Abstract
BACKGROUND:
The increasing prevalence of antibiotic-resistant strains of Helicobacter pylori (H. pylori) led to reduced success with traditional H. pylori treatments. This warrants further evaluation of other treatment options. One such treatment regimen of interest is nitazoxanide containing regimen. In this study, we evaluated the efficacy of the addition of nitazoxanide to clarithromycin-based triple therapy in patients with H. pylori infection.
METHODS:
In this single-center prospective observational trial, patients with H. pylori infection were treated with a regimen comprising of nitazoxanide 1000 mg, amoxicillin 2000 mg, clarithromycin 1000 mg, and esomeprazole 80 mg per day (NACE regimen) for14 days. Eradication of H. pylori infection was assessed 4 weeks after completion of therapy by using stool antigen assay. Treatment compliance and adverse effects were also evaluated.
RESULTS:
Out of 111 patients who entered into the study for final analysis, H. pylori eradication was achieved in 93.7% (104 out of 111) patients in per-protocol analysis and 90.4% (104 out of 115) patients in intention to treat analysis. The treatment regimen was well tolerated.
CONCLUSION:
The addition of nitazoxanide to standard clarithromycin-based triple therapy effectively eradicates H. pylori infection. This regimen is safe and well tolerated.
Keywords: H. pylori infection, Nitazoxanide, NACE, Gastritis, Eradication
INTRODUCTION
Helicobacter pylori (H. pylori) is a common bacterial infectious agent associated with peptic ulcers, gastritis, gastric carcinoma, and mucosa-associated lymphoid tissue lymphoma.1 The prevalence of H. pylori infection in India is about 58-68%.2-4H. pylori eradication is recommended in peptic ulcers disease, early gastric cancer, gastric mucosa-associated lymphoid tissue lymphomas, and uninvestigated dyspepsia.1 Evidence has shown that H. pylori eradicationcan reduce the risks of the above conditions.1
For the eradication of H. pylori infection, recent guidelines recommended a treatment regimen, mainly comprised of two or three antibiotics along with proton pump inhibitors (PPIs) given for 10-14 days. Drug allergy, recent use of antibiotics, and H. pylori resistance pattern are the main determining factors for choosing a specific treatment regimen.1 Recent data showed more than 15% resistance rate to clarithromycin, metronidazole, and levofloxacin found in many parts of the world, and thus the prevalence rate of antibiotic-resistant H. pylori infection is increasing.5 Several Indian studies also addressed the issue of drug resistance against H. pylori infection. In one study, the prevalence of drug-resistant H. pylori infection was seen in 70.6% of cases, including resistance to metronidazole (48.5%), amoxicillin (17.6%), tetracycline (16.2%), and clarithromycin (11.8%). Dual and multiple drug resistance were seen in 26.5% and 8.8% cases, respectively.6 Another Indian study showed that metronidazole, clarithromycin, amoxicillin, tetracycline, and levofloxacin resistance were seen in 83.8%, 58.8%, 72%, 53.8%, and 13.8% cases, respectively.7
Reduced efficacy of clarithromycin or metronidazole-based triple therapy against H. pylori infection has been explained by the rising prevalence of antibiotic-resistant strain of H. pylori, with some studies, including our own, which reported eradication in less than 50% of patients.4,8 Even efficacy of concomitant therapy (PPI + clarithromycin + amoxicillin + metronidazole) was also shown to be suboptimal in our previous study (per-protocol (PP) eradication rate - 77%, intention to treat (ITT) eradication rate - 70%).4 In regions with more than 20% clarithromycin resistance, the efficacy of concomitant or sequential therapy has been reduced to less than 80%.5 The important role of levofloxacin resistance to determine treatment failure has been shown in a recent meta-analysis, which demonstrated a significantly higher H. pylori eradication rate for levofloxacin susceptible strain than for resistant strain (81.1% vs. 36.3%, risk ratio -2.38, 95% CI: 1.6-3.0).9 Most of the recent guidelines recommended that the selection of treatment regimens should be based on regional resistance patterns.1,10 Even if useful, sensitivity tests have been challenging to be carried out as they are time-consuming, expensive, require endoscopy, and can only be performed in well-equipped laboratories. In view of the high resistance pattern, there is a need to evaluate other treatment options. One treatment regimen of interest is nitazoxanide containing regimen.
Nitazoxanide that has microbiological characteristics similar to metronidazole has been indicated in infection caused by cryptosporidium parvum and giardia lamblia.11 An in-vitro study suggested that nitazoxanide was a potent agent against H. pylori, including metronidazole-resistant H. pylori strain.12 Another study has shown 90% cure rate against H. pylori infection by using a treatment regimen comprising of levofloxacin, nitazoxanide, doxycycline, and esomeprazole.13 The aim of our study was to evaluate the efficacy of the addition of nitazoxanide to standard clarithromycin-based triple therapy in patients with H. pylori infection.
MATERIALS AND METHODS
This prospective single-center, open-label study for the treatment of H. pylori-positive patients was conducted in Indira Gandhi Institute of Medical Sciences, which is a tertiary level institute in the eastern part of India, from August 2018 to December 2019. The study was approved by the institutional ethics committee. Consecutive patients with dyspeptic symptoms undergoing upper gastrointestinal endoscopy (UGIE) were recruited for study participation. Patients, who underwent UGIE for other indications and were found to have mucosal hyperemia, erosions, or ulcers in the stomach or duodenal bulb incidentally, were also evaluated for H. pylori infection.
Patients with the following criteria were excluded from the study:
Prior H. pylori therapy
Allergy to drugs used in the treatment of H. pylori infection
Patients on PPIs, antibiotics, or anticoagulant treatment within 1 month of enrollment
Concomitant significant co-morbidities
Presence of gastrointestinal malignancy
Pregnancy or lactation
Age < 18 years or > 80 years
Refused to give consent
We evaluated the detailed clinical and endoscopic features of patients. Gastric or duodenal ulcer was said to be present if a breach in mucosal continuity of size 5 mm or more with apparent depth was seen during endoscopic examination. Gastritis or duodenitis was said to be present if localized or diffuse mucosal hyperemia or erosions were seen endoscopically in the stomach or duodenum, respectively. Rapid urease test (RUT) or gastric biopsy with the histological examination was used to diagnose H. pylori infection. We used RUT as a screening test as it is a rapid, cheap, and simple test with high sensitivity (88–95%), specificity (95–100%), positive predictive value (98.75%), negative predictive value (87.5%), and diagnostic accuracy (95%).14,15 We did not use a urea breath test to diagnose H. pylori infection as the facility of this test is not available in our institute. In RUT examination, we took one biopsy from the corpus and another from the antrum. Biopsied material was introduced into yellow media of RUT dry test kit along with one drop of distilled water. A positive test was indicated by a change in the color of media from yellow to red. Patients were considered as negative for H. pylori infection if the color of media remained yellow even after 24 hours of introducing biopsied material into media. We did a histological examination to diagnose H. pylori infection if a suspicious lesion was found during endoscopy or when RUT kit was not available. We took a biopsy from mid-body and mid-antrum along the greater and lesser curvature and from incisura in order to diagnose H. pylori infection. We took a biopsy from different parts of the stomach in order to enhance the detection of H. pylori infection, as shown by Lan and colleagues.16 Hematoxylin and eosin or Giemsa stains were used for histological examination.
Patients with H. pylori infections were treated with a regimen comprising of nitazoxanide 1000 mg (Nizonide® Lupin limited, Mumbai, India), amoxicillin 2000 mg (Mox®; Sun Pharmaceuticals Industries Limited, Mumbai, India), clarithromycin 1000 mg (Clarithro®; Alembic Pharmaceuticals Limited, Vadodara, India), and esomeprazole 80 mg (Nexpro®; Torrent Pharmaceuticals Limited, Ahmedabad, India) per day given orally in two divided doses (NACE therapy). The duration of treatment was 14 days. Telephone numbers were requested from all patients who participated in this study. Treatment compliance and adverse effects were evaluated during the treatment period. Patients were said to be compliant if the pill intake was more than 80% of total pills. Compliance was assessed by asking the patients and recovery of empty medication wrapper.
Patients were called by telephone 4 weeks after completion of therapy to confirm H. pylori eradication. Eradication of H. pylori infection was assessed by detection of H. pylori antigen in stool sample by immunochromatography method. PPI was stopped 2 weeks prior to stool antigen assay. We used The SD bioline H. pylori antigen test for the qualitative detection of H. pylori antigen in the human fecal specimen. The test kit uses mouse monoclonal anti- Helicobacter pylori antibody. The test kit result window has two precoated lines called the test and control lines. Neither of these lines is visible before applying a fecal sample. The control line is used for procedural control and should always appear if the test procedure is performed correctly. In stool antigen assay, after taking a portion of stool (about 50 mg) with a sterile swab, it was inserted into a specimen tube containing assay diluents to dissolve the sample. Three drops of the sample were inserted into the sample well of the test device. Test results were interpreted within 15 min. No interpretation was performed after 15 min. The test was said to be positive if both the control and test band appeared within the result window. The presence of only one band in the result window was considered as a negative result.
Primary Outcome
The primary outcome was the eradication of H. pylori infection 4 weeks after the completion of therapy. Eradication of H. pylori was said to be achieved if stool antigen assay was found negative for H. pylori infection.
Secondary Outcome
Treatment compliance and adverse effects were the secondary outcomes.
Statistical analysis
Descriptive analyses were presented as mean ± standard deviation (SD) for quantitative variables and absolute numbers or percentages for qualitative variables. Eradication rates and 95% confidence interval (CI) were determined. Analysis of H. pylori eradication was performed on ITT and on PP basis. ITT analysis included all patients who received at least one drug dose, and PP analysis included patients with complete adhesion to the trial protocol. SPSS software version 20 was used for all statistical analyses.
RESULTS
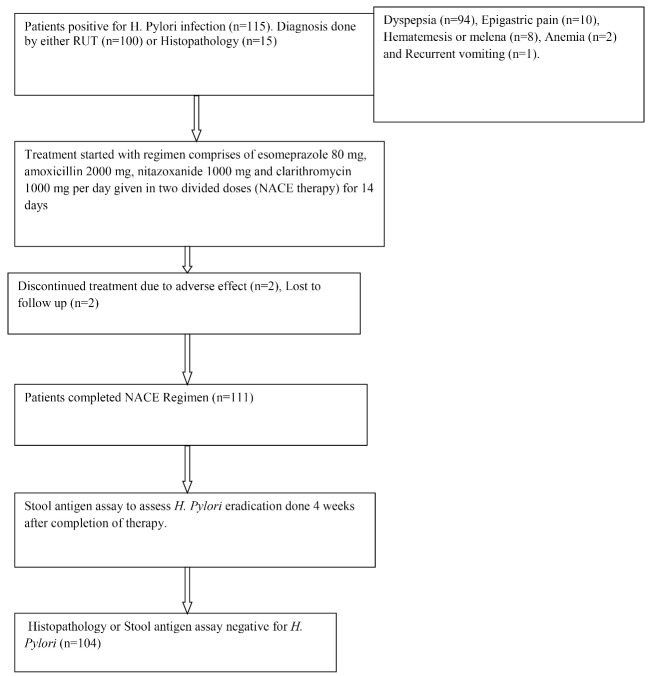
As shown in the flow chart (Figure 1), 115 consecutive patients positive for H. pylori infection were included. Diagnosis of H. pylori infection was made by either RUT (n = 100) or by histopathology (n = 15). Dyspeptic symptoms (n = 94), epigastric pain (n = 10), hematemesis or melena (n = 8), anemia (n = 2), and recurrent vomiting (n = 1) were the indications for UGIE. History of smoking, diabetes, and significant alcohol intake were seen in six, five, and three patients, respectively. Two patients discontinued treatment. Causes of discontinuation were dizziness and significant abdominal pain developed in one patient each. Two patients were lost to follow-up after completing therapy. Therefore 111 patients entered into study for final analysis.
Fig. 1:
Flow Chart of NACE Therapy in H. Pylori Infection.
The mean age of the patients was 41.80 ± 14.47 years, and 60.4% were male. Dyspepsia, epigastric pain and upper gastrointestinal bleeding were the main presenting features seen in 82%, 8.1% and 6.3% of cases, respectively. In baseline endoscopy, isolated gastric lesions were seen in 78.4% of the patients. 13.5% of the patients showed isolated duodenal lesions. Combined gastric and duodenal lesions were seen in 4.5% of the cases. Ulcerated lesions were seen in 17.1% of the cases. Table 1 shows the baseline endoscopic parameters of the patients. Endoscopic findings of the patients who underwent histopathological examination were as follows: antral ulcer; n = 6, antral gastritis; n = 4, pangastritis; n = 4, antral nodularity; n = 1. Histopathologic evaluation showed chronic inflammatory cell infiltrate in all patients.
Table 1: Endoscopic Parameters of Patients (n = 111) .
| Endoscopic Features | Number (%) |
| Isolated Gastric Lesion | 87(78.4%) |
| Antral Ulcer | 9 (8.1%) |
| Antral Gastritis | 48 (43.2%) |
| Fundal Gastritis | 1 (0.9%) |
| Pangastritis | 19 (17.1%) |
| Multifocal Gastritis | 9 (8.1%) |
| Antral Nodularity | 1 (0.9%) |
| Gastric Polyp | 1 (0.9%) |
| Deformed Pylorus | 2 (1.8%) |
| Isolated Duodenal Lesion | 15(13.5%) |
| Duodenal Ulcer | 10 (9.0%) |
| Duodenitis | 5 (4.5%) |
| Combined Duodenal and Gastric Lesion | 5(4.5%) |
| Normal UGI Endoscopy | 4 (3.6%) |
| Esophagitis | 2 (1.8%) |
| Gastroesophageal Varices/Portal Hypertensive Gastropathy | 5 (4.5%) |
H. pylori eradication rate
Table 2 shows the eradication rate of H. pylori infection by using NACE regimen. In PP analysis, 104 (93.7%; 95% CI: 89-98%) patients achieved H. pylori eradication. We have also done ITT analysis, which included patients who were lost to follow-up or who discontinued treatment due to the development of adverse effects and considered them as treatment failure. In ITT analysis, 90.4%; 95% CI: 85-96% (104 out of 115) of the patients achieved H. pylori eradication. All patients with duodenal ulcers or duodenitis as well as combined duodenal and gastric endoscopic lesions, achieved H. pylori eradication. Patients with isolated gastric lesions showed H. pylori eradication in 91.8% of the cases.
Table 2: Eradication Rate of Treatment Naive H. Pylori Infection using NACE Regimen .
| Variables | Eradication Achieved/Analyzed (Number) | Eradication Rate (95% confidence interval) |
| Per Protocol Therapy | 104/111 | 93.7% (89 - 98%) |
| Intention to Treat Analysis | 104/115 | 90.4% (85 - 96%) |
Compliance and Adverse effects
The treatment regimen was generally well tolerated. Two patients discontinued treatment.
Causes of discontinuation were dizziness and significant abdominal pain, developed in one patient each. Fifteen patients who developed mild adverse effects responded to conservative treatment. Five patients developed constipation requiring laxatives and six patients developed a mild abdominal cramp. Two patients each developed mild diarrhea and nausea and were responded to conservative treatment.
DISCUSSION
American College of Gastroenterology, Toronto Consensus, and Maastricht V/Florence Consensus have recommended bismuth quadruple therapy and concomitant therapy as the first-line treatment regimen against H. pylori infection.1,10,17 The use of bismuth quadruple therapy has been limited by less availability of bismuth salt and its relatively complex dosing schedule. Concomitant therapy has shown suboptimal response against H. pylori infection, possibly due to a high level of drug resistance.4 Eradication in more than 90% of treated patients has been suggested as the goal of therapy for H. pylori infection.18 In view of high levels of metronidazole resistance, nitazoxanide could be an acceptable alternative for patients with H. pylori infection.
Nitazoxanide and its major circulating metabolites tizoxanide interfere with pyruvate ferredoxin oxidoreductase enzyme-dependent electron transfer reaction resulting in cell swelling, membrane damage, and vacuole injury of bacteria and trophozoites.19 A study has shown improved effectiveness of nitazoxanide among metronidazole resistant H. pylori strain in patients with gastritis.20 Currently, there are no major data available regarding nitazoxanide resistance against H. pylori. Therefore, it seems more logical to replace metronidazole with nitazoxanide in a concomitant treatment regimen (PPI + amoxicillin + clarithromycin + metronidazole), which is currently recommended as the first-line treatment regimen. Nitazoxanide was tried as dual therapy along with omeprazole or sucralfate with a more than 80% eradication rate.12,21 Such results would be an excellent base for further studies using nitazoxanide in combination with PPI and other antibiotics.
In our study, we added nitazoxanide to clarithromycin-based triple therapy. Our study showed that the NACE regimen is a highly effective treatment to eradicate H. Pylori infection with an eradication rate of more than 90% (PP – 93.7%, ITT – 90.4%). Sehata and colleagues in a randomized controlled trial comparing nitazoxanide, clarithromycin, and omeprazole for 14 days with metronidazole, clarithromycin, and omeprazole for the same period found a significantly higher H. pylori eradication rate in nitazoxanide based therapy than metronidazole-based therapy (94.6% vs. 60.6%, P < 0.001).22 Abd-Elsalam and others assessed the effect of the treatment regimen comprising of nitazoxanide (500mg twice daily), levofloxacin (500mg once daily), omeprazole (40 mg twice daily), and doxycycline (100mg twice daily) for 14 days on H. pylori eradication. It demonstrated 83% eradication rate of H. pylori infection in patients who did not respond to triple therapy.23 A recently published review article demonstrated the eradication rate of more than 80% by nitazoxanide-containing regimen in 8 out of 10 studies. It has been shown to be effective in both the treatment-naive H. pylori infection and unresponsive to previous therapy.24 Most of the treatment regimens comprised nitazoxanide, PPI, fluoroquinolones, and doxycycline. However, as shown by a recent study, doxycycline is not considered to be as effective as tetracycline in the treatment of H. pylori infection.25 Therefore, the findings of our study are consistent with other studies in terms of H. Pylori eradication rate, as shown in table 3.
Table 3: Different Trials Evaluating Efficacy and Adverse Effects of Nitazoxanide Containing Regimen in Treatment of Helicobacter Pylori Infection .
| Studies | Regimen (Dose per day) | Eradication Rate | Adverse effect (%) |
| Shehata et al. 20 (n = 112) |
Nitazoxanide (1000mg), Clarithromycin (1000mg), Omeprazole (80 mg) daily Treatment Duration: 14 days |
94.6 (Both ITT & PP) | Constipation (8); Abdominal pain (5.4); Nausea (5.4); dizziness (1.8) |
| Abd-Elsalam et al. 21 (n = 100) |
Nitazoxanide (1000 mg). Levofloxacin (500 mg), Omeprazole (80 mg), Doxycycline(200mg) Treatment Duration: 14 days |
83(ITT) 88.3(PP) |
Constipation (12); Nausea (9); Abdominal pain (6); Headache (2); Dizziness (1) |
| Basu et al.13 (n = 152) |
Nitazoxanide (1000 mg), Levofloxacin (250 mg), Omeprazole (40 mg), Doxycycline (100 mg) Treatment Duration: 7 days |
90 (ITT) 93.1 (PP) |
Nausea (10); Headache (8.9); Abdominal pain (8.3); Bloating;(6.7); Diarrhoea (5.6); Constipation (5.6); Belching (5); Dizziness (3.3); Palpitations (2.2); Skin rash (1.1) |
| Ramos-Soriano et al.26(n = 111) Paediatric study |
Nitazoxanide - 3 days, 3rd generation cephalosporin - 7–10 days, Azithromycin - 7–10 days, PPI - 30 days | 89.2% | Nausea, vomiting or Abdominal cramps (10.8) |
| Sharma et al.27(n = 137) | Nitazoxanide (1000 mg)-5 days, Rabeprazole (20 mg)- 10 days, Doxycycline (100 mg)- 10 days, Levofloxacin (500 mg)- 10days | 82.4(ITT) 84.8(PP) |
Mild side effects (11.7) |
| Our Study (n = 115) | Nitazoxanide (1000mg), Clarithromycin (1000mg), Amoxycillin (2000mg), Esomeprazole (80 mg) daily Treatment Duration: 14 days |
90.4 (ITT) 93.7 (PP) |
Constipation (4.5), Mild abdominal cramp (5.4), Mild diarrhea (1.8), Nausea (1.8) Severe pain abdomen (0.9), Dizziness (0.9) |
NACE regimen was generally well tolerated. Profiles of adverse events seen in our study were comparable with those seen in previous studies, as mentioned in table 3.
Our study also has some limitations. A major limitation of this study was that it had no control arm. We have taken historical controls for comparison. In our recently published study, we compared the efficacy of concomitant therapy with standard clarithromycin-based triple therapy.4 In triple therapy, ITT eradication rate was shown to be about 50%, which is much lower than that of NACE regimen shown in this study. This was a single-center study, which may not reflect the general population. This is another limitation. Therefore, multicenter studies using larger numbers of patients are required to confirm the results. Another limitation of this study is that the H. pylori culture was not done.
CONCLUSION
The addition of nitazoxanide to standard clarithromycin-based triple therapy effectively eradicates H. pylori infection. This regimen is safe and well tolerated.
Acknowledgments
Sanjeev Kumar Jha drafted the manuscript, oversaw the study, assisted with data analysis, performed the statistical analysis, and prepared the manuscript; Ravikant Kumar participated in the design and oversight of the study; Amitesh Kumar helped in manuscript drafting and data analysis. Shubham Purkayastha, Saurabh Kumar, Ravi Keshari, and Aditya Vardhan Singh recruited the patients, were involved with data collection, and assisted with data analysis. All authors read and approved the final manuscript.
Please cite this paper as: K. Jha S, Kumar R, Kumar A, Purkayastha S, Keshri R, Kumar S, Vardhan Singh A. Addition of Nitazoxanide to Standard Clarithromycin Based Triple Therapy for 2 Weeks Effectively Eradicates Treatment-Naive Helicobacter Pylori Infection. A Single Centre prospective, open-label study. Middle East J Dig Dis 2022;14:77-84. doi: 10.34172/mejdd.2022.259.
Footnotes
FINANCIAL SUPPORT
Authors have nothing to disclose regarding financial support
INFORMED CONSENT STATEMENT
All study participants or their legal guardians provided written consent to participate in the study prior to enrolment.
ETHICAL APPROVAL
There is nothing to be declared.
CONFLICT OF INTEREST
The authors declare no conflict of interest related
to this work.
References
- 1.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 2.Adlekha S, Chadha T, Krishnan P, Sumangala B. Prevalence of helicobacter pylori infection among patients undergoing upper gastrointestinal endoscopy in a medical college hospital in Kerala, India. Ann Med Health Sci Res. 2013;3:559–63. doi: 10.4103/2141-9248.122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sodhi JS, Javid G, Zargar SA, Tufail S, Shah A, Khan BA. et al. Prevalence of Helicobacter pylori infection and the effect of its eradication on symptoms of functional dyspepsia in Kashmir, India. J Gastroenterol Hepatol. 2013;28:808–13. doi: 10.1111/jgh.12178. [DOI] [PubMed] [Google Scholar]
- 4.Jha SK, Mishra MK, Saharawat K, Jha P, Purkayastha S, Ranjan R. Comparison of concomitant therapy versus standard triple-drug therapy for eradication of Helicobacter pylori infection: A prospective open-label randomized controlled trial. Indian J Gastroenterol. 2019;38:325–331. doi: 10.1007/s12664-019-00949-4. [DOI] [PubMed] [Google Scholar]
- 5.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372–1382. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gehlot V, Mahant S, Mukhopadhyay AK, Das K, De R, Kar P. Antimicrobial susceptibility profiles of Helicobacter pylori isolated from patients in North India. J Glob Antimicrob Resist. 2016;5:51–6. doi: 10.1016/j.jgar.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Pandya HB, Agravat HH, Patel JS, Sodagar NR. Emerging antimicrobial resistance pattern of Helicobacter pylori in central Gujarat. Indian J Med Microbiol. 2014;32:408–13. doi: 10.4103/0255-0857.142256. [DOI] [PubMed] [Google Scholar]
- 8.Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33–45. doi: 10.1159/000350719. [DOI] [PubMed] [Google Scholar]
- 9.Chen PY, Wu MS, Chen CY, Bair MJ, Chou CK, Lin JT. et al. Taiwan Gastrointestinal Disease and Helicobacter Consortium Systematic review with meta-analysis: the efficacy of levofloxacin triple therapy as the first- or second-line treatments of Helicobacter pylori infection. Aliment Pharmacol Ther. 2016;44:427–37. doi: 10.1111/apt.13712. [DOI] [PubMed] [Google Scholar]
- 10.Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH. et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51–69. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Anderson VR, Curran MP. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs. 2007;67:1947–67. doi: 10.2165/00003495-200767130-00015. [DOI] [PubMed] [Google Scholar]
- 12.Megraud F, Occhialini A, Rossignol JF. Nitazoxanide, a potential drug for eradication of Helicobacter pylori with no cross-resistance to metronidazole. Antimicrob Agents Chemother. 1998;42:2836–40. doi: 10.1128/AAC.42.11.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu PP, Rayapudi K, Pacana T, James Shah N, Krishnaswamy N, Flynn M. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970–75. doi: 10.1038/ajg.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy AD, Deuri S, Dutta UC. The diagnostic accuracy of rapid urease biopsy test compared to histopathology in implementing “test and treat” policy for Helicobacter pylori. Int J Appl Basic Med Res. 2016;6:18–22. doi: 10.4103/2229-516X.174003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Trans Med. 2015;3:9. doi: 10.3978/j.issn.2305-5839.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan HC, Chen TS, Li AF, Chang FY, Lin HC. Additional corpus biopsy enhances the detection of Helicobacter pylori infection in a background of gastritis with atrophy. BMC Gastroenterol. 2012;12:182. doi: 10.1186/1471-230X-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT. et al. European Helicobacter and Microbiota Study Group and Consensus panel Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 18.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 19.Sisson G, Goodwin A, Raudonikiene A, Hughes JN, Mukhopadhyay KA, Berg ED. et al. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother. 2002;46:2116–23. doi: 10.1128/aac.46.7.2116-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baradaran Moghaddam A, Alebouyeh M, Farzi N, Bayati S, Amirmozafari N. Sensitivity to nitazoxanide among metronidazole resistant Helicobacter pylori strains in patients with gastritis. Med J Islam Repub Iran. 2016;30:405. [PMC free article] [PubMed] [Google Scholar]
- 21.Stuppy W. Dual therapy: nitazoxanide and sucralfate for the treatment of Helicobacter pylori. Am J Gastroenterol. 2010;105:S45–S46. [Google Scholar]
- 22.Shehata MA, Talaat R, Soliman S, Elmesseri H, Soliman S, Abd-Elsalam S. Randomized controlled study of a novel triple nitazoxanide (NTZ)-containing therapeutic regimen versus the traditional regimen for eradication of Helicobacter pylori infection. Helicobacter. 2017;22(5) doi: 10.1111/hel.12395. [DOI] [PubMed] [Google Scholar]
- 23.Abd-Elsalam S, Kobtan A, El-Kalla F, Elkhalawany W, Nawasany SE, Saif SA. et al. A 2-week Nitazoxanide-based quadruple treatment as a rescue therapy for Helicobacter pylori eradication: A single center experience. Medicine (Baltimore) 2016;95:e3879. doi: 10.1097/MD.0000000000003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Sneed GT, Brown JN. Treatment of Helicobacter pylori with nitazoxanide-containing regimens: a systematic review. Infect Dis (Lond) 2020;52:381–390. doi: 10.1080/23744235.2019.1708454. [DOI] [PubMed] [Google Scholar]
- 25.Liou JM, Chen PY, Luo JC, Lee JY, Chen CC, Fang YJ. et al. Efficacies of Genotypic Resistance-Guided vs Empirical Therapy for Refractory Helicobacter pylori Infection. Gastroenterology. 2018;155:1109–19. doi: 10.1053/j.gastro.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Soriano AG, Black J. Nitazoxanide use as part of an empiric multi-drug regimen in treating children with suspected Helicobacter pylori Infection. Case Rep Gastroenterol. 2015;9:36–42. doi: 10.1159/000375116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma V, Sharma R, Rizwani P. Initial experience with modified quadruple treatment regimen containing doxycycline, levofloxacin, nitazoxanide for Helicobacter pylori eradication. Indian J Gastroenterol. 2010;29:14. [Google Scholar]