Abstract
Clock gene expression in most organs of the living body exhibits a diurnal rhythm synchronized with the external 24 h light-dark (LD) cycle via circadian pacemaker suprachiasmatic nucleus (SCN). Disturbances in clock gene expression due to desynchronization of clock gene expression of the external LD cycle are risk factors for developing various diseases. Measuring the in vivo clock genes expression rhythm for a long duration under LD conditions can greatly contribute to understand the pathogenic mechanism of the disease caused by the disturbance of the biological rhythm. However, it is presently difficult to continuously measure gene expression for a long duration under LD conditions. In present study, we succeeded in measuring Period1 (Per1) gene expression under LD conditions using ultraviolet (UV) light with filter cut the visible light range. In addition, we succeeded in measuring the kinetic change of liver Per1 gene expression during the process of desynchronization of behavioral rhythm from the LD cycle by chronic administration of methamphetamine (MAP). In the future, by using this system to measure clock gene expression rhythms of brain tissues such as SCN and peripheral tissues under LD conditions, it could contribute to understand the onset mechanism of diseases induced by the desynchronization mechanism of biological rhythm to the LD cycle.
Keywords: Circadian rhythm, Period1, In vivo, Luciferin, Methamphetamine
Highlights
-
•
UV light around 370 nm wavelength has an important role in circadian system.
-
•
We succeeded in measuring period1 gene expression under LD conditions.
-
•
Visualization of effects of methamphetamine on circadian system.
1. Introduction
Organisms on the earth synchronize their biological clocks with the light-dark (LD) cycles of about 24 h in the external environment. The mammalian circadian clock, located in the SCN, controls the phase of daily rhythms and synchronizes these rhythmic responses to the local environment and coordinates peripheral tissue activity rhythm [1,2]. Molecular studies have revealed that clock genes have critical roles in expressing cellular circadian rhythms, primarily based on a cell autonomous molecular feedback loop, in which expression of the putative clock genes is suppressed by their own protein products, and entrainment results from their modification by light-induced signals. Clock genes express in most areas of the body and produce rhythms of each organ, all regulated by the SCN. Clock gene, Period 1(Per1) is reported to have an important role to photic entrainment [3].
Recent changes in work styles induce disturbances in synchronization with the 24 h LD cycle and disturbances in clock gene expression rhythms. These are reported to become risk factors for diabetes, cancer, sleep disorders, and the like [[4], [5], [6]]. Continuous analysis of gene expression under LD conditions is useful for understanding the mechanism of disease onset caused by the disturbance of biological rhythm that occurs in each tissue of the living body due to the difference from the cycle of the environmental LD cycle. However, at present, it is difficult to continuously measure gene expression for a long duration under LD conditions due to technical problems. The bioluminescent reporter enzyme firefly luciferase (luc) and substrate D-luciferin (luciferin) have been used to generate optical signals with high sensitivity in living animals and have been adapted successfully to measure the clock genes expression rhythm for long duration in constant dark conditions [7,8]. To record the luminescence signal from tissues in the whole-body, total darkness conditions are necessary due to the luminescence signal being very weak compared with the fluorescence signal [8,9]. In present studies, to measure gene expression under LD conditions, we tried to use UV light. It has been reported that UV light (λmax 365 nm) synchronizes behavioral rhythms under LD conditions in the absence of melanopsin (λmax 365 nm) in retinal ganglion cells (RGCs) [10,11] which is reported to be involved in LD entrainment [[12], [13], [14]]. UV light is short wavelength and difficult to reach to deep area of body. We therefore tried to analyze the gene expression rhythms in the deep part of the body of the moving animal under the conditions where the biological rhythm was synchronized with the UV LD conditions.
MAP has been reported to have the effect of extending the period of behavioral rhythms and desynchronizes the behavioral rhythm that is synchronized with the 24 h LD cycle in SCN independent manner [[15], [16], [17]]. The detailed mechanism of MAP for the biological clock has been studied by measuring the clock gene expression rhythm of each tissue in the living body by sampling at specific times of the day (PCR, in situ hybridization) and analyzing gene expression from a large number of animal tissues. Alternatively, gene expression in each tissue was investigated using tissue culture after administering MAP [[18], [19], [20], [21]]. With these methods there was no continuous analysis of clock gene expression of a single animal for a long duration after MAP administration.
In present studies, we succeeded in measuring gene expression in the liver under LD conditions using UV light (UV LD). We chose the liver as the main peripheral oscillator which plays a key role in maintain of metabolic homeostasis [4]. Using the UV LD conditions, we investigated whether it is possible to measure the temporal changes in the clock gene rhythm of the liver regulated by the SCN, in the process in which the behavioral rhythm due to MAP administration deviates from the LD cycle synchronization period. Period1 (Per1) expression was used as a marker of circadian activity of liver as we previously reported [8,[22], [23], [24]].
2. Materials and methods
2.1. Animals
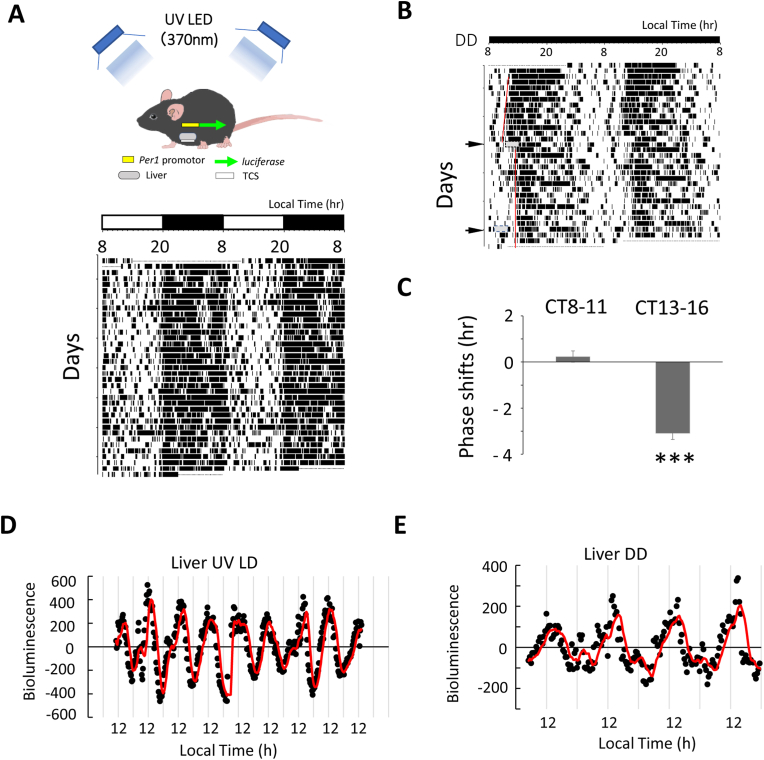
Per1-luc transgenic (Per1-luc) mice (Supplementary Information1) [25] were born and reared in our animal quarters where environmental conditions were controlled: 12 h light/12 h dark (LD) cycle with lights on 8:00–20:00 or 12:00–24:00 (Fig. 1B and C), 6:00–18:00 (Fig. 1D and E), temperature (23 ± 1 °C) and humidity (50 ± 5%). An ultraviolet-light-emitting diode (UV-LED, TUV550B-1,λMax = 370 nm) was used for recording the behavior rhythm and Per1 expression in the liver in UV LD conditions.
Fig. 1.
Locomotor activity rhythm and Per1 gene expression of Per1-luc mice in UV LD conditions.
(A). Locomotor activity rhythm under UV 12/12 light-dark conditions. Spontaneous locomotor activity records are expressed in black histograms of activity counts in 5 min bin and double-plotted so that 48 h are shown on the x-axis and consecutive days on the y-axis. (B). The effect of UV light on behavior rhythm in subjective day and night. Grey squares show the time zone when the UV light pulse was exposed (CT13-16 or CT8-11). Red lines show the regression lines fitted to the onset of activity time before and after UV light exposure. (C). The effect of UV light on locomotor activity rhythm by 3h UV light exposure in DD conditions. Each column indicates the mean ± SD (n = 3). Statistical significance was detected by Student’ t-test (***P < 0.05 vs. CT8∼11). (D, E).Per1 expression rhythm in the liver of Per1-luc mouse in UV LD conditions (D) and DD conditions (E). Luminescence (red curve) was computed by subtracting the 24 h moving average from the original data. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Three 5 mm cylindrical UV-LEDs (φ 5.1 × 3.6 mm) with a filter cutting the visible light region were set in a recording cage. The UV-LED spectrum is shown in Supplementary Figure S1 (from the data sheet of UV-LED TUV550B-1, Takatsuki Co. Ltd., Japan). Relative intensity was 0 at 345 and 390 nm, 0.2 at 360 and 381 nm, 0.4 at 363 and 378 nm, 0.6 at 365 and 376 nm, 0.8 at 367 and 374 nm. 1.0 at 370 nm. The full width half maximum (FWHM) of the UV-LED was 10 nm. The intensity of the UV-LED light was adjusted to entrain the locomotor activity rhythm of the UV LD cycle without affecting the measurement of bioluminescence of Per1 expression rhythm in freely moving mice [22,23]. Only three UV-LEDs were enough to entrain the locomotor activity rhythm to the LD cycle. Using UV recorder TR-72 Ui (recording range 0–30 mW/cm2, Measurement resolution; minimum 0.001 mW/cm2, T&D corporation, Nagano, Japan), the intensity of UV LED lights was 0.00 mW/cm2 at the floor in transparent plastic recording cage (W175 x D245 x H125 mm) which was at a distance of 250 mm from UV LD light sources. As for the UV-LED directivity, 50% power was measured at an angle of 50°.
All animal work was performed in accordance with Guidelines for the Care and Use of Laboratory Animals in International University of Health and Welfare with the permission #18014 from the Committee for Animal Experimentation.
2.2. Methamphetamine administration
Methamphetamine HCl (Dainippon Pharmaceutical Co., Osaka, Japan) was dissolved in water (0.005%) and given to the experimental male and female mice subjects for treatment. The effect of MAP on behavior activity rhythm has no significant differences between male and female mice [16].
2.3. Locomotor activity rhythm
Recording of locomotor activity rhythm was performed as our previous reports [8,[22], [23], [24]]. (Supplementary information2). For phase-shifting responses to UV light pulses, Per1-luc mice were maintained for at least 1 week in a UV LD cycle and transferred to constant dark (DD) conditions. The activity onset time was designated as Circadian time (CT) 12. Mice were exposed to 3-hr UV light pulses at CT13 in DD conditions. Actograms were prepared for each animal convering about 7 days both preceding and following UV light pulse. The phase of the locomotor activity rhythm was assessed visually by drawing a straight line through the onset of activity on successive days before the light pulse and again beginning about 2 days after UV light pulse. A phase shift was assessed as the horizontal distance between the two lines the day following UV light pulse [8,26,27].
2.4. Immunohistochemistry
Day–night variation of Per1 expression levels in the UV LD conditions were immunohistochemically identified in Per1-luc mice using anti luciferase antibody (Promega, Madison, WI). Immunostaining with free-floating section was performed as described previously [28].
2.5. Recording of Per1 gene expression in the liver of freely moving mice
Recording of Per1 gene expression in the liver of freely moving mice was performed as our previous reports [8,[22], [23], [24]](Supplementary information3).
3. Results
3.1. Locomotor activity rhythm and Per1 gene expression of the Per1-luc mice in UV LD conditions
UV-LED lights were used for the UV light entrained experiments (Fig. 1A). The intensity of the UV light was very low but enough to entrain the locomotor activity rhythm in LD conditions without affecting the bioluminescence of Per1 expression rhythm in freely moving mice. The UV-LED light was filtered with a peak at 370 nm, while light intensity was close to zero above 390 nm. In UV LD conditions, the period of locomotor activity rhythm was 24.0 ± 0.1 h (n = 7).
Melanopsin in the retinal ganglion cells (mRGC) has been reported to convey light information to the SCN [[12], [13], [14]]. The maximum absorption wavelength of melanopsin is 460–480 nm in blue light which did not overlap with the UV light used in our experiments [[12], [13], [14]]. The present results show that UV light (370 nm) (Supplementary FigureS1) has the ability to entrain behavioral activity rhythm to a LD cycle. We examined whether UV light has a different effect on behavior rhythm in subjective day and night. Fig. 1B,C shows the responses to UV light in the locomotor activity rhythms of Per1-luc mice. A 3 h UV light pulse was administered (CT13-16) to Per1-luc mice. The UV light pulse during subjective night produced the phase delay of behavior rhythm, but not subjective day (CT8-11) as previously reported [10]. These results support the theory that UV light has the ability to produce locomotor activity rhythm. Using the UV light, we succeeded in detecting Per1 expression rhythm in the liver in UV LD conditions (Fig. 1D and E). In the present study, the period of Per1 gene expression rhythm was 24.0 ± 0.5 h (n = 7 animals). In DD conditions, Per1 expression rhythm in the liver also was detected (24.2 ± 0.8 h, n = 7 animals) decreasing amplitude comparing with LD due to lack of photic signal via SCN [7,29].
3.2. Chronic application of methamphetamine in the drinking water affects the period of locomotor activity in UV LD conditions
The effects of MAP on circadian behavior were examined in UV LD conditions.
Chronic application of MAP in the drinking water was reported to increase the amount of total daily activity, the length of alpha (the duration between the onset and offset of daily activity) and the period length of the activity rhythm [15].
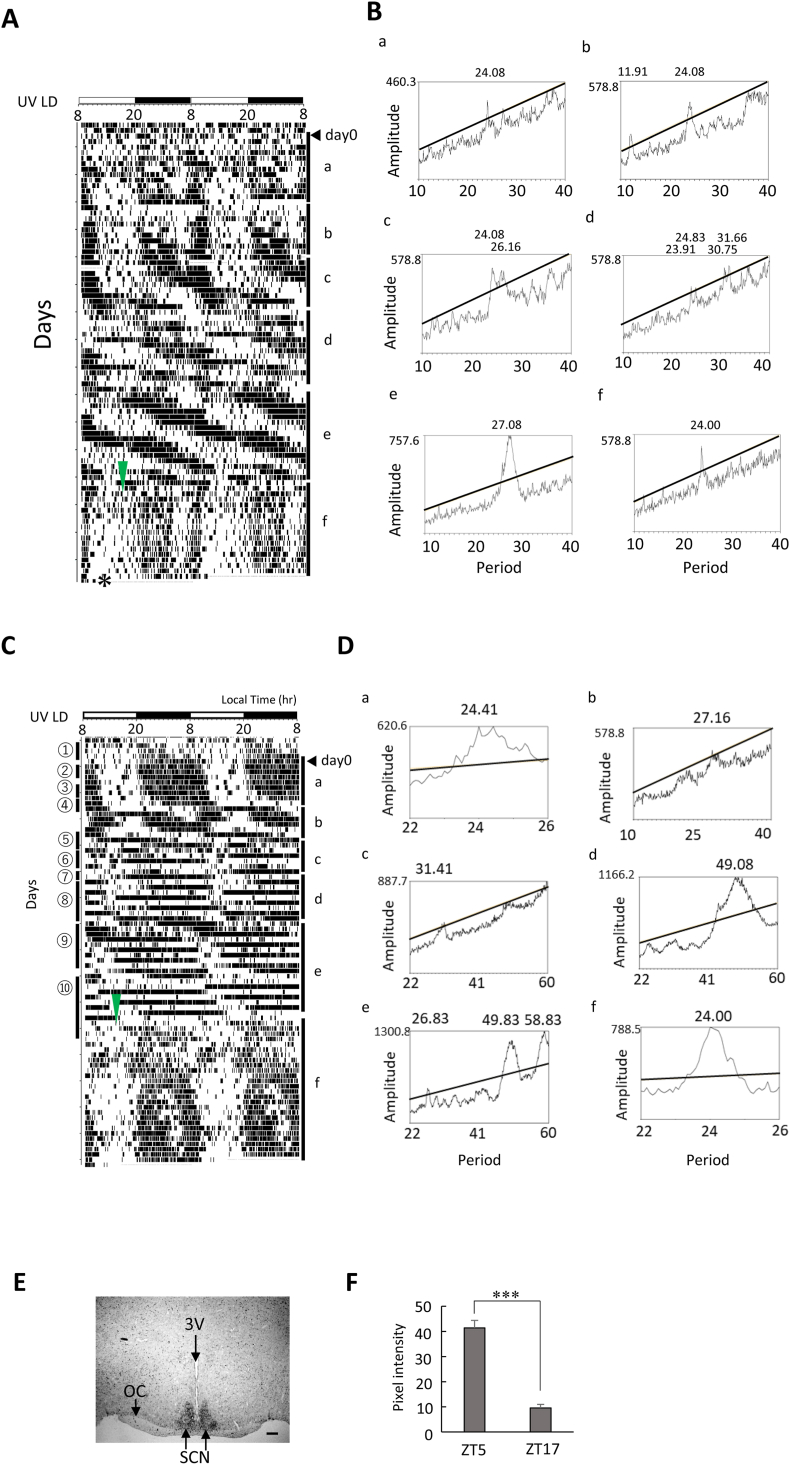
In UV LD conditions, Per1-luc mice were kept in LD and received 0.005% MAP at day 0 in Fig. 2A. Behavior activity rhythm was affected by 0.005% MAP as previously reported [16,17,[19], [20], [21]]. After a stable period of behavioral rhythm at b, the period became unstable and changed to a longer period at c and d when multiple free-running components appear. After disruption of period, a stable period of behavioral rhythm appeared again at e. MAP increased circadian period within 10.2 ± 2.7 days (n = 4) in DD conditions (data not shown) and 20.6 ± 7.8 days (n = 5) in UV LD conditions (Fig. 2A). Interestingly, after withdrawal of the MAP (pure water) at the triangle point, these effects disappeared immediately, and behavior rhythm entrained to LD cycle on next day (Fig. 2A, C). In UV LD conditions, Per1 expression was examined in the SCN (Fig. 2E and F). Per1 during the day was expressed in the whole SCN area. A day-night difference of Per1 expression was detected in the SCN (Fig. 2F).
Fig. 2.
Chronic methamphetamine administration affects the period of locomotor activity in UV LD conditions.
(A–D). Chronic MAP induced locomotor activity rhythm in UV LD conditions. Day 0 indicates the start day of MAP administration (0.005%). Each periodogram corresponds to a∼f in actograms. MAP was withdrawn at the time of triangle arrow in f (MAP-containing water bottle was replaced with the bottle containing pure water). Per1 expressions in the SCN were examined at * (ZT5) using LUC antibody in Fig. 2A. The period of locomotor activity rhythms are shown in a∼f. Per1 gene expression rhythms from ①∼⑩ in actogram in Fig. 2C are shown in Supplementary Figure S2. (E).Per1 expression in the SCN at ZT5 (* in Fig. 2A). Third ventricle (3V); optic chasm (OC); suprachiasmatic nucleus (SCN). (F). Day-night difference of Per1 expression in the SCN. Data are mean ± SDM (n = 3). Statistical significance as detected by student's t-test (***P < 0.001). The scale bar is 200 μm.
3.3. Kinetic-Per1 expression change during MAP administration in the liver of freely moving mouse in UV LD conditions
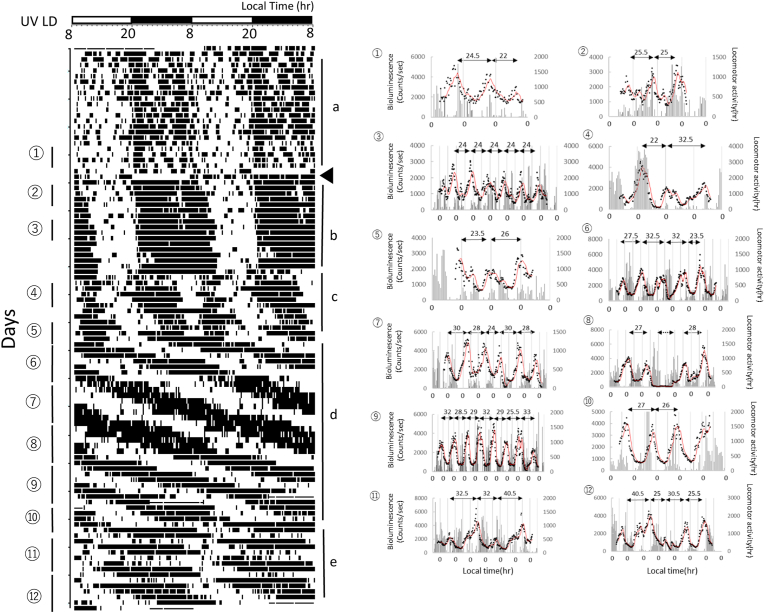
The SCN orchestrates synchrony among many peripheral oscillators and is required for circadian rhythms of locomotor activity and many physiological processes. In UV LD conditions, the SCN is thought to be entrained to the environmental LD cycle (Fig. 2E and F). In contrast, MAP lengthens behavior activity rhythm with the SCN in an independent manner [[15], [16], [17]]. MAP has no effect on neuronal activity rhythm in the SCN and Per1 gene expression [19,30]. Circadian gene expression is driven in the liver for both MAP and SCN derived signals. However, due to the lack of appropriate in vivo recording technologies, it has been difficult to study how MAP synchronizes oscillators in the liver. Recently we developed a TCS to measure Per1 expression rhythm in real time in freely moving mice [31]. Using the TCS, we examined the kineteic-Per1 expression change in the liver associated with the period change of behavioral rhythm in UV LD conditions to explore the relationship between Per1 expression rhythm and behavior rhythm during MAP administration (Figs. 2 and 3, Supplementary Figure S2 and S3).
Fig. 3.
Kinetic-Per1 expression change in the liver of a freely moving mouse in UV LD conditions.
Chronic MAP induced locomotor activity and Per1 gene expression rhythms in UV LD conditions. The black triangle indicates the start day of MAP administration (0.005%). Per1 gene expression rhythms were measured at ①∼⑫ in actogram. The two headed arrows and numbers at the top of the figure ①∼⑫ indicate the period of Per1 gene expression rhythm. The two headed dotted arrows in the figure ⑧ indicate the duration of no luciferin application. The periodograms from a∼e are shown in Supplementary Figure S3.
Before MAP administration, Per1 expression rhythm was measured at ① in Fig. 3 (Pre in Fig. 4A). Day 0 indicates the day when MAP administration was started. In the early stage of MAP administration, MAP increased the amount of total daily activity. The length of alpha Per1 expression rhythm at ②, ③ in Fig. 3 has a rhythmic expression with a period of 24.25 h while the period of behavioral activity rhythm is 24.0 h (MAPa in Fig. 4A).
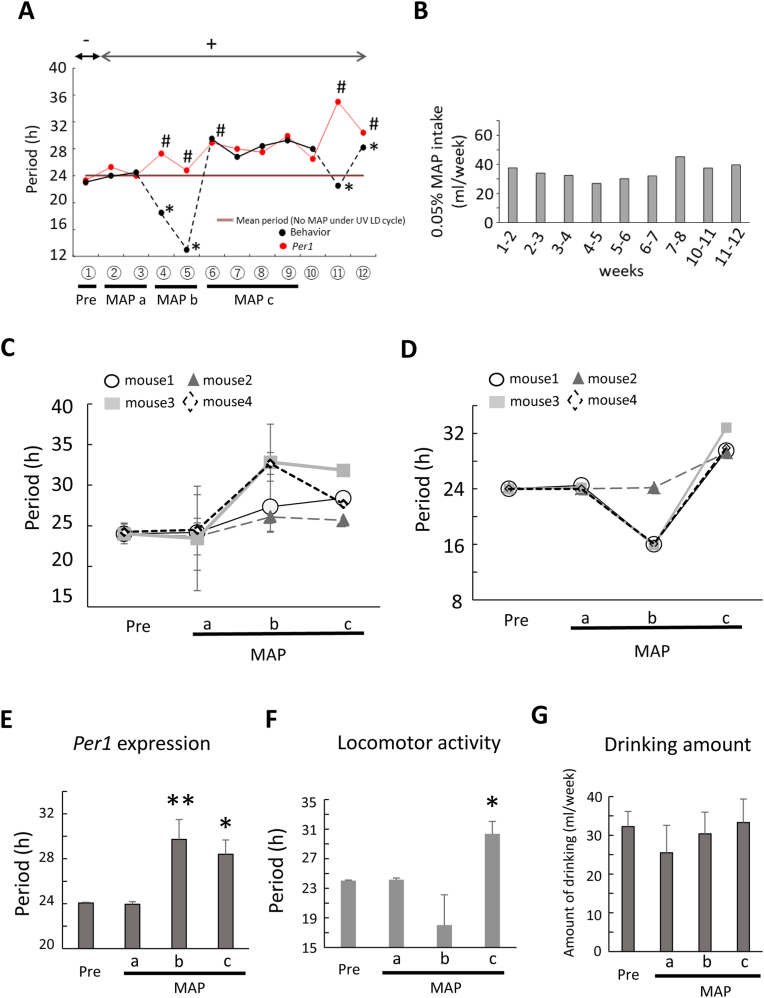
Fig. 4.
Relationship between Per1 gene expression and behavior activity under chronic methamphetamine administration.
(A). The period of Per1 gene expression in the liver and behavior activity rhythms in UV LD conditions. ①∼⑫ in the horizontal axis correspond to that of Fig. 3.
“-” indicates no MAP treatment. “+” indicates 0.005% MAP treatment. The slit peak (two peaks) of Per1 gene expression rhythm is shown with “#”. The mean periods of behavior activity and Per1 expression rhythms in UV LD cycle without MAP are shown as horizontal grey lines at 24.0 h “*” indicates the split of onset and offset of daily activity. The black dotted line suggests that the period of behavioral activity rhythms is unstable. (B). The amount of water intake under chronic MAP administration. (C). The period changes of Per1expression before and after methamphetamine treatment in individual mice. Data are mean ± SD. (D). The period changes of locomotor activity rhythm on the same mouse measured Per1 expression rhythm in Fig. 4C. (E, F). Effect of MAP on the period of Per1 expression rhythm in the liver and locomotor activity rhythm at each stage. Pre, MAP a, Map b and Map c in the horizontal axis correspond to that of Fig. 4A (n = 4 animals). Statistical significance as determined by one-way ANOVA followed by Dunnett's test (*P < 0.05 **P < 0.01 vs pre). (G). Effect of MAP on the drinking activity at each stage (n = 4 animals).
After the length of alpha was increased, the duration between the onset and offset of daily activity started to split in ④, ⑤ in Fig. 3 (MAP b in Fig. 4A). At this time, Per1 expression rhythms at ④, ⑤ in Fig. 3 were observed. The period of Per1 expression rhythms at ④ started to be longer and were then disrupted with 2 peaks at ⑤, ⑥.
The period of behavioral activity rhythm was stable with a long period at ⑥ and continued until ⑦, ⑧, ⑨ (MAP c in Fig. 4A). After ⑤, ⑥, the period of Per1 expression rhythm became longer and showed stable expression.
The period of behavioral activity rhythm changed at ⑩, ⑪ with a longer period.
With this change, the period of Per1 expression rhythm changed to be longer and then disturbed with 2 peaks. After ⑩, ⑪, the period of behavioral activity rhythm and the period of Per1 expression rhythm changed and showed a stable new rhythm.
3.4. The relationship between Per1 gene expression and behavior activity under chronic methamphetamine administration
The relationship between Per1 gene expression and behavior activity (the data of Per1-luc mouse in Fig. 3) was shown in Fig. 4A. The periods of behavior activity and Per1 expression rhythms in UV LD conditions without MAP are shown as a line at 24.0 h (see Results section 3.1). The period change of behavior activity rhythm by chronic MAP intake associated with the disturbance of Per1 gene expression rhythm (splitting of peak time at ④) following extension of period of Per1 gene expression rhythm and set in stable period of Per1 gene expression. Behavioral activity rhythm also changed the split of onset and offset of daily activity at ④, ⑤ or unstable at ⑪. After that, behavioral activity rhythm shows stable rhythm. During the long-term MAP administration, water intake had no change (Fig. 4B). Body weight also had no significant change (data not shown). The data of each animal from Pre, MAPa, MAPb, MAPc was shown in Fig. 4C and D. Pre, MAPa, MAPb and MAPc indicate that no treatment of MAP, early stage (entrained behavior activity rhythm to UV LD cycle), desynchronized stage (behavioral rhythm desynchronizes with UV LD cycle) and stable stage with extended period (behavioral rhythm with extended period due to desynchronization with UV LD cycle) after MAP administration respectively. We summarized the data from Pre, MAPa, MAPb, MAPc in each stage of Per1 expression rhythms and behavior activity in UV LD conditions in Fig. 4E and F and compared Pre with MAPa, MAPb, MAPc to clarify the effect of MAP on the period of Per1 expression rhythms and behavior activity rhythm. The period of Per1 expression in the stage of MAPb was significantly increased, but not that of locomotor activity. At the next stage of MAPc the period of these is significantly extended (Fig. 4E and F) {one-way ANOVA followed by Dunnett's test (*P < 0.05 **P < 0.01 vs pre)}. In the process of these step, water intake had no change (Fig. 4G).
4. Discussion
In the present study, we showed that UV light around 370 nm wavelength has an important role in the entrainment of environmental light-dark conditions. UV-LED with a filter cutting the visible light region is thought to not activate the melanopsin sensitive region (480 nm) which is reported to transmit light information to the SCN [14]. Using UV light, we succeeded in recording the luminescence signal showing Per1 expression from the liver in freely moving mice in LD entrained conditions. At present, to record the luminescence signal from tissues in the whole-body, total darkness conditions are necessary due to the luminescence signal being very weak compared with the fluorescence signal [8,9]. To detect the weak signal, a photon multiplier tube (PMT) or ultra-high sensitive charge-coupled device (CCD) camera are normally used, but are not suitable for measurement in normal white fluorescent light in LD conditions. Using a shorter wavelength of 370 nm UV light, our TCS sensor could detect Per1 expression in the liver without being affected by external UV light. Per1 in the liver showed circadian expression.
Next, we tried to measure the kinetic changes in the Per1 expression rhythm in the liver under conditions where the behavioral rhythm was asynchronous to the 24 h LD cycle. MAP treatment desynchronizes the behavior rhythms from LD cycle and tends to extend the period of circadian rhythms of body temperature, drinking, feeding, total activity, corticosterone independent of the SCN [15,17,32,33]. In this condition, the SCN was thought to be entrained to a LD cycle with a period of 24 h [19,30]. The amplitude of Per1 expression rhythm in the liver of the DD condition decreased comparing with that of LD condition. To explain the result, there is one possibility that the LD entrainment signal from SCN could amplify the Per1 expression in the liver. In present study, MAP had the effect of extending the period of locomotor activity rhythm over 26 h after administration on 20.6 days {vs. 10.2 days in DD conditions} in UV LD conditions. These result show LD entrainment signal from SCN conflict the signal from MAP. The Per1 expression in the liver can be adjusted by modifying the response properties of the own oscillator to an invariant signal from the SCN or from the environment [7,29]. After withdrawal, behavior rhythm immediately entrained to LD cycle on next day and Per1 expression in the SCN had day-night difference. These suggest that SCN might be entrained to UV LD cycle during MAP treatment.
For the persistence of the MAP effects on behavior rhythm, a previous study of intact SCN mice and SCN-lesioned mice showed that the effect of MAP disappeared within several cycles after withdrawal under DD conditions [16]. These results showed MAP-induced oscillator (MAO) system has an important role on MAP produced rhythm. However, our results cannot exclude an hourglass mechanism [33]. After withdrawal, behavior rhythm immediately entrained to LD cycle on next day and displayed no relative coordination (Fig. 2A, C). In present studies, we showed the kinetic change of liver Per1 gene expression during the process of desynchronization of behavioral rhythm from the LD cycle by chronic administration of MAP. During chronic MAP administration, the biological rhythm (pre or MAPa) has changed into stable longer-period rhythm (MAPc) through the process by first extending the period of Per1 expression rhythm (after splitting of peak time as like ④ in Fig. 4A)(MAPb) and then causing the behavioral rhythm to desynchronize (short period due to splitting of behavioral rhythm)(MAPb) to LD cycle. Finally, both period of Per1 expression and behavior activity rhythm were extended (MAPc). Considering the assumption that Per1 expression in the liver of the peripheral tissue is regulated by the SCN and MAO, firstly, after MAP administration, Per1 expression and behavioral rhythms are mainly regulated by the SCN. Second, before MAP produce a stable longer period, the length of alpha of the behavior activity rhythm is increased. Next, the peak of Per1 expression rhythm is split and then the period of Per1expression rhythm become longer. At that time, the alpha of behavioral activity rhythm is split and the period of locomotor activity rhythm changed to be short. Third, Per1 expression and behavioral rhythms become stable for longer periods (Fig. 4A, C,D,E,F).
In conclusion, we have shown that the UV-sensitive region around 370 nm has an important role in the entrainment of environmental light-dark conditions. This result suggests that seasonal changes in UV light on Earth might affect the function of circadian clock. In addition, using 370 nm UV LD, we succeed in recording the Per1 gene expression rhythm kinetics in the liver under LD cycle with MAP treatment.
In the future, using our measurement system to clarify how the gene expression rhythm is disturbed will be able to greatly contribute to understand the onset mechanism of sleep disorders, diabetes, cancer induced by internal desynchronization such as disturbance of biological rhythm [4].
Acknowledgments
This research was partially supported by JSPS KAKENHI Grant Number 17H04022, 20K06745, KAKENHI Grant Number 18H04724“Resonance Bio”, by a research fund from Tochigi industrial promotion center “The Grant-in-Aid for World-Class Technological Research and Development”. We are grateful to Dr K. Honma and Dr S. Honma for providing us with the Per1-luc transgenic mice.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bbrep.2022.101344.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Young M.W., Kay S.A. Time zones: a comparative genetics of circadian clocks. Nat. Rev. Genet. 2001;2(9):702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 2.Kriegsfeld L.J., LeSauter J., Hamada T., et al. In: Hormones, Brain and Behavior. Pfaff D., Etgen A., editors. Academic Press; NY: 2002. Circadian rhythms in the endocrine system; pp. 33–91. [Google Scholar]
- 3.Shigeyoshi Y., Taguchi K., Yamamoto S., et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91(7):1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 4.Green C.B., Takahashi J.S., Bass J. The meter of metabolism. Cell. 2008;134(5):728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahoney M.M. Shift work, jet lag, and female reproduction. J. Endocrinol. 2010 doi: 10.1155/2010/813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merchaeva B., Ramsey K.M., Buhr E.D., et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saini C., Liani A., Curie T., et al. Real-time recording of circadian liver gene expression in freely moving mice reveals the phase-setting behavior of hepatocyte clocks. Genes Dev. 2013;27(13):1526–1536. doi: 10.1101/gad.221374.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamada T., Sutherland K., Ishikawa M., et al. In vivo imaging of clock gene expression in multiple tissues of freely moving mice. Nat. Commun. 2016;7 doi: 10.1038/ncomms11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamada K., Ishii Y., Yoshida Y., et al. The analysis of Period1 gene expression in vivo and in vitro using a micro PMT system. Biochem. Biophys. Res. Commun. 2021;577:64–70. doi: 10.1016/j.bbrc.2021.08.084. [DOI] [PubMed] [Google Scholar]
- 10.van Oosterhout F., Fisher S.P., van Diepen H.C., et al. Ultraviolet light provides a major input to non-image-forming light detection in mice. Curr. Biol. 2012;22(15):1397–1402. doi: 10.1016/j.cub.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Diepen H.C., Schoonderwoerd R.A., Ramkisoensing A., et al. Distinct contribution of cone photoreceptor subtypes to the mammalian biological clock. Proc. Natl. Acad. Sci. U. S. A. 2021;118(22) doi: 10.1073/pnas.2024500118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berson D.M., Dunn D.M., Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 13.Hattar S., Liao H.W., Takao M., et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster R.G., Hughes S., Peirson S.N. Circadian photoentrainment in mice and humans. Biology. 2020;9(7):180. doi: 10.3390/biology9070180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honma K., Honma S., Hiroshige T. Disorganization of the rat activity rhythm by chronic treatment with methamphetamine. Physiol. Behav. 1986;38(5):687–695. doi: 10.1016/0031-9384(86)90265-9. [DOI] [PubMed] [Google Scholar]
- 16.Tataroglu O., Davidson A.J., Benvenuto L.J., et al. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. J. Biol. Rhythm. 2006;21(3):185–194. doi: 10.1177/0748730406287529. [DOI] [PubMed] [Google Scholar]
- 17.Honma K., Honma S. The SCN-independent clocks, methamphetamine and food restriction. Eur. J. Neurosci. 2009;30(9):1707–1717. doi: 10.1111/j.1460-9568.2009.06976.x. [DOI] [PubMed] [Google Scholar]
- 18.Iijima M., Nikaido T., Akiyama M., et al. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur. J. Neurosci. 2002;6(5):921–929. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- 19.Masubuchi S., Honma S., Abe H., K, et al. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur. J. Neurosci. 2000;12(12):4206–4214. [PubMed] [Google Scholar]
- 20.Mohawk J.A., Pezuk P., Menaker M. Methamphetamine and dopamine receptor D1 regulate entrainment of murine circadian oscillators. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0062463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natsubori A., Honma K., Honma S. Differential responses of circadian Per2 rhythms in cultured slices of discrete brain areas from rats showing internal desynchronisation by methamphetamine. Eur. J. Neurosci. 2013;38(4):2566–2571. doi: 10.1111/ejn.12265. [DOI] [PubMed] [Google Scholar]
- 22.Hamada K., Oota A., Ito R., et al. Double recording system of Period1 gene expression rhythm in the olfactory bulb and liver in freely moving mouse. Biochem. Biophys. Res. Commun. 2020;529(4):898–903. doi: 10.1016/j.bbrc.2020.05.224. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima K., Hamada K., Ito R., et al. Stability of d-luciferin for bioluminescence to detect gene expression in freely moving mice for long durations. Luminescence. 2021;36(1):94–98. doi: 10.1002/bio.3917. [DOI] [PubMed] [Google Scholar]
- 24.Kanou H., Nagasawa K., Ishii Y., et al. Period1 gene expression in the olfactory bulb and liver of freely moving streptozotocin-treated diabetic mouse. Biochem. Biophys. Res. Commun. 2021;560:14–20. doi: 10.1016/j.bbrc.2021.04.049. [DOI] [PubMed] [Google Scholar]
- 25.Hida A., Koike N., Hirose M., et al. The human and mouse Period1 genes: five well-conserved E-boxes additively contribute to the enhancement of mPer1 transcription. Genomics. 2000;65(3):224–233. doi: 10.1006/geno.2000.6166. [DOI] [PubMed] [Google Scholar]
- 26.Hamada T., Antle M.C., Silver R. The role of Period1 in non-photic resetting of the hamster circadian pacemaker in the suprachiasmatic nucleus. Neurosci. Lett. 2004;362(2):87–90. doi: 10.1016/j.neulet.2004.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamada T., Niki T., Ishida N. Role of p53 in the entrainment of mammalian circadian behavior rhythms. Gene Cell. 2014;19(5):441–448. doi: 10.1111/gtc.12144. [DOI] [PubMed] [Google Scholar]
- 28.Hamada T., LeSauter J., Venuti J.M., et al. Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J. Neurosci. 2001;21(19):7742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamazaki S., Numano R., Abe M., et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288(5466):682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 30.Moriya T., Fukushima T., Shimazoe T., et al. Chronic administration of methamphetamine does not affect the suprachiasmatic nucleus-operated circadian pacemaker in rats. Neurosci. Lett. 1996;208(2):129–132. doi: 10.1016/0304-3940(96)12565-9. [DOI] [PubMed] [Google Scholar]
- 31.Ito R., Hamada K., Kasahara S., et al. Mouse period1 gene expression recording from olfactory bulb under free moving conditions with a portable optic fibre device. Luminescence. 2020;35(8):1248–1253. doi: 10.1002/bio.3884. [DOI] [PubMed] [Google Scholar]
- 32.Morimasa T., Wirz-Justice A., Kraeuchi K., et al. Chronic methamphetamine and its withdrawal modify behavioral and neuroendocrine circadian rhythms. Physiol. Behav. 1987;39(6):699–705. doi: 10.1016/0031-9384(87)90253-8. [DOI] [PubMed] [Google Scholar]
- 33.Ruis J.F., Buys J.P., Cambras T., et al. Effects of T cycles of light/darkness and periodic forced activity on methamphetamine-induced rhythms in intact and SCN-lesioned rats: explanation by an hourglass-clock model. Physiol. Behav. 1990;47(5):917–929. doi: 10.1016/0031-9384(90)90020-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.