Abstract
Background:
A gluten-free diet (GFD) is the only effective treatment of celiac disease (CD) that is associated with body mass index (BMI) changes. This study aimed to determine how GFD duration affects the BMI of Iranian patients with CD.
Methods:
In this prospective study, 215 patients with CD, who were on a GFD, were categorized into three groups according to the duration of compliance to GFD: 1. patients with less than 6 months of diet, 2. Patients who had a diet for 6 months to 2 years, and 3. patients with more than 2 years of diet. The BMI changes were assessed before and after adherence to the GFD.
Results:
Most patients’ weight remains in the same BMI category during different courses of GFD adherence. Patients who were underweight showed significant changes in their BMI following the diet in less than 6 months (P=0.033) and more than 2 years (P<0.001), and the number of weight gain cases increased over time.
Conclusion:
There is a need for careful, updated, and personalized nutrition management of patients with CD in different periods of the diet. Conducting similar studies with larger sample sizes in different regions can lead to providing expert dietary counseling for patients with CD.
Keywords: Celiac disease, Gluten, Gluten-free diet, Body mass index
Introduction
Celiac disease (CD) is the most common chronic autoimmune disorder characterized by small intestinal villous atrophy in genetically predisposed subjects caused by gluten exposure.1,2 The prevalence of CD in the general population is about 1%, and 0.3%-2.9% in pediatrics.3-6 CD can present with different gastrointestinal and extraintestinal symptoms at any age, or it may remain asymptomatic.7,8 Common gastrointestinal manifestations of CD are chronic diarrhea, malabsorption, abdominal pain, weight loss, and steatorrhea. Extraintestinal presentations include arthritis, hair loss, mouth ulceration, fatigue, and neurological and psychiatric disorders.9 Important and challenging complications of CD include growth failure and weight loss, which affect patients’ body mass index (BMI) negatively and result from nutrient malabsorption.10,11 Nevertheless, several studies have reported normal weight, overweight, or even obesity in patients with CD, and diagnosis of CD can be delayed in these subjects.12-14 The lifelong, strict gluten-free diet (GFD) is the only effective treatment for patients with CD that can improve the absorption of the nutrients and subsequently eliminates CD-related malabsorption symptoms.15-18 It has been shown that the first year of following this diet is accompanied by significant improvement in the histological and clinical conditions of patients; although, some experts believe that complete histological recovery may achieve after more than one year of dieting.15-19 Studies have shown that GFD adherence is associated with significant changes in the BMI of patients with CD.20Little is known about the effect of different durations of compliance to GFD on weight and BMI changes in patients with CD. This study was conducted to evaluate this issue.
Materials and Methods
Participants and Procedure
We conducted a nationwide, questionnaire-based, prospective study that evaluated 250 patients with CD. These subjects were registered with the outpatient celiac clinic at Taleghani Hospital in Tehran, Iran, from different parts of the country. CD was diagnosed based on positive serological tests and histopathological findings of the small intestine according to the Marsh classification.21All patients had the following inclusion criteria: biopsy-proven CD, age of 18 years and above, adherence to a GFD, and available baseline and following GFD BMI (body weight in kg/height in m2) measurement. Inaccessibility to patient records, having only one recorded BMI, and refusal to participate in the study were considered exclusion criteria, and accordingly, 35 cases were excluded from this study and finally 215 subjects were studied. Subjects were assessed for the following variables: sex, age, marital status, GFD duration (< 6 months, 6 months to 2 years, more than 2 years), family history of CD, clinical symptoms, weight, and BMI (before and during the diet). BMIwas classified as underweight (< 18.5), normal (18.5–24.9), overweight (25–29.9), or obese (≥ 30) according to World Health Organization criteria.
Statistical Analysis
Statistical analysis was performed using the SPSS software for Windows (Version 21.0, SPSS Inc., Chicago, IL, USA). Comparisons were carried out by chi-square test, Student’s t test, Levene’s test, and ANOVA. Data are presented as mean ± SD and with a 95% confidence interval. P values < 0.05 were considered to be statistically significant.
Results
Patient Characteristics
Of the 215 biopsy-proven patients with CD, there were 77 men (35.8%) and 138 women (64.2%). The median age (mean ± SD) of the studied group was 38.96 ± 11.8 years and they were categorized into three age groups (18-30 years: 26%, 30-60 years: 68%, more than 60 years: 6%).
The majority of the participants were married (70.7%), and 23 (10.7%) of them had a family history of CD. In terms of duration of the GFD, 47 (21.8%) reported less than 6 months, 83 (38.6%) between 6 months and 2 years, and 85 (39.6%) were on the diet for more than 2 years. The most common gastrointestinal symptoms of patients were bloating (67%), weight loss (55.3%), and abdominal cramping (50.2%), and the most frequent extraintestinal manifestations were anemia (53%), musculoskeletal problems (48%), and neurological disorders (46%) at diagnosis (shown in Figure 1).
Figure 1.
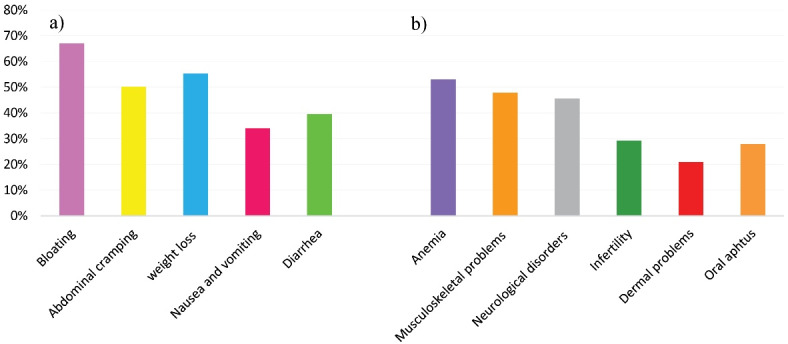
Frequencies of a) intestinal and b) extraintestinal symptoms of patients with CD.
Effect of Different GFD Durations on BMI Changes
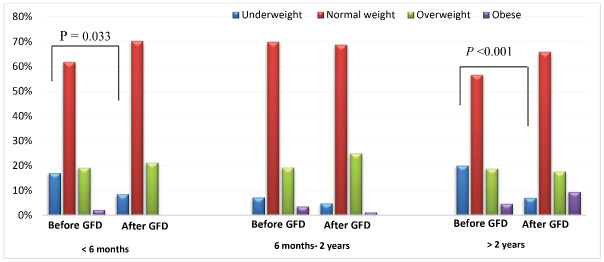
By following the GFD for less than 6 months, the BMI levels of four (50%) underweight subjects increased to normal levels. An obese person (100%) also lost weight by following the diet and was included in the overweight group.
By adherence to the diet for 6 months to 2 years, two (33.3%) underweight and three (5.17%) normal weight subjects gained weight, and their BMI category changed to normal and overweight, respectively. Two obese subjects (66.6%) also lost weight and were categorized into the overweight group.
Of 85 patients compliant with a GFD for more than 2 years, 11 (64.7%) underweight, 3 (6.25%) normal weight, and 4 (25%) overweight subjects returned to normal weight, overweight, and obese category, respectively (shown in Figure 2).
Figure 2.
Distribution of patients with CD according to their BMI levels (of GFD).
It is worthy to note that the changes in the underweight category were significant in two periods of less than 6 months (P = 0.033) and more than 2 years (P < 0.001) of dieting. This category did not show a significant change in 6 months to 2 years of adherence to GFD (P = 0.076). Moreover, normal weight, overweight and obese categories did not change significantly in less than 6 months, between 6 months and 2 years, and more than 2 years of adherence to the regimen, respectively (P = 0.326, 0.182, 0.096 for normal weight; P = 0.347, 0.333, 1 for overweight and P = 0.184, and 0.085 for the obese category; (P value was not calculated for obese subjects in less than 6 months of dieting due to lack of quorum).
The Relationship between the Patients’ Body Weight and BMI Changes with Different Variables During Three Courses of a GFD
According to our results, adherence to a GFD for less than 6 months was accompanied by significant changes in the body weight and BMI of women (P = 0.003 for both of them), patients in the age group of 31 to 60 years (P = 0.008 and 0.011, respectively), and who had a family history of CD (P = 0.033 and 0.035, respectively) (Tables 1 and 2).
Table 1. Relationship between patients’ body weight changes with different variables in three different periods of the diet (compared to the time before the start of the diet) .
| Under 6 months GFD | 6 months-2 years GFD | More than 2 years GFD | |||||||||||
| Mean | SD | P value (within groups) | P value (between groups) | Mean | SD | P value (within groups) | P value (between groups) | Mean | SD |
P
value
(within groups) |
P value (between groups) | ||
| Gender | Male | 1.50 | 2.96 | 0.108, | 0.937 | 1.51 | 4.86 | 0.083 | 0.687 | 2.25 | 4.53 | 0.009 | 0.749 |
| Female | 1.42 | 2.59 | 0.003 | 1.13 | 3.67 | 0.032 | 2.63 | 5.63 | 0.002 | ||||
| Age (y) | 18-30 | 1.80 | 3.01 | 0.091 | 0.606 | 1.90 | 3.61 | 0.026 | 0.490 | 3.48 | 3.20 | < 0.0001 | 0.503 |
| 31-60 | 1.25 | 2.63 | 0.008 | 1.20 | 4.39 | 0.040 | 2.10 | 6.03 | 0.016 | ||||
| > 61 | 3.00 | 0.00 | 0.083 | -0.75 | 2.98 | 0.650 | 1.50 | 4.27 | 0.430 | ||||
| Family history of CD | Yes | 3.60 | 2.50 | 0.033 | 0.191 | 1.11 | 4.34 | 0.465 | 0.563 | 1.00 | 4.40 | 0.524 | 0.742 |
| No | 1.00 | 2.88 | 0.137 | 1.00 | 3.40 | 0.071 | 2.38 | 3.88 | < 0.0001 | ||||
Table 2. The relationship between patients’ BMI changes with different variables in three different periods of the diet (compared to the time before the start of the diet) .
| Under 6 months GFD | 6 months-2 years GFD | More than 2 years GFD | |||||||||||
| Mean | SD | P value (within groups) | P value (between groups) | Mean | SD | P value (within groups) | P value (between groups) | Mean | SD | P value (within groups) | P value (between groups) | ||
| Gender | Male | 0.49 | 1.01 | 0.119 | 0.853 | 0.49 | 1.65 | 0.097 | 0.962 | 0.70 | 1.48 | 0.012 | 0.502 |
| Female | 0.55 | 1.03 | 0.003 | 0.47 | 1.44 | 0.023 | 1.01 | 2.33 | 0.003 | ||||
| Age (y) | 18-30 | 0.69 | 1.11 | 0.081 | 0.558 | 0.71 | 1.33 | 0.023 | 0.475 | 1.22 | 1.22 | < 0.0001 | 0.600 |
| 31-60 | 0.46 | 1.01 | 0.011 | 0.44 | 1.59 | 0.036 | 0.78 | 2.38 | 0.023 | ||||
| > 61 | 1.16 | 0.23 | 0.090 | -0.26 | 1.20 | 0.688 | 0.49 | 1.48 | 0.452 | ||||
| Family history of CD | Yes | 1.33 | 0.94 | 0.035 | 0.224 | 0.38 | 1.62 | 0.500 | 0.589 | 0.36 | 1.45 | 0.471 | 0.792 |
| No | 0.37 | 1.15 | 0.163 | 0.38 | 1.29 | 0.071 | 0.84 | 1.47 | 0.001 | ||||
Following the diet for 6 months to 2 years, significant changes in body weight and BMI of women (P = 0.032 and 0.023, respectively) and two age groups of 18-30 years (P = 0.026 and 0.023, respectively) and 31-60 years (P = 0.040 and 0.036, respectively) were observed (Tables 1 and 2).
Adherence to GFD for more than two years also caused significant changes in body weight and BMI in both men (P = 0.009 and 0.012, respectively) and women (P = 0.002 and 0.003, respectively), age groups of 18-30 years (P < 0.0001 for both of them) and 31-60 years (P = 0.016 and 0.023, respectively), and subjects without a family history of CD (P < 0.0001 and P = 0.001, respectively) (Tables 1 and 2).
Discussion
Adherence to a GFD is the only gold standard therapeutic pathway for CD, which helps intestinal mucosal healing and malabsorption correction. Previously, the general perception was that all patients with CD were underweight at the time of diagnosis, while later, either overweight or even obese newly diagnosed CD subjects were also reported.22 Accordingly, in a study from Minnesota, 27% of the patients with CD were overweight at diagnosis.23 Moreover, Dickey and Kearney reported that a large minority of Irish patients with CD were overweight before starting GFD.24 Similarly, in our study, despite the presence of gastrointestinal symptoms such as diarrhea and weight loss at the time of diagnosis, out of 215 patients, 135 (62.7%) were normal weight, and 49 (22.8%) were overweight and obese before GFD.
According to the reports, there are people without diagnosed gluten disorders who adopted a GFD (especially in the United States, where obesity is a systematic problem) to aid weight loss.25-27 However, studies have shown that gluten-free foods may lead to obesity in patients with CD due to the content of fat, sugars, and salt. Abnormal BMI (both low and high) is associated with an increased risk of mortality and morbidity.28,29 Little is known about the effect of different GFD durations on the BMI of patients with CD. We conducted this study to evaluate their association. According to our results, most patients’ weights remain in the same BMI category during different course duration of adherence to GFD. Patients who were underweight showed significant changes in their BMI (weight gain) following the diet in less than 6 months (P = 0.033) and more than 2 years (P < 0.001), and the number of weight gain cases increased over time. These results were in line with the result of Kabbani et al15 study of Israeli patients with CD who were on a GFD for an average of 39.5 months. On the contrary, in a United States study, Cheng and colleagues30 showed a positive effect of a GFD for an average of 2.8 years on patients with CD, which caused normalization of BMI in most subjects, whether patients were underweight or overweight at diagnosis.30 An Irish study conducted by Dickey and Kearney also showed that on a 2-year gluten exclusion, 81% of patients with CD gained weight.24 Moreover, Ukkola et al in a study in Finland showed that following GFD for 1 year, 69% of initially underweight patients with CD achieved normal weight, and 87% of those of normal weight remained in the same BMI category. Weight loss was noted in 18% of overweight and 42% of obese patients, respectively.20 The differences observed in the results of conducted studies can be justified by considering population-based genetic diversity and possible differences in the dietary consultations given to patients with CD in various countries.
Based on our findings, adherence to the GFD in the first months was associated with both weight loss and weight gain in patients, and increasing the duration of adherence to GFD was associated with an increase in weight gain. Our perception of these observations is that in the first months of starting the diet, patients lose weight because the diet is different and maybe unpalatable to adjust to. Thereafter, when they get used to this type of diet, BMI increases, and patients gain more weight.
In our study, women showed significant changes in both body weight and BMI in all three periods whilst on GFD; however, the differences became significant in men by adherence to the GFD for more than two years. The age group that showed significant changes in body weight and BMI by following the diet for less than 6 months was 31 to 60 years. Adherence to the diet for 6 months and more leads to significant changes in body weight and BMI in the age range of 18 to 60 years. A plausible explanation for these differences can be explained by considering the different eating habits of various age groups and the difference in the sex hormones.
Improving BMI, which was mainly shown by following the diet for less than 6 months, was mostly in people with a family history of CD, which could be due to receiving double counseling from the peers and the high awareness of these people compared with those without a family history of CD. Accordingly, changes in BMI and body weight after dieting for more than two years, which was mainly in the form of weight gain, were observed in people without a family history of CD.
Conclusion
We conclude that there is a need for careful, updated, and personalized nutrition management of patients with CD in different periods of the GFD. Patients in different regions respond differently to the diet; hence, it is suggested that similar studies be done in each region with a larger sample size so that expert dietary counseling will be provided for patients with CD.
Acknowledgments
We would like to thank the Gastroenterology and Liver Diseases Research Center, the Research Institute for Gastroenterology and Liver Diseases, and Shahid Beheshti University of Medical Sciences, Tehran, Iran, for all their support.
Please cite this paper as: Asri N, Taraghikhah N, Baniasadi R, Ishaq S, Rezaei-Tavirani M, Sadeghi A, et al. The effect of gluten-free diet duration on body mass index of Iranian patients with celiac disease. Middle East J Dig Dis 2022;14(3):323-329. doi: 10.34172/mejdd.2022.290.
Footnotes
Ethical Approval
The research was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran, with ethics code IR.SBMU.RETECH.REC.1398.866.
Conflict of Interest
The authors declare no conflict of interest related to this work.
References
- 1.Rostami-Nejad M, Taraghikhah N, Ciacci C, Pourhoseingholi MA, Barzegar F, Rezaei-Tavirani M. et al. Anxiety symptoms in adult celiac patients and the effect of a gluten-free diet: an Iranian Nationwide Study. Inflamm Intest Dis. 2020;5(1):42–7. doi: 10.1159/000505657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asri N, Rostami-Nejad M. The facts of celiac disease; a comprehensive review. Int J Celiac Dis. 2019;7(2):48–52. doi: 10.12691/ijcd-7-2-7. [DOI] [Google Scholar]
- 3.Mohammadibakhsh R, Sohrabi R, Salemi M, Taheri Mirghaed M, Behzadifar M. Celiac disease in Iran: a systematic review and meta-analysis. Electron Physician. 2017;9(3):3883–95. doi: 10.19082/3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rostami-Nejad M, Rostami K, Emami MH, Zali MR, Malekzadeh R. Epidemiology of celiac disease in Iran: a review. Middle East J Dig Dis. 2011;3(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- 5.Ludvigsson JF, Green PH. Clinical management of coeliac disease. J Intern Med. 2011;269(6):560–71. doi: 10.1111/j.1365-2796.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- 6.Cardo A, Churruca I, Lasa A, Navarro V, Vázquez-Polo M, Perez-Junkera G. et al. Nutritional imbalances in adult celiac patients following a gluten-free diet. Nutrients. 2021;13(8):2877. doi: 10.3390/nu13082877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rostami-Nejad M, Rostami K, Pourhoseingholi MA, Nazemalhosseini Mojarad E, Habibi M, Dabiri H. et al. Atypical presentation is dominant and typical for coeliac disease. J Gastrointestin Liver Dis. 2009;18(3):285–91. doi: 10.1088/2058-7058/3/8/4. [DOI] [PubMed] [Google Scholar]
- 8.Taraghikhah N, Ashtari S, Asri N, Shahbazkhani B, Al-Dulaimi D, Rostami-Nejad M. et al. An updated overview of spectrum of gluten-related disorders: clinical and diagnostic aspects. BMC Gastroenterol. 2020;20(1):258. doi: 10.1186/s12876-020-01390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leffler DA, Green PH, Fasano A. Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12(10):561–71. doi: 10.1038/nrgastro.2015.131. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti A, Capriati T, Basso MS, Panetta F, Di Ciommo Laurora VM, Bellucci F. et al. Celiac disease and overweight in children: an update. Nutrients. 2014;6(1):207–20. doi: 10.3390/nu6010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dommelen P, Grote FK, Oostdijk W, Keizer-Schrama SM, Boersma B, Damen GM. et al. Screening rules for growth to detect celiac disease: a case-control simulation study. BMC Pediatr. 2008;8:35. doi: 10.1186/1471-2431-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahraki T, Shahraki M, Hill ID. Frequency of overweight/obesity among a group of children with celiac disease in Iran. Prz Gastroenterol. 2018;13(2):127–31. doi: 10.5114/pg.2018.73347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nenna R, Mosca A, Mennini M, Papa RE, Petrarca L, Mercurio R. et al. Coeliac disease screening among a large cohort of overweight/obese children. J Pediatr Gastroenterol Nutr. 2015;60(3):405–7. doi: 10.1097/mpg.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 14.Rinninella E, Cintoni M, Raoul P, Triarico S, Dionisi T, Gasbarrini GB. et al. The healthy gluten-free diet: practical tips to prevent metabolic disorders and nutritional deficiencies in celiac patients. Gastroenterol Insights. 2021;12(2):166–82. doi: 10.3390/gastroent12020015. [DOI] [Google Scholar]
- 15.Kabbani TA, Goldberg A, Kelly CP, Pallav K, Tariq S, Peer A. et al. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment Pharmacol Ther. 2012;35(6):723–9. doi: 10.1111/j.1365-2036.2012.05001.x. [DOI] [PubMed] [Google Scholar]
- 16.Valletta E, Fornaro M, Cipolli M, Conte S, Bissolo F, Danchielli C. Celiac disease and obesity: need for nutritional follow-up after diagnosis. Eur J Clin Nutr. 2010;64(11):1371–2. doi: 10.1038/ejcn.2010.161. [DOI] [PubMed] [Google Scholar]
- 17.van der Pals M, Myléus A, Norström F, Hammarroth S, Högberg L, Rosén A. et al. Body mass index is not a reliable tool in predicting celiac disease in children. BMC Pediatr. 2014;14:165. doi: 10.1186/1471-2431-14-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asri N, Rostami-Nejad M, Rezaei-Tavirani M, Razzaghi M, Asadzadeh-Aghdaei H, Zali MR. Novel therapeutic strategies for celiac disease. Middle East J Dig Dis. 2020;12(4):229–37. doi: 10.34172/mejdd.2020.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sansotta N, Guandalini S, Romano S, Amirikian K, Cipolli M, Tridello G. et al. The gluten free diet’s impact on growth in children with celiac disease in two different countries. Nutrients. 2020;12(6):1547. doi: 10.3390/nu12061547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ukkola A, Mäki M, Kurppa K, Collin P, Huhtala H, Kekkonen L. et al. Changes in body mass index on a gluten-free diet in coeliac disease: a nationwide study. Eur J Intern Med. 2012;23(4):384–8. doi: 10.1016/j.ejim.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Rostami K, Marsh MN, Johnson MW, Mohaghegh H, Heal C, Holmes G. et al. ROC-king onwards: intraepithelial lymphocyte counts, distribution & role in coeliac disease mucosal interpretation. Gut. 2017;66(12):2080–6. doi: 10.1136/gutjnl-2017-314297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vereczkei Z, Farkas N, Hegyi P, Imrei M, Földi M, Szakács Z. et al. It is high time for personalized dietary counseling in celiac disease: a systematic review and meta-analysis on body composition. Nutrients. 2021;13(9):2947. doi: 10.3390/nu13092947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ 3rd. Trends in the identification and clinical features of celiac disease in a North American community, 1950-2001. Clin Gastroenterol Hepatol. 2003;1(1):19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 24.Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten-free diet. Am J Gastroenterol. 2006;101(10):2356–9. doi: 10.1111/j.1572-0241.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones AL. The gluten-free diet: fad or necessity? Diabetes Spectr. 2017;30(2):118–23. doi: 10.2337/ds16-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niland B, Cash BD. Health benefits and adverse effects of a gluten-free diet in non-celiac disease patients. Gastroenterol Hepatol (N Y) 2018;14(2):82–91. [PMC free article] [PubMed] [Google Scholar]
- 27.Garnweidner-Holme L, Sende K, Hellmann M, Henriksen C, Lundin KEA, Myhrstad MCW. et al. Experiences of managing a gluten-free diet on multiple levels of society: a qualitative study. BMC Nutr. 2020;6(1):65. doi: 10.1186/s40795-020-00390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R. et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG. et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–78. doi: 10.1016/s0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J, Brar PS, Lee AR, Green PH. Body mass index in celiac disease: beneficial effect of a gluten-free diet. J Clin Gastroenterol. 2010;44(4):267–71. doi: 10.1097/MCG.0b013e3181b7ed58. [DOI] [PubMed] [Google Scholar]