INTRODUCTION
Diabetes has been an independent risk factor for dementia and both conditions share pathophysiological mechanisms including impaired brain insulin signaling, inflammation, increased oxidative stress, and vascular damage.1 Evidence from both mechanistic and population studies has suggested that some glucose-lowering drugs (GLDs) might be beneficial in preventing or treating dementia.2, 3 However, existing evidence is primarily from either observational data that are susceptible to confounding and other biases, or disparate clinical trials that have inadequate power to examine such effects. Thus, we performed a meta-analysis of randomized outcome trials of dipeptidyl peptidase-4 (DPP4) inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1RAs), and sodium-glucose co-transporter-2 (SGLT2) inhibitors to evaluate their effects on dementia risk among individuals with and without type 2 diabetes (T2D).
METHODS
As described in our previous study,4 we systematically searched PubMed, Embase, and CENTRAL from inception to March 28, 2021. We included randomized placebo-controlled cardiovascular and renal outcome trials that reported all-cause dementia or vascular dementia (see definitions in Table S1) associated with DPP-4 inhibitors, GLP-1RAs, and SGLT2 inhibitors among adults with and without T2D. Two reviewers independently selected the studies, extracted the data, and assessed the risk of bias according to the Cochrane Risk of Bias Tool. The number of incident dementia cases was extracted from trial results published on clinicaltrials.gov. We calculated a pooled odds ratio (OR) and 95% confidence interval (CI) for dementia risk using Peto’s method.5 We also assessed the heterogeneity between trials using the I2 statistic 6 and evaluated the publication bias using a funnel plot, Begg’s test, and Egger’s test.7 Sensitivity analysis using a 0.5 continuity correction for each cell was conducted to test the robustness of the results. The statistical analyses were performed using Stata (version 16; Stata Corp., College Station, TX).
RESULTS
Of 9,648 citations retrieved from electronic databases (Figure S1), we included 21 randomized placebo-controlled trials that reported 108 all-cause dementia cases (including 22 vascular dementia cases) among 167,511 individuals during a median follow-up of 2.2 years (Table S2). Individuals with T2D account for 96.4% of clinical trial participants. Of the 21 trials included, five trials reported the dementia outcomes associated with DPP-4 inhibitors; 8 trials associated with GLP-1RAs; and 8 trials associated with SGLT2 inhibitors. Eighteen trials included participants with T2D while 3 trials included participants with chronic kidney disease or heart failure with reduced ejection fraction, regardless of the presence or absence of T2D. The risk of bias was judged as unclear because dementia was not the primary outcome.
Neither DPP-4 inhibitors, GLP-1RAs, nor SGLT2 inhibitors were significantly associated with a decrease in risk of all-cause dementia incidence as compared with placebo (Figure 1). SGLT2 inhibitors were significantly associated with a decreased risk for vascular dementia (OR, 0.11; 95% CI, 0.02 -0.66), compared with placebo. DPP-4 inhibitors (OR, 0.67; 95%CI, 0.12 – 3.85) and GLP-1RAs (OR, 0.37; 95%CI, 0.12-1.14) were associated with a decreased risk of vascular dementia, but no significant differences were observed (Figure 2). Our sensitivity analyses indicated the robustness of the results (Figure S2). There was no evidence of statistical heterogeneity (I2 <50%) and publication bias (Table S3 and Figure S3).
Figure 1.
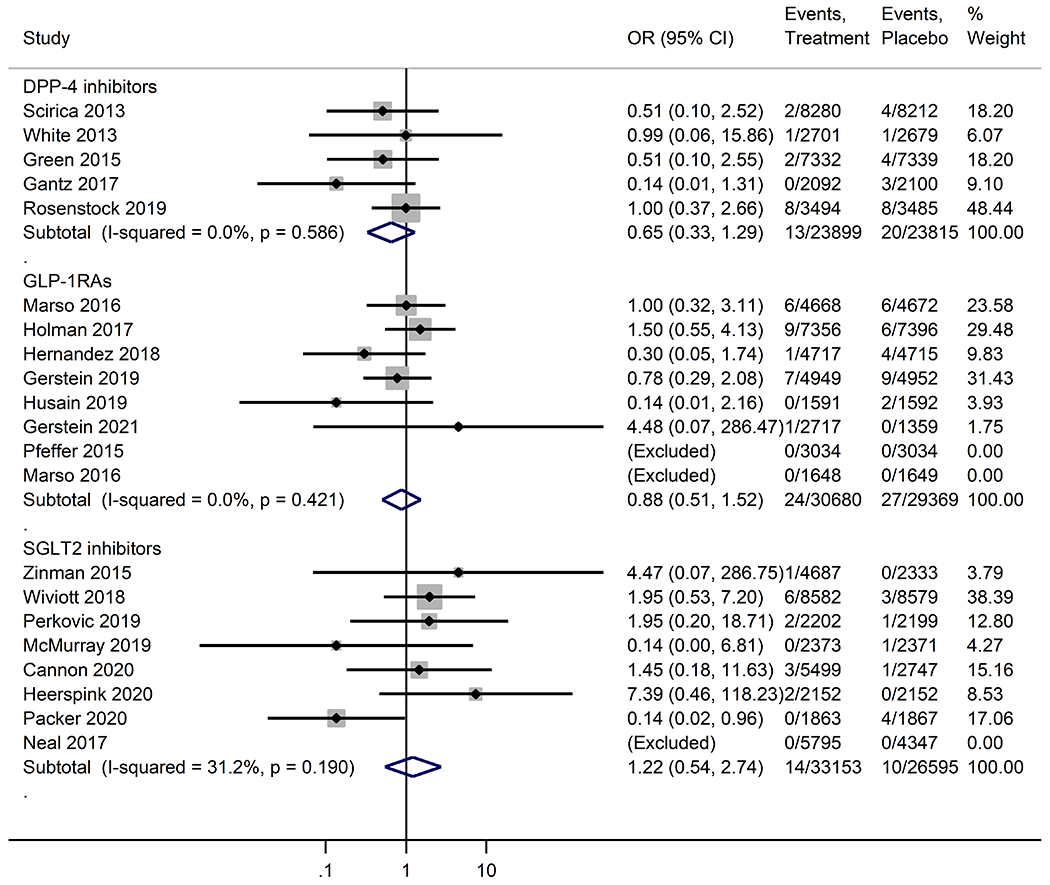
Meta-analysis of the effects of novel glucose-lowering drugs on the risk of all-cause dementia in participants with or without type 2 diabetes. OR, odds ratio; CI, confidence interval; DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors; GLP-1RAs, glucagon-like peptide-1 receptor agonists; SGLT2 inhibitors, sodium-glucose co-transporter-2 inhibitors.
Figure 2.
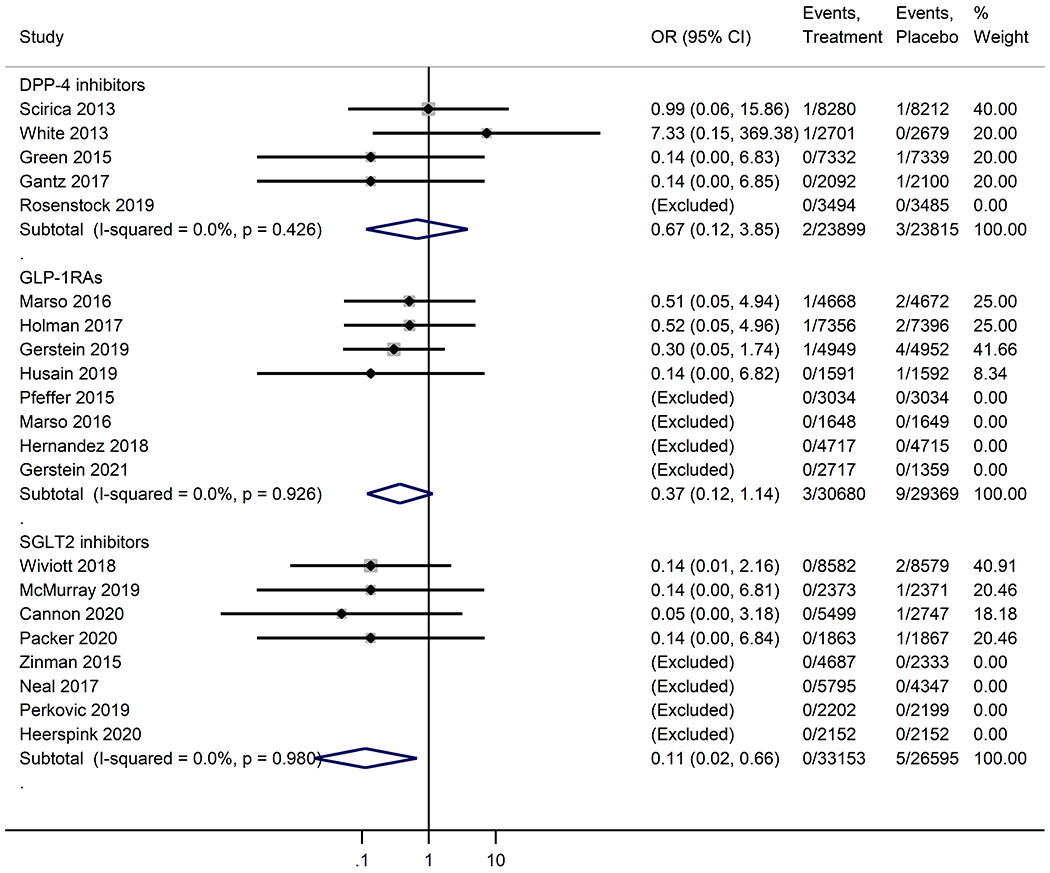
Meta-analysis of the effects of novel glucose-lowering drugs on the risk of vascular dementia in participants with or without type 2 diabetes. OR, odds ratio; CI, confidence interval; DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors; GLP-1RAs, glucagon-like peptide-1 receptor agonists; SGLT2 inhibitors, sodium-glucose co-transporter-2 inhibitors.
DISCUSSION
Previous studies have indicated that newer GLDs may decrease the risk of dementia.2, 3 Mechanistic and population studies showed that SGLT2 inhibitors could reduce risk factors (e.g., high cholesterol, high blood pressure, and obesity) directly related to vascular dementia,8, 9 improve insulin sensitivity, and involve the mammalian target of rapamycin (mTOR) signaling in the brain.2 DPP-4 inhibitors and GLP-1RAs may reduce endothelial dysfunction, cerebral oxidative stress, and ischemic brain damage and are shown to reduce amyloid-β deposition and tau phosphorylation.10 In this meta-analysis, we observed a reduction of vascular dementia risk associated with newer GLDs, especially for SGLT2 inhibitors. There was no association between newer GLDs and risk of all-cause dementia.
We acknowledged several limitations in this meta-analysis. First, because dementia was not the pre-specified outcome in these trials, there is likely an underestimation of the incidence of dementia. Second, given the relatively short follow-up period, there is likely insufficient time to observe the occurrence of dementia. Also, the long-term effect of newer GLDs on risk of dementia remains unknown. Third, our analyses are likely underpowered because of the low numbers of dementia cases in the clinical trials, which leads to wide CIs. Fourth, we were not able to obtain information on the baseline cognitive functions of study participants in these trials. Therefore, there is a possibility we included prevalent dementia cases that had a delayed diagnosis. Finally, the extremely low number of dementia cases precluded us from assessing the effect variation across individual drugs within one class of GLDs.
Overall, our findings suggest that newer GLDs do not appear to pose harm regarding dementia risk in individuals with T2D. Moreover, newer GLDs, particularly SGLT2 inhibitors, might be promising strategies for the prevention of vascular dementia in those with T2D. Nevertheless, future randomized controlled trials and real-world studies are warranted to evaluate the effects of newer GLDs on cognitive function and the risk of different types of dementia in individuals with T2D and whether the effect can be expanded to non-diabetes population.
Supplementary Material
Table S1. All-cause dementia was defined by a narrow Standardized Medical Dictionary for Regulatory Activities (MedDRA) Query (SMQ) of “dementia” including 21 preferred terms.
Table S2. Basic characteristics of 21 cardiovascular and renal outcome trials.
Table S3. Begg’s test and Egger’s test for the effects of DPP-4 inhibitors, GLP-1RAs, and SGLT2 inhibitors on risk of dementia.
Figure S1. The flowchart of the study selection. CENTRAL, Cochrane Central Register of Controlled Trials; DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors; GLP-1RAs, glucagon-like peptide-1 receptor agonists; SGLT2 inhibitors, sodium-glucose co-transporter-2 inhibitors.
Figure S2. Sensitivity analysis of the effects of novel glucose-lowering drugs on the risk of all-cause dementia (A) and vascular dementia (B) in participants with or without type 2 diabetes. OR, odds ratio; CI, confidence interval; DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors; GLP-1RAs, glucagon-like peptide-1 receptor agonists; SGLT2 inhibitors, sodium-glucose co-transporter-2 inhibitors.
Figure S3. The funnel plot for the effects of DPP-4 inhibitors, GLP-1RAs, and SGLT2 inhibitors on risk of dementia. DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors; GLP-1RAs, glucagon-like peptide-1 receptor agonists; SGLT2 inhibitors, sodium-glucose co-transporter-2 inhibitors.
ACKNOWLEDGMENT
Dr. Bian is partially supported by NIH Awards # R01AG076234, R21AG068717, and R56AG069880.
SPONSOR’S ROLE
No sponsorship was received for this study.
This paper was submitted to the 82nd Scientific Sessions | American Diabetes Association (2022)
No specific funding was received for this work.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vazquez A, Avila-Funes JA, Aguilar-Salinas CA. Pathophysiological Mechanisms Linking Type 2 Diabetes and Dementia: Review of Evidence from Clinical, Translational and Epidemiological Research. Curr Diabetes Rev. 2019;15(6): 456–470. [DOI] [PubMed] [Google Scholar]
- 2.Zhou JB, Tang X, Han M, Yang J, Simo R. Impact of antidiabetic agents on dementia risk: A Bayesian network meta-analysis. Metabolism. 2020;109154265. [DOI] [PubMed] [Google Scholar]
- 3.Wium-Andersen IK, Osler M, Jorgensen MB, Rungby J, Wium-Andersen MK. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur J Endocrinol. 2019;181(5): 499–507. [DOI] [PubMed] [Google Scholar]
- 4.Tang H, Kimmel SE, Hernandez I, et al. Are novel glucose-lowering agents’ cardiorenal benefits generalizable to individuals of Black race? A meta-trial sequential analysis to address disparities in cardiovascular and renal outcome trials enrolment. Diabetes Obes Metab. 2022;24(1): 154–159. [DOI] [PubMed] [Google Scholar]
- 5.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26(1): 53–77. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L, Chu H, Murad MH, et al. Empirical Comparison of Publication Bias Tests in Meta-Analysis. J Gen Intern Med. 2018;33(8): 1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(4): 262–274. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 2021;384(2): 129–139. [DOI] [PubMed] [Google Scholar]
- 10.Mousa SA, Ayoub BM. Repositioning of dipeptidyl peptidase-4 inhibitors and glucagon like peptide-1 agonists as potential neuroprotective agents. Neural Regen Res. 2019;14(5): 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. All-cause dementia was defined by a narrow Standardized Medical Dictionary for Regulatory Activities (MedDRA) Query (SMQ) of “dementia” including 21 preferred terms.
Table S2. Basic characteristics of 21 cardiovascular and renal outcome trials.
Table S3. Begg’s test and Egger’s test for the effects of DPP-4 inhibitors, GLP-1RAs, and SGLT2 inhibitors on risk of dementia.
Figure S1. The flowchart of the study selection. CENTRAL, Cochrane Central Register of Controlled Trials; DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors; GLP-1RAs, glucagon-like peptide-1 receptor agonists; SGLT2 inhibitors, sodium-glucose co-transporter-2 inhibitors.
Figure S2. Sensitivity analysis of the effects of novel glucose-lowering drugs on the risk of all-cause dementia (A) and vascular dementia (B) in participants with or without type 2 diabetes. OR, odds ratio; CI, confidence interval; DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors; GLP-1RAs, glucagon-like peptide-1 receptor agonists; SGLT2 inhibitors, sodium-glucose co-transporter-2 inhibitors.
Figure S3. The funnel plot for the effects of DPP-4 inhibitors, GLP-1RAs, and SGLT2 inhibitors on risk of dementia. DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors; GLP-1RAs, glucagon-like peptide-1 receptor agonists; SGLT2 inhibitors, sodium-glucose co-transporter-2 inhibitors.


