Abstract
Purpose:
This study evaluated the relationship between pain and depressive symptoms through pain self-efficacy and pain catastrophizing in breast cancer patients with pain.
Design:
Secondary analysis of a randomized trial investigating a cognitive-behavioral pain management protocol.
Sample:
Females (N = 327) with stage I–III breast cancer and report of at least moderate pain.
Methods:
Pain severity, pain self-efficacy, pain catastrophizing, and depressive symptoms were measured. The proposed model was assessed using structural equation modeling.
Results:
Higher pain severity was significantly related to lower pain self-efficacy and higher pain catastrophizing. Lower pain self-efficacy and higher pain catastrophizing were significantly related to more depressive symptoms. Higher pain severity was significantly associated with more depressive symptoms through lower pain self-efficacy and higher pain catastrophizing. The association between pain severity and depressive symptoms was not significant when specified as a direct effect.
Conclusion:
Pain severity related to depressive symptoms in breast cancer patients via pain self-efficacy and pain catastrophizing.
Implications for psychosocial providers:
Measurement of pain self-efficacy and pain catastrophizing should be incorporated into comprehensive pain assessments for women with breast cancer, as these variables may be relevant therapeutic targets. Psychosocial symptom management interventions should include strategies that increase pain self-efficacy and decrease pain catastrophizing because these pain-related cognitive variables appear to drive the relationship between pain severity and depressive symptoms.
Keywords: Breast cancer, depression, pain catastrophizing, pain self-efficacy, pain severity
Introduction
Women with breast cancer are living longer due to efforts in early detection and advancing treatments.1 This trend is encouraging, yet women surviving breast cancer face challenges with physical and emotional symptoms resulting from surgical and adjuvant treatments.2 In particular, pain is common and is often cited as one of the most distressing side effects of breast cancer and its treatment.3
Estimates suggest 30–60% of women with breast cancer experience significant pain related to their disease.3,4 Pain after breast surgery is especially bothersome, with 10.9% of women rating their post-surgical pain as “severe” (i.e., ≥ 7 on a 0–10 scale).3 The prevalence of pain among women who have recently completed adjuvant treatments (e.g., radiation, chemotherapy) remains high at 21.8%.3 Strikingly, though, rates of pain prevalence appear highest for women who are 3–6 months (53.6%) and 7–36 months (40.3%) post-treatment, underscoring that pain is a long-term symptom for women surviving breast cancer.3 The presence of pain is associated with a variety of poor outcomes for women with breast cancer.5,6 Approximately 32% of women with breast cancer endorse depressive symptoms,7 and survivors of breast cancer who endorse higher levels of pain severity are more likely to report higher levels of depressive symptoms, both concurrently and prospectively across the cancer continuum.8–12
Given the strength of the association between pain severity and depressive symptoms, it is critical to understand the mechanism(s) driving this relationship.13 The Biopsychosocial Theory of Pain11–13 offers a useful lens from which to explore the relationship between pain severity and symptoms of depression. This theory posits that within the context of a pain experience (i.e., breast cancer diagnosis and treatment), there are a complex set of interactions between biological (e.g., genetic predisposition, tissue damage), psychological (e.g., self-efficacy, coping), and social factors (e.g., social support, cultural practices), which can result in varied symptom profiles. That is, the pain experience may change based on how these factors present themselves for any given individual. Within the psychological domain of this theory, self-efficacy for pain management14 and pain catastrophizing15 are two pain-related cognitive processes that may be especially relevant to the relationship between pain severity and depressive symptoms.
The psychological variables of self-efficacy for pain management and pain catastrophizing have garnered much attention within the pain literature.16,17 Pain self-efficacy is defined as one’s confidence in their ability to control pain on their own (i.e., without medication or other medical intervention).17 Pain catastrophizing is the tendency to fixate on and magnify pain sensations, and to feel helpless when faced with pain.18 Both pain self-efficacy and pain catastrophizing are key constructs within the symptom experience for individuals coping with medical conditions wherein pain is common (e.g., arthritis, cancer).19–21 These pain-related cognitions have been linked to increased pain severity, stiffness, sleep disturbance, fatigue, and psychological disability.18–21 In breast cancer samples specifically, research has shown that lower pain self-efficacy and higher pain catastrophizing are separately associated with higher pain severity.6,22 Additionally, individuals who endorse low pain self-efficacy and/or high pain catastrophizing are more likely to also report more depressive symptoms.6,22–24
Importantly, though, these pain-related cognitive variables (i.e., self-efficacy for pain management and pain catastrophizing) have not been specified together in a single path model linking pain severity and depressive symptoms in the breast-cancer setting. Park and colleagues (2017) showed that more pain predicted higher distress across the first 12 months after breast cancer diagnosis.11 A prospective relationship was also observed by Jones et al. (2015), wherein report of pain among women with breast cancer predicted the incidence of depressive symptoms one year after diagnosis.12 Women with breast cancer who report elevated pain severity might be more likely to experience more depressive symptoms via the cognitive processes of pain self-efficacy and pain catastrophizing. Yet, there is little existing work exploring these mediated pathways. In a sample of heterogeneous cancer patients, self-efficacy for symptom management mediated the relationship between symptom severity and quality of life.25 Likewise, pain catastrophizing has been shown to prospectively mediate the relationship between pain severity and disability among patients with noncardiac chest pain.26 A similar relationship was seen in women with breast cancer, in which pain catastrophizing mediated the association between pain severity and emotional distress6
Yet, to our knowledge, no study has simultaneously explored the mediating potential of both pain self-efficacy and pain catastrophizing in one structural model linking pain severity with depressive symptoms. These relationships may be particularly relevant among survivors of breast cancer reporting moderate-to-severe pain. Clarifying the roles of pain self-efficacy and pain catastrophizing in relating to pain and depressive symptoms will support the development of efficacious psychosocial interventions that target these pain-related cognitions. Ultimately, improving self-efficacy for pain management and decreasing pain catastrophizing may attenuate the link between pain severity and depressive symptoms among survivors of breast cancer.
The purpose of the current study was to assess a theory-based,11,12 structural path model examining the relationship between pain severity and depressive symptoms via pain self-efficacy and pain catastrophizing, above and beyond relevant covariates. Our sample included women with breast cancer (N = 327) and at least moderate pain (i.e., worst pain ≥ 5/10). We hypothesized that: (1) higher pain severity would be related to lower pain self-efficacy and higher pain catastrophizing, (2) lower pain self-efficacy and higher pain catastrophizing would be related to more depressive symptoms, and (3) higher pain severity would be related to more depressive symptoms via lower pain self-efficacy and higher pain catastrophizing.
Methods
Participants
Women (N = 327) diagnosed with initial or recurrent stage I–IIIC breast cancer in the previous two years were enrolled in a randomized trial that investigated a cognitive-behavioral pain management protocol from 2017 to 2020 (NCT02791646). Additional inclusion criteria were: (1) pain severity rating of ≥ 5 out of 10 at screening, (2) ≥ 18 years of age, and (3) life expectancy of ≥ 12 months. Medical chart review and communication with the treating oncologist confirmed exclusion criteria and included: (1) brain metastases, (2) cognitive impairment, (3) current or past (< 6 months) engagement in behavioral symptom management for cancer pain, and/or (4) severe psychiatric disorder (e.g., psychotic disorder) or condition (e.g., suicidal intent) that would contraindicate safe participation. The trial protocol has been published previously.26 The Duke University Institutional Review Board approved the parent study and all procedures complied with HIPAA guidelines.
Procedures
Potential participants were recruited from a National Cancer Institute-designated comprehensive cancer center based on: (1) medical-record diagnosis of breast cancer during the last two years, (2) oncologist referral, or (3) self-referral in response to study advertisement. Following oncologist approval, the study staff mailed recruitment letters signed by the principal investigator and oncologist to inform potential participants that they may qualify for a randomized controlled trial of Pain Coping Skills Training (PCST).27 Potential participants who met all eligibility criteria completed informed consent in person, via phone, or online using Research Electronic Data Capture (REDCap). As part of the larger parent trial, participants completed an electronic baseline assessment consisting of self-report questionnaires measuring pain severity, pain self-efficacy, pain catastrophizing, and depressive symptoms. The present study is a secondary analysis of these baseline data.
Measures
Demographic and medical characteristics
Demographic and medical characteristics were collected by electronic medical record review and participant self-report. Information was recorded regarding demographics (e.g., age, race, ethnicity, education, and partner status) and medical history (e.g., cancer stage, surgeries and treatments received, use of antidepressant and/or pain medication).
Pain severity
Pain severity was assessed with four items from the Brief Pain Inventory (BPI).28 Participants were asked to rate their pain at its worst, least, and average over the past week, as well as their current pain. Response options ranged from 0 (no pain) to 10 (worst pain imaginable). These four items were averaged to obtain a composite score (Cronbach’s α = .86), with higher scores indexing higher levels of pain severity. The BPI is recommended for use in all clinical trials assessing pain, and is frequently used in cancer samples.29–31
Pain self-efficacy
Self-efficacy for pain management was assessed with the five-item Chronic Pain Self-Efficacy Scale.32 Participants were asked to rate their confidence in their ability to decrease their pain, continue their daily activities, keep pain from interfering with their sleep, make a small-to-moderate reduction in pain using methods other than taking medication, and make a large reduction in pain using methods other than taking medication. Response options ranged from 10 (very uncertain) to 100 (very certain), with higher scores reflecting more pain self-efficacy. These five items were averaged to obtain a composite score (Cronbach’s α = .85). The Chronic Pain Self-Efficacy Scale has been widely used in cancer samples.33,34
Pain catastrophizing
Pain catastrophizing was assessed with five items from the Coping Strategies Questionnaire (CSQ).35 Participants were asked about their tendencies to make negative self-statements and catastrophize when faced with pain (e.g., “When I feel pain, it is awful and it overwhelms me”). Response options ranged from 0 (never) to 6 (always), with higher scores indexing more pain catastrophizing. These five items were averaged to obtain a composite score (Cronbach’s α = .87). The Coping Strategies Questionnaire (CSQ) has been previously used in samples of patients with cancer.29
Depressive symptoms
Depressive symptoms were assessed utilizing the 20-item Center for Epidemiological Studies Depression Scale (CES-D).36 Participants were asked to rate the number of times during the previous week they experienced depressive symptoms (e.g., low mood, anhedonia, difficulty concentrating). Response options ranged from 0 (rarely or none of the time) to 3 (all of the time). These items were summed to obtain a total score with higher scores indicating higher levels of depressive symptoms. The CES-D is frequently used to assess depressive symptoms among survivors of breast cancer37,38 and demonstrated excellent reliability in the present sample (Cronbach’s α = .90).
Analytic strategy
The Statistical Package for the Social Sciences Version 27 (SPSS 27) was used to conduct preliminary descriptive analyses. Distributions for main study variables were screened for outliers and inspected for skewness, kurtosis, and multivariate assumptions of normality.39 Direct and indirect pathways relating pain severity, pain self-efficacy, pain catastrophizing, and depressive symptoms were tested using structural equation modeling in Mplus Version 7.40 A correlational path was specified between pain self-efficacy and pain catastrophizing. Indirect pathways were assessed using the delta method. Theoretically supported demographic (i.e., age) and medical factors (i.e., cancer stage, mastectomy, breast-conserving surgery, receipt of treatment during week before baseline assessment, use of antidepressant medication, and use of pain medication) were included as covariates and regressed on both mediators and the outcome variable. Mastectomy, breast-conserving surgery, and receipt of treatment (i.e., surgery, chemotherapy, radiation, endocrine therapy, immunotherapy) during the week before the baseline assessment were categorized dichotomously (no = 0; yes = 1). Missing data across main study variables were minimal and estimated using full-information maximum likelihood (FIML), which derives population estimates using all observed data.39 The cutoff values for accepted model fit indices are: chi-square test (χ2) p > .05, confirmatory fit index (CFI) > .95, root mean squared error of approximation (RMSEA) < .06, Tucker-Lewis Index (TLI) > .90, and standardized root mean square residual (SRMR) < .08 [39]. Effect sizes were indexed by standardized coefficients using the following criteria: ≥ .49 = small, .5–.79 = medium, ≥ .80 = large.41
Results
Participant characteristics
The average age of women enrolled in the parent trial was 57.19 (SD = 11.87) years old. More than one-third (35.6%) of the sample identified as a racial minority. The majority of participants were non-Hispanic (95.1%). Most participants reported a college education, with 31.2% obtaining a bachelor’s degree. Additional demographic characteristics are reported in Table 1.
Table 1.
Demographic characteristics (N = 327).
| N (%) | M (SD) | n Missing | |
|---|---|---|---|
| Age (years) | 57.19 (11.87) | 0 | |
| Race | 0 | ||
| White/Caucasian | 203 (62.1%) | ||
| Black/African American | 97 (29.7%) | ||
| Two or More Races | 9 (2.8%) | ||
| Asian | 9 (2.8%) | ||
| American Indian or Alaskan Native | 1 (0.3%) | ||
| Other | 3 (0.9%) | ||
| Not Reoorted/Declined | 5 (1.5%) | ||
| Ethnicity | 0 | ||
| Non-Hispanic | 311 (95.1%) | ||
| Hispanic or Latino | 5 (1.5%) | ||
| Hispanic Mexican | 2 (0.6%) | ||
| Hispanic Cuban | 3 (0.9%) | ||
| Hispanic Puerto Rican | 2 (0.6%) | ||
| Hispanic other | 4 (1.2%) | ||
| Education | 0 | ||
| Less than high school diploma | 7 (2.1%) | ||
| High school diploma | 41 (12.5%) | ||
| Some college | 106 (32.4%) | ||
| Bachelor’s degree | 102 (31.2%) | ||
| Graduate degree | 71 (21.7%) | ||
| Partner status | 0 | ||
| Single | 40 (12.2%) | ||
| Married | 191 (58.4%) | ||
| Divorced | 62 (19.0%) | ||
| Separated | 6 (1.8%) | ||
| Widowed | 24 (7.3%) | ||
| Life-/long-term partner | 4 (1.2%) |
Note. M = mean; SD = standard deviation.
At enrollment, mean time since diagnosis was approximately 10 months (SD = 6.21). For most women (97.2%), this was their first diagnosis of breast cancer. Over half the sample reported Stage I disease (56.0%). Breast-conserving surgery was common, with 56.9% of the sample having undergone lumpectomy, quadrantectomy, partial mastectomy, or segmental mastectomy. Only 8.3% (n = 27) reported receipt of chemotherapy during the week before the baseline assessment, while 10.8% (n = 35) reported receipt of radiation during that same time frame. At the time of the baseline assessment, 15.1% and 9.6% of women endorsed receipt of endocrine therapy or immunotherapy, respectively. Additional medical characteristics of the sample are reported in Table 2.
Table 2.
Medical characteristics (N = 327).
| N (%) | M (SD) | n (Missing) | |
|---|---|---|---|
| cancer Diagnosis | 1 | ||
| First/Initial | 317 (97.2%) | ||
| Recurrence | 9 (2.8%) | ||
| Months Since Diagnosis | 10.11 (6.21) | 0 | |
| Stage | 0 | ||
| I | 183 (56.0%) | ||
| II | 113 (34.6%) | ||
| III | 31 (9.5%) | ||
| Breast Surgery History | |||
| Mastectomy – one breast | 54 (16.7%) | 3 | |
| Mastectomy – two breasts | 65 (20.1%) | 3 | |
| Breast Conserving Surgery | 185 (56.9%) | 2 | |
| Lymph Node Removal | 286 (88.3%) | 3 | |
| Reconstruction | 73 (22.5%) | 3 | |
| Receipt of chemotherapy | 27 (8.3%) | 1 | |
| Receipt of Radiation | 35 (10.8%) | 2 | |
| Receipt of surgery | 22 (6.7%) | 1 | |
| Receipt of endocrine therapy | 49 (15.1%) | 3 | |
| Receipt of immunotherapy | 31 (9.6%) | 4 | |
| Antidepressant medication use | 123 (37.7%) | 1 | |
| Pain medication use | 212 (64.8%) | 0 | |
| Days with pain medication use | 4 | ||
| 0 | 118 (36.5%) | ||
| 1 | 15 (4.6%) | ||
| 2 | 22 (6.8%) | ||
| 3 | 34 (10.5%) | ||
| 4 | 28 (8.7%) | ||
| 5 | 18 (5.6%) | ||
| 6 | 3 (.9%) | ||
| 7 | 85 (26.3%) | ||
| Type of pain medication | |||
| OTC | 177 (54.1%) | 0 | |
| Opioid | 60 (18.3%) | 0 | |
| Corticosteroid | 8 (2,4%) | 0 | |
| Anti-epileptic (e.g, Neurontin) | 30 (9.2%) | 0 | |
| Anxiolytic | 48 (14.7%) | 0 |
Note. M = mean; SD = standard deviation; Breast-conserving Surgery includes lumpectomy, quadrantectomy, partial mastectomy, segmental mastectomy; Receipt of chemotherapy, radiation, surgery, endocrine therapy, and immunotherapy is for week before baseline assessment; OTC = over the counter.
Average pain severity using the BPI pain severity scale at the baseline assessment fell within the moderate range (M = 4.04, SD = 1.73). On average, participants reported moderate pain self-efficacy (M = 62.37, SD = 20.85) and low pain catastrophizing (M = 1.38, SD = 1.41). Approximately half of our sample (50.6%) obtained an average CES-D score (M = 17.60, SD = 10.30) at or above an established cutoff (≥ 16) that indicates risk for clinical symptoms of depression.42 Descriptive statistics and correlations for main study variables are reported in Table 3.
Table 3.
Means (M), Standard Deviations (SD), and Correlation Matrix for Main Study Variables.
| Variable | Pain Severity | Pain Self-Efficacy | Pain Catastrophizing | Depressive Symptoms |
|---|---|---|---|---|
| M (SD) | 4.04 (1.73) | 62.37 (20.85) | 1.38 (1.41) | 17.60 (10.30) |
| Pain severity | 1 | – | ||
| Pain self-efficacy | −.39** | 1 | – | – |
| Pain catastrophizing | .53** | −.45** | 1 | – |
| Depressive symptoms | .33** | −.44** | .59** | 1 |
Note. M = mean; SD = standard deviation; Fatigue scores shown as T-score;
p < .05;
p <. 01.
Relationships between pain severity, pain self-efficacy, pain catastrophizing, and depressive symptoms
A structural model assessed proposed relationships between pain severity, pain self-efficacy, pain catastrophizing, and depressive symptoms (Figure 1). Age, cancer stage, mastectomy, breast-conserving surgery, receipt of treatment during the week before baseline assessment, and use of antidepressant and/or pain medication were included as covariates. Fit indices suggested good model fit, χ2 (8) = 4.90, p = .77, RMSEA = .00, (90% CI [.00, .05]), CFI = 1.00, TLI = 1.00; SRMR = .01. Structural model parameters are reported in Table 4.
Figure 1.
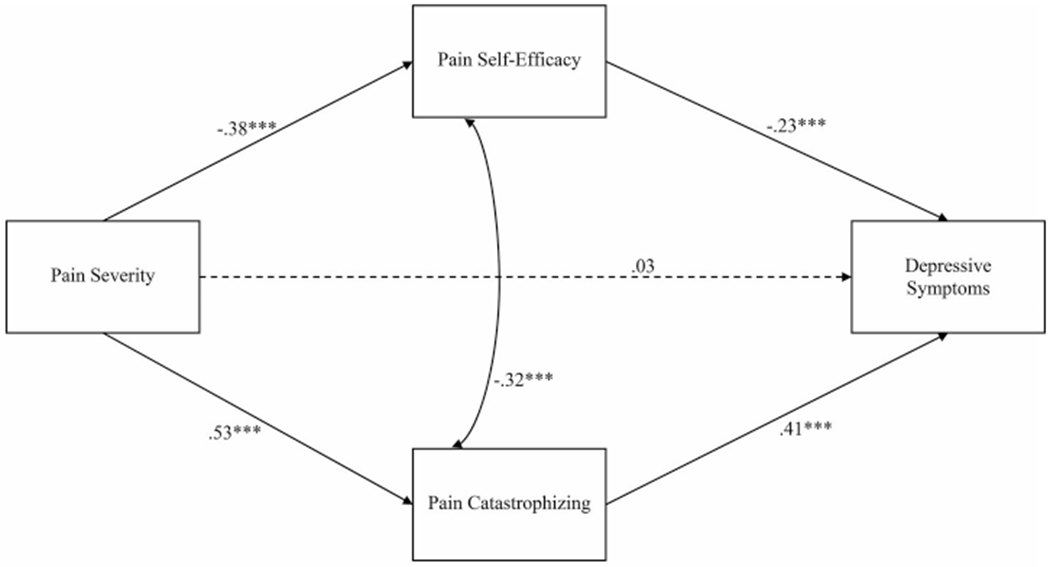
Structural model of the relationships between pain severity, pain self-efficacy, pain catastrophizing, and depressive symptoms
Note. Age, cancer stage, receipt of surgery and/or adjuvant treatment during week before baseline assessment, use of antidepressant medication, and use of pain medication were included as covariates; paths not shown for simplicity. Standardized parameter estimates are shown. Dashed lines indicated non-significant paths; Solid lines indicate significant paths; *p<.05; ***p<.001.
Table 4.
Structural model parameters.
| Pain Self-Efficacy |
Pain Catastrophizing |
Depressive Symptoms |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B | β | 95% CI | B | β | 95% CI | B | β | 95% CI | |
| Pain severity | −4.53** | −.38 | [−5.74, −3.32] | .43** | .53 | [.36,.51] | .20 | .03 | [−.36,.77] |
| Pain self-efficacy | – | – | – | – | – | – | −.11 | −.23 | [−.16,−.07] |
| Pain catastrophizing | – | – | – | – | – | – | 2.95 | .41 | [2.22,.3.68] |
| Covariates | |||||||||
| Age | −.03 | −.02 | [−.22, .17] | −.01 | −.08 | [−.02, .003] | −.09 | −.10 | [−.16,−.01] |
| Cancer stage | −1.23 | −.03 | [−5.74, 3.28] | .06 | .02 | [−.21, .34] | −.91 | −.04 | [−2.66,.84] |
| Mastectomy | 4.19 | .10 | [−1.64, 10.03] | .21 | .07 | [−.15, .56] | −.15 | −.01 | [−2.28,−1.98] |
| Breast-conserving surgery | 4.47 | .10 | [−1.55, 10.49] | −.15 | −.05 | [.52, .22] | −3.38 | −.15 | [−5.79,−.97] |
| Chemotherapy | −1.14 | −.02 | [−9.48, 7.19] | .18 | .04 | [−.33, .69] | 3.01 | .08 | [−.20,6.23] |
| Radiation therapy | −5.62 | −.08 | [−12.86, 1.63] | −.07 | −.02 | [−.51, .38] | −2.24 | −.07 | [−5.06,.58] |
| Endocrine therapy | −3.36 | −.06 | [−9.19, 2.47] | −.05 | −.01 | [−.41, .31] | −2.65 | −.09 | [−4.92,−.38] |
| Immunotherapy | 4.80 | .07 | [−2.49, 12.10] | −.08 | −.02 | [−.52, .37] | −.35 | −.01 | [−3.18,2.47] |
| Antidepressant medication | −1.36 | −.03 | [−5.69, 2.96] | .51** | .18 | [.25, .78] | 5.13 | .24 | [3.41,6.84] |
| Pain medication | −1.86 | −.04 | [−6.30, 2.58] | −.05 | −.02 | [−.32, .22] | .39 | .02 | [−1.33,2.11] |
Note: Unstandardized (B) and standardized estimates (β); p-values for unstandardized estimates;
p< .05;
p <.01.
Breast-conserving surgery includes lumpectomy, quadrantectomy, partial mastectomy, segmental mastectomy; Receipt of chemotherapy, radiation, surgery, endocrine therapy, and immunotherapy is for the week before baseline assessment.
Significant direct effects emerged linking more pain severity with less pain self-efficacy (B = −4.53, p < .001, 95% CI [−5.74, −3.32], β = −.38) and more pain catastrophizing (B = .43, p < .001, 95% CI [.36, .51], β = .53). Effect sizes for these associations were small and moderate, respectively. Less pain self-efficacy (B = −.11, p < .001, 95% CI [−.16, −.07], β = −.23) and more pain catastrophizing (B = 2.95, p < .001, 95% CI [2.22, 3.68], β = .41) were significantly associated with more depressive symptoms. Effect sizes for these associations small. The direct effect of pain severity on depressive symptoms was not significant (B = .20, p = .48, 95% CI [−.36, .77], β = .03).
The indirect effect relating more pain severity to more depressive symptoms via less pain self-efficacy was significant (B = .51, p < .001, 95% CI [.27, .76], β = .09). Likewise, a significant indirect effect emerged linking more pain severity to more depressive symptoms via more pain catastrophizing (B = 1.28, p < .001, 95% CI [.89, 1.66], β = .22). Effect sizes for these associations were small. After accounting for the effects of pain self-efficacy and pain catastrophizing, the direct path connecting pain severity and depressive symptoms was not significant (p = .48). The total effect of pain severity on depressive symptoms was significant (B = 1.99, p < .001, 95% CI [1.44, 2.55], β = .34), and 89.9% was accounted for by the total indirect effect (B = 1.79, p < .001, 95% CI [1.36, 2.22], β = .30).
Discussion
This was an observational study using data from a randomized trial of a cognitive-behavioral pain management protocol for women with breast cancer and pain.27 Our structural path model exhibited strong fit, suggesting that specified relationships between pain severity and depressive symptoms through pain self-efficacy and pain catastrophizing were consistent with the data. First, we observed that women with breast cancer who reported higher levels of pain severity were more likely to report significantly lower levels of pain self-efficacy and higher levels of pain catastrophizing. Second, we found that women with breast cancer who endorsed low pain self-efficacy and/or high pain catastrophizing were more likely to report significantly higher levels of depressive symptoms. Finally, we observed significant associations linking higher levels of pain severity to higher levels of depressive symptoms via low pain self-efficacy and high pain catastrophizing. The association between pain severity and depressive symptoms was not significant when specified as a direct effect.
To our knowledge, no study has explored the relationships between pain severity, pain self-efficacy, pain catastrophizing, and depressive symptoms together in a single structural path model in a sample of women with breast cancer and at least moderate pain. Results from the current study extend previous work19–21 in other medical populations with pain (e.g., arthritis, autoimmune disease) by showing that higher pain severity is significantly related to both lower pain self-efficacy and higher pain catastrophizing among women with breast cancer and pain. Effect sizes for these relationships were small and moderate, respectively, suggesting robust links between pain and both these pain-related cognitive variables. This finding underscores the priority of attending to self-efficacy for pain management and pain catastrophizing when supporting survivors of breast cancer coping with pain.
We also observed that lower pain self-efficacy and higher pain catastrophizing were significantly associated with higher levels of depressive symptoms. This is a valuable contribution to the literature, wherein there is minimal research exploring the role of pain self-efficacy and pain catastrophizing, taken together, on symptoms of depression among women with breast cancer and pain.19–21,25,26 The effect size for the association between pain catastrophizing and depressive symptoms was larger than that of the association between pain self-efficacy and depressive symptoms, highlighting the strength of the relationship between this particular pain-related cognitive variable and depressive symptoms. Our finding may be unique to this sample and should be explored further. If replicated, this observation might be used to refine psychosocial interventions to target the most relevant variables for coping with pain (i.e., pain catastrophizing) due to a medical condition such as breast cancer.
Finally, there were significant associations linking higher levels of pain severity to higher levels of depressive symptoms via lower pain self-efficacy and higher pain catastrophizing, as evidenced by significant indirect effects. Notably, the association between pain severity and depressive symptoms was not significant when specified as a direct effect. This suggests that the relationship between pain and depressive symptoms is mostly relayed through pain self-efficacy and pain catastrophizing. Moreover, the associations between pain and depressive symptoms through pain self-efficacy and pain catastrophizing remained significant, above and beyond the effects of relevant covariates (i.e., age, cancer stage, mastectomy, breast-conserving surgery, receipt of treatment during the week before baseline assessment, use of antidepressant medication, and use of pain medication).
To our knowledge, this is the first study to explore the role of self-efficacy for pain management and pain catastrophizing, assessed together in a structural path model applied to a breast cancer sample. It is noteworthy that both pain self-efficacy and pain catastrophizing may drive the relationship between pain and depression. It is plausible that these cognitive processes interact with each other; for example, a woman with breast cancer may experience low confidence in her ability to manage her pain, which may in turn yield more catastrophic thoughts about her overall pain experience and further reduce confidence levels in pain management. Still, our finding that pain severity is related to depressive symptoms through pain self-efficacy and pain catastrophizing can be leveraged to optimize psychosocial interventions aimed at improving psychosocial adaptation to breast cancer.
Strengths and limitations
This study has notable strengths to highlight. We employed rigorous statistical methods in a large sample of breast cancer survivors to evaluate a structural path model grounded in the Biopsychosocial Theory of Pain.11,12 Structural equation modeling in Mplus allowed for the use of FIML to account for missing data, which reinforced confidence in parameter estimates. Our structural path model was further strengthened by including multiple relevant covariates (e.g., age, cancer stage, mastectomy, breast-conserving surgery, receipt of treatment during the week before baseline assessment, use of antidepressant medication, and use of pain medication). Associations in the model remained significant above and beyond the effect of these covariates, further underscoring the robust nature of the relationship between pain severity, pain self-efficacy, pain catastrophizing, and depressive symptoms.
The following limitations should be considered when interpreting these findings. This study only explored variables within the psychological domain of the biopsychosocial framework. Future work should examine other relevant domains (i.e., biological, social), both separately and in conjunction with the psychological domain. Advanced statistical methods such as confirmatory factor analysis could allow for such explorations.39 This was a cross-sectional analysis of baseline data, thus formal mediation could not be confirmed. Future research should investigate these associations across time to establish temporal precedence. Furthermore, the current model assessed only one specification of the relationships between pain severity, pain self-efficacy, pain catastrophizing, and depressive symptoms. Reverse directionality is possible wherein more depressive symptoms lead to higher pain severity through pain self-efficacy and pain catastrophizing.43 Future research should explore this alternative model in breast cancer samples to strengthen confidence in the ordering of these constructs. Finally, participants were mostly White, non-Hispanic women who self-selected into the parent trial. Women who opted into the trial might have differed from women who did not, particularly on the basis of pain self-efficacy and pain catastrophizing. This selection bias might explain the low levels of pain catastrophizing observed in the current study. This relatively homogenous sample may limit generalizability to less-represented groups in nonacademic medical center environments.
Clinical implications
Our findings have meaningful clinical implications. Given that both pain self-efficacy and pain catastrophizing emerged as relevant variables linking pain and depressive symptoms, these variables should be included in comprehensive pain assessments for cancer patients. Brief, validated scales of pain self-efficacy and pain catastrophizing are readily available,32,35 and may help health-care professionals identify those patients most in need of pain coping support. Survivors of breast cancer reporting low self-efficacy for pain management and high pain catastrophizing might especially benefit from psychosocial interventions that include strategies for improving these pain-related cognitive processes (e.g., progressive muscle relaxation, activity-rest cycle, pleasant activity planning, cognitive restructuring, and calming self-statements).44–46 One such protocol is pain coping skills training (PCST), which has been shown to increase pain self-efficacy and decrease pain catastrophizing among patients with pain conditions (i.e., arthritis) and cancer.29,47,48 Routine use of PCST among women surviving breast cancer may lead to improvements in pain self-efficacy and pain catastrophizing, and consequently reduce pain severity and depressive symptoms.
Conclusion
In this sample of women with breast cancer, the relationship between pain severity and depressive symptoms was largely explained by pathways through pain self-efficacy and pain catastrophizing. Future research should assess these relationships longitudinally to confirm formal mediation. Health-care professionals working with survivors of breast cancer reporting pain and depression should evaluate levels of pain self-efficacy and pain catastrophizing. It is critical that psychosocial interventions include techniques to increase self-efficacy for pain management and decrease pain catastrophizing because this may attenuate the link between pain and depressive symptoms among women with breast cancer.
Funding
This study was funded through NIH/NCI 1R01CA202779-01 awarded to senior author, Tamara J. Somers, PhD. The work of Joseph G. Winger, PhD, was supported, in part, by a Kornfeld Scholars Program Award from the National Palliative Care Research Center.
Footnotes
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the data included in this publication.
Disclosure statement
The authors have no relevant financial or nonfinancial interests to disclose.
Ethics approval
Procedures complied with ethical guidelines and received Duke University Institutional Review Board approval (Pro00070823).
References
- 1.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 2.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34(6):611–635. doi: 10.1200/JCO.2015.64.3809 [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Yee C, Tam S, et al. Prevalence of pain in patients with breast cancer post-treatment: a systematic review. Breast. 2018;42:113–127. doi: 10.1016/j.breast.2018.08.105 [DOI] [PubMed] [Google Scholar]
- 4.Ilhan E, Chee E, Hush J, Moloney N. The prevalence of neuropathic pain is high after treatment for breast cancer: a systematic review. Pain. 2017;158(11):2082–2091. doi: 10.1097/j.pain.0000000000001004 [DOI] [PubMed] [Google Scholar]
- 5.Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat. 2018;167(1):157–169. doi: 10.1007/s10549-017-4485-0 [DOI] [PubMed] [Google Scholar]
- 6.Bovbjerg DH, Keefe FJ, Soo MS, et al. Persistent breast pain in post-surgery breast cancer survivors and women with no history of breast surgery or cancer: associations with pain catastrophizing, perceived breast cancer risk, breast cancer worry, and emotional distress. Acta Oncol. 2019;58(5):763–768. doi: 10.1080/0284186X.2019.1574023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilevarzadeh M, Amirshahi M, Afsargharehbagh R, et al. Global prevalence of depression among breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;176(3):519–533. doi: 10.1007/s10549-019-05271-3 [DOI] [PubMed] [Google Scholar]
- 8.Bamonti PM, Moye J, Naik AD. Pain is associated with continuing depression in cancer survivors. Psychol Health Med. 2018;23(10):1182–1195. doi: 10.1080/13548506.2018.1476723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandao T, Schulz MS, Matos PM. Psychological adjustment after breast cancer: a systematic review of longitudinal studies. Psychooncology. 2017;26(7):917–926. doi: 10.1002/pon.4230 [DOI] [PubMed] [Google Scholar]
- 10.Jafari A, Goudarzian AH, Bagheri Nesami M. Depression in women with breast cancer: a systematic review of cross-sectional studies in Iran. Asian Pac J Cancer Prev. 2018;19(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Chun M, Jung Y-S, Bae SH. Predictors of psychological distress trajectories in the first year after a breast cancer diagnosis. Asian Nurs Res (Korean Soc Nurs Sci). 2017;11(4):268–275. doi: 10.1016/j.anr.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 12.Jones SM, LaCroix AZ, Li W, et al. Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. J Cancer Surviv. 2015;9(4):620–629. doi: 10.1007/s11764-015-0438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399–409. doi: 10.1016/S0006-3223(03)00545-6 [DOI] [PubMed] [Google Scholar]
- 14.Bandura A Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- 15.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid-arthritis pain—catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51–56. doi: 10.1016/0304-3959(89)90152-8 [DOI] [PubMed] [Google Scholar]
- 16.Keefe FJ, Shelby RA, Somers TJ. Catastrophizing and pain coping: moving forward. Pain. 2010; 149(2): 165–166. doi: 10.1016/j.pain.2010.02.030 [DOI] [PubMed] [Google Scholar]
- 17.Somers TJ, Wren AA, Shelby RA. The context of pain in arthritis: self-efficacy for managing pain and other symptoms. Curr Pain Headache Rep. 2012;16(6):502–508. doi: 10.1007/s11916-012-0298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somers TJ, Keefe FJ, Pells JJ, et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J Pain Symptom Manage. 2009;37(5):863–872. doi: 10.1016/j.jpainsymman.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somers TJ, Kurakula PC, Criscione-Schreiber L, Keefe FJ, Clowse MEB. Self-efficacy and pain catastrophizing in systemic lupus erythematosus: relationship to pain, stiffness, fatigue, and psychological distress. Arthritis Care Res (Hoboken). 2012;64(9): 1334–1340. doi: 10.1002/acr.21686 [DOI] [PubMed] [Google Scholar]
- 20.Van Denburg AN, Shelby RA, Caldwell DS, O’Sullivan ML, Keefe FJ. Self-efficacy for pain communication moderates the relation between ambivalence over emotional expression and pain catastrophizing among patients with osteoarthritis. J Pain. 2018; 19(9): 1006–1014. doi: 10.1016/j.jpain.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 21.Tighe CA, Youk A, Ibrahim SA, et al. Pain catastrophizing and arthritis self-efficacy as mediators of sleep disturbance and osteoarthritis symptom severity. Pain Med. 2020;21(3):501–510. doi: 10.1093/pm/pnz187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster C, Breckons M, Cotterell P, et al. Cancer survivors’ self-efficacy to self-manage in the year following primary treatment. J Cancer Surviv. 2015;9(1):11–19. doi: 10.1007/s11764-014-0384-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop SR, Warr D. Coping, catastrophizing and chronic pain in breast cancer. J Behav Med. 2003;26(3):265–281. doi: 10.1023/A:1023464621554 [DOI] [PubMed] [Google Scholar]
- 24.Mosher CE, Duhamel KN, Egert J, Smith MY. Self-efficacy for coping with cancer in a multiethnic sample of breast cancer patients: associations with barriers to pain management and distress. Clin J Pain. 2010;26(3):227–234. doi: 10.1097/AJP.0b013e3181bed0e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omran S, McMillan S. Symptom severity, anxiety, depression, self-efficacy and quality of life in patients with cancer. Asian Pac J Cancer Prev. 2018;19(2):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shelby RA, Somers TJ, Keefe FJ, et al. Pain catastrophizing in patients with noncardiac chest pain: relationships with pain, anxiety, and disability. Psychosom Med. 2009;71(8):861–868. doi: 10.1097/PSY.0b013e3181b49584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelleher SA, Dorfman CS, Vilardaga JCP, et al. Optimizing delivery of a behavioral pain intervention in cancer patients using a sequential multiple assignment randomized trial SMART. Contemp Clin Trials. 2017;57:51–57. doi: 10.1016/j.cct.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 29.Somers TJ, Abernethy AP, Edmond SN, et al. A pilot study of a mobile health pain coping skills training protocol for patients with persistent cancer pain. J Pain Symptom Manage. 2015;50(4):553–558. doi: 10.1016/j.jpainsymman.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 31.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003; 106(3) :337–345. doi: 10.1016/j.pain.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 32.Anderson KO, Dowds BN, Pelletz RE, Edwards TW, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63(1):77–83. doi: 10.1016/0304-3959(95)00021-J [DOI] [PubMed] [Google Scholar]
- 33.Porter LS, Keefe FJ, Garst J, McBride CM, Baucom D. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain. 2008;137(2):306–315. doi: 10.1016/j.pain.2007.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelleher SA, Fisher HM, Winger JG, et al. Feasibility, engagement, and acceptability of a behavioral pain management intervention for colorectal cancer survivors with pain and psychological distress: data from a pilot randomized controlled trial. Support Care Cancer. 2021;29:5361–5369. doi: 10.1007/s00520-021-06126-8 [DOI] [PubMed] [Google Scholar]
- 35.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17(1):33–44. [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 37.Stagl JM, Bouchard LC, Lechner SC, et al. Long-term psychological benefits of cognitive-behavioral stress management for women with breast cancer: 11-year follow-up of a randomized controlled trial. Cancer. 2015;121(11):1873–1881. doi: 10.1002/cncr.29076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res. 1999;46(5):437–443. doi: 10.1016/S0022-3999(99)00004-5 [DOI] [PubMed] [Google Scholar]
- 39.Kline RB. Principles and Practice of Structural Equation Modeling. 2nd ed. New York, NY: Guildford Press; 2005. [Google Scholar]
- 40.Muthén L, Muthén B. MPlus User’s Guide. 6th ed. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- 41.Cohen J A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 42.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–287. doi: 10.1037/0882-7974.12.2.277 [DOI] [PubMed] [Google Scholar]
- 43.Skidmore JR, Koenig AL, Dyson SJ, Kupper AE, Garner MJ, Keller CJ. Pain self-efficacy mediates the relationship between depressive symptoms and pain severity. Clin J Pain. 2015;31(2):137–144. doi: 10.1097/AJP.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 44.Keefe FJ, Kashikar-Zuck S, Robinson E, et al. Pain coping strategies that predict patients’ and spouses’ ratings of patients’ self-efficacy. Pain. 1997;73(2):191–199. doi: 10.1016/S0304-3959(97)00109-7 [DOI] [PubMed] [Google Scholar]
- 45.Porter LS, Steel JL, Fairclough DL, et al. Caregiver-guided pain coping skills training for patients with advanced cancer: results from a randomized clinical trial. Palliat Med. 2021;35(5):952–961. doi: 10.1177/02692163211004216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Zhang L, Yang L, Cheng-Qi H. Effectiveness of pain coping skills training on pain, physical function, and psychological outcomes in patients with osteoarthritis: a systemic review and meta-analysis. Clin Rehabil. 2021;35(3):342–355. doi: 10.1177/0269215520968251 [DOI] [PubMed] [Google Scholar]
- 47.Dorfman CS, Kelleher SA, Winger JG, et al. Development and pilot testing of an mHealth behavioral cancer pain protocol for medically underserved communities. J Psychosoc Oncol. 2019;37(3):335–349. doi: 10.1080/07347332.2018.1479327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rini C, Porter LS, Somers TJ, et al. Automated Internet-based pain coping skills training to manage osteoarthritis pain: a randomized controlled trial. Pain. 2015;156(5):837–848. doi: 10.1097/j.pain.0000000000000121 [DOI] [PMC free article] [PubMed] [Google Scholar]


