Abstract
Background:
Surveillance colonoscopy is recommended to reduce colorectal cancer (CRC)-related morbidity and mortality in patients with inflammatory bowel disease (IBD). The comparative effectiveness of varying colonoscopy intervals on CRC outcomes among IBD patients is unknown.
Method:
We performed a retrospective cohort study of patients with confirmed CRC within a cohort of 77,824 IBD patients during 2000–2015 in the National Veterans Health Administration. We examined the association between colonoscopy surveillance intervals on CRC stage, treatment, or all-cause and cancer-specific mortality. The interval of colonoscopy prior to CRC diagnosis was categorized as those performed within <1 year, 1–3 years, 3–5 years, or none within 5 years.
Results:
Among 566 patients with CRC IBD, most (69.4%) did not have colonoscopy within 5 years prior to CRC diagnosis, while 9.7% had colonoscopy within one year prior to diagnosis, 17.7% within 1–3 years, and 3.1% between 3–5 years. Compared to no surveillance, colonoscopy within one year [adjusted odds ratio (aOR) 0.40 (95% CI 0.20–0.82)], and 1–3 years [aOR 0.56 (95% CI 0.32–0.98)] were less likely to be diagnosed at late stage. Regardless of IBD type and duration, colonoscopy within one year was associated with a lower all-cause mortality [adjusted hazard ratio 0.56 (95% CI 0.36–0.88].
Conclusions:
In a national cohort of IBD patients with CRC, colonoscopy within 3 years prior to CRC diagnosis was associated with early tumor stage at diagnosis, and colonoscopy within one year was associated with a reduced all-cause mortality compared to no colonoscopy. Our findings support colonoscopy intervals of 1–3 years in IBD patients to reduce late-stage CRC and all-cause mortality.
Keywords: Colonoscopy interval, Colorectal Cancer, Inflammatory Bowel Disease
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC), collectively referred to as inflammatory bowel disease (IBD), are associated with an increased risk of colorectal cancer (CRC).1–5 Current US and European Gastrointestinal society practice guidelines recommend colonoscopy for CRC surveillance in IBD patients at recurring periods ranging from 1 to 3 years (and 5 years by the British Society of Gastroenterology).1–4 Approximately one-quarter of IBD patients in clinical practice receive guideline-recommended colonoscopy surveillance.6, 7 Frequent colonoscopy surveillance places a substantial discomfort and financial burden on patients with IBD.8 In addition, the evidence base supporting the effectiveness of surveillance colonoscopy in IBD is relatively weak. There have been no randomized controlled trials, and it is unlikely that such trials could be feasibly or ethically performed. A meta-analysis of five observational studies suggests that CRC in IBD patients who had undergone surveillance colonoscopy was detected at an earlier stage than those without surveillance colonoscopy.9 Few studies have evaluated the effectiveness of varying colonoscopy intervals on other CRC outcomes in IBD patients, such as stage at detection, receipt of CRC treatment, or reducing mortality. Therefore, an additional well-powered study addressing these outcomes is needed.
In this study, we examined the association of surveillance colonoscopy interval prior to CRC diagnosis and CRC outcomes (stage at the diagnosis, treatment, and mortality) in a national cohort of IBD identified in the national Veterans Health Administration (VHA) datasets.
Method
Data Sources
We used the national VHA Corporate Data Warehouse (CDW) as the study’s IBD cohort data source. CDW contains patient demographics, outpatient and inpatient diagnosis and procedure codes, pharmacy and lab records. In addition, we performed structured reviews of complete electronic medical records of patients with IBD and colonoscopy, and comorbidities. Medical records review for each patient was performed by two independent reviewers (HK, SS), and discrepancies were resolved by a third expert IBD reviewer (JKH).
Study Population
We identified Veterans with IBD who developed CRC within our study period of 2000–2015. Patients with IBD were identified using International Classification of Diseases (ICD-9) diagnosis codes for CD (555.x) or UC (556.x), with at least two VA encounters, one of them outpatient with an IBD ICD-9 code. Patients with both CD and UC codes were categorized as IBD unclassified. We previously validated this approach to have 83% and 89% positive predictive values for CD and UC, respectively.10 IBD index date was defined as the date of first encounter with IBD code. Among the patients included in the IBD cohort, we identified patients with CRC using ICD-9 codes (153.xx or 154.xx) or ICD-10 codes (C18.xx, C19.xx, C20.xx or C21.xx), and included only those with confirmed both IBD and CRC diagnoses and diagnosis date by chart review. The date of CRC diagnosis was considered the index date. We included only those with a CRC diagnosis made during the study period and had at least one year of follow-up after their CRC diagnosis visit (or death within the first year). We excluded patients with CRC index date prior IBD index date, and those who have undergone any colectomy prior to IBD index date, inadequate pre-CRC colonoscopy data, dysplasia diagnosis prior to CRC, carcinoma-in-situ, or second CRC diagnosis.
Colonoscopy Interval Prior CRC diagnosis
Our main study exposure was colonoscopy before CRC diagnosis date. Colonoscopy within 6 months of CRC diagnosis was excluded because it may have been related to the identification of high-risk lesions and not performed for surveillance. We classified the colonoscopy interval preceding CRC diagnosis into: none, annual colonoscopy (most recent colonoscopy pre-CRC diagnosis 6 months to 1 year prior to CRC diagnosis), within 3-year interval (most recent colonoscopy >1 to 3 years prior to CRC diagnosis), and within 5-year interval (most recent colonoscopy >3 to 5 years prior to CRC diagnosis). Colonoscopy 5 or more years prior to CRC index was not considered. We defined colonoscopy intervals based on existing CRC guidelines with 1- and 1–3-year interval from US based guidelines and 5-year interval based on the British Society of Gastroenterology guidelines.1–4
Study Outcome
There were four study outcomes in our study: CRC stage at diagnosis, receipt of CRC treatment, overall mortality and CRC-specific mortality. CRC stage at diagnosis was determined by reviewing tumor board reports for American Joint Committee on Cancer staging or, when absent, by examining reports from pathology, endoscopy, imaging, and notes of consultants. The stage at CRC diagnosis was examined as early-stage (0–2 stage) or late-stage (3–4). Models with CRC stage as the outcome variable excluded patients with missing CRC stage. CRC treatment included complete or partial colectomy, endoscopic resection, chemotherapy or radiation. Death, if any, was identified in the VHA Vital Status file. Cause of death was defined as CRC-related death if caused by local or metastatic complications resulting from CRC or its treatment based on chart reviews. Survival duration was calculated from the date of CRC diagnosis to death or last VA encounter prior to July 2019.
Potential Confounders
The following variables were considered potential confounders for the association of CRC surveillance and our study outcomes: age at CRC diagnosis, gender, race/ethnicity, IBD type, IBD extent (For CD: IBD extension was positive if there was colonic involvement vs. isolated ileitis, For UC: positive if UC extended to left side or pancolitis vs. isolated proctitis), IBD duration (time from IBD diagnosis to CRC diagnosis), presence of PSC, tobacco or alcohol abuse, facility IBD volume, and comorbidity score. We also examined a variable indicative of the colonoscopy’s documented intention based on chart reviews (surveillance vs. not). Comorbidity score was calculated using a modified Deyo score of available chart review confirmed variables (myocardial Infarction, congestive heart failure, chronic obstructive pulmonary disease, peripheral vascular disease, PSC, and kidney failure). To assess the potential effect of patients receiving care outside of VA, we included VA priority level, a proxy for coverage, and out-of-pocket cost.11
Statistical Analysis
We evaluated the missingness pattern for all variables of interest. Missing values for race, alcohol abuse, and tobacco use, missing at random (from 12–20%), were imputed using multiple imputations with 10 iterations using proc MI and MIANALYZE statement in SAS®.12 All available variables went into the model for calculating missing values. About 40% of values were missing for CRC stage, which was not imputed since we believed CRC stage is an important variable that affected CRC outcome. Models with CRC stage as the outcome variable excluded all with unknown stage, whereas models that had CRC stage as a predictor variable contained an “unknown/missing” variable level. The propensity score for receiving a colonoscopy before CRC diagnosis was calculated using a conditional logistic model.
We compared socio-demographic and clinical features of IBD patients with CRC by the colonoscopy presence and intervals prior to the CRC diagnosis. Bivariate models were also run for all variables used in the multivariable models. We used multivariable logistic models stratified by quintiles of the propensity score to evaluate the possible effect of surveillance colonoscopy interval on the CRC stage at diagnosis, or receipt of CRC treatment as two separate outcomes. We used the Cox proportional hazards (PH) model to examine the possible effect of surveillance colonoscopy on mortality risk. We adjusted the models for all potential confounders listed earlier and retained variables based upon p-value <0.25 in the univariate test and clinical relevance.13 The Cox PH models were adjusted for lead time bias14, using an assumed CRC sojourn time (time from cancer initiation to clinical detection)14 of 5 years based on the literature showing a range from 4.5 (95% CI 4.1,4.8) to 5.8 years (95% CI 5.3, 6.3).15 A stepped approach was used in analyzing Cox proportional hazard models for all-cause mortality and competing risk model for CRC-specific mortality. Tumor stage and receipt of treatment variables were considered as potential explanatory variables for colonoscopy interval. Six separate models were run for each, all-cause mortality and CRC-specific mortality. In Model 1, the colonoscopy interval was not adjusted for lead-time bias, whereas models 2 through 6 were all adjusted for lead time. Model 2 contained colonoscopy interval adjusted for leadtime bias. Models 3 and 4 adjusted colonoscopy intervals for CRC stage or receipt of treatment, respectively; model 5) colonoscopy interval with both CRC stage and receipt of treatment 6) adjusted with all variables including confounders. Lastly, we conducted several sensitivity analyses to investigate the robustness of our results; these included various sojourn time between 4.1 – 6.3 years14 to investigate the robustness of lead time bias adjustment and the VA priority level [low (1–6) vs high (7–8)] to account for potential use of non-VA facilities. In a sensitivity analysis, we performed additional Cox PH analyses restricting to those with disease duration >8 years since most guidelines suggest starting colonoscopy surveillance 8–10 years after diagnosis or symptom onset.1–4 P-value <0.05 was considered significant in all analyses and all analyses were conducted using SAS® 9.4 statistical package.
This study was approved by the Institutional Review Boards of Baylor College of Medicine in Houston, Texas.
Results
CRC Cohort Characteristics
Within a cohort of 77,824 patients with IBD, we identified 714 unique patients with diagnosis codes for IBD and CRC, of whom 566 cases of incident CRC were confirmed by chart review (358 patients with underlying UC, 195 with CD, and 13 with IBD unclassified) (Supplemental Figure 1, Table 1). The mean age of IBD onset was 53.6 (±19.2). The mean age at CRC diagnosis was 67.5 (±12.5) years, and the majority were male (97.5%) and Caucasian (77.2%). Most (69.4%) did not receive colonoscopy within 5 years of CRC diagnosis, while 9.7% had colonoscopy within 1 year, 17.7% within >1–3 years, and 3.1% within >3–5 years prior to CRC diagnosis. The mean IBD duration for the no colonoscopy group was significantly shorter than surveillance group. The duration was 12.4 years for those without colonoscopy prior to CRC diagnosis vs. 15.7, 17.1, and 17.7 years for those with colonoscopy within 3–5 years, 1–3 years, and less than 1 year of CRC diagnosis, respectively, p-value <0.01.
Table 1.
Cohort characteristics of colorectal cancer (CRC) patients (n=566)
| Total (n) | No Colonoscopy$ Pre-CRC diagnosis n=393 (N, %) | Colonoscopy within >3–5yrs of CRC diagnosis n=18 (N, %) | Colonoscopy within >1–3yrs of CRC diagnosis n=100 (N, %) | Colonoscopy within <=1yr of CRC diagnosis n=55 (N, %) | P-value | |
|---|---|---|---|---|---|---|
| Age at CRC Diagnosis (years) (mean, SD) | 566 | 68.4 (12.8) | 66.1 (12.1) | 67.2 (10.5) | 61.7 (12.3) | <0.01 |
| Gender | 0.13 | |||||
| Male | 552 | 383 (97.5) | 16 (88.9) | 99 (99.0) | 54 (98.2) | |
| Female | 14 | 10 (2.5) | 2 (11.1) | 1 (1.0) | 1 (1.8) | |
| Race | 0.04 | |||||
| Caucasian | 437 | 300 (76.3) | 15 (83.3) | 82 (82.0) | 40 (72.7) | |
| African American | 30 | 16 (4.1) | 3 (16.7) | 5 (5.0) | 6 (10.9) | |
| Hispanics | 15 | 12 (3.1) | 0 (0.0) | 1 (1.0) | 2 (3.6) | |
| Asian/Native American/Others | 9 | 5 (1.3) | 0 (0.0) | 4 (4.0) | 0 (0.0) | |
| Unknown | 75 | 60 (15.3) | 0 (0.0) | 8 (8.0) | 7 (12.7) | |
| IBD type | 0.63 | |||||
| Ulcerative Colitis | 358 | 247 (62.9) | 12 (66.7) | 68 (68.0) | 31 (56.4) | |
| Crohn’s Disease | 195 | 137 (34.9) | 6 (33.3) | 31 (31.0) | 21 (38.2) | |
| IBD unclassified | 13 | 9 (2.3) | 0 (0.0) | 1 (1.0) | 3 (5.5) | |
| Colonic IBD Extent * | <.01 | |||||
| No | 330 | 270 (68.7) | 5 (27.8) | 37 (37.0) | 18 (32.7) | |
| Yes | 236 | 123 (31.3) | 13 (72.2) | 63 (63.0) | 37 (67.3) | |
| Age at IBD Diagnosis (years) (mean, SD) | 566 | 56.0 (18.9) | 50.4 (21.0) | 50.1 (18.7) | 44.0 (17.5) | <.01 |
| IBD duration (years) | 566 | 12.4 (13.8) | 15.7 (15.4) | 17.1 (14.2) | 17.7 (12.9) | <0.01 |
| CRC stage** | <.01 | |||||
| 0–2 | 168 | 67 (17.1) | 8 (44.4) | 59 (59.0) | 34 (61.8) | |
| 3–4 | 169 | 97 (24.7) | 10 (55.6) | 41 (41.0) | 21 (38.2) | |
| Unknown | 229 | 229 (58.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Colonoscopy surveillance indication | <.01 | |||||
| No | 452 | 339 (86.3) | 13 (72.2) | 62 (62.0) | 38 (69.1) | |
| Yes | 114 | 54 (13.7) | 5 (27.8) | 38 (38.0) | 17 (30.9) | |
| Primary Sclerosing Cholangitis | 0.01 | |||||
| No | 547 | 386 (98.2) | 18 (100.0) | 92 (92.0) | 51 (92.7) | |
| Yes | 19 | 7 (1.8) | 0 (0.0) | 8 (8.0) | 4 (7.3) | |
| Tobacco Use | 0.03 | |||||
| No | 216 | 141 (35.9) | 13 (72.3) | 40 (40.0) | 22 (40.0) | |
| Yes | 283 | 198 (50.4) | 5 (27.8) | 50 (50.0) | 30 (54.6) | |
| Unknown | 67 | 54 (13.7) | 0 (0.0) | 10 (10.0) | 3 (5.5) | |
| Alcohol abuse | 0.29 | |||||
| No | 375 | 256 (65.1) | 14 (77.8) | 71 (71.0) | 34 (61.3) | |
| Yes | 75 | 56 (14.3) | 1 (5.6) | 7 (7.0) | 11 (20.0) | |
| Unknown | 116 | 81(20.6) | 3 (16.7) | 22 (22.0) | 10 (18.2) | |
| Comorbidity Score | 0.11 | |||||
| 0 | 385 | 272 (73.3) | 34 (66.7) | 66 (70.2) | 13 (72.2) | |
| 1 | 96 | 69 (18.6) | 9 (17.7) | 13 (13.8) | 5 (27.8) | |
| ≥2 | 53 | 30 (8.1) | 8 (15.7) | 15 (16.0) | 0 (0.0) | |
| IBD facility volume | 566 | 2372.3 (1560.5) | 2163.6 (1027.2) | 2493.7 (1320.5) | 2685.2 (1681.5) | 0.42 |
| Priority level | ||||||
| 1–6 | 441 | 298 (76.0) | 46 (83.6) | 82 (82.0) | 15 (83.3) | 0.37 |
| 7–8 | 124 | 94 (24.0) | 9 (16.4) | 18 (18.0) | 3 (16.7) |
Time from most recent pre-cancer colonoscopy to CRC Diagnosis (Note: Colonoscopy within 6m or >5yrs pre-CRC diagnosis was grouped as No colonoscopy);
IBD extent (Yes or No) was determined separately for CD and UC as below:
For CD, IBD extension was positive if CD involves colonic area vs isolated ileitis, and For UC, IBD extension was positive if UC extended to left sided or pancolitis vs limited proctitis
IBD (Inflammatory Bowel Disease), CRC (Colorectal Cancer)
Colonoscopy Interval and CRC Stage
CRC was diagnosed at an early stage in 29.7% of cases, late-stage in 29.9% of cases, while in 40.4% cases stage could not be determined. All analyses for the stage were performed only in cases with a confirmed stage (n=337). Colonoscopy interval less than 1 year [Unadjusted odds ratio (OR) 0.46, 95% CI 0.24–0.88] or >1–3 years (OR 0.53, 95% CI 0.31–0.88) had a lower likelihood of being diagnosed with late-stage compared with no colonoscopy. In the multivariate analyses, colonoscopy interval less than 1 year (adjusted OR 0.40, 95% CI 0.20–0.82) and >1–3 years (adjusted OR 0.56, 95% CI 0.32–0.98) had a lower likelihood of being diagnosed with late-stage when compared with no colonoscopy (Table 2). Race/ethnicity, IBD type, IBD extent, PSC status, smoking or alcohol use, comorbidity score, and IBD facility volume were not associated with tumor stage.
Table 2.
Late stage colorectal cancer (CRC) diagnosis (III/IV) when compared with early stage (0/I/II) in patients with known stage (n=337)
| Adjusted Model | ||
|---|---|---|
| OR [95% CI] | P-value | |
| Colonoscopy Interval (years) | ||
| > 5 pre-CRC diagnosis | Ref | |
| ≤1 pre-CRC diagnosis | 0.40 (0.20–0.82) | 0.01 |
| >1–3 pre-CRC diagnosis | 0.56 (0.32–0.98) | 0.04 |
| >3–5 pre-CRC diagnosis | 1.13 (0.39–3.33) | 0.82 |
| Age at CRC index (per one year) | 0.98 (0.96–1.00) | 0.07 |
| Race/ethnicity | ||
| Caucasian | Ref | |
| African American | 0.93 (0.37–2.33) | 0.88 |
| Hispanics | 0.43 (0.11–1.70) | 0.22 |
| Asian/Native American/Others | 1.82 (0.36–9.18) | 0.47 |
| IBD type | ||
| Ulcerative Colitis | Ref | |
| Crohn’s Disease | 1.45 (0.87–2.44) | 0.16 |
| IBD unclassified | 4.11 (0.74–22.87) | 0.11 |
| Colonic IBD Extent | ||
| No | Ref | |
| Yes | 0.84 (0.45–1.56) | 0.58 |
| Colonoscopy surveillance indication | ||
| No | Ref | |
| Yes | 0.70 (0.42–1.20) | 0.20 |
| Primary Sclerosing Cholangitis | ||
| No | Ref | |
| Yes | 1.43 (0.31–6.70) | 0.65 |
| Tobacco Use | ||
| No | Ref | |
| Yes | 1.33 (0.76–2.35) | 0.31 |
| Alcohol abuse | ||
| No | Ref | |
| Yes | 0.92 (0.44–1.91) | 0.82 |
| Comorbidity Score | ||
| 0 | Ref | |
| 1 | 0.88 (0.45–1.69) | 0.70 |
| ≥2 | 0.50 (0.19–1.3) | 0.16 |
| IBD facility volume | 1.00 (1.00–1.00) | 0.13 |
IBD (Inflammatory Bowel Disease), CRC (Colorectal Cancer)
Colonoscopy Interval and Receipt of CRC Treatment
Treatment information was available for all included patients. Approximately 87% received CRC treatment; 96% in early and 86% in late-stage CRC. Bivariate logistic models indicated that colonoscopy interval less than one year (OR 3.08, 95% CI 1.04–9.07) or >1–3 years (OR 3.00, 95% CI 1.30–6.93) had higher odds of receiving treatment when compared with no colonoscopy. Patients with CRC diagnosis at a late stage had lower odds (OR 0.24, 95% CI 0.10–0.58) of receiving treatment when compared with diagnosis at an early stage. Comorbidity scores 1 (OR 0.54, 95% CI 0.30–0.97) or ≥2 (OR 0.69, 95% CI 0.31–1.51) were associated with lower odds of receiving CRC treatment than no comorbidity. Adjusting for differences in CRC stage and comorbidity, colonoscopy interval was no longer significant (p>0.05) in the multiple logistic models (Supplemental Table 1); the only significant variable was CRC stage (adjusted OR 0.2, 95% CI 0.08–0.54). Age, gender, race/ethnicity, IBD type, IBD extent, PSC status, smoking or alcohol use, comorbidity score, and IBD facility volume were not associated with CRC treatment receipt.
Colonoscopy Interval and All-cause and CRC-Specific Mortality
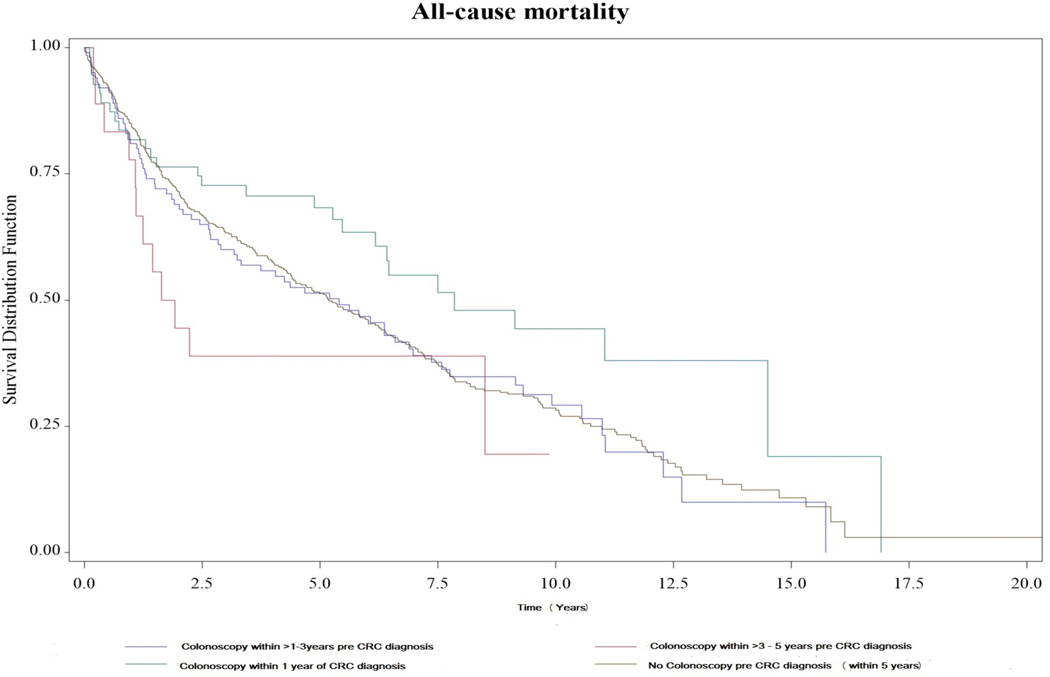
Approximately 70% of patients died during the study period, with approximately 23% deaths being CRC-related. A stepped approach was applied to modeling all-cause and CRC-specific mortality. The unadjusted model indicated colonoscopy less than one year had approximately 40% decreased in hazard rate when compared with no colonoscopy (HR 0.61, 95% CI 0.40–0.92) (Table 5). This result was maintained (HR 0.62, 95% CI 0.41–0.93) when adjusted for lead-time bias. The results were also maintained when further adjusting for tumor stage alone (HR 0.54, 95% CI 0.35–0.83), both stage and treatment (HR 0.55, 95% CI 0.36–0.85) or all covariates (HR 0.56, 95% CI 0.36–0.88) to the model (Table 5). Compared to the group in whom colonoscopy was performed within 1 year of CRC diagnosis, the risk of death was higher in the no colonoscopy group (aHR 1.59, 95% CI: 1.05–2.42), the colonoscopy within 1–3 years group (aHR 1.59, 95% CI 1.01–2.53), and the colonoscopy within 3–5 years group (aHR 2.29, 95% CI: 1.14–4.60). (Supplemental Table 2). As expected, late-stage CRC at diagnosis was associated with higher mortality when compared with early-stage CRC (adjusted HR 2.99, 95% CI 2.26–3.95) (Table 3) (Figure 1). After adjusted for covariates, gender, race/ethnicity, IBD type, IBD extent, PSC status, smoking or alcohol use, comorbidity score and IBD facility volume were not associated with mortality. When restricting the analysis to only those with IBD duration greater than 8 years, colonoscopy performed within one year of CRC diagnosis was significantly associated with a reduced risk of overall mortality. (Supplemental Table 3)
Table 5.
Stepped models stratified by propensity score: All-cause mortality
| Predictor: Colonoscopy Interval | HR (95% CI) | p-value |
|---|---|---|
| Unadjusted for lead time bias | ||
| Only Colonoscopy interval | ||
| >5 pre-CRC diagnosis | Ref | |
| ≤1 pre-CRC diagnosis | 0.61 (0.40–0.92) | 0.02 |
| >1–3 pre-CRC diagnosis | 0.95 (0.72–1.26) | 0.73 |
| >3–5 pre-CRC diagnosis | 1.43 (0.78–2.61) | 0.25 |
| Adjusted for lead time bias | ||
| Only Colonoscopy interval | ||
| >5 pre-CRC diagnosis | Ref | |
| ≤1 pre-CRC diagnosis | 0.62 (0.41–0.93) | 0.02 |
| >1–3 pre-CRC diagnosis | 0.96 (0.73–1.27) | 0.79 |
| >3–5 pre-CRC diagnosis | 1.42 (0.77–2.61) | 0.26 |
| Adjusted for Stage | ||
| >5 pre-CRC diagnosis | Ref | |
| ≤1 pre-CRC diagnosis | 0.54 (0.35–0.83) | <0.01 |
| >1–3 pre-CRC diagnosis | 0.90 (0.67–1.22) | 0.49 |
| >3–5 pre-CRC diagnosis | 0.97 (0.53–1.80) | 0.93 |
| Adjusted for treatment | ||
| >5 pre-CRC diagnosis | Ref | |
| ≤1 pre-CRC diagnosis | 0.67 (0.45–1.02) | 0.06 |
| >1–3 pre-CRC diagnosis | 1.03 (0.78–1.36) | 0.84 |
| >3–5 pre-CRC diagnosis | 1.57 (0.85–2.88) | 0.15 |
| Adjusted for both stage and treatment | ||
| >5 pre-CRC diagnosis | Ref | |
| ≤1 pre-CRC diagnosis | 0.55 (0.36–0.85) | 0.01 |
| >1–3 pre-CRC diagnosis | 0.90 (0.66–1.21) | 0.47 |
| >3–5 pre-CRC diagnosis | 1.03 (0.56–1.90) | 0.93 |
| Full model adjusted for all variables | ||
| >5 pre-CRC diagnosis | Ref | |
| ≤1 pre-CRC diagnosis | 0.56 (0.36–0.88) | 0.01 |
| >1–3 pre-CRC diagnosis | 0.81 (0.59–1.12) | 0.21 |
| >3–5 pre-CRC diagnosis | 0.82 (0.43–1.54) | 0.53 |
Table 3.
All-cause mortality in IBD patients with CRC by different colonoscopy intervals adjusted for lead time bias
| Adjusted Model | ||
|---|---|---|
| HR [95% CI] | P-value | |
| Colonoscopy Interval (years) | ||
| > 5 pre-CRC diagnosis | Ref | |
| ≤1yr pre-CRC diagnosis | 0.56 (0.36–0.88) | 0.01 |
| >1–3 pre-CRC diagnosis | 0.81 (0.59–1.12) | 0.22 |
| >3–5 pre-CRC diagnosis | 0.82 (0.43–1.54) | 0.53 |
| Age at CRC index (years) | 1.03 (1.01–1.04) | <.0001 |
| Gender | ||
| Male | Ref | |
| Female | 1.14 (0.57–2.27) | 0.71 |
| Race | ||
| Caucasian | Ref | |
| African American | 1.24 (0.73–2.11) | 0.43 |
| Hispanics | 0.81 (0.41–1.63) | 0.55 |
| Asian/Native American/Others | 0.49 (0.14–1.68) | 0.25 |
| IBD type | ||
| Ulcerative Colitis | Ref | |
| Crohn’s Disease | 1.11 (0.88–1.39) | 0.38 |
| IBD unclassified | 0.70 (0.33–1.45) | 0.33 |
| IBD extension | ||
| No | Ref | |
| Yes | 1.22 (0.91–1.65) | 0.19 |
| IBD duration (years) | 1.00 (1.00–1.01) | 0.38 |
| CRC stage | ||
| 0–2 | Ref | |
| 3–4 | 2.99 (2.26–3.95) | <.0001 |
| Unknown | 0.91 (0.67–1.27) | 0.57 |
| Colonoscopy surveillance indication | ||
| No | Ref | |
| Yes | 0.87 (0.66–1.15) | 0.33 |
| Primary Sclerosing Cholangitis | ||
| No | Ref | |
| Yes | 1.10 (0.53–2.27) | 0.80 |
| Tobacco Use | ||
| No | Ref | |
| Yes | 1.00 (0.79–1.26) | 0.99 |
| Alcohol abuse | ||
| No | Ref | |
| Yes | 1.07 (0.77–1.48) | 0.70 |
| Comorbidity Score | ||
| 0 | Ref | |
| 1 | 1.28 (0.97–1.69) | 0.08 |
| ≥2 | 1.22 (0.82–1.84) | 0.33 |
| IBD facility volume | 1.0 (1.0–1.0) | 0.20 |
IBD (Inflammatory Bowel Disease), CRC (Colorectal Cancer)
Figure 1.
Kaplan-Meier survival curves of all-cause mortality by colonoscopy interval pre- colorectal cancer diagnosis
Colonoscopy interval did not significantly affect CRC-related mortality compared with no colonoscopy in any of the models (Supplemental Table 4). Colonoscopy within one year of CRC diagnosis was associated with a trend toward reduced CRC-related mortality, it did not reach a statistically significant level (adjusted HR 0.53, 95% CI 0.30–1.05, p 0.07) (Supplemental Table 4). A sensitivity analysis adding priority group to the model did not change any results for colonoscopy interval.
Discussion
In our study of a patients with CRC in a national cohort of IBD, we observed that colonoscopy within 3 years prior to CRC diagnosis was inversely associated with late-stage CRC at diagnosis and with an increased likelihood of receiving CRC treatment compared with no colonoscopies. Colonoscopy within one year was also associated with a trend toward lower all-cause mortality. Our findings support current practice guidelines that recommend colonoscopy intervals from 1 year to 3 years among patients with IBD who have extensive colitis or left-sided colitis.1–3 Current guideline recommended CRC screening intervals for IBD patients were based on estimates of tumor progression; no comparison of IBD-CRC outcomes associated with colonoscopy intervals has been previously reported.1–3 Hereby, we provide further evidence that current practice guidelines surveillance intervals in IBD patients may provide several clinical benefits.
There is a paucity of data on how patients with IBD understand and value CRC surveillance. Only a minority of IBD patients (~24%) for whom surveillance is indicated receive surveillance colonoscopy.6,7 IBD patients have a high level of concern about developing cancer, but many patients lack awareness of individualized surveillance interval recommendations. In a survey of 101 patients with IBD, 61% of patients responded “I don’t know” regarding when CRC surveillance is recommended among patients with IBD, with only 5% correctly identifying recommended surveillance intervals.16 Furthermore, colonoscopy is associated with significantly more embarrassment and pain among patients with IBD than in non-IBD patients.17 Painful memories from colonoscopy have been associated with less willingness to return for subsequent colonoscopy. Therefore, the burden of intense colonoscopy surveillance needs to be carefully weighed against the data to support colonoscopy intervals. Our study provides additional data to support the guideline-recommended intervals that can help patients appreciate the impact of colonoscopy on CRC outcomes.
We did not observe statistically significant differences in surveillance colonoscopy effectiveness by IBD type, extent or duration, in tumor stage, receipt of cancer treatment, or mortality. While early perceptions of CRC risk in IBD have focused on UC, there is growing literature that CD with colonic involvement may have similar CRC risk as UC which we similarly demonstrated in our study.18 In regards to IBD extent, patients with isolated ileal or rectal disease were less likely to get surveillance colonoscopy as expected likely related to current guideline recommendations that patients with limited colonic involvement do not need to be included in CRC surveillance programs; our study was likely underpowered to detect differences in outcomes based on IBD extent. IBD-CRC patients without surveillance colonoscopy were older with limited disease and shorter duration of IBD compared to the surveillance group. Due to the relatively old age of the study cohort, most patients, even without colonic IBD extent, were eligible for de novo CRC colonoscopy surveillance, and this may have diminished the measured benefit of CRC surveillance. To minimize selection bias, we used a propensity score in our analyses. However, the propensity score is calculated based on only known, identified, and available variables, thus this score may not fully account for other competing IBD-CRC risk factors, such as severity and activity of IBD, and medication use.19
This is the first national IBD cohort based study to evaluate the comparative effectiveness of colonoscopy interval for several CRC-related outcomes in IBD. This design maximized the likelihood of studying all comers with CRC in a well-defined cohort and thus considerably reduced ascertainment bias. There was an inverse/protective association between colonoscopy and risk of early-stage CRC and mortality, and increased CRC treatment. The association with the risk of CRC stage persisted with the adjustment for healthy volunteer bias (i.e., stratification by propensity score).The increase in CRC treatment was almost completely explained away by the increase in early-stage diagnosis, a finding that is compatible with a beneficial effect of colonoscopy rather than selection bias. The reduction in mortality can be related to true beneficial effect and/or a confounding effect of health volunteer bias and lead-time bias. In a hypothetical scenario where surveillance is associated with a true reduction in mortality, the beneficial effect would persist after adjustments for these two types of bias; further, based on our knowledge of how surveillance works, the benefit would be explained mostly by early-stage diagnosis coupled with treatment. In our study, we adjusted for health volunteer/selection bias by propensity score stratification and for lead-time bias by adjusting for a range of sojourn times. The inverse effect on CRC stage and treatment largely persisted through these adjustments. However, adjusting for stage and treatment did not explain away the findings either. Therefore, it is still possible that some of our findings could be explained by a yet unexplained selection or ascertainment bias. Stage was limited by a large 40% proportion of patients with a missing stage that may have affected the findings of the main analysis and weakened the adjustment/explanatory effect of this variable.
In addition to the analytic techniques described above, our study has many strengths. We utilized the national VA database, the only national comprehensive, integrated health care system with data available on inpatient and outpatient encounters available from all VA facilities nationwide with access to electronic medical records to confirm exposure and outcomes. However, our study has several limitations. This study is a retrospective cohort study that still contains potential biases such as selection and confounders, which can be ideally minimized in a randomized clinical trial study design. However, conducting a randomized clinical trial of the effectiveness of colonoscopy intervals on CRC survival among patients with IBD would not only be impractical due to cost, and length of follow-up required. CRC stage was missing in 40% of our study population, largely due to patients who obtained care outside of VA. To minimize bias related to missing CRC stage, no imputation was performed for missing stage and instead patients with missing CRC stage were excluded from the CRC outcome analyses. Furthermore, we performed sensitivity analyses in which we excluded patients likely to get care outside of VA based on their priority level, and found no significant difference in results, however residual bias from non-VA care or from missing stage data may not be accounted for. We could not account for the IBD treatment history, colonoscopy quality measures, and biopsy results due to the retrospective study design. To address potential limitations in diagnostic codes, we used a previously validated diagnostic algorithm of ICD codes for IBD, and confirmed key exposures and outcomes with chart review in every case.20 However, screening of related outcomes that occurred outside the VA could not be fully captured in VA data sets, and therefore there is a potential to underestimate the CRC cases diagnoses and mortality and the risk of misclassification of colonoscopy intervals. Adjustment for priority level, a proxy of the probability that a veteran may get care outside of VA did not change our main results. The VA population is predominantly male and with a later onset of IBD compared to the general population, and therefore our findings may not be generalizable to females with IBD or patients with younger IBD onset. Lastly, our analyses does not address the role of high definition colonoscopy or white-light vs. chromoendoscopy. The study period was prior to the SCENIC Consensus statement and therefore random biopsy was the predominant method of surveillance.21
In conclusion, colonoscopy within 3 years prior to CRC diagnosis compared with no colonoscopy was less likely to be diagnosed with late tumor stage Colonoscopy within one year was associated with lower all-cause mortality than no colonoscopy. Our findings support the use of surveillance colonoscopy to improve CRC outcomes in IBD patients.
Supplementary Material
Supplemental Figure 1. Flow chart of cohort
Table 4.
Colorectal cancer (CRC) specific mortality in IBD patients with CRC by different colonoscopy intervals adjusted for lead time bias
| Adjusted Model | ||
|---|---|---|
| HR [95% CI] | P-value | |
| Colonoscopy Interval (years) | ||
| > 5 pre-CRC diagnosis | ref | |
| ≤1yr pre-CRC diagnosis | 0.53 (0.30–1.05) | 0.07 |
| >1–3 pre-CRC diagnosis | 0.81 (0.48–1.37) | 0.43 |
| >3–5 pre-CRC diagnosis | 0.88 (0.38–2.00) | 0.76 |
| Age at CRC index (years) | 1.00 (0.98–1.02) | 0.87 |
| Gender | ||
| Male | Ref | |
| Female | 1.78 (0.65–4.90) | 0.26 |
| Race | ||
| Caucasian | Ref | |
| African American | 1.53 (0.74–3.12) | 0.25 |
| Hispanics | 1.20 (0.38–3.73) | 0.76 |
| Asian/Native American/Others | 0.46 (0.12–1.75) | 0.25 |
| IBD type | ||
| Ulcerative Colitis | Ref | |
| Crohn’s Disease | 1.06 (0.70–1.61) | 0.77 |
| IBD unclassified | 0.88 (0.30–2.60) | 0.82 |
| IBD unclassified | 0.88 (0.30–2.60) | 0.82 |
| IBD extension | ||
| No | Ref | |
| Yes | 1.60 (0.99–2.65) | 0.07 |
| IBD duration (years) | 0.995 (0.98–1.01) | 0.47 |
| CRC stage | ||
| 0–2 | ||
| 3–4 | 6.79 (4.16–11.10) | <.01 |
| Unknown | 0.87 (0.46–1.68) | 0.69 |
| Colonoscopy surveillance indication | ||
| No | Ref | |
| Yes | 0.79 (0.50–1.24) | 0.31 |
| Primary Sclerosing Cholangitis | ||
| No | Ref | |
| Yes | 1.29 (0.23–−7.32) | 0.77 |
| Tobacco Use | ||
| No | Ref | |
| Yes | 1.02 (0.69–1.52) | 0.91 |
| Alcohol abuse | ||
| No | ||
| Yes | 1.13 (0.65–1.97) | 0.67 |
| Comorbidity Score | ||
| 0 | Ref | |
| 1 | 1.06 (0.64–1.77) | 0.82 |
| ≥2 | 0.40 (0.16–1.04) | 0.06 |
| IBD facility volume | 1.00 (1.00–1.00) | 0.70 |
IBD (Inflammatory Bowel Disease), CRC (Colorectal Cancer)
What you need to know:
Background:
Surveillance colonoscopy is recommended to reduce colorectal cancer (CRC)-related morbidity and mortality in inflammatory bowel disease (IBD). Most society practice guidelines have recommended colonoscopy between 1–5 years, however the comparative effectiveness of varying colonoscopy intervals on CRC outcomes among IBD patients is not known.
Findings:
We found that colonoscopy within 3 years prior to CRC diagnosis was associated with early tumor stage at diagnosis, and colonoscopy within one year was associated with reduced all-cause mortality compared to no colonoscopy Our findings support colonoscopy intervals of 1–3 years in IBD patients to reduce late-stage CRC and all-cause mortality.
Implications for patient care:
Although the study has limitation inherent to retrospective studies, it provides new data to support the recommendation of 1–3 year intervals for surveillance colonoscopy among patients with IBD.
Acknowledgments/Funding Source:
The research reported here was supported by a grant from the Agency for Healthcare Research and Quality (K08 HS24122-02)(Hou), a Career Development Award from the Crohn’s and Colitis Foundation (Hou), and in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX (Hou).
Footnotes
Disclosures:
JH has received research funding from Redhill Biosciences, Janssen, Abbvie, Celgene, Genentech, Eli-Lily, Lycera, and Pfizer Inc. JH has served as a consultant for Abbvie, Janssen, and Pfizer. Dr. Lewis consulted or served on an advisory board for Eli Lilly and company, Samsung Bioepis, UCB, Bristol-Myers Squibb, Nestle Health Science, Merck, Celgene, Janssen Pharmaceuticals, Bridge Biotherapeutics, Entasis Therapeutics, AbbVie, Pfizer, Gilead, Arena Pharmaceuticals, Protagonist Therapeutics, Amgen and Scipher Medicine. He has had research funding from Nestle Health Science, Takeda, Janssen Pharmaceuticals, and AbbVie. He has performed legal work on behalf of generic manufacturers of ranitidine, including L. Perrigo Company, Glenmark Pharmaceuticals Inc., Amneal Pharmaceuticals LLC, Aurobindo Pharma USA, Inc., Dr. Reddy’s Laboratories, Inc., Novitium Pharma, Ranbaxy Inc. and Sun Pharmaceutical Industries, Inc., Strides Pharma, Inc., and Wockhardt USA LLC.
The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Annese V, Beaugerie L, Egan L, et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis 2015;9:945–65. [DOI] [PubMed] [Google Scholar]
- 2.Itzkowitz SH, Present DH, Crohn’s, et al. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis 2005;11:314–21. [DOI] [PubMed] [Google Scholar]
- 3.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:738–45. [DOI] [PubMed] [Google Scholar]
- 4.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biancone L, Armuzzi A, Scribano ML, et al. Cancer Risk in Inflammatory Bowel Disease: A 6-Year Prospective Multicenter Nested Case-Control IG-IBD Study. Inflamm Bowel Dis 2020;26:450–459. [DOI] [PubMed] [Google Scholar]
- 6.Velayos FS, Liu L, Lewis JD, et al. Prevalence of colorectal cancer surveillance for ulcerative colitis in an integrated health care delivery system. Gastroenterology 2010;139:1511–8. [DOI] [PubMed] [Google Scholar]
- 7.Ananthakrishnan AN, Cagan A, Cai T, et al. Colonoscopy is associated with a reduced risk for colon cancer and mortality in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2015;13:322–329 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultan S, Partin MR, Shah P, et al. Barriers and facilitators associated with colonoscopy completion in individuals with multiple chronic conditions: a qualitative study. Patient Prefer Adherence 2017;11:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bye WA, Nguyen TM, Parker CE, et al. Strategies for detecting colon cancer in patients with inflammatory bowel disease. Cochrane Database Syst Rev 2017;9:CD000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou JK, Tan M, Stidham RW, et al. Accuracy of diagnostic codes for identifying patients with ulcerative colitis and Crohn’s disease in the Veterans Affairs Health Care System. Dig Dis Sci 2014;59:2406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen LA, Byrne MM, Daw CN, et al. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare-enrolled veterans. Health Serv Res 2010;45:762–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, Stuart EA, Allison DB. Multiple Imputation: A Flexible Tool for Handling Missing Data. JAMA 2015;314:1966–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendel RE, Afifi A. Comparison of Stopping Rules in Forward “Stepwise” Regression. Journal of the American Statistical Association 1977;72:46–53. [Google Scholar]
- 14.Duffy SW, Nagtegaal ID, Wallis M, et al. Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. Am J Epidemiol 2008;168:98–104. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Altenhofen L, Katalinic A, et al. Sojourn time of preclinical colorectal cancer by sex and age: estimates from the German national screening colonoscopy database. Am J Epidemiol 2011;174:1140–6. [DOI] [PubMed] [Google Scholar]
- 16.Hou JK, Turkeltaub JA, McCarty Iii TR, et al. Assessment of disease specific knowledge and health-related quality of life among United States military veterans with inflammatory bowel disease. World J Gastroenterol 2015;21:6001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denters MJ, Schreuder M, Depla AC, et al. Patients’ perception of colonoscopy: patients with inflammatory bowel disease and irritable bowel syndrome experience the largest burden. Eur J Gastroenterol Hepatol 2013;25:964–72. [DOI] [PubMed] [Google Scholar]
- 18.Olen O, Erichsen R, Sachs MC, et al. Colorectal cancer in Crohn’s disease: a Scandinavian population-based cohort study. Lancet Gastroenterol Hepatol 2020;5:475–484. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thirumurthi S, Chowdhury R, Richardson P, et al. Validation of ICD-9-CM diagnostic codes for inflammatory bowel disease among veterans. Dig Dis Sci 2010;55:2592–8. [DOI] [PubMed] [Google Scholar]
- 21.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148:639–651 e28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flow chart of cohort