Abstract
BACKGROUND:
In recent decades, it has been shown that the association between intestinal bacterial imbalance (dysbiosis) and various diseases such as type 2 diabetes can play a role in the development of Alzheimer's and Parkinson's diseases. In this study, the beneficial effects of intestinal microbiota glucagon-like peptide 1 (GLP-1) in cognitive disorders were investigated.
METHODS:
PubMed-Medline, Web of Science, and Scopus were searched to identify experimental studies based on the bacterial strains along with GLP-1 1 expression in preventing or reducing cognitive impairment. Of the 233 studies, six were eligible for inclusion, and the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) risk of bias tool was used to evaluate the risk of bias in individual studies.
RESULTS:
The results showed that intestinal expression of GLP-1 1 could reduce the intestinal pathogenic genus such as Enterobacteriaceae and was obviously associated with a greater number of beneficial genera such as Lactobacillus and Akkermansia. Also, the neuroprotective effects of Clostridium butyricum with GLP-1 1 in a mice were approved. Therefore, the modulation of the intestinal microbiota, mediated by an increase in the intestinal GLP-1 1 level, consequently improved cognitive function.
CONCLUSION:
In this review, we have indicated that the gut microbiota, by stimulating the expression of the intestinal hormones like GLP-1 1, and also with a beneficial effect in inhibiting some involved genes in inflammation, can declined the development of cognitive disorders.
Keywords: Alzheimer disease, Parkinson disease, Neurodegenerative diseases
INTRODUCTION
Recent technological advances have proposed the relationship between microorganisms of the intestine, gut microbiota, and human health, and the disease situation. The gastrointestinal tract is home to over 100 trillion microorganisms, including at least 1000 different species of bacteria.1,2 Interestingly, one-third of an individual's gut microbiota is common to most people, while the remaining two-thirds is specific to the individual; which means that each person's microbiotics are noticeably different from others.3 More specifically, regardless of lifestyle and dietary variations, Firmicutes (such as Lactobacillus) and Bacteroides represent the main bacterial phyla in the intestine and then Proteobacteria, Actinobacteria (such as Bifidobacterium), and Cyanobacteria, which constitute a natural community that maintains a beneficial community relationship with the host.4
However, an imbalance in the intestinal bacterial picture (dysbiosis) is associated with various diseases such as inflammatory bowel disease, obesity, type 2 diabetes (T2D), cancer, asthma, as well as Parkinson's disease, Alzheimer's disease (AD), autism, and depression.5-7 There is a strong link between inflammation, intestinal microbiota, and metabolic disorders.8 It is also characterized by the fact that neurodegenerative disorders associated with aging, such as Alzheimer's or Parkinson's diseases, are complications of diabetes.9,10 Through the complexity of the gut-brain axis, the intestinal tract has the ability to produce glucagon-like peptide-1 (GLP-1) or gastric inhibitory polypeptide (GIP) in response to nutrients and bacterial factors. GLP-1 is an incretin that is preserved in patients with type 2 diabetes and may decrease blood sugar levels by increasing insulin secretion.11 Pharmaceutical research has therefore moved towards the advancement of GLP-1 therapy. GLP-1 considerably and in a dose-dependent manner increased the neuronal survival of myenteric neurons. Intestinal neurohormone GLP-1 has shown to have the potential for neuroprotection and neurogenesis in primary neurons culture.12,13 Probiotic treatment in diabetic mice has been revealed to restore the GLP-1 level for improved glucose metabolism, which positively affects behavioral performance and cognitive impairment.14,15
Various studies have presented convincing evidence that the gut and central nervous system influence each other in the gut-brain axis.16 Neurons have a long life, and consequently, the lesions can accumulate in excess for decades with consequent synaptic loss and neuronal dysfunction.
Therefore, considering that the microbiome and regulation of intestinal flora can be a promising treatment for neurological diseases, in this study the beneficial effects of intestinal microbiota composition on cognitive disorders with GLP-1 intervention are discussed.
MATERIALS AND METHODS
Protocol
This systematic review was designed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes guideline (PRISMA)17 to investigate the relationship between gastrointestinal microbiome affecting GLP-1 production and cognitive disorders.
Eligibility Criteria
Two researchers independently screened titles and abstracts of all identified studies to find potentially relevant articles. The studies were considered qualified if they met all of the following inclusion criteria: (1) studies that assessed microbial changes (microbiome depletion or dysbiosis and so on) in the intestine. The study was not limited to one type of microbial flora, (2) studies that were measured concentrations of GLP 1, (3) studies that assessment the cognitive deficits including Alzheimer's, Parkinson's, schizophrenia, social behavior, bipolar disorder and so on, (4) studies with quantitative data. The exclusion criteria were: (1) studies that their full texts were not available, (2) studies published in languages other than English or Persian, (3) duplicate publications, (4) studies presented insufficient data.
Information Sources
An extended, systematic search was carried out on international databases such as EMBASE, PubMed, Scopus, Web of Science, and Cochrane Library for cohort databases and also, Persian databases such as SID, Irandoc, Hayat, Magiran, Iran MEDEX. In addition, the search was done to find unpublished studies and ongoing studies published until September 2020.
Search Strategy
In order to recover the maximum number of articles with maximum sensitivity, the search was carried out in the form of vocabulary research, subjective search strategy, and subject heading. Controlled vocabulary terms and free text terms were also used. The search strategy was performed by keywords and Medical Subject Heading (MeSH) terms such as: GLP-1, glucagon-like peptide 1, incretin mimetics, microbiome-gut-brain, microbiome gut, gastrointestinal microbiome, gut-brain axis, germ-free mice, dysbiosis, cognitive disorders, cognitive diseases, Alzheimer, Parkinson, schizophrenia, social behavior, neuroinflammation, brain function, bipolar disorder. Scope and advanced search were performed using the wildcard operator ("*") and Boolean operators (‘AND’, ‘OR’, ‘NOT’). In addition, a manual search was performed to find references from all related reviews and main articles.
Study Selection and Data Extraction
Screening of 233 studies yielded six studies through electronic and manual searches in national and international databases. Two researchers separately screened the titles and abstracts of all identified studies to find potentially relevant articles. Finally, data were extracted from six articles by two reviewers using a standard form containing general study information (title, first author's name, year of publication, place of study) and the type of intervention on the gastrointestinal microbiome, the type of cognitive disorders, the study population, the mouse/rat line, molecular assessments, behavioral assessments, electrophysiological assessment, and main results.
Risk of Bias in Individual Studies (Quality Assessment)
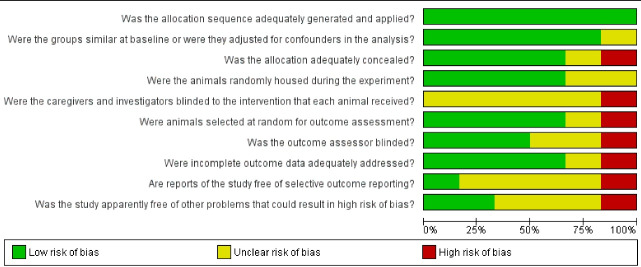
The Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) risk of bias tool was used to evaluate the risk of bias in individual studies. This tool consists of 10 questions, which are scored as “yes”, “no”, or “unclear”: “yes” means low risk of bias, “no” answers mean a high risk of bias, and “unclear” means insufficient details to adequately assess (Figure 1).
Fig. 1:
Risk of bias in individual studies (quality assessment) based on SYRCLE tool.
RESULTS
Study Selection
A total of 233 studies from 13 databases were independently assessed for eligibility. Literature was combined in EndNote version X8 to manage the research process and remove duplication. Irrelevant literature, review articles, studies in other language, case-reports, and articles that evaluated a population with the systematic disease were excluded from our study. Finally, 36 papers were selected for the accurate evaluation of full texts. Twenty-five articles were omitted due to insufficient information, lack of full text, and studies evaluating the association between cognitive impairment and gastrointestinal microbiome without mentioning the correlation associated with GLP-1. In the end, six articles were selected for this review. The study’s flowchart is shown in Figure 2.
Fig. 2:
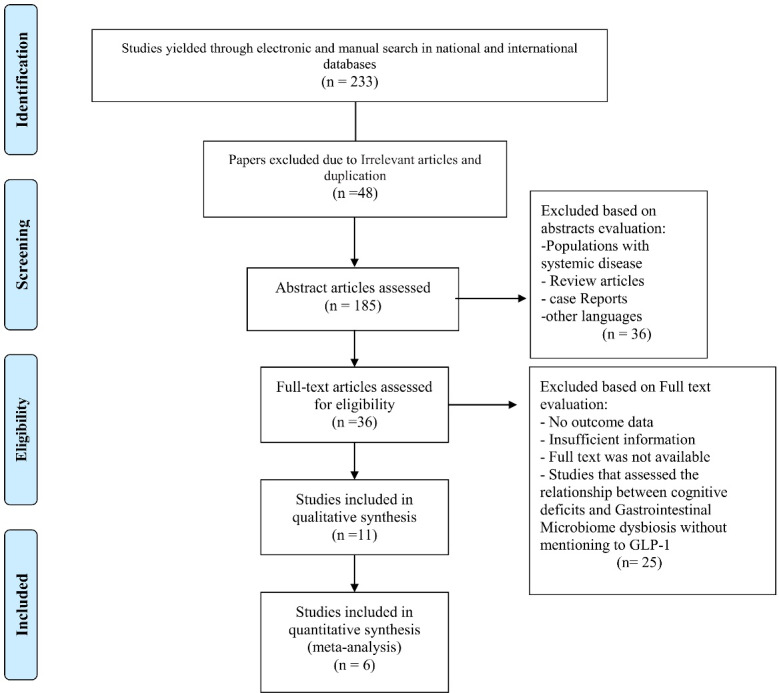
Flow chart of study design.
Risk of Bias Report
Selection bias was present in one out of the six studies.15 Performance bias and detection were present in one study.15 Attrition bias was found in two of the studies.14,15 Reporting bias was not detected. Other biases were found in all studies.
Description of the Included Studies and the Study Characteristics
The basic characteristics of each study included in the systematic review are demonstrated in Table 1. Since studies were conducted on heterogeneous groups of animals and on clinical samples with various focus on the results, it was not possible to conduct a meta-analysis of the data.
Table 1: Characteristics of the included studies .
| First author (ref) | Bonfili, L 18 | Chen, T 14 | SUN, J19 | Fang X 15 | SUN, J 20 | Li, H 21 |
| Sample Size | 128 | 50 | 30 | 72 | 32 | 75 |
|
Country
(year of publication) |
Italy (2017) | China (2018) | China (2018) | China (2019) | China (2019) | China (2020) |
| Subjects AND Age (weight) | 8-week-old male 3xTg-ADmice AND wild types mice (weight 15–25 g) | Three-month-old C57BL/6J male mice | Adult (age 6−8 weeks, 18−22 g) male C57BL/6J mice | Male C57BL/6 mice (25–30 g) | Male APPswe/PS 1dE9 (APP/ PS1) transgenic (Tg) mice (6 months old) and matched WT C57BL/6J mice |
Male C57BL/6 mice (18-22 g, 6-8 weeks old) |
| Cognition disorders type | AD | AD | Chronic unpredictable mild stress | Parkinson disease | AD | Traumatic brain injury |
| Intervention type | Microbial intervention | Gut intervention and Microbial intervention | Microbial intervention | Gut intervention | Microbial intervention | Microbial intervention |
| Administration type | SLAB51* administration for 4 months with in water | LPS** for 9 days +GLP-1 (MG1363-pMG36e-GLP-1 and VNP20009-pLIVE-GLP-1) for 4 weeks |
C. butyricum WZMC1018 for 28 days | MG1363- pMG36e-GLP-1 -1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) |
Prebiotic fructooligosaccharides (FOS) for 6 weeks |
Clostridium butyricum for 14 days |
| Administration dose | 200 bn bacteria/kg/day | LPS = 0.25 mg/kg/day GLP-1 = 100 μL/day |
5 × 108 CFU/0.5 mL/day/mice | - MPTP: 20 mg/kg body weight for 7 days - GLP-1: 109CFU |
2% (w/w) FOS | 109 CFU/mL once daily |
| Microbiota assessment | fecal samples AND bacterial DNA extraction | fecal samples AND bacterial DNA extraction | DNA extraction | fecal samples AND bacterial DNA extraction | fecal samples AND bacterial DNA extraction | |
| Behavioral assessments | 1-Locomotor evaluation: The OF test 2- Recognition memory evaluation: The NOR) test 3- Memory function evaluation: passive avoidance test 4- Anxiety-related behavior assessment: EPM |
A spatial learning and memory: Barnes maze | Depression-related behaviors: OF test 2- TST 3- FST |
1- Bradykinesia evaluation: pole test 2- Locomotor evaluation: The OF test |
1- Locomotor evaluation: OF test OFT 2- MWM test 3- ORT |
Post-trauma neurological deficits evaluation: blinded fashion using a 10-point NSS (assessment motor functions, balance, and alertness of the mice) |
| GLP-1 analyses | ELISA kit | IHC | ELISA kit | MG 1363‐pMG 36e‐GLP ‐1 was engineered to continuously express GLP ‐1 | IHC | IHC |
| Additional tests | - Measurement of the cortex - Ventricular sizes evaluation in the brain sections - ELISA assay for Aβ levels determination - Brian cytokine measurement plasma levels of pro- and anti- inflammatory cytokines were measured through ELISA ELISA assay for Aβ levels determination |
- Brain quantitative PCR - Brain HE staining, immunohistochemistry, and Congo red staining - Brian cytokine measurement pro-inflammatory factors of COX-2, TLR-4, TNF-a, and IL-1β |
- Brain Western Blot analysis |
- Brian Cytokine measurement | - Brain Congo Red staining and immunohistochemistry - Analysis of the Aβ42 level in the brain by ELISA |
- EB assay - brain Western blot analysis - ELISA assay |
Abbreviations: WT, wild type; AD, Alzheimer's disease; OF, open field; NOR, novel-object recognition; EPM, elevated plus maze; TST, Tail suspension test; FST, forced swim test; MWM, Morris water maze; ORT, Object recognition test; IHC, immunohistochemistry; IL-1, interleukin-1; TLR-4, Toll-like receptor-4; COX-2, cyclooxygenase-2; TNF-α, Tumor necrosis factor-α.
*SLAB51; a formulation made of nine live bacterial strains: Streptococcus thermophilus, bifidobacteria (B. longum, B. breve, B. infantis), lactobacilli (L. acidophilus, L plantarum, L. paracasei, L. delbrueckii subsp. bulgaricus, L. brevis). **LPS; endotoxin from the outer membrane of gram-negative bacteria.
DISCUSSION
The effects of GLP-1 on behavior in AD mice models after the intestinal microbial intervention was assessed in three out of the six studies.14,18,20 Oral administration of SLAB51, a new formulation of nine live bacterial strains: Streptococcus thermophilus, bifidobacteria (B. longum, B. breve, B. infantis), lactobacilli (L. acidophilus, L, plantarum, L. paracasei, L. delbrueckii subsp. bulgaricus and L. brevis), in animal models of AD, negatively modulated mRNA levels of pro-inflammatory cytokines produced by LPS-stimulated macrophages. Based on the Bonfili et al study, SLAB51 treatment improved plasma concentrations of GLP-1 and GIP in mice with Alzheimer, which reduced amyloid load in their brain and caused modulation of neuronal functions such as learning and memory.18 Behavioral tests showed a positive influence of SLAB51 oral treatment on behavioral performance in AD mice model.
In the study, Chen et al showed a decrease in LPS-induced learning, significantly associated with GLP-1 expression in a model of AD mice. They found that GLP-1 increased the beneficial genus of Lactobacillus and reduced the pathogenic genus of Enterobacteriaceae, Fusobacterium spp., and Clostridium perfringens. The fecal microbiota of the GLP-1 treated groups showed an increase in the number of Lactobacillus, Enterobacteriaceae, Fusobacterium spp., C. perfringens, Enterococcus and Bacteroides.14
The execution of the MG1363-pMG36e-GLP-1 strain has been safe and effective for diseases related to neuroinflammation, such as AD. It suppressed glial activation and accumulation of beta-amyloid in the brain and reduced the inflammatory expressions of cyclooxygenase-2 (COX-2), toll-like receptor-4 (TLR-4), tumor necrosis factor alpha (TNF-α), and Interleukin 1 beta (IL-1β). They found that GLP-1 increased the beneficial genus of Lactobacillus and reduced the pathogenic genus of Enterobacteriaceae, Fusobacterium spp., and C. perfringens. The fecal microbiota of the GLP-1 treated groups showed an increase in the number of Lactobacillus, Enterobacteriaceae, Fusobacterium spp., C. perfringens, Enterococcus, and Bacteroides.14
Fructooligosaccharide prebiotics (FOS) have been used by Sun et al., to regulate the gut microbiota as a protective mechanism against AD. They reported that treatment with prebiotic FOS improved cognitive impairment in tg mice model by increasing the level of GLP-1, but reduced the level of GLP-1R.20
In the following, the protective effect of GLP-1 on Parkinson's disease through the intestinal microbiome replacement has been review. In study by Fang et al, in MPTP mice model of PD, the bacterial strains MG1363-pMG36e-GLP-1, leads to reduced locomotors damage, enriched tyrosine hydroxylase neurons, disabled microglia and astrocytes, and finally suppressed the expression of a series of inflammation-related factors. Furthermore, the use of the bacterial strain MG1363-pMG36e-GLP-1 has been associated with reduced intestinal pathogenic Enterobacteriaceae and obviously with a greater number of probiotics Lactobacillus, and Akkermansia. Therefore MG 1363 - pMG 36e - GLP‐1 has been suggested as a new therapeutic target for PD.15
The effects of GLP-1 and Clostridium butyricum, as an intestinal bacterium, in two types of cognitive impairment resulting from unpredictable chronic stress and cerebral head injury, were assessed in the Sun et al, and Li et al studies, respectively.7,8C. butyricum has been used an antidepressant drug in the unpredictable chronic stress in mice, and found that C. butyricum attenuated unpredictable chronic stress-induced depressive behavior by increasing 5-hydroxytryptamine and GLP-1 and up-regulate the expression of the brain-derived neurotrophic factor in the gut-brain axis. Mice treated with Clostridium butyricum showed increased intestinal secretion of GLP-1 and up-regulate the brain GLP-1R expression.19
Furthermore, treatment with C. butyricum in mice models of brain failure ameliorated neurological dysfunction, brain edema, neurodegeneration, and blood-brain barrier deficiency throughout the increased expression of TJ proteins (occludin and zonula occluden-1). treatment with C. butyricum increased intestinal GLP-1 secretion and brain GLP-1R expression while decreased plasma d-lactate and colon-IL-6 levels.21 These results, highlighted the neuroprotective effects of C. butyricum in the gut-brain axis with GLP-1 intervention in a mice TBI models.
Interestingly, the role of microbes in the onset and progression of cognitive disorders related to aging has recently developed.10 It has been shown that modulation of the gut microbiota is consequently associated with the improvement of cognitive function mediated by increased plasma concentration of the intestine hormones such as GLP1.13 The intestinal microbiota plays an important role in maintaining intestinal health and human metabolism; therefore, any dysbiosis plays a role in metabolic diseases such as T2D and neurological diseases such as Parkinson's and Alzheimer's diseases.22,23 Parkinson's disease is typically classified as a neurodegenerative disease affecting the motor system.24 AD is a common and progressive neurodegenerative disorder with pro-inflammatory signaling, brain cell atrophy, and brain accumulation of amyloid-β (Aβ) peptides.25A number of studies indicated that impaired insulin signaling increased the risk of both diseases.
The use of SLAB51 (a mixture of lactic acid bacteria and bifidobacteria) to modulate the composition of the intestinal microbiota has beneficial effects on behavioral performance in mice model of AD. In fact, treatment with SLAB51 increased the plasma concentration of GLP-1 with a positive impact on neuronal function,18 In this way, the use of bacterial strains that constantly express GLP-1 restores learning and has neuroprotective effects in this model. Therefore, the management of AD through the administration of beneficial genus such as Lactobacillus and the reduction of the pathogenic genus such as Enterobacteriaceae, Fusobacterium spp. and C. perfringens helped to reduce the amyloid load in the brain of mice.14 The increase in Bifidobacterium spp. and the reduction of Campylobacterales observed in AD mice after administration with SLAB51 is important for the role of these bacteria in the inflammatory pathways. Consequently, oral administration of SLAB51 improved the plasma concentrations of GLP-1 and GIP in AD mice, resulting the neuroprotective effect. Indeed, Bifidobacterium strains have anti-inflammatory properties.26 Furthermore, immune-stimulatory effects of Campylobacter jejuni and Campylobacter coli have been observed on peripheral blood mononuclear cells.27 During the administration of probiotics, reduced the plasma concentrations of pro-inflammatory cytokines have been observed in AD mice, confirming the anti-inflammatory effects of microbiota modification.6 Although, probiotic management has been associated with reduced intestinal pathogen Enterobacteriaceae and obviously with a larger number of Lactobacillus probiotics and Akkermansia. Therefore MG 1363-pMG 36e-GLP‐1 has been suggested as a new therapeutic resource for PD.15
The production of SCFAs by intestinal bacteria has shown numerous neuromodulatory benefits that act directly on gastrointestinal cells to stimulate the synthesis of hormones such as leptin and GLP-1.28 Intestinal microbiota dysbiosis was related to the reduction of the abundance of some SCFAs such as butyrate, known as anti-inflammatory bioactive metabolites, result in progressive neurodegenerative diseases. So, the effect of oral administration of probiotics, including butyrate-producing bacteria, on the development of neuroplasticity and the inhibition of genes involved in inflammation is mediated by intestinal hormones such as GLP-1.29 In the mice model, the intake of GLP-1 caused a decrease in nerve cell destruction that triggered by neurotoxic agents; therefore, GLP-1 was considered an innovative drug for cognitive impairment.13
CONCLUSION
In this review, we have indicated that the gut microbiota, by stimulating the expression of the intestinal hormones GLP-1 and also with a beneficial effect in inhibiting the genes involved in neuroinflammation, can inhibit the development of cognitive disorders. So, the gut microbiota could as a potential target for therapeutic intervention in neurodegenerative diseases
Acknowledgments
The study is related to project No. 1398/10481 from student research committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We also appreciate the "Student Research Committee" and "Research & Technology Chancellor" in Shahid Beheshti University of Medical Sciences for their financial support.
Please cite this paper as: Sayehmiri F, Samadian M, Mohamadkhani A, Tafakhori A, Haghighat S, Rahmatian A, et al. Gut microbiota modification via glucagon-like peptide-1 with beneficial neuroprotective effects. Middle East J Dig Dis 2022;14(2):235- 243. doi: 10.34172/mejdd.2022.278.
Footnotes
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed during this study are included in this published article.
ETHICS APPROVAL
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, student research committee (IR.SBMU.RETECH.REC.1398/10481).
AUTHORS’ CONTRIBUTION
FS and AM designed the conception of the study. SH and HRF searched and did the quality assessment. MS, AT, and MRT did the technical support and conceptual advice. All authors contributed to the drafting of the manuscript, revising it, and approving the final version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Cani PD. Interactions between gut microbes and host cells control gut barrier and metabolism. Int J Obes Suppl. 2016;6(Suppl 1):S28–S31. doi: 10.1038/ijosup.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogtmann E, Han Y, Caporaso JG, Bokulich N, Mohamadkhani A, Moayyedkazemi A. et al. Oral microbial community composition is associated with pancreatic cancer: a case-control study in Iran. Cancer Med. 2020;9(2):797–806. doi: 10.1002/cam4.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Şerban DE. The gut microbiota in the metagenomics era: sometimes a friend, sometimes a foe. Roum Arch Microbiol Immunol. 2011;70(3):134–40. [PubMed] [Google Scholar]
- 4.Sirisinha S. The potential impact of gut microbiota on your health: current status and future challenges. Asian Pac J Allergy Immunol. 2016;34(4):249–64. doi: 10.12932/ap0803. [DOI] [PubMed] [Google Scholar]
- 5.Xu WT, Nie YZ, Yang Z, Lu NH. The crosstalk between gut microbiota and obesity and related metabolic disorders. Future Microbiol. 2016;11:825–36. doi: 10.2217/fmb-2015-0024. [DOI] [PubMed] [Google Scholar]
- 6.Mohamadkhani A. Gut microbiota and fecal metabolome perturbation in children with autism spectrum disorder. Middle East J Dig Dis. 2018;10(4):205–12. doi: 10.15171/mejdd.2018.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohamadkhani A. On the potential role of intestinal microbial community in hepatocarcinogenesis in chronic hepatitis B. Cancer Med. 2018;7(7):3095–100. doi: 10.1002/cam4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greiner TU, Bäckhed F. Microbial regulation of GLP-1 and L-cell biology. Mol Metab. 2016;5(9):753–8. doi: 10.1016/j.molmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caballero-Villarraso J, Galvan A, Escribano BM, Tunez I. Interrelationships among gut microbiota and host: paradigms, role in neurodegenerative diseases and future prospects. CNS Neurol Disord Drug Targets. 2017;16(8):945–64. doi: 10.2174/1871527316666170714120118. [DOI] [PubMed] [Google Scholar]
- 10.Vuotto C, Battistini L, Caltagirone C, Borsellino G. Gut microbiota and disorders of the central nervous system. Neuroscientist. 2020;26(5-6):487–502. doi: 10.1177/1073858420918826. [DOI] [PubMed] [Google Scholar]
- 11.Lang S, Yang J, Yang K, Gu L, Cui X, Wei T. et al. Glucagon receptor antagonist upregulates circulating GLP-1 level by promoting intestinal L-cell proliferation and GLP-1 production in type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001025. doi: 10.1136/bmjdrc-2019-001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voss U, Sand E, Hellström PM, Ekblad E. Glucagon-like peptides 1 and 2 and vasoactive intestinal peptide are neuroprotective on cultured and mast cell co-cultured rat myenteric neurons. BMC Gastroenterol. 2012;12:30. doi: 10.1186/1471-230x-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filchenko I, Simanenkova A, Chefu S, Kolpakova M, Vlasov T. Neuroprotective effect of glucagon-like peptide-1 receptor agonist is independent of glycaemia normalization in type two diabetic rats. Diab Vasc Dis Res. 2018;15(6):567–70. doi: 10.1177/1479164118788079. [DOI] [PubMed] [Google Scholar]
- 14.Chen T, Tian P, Huang Z, Zhao X, Wang H, Xia C. et al. Engineered commensal bacteria prevent systemic inflammation-induced memory impairment and amyloidogenesis via producing GLP-1. Appl Microbiol Biotechnol. 2018;102(17):7565–75. doi: 10.1007/s00253-018-9155-6. [DOI] [PubMed] [Google Scholar]
- 15.Fang X, Tian P, Zhao X, Jiang C, Chen T. Neuroprotective effects of an engineered commensal bacterium in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine Parkinson disease mouse model via producing glucagon-like peptide-1. J Neurochem. 2019;150(4):441–52. doi: 10.1111/jnc.14694. [DOI] [PubMed] [Google Scholar]
- 16.Bhattarai Y. Microbiota-gut-brain axis: Interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol Motil. 2018;30(6):e13366. doi: 10.1111/nmo.13366. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C. et al. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7(1):2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Wang F, Hu X, Yang C, Xu H, Yao Y. et al. Clostridium butyricum attenuates chronic unpredictable mild stress-induced depressive-like behavior in mice via the gut-brain axis. J Agric Food Chem. 2018;66(31):8415–21. doi: 10.1021/acs.jafc.8b02462. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Liu S, Ling Z, Wang F, Ling Y, Gong T. et al. Fructooligosaccharides ameliorating cognitive deficits and neurodegeneration in APP/PS1 transgenic mice through modulating gut microbiota. J Agric Food Chem. 2019;67(10):3006–17. doi: 10.1021/acs.jafc.8b07313. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Sun J, Du J, Wang F, Fang R, Yu C. et al. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil. 2018;30(5):e13260. doi: 10.1111/nmo.13260. [DOI] [PubMed] [Google Scholar]
- 22.Harsanyiova J, Buday T, Kralova Trancikova A. Parkinson's disease and the gut: future perspectives for early diagnosis. Front Neurosci. 2020;14:626. doi: 10.3389/fnins.2020.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Fang L, Chen S, Zhou H, Fan Y, Lin L. et al. Gut microbiome alterations precede cerebral amyloidosis and microglial pathology in a mouse model of Alzheimer's disease. Biomed Res Int. 2020;2020:8456596. doi: 10.1155/2020/8456596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Athauda D, Maclagan K, Budnik N, Zampedri L, Hibbert S, Skene SS. et al. What effects might exenatide have on non-motor symptoms in Parkinson's disease: a post hoc analysis. J Parkinsons Dis. 2018;8(2):247–58. doi: 10.3233/jpd-181329. [DOI] [PubMed] [Google Scholar]
- 25.Bomba M, Granzotto A, Castelli V, Onofrj M, Lattanzio R, Cimini A. et al. Exenatide reverts the high-fat-diet-induced impairment of BDNF signaling and inflammatory response in an animal model of Alzheimer's disease. J Alzheimers Dis. 2019;70(3):793–810. doi: 10.3233/jad-190237. [DOI] [PubMed] [Google Scholar]
- 26.Khokhlova EV, Smeianov VV, Efimov BA, Kafarskaia LI, Pavlova SI, Shkoporov AN. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol. 2012;56(1):27–39. doi: 10.1111/j.1348-0421.2011.00398.x. [DOI] [PubMed] [Google Scholar]
- 27.Hamza E, Kittl S, Kuhnert P. Temporal induction of pro-inflammatory and regulatory cytokines in human peripheral blood mononuclear cells by Campylobacter jejuni and Campylobacter coli. PLoS One. 2017;12(2):e0171350. doi: 10.1371/journal.pone.0171350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. J Neuroinflammation. 2008;5:19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niccolai E, Boem F, Russo E, Amedei A. The gut-brain axis in the neuropsychological disease model of obesity: a classical movie revised by the emerging director "microbiome". Nutrients. 2019;11(1):156. doi: 10.3390/nu11010156. [DOI] [PMC free article] [PubMed] [Google Scholar]