Abstract
Periodontitis is a common inflammatory disease in dentistry that may lead to tooth loss and aesthetic problems. Periodontal tissue has a sophisticated architecture including four sections of alveolar bone, cementum, gingiva, and periodontal ligament fiber; all these four can be damaged during periodontitis. Thus, for whole periodontal regeneration, it is important to form both hard and soft tissue structures simultaneously on the tooth root surface without forming junctional epithelium and ankylosis. This condition makes the treatment of the periodontium a challenging process. Various regenerative methods including Guided Bone/Tissue Regeneration (GBR/GTR) using various membranes have been developed. Although using such GBR/GTR membranes was successful for partial periodontal treatment, they cannot be used for the regeneration of complete periodontium. For this purpose, multilayered scaffolds are now being developed. Such scaffolds may include various biomaterials, stem cells, and growth factors in a multiphasic configuration in which each layer is designed to regenerate specific section of the periodontium. This article provides a comprehensive review of the multilayered scaffolds for periodontal regeneration based on natural or synthetic polymers, and their combinations with other biomaterials and bioactive molecules. After highlighting the challenges related to multilayered scaffolds preparation, features of suitable scaffolds for periodontal regeneration are discussed.
Keywords: Biomaterials, Layered scaffolds, Periodontitis, Periodontal regeneration, Polymer
Graphical abstract
Abbreviations
- CM
Cementum
- AB
Alveolar Bone
- GTR
Guided Tissue Regeneration
- GBR
Guided Bone Regeneration
- PCL
polycaprolactone
- PLCL
Poly lactic acid-co-ε-caprolactone
- PGA
Polyglycolic acid
- PLGA
Polylactic-co-glycolic acid
- PVA
Polyvinyl alcohol
- HA
Hydroxyapatite
- β-TCP
β-tricalcium phosphate
- PDLCs
Periodontal Ligament Cell
- MMP
Metalloproteases
- HPMC
Hydroxypropyl Methyl Cellulose
- ECM
Extracellular Matrix
- FeHA
iron-doped hydroxyapatite
- Si-nHAp
Si-doped nanohydroxyapatite
- CTGF
Connective Tissue Growth Factor
- BMP-2
Bone Morphogenic Protein 2
- MSCs
Mesenchymal Stem Cell
- CP
Calcium Phosphate
- BG
Bioactive GLass
- rhCEMP1
recombinant human cementum protein
- rhFGF2
recombinant human fibroblast growth factor
- PRP
Platelet-Rich Plasma
- BMP-7
Bone Morphogenic Protein 7
- Sr-nHA
Sr-doped nanohydroxyapatite
1. Introduction
Periodontium is tooth-supporting tissue created by, gingiva, periodontal ligament (PDL), cementum (CM), and alveolar bone (AB).1 One of the most inflammatory diseases in dentistry is periodontitis that originated from plaque and oral microorganisms. In the U.S.A, approximately 50% of adults have chronic periodontitis, which was consistent with other areas of the world. Bacteria and their elements, like lipopolysaccharide, caused periodontitis and inflammation in the host body that can destruct the periodontium.2 Periodontitis is the major reason for loosening, movement, and tooth loss, it can also affect the function and aesthetic aspects of humans and finally make periodontitis a global concern in oral diseases. Periodontitis is also, related to different systemic conditions such as; diabetes Mellitus, cardiovascular diseases, respiratory diseases, chronic kidney diseases, and rheumatoid arthroses. Therefore, periodontal treatment is very important for humans not only for reducing periodontal symptoms in the whole body but also for improving quality of life.3
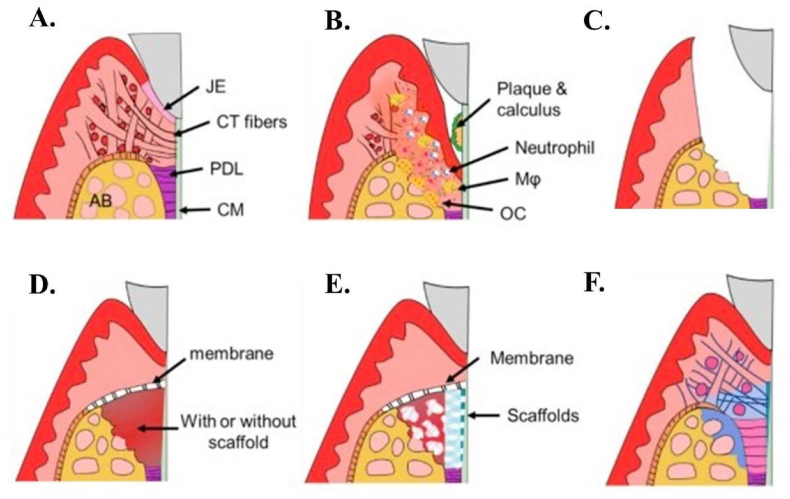
Inhibiting inflammation and attempting to regenerate functional periodontium is the aim of periodontitis therapy.3 The goal of this treatment is to regenerate periodontal tissues in harmony, with PDL, CM, and AB forming in their correct locations; PDL fibers should be oriented longitudinally between CM and AB (Fig. 1A).4 Periodontal tissues have a low capacity to regenerate without treatment. Common treatments for periodontal diseases include plaque removal and local inflammation management, scaling and root planning, antibiotic administration, and surgery. Such therapies are used to decrease signs and symptoms and arrest disease progression. However, these treatments are not able to reattach the periodontal tissue to teeth, or the root surface and the native tissues also these treatments have some drawbacks like, pain and cost of treatment (Fig. 1 B, C).4,5 The treatment of antibiotic application has disadvantages such as insufficient antibiotic concentration at the applied site, a fast reduction of the plasma antibiotic concentration to subtherapeutic levels, and increased microbial resistance. Therefore, antibiotics should only be used after proper patient assessment.6 The function of tooth-supporting architecture remains weak after these therapies.
Fig. 1.
In this picture healthy periodontium, periodontitis, and regeneration process are presented: (A)healthy periodontium, (B) Periodontitis, (C)Root planning and removal of inflamed tissue, (D)GTR with or without the scaffold, (E) Multi-phase scaffold for cementum-PDL-bone and (F) Scaffold degradation and periodontal regeneration. Adopted from reference 4 with permission.
Different regenerative methods including guided tissue regeneration (GTR) and guided bone regeneration (GBR) have recently replaced the previous methods to obtain better periodontal regeneration. Karring and Nyman first introduced the concept of GTR in the 1980s as an alternative regenerative method for periodontium regeneration. During the GTR process, Millipore membrane was applied to treat periodontitis by forming a new periodontal ligament attachment without the formation of long junctional epithelium or ankylosis (Fig. 1D).4,7 Despite the successful results of GTR, sometimes root resorption and ankylosis are reported. Moreover, continuous periodontitis can dramatically reduce the regeneration capacity of PDL and CM and also, affect the regeneration of the whole multiple periodontal tissues.8 In addition, the clinical outcomes of those techniques are variable and uncertain.6
Therefore, it is critical to investigate new techniques for the formation of periodontal tissue. One of the newest methods is the tissue engineering approach that can reestablish the new microenvironment and cause the formation of functional tissues. Thereby, when the tissue engineering concept is used, two important factors are considerable: scaffold (biomaterials, stem cell and method of scaffold fabrication) and controlled drug delivery strategy (biologic molecules, methods of drug releasing and their suitable concentration).9,10 Some results of bioengineered scaffolds yielded similar results to those obtained with GTR. These approaches are candidates for use in a limited clinical range such as mandibular molar class II furcations, and infrabony defect reconstruction, so obtaining complete regeneration in the imperative tissue remains to be difficult.
Regeneration of the indigenous structure and function of the periodontal intricate tissue is necessary. Because new cementum should form on the tooth root surface, with the correct position of periodontal attachment between newly formed bone and cementum. Ultimate regeneration of periodontal tissue could not be achieved by traditional methods due to the presence of both soft (gingiva, periodontal ligament) and hard (bone, cementum) tissues.11 Therefore, for complete regeneration of this sophisticated structure, new strategies including stem cells, biomaterials, and advanced scaffold design should be investigated.
Due to the use of the multilayered/multiphasic scaffold strategy, the simultaneous regeneration of two or more tissues is becoming feasible. Multiphasic scaffolds are vital to form the biomimetic functionality structures for bone and soft tissue grafts. They are recognized for their substantial ability to emerge in the orthopedic tissue engineering field. In recent years, this type of advanced scaffold emerged in periodontal regeneration.12,13 Periodontal regeneration, like orthopedic reconstruction, often needs soft to hard tissue interface, including, ligament, tendon, and cartilage to bone. Obtaining functional integration between soft and hard tissue elements and their environment is the major clinical challenge of periodontal regeneration. It has been proposed that the application of multilayered scaffold architecture is required to enhance the strong connection among soft and hard tissue. Strategic biomimicry could be used by multilayered scaffolds to reconstruct the critical structure-function of native soft tissue–bone interface. Multiphasic scaffolds provide the pore size and suitable interconnection between the layers of structure. Obtaining a strong cohesion among the different layers is the most important aspect of multiphasic scaffolds to prevent the destruction of whole regenerated layers, especially during surgical process and physiologic loading.14,15
The use of advanced scaffolds such as multilayered scaffolds are essential in preparing the pore sizes in macro and micro scales, anisotropic pore division, and the management of structural fabrication. Due to periodontium role in teeth support, improving the physiologic loading and homeostasis regulation of this tissue is required. These multilayered structures are required to mimic the interface between the periodontal ligament and the tooth root surface. In addition, the formation of cementum with functional periodontal ligament fibers is essential and preparing enough space for GBR and the provision of the obstacles that prevent epithelial migration along the root surface are some supplementary requirements in this field (Fig. 1E and F).4 Recently, scientists have attempted to combine multilayered scaffold with certain periodontal regenerative techniques, including GTR, growth factors, and drug and stem cell technology to improve regeneration goal.16
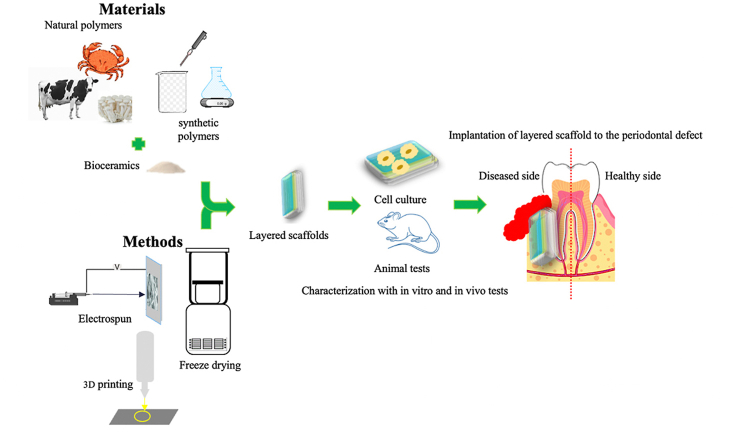
Various articles focused on periodontal regeneration with emphasis on scaffolds and their synthesis method have been published. Despite the many experimental studies on layered scaffolds, a comprehensive review that include a summary of layered scaffolds fabrication, suitable features of biomaterials, drug delivery system, stem cells for each layer, pivotal roles of each layer in periodontal regeneration and results of experiments, is not recently published. Although a small number of reviews have been published before. For example, Liang et al.1 reviewed different biomaterial and drug control systems for periodontal regeneration. Bittner et al.17 reviewed 3D printed multilayered scaffold for different body organs with a short review on oral and periodontal regeneration scaffolds. Ivanovski et al.16 reviewed multiphasic periodontal scaffolds categorized in membrane scaffolds, biphasic scaffolds (with fiber guiding properties and cell incorporated ones) and triphasic scaffolds. But, in current review article, we aim to study developed layered scaffolds for periodontal regeneration in order to summarize previous scientific studies in layered scaffolds synthesis methods, biomaterials features and challenges regarding to this complex tissue which could help future scientists in this field to enhance their research outcomes and improve strong connection between hard and soft tissue to repair damaged periodontium. Beside this, in most studies, polymers have been used to provide the essential features of periodontal tissue complex structure and layered scaffolds. Herein, We will describe important properties of polymers which make them the best candidates for periodontal regeneration and categorize experimental studies based on the main polymeric components into three general groups: natural (collagen and chitosan), synthetic polymers including Polycaprolactone (PCL), poly lactic acid-co-ε-caprolactone (PLCL), polyglycolic acid (PGA), polylactic-co-glycolic acid (PLGA), and a combination of natural and synthetic polymers. Along with a short review on bone grafting methods, significant material properties for these scaffolds, a short explanation on different multilayered scaffold fabrication methods, explain the major problems in this field, and future perspective of this science.
2. Bone grafting for periodontal regeneration
Bone grafting is useful in the periodontal regeneration to provide a supplement for alveolar bone and increase the mechanical features of the scaffold.13,18 Materials used for bone grafting are typically biological materials like bone transplants, or synthetic materials with similar properties to that of bone. Studies have used these materials in different ways like scaffolds, composite with other polymers, and nano or micro particles.19 The main problems of bone grafting are second surgery and pain and infection (for autograft method),20 risk of disease transmission and immune responses (for allograft method),21,22 clinical results are different and unforeseeable (for xenograft method).23 This problems demonstrate the need for materials with appropriate degradability, and great mechanical and biological properties for alveolar bone regeneration.24,25 New studies on periodontal regeneration usually use more than two materials, specific ceramics for bone regeneration and polymers for PDL and cementum regeneration.26,27 The most used bioceramics are explained in Table 1. Hydroxyapatite (HA), tricalcium phosphate (TCP), β-tricalcium phosphate (β-TCP), and bioactive are the most popular bioceramics in periodontal regeneration. There are other examples of bioceramics with different properties such as silicate-based bioceramics, which have even better properties in some cases.28,29 The most important feature of HA, TCP, β-TCP, and bioactive glass is their similarity to the inorganic nature of bone. Due to this feature, they can improve the cellular differentiation and the alveolar bone formation. Additionally, periodontium's complex structure, cementum regeneration is required. Overall, bioceramics can improve the cementum formation, but silicate-based ones demonstrated more cementum formation.30
Table 1.
Bioceramics for alveolar bone and cementum regeneration.
| Bioceramics | Target tissue | Features | Ref |
|---|---|---|---|
| Hydroxyapatite (HA) | Cementum, Alveolar bone | Similar composition to the inorganic phase of bone Osteoconductive Slow degradation |
29,31 |
| tricalcium phosphates (TCP) | Alveolar bone, cementum | Similar composition to the inorganic phase of bone Higher stability Osteoconductive Bioabsorbable Β-TCP: Similar degradation kinetic to the autologous grafts |
32, 33, 34 |
| Bioactive glass | Alveolar bone, cementum | Osteoconductive and osteoblast cell differentiation Similar composition to the inorganic phase of bone Biocompatible with the different degradation rate Degradation products improve the osteogenesis, angiogenesis, and antibacterial activities |
28,35,36 |
| Nagelschmidtite (Ca7Si2P2O16) | Alveolar bone, cementum | Suitable mechanical properties and degradation rate for spongy bone regeneration Favorable apatite formation and angiogenesis Improved osteogenesis and cementogenesis compared to HA and TCP |
37 |
| Silicocarnotite (Ca2SiO4.Ca(PO4)2) | Alveolar bone, cementum | Better mechanical properties and manufacturability compared to Nagelschmidtite Better bone formation, osteogenesis, and cementogenesis compared to the HA |
37 |
| Nurse's Ass-phase (2Ca2SiO4.Ca3(PO4)2) | Alveolar bone, cementum | Favorable bone formation and degradation rate Lower bone formation compares to other Silicate based bioceramics |
37 |
| Other silicate-based bioceramics: | Alveolar bone, cementum | Great cellular properties Excellent ability in improving cell proliferation and differentiation to osteogenic of PDLCs Great cementum formation compares to other bioceramics Increasing expression of osteocalcin and bone sialoprotein |
30 |
| Akermanite (Ca2MgSi2O7) | |||
| Bredigite (Ca7MgSi4O16) | |||
| Baghdadite (Ca3ZrSi2O9) | |||
| Diopside (CaMgSi2O6) |
3. Layered periodontal scaffolds based on natural polymers
The unique benefit of natural polymers is their ability of mimicking the extracellular matrix of tissue leading to the promotion of cell proliferation and differentiation such as osteoblastic-like cell, mesenchymal and periodontal ligament stem cells, and new tissue formation. Natural polymers are biocompatible and biodegradable, and show more elasticity and hydrophilicity compared to synthetic ones, which make them the most useful materials for tissue engineering.13,38 Different natural polymers have been used in periodontal regeneration. However, problems such as unsuitable immune responses due to crosslinking agents and sources have been reported in previous studies.39,40 Based on previous research, collagen, chitosan, pectin, alginate, and hyaluronic acid are the most used natural polymers. Collagen is a great candidate for PDL regeneration, but crosslinking methods are necessary due to its fast degradation rate.41 In some cases, degradation methods can lead to toxic responses. The main challenge in collagen scaffold research is to provide suitable mechanical and degradation properties without inflammation and toxic responses.42,43 Chitosan demonstrates great cellular properties and can be used to provide antibacterial properties for periodontal regeneration. However, providing the appropriate porosities and suitable mechanical properties are still challenging.39,43 Due to the wide use of collagen and chitosan in layered scaffolds, they are described below. Other useful natural polymers such as pectin (for Alveolar bone regeneration),44, 45, 46 alginate (for Alveolar bone, PDL regeneration),47, 48, 49, 50 and hyaluronic acid (for PDL, gingival, cementum regeneration)51, 52, 53 demonstrated great biocompatibility and biodegradability, and good cell adhesion and proliferation properties. In addition, alginate and hyaluronic acid are popular for their great antibacterial properties, but, due to their poor mechanical and stability, they are usually not commonly used in layered scaffold research.54
4. Collagen-based layered scaffolds
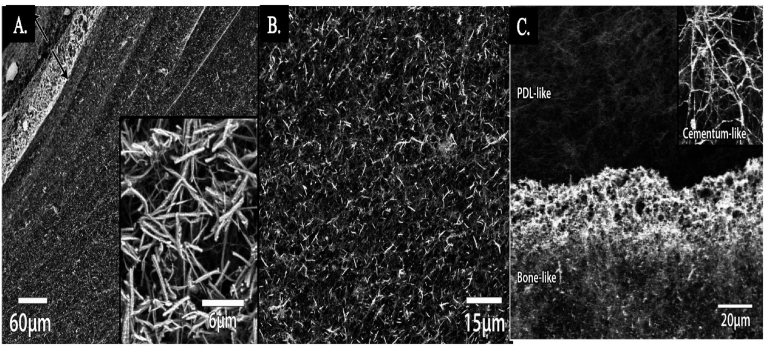
Collagen scaffolds, widely used for soft and hard tissue engineering, and drug and gene delivery show the potential for periodontal regeneration. Collagen is the most available protein in the human body. The main reaction of its biodegradability is related to the metalloproteases (MMP), which is released in the wound healing process by fibroblast and monocytes cells.55 Each type of collagen has unique advantages. For instance, collagen type I shows angiogenic features and enhances the growth of healthy tissue in a defect. Based on the area and type of implant used, the crosslinking process is needed in some cases. For example, membrane and barrier functions are long-term, so crosslinking methods are required for reducing biodegradation rate, increasing mechanical properties, and stabilizing the membrane.56 Crosslinked collage shows less attachment and proliferation of osteoblasts and periodontal ligament fibroblast compared to non-cross linked collagen.57 Also, more foreign body reactions are reported in in vivo research.58 Regarding this, a combination of collagen types is a way to achieve both stability and mechanical properties without harmful biological effects.59 Laucsh et al.60 synthesized a multilayered collagen scaffold for soft-hard tissue regeneration. One of the important factors in multilayered scaffolds is cohesion between layers, which provides the diffusion of cells over layers. To provide suitable cohesion between layers, they used a mineralized system. They designed the unique tri-layered intrafibrillar mineralized collagen and HA to mimic periodontium, including bone, ligament, and cementum. Fig. 2A demonstrates the background electron- SEM (BSE-SEM) image of collagen scaffolds after 6 days of mineralization with different rates of surface and bulk mineralization. Around 120 μm of thickness highly mineralized after 6 days. Also, the energy dispersive X-ray spectroscopy (EDX) analysis of this part shows the Ca/P ratio to be around 1.76, which confirms the presence of HA. Fig. 2B shows the homogenous mineralization in bulk scaffold. Finally, Fig. 2C shows the BSE image of interface between two different mineralized parts, the upper one with unmineralized part for PDL regeneration and the lower part for bone regeneration which is mineralized for 10 days. The inset image demonstrates the cementum layer with 4 days of collagen layer mineralization.
Fig. 2.
Multilayered collagen scaffold for soft-hard tissue regeneration A. Overview of cross-section with highly mineralized parts at upper left and lower, and a slower mineralized part at the middle. B. Higher magnification of homogenous mineralization in the middle. Adopted from reference 60 with permission.
Sprio et al.61 designed a tri-layered scaffold based on collagen and hydroxyapatite with new properties. They mentioned that hybrid magnetic structures could improve the tissue regeneration by magnetic stimulation on ontogenesis and drug delivery with the help of nanoparticles or bioactive molecules.62,63 Their unique structure was made of three-layers to mimic cementum tissue, PDL, and alveolar bone. For the first layer, collagen type I was used in the mineralization process in the presence of Ca2+, Fe2+/Fe3+ and PO43−. After this step, the iron-doped hydroxyapatite (FeHA) with heterogeneous structure was obtained. This structure was similar to the mineral construction of alveolar bone. To achieve cementum like structure, a thin mineralized layer of collagen fibers and FeHA was developed using the electrospinning method. Also, for PDL regeneration, a layer of nano-fibril type I collagen was used. Their method was pH-driving, self-assembling, and super molecular operation and then crosslinking and freeze-drying to achieve the best porosity and mechanical properties. Similar researches are shortly described in Table 2.
Table 2.
Researches on natural polymer-based layered scaffolds for periodontal regeneration.
| Biomaterials | Target periodontal tissue | Bilayered/Trilayered/method of fabrication | Significant results | Ref |
|---|---|---|---|---|
| Non-cross linked collagen type I and III | Soft tissue (gingiva, PDL) Hard tissue (alveolar bone, cementum) |
Bilayered lyophilization |
Combination of collagen I and III: improve the stability, mechanical properties, and cellular properties No chemical crosslinking: improve the cell attachment and proliferation Low porosity, smooth and thin layer with elastic properties improves the suturing of host mucosal margins High porosity layer increases the tissue adherence, improve cell integration, and improve wound healing process. High porosity side can face to bone or soft tissue and improve each side regeneration |
68 |
| Non-cross linked collagen type I and III | Soft tissue (gingiva PDL) Hard tissue (alveolar bone, cementum) |
Bilayered Lyophilization |
One dense layer and high porosity layer to improve cell attachment Use two different positions, one dense layer faces with soft tissue and other one upside-down same Radiological and histomorphometric results in both positions show no orientation preference in bone defects |
69 |
| Collagen and calcium silicate with strontium doped | Hard tissue (Alveolar bone and cementum) | Bilayered 3D printing |
Calcium silicate (CS): increases the bonding between surrounding bone and new scaffolds because of hydroxyapatite formation on the surface of scaffold, promoting the dentin metabolism and increasing secretion of cementum, supporting bone tissue for soft tissue was formed great bone formation of bilayered cell laden structure after 12 weeks Excellent improvement in bone formation in presence of Sr significant improvement in cell laden bilayered scaffold (∼20%) while bone volume fraction in nest bilayered scaffold is 13% and in SrCS is 9%. Higher and trabecular thickness in cell laden bilayered scaffold is comparable to others |
70 |
| Different molecular wight Chitosan with genipin crosslinking | Alveolar bone, gingiva and PDL | trilayered freeze drying |
Different molecular weight chitosan: match degradation rate and mechanical properties with target tissue Controllable degradation rate and great PDL regeneration |
71 |
| Chitosan membranes with Doxycycline hyclate | Soft tissue (gingiva, PDL) Hard tissue (alveolar bone, cementum) |
Bilayered and trilayered | Doxycycline hyclate: decrease the bacteria infection in the periodontal defect site Suitable drug release especially in the first stage and efficient dosage at long term Appropriate mechanical properties were seen |
72 |
| Collagen and chitosan First layer: two solid layers of chitosan. And collagen Second layer: electrospined collagen nanofiber on the chitosan sublayer |
Hard tissue (alveolar bone, cementum) | Bilayered Electrospinning |
Collagen: great biocompatibility, low tissue morbidity, good resorbability, bio-affinity, poor effective shield in bone defect, rapid degradation, early collapse, without any effective blood clot transformation into the bone significant increase in rabbit MSCs activity for 2 weeks More metabolic activity of MSCs cells after 3 days Higher cellular activities on the second layer due to higher surface area of collagen fibers considerable difference for Colα1 and Runx-2 between two layers after three weeks More bone formation and no inflammation responses were seen |
73 |
| Chitosan and gelatin | Soft tissue (gingiva, PDL) | Bilayered Solvent casting and freeze drying And chemical reaction between layers with genipin |
Genipin: increasing the interactions between layer and increasing mechanical properties and stability Gelatin: increasing mechanical properties of chitosan membrane Suitable mechanical properties: Yield stress in the range of 10 kPa and 19 kPa, elastic modules: 26–34 kPa Rapid mineralization |
74 |
5. Chitosan-based layered scaffolds
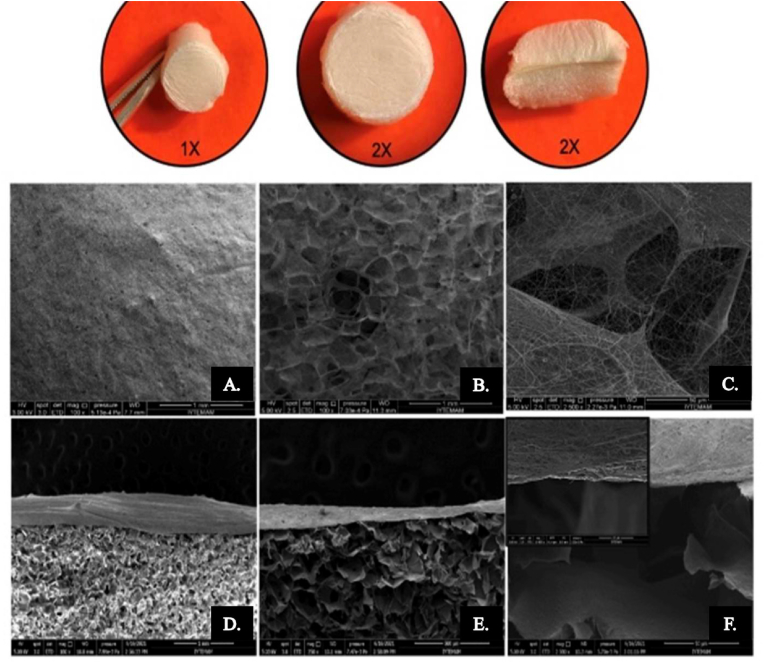
Chitosan, a naturally occurring deacetylated product of chitin, shows essential properties required in biomedical applications including drug delivery, wound healing, gene delivery, and regenerative medicine. Some of its features include biocompatibility, biodegradability, antimicrobial nature, non-toxicity, hemostatic nature, antioxidant property, muco-adhesiveness, and structural similarity with extracellular matrix components, and protein degradability, makes it suitable for periodontal regeneration.64,65 Different novel bio-scaffolds from chitosan can be created via surface alteration and lyophilization. Also, chitosan can easily be processed into different functional forms such as gels, nanofibers, membranes, beads, nanofibrils, nanoparticles, microparticles, scaffolds, and sponge-like structures.66 In the layered scaffold area, Tamburaci et al.67 formed a novel bi-layered scaffold from chitosan and Si-doped nanohydroxyapatite as a nanocomposite structure. This layered scaffold was formed to mimic periodontal tissue and dental defects. The upper layer of the scaffold was compromised by chitosan nanofiber structure to provide the suitable ECM environment for soft tissue and to prevent fibroblast migration to the soft tissue. The lower layer prepared suitable porous structure for bone tissue regeneration inducing bioactivity via Si-doped nanohydroxyapatite modification (Fig. 3). Functional barrier membranes aimed to enhance bone defect areas in periodontal regeneration applications with antibacterial and resorbable properties of chitosan, as well as developing biomineralization with a Si-doped nanohydroxyapatite component. The porous lower layer was fabricated with a lyophilization method and the nanofibrous upper layer was fabricated with the electrospinning method. The porous layer, with a high porosity range of 81–85% was appropriate for cell proliferation. The nanofiber layer with an average fiber diameter of 107 nm showed barrier properties to prevent fibroblast migration to the defect site. Moreover, Si-nHAp incorporation to a porous layer of membrane enhanced compression modulus, protein adsorption, and improved biodegradation rate with increasing concentrations. Antimicrobial tests also indicated that 40% and 50% Si-nHAp incorporated chitosan scaffolds inhibited both gram-negative and positive bacteria. Despite great Biocompatibility, great cell adhesion and differentiation, antibacterial properties of chitosan membrane, mechanical properties, and suitable stability of chitosan scaffolds in body condition, providing the best porosity for cells is still challenging.
Fig. 3.
Stereomicroscopy images of bi-layered membranes (1 × , 2 × ) and SEM images of chitosan/PEO nanofiber coated porous layer surface (A,B,C) with 250 × , 1000 × and 2500 × magnifications; cross-sectional view of bi-layered structure (D,E,F) with 250 × , 500 × and 10,000 × magnifications. Adopted from reference 67 with permission.
6. Synthetic polymer-based layered scaffolds for periodontal regeneration
Different synthetic polymers have been used in biomedical and in periodontal regeneration. Compared to natural polymers, synthetic ones are cost-efficient, present suitable mechanical properties, and show consistent results.75 From a wide range of synthetic polymers, Poly lactic acid (PLA), PCL, PLCL, PGA, PLGA and Poly vinyl alcohol (PVA) are the most used in periodontal research.76 PLA, which is used as a bioabsorbable membrane in some research, demonstrates high hydrophilicity, low cytotoxicity, high alkalinity and due to its predictable results, it is widely used in different periodontal studies.39,77,78 In addition, PCL is another popular synthetic polymer with biocompatibility and biodegradability features. Due to its favorable mechanical properties for dental applications and great bone formation it has been widely used in layered scaffold research [91–93]. Low degradation rates are still proven to be a challenge for both PLA and PCL, thereafter, to solve this problem PLGA is used to enhance degradation rate. PLCL (for Alveolar bone regeneration),79 PGA (for Alveolar bone, PDL and Cementum regeneration),80,81 PLGA (for PDL, Alveolar bone regeneration)39 and PVA (for PDL, Alveolar bone regeneration)82,83 are also useful synthetic polymers for periodontal regeneration. Research demonstrates cellular properties for all synthetic polymers is still a big challenge. These polymers usually show low cell adhesion due to their surface properties. In combination with other polymers, especially natural polymers or the use of nanoparticles and surface modification, have overcome the mentioned problems.
7. PCL and PLCL-based layered scaffolds
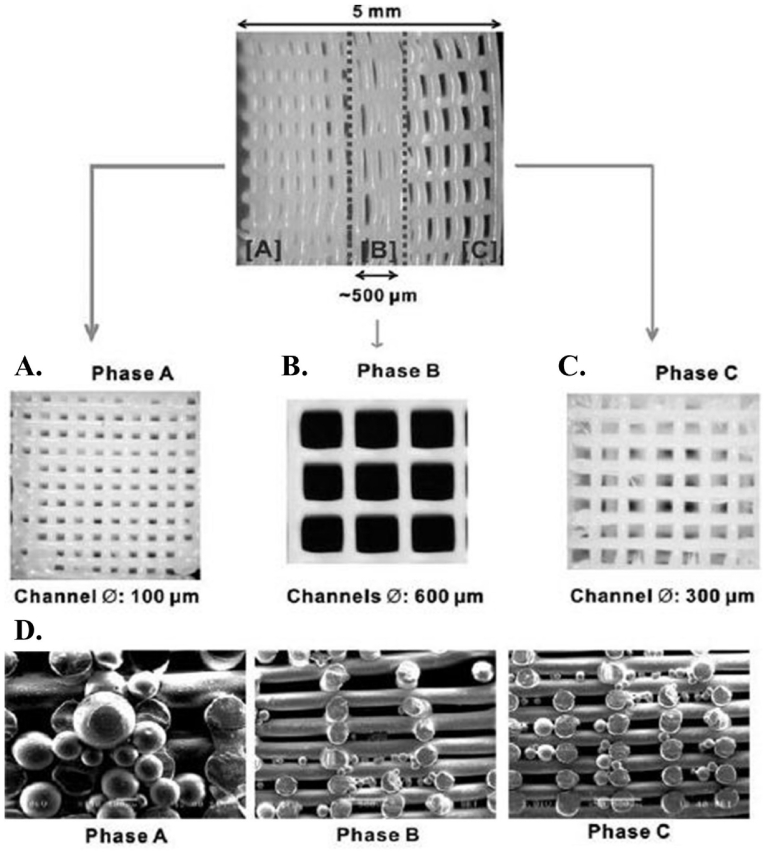
PCL is used as a biomaterial via the membrane in dentistry. The PCL membrane has been reported to be biodegradable and biocompatible with enhanced mechanical properties to stabilize the initial clot that is essential for regeneration. PCL membranes are bio-resorbable and have been shown to effectively induce bone84 and periodontal tissue regeneration.85 Also, they show better adhesion/growth of cells than collagen membranes, which are commonly used for periodontal regeneration.86 In addition, biocompatibility of PCL in both in vitro and in vivo situations were approved by the Food & Drug Administration (FDA). In one of the PCL based researches, Lee et al.86 fabricated the multiphasic scaffold for periodontal complex tissue regeneration by PCL-hydroxyapatite (PCL-HA). Using a 3D printing method, they used layer-by-layer deposition in three phases: 100-mm microchannels in Phase A prepared for cementum/dentin interface, 600-mm microchannels in Phase B prepared for the PDL, and 300-mm microchannels in Phase C prepared for alveolar bone. To create interconnected microchannels, PCL/HA were melted simultaneously at 120 °C and dispensed through a 28-gauge metal needle (Fig. 4). For cell differentiation, PLGA encapsulating recombinant human amelogenin, connective tissue growth factor (CTGF), and bone morphogenic protein 2 (BMP2) were integrated into all three scaffold phases. CTGF was required to promote fibroblastic differentiation, BMP2 enhanced osteogenesis, and amelogenin was used to develop odontogenesis and/or cementogenesis. The results showed scaffolds with spatiotemporal delivery of bioactive materials which formed distinguishable multiphase tissues containing original PDL-like collagen fibers in Phase B. These tissues interfaced between mineralized bone-like tissues of Phases A and C which simulated dentin/cementum following 4-weeks.
Fig. 4.
3D-printed scaffold with distinctive region microstructure and providing delivery system for protein releasing. Adopted from reference 86 with permission.
Aldemir dikici et al.87 synthesized a bi-layered scaffold based on PCL. Their unique structure was based on two different synthesis methods: the electrospinning and emulsion templating methods. First, the PCL layer was fabricated with high internal phase emulsion and treated with air plasma (poly HIPE). For the upper layer, PCL nanofibrous was electrospun on the PCL polyHIPE layer. Mechanical features including stretching and torsion and stability of the scaffold were appreciated for periodontal barriers. Biological properties were evaluated with an in vivo test over 4 weeks. Interconnected porosity, great cell viability, infiltration, and migration on murine long-bone osteocytes and human dermal fibroblast cells were reported. Higher metabolic activity on the electrospun PCL layer was reported from 1 to 28 days. Also, they showed PCL nanofibrous have some limitation on cell infiltration because of small pore size and random distribution. On the other hand, the PCL polyHIPE layer can increase the cellular properties, vessel formation and mineral and calcium deposition for improving bone regeneration. Other important studies based on PCL polymer are summarized in Table 3.
Table 3.
Researches on synthetic polymer-based layered scaffolds for periodontal regeneration.
| Biomaterial | Target periodontal tissue | Method of fabrication | Significant features and results | Ref |
|---|---|---|---|---|
| PCL/(β-TCP) | Alveolar bone/other periodontal component: PDL, cementum | cell-seeded biphasic Fused Deposition Modeling (FDM) for lower layer (bone component + osteoblast culture) Electrospinning for upper layer (PDL cell sheets) |
β-TCP: suitable for bone formation Deposition of thin mineralized cementum-like tissue on the dentin surface by incorporating the multiple PDL cell sheets Better attachment onto the dentin surface compared to no attachment when no cell sheets. New approach by using both multiple PDL cell sheets and a biphasic scaffold for enhancement delivery of the cells. New formation of alveolar bone, PDL and cementum was observed in in-vivo test. |
94 |
| PCL/Sr-doped nano hydroxyapatite (Sr-nHA) | Alveolar bone cementum |
Trilayered 3D printing |
Sr-nHA: suitable for bone tissue due to the resemblance to inorganic phase. Great approach for 3D printing multilayered scaffolds and complex structure with high mechanical properties. Suitable for patient specific scaffolds. |
95 |
| Bottom layer: 20% Sr-nHA (bone component) | ||||
| Upper layer: 10% Sr-nHA (cementum) | ||||
| Starch/PCL (30:70 wt%; SPCL) | Periodontal tissue (specially for alveolar bone) | Bilayered Solvent casting Wet spinning |
SPCL solvent casting membrane: suitable obstacle for migration of gingival epithelium into the periodontal defect. SPCL fiber has enough biological, physical and chemical properties also suitable for periodontal tissue engineering. |
96 |
| 3D fiber mesh functionalized by silanol groups With Starch/PCL membrane (30:70 wt%; SPCL) | Alveolar bone | Bilayered Wet spinning |
Silanol group improve osteogenic properties. Promoting colonization with a distinct cellular population of the periodontium and prevent migration of endothelial cells to defect. |
97 |
| PCL/calcium phosphate (CP) | Bone/PDL | Bilayered Fused deposition modeled for bone. Melt electrospinning for periodontal compartment |
CP: Increase osteoconductivity Great attachment to dentin block and move to the rats for 8 weeks. New method to overcome challenges in layered scaffolds by combining the cell sheet and bilayered scaffolds |
98 |
| PCL/polyurethane (PU)/bioactive glass | Alveolar bone/other periodontal component: PDL, cementum | Bilayered Freeze drying |
Upper layer: PU: no porosity, excluding epithelial growth for bone regeneration Lower layer: PCL and bioactive glass: with suitable porosity for supporting metabolic activates Bioactive glass: increasing stability and mechanical properties of scaffold during healing process Great compatibility in both in vivo and in vitro tests No inflammation after 6 weeks implantation in rats No accumulation of host immune system after6 weeks |
99 |
| PGA/β-TCP | Alveolar bone Cementum PDL |
Trilayered Cell sheet technology |
β-TCP: has suitable biocompatibility, osteoconduction and resorption features promoted bone tissue formation in upper layer by incorporation of transplanted PDL cell sheets No significant harmful side effects No remarkable inflammation during 6 weeks of healing period in in vivo tests |
100 |
| PLGA and CP | Alveolar bone Cementum PDL |
Bilayered Solvent casting and deposition |
Macroprosities by CP in inner layer: improvement in osteoconductive properties and clot retention (because of PLGA). No collapse results in periodontal defect observed. Retained blood clot in buccal side. New bone, cementum, and fine PDL fiber in in vivo analysis was obtained. |
101 |
| PLGA Solid layer/porous layer | Alveolar bone | Bilayered Lyophilization |
Solid layer: inhibit the cell proliferation and subsequent connective tissue invasion Porous layer: improve proliferation and osteogenic differentiation Great in vivo results and facilitating tissue regeneration |
102 |
8. PGA- and PLGA-based layered scaffolds
PGA scaffold materials are optimal with respect to their easy processing and adjustable degradation rates. They have been widely used in business and clinical applications such as degradable sutures, which have been approved by the US FDA.88 In addition, a study has demonstrated that PGA did not cause any significant harmful side effects such as inflammation during a six-weeks healing period in experimental dogs.89 Among the materials studied, PLGA stands out due to its rapid degradation rate, which is very useful when developing a membrane that requires an adequate degradation profile. In addition, its degradation products are non-toxic and are metabolized by natural processes of the organism. Therefore, PLGA has a wide variety of applications in the biomedical area. However, when neat PLGA is used, its degradation products can acidify the medium and cause adverse reactions to the patient. Thus, the incorporation of bioceramics – such as hydroxyapatite and β-tricalcium phosphate to this polymer aims to improve its bioactivity and osteoconductivity.90, 91, 92
Santos et al.92 developed bi-layered membranes of 1- a dense layer of PLGA and Hydroxy apatite particle (Hap) produced to be occlusive and resist fibroblast infiltration via dry phase inversion, and 2-electrospune layer of PLGA/HAp/β-TCP, with three different concentrations of HAp: β-TCP, to increase surface interaction with osteoblasts through the formation of the ECM nanofiber structure (Fig. 5). Such a membrane aims to overcome the drawbacks of the current GBR membrane generation in periodontal formation by achieving a balance between stiffness and elasticity, suitable degradation profile, and adequate bone regenerative capacity. This study confirmed the higher storage modulus of bi-layered membranes compared to neat dry phase inversion and electrospun membranes. It should be noted that in this study, the results demonstrated that a bi-layered membrane with a well-adhered interface was feasible and presented excellent outcomes regarding proper degradation profile, mechanical behavior, and morphological characteristics such as pore structure and size. The incorporation of calcium phosphates in bone regeneration improved osteoblast attachment and migration. In addition, the small pore sizes of dense layer avoided fibroblast infiltration, as well as an electrospun layer with nanofiber structure which mimicked bone ECM, allowing osteoblast adhesion, migration, and nutrient permeation. In addition, the top layer of membranes had the adequate mechanical properties (not too stiff, nor too flexible) to prevent collapse of the membrane in use.
Fig. 5.
Bi-layered membrane for GBR and dry phase Inversion prevents fibroblast growth, and the electrospun layer enhances osteoblastic adhesion and proliferation. Adopted from reference 92 with permission.
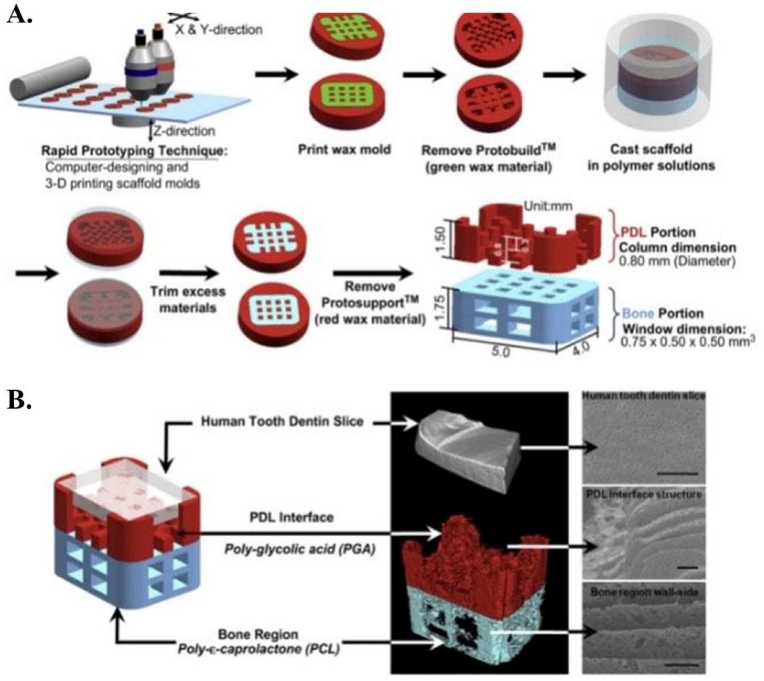
Park et al.93 made the multi-scale composite hybrid polymer scaffold, by using synthetic polymers PGA and PCL. A PCL-acetone was used for casting of the bone region of the hybrid scaffold. PCL-acetone was pasted on the PCL-casted mold and PDL interface architectures were placed on it (Fig. 6). 15% PGA was then used to fuse together the PDL and bone components to form a single hybrid scaffold structure. They demonstrated the consistent generation of newly formed tissues possessing interfacial neogenesis of parallel- and obliquely- oriented ligamentous fibers that sprout and traverse through the polymer designed constructs. This formed tooth cementum-like tissue, ligament, and bone structures. Other related researches about using only one synthetic polymer in scaffold structure are reported in Table 3.
Fig. 6.
Composite hybrid polymer scaffold, by using synthetic polymers PGA and PCL. On the left is the 3-D designed hybrid scaffold, on the right is the micro-CT scan, 3-D reconstructed hybrid scaffold, and a dentin slice. Scale bar: 50 mm. Adopted from reference 93 with permission.
9. Combination of synthetic and natural polymers
Polymeric materials used in biomedical applications should have the appropriate physio-chemical and mechanical properties and be suitable candidates for use in biomedical applications. Synthetic polymers are utilized in different areas of medicine such as dentistry. Many synthetic polymers that are already commercially available present better physicochemical, and mechanical properties than those of the biological tissue that are required to substitute, but are not sufficiently biocompatible. Whereas many biological polymers have good biocompatibility, their mechanical properties are often insufficient. Also, development of the characteristics of synthetic biomaterials could be gained by the addition of biological macromolecules.103 In some studies, researchers combine natural and synthetic polymers to gain both ideal biological and mechanical properties. Herein, we shortly describe some research with combination of natural and synthetic polymers and their quince structure, fabrication method, and significant properties.
To improve periodontal regeneration, Jiang et al.104 synthesized a multilayered scaffold include embedded highly aligned PCL and poly ethylene glycol (PEG) copolymer which were electrospun and mated in chitosan membrane (Fig. 7). PCL demonstrates slow degradation rate and strong hydrophobicity. Copolymerization of PCL and PEG can affect the degradation rate in suitable range and improve the water wettability. Presence of aligned nanofibers increased the biological properties of the scaffold compared to the pure chitosan membrane and the control group. In vitro studies demonstrated the great viability of the cell; infiltration and gene expression related to the periodontal ligament. In vivo tests also confirmed the PDL structure near perpendicular and more organized in multilayered scaffolds compared to sample and chitosan membranes. Additionally, higher gene expression and more supporting tissue formation were observed in multilayered scaffolds.
Fig. 7.
A. Micro-CT image scan of transverse view in rat periodontal defect, B. structure of multilayered scaffold, C. surgical procedure, which is used in periodontal fenestration defect, D. schematic of multilayered structure near the root and bone grafts, E. arrangement of PDL-like fibrous in tissue against root. (Black lines show the orientation of PDL-like structure and yellow line demonstrates the formation of cementum). Adopted from reference 104 with permission.
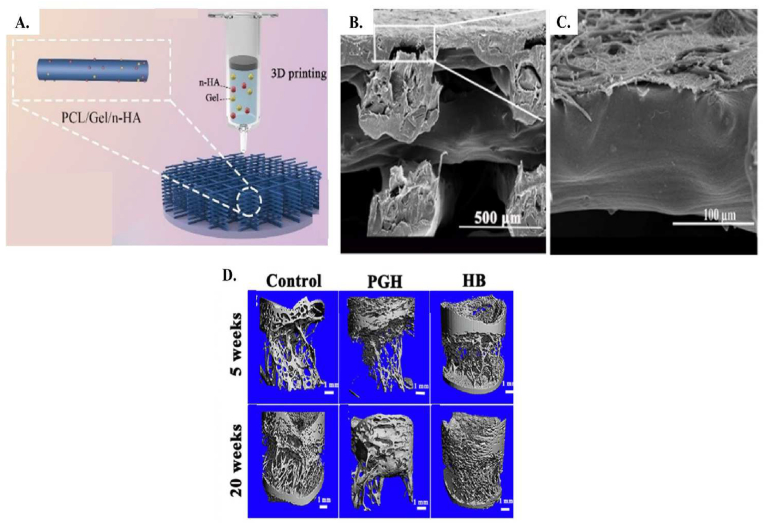
One of the newest studies on layered scaffolds was conducted by Liu et al.105 They fabricated a bi-layered bone membrane based on heparin conjugated PCL/gelation nanofiber with the electrospinning method for the upper layer and PCL/gelatin and nano hydroxyapatite scaffold with 3d printing for the lower layer (Fig. 8A). in this research, the bonding between layers was completed by dissolving PCL/gelatin fibers on PCL/gelatin/nano hydroxyapatite scaffold. SEM images in Fig. 8B, C demonstrates the good connectivity between layers and uniform pores in the lower side. Mechanical properties were estimated by compressive strength test. PCL/gelatin and nano hydroxyapatite had the highest comprehensive strength between samples, which was near 13.8 MPa and was close to cancellous strength. Based on their results, great adhesion and proliferation of L929 cells in the upper layer was reported. In the lower layer attachment and osteogenic differentiation of BMSCs was revealed. The optical density between BMSC cells, which was cultured for different samples (control, PCL, PCL/gelatin (PG) and PCL/gelatin and nano hydroxyapatite (PGH)), demonstrated an insignificant difference. Metabolic activity after 4 days was significantly increased in PG and PGH compared to the PCL samples. The same trend was also obvious after 7 days of culture and cell proliferation increased. Residual solvent present in the structure can affect the metabolic activity and cause faster cell proliferation in anaphase compared to prophase. BMSCs osteogenic gene expression was evaluated by three different factors (RunX2, COL1A1 and BMP-2). COL1A1 and BMP-2 results showed the significant difference between PCL/gelatin with PCL group after 7 and 14 days, but no significant difference was reported in RunX2 at the same time between samples. PGH samples had the highest amount of difference between the 3 gene expression factors, which was related to the enhancement in the osteogenic differentiation because of nano hydroxyapatite particles. In addition, the in vivo results demonstrated significant new bone formation in the bi-layered scaffold implanted group compared to the control one. Micro-TC images (Fig. 8D) confirmed more new bone formation in bi-layered structure compared to PGH and control samples.
Fig. 8.
Schematic structure of the bi-layered scaffold (heparin conjugated PCL/gelation nanofibers and PCL/gelatin/nano-hydroxyapatite). Adopted from reference 105 with permission.
Lian et al.106 designed a bi-layered scaffold based on incorporated copper loaded on silica nanoparticles (with 121 nm average size) on PLGA and gelatin (PG) matrix with great biological and mechanical properties by solution electrospinning writing (SEW) printer. Synthesized Cu loaded silicate nanoparticles were dispersed in PG matrix and used as spinning solution. The electrospinning method produced a dense sheet-like structure. A combination of the electrospinning method and 3D printing to control and improve the porous dependent properties like cellular features is a new method. Solution electrospinning (SES) and SEW methods produced different layers for bone and tissue regeneration. The loose layer has excellent and completely organized porosity and demonstrated a fibrous network with an average diameter of 10.2 ± 0.5 μm. Due to the improved osteoconductive properties, the fiber spacing was selected on 400 μm. Comparing the dense and loose layer highlighted the average fiber diameter size, which was decreased in density pattern (∼96.5 ± 11.8 nm). Comparing integrations between layers showed the loose layer possesses larger integrated porosities. To determine mechanical properties, a tensile test was carried out. Bi-layered scaffold with 3.7 MPa tensile strength, 15.7 MPa Young's modules and ∼46% elongation meets the suitable mechanical features needed in periodontal GTRs. SEW layer had more porosity and can support the bone tissue regeneration. SES layer was dense and compact and resisted the interference of non-osteoblast cells. As cellular tests confirm the in-depth infiltration of BMCSs in loose layer and L929 cells in dense layer. Due to the presence of Cu and Si nanoparticles, antibacterial properties and osteogenic properties were favorable. Osteogenic gene expression (RUNX2, Col1, ALP and OCN) showed the great increase in the level of gene expression in bi-layered scaffolds. Antibacterial properties also showed the 40% and 50% of bacteriostatic rate over E. Coli and S. aureu after 12 h, respectively. In vivo studies on rats' periodontal problems confirmed the excellent periodontal regeneration properties of this scaffold. The significant increase is obvious in defect treatment with bi-layered structure. In addition, new bone volume formation and bone mineralization degree confirmed these increases. More studies about combination of both natural and synthetic polymers in the scaffold structures are described in Table 4.
Table 4.
Different studies based on combining natural and synthetic polymers for layered scaffolds to improve periodontal regeneration.
| Biomaterials | Target periodontal tissue | Bilayered/Trilayered/method of fabrication | Significant characteristic of Biomaterial in the multiphasic scaffold | Significant results | Ref |
|---|---|---|---|---|---|
| Upper layer: PCL/gelatin Lower layer PCL/gelatin/nano-HA Heparin in both layer |
Soft tissue (gingiva, PDL) Hard tissue (alveolar bone, cementum) |
Bilayered electrospinning |
HA: osteoblast proliferation and osteointegration/make filaments thinner, increase surface area/effect on conductivity of solution also decrease tensile forces the filament dimeter Heparin: Improve biological properties/increase the hydrophilicity also prepare suitable cell growth culture |
significant cell proliferation and differentiation and increase cell adhesion | 107 |
| layer of electrospun silk fibroin/PCL-PEG-PCL incorporating nano calcium phosphate (SPCA) layer of PCL membrane |
Alveolar bone | Bilayered Electrospinning Flame Spray Pyrolysis for incorporate phosphate Solvent casting |
Calcium phosphate: osteoconductive/enhance mechanical strength/improve water uptake capacity | After 10 days nucleation and growth of apatite around fibers were apparent | 108 |
| PCL/PLGA | Alveolar bone | Bilayered carbon dioxide solvent free forming |
PCL: lower viscosity and gain highly interconnected pores rather than PLGA | the PCL layer suited for the proliferation of osteoblasts and the PLGA layer inhibited the ingrowth of fibroblasts. | 109 |
| Upper layer: PLGA Lower layer: hydronic acid- acid dihyrazide (HA-ADH) |
Alveolar bone | Bilayered Chemical modification |
Chemical modification: increase the HA stability | In-vivo evaluation in rats showed new bone formation | 110 |
| Inner layer: fish collagen/outer layer: polyvinyl alcohol (Col/PVA) | Hard and soft tissue | Bilayered freezing/thawing for PVA Collagen coat into pre-set PVA without chemical crosslinker |
Fish collagen: stimulate human vascular endothelial cell proliferation, showed higher fibroblast viability than other natural biomaterials PVA: improve mechanical properties, physical barrier, prevent fast adhesion of epithelium |
Col/PVA dual layered was suitable membrane for GTR. The Col/PVA bilayered membrane had an obvious contact boundary line between layers. Layers also have hydrophilic property. This membrane could induce osteogenic effect on BMSC |
111 |
| Gelatin/PCL fiber | PDL Alveolar bone |
Bilayered electrospinning |
Aligned (fiber) PCL: facilitated to form and maturation collagen at periodontal defects than amorphous PCL | This scaffold could provide good attachment and tissue-mimicking microenvironments for “seeding cells”, that is, human periodontal ligament mesenchyme cells (PDLSCs) and may become potential for periodontal regenerative medicine. | 112 |
| magnesium (Mg)and hydroxyapatite (HA) and bromelain/PVA/collagen/sericin | Soft and hard tissue | Bilayered electrospinning |
Mg/HA/bromelain: enhanced the mechanical, Physico-chemical, thermal, and biological features of the scaffold and. Also mimicking the complex structure of extracellular matrix/bromelain has an antibacterial effect |
fabricated scaffold has provided a good support in early healing of damaged periodontium with multiple tissue type by promoting cellular attachment, growth, and migration both in vitro and vivo studies | 82 |
| Upper layer: chitosan, Pluronic F127, and crosslinking agent Hydroxypropyl Methyl Cellulose (HPMC) Middle layer: chitosan/HPMC/Bioactive glass25% (BG) Lower layer: chitosan/BG 50%/HPMC |
Alveolar bone | trilayered lyophilization |
Upper layer: prevented the invasion of cells/not cell adhesion due to the not BG Lower layer: formed the porous structure/form alveolar bone/cell proliferation |
It is concluded that the trilayered membrane with bioactive glass gradient (0–50 wt%)could be applied asGTR/GBR membranes for the treatment of periodontitis. | 113 |
| Chitosan/PCL/gelatin | Periodontal tissue | Multilayered electrospinning |
Gelatin: biological properties PCL: mechanical strength Gelatin/PCL: hydrophilicity and suitable degradation rate Chitosan: improve the hemostasis properties/antibacterial/cell proliferation, differentiation |
multifunctional composite scaffolds showed optimized structure, enhanced regenerative capabilities, accelerating blood clotting and serve as a basis for approaches to improve GTR designs for periodontal regeneration. | 114 |
| Chitosan/PLGA/nano -bio active glass (n BG)/rhCEMP1/rhFGF2/PRP/ | Alveolar bone (chitin + PLGA + n-BG + PRP) Cementum (chitin _ PLGA + n-BG + rhCEMP1 PDL (chitin + PLGA + rhFGF2) |
Trilayered lyophilization |
chitosan: mimic extracellular matrix PLGA: improve mechanical properties/degradation rate Nano bioactive glass: regenerate hard tissue Growth factor: obtain successful result |
trilayered scaffold compromise nanocomposite hydrogel and growth factors can enhance absolute periodontal regeneration based on in vivo and in vitro studies | 115 |
| Core layer: PCL/nano-hydroxyapatite (n HA) Outer layer: PCL/collagen-PCL/collagen/BMP7 |
Alveolar bone | Multilayered Core layer: solvent casting/particulate leaching technique Outer layer: electrospinning |
n HA: increase bioactivity/mechanical integrity of bone tissue Collagen: mimic natural extracellular matrix BMP7: osteoblastic differentiation |
The structure and integrity of this novel multilayered scaffold are maintained without any separation and disruption. Osteogenic differentiation was observed in pre-osteoblastic cells | 116 |
10. Conclusion and future perspective
Periodontium is the main tissue that surrounds and supports tooth structure both in the maxilla and mandible. tooth loss, losing oral cavity function, and aesthetic problems are the results of periodontal tissue disorders.117 Previously researchers used only GTR and GBR methods to enhance regeneration but they face some problems, herein, finding the proper tissue engineering approach by using suitable biomaterials, stem cells, bioactive molecules, and novel scaffold design is very necessary for this area. Although many advances have been reported for this complex structure regeneration, they are still some doubts and challenges to receive the ultimate and ideal regeneration purpose. The first challenge is related to biomaterials, in most of the articles traditional biomaterials include HA, β-TCP, CP, PGA, PLLA, PCL, and PLGA were used. Although these biomaterials could mimic the tissue composition and have been approved in researches, but, the degradation rate for natural materials and biocompatibility for synthetic material is critical and also, for forming functional and native architecture tissue such as various kinds of PDL fiber, different cementum structures such as cellular and acellular cementum, and vascular networks they were not suitable enough, to solve this challenge, recent articles try to modify different natural and synthetic biomaterials with novel nanomaterials. The second challenge is about the formation of sharp and fine fibers including functional and correct positioned PDL, cementum, and alveolar bone is difficult to achieve in this field. The lack of these fibers results in a weak connection between cementum-PDL-alveolar bones and teeth or occlusal function support will not be provided. Therefore, newly designed materials and strategies such as nanomaterials and nanotechnology are needed for creating periodontal tissue architecture. The third challenge is related to the biological nature of periodontal tissue which makes it difficult for scientists to achieve a solid structure with the separated alveolar bone, PDL, and cementum simultaneously. In spite of much progress in the multidrug-delivery system, researchers face a lot of obstacles due to the lack of comprehensive basic biology about periodontium restoration, for example; they cannot completely identify certain factors that affect this complex tissue regeneration; therefore, the bioactive molecules cannot obtain the expected regenerative purpose. Concentrations of bioactive molecules are another gap because overuse or not enough drugs/growth factors could change the results and scientists should try to achieve proper concentrations of these molecules. In addition, the critical disadvantage associated with the local delivery of growth factors is their short biological half-life in vivo as well as high cost.118 Even most importantly, the application of a high dosage of bioactive molecules is needed to enhance tissue regeneration, which could cause uncertain reactions and side effects; therefore, a novel strategy for local releasing of growth factors is the use of gene therapy that upregulates the expression of a related gene such as BMP-2, RunX2, COL1A1 for periodontal regeneration instead of a high number of bioactive molecules. Also, regenerating horizontal alveolar bone such as the natural tissue is another problem related to biological issues. Another challenge is related to the mechanical strength of the scaffold that mimics periodontium. Because periodontium is the tooth-supporting tissue, the mechanical performance of regenerated methods is critical. In periodontal tissue regeneration, mechanical cues were rarely considered. Therefore, cooperation of mechanical cues to biomaterial scaffold and assessment of the regenerative results will affect the future science of periodontal regeneration. The final challenge considers the fabrication scaffolds methods, the fabrication method is also effective on the different properties of layered scaffolds. Traditional methods like solvent casting, freeze-drying, electrospinning, and gas-forming have been used in most studies. Suitable cost, high porosity, and interconnected pores are advantageous of these methods. But the complex structure of periodontal tissue and also need for personalized scaffolds are shown the requirement for new fabrication methods. Printing fabrication methods are new great processes for various biomedical applications and especially for layered scaffolds and can provide great adhesion between layers, and produce complex and personalized structures. The main challenge in this new method field is producing the appropriate ink with suitable printing properties and in the next step suitable mechanical and cellular features for periodontal regeneration.
Regarding the challenges mentioned above, scientists fabricated multi-layered scaffolds that may include specific layers for regeneration of PDL, cementum, and alveolar bone. However, with the best of our knowledge, no report presented a scaffold for simultaneous regeneration of all four sections of periodontium (gingiva, PDL, cementum, and alveolar bone). Despite many in-vitro and in-vivo studies on multi-layered scaffolds, no human clinical trials have been yet reported in this field, which is expected to be conducted in near future.
In summary, although progress has been made in the field of periodontal regeneration, it remains a major challenge in tissue engineering. Incorporation of relevant biomaterials, stem cells, and growth factor in layered configuration shows promising outcome as an effective approach to facilitate the regeneration of the multi-tissue construct of periodontium.
Declaration of competing interest
The authors have no conflicts of interest relevant to the publication of this article.
Acknowledgment
Part of the research reported in this paper was supported by National Institute of Dental and Craniofacial Research of the National Institutes of Health under award numbers R15DE027533, 1 R56 DE029191-01A1 and 3R15DE027533-01A1W1.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Liang Y., Luan X., Liu X. Recent advances in periodontal regeneration: a biomaterial perspective. Bioact Mater. 2020;5:297–308. doi: 10.1016/j.bioactmat.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sculean A., Chapple I.L., Giannobile W.V. Wound models for periodontal and bone regeneration: the role of biologic research. Periodontol. 2015;68:7–20. doi: 10.1111/prd.12091. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo H.N., Cho Y.J., Tarafder S., Lee C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact Mater. 2021;6:3328–3342. doi: 10.1016/j.bioactmat.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao R.T., Nares S., Reynolds M.A. Periodontal regeneration–intrabony defects: a systematic review from the AAP regeneration workshop. J Periodontol. 2015;86:S77–S104. doi: 10.1902/jop.2015.130685. [DOI] [PubMed] [Google Scholar]
- 6.Lin Z., Rios H.F., Cochran D.L. Emerging regenerative approaches for periodontal reconstruction: a systematic review from the AAP Regeneration Workshop. J Periodontol. 2015;86:S134–S152. doi: 10.1902/jop.2015.130689. [DOI] [PubMed] [Google Scholar]
- 7.Kantarci A. Biological basis of periodontal regeneration. Dent Clin. 2022;66:1–9. doi: 10.1016/j.cden.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Abdul Rahman N., Nickles K., Gallenbach K., et al. Five‐year stability of clinical attachment after regenerative treatment of infrabony defects compared to controls. J Clin Pedriodontol. 2019;46:650–658. doi: 10.1111/jcpe.13105. [DOI] [PubMed] [Google Scholar]
- 9.Seciu A.-M., Craciunescu O., Stanciuc A.-M., Zarnescu O. Tailored biomaterials for therapeutic strategies applied in periodontal tissue engineering. Stem Cell Dev. 2019;28:963–973. doi: 10.1089/scd.2019.0016. [DOI] [PubMed] [Google Scholar]
- 10.Ramseier C.A., Rasperini G., Batia S., Giannobile W.V. Advanced reconstructive technologies for periodontal tissue repair. Periodontol. 2012;59:185–202. doi: 10.1111/j.1600-0757.2011.00432.x. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman M.G., Takei H., Klokkevold P.R., Carranza F.A. Elsevier Health Sciences; 2018. Newman and Carranza's Clinical Periodontology E-Book. [Google Scholar]
- 12.Yousefi A.M., Hoque M.E., Prasad R.G., Uth N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J Biomed Mater Res. 2015;103:2460–2481. doi: 10.1002/jbm.a.35356. [DOI] [PubMed] [Google Scholar]
- 13.Iviglia G., Kargozar S., Baino F. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J Funct Biomater. 2019;10:3. doi: 10.3390/jfb10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaquette C., Cooper-White J. A simple method for fabricating 3-D multilayered composite scaffolds. Acta Biomater. 2013;9:4599–4608. doi: 10.1016/j.actbio.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Asa’ad F., Pagni G., Pilipchuk S.P., Giannì A.B., Giannobile W.V., Rasperini G. 3D-printed scaffolds and biomaterials: review of alveolar bone augmentation and periodontal regeneration applications. Int J Dent. 2016;2016:1–15. doi: 10.1155/2016/1239842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanovski S., Vaquette C., Gronthos S., Hutmacher D., Bartold P. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res. 2014;93:1212–1221. doi: 10.1177/0022034514544301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bittner S.M., Guo J.L., Melchiorri A., Mikos A.G. Three-dimensional printing of multilayered tissue engineering scaffolds. Mater Today Commun. 2018;21:861–874. doi: 10.1016/j.mattod.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohd N., Razali M., Ghazali M.J., Abu Kasim N.H. 3D-Printed hydroxyapatite and tricalcium phosphates-based scaffolds for alveolar bone regeneration in animal models: a scoping review. Materials. 2022;15:2621. doi: 10.3390/ma15072621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siaili M., Chatzopoulou D., Gillam D. An overview of periodontal regenerative procedures for the general dental practitioner. Saudi Dent J. 2018;30:26–37. doi: 10.1016/j.sdentj.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omar O., Elgali I., Dahlin C., Thomsen P. Barrier membranes: more than the barrier effect? J Clin Pedriodontol. 2019;46:103–123. doi: 10.1111/jcpe.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badran Z., Abdallah M.-N., Torres J., Tamimi F. Platelet concentrates for bone regeneration: current evidence and future challenges. Platelets. 2018;29:105–112. doi: 10.1080/09537104.2017.1327656. [DOI] [PubMed] [Google Scholar]
- 22.Ducic I., Yoon J. Reconstructive options for inferior alveolar and lingual nerve injuries after dental and oral surgery: an evidence-based review. Ann Plast Surg. 2019;82:653–660. doi: 10.1097/SAP.0000000000001783. [DOI] [PubMed] [Google Scholar]
- 23.Goyal S, Masood M, Le C, Rajendran Y, Nanjapa S, Vaderhobli R. Comparative evaluation of different types of bone grafts for dental implants success: an evidence-based review. J J Long Term Eff Med Implants. 2021;31(3):33–44. doi: 10.1615/JLongTermEffMedImplants.2021038292. [DOI] [PubMed] [Google Scholar]
- 24.Özcan M., Hotza D., Fredel M.C., Cruz A., Volpato C.A.M. Materials and manufacturing techniques for polymeric and ceramic scaffolds used in implant dentistry. J Compos Sci. 2021;5:78. [Google Scholar]
- 25.Huang J., Best S.M. Ceramic biomaterials for tissue engineering. Tissue engineering using ceramics and polymers. 2022:3–40. [Google Scholar]
- 26.Ma H., Feng C., Chang J., Wu C. 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomater. 2018;79:37–59. doi: 10.1016/j.actbio.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Millan C., Vivanco J.F., Benjumeda-Wijnhoven I.M., Bjelica S., Santibanez J.F. Mesenchymal stem cells and calcium phosphate bioceramics: implications in periodontal bone regeneration. Cells Bio Transl Med. 2018:91–112. doi: 10.1007/5584_2018_249. [DOI] [PubMed] [Google Scholar]
- 28.Moussa D.G., Aparicio C. Present and future of tissue engineering scaffolds for dentin‐pulp complex regeneration. J Tissue Eng Regen Med. 2019;13:58–75. doi: 10.1002/term.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samavedi S., Whittington A.R., Goldstein A.S. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater. 2013;9:8037–8045. doi: 10.1016/j.actbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y., Wu C., Xiao Y. Silicate-based bioceramics for periodontal regeneration. J Mater Chem B. 2014;2:3907–3910. doi: 10.1039/c4tb00377b. [DOI] [PubMed] [Google Scholar]
- 31.Bohner M., Santoni B.L.G., Döbelin N. β-tricalcium phosphate for bone substitution: synthesis and properties. Acta Biomater. 2020;113:23–41. doi: 10.1016/j.actbio.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Liu C.C., Solderer A., Heumann C., Attin T., Schmidlin P.R. Tricalcium phosphate (-containing) biomaterials in the treatment of periodontal infra-bony defects: a systematic review and meta-analysis. J Dent. 2021;114 doi: 10.1016/j.jdent.2021.103812. [DOI] [PubMed] [Google Scholar]
- 33.Jeong J., Kim J.H., Shim J.H., Hwang N.S., Heo C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23:1–11. doi: 10.1186/s40824-018-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahbazi M.-A., Faghfouri L., Ferreira M.P., et al. The versatile biomedical applications of bismuth-based nanoparticles and composites: therapeutic, diagnostic, biosensing, and regenerative properties. Chem Soc Rev. 2020;49:1253–1321. doi: 10.1039/c9cs00283a. [DOI] [PubMed] [Google Scholar]
- 35.Skallevold H.E., Rokaya D., Khurshid Z., Zafar M.S. Bioactive glass applications in dentistry. Int J Mol Sci. 2019;20:5960. doi: 10.3390/ijms20235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribas R.G., Schatkoski V.M., do Amaral Montanheiro T.L., et al. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: a review. Ceram. 2019;45:21051–21061. [Google Scholar]
- 37.Sepantafar M., Mohammadi H., Maheronnaghsh R., Tayebi L., Baharvand H. Single phased silicate-containing calcium phosphate bioceramics: promising biomaterials for periodontal repair. Ceram. 2018;44:11003–11012. [Google Scholar]
- 38.Sharifianjazi F., Khaksar S., Esmaeilkhanian A., et al. Advancements in fabrication and application of chitosan composites in implants and dentistry: a review. Biomolecules. 2022;12:155. doi: 10.3390/biom12020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shue L., Yufeng Z., Mony U. Biomaterials for periodontal regeneration: a review of ceramics and polymers. Biomatter. 2012;2:271–277. doi: 10.4161/biom.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada S., Shanbhag S., Mustafa K. Scaffolds in periodontal regenerative treatment. Dent Clin. 2022;66:111–130. doi: 10.1016/j.cden.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Das E.C., Komath M., Kumar P.A. Elsevier; 2022. Dental Tissue Engineering; pp. 493–529. Tissue Engineering. [Google Scholar]
- 42.Dong C., Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers. 2016;8:42. doi: 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X., Black L., Santacana‐Laffitte G., Patrick C.W., Jr. Preparation and assessment of glutaraldehyde‐crosslinked collagen–chitosan hydrogels for adipose tissue engineering. J Biomed Mater Res. 2007;81:59–65. doi: 10.1002/jbm.a.31003. [DOI] [PubMed] [Google Scholar]
- 44.Morris G.A., Kök S.M., Harding S.E., Adams G.G. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol Genet Eng Rev. 2010;27:257–284. doi: 10.1080/02648725.2010.10648153. [DOI] [PubMed] [Google Scholar]
- 45.Munarin F., Guerreiro S., Grellier M., et al. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules. 2011;12:568–577. doi: 10.1021/bm101110x. [DOI] [PubMed] [Google Scholar]
- 46.Martins A.F., Vlcek J., Wigmosta T., et al. Chitosan/iota-carrageenan and chitosan/pectin polyelectrolyte multilayer scaffolds with antiadhesive and bactericidal properties. Appl Surf Sci. 2020;502 [Google Scholar]
- 47.Prakash J., Kumar T.S., Venkataprasanna K., et al. PVA/alginate/hydroxyapatite films for controlled release of amoxicillin for the treatment of periodontal defects. Appl Surf Sci. 2019;495 [Google Scholar]
- 48.Elango J., Selvaganapathy P.R., Lazzari G., Bao B., Wenhui W. Biomimetic collagen-sodium alginate-titanium oxide (TiO2) 3D matrix supports differentiated periodontal ligament fibroblasts growth for periodontal tissue regeneration. Int J Biol Macromol. 2020;163:9–18. doi: 10.1016/j.ijbiomac.2020.06.173. [DOI] [PubMed] [Google Scholar]
- 49.Yang S., Zhu B., Yin P., et al. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater Sci Eng. 2020;6:1590–1602. doi: 10.1021/acsbiomaterials.9b01363. [DOI] [PubMed] [Google Scholar]
- 50.Madhumathi K., Rekha L.J., Kumar T.S. Tailoring antibiotic release for the treatment of periodontal infrabony defects using bioactive gelatin-alginate/apatite nanocomposite films. J Drug Deliv Sci Technol. 2018;43:57–64. [Google Scholar]
- 51.Shirakata Y., Imafuji T., Nakamura T., et al. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: a preclinical study in dogs. Quintessence Int. 2021;52:2–10. doi: 10.3290/j.qi.b937003. [DOI] [PubMed] [Google Scholar]
- 52.Pilloni A., Rojas M.A., Marini L., et al. Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either hyaluronic acid or an enamel matrix derivative: a 24-month randomized controlled clinical trial. Clin Oral Invest. 2021;25:5095–5107. doi: 10.1007/s00784-021-03822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bansal J., Kedige S.D., Anand S. Hyaluronic acid: a promising mediator for periodontal regeneration. Indian J Dent Res. 2010;21:575. doi: 10.4103/0970-9290.74232. [DOI] [PubMed] [Google Scholar]
- 54.Hurtado A., Aljabali A.A., Mishra V., Tambuwala M.M., Serrano-Aroca Á. Alginate: enhancement strategies for advanced applications. Int J Mol Sci. 2022;23:4486. doi: 10.3390/ijms23094486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong D.G., Jude E.B. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc. 2002;92:12–18. doi: 10.7547/87507315-92-1-12. [DOI] [PubMed] [Google Scholar]
- 56.Yu X., Zhang H., Miao Y., Xiong S., Hu Y. Recent strategies of collagen-based biomaterials for cartilage repair: from structure cognition to function endowment. J Leather Sci Eng. 2022;4:1–23. [Google Scholar]
- 57.Rothamel D., Schwarz F., Sculean A., Herten M., Scherbaum W., Becker J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast‐like cells. Clin Oral Implants Res. 2004;15:443–449. doi: 10.1111/j.1600-0501.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 58.Rothamel D., Schwarz F., Sager M., Herten M., Sculean A., Becker J. Biodegradation of differently cross‐linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res. 2005;16:369–378. doi: 10.1111/j.1600-0501.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 59.Schwarz F., Rothamel D., Herten M., Sager M., Becker J. Angiogenesis pattern of native and cross‐linked collagen membranes: an immunohistochemical study in the rat. Clin Oral Implants Res. 2006;17:403–409. doi: 10.1111/j.1600-0501.2005.01225.x. [DOI] [PubMed] [Google Scholar]
- 60.Lausch A.J., Chong L.C., Uludag H., Sone E.D. Multiphasic collagen scaffolds for engineered tissue interfaces. Adv Funct Mater. 2018;28 [Google Scholar]
- 61.Sprio S., Campodoni E., Sandri M., et al. A graded multifunctional hybrid scaffold with superparamagnetic ability for periodontal regeneration. Int J Mol Sci. 2018;19:3604. doi: 10.3390/ijms19113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tampieri A., Iafisco M., Sandri M., et al. Magnetic bioinspired hybrid nanostructured collagen–hydroxyapatite scaffolds supporting cell proliferation and tuning regenerative process. ACS Appl Mater Interfaces. 2014;6:15697–15707. doi: 10.1021/am5050967. [DOI] [PubMed] [Google Scholar]
- 63.Panseri S., Russo A., Giavaresi G., et al. Innovative magnetic scaffolds for orthopedic tissue engineering. J Biomed Mater Res. 2012;100:2278–2286. doi: 10.1002/jbm.a.34167. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed S., Ali A., Sheikh J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int J Biol Macromol. 2018;116:849–862. doi: 10.1016/j.ijbiomac.2018.04.176. [DOI] [PubMed] [Google Scholar]
- 65.Hamedi H., Moradi S., Hudson S.M., Tonelli A.E., King M.W. Chitosan based bioadhesives for biomedical applications: a review. Carbohydr Polym. 2022 doi: 10.1016/j.carbpol.2022.119100. [DOI] [PubMed] [Google Scholar]
- 66.Baranwal A., Kumar A., Priyadharshini A., et al. Chitosan: an undisputed bio-fabrication material for tissue engineering and bio-sensing applications. Int J Biol Macromol. 2018;110:110–123. doi: 10.1016/j.ijbiomac.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Tamburaci S., Tihminlioglu F. Development of Si doped nano hydroxyapatite reinforced bilayer chitosan nanocomposite barrier membranes for guided bone regeneration. Mater Sci Eng C. 2021;128 doi: 10.1016/j.msec.2021.112298. [DOI] [PubMed] [Google Scholar]
- 68.Ghanaati S., Schlee M., Webber M.J., et al. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed Mater. 2011;6 doi: 10.1088/1748-6041/6/1/015010. [DOI] [PubMed] [Google Scholar]
- 69.Feher B., Apaza Alccayhuaman K.A., Strauss F.J., et al. Osteoconductive properties of upside-down bilayer collagen membranes in rat calvarial defects. Int J Implant Dent. 2021;7:1–10. doi: 10.1186/s40729-021-00333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang C.-Y., Chiu Y.-C., Lee A.K.-X., Lin Y.-A., Lin P.-Y., Shie M.-Y. Biofabrication of gingival fibroblast cell-laden collagen/strontium-doped calcium silicate 3D-printed bi-layered scaffold for osteoporotic periodontal regeneration. Biomedicines. 2021;9:431. doi: 10.3390/biomedicines9040431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varoni E., Vijayakumar S., Canciani E., et al. Chitosan-based trilayer scaffold for multitissue periodontal regeneration. J Dent Res. 2018;97:303–311. doi: 10.1177/0022034517736255. [DOI] [PubMed] [Google Scholar]
- 72.Ahila I. Edayathangudy GS Pillay College of Pharmacy; Nagapattinam: 2014. Designand Development of Novel Multilayer Chitosaninserts Containing Doxycycline Hyclate. [Google Scholar]
- 73.Lotfi G., Shokrgozar M.A., Mofid R., et al. Biological evaluation (in vitro and in vivo) of bilayered collagenous coated (nano electrospun and solid wall) chitosan membrane for periodontal guided bone regeneration. Ann Biomed Eng. 2016;44:2132–2144. doi: 10.1007/s10439-015-1516-z. [DOI] [PubMed] [Google Scholar]
- 74.Gorgieva S., Vuherer T., Kokol V. Autofluorescence-aided assessment of integration and μ-structuring in chitosan/gelatin bilayer membranes with rapidly mineralized interface in relevance to guided tissue regeneration. Mater Sci Eng C. 2018;93:226–241. doi: 10.1016/j.msec.2018.07.077. [DOI] [PubMed] [Google Scholar]
- 75.Kannan R., Wei G., Ma P.X. Synthetic polymeric biomaterials for tissue engineering. Tissue Engineering Using Ceramics and Polymers. 2022:41–74. Elsevier. [Google Scholar]
- 76.Alauddin M.S., Abdul Hayei N.A., Sabarudin M.A., Mat Baharin N.H. Barrier membrane in regenerative therapy: a narrative review. Membranes. 2022;12:444. doi: 10.3390/membranes12050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye Z., Xu W., Shen R., Yan Y. Emulsion electrospun PLA/calcium alginate nanofibers for periodontal tissue engineering. J Biomater Appl. 2020;34:763–777. doi: 10.1177/0885328219873561. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H.Y., Jiang H.B., Ryu J.-H., Kang H., Kim K.-M., Kwon J.-S. Comparing properties of variable pore-sized 3D-printed PLA membrane with conventional PLA membrane for guided bone/tissue regeneration. Materials. 2019;12:1718. doi: 10.3390/ma12101718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abe G.L., Sasaki J.-I., Katata C., et al. Fabrication of novel poly (lactic acid/caprolactone) bilayer membrane for GBR application. Dent Mater J. 2020;36:626–634. doi: 10.1016/j.dental.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 80.d'Avanzo N., Bruno M.C., Giudice A., et al. Influence of materials properties on bio-physical features and effectiveness of 3D-scaffolds for periodontal regeneration. Molecules. 2021;26:1643. doi: 10.3390/molecules26061643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y., Xia H., Zhang B., Zhao Y., Wang Y. Assessment of polyglycolic acid scaffolds for periodontal ligament regeneration. Biotechnol Biotechnol Equip. 2018;32:701–706. [Google Scholar]
- 82.Shoba E., Lakra R., Kiran M.S., Korrapati P.S. 3 D nano bilayered spatially and functionally graded scaffold impregnated bromelain conjugated magnesium doped hydroxyapatite nanoparticle for periodontal regeneration. J Mech Behav Biomed Mater. 2020;109 doi: 10.1016/j.jmbbm.2020.103822. [DOI] [PubMed] [Google Scholar]
- 83.Chen X., Fan H., Deng X., et al. Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. J Nanomater. 2018;8:960. doi: 10.3390/nano8110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferrand A., Eap S., Richert L., et al. Osteogenetic properties of electrospun nanofibrous PCL scaffolds equipped with chitosan‐B ased nanoreservoirs of growth factors. Macromol Biosci. 2014;14:45–55. doi: 10.1002/mabi.201300283. [DOI] [PubMed] [Google Scholar]
- 85.Bashutski J.D., Wang H.-L. Periodontal and endodontic regeneration. J Endod. 2009;35:321–328. doi: 10.1016/j.joen.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 86.Lee C.H., Hajibandeh J., Suzuki T., Fan A., Shang P., Mao J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. 2014;20:1342–1351. doi: 10.1089/ten.tea.2013.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aldemir Dikici B., Dikici S., Reilly G.C., MacNeil S., Claeyssens F. A novel bilayer polycaprolactone membrane for guided bone regeneration: combining electrospinning and emulsion templating. Materials. 2019;12:2643. doi: 10.3390/ma12162643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alinasab B., Haraldsson P.-O. Rapid resorbable sutures are a favourable alternative to non-resorbable sutures in closing transcolumellar incision in rhinoplasty. Aesthetic Plast Surg. 2016;40:449–452. doi: 10.1007/s00266-016-0649-2. [DOI] [PubMed] [Google Scholar]
- 89.Iwata T., Yamato M., Tsuchioka H., et al. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009;30:2716–2723. doi: 10.1016/j.biomaterials.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 90.Farokhi M., Mottaghitalab F., Ai J., Shokrgozar M.A. Sustained release of platelet-derived growth factor and vascular endothelial growth factor from silk/calcium phosphate/PLGA based nanocomposite scaffold. Int J Pharm. 2013;454:216–225. doi: 10.1016/j.ijpharm.2013.06.080. [DOI] [PubMed] [Google Scholar]
- 91.Gentile P., Chiono V., Tonda‐Turo C., Ferreira A.M., Ciardelli G. Polymeric membranes for guided bone regeneration. Biotechnol J. 2011;6:1187–1197. doi: 10.1002/biot.201100294. [DOI] [PubMed] [Google Scholar]
- 92.Dos Santos V.I., Merlini C., Aragones Á., Cesca K., Fredel M.C. In vitro evaluation of bilayer membranes of PLGA/hydroxyapatite/β-tricalcium phosphate for guided bone regeneration. Mater Sci Eng C. 2020;112 doi: 10.1016/j.msec.2020.110849. [DOI] [PubMed] [Google Scholar]
- 93.Park C.H., Rios H.F., Jin Q., et al. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31:5945–5952. doi: 10.1016/j.biomaterials.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vaquette C., Fan W., Xiao Y., Hamlet S., Hutmacher D.W., Ivanovski S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials. 2012;33:5560–5573. doi: 10.1016/j.biomaterials.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 95.Porta M., Tonda-Turo C., Pierantozzi D., Ciardelli G., Mancuso E. Towards 3D multi-layer scaffolds for periodontal tissue engineering applications: addressing manufacturing and architectural challenges. Polymers. 2020;12:2233. doi: 10.3390/polym12102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Requicha J.F., Viegas C.A., Hede S., Leonor I.B., Reis R.L., Gomes M.E. Design and characterization of a biodegradable double‐layer scaffold aimed at periodontal tissue‐engineering applications. J Tissue Eng Regen Med. 2016;10:392–403. doi: 10.1002/term.1816. [DOI] [PubMed] [Google Scholar]
- 97.Requicha J.F., Viegas C.A., Muñoz F., et al. A tissue engineering approach for periodontal regeneration based on a biodegradable double-layer scaffold and adipose-derived stem cells. Tissue Eng. 2014;20:2483–2492. doi: 10.1089/ten.tea.2013.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Costa P.F., Vaquette C., Zhang Q., Reis R.L., Ivanovski S., Hutmacher D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J Clin Pedriodontol. 2014;41:283–294. doi: 10.1111/jcpe.12214. [DOI] [PubMed] [Google Scholar]
- 99.Zahid S., Khan A.S., Chaudhry A.A., et al. Fabrication, in vitro and in vivo studies of bilayer composite membrane for periodontal guided tissue regeneration. J Biomater Appl. 2019;33:967–978. doi: 10.1177/0885328218814986. [DOI] [PubMed] [Google Scholar]
- 100.Flores M.G., Yashiro R., Washio K., Yamato M., Okano T., Ishikawa I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J Clin Pedriodontol. 2008;35:1066–1072. doi: 10.1111/j.1600-051X.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 101.Reis E.C.C., Borges A.P., Araújo M.V., Mendes V.C., Guan L., Davies J.E. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials. 2011;32:9244–9253. doi: 10.1016/j.biomaterials.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 102.Yoshimoto I., Sasaki J.-I., Tsuboi R., Yamaguchi S., Kitagawa H., Imazato S. Development of layered PLGA membranes for periodontal tissue regeneration. Dent Mater J. 2018;34:538–550. doi: 10.1016/j.dental.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 103.Giusti P., Lazzeri L., Lelli L. Bioartificial polymeric materials: a new method to design biomaterials by using both biological and synthetic polymers. Trends Polym Sci. 1993;1:261–267. [Google Scholar]
- 104.Jiang W., Li L., Zhang D., et al. Incorporation of aligned PCL–PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015;25:240–252. doi: 10.1016/j.actbio.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 105.Liu J., Zou Q., Wang C., et al. Electrospinning and 3D printed hybrid bi-layer scaffold for guided bone regeneration. Mater Des. 2021;210 [Google Scholar]
- 106.Lian M., Han Y., Sun B., et al. A multifunctional electrowritten bi-layered scaffold for guided bone regeneration. Acta Biomater. 2020;118:83–99. doi: 10.1016/j.actbio.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 107.Liu J., Zou Q., Cai B., Wei J., Yuan C., Li Y. Heparin conjugated PCL/Gel–PCL/Gel/n-HA bilayer fibrous membrane for potential regeneration of soft and hard tissues. J Biomater Sci Polym Ed. 2020;31:1421–1436. doi: 10.1080/09205063.2020.1760700. [DOI] [PubMed] [Google Scholar]
- 108.Türkkan S., Pazarçeviren A.E., Keskin D., Machin N.E., Duygulu Ö., Tezcaner A. Nanosized CaP-silk fibroin-PCL-PEG-PCL/PCL based bilayer membranes for guided bone regeneration. Mater Sci Eng C. 2017;80:484–493. doi: 10.1016/j.msec.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 109.Song C., Li S., Zhang J., et al. Controllable fabrication of porous PLGA/PCL bilayer membrane for GTR using supercritical carbon dioxide foaming. Appl Surf Sci. 2019;472:82–92. [Google Scholar]
- 110.Park J.K., Yeom J., Oh E.J., et al. Guided bone regeneration by poly (lactic-co-glycolic acid) grafted hyaluronic acid bi-layer films for periodontal barrier applications. Acta Biomater. 2009;5:3394–3403. doi: 10.1016/j.actbio.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 111.Zhou T., Chen S., Ding X., Hu Z., Cen L., Zhang X. Fabrication and characterization of collagen/PVA dual-layer membranes for periodontal bone regeneration. Front Bioeng Biotechnol. 2021;9:437. doi: 10.3389/fbioe.2021.630977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang M., Gao X., Shen Z., Shi X., Lin Z. Gelatin-assisted conglutination of aligned polycaprolactone nanofilms into a multilayered fibre-guiding scaffold for periodontal ligament regeneration. RCS Adv. 2019;9:507–518. doi: 10.1039/c8ra09073d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shah A.T., Zahid S., Ikram F., et al. Tri-layered functionally graded membrane for potential application in periodontal regeneration. Mater Sci Eng C. 2019;103 doi: 10.1016/j.msec.2019.109812. [DOI] [PubMed] [Google Scholar]
- 114.Zhang L., Dong Y., Zhang N., et al. Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane. Mater Sci Eng C. 2020;109 doi: 10.1016/j.msec.2019.110618. [DOI] [PubMed] [Google Scholar]
- 115.Sowmya S., Mony U., Jayachandran P., et al. Tri‐layered nanocomposite hydrogel scaffold for the concurrent regeneration of cementum, periodontal ligament, and alveolar bone. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201601251. [DOI] [PubMed] [Google Scholar]
- 116.Gürbüz S., Demirtaş T.T., Yüksel E., Karakeçili A., Doğan A., Gümüşderelioğlu M. Multi-layered functional membranes for periodontal regeneration: preparation and characterization. Mater Lett. 2016;178:256–259. [Google Scholar]
- 117.Nanci A., Bosshardt D.D. Structure of periodontal tissues in health and disease. Periodontol. 2006;40:11–28. doi: 10.1111/j.1600-0757.2005.00141.x. 2000. [DOI] [PubMed] [Google Scholar]
- 118.Taba M., Jr., Jin Q., Sugai J., Giannobile W. Current concepts in periodontal bioengineering. Orthod Craniofac Res. 2005;8:292–302. doi: 10.1111/j.1601-6343.2005.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]