Abstract
Social play in rats is a highly rewarding, energetic form of social interaction and important for development of the brain and social skills. The 50 kHz ultrasonic vocalizations (USV) emitted during social play are thought to be an expression of a positive affective state (laughter), which in some situations may also function as communication signals. Heterospecific play, ‘tickling’ by an experimenter, is thought to simulate conspecific play, and has been used to improve welfare and to study the neurobiology of positive affect. Given that tickling evokes substantial amounts of USV, we investigated whether heterospecific play is simulating conspecific play by comparing USV-behaviour associations in both contexts. If the 50 kHz calls are merely an expression of ‘laughter’ then the pattern and type of emission in both contexts should be similar. By contrast, as playing with a conspecific involves a two-way exchange of signalling, the additional demands on communication should lead to a different pattern of calling. While calling was prevalent in both types of play, how the different types of 50 kHz calls are used in the two contexts differed markedly. The findings suggest that while conspecific and heterospecific play are positive experiences, tickling is not the equivalent of conspecific play.
This article is part of the theme issue ‘Cracking the laugh code: laughter through the lens of biology, psychology and neuroscience’.
Keywords: ultrasonic vocalizations, social play, heterospecific play
1. Introduction
In rats and several other species of mammals, social play behaviour during the juvenile period is critical for the development of skills that are important for navigating the social world [1,2]. Engaging in social play is highly rewarding, as has been demonstrated with place preference and operant conditioning paradigms [3]. In rats, social play mainly involves play fighting, a vigorous form of behaviour containing components of other social behaviour, especially that from sex, that are displayed in an altered and/or out-of-context form [4–6]. Social play mostly involves attack and defence of the nape of the neck, which is nuzzled with the snout if contacted [7,8]. After a successful pounce that leads to nape contact, the recipient may protect its nape by rotating to supine, with the partner standing on top (i.e. pinning). From the pinned position, the rat can launch a counterattack for access to the nape. The back-and-forth interaction leads to reciprocity, thus alternating which partner has the advantage. This requires not only cooperation between the playing rats but also seems to be one of the factors that makes play enjoyable [9].
Because social play is unpredictable, dynamic and complex, many species rely on play signals to prevent misinterpretation of each other's actions and potential escalation to serious fighting [10]. Given that rats mostly play in low light conditions, visual cues are of limited use, as are olfactory cues, which quickly saturate the play arena [11]. However, rats have a rich repertoire of vocalizations, especially ultrasonic vocalizations (USV) in the 50 kHz range [12–14], many of which are emitted both when anticipating the arrival of a play partner [15,16] and during social play [17,18]. While 50 kHz calls are emitted in both social and non-social positive affective contexts [19,20], during play many calls are emitted in contexts that suggest that they are being used to communicate with one another [21,22]. Indeed, if both partners are devocalized, the amount of play initiated declines, as do the role reversals indicative of reciprocity [23]. Moreover, when adult male rats, who are unfamiliar with one another, play together in a neutral arena, if one of the pair is devocalized, there is an increased risk of escalation to serious fighting [23], which seems to arise from the failure to use specific ultrasonic calls to de-escalate the encounter [24].
Therefore, USV seem important as play signals that are used to negotiate interactions and avoid misinterpretation, much as is the case for some of the more intensively studied visual signals used by canids and primates, such as play bows and open mouth facial expressions [15]. Some signals used during play may simply have a tonic effect, that of sustaining the playful mood of emitters and receivers [25,26]. A good example of such a mood effect emanating from a vocal play signal has recently been shown in keas (Nestor notabilis). When keas were exposed to the playback of play-typical warbles of keas, play behaviour was increased [27]. In this regard, some play signals can be like human laughter, a signal that induces a positive affective state [28]. Indeed, by focusing on the types of 50 kHz calls most commonly emitted during play, Jaak Panksepp and Jeff Burgdorf emphasized the role of calling as an expression of a positive affective state, and suggested that this could be a homologue of human laughter [29,30]. That juvenile rats, when ‘tickled’ by an experimenter, emit the same types of 50 kHz calls as they do when playing with other rats [31], supports this view. Since these initial insights and findings, rat tickling or ‘heterospecific play’ has been widely viewed as mimicking social play and has been used extensively to assess and improve animal welfare [32,33] and to study the neurobiology of positive affect and communication [34–37].
However, we think there are three problems with regarding heterospecific play as mimicking conspecific play, two of which have also been raised in a recent opinion paper by Bombail et al. [33]. First, there are individual differences among rats in both their motivation to engage in conspecific and heterospecific play, with rats that engage in more conspecific play not being the same as those that prefer to engage in heterospecific play [38]. This suggests that the rats do not consider these two forms of play as the same.
Second, part of heterospecific tickling involves turning the rat onto its back to simulate the pinning typical of conspecific play [31], the implication being that the rat emits positively affective 50 kHz calls in this context. Using pairs of conspecifics, in which one member is devocalized the calling by the rats in the pin configuration could be assessed [39]. It is the pinning rat, not the partner being pinned that emits most of 50 kHz calls. Again, this would suggest that heterospecific play does not directly mimic conspecific play. Bombail et al. [33] argue that tickling methods focusing on the pin configuration and following a protocol that loosely resembles social play loses some essential elements of play, unpredictability and the choice to use the pin configuration as a defence mechanism against the neck area being touched.
We add a third problem, that clustering together all 50 kHz vocalizations as an index of the positively affective state of the rat during heterospecific play as is typically done [31,33], may be misleading. During conspecific play different types of frequency modulated (FM) 50 kHz calls, as well as 50 kHz flat calls, are emitted in context specific ways [17,22]. For example, the most frequently emitted 50 kHz calls are not the ones significantly associated with the pin configuration. Therefore, it cannot be assumed that how vocalizations are used in heterospecific and conspecific play are the same. Both may include a certain amount of calling that reflects a common positive affective state, but which calls are used in different phases of the encounter may differ markedly.
In this paper, we address the following question: are the emitted USV during play with a conspecific similar to those emitted during heterospecific tickling? If 50 kHz calls primarily arise as an expression of positive affect as hypothesized by Panksepp & Burgdorf [31], we predict that the pattern and type of call emission between conspecific and heterospecific play should not differ substantially. However, if such calling is not a generalized expression of state, reflecting the playful mood of the subjects (see the example of keas above), but rather primarily used for communication [17,22], we predict conspecific play and heterospecific play should have a distinct pattern of USV emission. This is because demands on communication would differ markedly between the two types of play.
2. Material and methods
(a) . Subjects
In total, 30 juvenile male Lister hooded rats (Charles River, Sulzfeld, Germany) arrived at postnatal day (PND) 21 ± 2 days in the facility and were group housed in same sex cages (three or four animals per cage) under controlled conditions (i.e. temperature 20–21°C, 55–65% relative humidity and 12 L : 12 D cycle with lights on at 7.00) and ad libitum access to water and food. The rats were acclimatized to the facility for 5 days upon arrival and were handled at least 2 days prior to testing. Animals were weighed the day before each test. All experiments were approved by the Dutch Central Authority for Scientific Procedures on Animals (CCD) and the Animal Ethics Committee of Utrecht University (licence: AVD108002015189 with protocols 189-1-07, 189-1-08 and 189-1-09) and conducted in agreement with Dutch laws (Wet op de Dierproeven, 1996) and European regulations (guideline 86/609/EEC).
(b) . Experimental procedures
(i) . Conspecific play procedure
The 30 rats were tested for their peer-peer social play in dyads on PNDs 26, 28, 30, 34, 36, 41 and 43. The animals were paired with an unfamiliar partner (i.e. not a cage mate). Trials were performed in a sound attenuated chamber under red light conditions. The testing arena was a Plexiglas cage (40 × 40 × 60 cm; l × w × h) with approximately 2 cm of cellulose fibres (ALCarefresh). Animals were habituated to the test cage for 10 min on PNDs 22 and 23 with their cage mates. On the test days, each rat was isolated for 2.5 h prior to testing, in a Eurostandard type III cage in a room different from the housing room, to increase their motivation to play [40,41]. From the five trials between PNDs 26–36 (15 test pairs per day, 75 pairs in total), the two most playful and the two least playful pairs of each test day were selected, resulting in the 10 most and 10 least playful pairs. The number of nape attacks was used to determine the magnitude of playfulness.
(ii) . Tickling procedure
From the 30 rats tested for social play, 12 were randomly selected to assess their responses to being tickled by an experimenter. Nine out of these 12 animals were part of the 10 most and least playful dyads. Selected animals were subjected to tickling on PND 35. The testing arena was a Plexiglas cage (Macrolon type 4) that is normally used as a home-cage, with sawdust bedding of approximately 2 cm thick. The bedding consisted of sawdust from all home-cages of the tested animals. Animals were habituated to the testing arena for 1 min before the tickling procedure started, with the test lasting 3 min. During these 3 min, the animal was subjected to a tickling procedure as described by LaFollette [42] and adapted from Panksepp & Burgdorf [43]. After completion of testing the rats were returned to their respective home-cages.
Each session of both types of interaction was recorded using a digital camera (Logitech C922 pro stream webcam, Lausanne, Switzerland). The behaviour of both partners in the rat dyads and of the rat and the human ‘tickler’ was assessed afterwards to perform detailed scoring by a trained observer using Avidmux software (free online software). Vocalizations were collected using a Pettersson M500-384 USB Ultrasound Microphone (Pettersson Elektronik AB, Sweden) that was suspended roughly 65 cm above the floor of the test-cage.
(c) . Behavioural analyses
(i) . Conspecific play
Social play in rats involves attack and defence of the nape of the neck, with several options to avoid such contact [44]. One rat can approach and pounce on the other, aiming at the nape, and the recipient can defend itself by evading or turning to face the attacker. The latter option often involves the defender rotating to supine, leading the attacker to stand over it in the ‘pin’ configuration. During test trials, the rats may also explore their surroundings while seemingly ignoring the presence of the partner and periodically, especially when first introduced to the test chamber, approach the partner and sniff it, most often in the anogenital area. So, during the trials, the rats can behave non-socially, engage in social investigation and in social play that can involve multiple actions. Given that different USV can be emitted while performing different actions [15,17], the different actions that could occur during the play sessions were scored (table 1).
Table 1.
Description of conspecific play behaviours scored adapted from [17].
| behaviour | description |
|---|---|
| playful | |
| nape | when the rat moves towards the nape of its partner's neck with its snout |
| chase | when, following an interaction, one rat chases its fleeing partner |
| pin active | when one rat stands over its supine partner, attempts to free itself or counterattack. The partner standing on top may move to block the supine rat's manoeuvres |
| pin passive | when one rat stands over its supine partner, but the supine rat remains relatively immobile |
| mutual upright (m.u.) | when both rats face one another while rearing on to their hind feet, usually holding each other with their forepaws. From this position, they can sniff or push one another |
| evade | when the recipient of a nape attack protects against contact on its nape by dodging, running or jumping away |
| non-playful | |
| sniff | when one rat sniffs the face and flanks of its partner |
| sniff (anogenital) | when one rat sniffs the anogenital area of its partner |
| approach | when one rat moves toward its partner, but without targeting the nape |
| follow | when one rat follows its partner, but unlike chasing, is not preceded by an interaction |
| solo | when the rats are not engaged with each other |
(ii) . Heterospecific play
Tickling play involves the experimenter approaching, touching and flipping the rat onto its back and gently rubbing it with their fingers [31]. However, as pointed out by Bombail et al. [33], the procedure does not truly reflect the dynamics of conspecific social play. The tickling procedure combined aspects from previously described methodologies to mimic rough and tumble play between the rat and the hand [43,45]. To more closely resemble conspecific play, both the animal and the hand initiated play but not continuously. Thus, the hand could chase the rat and the rat could chase the hand, the hand could contact the rat's nape and the rat could pounce on the hand like it pounces on a partner to contact the nape. In the latter, the rat could nuzzle the wrist as if it were the nape of another rat. Moreover, as well as tickling its belly when the rat was flipped on its back, when not on its back, it was also tickled on its flanks. As for conspecific play, the behaviour was manually scored according to the behaviours described in table 2.
Table 2.
Description of the heterospecific play (tickling) behaviours scored.
| behaviour | description |
|---|---|
| playful | |
| nape | when, following an approach or pounce, the rat moves toward the experimenter's wrist or upper palm and nuzzles these areas with its snout |
| naped | when the experimenter uses their thumb and index finger to rub the nape of the rat's neck and then flips it onto its back |
| follow | when the rat follows the experimenter's hand around the enclosure |
| pin | when the rat stands on the fingers and palm of the experimenter's hand as the experimenter tickles the rat's ventral surface, as the rat lays on its back |
| pinned | when experimenter places their fingers on top of the rat's ventral surface, moving their fingers in a tickling motion |
| evade | when the rat protects against contact on its nape by dodging, running or jumping away |
| tickle ventral | when, following nape contact, the experimenter tickles the standing rat's ventral surface [31] |
| tickle dorsal | when the experimenter tickles the rat's dorsal surface especially its neck [31] |
| tickle double | when the experimenter tickles the rat on its dorso-lateral surfaces, using both hands |
| non-playful | |
| approach | when the rat moves towards the hand, but without any clear indication that the wrist is being targeted |
| solo | when the rat is not interacting with the experimenter's hand |
| sniff | when the rat sniffs the experimenter's hand |
| other | when the rat is not engaging with the experimenter's hand and is exploring the side of the enclosure |
The various behaviours shown in tables 1 and 2 were scored manually, so that every second of the 4 min of each conspecific play trial and the 3 min of the tickling trial was assigned with a specific behaviour. The start and stop of each behavioural event were recorded. Rats, on average, start playing 60.86 s ± 7.34 s (mean ± s.e.m.) after a trial begins, so the majority of the play occurs during the last 3 min sampled. Therefore, we chose not to include the first minute of each conspecific play trial, thus matching the time sampled in heterospecific play. The vocalizations were manually selected and the calls were categorized using previously established spectrographic criteria [14]. The time each call began and ended was then superimposed on the behavioural events to identify the calls and behaviours that co-occurred (see below).
(d) . Vocalization analyses
Acoustic data were analysed using Raven Pro 1.4 software (Bioacoustics Research Program, Cornell Lab of Ornithology, Ithaca, NY, USA). The Raven Pro software generated spectrograms with a 256-sample Hann window from which the experimenter manually selected 50 kHz vocalizations. After manual scoring, the Raven Pro software provided the beginning and end times for each vocalization and the overall number of vocalizations. Based on its frequency over time, each call was categorized according to the schema provided by Wright et al. [14], in which 14 distinct categories of 50 kHz calls are recognized (see fig. 1 in [15]). These data were used to compare the overall number of calls, the production of different types of calls, and how different types of calls were associated with different types of behaviours during the two types of play.
(e) . Statistical analyses
(i) . Vocalization counts
During conspecific play interactions, both partners emit USV, whereas during tickling, there is only one rat present to contribute to the number of calls emitted. However, when the rate of calling per unit time was compared between pairs of intact rats with pairs in which one partner was surgically devocalized, there was no statistical difference [17]. Whether one or two rats are calling during conspecific play, the average amount of calling is the same; therefore, in the present study, the rate per pair was compared to the rate of calling in the tickling condition. Even though some of the same rats were tested in the two play paradigms, we could not discern which partner in the dyad contributed to calls, so it was not possible to compare the same rats in the two conditions. Therefore, an independent t-test, rather than matched-pairs t-test, was used. To evaluate whether the number of calls was correlated with the number of nape attacks (conspecific play) or tickling events (heterospecific play), linear regression analyses were used.
To compare the types of calls produced, the percentage of occurrence of each call type was scored for each pair (conspecific play) or individual rat (heterospecific play) to produce the mean for each call type. Call types that occurred less than 1%, which included step down, step up, multi, inverted u and complex calls were grouped together in an ‘other’ category. To assess the overall pattern, all call types containing a trill component were lumped together and the average use of these was compared between the two types of play using an independent t-test. Similarly, each of the 10 call types compared was tested between the two play types using independent t-tests. The α value for significance was set at 0.05. Given that comparing call types involved 10 separate t-tests, a Bonferroni correction was used yielding a revised significance level of 0.005.
(ii) . Vocal-behavioural correlations
To evaluate the associations between all the conspecific and heterospecific interactions and call types, a Monte Carlo shuffling method was used [15]. First, we counted the number of co-occurrences of each vocalization type with each of the coded behavioural categories. Next, for each animal pair, vocalizations were repeatedly shuffled and the number of behaviour-vocalization co-occurrences computed. A vocalization was counted as occurring during a particular behaviour if the mid-point of the call occurred between the beginning and end of the behaviour. Shuffling was achieved by assigning each vocalization a random time within the duration of the observation periods. Hence, the relative frequency of vocalizations was kept the same for each shuffle. Shuffling was done 10 000 times and the total number of co-occurrences of each vocalization type with each type of behaviour was tabulated (for a graphical illustration of the method, [22] fig. 2). Based on the distribution of these random-shuffle counts, we assigned a z-score to the actual number of occurrences. The higher the z-score, the less likely that a specific combination of vocalization and behaviour could have occurred by chance (i.e. a z-value of 1.96 gives a p-value of less than 0.05 and a z-value of 2.58 gives a p-value of <0.01). Shuffling was performed separately for each trial (conspecific and heterospecific), and then the z-scores were averaged across each trial to generate the final z-score values.
3. Results
(a) . Vocalization counts
Overall vocal production per minute was significantly higher in the heterospecific (M = 97.0; s.d. = 64.4) compared to the conspecific (M = 34.3; s.d. = 18.3) context (t30 = 4.12, p = 0.001). No relationship between the number of USV and the number of nape attacks that occurred was found (F1,19 = 1.87, p = 0.335) in conspecific play. Similarly, no relationship between the number of USV and the number of tickles (dorsal, ventral and double tickles) was found (F3,8 = 1.05, p = 0.421) in heterospecific play.
(b) . Vocalization types
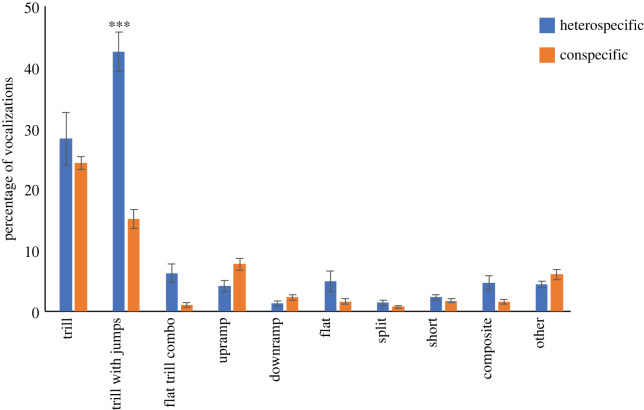
The distribution of producing different types of calls was similar in the two forms of play (figure 1). In both cases, the majority of calls were those that contained trills (mean ± s.d.: heterospecific play 76.88% ± 12.01 versus conspecific play 68.4% ± 2.90), and these percentages did not differ significantly between the two contexts (t30 = 1.84, p = 0.075). Visual inspection of figure 1 indicates that some calls were used more in one type of play than the other, but pairwise comparisons between the two types of play only revealed one significant difference, more trill with jumps were emitted during heterospecific play (t30 = 8.57, p < 0.001).
Figure 1.
The different types of USV emitted are compared between heterospecific and conspecific play as percentages (mean ± s.d.). ***p < 0.001. (Online version in colour.)
(c) . Vocal-behavioural correlates
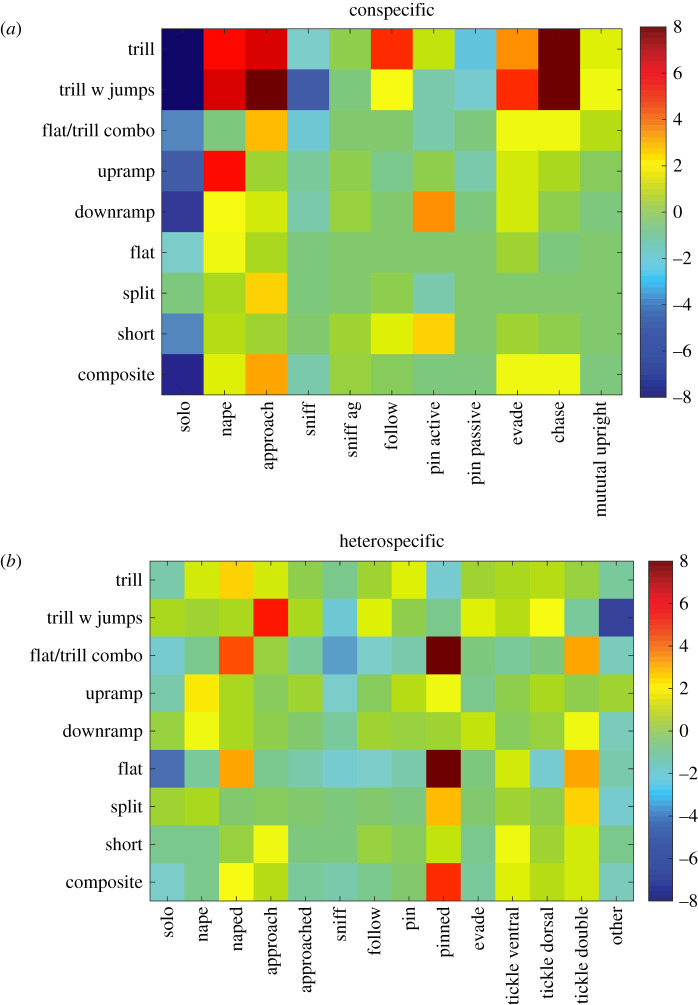
As during conspecific play, calls are emitted by both partners. However, it cannot be ascertained whether it is the attacking rat, defending rat or both that contribute to the significant associations between specific calls and specific behaviours. By contrast, in heterospecific play, the partner producing a call in a particular context is always the rat. Therefore, caution needs to be taken in interpreting differences in association patterns between calls and behaviours. Nonetheless, some key similarities and differences can be unambiguously identified (figure 2).
Figure 2.
The matrix shows call types on one axis and the types of behaviour on the other, with z-scores showing the strength of the associations between particular calls and particular behaviours, spanning from deep red for the strongest positive association, green for no association and deep blue for the strongest negative association. A z-value of 1.96 gives a p-value of <0.05 and a z-value of 2.58 gives a p-value of <0.01. Because some of the behaviours scored in the two play contexts were different, some of the behaviours in (a,b) differ. The description of the relevant behaviours scored can be found in tables 1 and 2. (Online version in colour.)
In both types of play, when the rats engaged in solitary, non-playful behaviour, they were unlikely to emit any calls, as shown by the green (no significant associations) and blue (significant negative associations) boxes. That is, rats are more likely to integrate calling in a patterned manner when interacting with another rat or with an experimenter's hand. For positive associations, there were two striking differences between the two types of play. First, in conspecific play, the strongest associations were with approaching, following, chasing and contacting the nape, whereas, in heterospecific play, the strongest associations were when the rat was pinned. Second, most of the strongest associations in conspecific play involved calls with trills, whereas, in heterospecific play, several of the strongest associations involved non-trill calls, including flat calls. Interestingly, far less strong associations with call patterns were found for evading, approaching and nape attacks in heterospecific play compared to the conspecific play context. The opposite was true for being pinned. In summary, while in both forms of play, rats are more likely to produce particular calls, in particular behavioural contexts, the pattern of such calling differs markedly between the two types of play.
4. Discussion
The evidence that heterospecific play, in the form of tickling, induces a positive effective state in rats and can have a positive effect on their welfare is very strong [32,46–48]. Indeed, we detected no aversive 22 kHz calls [12,13] in either type of play, indicating both were rewarding to the rats. Our goal in this paper was not to challenge these positive associations between the tickling of rats by humans, but whether, as is often claimed, heterospecific play simulates conspecific play [33]. As 50 kHz calls are emitted during both tickling and playing with another rat [18,29], this has been taken as evidence of a common positive effective state [46,47]. Therefore, in the present study, we conducted a detailed analysis of the contextual use of the different types of 50 kHz calls [14] in hetero- and conspecific play. Given that heterospecific play aims to mimic the ‘tickling’ that occurs when rats nuzzle each others' napes following a pounce and each others' ventrums when in the pin configuration [46], it would be predicted that the pattern of calling should be most similar in these contexts.
As previously reported [31], in terms of overall 50 kHz call production, we found that more calls were emitted per unit time during heterospecific play than conspecific play. Also consistent with previous findings [17,18,29,31,46], it was the FM 50 kHz calls that were most frequently emitted during both types of play, especially the calls including trills. Even though trill based calls constituted 70–80% of all calls emitted in both types of play, one difference was that there was a significant increase in the production of trill with jumps in heterospecific play (figure 1). Unlike previous findings [18,20,47] neither type of play had a positive correlation between the amount of playful contact and the number of calls produced. At present, this inconsistency remains to be explained. In terms of the overall pattern of producing calls, our data are consistent with previous findings, and where they were not, we found no differences between the two types of play. It is in how the calls are contextualized that the two types of play differ.
Whether the trial involved interacting with another rat or with an experimenter's hand, when not engaged socially, the rats were highly unlikely to emit calls (figure 2), supporting growing evidence that while 50 kHz calls reflect a positive affective state, they are more strongly associated with social stimuli [49–51]. During conspecific play, rats are most likely to emit 50 kHz calls immediately prior to making playful contact, and by using pairs in which one partner is devocalized, it was shown that the calls are emitted both by the initiator and the receiver [23], with the strongest positive associations being between trill containing calls and approaching, chasing, evading and contacting the nape, with little or no association between such calls and pinning [17,22]. This is the same pattern as was found in the current study (figure 2a).
The pattern of call emission during heterospecific play differed markedly from that in conspecific play (figure 2b). The strongest positive associations were when the rat was pinned. Indeed, one of the strongest associations was between the flat 50 kHz call and pinning. Moderately strong associations occurred between both flat and trill calls when contacted on the nape by the experimenter, as is the case in conspecific play. However, when one partner is devocalized, so that the emitter can be identified, typically the attacker, not the recipient emits these calls [17]. As is the case with conspecific play, there is a moderate association with the rat attacking the partner's nape, which in the case of the experimenter's hand is the wrist. To further differentiate tickling from the rat's postural configuration, the rat was tickled with one or both hands on the dorsum and the ventrum when standing prone (table 2). Only tickling the dorsum with both hands produced some significant positive associations, but surprisingly not when tickled on the ventrum, as the hand pinning the rat and tickling the ventrum is thought to be the hallmark of heterospecific play being a simulation of conspecific play [46]. This suggests that the high production of calls when pinned is not because of the ventrum being rubbed, as rubbing the ventrum in another context does not produce this effect. Rather, it is either being pinned that is crucial or the combination of being tickled when pinned. This is unlike conspecific play in which it is the rat standing over the pinned rat, not the pinned rat, which emits the vast majority of calls [39].
Given the similarity in the pattern of contextual call emission in the present study with that found in [17], in which a different strain of rat was studied in a different laboratory with some environmental differences (e.g. size of housing cage, size of testing cage, number of rats housed together, familiarity of the play partners), it is likely that the general pattern reflects the typical case for conspecific play. While similar to each other, the patterns are highly divergent from that found for heterospecific play (figure 2). There are some similarities, but the difference in which calls are emitted and how strongly they are associated is marked—the pattern associated with nape contact is only partially similar and the pattern for pinning is completely dissimilar. Based on these differences, the present findings support the view that heterospecific play is not a simulation of conspecific play [33,39], but rather a different kind of play experience the positive aspects of which are not valued to the same extent by different rats [38].
While our data are consistent with the view that hetero- and conspecific play are not viewed by rats as equivalent experiences, some of the differences that emerged require explanation. Most striking was that during heterospecific play, calling was around two times more frequent than in conspecific play. Given that the majority of those calls were ones involving trills, it was surprising that there were so few positive associations between trill calls and specific behavioural contexts (figure 2b). This means that many of these calls were emitted more indiscriminately during the test trial, rather than coupled with particular actions. Feedback from the partner, so that they exchange signals back and forth, has been shown to be important for initiating and sustaining play fighting in several species in which visual signals are used [52–54]. Such signalling feedback is not available to rats interacting with an experimenter's hand, and this may account for the increased calling in heterospecific play.
Devocalized rats are less likely to reciprocate during play, suggesting that some of the 50 kHz calls are used to signal to the partner to ensure continued play [23]. In some situations, it may be that it is the exchanging of calls between partners that is critical to communicate effectively and to prevent the encounter from escalating to aggression [17,22,24]. Alternatively, since pairs of devocalized rats also play less than vocal pairs [23], it is possible that some calls, such as trills [17], have a tonic function, that of stimulating a playful mood, as some data suggest for some visual signals [25,26], and has been experimentally demonstrated for an acoustic play signal [27]. When interacting with an experimenter's hand, no auditory signalling is possible, as the hand responded flexibly to the rat's behaviour but not as flexibly as a conspecific would do, so from the rat's perspective, the hand is not responding to calls so as to modify its behaviour, as would a conspecific [22]. This could account for the strong positive associations of some calls, mostly non-trill ones, when pinned (figure 2)—signals are repeated over and over because the hand is failing to change its behaviour! The only signalling possible is with itself, that is emitting signals that maintain its own playful mood, as appears to be the case for the emission of some play signals in other species [25,26]. In rats, trill-based calls are the most frequently emitted calls in both hetero- and conspecific play, and are the likeliest candidate as signals for maintaining the playful mood. The increased emission of calls during heterospecific play, and the less structured emission of these calls with specific actions (figure 2b), suggests that greater non-associated emission of trills throughout the trial may help sustain the rat's playful mood. Indeed, when playing with a devocalized partner, the vocal partner doubles its production of calls [55], so the tripling of call production in heterospecific play may be the way that the rat compensates for the muteness of its ‘partner’. That is, ‘playing’ with a non-communicating hand may stimulate the production of non-directed ‘laughter’.
More needs to be known about the signalling and non-signalling functions of USV during playful encounters to fully understand the differences in calling present in hetero- compared to conspecific play. Indeed, the study needs to be replicated with females and other strains of rats to determine the generality of the present findings. Nonetheless, the striking difference between the two types of play in how different calls are inserted into particular actions suggest that for rats, playing with a human hand is not the same as playing with a conspecific.
Acknowledgments
We thank Vivien Pellis for her comments.
Ethics
All experiments were approved by the Dutch Central Authority for Scientific Procedures on Animals (CCD) and the Animal Ethics Committee of Utrecht University (licence: AVD108002015189 with protocols 189-1-07, 189-1-08 and 189-1-09) and conducted in agreement with Dutch laws (Wet opde Dierproeven, 1996) and European regulations (guideline 86/609/EEC).
Data accessibility
All relevant data, codes and materials used in this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.fn2z34tvc [56].
Authors' contributions
C.J.B.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; S.M.P.: conceptualization, funding acquisition, investigation, methodology, project administration, supervision, writing—original draft, writing—review and editing; E.J.M.A.: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
The work was supported by a NWO-ENW Veni grant no. (016.Veni.181.039) to E.J.M.A. and grants from the Natural Science and Engineering Research Council of Canada to C.J.B. and S.M.P.
References
- 1.Pellis M, Pellis VC, Himmler BT. 2014. How play makes for a more adaptable brain: a comparative and neural perspective. Am. J. Play 7, 73-98. [Google Scholar]
- 2.Vanderschuren LJMJ, Trezza V. 2014. What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. Curr. Top. Behav. Neurosci. 16, 189-212. ( 10.1007/978-3-662-45758-0_268) [DOI] [PubMed] [Google Scholar]
- 3.Vanderschuren LJMJ, Achterberg EJM, Trezza V. 2016. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 70, 86-105. ( 10.1016/j.neubiorev.2016.07.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baarendse PJJ, Counotte DS, O'Donnell P, Vanderschuren LJMJ. 2013. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacol 38, 1485-1494. ( 10.1038/npp.2013.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burghardt GM. 2005. The genesis of animal play: testing the limits. Cambridge, MA: MIT Press. [Google Scholar]
- 6.Pellis SM, Pellis VC. 2009. The playful brain . Oxford, UK: OneWorld Publications. [Google Scholar]
- 7.Pellis SM, Pellis VC. 1987. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggress. Behav. 13, 227-242. () [DOI] [Google Scholar]
- 8.Siviy SM, Panksepp J. 1987. Sensory modulation of juvenile play in rats. Dev. Psychobiol. 20, 39-55. ( 10.1002/dev.420200108) [DOI] [PubMed] [Google Scholar]
- 9.Pellis SM, Pellis VC. 2017. What is play fighting and what is it good for? Learn. Behav. 45, 355-366. ( 10.3758/s13420-017-0264-3) [DOI] [PubMed] [Google Scholar]
- 10.Palagi E, Burghardt GM, Smuts B, Cordoni G, Dall'Olio S, Fouts HN, Řeháková-Petrů M, Siviy SM, Pellis SM. 2016. Rough-and-tumble play as a window on animal communication. Biol. Rev. 91, 311-327. ( 10.1111/brv.12172) [DOI] [PubMed] [Google Scholar]
- 11.Hole GJ, Einon DF. 1984. Play in rodents. In Play in animals and humans (ed. Smith PK), pp. 95-117. Oxford, UK: Blackwell. [Google Scholar]
- 12.Brudzynski SM. 2013. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr. Opin Neurobiol. 14, 310-317. ( 10.1016/j.conb.2013.01.014) [DOI] [PubMed] [Google Scholar]
- 13.Wöhr M, Schwarting RKW. 2013. Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 354, 81-97. ( 10.1007/s00441-013-1607-9) [DOI] [PubMed] [Google Scholar]
- 14.Wright JM, Gourdon JC, Clarke PB. 2010. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacol 211, 1-13. ( 10.1007/s00213-010-1859-y) [DOI] [PubMed] [Google Scholar]
- 15.Burke CJ, Kisko TM, Swiftwolfe H, Pellis SM, Euston DR. 2017. Specific 50-kHz vocalizations are tightly linked to particular types of behavior in juvenile rats anticipating play. PLoS ONE 125, e0175841. ( 10.1371/journal.pone.0175841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson B, Burgdorf J, Panksepp J. 1998. Anticipation of play elicits high frequency ultrasonic vocalizations in young rats. J. Comp. Psychol. 112, 65-73. ( 10.1037/0735-7036.112.1.65) [DOI] [PubMed] [Google Scholar]
- 17.Burke CJ, Kisko TM, Euston DR, Pellis SM. 2018. Do juvenile rats use specific ultrasonic calls to coordinate their social play? Anim. Behav. 140, 81-92. ( 10.1016/j.anbehav.2018.03.019) [DOI] [Google Scholar]
- 18.Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. 2008. Ultrasonic vocalizations of rats Rattus norvegicus during mating play and aggression: behavioral concomitants relationship to reward and self-administration of playback. J. Comp. Psychol. 122, 357-367. ( 10.1037/a0012889) [DOI] [PubMed] [Google Scholar]
- 19.Brudzynski SM. 2009. Communication of adult rats by ultrasonic vocalization: biological sociobiological and neuroscience approaches. ILAR J. 501, 43-50. ( 10.1093/ilar.50.1.43) [DOI] [PubMed] [Google Scholar]
- 20.Knutson B, Burgdorf J, Panksepp J. 2002. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 128, 961-977. ( 10.1037/0033-2909.128.6.961) [DOI] [PubMed] [Google Scholar]
- 21.Wöhr M, Schwarting RKW. 2007. Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLoS ONE 2, 12e1365. ( 10.1371/journal.pone.0001365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke CJ, Euston DR, Pellis SM. 2020. What do you hear what do you say? Ultrasonic calls as signals during play fighting in rats. Int. J. Play 9, 92-107. ( 10.1080/21594937.2020.1720126) [DOI] [Google Scholar]
- 23.Kisko TM, Himmler BT, Himmler SM, Euston DR, Pellis SM. 2015. Are 50-kHz calls used as play signals in the playful interactions of rats? II Evidence from the effects of devocalization. Behav. Process. 111, 25-33. ( 10.1016/j.beproc.2014.11.011) [DOI] [PubMed] [Google Scholar]
- 24.Burke CJ, Kisko TM, Pellis SM, Euston DR. 2017. Avoiding escalation from play to aggression in adult male rats: the role of ultrasonic calls. Behav. Process. 144, 72-81. ( 10.1016/j.beproc.2017.09.014) [DOI] [PubMed] [Google Scholar]
- 25.Pellis SM, Pellis VC, Reinhart RJ, Thierry B. 2011. The use of the bared-teeth display during play fighting in Tonkean macaques Macaca tonkeana: sometimes it is all about oneself. J. Comp. Psychol. 125, 393-403. ( 10.1037/a0024514) [DOI] [PubMed] [Google Scholar]
- 26.Scopa C, Palagi E. 2016. Mimic me while playing! Social tolerance and rapid facial mimicry in macaques Macaca tonkeana and Macaca fuscata. J. Comp. Psychol. 130, 153-161. ( 10.1037/com0000028) [DOI] [PubMed] [Google Scholar]
- 27.Schwing R, Nelson XJ, Wein A, Parsons S. 2017. Positive emotional contagion in a New Zealand parrot. Curr. Biol. 27, R213-R214. ( 10.1016/j.cub.2017.02.020) [DOI] [PubMed] [Google Scholar]
- 28.Provine RR. 2000. Laughter: a scientific investigation. New York, NY: Viking. [Google Scholar]
- 29.Panksepp J, Burgdorf J. 2003. Laughing rats and the evolutionary antecedents of human joy? Physiol. Behav. 79, 533-547. ( 10.1016/S0031-9384(03)00159-8) [DOI] [PubMed] [Google Scholar]
- 30.Panksepp J. 2007. Neuroevolutionary sources of laughter and social joy: modeling primal human laughter in laboratory rats. Behav. Brain Res. 1822, 231-244. ( 10.1016/j.bbr.2007.02.015) [DOI] [PubMed] [Google Scholar]
- 31.Panksepp J, Burgdorf J. 2000. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav. Brain Res. 115, 25-38. ( 10.1016/S0166-4328(00)00238-2) [DOI] [PubMed] [Google Scholar]
- 32.LaFollette MR, O'Haire ME, Cloutier S, Blankenberger WB, Gaskill BN. 2017. Rat tickling: a systematic review of applications outcomes and moderators. PLoS ONE 12, e0175320. ( 10.1371/journal.pone.0175320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bombail V, Brown SM, Hammond TJ, Meddle SL, Nielsen BL, Tivey EKL, Lawrence AB. 2021. Crying with laughter: adapting the tickling protocol to address individual differences among rats in their response to playful handling. Front. Vet. Sci. 24, 677872. ( 10.3389/fvets.2021.677872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. 2007. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping lesion and pharmacology studies. Behav. Brain Res. 182, 274-283. ( 10.1016/j.bbr.2007.03.010) [DOI] [PubMed] [Google Scholar]
- 35.Yamamuro T, et al. 2013. Tickling stimulation causes the up-regulation of the kallikrein family in the submandibular gland of the rat. Behav. Brain Res. 2361, 236-243. ( 10.1016/j.bbr.2012.09.001) [DOI] [PubMed] [Google Scholar]
- 36.Wöhr M, Kehl M, Borta A, Schanzer A, Schwarting RKW, Hoglinger GU. 2009. New insights into the relationship of neurogenesis and affect: tickling induces hippocampal cell proliferation in rats emitting appetitive 50-kHz ultrasonic vocalizations. Neuroscience 1634, 1024-1030. ( 10.1016/j.neuroscience.2009.07.043) [DOI] [PubMed] [Google Scholar]
- 37.Ishiyama S, Brecht M. 2016. Neural correlates of ticklishness in the rat somatosensory cortex. Science 354, 757-760. ( 10.1126/science.aah5114) [DOI] [PubMed] [Google Scholar]
- 38.Melotti L, Bailoo JD, Murphy E, Burman O, Würbel H. 2014. Play in rats: association across contexts and types and analysis of structure. Anim. Behav. Cogn. 1, 489-501. ( 10.12966/abc.11.06.2014) [DOI] [Google Scholar]
- 39.Kisko TM, Wöhr M, Pellis VC, Pellis SM. 2017. From play to aggression: high frequency 50 kHz vocalizations as play and appeasement signals in rats. Curr. Top. Behav. Neurosci. 30, 91-108. ( 10.1007/7854_2015_432) [DOI] [PubMed] [Google Scholar]
- 40.Achterberg EJM, Trezza V, Siviy SM, Schrama L, Schoffelmeer ANM, Vanderschuren LJMJ. 2014. Amphetamine and cocaine suppress social play behavior in rats through distinct mechanisms. Psychopharmacology 231, 1503-1515. ( 10.1007/s00213-013-3272-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Achterberg EJM, van Kerkhof L, Servadio M, Van Swieten MMH, Houweling DJ, Aalderink M, Driel NV, Trezza V, Vanderschuren LJMJ. 2016. Contrasting roles of dopamine and noradrenaline in the motivational properties of social play behavior in rats. Neuropsychopharmacology 41, 858-868. ( 10.1038/npp.2015.212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaFollette MR, O'Haire ME, Cloutier S, Gaskill BN. 2018. Practical rat tickling: determining an efficient and effective dosage of heterospecific play. Appl. Anim. Behav. Sci. 208, 82-91. ( 10.1016/j.applanim.2018.08.005) [DOI] [Google Scholar]
- 43.Panksepp J, Burgdorf J. 2010. Laughing rats? Playful tickling arouses high-frequency ultrasonic chirping in young rodents. Am. J. Play 2, 357-372. (Reprinted from: Toward a science of consciousness III: the third Tucson Discussions and Debates (eds S Hameroff, A Kaszniak, D Chalmers), pp. 231–244. Cambridge, MA: MIT Press.) [Google Scholar]
- 44.Himmler BT, Pellis VC, Pellis SM. 2013. Peering into the dynamics of social interactions: measuring play fighting in rats. JoVE 71, e4288. ( 10.3791/4288). See http://wwwjovecom/video/4288/peering-into-dynamics-social-interactions-measuring-play-fighting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarting RKW, Jegan N, Wöhr M. 2007. Situational factors conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav. Brain Res. 182, 208-222. ( 10.1016/j.bbr.2007.01.029) [DOI] [PubMed] [Google Scholar]
- 46.Cloutier S, LaFollette MR, Gaskill BN, Panksepp J, Newberry RC. 2018. Tickling a technique for inducing positive affect when handling rats. JoVE 135, e57190. ( 10.3791/57190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinchcliffe JK, Mendl M, Robinson ESJ. 2020. Rat 50 kHz calls reflect graded tickling-induced positive emotion. Curr. Biol. 30, R1034-R1035. ( 10.1016/j.cub.2020.08.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaFollette MR, O'Haire ME, Cloutier S, Gaskill BN. 2008. A happier rat pack: the impacts of tickling pet store rats on human-animal interactions and rat welfare. Appl. Anim. Behav. Sci. 203, 92-102. ( 10.1016/j.applanim.2018.02.006) [DOI] [Google Scholar]
- 49.Burke CJ, Markovina M, Pellis SM, Euston DR. 2021. Rat 50-kHz calls are tied to the expectation of social interaction. Brain Sci. 11, 1142. ( 10.3390/brainsci11091142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laplagne DA, Costa ME. 2016. Rats synchronize locomotion with ultrasonic vocalisations at the subsecond time scale. Front. Behav. Neurosci. 10, 184. ( 10.3389/fnbeh.2016.00184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulvihill KG, Brudzynski SM. 2018. Non-pharmacological induction of rat 50 kHz ultrasonic vocalization: social and non-social contexts differentially induce 50 kHz call subtypes. Physiol. Behav. 196, 200-207. ( 10.1016/j.physbeh.2018.09.005) [DOI] [PubMed] [Google Scholar]
- 52.Mancini G, Ferrari PF, Palagi E. 2013. Rapid facial mimicry in geladas. Sci. Rep. 3, 1527. ( 10.1038/srep01527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palagi E, Marchi E, Cavicchio P, Bandoli F. 2019. Sharing playful mood: rapid facial mimicry in Suricata suricata. Anim. Cog. 22, 719-732. ( 10.1007/s10071-019-01269-y) [DOI] [PubMed] [Google Scholar]
- 54.Palagi E, Nicotra V, Cordoni G. 2015. Rapid mimicry and emotional contagion in domestic dogs. R. Soc. Open Sci. 2, 150505. ( 10.1098/rsos.150505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kisko TM, Euston DR, Pellis SM. 2015. Are 50-kHz calls used as play signals in the playful interactions of rats? III The effects of devocalization on play with unfamiliar partners as juveniles and as adults. Behav. Process. 113, 113-121. ( 10.1016/j.beproc.2015.01.016) [DOI] [PubMed] [Google Scholar]
- 56.Burke CJ, Pellis SM, Achterberg EJM. 2022. Data from: Who's laughing? Play, tickling and ultrasonic vocalisations in rats. Dryad Digital Repository. ( 10.5061/dryad.fn2z34tvc) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Burke CJ, Pellis SM, Achterberg EJM. 2022. Data from: Who's laughing? Play, tickling and ultrasonic vocalisations in rats. Dryad Digital Repository. ( 10.5061/dryad.fn2z34tvc) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All relevant data, codes and materials used in this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.fn2z34tvc [56].