Abstract
Background:
Low tidal volume and adequate positive end-expiratory pressure (PEEP) are evidence-based approaches for pediatric acute respiratory distress syndrome (pARDS), however data are limited regarding their use since pARDS guidelines were revised in 2015.
Objective:
To identify prevalence of, and factors associated with, nonadherence to appropriate tidal volume and PEEP in children with pARDS.
Methods:
Retrospective cohort study of children 1 month-<18 years with pARDS who received invasive mechanical ventilation from 2016–2018 in a single pediatric intensive care unit (PICU).
Results:
At 24 hours after meeting pARDS criteria, 48/86 (56%) patients received tidal volume ≤8 mL/kg of ideal body weight and 45/86 (52%) received appropriate PEEP, with 22/86 (26%) receiving both. Among patients ≥2 years of age, a lower proportion of patients with overweight/obesity (9/25, 36%) had appropriate tidal volume vs. those in the normal or underweight category (16/22, 73%, p=0.02). When FIO2 was ≥50%, PEEP was appropriate in 19/60 (32%) cases vs. 26/26 (100%) with FIO2 <50% (p<0.0001). pARDS was documented in the progress note in 7/86 (8%) patients at 24 hours. Severity of pARDS, documentation in the progress note, and other clinical factors were not significantly associated with use of appropriate tidal volume and PEEP, however pARDS was documented more commonly in patients with severe pARDS.
Conclusions:
In a single PICU in the United States, children with pARDS did not receive appropriate tidal volume for ideal body weight nor PEEP. Targets for improving tidal volume and PEEP adherence may include overweight patients and those receiving FIO2≥50%, respectively.
Keywords: acute respiratory distress syndrome, pediatric, quality, positive end expiratory pressure, diagnosis, respiratory failure
Introduction
Pediatric acute respiratory distress syndrome (pARDS) occurs in 3% of patients overall and 6% of those on mechanical ventilation1 in the Pediatric Intensive Care Unit (PICU), and is associated with high in-hospital mortality (13–35%)1–4 and new morbidity (23% of survivors)2. Low tidal volume ventilation and use of positive end expiratory pressure (PEEP) are key elements of ventilator management in ARDS and have been associated with decreased mortality in both adults and children4–6. Ventilator parameters in the first 24–72 hours after ARDS diagnosis have been shown to affect outcome4–5, 7, emphasizing the need for rapid recognition and evidence-based management.
Despite existing evidence, use of low tidal volume ventilation and PEEP appropriate for fraction of inspired oxygen (FIO2) deviate from recommendations 29–87% of the time in adults8–12 and children4–5, 13–15. Reasons for these variations in care are incompletely described, but could include patient demographics12, intensive care unit or hospital organizational characteristics10, 15–16, usage of actual rather than ideal weight14–15, 17, delayed identification of ARDS9,18, severity of disease5,8,10,11–12, 18, provider perceptions and knowledge17–19, ventilator mode5, or other patient or illness characteristics11, 14–15, l7, 18–19.
New guidelines for diagnosis and management of pARDS were published in 201520, resulting in an increased percentage of patients meeting criteria for pARDS earlier in their PICU course1. Furthermore, studies of adherence to evidence-based ventilator management in children included some or all of their patients in the context of prospective studies of pARDS4–5, 14, which may not reflect usual care. The objective of this study was to identify prevalence of, and factors associated with, nonadherence to 1) use of tidal volume ≤8 milliliters per kilogram (mL/kg) of ideal body weight and 2) PEEP appropriate for FIO2 in children with pARDS in usual care in a tertiary PICU since publication of the 2015 guidelines.
Methods
Study population
Patient admissions 1 month to <18 years of age to the Wake Forest Brenner Children’s Hospital PICU who received invasive mechanical ventilation during their stay, excluding those with cyanotic heart disease or receiving cardiac bypass, were identified over the time period January 1st, 2016 through December 31st, 2018. The study PICU has 11 beds with an adjacent intermediate care unit, located in a 193-bed Children’s Hospital within a tertiary care academic medical center designated as a level 1 adult and pediatric trauma center. At least one dedicated respiratory therapist is present in the PICU at all times. There is also a pediatric intensive care board-certified or board-eligible attending physician present in the PICU 24 hours per day along with resident providers, with no pediatric critical care fellows. The ventilator in use was the Servo-i (Getinge, Gothenburg, Sweden), and ventilator changes were made at the discretion of the care team with no formal mechanical ventilation protocol in place. No prepopulated fields for oxygenation index, oxygen saturation index, or ideal body weight were present in the electronic health record during the study period, nor were any decision support tools for tidal volume or peep titration.
Study Design
This was a single center retrospective cohort study designed to identify factors associated with evidence-based management of pARDS. The exposure was first occurrence of pARDS during a PICU stay in a patient who received mechanical ventilation. The primary outcomes were a) use of tidal volume ≤8 mL/kg ideal body weight (actual body weight if <2 years old) and b) use of minimum PEEP per fraction of inspired oxygen (FIO2) by the ARDSnet lower PEEP/higher FIO2 table (Supplemental Content Table s1)6 at 24 hours after the patient met criteria for ARDS. The secondary outcomes were parameters a) and b) at 72 hours after meeting criteria for ARDS. A pre-specified additional analysis was performed to evaluate presence of pARDS in the progress note at 24 and 72 hours, given that physician recognition of ARDS was perceived as the top barrier to initiation of lung-protective ventilation in a previous survey study18. Post-hoc evaluations of the progress note were also performed for presence of a) weight or obesity (if overweight or obese) and b) “respiratory failure” to evaluate further for possible indications of physician awareness of these conditions.
Data Collection
Patient demographics, clinical features, and outcomes, including severity of illness by Pediatric Index of Mortality 3 (PIM 3)21 and Pediatric Risk of Mortality 3 (PRISM III)22, were identified from local data previously collected and submitted to the Virtual Pediatric Systems (VPS, LLC) database. No additional data were provided by VPS, LLC; no endorsement or editorial restriction of the interpretation of these data or the opinions of the authors has been implied or stated.
Ventilator, blood gas, and vital sign data were obtained from the electronic medical record and time-linked. Patients were evaluated for inclusion if they received mechanical ventilation and met hypoxemia criteria for pARDS during their PICU stay. Patients meeting criteria while on non-invasive ventilation were required to be on conventional invasive mechanical ventilation by 24 hours so that the primary outcome could be assessed. Hypoxemia criteria for pARDS were assigned per 2015 PALICC guidelines20. Oxygenation index (OI) or oxygen saturation index (OSI) was used for invasively ventilated patients, with OI used preferentially in cases in which a PaO2 was recorded. PaO2:FIO2 (P:F) ratio or Saturation:FIO2 (S:F) ratio was used for patients non-invasively ventilated with full face-mask continuous positive airway pressure (CPAP) or bi-level ventilation. Peak inspiratory pressure (PIP) was recorded as plateau pressure was not documented. Any values for OSI based on a saturation <88% were reviewed to ensure that a sustained value rather than an isolated value was used. All patient charts were also reviewed by one of the authors (AGW, ALB, or MCM) for additional pARDS criteria (acute insult within 7 days, respiratory failure not fully explained by cardiac failure or fluid overload, chest imaging findings). Patients on extracorporeal support or on ventilator modalities other than conventional ventilation (high frequency or airway pressure release) at 24 hours after meeting pARDS criteria were also excluded from the primary analysis, as tidal volume evaluation would be systematically different. Inhaled nitric oxide use was not evaluated in this study, as it is not recommended for routine use in pARDS20.
Body weight and height (length) were obtained from the medical record. Ideal body weight was determined using the McLaren method23–24 and age and gender-specific Centers for Disease Control and Prevention (CDC) growth charts25. Ideal body weight was only calculated for children ≥2 years in this study given that ideal body weight calculations are less reliable in younger age groups26 and CDC guidelines only define overweight/obesity categories in children ≥2 years27. For patients ≥2 years old, age, sex, height, and weight were used to categorize patients as having underweight, normal weight, overweight, and obesity according to Center for Disease Control (CDC) and Prevention guidelines27. Patients were classified based on BMI percentile for age and sex as follows: less than 5% underweight, 5% to less than 85% normal weight, 85% to less than 95% overweight, and greater than or equal to 95% obesity. Patients with obesity were further categorized into class 1 obesity (100-<120% of 95th percentile BMI) or class 2–3 obesity (≥120%of 95th % BMI)28. For those classified in a category other than normal weight, predicted body weight was determined using the value for the closest percentile in the normal weight category for age/sex/height (i.e. 5th percentile if underweight and 85th % if overweight or obese).
Primary and secondary outcomes were collected from the electronic medical record as the point-in-time (steady state) tidal volume and PEEP at 24 and 72 hours after pARDS hypoxemia criteria were met, respectively. These time points were chosen for comparability with previous studies that have evaluated low tidal volume or PEEP use in acute lung injury or ARDS at or within the first 244,9.14 or 725,13,15 hours, as well as data indicating that ventilator settings in the first few days may affect outcome7. The 24-hour point was chosen as the primary outcome for direct comparability with the largest study of tidal volume adherence in pARDS prior to the 2015 guidelines14, as well as to allow time for recognition of pARDS and ventilator adjustment after hypoxemia criteria were met. Tidal volume was recorded as either the set (if in volume control mode) or measured (if in pressure control mode) value, then divided by either the measured (if <2 years of age) or ideal body weight (if ≥2 years of age) to determine mL/kg. PEEP and FIO2 were recorded at the predetermined time points (24 or 72 hours after meeting pARDS criteria) and designated as appropriate if PEEP was equal to or greater than the minimum recommended PEEP in the ARDSnet low PEEP/high FIO2 table6 (Supplemental Content Table s1). Any FIO2 value in between values in the FIO2/PEEP table was rounded down to the nearest decile if ≤5% above and up if >5% above (e.g. 45% FIO2 was considered as 40%, but 48% FIO2 was considered as 50%). The recommendations from the 2015 PALICC guidelines20 of a) allowing permissive hypercapnia while maintaining pH between 7.15–7.30, excepting clinical circumstances such as elevated intracranial pressure or hemodynamic instability, and b) maintaining PEEP ≥10 cm H20 for patients with severe pARDS were also evaluated. Documentation of pARDS was evaluated by review of the daily progress note for “acute respiratory distress syndrome”, “pARDS”, or “ARDS” on the calendar day of the primary and secondary outcomes (24 or 72 hours after meeting pARDS criteria) by one of the investigators (AGW, ALB, or MCM). Years of experience for each attending was calculated as the time from completion of fellowship to the first year of the study. Vasopressors were considered present if a continuous infusion of dopamine (dose ≥5 mcg/kg/min), epinephrine, norepinephrine, phenylephrine, or vasopressin (dose ≥0.0005 units/kg/min) was documented.
Analyses
Continuous data were compared using the Student’s t-test if normally distributed and Wilcoxon rank-sum test if non-normally distributed. Proportions were compared using Pearson’s chi-square test or Fisher’s exact test, as appropriate. A two-tailed p value of <0.05was considered statistically significant. Data were analyzed using SAS software version 9.4 (SAS Institute, Cary, NC). The Institutional Review Board of Wake Forest University School of Medicine approved this study (approval # IRB00059600).
Results
At 24 hours after meeting pARDS criteria, 86 patient admissions in 80 unique patients were included in the primary analysis (Figure 1), representing 4.8% of total admissions and 15.6% of those with invasive ventilation. Demographic and clinical features of included patient admissions are in Table 1. Median length of PICU stay was 14.3 days (IQR 7.4–22.5) and 13 patients (15.1%) died in the PICU.
Figure 1:
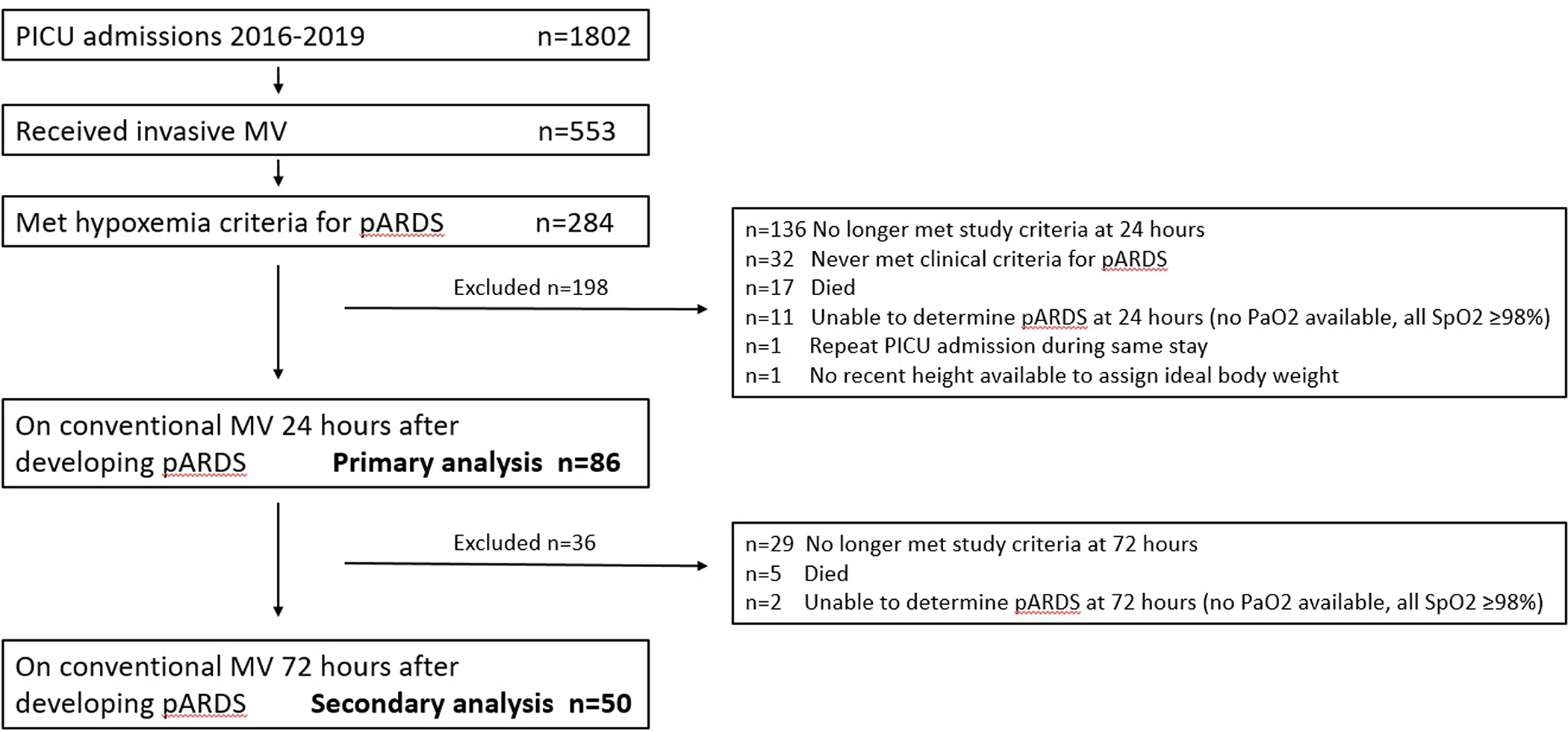
Study flow diagram. MV: Mechanical Ventilation. pARDS: pediatric acute respiratory distress syndrome. PICU: pediatric intensive care unit. Among those (n=136) who did not meet study criteria at 24 hours: Conventional ventilation without ARDS n=79, extubated or off of positive pressure ventilation by tracheostomy n=32, transferred/discharged/not in PICU n=15, never intubated n=4, other ventilator modality (airway pressure release or high frequency) n=4, on extracorporeal support n=2.
Table 1 –
Characteristics of included patient admissions
| Category | Total n=86 |
|---|---|
|
| |
| Age months, median(IQR) | 33.5 (7.0–103.0) |
|
| |
| Age category, n(%) | |
| 1 month–<2 years | 39 (45%) |
| 2–5 years | 16 (19%) |
| 6–12 years | 19 (22%) |
| 13–<18 years | 12 (14%) |
|
| |
| Gender=male, n(%) | 46 (53%) |
|
| |
| Race, n(%) | |
| White | 43 (50%) |
| Black or African American | 20 (23%) |
| Other/Mixed/Unspecified | 16 (19%) |
| Hispanic or Latino | 7 (8%) |
|
| |
| Weight category, n(%) (among n=47 ≥2 yrs old) | |
| Normal/underweight | 22 (47%) |
| Overweight | 5 (11%) |
| Obese | 20 (43%) |
|
| |
| Tracheostomy tube in place | 11 (13%) |
|
| |
| Risk of mortality (probability of death) on admission, mean (SD) | |
| PIM 3 | 15.3 (27.3) |
| PRISM-III | 16.6 (28.5) |
|
| |
| Primary Cause of pARDS, n(%) | |
| Respiratory | 49 (57%) |
| Systemic | 37 (43%) |
|
| |
| Initial pARDS hypoxia criterion met n(%) | |
| Invasive Ventilation | |
| Oxygenation Saturation Index (OSI) | 55 (64%) |
| Oxygenation Index (OI) | 28 (33%) |
| Non-invasive Ventilation | |
| PaO2: FIO2 (P:F) ratio | 2 (2%) |
| Saturation: FIO2 (S:F) ratio | 1 (1%) |
|
| |
| Initial pARDS Severity | |
| Mild | 36 (42%) |
| Moderate | 19 (22%) |
| Severe | 28 (33%) |
| Not applicable (non-invasive ventilation) | 3 (3%) |
| Year of Admission | |
| 2016 | 40 (47) |
| 2017 | 19 (22) |
| 2018 | 27 (31) |
At 24 hours after meeting pARDS criteria, tidal volume ≤8 mL/kg of ideal body weight (measured body weight if <2 years of age) was present in 48/86 (56%) patients, and PEEP was appropriate for FIO2 in 45/86 (52%) patients. In patients with severe pARDS, PEEP≥10 was present in 9/18 (50%) cases at 24 hours. PICU mortality occurred in 4/48 (8.3%) patients with tidal volume ≤8 mL/kg vs. 9/38 (23.7%) with tidal volume >8mL/kg (p=0.05) and 3/45 (6.7%) patients with appropriate PEEP vs 10/41 (24.4%) without (p=0.02). Median tidal volume was 7.7 mL/kg (IQR 6.5–9.0) and median PEEP was 8 (IQR 7–9). Both tidal volume and PEEP were appropriate in 22/86 (26%) cases. Tidal volume and PEEP status by patient characteristic at 24 hours is displayed in Table 2.
Table 2 –
Tidal volume and PEEP at 24 hours
| Characteristic Total n=86 | TV ≤ 8 mL/kg | p | PEEP appropriate for FIO2 | p |
|---|---|---|---|---|
|
| ||||
| Age group | ||||
| 1 month-<2 years | 23/39 (59%) | 0.04 | 17/39 (44%) | 0.15 |
| 2–5 years | 13/16 (81%) | 7/16 (44%) | ||
| 6–12 years | 7/19 (37%) | 14/19 (74%) | ||
| 13–<18 years | 5/12 (42%) | 7/12 (58%) | ||
|
| ||||
| Gender | ||||
| Female | 24/40 (60%) | 0.47 | 20/40 (50%) | 0.69 |
| Male | 24/46 (52%) | 25/46 (47%) | ||
|
| ||||
| Race | ||||
| White | 21/43 (49%) | 0.64 | 21/43 (49%) | 0.74 |
| Black or African American | 13/20 (65%) | 10/20 (50%) | ||
| Other/Mixed/Unspecified | 10/16 (63%) | 9/16 (56%) | ||
| Hispanic or Latino | 4/7 (57%) | 5/7 (71%) | ||
|
| ||||
| Weight category (≥2 years old, n=47) | ||||
| Normal/underweight | 16/22 (73%) | 0.046 | 13/22 (59%) | 0.76 |
| Overweight | 2/5 (40%) | 4/5 (80%) | ||
| Obese | 7/20 (35%) | 11/20 (55%) | ||
|
| ||||
| Tracheostomy tube present | ||||
| No | 40/75 (53%) | 0.33 | 40/75 (53%) | 0.75 |
| Yes | 8/11 (73%) | 5/11 (45%) | ||
|
| ||||
| pARDS cause | ||||
| Respiratory | 30/49 (61%) | 0.25 | 26/49 (53%) | 0.88 |
| Systemic | 18/37 (49%) | 19/37 (51%) | ||
|
| ||||
| pARDS severity | ||||
| Initial Mild | 19/36 (53%) | 0.55 | 25/43 (58%) | 0.49 |
| Moderate | 11/19 (58%) | 13/25 (52%) | ||
| Severe | 15/28 (54%) | 10/18 (56%) | ||
| N/A (non-invasive vent) | 3/3 (100%) | 3/3 (100%) | ||
| 24 hours Mild | 25/43 (58%) | 0.89 | 28/43 (65%) | 0.35 |
| Moderate | 13/25 (52%) | 11/25 (44%) | ||
| Severe | 10/18 (56%) | 6/18 (33%) | ||
|
| ||||
| Ventilator mode | ||||
| Pressure regulated volume control | 38/73 (52%) | 0.08 | 40/73 (55%) | 0.11 |
| Pressure control/pressure support | 9/11 (82%) | 3/11 (27%) | ||
| CPAP with pressure or volume support | 1 /2 (50%) | 2/2 (100%) | ||
|
| ||||
| pARDS in progress note at 24 hours | ||||
| No | 45/79 (57%) | 0.69 | 40/79 (51%) | 0.44 |
| Yes | 3/7 (43%) | 5/7 (71%) | ||
Notes: All were on conventional mechanical ventilation using Servo-i (Maquet corporation) in synchronized intermittent mechanical ventilation (SIMV) setting. Bold indicates p<0.05.
Peak inspiratory pressure was similar between patients with tidal volume ≤8 mL/kg (Median peak inspiratory pressure 24.2, IQR 20.1–29.1) vs tidal volume >8 mL/kg (Median peak inspiratory pressure 26.0, IQR 22.6–29.4, p=0.40) at 24 hours. Delta pressure (PIP-PEEP) was also similar [tidal volume ≤8 mL/kg: 17.6 (IQR 14–21), tidal volume >8mL/kg: 17.7 (IQR 14–22), p=0.68). In 84 patients with blood gas data available at 24 hours, median pH was 7.37 (IQR 7.31–7.42) and pCO2 was 44.5 (IQR 37.0–52.5). pH was <7.15 in 3/84 (3.6%), between 7.15–7.30 in 13/84 (15.5%), and >7.30 in 68/84 (81%), while pCO2 was <60 mm Hg in 74/84 (88%). In patients with tidal volume nonadherence, 0/38 (0%) had pH<7.15, 10/38 (26.3%) had pH between 7.15–7.30, and 28/38(74%) had pH>7.30Median SpO2 at 24 hours was 95% (IQR 92–97) and PaO2 (n=50) was 74 mm Hg (IQR 62–102). SpO2 was <92% in 19/86 (22%), 92–97% in 56/86 (65%), and >97% in 11/86 (13%) patients; among those with PEEP ≥10 cm H20 (n=18), SpO2 was <88% in 2/18 (11%), 88–92% in 7/18 (39%), and >92% in 9/18 (50%). There were no significant differences in tidal volume or PEEP adherence at 24 hours by pARDS hypoxemia criterion used, study year, oxygenation data, or use of vasopressor support; however, pCO2 was significantly higher and proportion with pH between 7.15–7.30 was significantly lower in patients with tidal volume ≤8 mL/kg, while PIM3 risk of mortality was significantly lower in patients with appropriate PEEP at 24 hours. (Supplemental Content Table s2) In patients with head trauma and intracranial pressure monitoring in place at 24 hours, 5/6 (83%) had appropriate tidal volume and 3/6 (50%) had appropriate PEEP. pH was >7.30 and pCO2 was <40 in 4/5 (80%) of head trauma patients with tidal volume adherence. Among patients declared brain dead by 72 hours, 2/5 (40%) had appropriate tidal volume and 1/5 (20%) had appropriate PEEP at 24 hours.
Among patients for whom an ideal body weight and weight category was assigned (≥2 years of age, n=47), a lower proportion of patients with overweight/obesity (9/25, 36%) had a tidal volume ≤8 mL/kg of ideal body weight vs. those with normal or underweight category (16/22, 73%, p=0.02). Tidal volume per kg of ideal body weight was highest for the patients with Class 2–3 obesity (n=8, median 9.6 mL/kg, IQR 7.8–10.0), followed by Class 1 obesity (n=12, 9.1 mL/kg, IQR 7.5–10.1), and overweight patients (n=5, 8.5 mL/kg, IQR 7.7–8.7), as shown in Figure 2. When tidal volume was evaluated based on measured body weight for all patients, median tidal volume was 7.1 mL/kg (IQR 6.3–8.1) and 59/86 (69%) patients had tidal volume ≤8 mL/kg, with 11 patients (13%) appearing to have tidal volume ≤8 ml/kg when this was not the case by ideal body weight. Age was no longer significantly associated with use of appropriate tidal volume ≤8 ml/kg when actual body weight was used {tidal volume ≤8 ml/kg: 1–23 months 23/39 (59%), 2–5 years 13/16 (81%), 6–12 years 14/19 (74%), 13-<18 years 9/12 (75%), p=0.34}.
Figure 2:
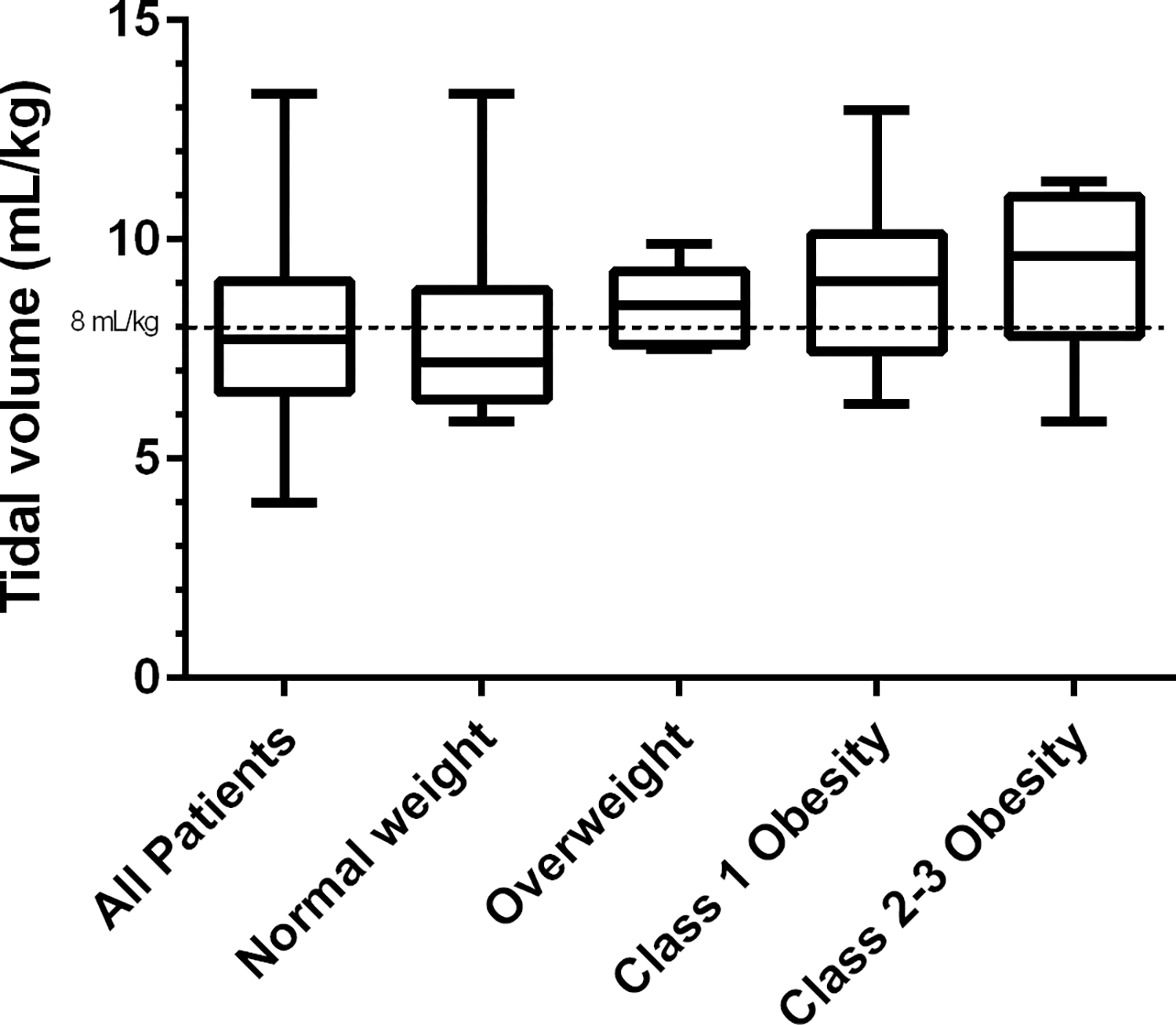
Tidal volume (mL/kg of ideal weight if ≥2 years of age, actual weight if <2 years) by weight category. All patients: n=86; Normal weight: n=18; Overweight n=5; Class 1 Obesity: n=12; Class 2–3 Obesity n=18
PEEP used for each FIO2 value is displayed in Figure 3. Median PIP for patients with appropriate PEEP was 25.7 (IQR 22.0–29.0) vs. 25.0 (IQR 22.7–30.0) in patients with insufficient PEEP (p=0.72). Among those observations with FIO2≥50%, PEEP was appropriate for FIO2 in 19/60 (32%) cases, vs 26/26 (100%) when FIO2 was <50% (p<0.0001). In patients with FIO2 ≥80%, 0/15 (0%) had an appropriate PEEP.
Figure 3:
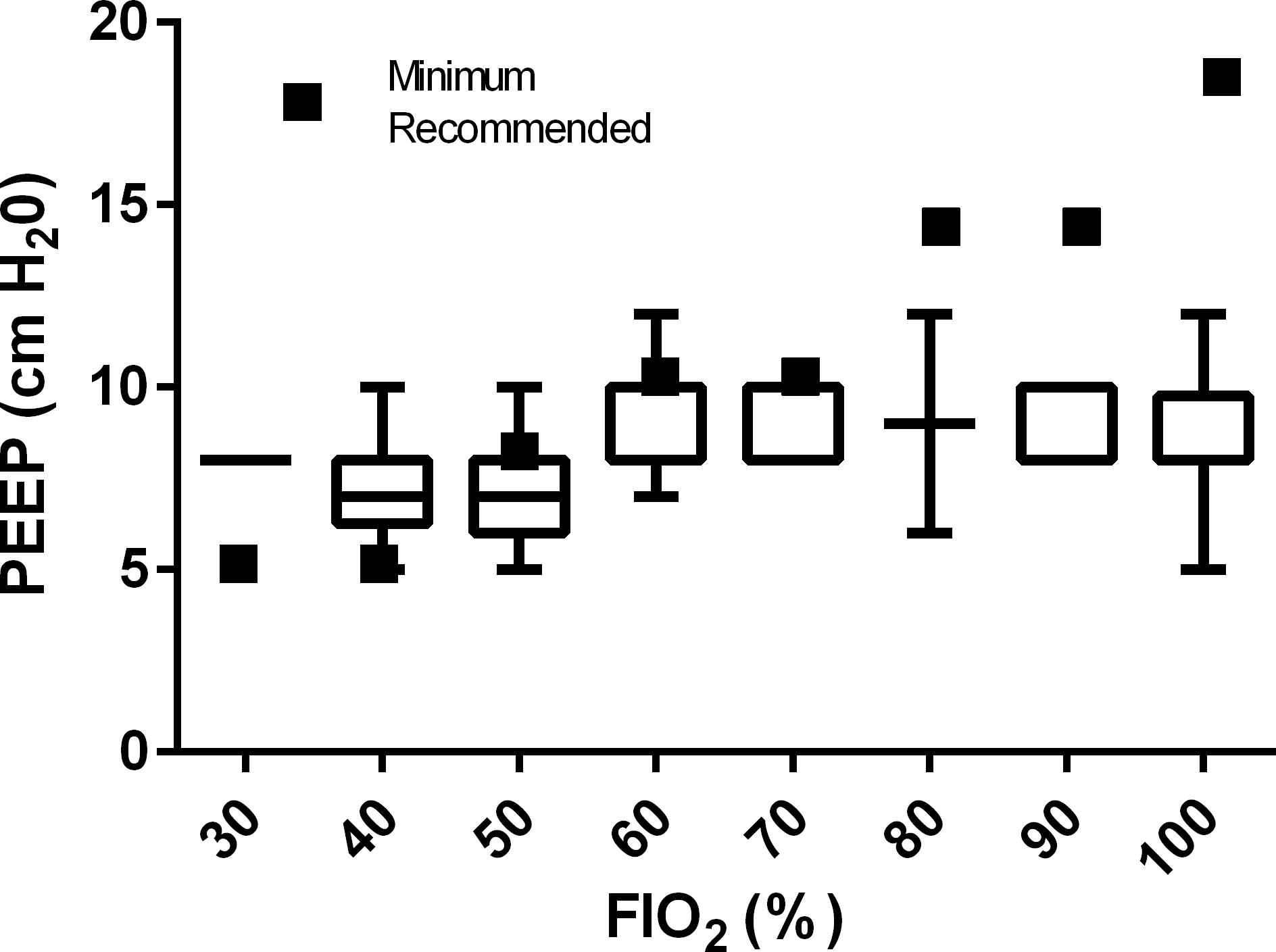
PEEP (cm H20) by FIO2 at 24 hours after meeting ARDS hypoxemia criteria. All patients: n=86; FIO2 30%: n=2, FIO2 40%: n=24, FIO2 50%: n=27, FIO2 60%: n=13, FIO2 70%: n=5, FIO2 80%: n=2, FIO2 90%: n=5, FIO2 100%: n=8.
At 72 hours after meeting ARDS criteria, 50/86 (58%) patients continued to meet study criteria (Figure 1) and were included in the secondary analysis. Tidal volume ≤ 8 mL/kg was present in 29/50 (58%) cases and PEEP was appropriate for FIO2 in 34/50 (68%) cases, with both parameters met in 19/50 (38%) cases. In patients with severe pARDS, PEEP≥10 was present in 5/7 (71%) patients at 72 hours. Documentation in the progress note was poor for both pARDS (7/86=8% of progress notes at 24 hours, 6/50=12% at 72 hours) and overweight/obesity (3/25=12% at 24 hours), while “respiratory failure” was often documented (70/86=89% at 24 hours). Documentation occurred in a higher proportion of patients with severe pARDS at both 24 (severe 4/18, 22% vs mild or moderate 3/68, 4%, p=0.01) and 72 hours (severe 4/7, 57% vs mild or moderate 2/43, 5%, p=0.002). Characteristics of patients with tidal volume ≤ 8 mL/kg or PEEP appropriate for FIO2 at 72 hours are displayed in Supplemental Content Table s3.
Discussion
In this single center retrospective study evaluating evidence-based ventilator management 24 hours after criteria were met for pARDS, just over half of patient admissions had tidal volume ≤8 ml/kg ideal body weight (56%) or PEEP appropriate for FIO2 (52%), with a minority (26%) having both. The percentage of patients achieving these parameters were only marginally better at 72 hours for tidal volume (58%), PEEP (68%), and both (38%).
The incidence of pARDS, mortality, and proportion of patients with tidal volume and PEEP adherence under usual care in this single tertiary PICU were similar to previous studies1,4–5,14, suggesting that these findings may be comparable in many PICUs, that nonadherence persists since publication of the 2015 PALICC guidelines20, and that any Hawthorne effect on adherence in the context of previous prospective studies was minimal. Furthermore, the additional data points collected in this study such as severity of illness, vasopressor use, head trauma, documentation in progress notes, and goal pH and SpO2 ranges may provide further hypotheses to guide quality improvement efforts.
Although the PALICC guidelines identify 40% more children as having ARDS at a median of 12.8 hours sooner than prior criteria1, adherence to tidal volume and PEEP goals were not significantly different by severity of ARDS, hypoxemia criterion, duration since meeting criteria (24 vs 72 hours), or documentation of ARDS in the progress note (although documentation was only present in 8% of patients at 24 hours) in this study. However, the small number of patients (n=7) with severe ARDS at 72 hours had both the highest adherence to tidal volume ≤8 mL/kg (86%) and the highest proportion with ARDS documented in the progress note (57%). The lack of association in this study between pARDS severity and ventilator management at 24 hours was similar to one prior study14, however in another study5 severe pARDS was actually associated with worse adherence to tidal volume ventilation and PEEP goals, suggesting that adherence may be hampered by actual or perceived patient instability.
Provider management of tidal volume and PEEP may relate to multiple aspects of organ system dysfunction in a critically ill child besides lung disease, such as neurologic (impact of pH/PaCO2 on cerebral bloodflow) or cardiovascular (impact of pH or mean airway pressure on cardiac output) considerations. Patients with PEEP non-adherence at 24 hours had significantly higher PIM3 risk of mortality, suggesting that overall severity of illness may have been related to PEEP titration. Patients with head trauma and intracranial pressure monitoring actually had a relatively high adherence (5/6, 83%) to tidal volume ≤8 ml/kg in this study, however the pCO2 remained <40 in the majority (80%) of head trauma patients with tidal volume adherence, suggesting that permissive hypercapnia was not necessary. In patients on vasopressor infusions, there was lower observed adherence to both tidal volume (42% adherence in patients on vasopressors vs 62% in those not on vasopressors) and PEEP (42% adherence on vasopressors vs 57% not) at 24 hours, but these results were not statistically significant. These findings could indicate concerns about hypercapnia and acidosis with low tidal volume use, consistent with a survey of providers for adults with ARDS18. Among those with tidal volume non-adherence there was a significantly higher percentage of patients with pH between 7.15–7.30 (26% vs 6% of those with tidal volume adherence), with a significantly lower pCO2 (median 40 vs. 48 in those with tidal volume adherence). However, the pH was similar and within normal parameters in patients on vasopressors regardless of tidal volume or PEEP adherence, suggesting that cardiovascular stability may not have been compromised by lowering tidal volume or increasing PEEP even on vasopressors. Finally, in study patients with brain death by 72 hours after meeting ARDS hypoxemia criteria (n=5), only 40% met tidal volume goal and 20% met PEEP goal at 24 hours, suggesting that de-prioritization of lung protection may have taken in place in patients with non-survivable neurologic injury.
The strongest association with use of tidal volume ≤8 ml/kg in our study was weight category, suggesting that practitioners may not be routinely recognizing or using ideal body weight for ventilator management, consistent with previous studies in children with ARDS5,14. The poor documentation of overweight/obesity (3/25, 12%) in the progress notes is consistent with previous studies reporting weight status documentation in only 8.3–26% pediatric inpatients with obesity29–30. Furthermore, there was a higher observed tidal volume adherence (though not statistically significant) in patients on a pressure control mode of ventilation, suggesting that when actual body weight was used instead of ideal, a volume control setting (presumably used to attempt to actively maintain appropriate tidal volumes) was actually more likely to deliver potentially injurious tidal volumes. The association between age and lower tidal volume use disappeared when actual body weight was used, with a particular difference in patients 6–18 years old. These findings suggest that increasing provider awareness of overweight/obesity and ideal body weight, especially in children ≥6 years old, may be an attractive target to improve use of lower tidal volume ventilation.
The strengths of this study include the rigorous approach to identifying cases of pARDS using medical record data combined with chart review. Many features of care were able to be extracted that have been rarely evaluated in other studies, including documentation of pARDS or weight/obesity status in the progress note, tracheostomy status, pH, severity of illness by both PIM3 and PRISM III, vasopressor infusions, attending years of experience, and head trauma. Furthermore, since these data were collected under usual care, any potential impact of the Hawthorne effect in the setting of a prospective study of pARDS was not present.
The primary weakness of this study is its single center nature, however, the similarity of the population characteristics as well as the use of tidal volume and PEEP to other reports1, 4–5, 14 suggest that associations reported here may be generalizable. Nonetheless, given the relatively low numbers from a single center, these results can primarily be considered as hypothesis-generating to guide future quality improvement investigations, especially with respect to documentation in the daily progress note since this may not be reflective of care team awareness of pARDS. Additionally, although the PALICC guidelines recommend tidal volume use ≤8 mL/kg, the association between tidal volume and outcome in children with pARDS31–33, as well as the appropriate calculation of ideal body weight in children24, are sources of substantial inconsistency in the literature, and it is unclear what effect this ambiguity may have had on provider decision-making especially when peak inspiratory pressure was not high. Similarly, the ARDSnet tables for PEEP and FIO2 have been available since their publication in 20006, but these PEEP parameters are not explicitly recommended in the pARDS guidelines and the strongest data supporting their use in children were published during the last year of the data collected in this study4. The study was designed to evaluate adherence, and there was lower observed severity of illness in patients with tidal volume and PEEP adherence, therefore the lower mortality in these patients should be interpreted with caution. The outcomes were also evaluated as point-in-time rather than continuous or average values for tidal volume and PEEP, which facilitated comparison with some previous studies9,14 allowed time for clinicians to recognize pARDS and titrate settings, and corresponded with other data points such as progress note documentation, but may have not have reflected true values for these parameters if settings were changed frequently. A ventilator protocol was not used in this study, although use of such protocols has not been quantified in previous studies of lung-protective ventilation in pARDS5,14, and their utility is unknown. Finally, while alternate ventilator modalities and use of inhaled nitric oxide are not routinely recommended in pARDS20and were not evaluated in this study, both can be considered in certain clinical situations and their use may influence variations in provider decisions regarding escalation in tidal volume and PEEP. The relatively low number of patients on alternate ventilator modalities at 24 hours (n=4) may reflect different practice patterns than some other PICUs.
Overall, it appears that providers in this study had a usual comfort range of ventilator parameters and set the tidal volume and PEEP within those ranges, around 7–8 mL/kg (IQR 6.3–8.1 based on actual body weight) and PEEP 5–10, but were not responsive to patient factors that should have prompted adjustment, especially overweight/obesity and escalating FIO2. There also appeared to be a usual comfort range of pH (>7.30), pCO2 (<60), and SpO2 (≥92%) that providers often accepted without adjusting settings when pARDS was present. Additionally, providers did not routinely document when a patient had pARDS; documentation was slightly improved in the presence of severe pARDS. Progress notes in this study included “respiratory failure” in the note much more frequently than “pARDS”, perhaps because this phrase is considered sufficient to justify critical care billing 34 and thus becomes routine unless more severe hypoxemia prompts pARDS recognition. Patients with tidal volume and PEEP nonadherence generally had higher observed severity of illness scores and lower pH, suggesting that overall critical status led to decreased recognition or prioritization of tidal volume and PEEP adherence, not necessarily attributable to elevated PIP, severe acidosis with pH <7.15, hemodynamic instability, or head trauma. We hypothesize that the system of care in place, specifically the lack of prepopulated fields to aid the provider in identifying pARDS hypoxemia criteria or ideal body weight, as well as lack of decision tools to streamline evidence-based management (such as PEEP/FIO2 tables) could be an important reason for the observed tidal volume and PEEP nonadherence. Integration of such tools, streamlined into workflow either in the electronic medical record or at the bedside, could ease cognitive burden on providers especially in the setting of more critically unstable patients and improve evidence-based management of pARDS.
Conclusions
In a single PICU in the United States, children with pARDS did not receive appropriate tidal volume for ideal body weight nor PEEP. Interventions to increase the use of appropriate tidal volume may include improving provider awareness and documentation of pARDS and ideal body weight, while interventions for improving the use of appropriate PEEP may target patients receiving FIO2≥50%.
Supplementary Material
Financial Support:
This project was supported by a small pilot grant from the Wake Forest Center for Biomedical Informatics via the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: The authors declare that there are no conflicts of interest.
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Khemani RG, Smith L, Lopez-Fernandez YM, Kwok J, Morzov R, Klein MJ, Yehya N, Willson D, Kneyber MCJ, Lillie J, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Resp Med 2019; 7(2):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keim G, Watson RS, Thomas NJ, Yehya N. New morbidity and discharge disposition of pediatric acute respiratory distress syndrome survivors. Crit Care Med 2018; 46:1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, Wilkins B. Acute lung injury in pediatric intensive care in Australia and New Zealand – A prospective, multicenter, observational study. Pediatric Crit Care Med 2007; 8(4):317–323. [DOI] [PubMed] [Google Scholar]
- 4.Khemani RG, Parvathaneni K, Yehya N, Bhalla AK, Thomas NJ, Newth CJL. Positive end-expiratory pressure lower than the ARDS Network protocol is associated with higher pediatric acute respiratory distress syndrome mortality. Am J Respir Crit Care Med 2018; 198(1):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhalla AK, Klein MJ, Emeriaud G, Lopez-Fernandez YM, Napolitano N, Fernandez A, Al-Subu AM, Gedeit R, Shein SL, Nofziger R, et al. Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology (PARDIE) V.2. Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Adherence to Lung-Protective Ventilation Principles in Pediatric Acute Respiratory Distress Syndrome: A Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology Study. Crit Care Med 2021; 49(10):1779–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342(18):1301–1308. [DOI] [PubMed] [Google Scholar]
- 7.Needham DM, Yang T, Dinglas VD, Mendez-Tellez PA, Shanholtz C, Sevransky JE, Brower RG, Pronovost PJ, Colantuoni E. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med 2015; 191(2):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, Finkel B, Gallop R, Fuchs BD. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med 2006; 34(2):300–306. [DOI] [PubMed] [Google Scholar]
- 9.Young MP, Manning HL, Wilson DL, Mette SA, Riker RR, Leiter JC, Liu SK, Bates JT, Parsons PE. Ventilation of patients with acute lung injury and acute respiratory distress syndrome: has new evidence changed clinical practice? Crit Care Med 2004;32(6):1260–1265. [DOI] [PubMed] [Google Scholar]
- 10.Umoh NJ, Fan E, Mendez-Tellez PA, Sevransky JE, Dennison CR, Shanholtz C, Pronovost PJ, Needham DM. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med 2008;36(5):1463–1468. [DOI] [PubMed] [Google Scholar]
- 11.Chen YF, Lim CK, Ruan SY, Jerng JS, Lin JW, Kuo PH, Wu HD, Yu CJ. Factors associated with adherence to low-tidal volume strategy for acute lung injury and acute respiratory distress syndrome and their impacts on outcomes: an observational study and propensity analysis. Minerva Anestesiol 2014;80(11):1158–1168. [PubMed] [Google Scholar]
- 12.Santamaria JD, Tobin AE, Reid DA. Do we practise low tidal-volume ventilation in the intensive care unit? a 14-year audit. Crit Care Resusc 2015;17(2):108–112. [PubMed] [Google Scholar]
- 13.Khemani RG, Sward K, Morris A, Dean JM, Newth CJL; NICHD Collaborative Pediatric Critical Care Research Network (CPCCRN). Variability in usual care mechanical ventilation for pediatric acute lung injury: the potential benefit of a lung protective computer protocol. Intensive Care Med 2011;37(11):1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward SL, Quinn CM, Valentine SL, Sapru A, Curley MAQ, Willson DF, Liu KD, Matthay MA, Flori HR. Poor adherence to lung-protective mechanical ventilation in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med 2016; 17: 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newth CJL, Sward KA, Khemani RG, Page K, Meert KL, Carcillo JA, Shanley TP, Moler FW, Pollack MM, Dalton HJ, et al. Variability in usual care mechanical ventilation for acute respiratory distress syndrome: time for a decision support protocol? Pediatr Crit Care Med 2017; 18(11):e521–e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Midega TD, Bozza FA, Machado FR, Guimaraes HP, Salluh JI, Nassar AP Jr, Normilio-Silva K, Schultz MJ, Cavalcanti AB, Neto AS. Organizational factors associated with adherence to low tidal volume ventilation: a secondary analysis of the CHECKLIST-ICU database. Ann Intensive Care 2020;10(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sward KA, Newth CJL, Khemani RG, Page K, Meert KL, Carcillo JA, Shanley TP, Moler FW, Pollack MM, Dalton HJ, et al. Potential acceptability of a pediatric ventilator management computer protocol. Pediatr Crit Care Med 2017; 18:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med 2004; 32(6):1289–1293. [DOI] [PubMed] [Google Scholar]
- 19.Dennison CR, Mendez-Tellez PA, Wang W, Pronovost PJ, Needham DM. Barriers to low tidal volume ventilation in acute respiratory distress syndrome: survey development, validation, and results. Crit Care Med 2007; 35(12):2747–2754. [DOI] [PubMed] [Google Scholar]
- 20.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16(5):428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straney L, Clements A, Parslow RC, Pearson G, Shann F, Alexander J, Slater A; ANZICS Paediatric Study Group and the Paediatric Intensive Care Audit Network. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Me. 2013; 14(7):673–81. [DOI] [PubMed] [Google Scholar]
- 22.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996; 24(5):743–752. [DOI] [PubMed] [Google Scholar]
- 23.McLaren DS, Read WW. Classification of nutritional status in early childhood. Lancet 1972; 2: 146–148. [DOI] [PubMed] [Google Scholar]
- 24.Ward SL, Quinn CM, Steurer MA, Liu KD, Flori HR, Matthay MA. Variability in Pediatric Ideal Body Weight Calculation: Implications for Lung-Protective Mechanical Ventilation Strategies in Pediatric Acute Respiratory Distress Syndrome. Pediatr Crit Care Med 2018; 19(12):e643–e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention, National Center for Health Statistics. Growth Charts. Available at https://www.cdc.gov/growthcharts/index.htm. Accessed January 5th, 2022.
- 26.Phillips S, Edlbeck A, Kirby M, Goday P. Ideal body weight in children. Nutr Clin Pract 2007; 22(2):240–245. [DOI] [PubMed] [Google Scholar]
- 27.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000. CDC growth charts for the United States: Methods and development. Vital Health Stat 11 2002; (246):1–190. [PubMed] [Google Scholar]
- 28.Kelly AS Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013; 128(15): 1689–1712. [DOI] [PubMed] [Google Scholar]
- 29.King MA, Nkoy FL, Maloney CG, Mihalopoulos NL. Physicians and Physician Trainees Rarely Identify or Address Overweight/Obesity in Hospitalized Children. J Pediatr 2015;167(4):816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzow M, Homel P, Rhee K. Factors Associated With Documentation of Obesity in the Inpatient Setting. Hosp Pediatr 2017;7(12):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imber DA, Thomas NJ, Yehya N. Association Between Tidal Volumes Adjusted for Ideal Body Weight and Outcomes in Pediatric Acute Respiratory Distress Syndrome. Pediatr Crit Care Med 2019;20(3):e145–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shein SL, Rotta AT, Piva JP. What Is Weighing Us Down From Elucidating Ideal Ventilation Strategies in Pediatric Acute Respiratory Distress Syndrome? Pediatr Crit Care Med 2019;20(3):303–305. [DOI] [PubMed] [Google Scholar]
- 33.de Jager P, Burgerhof JG, van Heerde M, Albers MJ, Markhorst DG, Kneyber MC. Tidal volume and mortality in mechanically ventilated children: a systematic review and meta-analysis of observational studies. Crit Care Med 2014;42(12):2461–72. [DOI] [PubMed] [Google Scholar]
- 34.Moss MM, Schexnayder SM. Billing and coding in the intensive care unit. Pediatr Clin N Amer 2001; 48(3):783–794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


