Abstract
We determined incidence of post-traumatic epilepsy (PTE) after severe traumatic brain injury (TBI). Of 392 patients surviving to discharge, cumulative incidence of PTE was 25% at five years and 32% at fifteen years, an increase compared to historical reports. Among patients with one late seizure (>7 days post-trauma), risk of seizure recurrence was 62% after one year and 82% at ten years. Competing hazards regression identified age, decompressive hemicraniectomy, and intracranial infection as independent predictors of PTE. Patients with severe TBI and a single late post-traumatic seizure will likely require long-term antiseizure medicines.
Keywords: brain trauma, post-traumatic epilepsy, epilepsy, outcome research, seizures
Introduction
Epilepsy affects nearly 70 million people worldwide,1 and reduces quality of life due to cognitive, behavioral, and psychosocial effects.2 In the United States, care for a single patient with epilepsy costs approximately $50,000 annually.3,4
Traumatic brain injury (TBI) is an important epilepsy risk factor. Post-traumatic epilepsy (PTE) accounts for 5% of all cases of epilepsy and 20% of all symptomatic epilepsy4. Risk for PTE is highest after severe TBI, defined as a post-resuscitation Glasgow Coma Scale (GCS) score of <=8,4 developing in 10-20% of patients who survive their initial injury.5-7 PTE may develop years after initial injury.5
The incidence and risk factors of PTE have not been well characterized in modern cohorts, and definitions have varied.8-11 According to the International League Against Epilepsy (ILAE), epilepsy is defined as two seizures more than 24 hours apart or one seizure with a risk of seizure recurrence of >60% after 10 years.12 Under this operational definition, a single non-acute (i.e., >7d from initial injury) post-traumatic seizure (PTS) after severe TBI would qualify as epilepsy.13 We reviewed a large modern cohort of severe TBI patients with extended outpatient follow-up to determine incidence and risk factors of PTE.
Subjects/Materials and Methods
Study Cohort
The University of Pittsburgh Human Research Protection Office approved this study.
We performed a retrospective analysis of a prospective database including consecutive patients with severe TBI treated at a single Level 1 trauma center from 2002 through 2018.14 We excluded patients who died during their index hospitalization and those with a past medical history of seizures, regardless of the etiology. Patients were followed at outpatient clinic appointments at 3-, 6-, 12-, and 24-months post-injury and those with persistent neurological problems had continued follow up. We extracted demographic, clinical, and imaging variables from the database.
We retrospectively identified occurrence and timing of first and second PTS. Table 1.A lists operational definitions for early and late PTS, and PTS recurrence. Since many PTS are expected to occur after the index hospitalization, we anticipated most diagnoses to be made clinically rather than electrographically.
Table 1.A.
Definitions of terms used in the manuscript
| Term | Abbreviation | Definition |
|---|---|---|
| Post-traumatic seizure | PTS | A clinical event deemed to be a seizure by the treating healthcare team or an electrographic seizure on electroencephalography (EEG) |
| Early PTS | PTS occurring within 7 days from the date of trauma | |
| Late PTS | Any PTS after 7 days from the date of trauma | |
| Post-traumatic epilepsy | PTE | A single late post-traumatic seizure |
| PTS recurrence | A second late PTS among patients who have already had a late PTS |
Patients typically received 7 days of a prophylactic antiseizure medicines (ASM), usually phenytoin. Continuous video-EEG (cEEG) monitoring was utilized for 3-5 days post-injury and extended at discretion of the treating clinician. A board-certified epileptologist interpreted all cEEG and recorded dates and times of PTS in the electronic health record (EHR). In cases where a seizure was reported in the distant past, we estimated the date of seizure as an average of the period in question (i.e., a seizure occurred “2-3 years ago” would be 2.5 years prior to date of evaluation). After the first late PTS, we recorded new ASM prescriptions and duration of use, excluding subtherapeutic epilepsy dosages for other indications.
To determine last date of follow up and date of death, we searched the EHR of our system and health systems of nearby states. The date of last follow up was the last visit to a healthcare provider, regardless of specialty, that included a medication list. We determined date of death from the EHR. If a patient had ASMs listed in the EHR but no seizure reported, we contacted the patient to clarify medication and seizure history.
Statistical Analysis
We summarized baseline clinical characteristics using descriptive statistics. We reported the number of patients with early and late PTS. To identify characteristics associated with late PTS, we used 2-sided t-tests for normally distributed continuous variables and a chi-squared or Fisher’s exact test for categorical variables. We described the cumulative incidence of late PTS and recurrent PTS. We compared cumulative incidence of late PTS between patients with and without early PTS.15 We then used adjusted Cox regression to identify risk factors for the first late PTS using backwards selection. As a sensitivity analysis, we developed a competing risks regression (CRR) model with the same model inputs using the competing hazard of death. To identify risk factors for recurrent PTS, we used a multivariable Cox regression model, treating the first seizure as a time-varying covariate. We did not use CRR because estimates of subdistribution hazards from time-varying covariates are unreliable.16 We tested our model assumptions inspecting Schoenfeld and Martingale residuals and the proportional hazards assumption. For all analysis, we used R (Vienna, Austria) and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist.17
Results
Overall, 598 patients presented with severe TBI, of whom 192 died before discharge, 12 had pre-existing epilepsy, and 2 had a history of alcohol withdrawal seizures (Figure 1). Among 392 included patients, 18 had early PTS; 98 had late PTS (72/98 had recurrent PTS, 26/98 had a single late PTS); and 55 died during the study period. Overall, median follow up was 3.5 years (interquartile range [IQR]: 0.8 – 8.1 years), during which patients had a median of 11 follow-up encounters (IQR: 4 – 23), excluding the initial inpatient rehabilitation stay.
Figure 1.
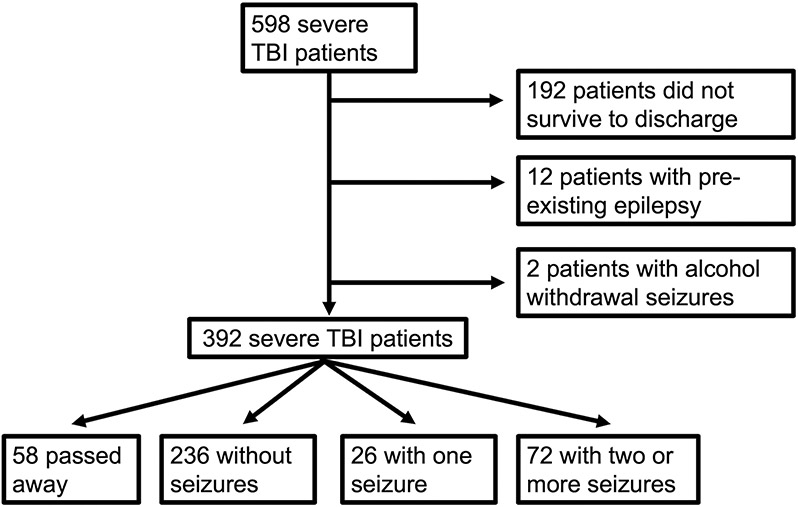
Consort Diagram. Outline of patient accrual and exclusions.
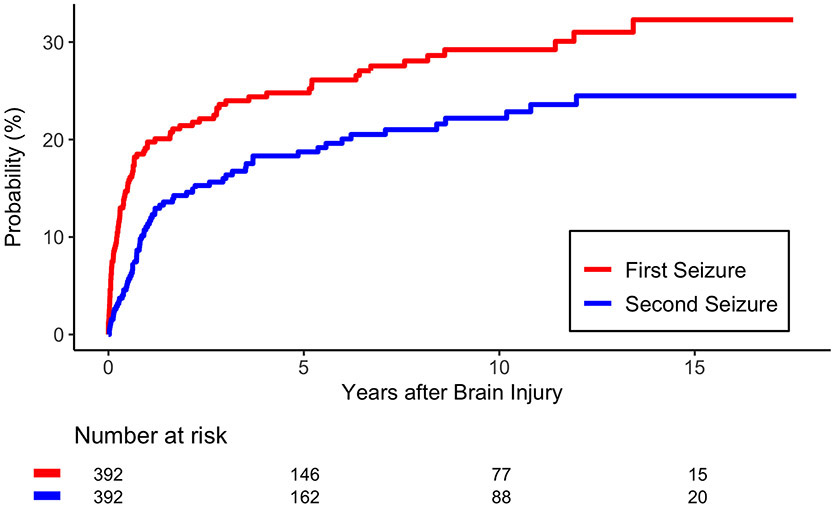
The rate of early PTS was 5% (n=18). Cumulative incidence amongst TBI survivors for late PTS was 25% (95% Confidence Interval [CI]: 22–27%) at five years and 32% (95% CI: 29–35%) at fifteen years (Figure 2). Risk of seizure recurrence was 61% (95% CI: 57–66%) at two years and 82% (95% CI: 77-87) at ten years. Among patients with early PTS, the risk of a late PTS was 44% (95% CI: 32-57%) at 2 years, which was higher than the rate among patients without early PTS (p=0.01).
Figure 2.A.
Cumulative Incidence of the first PTS and second PTS. Time zero is the date of trauma.
All but seven patients were started on ASMs after their first late PTS. These seven patients, all of whom had PTS recurrence, were not started on an ASMs because they did not seek medical attention until after repeat PTS (4); had seizures in the setting of cocaine use (1) or shunt malfunction (1); and one had a questionable PTS and was started on ASMs after a repeat PTS. Nine patients were started on multiple ASMs due to pharmacoresistance. Of patients with recurrent seizures, 25 were off ASMs of the time of PTS recurrence. These patients were not on ASMs because they were never started (7), ran out of ASMs or self-discontinued (6), or were weaned by their healthcare provider (12). Overall, 23/98 patients with PTS were on multiple ASMs at the time of last follow up.
Table 1.B and 1.C summarize clinical characteristics and unadjusted associations with the first late PTS and PTS recurrence, respectively. Our multivariate models found age, DHC, and CNS infection to increase the risk for PTS (Table 1.D). Shunt dependency and female gender decreased the risk for PTS recurrence.
Table 1.B.
Predictors of late PTS. This table reflects timing of the first late PTS and not PTS recurrence. Although the likelihood of observing a late PTS is affected by length of follow up, the effect is minimal and explored in Supplementary Table 1. For p-values, we used 2-sided t-tests for normally distributed continuous variables and a chi-squared or Fisher’s exact test for categorical variables. CNS infection is any culture positive infection of the CNS that occurred within 3 months of trauma.
| Description | No Late PTS (n=294) | Late PTS (n=98) | p-value | |||
|---|---|---|---|---|---|---|
| Demographic | Age | 38 +/− 16 | 31 +/− 14 | <0.001 | ||
| Race | American Indian | 1/294 (0.3%) | 1/98 (1%) | 0.22 | ||
| Asian/Indian | 4/294 (1.4%) | 0/98 (0%) | ||||
| Black | 24/294 (8%) | 3/98 (3%) | ||||
| White | 264/294 (90%) | 94/98 (96%) | ||||
| Other | 1/294 (0.3%) | 0/98 (0%) | ||||
| Clinical | GCS | 3 | 37/292 (13%) | 13/98 (13%) | 0.29 | |
| 4 | 22/292 (7%) | 7/98 (7%) | ||||
| 5 | 33/292 (11%) | 6/98 (6%) | ||||
| 6 | 49/292 (17%) | 23/98 (24%) | ||||
| 7 | 121/292 (41%) | 44/98 (45%) | ||||
| 8 | 30/292 (11%) | 5/98 (5%) | ||||
| Hypoxia | 37/284 (13%) | 12/92 (13%) | 0.99 | |||
| Hypotension | 68/282 (24%) | 17/93 (18%) | 0.24 | |||
| Major extracranial injury | 45/281 (16%) | 9/93 (10%) | 0.13 | |||
| EVD hemorrhage | 33/288 (11%) | 11/96 (11%) | 0.99 | |||
| CNS Infection | 23/294 (8%) | 19/98 (19%) | 0.001 | |||
| DHC | 62/294 (21%) | 55/98 (56%) | <0.001 | |||
| Craniotomy | 89/294 (30%) | 65/98 (66%) | <0.001 | |||
| Shunt | 35/294 (12%) | 30/98 (31%) | <0.001 | |||
| Lobectomy | 6/294 (2%) | 17/98 (17%) | <0.001 | |||
| LOS | 24 +/− 15 | 28 +/− 13 | 0.002 | |||
| ICU LOS | 17 +/− 9 | 21 +/− 10 | 0.001 | |||
| Laboratory | Glucose | 156 +/− 61 | 158 +/− 60 | 0.79 | ||
| Hemoglobin | 13.2 +/− 2.2 | 13.4 +/− 1.8 | 0.57 | |||
| INR | 1.2 +/− 0.3 | 1.2 +/− 0.3 | 0.47 | |||
| Intoxicated | 117/245 (48%) | 38/76 (50%) | 0.78 | |||
| Radiographic | SDH | 104/290 (36%) | 50/96 (52%) | 0.005 | ||
| EDH | 30/286 (10%) | 13/93 (14%) | 0.36 | |||
| tSAH | 222/286 (78%) | 72/93 (77%) | 0.97 | |||
| IVH | 53/288 (18%) | 20/95 (21%) | 0.57 | |||
| Contusion | 157/288 (55%) | 61/96 (64%) | 0.12 | |||
| Depressed Skull Fracture | 35/288 (12%) | 23/96 (24%) | 0.005 | |||
| Petechial hemorrhage | 184/288 (69%) | 58/87 (67%) | 0.70 | |||
| Obliteration of the 3rd ventricle | 36/267 (13%) | 27/87 (31%) | <0.001 | |||
| Midline Shift | 73/267 (13%) | 27/87 (31%) | <0.001 | |||
| MLS > 5mm | 45/290 (16%) | 28/96 (29%) | <0.01 | |||
| Marshall CT score | 1 | 19/285 (7%) | 1/93 (1%) | <0.001 | ||
| 2 | 186/285 (65%) | 45/93 (48%) | ||||
| 3 | 18/285 (6%) | 10/93 (11% | ||||
| 4 | 14/285 (5%) | 8/93 (9%) | ||||
| 5 | 44/285 (15%) | 29/93 (31%) | ||||
| 6 | 4/285 (2%) | 0/93 (0%) | ||||
EVD – External ventricular drain
DHC – Decompressive hemicraniectomy
LOS – Length of Stay
ICU – Intensive Care Unit
INR – International Normalized Ratio
SDH – Subdural hematoma
EDH – Epidural hematoma
tSAH – Traumatic subarachnoid hemorrhage
IVH – Intraventricular hemorrhage
MLS – Midline shift
Table 1.C.
Predictors of PTS Recurrence. We report predictors of PTS recurrence (i.e., a second PTS). Although the likelihood of PTS recurrence varies by follow-up time, the effect is minimal and explored in Supplementary Table 2. Time zero is the time of the first late PTS. For p-values, we used 2-sided t-tests for normally distributed continuous variables and a chi-squared or Fisher’s exact test for categorical variables.
| Description | No PTS Recurrence (n=26) |
PTS Recurrence (n=72) |
p-value | |||
|---|---|---|---|---|---|---|
| Demographic | Age | 36 +/− 16 | 30 +/− 12 | 0.07 | ||
| Race | American Indian | 1/26 (4%) | 0/72 (0%) | 0.39 | ||
| Asian/Indian | 0/26 (0%) | 0/72 (0%) | ||||
| Black | 1/26 (4%) | 2/72 (3%) | ||||
| White | 24/26 (92%) | 70/72 (97%) | ||||
| Other | 0/26 (0%) | 0/72 (0%) | ||||
| Sex (male) | 18/26 (69%) | 62/72 (86%) | 0.08 | |||
| Clinical | GCS | 3 | 5/26 (19%) | 8/72 (11%) | 0.35 | |
| 4 | 2/26 (8%) | 5/72 (7%) | ||||
| 5 | 3/26 (11%) | 3/72 (4%) | ||||
| 6 | 7/26 (27%) | 16/72 (22%) | ||||
| 7 | 9/26 (35%) | 35/72 (49%) | ||||
| 8 | 0/26 (0%) | 5/72 (7%) | ||||
| Hypoxia | 5/25 (20%) | 7/67 (10%) | 0.30 | |||
| Hypotension | 6/26 (23%) | 11/67 (16%) | 0.55 | |||
| Major extracranial injury | 3/26 (12%) | 6/67 (9%) | 0.71 | |||
| EVD hemorrhage | 3/25 (12%) | 8/71 (11%) | 0.99 | |||
| CNS Infection | 5/26 (19%) | 14/72 (19%) | 0.98 | |||
| DHC | 11/26 (42%) | 44/72 (61%) | 0.10 | |||
| Craniotomy | 15/26 (58%) | 50/72 (69%) | 0.28 | |||
| Shunt | 10/26 (38%) | 20/72 (28%) | 0.31 | |||
| Lobectomy | 3/26 (12%) | 14/72 (19%) | 0.55 | |||
| LOS | 29 +/− 13 | 27 +/− 13 | 0.59 | |||
| ICU LOS | 23 +/− 9 | 21 +/− 10 | 0.33 | |||
| Laboratory | Glucose | 136 +/− 40 | 165 +/− 65 | 0.01 | ||
| Hemoglobin | 13.0 +/− 1.8 | 13.5 +/− 1.8 | 0.27 | |||
| INR | 1.2 +/− 0.3 | 1.2 +/− 0.2 | 0.60 | |||
| Intoxicated | 13/20 (65%) | 25/56 (45%) | 0.12 | |||
| Radiographic | SDH | 14/26 (54%) | 36/70 (51%) | 0.83 | ||
| EDH | 4/25 (16%) | 9/68 (13%) | 0.74 | |||
| tSAH | 19/25 (76%) | 53/68 (78%) | 0.84 | |||
| IVH | 5/25 (20%) | 15/70 (21%) | 0.88 | |||
| Contusion | 16/26 (62%) | 45/70 (64%) | 0.80 | |||
| Depressed Skull Fracture | 7/26 (27%) | 16/70 (23%) | 0.68 | |||
| Petechial hemorrhage | 14/23 (61%) | 44/64 (69%) | 0.49 | |||
| Obliteration of the 3rd ventricle | 7/23 (30%) | 20/64 (31%) | 0.94 | |||
| Midline Shift | 9/26 (35%) | 31/70 (44%) | 0.45 | |||
| MLS > 5mm | 6/26 (23%) | 22/70 (31%) | 0.42 | |||
| Marshall CT score | 1 | 1/25 (4%) | 0/68 (0%) | 0.36 | ||
| 2 | 12/25 (48%) | 33/68 (49%) | ||||
| 3 | 1/25 (4%) | 9/68 (13% | ||||
| 4 | 3/25 (12%) | 5/68 (7%) | ||||
| 5 | 8/25 (32%) | 21/68 (31%) | ||||
| 6 | 0/25 (0%) | 0/68 (0%) | ||||
Table 1.D.
Models for risk factors for first late PTS (i.e., PTE) and PTS Recurrence. The first model is a Cox Proportional Hazards (CPH) model predicting the first late PTS (i.e., PTE). As a sensitivity analysis, we developed a competing risks regression using the same inputs as our first model and modeling the competing hazard of death. Both models predict the occurrence of the first late PTS. Our third model predicts PTS recurrence, or the second PTS. In this last model, we include the occurrence of a first late PTS as a time-varying covariate, as the first late PTS occurs during follow up and, by definition, prior to PTS recurrence. *The CRR model reports the subdistribution hazard.
| Cumulative Incidence | Model Type | Variable | Hazard Ratio* | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| First late PTS (PTE) | CPH | DHC | 4.1 | 2.2 | 6.1 | <0.001 |
| CNS Infection | 1.7 | 1.2 | 2.3 | 0.04 | ||
| Age (decade) | 0.7 | 0.6 | 0.9 | <0.001 | ||
| First late PTS (PTE) | CRR | DHC | 4.4 | 3.7 | 4.9 | <0.001 |
| CNS Infection | 1.7 | 1.2 | 2.2 | 0.03 | ||
| Age (decade) | 0.7 | 0.6 | 0.9 | <0.001 | ||
| PTS Recurrence | CPH with time-varying coefficient | Shunt Dependence | 0.6 | 0.3 | 1.0 | 0.04 |
| Sex (male) | 2.7 | 1.4 | 5.3 | <0.01 | ||
Discussion
In a modern cohort of severe TBI patients, we identified a cumulative incidence of PTS of 25% at five years and 32% at fifteen years. Including patients from the 1930s to 1980s, Annengers et al. first described the rates of PTS after severe TBI at approximately 10% at five years and increasing to 17% at twenty years.5 Subsequent groups have described higher rates of PTS of 14-17% from 2-3 years post-injury6,8 and 21% at five years.9 Previous evaluations have had several drawbacks: (1) cohorts from prior decades with presumably worse survival and more limited means of monitoring for seizures;6,8,9 (2) short-term follow up;6,8,10,18 (3) non-standardized definitions of severe TBI, PTS and PTE;8,11 and (4) small sample sizes.18 Our reported rates of PTS (25% at five years and 32% at fifteen years) are substantially higher than previous reports.
Additionally, we identified several risk factors including DHC, age, and CNS infection for the first late PTS and male gender and shunt independence for PTS recurrence. Previous reports found penetrating injury, depressed skull fractures, and cerebral contusions to be associated with PTE.1,2 These are markers of injury severity, similar to our findings. The increased incidence of PTE, coupled with reliable risk factors, encourages the utilization of severe TBI as not only a model of epileptogenesis but also as a screen for anti-epileptogenic therapeutics.
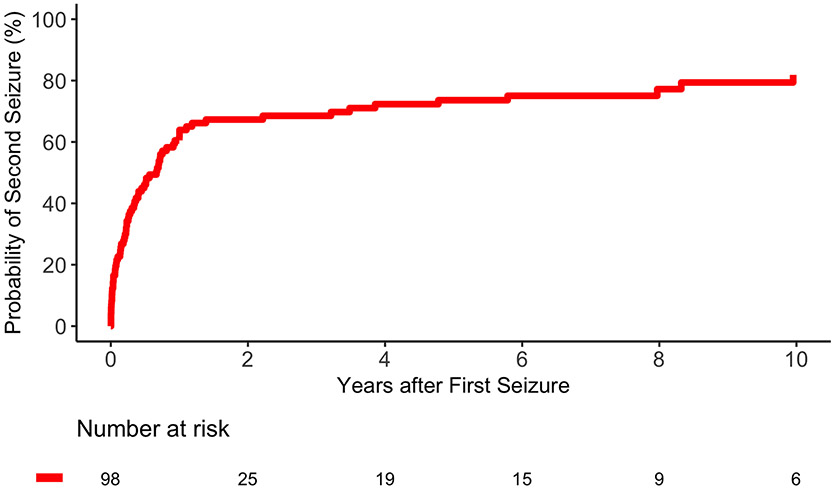
We identified a long-term, high risk (82% at 10 years) of seizure recurrence, with a majority (62%) of patients having seizure recurrence within one year. Within the context of current ILAE definitions,19 this validates our proposed definition of post-traumatic epilepsy as a single late seizure after severe TBI. Similarly, patients with an early PTS were at increased risk of late PTE (44% by two years). This finding contradicts previous reports9 stating that only immediate PTS (i.e., within 24 hours of trauma), and not early PTS, predict late PTS. Taken together, patients with any PTS should be carefully monitored for seizure recurrence, especially in the setting of aforementioned risk factors. Patients with a single late PTS should be diagnosed with epilepsy and promptly treated with ASMs.
Our study has several limitations, including retrospective nature and single center. We employed a passive surveillance for seizures, which may under-report the true incidence of PTE.
In conclusion, we demonstrated the incidence of post-traumatic epilepsy may be increasing over time with improvements in acute and post-acute care, as well as overall patient survival. After a single post-traumatic seizure, patients with severe TBI should be referred to an epileptologist for consideration of long-term anti-epileptic therapy and close follow-up.
Supplementary Material
Figure 2.B.
Cumulative Incidence of Seizure Recurrence. Figure 2.B shows the cumulative incidence of PTS recurrence with time zero as the time of first late PTS. The international league against epilepsy (ILAE) defines epilepsy as two seizures more than 24 hours apart or one seizure with a risk of seizure recurrence of >60% after 10 years. With a risk of seizure recurrence of 82% after ten years, a single late PTS meets the ILAE definition of epilepsy.
Acknowledgements
Dr. Elmer’s research time is supported by the NIH through grant 5K23NS097629.
Footnotes
Potential Conflicts of Interest
Nothing to report
Data Availability
All data and statistical analyses are available from the authors upon request of qualified researchers.
References
- 1.Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689–701. doi: 10.1016/S0140-6736(18)32596-0 [DOI] [PubMed] [Google Scholar]
- 2.Baranowski CJ. The quality of life of older adults with epilepsy: A systematic review. Seizure. 2018;60(May):190–197. doi: 10.1016/j.seizure.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 3.Begley CE, Durgin TL. The direct cost of epilepsy in the United States: A systematic review of estimates. Epilepsia. 2015;56(9):1376–1387. doi: 10.1111/epi.13084 [DOI] [PubMed] [Google Scholar]
- 4.Fordington S, Manford M. A review of seizures and epilepsy following traumatic brain injury. J Neurol. 2020;267(10):3105–3111. doi: 10.1007/s00415-020-09926-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338(1):20–24. doi: 10.1056/NEJM199801013380104 [DOI] [PubMed] [Google Scholar]
- 6.Englander J, Bushnik T, Duong TT, et al. Analyzing risk factors for late posttraumatic seizures: A prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84(3 SUPPL. 1):365–373. doi: 10.1053/apmr.2003.50022 [DOI] [PubMed] [Google Scholar]
- 7.Karlander M, Ljungqvist J, Zelano J. Post-traumatic epilepsy in adults: A nationwide register-based study. J Neurol Neurosurg Psychiatry. 2021;92(6):617–621. doi: 10.1136/jnnp-2020-325382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson PL, Smith GM, Wannamaker BB, Thurman DJ, Pickelsimer E, Selassie AW. A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia. 2010;51(5):891–898. doi: 10.1111/j.1528-1167.2009.02384.x [DOI] [PubMed] [Google Scholar]
- 9.Ritter AC, Wagner AK, Fabio A, et al. Incidence and risk factors of posttraumatic seizures following traumatic brain injury: A Traumatic Brain Injury Model Systems Study. Epilepsia. 2016;57(12):1968–1977. doi: 10.1111/epi.13582 [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Wu H, Wang X, Li J, Zhang S. Clinical epidemiology of posttraumatic epilepsy in a group of Chinese patients. Seizure. 2012;21(5):322–326. doi: 10.1016/j.seizure.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 11.Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373(9669):1105–1110. doi: 10.1016/S0140-6736(09)60214-2 [DOI] [PubMed] [Google Scholar]
- 12.Christensen J The epidemiology of posttraumatic epilepsy. Semin Neurol. 2015;35(3):218–222. doi: 10.1055/s-0035-1552923 [DOI] [PubMed] [Google Scholar]
- 13.Haltiner AM, Temkin NR, Dikmen SS. Risk of seizure recurrence after the first late posttraumatic seizure. Arch Phys Med Rehabil. 1997;78(8):835–840. doi: 10.1016/S0003-9993(97)90196-9 [DOI] [PubMed] [Google Scholar]
- 14.Goldschmidt E, Deng H, Puccio AM, Okonkwo DO. Post-traumatic hydrocephalus following decompressive hemicraniectomy: Incidence and risk factors in a prospective cohort of severe TBI patients. J Clin Neurosci. 2020;73:85–88. doi: 10.1016/j.jocn.2020.01.027 [DOI] [PubMed] [Google Scholar]
- 15.Li J, Le-Rademacher J, Zhang MJ. Weighted comparison of two cumulative incidence functions with R-CIFsmry package. Comput Methods Programs Biomed. 2014;116(3):205–214. doi: 10.1016/j.cmpb.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poguntke I, Schumacher M, Beyersmann J, Wolkewitz M. Simulation shows undesirable results for competing risks analysis with time-dependent covariates for clinical outcomes. BMC Med Res Methodol. 2018;18(1):79. doi: 10.1186/s12874-018-0535-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England). 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 18.Tubi MA, Lutkenhoff E, Blanco MB, et al. Early seizures and temporal lobe trauma predict post-traumatic epilepsy: A longitudinal study. Neurobiol Dis. 2019;123:115–121. doi: 10.1016/j.nbd.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beretta S, Carone D, Zanchi C, et al. Long-term applicability of the new ILAE definition of epilepsy. Results from the PRO-LONG study. Epilepsia. 2017;58(9):1518–1523. doi: 10.1111/epi.13854 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and statistical analyses are available from the authors upon request of qualified researchers.