Abstract
Purpose
Radiation treatment interruption associated with unplanned hospitalization remains understudied. The intent of this study was to benchmark the frequency of hospitalization-associated radiation therapy interruptions (HARTI), characterize disease processes causing hospitalization during radiation, identify factors predictive for HARTI, and localize neighborhood environments associated with HARTI at our academic referral center.
Methods and Materials
This retrospective review of electronic health records provided descriptive statistics of HARTI event rates at our institutional practice. Uni- and multivariable logistic regression models were developed to identify significant factors predictive for HARTI. Causes of hospitalization were established from primary discharge diagnoses. HARTI rates were mapped according to patient residence addresses.
Results
Between January 1, 2015, and December 31, 2017, 197 HARTI events (5.3%) were captured across 3729 patients with 727 total missed treatments. The 3 most common causes of hospitalization were malnutrition/dehydration (n = 28; 17.7%), respiratory distress/infection (n = 24; 13.7%), and fever/sepsis (n = 17; 9.7%). Factors predictive for HARTI included African-American race (odds ratio [OR]: 1.48; 95% confidence interval [CI], 1.07-2.06; P = .018), Medicaid/uninsured status (OR: 2.05; 95% CI, 1.32-3.15; P = .0013), Medicare coverage (OR: 1.7; 95% CI, 1.21-2.39; P = .0022), lung (OR: 5.97; 95% CI, 3.22-11.44; P < .0001), and head and neck (OR: 5.6; 95% CI, 2.96-10.93; P < .0001) malignancies, and prescriptions >20 fractions (OR: 2.23; 95% CI, 1.51-3.34; P < .0001). HARTI events clustered among Medicaid/uninsured patients living in urban, low-income, majority African-American neighborhoods, and patients from middle-income suburban communities, independent of race and insurance status. Only the wealthiest residential areas demonstrated low HARTI rates.
Conclusions
HARTI disproportionately affected socioeconomically disadvantaged urban patients facing a high treatment burden in our catchment population. A complementary geospatial analysis also captured the risk experienced by middle-income suburban patients independent of race or insurance status. Confirmatory studies are warranted to provide scale and context to guide intervention strategies to equitably reduce HARTI events.
Introduction
Radiation therapy (RT) is an integral component of cancer care. Approximately 50% of all patients with cancer will undergo at least 1 course of radiation treatment.1 Optimal tumor control requires strict adherence to daily treatment scheduling. Unplanned interruptions are associated with inferior outcomes, including reduced overall survival.2, 3, 4, 5, 6, 7, 8
Hospitalization during RT is a severe, potentially preventable complication of treatment.9,10 Limited data are available to identify specific causes for hospitalization during RT.10 The purpose of this study was to catalog hospitalization rates during RT and identify patient-specific demographic, clinical, and treatment factors predictive for hospitalization-associated RT interruptions (HARTI) at our academic referral center. We also employed a secondary geospatial analysis to identify residential neighborhood environments most closely associated with interruption events to localize the potential need for interventional support to protect vulnerable populations.11
Methods and Materials
Patient population
Institutional review board approval was obtained to examine the electronic health records of patients receiving radiation treatment. Patients were included in the study if they were scheduled to begin RT between January 1, 2015, and December 31, 2017. Only those who initiated therapy were included in the study.
Outcome measures
The primary outcome of the study was frequency of HARTI, defined as any unplanned cancellation of scheduled RT associated with hospitalization during RT. Hospitalization was defined as emergency department visits, as well as inpatient admissions with levels of care ranging from observation to the intensive care unit.
Secondary outcomes included causes of hospitalization defined by patient primary diagnoses at the time of discharge, as well as identification of patient-specific demographic, clinical, and treatment factors significantly predictive for HARTI.
Data collection
Patient demographic, clinical, and treatment information was compiled from electronic medical records. Patient predicted income was categorized according to median household income from the 2016 federal census information at the census tract level, and stratified into low (<$34,000), middle ($34,000-$67,000), and high (>$67,000) thirds for the statistical analysis. Residence addresses were mapped at the ZIP code level for the purpose of geospatial analysis. The season in which patients began treatment was divided into winter (November-February) or nonwinter (March-October). Travel distance to the treating facility for individual patients was measured from residence ZIP code centroids. Rurality of patient home address was defined according to the U.S. Department of Agriculture 2013 Rural–Urban Continuum Codes.
Statistical analysis
Descriptive statistical analyses were performed to classify the frequency of HARTI events across demographic, clinical, and treatment variables.2 tests were performed to determine significance. Post hoc pairwise χ2d tests were used to further identify significant factors within each categorical variable. Univariable logistic regression models were developed to determine significant factors among those previously identified to predict any interruption event.12, 13, 14 Subsequent stepwise logistic regression models identified those variables most predictive for HARTI, and a multivariable logistic regression model was created to determine significant independent predictors. P-values were 2-sided, and P < .05 was considered statistically significant. All analyses were performed using RStudio, version 1.3.959 (PBC, Boston, MA), and SAS, version 9.4 (SAS Institute, Inc, Cary, NC).
Geospatial analysis
Frequency of HARTI was mapped at the level of residence ZIP codes and stratified according to patient race and insurance status to identify HARTI hotspots. Geographic data were plotted with RStudio, version 1.3.959 (PBC, Boston, MA), using the GIS package, and ggmap: Spatial Visualization was performed with ggplot2.15
Results
Cohort characteristics
A total of 3729 patients received 72,964 fractions of EBRT between January 1, 2015, and December 31, 2017, with an average prescription of 20 fractions. Of the 3729 patients, 3487 (93.5%) completed the entire prescribed regimen. Patient characteristics are described in Table 1. Average patient age was 61.2 years, 2065 patients (55.4%) were female, and 2195 (58.9%) were age <65 years. In addition, 2032 patients (54.5%) were White, 1577 (43.3%) African-American, and 120 (3.2%) reported a race other than White or African-American. Hispanic ethnicity was reported by 50 patients (1.3%). A total of 1967 patients (52.7%) were married.
Table 1.
Study cohort characteristics and hospitalization-associated RT interruption likelihood
| Number (%) | Frequency of hospitalization-associated RT interruption, n (%) | |
|---|---|---|
| Total | 3729 | 197 (5.3) |
| Sex | ||
| Male | 1664 (44.6) | 6.0 |
| Female | 2065 (55.4) | 4.7 |
| Age, mean, y | 61.2 | |
| <65 | 2195 (58.9) | 5.1 |
| ≥65 | 1534 (41.1) | 5.6 |
| Race | ||
| White | 2032 (54.5) | 4.4* |
| African-American | 1577 (42.3) | 6.3* |
| Other | 120 (3.2) | 6.7 |
| Ethnicity | ||
| Hispanic | 50 (1.3) | 6.0 |
| Non-Hispanic | 3540 (94.9) | 5.2 |
| Unknown | 139 (3.7) | 7.9 |
| Marital status | ||
| Married | 1967 (52.7) | 4.3* |
| Unmarried | 1648 (44.2) | 6.4* |
| Unknown | 114 (3.1) | 5.3 |
| Patient predicted income | ||
| Low (<$34k) | 1012 (27.1) | 4.4 |
| Middle ($34-67k) | 1532 (41.1) | 5.6 |
| High (>$67k) | 1149 (30.8) | 5.7 |
| Unknown | 36 (1.0) | 5.6 |
| Geography of residence | ||
| Rural not by metro | 114 (3.0) | 0.9 |
| Rural by metro | 201 (5.4) | 3.0 |
| Metro | 3428 (91.6) | 5.6 |
| Distance from RT, mile | ||
| 0-5 | 1108 (29.9) | 6.6 |
| 6-10 | 1175 (31.7) | 5.6 |
| 11-15 | 503 (13.6) | 3.8 |
| 16-20 | 220 (5.9) | 6.9 |
| 21-30 | 253 (6.8) | 4.8 |
| 31-40 | 110 (3.0) | 1.9 |
| >40 | 337 (9.1) | 3.3 |
| Insurance type | ||
| Commercial | 1794 (48.1) | 3.5* |
| Medicare | 1503 (40.3) | 6.3* |
| Medicaid/no insurance | 432 (11.6) | 9.0* |
| Medicaid | 221 (5.9) | 7.7 |
| No insurance | 211 (5.7) | 10.4* |
| Diagnosis | ||
| Breast | 974 (26.1) | 1.7* |
| Prostate | 413 (11.1) | 3.4 |
| Lung | 353 (9.5) | 10.8* |
| Gynecologic | 226 (6.1) | 6.2 |
| Head and neck | 402 (10.8) | 10.7* |
| Gastrointestinal | 238 (6.4) | 5.0 |
| Central nervous system | 148 (4.0) | 4.7 |
| Metastasis | 490 (13.1) | 4.1 |
| Skin | 123 (3.3) | 5.7 |
| Soft tissue | 53 (1.4) | 3.8 |
| Hematologic | 146 (3.9) | 2.7 |
| Other | 163 (4.4) | 13.4 |
| Treatment season | ||
| Nonwinter (March-October) | 2455 (65.8) | 5.7 |
| Winter (November-February) | 1274 (34.2) | 4.6 |
| Prescribed fractions | ||
| 1-5 | 421 (11.3) | 1.2* |
| 6-10 | 494 (13.2) | 5.7 |
| 11-15 | 195 (5.2) | 5.1 |
| 16-20 | 586 (15.7) | 2.2* |
| 21-25 | 368 (9.9) | 4.6 |
| 26-30 | 976 (26.2) | 7.0* |
| >30 | 689 (18.5) | 8.1* |
RT, radiation therapy
Denotes statistical significance
Insurance status was recorded as commercial for 1794 (48.1%), Medicare for 1503 (40.3%), and Medicaid/uninsured for 432 (11.6%; 221 Medicaid and 211 uninsured patients; 5.9% and 5.7%, respectively) patients. In addition, 1012 patients (27.1%) fell within the low, 1532 (41.1%) within the medium, and 1149 (30.8%) within the high patient predicted-income categories. The most common sites treated were breast (n = 974; 26.1%), metastases (n = 490; 13.1%), and prostate (n = 413; 11.1%) with 976 patients (26.1%) receiving most commonly 26 to 30 fractions, 689 (18.5%) receiving >30 fractions, and 586 receiving (15.7%) 16 to 20 fractions.
Most patients (n = 2455; 65.8%) began treatment in nonwinter months. The mean distance between patient residence and treatment facility was 23.7 miles, with a median of 9.1 miles (standard deviation: 103.3; interquartile range, 5.4-15.6).
Hospitalization-associated radiation therapy interruption
HARTI was observed in 197 patients (5.3%) with a total of 727 scheduled treatments missed. Patients missed between 1 and 21 treatments, with a median of 2 treatments and mean of 3.69 treatments (standard deviation: 4.13; interquartile range, 1-5). Of the 197 patients, 83 (42.1%) missed only 1 treatment.
Causes of hospitalization
Of the 197 patients to experience HARTI, an identifiable cause of hospitalization was found in 175 patients. Table 2 details the most common principal problems associated with hospitalization as determined by primary discharge diagnoses. The most common primary disease processes leading to hospitalization were malnutrition/dehydration (n = 28; 28.7%), respiratory distress or infection (n = 24; 13.7%), fever/sepsis (n = 17; 9.7%), inadequate pain control (n = 16; 9.1%), renal dysfunction (n = 15; 8.6%), and chest pain (n = 15; 8.6%).
Table 2.
Primary causes of hospitalization
| Number (%) | |
|---|---|
| Total | 175 (100) |
| Principal problem | |
| Malnutrition/dehydration | 28 (17.7) |
| Respiratory distress/infection | 24 (13.7) |
| Fever/sepsis | 17 (9.7) |
| Pain control | 16 (9.1) |
| Renal dysfunction | 15 (8.6) |
| Chest pain | 15 (8.6) |
| Neurologic dysfunction | 10 (5.7) |
| Percutaneous endoscopic gastrostomy tube complication | 9 (5.1) |
| Radiation mucositis/dermatitis | 9 (5.1) |
| Acute bleeding episode | 7 (4) |
| Urinary tract infection | 5 (2.1) |
| Soft tissue infection | 5 (2.1) |
| Other | 15 (8.6) |
Predictive factors for hospitalization-associated radiation therapy interruptions
Table 1 details the proportion of patients who experienced HARTI among several demographic, clinical, and treatment factors. A ꭓ2 analysis identified statistically significant differences in the proportion of patients experiencing HARTI among each factor. Increased likelihood of HARTI was seen among African-American (6.3% vs 4.4% for White; P = .016) and unmarried (6.4% vs 4.3% for married; P = .007) patients. A pairwise χ2d analysis further demonstrated that patients treated for lung (10.8%) and head and neck (10.7%) malignancies were significantly more likely to experience HARTI compared with patients treated for breast (1.7%) malignancies.
Patients treated for malignancies with regimens composed of 26 to 30 (7.0%) and >30 (8.1%) fractions were more likely to experience HARTI compared with those with treatment regimens composed of either 1 to 5 (1.2%) or 16 to 20 (2.2%) fractions. Medicare patients were almost twice as likely to experience HARTI (6.3% vs 3.5% for commercially insured; P = .0002), and Medicaid/uninsured patients almost 3 times as likely (9.0% vs 3.5%; P < .0001) with 10.4% of uninsured patients experiencing HARTI (P < .0001 vs commercial).
Uni- and multivariable analyses of hospitalization-associated radiation therapy interruptions
Findings for both uni- and multivariable analyses models predicting HARTI are described in Table 3. On multivariable analysis, African-American patients had an almost 50% greater odds to experience HARTI (odds ratio [OR]: 1.48; 95% confidence interval [CI], 1.07-2.06; P = .018) compared with White patients. Additionally, both Medicare (OR: 1.7; 95% CI, 1.21-2.39; P = .0022) and Medicaid/uninsured (OR: 2.05; 95% CI, 1.32-3.15; P = .0013) patients had greater odds of HARTI compared with patients with commercial insurance. Patients treated with >20 fractions were more likely (OR: 2.23; 95% CI, 1.51-3.34; P < .0001) to experience HARTI than those receiving <20 fractions. Compared with patients treated for breast cancer, significantly higher odds of HARTI were seen among patients treated for lung (OR: 5.97; 95% CI, 3.22-11.44; P < .0001), head and neck (OR: 5.6; 95% CI, 2.96-10.93; P < .0001), gynecologic (OR: 2.57; 95% CI, 1.20-5.40; P = .013), gastrointestinal (OR: 2.55; 95% CI, 1.13-5.56; P = .02), central nervous system (OR: 2.71; 95% CI, 1.00-6.60; P = .036), metastatic (OR: 3.46; 95% CI, 1.69-7.13; P = .0007), and skin malignancies (OR: 4.37; 95% CI, 1.58-11.01; P = .0026).
Table 3.
Analysis of hospitalization-associated radiation therapy interruptions by study cohort characteristics
| Univariable model |
Multivariable model |
|||
|---|---|---|---|---|
| Unadjusted odds ratio (95% confidence interval) | P-value | Adjusted odds ratio (95% confidence interval) | P-value | |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.77 (0.58-1.03) | .076 | 1.33 (0.93-1.88) | .12 |
| Age, y, mean | ||||
| ≥65 | Reference | Reference | ||
| <65 | 0.9 (0.67-1.20) | .46 | 1.1 (0.71-1.70) | .68 |
| Race | ||||
| White | Reference | Reference | ||
| African-American | 1.48 (1.10-1.98) | .009* | 1.48 (1.07-2.06) | .018* |
| Other | 1.56 (0.68-3.11) | .24 | 1.49 (0.64-3.06) | .32 |
| Marital status | ||||
| Married | Reference | Reference | ||
| Unmarried | 1.38 (1.03-1.85) | .032* | 0.4 (0.02-4.36) | .48 |
| Patient predicted income | ||||
| High (>$67k) | Reference | Reference | ||
| Middle ($34-67k) | 0.98 (0.71-1.37) | .92 | 0.97 (0.69-1.38) | .88 |
| Low (<$34k) | 0.77 (0.52-1.13) | .19 | 0.85 (0.56-1.28) | .43 |
| Geography of residence | ||||
| Metro | Reference | Reference | ||
| Rural not by metro | 0.15 (0.01-0.68) | .061 | 0.15 (0.008-0.67) | .058 |
| Rural by metro | 0.52 (0.20-1.09) | .12 | 0.51 (0.20-1.09) | .12 |
| Distance from radiation therapy, mile | ||||
| 0-5 | Reference | Reference | ||
| 6-10 | 0.83 (0.59-1.17) | .29 | 0.82 (0.57-1.17) | .27 |
| 11-15 | 0.56 (0.32-0.91) | .026* | 0.7 (0.40-1.16) | .18 |
| 16-20 | 1.04 (0.57-1.81) | .88 | 1.26 (0.67-2.24) | .46 |
| 21-30 | 0.71 (0.36-1.27) | .28 | 0.76 (0.38-1.40) | .4 |
| 31-40 | 0.27 (0.043-0.86) | .068 | 0.32 (0.05-1.09) | .13 |
| >40 | 0.48 (0.24-0.87) | .025* | 1.32 (0.46-3.21) | .58 |
| Insurance type | ||||
| Commercial | Reference | Reference | ||
| Medicare | 1.85 (1.34-2.58) | .0002* | 1.7 (1.21-2.39) | .0022* |
| Medicaid/no insurance | 2.73 (1.79-4.11) | < .0001* | 2.05 (1.32-3.15) | .0013* |
| Medicaid | 2.29 (1.28-3.90) | .0034* | 1.56 (0.85-2.73) | .13 |
| No insurance | 3.20 (1.89-5.24) | < .0001* | 2.64 (1.53-4.43) | .0003* |
| Diagnosis | ||||
| Breast | Reference | Reference | ||
| Prostate | 1.98 (0.95-4.06) | .061 | 1.73 (0.76-3.89) | .19 |
| Lung | 6.82 (3.86-12.55) | < .0001* | 5.97 (3.22-11.44) | < .0001* |
| Gynecologic | 3.73 (1.79-7.69) | .0004* | 2.57 (1.20-5.40) | .013* |
| Head and neck | 6.77 (3.88-12.34) | < .0001* | 5.6 (2.96-10.93) | < .0001* |
| Gastrointestinal | 3.00 (1.38-6.33) | .0042* | 2.55 (1.13-5.56) | .02* |
| Central nervous system | 2.81 (1.07-6.63) | .024* | 2.71 (1.00-6.60) | .036* |
| Metastasis | 2.41 (1.25-4.69) | .0087* | 3.46 (1.69-7.13) | .0007* |
| Skin | 3.41 (1.30-8.09) | .0076* | 4.37 (1.58-11.01) | .0026* |
| Soft tissue | 2.22 (0.35-8.02) | .3 | 1.94 (0.30-7.23) | .39 |
| Hematologic | 1.59 (0.45-4.37) | .41 | 2.28 (0.63-6.57) | .16 |
| Other | 7.67 (3.89-15.25) | < .0001* | 8.4 (4.07-17.45) | < .0001* |
| Treatment season | ||||
| Nonwinter (March-October) | Reference | Reference | ||
| Winter (November-February) | 0.77 (0.56-1.06) | .11 | 0.81 (0.58-1.12) | .22 |
| Prescribed fractions | ||||
| 1-20 | Reference | Reference | ||
| >20 | 2.18 (1.60-3.02) | < .0001* | 2.23 (1.51-3.34) | < .0001* |
*Denotes statistical significanceasd
Variables predictive for HARTI on univariable analysis that did not reach statistical significance on multivariable analysis included marriage status and travel distance to treatment facility.
Geospatial analysis of hospitalization-associated radiation therapy interruptions
The greater Memphis metropolitan region has been historically shaped by racial and socioeconomic segregation. Central Memphis is comprised predominantly of African-American neighborhoods, clustered into areas with limited social resources apart from a smaller majority of White neighborhoods. Suburban/exurban Memphis has gradually become more racially diverse, but remains the majority White with affluent regions interspersed with middle and low-income rural ZIP codes.
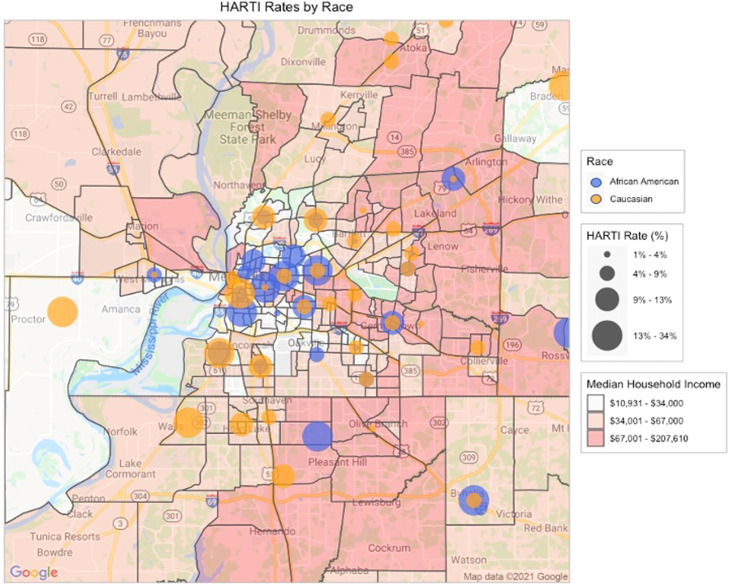
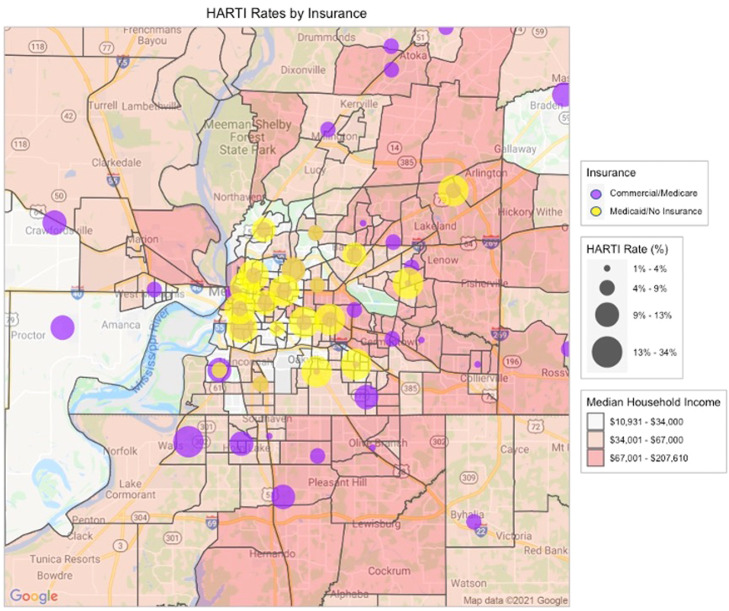
A geospatial analysis of our patient's reported home addresses mapped at the ZIP code level (Figure 1, Figure 2) identified associations between HARTI and patient home location. The highest rates were observed in Medicaid/uninsured patients living in urban, low-income, majority African-American neighborhoods. Moving outward from downtown, elevated HARTI rates were observed in middle-income suburban ZIP codes independent of patient race and insurance coverage. Only the wealthiest ZIP codes (so-called “Poplar Corridor” of East Memphis) demonstrated low rates of HARTI events.
Figure 1.
Geospatial analysis of hospitalization-associated radiation therapy interruption rates, stratified according to patient race. Median household income is mapped at census tract level according to prespecified categories. Greater hospitalization-associated radiation therapy interruption rates are denoted by larger bubbles plotted at ZIP code centroids.
Figure 2.
Geospatial analysis of hospitalization-associated radiation therapy interruption rates stratified according to patient insurance. Median household income is mapped at census tract level according to prespecified categories. Greater hospitalization-associated radiation therapy interruption rates are denoted by larger bubbles plotted at ZIP code centroids.
Discussion
The Southeastern United States experiences some of the worst cancer outcomes in the country,16 attributable in part to socioeconomic burdens endemic to the region, such as increased rurality and poverty, which are predictive for increased cancer mortality burden.17, 18, 19 In our Mid-Southern academic referral practice, we have identified candidate risk factors for RT interruption associated with unplanned hospitalization. African-American race, Medicaid/uninsured status, Medicare coverage, longer treatment regimens of >20 fractions, and disease sites associated with high radiation toxicity were predictive for HARTI. Government-based coverage or lack of insurance was associated with up to a 200% greater risk of HARTI compared with commercial insurance. African-American patients faced a nearly 50% increased risk, and patients with head and neck or lung cancer experienced almost 6 times the risk experienced by patients with breast malignancies.
The mechanistic pathways by which upstream social risk and health status factors affect radiation treatment quality are complex and difficult to disentangle. Various theories have been proposed to simplify the explanation of persistent associations between social risk factors and health disparities in the face of ongoing improvement in public health and medical interventions over time.20 The fundamental cause theory is specifically relevant to our current study. As proposed and tested by Link and Phelan,21 this theory in simplest terms postulates that improvements in disease control fuel paradoxical health shortfalls in disadvantaged groups, because advantaged individuals enjoy preferential access to such improvements. Privileged populations are less exposed to the causes of preventable disease and, when affected, are better treated by virtue of better access to resources.
Empirical data support the explanatory value of this model across numerous infectious, chronic disease, and mortality rate case examples,22, 23, 24, 25, 26 including race/ethnicity-specific COVID-19 transmission patterns observed in the United States.27 In the case of HARTI, disadvantaged groups (eg, minority race and/or those without commercial insurance) potentially face the greatest risk exposure to preventable chronic disease, including cancer. When faced with the need for full-course RT for high-burden cancer diagnoses (eg, head and neck, lung), these patients lack social, financial, and medical support to manage toxicity and comorbidities at home. Hospitalization and RT interruptions ensue, leading to preventable financial cost, morbidity, and outcome disparities.
Although identification of specific root causes responsible for HARTI events in this study were outside the scope of work, a secondary geospatial analysis provided insight into residential environments associated with risk. HARTI affected more patients living in urban, low-income, majority–minority neighborhoods, as well as suburban, low–middle income areas. Expected income appeared to be more tightly associated with HARTI risk than race or insurance in the suburban setting, which echoes data demonstrating a tight geographic association between entrenched county/ZIP code-level poverty and cancer mortality,18 even in the cooperative group trial setting.28 Access to local assets and social networks intertwine with patient-level socioeconomic factors to determine individual vulnerability.11, 12, 13,29 Validated identification of specific social, financial, environmental, and health risks mechanistically responsible for HARTI risk in specific patients will be required to effectively triage supportive intervention strategies. Automated warehousing and linkage of high-dimensional, population-level, social risk data to individual-level electronic health record data are a realistic, testable strategy to achieve these strategies.30,31
Previous studies have focused on Medicaid status or lack of insurance as predictive risk factors for RT interruption.12,13 We identified a novel risk for HARTI in our Medicare patient population, potentially attributable to coexisting health issues in this older population. Up to 70% of Medicare beneficiaries have at least 2 chronic conditions and 14% have ≥6 comorbidities requiring treatment.32 Such comorbidities can be reasonably presumed to predispose Medicare patients to increased radiation-related toxicity, thereby increasing their risk of hospitalization,33, 34, 35 which would be a testable hypothesis in confirmatory studies.
Progression of toxicity to the point of unplanned hospitalization may signal inadequacies in supportive management and/or coordination with primary care providers. Individualized supportive care strategies employing the real-time, automated collection of patient-reported toxicities and responsive supportive care have been shown to be effective during chemotherapy,36, 37, 38 and could be formally investigated in the radiation treatment setting. Many of the causes for hospitalization we found were preventable, and could be identified by upfront patient risk stratification. Many hospitalizations could be preempted by primary care teams already familiar with the patients. Unfortunately, formal coordination pathways between cancer and primary providers remain relatively understudied, and are a straightforward path toward holistic care.39, 40, 41 Other institutions have investigated the implementation of other interventions, such as patient symptom inventories and intensified visit schedules, and have found these to significantly reduce hospitalizations during cancer treatment.9,42
Reducing hospitalization during RT would potentially provide a significant value. Patients would benefit from optimized cancer treatment outcomes and reduced suffering. All stakeholders, notably provider systems and insurers, would directly benefit from cost savings and improved capacity. From the perspective of Medicare, the average cancer diagnosis-related hospitalization can generate costs totaling more than twice the expected charges for a standard-fractionated radiation treatment course.43,44 All patients, insured or uninsured, share direct out-of-pocket expenses from hospitalizations. Stressed families and caregivers are additionally affected by indirect costs and lost income opportunities.
Our study has limitations affecing the interpretation and generalizability of our findings. First, this study has a relatively small study population sampled from a single metropolitan region and managed by 1 provider system, with only 197 HARTI events captured. Of note, the full Memphis region surrounding our academic care center is served by several hospital systems, each with siloed electronic health record platforms. If any patients were admitted to outside hospitals in the area, their respective HARTI events would not have been captured. Second, causes for hospitalization were cataloged retrospectively from discharge diagnoses; thus, conclusive associations (or lack of association) of hospitalization events with cancer-specific treatment was not possible to establish.
Third, effect specific to hospitalization on downstream cancer outcomes relative to RT interruption was not addressed by this data. Finally, some ZIP codes in outlying suburban areas contained small total numbers of captured patients, so any single event would disproportionately affect the risk metric. Full regional sampling of all patients with cancer treated with RT in the region would be required to correct for this. Prompted by COVID-19, we are creating a unified public health observatory for the full Memphis region to achieve this goal in future studies.45 We expect our baseline results to focus more definitive work toward candidate neighborhoods and patient populations most in need.
Conclusions
At our academic referral practice, we found that RT interruptions during unplanned hospitalizations were associated with Medicaid/uninsured or Medicare coverage, African-American race, prolonged treatment course, and treatment of sites with high symptomatic burden requiring intensive treatment. A complementary geospatial analysis identified risk hotspots in low-income, urban, majority African-American neighborhoods, as well as suburban low–middle income areas independent of race or insurance coverage. These findings are hypothesis-generating, and require additional context via scaled-up sampling from a wider assortment of U.S. cities. Nonetheless, this work promises to guide design and validation of individualized social interventions to meaningfully reduce RT outcome disparities.
Footnotes
Sources of support: No outside funding was used for this study.
Disclosures: none.
Data sharing statement: Research data are not available at this time.
References
- 1.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 2.Ohri N, Rapkin BD, Guha C, Kalnicki S, Garg M. Radiation therapy noncompliance and clinical outcomes in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2016;95:563–570. doi: 10.1016/j.ijrobp.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Yao JJ, Jin YN, Wang SY, et al. The detrimental effects of radiotherapy interruption on local control after concurrent chemoradiotherapy for advanced T-stage nasopharyngeal carcinoma: An observational, prospective analysis. BMC Cancer. 2018;18:740. doi: 10.1186/s12885-018-4495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter AJ, Hendrikse AS. Estimation of the effects of radiotherapy treatment delays on tumour responses: A review. S African J Oncol. 2020;4:a91. [Google Scholar]
- 5.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta S, Ramey SJ, Kwon D, et al. Impact of radiotherapy duration on overall survival in squamous cell carcinoma of the anus. J Gastrointest Oncol. 2020;11:277–290. doi: 10.21037/jgo.2020.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giddings A. Treatment interruptions in radiation therapy for head-and-neck cancer: Rates and causes. J Med Imaging Radiat Sci. 2010;41:222–229. doi: 10.1016/j.jmir.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 8.McCloskey SA, Jaggernauth W, Rigual NR, et al. Radiation treatment interruptions greater than one week and low hemoglobin levels (12 g/dL) are predictors of local regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2009;32:587–591. doi: 10.1097/COC.0b013e3181967dd0. [DOI] [PubMed] [Google Scholar]
- 9.Hong JC, Niedzwiecki D, Palta M, Tenenbaum JD. Predicting emergency visits and hospital admissions during radiation and chemoradiation: An internally validated pretreatment machine learning algorithm. JCO Clin Cancer Inform. 2018;2:1–11. doi: 10.1200/CCI.18.00037. [DOI] [PubMed] [Google Scholar]
- 10.Waddle MR, Chen RC, Arastu NH, et al. Unanticipated hospital admissions during or soon after radiation therapy: Incidence and predictive factors. Pract Radiat Oncol. 2015;5:e245–e253. doi: 10.1016/j.prro.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Holzer J, Canavan ME, Cherlin E, Bradley E. Health hot spots: Mapping hospital costs and social determinants of health. Open J Prevent Med. 2014;4:717–722. [Google Scholar]
- 12.Wakefield D.V., et al. Location as Destiny: Identifying Geospatial Disparities in Radiation Treatment Interruption by Neighborhood, Race, and Insurance. Int J Radiat Oncol Biol Phys. 2020;107:815–826. doi: 10.1016/j.ijrobp.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Ohri N, Rapkin BD, Guha D, et al. Predictors of radiation therapy noncompliance in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2015;91:232–238. doi: 10.1016/j.ijrobp.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Thomas K, Martin T, Gao A, Ahn C, Wilhelm H, Schartz DL. Interruptions of head and neck radiotherapy across insured and indigent patient populations. J Oncol Pract. 2017;13:e319–e328. doi: 10.1200/JOP.2016.017863. [DOI] [PubMed] [Google Scholar]
- 15.Kahle D, Wickam H. Spatial Visualization with ggplot2. R Journal. 2013;5:144–161. [Google Scholar]
- 16.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 17.Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: An analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26:992–997. doi: 10.1158/1055-9965.EPI-17-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss JL, Pinto CN, Srinivasan S, Cronin KA, Croyle RT. Persistent poverty and cancer mortality rates: An analysis of county-level poverty designations. Cancer Epidemiol Biomarkers Prev. 2020;29:1949–1954. doi: 10.1158/1055-9965.EPI-20-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erhunmwunsee L, Joshi MBM, Conlon DH, Harpole DH., Jr. Neighborhood-level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118:5117–5123. doi: 10.1002/cncr.26185. [DOI] [PubMed] [Google Scholar]
- 20.Cockerham WC, Hamby BW, Oates GR. The social determinants of chronic disease. Am J Prev Med. 2017;52:S5–S12. doi: 10.1016/j.amepre.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. J Health Soc Behav. 2010;51:S28–S40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- 22.Noppert GA, Malosh RE, Moran EB, Ahuja SD, Zelner J. Contemporary social disparities in TB infection and disease in the USA: A review. Curr Epidemiol Rep. 2018;5:442–449. doi: 10.1007/s40471-018-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang VW, Lauderdale DS. Fundamental cause theory, technological innovation, and health disparities: The case of cholesterol in the era of statins. J Health Soc Behav. 2009;50:245–260. doi: 10.1177/002214650905000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phelan JC, Link BG, Diez-Roux A, Kawachi I, Levin B. Fundamental causes" of social inequalities in mortality: A test of the theory. J Health Soc Behav. 2004;45:265–285. doi: 10.1177/002214650404500303. [DOI] [PubMed] [Google Scholar]
- 25.Lutfey KE, Ketcham JD. Patient and provider assessments of adherence and the sources of disparities: Evidence from diabetes care. Health Serv Res. 2005;40:1803–1817. doi: 10.1111/j.1475-6773.2005.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daw J. Explaining the persistence of health disparities: Social stratification and the efficiency-equity trade-off in the kidney transplantation system. AJS. 2015;120:1595–1640. doi: 10.1086/681961. [DOI] [PubMed] [Google Scholar]
- 27.Clouston SAP, Natale G, Link BG. Socioeconomic inequalities in the spread of coronavirus-19 in the United States: A examination of the emergence of social inequalities. Soc Sci Med. 2021;268 doi: 10.1016/j.socscimed.2020.113554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unger JM, Moseley AB, Cheung CK, et al. Persistent disparity: Socioeconomic deprivation and cancer outcomes in patients treated in clinical trials. J Clin Oncol. 2021;39:1339–1348. doi: 10.1200/JCO.20.02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shavers VL. Racial and ethnic disparities in the receipt of cancer treatment. CancerSpectrum Knowledge Environ. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 30.Shaban-Nejad A., et al. Seven pillars of precision digital health and medicine. Artif Intell Med. 2020;103:101793. doi: 10.1016/j.artmed.2020.101793. [DOI] [PubMed] [Google Scholar]
- 31.Shin EK, Kwon Y, Shaban-Nejad A. Geo-clustered chronic affinity: Pathways from socio-economic disadvantages to health disparities. JAMIA Open. 2019;2:317–322. doi: 10.1093/jamiaopen/ooz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Centers for Medicare & Medicaid Services. Chronic conditions among Medicare beneficiaries chartbook. Baltimore, MD: U.S. Centers for Medicare & Medicaid Services; 2012.
- 33.Nalbantov G, Kietselaer B, Vandecasteele K, et al. Cardiac comorbidity is an independent risk factor for radiation-induced lung toxicity in lung cancer patients. Radiother Oncol. 2013;109:100–106. doi: 10.1016/j.radonc.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Wu HG, Kim HJ, et al. Influence of comorbidities on the efficacy of radiotherapy with or without chemotherapy in elderly stage III non-small cell lung cancer patients. Cancer Res Treat. 2012;44:242–250. doi: 10.4143/crt.2012.44.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazell SZ, Mai N, Fu W, et al. Hospitalization and definitive radiotherapy in lung cancer: Incidence, risk factors and survival impact. BMC Cancer. 2020;20:334. doi: 10.1186/s12885-020-06843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Absolom K, Warrington L, Hudson E, et al. Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol. 2021;39:734–747. doi: 10.1200/JCO.20.02015. [DOI] [PubMed] [Google Scholar]
- 37.Doolin JW, Berry JL, Forbath NS, et al. Implementing electronic patient-reported outcomes for patients with new oral chemotherapy prescriptions at an academic site and a community site. JCO Clin Cancer Inform. 2021;5:631–640. doi: 10.1200/CCI.20.00191. [DOI] [PubMed] [Google Scholar]
- 38.Patt D, Wilfong L, Hudson KE, et al. Implementation of electronic patient-reported outcomes for symptom monitoring in a large multisite community oncology practice: Dancing the Texas two-step through a pandemic. JCO Clin Cancer Inform. 2021;5:615–621. doi: 10.1200/CCI.21.00063. [DOI] [PubMed] [Google Scholar]
- 39.Grunfeld E. It takes a team: CanIMPACT: Canadian team to improve community-based cancer care along the continuum. Can Fam Physician. 2016;62:781–782. [PMC free article] [PubMed] [Google Scholar]
- 40.Tomasone JR, Brouwers MC, Vukmirovic M, et al. Interventions to improve care coordination between primary healthcare and oncology care providers: A systematic review. ESMO Open. 2016;1 doi: 10.1136/esmoopen-2016-000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson CE, Saunders CM, Phillips M, et al. Randomized controlled trial of shared care for patients with cancer involving general practitioners and cancer specialists. J Oncol Pract. 2015;11:349–355. doi: 10.1200/JOP.2014.001569. [DOI] [PubMed] [Google Scholar]
- 42.Noel C.W., et al. Patient-Reported Symptom Burden as a Predictor of Emergency Department Use and Unplanned Hospitalization in Head and Neck Cancer: A Longitudinal Population-Based Study. Journal of Clinical Oncology. 2021;39:675–684. doi: 10.1200/JCO.20.01845. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Department of Health and Human Services . U.S. Department of Health and Human Services; Washington, DC: 2017. Report to Congress: Episodic alternative payment model for radiation therapy services. [Google Scholar]
- 44.Agency for Healthcare Research and Quality . Agency for Healthcare Research and Quality; Rockville, MD: 2020. Statistical brief #262: Healthcare cost and utilization project (HCUP) [PubMed] [Google Scholar]
- 45.Brakefield WS, Ammar N, Olusanya OA. Shaban-Nejad A. An urban population health observatory system to support COVID-19 pandemic preparedness, response, and management: Design and development study. JMIR Public Health Surveill. 2021;7:e28269. doi: 10.2196/28269. [DOI] [PMC free article] [PubMed] [Google Scholar]