed, number of ICI cycles preceding surgery, prior receipt of other therapies, and resumption of ICI postoperatively were not standardized across all patients. The cohort was also heterogeneous, including differences in histopathology, metastatic burden, surgical approach, performance of lymphadenectomy, and performance of metastasectomy, which may complicate the interpretation of results. Furthermore, our ability to assess oncologic outcomes is limited by a relatively short postoperative follow-up (median 6 months). Nonetheless, to our knowledge, this is the first study to systematically report the feasibility and safety of nephrectomy after prior exposure to ICI for RCC. Our encouraging experience may form the impetus for larger studies exploring patient selection and oncologic outcomes in these patients. Novel clinical trials are needed to further elucidate the role and timing of nephrectomy in the setting of ICI. We eagerly await the results from ongoing prospective trials, such as the phase III PROSPER trial comparing perioperative nivolumab versus observation in patients with localized RCC undergoing nephrectomy (NCT03055013) and another trial comparing nivolumab with or without bevacizumab or ipilimumab before nephrectomy in patients with metastatic RCC (NCT02210117), to shed light on nephrectomy after ICI.
5. Conclusion
In our experience, nephrectomy following ICI for RCC is both safe and technically feasible. Surgical and postoperative outcomes are encouraging, and pathologic response to ICI is strikingly favorable in both the primary tumor and metastatic sites. Biopsies of lesions responding radiographically to ICI should be considered prior to surgical excision. As multimodal management in the immunotherapy era continues to evolve, the utility and timing of nephrectomy combined with ICI in selected patients warrants further study.
Acknowledgment
N.S. is receiving funding support from the Ruth L. Kirschstein National Research Service Award T32 CA136515-09 and the University of Texas Southwestern Medical Center Physician Scientist Training Program.
Footnotes
Conflicts of interest
None
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(l):7–30. [DOI] [PubMed] [Google Scholar]
- [2].Howlader NNA, Krapcho M, et al. (eds). SEER cancer statistics review, 1975–2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- [3].Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27(34):5794–9. [DOI] [PubMed] [Google Scholar]
- [4].Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan—Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23(4):832–41. [DOI] [PubMed] [Google Scholar]
- [5].Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for Sunitinib compared with interferon Alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27 (22):3584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30 (4):843–52. [DOI] [PubMed] [Google Scholar]
- [7].Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving Sunitinib: the SURTIME randomized clinical trial. JAMA Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Borregales LD, Adibi M, Thomas AZ, Wood CG, Karam JA. The role of neoadjuvant therapy in the management of locally advanced renal cell carcinoma. Ther Adv Urol 2016;8(2): 130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jonasch E, Wood CG, Matin SF, et al. Phase II presurgical feasibility study of Bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27(25):4076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Karam JA, Devine CE, Urbauer DL, et al. Phase 2 trial of Neoadjuvant Axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol 2014;66(5):874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lebacle C, Bensalah K, Bernhard JC, et al. Evaluation of axitinib to downstage cT2a renal tumours and allow partial nephrectomy: a phase II study. BJU Int 2018. [DOI] [PubMed] [Google Scholar]
- [12].McCormick B, Meissner MA, Karam JA, Wood CG. Surgical complications of presurgical systemic therapy for renal cell carcinoma: a systematic review. Kidney Cancer 2017;1 (2): 115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Powles T, Kayani I, Blank C, et al. The safety and efficacy of Sunitinib before planned nephrectomy in metastatic clear cell renal cancer. Ann Oncol 2011;22(5):1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Powles T, Sarwar N, Stockdale A, et al. Safety and efficacy of Pazopanib therapy prior to planned nephrectomy in metastatic clear cell renal cancer. JAMA Oncol 2016;2(10): 1303–9. [DOI] [PubMed] [Google Scholar]
- [15].Harshman LC, Yu RJ, Allen GI, Srinivas S, Gill HS, Chung BI. Surgical outcomes and complications associated with presurgical tyrosine kinase inhibition for advanced renal cell carcinoma (RCC). Urol Oncol 2013;31(3):379–85. [DOI] [PubMed] [Google Scholar]
- [16].Margulis V, Matin SF, Tannir N, et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. J Urol 2008;180(l):94–8. [DOI] [PubMed] [Google Scholar]
- [17].Thomas AA, Rini BI, Stephenson AJ, et al. Surgical resection of renal cell carcinoma after targeted therapy. J Urol 2009; 182(3):881–6. [DOI] [PubMed] [Google Scholar]
- [18].Shaw GL, Hussain M, Nair R, et al. Performing cytoreductive nephrectomy following targeted sunitinib therapy for metastatic renal cell carcinoma: a surgical perspective. Urol Int 2012;89(1):83–8. [DOI] [PubMed] [Google Scholar]
- [19].Patel N, Woo J, Liss MA, et al. Does timing of targeted therapy for metastatic renal cell carcinoma impact treatment toxicity and surgical complications? A comparison of primary and adjuvant approaches. Can J Urol 2016;23(2):8227–33. [PubMed] [Google Scholar]
- [20].Hanna N, Sun M, Meyer CP, et al. Survival analyses of patients with metastatic renal cancer treated with targeted therapy with or without cytoreductive nephrectomy: a national cancer data base study. J Clin Oncol 2016;34(27):3267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373(19): 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378(14):1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ascierto PA, Addeo R, Carteni G, et al. The role of immunotherapy in solid tumors: report from the Campania Society of Oncology Immunotherapy (SCITO) meeting, Naples 2014. J Transl Med. 2014; 12:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Woldu SL, Brugarolas J, Kapur P, Margulis V. What is the role of nephrectomy following complete response to checkpoint inhibitors? Urol Case Rep 2018;18:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ikarashi D, Kato Y, Katagiri H, et al. Case of complete response to neoadjuvant therapy using nivolumab in a patient with metastatic renal cell carcinoma. Int J Urol 2018;25(6):630–2. [DOI] [PubMed] [Google Scholar]
- [26].Jackson BL, Fowler S, Williams ST. British association of urological surgeons - section of O. Perioperative outcomes of cytoreductive nephrectomy in the UK in 2012. BJU Int 2015;116(6):905–10. [DOI] [PubMed] [Google Scholar]
- [27].Rinott Mizrahi G, Freifeld Y, Klein I, et al. Comparison of partial and radical laparascopic nephrectomy: perioperative and oncologic outcomes for clinical T2 renal cell carcinoma. J Endourol 2018;32(10):950–4. [DOI] [PubMed] [Google Scholar]
- [28].Jeong IG, Khandwala YS, Kim JH, et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA 2017;318(16): 1561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Elias AW, Kasi PM, Stauffer JA, et al. The feasibility and safety of surgery in patients receiving immune checkpoint inhibitors: a retrospective study. Front Oncol 2017;7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rini BI, Plimack ER, Takagi T, et al. A phase II study of pazopanib in patients with localized renal cell carcinoma to optimize preservation of renal parenchyma. J Urol 2015;194(2):297–303. [DOI] [PubMed] [Google Scholar]
- [31].Kluger HM, Zito CR, Turcu G, et al. PD-L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors. Clin Cancer Res 2017;23(15):4270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Panda A, de Cubas AA, Stein M, et al. Endogenous retrovirus expression is associated with response to immune checkpoint blockade in clear cell renal cell carcinoma. JCI Insight 2018;3(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018;359(6377):801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


