Summary
The blood–brain barrier (BBB) is a selectively permeable barrier separating the periphery from the central nervous system (CNS). The BBB restricts the flow of most material into and out of the CNS, including many drugs that could be used as potent therapies. BBB permeability is modulated by several cells that are collectively called the neurovascular unit (NVU). The NVU consists of specialized CNS endothelial cells (ECs), pericytes, astrocytes, microglia, and neurons. CNS ECs maintain a complex “seal” via tight junctions, forming the BBB; breakdown of these tight junctions leads to BBB disruption. Pericytes control the vascular flow within capillaries and help maintain the basal lamina. Astrocytes control much of the flow of material that has moved beyond the CNS EC layer and can form a secondary barrier under inflammatory conditions. Microglia survey the border of the NVU for noxious material. Neuronal activity also plays a role in the maintenance of the BBB. Since astrocytes, pericytes, microglia, and neurons are all able to modulate the permeability of the BBB, understating the complex contributions of each member of the NVU will potentially uncover novel and effective methods for delivery of neurotherapies to the CNS.
Keywords: blood–brain barrier, endothelial cell, glia, neuroinflammatory disease, pericyte, tight junction
1. INTRODUCTION
The blood–brain barrier (BBB) is a complex, highly regulated system with multiple cell types influencing its maintenance and formation. The BBB forms a privileged environment for the central nervous system (CNS), restricting entry of a wide breadth of potential hazards, including pathogens, immune cells, antibodies, and pharmaceuticals. This tight regulation protects the brain from external insults, but simultaneously prevents access of many therapeutics meant to treat neuroinflammatory and neurodegenerative disorders. As a result, multiple clinical trials have failed despite promise in preclinical studies, underscoring the need for a more complete understanding of the BBB and its modulatory mechanisms. In this review, we discuss the composition of the neurovascular unit (NVU), known mechanisms of BBB modulation, and potential therapeutic targets for neuroinflammatory disorders.
The existence of a selectively permeable BBB was first postulated at the turn of the 20th century when it was noted that water‐soluble dyes injected into the periphery did not permeate the CNS, 1 , 2 and dyes injected into the CNS parenchyma did not exit to the periphery. 2 , 3 Further complexity of the BBB was demonstrated by seminal evidence indicating immune privilege, wherein immune cells are excluded and unable to surveille the CNS, over 100 years ago by Shirai 4 and later confirmed by Murphy and Sturm. 5 However, even now, the permeability, complexity, and dynamics of the BBB are incompletely understood. 6 , 7
The BBB acts as a robust biological gateway, responsible for the flow of nearly all molecules and cells passing into and out of the CNS. 6 , 8 , 9 This selective permeability is critical in sparing the CNS from many circulating toxins and pathogens and allowing for availability of necessary nutrients to the CNS. 2 , 10 , 11 Instead of passing through fenestrations in endothelial cells (ECs) as it does in the peripheral vasculature, material is transported and scrutinized by one or more cells closely associated with the BBB. 11 , 12 However, it is this very system, designed to protect the CNS, that also hinders therapeutic development to treat CNS disorders including multiple sclerosis (MS), Alzheimer's disease (AD), Parkinson's disease, traumatic brain injuries, and brain cancers. 10 , 11 , 13 Several researchers have even employed artificial intelligence to determine the likelihood of small molecules to penetrate the BBB. 13 , 14
This review will examine some of the NVU cell‐specific effects on BBB permeability as well as the intercellular communication that is critical for proper function of the NVU. While other complex CNS barriers, including the blood‐retinal barrier, the blood‐cerebrospinal fluid barrier, and the arachnoid layer, 15 are also important to consider in therapeutic availability to the CNS, we will only focus on the BBB for the purposes of this review.
2. STRUCTURE AND ORGANIZATION OF THE NVU
The NVU is the fundamental multicellular unit supporting the BBB and consists of ECs, pericytes, astrocytes, microglia, and neurons (Figure 1). 6 , 7 The capillary lumen is the primary area of contact between the NVU and the periphery, and interfacing factors vary from cytokines and hormones to immune cells that have the potential to extravasate. 16 , 17 Forming the vessel lumen is specialized CNS ECs, which are semipermeable under homeostatic conditions due to the presence of tight junctions. 6 , 16 , 18 , 19 , 20 Perivascular mural cells, or pericytes, have recently been shown to be more complex and varied than initially assumed. Pericytes are functionally heterogeneous 21 , 22 and further understanding of their diverse roles in the CNS is an active area of research. Pericytes were classically described to help govern vasoconstriction and dilation of capillaries, 23 , 24 which is highly regulated in the NVU, to limit BBB permeability. Recent studies are beginning to uncover pericyte coordination with other members of the NVU, particularly ECs and astrocytes. 21 , 24 Astrocytes are vital to the NVU and while the other members of the NVU have critical roles, it is largely the astrocytes that set the NVU apart from other capillary layers in the body. 25 , 26 Understanding the specific mechanisms by which astrocytes regulate tight junctions in CNS ECs could prove incredibly beneficial to optimizing BBB permeability for drug delivery. Astrocytes are relatively large and abundant CNS trophic cells 27 and their endfeet form an additional barrier around CNS capillaries. 6 , 25 The perivascular, or Virchow‐Robin, space refers to the area between ECs and astrocytes and is a key checkpoint prior to entering the CNS parenchyma. 6 , 28 Microglia are a recent addition to our understanding of the NVU. Although astrocytes cover the majority of the perivascular space, there are some gaps in coverage. Recently, Kisler et al. used two‐photon in vivo imaging to observe microglial processes covering many of these gaps. 29 How involved microglia are in the structure of the NVU is still being investigated. By area, neurons have a relatively minor role in the structure of the NVU but can modulate BBB permeability via neuronal activity. 30 , 31 Due to the complex nature of the NVU, it has proven difficult to study. In vitro models have been useful; however, the heterogeneity between species, individuals, and even CNS regions have added to this investigational barrier. 32 , 33 Here, we will discuss the components of the NVU and how each cell type may impact BBB permeability.
FIGURE 1.
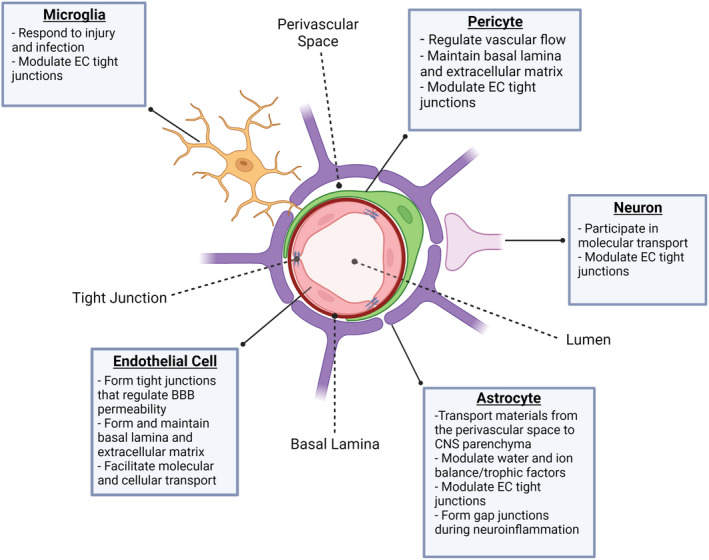
Cellular components of the neurovascular unit. The NVU is composed of a complex network of cells that are functionally diverse. ECs form the walls of blood vessels and capillaries and contribute to the formation and maintenance of the basal lamina and extracellular matrix. In addition, tight junctions formed between ECs and expression of adhesion molecules regulate BBB permeability. Pericytes reside in the capillary bed and, with regard to BBB integrity, are primarily responsible for modulation of vascular flow as well as structural changes in tight junctions and the extracellular matrix. Astrocyte endfeet cover 90%–95% of the area surrounding the BBB and contribute to a variety of processes that include, but are not limited to, osmotic homeostasis, trophic factor concentration, molecular transport into and out of the perivascular space, and formation of gap junctions under neuroinflammatory conditions. Microglia have been found to cover the remaining space around BBB ECs, respond to injury and infection, and regulate tight junction formation between ECs. Neurons predominantly communicate with astrocyte endfeet and aid in regulation of tight junctions and molecular transport.
3. ENDOTHELIAL CELLS
ECs are found throughout the body and make up the walls of arteries, veins, and capillaries. They form a tube‐like structure to allow passage of various blood cells and proteins. 34 ECs secrete and maintain the basal lamina—the extracellular matrix on which they reside. 35 , 36 The CNS ECs differ in several ways from peripheral ECs. Most notably, they lack the fenestrations found in peripheral vessels and instead create a continuous cellular barrier with significantly reduced permeability along the capillary lumen. 37 Instead, CNS ECs form tight junctions which prevent hydrophobic molecules from penetrating the endothelial layer unless transported through the cell. These tight junctions are responsible for much of the impermeability of the BBB. Tight junctions are composed primarily of claudin‐5, occludin, and other junctional adhesion molecules 38 and bind to the actin cytoskeleton via ZO‐1, ZO‐2, or ZO‐3. 39 Tight junctions are highly complex and can vary depending on the proteins coupled. 40 , 41 Transport of nutrients, waste, and signaling molecules between the CNS and the periphery is necessary under physiological conditions and is typically achieved by a myriad of EC transporters. 6 , 42 In fact, these transporters make up 10–15% of the total protein in CNS ECs and allow specific molecules, peptides, and even cells to cross into the perivascular space, bypassing tight junctions. 33 , 43
3.1. ECs and immunity
Classically, it was thought that immune cells could not access the CNS parenchyma. 18 , 44 While mostly impermeable during homeostatic conditions, CD4+ and CD8+ T cells are allowed passage for immune surveillance of the CNS, facilitated by a highly regulated, multistep process through a tricellular junction. 45 This process involves endothelial ligands vascular cell adhesion molecule (VCAM)‐1 and intercellular adhesion molecule (ICAM)‐1 which recognize lymphocyte function‐associated antigen 1 (LFA‐1) and very late antigen‐4 (VLA‐4) on T cells. These interactions arrest T cells on the endothelium and allow migration against the flow of blood to these tricellular junctions and ultimately extravasation. 45 , 46 These tricellular junctions contain the proteins tricellulin and angulin‐1 which direct and aid T‐cell diapedesis. 45 While CNS ECs express VCAM‐1 and ICAM‐1 under normal conditions, the expression is sparse, limiting the number of leukocytes that can traverse tricellular junctions. 45 , 47
In response to infection, autoimmunity, or injury, the permeability of the BBB is significantly enhanced and can lead to severe inflammation. 48 This is partially due to an increase in the expression of adhesion molecules on the EC surface, primarily ICAM‐1 and VCAM‐1, which can arrest a greater number of immune cells for extravasation. 49 Inflammation can lead to break down of the tight junctions, allowing leukocytes to invade the perivascular space. 48 Many secreted inflammatory factors, cell damage signals, and pathogen components can alter tight junction integrity including CCL2 and transforming growth factor (TGF)‐β, which is known to alter expression of claudin‐5, occludin, and ZO‐1. 39 , 50 , 51 Likewise, tumor necrosis factor (TNF)‐α, lipopolysaccharide (LPS), and mitochondrial damage can induce BBB permeability via actin filament rearrangement. 52 , 53 , 54 , 55 Prolonged dysfunction of the BBB, as in chronic inflammatory CNS diseases such as MS or AD, can lead to permanent CNS tissue damage and neuroaxonal loss. 56 , 57 , 58 However, although overt and/or chronic inflammation can lead to the loss of BBB integrity and extensive damage to the CNS, low‐to‐moderate amounts of inflammation can partially restore the BBB and limit peripheral immune cell infiltration into the CNS parenchyma, mitigating injury, or infection. 56 , 59 , 60 , 61 Taking advantage of this immunological state and exploiting molecular signals used in the formation of tricellular junctions may be a novel avenue for therapeutic development.
3.2. Targeting ECs
It is possible to permeate the BBB to treat neurological disorders, but the potential bystander effects of leaky BBB during diseases such as glioblastoma, MS, AD, and others imposes significant risk. The BBB is disrupted in many neurodegenerative and psychological disorders. Additional access of immune cells, toxins, or other inflammatory mediators could enhance inflammation‐mediated damage to the CNS. Additionally, BBB dysregulation disrupts homeostatic transport across the barrier, which provides trophic factors and controls osmotic regulation during physiological conditions. 62 Bypassing the ECs of the BBB is a difficult challenge; however, there are several other members of the NVU that introduce potentially novel avenues for drug targeting.
4. PERICYTES
Proportionally, there is a higher density of pericytes in the CNS relative to peripheral organs, 24 suggesting they have a critical role in the CNS. As vascular mural cells, pericytes play a large role in the dilation of capillaries in the NVU. Of note, pericytes are found in the capillary bed and not surrounding arteries or veins, which house similar, but distinct, vascular mural cells, and vascular smooth muscle cells. 63 In addition to vasodilation and vasoconstriction, pericytes also help form and maintain the basal lamina. 64 , 65 Control of vascular flow by pericytes can indirectly impact the BBB as changes in blood flow can allow for more or less cellular contact in the capillary lumen. Increases in cellular interaction can stretch and stress CNS ECs, 66 , 67 , 68 causing pericyte dysfunction, which is associated with aberrant immune cell trafficking. 69 Further, in mouse models deficient in functional pericytes, there is a loss of vascular control, dysfunctional tight junction regulation, and aberrant angiogenic sprouting, highlighting the importance of pericytes in maintaining a healthy BBB. 69 , 70 , 71 , 72 , 73
New roles for pericytes continue to be uncovered, including their ability to maintain and produce elements of the basal lamina 74 , 75 , 76 , 77 and intricate regulation of tight junctions. Contrary to ECs, TGF‐β and angiopoietin‐1 (Ang‐1) signaling in pericytes enhances the expression of occludin on CNS ECs, reducing the permeability of the BBB. TGF‐β from pericytes also activates Smad4 signaling to upregulate bone morphogenic proteins (BMPs). BMPs can then ensure tight adherence of pericytes to ECs via N‐cadherins, which is upregulated by vascular endothelial growth factor (VEGF), reinforcing the communication and physical interaction between pericytes and CNS ECs. 78 , 79 , 80 While basal levels of VEGF signaling can reduce BBB permeability, excessive VEGF signaling to pericytes can lead to downregulation of claudin‐5 on ECs and dysregulate BBB tight junctions (Figure 2). 81 , 82 , 83 , 84
FIGURE 2.
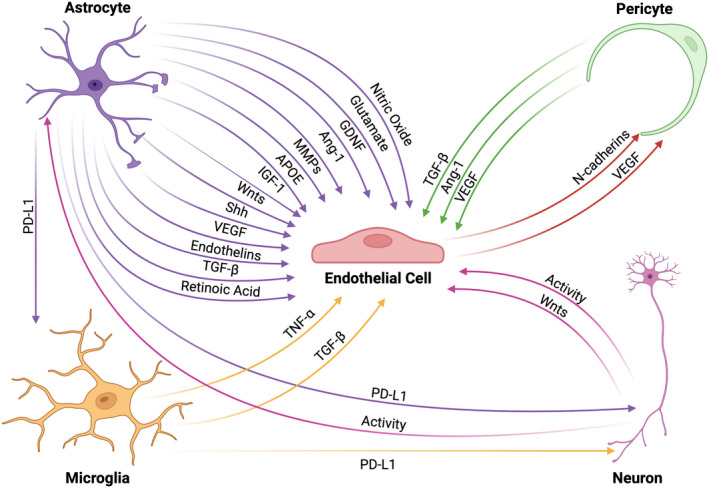
NVU interaction network. Cells within the NVU interact through intricate signaling mechanisms that allow for proper functioning of the BBB. ECs receive a variety of protective signals from other NVU cells that upregulate tight junction formation thus enhancing BBB integrity. These factors include, but are not limited to, TGFβ, Ang‐1, APOE, Shh, Wnts, glial‐derived neurotrophic factor, insulin‐like growth factor (IGF)‐1, and retinoic acid. Contrastingly, ECs may also receive signals that downregulate tight junction proteins, particularly during inflammatory events, such as TNFα, NO, MMPs, endothelins, and glutamate that lead to increased BBB permeability. Importantly, ECs also maintain the ability to signal to pericytes through VEGF and N‐cadherin, which are necessary for BBB maintenance. Immune checkpoint proteins, such as the PD‐1/PD‐L1 complex, regulate the cellular activity of microglia and neurons to modulate BBB integrity as well as dampen the inflammatory activity of infiltrating peripheral immune cells, which increases BBB permeability. Finally, neuronal activity is a critical modulator of cellular and molecular transport across the BBB.
4.1. Heterogeneity of pericytes
Similar to ECs, pericytes respond robustly to various signaling molecules. Inflammatory and non‐inflammatory pericytes have been described and subdivided into Type‐1 pericytes (PC1) and Type‐2 pericytes (PC2). PC1s are non‐inflammatory and tend to be the resting state of pericytes without injury or infection. PC2s tend to increase in frequency with and are highly responsive to inflammation. 73 , 85 It is likely, as is the case with astrocytes and microglia that there is a spectrum of activation states, but as the study of pericytes is still in its infancy, this has not yet been fully elucidated. In young healthy patients, nearly all pericytes exhibit a non‐inflammatory morphology. 71 , 85 , 86 However, aging results in an increase in the population of inflammatory pericytes, which is consistent with enhanced inflammation and BBB permeability associated with age. 85 Both pericyte subtypes contribute to the basal lamina, but PC2s tend to produce a more irregular basal lamina, impacting both ECs and astrocytes. 21 , 85 Additionally, PC2s produce less laminin‐111 and laminin‐211, which leads to cell hypertrophy and BBB disruption. 87 , 88 Exposing pericytes to inflammatory cytokines, LPS, or reactive oxygen species induces immunoreactivity in pericytes, altering their morphology, as well as inducing their separation from the basal lamina. 87 , 88 , 89 The dynamic morphology of pericytes suggests they have a critical role in the integrity and function of the BBB, which is altered in pathological states and in aging.
4.2. Targeting pericytes
Examining the targetability of pericytes and its potential influence on the BBB is difficult but may prove promising. Affecting pericytes may be a subtler approach to drug penetrance rather than reducing the integrity of the EC layer itself. A deeper understanding of how pericytes communicate with CNS ECs and astrocytes could reveal potential nuanced approaches to bypass the BBB. While pericytes may be a promising target to leverage BBB permeability, much about pericyte mechanisms of BBB control remains unknown.
5. ASTROCYTES
Astrocytes are large stellate cells with extensive processes that extend throughout the CNS. 27 , 90 However, unlike CNS ECs and pericytes, astrocytes are unique in that only the ends of their processes are considered part of the NVU (Figure 1). These endfeet cover roughly 90–95% of the area surrounding the BBB and have properties that are unique from the rest of the astrocyte. 91 , 92 , 93 Endfeet contain aquaporin‐4 and the potassium channel Kir4.1 to modulate water and ion balance and, lacking tight junctions, astrocytic endfeet permit immune cell extravasation. 92 , 94 , 95 Although endfeet lack the barrier that tight junctions provide, ablation of astrocytes leads to rapid and extensive BBB deterioration. 25 , 93 And interestingly, early studies transplanting astrocytes outside of the CNS demonstrated that transplanted astrocytes develop a BBB‐like morphology surrounding the peripheral vasculature. 96 , 97 In addition to the physical barrier that astrocytes provide, during homeostasis, astrocytes are responsible for the transport of material from the perivascular space into the parenchyma. 6 , 25 This function makes them excellent and critical supportive cells in the maintenance of the BBB.
Astrocytes form gap junctions between endfeet forming a much “looser” network of connections compared with that of CNS ECs. 98 Gap junctions are formed by a hexamer of proteins called connexins. The material that is transported by a given gap junction is largely dependent on the specific combination of connexins that make up the junction. 99 , 100 Notable gap junctions in astrocytes are formed by connexin 30 and connexin 43, which help mediate glucose and lactate transport to distal neurons. 101 Astrocytes use these gap junctions to communicate with other cells of the NVU including neurons, microglia, and other astrocytes using ion gradients, electrical signals, and signaling molecules. 102
5.1. Tight junction modulation
Astrocytes release a variety of trophic factors that help maintain a functional NVU. 103 , 104 , 105 Many of the factors released under physiologic conditions increase the amount and order of tight junction proteins between BBB ECs. 106 , 107 For instance, astrocytes secrete sonic hedgehog (Shh), which increases tight junctions in CNS ECs by inducing Patch1 signaling. 107 Other astrocytic factors that can enhance tight junctions in ECs include Wnt signaling, TGFβ, and apolipoprotein E (ApoE). 104 , 106 , 108 , 109 , 110 ApoE and TGFβ may work indirectly through pericytes to impact EC tight junctions. 111 , 112 Additionally, astrocytes produce Ang‐1, which signals to EC Tie2 to further upregulate tight junction proteins and reduce adhesion molecules, reducing the likelihood of leukocyte entry. 113 , 114 Further, astrocytes are incredibly responsive to inflammatory stimuli, 115 , 116 and during inflammation, it has been shown that BBB ECs downregulate claudin‐5, while astrocytes upregulate tight junction proteins claudin‐1, claudin‐4, and JAM‐A. 117 , 118 This suggests that astrocyte endfeet form a secondary barrier to prevent excessive immune cell entry past the perivascular space, regulating access to the CNS parenchyma. 117 This accumulation of peripheral immune cells in the perivascular space is often referred to as perivascular cuffing and is commonly seen in MS lesions. 117 , 119
However, although astrocytes provide an impressive physical barrier, activated astrocytes can also secrete trophic factors, chemokines, and cytokines that dysregulate EC tight junctions and recruit peripheral immune cells (Figure 2). 25 , 39 , 119 , 120 These factors can include VEGF, nitric oxide, MMPs, endothelins, and glutamate; all of which can downregulate endothelial tight junction proteins. 39 , 121 , 122 Importantly, astrocytes also promote a return to homeostasis following an inflammatory event, secreting several beneficial trophic factors including Shh, astrocyte‐derived Ang‐1, glial‐derived neurotrophic factor (GDNF), insulin‐like growth factor‐1, ApoE, and retinoic acid. These factors not only prevent EC apoptosis, but also stimulate tight junction formation and a return to a homeostatic state. 116 , 121 Taken together, astrocytes orchestrate a complex modulation of the BBB and may represent an amenable cell type for drug manipulation.
5.2. Astrocytes as an immunologic barrier
Since astrocytes are highly responsive to immune stimuli, they have the ability to upregulate a wide array of chemokines and adhesion molecules that can serve as an immunologic barrier to prevent excessive influx of inflammatory cells during a CNS insult (Figure 2). 120 , 123 Interestingly, in the event immune cells breach the BBB, enter the perivascular space, and cross astrocyte endfeet into the parenchyma, astrocytes can express immune checkpoint molecules, or inhibitory receptors, which can induce exhaustion and death of leukocytes, 124 , 125 providing the CNS multiple layers of immunologic protection. As an example, astrocytes are known to upregulate the immune checkpoint protein programmed death ligand 1 (PD‐L1) in response to inflammatory cytokines, primarily interferons. 126 PD‐L1 typically signals to cells via programmed death 1 (PD‐1) to reduce activation and induce apoptosis. 127 , 128 While many CNS cells, including microglia and even neurons, express PD‐1, it is highly enriched on immune cells, making them particularly susceptible to exhaustion. 126 , 127 , 128 , 129 In summary, astrocytes act not only as a physical barrier, but also a trophic and immunologic barrier to limit and control the activation state of immune cells that enter the CNS during neuroinflammation.
5.3. Targeting astrocytes
Astrocytes have great potential as a therapeutic target for BBB maintenance. Their ability to weaken EC tight junctions while maintaining a barrier could prove beneficial in the development of drugs to bypass traditional CNS barriers. There are many examples of astrocytes guiding various cell types and molecules across the BBB. This principle—if better understood—could be leveraged to advance CNS drug permeability. Similar to pericytes, astrocytes have great influence on the maintenance of EC tight junctions. Elucidating the molecular control that astrocytes have over BBB permeability will inevitably lead to more avenues of drug delivery.
6. MICROGLIA
Microglia are the resident immune cells of the CNS. As such, their functions are multifaceted. While they share many similarities with peripheral macrophages, they have many distinct characteristics, including their origins. Macrophages are generated in the bone marrow and circulate in the blood, while microglia migrate from the embryonic yolk sac. 130 In general, microglia are responsible for initial responses to injury and infection, clearance of waste, synaptic pruning and maintenance, as well as providing several trophic factors to other cells of the NVU. 130 Although it has been long appreciated that microglia is vital to the health of the CNS, surveying along the BBB and responding quickly to breaches, they are a relatively recent addition to the NVU and provide coverage of BBB areas not wrapped by astrocytes. 29 , 131 In addition, microglia are known to signal to other members of the NVU and communicate with peripheral immune cells, 6 attracting and/or activating them within the CNS parenchyma during injury and infection as sentinels of the CNS. 132 , 133 , 134 , 135 Activated microglia and peripheral immune cells can secrete a number of factors that modulate BBB integrity (Figure 2). This is discussed in a number of reviews, but as an example, TNF‐α and TGF‐β can be secreted from reactive microglia under certain conditions which can either increase or decrease BBB integrity, respectively. 123 , 136 , 137 While ablation or depletion of microglia did not result in overt BBB breakdown, 138 recently, microglia was described to intricately associate with CNS capillaries and contribute to blood flow regulation and vasodilation. 139 , 140 , 141 Additionally, microglia are incredibly motile and migrate quickly in response to injury, BBB leakage, and/or inflammation. 142 Finally, similar to astrocytes, microglia can upregulate PD‐L1 in response to neuroinflammation, 143 , 144 providing multiple mechanisms of potential control of the BBB by microglia, although there is still much to learn about the intricacies of microglia and their impact on BBB permeability.
Given their emerging role in the NVU, the mechanisms underlying microglial regulation of the BBB remain incompletely described. Targeting microglia may modify CNS capillary permeability or other NVU cells to temporarily allow access to the CNS, but there is still much to learn about this exciting new potential target.
7. NEURONS
Neurons are the functional unit of the CNS and most other cells in the CNS support them, either directly or indirectly. 27 Despite their indispensable role in the CNS, as a member of the NVU, they do not directly provide a physical barrier, but instead release a number of factors that modulate other NVU cells 27 , 145 using primarily their axons and dendrites. 6 Interestingly, like astrocytes, neurons can induce a BBB‐like barrier in neighboring ECs, 96 , 97 suggesting neurons influence the formation and maintenance of the BBB in vivo. 146 , 147 Astrocytes and neurons are highly communicative, 148 , 149 providing a potential route of BBB control. Additionally, neural activity can influence the integrity of the BBB, 150 , 151 , 152 although it is unclear if neural activity directly impacts ECs or if neuronal signals are propagated through astrocytes. 153
Neuroinflammation can alter the activity of neurons, which in turn can lead to alterations in the BBB. 150 , 154 As mentioned above, neurons express PD‐1, to which astrocytes and microglia can signal via PD‐L1 expression. 127 , 128 , 129 Neuronal PD‐1 signaling does not induce apoptosis, but instead hyperpolarizes the neuronal membrane to inhibit action potential frequency. 129 This in turn could lead to a reduction in activity‐dependent transport across the BBB. 155 Neurons also produce Wnt ligands which reduce BBB permeability by increasing tight junction proteins in ECs. 156 While neurons partially modulate the BBB via activity, they largely rely on communication with other NVU members (Figure 2). While more information is necessary to determine how neurons might control the BBB, neurons represent an unlikely target for BBB permeability modulation given their moderate impact on the BBB and other essential functions.
8. FUTURE DIRECTIONS
Each cellular component of the NVU has both unique and overlapping roles in maintaining BBB permeability. ECs are the initial barrier to the periphery, reinforced with tight junctions, specialized to transport material between the periphery and the CNS. Pericytes, and to some extent, microglia, regulate vascular flow and, while they may also have a structural role, pericytes provide critical trophic support to ECs. Astrocytes, and potentially neurons, are critical to the formation of the BBB, aiding in tight junction formation. Microglia and neurons also potentially modulate BBB permeability through complex signaling networks, although this is an emerging field. The intricate dynamics of BBB formation, function, and maintenance has created a literal and figurative barrier when it comes to therapy development for the treatment of chronic neurologic diseases such as glioblastoma, MS, and AD. While it is possible to increase the permeability of the BBB, this typically results in detrimental off target effects. The BBB is disrupted in many neurological diseases, thus additional interventional disruption would likely prove deleterious as unwanted CNS “intruders” including immune cells and large molecules are able to enter the CNS without proper scrutiny, leading to a cascade of osmotic, trophic, and inflammatory dysregulation. Thus, optimizing targeted and temporary entry of therapeutics into the CNS with minimal BBB dysregulation is the holy grail of next‐generation neurotherapeutics. Promising modalities include encapsulating drugs of interest in a form that allows them to be transported through the BBB and astrocyte endfeet or creating a transient passage that is quickly repaired. Both of these methods are active areas of research using nanoparticles and ultrasonic disruption of the BBB, respectively. 12 , 157 , 158 Ultimately, a deeper understanding of how each of the NVU components modulate CNS drug accessibility may shed new light on actionable therapeutic modalities.
AUTHOR CONTRIBUTIONS
All authors contributed to the writing, editing, and review of the manuscript.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGEMENTS
We apologize to authors whose work could not be referenced in this review due to scope and space limitations. Graphical illustrations were made using BioRender (https://biorender.com/). This work was supported by grants from the CSU CD‐CAVS training grant NIH T32 HL150389 (awarded to Brandon C. Smith), NIH K00 NS120365 (awarded to Benjamin C. Shaw), NIH R01 NS119178 (awarded to Jessica L. Williams).
Smith BC, Tinkey RA, Shaw BC, Williams JL. Targetability of the neurovascular unit in inflammatory diseases of the central nervous system. Immunol Rev. 2022;311:39‐49. doi: 10.1111/imr.13121
This article is part of a series of reviews covering Neuroimmunology appearing in Volume 311 of Immunological Reviews
DATA AVAILABILITY STATEMENT
There are no data in this manuscript.
REFERENCES
- 1. Biedl A, Kraus R. Über eine bisher unbekannte toxische Wirkung der Gallensäuren auf das Zentralnervensystem. Zentralblatt Inn Med. 1898;19:1185‐1200. [Google Scholar]
- 2. Goldmann EE. Vitalfärbung am Zentralnervensystem: Beitrag zur Physio‐Pathologie des Plexus chorioideus und der Hirnhäute. Königl. Akademie der Wissenschaften; 1913. [Google Scholar]
- 3. Dreser H. Ueber den experimentellen Nachweis der Vertiefung und Verlangsamung der Athemzüge nach therapeutischen Heroingaben. Archiv für Die Gesamte Physiologie Des Menschen Und der Tiere. 1900;80:86‐95. doi: 10.1007/BF01661930 [DOI] [Google Scholar]
- 4. Shirai Y. On the transplantation of the rat sarcoma in adult heterogenous animals. Jap Med World. 1921;1:14‐15. [Google Scholar]
- 5. Murphy JB, Sturm E. Conditions determining the transplantability of tissues in the brain. J Exp Med. 1923;38:183‐197. doi: 10.1084/jem.38.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Segarra M, Aburto MR, Acker‐Palmer A. Blood–brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021;44:393‐405. doi: 10.1016/j.tins.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 7. Salmina AB, Komleva YK, Malinovskaya NA, et al. Blood–brain barrier breakdown in stress and neurodegeneration: biochemical mechanisms and new models for translational research. Biochemistry (Mosc). 2021;86:746‐760. doi: 10.1134/S0006297921060122 [DOI] [PubMed] [Google Scholar]
- 8. Kolářová H, Ambrůzová B, Švihálková Šindlerová L, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm. 2014;2014:694312. doi: 10.1155/2014/694312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood‐brain barrier. Proc Natl Acad Sci U S A. 2018;115:E9429‐E9438. doi: 10.1073/pnas.1802155115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P‐glycoprotein in the blood‐brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517‐2524. doi: 10.1172/JCI118699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li J, Zheng M, Shimoni O, et al. Development of novel therapeutics targeting the blood–brain barrier: from barrier to carrier. Adv Sci (Weinh). 2021;8:2101090. doi: 10.1002/advs.202101090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anthony DP, Hegde M, Shetty SS, Rafic T, Mutalik S, Rao BSS. Targeting receptor‐ligand chemistry for drug delivery across blood‐brain barrier in brain diseases. Life Sci. 2021;274:119326. doi: 10.1016/j.lfs.2021.119326 [DOI] [PubMed] [Google Scholar]
- 13. Nicolazzo JA, Charman SA, Charman WN. Methods to assess drug permeability across the blood‐brain barrier. J Pharm Pharmacol. 2006;58:281‐293. doi: 10.1211/jpp.58.3.0001 [DOI] [PubMed] [Google Scholar]
- 14. Liu L, Zhang L, Feng H, et al. Prediction of the blood–brain barrier (BBB) permeability of chemicals based on machine‐learning and ensemble methods. Chem Res Toxicol. 2021;34:1456‐1467. doi: 10.1021/acs.chemrestox.0c00343 [DOI] [PubMed] [Google Scholar]
- 15. Strazielle N, Ghersi‐Egea JF. Physiology of blood–brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol Pharm. 2013;10:1473‐1491. doi: 10.1021/mp300518e [DOI] [PubMed] [Google Scholar]
- 16. Ando Y, Okada H, Takemura G, et al. Brain‐specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep. 2018;8:17523. doi: 10.1038/s41598-018-35976-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaragozá R. Transport of amino acids across the blood‐brain barrier. Front Physiol. 2020;11:973. doi: 10.3389/fphys.2020.00973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pachter JS, de Vries HE, Fabry Z. The blood‐brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62:593‐604. doi: 10.1093/jnen/62.6.593 [DOI] [PubMed] [Google Scholar]
- 19. Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280:H434‐H440. doi: 10.1152/ajpheart.2001.280.1.H434 [DOI] [PubMed] [Google Scholar]
- 20. Liu W‐Y, Wang Z‐B, Zhang L‐C, Wei X, Li L. Tight junction in blood‐brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther. 2012;18:609‐615. doi: 10.1111/j.1755-5949.2012.00340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bohannon DG, Long D, Kim W‐K. Understanding the heterogeneity of human pericyte subsets in blood–brain barrier homeostasis and neurological diseases. Cell. 2021;10:890. doi: 10.3390/cells10040890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rustenhoven J, Aalderink M, Scotter EL, et al. TGF‐beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation. 2016;13:37. doi: 10.1186/s12974-016-0503-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmermann KW. Der feinere Bau der Blutcapillaren. Z Anat Entwicklungsgesch. 1923;68:29‐109. doi: 10.1007/BF02593544 [DOI] [Google Scholar]
- 24. Hamilton N, Attwell D, Hall C. Pericyte‐mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2:5. doi: 10.3389/fnene.2010.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heithoff BP, George KK, Phares AN, Zuidhoek IA, Munoz‐Ballester C, Robel S. Astrocytes are necessary for blood–brain barrier maintenance in the adult mouse brain. Glia. 2021;69:436‐472. doi: 10.1002/glia.23908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubin LL, Hall DE, Porter S, et al. A cell culture model of the blood‐brain barrier. J Cell Biol. 1991;115:1725‐1735. doi: 10.1083/jcb.115.6.1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bear M, Connors B, Paradiso MA. Neuroscience: Exploring the Brain, Enhanced Edition. Jones & Bartlett Learning; 2020. [Google Scholar]
- 28. Ueno M, Akiguchi I, Hosokawa M, Kotani H, Kanenishi K, Sakamoto H. Blood‐brain barrier permeability in the periventricular areas of the normal mouse brain. Acta Neuropathol. 2000;99:385‐392. doi: 10.1007/s004010051140 [DOI] [PubMed] [Google Scholar]
- 29. Kisler K, Nikolakopoulou AM, Zlokovic BV. Microglia have a grip on brain microvasculature. Nat Commun. 2021;12:5290. doi: 10.1038/s41467-021-25595-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cauli B, Tong XK, Rancillac A, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940‐8949. doi: 10.1523/jneurosci.3065-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McConnell HL, Kersch CN, Woltjer RL, Neuwelt EA. The translational significance of the neurovascular unit. J Biol Chem. 2017;292:762‐770. doi: 10.1074/jbc.R116.760215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bagchi S, Chhibber T, Lahooti B, Verma A, Borse V, Jayant RD. In vitro blood‐brain barrier models for drug screening and permeation studies: an overview. Drug Des Devel Ther. 2019;13:3591‐3605. doi: 10.2147/DDDT.S218708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wong A et al. The blood‐brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaffe EA. Cell biology of endothelial cells. Hum Pathol. 1987;18:234‐239. doi: 10.1016/S0046-8177(87)80005-9 [DOI] [PubMed] [Google Scholar]
- 35. Kramer RH, Bensch KG, Davison PM, Karasek MA. Basal lamina formation by cultured microvascular endothelial cells. J Cell Biol. 1984;99:692‐698. doi: 10.1083/jcb.99.2.692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolburg H, Noell S, Mack A, Wolburg‐Buchholz K, Fallier‐Becker P. Brain endothelial cells and the glio‐vascular complex. Cell Tissue Res. 2009;335:75‐96. doi: 10.1007/s00441-008-0658-9 [DOI] [PubMed] [Google Scholar]
- 37. Ruck T, Bittner S, Meuth SG. Blood‐brain barrier modeling: challenges and perspectives. Neural Regen Res. 2015;10:889‐891. doi: 10.4103/1673-5374.158342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shayan G, Choi YS, Shusta EV, Shuler ML, Lee KH. Murine in vitro model of the blood–brain barrier for evaluating drug transport. Eur J Pharm Sci. 2011;42:148‐155. doi: 10.1016/j.ejps.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 39. Greene C, Hanley N, Campbell M. Claudin‐5: gatekeeper of neurological function. Fluids Barriers CNS. 2019;16:3. doi: 10.1186/s12987-019-0123-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Branca JJV, Maresca M, Morucci G, et al. Effects of cadmium on ZO‐1 tight junction integrity of the blood brain barrier. Int J Mol Sci. 2019;20:6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lochhead JJ, Yang J, Ronaldson PT, Davis TP. Structure, function, and regulation of the blood‐brain barrier tight junction in central nervous system disorders. Front Physiol. 2020;11:914. doi: 10.3389/fphys.2020.00914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nitta T, Hata M, Gotoh S, et al. Size‐selective loosening of the blood‐brain barrier in claudin‐5‐deficient mice. J Cell Biol. 2003;161:653‐660. doi: 10.1083/jcb.200302070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Enerson BE, Drewes LR. The rat blood—brain barrier transcriptome. J Cereb Blood Flow Metab. 2006;26:959‐973. doi: 10.1038/sj.jcbfm.9600249 [DOI] [PubMed] [Google Scholar]
- 44. Fabry Z, Sandor M, Gajewski TF, et al. Differential activation of Th1 and Th2 CD4+ cells by murine brain microvessel endothelial cells and smooth muscle/pericytes. J Immunol. 1993;151:38‐47. [PubMed] [Google Scholar]
- 45. Castro Dias M, Odriozola Quesada A, Soldati S, et al. Brain endothelial tricellular junctions as novel sites for T cell diapedesis across the blood–brain barrier. J Cell Sci. 2021;134:jcs253880. doi: 10.1242/jcs.253880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greenwood J, Wang Y, Calder VL. Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA‐1, ICAM‐1, VLA‐4 and VCAM‐1. Off. Immunology. 1995;86:408‐415. [PMC free article] [PubMed] [Google Scholar]
- 47. Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001;2:515‐522. doi: 10.1038/88710 [DOI] [PubMed] [Google Scholar]
- 48. Ronaldson PT, Demarco KM, Sanchez‐Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor‐beta signaling alters substrate permeability and tight junction protein expression at the blood‐brain barrier during inflammatory pain. J Cereb Blood Flow Metab. 2009;29:1084‐1098. doi: 10.1038/jcbfm.2009.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Majerova P, Michalicova A, Cente M, et al. Trafficking of immune cells across the blood‐brain barrier is modulated by neurofibrillary pathology in tauopathies. PLoS One. 2019;14:e0217216. doi: 10.1371/journal.pone.0217216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arima M, Nakao S, Yamaguchi M, et al. Claudin‐5 redistribution induced by inflammation leads to anti‐VEGF–resistant diabetic macular edema. Diabetes. 2020;69:981‐999. doi: 10.2337/db19-1121 [DOI] [PubMed] [Google Scholar]
- 51. Wang J, Zhang C, Zhu J, Ding J, Chen Y, Han X. Blood‐brain barrier disruption and inflammation reaction in mice after chronic exposure to microcystin‐LR. Sci Total Environ. 2019;689:662‐678. doi: 10.1016/j.scitotenv.2019.06.387 [DOI] [PubMed] [Google Scholar]
- 52. Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood–brain barrier to insulin. Brain Res. 2001;896:36‐42. doi: 10.1016/S0006-8993(00)03247-9 [DOI] [PubMed] [Google Scholar]
- 53. Hansson E. Actin filament reorganization in astrocyte networks is a key functional step in neuroinflammation resulting in persistent pain: novel findings on network restoration. Neurochem Res. 2015;40:372‐379. doi: 10.1007/s11064-014-1363-6 [DOI] [PubMed] [Google Scholar]
- 54. Descamps L, Coisne C, Dehouck B, Cecchelli R, Torpier G. Protective effect of glial cells against lipopolysaccharide‐mediated blood‐brain barrier injury. Glia. 2003;42:46‐58. doi: 10.1002/glia.10205 [DOI] [PubMed] [Google Scholar]
- 55. Hoshi Y, Uchida Y, Tachikawa M, Ohtsuki S, Terasaki T. Actin filament‐associated protein 1 (AFAP‐1) is a key mediator in inflammatory signaling‐induced rapid attenuation of intrinsic P‐gp function in human brain capillary endothelial cells. J Neurochem. 2017;141:247‐262. doi: 10.1111/jnc.13960 [DOI] [PubMed] [Google Scholar]
- 56. Van Skike CE, Jahrling JB, Olson AB, et al. Inhibition of mTOR protects the blood‐brain barrier in models of Alzheimer's disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2018;314:H693‐H703. doi: 10.1152/ajpheart.00570.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kurz C, Walker L, Rauchmann B‐S, Perneczky R. Dysfunction of the blood–brain barrier in Alzheimer's disease: evidence from human studies. Neuropathol Appl Neurobiol. 2022;48:e12782. doi: 10.1111/nan.12782 [DOI] [PubMed] [Google Scholar]
- 58. Nishihara H, Perriot S, Gastfriend BD, et al. Intrinsic blood–brain barrier dysfunction contributes to multiple sclerosis pathogenesis. Brain. 2022;awac019. doi: 10.1093/brain/awac019. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pan W, P. Stone K, Hsuchou H, K. Manda V, Zhang Y, J. Kastin A. Cytokine signaling modulates blood‐brain barrier function. Curr Pharm Des. 2011;17:3729‐3740. doi: 10.2174/138161211798220918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zamudio F, Loon AR, Smeltzer S, et al. TDP‐43 mediated blood‐brain barrier permeability and leukocyte infiltration promote neurodegeneration in a low‐grade systemic inflammation mouse model. J Neuroinflammation. 2020;17:283. doi: 10.1186/s12974-020-01952-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saand AR, Yu F, Chen J, Chou SHY. Systemic inflammation in hemorrhagic strokes ‐ a novel neurological sign and therapeutic target? J Cereb Blood Flow Metab. 2019;39:959‐988. doi: 10.1177/0271678X19841443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Villaseñor R, Lampe J, Schwaninger M, Collin L. Intracellular transport and regulation of transcytosis across the blood–brain barrier. Cell Mol Life Sci. 2019;76:1081‐1092. doi: 10.1007/s00018-018-2982-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Grubb S, Lauritzen M, Aalkjær C. Brain capillary pericytes and neurovascular coupling. Comp Biochem Physiol A Mol Integr Physiol. 2021;254:110893. doi: 10.1016/j.cbpa.2020.110893 [DOI] [PubMed] [Google Scholar]
- 64. Kim S, Lee S, Lim J, et al. Human bone marrow‐derived mesenchymal stem cells play a role as a vascular pericyte in the reconstruction of human BBB on the angiogenesis microfluidic chip. Biomaterials. 2021;279:121210. doi: 10.1016/j.biomaterials.2021.121210 [DOI] [PubMed] [Google Scholar]
- 65. Xu L, Nirwane A, Yao Y. Basement membrane and blood‐brain barrier. Stroke Vasc Neurol. 2018;4:78‐82. doi: 10.1136/svn-2018-000198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700‐704. doi: 10.1038/nature05193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sá‐Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012;45:327‐347. doi: 10.1007/s12035-012-8244-2 [DOI] [PubMed] [Google Scholar]
- 68. Ferro MP, Heilshorn SC, Owens RM. Materials for blood brain barrier modeling in vitro. Mater Sci Eng R Rep. 2020;140:100522. doi: 10.1016/j.mser.2019.100522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Török O, Schreiner B, Schaffenrath J, et al. Pericytes regulate vascular immune homeostasis in the CNS. Proc Natl Acad Sci U S A. 2021;118:e2016587118. doi: 10.1073/pnas.2016587118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mäe MA, He L, Nordling S, et al. Single‐cell analysis of blood‐brain barrier response to pericyte loss. Circ Res. 2021;128:e46‐e62. doi: 10.1161/CIRCRESAHA.120.317473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dubrac A, Künzel SE, Künzel SH, et al. NCK‐dependent pericyte migration promotes pathological neovascularization in ischemic retinopathy. Nat Commun. 2018;9:3463. doi: 10.1038/s41467-018-05926-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Warmke N, Platt F, Bruns AF, et al. Pericyte insulin receptors modulate retinal vascular remodeling and endothelial angiopoietin signaling. Endocrinology. 2021;162:bqab182. doi: 10.1210/endocr/bqab182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vazquez‐Liebanas E, Nahar K, Bertuzzi G, Keller A, Betsholtz C, Mäe MA. Adult‐induced genetic ablation distinguishes PDGFB roles in blood‐brain barrier maintenance and development. J Cereb Blood Flow Metab. 2022;42:264‐279. doi: 10.1177/0271678x211056395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rudziak P, Ellis CG, Kowalewska PM. Role and molecular mechanisms of pericytes in regulation of leukocyte diapedesis in inflamed tissues. Mediators Inflamm. 2019;2019:4123605. doi: 10.1155/2019/4123605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nag TC, Gorla S, Kumari C, Roy TS. Aging of the human choriocapillaris: evidence that early pericyte damage can trigger endothelial changes. Exp Eye Res. 2021;212:108771. doi: 10.1016/j.exer.2021.108771 [DOI] [PubMed] [Google Scholar]
- 76. Kisler K, Nikolakopoulou AM, Sweeney MD, Lazic D, Zhao Z, Zlokovic BV. Acute ablation of cortical pericytes leads to rapid neurovascular uncoupling. Front Cell Neurosci. 2020;14:27. doi: 10.3389/fncel.2020.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nikolakopoulou AM, Montagne A, Kisler K, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22:1089‐1098. doi: 10.1038/s41593-019-0434-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gerhardt H, Wolburg H, Redies C. N‐cadherin mediates pericytic‐endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472‐479. [DOI] [PubMed] [Google Scholar]
- 79. Schofield CL, Rodrigo‐Navarro A, Dalby MJ, Van Agtmael T, Salmeron‐Sanchez M. Biochemical‐ and biophysical‐induced Barriergenesis in the blood–brain barrier: a review of Barriergenic factors for use in in vitro models. Adv NanoBiomed Res. 2021;1:2000068. doi: 10.1002/anbr.202000068 [DOI] [Google Scholar]
- 80. Tillet E, Vittet D, Féraud O, Moore R, Kemler R, Huber P. N‐cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell‐derived angiogenesis. Exp Cell Res. 2005;310:392‐400. doi: 10.1016/j.yexcr.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 81. Kuo Y‐C, Lee C‐L, Rajesh R. Regulation of human brain vascular pericytes and human astrocytes in a blood–brain barrier model using human brain microvascular endothelial cells: expression of TGF‐β1, VEGF, MMP‐9 and P‐gp. J Taiwan Inst Chem Eng. 2018;86:9‐17. doi: 10.1016/j.jtice.2018.03.003 [DOI] [Google Scholar]
- 82. Zheng Z, Chopp M, Chen J. Multifaceted roles of pericytes in central nervous system homeostasis and disease. J Cereb Blood Flow Metab. 2020;40:1381‐1401. doi: 10.1177/0271678X20911331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Laredo F, Plebanski J, Tedeschi A. Pericytes: problems and promises for CNS repair. Front Cell Neurosci. 2019;13:546. doi: 10.3389/fncel.2019.00546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Geranmayeh MH, Rahbarghazi R, Farhoudi M. Targeting pericytes for neurovascular regeneration. Cell Commun Signal. 2019;17:26. doi: 10.1186/s12964-019-0340-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bohannon DG, Okhravi HR, Kim J, Kuroda MJ, Didier ES, Kim WK. A subtype of cerebrovascular pericytes is associated with blood‐brain barrier disruption that develops during normal aging and simian immunodeficiency virus infection. Neurobiol Aging. 2020;96:128‐136. doi: 10.1016/j.neurobiolaging.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24:312‐325. doi: 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gautam J, Cao Y, Yao Y. Pericytic laminin maintains blood‐brain barrier integrity in an age‐dependent manner. Transl Stroke Res. 2020;11:228‐242. doi: 10.1007/s12975-019-00709-8 [DOI] [PubMed] [Google Scholar]
- 88. Yao Y, Chen Z‐L, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5:3413. doi: 10.1038/ncomms4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yamanaka G, Takata F, Kataoka Y, et al. The neuroinflammatory role of pericytes in epilepsy. Biomedicine. 2021;9:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Miller SJ. Astrocyte heterogeneity in the adult central nervous system. Front Cell Neurosci. 2018;12:401. doi: 10.3389/fncel.2018.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094‐1103. doi: 10.1002/glia.20990 [DOI] [PubMed] [Google Scholar]
- 92. Wolburg‐Buchholz K, Mack AF, Steiner E, Pfeiffer F, Engelhardt B, Wolburg H. Loss of astrocyte polarity marks blood–brain barrier impairment during experimental autoimmune encephalomyelitis. Acta Neuropathol. 2009;118:219‐233. doi: 10.1007/s00401-009-0558-4 [DOI] [PubMed] [Google Scholar]
- 93. Kubotera H, Ikeshima‐Kataoka H, Hatashita Y, Allegra Mascaro AL, Pavone FS, Inoue T. Astrocytic endfeet re‐cover blood vessels after removal by laser ablation. Sci Rep. 2019;9:1263. doi: 10.1038/s41598-018-37419-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Higashi K, Fujita A, Inanobe A, et al. An inwardly rectifying K+ channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol. 2001;281:C922‐C931. doi: 10.1152/ajpcell.2001.281.3.C922 [DOI] [PubMed] [Google Scholar]
- 95. Warth A, Mittelbronn M, Wolburg H. Redistribution of the water channel protein aquaporin‐4 and the K+ channel protein Kir4.1 differs in low‐ and high‐grade human brain tumors. Acta Neuropathol. 2005;109:418‐426. doi: 10.1007/s00401-005-0984-x [DOI] [PubMed] [Google Scholar]
- 96. Kaisar MA, Sajja RK, Prasad S, Abhyankar VV, Liles T, Cucullo L. New experimental models of the blood‐brain barrier for CNS drug discovery. Expert Opin Drug Discovery. 2017;12:89‐103. doi: 10.1080/17460441.2017.1253676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ponio JB‐D, el‐Ayoubi F, Glacial F, et al. Instruction of circulating endothelial progenitors in vitro towards specialized blood‐brain barrier and arterial phenotypes. PLoS One. 2014;9:e84179. doi: 10.1371/journal.pone.0084179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Huang X, Su Y, Wang N, et al. Astroglial connexins in neurodegenerative diseases. Front Mol Neurosci. 2021;14:657514. doi: 10.3389/fnmol.2021.657514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Van Campenhout R, Gomes AR, TWM DG, Muyldermans S, Devoogdt N, Vinken M. Mechanisms underlying connexin hemichannel activation in disease. Int J Mol Sci. 2021;22:3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Peng B, Xu C, Wang S, Zhang Y, Li W. The role of connexin hemichannels in inflammatory diseases. Biology. 2022;11:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551‐1555. doi: 10.1126/science.1164022 [DOI] [PubMed] [Google Scholar]
- 102. Xing L, Yang T, Cui S, Chen G. Connexin hemichannels in astrocytes: role in CNS disorders. Front Mol Neurosci. 2019;12:23. doi: 10.3389/fnmol.2019.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Spampinato SF, Merlo S, Sano Y, Kanda T, Sortino MA. Astrocytes contribute to Aβ‐induced blood‐brain barrier damage through activation of endothelial MMP9. J Neurochem. 2017;142:464‐477. doi: 10.1111/jnc.14068 [DOI] [PubMed] [Google Scholar]
- 104. Song S, Huang H, Guan X, et al. Activation of endothelial Wnt/β‐catenin signaling by protective astrocytes repairs BBB damage in ischemic stroke. Prog Neurobiol. 2021;199:101963. doi: 10.1016/j.pneurobio.2020.101963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Han RT, Kim RD, Molofsky AV, Liddelow SA. Astrocyte‐immune cell interactions in physiology and pathology. Immunity. 2021;54:211‐224. doi: 10.1016/j.immuni.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 106. Fu J, Li L, Huo D, et al. Astrocyte‐derived TGFβ1 facilitates blood–brain barrier function via non‐canonical hedgehog signaling in brain microvascular endothelial cells. Brain Sci. 2021;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Feng S, Zou L, Wang H, He R, Liu K, Zhu H. RhoA/ROCK‐2 pathway inhibition and tight junction protein upregulation by Catalpol suppresses Lipopolysaccaride‐induced disruption of blood‐brain barrier permeability. Molecules. 2018;23:2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Liu C‐C, Yamazaki Y, Heckman MG, et al. Tau and apolipoprotein E modulate cerebrovascular tight junction integrity independent of cerebral amyloid angiopathy in Alzheimer's disease. Alzheimers Dement. 2020;16:1372‐1383. doi: 10.1002/alz.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jackson RJ, Meltzer JC, Nguyen H, et al. APOE4 derived from astrocytes leads to blood–brain barrier impairment. Brain. 2021;awab478. doi: 10.1093/brain/awab478. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Guérit S, Fidan E, Macas J, et al. Astrocyte‐derived Wnt growth factors are required for endothelial blood‐brain barrier maintenance. Prog Neurobiol. 2021;199:101937. doi: 10.1016/j.pneurobio.2020.101937 [DOI] [PubMed] [Google Scholar]
- 111. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood‐brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71‐76. doi: 10.1038/s41586-020-2247-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yamazaki Y, Shinohara M, Yamazaki A, et al. ApoE (apolipoprotein E) in brain pericytes regulates endothelial function in an isoform‐dependent manner by modulating basement membrane components. Arterioscler Thromb Vasc Biol. 2020;40:128‐144. doi: 10.1161/ATVBAHA.119.313169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin‐1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683‐687. doi: 10.1016/S0306-4522(02)00175-6 [DOI] [PubMed] [Google Scholar]
- 114. Venkat P, Yan T, Chopp M, et al. Angiopoietin‐1 mimetic peptide promotes neuroprotection after stroke in type 1 diabetic rats. Cell Transplant. 2018;27:1744‐1752. doi: 10.1177/0963689718791568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Giovannoni F, Quintana FJ. The role of astrocytes in CNS inflammation. Trends Immunol. 2020;41:805‐819. doi: 10.1016/j.it.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Smith BC, Sinyuk M, Jenkins JE, Psenicka MW, Williams JL. The impact of regional astrocyte interferon‐γ signaling during chronic autoimmunity: a novel role for the immunoproteasome. J Neuroinflammation. 2020;17:184. doi: 10.1186/s12974-020-01861-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Horng S, Therattil A, Moyon S, et al. Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J Clin Invest. 2017;127:3136‐3151. doi: 10.1172/JCI91301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Neuhaus W, Gaiser F, Mahringer A, Franz J, Riethmüller C, Förster C. The pivotal role of astrocytes in an in vitro stroke model of the blood‐brain barrier. Front Cell Neurosci. 2014;8:352. doi: 10.3389/fncel.2014.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053‐8064. doi: 10.4049/jimmunol.177.11.8053 [DOI] [PubMed] [Google Scholar]
- 120. Williams JL, Manivasagam S, Smith BC, et al. Astrocyte‐T cell crosstalk regulates region‐specific neuroinflammation. Glia. 2020;68:1361‐1374. doi: 10.1002/glia.23783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Michinaga S, Koyama Y. Dual roles of astrocyte‐derived factors in regulation of blood‐brain barrier function after brain damage. Int J Mol Sci. 2019;20:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kim GW, Gasche Y, Grzeschik S, Copin JC, Maier CM, Chan PH. Neurodegeneration in striatum induced by the mitochondrial toxin 3‐nitropropionic acid: role of matrix metalloproteinase‐9 in early blood‐brain barrier disruption? J Neurosci. 2003;23:8733‐8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Psenicka MW, Smith BC, Tinkey RA, Williams JL. Connecting neuroinflammation and neurodegeneration in multiple sclerosis: are oligodendrocyte precursor cells a nexus of disease? Front Cell Neurosci. 2021;15:654284. doi: 10.3389/fncel.2021.654284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gao X., Li W., Syed F., Yuan F., Li P., Yu Q.. PD‐L1‐expressing astrocytes act as a gate‐keeper for neuroinflammation in the central nervous system of mice with traumatic brain injury. bioRxiv. 2021. doi: 10.1101/2021.11.04.467368 [DOI]
- 125. Gao X, Li W, Syed F, Yuan F, Li P, Yu Q. PD‐L1 signaling in reactive astrocytes counteracts neuroinflammation and ameliorates neuronal damage after traumatic brain injury. J Neuroinflammation. 2022;19:43. doi: 10.1186/s12974-022-02398-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kummer MP, Ising C, Kummer C, et al. Microglial PD‐1 stimulation by astrocytic PD‐L1 suppresses neuroinflammation and Alzheimer's disease pathology. EMBO J. 2021;40:e108662. doi: 10.15252/embj.2021108662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677‐704. doi: 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Latchman Y, Wood CR, Chernova T, et al. PD‐L2 is a second ligand for PD‐1 and inhibits T cell activation. Nat Immunol. 2001;2:261‐268. doi: 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- 129. Chen G, Kim YH, Li H, et al. PD‐L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD‐1. Nat Neurosci. 2017;20:917‐926. doi: 10.1038/nn.4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18:225‐242. doi: 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- 131. Mondo E, Becker SC, Kautzman AG, et al. A developmental analysis of Juxtavascular microglia dynamics and interactions with the vasculature. J Neurosci. 2020;40:6503‐6521. doi: 10.1523/jneurosci.3006-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shigemoto‐Mogami Y, Hoshikawa K, Sato K. Activated microglia disrupt the blood‐brain barrier and induce chemokines and cytokines in a rat in vitro model. Front Cell Neurosci. 2018;12:494. doi: 10.3389/fncel.2018.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. da Fonseca ACC, Matias D, Garcia C, et al. The impact of microglial activation on blood‐brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362. doi: 10.3389/fncel.2014.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Halder SK, Milner R. Mild hypoxia triggers transient blood–brain barrier disruption: a fundamental protective role for microglia. Acta Neuropathol Commun. 2020;8:175. doi: 10.1186/s40478-020-01051-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ulvestad E, Williams K, Vedeler C, et al. Reactive microglia in multiple sclerosis lesions have an increased expression of receptors for the fc part of IgG. J Neurol Sci. 1994;121:125‐131. doi: 10.1016/0022-510X(94)90340-9 [DOI] [PubMed] [Google Scholar]
- 136. Chen A‐Q, Fang Z, Chen XL, et al. Microglia‐derived TNF‐α mediates endothelial necroptosis aggravating blood brain–barrier disruption after ischemic stroke. Cell Death Dis. 2019;10:487. doi: 10.1038/s41419-019-1716-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kuroda E, Nishimura K, Kawanishi S, et al. Mouse bone marrow‐derived microglia‐like cells secrete transforming growth factor‐β1 and promote microglial Aβ phagocytosis and reduction of brain Aβ. Neuroscience. 2020;438:217‐228. doi: 10.1016/j.neuroscience.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 138. Kerkhofs D, van Hagen BT, Milanova IV, et al. Pharmacological depletion of microglia and perivascular macrophages prevents vascular cognitive impairment in ang II‐induced hypertension. Theranostics. 2020;10:9512‐9527. doi: 10.7150/thno.44394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bisht K, Okojie KA, Sharma K, et al. Capillary‐associated microglia regulate vascular structure and function through PANX1‐P2RY12 coupling in mice. Nat Commun. 2021;12:5289. doi: 10.1038/s41467-021-25590-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Császár E, Lénárt N, Cserép C, et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J Exp Med. 2022;219:e20211071. doi: 10.1084/jem.20211071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Halder SK, Milner R. A critical role for microglia in maintaining vascular integrity in the hypoxic spinal cord. Proc Natl Acad Sci U S A. 2019;116:26029‐26037. doi: 10.1073/pnas.1912178116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Davalos D, Kyu Ryu J, Merlini M, et al. Fibrinogen‐induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chen Q, Xu L, du T, et al. Enhanced expression of PD‐L1 on microglia after surgical brain injury exerts self‐protection from inflammation and promotes neurological repair. Neurochem Res. 2019;44:2470‐2481. doi: 10.1007/s11064-019-02864-8 [DOI] [PubMed] [Google Scholar]
- 144. Wang L‐L, Li ZH, Hu XH, Muyayalo KP, Zhang YH, Liao AH. The roles of the PD‐1/PD‐L1 pathway at immunologically privileged sites. Am J Reprod Immunol. 2017;78:e12710. doi: 10.1111/aji.12710 [DOI] [PubMed] [Google Scholar]
- 145. Zhang Y, Chen K, Sloan SA, et al. An RNA‐sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929‐11947. doi: 10.1523/jneurosci.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tontsch U, Bauer H‐C. Glial cells and neurons induce blood‐brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res. 1991;539:247‐253. doi: 10.1016/0006-8993(91)91628-E [DOI] [PubMed] [Google Scholar]
- 147. Stanness KA, Neumaier JF, Sexton TJ, et al. A new model of the blood–brain barrier: co‐culture of neuronal, endothelial and glial cells under dynamic conditions. Neuroreport. 1999;10:3725‐3731. doi: 10.1097/00001756-199912160-00001 [DOI] [PubMed] [Google Scholar]
- 148. Barreto GE, Gonzalez J, Torres Y, Morales L. Astrocytic‐neuronal crosstalk: implications for neuroprotection from brain injury. Neurosci Res. 2011;71:107‐113. doi: 10.1016/j.neures.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 149. Mulica P, Grünewald A, Pereira SL. Astrocyte‐neuron metabolic crosstalk in neurodegeneration: a mitochondrial perspective. Front Endocrinol (Lausanne). 2021;12:668517. doi: 10.3389/fendo.2021.668517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kaplan L, Chow BW, Gu C. Neuronal regulation of the blood–brain barrier and neurovascular coupling. Nat Rev Neurosci. 2020;21:416‐432. doi: 10.1038/s41583-020-0322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yang G, Zhang Y, Ross ME, Iadecola C. Attenuation of activity‐induced increases in cerebellar blood flow in mice lacking neuronal nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2003;285:H298‐H304. doi: 10.1152/ajpheart.00043.2003 [DOI] [PubMed] [Google Scholar]
- 152. Hogan‐Cann AD, Lu P, Anderson CM. Endothelial NMDA receptors mediate activity‐dependent brain hemodynamic responses in mice. Proc Natl Acad Sci U S A. 2019;116:10229‐10231. doi: 10.1073/pnas.1902647116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lia A, Henriques VJ, Zonta M, et al. Calcium signals in astrocyte microdomains, a decade of great advances. Front Cell Neurosci. 2021;15:673433. doi: 10.3389/fncel.2021.673433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Semmler A, Hermann S, Mormann F, et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38. doi: 10.1186/1742-2094-5-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Pulido RS, Munji RN, Chan TC, et al. Neuronal activity regulates blood‐brain barrier efflux transport through endothelial circadian genes. Neuron. 2020;108:937‐952. doi: 10.1016/j.neuron.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339‐358. [DOI] [PubMed] [Google Scholar]
- 157. Gasca‐Salas C, Fernández‐Rodríguez B, Pineda‐Pardo JA, et al. Blood‐brain barrier opening with focused ultrasound in Parkinson's disease dementia. Nat Commun. 2021;12:779. doi: 10.1038/s41467-021-21022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang W, Mehta A, Tong Z, Esser L, Voelcker NH. Development of polymeric nanoparticles for blood–brain barrier transfer—strategies and challenges. Adv Sci. 2021;8:2003937. doi: 10.1002/advs.202003937 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data in this manuscript.


