Abstract
Background:
Due to underrepresentation of racial/ethnic minority and low-income groups in clinical studies, there is a call to improve the recruitment and retention of these populations in research. Pilot studies can test recruitment and retention practices for better inclusion of medically underserved children and families in subsequent clinical trials. We examined this using a school-based asthma intervention, in preparation for a larger clinical trial in which our goal is to include an underserved study population.
Methods:
We recruited children with poorly controlled asthma in a two-site pilot cluster randomized controlled trial of school-supervised asthma therapy versus enhanced usual care (receipt of an educational asthma workbook). We sought a study population with a high percentage of children and families from racial/ethnic minority and low-income groups. The primary outcome of the pilot trial was recruitment/retention over 12 months. Strategies used to facilitate recruitment/retention of this study population included engaging pre-trial multi-level stakeholders, selecting trial sites with high percentages of underserved children and families, training diverse medical providers to recruit participants, conducting remote trial assessments, and providing multi-lingual study materials.
Results:
Twenty-six children [42.3% female, 11.5% Black, 30.8% Multiracial (Black & other), 76.9% Hispanic, and 92.3% with family income below $40,000] and their caregivers were enrolled in the study, which represents 55.3% of those initially referred by their provider, with 96.2%, 92.3%, and 96.2% retention at 3-, 6-, and 12-month follow-up, respectively.
Conclusion:
Targeted strategies facilitated the inclusion of a medically underserved population of children and families in our pilot study, prior to expanding to a larger trial.
Keywords: Pilot test, Recruitment, Retention, Asthma, Clinical trials, Underserved
1. Introduction
Many clinical trials struggle to recruit and retain participants from medically underserved populations, defined as those who may face “economic, cultural, or linguistic barriers to healthcare,” [1] due to factors including language and access barriers, as well as mistrust in research [2–5]. This threatens health equity [6–11] because underserved populations experience disproportionate morbidity from chronic diseases and would greatly benefit from innovative interventions [12]. Moreover, it leaves clinicians and policy makers uncertain of the applicability of the studies’ results to their patients, practices, and communities [13].
The scientific community has been called to improve the recruitment and retention of medically underserved populations into all clinical trials [6,14,15] and specific strategies have been proposed to achieve this goal [2,6]. Pilot studies can field test recruitment and retention strategies to promote the inclusion of underserved populations [16]. While there are a few examples of the use of pilot studies to improve recruitment and retention of adults and adolescents into clinical trials [17,18], there have yet to be examples of pilot studies to test recruitment and retention strategies of medically underserved children and families. This is especially important for asthma interventions, given the grave health inequities in pediatric asthma among racial/ethnic minority and low-income groups [2,19–23]. Herein, we present an example of a pilot clinical trial of a school-supervised asthma intervention and describe our strategies for the recruitment and retention of medically underserved children and families.
2. Materials and methods
2.1. Pilot study design
A cluster pilot randomized controlled trial was conducted starting in April 2019 in two pediatric primary care practices in central Massachusetts. These practices serve high numbers of children from low-income and minority backgrounds, reflecting the population that experiences the greatest morbidity from asthma [21,22,24], and were pair-matched based on size and sociodemographics. In both practices, pediatric clinic staff received a 1-h training on how to identify children with poorly controlled asthma and poor medication adherence. One practice was recruited into the Asthma Link condition, in which providers were taught to enroll children to receive school-supervised asthma therapy. The other practice was recruited into the Enhanced Usual Care condition, in which providers gave families an educational workbook with medication adherence strategies. After providers identified potential participants, their information was transmitted to a research coordinator for consent and enrollment into the study. Poorly controlled asthma was defined as an Asthma Control Test [25] score of 19 or less or one emergency department visit, hospital stay, or oral corticosteroid course for asthma in the last year. Poor medication adherence was defined as the family reporting frequently missed doses of the inhaled corticosteroid (ICS) or the pharmacy reporting an inconsistent ICS refill history in the past six months [26].
Child-caregiver dyads completed surveys at the time of enrollment (baseline), and at 3, 6, and 12 months after enrollment. These surveys assessed sociodemographics, quality of life (measured by validated questionnaire [27]), and asthma health outcomes based on validated measures including asthma symptoms (measured by the Asthma Control Test [25]), spirometry (measured using portable EasyOne spirometer), asthma-related emergency room visits, hospital admissions, and oral steroid courses (by caregiver report and medical record review). All assessments were performed remotely with the caregiver/child, except spirometry was conducted at school in the school health office.
Inclusion and exclusion criteria are noted in Fig. 1. Recruitment from each practice continued until at least 10 participants were enrolled from each practice. This sample size was based on pragmatics of examining feasibility and providing information for design and recruitment for the future larger clinical trial.
Fig. 1.
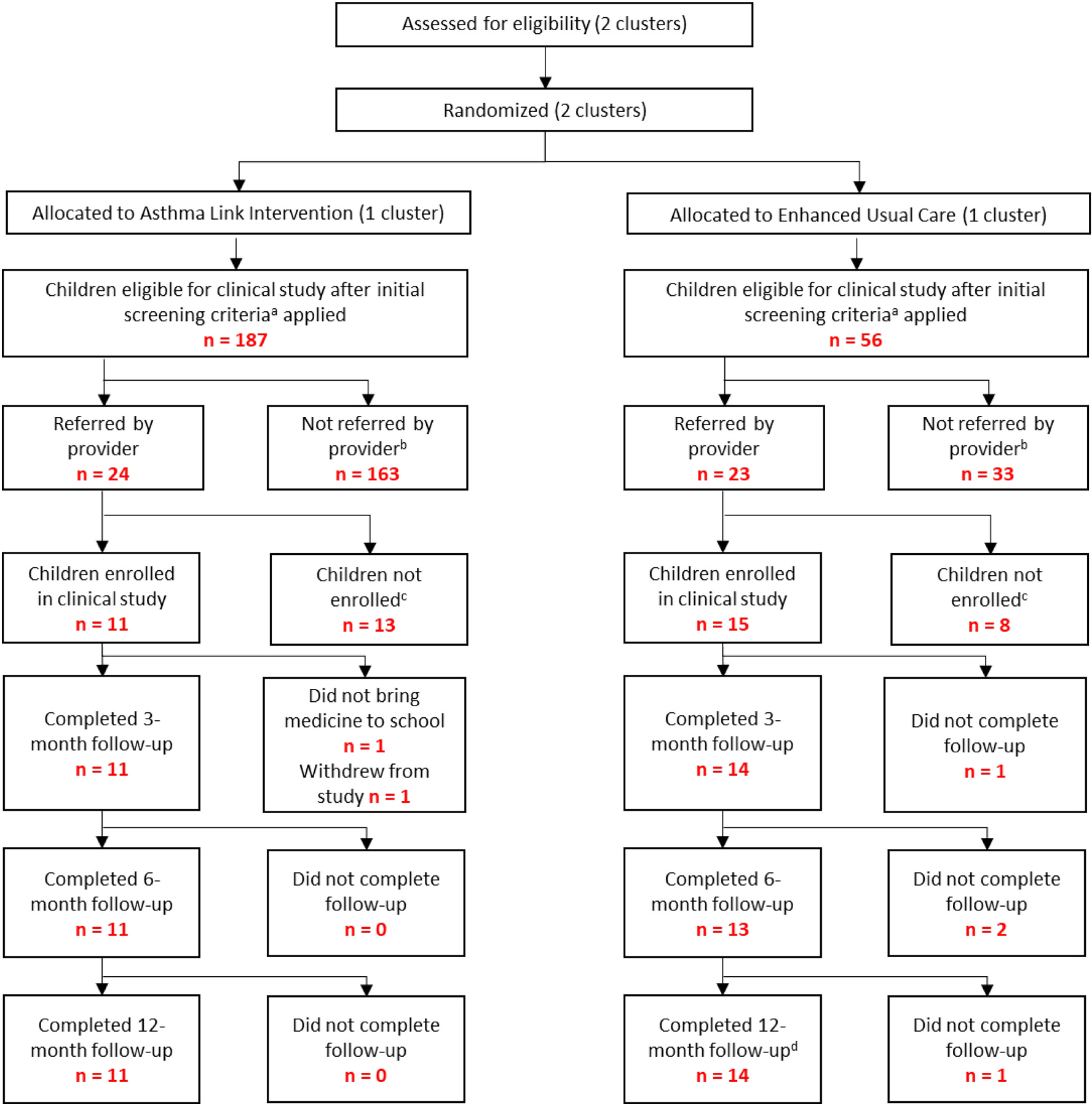
Recruitment, Enrollment, and Retention.
a Grade 1–6, prescription of daily ICS, ACT score < 19 or 1 or more hospitalizations/ED visits/prescriptions for oral steroids in the past year, and poor adherence to daily ICS therapy, ability to speak English or Spanish.
b Reasons why providers did not refer children to Asthma Link and/or clinical study: family did not attend pediatric office visit, provider did not have enough time to bring up Asthma Link and the clinical study, child’s asthma already well-controlled, family not interested in participating in Asthma Link and/or clinical study.
c Reasons why children were not enrolled into clinical study: Unable to reach child’s family by phone to enroll in study, serious co-morbid illness in the last 5 years, planning to move out of the school district within the next year, having a sibling in the study, and having a developmental delay that would prevent participation.
d Families that did not complete an earlier follow-up survey were permitted to complete later follow-up surveys.
2.2. Recruitment and retention strategies to recruit an underserved study population
Multiple strategies were utilized to recruit our study population of underserved children and families. First, prior to trial execution, in work previously described [28,29], we elicited iterative qualitative feedback from multi-level stakeholders (children and families from racial/ethnic and low-income minority groups, school nurses, medical providers) to adapt our community-based asthma intervention to meet the needs of our target population. Second, we trained medical providers from diverse racial/ethnic backgrounds to introduce the study to families. Third, we conducted all assessments remotely via phone, avoiding the need for families to travel or have internet access to participate. Fourth, all study materials, including consent material and study surveys, were available in English and Spanish, and interpreter services or Spanish-speaking study staff was available for all study assessments. Additional strategies used in our pilot study are shown in Table 1.
Table 1.
Strategies used in our pilot study to facilitate recruitment and retention of a study population of underserved children and families.
| Goal | Potential barrier | Strategy to overcome barrier |
|---|---|---|
|
| ||
| Elicit the perspectives of traditionally underserved children and families on the intervention of interest | Lack of end-user and community engagement in intervention protocol development | Conduct iterative, qualitative interviews with multi-level stakeholders (children and families, school nurses, clinical staff, etc.) to adapt intervention to meet needs of children and families |
| Recruit traditionally underserved populations into clinical trials | Medical practices without racial, ethnic, or income diversity | Select practices with higher percentage of children and families that are medically underserved for study participation |
| Patients from minority groups prefer to learn about studies from providers of their own race or ethnicity | Recruit physicians from minority racial and ethnic groups to participate in clinical trial recruitment | |
| Medical provider without enough time to identify children at higher risk for asthma morbidity | Practice managers flag children with poorly controlled asthma, thus identifying a population disproportionally impacted by morbidity | |
| Mistrust in medical research and recruitment personnel | Allow the child’s pediatrician to initially introduce the study to families Emphasize the intervention partnership with trusted community members (i.e. school nurses) Maintain consistent study staff for longitudinal assessments with the family to build trust and rapport |
|
| Recruit Spanish-speaking populations | Lack of Spanish language study materials/services | Translate all study materials (consent, surveys, etc.) into Spanish Conduct phone assessments with Spanish-speaking study staff or interpreter |
| Retain underserved populations to study completion | Inadequate access to childcare, reliable transportation, and inability to miss work for in-person study assessments | Conduct surveys remotely, eliminating need for office visits |
| Compensation occurs a long time after study involvement | Send out electronic gift cards immediately after follow-up surveys completed | |
| Compensation not useful to participants | Ensure payment occurs via electronic gift cards to retailers present in the community (e.g. Walmart or Target), asking the participants about their preference | |
| Include populations without stable Wi-Fi | Lack of stable Wi-Fi | Conduct interactions with research staff via phone rather than video conferencing or email. Allow for sending of REDCap surveys using text links, as most families have text-enabled phones |
2.3. Ethics approval
This research was approved by the University of Massachusetts Chan Medical School Institutional Review Board (IRB). Verbal assent and written consent was obtained from all participants and their guardians, with adherence to IRB guidelines.
2.4. COVID-19 disruption
Notably, this study was ongoing in March 2020 when COVID-19 forced abrupt school closures across the state and nation. Thus, children in the Asthma Link intervention group could not continue to receive school-supervised asthma therapy, and medication administration responsibility was transferred to the participants’ caregivers. To continue supporting families while monitoring medication adherence, we elicited feedback from caregivers in Asthma Link, who preferred to receive text message reminders during school closures. Therefore, caregivers in the intervention group were sent daily text message reminders for the remainder of the 2019–2020 school year. Details of this text message intervention are previously described [30]. To maintain the relationship between schools and families, school nurses performed weekly virtual visits with families. Spirometry was not conducted after school closures, but all other study assessments proceeded as planned due to their remote nature (via phone).
3. Results
After initial screening criteria were applied for study eligibility, pediatric providers referred 47 children into the pilot trial (Fig. 1). Between April 2019 and February 2020, 11 child-caregiver dyads were recruited into the Asthma Link intervention condition; 15 child-caregiver dyads were recruited into the Enhanced Usual Care condition. Reasons for non-recruitment are noted in Fig. 1. At the 3-, 6-, and 12-month follow-up, we retained 25 (96.2%), 24 (92.3%), and 25 (96.2%) participants, respectively. Families that did not complete a prior follow-up survey were permitted to complete subsequent follow-up surveys. As such, all 26 families completed at least one survey at some point during the study.
Of the children enrolled, 11 (42.3%) were female, 10 (38.5%) were White, 3 (11.5%) were Black, 5 (19.2%) were Other/Multiracial, and 8 (30.8%) did not self-report race. Of note, all of the participants that were recorded as Multiracial, self-reported as Black in addition to another race; therefore the total number of Black participants was 8 (30.8%). Of the 26 children enrolled, 20 (76.9%) reported Hispanic as their ethnicity. Most children included in the study (n = 18, 69.2%) were from families that earned less than $25,000 per year, and 24 (92.3%) of families earned less than $40,000. (Table 2).
Table 2.
Demographic characteristics of study participants (n = 26).
| Child | |
|---|---|
|
| |
| Age in years, range (mean) | 6.6–12.5 (9.4) |
| Race, n (%) | |
| White | 10 (38.5) |
| African American/Black only | 3 (11.5) |
| Multiracial (Black plus another race) | 5 (19.2) |
| Not Reported | 8 (30.8) |
| Ethnicity, n (%) | |
| Hispanic/Puerto Rican | 20 (76.9) |
| Sex, n (%) | |
| Female | 11 (42.3) |
| Caregiver | |
| Annual Income, n (%) | |
| Under $25,000 | 18 (69.2) |
| Between $25,000 and $40,000 | 6 (23.1) |
| Between $40,001 and $60,000 | 1 (3.9) |
4. Discussion
In our case example of a school-based asthma pilot trial, we demonstrate the use of specific strategies that facilitated the successful recruitment and retention of a study population of children and families from racial/ethnic minority and low-income groups for 12 months. These strategies allowed this small-scale trial to serve as a model for a planned future larger clinical trial in which we aim to include a similar study population.
Most clinical trials, including those seeking to improve asthma health outcomes, do not recruit highly diverse study populations: across the 32,000 study participants in 2020 clinical trials, 75% of participants identified as white race [20]. Moreover, retention rates of racial/ethnic minority study participants can be as low as 52% [31–33], compared to our >90% retention rate. We propose several explanations for why our strategies led to successful recruitment and retention of a diverse study population of children and families. Through eliciting the perspectives of children, families, and trusted community partners (school nurses), we were able to better develop an intervention that met the needs of our target population, promoting interest in the study. Given the fact that medical providers from racial and ethnic minority groups are more likely to serve patients from similar backgrounds [34], we focused on engaging pediatric practices who serve a high proportion of children from racial/ethnic minorities and low-income groups and then training providers at these practices to introduce the study. In doing so, we included more diverse medical providers and participants and may have overcome some mistrust in research that is experienced by underserved children and families [2]. With remote study assessments, children and families could avoid traveling, relying on stable internet access, or additional time or financial burden, known barriers to study participation from minority/underserved populations [3,6,9,35]. Our availability of study materials and staff in both English and Spanish likely contributed to our ability to include a large percentage of Hispanic children and families in our study. Providing quick, accessible compensation after study visits may have incentivized participants with less resources to remain in the study to completion.
We did find that certain strategies were more effective than others for inclusion of underserved populations in our trial, including selecting practices with high populations of medically underserved patients, translating study materials into Spanish, and conducting remote assessments. For some clinical trials, remote assessments may not be possible, and, in these cases, researchers can consider the following strategies: scheduling lab draws closer to home rather than in hospital settings hosting clinical trials and utilizing home health equipment, with appropriate training, for assessments (i.e. home spirometers, pulse oxygen monitors, activity monitors, scales).
Because the COVID-19 pandemic and its unanticipated school closures interfered with intervention fidelity, it is important for future trials to deploy this intervention under regular, in-person school conditions. However, our COVID-19 adaptation produced an innovative text reminder system that could supplement our school-based intervention during school breaks [30]. Excluding participants above grade 6 was an additional limitation of the study. We targeted younger children because prior studies showed children under fourteen years were most accepting of the program, but there is an opportunity to expand to include older age groups [29]. Considering the significant resources invested into large clinical trials, field-testing recruitment/retention strategies and adaptability of protocols on a small scale can help to ensure inclusion of medically underserved populations in future larger trials.
5. Conclusion
Our case example of a school-based asthma therapy pilot demonstrated successful strategies used to recruit and retain a study population of underserved children and families. As we strive for better inclusion of diverse study populations in clinical research, these strategies can be applied to future trials to promote better representation of underserved children and families in clinical research.
Funding
Dr. Trivedi was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL15034 and the National Center for Advancing Translational Science under Award number KL2TR001454. The funding sources were not involved in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations:
- ICS
Inhaled corticosteroid
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- [1].Medically Underserved Areas and Populations (MUA/Ps), Health Resources & Services Administration, Accessed April 26, 2022, at, https://bhw.hrsa.gov/shortage-designation/muap, 2021. [Google Scholar]
- [2].Coakley M, Fadiran EO, Parrish LJ, Griffith RA, Weiss E, Carter C, Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials, J. Women’s Health 21 (2012) 713–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Warren-Findlow J, Prohaska TR, Freedman D, Challenges and opportunities in recruiting and retaining underrepresented populations into health promotion research, Gerontologist 43 (1) (2003) 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nicholson LM, Schwirian PM, Groner JA, Recruitment and retention strategies in clinical studies with low-income and minority populations: Progress from 2004–2014, Contemp Clin Trials 45 (2015) 34–40. [DOI] [PubMed] [Google Scholar]
- [5].Fogel DB, Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review, Contemporary Clinical Trials Communications 11 (2018) 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Warren CM, Brown E, Wang J, Matsui EC, Increasing representation of historically marginalized populations in allergy, asthma, and immunologic research studies: challenges and opportunities, J Allergy Clin Immunol Pract 10 (4) (2022) 929–935. [DOI] [PubMed] [Google Scholar]
- [7].Whyte J, Racial and ethnic representation of participants in US clinical trials of new drugs and biologics, JAMA 327 (2022) 985. [DOI] [PubMed] [Google Scholar]
- [8].Lolic M, Araojo R, Okeke M, Temple R, racial US and ethnic participation in global clinical trials by therapeutic areas, J. Clin. Pharm. Ther. 46 (2021) 1576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Niranjan SJ, Wenzel JA, Martin MY, et al. , Perceived institutional barriers among clinical and research professionals: minority participation in oncology clinical trials, JCO Oncol Pract 17 (2021) (e666–e75). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Legor KA, Hayman LL, Foust JB, Blazey ML, The role of clinical research nurses in minority recruitment to cancer clinical trials, Contemp Clin Trials 110 (2021), 106590. [DOI] [PubMed] [Google Scholar]
- [11].Saltzman RG, Jayaweera DT, Caceres LV, et al. , Demographic representation in clinical trials for cell-based therapy, Contemp Clin Trials Commun 21 (2021), 100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Striving for diversity in research studies, N. Engl. J. Med. 385 (2021) 1429–1430. [DOI] [PubMed] [Google Scholar]
- [13].Kagawa-Singer M, Improving the validity and generalizability of studies with underserved U.S. populations expanding the research paradigm, Ann. Epidemiol. 10 (2000) S92–S103. [DOI] [PubMed] [Google Scholar]
- [14].Wallace DC, Bartlett R, Recruitment and retention of African American and Hispanic girls and women in research, Public Health Nurs. 30 (2013) 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rhodes SD, Alonzo J, Mann-Jackson L, et al. , Selling the product: strategies to increase recruitment and retention of Spanish-speaking Latinos in biomedical research, J Clin Transl Sci 2 (2018) 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kistin C, Silverstein M, Pilot studies: a critical but potentially misused component of interventional research, Jama 314 (2015) 1561–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fortune T, Wright E, Juzang I, Bull S, Recruitment, enrollment and retention of young black men for HIV prevention research: experiences from the 411 for safe text project, Contemp Clin Trials 31 (2010) 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Taani MH, Zabler B, Fendrich M, Schiffman R, Lessons learned for recruitment and retention of low-income African Americans, Contemporary clinical trials communications 17 (2020) 100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Crocker D, Brown C, Moolenaar R, et al. , Racial and ethnic disparities in asthma medication usage and health-care utilization: data from the National Asthma Survey, Chest 136 (2009) 1063–1071. [DOI] [PubMed] [Google Scholar]
- [20].Drug Trials Snapshot Summary Report, U.S. Food & Drug Administration, 2020. [Google Scholar]
- [21].Akinbami LJ, Moorman JE, Bailey C, et al. , Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010, NCHS Data Brief (2012) 1–8. [PubMed] [Google Scholar]
- [22].Akinbami LJ, Simon AE, Rossen LM, Changing trends in asthma prevalence among children, Pediatrics 137 (2016) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Davis CM, Apter AJ, Casillas A, et al. , Health disparities in allergic and immunologic conditions in racial and ethnic underserved populations: a work group report of the AAAAI Committee on the underserved, J. Allergy Clin. Immunol. 147 (2021) 1579–1593. [DOI] [PubMed] [Google Scholar]
- [24].Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS, Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases, J. Allergy Clin. Immunol. 125 (2010) 540–544. [DOI] [PubMed] [Google Scholar]
- [25].Liu AH, Zeiger RS, Sorkness CA, et al. , The Childhood Asthma Control Test: retrospective determination and clinical validation of a cut point to identify children with very poorly controlled asthma, J. Allergy Clin. Immunol. 126 (267–73) (2010) 73.e1. [DOI] [PubMed] [Google Scholar]
- [26].Trivedi M, Patel J, Lessard D, et al. , School nurse asthma program reduces healthcare utilization in children with persistent asthma, J Asthma 55 (2018) 1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bukstein DA, McGrath MM, Buchner DA, Landgraf J, Goss TF, Evaluation of a short form for measuring health-related quality of life among pediatric asthma patients, J. Allergy Clin. Immunol. 105 (2000) 245–251. [DOI] [PubMed] [Google Scholar]
- [28].Trivedi M, Hoque S, Shillan H, et al. , CENTER-IT: a novel methodology for adapting multi-level interventions using the consolidated framework for implementation research—a case example of a school-supervised asthma intervention, Implementation Science Communications 3 (2022) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Trivedi M, Patel J, Hoque S, et al. , Alignment of stakeholder agendas to facilitate the adoption of school-supervised asthma therapy, Pediatr. Pulmonol 55 (2020) 580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arenas J, Becker S, Seay H, et al. , A response to COVID-19 school closures: the feasibility of a school-linked text message intervention as an adaptation to school-supervised asthma therapy, Pediatr. Pulmonol 57 (5) (2022) 1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mahmood B, Afshar R, Tang TS, Recruitment and retention of south Asian ethnic minority populations in behavioral interventions to improve type 2 diabetes outcomes, Curr Diab Rep 17 (2017) 25. [DOI] [PubMed] [Google Scholar]
- [32].Cui Z, Seburg EM, Sherwood NE, Faith MS, Ward DS, Recruitment and retention in obesity prevention and treatment trials targeting minority or low-income children: a review of the clinical trials registration database, Trials 16 (2015) 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nicholson LM, Schwirian PM, Klein EG, et al. , Recruitment and retention strategies in longitudinal clinical studies with low-income populations, Contemporary Clinical Trials 32 (2011) 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brotherton SE, Stoddard JJ, Tang SS, Minority and nonminority pediatricians’ care of minority and poor children, Arch Pediatr Adolesc Med 154 (2000) 912–917. [DOI] [PubMed] [Google Scholar]
- [35].Janson SL, Alioto ME, Boushey HA, Attrition and retention of ethnically diverse subjects in a multicenter randomized controlled research trial, Control. Clin. Trials 22 (2001) (S236–S43). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


