Abstract
Background
Heart failure (HF) is a complex clinical syndrome with symptoms and signs that result from any structural or functional impairment of ventricular filling or ejection of blood. Limited data is available regarding the in-hospital outcomes of TAVR compared to SAVR in the octogenarian population with HF.
Methods
The National Inpatient Sample (NIS) database was used to compare TAVR versus SAVR among octogenarians with HF. The primary outcome was in-hospital mortality. The secondary outcome included acute kidney injury (AKI), cerebrovascular accident (CVA), post-procedural stroke, major bleeding, blood transfusions, sudden cardiac arrest (SCA), cardiogenic shock (CS), and mechanical circulatory support (MCS).
Results
A total of 74,995 octogenarian patients with HF (TAVR-HF n = 64,890 (86.5%); SAVR n = 10,105 (13.5%)) were included. The median age of patients in TAVR-HF and SAVR-HF was 86 (83–89) and 82 (81–84) respectively. TAVR-HF had lower percentage in-hospital mortality (1.8% vs. 6.9%;p < 0.001), CVA (2.5% vs. 3.6%; p = 0.009), SCA (9.9% vs. 20.2%; p < 0.001), AKI (17.4% vs. 40.8%); p < 0.001), major transfusion (26.4% vs 67.3%; p < 0.001), CS (1.8% vs 9.8%; p < 0.001), and MCS (0.8% vs 7.3%; p < 0.001) when compared to SAVR-HF. Additionally, post-procedural stroke and major bleeding showed no significant difference. The median unmatched total charges for TAVR-HF and SAVR-HF were 194,561$ and 246,100$ respectively.
Conclusion
In this nationwide observational analysis, TAVR is associated with an improved safety profile for octogenarians with heart failure (both preserved and reduced ejection fraction) compared to SAVR.
Keywords: Transcatheter aortic valve replacement, Surgical aortic valve replacement, Heart failure, Heart Failure with reduced ejection fraction, Heart Failure with preserved ejection fraction
Abbreviations: TAVR, Transcatheter aortic valve replacement; SAVR, Surgical aortic valve Replacement; HFrEF, Heart Failure with reduced Ejection fraction; HFpEF, Heart Failure with a Preserved ejection fraction; AS, Aortic Stenosis; CVD, cardiovascular disease; RCT, Randomized Controlled Trial; HF, Heart Failure; AKI, Acute Kidney Injury; CVA, Cerebrovascular Accident; LVAD, Left ventricular assist device; LOS, Length of hospital stay; SCA, sudden cardiac arrest; CS, Cardiogenic Shock; MCS, Mechanical Circulatory Support; PSM, Propensity Matched
1. Introduction
Aortic stenosis (AS) is the most common valvular heart disease in the United States' geriatric population [1]. According to the European and American guidelines, the prevalence of severe AS reaches up to 10% among patients aged 80 and older [2]. Transcatheter aortic valve replacement (TAVR) is a favorable alternative over surgical aortic valve replacement (SAVR) in patients aged 75 and older who; have low, intermediate, or high Society of Thoracic Surgeons (STS) scores or a EuroSCORE II score ≥ 4%; and have a history of organ dysfunction according to the European Society of Cardiology (ESC) and the American College of Cardiology (ACC) [3]. According to current guidelines, in octogenarian patients (individuals aged 80–89), surgical risk constitutes the primary factor in determining the therapeutic route as assessed by a multidisciplinary heart team [2], [3]. However, the surgical risk may be underestimated in elderly patients due to a lower physiologic reserve, thus providing a rationale for suggesting TAVR over SAVR in this population. Furthermore, TAVR has proven to have similar outcomes compared to surgical repair in severe AS in high and intermediate-risk surgical patients [4]. The less invasiveness of the procedure and faster recovery are likely to be of significant advantage in the octogenarian population.
Cardiovascular Health Study (CHS) criteria can be used to identify higher-risk surgical candidates. Frailty is a geriatric syndrome resulting in decline across multiple physiological systems and serves as a predictor of operative complications and mortality, especially in the context of cardiovascular disease (CVD). Per Kotajarvi et al. and Green et al., despite generally comparable age, disease severity, cardiac function, and comorbid disease burden, re-hospitalizations and death were twice as common in frail compared to non-frail older adults receiving SAVR or TAVR [5], [6]. In summary, frailty is prevalent in older adults with severe AS and is associated with increased adverse outcomes and mortality risk following both SAVR and TAVR [5], [6].
Heart Failure (HF), as per ACC/AHA heart failure 2022 guidelines, is a complex clinical syndrome with symptoms and signs that result from any structural or functional impairment of ventricular filling or ejection of blood [7]. Guideline-directed medical treatment (GDMT) of HF is directed toward neurohormonal modulation and afterload reduction. However, medical treatment alone does not address the mechanical increase in afterload related to the stenotic valve. Current randomized control trials (RCT) are underway to evaluate the value of unloading the left ventricle through TAVR [8]. In the current study, we used the National Inpatient Sample (NIS) database to evaluate and compare clinical outcomes in octogenarian patients with HF who underwent either TAVR or SAVR. We further investigated whether differences in clinical outcomes exist between patients with a reduced ejection fraction (HFrEF) and those with a preserved ejection fraction (HFpEF) in either group.
2. Methods
2.1. Data source
We analyzed data from the NIS database from 2015 to 2018. NIS is part of the healthcare cost and utilization project (HCUP) databases. The Agency for Healthcare Research and Quality (AHRQ) sponsors these databases [9]. The NIS database represents nearly 95% of the US population and includes 20% of discharge patient data from nearly 1000 hospitals. The NIS undergoes annual quality assessments confirming its internal validity. Additionally, the NIS is a publicly available database with de-identified data; therefore, Institutional Review Board approval was not required for our study.
2.2. Study population
We selected a HF cohort of TAVR and SAVR using the International Classification of Disease, Tenth Edition, Clinical Modification (ICD-10-CM) codes for demographics, baseline comorbidities, matching variables, and outcomes. The codes to generate cohorts are summarized in Supplemental S1. Further, TAVR-HF and SAVR HF were created using baseline TAVR and SAVR index cases in NIS samples. HFrEF and HFpEF are defined per 2022 ACC/AHA/Heart failure guidelines as left ventricular ejection fraction (LVEF) ≤ 40% and LVEF ≥ 50% respectively. Furthermore, the guideline also further sub-classify HF into HF with mildly reduced EF (HFmrEF) and HF with improved EF (HFimpEF) as LVEF 41%-49% and previous LVEF < 40% and a follow up measurement of LVEF > 40% [7].
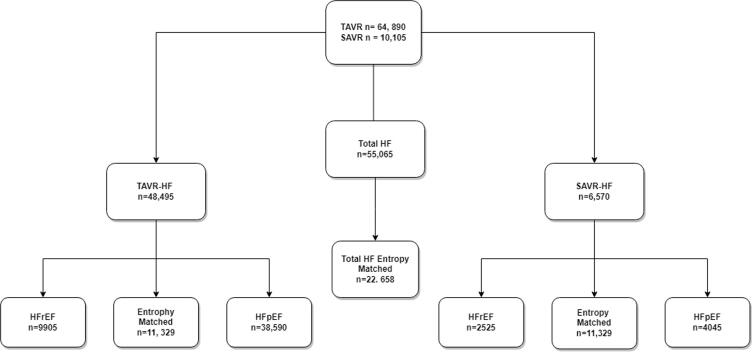
The cohort selection flow diagram is shown in Fig. 1. Both STS and Euroscore II cannot be calculated from the NIS. As a result, we used the elixhauser index, AHRQ risk severity, and mortality index to calculate the combined estimate of the patients’ risk profiles [10].
Fig. 1.
Selection of cohort for crude and matched cohort.
The inclusion criteria for our study consisted of patients aged 80–90 with a history of HF who underwent TAVR or SAVR. Patients were then sub-grouped into HFrEF and HFpEF groups. Exclusion criteria consisted of all patients under 80 or greater than 90 years of age. Octogenarians who received both SAVR and TAVR or had AS in the setting of congenital rheumatic heart disease were also excluded.
2.3. Outcomes measured
The primary outcome was in-hospital mortality. Secondary outcomes included acute kidney injury (AKI), cerebrovascular accident (CVA), post-procedural stroke, major bleeding-bleeding as defined by the Valve Academic Research Consortium (VARC), blood transfusions, sudden cardiac arrest (SCA), cardiogenic shock (CS), mechanical circulatory support (MCS: including left ventricular assist device, pVAD, and ECMO). Tertiary outcomes included quality measures such as length of hospital stay (LOS) and cost of hospitalization. The common variable definitions are shown in Supplementary S2.
2.4. Statistical analysis
Categorical variables were reported as frequencies with percentages using Pearson’s chi-square test and compared using logistic regression for accurate documentation. In contrast, continuous variables were reported as weighted means with standard deviation (normal distribution) or median with interquartile ranges (IQR) for skewed distribution. Outcome’s frequency and percentages of the unmatched cohort were reported using Pearson’s chi-square test and logistic regression. Propensity matching (PSM) was done using Entropy near matching balance for mean, median, and skewness weighted using the STATA ebalance module (Supplemental S3). PSM Entropy balance is superior to another propensity matching of any kind including nearest neighbor matching, pruning, or inverse probability treatment weighting [11]. PSM was done for cohorts TAVR-HF and SAVR-HF. The matching was done to eliminate confounding effects secondary to baseline demographics, comorbidities, and STS score components (Supplemental S3). Matched cohort data including characteristics and outcomes were as percentages, frequencies, and p-values using Pearson's chi-square and logistic regression. Further subgroup analysis was performed for HF with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF) in terms of TAVR and SAVR. Trend analysis was also performed for all outcomes for TAVR-HF and SAVR-HF using Pearson’s chi-square. All analyses were conducted using appropriate stratifying, clustering, and weighting samples provided by Healthcare Cost and Utilization Project regulations [12], [13]. Discharge weights provided by NIS were applied for all analyses to develop national representative procedures for this study. Statistical analysis was performed using STATA Version 16.1, College Station, TX: StataCorp LLC [14].
3. Results
3.1. Demographic and baseline comorbidities
A total of 74,995 octogenarian HF patients (TAVR-HF n = 64,890 (86.5%); SAVR-HF n = 10,105 (13.5%) were included in our study. Patients' median age for TAVR-HF and SAVR-HF was 86 (IQR: 83–89) and 82 (IQR: 81–84); 48.8% and 36.8% were females, respectively. The most common procedure setting in TAVR and SAVR was elective and accounted for 79.7% and 63.4%, respectively. The baseline demographics, hospital characteristics, and comorbidities are shown in (Table 1). Among the population stratified based on ejection fraction of the left ventricle in the HF octogenarian cohort, a total of 38,590 (79.6%) HFpEF and 9905 (20.4%) HFrEF patients underwent TAVR. In contrast, in the SAVR cohort, 4045 (61.6%) and 2525 (38.4%) patients had HFpEF and HFrEF, respectively (Table 1). After PSM, we included 11,329 patients in each study cohort (TAVR-HF and SAVR-HF) (Table 1).
Table 1.
Showing Baseline demographics, comorbidities, and descriptive complications among TAVR and SAVR with Heart Failure Octogenarian groups for both unmatched and propensity matched cohorts.
| Analyte |
Unmatched Cohort |
Propensity Matched Cohort |
||||
|---|---|---|---|---|---|---|
| TAVR-HF (n = 64,890) | SAVR-HF (n = 10,105) | p-value | TAVR-HF (n = 11,329) | SAVR-HF (n = 11,329) | p-value | |
| Age (median; IQRS) years | 86 (83–89) | 82 (81–84) | 86(23–89) | 82(81–84) | ||
| Year n (%) | ||||||
| 2015 | 3,480 (5.4) | 1,270 (12.6) | 0.000 | – | – | |
| 2016 | 17,230 (26.6) | 3,580 (35.4) | 0.000 | 0.000 | ||
| 2017 | 21,510 (33.1) | 2,825 (28) | 0.000 | 0.000 | ||
| 2018 | 22,675 (34.9) | 2,430 (24) | 0.000 | 0.000 | ||
| Sex n (%) | ||||||
| Male | 33,475 (51.6) | 6,455 (63.9) | 0.000 | 5,816 (51.3) | 5,816 (51.3) | 1.000 |
| Female | 31,415 (48.4) | 3,650 (36.1) | 0.000 | 5,513 (48.7) | 5,513 (48.7) | 1.000 |
| Race n (%) | ||||||
| White | 55,190 (90.5) | 8,160 (87.6) | 0.000 | 10,252 (90.5) | 10,272 (90.7) | 0.449 |
| Black | 2,175 (3.6) | 315 (3.4) | 0.000 | 402 (3.5) | 321.9 (2.8) | 0.449 |
| Hispanic | 2,700 (4.4) | 640 (6.9) | 0.000 | 502 (4.4) | 585.8 (5.2) | 0.449 |
| Asian/PI | 815 (1.3) | 185 (2) | 0.000 | 152 (1.3) | 143.8 (1.3) | 0.449 |
| Transfers (%) | ||||||
| Not Transferred | 59,770 (92.3) | 8,665 (86.1) | 0.000 | 10,476 (92.5) | 10,531 (93) | 0.391 |
| Transferred | 3,990 (6.2) | 1,235 (12.3) | 0.000 | 678 (6) | 567.9 (5) | 0.391 |
| Elective (%) | ||||||
| Non-elective | 13,110 (20.3) | 3,690 (36.6) | 0.000 | 2,273 (20.1) | 2,273 (20.1) | 1.000 |
| Elective | 51,380 (79.7) | 6,385 (63.4) | 0.000 | 9,056 (79.9) | 9,056 (79.9) | 1.000 |
| Hospital Bed Size n (%) [Values vary by Region & Control] | ||||||
| Small | 4,275 (7) | 850 (9.6) | 0.003 | 771 (6.8) | 788 (7) | 0.973 |
| Medium | 12,525 (20.4) | 1,940 (22) | 0.003 | 2,303 (20.3) | 2,269 (20) | 0.973 |
| Large | 61,415 (72.6) | 8,835 (68.4) | 0.003 | 8,255 (72.9) | 8,272 (73) | 0.973 |
| Hospital Location & Teaching Status n (%) | ||||||
| Rural | 645 (1.1) | 175 (2) | 0.000 | 115 (1) | 100.8 (0.9) | 0.861 |
| Urban Non-Teaching | 6,915 (10.1) | 1,320 (14.9) | 0.000 | 1,071 (9.5) | 1,099.4 (9.7) | 0.861 |
| Urban Teaching | 54,575 (88.9) | 7,340 (83.1) | 0.000 | 10,143 (89.5) | 10,128.8 (89.4) | 0.861 |
| Hospital Region n (%) | ||||||
| Northeast | 14,875 (24.2) | 2,130 (24.1) | 0.266 | 2,773 (24.5) | 2,797.3 (24.7) | 0.003 |
| Midwest | 14,255 (23.2) | 2,135 (24.2) | 0.266 | 2,549 (22.5) | 2,916.9 (25.7) | 0.003 |
| South | 19,825 (32.3) | 2,600 (29.4) | 0.266 | 3,748 (33.1) | 2,939.2 (25.9) | 0.003 |
| West | 12,460 (20.3) | 1,970 (22.3) | 0.266 | 2,259 (19.9) | 2,675 (23.6) | 0.003 |
| Weekend Admission n (%) | ||||||
| Monday-Friday | 62,365 (96.1) | 9,320 (92.2) | 0.000 | 10,908 (96.3) | 10,908 (96.3) | 1.000 |
| Saturday-Sunday | 2,530 (3.9) | 785 (7.8) | 0.000 | 421(3.7) | 421(3.7) | 1.000 |
| Comorbidities | ||||||
| Pulmonary Circulation Disorders | 13,000 (20) | 2190 (21.7) | 0.117 | 2234 (19.7) | 2234 (19.7) | 1.000 |
| Chronic Pulmonary Disease | 16,825 (25.9) | 2,200 (21.8) | 0.000 | 2932 (25.9) | 2932 (25.9) | 1.000 |
| Diabetes Uncomplicated | 9,325 (14.4) | 1,215 (12) | 0.006 | 1573 (13.9) | 1573 (13.9) | 1.000 |
| Diabetes Complicated | 11,190 (17.2) | 1,665 (16.5) | 0.423 | 2001 (17.7) | 2001(17.7) | 1.000 |
| Hypothyroidism | 14,670 (22.6) | 1,665 (16.5) | 0.000 | 2590 (22.9) | 2590 (22.9) | 1.000 |
| Renal Failure | 26,125 (40.3) | 3,190 (31.6) | 0.000 | 4513 (39.8) | 4513 (39.8) | 1.000 |
| Peptic Ulcer Disease (excluding bleeding) | 410 (0.6) | 100 (1) | 0.066 | 71 (0.6) | 71 (0.6) | 1.000 |
| Lymphoma | 475 (0.7) | 50 (0.5) | 0.233 | 89 (0.8) | 89 (0.8) | 1.000 |
| Metastatic Cancer | 325 (0.5) | 20 (0.2) | 0.058 | 52 (0.5) | 52 (0.5) | 1.000 |
| Solid Tumor Without Metastasis | 1,475 (2.3) | 180 (1.8) | 0.190 | 251 (2.2) | 251 (2.2) | 1.000 |
| Rheumatoid Arthritis/Collagen Vascular | 2,910 (4.5) | 305 (3) | 0.003 | 513 (4.5) | 513 (4.5) | 1.000 |
| Coagulopathy | 8,420 (13) | 4,455 (44.1) | 0.000 | 1421 (12.5) | 1421 (12.5) | 1.000 |
| Obesity | 7,605 (11.7) | 1,360 (13.5) | 0.028 | 1345 (11.9) | 1345 (11.9) | 1.000 |
| Weight Loss | 2,495 (3.8) | 965 (9.5) | 0.000 | 442 (3.9) | 442 (3.9) | 1.000 |
| Fluid and Electrolyte Disorders | 10,170 (15.7) | 4,500 (44.5) | 0.000 | 1758 (15.5) | 1758 (15.5) | 1.000 |
| Blood Loss Anemia | 805 (1.2) | 120 (1.2) | 0.843 | 137 (1.2) | 137 (1.2) | 1.000 |
| Deficiency Anemia | 2,765 (4.3) | 390 (3.9) | 0.416 | 479 (4.2) | 479 (4.2) | 1.000 |
| Alcohol Abuse | 395 (0.6) | 135 (1.3) | 0.000 | 69 (0.6) | 69 (0.6) | 1.000 |
| Drug Abuse | 75 (0.1) | 15 (0.1) | 0.691 | 15 (0.1) | 15 (0.1) | 1.000 |
| HD | 545 (0.8) | 135 (1.3) | 0.024 | 105 (0.9) | 105 (0.9) | 1.000 |
| HTN | 10,990 (16.9) | 2,670 (26.4) | 0.000 | 1,694 (15) | 1,694 (15) | 1.000 |
| PAD | 7,990 (12.3) | 860 (8.5) | 0.000 | 1,384 (12.2) | 1,384 (12.2) | 1.000 |
| Family History of CAD | 4,605 (7.1) | 730 (7.2) | 0.834 | 790 (7) | 790 (7) | 1.000 |
| OSA | 6005 | 965 | 0.600 | 1,032 (9.1) | 1,032 (9.1) | 1.000 |
| Liver Disease | 795 (1.2) | 155 (1.5) | 0.279 | 139 (1.2) | 139 (1.2) | 1.000 |
| Alcohol | 355 (0.5) | 135 (1.3) | 0.000 | 62 (0.5) | 62 (0.5) | 1.000 |
| Smoking | 23,215 (35.8) | 3,485 (34.5) | 0.268 | 4,103 (36.2) | 4,103 (36.2) | 1.000 |
| Pneumonia | 995 (1.5) | 575 (5.7) | 0.000 | 169 (1.5) | 169 (1.5) | 1.000 |
| Hx of PCI | 1,790 (2.8) | 135 (1.3) | 0.000 | 302 (2.7) | 302 (2.7) | 1.000 |
| Hx of CABG | 10,665 (16.4) | 465 (4.6) | 0.000 | 1,837 (16.2) | 1,837 (16.2) | 1.000 |
| Previous MI | 8,700 (13.4) | 760 (7.5) | 0.000 | 1,530 (13.5) | 1,530 (13.5) | 1.000 |
| CAD | 46,430 (71.5) | 7,105 (70.3) | 0.279 | 8,106 (71.6) | 8,106 (71.6) | 1.000 |
| Syncope | 715 (1.1) | 115 (1.1) | 0.889 | 126 (1.1) | 126 (1.1) | 1.000 |
| Endocarditis | 215 (0.3) | 290 (2.9) | 0.000 | 42 (0.4) | 42 (0.4) | 1.000 |
*Abbreviations: PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Graft; CAD: Coronary Artery Disease; MI: Myocardial infarction; AIDS: Acquired Immunodeficiency Syndrome; HIV: Human Immunodeficiency Virus; Peripheral Artery Disease (PAD); Hypertension (HTN); Hemodialysis (HD); CVA (cerebral vascular accident); SCA (sudden cardiac arrest); MCS (mechanical circulatory support); AKI (acute kidney injury); OSA (obstructive sleep apnea).
3.2. Comparison of primary and secondary outcomes between TAVR and SAVR HF
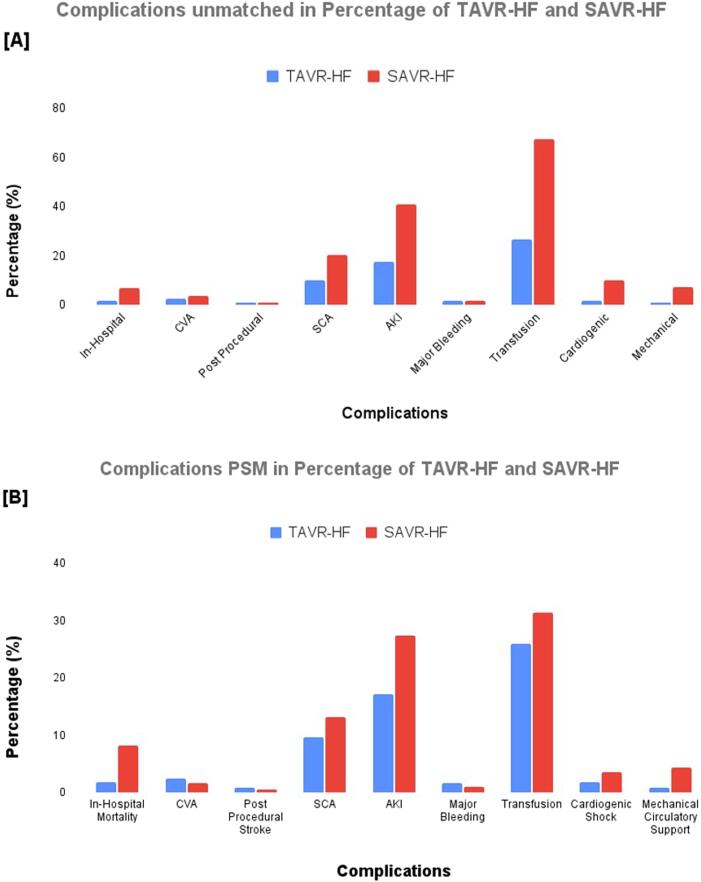
Patients undergoing TAVR-HF as compared to SAVR-HF have lower percentage in-hospital mortality (1.8% vs. 6.9%; p = 0.000), CVA (2.5% 3.6%; p = 0.009), SCA (9.9% vs. 20.2%; p < 0.001), AKI (17.4% vs. 40.8%; p < 0.001), major transfusion (26.4% vs. 67.3%; p < 0.000), CS (1.8% vs. 9.8%; p < 0.001), and MCS (0.8% vs. 7.3%; p < 0.001). Additionally, post-procedural stroke (0.9% vs. 1.0%; p = 0.08), and major bleeding (1.8% vs. 1.8%; p = 0.89) showed no significant difference (Table 2).
Table 2.
Unmatched Cohort and Propensity Matched Outcomes of SAVR vs TAVR Among Heart Failure, Heart Failure with reduced and preserved ejection fraction in Octogenarian Population.
| Outcomes |
Unmatched Cohort |
Propensity Matched Cohort |
||||
|---|---|---|---|---|---|---|
| TAVR-HF n (%) | SAVR-HF n (%) | p-value | TAVR-HF n (%) | SAVR-HF n (%) | p-value | |
| In-Hospital Mortality | 1145 (1.8) | 695 (6.9) | 0.000 | 206 (1.8) | 926.6 (8.2) | 0.000 |
| HFrEF | 185 (1.9) | 195 (7.7) | 0.000 | 33 (2) | 146 (8.7) | 0.001 |
| HFpEF | 580 (1.5) | 210 (5.2) | 0.000 | 107 (1.6) | 325.9 (4.8) | 0.006 |
| Post-procedural stroke | 605 (0.9) | 100 (1) | 0.080 | 104 (0.9) | 54.6 (0.5) | 0.251 |
| SCA | 6450 (9.9) | 2045 (20.2) | 0.000 | 1087 (9.6) | 1495.5 (13.2) | 0.018 |
| HFrEF | 990 (10) | 490 (19.4) | 0.000 | 161 (9.5) | 238.2 (14.1) | 0.362 |
| HFpEF | 3795 (9.8) | 800 (19.8) | 0.000 | 651 (9.5) | 798.6 (11.7) | 0.346 |
| AKI | 11,265 (17.4) | 4125 (40.8) | 0.000 | 1951 (17.2) | 3099.2 (27.4) | 0.000 |
| HFrEF | 2080 (21) | 1140 (45.1) | 0.000 | 357 (21.2) | 681.7 (40.4) | 0.002 |
| HFpEF | 6040 (15.7) | 1495 (37) | 0.000 | 1053 (15.4) | 2134.8 (31.3) | 0.000 |
| Major Bleeding | 1185 (1.8) | 180 (1.8) | 0.891 | 195 (1.7) | 111.7 (1) | 0.104 |
| HFrEF | 220 (2.2) | 60 (2.4) | 0.831 | 34 (2) | 17.1 (1) | 0.418 |
| HFpEF | 675 (1.7) | 65 (1.6) | 0.781 | 111 (1.6) | 60.3 (0.9) | 0.379 |
| Transfusion | 17,110 (26.4) | 6805 (67.3) | 0.000 | 2931 (25.9) | 3559.6 (31.4) | 0.026 |
| HFrEF | 2995 (30.2) | 1810 (71.7) | 0.000 | 513 (30.4) | 561 (33.3) | 0.651 |
| HFpEF | 9430 (24.4) | 2570 (63.5) | 0.000 | 1632 (23.9) | 1943.2 (28.5) | 0.232 |
| Cardiogenic Shock | 1180 (1.8) | 995 (9.8) | 0.000 | 202 (1.8) | 391.4 (3.5) | 0.000 |
| HFrEF | 310 (3.1) | 375 (14.9) | 0.000 | 53 (3.1) | 105.9 (6.3) | 0.078 |
| HFpEF | 410 (1.1) | 270 (6.7) | 0.000 | 75 (1.1) | 224.8 (3.3) | 0.001 |
| Mechanical Circulatory Support (LVAD or pVAD or ECMO) | 535 (0.8) | 735 (7.3) | 0.000 | 90 (0.8) | 495.5 (4.4) | 0.000 |
| HFrEF | 165 (1.7) | 315 (12.5) | 0.000 | 28 (1.7) | 201.4 (11.9) | 0.000 |
| HFpEF | 175 (0.5) | 130 (3.2) | 0.000 | 29 (0.4) | 209.4 (3.1) | 0.000 |
Abbreviations: CVA: Cerebral Vascular Accident; TAVR: Transaortic Valve Replacement; SAVR: Surgical Aortic Valve Replacement; SCA: Sudden Cardiac Arrest; AKI: Acute Kidney Injury; LVAD: Left Ventricular Assist Device; pVAD: Percutaneous Ventricular Assist Device; ECMO: Extracorporeal Membrane Oxygenation; HF: Heart Failure; HFrEF: Heart Failure with reduced ejection fraction; HFpEF: Heart Failure with preserved ejection fraction.
PSM results for TAVR-HR in comparison to SAVR-HF were consistent in terms of in-hospital mortality (1.8% vs. 8.2%; p < 0.001), SCA (9.6% vs. 13.2%; p = 0.02), AKI (17.2% vs. 27.4%); p < 0.001), transfusion (25.9% vs. 31.4%; p = 0.03), CS (1.8% vs. 3.5%; p < 0.001) and MCS (0.8% vs. 4.4%; p < 0.001). However, CVA (2.5% vs. 1.7%; p = 0.17), post-procedural stroke (0.9% vs. 0.5%; p = 0.25) and major bleeding (1.7% vs. 1.0%; p = 0.1) showed no significant difference (Table 2). The overall frequency and percentages of weighted unmatched and PSM outcomes are shown in Table 1; Table 2.
Further subgrouping to compare frequency and percentage of complications for HFrEF and HFpEF group showed that in TAVR-HFrEF had lower in-hospital mortality (1.9% vs. 7.7%; p < 0.001), SCA (10% vs. 19.4%; p < 0.001), AKI (21% vs. 45.1%; p < 0.001), transfusion (30.2% vs. 71.7%; p < 0.001), CS (3.1% vs. 14.9%; p < 0.001), and MCS (1.7% vs. 12.5%; p < 0.001); while there was no significant difference in CVA (2.6% vs. 3.4%; p = 0.33), post-procedural CVA (0.8% vs. 1.2%; p = 0.35), and major bleeding (2.2% vs. 2.4%; p = 0.83) when compared to SAVR-HFrEF (Table 2).
Similarly, TAVR-HFpEF also had a lower percentage of in-hospital mortality, SCA, AKI, transfusion, CS, and MCS, where there was no significant difference in CVA, post-procedural stroke, and major bleeding when compared to SAVR-HFpEF (Table 2).
3.3. The trend of complications between TAVR-HF and SAVR-HF
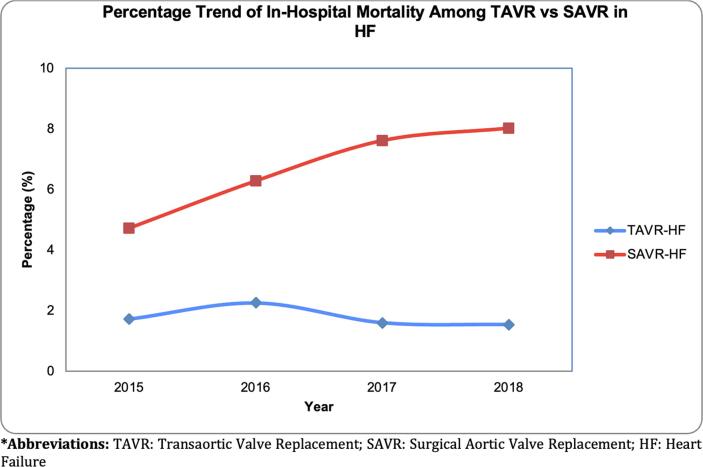
TAVR-HF had lower trends from 2015 to 2018 regarding in-hospital mortality, post-procedural stroke, SCA, AKI, major bleeding, transfusion, CS, and MCS (Fig. 2). On the other hand, SAVR-HF had high trends of in-hospital mortality, AKI, CS, and a lower trend of CVA, post-procedural CVA, and major bleeding (Fig. 2). There was no significant difference in trend in SAVR-HF among SCA, transfusion, and MCS (see Fig. 3).
Fig. 2.
(A) Complications of the unmatched percentage of TAVR-HF and SAVR-HF. (B) Complications of the propensity-matched percentage of TAVR HF and SAVR HF.
Fig. 3.
Trend Percentage of In-Hospital Mortality in unmatched TAVR-HF and SAVR-HF.
3.4. Comparison of quality measures between TAVR and SAVR with HF
The median length of stay in TAVR-HF and SAVR-HF was three days and ten days, respectively. The median unmatched total charges for TAVR-HF and SAVR-HF were 194,561$ and 246,100$, respectively. The median Elixhauser index was 6, and the median AHRQ risk mortality index was 3 for both cohorts (Table 1). TAVR and SAVR trends of quality measure showed a high trend of increasing hospital stay cost of SAVR-HF compared to TAVR-HF (Table 3). LOS trend comparison between TAVR-HF and SAVR-HF showed high LOS among SAVR cohorts, but the trend of the SAVR intragroup from 2015 to 2018 was stable (Table 3).
Table 3.
Trend of hospital cost and length of stay between TAVR and SAVR with HF in Octogenarian Population.
| variable | TAVR-HF Median Cost (IQR) | SAVR-HF Median Cost (IQR) | TAVR-HF LOS [Median (IQR)] days | SAVR-LOS [Median (IQR)] days |
|---|---|---|---|---|
| 2015 | 195,788$ (141 282) | 217,983$ (184 796) | 4 (4) | 10 (8) |
| 2016 | 198,450$ (139 528) | 233,911$ (186 586) | 3 (4) | 10 (9) |
| 2017 | 187,849$ (137 886) | 245,692$ (207 935) | 3 (3) | 10 (8) |
| 2018 | 196,157$ (141 501) | 286,817$ (256 135) | 2 (3) | 10 (9) |
| Pooled | 193,819 $ (142,201$- 282,323$) | 244,369$ (168,669$-386,322$) | 3 (2–5) | 10 (7–16) |
Abbreviations: TAVR: transcatheter aortic valve replacement; HF: Heart Failure; LOS: Length of Stay; IQR: Interquartile Range; SAVR: surgical aortic valve replacement.
4. Discussion
This national cohort included 74,995 octogenarian patients with HF. Major findings of the study include: 1) TAVR-HF, compared to SAVR-HF, had a significantly lower percentage of in-hospital mortality, SCA, CVA, AKI, major transfusion, CS, and MCS. 2) There was no significant difference in the percentage of post-procedural stroke and major bleeding in TAVR-HF and SAVR-HF. 3) In the subgroups, TAVR-HFrEF and TAVR-HFpEF had a lower risk of in-hospital mortality, SCA, AKI, transfusion, CS, and MCS compared to SAVR-HFrEF and SAVR-HFpEF. 4) There was no significant difference in CVA incidence, post-procedural stroke, and major bleeding between the subgroups. 5) Lower trends were observed for in-hospital mortality, post-procedural stroke, SCA, AKI, major bleeding, transfusion, CS, and MCS in the TAVR-HF. 6) Higher trends were observed for in-hospital mortality, AKI, and CS in SAVR-HF. As a result, our findings suggest that TAVR may be a more promising approach to treating AS in this vulnerable population.
After the onset of symptomatic valvular HF, the average survival in untreated AS is approximately two years [1]. Currently, recommended evidence-based operative treatments for AS are TAVR and SAVR [15]. It has been demonstrated that for high surgical risk patients, utilization of TAVR is associated with better outcomes [16]. Octogenarians are a unique subset of patients with high surgical risk due to depleting physiological reserves, age, and pathologic processes, including associated comorbidities [17], [18]. In this population, a recent study comparing the outcome of in-hospital mortality in patients undergoing TAVR and SAVR found no significant difference [19]. However, no studies have investigated whether TAVR has lower rates of in-hospital mortality in patients who are both octogenarians and at high surgical risk due to coexisting HF. This is important since approximately 10% of octogenarians may have HF, placing them at very high surgical risk [20].
Our study found that in-hospital mortality in octogenarians with HF was 1.8% in patients undergoing TAVR. The in-hospital mortality rate in our analysis is lower than previous studies comparing octogenarians undergoing TAVR reporting in-hospitality mortality ranging between 3 and 4.2% [21], [22], [23], [24]. Our lower in-hospital mortality rate in the TAVR group likely reflects improved design and delivery of the prosthesis [16], [23], [24], [25]. Additionally, lower in-hospital mortality in our study may be due to the positive effects of aortic valve replacement on decreasing afterload in HF patients with significant AS [16]. In our study, the in-hospital mortality rate for SAVR was 6.9%. This is consistent with the previous studies suggesting an in-hospital mortality rate ranging between 2 and 7.1% [21], [22], [23], [24]. Higher rates of in-hospital mortality of SAVR in our study are likely secondary to our sample consisting of HF patients only, compared to previous studies including healthier octogenarians at lower surgical risk, particularly for open-heart surgery. Finally, we also found significant differences in in-hospital mortality in octogenarians with either HFpEF or HFrEF undergoing TAVR compared to SAVR. The in-hospital mortality of octogenarians in TAVR-HFrEF and TAVR-HFpEF was 1.9% and 7.7% compared to SAVR-HFrEF, and SAVR-HFpEF 7.7% and 5.2%, respectively. Our findings on in-hospital mortality are in parallel with the recent findings of Sheng et al. that TAVR may be of benefit over SAVR in all octogenarians regardless of comorbidity burden [24].
Our study also found an association between the use of TAVR in octogenarians with HF and lower in-hospital complications such as CVA, post-procedural stroke, AKI, transfusion, CS, and MCS compared to SAVR. Compared to the lower incidence of stroke in HF patients undergoing TAVR in our analysis, Hijri et al. and Brennan et al. demonstrated no change in stroke between TAVR and SAVR groups [4], [19]. Leon et al. demonstrated a lower risk of stroke in the transfemoral access cohort undergoing TAVR when compared with SAVR, whereas in the transthoracic access cohort, no significant difference was observed between the two groups [23]. According to Hijri et al., TAVR demonstrated a lower incidence of AKI, similar to our analysis [19]. Our findings of lower incidence of AKI in HF patients undergoing TAVR are consistent with Reardon et al. and Leon et al., who demonstrated similar findings in patients at intermediate risk undergoing TAVR vs. SAVR [23], [26]. Increased requirement of blood transfusions in HF patients undergoing SAVR as compared to those undergoing TAVR according to our analysis is also consistent with higher transfusion requirement in intermediate-risk patients undergoing SAVR as compared to TAVR as illustrated by Reardon et al. and Leon et al. [23], [26]. Decreased LOS demonstrated by Hijri et al. is also consistent with our analysis in patients with HF undergoing TAVR [19]. Our findings suggesting that TAVR is associated with better in-hospital outcomes in octogenarians with HF are likely related to the recent improved design and delivery of the prosthesis [16], [23], [24], [25]. A recent study suggests that older patients and patients with significant comorbidities were more likely to undergo transapical than transfemoral access for TAVR [4]. However, emerging evidence shows that transfemoral access may be associated with lower in-hospital mortality, LOS, AKI, and CS [27]. An increase in transfemoral access may, therefore, potentially explain lower complications compared to the past. A comparison of quality measures between TAVR-HF and SAVR-HF groups demonstrated decreased LOS (3 vs. ten days) and median unmatched total charges (194,561$ vs. 246,100$) for the TAVR-HF group in our analysis. Our analysis also demonstrated lower trends of in-hospital mortality, post-procedural CVA, SCA, AKI, major bleeding, transfusion, CS, and MCS in the TAVR-HF group compared to SAVR-HF.
Subgroup analysis of our patients revealed that TAVR-HFrEF had lower in-hospital mortality, SCA, AKI, transfusion, CS, and MCS when compared with SAVR- HFrEF. In contrast, there was no significant difference in CVA, post-procedural CVA, and major bleeding between TAVR-HFrEF and SAVR-HFrEF. Whereas TAVR-HFpEF also had a lower percentage of in-hospital mortality, SCA, AKI, transfusion, CS, and MCS compared to SAVR-HFpEF. There was no significant difference in CVA, post-procedural CVA, and major bleeding in patients with HFpEF undergoing TAVR or SAVR. As a result, TAVR use in octogenarians with HF (both in HFrEF and HFpEF subgroups) compared to SAVR is a promising option.
5. Limitations
Our study's main limitation was that given data was selected from NIS, the observational and retrospective data had some inherent selection bias. We attempted to decrease the selection bias by entropy matching to match the mean, median, and variance of nearby matching. Secondly, NIS outcomes are in-hospital, and it does not capture the procedural details, the frailty of the patients included, and long-term outcomes or medication-induced changes in outcomes. Furthermore, given the cross-sectional snapshot data nature, we could not do a long-term follow-up to report improvement in ejection fraction after TAVR.
6. Conclusion
In our study of octogenarians, TAVR-HF had lower odds of in-hospital mortality, CVA, AKI, major transfusion, CS, and MCS than SAVR-HF. In the subgroup analysis of HFrEF and HFpEF for TAVR and SAVR, TAVR in both subgroups had a lower risk of in-hospital mortality, SCA, AKI, transfusion, CS, and MCS. Therefore, our study suggests that octogenarians with HF may benefit from TAVR in both HFrEF and HFpEF as the first-line treatment of their AS.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement of grant support
N/A.
Registration number of clinical studies
N/A.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2022.101119.
Contributor Information
Talal Almas, Email: talalalmas.almas@gmail.com.
M. Chadi Alraies, Email: alraies@hotmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Kodali S.K., Velagapudi P., Hahn R.T., Abbott D., Leon M.B. Valvular Heart Disease in Patients ≥80 Years of Age. J. Am. Coll. Cardiol. 2018;71(18):2058–2072. doi: 10.1016/j.jacc.2018.03.459. [DOI] [PubMed] [Google Scholar]
- 3.Otto C.M., Kumbhani D.J., Alexander K.P., Calhoon J.H., Desai M.Y., Kaul S., Lee J.C., Ruiz C.E., Vassileva C.M. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults With Aortic Stenosis: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2017;69(10):1313–1346. doi: 10.1016/j.jacc.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Brennan J.M., Thomas L., Cohen D.J., Shahian D., Wang A., Mack M.J., Holmes D.R., Edwards F.H., Frankel N.Z., Baron S.J., Carroll J., Thourani V., Tuzcu E.M., Arnold S.V., Cohn R., Maser T., Schawe B., Strong S., Stickfort A., Patrick-Lake E., Graham F.L., Dai D., Li F., Matsouaka R.A., O’Brien S., Li F., Pencina M.J., Peterson E.D. Transcatheter Versus Surgical Aortic Valve Replacement: Propensity-Matched Comparison. J. Am. Coll. Cardiol. 2017;70(4):439–450. doi: 10.1016/j.jacc.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotajarvi B.R., Schafer M.J., Atkinson E.J., et al. The impact of frailty on patient-centered outcomes following aortic valve replacement. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72(7):917–921. doi: 10.1093/gerona/glx038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green P., Woglom A.E., Genereux P., Daneault B., Paradis J.-M., Schnell S., Hawkey M., Maurer M.S., Kirtane A.J., Kodali S., Moses J.W., Leon M.B., Smith C.R., Williams M. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc. Interv. 2012;5(9):974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidenreich P.A., Bozkurt B., Aguilar D., Allen L.A., Byun J.J., Colvin M.M., Deswal A., Drazner M.H., Dunlay S.M., Evers L.R., Fang J.C., Fedson S.E., Fonarow G.C., Hayek S.S., Hernandez A.F., Khazanie P., Kittleson M.M., Lee C.S., Link M.S., Milano C.A., Nnacheta L.C., Sandhu A.T., Stevenson L.W., Vardeny O., Vest A.R., Yancy C.W. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18) doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 8.Spitzer E., Van Mieghem N.M., Pibarot P., Hahn R.T., Kodali S., Maurer M.S., Nazif T.M., Rodés-Cabau J., Paradis J.-M., Kappetein A.-P., Ben-Yehuda O., van Es G.-A., Kallel F., Anderson W.N., Tijssen J., Leon M.B. Rationale and design of the Transcatheter Aortic Valve Replacement to UNload the Left ventricle in patients with ADvanced heart failure (TAVR UNLOAD) trial. Am. Heart. J. 2016;182:80–88. doi: 10.1016/j.ahj.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Sample N.I. Agency for Healthcare Research and Quality; Rockville, MD: 2003. Healthcare Cost and Utilization in Project (HCUP) [PubMed] [Google Scholar]
- 10.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med. Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Matschinger H., Heider D., König H.-H. A comparison of matching and weighting methods for causal inference based on routine health insurance data, or: what to do if an RCT is impossible. Das Gesundheitswesen. 2020;82(S 02):S139–S150. doi: 10.1055/a-1009-6634. [DOI] [PubMed] [Google Scholar]
- 12.Khera R., Angraal S., Couch T., Welsh J.W., Nallamothu B.K., Girotra S., Chan P.S., Krumholz H.M. Adherence to Methodological Standards in Research Using the National Inpatient Sample. Jama. 2017;318(20):2011. doi: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houchens R., Elixhauser A. US Agency for Healthcare Research and Quality; Rockville, MD: 2006–2005 (2006).. Using the HCUP Nationwide Inpatient Sample to estimate trends (updated for 1988–2004) [Google Scholar]
- 14.StataCorp L. Stata statistical software. College. Station. TX. 2009 [Google Scholar]
- 15.Osnabrugge R.L.J., Mylotte D., Head S.J., Van Mieghem N.M., Nkomo V.T., LeReun C.M., Bogers A.J.J.C., Piazza N., Kappetein A.P. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013;62(11):1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Stewart B.F., Siscovick D., Lind B.K., et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997;29(3):630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 17.Vaes B., Rezzoug N., Pasquet A., Wallemacq P., Van Pottelbergh G., Mathe C., Vanoverschelde J.-L., Degryse J. The prevalence of cardiac dysfunction and the correlation with poor functioning among the very elderly. Int. J. Cardiol. 2012;155(1):134–143. doi: 10.1016/j.ijcard.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner H., Falk V., Bax J.J., et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart. J. 2017;38(36):2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 19.Hirji S.A., Ramirez-Del Val F., Kolkailah A.A., Ejiofor J.I., McGurk S., Chowdhury R., Lee J., Shah P.B., Sobieszczyk P.S., Aranki S.F., Pelletier M.P., Shekar P.S., Kaneko T. Outcomes of surgical and transcatheter aortic valve replacement in the octogenarians-surgery still the gold standard? Ann. Cardiothorac. Surg. 2017;6(5):453–462. doi: 10.21037/acs.2017.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coats A.J.S. Ageing, demographics, and heart failure. Eur. Heart. J. Suppl. 2019;21(Suppl L):L4–l7. doi: 10.1093/eurheartj/suz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Himbert D., Vahanian A. Transcatheter aortic valve replacement for patients with heart failure. Heart. Fail. Clin. 2015;11(2):231–242. doi: 10.1016/j.hfc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., Fleisher L.A., Jneid H., Mack M.J., McLeod C.J., O’Gara P.T., Rigolin V.H., Sundt T.M., Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017;70(2):252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Leon M.B., Smith C.R., Mack M.J., et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016;374(17):1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 24.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., Kapadia S.R., Malaisrie S.C., Cohen D.J., Pibarot P., Leipsic J., Hahn R.T., Blanke P., Williams M.R., McCabe J.M., Brown D.L., Babaliaros V., Goldman S., Szeto W.Y., Genereux P., Pershad A., Pocock S.J., Alu M.C., Webb J.G., Smith C.R. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 25.Onorati F., D’Errigo P., Grossi C., Barbanti M., Ranucci M., Covello D.R., Rosato S., Maraschini A., Santoro G., Tamburino C., Seccareccia F., Santini F., Menicanti L. Effect of severe left ventricular systolic dysfunction on hospital outcome after transcatheter aortic valve implantation or surgical aortic valve replacement: results from a propensity-matched population of the Italian OBSERVANT multicenter study. J. Thorac. Cardiovasc. Surg. 2014;147(2):568–575. doi: 10.1016/j.jtcvs.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Reardon M.J., Van Mieghem N.M., Popma J.J., Kleiman N.S., Søndergaard L., Mumtaz M., Adams D.H., Deeb G.M., Maini B., Gada H., Chetcuti S., Gleason T., Heiser J., Lange R., Merhi W., Oh J.K., Olsen P.S., Piazza N., Williams M., Windecker S., Yakubov S.J., Grube E., Makkar R., Lee J.S., Conte J., Vang E., Nguyen H., Chang Y., Mugglin A.S., Serruys P.W.J.C., Kappetein A.P. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017;376(14):1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 27.Doshi R., Shah P., Meraj P.M. In-hospital outcomes comparison of transfemoral vs transapical transcatheter aortic valve replacement in propensity-matched cohorts with severe aortic stenosis. Clin. Cardiol. 2018;41(3):326–332. doi: 10.1002/clc.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.