Abstract
In decompensated cirrhosis, massive ascites and pleural effusion (hepatic hydrothorax) can be complicated by infection, which manifests either as spontaneous bacterial peritonitis (SBP) or spontaneous bacterial empyema (SBE). SBE is a distinct and often underdiagnosed complication having different pathogenesis and treatment strategy when compared with parapneumonic empyema. Hepatic hydrothorax in the absence of ascites is rare in patients with cirrhosis. The occurrence of SBE without SBP or ascites is even more of a rarity in cirrhosis and carries great morbidity and mortality. Here we report a case of an elderly female patient with cirrhosis (Child-Pugh Class B) who had unusual features of isolated right-sided hepatic hydrothorax without clinically evident ascites and was later diagnosed as having SBE based on imaging of the thorax, pleural fluid analysis, and cultures. The patient was initially treated conservatively with antibiotics, and diuretics, and later pigtail insertion and drainage was done.
Keywords: Hepatic hydrothorax, Spontaneous bacterial empyema, Cirrhosis liver
INTRODUCTION
Hepatic hydrothorax has an estimated prevalence of 5%-10% among patients with cirrhosis.1 Spontaneous bacterial empyema (SBE) is a rare entity in which hepatic hydrothorax gets infected without concomitant pneumonia. SBE occurs in 2%-2.4% of patients with cirrhosis and 13% among those with hepatic hydrothorax.2 the presence of isolated hydrothorax without ascites is reported to be only 4%.3In most studies, SBE is reported in cirrhotic patients with ascites. Reports on SBE without ascites are lacking in the literature and seem to be rare. Among those with SBE, more than half of the patients usually have co-existing spontaneous bacterial peritonitis (SBP).4 Our case is a cirrhotic patient (Child-Pugh Class B) with isolated hydrothorax without any clinically evident ascites or SBP who developed SBE.
CASE REPORT
An elderly female patient with liver cirrhosis had complaints of right-sided upper abdominal pain, fever with chills, and a history of passing loose stools (four episodes a day) for two days. The patient had no history of melena, dysuria, vomiting or abdominal distension. She was on levothyroxine 75 microgram once a day for hypothyroidism. She had no other medical comorbid conditions. Personal history included vegetarian with no history of tobacco use or alcohol intake or any other high-risk behavior. The patient was admitted to another hospital one week prior to the current hospital admission, where she was treated with empirical antibiotics based on pleural fluid analysis. Since shortness of breath worsened, she was referred to our hospital. On examination, she had tachycardia, tachypnoea, and decreased breath sounds in the right infra-scapular and infra-axillary regions. The abdomen was soft, with no clinically evident ascites. Glasgow Coma Scale (GCS) was 15/15. Other system examinations were normal with no features of hepatic encephalopathy.
Investigations
Hemogram showed white cell count 7900 cells/mm3 with 89.4% polymorphs, and platelet count 85 000 cells/mm3. Renal function tests were within normal limits. Liver function tests showed total bilirubin 4.73 mg/dL, direct bilirubin 2.45 mg/dL, total protein 6.5 g/dL, albumin 3.1 g/dL, international normalized ratio (INR) 1.52 and calculated Child-Pugh Score 9 (Class B). Cultures from blood and urine showed no growth. Tests for hepatitis B surface antigen and antibody against hepatitis C virus were negative. Chest radiograph showed a moderate to massive right-sided pleural effusion (Figure 1).
Fig. 1:
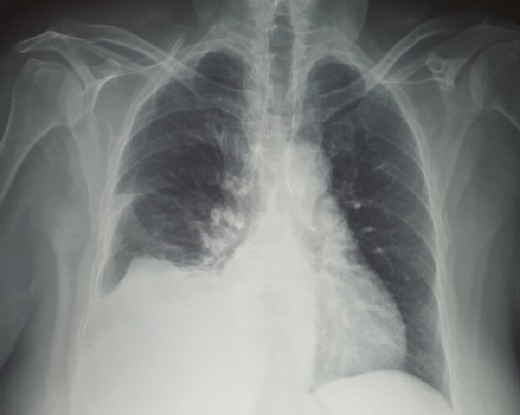
The chest radiograph on admission.
Thyroid function tests were within normal limits. Electrocardiogram and Echocardiogram were normal. Ultrasound of the abdomen showed a shrunken liver with coarse echotexture, mild splenomegaly, and minimal ascites. The liver elastography result was 18.1 kPa, suggestive of severe cirrhosis. Computed tomography (CT) of the thorax showed right-sided moderate to massive pleural effusion and collapse of the underlying right lung. No underlying lung consolidation was seen (Figure 2). CT of the abdomen revealed cirrhotic liver with portal hypertension and minimal ascites (Figure 3). Upper gastrointestinal endoscopy showed no varices.
Fig. 2:
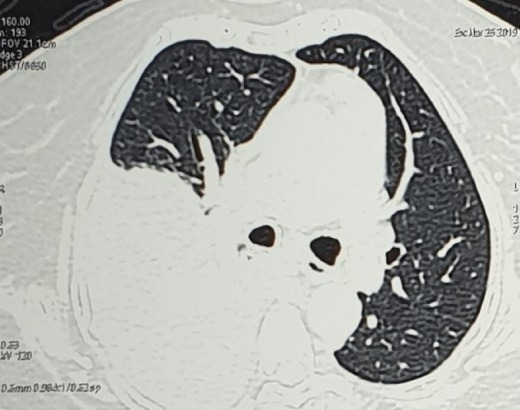
Computed tomogram of the thorax showing right side pleural effusion with no underlying parenchymal consolidation.
Fig. 3:
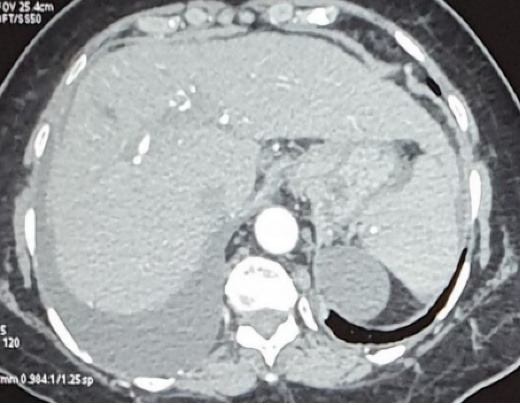
Tomogarm of the abdomen shows cirrhotic liver with minimal ascites.
Therapeutic thoracentesis was done on day one of admission, during which about 1200 mL of straw-colored pleural fluid was removed. On analysis, it was a transudate with protein 1 g/dL, LDH 35 U/L (serum LDH 298 U/L), Glucose 118 mg/dL. Blood investigation results and pleural fluid analysis values are shown in Table 1.
Table 1: Blood investigations and values of pleural fluid analysis .
| Blood tests | Pleural fluid parameters | On admission | Day 8, after antibiotic therapy | |
| TC (cells/mm3) | 7900 | Cell count (cells/mm3) | 4500 | 150 |
| Polymorphs (%) | 89.4 | Polymorphs (%) | 90 | 60 |
| Platelet count (cells/mm3) | 85 000 | Polymorphonuclear count (cells/mm3) | 4050 | 90 |
| Total bilirubin (mg/dL) | 4.73 | Serum/pleural fluid albumin gradient | 2.1 | - |
| Direct bilirubin (mg/dL) | 2.75 | Smear for AFB | Negative | - |
| Total protein (g/dL) | 6.5 | CB-NAAT for MTBa | Negative | - |
| Albumin (g/dL) | 3.1 | Pleural fluid Cytology | Negative for malignant cells | - |
| INR | 1.52 | Pleural fluid culture | Negative | Negative |
| HbsAg | Negative | |||
| Anti-hepatitis C virus serology | Negative |
Abbreviations: AFB, acid fast bacilli; HbsAg, hepatitis B surface antigen; INR, International normalized ratio.
a Cartridge based nucleic acid amplification test for mycobacterium tuberculosis (CB-NAAT for MTB).
Acid-fast staining and CB-NAAT (Cartridge based nucleic acid amplification test) analysis showed no acid-fast bacilli. Cytology was negative for malignant cells, with features of suppurative inflammation. The pleural fluid culture showed no growth. Repeat chest radiography after thoracentesis showed expanded lung with no underlying parenchymal disease. Table 2 shows the trend in pleural fluid cell count over the course of in-hospital stay.
Table 2: Trends in pleural fluid cell count over the course of hospital stay .
| Pleural fluid parameters | Previous admission in a different hospital (7 days earlier to current admission) | Day 1 of admission in our hospital | Day 8, after antibiotic therapy |
| Cell count (cells/mm3) | 4500 | 2500 | 150 |
| Polymorphs (%) | 90 | 70 | 60 |
| Absolute neutrophil count (cells/mm3) | 4050 | 1750 | 90 |
Diagnosis
The diagnostic criteria for SBE include: (i) polymorphonuclear (PMN) count > 250 cells/mm3 with a positive culture or (ii) PMN > 500 cells/mm3 in cases of negative culture and no evidence of pneumonia. Our patient had PMN 1750 cells/mm3 with negative pleural fluid culture, so a diagnosis of SBE was confirmed.
Treatment
She was treated with intravenous (IV) antibiotics (piperacillin-tazobactam), albumin infusion, and diuretics. She improved symptomatically after thoracentesis on day one, but later, on day four, she developed respiratory distress. Repeat chest radiography showed the presence of massive right pleural effusion. In view of re-accumulation despite antibiotic therapy for more than 72 hours, an ultrasound-guided pigtail catheter was inserted. Daily fluid drainage was monitored. Repeat pleural fluid analysis after 7 days of IV antibiotics showed a significant decrease in white cell count, as shown in Table 2. On ultrasonography of the thorax, resolution of pleural effusion was noted, so the pigtail catheter was removed. After two weeks at the follow-up visit, the patient was symptomatically better with complete resolution of pleural effusion on chest radiogram.
DISCUSSION
Hepatic hydrothorax was first described by Morrow and colleagues in 1958 while describing a rapid accumulation of massive right pleural effusion in a patient with cirrhosis.5It is defined as a significant pleural effusion, usually greater than 500 mL, in a cirrhotic patient, without an underlying pulmonary or cardiac diseases.6It has an estimated prevalence of 5%-10% among patients with cirrhosis.1,6-8
SBE is a rare complication that occurs in 2%-2.4% of patients with cirrhosis.2,9 Flaum, in 1976, first described SBE in a patient with cirrhosis and pre-existing right hepatic hydrothorax who developed empyema in the setting of sterile ascites and absence of pneumonia.10Makhlouf and others, in their study, reported 93.8% of the patients with cirrhosis and SBE had Child-Pugh Class C.11
Two unique and rare features observed in our patient were alone hepatic hydrothorax with clinically no ascites and SBE without SBP. Also, our patient developed SBE while having Child-Pugh Class B cirrhosis. Though the exact mechanism remains unclear, it is suggested that fluid gains access to the pleural cavity through diaphragmatic defects.9The driving force for the collection of ascitic fluid in the pleural space is the pressure gradient determined by negative pleural pressure and positive intra-abdominal pressure. When the pleural filling pressure attains a state of balance with negative pleural pressure and positive abdominal pressure, ascitic fluid starts accumulating in the peritoneal cavity. Isolated hepatic hydrothorax without ascites is possible if the pleural re-absorption rate of fluid equilibrates with the production rate of ascites in the peritoneal cavity, thereby causing minimal or no ascites.12
The source of infection in SBE could be either contiguous spread from SBP, which occurs in nearly half of the patients with SBE, or hematogenous seeding of pleural space by bacteremia originating from gut flora. Major etiological agents implicated are gram-negative bacilli, including Escherichia coli, Klebsiella, or Pseudomonas.13The milieu of low complement and protein levels in pleural fluid results in decreased opsonic activity and hence predisposes to the development of empyema. Thus, spontaneous infection of pleural fluid is a plausible mechanism.
Management of SBE is mainly with antibiotics covering gram-negative pathogens with third-generation cephalosporins as the first-line treatment, and supportive measures including albumin infusion and diuretics. Ideally, chest tube placement is not required, but in our patient, due to recurrent massive pleural effusion resistant to diuretics and antibiotic therapy causing respiratory distress, pigtail drainage was done. Despite adequate treatment, SBE is reported to have a mortality rate of 20-38%.2 Liver transplantation is the only curative treatment.9
CONCLUSION
Suspect SBE in febrile cirrhotic patients with new-onset respiratory distress or unexplained acute kidney injury or altered mental status.
SBE is a rare complication reported in 2%-2.4% of patients with cirrhosis.
Empirical antibiotics should cover gram-negative pathogens since bacteremia from gut flora is implicated as the source of seeding in hepatic hydrothorax resulting in SBE.
Please cite this paper as: Dharmalingam AK, Pandurangan V, Ramadurai S, Arthur P, Lakshmanan S, M Nair A. Rare presentation of isolated spontaneous bacterial empyema without concomitant ascites in a patient with cirrhosis. Middle East J Dig Dis 2022;14(2):261-264. doi: 10.34172/mejdd.2022.282.
Footnotes
ETHICAL APPROVAL
Written informed consent was obtained from the patient for the publication of any data included in this report.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
References
- 1.Cardenas A, Kelleher T, Chopra S. Review article: hepatic hydrothorax. Aliment Pharmacol Ther. 2004;20(3):271–9. doi: 10.1111/j.1365-2036.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen CH, Shih CM, Chou JW, Liu YH, Hang LW, Hsia TC. et al. Outcome predictors of cirrhotic patients with spontaneous bacterial empyema. Liver Int. 2011;31(3):417–24. doi: 10.1111/j.1478-3231.2010.02447.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen TA, Lo GH, Lai KH. Risk factors for spontaneous bacterial empyema in cirrhotic patients with hydrothorax. J Chin Med Assoc. 2003;66(10):579–86. [PubMed] [Google Scholar]
- 4.Xiol X, Castellví JM, Guardiola J, Sesé E, Castellote J, Perelló A. et al. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology. 1996;23(4):719–23. doi: 10.1002/hep.510230410. [DOI] [PubMed] [Google Scholar]
- 5.Morrow CS, Kantor M, Armen RN. Hepatic hydrothorax. Ann Intern Med. 1958;49(1):193–203. doi: 10.7326/0003-4819-49-1-193. [DOI] [PubMed] [Google Scholar]
- 6.Strauss RM, Boyer TD. Hepatic hydrothorax. Semin Liver Dis. 1997;17(3):227–32. doi: 10.1055/s-2007-1007200. [DOI] [PubMed] [Google Scholar]
- 7.Lazaridis KN, Frank JW, Krowka MJ, Kamath PS. Hepatic hydrothorax: pathogenesis, diagnosis, and management. Am J Med. 1999;107(3):262–7. doi: 10.1016/s0002-9343(99)00217-x. [DOI] [PubMed] [Google Scholar]
- 8.Kinasewitz GT, Keddissi JI. Hepatic hydrothorax. Curr Opin Pulm Med. 2003;9(4):261–5. doi: 10.1097/00063198-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Xiol X, Castellote J, Baliellas C, Ariza J, Gimenez Roca A, Guardiola J. et al. Spontaneous bacterial empyema in cirrhotic patients: analysis of eleven cases. Hepatology. 1990;11(3):365–70. doi: 10.1002/hep.1840110306. [DOI] [PubMed] [Google Scholar]
- 10.Flaum MA. Spontaneous bacterial empyema in cirrhosis. Gastroenterology. 1976;70(3):416–7. [PubMed] [Google Scholar]
- 11.Makhlouf HA, Morsy KH, Makhlouf NA, Eldin EN, Khairy M. Spontaneous bacterial empyema in patients with liver cirrhosis in Upper Egypt: prevalence and causative organisms. Hepatol Int. 2013;7(1):274–9. doi: 10.1007/s12072-012-9372-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Kim CW, Nam HS, Cho JH, Ryu JS, Lee HL. Hepatic hydrothorax without ascites as the first sign of liver cirrhosis. Respirol Case Rep. 2016;4(1):16–8. doi: 10.1002/rcr2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falchuk KR, Jacoby I, Colucci WS, Rybak ME. Tetracycline-induced pleural symphysis for recurrent hydrothorax complicating cirrhosis A new approach to treatment. Gastroenterology. 1977;72(2):319–21. [PubMed] [Google Scholar]


