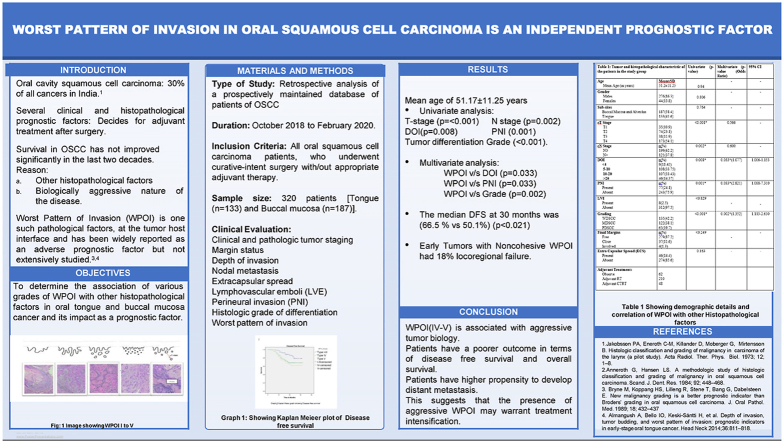
Abstract
Introduction
Biologic aggressiveness of OSCC (Oral Cavity Squamous Cell Carcinoma), has intrigued research in various prognosticating histopathological markers over past few decades. DOI (Depth of Invasion) is one such histopathological factor which affects outcomes and was included in the AJCC 8th edition TNM staging. Pattern of Invasion (POI) has been widely reported as an adverse prognostic factor associated with higher locoregional failure and poor prognosis. However, these factors are not utilized for treatment decision making and for outcome assessment.
Materials and methods
This is a retrospective analysis of 320 patients with OSCC who underwent treatment, from October 2018–February 2020. Clinic demographic details were extracted from electronic medical records. Univariate and multivariate analysis was done for the parameters. WPOI (Worst Pattern of Invasion) was correlated with all histopathological prognostic factors. Survival analysis was done using Kaplan Meier for WPOI type's I–V. DFS (Disease free Survival) was evaluated for different grades of WPOI.
Results
We analyzed the results comparing, early and advanced T (Tumor) stages, cohesive WPOI I-III, non-cohesive WPOI IV-V. Univariate analysis showed a significant association of T-stage (p = 0.001), N (Nodal) -stage (p = 0.002), DOI (p = 0.008), PNI (Peri-neural invasion) (0.001) and Tumor differentiation Grade (p = 0.001). On multivariate analysis, non-cohesive WPOI (IV & V) showed significant association with grade, PNI, DOI (0.002, 0.033 & 0.033 respectively). Non-cohesive WPOI had significantly higher locoregional failures and short DFS.
Conclusion
Presence of invasive WPOI is associated with advanced T stage, poor differentiation, PNI, greater depth of invasion, and higher chances of nodal metastasis. WPOI is associated with poor DFS, treatment intensification in early stage disease with WPOI type IV & V may improve survival.
Keywords: Worst Pattern of Invasion, Histopathological features, Aggressive Tumor Biology, Oral Squamous Cell Carcinoma
Abbreviations: WPOI, Worst Pattern of Invaion; OSCC, Oral Cavity Squamous Cell Carcinoma; DOI, Depth of Invasion; PNI, Peri neural invasion; T-stage, Tumor Stage; N- Stage, N stage; POI, Pattern of Invasion; LVE, Lymphovascular emboli; DFS, Disease Free Survival
Graphical abstract
1. Introduction
Oral cavity squamous cell carcinoma (OSCC) has been prognosticated clinically as well as histopathologically. Adjuvant therapy is planned based upon several factors like T-stage, nodal status, extra nodal extension etc. Survival of OSCC has not improved significantly in the last two decades despite several advances in the treatment strategies.1 There are several prognostic factors like peri-neural invasion (PNI), lympho-vascular emboli (LVE) and molecular studies etc. however, the biological aggressiveness of OSCC, necessitates further evaluation for factors affecting the prognosis.2 There are studies analyzing OSCC at the tumor host interphase to determine the pathological factors affecting outcomes. Pattern of Invasion (POI) is one such histopathological factor, at the tumor host interface and has been widely reported as an adverse prognostic factor.3, 4, 5, 6 Studies have found that WPOI is associated with higher locoregional failure and poor prognosis.7,8 However, there are very few studies analyzing the types of WPOI and its correlation with other histopathological factors.
We have previously evaluated the role of margin and the role microscopic spread of tumor beyond the gross disease. However, we considered only the spread of disease as separate island (Type-V WPOI) as microscopic spread in that study. These patients with microscopic spread had poor survival as compared to patients without microscopic spread.9 Subsequently after the AJCC 8th edition staging, incorporation of the types of POI (I–V) has been emphasized.10 We therefore, performed this analysis to evaluate the role of WPOI and its impact as a prognostic factor. We aim to analyze the correlation of different histopathological factors with different types of WPOI and its impact on survival.
2. Materials & methods
2.1. Type of study
This was a retrospective analysis of a prospectively maintained database of patients with OSCC who underwent surgery between October 2018 to February 2020. Clinico-demographic and histopathological details were recorded from electronic medical records. The data was de-identified after abstraction. Institutional ethical committee clearance was obtained for analysis. Waiver of Consent was taken as this was a retrospective analysis, and the study was compliant with the Helsinki declaration.
Inclusion Criteria: All oral squamous cell carcinoma patients, who underwent curative-intent surgery with/without appropriate adjuvant therapy were included in the analysis. Patient's demographic details, tumor, and treatment details were recorded. Histopathological details of each patient were evaluated and recorded. A head and neck pathologist reviewed the histopathology slides of the patients for worst pattern of invasion and other adverse prognostic factors.
Exclusion Criteria: Patients with incomplete medical records of clinical or pathological details, histology other than squamous carcinoma, and recurrent disease or second primary tumor were excluded. Patients who did not complete treatment or were lost to follow up were excluded.
Sample size: 412 patients with Oral cancer underwent surgery during the period of study. Out of which 48 had recurrent disease, 28 had histology other than squamous cell carcinoma and 16 patients had incomplete surgical records, pathological data or follow-up details and therefore were excluded from the analysis. Thus, 320 patients were included for final analysis [Tongue (n = 133) and Buccal mucosa (n = 187)].
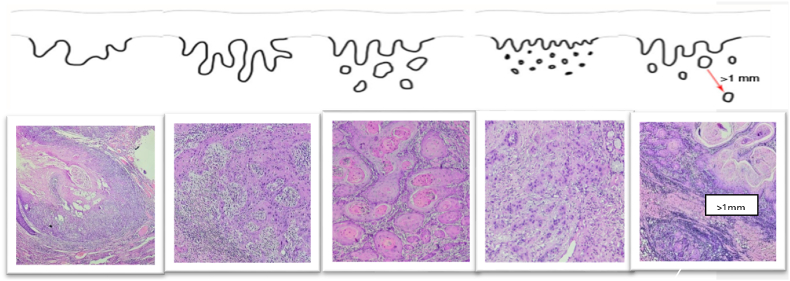
Clinicopathologic Evaluation: The following clinico-demographic and pathologic findings were recorded: age, gender, primary site of disease (oral tongue, buccal mucosa). Standardized synoptic reporting is followed at our center to ensure the adequacy of pathology reporting. Sections from paraffin blocks were taken such that the slides included the deepest portion in terms of tumor-host interface or invasive front. Clinical and pathologic tumor staging; and histopathology parameters, such as margin status, depth of invasion, nodal metastasis, extra nodal extension (ENE), lympho vascular emboli etc. was recorded. Histologic grade of differentiation was reported as mild, moderate and severe differentiation. This was reported as per following well-differentiated- mild pleomorphism good amount of intracellular and extracellular keratinization in the form of keratin pearls and occasional mitotic figures, moderately differentiated - few keratin pearl formation moderate nuclear pleomorphism and mitotic rate of 3/10 Hpf, poorly differentiated - moderate to marked nuclear pleomorphism, absence of intracellular and extracellular keratinization and increased mitotic rate of 10/10 Hpf. Margin of resection was considered free if > 5 mm, close if 2–5 mm, and involved <1 mm. DOI was sub grouped as <4 mm, 5–10 mm, 11–20 mm and >20 mm. Worst pattern of invasion (Type I– V) was analyzed by the pathologist as per CAP protocol.10 WPOI was graded as follows (Figure-1).
Fig. 1.
A. Pattern Of Invasion Type 1: Tumor invasion is in broad pushing manner (40x), B. POI Type 2 represents tumor invading in solid cords and strands (“finger-like")(100x), C. POI Type 3 shows invasive islands of the tumor with >15 cells cluster (100x), D. POI Type 4 represents invasive tumor islands with <15 cells cluster (100x), E. POI Type 5 is tumor islands more than 1 mm away from the progressive end of the tumor (40x).
Clinicodemographic details were segregated between two subsites tongue and buccal mucosa. The histopathological details were tabulated and analysis was done for correlation of WPOI with other adverse prognostic factors. Adjuvant treatment was administered when indicated. Adjuvant radiotherapy was administered to patients with advanced T3-T4 disease, Nodal metastasis, Perineural invasion and LVE present or DOI more than 10 mm. Adjuvant chemo radiotherapy was administered to patients with involved margins and/or extra nodal extension.
Association of WPOI with nodal metastasis and clinicopathological features like (pathological TNM stage, tumor size, grade of differentiation, depth of invasion, perineural invasion, lymphovascular emboli, and extracapsular spread) was determined.
2.2. Statistical analysis
Data analysis was done using IBM SPSS 25 software. To identify factors associated with WPOI, univariate analysis was done using the chi-square test. Multivariate analysis was done using binary logistic regression. Information regarding patient survival and disease status was also retrieved from medical records. Disease-free (DFS) were calculated by the Kaplan-Meier method. We defined DFS as the period between date of the first recurrence: loco‐regional or distant from the date of surgery. Date of disease recurrence was collected from the medical records when they were diagnosed histologically or radiologically during the follow up. For survival analysis, the variables for univariate analysis were selected based on their clinical relevance as well as those previously described in the literature and analyzed using the log‐rank test. All significant (p < 0.05) variables were subsequently tested (multivariate) with cox‐regression analysis using forward stepwise selection.
3. Results
There were 320 participants in the study with a Mean age of 51.17 ± 11.25 years (Range: 25–86 years). The majority of the patients were male (86.3%) with a male to female ratio of 1:0.15. Bucco-alveolar complex was the most common subsite (58.4%) and tongue was 41.6%. The clinic-demographic and tumor characteristics of the patients in the study group are given in Table 1. T stage distribution in the study was as follows T1-35(10.9%), T2-74(23.1%) T3-38(11.9%) and T4-173(54.1%). Patients with T1-T2 were grouped as early T stage and those with T3-T4 tumors were grouped as advanced T Stage. On histopathology slides, Majority of study patients i.e 42.2% (n = 135) showed well differentiated tumors, while 38.1% (n = 122) were moderately differentiated tumors and 19.7% (n = 63) were poorly differentiated tumors. When resected margins were evaluated, 87.1% (n = 279) were found to be free, 11.6% (n = 37) had close margins and 1.3% (n = 4) had involved margins. 62.2% (n = 199) of our study patients had N0 nodal stage. Of the 37.8% (n = 121) patients who had N+ disease, 14.4% (n = 46) had extranodal extension. 24.1% (n = 77) had Perineural invasion. Lympho vascular emboli was noted in 2.5% (n = 8) patients. Depth of invasion (DOI) of 5–10 mm was noted in 33.75% (n = 108) cases, 10–20 mm in 107(33.43%), while >20 mm was seen in 14.37% (n = 46) and <4 mm in 1843% (n = 59) cases.
Table 1.
Demographic details and Histopathologic Characteristics of the Study group.
| Patient and Tumor Characteristics | Univariate (p-vaue) | Multivariate (p-value (Odds Ratio)) | 95% CI | |
|---|---|---|---|---|
| Demographics: | ||||
| Age | Mean ± SD | 0.94 | – | – |
| Mean Age (in years) | 51.2 ± 11.25 | |||
| Gender | n(%) | 0.806 | - | - |
| Males | 276(86.3) | |||
| Females | 44(13.8) | |||
| Sub-sites | 0.764 | - | - | |
| Buccal Mucosa and Alveolus | 187(58.4) | |||
| Tongue | 133(41.6) | |||
| Tumor Factors and WPOI I-III vs IV,V Correlation: | ||||
| cT Stage | n(%) | 0.000 | 0.566 | - |
| T1 | 35(10.9) | |||
| T2 | 74(23.1) | |||
| T3 | 38(11.9) | |||
| T4 | 173(54.1) | |||
| cN Stage | n(%) | 0.0018 | 0.600 | - |
| N0 | 199(62.2) | |||
| N+ | 121(37.8) | |||
| DOI | n(%) | 0.000 | 0.033*(1.077) | 1.006–1.153 |
| <4 | 59(18.43) | |||
| 5-10 | 108(33.75) | |||
| 10-20 | 107(33.43) | |||
| >20 | 46(14.37) | |||
| PNI | n(%) | 0.000 | 0.033*(2.821) | 1.088–7.310 |
| Present | 77(24.1) | |||
| Absent | 243(75.9) | |||
| LVI | 0.829 | |||
| Present | 8(2.5) | – | – | |
| Absent | 312(97.5) | |||
| Grading | 0.000 | 0.002*(1.352) | 1.113–2.630 | |
| WDSCC | 135(42.2) | |||
| MDSCC | 122(38.1) | |||
| PDSCC | 63(19.7) | |||
| Final Margins | n(%) | <0.249 | – | – |
| Free | 279(87.2) | |||
| Close | 37(11.6) | |||
| Involved | 4(1.3) | |||
| Extra Nodal Extension (ENE) | 0.163 | - | - | |
| Present | 46(14.4) | |||
| Absent | 274(85.6) | |||
| Adjuvant Treatment: | ||||
| Observe | 62 | |||
| Adjuvant RT | 210 | |||
| Adjuvant CTRT | 48 | |||
3.1. Histopathological factors and WPOI (Table 1)
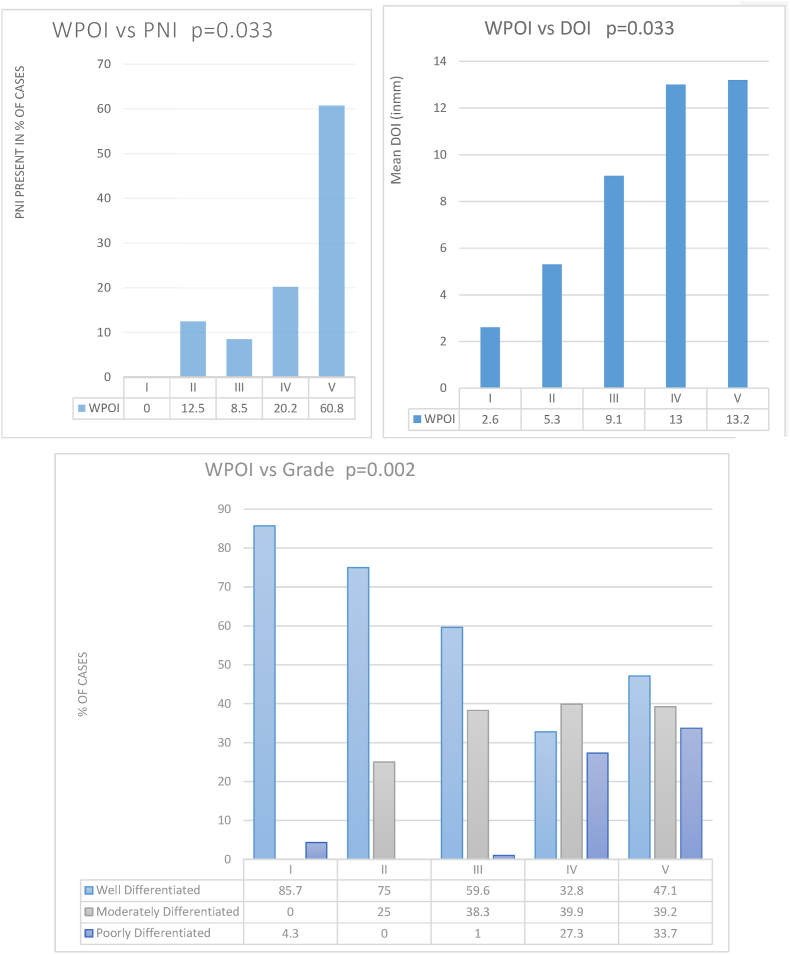
WPOI was correlated with other histopathological factors. Around 71.4% of (T3-T4) tongue cancer and 87.8% of (T3-T4) buccal mucosa cancer had WPOI IV-V. 92.20% (71/77) Patients with WPOI IV-V had PNI positivity. 96.82% (61/63) patients with poorer differentiation had WPOI IV-V. 89.13% (41/46) with DOI >20 mm had WPOI IV-V (p < 0.001).
Univariate analysis was done to check whether T-stage, N stage, PNI, LVE, Grade of differentiation had any association with WPOI. It showed a significant association of T-stage (p < 0.001), N stage (p = 0.002), DOI (p < 0.008), PNI (p < 0.001) and Tumor differentiation Grade (<0.001). Binomial Logistic regression used for multivariate analysis comparing WPOI (I-III) against WPOI (IV and V) showed DOI (p < 0.033), PNI (p < 0.033), and Grade of differentiation (p < 0.002), had a statistically significant association with non-cohesive or aggressive WPOI. However, Tumor Stage (p < 0.56) and Nodal stage (p = 0.60) were not statistically significant (Graph 1).
Graph 1.
WPOI association with DOI, PNI and Tumor differentiation.
3.2. WPOI I– III vs WPOI IV vs WPOI V
Cohesive WPOI I-III when compared with WPOI IV and WPOI V individually showed statistical significance on univariate analysis T-stage (p < 0.000), N stage (p < 0.018), DOI (p,0.000), PNI (p < 0.000) and Tumor differentiation Grade (p < 0.000).
Adjuvant treatment was indicated and received by 258 patients, 210 patients underwent adjuvant RT alone, while 48 patients received adjuvant CTRT (Chemo radiotherapy). 62 patients were on observation as they did not warrant any adjuvant treatment.
3.3. Survival analysis
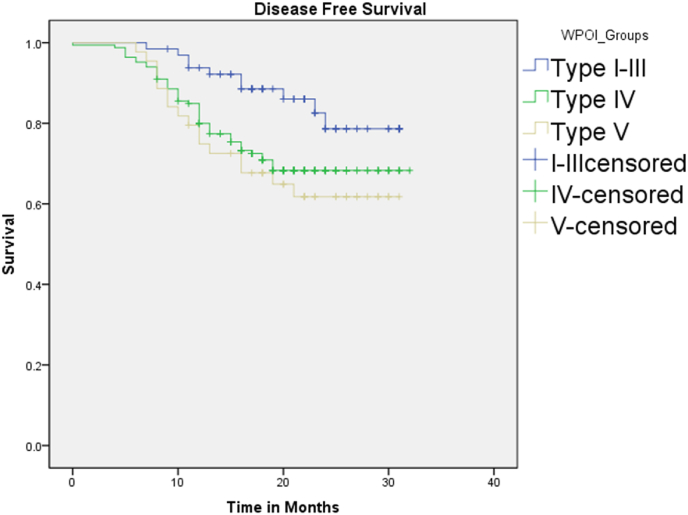
Survival analysis was done for different grades of WPOI. Mean follow-up time in our study was 1.66 years. (Range 0.5–3.08 years). Kaplan Meier plot was done for disease-free survival comparing cohesive WPOI I-III and non-cohesive WPOI IV & V groups separately. Median DFS at 36 months was 28.7 months and 20.9 months (66.5% vs 50.1%) was noted in cohesive and non -cohesive WPOI respectively. Graph 2.
Graph 2.
Kaplan Meier graph showing Disease free survival.
Cohesive WPOI had a significantly better DFS (p < 0.021 Log rank test) compared to non-cohesive WPOI IV and V (0.046, 0.031) respectively. Total of 36.3% (n = 77) of the study patients had locoregional failure, of which 59.6% (n = 45) were in tongue carcinoma and 29.7% (n = 22) associated with buccal cancer. There was no Loco-regional failure in WPOI I –III in tongue carcinoma, 4.08% (n = 3) with loco regional failure in buccal cancer in WPOI I-III. In non-cohesive WPOI group, 83.67% (n = 38) of patients with Buccal cancer and 92.8% (n = 19) of patients with tongue cancer had locoregional failure. (Table 2)
Table 2.
Log Rank test comparing DFS vs WPOI Survival analysis between WPOI I-III vs IV vs V.
| WPOI GROUP | I-III | IV | V | ||||
|---|---|---|---|---|---|---|---|
| DISEASE FREE SURVIVAL | Chi-sq | P value | Chi-sq | P value | Chi-sq | P value | |
| I-III | 3.993 | 0.046 | 5.355 | 0.021 | |||
| IV | 3.993 | 0.046 | 0.599 | 0.439 | |||
| V | 5.355 | 0.021 | 0.599 | 0.439 | |||
There were 72 patients who developed locoregional recurrence despite Adjuvant therapy, 89% (n = 64) of them had WPOI IV-V, as compared to 11% (n = 8) with WPOI I-III. This suggest that WPOI IV-V makes the tumor biologically aggressive and may warrant aggressive adjuvant treatment.
3.4. Pathologic tumor stage and survival analysis
In early tumors with cohesive WPOI 1/18 (5.5%) patients had locoregional failure compared to 10/53 (18%) patients with non-cohesive WPOI. In advance tumors with Cohesive WPOI 2/9 (22%) patients had loco regional failure compared to 19/47 (40%) patients with non-cohesive WPOI. These findings suggest that early tumors with non-cohesive WPOI may need treatment intensification. However, there is a need for further prospective studies, with larger sample size to validate the above findings. (Table 3)
Table 3.
Tumor stage vs WPOI Survival analysis between Cohesive and Non cohesive WPOI.
| Locoregional Failure | Total Deaths | ||
|---|---|---|---|
| Pathologic T Stage | Cohesive WPOI | Non-cohesive WPOI | |
| T1 | 0/12 | 2/21 | 2/33 |
| T2 | 1/6 | 8/32 | 9/38 |
| T3 | 1/7 | 13/37 | 14/44 |
| T4a | 1/2 | 6/10 | 7/12 |
| Total | 3/27 | 29/100 | 32/127 |
4. Discussion
Surgery with or without adjuvant therapy remains the mainstay of treatment in the OSCC. In oral cavity, tongue and buccal mucosa cancers are the most common subsites and they have been shown to have varied biological aggressiveness.11, 12, 13, 14 Despite modern therapeutic advances, survival has not significantly improved in these cancers. Clinical assessment by tumor node metastasis (TNM) system is widely and routinely used to define the extent of tumor load and thus determine treatment options for patients with OSCC. One of the major criticisms of TNM system is that it ignores the individual histological characteristics of tumors.
To aid this, several histopathological factors have been studied and taken into consideration for adjuvant treatment decision making. There may be several prognosticators that may affect survival. The Pattern of invasion is one such factor. It is described as the invasive front of the tumor at the tumor-host interface.15,16
Many histological prognostic models and scoring systems have been developed in past for predicting the biological behavior of OSCC. POI remains an important factor in all these grading systems. In 1973, Jakobsson et al.3 developed a multifactorial grading system that had the advantage of scoring tumor-host interactions and tumor characteristics but eventually proved to be useful only when applied to tongue cancers.17 Later, Anneroth et al. proposed a modification of Jakobsson's system based on the assessments of six histo-morphological parameters.4 Bryne modified Anneroth's grading system and developed a malignancy grading system focusing only on the invasive front of the tumor.5 A risk assessment score has been proposed by Brandwein which includes PNI, Pattern of invasion and Lymphocytic response as a predictor of local recurrence and survival.6
The pattern of invasion reflects biological mechanisms of malignancy, such as loss of contact inhibition, increased tumor cell motility and secretion of proteolytic enzymes. Its observation in routine histological preparations provides a simple measure of tumor behavior. Molecular studies on the pattern of invasion have shown that deep invasive tumor fronts have higher expression of Ki-67 and cyclin B1 markers with reduced E-cadherin expression.18 Thus, having a higher propensity for malignant cells to metastasize. This is similar to our study findings wherein WPOI IV-V was associated with higher incidence of lymph node metastasis (p < 0.001) and distant metastasis (p < 0.001).
In the present study, we intended to correlate and evaluate the types of WPOI at the tumor-host interface with other clinicopathologic prognostic factors in buccal mucosa and tongue carcinomas. WPOI type IV remains the most common pattern. Majority of the patients with involved margin were associated with WPOI type IV, a similar finding has been shown in other studies.19, 20, 21, 22 This shows that disease can microscopically spread beyond clinical margins in invasive or non-cohesive WPOI. It appears logical to include wider margins of excision during surgery in cases shown to have pathologically defined non-cohesive/invasive WPOI in pre-operative biopsy. This may not improve survival but may reduce the local recurrence rate.23,24
In our study, patients who did not receive adjuvant therapy, as they did not warrant adjuvant therapy as per the guidelines, showed higher incidence of loco regional failure. This group of patients may have benefitted with adjuvant therapy. As the depth of invasion increase from 5 mm onwards, the chances of having WPOI of grade IV and V increases.25 The superficial tumors are less aggressive as compared to tumor with greater DOI. Having said that, there are several studies which have shown improved survival outcomes by adding adjuvant therapy based on DOI. This may point towards the hypothesis that patients with higher WPOI IV-V may benefit by treatment intensification. Similarly, DOI less than 4 mm with WPOI IV and V may be proposed to be treated more aggressively. We analyzed that, tumors with invasive fronts tend to spread through perineural invasion defining it as biologically aggressive feature in OSCC.26 Similarly, penetration of invasive tumors in vascular and lymphatic vessels will release tumor cells aggregates or emboli into circulation leading to metastasis formation and would have an inferior prognosis.
High risk patients stratified by Adjuvant treatment may warrant treatment intensification with non-cohesive WPOI. In our study patients, we also compared WPOI with early and advanced tumor stages, tumor subsites: tongue and buccal cancers. Early tumors with non-cohesive WPOI, and patients with tongue cancers had higher chances of locoregional failure, than those with cohesive WPOI. Median Disease-free survival at a 36 month follow up of patients showed better survival outcomes with cohesive WPOI (p < 0.03) and early tumors (p < 0.04) respectively. However, when WPOI IV and WPOI V were compared individually for survival outcomes we did not find any statistically significant difference among both groups. Higher number of locoregional failures were noted in patients with WPOI IV and V, irrespective of adjuvant treatment and tumor stage. This signifies that, early tumors with non-cohesive WPOI need more stringent follow up and probably treatment intensification or modification to reduce chances of locoregional failure and improve oncological outcomes.
5. Conclusion
This study is one of its kind where role of WPOI as a separate prognostic marker has been evaluated in oral squamous cell carcinoma. We have found that WPOI (IV & V) is associated with aggressive tumor biology. The presence of type IV and V WPOI is associated with larger tumor size, poor differentiation, PNI, greater depth of invasion, and higher incidence of nodal metastasis. Patients with WPOI IV, V have a poorer oncological outcome in terms of disease-free survival and overall survival irrespective of stage and adjuvant treatment. Patients with WPOI IV & V may be considered for treatment intensification especially in early stage tumors.
Future treatment
There is a paucity in the available literature particularly from the Indian subcontinent on WPOI as a reliable histopathological parameter for determining tumor biologic behavior and predicting prognosis. With over 2 lakh cases occurring yearly and 60–80% cases presenting with advanced disease, mandates further prospective evaluation on WPOI at invasive tumor front and evaluation of other prognostic factors. Identifying areas of adding adjuvant treatment to improve survival especially in early tumors will impact treatment in the long run. Inclusion of such parameters in tumor staging may help better treatment planning, reduce locoregional failure and improve long-term survival in OSCC.
Drawbacks
Since it is a retrospective study, with data collected from Electronic medical records, some of the relevant clinical or histopathological information may have been missed. Survival data is difficult to interpret accurately due to shorter mean follow-up period.
Ethical considerations
Not required.
Financial disclosure
Nil.
Funding source
This work was financially supported by Intramural Grant from Tata Memorial Centre- TMC Research Administration Council (TRAC).
Role of funder
The TMC-TRAC reviewed the study and supported the funding. The funding body had no role in design and conduct of the study, data collection, management or analysis.
Declaration of competing interest
None.
References
- 1.Warnakulasuriya S. Prognostic and predictive markers for oral squamous cell carcinoma: the importance of clinical, pathological and molecular markers. Saudi J Med Med Sci. 2014;2:12–16. doi: 10.4103/1658-631X.128400. [DOI] [Google Scholar]
- 2.Amin M.B., Edge S.B., Greene F.L., et al., editors. AJCC Cancer Staging Manual. eighth ed. Springer; New York: 2017. [DOI] [Google Scholar]
- 3.Jakobsson P.A., Eneroth C.-M., Killander D., Moberger G., Mirtensson B. Histologic classification and grading of malignancy in carcinoma of the larynx (a pilot study) Acta Radiol Ther Phys Biol. 1973;12:1–8. doi: 10.3109/02841867309131085. [DOI] [PubMed] [Google Scholar]
- 4.Anneroth G., Hansen L.S. A methodologic study of histologic classification and grading of malignancy in oral squamous cell carcinoma. Scand J Dent Res. 1984;92:448–468. doi: 10.1111/j.1600-0722.1984.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 5.Bryne M., Koppang H.S., Lilleng R., Stene T., Bang G., Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders' grading in oral squamous cell carcinoma. J Oral Pathol Med. 1989;18:432–437. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 6.Brandwein-Gensler M., Teixeira M.S., Lewis C.M., et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 7.Spiro Ronald H., Guillamondegui Oscar, Jr., Paulino Augusto F., Huvos Andrew G. Vol. 21. 1999. Pattern of invasion and margin assessment in patients with oral tongue cancer; pp. 408–413. 5. (199908)21:5<408::aid-hed5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Manjula B.V., Augustine Suni, Selvam Sumithra, Mohan, Mathan A. Prognostic and predictive factors in gingivo buccal complex squamous cell carcinoma: role of tumor budding and pattern of invasion. Indian J Otolaryngol. 2015;67(1 Supplement):98–104. doi: 10.1007/s12070-014-0787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra A., et al. Role of microscopic spread beyond gross disease as an adverse prognostic factor in oral squamous cell carcinoma. Eur J Surg Oncol. 2017 doi: 10.1016/j.ejso.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Ridge J.A., Lydiatt W.M., Patel S.G., et al. In: AJCC Cancer Staging Manual. eighth ed. Amin M.B., editor. Springer; New York, NY: 2017. Lip and oral cavity. [DOI] [Google Scholar]
- 11.Heerema M.G., Melchers L.J., Roodenburg J.L., Schuuring E., de Bock G.H., van der Vegt B. Reproducibility and prognostic value of the pattern of invasion scoring in low-stage oral squamous cell carcinoma. Histopathology. 2016;68(3):388–397. doi: 10.1111/his.12754. [DOI] [PubMed] [Google Scholar]
- 12.Li Y., Bai S., Carroll W., et al. Validation of the risk model: highrisk classification and tumor pattern of invasion predict the outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013;7(3):211–223. doi: 10.1007/s12105-012-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velosa Claudia, Shi Qiuying, Stevens Todd, et al. Worst Pattern of Invasion and occult cervical metastases for oral squamous carcinoma. Head Neck. 2017 doi: 10.1002/hed.24754. 10.1002/hed.24754. [DOI] [PubMed] [Google Scholar]
- 14.Nair Sudhir, Singh Bikramjit, Pawar Prashant V., et al. Squamous cell carcinoma of tongue and buccal mucosa: clinico-pathologically different entities. Eur Arch Oto-Rhino-Laryngol. 2016;273(11):3921–3928. doi: 10.1007/s00405-016-4051-0. [DOI] [PubMed] [Google Scholar]
- 15.Liao C.T., Huang S.F., Chen I.H., et al. Tongue and buccal mucosa carcinoma: is there a difference in outcome? Ann Surg Oncol. 2010;17(11):2984–2991. doi: 10.1245/s10434-010-1174-1. [DOI] [PubMed] [Google Scholar]
- 16.Sathyan K.M., Sailasree R., Jayasurya R., et al. Carcinoma of the tongue and buccal mucosa represent different biological subentities of the oral carcinoma. J Cancer Res Clin Oncol. 2006;132:601–609. doi: 10.1007/s00432-006-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holm L.E., Lundquist P.G., Silfversward C., Sobin A. Histological grading of malignancy in squamous cell carcinoma of the oral tongue. Acta Otolaryngol. 1982;94:185–192. doi: 10.3109/00016488209128904. [DOI] [PubMed] [Google Scholar]
- 18.Lund C., Sogaard H., Elbrond D., Jorgensen K., Petersen A.P. Epidermoid carcinoma of the tongue: histologic grading in the clinical evaluation. Acta Radiol Ther Phys Biol. 1975;14:513–521. doi: 10.3109/02841867509132692. [DOI] [PubMed] [Google Scholar]
- 19.Spiro R.H., Guillamondegui O., Jr., Paulino A.F., Huvos A.G. The pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck. 1999 Aug;21(5):408–413. doi: 10.1002/(sici)1097-0347. (199908)21:5<408::aid-hed5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Kane S.V., Gupta M., Kakade A.C., D’ Cruz A. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur J Surg Oncol. 2006;32(7):795–803. doi: 10.1016/j.ejso.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues P.C., Miguel M.C., Bagordakis E., et al. Clinicopathological prognostic factors of oral tongue squamous cell carcinoma: a retrospective study of 202 cases. Int J Oral Maxillofac Surg. 2014;43:795–801. doi: 10.1016/j.ijom.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Almangush A., Bello I.O., Keski-Säntti H., et al. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36:811–818. doi: 10.1002/hed.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson A.R., Kemmer J., Formeister E., et al. Beyond depth of invasion: adverse pathologic tumor features in early oral tongue squamous cell carcinoma. Laryngoscope. 2020 Jul;130(7):1715–1720. doi: 10.1002/lary.28241. Epub 2019 Aug 14. PMID: 31411752. [DOI] [PubMed] [Google Scholar]
- 24.Nadaf A., Bavle R.M., Soumya M., D'mello S., Kuriakose M.A., Govindan S. Analysis of the invasive edge in primary and secondary oral squamous cell carcinoma: an independent prognostic marker: a retrospective study. J Oral Maxillofac Pathol. 2016;20:239–245. doi: 10.4103/0973-029X.185931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu K., Wei J., Liu Z., et al. Can pattern and depth of invasion predict lymph node relapse and prognosis in tongue squamous cell carcinoma. BMC Cancer. 2019;19:714. doi: 10.1186/s12885-019-5859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundqvist L., Stenlund H., Laurell G., Nylander K. The importance of stromal inflammation in squamous cell carcinoma of the tongue. J Oral Pathol Med. 2012;41(5):379–383. doi: 10.1111/j.1600-0714.2011.01107.x. [DOI] [PubMed] [Google Scholar]