Abstract
Background:
While identifying risk factors for adolescent depression is critical for early prevention and intervention, most studies have sought to understand the role of isolated factors rather than across a broad set of factors. Here, we sought to examine multi-level factors that maximize the prediction of depression symptoms in U.S. children participating in the Adolescent Brain and Cognitive Development (ABCD) study.
Methods:
7,995 participants from ABCD (version 3.0 release) provided complete data at baseline and one-year follow-up data. Depression symptoms were measured with the Child Behavior Checklist. Predictive features included child demographic, environmental, and structural and resting-state fMRI variables, parental depression history and demographic characteristics. We used linear (elastic net regression, EN) and non-linear (gradient boosted trees, GBT) predictive models to identify which set of features maximized prediction of depression symptoms at baseline and, separately, at one-year follow-up.
Results:
Both linear and non-linear models achieved comparable results for predicting baseline (EN: MAE=3.757; R2=0.156; GBT: MAE=3.761; R2=0.147) and one-year follow-up (EN: MAE=4.255; R2=0.103; GBT: MAE=4.262; R2=0.089) depression. Parental history of depression, greater family conflict, and shorter child sleep duration were among the top predictors of concurrent and future child depression symptoms across both models. Although resting-state fMRI features were relatively weaker predictors, functional connectivity of the caudate was consistently the strongest neural feature associated with depression symptoms at both timepoints.
Conclusions:
Consistent with prior research, parental mental health, family environment, and child sleep quality are important risk factors for youth depression. Functional connectivity of the caudate is a relatively weaker predictor of depression symptoms but may represent a biomarker for depression risk.
Introduction
Depression is a leading contributor of disability worldwide, increasingly prevalent, and highly recurrent (1). Its onset typically occurs during adolescence (2, 3); moreover, because adolescent-onset depression is associated with more severe functional impairment, greater risk for other mental and physical illnesses, and more recurrent episodes that are often resistant to treatment (4, 5), identifying risk factors for adolescent depression is critical for early intervention. While research studies have identified multiple biomarkers and putative risk factors for depression in adolescence that span different domains, including demographic, clinical, psychosocial, and neurobiological factors (6–11), most studies have sought to understand the role of isolated factors that each explain a small portion of variance rather than maximizing prediction across a broad set of factors. One recent exception, however, is an investigation by Toenders et al., which focused on structural MRI markers in addition to clinical, cognitive, and environmental factors (12). Here, the authors found that baseline depression severity at age 14, female sex, neuroticism, stressful life events, and surface area of the supramarginal gyrus were the strongest contributors to a model that predicted onset of depression 2–5 years later (12). This study, however, did not examine the role of functional MRI markers as a predictor of adolescent-onset depression.
Two analytic barriers have hindered our ability to integrate across a diverse set of distinct yet related risk factors: 1) large sample sizes are needed to accommodate statistical models with more predictors; and 2) risk factors for depression are often highly collinear. In this context, Adolescent Brain Cognitive Development (ABCD), an ongoing multi-site longitudinal study of brain development and mental health in nearly 12,000 U.S. children ages 9–10 collecting comprehensive demographic, clinical, psychosocial, and neurobiological information, represents an ideal opportunity to leverage machine learning methods that address instances of multicollinearity. Here, we utilized two complementary machine learning techniques—regularized linear regressions (elastic-net) and non-linear ensemble learning (gradient boosted trees) to identify the strongest statistical predictors of depression symptoms in the first waves of ABCD.
Because prior work from “big data” consortia has focused on task-independent MRI markers of depression, including morphological characteristics (i.e., surface area, cortical thickness) derived from structural MRI (13–16) and intrinsic resting-state functional connectivity (FC) obtained from fMRI signals (17, 18), we limited our feature set (i.e., statistical predictors) of brain-based variables to those derived from these imaging modalities for the purposes of comparability with other large-scale efforts, as well as for computational tractability and potential clinical utility.
Based on previous studies examining factors associated with the onset and presence of depression in adolescence (3, 19–22), we hypothesized that female gender, parental depression, morphology and functional connectivity of regions comprising affective, salience (e.g., cingulo-opercular), and default mode networks would constitute the most important features in the best performing models.
Methods
Participants and Ethical Considerations
Baseline and follow-up data used for the present investigation were obtained from the Annual Curated Data Release 3.0 from the ABCD consortium (https://abcdstudy.org/index.html), which is a population-based cohort of 11,878 children ages 8–11 years recruited from 21 sites throughout the U.S. (23). Each recruitment site obtained full assent and consent from the children and their parent(s)/legal guardian(s), respectively in accordance with local Institutional Reviews Boards. Out of the 11,878 children with data at baseline, we excluded those without usable structural and functional MRI based on initial quality control, with familial participants (e.g., twins), and those with substantial missing postprocessed data, resulting in 8,507 unique participants at baseline. Of these, 7,995 provided one-year follow-up Child Behavior Checklist (CBCL) data along with all relevant predictive features. Therefore, we conducted all analyses in the 7,995 with data at both timepoints. See Figure S1 for a flowchart showing the initial sample size and final sample size.
Depression Symptoms (Outcome Measure)
Children and their parent/guardian completed the computerized Kiddie-Schedule for Affective Disorders–5 (K-SADS-5; 23–25) to assess current and lifetime history of Axis I disorders. Based on endorsement from either child- or parent-report, only 6.3% of the sample met lifetime criteria for any depressive disorder (i.e., Major Depressive Disorder, dysthymia, and depression not otherwise specified). Because a history of depression does not necessarily reflect current depressive symptomatology and because classification models for imbalanced datasets tend to introduce bias and misclassification of the minority (less represented) class (27), we did not use the K-SADS data in any of our statistical models. Instead, we elected to assess current depression symptoms dimensionally. Specifically, we used scores from the “Depressive Problems” subscale of the CBCL which comprises items consistent with the DSM-5 criteria for depression in youth from parent report. The CBCL is one of the most widely used measures of emotional problems and provides standardized scores based on national norms in children ages 6–18 (28).
Features (Predictors)
Non-brain features included history of parental depression, family conflict, parent-reported demographic information (including child age, child sex assigned at birth, child race, child education level, parental marital status, parental income, and parental education), average hours of sleep per night (herein referred to as sleep duration), and substances taken in the past 24 hours. See Table S1 and Appendix S1 in the Supporting Information for more information on how family conflict and sleep duration were defined. Site, scanner type (Siemens, GE, or Philips), and MRI device serial number were used as regressors for the brain-based features only (see Machine Learning Analyses, below, for more information).
All structural MRI data were preprocessed using FreeSurfer v. 5.30 (29) (http://surfer.nmr.mgh.harvard.edu/) and underwent automated and manual quality control procedures. All resting-state fMRI data were preprocessed using AFNI (30) and rigorous motion censoring was applied. See (31) and Appendix S2 in the Supporting Information for more details.
Brain features derived from structural MRI included subcortical gray matter volumes, cortical thickness, and cortical surface area from regions defined by the Desikan atlas (32). Because over 100 subjects were missing data from morphometry metrics based on the ventricles, we did not include ventricles in our analyses (see Appendix S3 in the Supporting Information). Participants with low-quality control scores on their MRIs were excluded from all analyses (see Appendix S2 in the Supporting Information).
Brain features derived from resting-state fMRI included functional connectivity between networks defined by the Gordon parcellation (33) and also between each of the subcortical structures segmented by FreeSurfer with each of the cortical networks (see 28 for more details). These eight cortical networks included the auditory network, the cingulo-parietal network, the cingulo-opercular network, the default mode network, the frontoparietal network, the retrosplenial-temporal network, the ventral attention network, and the visual network. Specifically, regions within the Gordon parcellation were classified as belonging to a particular network or community (e.g., retrosplenial temporal, cingulo-opercular network); thus, average correlations within a network were computed as the average of correlations (after Fisher-r-to-z transformation) for each pairwise combination of regions with membership to said network (i.e., network-to-network). Similarly, correlations between each of the networks identified in the Gordon parcellation and each of the subcortical structures segmented by FreeSurfer (amygdala, caudate, nucleus accumbens, pallidum, putamen, and thalamus; left and right, separately) were computed from the resulting Gordon network correlations (described previously) and the average time series from the respective subcortical structure. Participants for whom more than 10% of their timeseries framewise displacement > 0.2 mm or for whom there were fewer than 375 usable timepoints for modeling were excluded from the present analyses (see Appendix S2 in the Supporting Information for more details). See Table S2 for a summary of the filenames and variables used as features and outcomes in our models.
Machine Learning Analyses
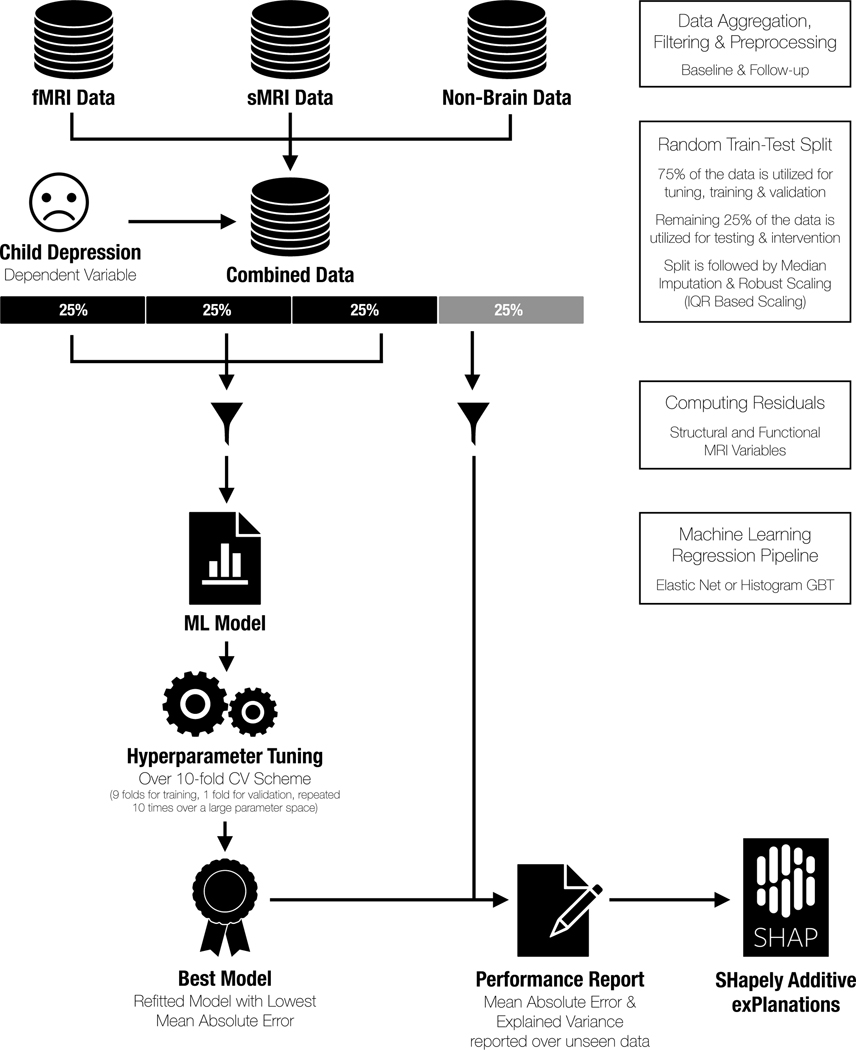
We used two different machine learning approaches in order to identify features that converged across both strategies. First, we used elastic net (EN) to perform regularized linear regression, which combines L1 (least absolute shrinkage and selection operator, or LASSO), and L2 (ridge) penalties (34). Second, we used histogram gradient boosted trees (light GBT), an ensemble method of supervised machine learning that does not assume linearity (35). For both models, we utilized the sklearn package in Python. We first randomly split the data into 4-folds (25% data in each fold). Three randomly chosen folds (75% of the total data) were used for training (i.e., hyperparameter tuning and validation). The remaining 25% of the data was used solely for testing model performance, thereby maintaining independence in our training and test sets. That is, 75% of the data was used for training and validation and the remaining 25% of the data represented an independent sample used solely for testing purposes to yield the most conservative results (i.e., all model performance results are based on this independent test set). Selecting the optimal ratio for splitting the data set comes from Pareto’s principle (36), which suggests that ~20% of the data constitute the out-of-sample data set for testing. However, as increasing the sample size of the test set increases generalizability (37), and given the large sample size of the ABCD data, we elected to have 25% of the data constitute the test set. We note that the training and validation procedures (i.e., on the initial 75% of the data) were still conducted using a standard 10-fold cross-validation scheme.
For all structural and functional MRI features, we first regressed out the effects of site, scanner type, and MRI serial number (after dummy coding each of these categorical variables) using ordinary least squares linear regression; for all functional MRI features, mean framewise displacement and the number of outlier volumes were also regressed out. For all continuous features, missing values were imputed using the median value of the respective feature; for all categorical features, missing values were imputed using the modal value of the respective feature. See Appendix S2 and Table S3 in the Supporting Information for more details. Critically, data were imputed after splitting the data to prevent the risk of leakage between the training/validation and test sets.
In the training data (75% of the total data), we conducted hyperparameter tuning using training and validation over a 10-fold cross-validation scheme. In other words, 9-folds (67.5% of the total data) were used to identify a set of hyperparameters, using grid search, that best fit each model based on minimizing mean absolute error (MAE), and 1-fold (7.5% of the total data) was used for validation. This process was repeated ten times for each fold to identify a model with the optimal set of hyperparameters and then applied to the training set (75% of the total data). This final model was then evaluated on the remaining left-out test set (25% of the total data), from which we report our model performance metrics. See Figure 1.
Figure 1.
Visualization of the machine learning approach used to identify features contributing to the prediction of concurrent depression symptoms.
To determine the importance of each relative feature within the best-performing model, we used the Shapley Additive exPlanations (SHAP) method (38). Briefly, a Shapley value for a feature quantifies how well a particular feature contributes to overall model performance even in the presence of correlated features. In more technical terms, a Shapely value is the average marginal contribution of a feature value across all possible coalitions or combinations of features other than the given feature. SHAP is a computationally efficient way to generate Shapley values, given the number of features in our models (almost 400 total). One significant advantage of SHAP is that it assigns correct feature importance values to each feature even in the presence of correlated features. See Appendix S4 in the Supporting Information for more details.
Code Availability
All code for performing data cleaning, organization, and predictive modeling can be found here: https://github.com/tiffanycheingho/ABCD
Results
The final analytic sample contained 7,995 participants: 49.51% were assigned female at birth and 76.36% identified as being White. Distributions of parental income and educational attainment were consistent with percentages reported from recent epidemiological data (39, 40). See Table 1 for a summary of the descriptive statistics of the final sample at baseline. See Table S4 in the Supporting Information for a summary of the descriptive statistics in the one-year follow-up (which were comparable to the full baseline sample).
Table 1. Demographics of final analytic sample (N=7,995).
Continuous variables are reported as mean ± standard deviation (min – max) or median ± IQR for variables with outliers or likely errors. All variables unless otherwise noted are from the baseline assessment.
| Total N | 7995 |
|---|---|
| Sex (% Female) | 49.52% |
| Age (in years) | 9.49 ± 0.51 (8–11) |
| Race (% White) | 76.36% |
| Puberty Development Score | 6.74 ± 2.87 (1–20) |
| Body mass index | 17.51 (15.89–20.34) |
| % with caffeine consumption in past 24 hours | 17.44% |
| % with prescription medication use in past 24 hours | 14.25% |
| % with over-the-counter medications use in past 24 hours | 15.25% |
| Sleep duration (hours per night) | 9–11 hours: 48.73% 8–9 hours: 37.19% 7–8 hours: 10.88% 5–7 hours: 2.96% <5 hours: 0.24% |
|
Current grade
|
1st grade: 0.025% 2nd grade: 0.39% 3rd grade: 17.12% 4th grade: 44.61% 5th grade: 34.75% 6th grade: 3.08% 7th grade: 0.025% |
| Highest parental education | 3rd grade: 0.038% 4th grade: 0.026% 5th grade: 0.026% 6th grade: 0.34% 7th grade: 0.12% 8th grade: 0.35% 9th grade: 0.70% 10th grade: 0.69% 11th grade: 0.84% 12th grade (no diploma): 1.27% High school graduate: 6.26% GED or equivalent: 2.36% Some College Degree: 12.29% Associate Degree (Occupational, Technical, or Vocational): 7.29% Associate Degree (Academic Program): 5.11% Bachelor’s Degree: 25.83% Master’ s Degree: 25.11% Professional School Degree: 5.34% Doctoral Degree: 6.01% |
| Weekend daily screen time (hours) | 3.78 ± 2.58 (0–24) |
| Parental marital status | Married: 70.58% Never Married: 10.96% Divorced: 8.63% Living with Partner: 5.45% Separated: 3.61% Widowed: 0.77% |
| Combined family income, past year | <$5k: 2.82% $5k – $12k: 2.99% $12k – $16k: 2.31% $16k – $25k: 4.06% $25k – $35k: 5.50% $35k – $50k: 7.65% $50k – $75k: 12.68% $75k – $100k: 21.94% $100k – $200k: 28.86% >$200k: 11.19% |
| % parental history of depression | 30.68% |
| Family Conflict Scores | 2.50 ± 1.96 (0–9) |
|
CBCL (Youth) Depression (t-score) Baseline Follow-up |
53.54 ± 5.67 (50–89) 53.87 ± 6.02 (50–87) |
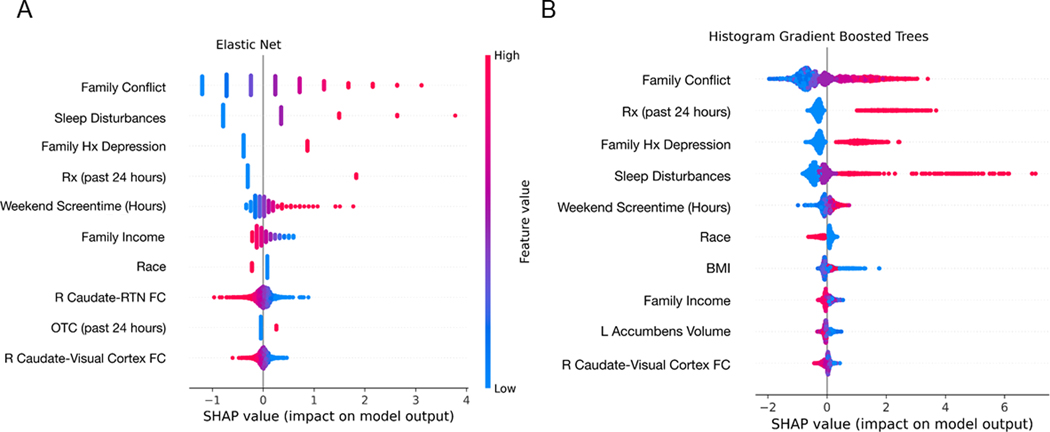
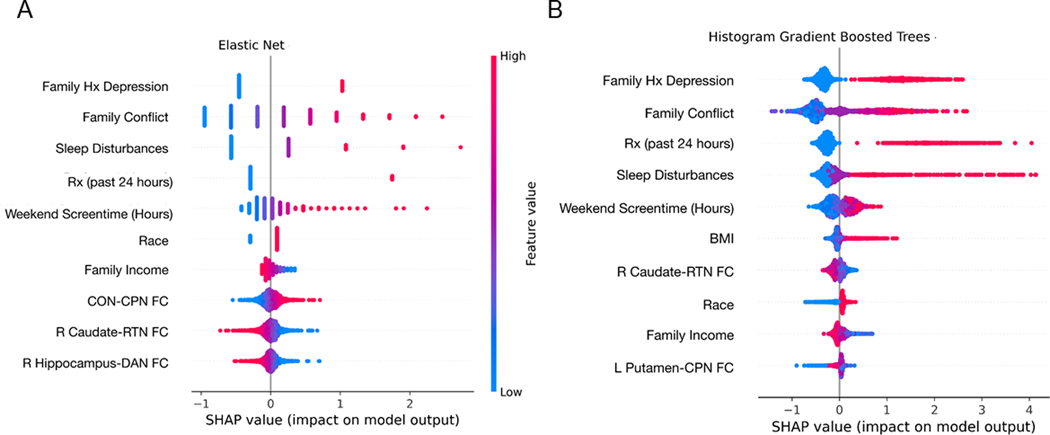
Both EN and GBT models yielded comparable results in terms of model performance (see Table 2). Across both models, family conflict was the strongest predictor of youth depression symptoms at baseline while parental history of depression was the strongest predictor of youth depression at follow-up. Both models also identified child sleep duration, prescription medication usage, hours of screentime on the weekends as important features, although the rank order of these features in their contribution to their respective models differed slightly (see Figures 2–3).
Table 2. Summary of model performance metrics.
EN=Elastic Net; GBT=Gradient Boosted Trees; MAE=Mean Absolute Error.
| Outcome | Model Type | MAE | R2 |
|---|---|---|---|
| Baseline CBCL | EN (linear) | 3.757 | 0.156 |
| Baseline CBCL | GBT (non-linear) | 3.761 | 0.147 |
| Follow-up CBCL | EN (linear) | 4.255 | 0.103 |
| Follow-up CBCL | GBT (non-linear) | 4.262 | 0.089 |
Figure 2. Shapley values of the top 10 features in the elastic net (A) and gradient boosted trees (B) models predicting baseline depression symptoms.
The summary plots indicate the relationship between the value of a feature and the impact on the prediction, thus combining feature importance with feature effects. Each point on the summary plot is a Shapley value for a feature and an instance. The position on the y-axis is determined by the feature and on the x-axis by the Shapley value. The color represents the value of the feature from low (blue) to high (pink). The features are ordered according to their importance. See Figure S2 for a summary of the magnitude of Shapley values per feature in each model. FC=functional connectivity; Hx=history; RTN=retrosplenial temporal network; Rx=prescription medication
Figure 3. Shapley values of the top 10 features in the elastic net (A) and gradient boosted trees (B) models predicting 1-year follow-up depression symptoms.
The summary plots indicate the relationship between the value of a feature and the impact on the prediction, thus combining feature importance with feature effects. Each point on the summary plot is a Shapley value for a feature and an instance. The position on the y-axis is determined by the feature and on the x-axis by the Shapley value. The color represents the value of the feature from low (blue) to high (pink). The features are ordered according to their importance. See Figure S3 for a summary of the magnitude of Shapley values per feature in each model. BMI=body mass index; CON=cingulo-opercular network; CPN=cingulo-parietal network; FC=functional connectivity; Hx=history; RTN=retrosplenial temporal network; Rx=prescription medication
With respect to neural features predicting baseline levels of depression symptoms, resting-state functional connectivity between the right caudate and retrosplenial-temporal network (RTN) was the 8th most important feature in the EN model whereas gray matter volumes of the nucleus accumbens and resting-state functional connectivity between the right caudate and visual network were the 8th and 9th overall most important features, respectively, in the GBT model. With respect to neural features predicting depression symptoms at one-year follow-up, resting-state functional connectivity across a broader set of networks was identified in the EN model, with resting-state functional connectivity between the cingulo-opercular network and the cingulo-parietal network and between the right caudate and RTN as the 8th and 9th overall most important features, respectively, whereas in the GBT model, resting-state functional connectivity between the right caudate and RTN remained the most important neural feature and the 7th overall most important feature (see Figures 2–3and Figures S2–S3). Parental depression and family conflict were moderately correlated (r=0.23), as was sleep duration and weekend screen time (r=0.21); the remaining top features mostly showed weaker or null correlations, suggesting that the family environment (parental depression and family conflict), sleep quality (sleep duration and weekend screen time), and functional neural factors represent distinct predictors of depression. See Figure S4 for a zero-order correlation matrix among the top features across both models. See Figures S5–S6 for partial dependence plots which displays the marginal effect of a given feature on the outcome measure (CBCL depression score at baseline and follow-up, respectively). Finally, as a specificity analysis, we also computed Pearson’s correlations among the shared top features across both models with CBCL externalizing scores and found that with the exception of resting-state functional connectivity between right caudate and RTN, all other top features had similar effect sizes (see Table S5).
With the exception of gray matter volumes of the nucleus accumbens in the GBT model predicting baseline depression symptoms, structural MRI features were not identified among the top contributing features in our predictive models. In a supplemental analysis, we ran an EN model and a GBT model with demographic, clinical, and resting-state fMRI features only to predict baseline and follow-up CBCL scores. These models performed similarly to their full model counterparts (baseline EN: MAE=3.772, R2=0.157; follow-up EN: MAE=4.258; R2=0.108; baseline GBT: MAE=3.778; R2=0.140; follow-up GBT: MAE=4.319; R2=0.075). Moreover, many of the same top features, including parental depression, child sleep duration, prescription medication usage in the past 24 hours, weekend screen time, family support, being of mixed race, and resting-state fMRI connectivity between the right caudate and the RTN, were identified in these models. Our results, therefore, suggest that structural MRI features did not contribute meaningfully to the prediction of CBCL depression scores (either at baseline or follow-up).
Discussion
This is the first investigation utilizing the ABCD data to examine statistical predictors of concurrent and subsequent (one-year follow-up) child depression symptoms across several domains. We found that history of parental depression, higher levels of family conflict, fewer hours of sleep per night (i.e., sleep duration), and medication usage were among the strongest features contributing to the statistical prediction of depression symptoms across two distinct machine learning modeling approaches. While resting-state functional connectivity of several cortical networks were weak contributors in the elastic net model, lower resting-state functional connectivity between the right caudate and retrosplenial-temporal network (RTN) was the most important neural feature (and 8th most important overall feature) in the elastic net (EN) model, while resting-state functional connectivity between the right caudate and visual network was the most important neural feature (and 9th most important overall feature) in the gradient boosted trees (GBT) model. Interestingly, lower connectivity between the right caudate and RTN was consistently identified across both modeling approaches (the second most important neural feature in the EN model and the most important neural feature in the GBT model) in predicting depression severity in this sample one year later. Parental depression and family conflict were moderately correlated (r=0.23), as was sleep duration and weekend screen time (r=0.21); the remaining top features mostly showed weaker or null correlations, suggesting that the family environment (parental depression and family conflict), sleep quality (sleep duration and weekend screen time), and functional neural factors represent distinct predictors of depression. Together, our results highlight family environment (which is arguably shaped strongly by parental mental health status) and sleep quality—both of which are modifiable processes that may be potentially important intervention targets for mental health more generally—as important risk factors for adolescent depression. Moreover, our results also demonstrate that lower resting-state functional connectivity of the right caudate with regions comprising visual attention and default mode network functioning—including processing underlying autobiographical memories and self-reflection—may be a promising biomarker of early depression symptoms in adolescence and that this biomarker may be specific to depression given the absence of associations with externalizing symptoms.
Parental mental health and relationship quality between the child and parent remain strong predictors of mental health outcomes in adolescents, particularly for depression (41). Researchers have identified a multitude of pathways that may explain the intergenerational transmission of depression—including genetic as well as environmental (e.g., caregiving quality, epigenetic) factors—although the relative contribution of these purported mechanisms still remains unknown (for reviews, see references (19, 42, 43). Critically, there is evidence of biological alterations in youth whose parents have a history of depression, including smaller gray matter volumes in subcortical structures (44, 45), HPA-axis dysfunction (for a review see 40), and alterations in epigenetic markers (47, 48), even when youth whose parents have a history of depression but do not endorse current depression symptoms themselves. That higher levels of family conflict were identified as an important predictor of both current and prospective symptoms of depression further supports the formulation that parental behaviors, which are informed by mental health status, are critical for scaffolding offspring brain development. It will be important for future investigations of the ABCD data to examine possible mechanisms (e.g., caregiving style) that could be targeted to mitigate risk for depression.
Our investigation also identified shorter sleep duration and longer weekend screen time usage as important features for predicting depression symptoms. Adolescence is an important time of development that includes changes in chronobiological processes that regulate circadian rhythms, as well as changes in sleep homeostasis that, together, affect the timing and duration of sleep (49). Our findings are consistent with several studies that have found that shorter sleep duration is associated with higher depression and other mental health symptoms in adolescents (50, 51). Indeed, previous analyses from the ABCD dataset using non-regularized linear models found that depressive symptoms were significantly correlated with shorter sleep duration and, furthermore, that greater depression symptoms at baseline predicted shorter sleep duration at follow-up (52). We replicate these findings in a larger sample and further highlight the potential role of screen time. Although there has been controversy regarding the extent to which screen time affects mental health in adolescents (see 42–44), our results indicate that more weekend screen time, which will likely reflect more leisure usage, is related to more depression symptoms at baseline and is predictive of greater depression symptoms one-year later. Together, our results suggest that sleep quality may be targetable processes in reducing depression risk. An important next step in this line of research will be to obtain richer contextual information to understand the different sources of shorter sleep duration and longer screen time usage (e.g., academic pressure), and if these sources moderate the associations between sleep quality and depression.
Our findings are broadly consistent with our hypotheses insofar as functional connectivity among portions of the affective (caudate) and default mode (retrosplenial-temporal) networks are implicated in depression risk; however, regions comprising the so-called salience network (amygdala, cingulo-opercular network, etc) were not identified as robust predictive features. Specifically, lower resting-state functional connectivity between the right caudate and retrosplenial-temporal and visual networks remained the most consistent neural features in predicting depression symptoms (albeit their contribution to the predictive model relative to other non-neural features was weaker). With respect to the role of the caudate in depression risk, our results cohere with a meta-analytic investigation that identified modest gray matter volume reductions in the caudate (Cohen’s d=−0.31) in depressed versus psychiatrically healthy adults (56) and in empirical studies that have identified lower structural connectivity of a caudate-based network in depressed versus psychiatrically healthy adults (57) and adolescents (58). As a component of the dorsal striatum, the caudate plays an important role in reward processing and stimulus-behavior mapping, particularly in contexts where stimulus outcomes are perceived to be contingent on one’s own behavioral actions (59). While previous studies have identified that node strength of the left ventral striatum to other reward-related regions based on resting-state signals were informative for predicting depression symptoms in a relatively large sample (N=637) of Brazilian youth ages 6–12 years recruited from the community (10), our results suggest that intrinsic connectivity of the right caudate with regions outside of the putative reward network—namely, visual attention and default mode network regions—are also useful risk markers for depression during late childhood and early adolescence.
In contrast, structural MRI features largely did not contribute to any of the models predicting concurrent or future depression symptoms, with the exception of smaller gray matter volumes of the nucleus accumbens in the EN model predicting baseline depression symptoms (which is consistent with a recent study examining nucleus accumbens alterations in depressed and anxious adolescents, 60). Previous investigations of the ABCD data using non-regularized linear models found that smaller cortical surface area (but not cortical thickness) and volumes of regions in the default mode network (ventromedial prefrontal cortex, precuneus, and posterior cingulate cortex) in the lateral and medial orbitofrontal cortex (OFC), and in frontal, temporal, and motor regions were associated with greater concurrent depression symptoms (52). Similarly, studies from the ENIGMA Major Depressive Disorder (MDD) consortium (13), which utilizes meta-analytic techniques to estimate the effects of depression on FreeSurfer-derived brain morphometry across different sites worldwide, have also reported similar findings. Specifically, in one subanalysis that compared 294 healthy controls to 213 depressed adolescents, the investigators found that depressed adolescents exhibited lower surface area (but no differences in cortical thickness), with the strongest effect sizes in the medial OFC, superior frontal gyrus, and visual, somatosensory, and motor areas (13). Consistent with our results, the studies from ENIGMA MDD did not find evidence that subcortical and cortical morphometry was associated with current depression symptoms (13, 14, 16). Importantly, our study extends these previous “big data” investigations that focused on examining associations between regional surface area and adolescent depression by demonstrating that functional connectivity patterns of the right caudate with visual attention and default mode network regions contribute to the prediction of concurrent and future symptoms of depression and may, thus, represent potentially more sensitive biomarkers of early depression symptoms than structural markers (although see 60).
Several limitations must be considered when interpreting our findings. First, while the CBCL provides dimensional measures of depression symptoms, it is a measure based on parent-report, which may not necessarily be concordant with child-report, particularly for depression and other internalizing disorders (61, 62). Future assessments of the ABCD Study will also include the Youth Self-Report, a child-reported questionnaire that captures the same symptom dimensions as the CBCL, which will provide us an opportunity to replicate the analyses in the present investigation. Similarly, sleep duration and screen time were measured based on parent-report from questionnaires; future assessments of the ABCD Study will seek to comprehensively assess these constructs using more objective measures (e.g., actigraphs or other wearables, sensor data from smartphones) in order to understand the role of sleep quality on depression risk in adolescents. Second, the sample composition of ABCD limits generalizability of our findings, particularly with respect to important racial, ethnic, and socioeconomic factors. It will be critical to apply predictive modeling to test the extent to which our findings replicate in other cohorts that are enriched in these factors, as well as cohorts outside of the United States, which are needed to shed critical insight into cultural factors that undoubtedly play an important role in the development of adolescent depression. Third, it is important to note that our analytic approaches were data-driven for the purposes of constraining the wide parameter space, which we believe will be informative for researchers designing and conducting future studies examining adolescent depression risk where it may not be possible to acquire data as broad in scope as those that are being collected in ABCD. Fourth, even though the results from the EN (linear) models performed better than the GBT (non-linear) on several metrics of model performance (including mean absolute error and explained variance, although GBT performed slightly better in median absolute error), it is nonetheless worth examining non-linear associations between the features and outcomes of interest. Given the complexity of ensemble learning models such as GBT, it is difficult to interpret the results of the GBT analyses, as the non-linear results may be capturing higher-order interaction effects. That said, our partial dependence plots indicate that while there is a strong linear association between family history of depression and prescription medication usage with CBCL depression scores, there is an increasing monotonic association between family conflict and CBCL depression scores that appears to plateau, and an exponential association between sleep disturbances and CBCL depression scores. Future research with study designs targeting these processes of interest is needed to disentangle the nature of these associations.
In conclusion, our study identified multi-level predictors of depression symptoms in a nationally representative sample of almost 8,000 youth ages 9–10. We found that higher parental depression symptoms, higher levels of family conflict, shorter sleep duration, longer weekend screen time, and lower resting-state functional connectivity of the caudate were associated with greater concurrent and one-year depression symptoms. Our findings point to two modifiable processes for depression risk that may be important considerations for depression prevention—family environment and sleep quality—as well as highlight the potential of caudate-based functional connectivity patterns to be biomarkers of early depression emergence in youth.
Supplementary Material
Appendix S1. Features (Predictors).
Appendix S2. Image Processing.
Appendix S3. Missing Data.
Appendix S4. Feature Importance.
Figure S1. Flowchart of study participants included in final analytic sample (N=7,995).
Figure S2. Magnitude of feature importance values for top 10 features in the elastic net (A) and gradient boosted trees (B) models predicting baseline depression symptoms.
Figure S3. Magnitude of feature importance values for top 10 features in the elastic net (A) and gradient boosted trees (B) models predicting 1-year depression symptoms.
Figure S4. Correlation values among the shared top predictive features in both models.
Figure S5. Partial dependence plots for the top 10 features in predicting baseline CBCL depression scores.
Figure S6. Partial dependence plots for the top 10 features at baseline predicting CBCL follow-up scores.
Table S1. Items from the Family Environment Scale.
Table S2. Summary of ABCD 3.0 file names where features and outcome variables were extracted.
Table S3. Overview of number of participants missing non-brain features.
Table S4. Demographic values of the sample split by training versus test set.
Table S5. Summary of Pearson’s correlations between the top features in each model with CBCL Externalizing t-scores.
Key points.
While identifying risk factors for adolescent depression is critical for early prevention and intervention, most studies have sought to understand the role of isolated factors rather than across a broad set of factors.
The significance of the present study is that we sought to examine multi-level factors that maximize the prediction of depression symptoms in a large epidemiological sample of almost 8,000 children in the U.S. who were participating in the Adolescent Brain and Cognitive Development (ABCD) study.
We found that parental mental health, family environment, and child sleep quality are potentially modifiable risk factors for youth depression.
Unlike previous large-scale studies examining MRI correlates of depression, we did not find evidence of robust structural MRI patterns contributing to depressive risk. In contrast, resting-state functional connectivity of the caudate was a relatively weaker predictor of depression symptoms but may represent a biomarker for depression risk.
Acknowledgments
The ABCD Study is funded by NIH and other federal partners under grants DA-041048, DA-050989, DA-051016, DA-041022, DA-051018, DA-051037, DA-050987, DA-041174, DA-041106, DA-041117, DA-041028, DA-041134, DA-050988, DA-051039, DA-041156, DA-041025, DA-041120, DA-051038, DA-041148, DA-041093, DA-041089, DA-041123, and DA-041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive. The ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This article reflects the views of the authors and may not reflect the opinions or views of NIH or of the ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from https://dx.doi.org/10.15154/1504431.
This research was supported by the National Institutes of Health (K01MH117442 to T.C.H.), the Ray and Dagmar Dolby Family Fund (to T.C.H.), and the National Institute on Alcohol Abuse and Alcoholism (F31AA027169 to A.C.M.). The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. All authors report no biomedical conflicts of interest. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article:
Conflict of interest statement: No conflicts declared.
References
- 1.Organization WH: Depression and other common mental disorders: global health estimates. World Health Organisation. World Heal Organ; 2017; [Google Scholar]
- 2.Avenevoli S, Swendsen J, He JP, et al. : Major Depression in the National Comorbidity Survey–Adolescent Supplement: Prevalence, Correlates, and Treatment. J Am Acad Child Adolesc Psychiatry 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breslau J, Gilman SE, Stein BD, et al. : Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl Psychiatry 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewinsohn PM, Allen NB, Seeley JR, et al. : First onset versus recurrence of depression: Differential processes of psychosocial risk. J Abnorm Psychol 1999; [DOI] [PubMed] [Google Scholar]
- 5.Balázs J, Miklõsi M, Keresztény Á, et al. : Adolescent subthreshold-depression and anxiety: Psychopathology, functional impairment and increased suicide risk. J Child Psychol Psychiatry Allied Discip 2013; [DOI] [PubMed] [Google Scholar]
- 6.Carter JS, Garber J: Predictors of the first onset of a major depressive episode and changes in depressive symptoms across adolescence: Stress and negative cognitions. J Abnorm Psychol 2011; [DOI] [PubMed] [Google Scholar]
- 7.Colich NL, Kircanski K, Foland-Ross LC, et al. : HPA-axis reactivity interacts with stage of pubertal development to predict the onset of depression. Psychoneuroendocrinology 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foland-Ross LC, Sacchet MD, Prasad G, et al. : Cortical thickness predicts the first onset of major depression in adolescence. Int J Dev Neurosci 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeMoult J, Ordaz SJ, Kircanski K, et al. : Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. J Abnorm Psychol 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan PM, Sato JR, Salum GA, et al. : Ventral striatum functional connectivity as a predictor of adolescent depressive disorder in a longitudinal community-based sample. Am J Psychiatry 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stringaris A, Belil PVR, Artiges E, et al. : The brain’s response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toenders YJ, Kottaram A, Dinga R, et al. : Predicting Depression Onset in Young People Based on Clinical, Cognitive, Environmental, and Neurobiological Data. Biol Psychiatry Cogn Neurosci Neuroimaging 2021; [DOI] [PubMed] [Google Scholar]
- 13.Schmaal L, Hibar DP, Sämann PG, et al. : Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmaal L, Veltman DJ, Van Erp TGM, et al. : Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen X, Reus LM, Cox SR, et al. : Subcortical volume and white matter integrity abnormalities in major depressive disorder: Findings from UK Biobank imaging data. Sci Rep 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho TC, Colich NL, Sisk LM, et al. : Sex differences in the effects of gonadal hormones on white matter microstructure development in adolescence. Dev Cogn Neurosci 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia M, Si T, Sun X, et al. : Reproducibility of functional brain alterations in major depressive disorder: Evidence from a multisite resting-state functional MRI study with 1,434 individuals. Neuroimage 2019; [DOI] [PubMed] [Google Scholar]
- 18.Drysdale AT, Grosenick L, Downar J, et al. : Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotlib IH, Goodman SH, Humphreys KL: Studying the Intergenerational Transmission of Risk for Depression: Current Status and Future Directions. Curr Dir Psychol Sci 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichenstein SD, Verstynen T, Forbes EE: Adolescent brain development and depression: A case for the importance of connectivity of the anterior cingulate cortex. Neurosci Biobehav Rev 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luking KR, Pagliaccio D, Luby JL, et al. : Reward Processing and Risk for Depression Across Development. Trends Cogn Sci 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerestes R, Davey CG, Stephanou K, et al. : Functional brain imaging studies of youth depression: A systematic review. NeuroImage Clin 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barch DM, Albaugh MD, Avenevoli S, et al. : Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, et al. : Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; [DOI] [PubMed] [Google Scholar]
- 25.Kobak KA, Kaufman J: KSADS-COMP 2015; [Google Scholar]
- 26.Townsend L, Kobak K, Kearney C, et al. : Development of Three Web-Based Computerized Versions of the Kiddie Schedule for Affective Disorders and Schizophrenia Child Psychiatric Diagnostic Interview: Preliminary Validity Data. J Am Acad Child Adolesc Psychiatry 2020; [DOI] [PubMed] [Google Scholar]
- 27.Blagus R, Lusa L: Improved shrunken centroid classifiers for high-dimensional class-imbalanced data. BMC Bioinformatics 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achenbach TM, Rescorla LA: Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children. 2004 [Google Scholar]
- 29.Fischl B, Salat DH, Busa E, et al. : Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002; [DOI] [PubMed] [Google Scholar]
- 30.Cox RW: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; [DOI] [PubMed] [Google Scholar]
- 31.Hagler DJ, Hatton S, Cornejo MD, et al. : Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desikan RS, Ségonne F, Fischl B, et al. : An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; [DOI] [PubMed] [Google Scholar]
- 33.Gordon EM, Laumann TO, Adeyemo B, et al. : Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou H, Hastie T: Regression Shrinkage and Selection via the Elastic Net, with Applications to Microarrays. J R Stat Soc Ser B 2003; [Google Scholar]
- 35.Zhou Z-H: Ensemble methods: foundations and algorithms. CRC press, 2012 [Google Scholar]
- 36.Chen YS, Chong PP, Tong MY: Mathematical and computer modelling of the Pareto principle. Math Comput Model 1994; 19 [Google Scholar]
- 37.Raykar VC, Saha A: Data split strategies for evolving predictive models, in Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics; ). 2015 [Google Scholar]
- 38.Lundberg SM, Lee SI: A unified approach to interpreting model predictions, in Advances in Neural Information Processing Systems. 2017 [Google Scholar]
- 39.Ryan C, Bauman K: Educational Attainment in the United States: 2015. Curr Popul Reports 2016; [Google Scholar]
- 40.Semega J, Kollar M, Creamer J, et al. : Income and Poverty in the United States: 2018. 2019 [Google Scholar]
- 41.Lieb R, Isensee B, Höfler M, et al. : Parental major depression and the risk of depression and other mental disorders in offspring: A prospective-longitudinal community study. Arch Gen Psychiatry 2002; [DOI] [PubMed] [Google Scholar]
- 42.Goodman SH: Intergenerational Transmission of Depression. Annu Rev Clin Psychol 2020; [DOI] [PubMed] [Google Scholar]
- 43.Sawyer KM, Zunszain PA, Dazzan P, et al. : Intergenerational transmission of depression: clinical observations and molecular mechanisms. Mol Psychiatry 2019; [DOI] [PubMed] [Google Scholar]
- 44.Foland-Ross LC, Gotlib IH: Cognitive and neural aspects of information processing in major depressive disorder: An integrative perspective. Front Psychol 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pagliaccio D, Alqueza KL, Marsh R, et al. : Brain Volume Abnormalities in Youth at High Risk for Depression: Adolescent Brain and Cognitive Development Study. J Am Acad Child Adolesc Psychiatry 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerry JD, Hastings PD: In Search of HPA Axis Dysregulation in Child and Adolescent Depression. Clin Child Fam Psychol Rev 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gotlib IH, Lemoult J, Colich NL, et al. : Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humphreys KL, Moore SR, Davis EG, et al. : DNA methylation of HPA-axis genes and the onset of major depressive disorder in adolescent girls: a prospective analysis. Transl Psychiatry 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carskadon MA, Tarokh L: Developmental changes in sleep biology and potential effects on adolescent behavior and caffeine use. Nutr Rev 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ojio Y, Nishida A, Shimodera S, et al. : Sleep duration associated with the lowest risk of depression/anxiety in adolescents 2016; 39:1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Short MA, Gradisar M, Lack LC, et al. : The impact of sleep on adolescent depressed mood, alertness and academic performance. J Adolesc 2013; [DOI] [PubMed] [Google Scholar]
- 52.Cheng W, Rolls E, Gong W, et al. : Sleep duration, brain structure, and psychiatric and cognitive problems in children. Mol Psychiatry 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Twenge JM, Campbell WK: Associations between screen time and lower psychological well-being among children and adolescents: Evidence from a population-based study. Prev Med Reports 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boers E, Afzali MH, Newton N, et al. : Association of Screen Time and Depression in Adolescence. JAMA Pediatr 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orben A, Przybylski AK: The association between adolescent well-being and digital technology use. Nat Hum Behav 2019; [DOI] [PubMed] [Google Scholar]
- 56.Koolschijn PCMP, Van Haren NEM, Lensvelt-Mulders GJLM, et al. : Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 2009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korgaonkar MS, Fornito A, Williams LM, et al. : Abnormal structural networks characterize major depressive disorder: A connectome analysis. Biol Psychiatry 2014; [DOI] [PubMed] [Google Scholar]
- 58.Tymofiyeva O, Connolly CG, Ho TC, et al. : DTI-based connectome analysis of adolescents with major depressive disorder reveals hypoconnectivity of the right caudate. J Affect Disord 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haber SN, Knutson B: The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Auerbach RP, Pagliaccio D, Hubbard NA, et al. : Reward-Related Neural Circuitry in Depressed and Anxious Adolescents: A Human Connectome Project. J Am Acad Child Adolesc Psychiatry 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cleridou K, Patalay P, Martin P: Does parent-child agreement vary based on presenting problems? Results from a UK clinical sample. Child Adolesc Psychiatry Ment Health 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis AJ, Bertino MD, Bailey CM, et al. : Depression and suicidal behavior in adolescents: A multi-informant and multi-methods approach to diagnostic classification. Front Psychol 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Features (Predictors).
Appendix S2. Image Processing.
Appendix S3. Missing Data.
Appendix S4. Feature Importance.
Figure S1. Flowchart of study participants included in final analytic sample (N=7,995).
Figure S2. Magnitude of feature importance values for top 10 features in the elastic net (A) and gradient boosted trees (B) models predicting baseline depression symptoms.
Figure S3. Magnitude of feature importance values for top 10 features in the elastic net (A) and gradient boosted trees (B) models predicting 1-year depression symptoms.
Figure S4. Correlation values among the shared top predictive features in both models.
Figure S5. Partial dependence plots for the top 10 features in predicting baseline CBCL depression scores.
Figure S6. Partial dependence plots for the top 10 features at baseline predicting CBCL follow-up scores.
Table S1. Items from the Family Environment Scale.
Table S2. Summary of ABCD 3.0 file names where features and outcome variables were extracted.
Table S3. Overview of number of participants missing non-brain features.
Table S4. Demographic values of the sample split by training versus test set.
Table S5. Summary of Pearson’s correlations between the top features in each model with CBCL Externalizing t-scores.