Abstract
A quorum-sensing mechanism involving the pheromone ComX and the ComP-ComA two-component system controls natural competence in Bacillus subtilis. ComX is expressed as a cytoplasmic inactive precursor that is released into the extracellular medium as a cleaved, modified decapeptide. This process requires the product of comQ. In the presence of ComX, the membrane-localized ComP histidine kinase activates the response regulator ComA. We compared the sequences of the quorum-sensing genes from four closely related bacilli, and we report extensive genetic polymorphism extending through comQ, comX, and the 5′ two-thirds of comP. This part of ComP encodes the membrane-localized and linker domains of the sensor protein. We also determined the sequences of the comX genes of four additional wild-type bacilli and tested the in vivo activities of all eight pheromones on isogenic strains containing four different ComP receptor proteins. A striking pattern of specificity was discovered, providing strong evidence that the pheromone contacts ComP directly. Furthermore, we show that coexpression of comQ and comX in Escherichia coli leads to the production of active pheromone in the medium, demonstrating that comQ is the only dedicated protein required for the processing, modification, and release of active competence pheromone. Some of the implications of these findings for the evolution and the mechanism of the quorum-sensing system are discussed.
Under certain growth conditions, Bacillus subtilis expresses a set of proteins involved in the uptake and integration of extracellular DNA (reviewed in reference 3). The expression of these proteins in the competent state is tightly controlled and initially regulated by a quorum-sensing (QS) system involving the ComX pheromone (14–17, 30, 31) and the ComP-ComA two-component system (38, 40). The membrane-localized histidine kinase ComP, in the presence of ComX, activates the response regulator ComA, permitting the expression of downstream regulatory genes (4). This signal transduction cascade culminates with the expression of the competence transcription factor ComK (35, 36), resulting in the synthesis of the DNA uptake and recombination proteins. A convergent pathway utilizes a second pheromone, the competence-stimulating factor (CSF) peptide, to modulate this response (16, 31). This second pathway, which exerts a relatively minor effect, probably acts by inhibiting a phosphatase specific for ComA-ComP (22, 23). The two pathways thus appear to converge, both controlling the level of ComA phosphorylation (14, 15, 30).
The ComX pheromone is translated as an inactive propeptide, which is then cleaved and released in the extracellular medium as a modified decapeptide, with the addition of an uncharacterized lipid moiety to a tryptophan residue (17). At least one other gene, comQ (39), is required for the production of modified, active ComX (17), but the precise role of ComQ is not understood.
In the laboratory strains of B. subtilis, derived from strain 168, the comQ, comX, comP, and comA genes are clustered on the chromosome in the order given. Interestingly, a similar genetic organization has been reported for two other bacterial QS systems involving peptide pheromones: the agrBDCE and the comCDE systems, regulating virulence in Staphylococcus aureus and competence in Streptococcus pneumoniae, respectively (9, 12, 41). In these cases, substantial genetic polymorphism of the pheromones as well as of the sensor histidine kinases has been reported. This genetic polymorphism is associated with the specificity of each pheromone for its cognate receptor (9, 26), pointing to the presence of distinct pherotypes among staphylococcal and streptococcal species.
A suggestion that such extensive polymorphism may also exist in Bacillus has been provided by the recent comparison of the QS loci of B. subtilis 168 and B. natto NAF4 (34). Since the evolution, ecology, and mechanistic aspects of this polymorphism are of interest, we have extended the initial observations reported in this paper, sequencing all or part of the comQXPA loci from a number of closely related bacilli and comparing their pherotype cross-specificities. We report a striking polymorphism within the DNA sequences encoding ComQ, ComX, and the N-terminal two-thirds of ComP. We constructed a set of isogenic B. subtilis “producer” strains that release different pheromone molecules into the medium and a set of isogenic “tester” strains expressing different ComP sensors and ComQ proteins. We show that each ComP sensor is specifically activated in vivo by its cognate pheromone and in some cases by a limited set of pheromones from other strains, indicating that ComX-ComP pairs determine different pherotypes in Bacillus. In addition, we show that the competence pheromone can be produced in Escherichia coli in the presence of ComQ. Our results show that ComQ and ComX are the only dedicated proteins required for production of the competence pheromone, and that comQ, comX, and comP are the sole determinants of the QS pherotype.
MATERIALS AND METHODS
Growth conditions and general methods.
E. coli KC8 (Clontech Laboratories, Inc.) was used for cloning, and transformants were selected on Luria-Bertani (LB) agar supplemented with ampicillin, (100 μg/ml). E. coli KC8 ComX producer strains were grown in liquid competence medium (1) supplemented with histidine, methionine, tryptophan, uracil (20 μg/ml), and isopropyl-β-d-thiogalactopyranoside (IPTG) (2 mM). B. subtilis was grown either in liquid competence medium, on minimal medium (2) plates supplemented with glucose and the required amino acids (50 μg/ml), or on Tryptose Blood Agar Base (Difco) plates supplemented with chloramphenicol (5 μg/ml), erythromycin (5 μg/ml), kanamycin (5 μg/ml), phleomycin (1 μg/ml), spectinomycin (100 μg/ml), or tetracycline (20 μg/ml). 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was used at a final concentration of 0.004%. B. subtilis competent cells were prepared as described previously (1). DNA manipulations, cloning, and standard molecular biological procedures were performed as described previously (29). The strains used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| B. subtilis | ||
| OKB125 | his leu met srfA::Tn917Ω OK120 (pTV55) Cmr | 21 |
| BD1658 | his leu met comP::cat | This work |
| BD2833 | his leu met srfA-lacZ (tet) | This work |
| BD2834 | his leu met srfA-lacZ (tet) orf-2::kan | This work |
| BD2847 | his leu met srfA-lacZ (tet) (comQ comX comP replaced by genes from B. natto NAF4) | |
| BD2876 | his leu met srfA-lacZ (tet) comQ::Km | This work |
| BD2877 | his leu met srfA-lacZ (tet) (comQ::phl comX comP replaced by genes from B. natto NAF4) | This work |
| BD2911 | his leu met srfA-lacZ (tet) amyE::xylR Pxyl-comK (ery) (ΔcomQXP)::Km | This work |
| BD2913 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (comQ comX comP replaced by genes from B. mojavensis RO-H-1) | This work |
| BD2914 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (comQ comX comP replaced by genes from B. subtilis RS-B-1) | This work |
| BD2915 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (comQ comX comP replaced by genes from B. natto NAF4) | This work |
| BD2935 | his leu met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (ΔcomQXP)::Km | This work |
| BD2936 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ comX comP replaced by genes from B. mojavensis RO-H-1) | This work |
| BD2937 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ comX comP replaced by genes from B. mojavensis RO-C-2) | This work |
| BD2939 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ comX comP replaced by genes from B. subtilis RO-FF-1) | This work |
| BD2947 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ comX comP replaced by genes from B. subtilis RO-O-2) | This work |
| BD2962 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ::pED345 comX comP replaced by genes from B. mojavensis RO-H-1) | This work |
| BD2963 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ::pED346 comX comP replaced by genes from B. mojavensis RO-C-2) | This work |
| E. coli | ||
| ED318 | KC8 (PCR script with comQ comX from B. subtilis 168 in + orientation) | This work |
| ED319 | KC8 (PCR script with comQ comX from B. subtilis 168 in − orientation) | This work |
| KC8 | hsdR leuB600 trpC9830 pyrF::Tn5 hisB463 lacΔX74 strA galU galK | Clontech |
| Plasmids | ||
| pED302 | PCR script with kan cassette between degQ and comA | This work |
| pED345 | pUS19 with internal comQ fragment of B. mojavensis RO-H-1 in EcoRI/HindIII sites | This work |
| pED346 | pUS19 with internal comQ fragment of B. mojavensis RO-C-2 in EcoRI/HindIII sites | This work |
| pUS19 | pUC19 with spc cassette between the NdeI and NarI sites | This work |
Preparation and assay of conditioned medium.
Conditioned medium was prepared by growing producer strains to 2 h past the end of exponential growth. Cells were removed by centrifugation, and the supernatants were sterilized by filtration through 0.2-μm-pore-size filters. Conditioned medium was stored at −20°C. Overnight cultures of tester strains were grown at 32°C in competence medium and then diluted 50-fold into fresh competence medium. An equal volume of prewarmed conditioned medium was added, and aliquots were removed for assay of β-galactosidase at intervals during growth at 37°C. β-Galactosidase assays were carried out as described previously (1) except that the enzyme reactions were carried out in microtiter plates and the course of the reactions were followed using a Tecan Rainbow plate reader. β-Galactosidase activities were calculated from the slopes of the reaction curves.
Sequencing of the RO-H-1 and RS-B-1 comQXPA loci.
The entire comQXP locus from RS-B-1 and RO-H-1 was amplified by PCR with primers RS comB-Pst (CGAATTCCTCGACCTCATAACCGG) and RS comA-Eco (CGGAATTCACGAATCGCTTCCTC) and with primers Q3 (GTGTCCGGATCAAGGAGAAA) and P1 (AAGAACCGAATCGTGGAGATCGGC), respectively. The PCR fragments were then directly sequenced. As sequences were collected, additional primers were synthesized. The sequencing of the B. natto NAF4 comQXP locus has been described elsewhere (34).
Sequencing of the comX genes from RO-O-2, RO-C-2, RO-FF-1, and RO-B-2.
PCR products corresponding to the entire comQ and comX genes and the first 1.7 kb of comP were obtained with the primers unicomQ-Xba (GTCTAGAGGAATGGGAGGGGGGAAG) and P1 (see above), located just upstream of comQ and within a conserved part of comP, respectively. Chromosomal DNA was used as the template. The 3-kb PCR products were purified and cloned into pPCR-Script Amp (SK+) (Stratagene). The resulting inserts were sequenced using the M13 forward and reverse primers and then using primers designed on the basis of the initial sequence obtained.
Plasmid constructions.
Plasmid pED302, used to replace the comQXP locus from B. subtilis BD2833 with a Kmr cassette, was constructed in three steps. A PCR fragment containing the 3′ end of comP and the 5′ end of comA was obtained by amplification of B. subtilis DNA with SalI-′comP (ACTGTCGACAGATGAATGAGCAGATGTC) and comA′-Pst (CCCTGCAGATCCATCTCTACACCCCCC) primers, and cloned into the SrfI site of pPCR-script (Stratagene) to give pED300. A second PCR fragment contained the upstream degQ gene and was obtained with the primers Eco-degQ (AGAATTCGGCTGCGGTCAGAATG) and degQ-Xma (AACCCGGGCTGCTCAATAACGACTTCC). This fragment was cloned into pED300, giving rise to pED301. A HincII kanamycin resistance cassette from pKM1 (11) was then inserted into pED300 at the SmaI restriction site, between the two cloned fragments, giving rise to pED302, in which the resistance cassette was flanked by sequences derived from upstream and downstream of the region to be deleted.
The pED318 pheromone production plasmid was derived from the PCR script vector by insertion of PCR-amplified DNA fragments encoding the B. subtilis 168 comQ and comX genes at the SrfI restriction site. Primers used for the PCR were uni-comQ-Xba (see above) and 168-comX/Sph (CGCATGCCACCTATTAATCACCCC). pED319 is the same as pED318 but with comQX cloned in the opposite (unexpressed) orientation.
Construction of Bacillus strains.
BD2833 was constructed by transforming a strain carrying the Tn917 lacZ OK120 insertion in srfA (21) with a Cm-to-Tc switching plasmid (32), replacing the Cmr marker with a Tcr marker. This insertion was used because it occurred downstream from comS and therefore does not interfere with transformation, facilitating further strain construction. The B. natto NAF4 QS system was introduced into BD1658 by transformation with pNAF193. This plasmid carries the entire B. natto comQXPA locus with an Spr cassette inserted in the SacI site of yuzC, a small gene of unknown function to the left of degQ. BD1658 has a Cmr cassette inserted in comP. Selection for Spr was carried out, and transformants were screened for Cms. DNA from such a transformant was used to introduce the B. natto QS system into BD2834 to create BD2847. BD2834 carries a Kmr marker in an irrelevant open reading frame downstream of comA as well as a srfA-lacZ reporter construct. Selection for Spr was carried out, and transformants were screened for loss of Kmr. The loss of this marker selects for introduction of the entire B. natto NAF4 QS locus. A comX derivative of BD2847 (BD2877) was made by transformation with a plasmid carrying the QS locus of B. natto with a Phr cassette inserted in the MluI site of comX. BD2911 was obtained by transforming B. subtilis BD2833 with the linearized pED302 plasmid with selection for Kmr. Double recombination leads to a deletion of comQ, comX, and the first 613 codons of comP. BD2911 also contains a copy of comK in the amyE locus, under Pxyl control (6). Since BD2911 was a comQ, comX, and comP mutant, it was made competent by growth in the presence of xylose. To introduce the QS locus of heterologous Bacillus strains by congression, BD2911 was transformed with chromosomal DNA from these strains. In these transformations, selection was for the unlinked leu marker on X-Gal plates to score for the introduction of the donor QS system. For most of these congression experiments, BD2935, a derivative of BD2911, was used. BD2935 has a Cmr marker associated with the Pxyl-comK construct at the amyE locus, instead of Emr.
Tester strains for RO-H-1 (BD2962) and RO-C-2 (BD2963) were obtained by disruption of the comQ genes of the respective producer strains (BD2913 and BD2937). An internal fragment of each comQ gene (0.45 kb for RO-C-2 and 0.52 kb for RO-H-1) was PCR amplified by PCR using primers with EcoRI and HindIII sites and was cloned into the EcoRI and HindIII restriction sites of pUS19. This vector consists of pUC19 with a Spr cassette cloned between the NdeI and NarI restriction sites. The resulting plasmids (pED345 for RO-H-1 and pED346 for RO-C-2) were then used to transform the producer strains (BD2913 and BD2937) with selection for Spr. This resulted in inactivation of the chromosomal comQ genes by Campbell-like recombination.
PCR-RFLP genotyping of the ComX producer strains.
Chromosomal DNA was prepared from BD2913, BD2914, and BD2915, and PCR amplification of comQ, comX, and the 5′ end of comP was performed by using primers uni-comQ-Xba and P1, hybridizing upstream from comQ and in a conserved region of comP, respectively. These PCR fragments were then digested with either HindIII or EcoRV restriction enzyme. This restriction analysis allowed the four QS loci to be distinguished (not shown) and confirmed that BD2913, BD2914, and BD2915 were carrying RO-H-1, RS-B-1, and B. natto NAF4 comQXP loci, respectively.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the sequences of the B. mojavensis RO-H-1 and B. subtilis RS-B-1 comQXPA loci are AY003901 and AY003900, respectively. The GenBank accession numbers of the comX sequences obtained in this study are AY003902 (RO-OO-2), AY003904 (RO-C-2), AY003903 (RO-FF-1), and AY003905 (RO-B-2).
RESULTS
Bacillus strains.
A group of Bacillus isolates was used for analysis of polymorphism at the QS locus. These are listed and described in Table 2. Most were collected in the Mojave desert by Roberts and Cohan (27), who also described their phylogenetic relationships. Our selected group of strains also includes B. natto NAF4 (34) and laboratory strains of B. subtilis, derived from strain 168. The B. subtilis strains have been assigned to the 168 and W23 subspecies (20, 27) (Table 2).
TABLE 2.
Wild-type Bacillus strains
The comQ, comX, and comP genes are highly polymorphic.
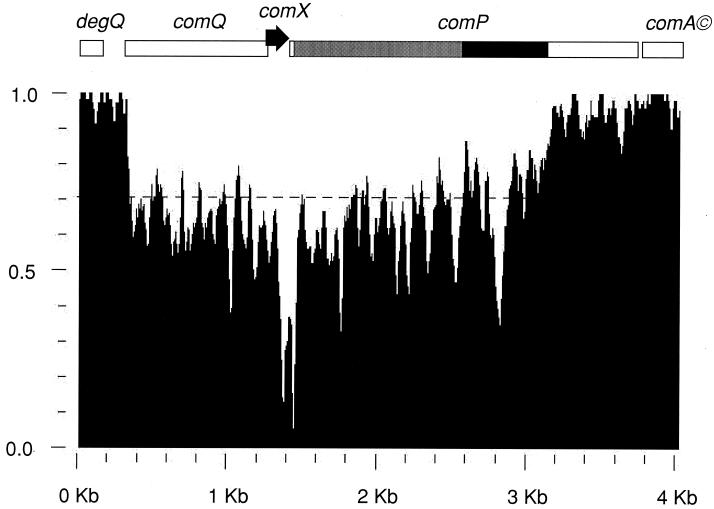
The comQ, comX, comP, and comA genes from B. mojavensis RO-H-1 and B. subtilis RS-B-1 were sequenced and compared to those of B. subtilis 168 and B. natto NAF4 (34). We compared the four degQ comQXPA DNA sequences using the PLOTSIMILARITY program (Genetics Computer Group [GCG] Wisconsin package). degQ is a flanking gene that is not involved in the QS mechanism. The sequences used for this analysis extended from the degQ start codon up to the 63rd codon of comA. The resulting plot (Fig. 1) displays the average similarity among the four aligned degQ comQ comX comP comA DNA sequences at each position in the alignment, using a moving window of 25 nucleotides. A strong polymorphism is evident, extending from the beginning of the comQ open reading frame (starting at the 6th codon) through the first two-thirds of comP. In contrast, degQ, comA, and the C-terminal third of comP, corresponding to the histidine kinase domain, are highly conserved among the four strains. The degQ and comA nucleotide sequences display more than 90% identity. Interestingly, the polymorphism is hypervariable in several regions. One corresponds to the DNA sequences encoding the C-terminal end of ComX extending through the first 20 codons of comP. Another hypervariable region is located in the central portion of the DNA that encodes the comP linker. We define this linker as extending from the membrane domain of comP to the DNA encoding the conserved H box (5) of ComP (position 564 in the B. subtilis 168 ComP sequence). In summary, this sequence comparison shows that the DNA sequences are almost identical among the four Bacillus strains until the 6th codon of comQ. Then a dramatic polymorphism extends roughly up to the ComP histidine kinase domain, where the divergence weakens, so that the comA sequences are almost identical in all four strains. A pairwise comparison of the four strains revealed that the entire sequences of B. mojavensis RO-H-1 and B. subtilis RS-B-1 are quite similar to one another, although distinct from those of B. natto NAF4 and B. subtilis 168.
FIG. 1.
Polymorphism at the Bacillus QS locus. DNA sequences of B. subtilis 168 and RS-B-1, and of B. mojavensis RO-H-1 and of B. natto NAF4, extending from the degQ start codon to codon 63 of comA, were aligned using the PILEUP alignment program (GCG Wisconsin package). This alignment was used as an input sequence for the PLOTSIMILARITY program. A 25-nucleotide window of comparison was moved one position at a time along the sequence, and the average similarity within the window is plotted at the middle position of the window. The overall average similarity is plotted as a dotted line. A genetic map is also shown above the plot. The membrane and linker domains of ComP are shown as shaded and solid bars, respectively. The linker domain is defined as extending from the C-terminal end of the 8th putative membrane-spanning segment to the 1st residue of the ComP H box.
In addition to these comparisons, we have sequenced the comX genes from the four additional wild-type Bacillus isolates listed in Table 2. An alignment of the predicted sequences of all eight ComX proteins is displayed in Fig. 2. The only constraint placed on these comparisons is that we forced alignment of the tryptophan residues, known to be the site of modification in B. subtilis 168 (17). Several of the predicted ComX sequences are closely similar to one another (e.g., RO-H-1 and RS-B-1, RO-B-2 and RO-OO-2, and RO-C-2 and 168). The B. natto NAF4 sequence is remarkable in that it has both N- and C-terminal extensions. The mature B. subtilis 168 ComX molecule is processed to yield an N-terminal alanine (Fig. 2) or aspartate residue (17). It is striking that the most extreme polymorphism occurs downstream of the presumptive processing sites, with the sole exception of the tryptophan residue that bears the uncharacterized posttranslational modification.
FIG. 2.
Alignment of ComX amino acid sequences. The conserved W residue is marked in boldface and was used to align the sequences with the ZEGA program (http://molsoft.com/services/help/intro.htm). The following symbols indicate similar residues: #, hydrophobic residues; ∼, polar residue; ^, small residue; %, aromatic residue. Nonconserved residues are indicated by dots. The N-terminal alanine in B. subtilis 168 ComX is underlined.
Replacement of comQXP genes from B. subtilis 168 with their orthologs from other bacilli.
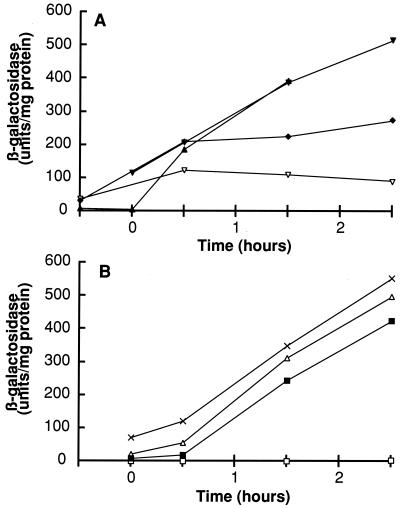
In order to compare the specificities of these QS systems, it was necessary to construct isogenic strains that produce pheromones (producer strains) and can respond to them (tester strains). This requirement for isogenicity is more than just good genetic practice. The addition of conditioned media from heterologous strains generally resulted in pronounced growth inhibition (not shown), most likely due to the presence of defective phages (8), bacteriocins (42), antibiotics, or other toxic substances (not shown). Two genetic methods were employed for the construction of these strains, and the results obtained with them were the same. We first constructed strains BD2911 and BD2935. These B. subtilis 168 strains carry a srfA-lacZ reporter for QS activity, and a replacement of the comQXP genes by a Kmr marker. At the amyE locus is a copy of comK under the control of a xylose-inducible promoter. BD2911 and BD2935 differ only in the antibiotic resistance markers associated with the Pxyl-comK constructs at the amyE locus. When they are grown in competence medium, the addition of 2% xylose enables transformation to occur in the comQXP deletion strains. BD2911 or BD2935 were transformed with chromosomal DNA prepared from a given heterologous strain, with selection for leucine prototrophy on plates containing X-Gal. Those Leu transformants that had incorporated foreign comQXP genes by congression were visualized as blue colonies due to the expression of sfrA-lacZ. These strains were checked for Kms, which results from the replacement of the Kmr marker by the foreign comQXP genes, and were further characterized by PCR genotyping (see Materials and Methods). In addition to strain 168 itself, seven such additional producer strains were constructed from RO-OO-2, RO-FF-1, RS-B-1, RO-B-2, RO-H-1, RO-C-2, and B. natto NAF4. As shown in Fig. 3, all of these strains except RO-OO-2 expressed significant levels of srfA, although the amounts of β-galactosidase expressed by the various strains varied somewhat. For instance, the RS-B-1 producer strain consistently exhibited lower β-galactosidase activity than the others, and the B. natto NAF4 producer exhibited an intermediate level. To exclude the possibility that a relevant unsuspected gene was integrated into B. subtilis by congression during the construction of these producer strains, we employed a second strategy to construct producer and tester strains carrying the B. natto NAF4 system as described in Materials and Methods. A plasmid carrying only the QS locus and a few flanking genes from B. natto was used to replace the QS locus of B. subtilis 168 as described in Materials and Methods. A tester strain was derived from this producer by inactivation of comX. This producer strain expressed a level of β-galactosidase comparable to that shown in Fig. 3 (data not shown) and produced a conditioned medium that could activate the tester strain. Taken together, these results demonstrate that the QS systems from other bacilli can replace that of B. subtilis 168, strongly suggesting that all of the strains, with the possible exception of RO-OO-2, possess active ComX-mediated QS systems.
FIG. 3.
Expression of srfA-lacZ by producer strains. β-Galactosidase activities were measured in a set of isogenic B. subtilis strains expressing heterologous comQXP genes. Time is in hours before or after the time of transition from exponential to stationary phase. The specificities of the producer strains in the two panels were as follows. (A) ▴, 168 (BD2833); ▾, RO-H-1 (BD2913); ▿, RS-B-1 (BD2914); ⧫, B. natto NAF4 (BD2915). (B) ×, RO-FF-1 (BD2939); ▵, RO-B-2 (BD2936); ■, RO-C-2 (BD2937); □, RO-OO-2 (BD2947).
Specificity of pheromone recognition.
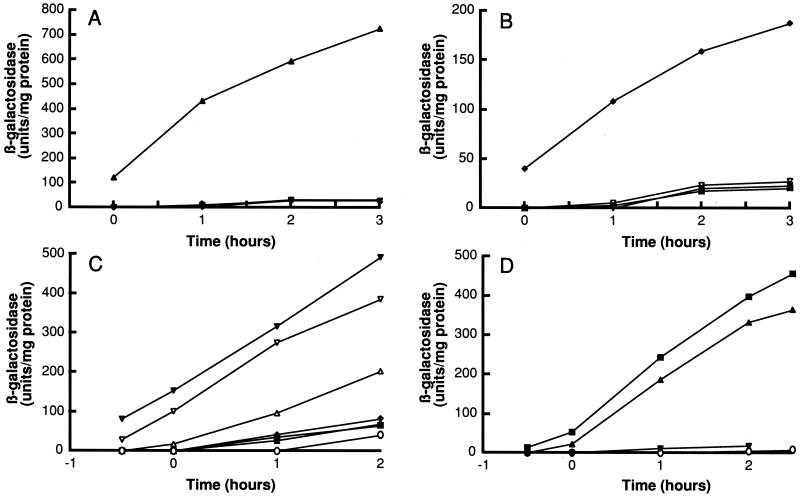
The striking polymorphism noted above suggests that there is specificity of interaction between a given pheromone, its receptor protein ComP, and the processing protein ComQ. We have investigated this predicted specificity with regard to ComX and ComP. For this, the isogenic producer strains were grown in competence medium to prepare conditioned medium. Four tester strains were constructed by inactivating the comQ or comX genes of the 168, RO-H-1, RO-C-2, and B. natto NAF4 producer strains, as described in Materials and Methods. The tester strains cannot produce active pheromone but are expected to express ComP sensor proteins. Each conditioned medium was mixed with an equivalent volume of fresh competence medium and used to grow each of the isogenic tester strains. β-Galactosidase activities were measured at different times for each of the producer-tester combinations, and representative experiments are reported in Fig. 4. A summary of the pattern of responses is reported in Table 3, which includes results for RO-FF-1 that are not shown in Fig. 4. Since the growth of the tester strains in diluted conditioned medium was slightly less than in fresh medium, in some experiments we used conditioned medium from the tester strain itself as a control, instead of fresh medium. This seemed to give a slightly higher “baseline” response, probably due to the presence of CSF (31), but the qualitative pattern was the same when either control was used. In all cases, although conditioned medium was added at the onset of the experiment, the specific activity of β-galactosidase increased during growth. This was expected, since additional control mechanisms for srfA are known to exist (4). Conditioned medium from the RO-OO-2 “producer” strain failed to induce expression of srfA-lacZ from any of the tester strains, consistent with the absence of activity of this strain when tested by itself (Fig. 3). Several examples of specificity are evident from the results in Table 3 and Fig. 4. For example, the B. natto NAF4 pheromone induces only the cognate tester, and conversely, the B. natto NAF4 tester is induced only by its cognate pheromone. Some cross talk is also evident. For instance, the 168 pheromone induces the RO-C-2 tester, and the RO-H-1, RS-B-1, and the RO-B-2 pheromones induce the RO-H-1 tester. It should be noted that this is not a complete response matrix, and the full pattern of specificity is therefore not evident.
FIG. 4.
Specificity of the QS response. Isogenic B. subtilis tester strains that do not produce ComX were grown in the presence of conditioned media prepared from isogenic producer strains. β-Galactosidase expressed from a srfA-lacZ reporter construct was assayed during growth. The following tester strains were used: BD2876 (derived from B. subtilis 168) (A), BD2877 (derived from B. natto NAF4) (B), BD2962 (derived from B. mojavensis RO-H-1) (C), and BD2963 (derived from B. mojavensis RO-C-2) (D). Conditioned media were from strain 168 (BD2833) (▴), RO-H-1 (BD2913) (▾), RS-B-1 (BD2914) (▿), B. natto NAF4 (BD2915) (⧫), RO-B-2 (BD2936) (▵), and RO-C-2 (BD2937) (■). The open circles in panels C and D show the effect of adding fresh medium to the tester (panel D) or conditioned medium from the tester itself (panel C). Time is in hours before or after the time of transition from exponential to stationary phase. Note that the ordinate scales differ in the four panels.
TABLE 3.
Summary of QS specificities
| Producer specificity | Response of the indicated tester strain to conditioned medium from the producer straina
|
|||
|---|---|---|---|---|
| 168 | RO-C-2 | RO-H-1 | NAF4 | |
| B. subtilis 168 | ++ | ++ | − | − |
| B. mojavensis RO-C-2 | +/−b | ++ | − | − |
| B. subtilis RO-OO-2 | − | − | − | − |
| B. subtilis RS-B-1 | − | − | ++ | − |
| B. mojavensis RO-B-2 | − | − | + | − |
| B. mojavensis RO-H-1 | − | − | ++ | − |
| B. natto NAF4 | − | − | − | ++ |
| B. subtilis RO-FF-1 | − | − | − | − |
Determined as described in the text. The approximate magnitude of each response is indicated.
This response is very weak but was noted in several experiments. It is not evident in Fig. 4.
Production of active ComX pheromone from E. coli.
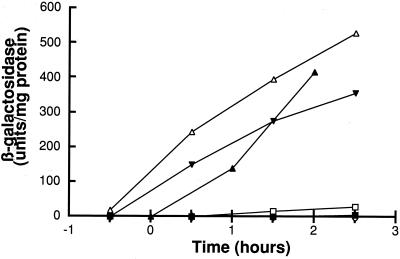
The experiments reported above, as well as unpublished work described below, demonstrate that comX, comQ, and comP are sufficient to specify the pherotype. We next determined whether comQ and comX are sufficient for production of active ComX. For this, the B. subtilis comQ and comX genes were cloned into an E. coli plasmid under the control of the lac promoter. A second plasmid containing the same cloned fragment inserted in the opposite (unexpressed) direction was used as a control. E. coli strains carrying these plasmids were grown for 5 h in liquid competence medium supplemented with IPTG in order to induce comQX expression. Cells were harvested by centrifugation, and the supernatants were collected and filter sterilized. These supernatants were diluted into fresh competence medium, and B. subtilis BD2876 was used as a tester strain. As shown in Fig. 5, srfA-lacZ expression was induced to a level comparable to that of the BD2833 reference strain. The activity detected was dose dependent. The supernatant prepared from the reversed-orientation control did not lead to detectable sfrA expression. These data demonstrate that the ComX pheromone can be produced and secreted in E. coli, and that no accessory gene from B. subtilis, other than comQ, is required for this production. In other experiments we have shown that the active pheromone produced by E. coli does not appear to accumulate in the periplasm (not shown).
FIG. 5.
Competence pheromone produced in E. coli. The B. subtilis 168 tester strain (BD2876) was grown in the presence of supernatant prepared from E. coli (ED318) carrying a plasmid with the B. subtilis 168 comQ and comX genes. β-Galactosidase activities produced from a srfA-lacZ reporter construct were assayed during growth. BD2876 was grown in the presence of 10-fold (▵), 20-fold (▾), and 100-fold (□) dilutions of the E. coli supernatant. The activities of the B. subtilis producer (BD2833) (▴) and tester (○) strains grown in fresh medium are included for comparison. (The open circles are obscured by the solid squares). Conditioned medium from an isogenic E. coli strain (ED319) carrying the comQ and comX genes in reversed (nonexpressing) orientation (■) exhibited no activity.
DISCUSSION
The QS system that governs srfA and competence gene expression in B. subtilis joins the Agr system of S. aureus (9, 10, 18) and competence regulation in S. pneumoniae (7, 24, 26, 41) in exhibiting striking polymorphism at the relevant loci and a corresponding variability in pherotype specificity. Bacillus and Staphylococcus QS systems represent a class of gram-positive mechanisms that involve peptide pheromones and membrane-localized histidine kinase sensors with complex topologies (12). It is likely that other QS systems in this group will also exhibit polymorphism at the relevant loci.
In the Bacillus system it appears that the comQ, comX, and comP genes are sufficient to determine specificity and that the strain 168 ComA can interact well with any of the ComP molecules. This was expected from the pattern of polymorphism in these genes (Fig. 1), since both ComA and the C-terminal domain of ComP with which it interacts are highly conserved. It has been confirmed by unpublished experiments (M. Ansaldi and D. Dubnau) in which chimeras containing all or part of the N-terminal domain of a given ComP respond to the cognate pheromone even if the comA gene is from strain 168.
The mechanism of transmembrane signaling in these systems is not understood, nor is the role of the unusual polytopic histidine kinase membrane domains characteristic of this group of sensor proteins. ComP for instance, has six to eight membrane-spanning segments and two large extracellular loops (25). Sequence comparisons may assist in making testable predictions concerning the interacting residues in ComX and ComP, as well as those in ComX and ComQ. For instance, it is interesting that hypervariable regions seem to exist in ComQ, in the C terminus of ComX, in the center of the linker region of ComP, and in about five additional segments in the N-terminal domain of ComP (Fig. 1). The C-terminal ComX variations presumably determine the observed variations in pheromone specificity, since they encode the mature pheromones. This is supported by the correspondence between the predicted amino acid sequences of the mature ComX molecules and the pherotype specificities (see below). Hypervariability in the N-terminal region of ComP suggests that some or all of these segments may be involved in determining response specificity. We have reported that deletion of one of the extracellular loops and two of the membrane-spanning segments of ComP confers complete ComX independence on srfA transcription (25). Clearly, the ComX-ComP interaction and the mechanism of transmembrane signaling are complex. The apparent conservation of the tryptophan residue in the eight ComX sequences, together with the many conserved residues in the four available ComQ sequences, strongly suggest that the uncharacterized modification of ComX is the same in all the strains. The conserved residues upstream of the presumptive cleavage site in ComX probably reflect common features in ComQ recognition.
In the staphylococcal Agr system, the peptide pheromone produced by one strain inhibits the response of another (9). We have tested the B. subtilis 168 tester strain for evidence of such interference (not shown). The homologous (168 specificity) conditioned media were mixed with equal amounts of each of the producer-conditioned media, and the srfA-lacZ expression was compared to that obtained by diluting the 168 conditioned medium with an equal volume of tester conditioned medium. In contrast to the staphylococcal results, we observed no dramatic interference. Dilution of the 168 conditioned medium with tester strain conditioned medium produced a reduction in srfA-lacZ activity. Dilution with the heterologous conditioned media gave approximately the same response. At most, an inhibition of about 25% was noted when the B. mojavensis RO-H-1 conditioned medium was used. However, since we do not know the concentrations of the various pheromones in the conditioned media, the absence of a strong effect makes this experiment somewhat inconclusive. Tran et al. (34) have also failed to detect interference between B. natto NAF4 and B. subtilis 168.
Several additional features of the pherotype specificity pattern deserve discussion. First, the RO-OO-2 producer strain exhibits no β-galactosidase activity, indicating that the system fails to induce srfA transcription, and the conditioned medium from this strain fails to induce any of the testers we have used. This has been observed with independently made RO-OO-2 producer constructs. We have no explanation for this failure, except to suggest that the original B. subtilis RO-OO-2 strain may carry an inactivating mutation in the QS locus. We have sequenced the comX gene from this strain, and it is evident from Fig. 2 that it is nearly identical to that of the fully active comX from B. mojavensis RO-B-2. There are however, three differences in the predicted amino acid sequences of these two precursor ComX molecules. These differences, or a mutation in comQ, may be responsible for the failure of the RO-OO-2 producer strain to synthesize detectable pheromone.
Second, the RO-FF-1 producer strain exhibits robust expression of srfA-lacZ, while conditioned medium from this strain does not activate any of the tester strains. Inspection of the C-terminal sequence of the RO-FF-1 pheromone confirms that its predicted mature pheromone differs from the others at every position except the conserved tryptophan residue (Fig. 2).
A third point concerns B. mojavensis RO-H-1 and B. subtilis RS-B-1. The entire QS loci of these two strains are quite similar (not shown), and conditioned media from both the RO-H-1 and RS-B-1 producer strains activate the RO-H-1 tester equally well. The RS-B-1 producer, however, exhibits low activity (Fig. 3). We have constructed an RS-B-1 tester strain and have failed to demonstrate a response to conditioned medium from either the RS-B-1 or RO-H-1 producers (not shown). We have no explanation for this anomaly except that insertion of an inactivating cassette in the comQ gene of RS-B-1 may exert a polar effect on comP expression in this strain if the locations of promoter sequences in RS-B-1 differ from those of the other strains.
Fourth, an asymmetry is apparent in the responses of B. subtilis 168 and B. mojavensis RO-C-2. The RO-C-2 tester is activated by conditioned medium from both producer strains, whereas the 168 tester is activated by the homologous conditioned medium but very poorly by the RO-C-2 pheromone (not shown). This is likely due to the nature of the presumed ComP binding sites for pheromone. The mature pheromones of RO-C-2 and 168 are similar, sharing 4 out of 10 identities (Fig. 2). It is therefore apparent that the patterns of pheromone and receptor activities need not be identical.
Our analysis demonstrates the existence of at least four pherotype specificities in these Bacillus strains. One group consists of B. subtilis 168 and B. mojavensis RO-C-2, although these strains exhibit asymmetric responses, as just noted. A second group consists of B. subtilis RS-B-1 and the B. mojavensis isolates RO-H-1 and RO-B-2. The predicted mature pheromone sequences of RO-H-1 and RS-B-1 are nearly identical, exhibiting only two conservative replacements. The pheromone of RO-B-2 is predicted to share 5 out of 10 identical amino acid residues and 1 similar residue with the two other members of this group. The third group contains a single member, B. subtilis RO-FF-1, that exhibits little similarity to the others in the predicted sequence of its mature pheromone. The fourth group consists only of B. natto NAF4, with its unique predicted mature pheromone sequence. The long C-terminal extension on the B. natto NAF4 ComX precursor protein is notable. Tran et al. (34) have also tested the responses of B. natto NAF4 and B. subtilis 168 to one another, using transformability as a downstream reporter, and detected a low degree of cross-activation (∼15%), in contrast to the present results (Fig. 4). This discrepancy might be due to the higher sensitivity of the transformation assay. Additionally, they introduced the heterologous genes on multicopy plasmids, which may have increased the sensitivity of the system by overexpression of ComP. Finally, detection of low-level cross-activation will depend on the concentration of active pheromone in conditioned medium in a given experiment, over which we have little control. In general, the predicted sequences of the mature pheromones are consistent with the observed pattern of responses. Clearly the variations in ComP sequence must determine response specificity, and our data provide very strong evidence that ComX must directly contact ComP. We have not shown that ComQ variation establishes specificity with respect to processing of ComX, although this is likely to be true, and has been demonstrated for B. natto NAF4 and B. subtilis 168 (34).
Our data raise questions concerning the evolution of the QS system and also provide some insights. For instance it is likely that the polymorphic regions of the QS loci have been acquired recently by the host organisms. The average G+C contents of the sequenced portions of the comQ, comX, comP, and comA genes regions are lower than the value of 43.5% reported for the entire B. subtilis genome (13). The G+C averages for the QS genes are 34.0% for B. subtilis 168, B. subtilis RS-B-1, and B. mojavensis RO-H-1 and 35.5% for B. natto NAF4.
How was QS polymorphism generated? It is formally possible that QS polymorphism reflects a series of independent recent horizontal-transfer events from distinct donors. This appears unlikely and would merely displace discussion of the evolutionary forces driving polymorphism to other organisms. It seems more reasonable to assume that a single transfer event took place in an ancestor of the contemporary strains. Any model for the evolution of divergent pherotypes should be general. It should be adaptable not only to the transformation systems of B. subtilis and S. pneumoniae, but also to the Agr system of S. aureus. We assume that each bacterial type tends to evolve a QS system that senses population density, resulting in the activation of downstream genes so as to increase its own fitness. The activation of these target genes will then occur optimally only in response to the needs of the given strain and not when another, possibly competing organism releases pheromone. Given this assumption, if another such strain is in the environment and produces a pheromone with cross-specificity, mutations that minimize this heterologous response without eliminating the homologous response would be advantageous. If the heterologous pheromone can actually interfere with response to the homologous molecule, as reported for the Agr system (9), then the selective pressure for change would be even greater. This situation would favor evolution toward greater specificity in the presence of foreign pheromones that possess partial or complete cross-activating activity. The result would be the evolution of polymorphism at the QS locus. A first step in this evolution might be an alteration in the sensor protein that reduces activation or interference by the foreign pheromone without eliminating homologous activation. Such a situation may obtain in the case of B. subtilis 168 and B. mojavensis RO-C-2, since the RO-C-2 pheromone activates the 168 tester very weakly (not shown), whereas in the reverse situation, robust activation occurs (Fig. 4D). The 168 comP may carry mutations that mitigate its response to the heterologous pheromone. Such restrictive mutations may sometimes decrease the extent of the homologous response, creating a situation in which selective pressure exists for compensating mutations in comX and then in comQ. This scenario predicts the coevolution of the three genes. Since the C-terminal domain of ComP that presumably interacts with ComA does not determine pherotype, there would be no pressure for divergence of this domain or of comA, as observed.
This model has the virtue of generality but is not concrete. For each system we must further consider the specific forces that select for function. In the case of the Agr system it has been postulated that there is competition for colonization of a new host, and each strain evolves to minimize the chances that a competing strain will gain a foothold (9). In our case, it would be premature to argue that transformability, whatever its adaptive role (33), provides the sole driving force for the evolution of the QS system, because it is clear that the Bacillus QS system does more than regulate competence. Although the QS system was first identified on the basis of its function in competence, it is certainly involved in regulating many genes, among them at least several that are involved with survival-enhancing stationary-phase adaptations. ComA-PO4 activates the transcription of srfA (encoding surfactin synthesis). In addition degQ, rapA, and rapC are ComA activated (19), and these genes are known to regulate the synthesis of degradative enzymes, sporulation, and competence. In B. natto NAF4, comA also regulates the synthesis of capsular γ-polyglutamate, postulated to provide protection against bacteriophages (34). A computer analysis revealed more than 400 potential ComA-binding DNA sequences in the B. subtilis genome (15). We can explain the selective pressure driving the evolution of polymorphism by supposing that it would be disadvantageous for the ensemble of genes activated by ComA-PO4 (including competence genes) to be expressed inappropriately in response to foreign QS signals.
It is striking that the pherotype and phylogenetic classifications are not congruent. In fact, individual members within the first and second pherotype groups described above have been assigned to different species based on restriction site differences at three housekeeping loci, fatty acid composition differences, genetic transformation between the species, and DNA hybridization experiments (27, 28). The lack of congruence between the QS specificities and the phylogenetic relationships among the Bacillus strains suggests that the QS loci have passed horizontally among these strains, most likely by transformation, and that this horizontal transmission must have occurred more frequently than for most other genes. Alternatively, as noted above, they may have entered this group of bacilli from another source in a number of independent transmission events, again more frequently than transfer of other genes. Why the QS genes would spread so readily is mysterious. Perhaps pherotype switching transiently enhances fitness. This would be a Red Queen situation (37), defined as an evolutionary mechanism in which there is selection for change, usually in response to alterations in the biological environment, but without any long-term increase in fitness. Another, more gene-centered view is that the QS locus behaves like selfish DNA and that a special pheromone-activated mechanism exists for the transfer of this locus. Possibly an answer will present itself when the complete catalog of genes activated and repressed in response to ComX are identified by genomic approaches.
ACKNOWLEDGMENTS
We thank members of our laboratories for frequent discussions. We also thank F. Cohan for the kind gift of wild-type Bacillus strains.
This work was supported by NIH grant GM57720.
REFERENCES
- 1.Albano M, Hahn J, Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987;169:3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubnau D. DNA uptake in bacteria. Annu Rev Microbiol. 1999;53:217–244. doi: 10.1146/annurev.micro.53.1.217. [DOI] [PubMed] [Google Scholar]
- 4.Dubnau D, Turgay K. The regulation of competence in Bacillus subtilis and its relation to stress response. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: American Society for Microbiology; 2000. pp. 249–260. [Google Scholar]
- 5.Grebe T W, Stock J B. The histidine protein kinase superfamily. Adv Microb Physiol. 1999;41:139–227. doi: 10.1016/s0065-2911(08)60167-8. [DOI] [PubMed] [Google Scholar]
- 6.Hahn J, Luttinger A, Dubnau D. Regulatory inputs for the synthesis of ComK, the competence transcription factor of Bacillus subtilis. Mol Microbiol. 1996;21:763–775. doi: 10.1046/j.1365-2958.1996.371407.x. [DOI] [PubMed] [Google Scholar]
- 7.Havarstein L S, Morrison D A. Quorum sensing and peptide pheromones in streptococcal competence for genetic transformation. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 9–26. [Google Scholar]
- 8.Huang W M, Marmur J. The 5′-ends of the DNA of defective bacteriophages of Bacillus subtilis. J Mol Biol. 1970;47:591–593. doi: 10.1016/0022-2836(70)90326-8. [DOI] [PubMed] [Google Scholar]
- 9.Ji G, Beavis R, Novick R P. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 10.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiel J A K W, Vossen J P M J, Venema G. A general method for the construction of Escherichia coli mutants by homologous recombination and plasmid segregation. Mol Gen Genet. 1987;207:294–301. doi: 10.1007/BF00331592. [DOI] [PubMed] [Google Scholar]
- 12.Kleerebezem M, Quadri L E, Kuipers O P, de Vos W M. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 13.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 14.Lazazzera B A, Grossman A D. The ins and outs of peptide signaling. Trends Microbiol. 1998;6:288–294. doi: 10.1016/s0966-842x(98)01313-4. [DOI] [PubMed] [Google Scholar]
- 15.Lazazzera B A, Palmer T, Quisel J, Grossman A D. Cell density control of gene expression and development in Bacillus subtilis. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 27–46. [Google Scholar]
- 16.Lazazzera B A, Solomon J M, Grossman A D. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell. 1997;89:917–925. doi: 10.1016/s0092-8674(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 17.Magnuson R, Solomon J, Grossman A D. Biochemical and genetic characterization of a competence pheromone. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 18.Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick R P, Muir T W. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci USA. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Msadek T. When the going gets tough: survival strategies and environmental signaling networks in Bacillus subtilis. Trends Microbiol. 1999;7:201–207. doi: 10.1016/s0966-842x(99)01479-1. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura L K, Roberts M S, Cohan F M. Relationship of Bacillus subtilis clades associated with strains 168 and W23: a proposal for Bacillus subtilis subsp. subtilis subsp. nov. and Bacillus subtilis subsp. spizizenii subsp. nov. Int J Syst Bacteriol. 1999;49:1211–1215. doi: 10.1099/00207713-49-3-1211. [DOI] [PubMed] [Google Scholar]
- 21.Nakano M M, Xia L, Zuber P. Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis. J Bacteriol. 1991;173:5487–5493. doi: 10.1128/jb.173.17.5487-5493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perego M. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc Natl Acad Sci USA. 1997;94:8612–8617. doi: 10.1073/pnas.94.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perego M. Self-signaling by Phr peptides modulates Bacillus subtilis development. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 243–258. [Google Scholar]
- 24.Pestova E V, Håvarstein L S, Morrison D A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 25.Piazza F, Tortosa P, Dubnau D. Mutational analysis and membrane topology of ComP, a quorum-sensing histidine kinase of Bacillus subtilis controlling competence development. J Bacteriol. 1999;181:4540–4548. doi: 10.1128/jb.181.15.4540-4548.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pozzi G, Masala L, Iannelli F, Manganelli R, Havarstein L S, Piccoli L, Simon D, Morrison D A. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J Bacteriol. 1996;178:6087–6090. doi: 10.1128/jb.178.20.6087-6090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts M S, Cohan F M. Recombination and migration rates in natural populations of Bacillus subtilis and Bacillus mojavensis. Evolution. 1995;49:1081–1094. doi: 10.1111/j.1558-5646.1995.tb04435.x. [DOI] [PubMed] [Google Scholar]
- 28.Roberts M S, Nakamura L K, Cohan F M. Bacillus mojavensis sp. nov., distinguishable from Bacillus subtilis by sexual isolation, divergence in DNA sequence, and differences in fatty acid composition. Int J Syst Bacteriol. 1994;44:256–264. doi: 10.1099/00207713-44-2-256. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Solomon J, Magnuson R, Srivastava A, Grossman A D. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 1995;9:547–558. doi: 10.1101/gad.9.5.547. [DOI] [PubMed] [Google Scholar]
- 31.Solomon J M, Lazazzera B A, Grossman A D. Purification and characterization of an extracellular peptide factor that affects two developmental pathways in Bacillus subtilis. Genes Dev. 1996;10:2014–2024. doi: 10.1101/gad.10.16.2014. [DOI] [PubMed] [Google Scholar]
- 32.Steinmetz M, Richter R. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene. 1994;142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 33.Tortosa P, Dubnau D. Competence for transformation: a matter of taste. Curr Opin Microbiol. 1999;2:588–592. doi: 10.1016/s1369-5274(99)00026-0. [DOI] [PubMed] [Google Scholar]
- 34.Tran L-S P, Nagai T, Itoh Y. Divergent structure of the ComQXPA quorum sensing components: molecular basis of strain-specific communication mechanism in Bacillus subtilis. Mol Microbiol. 2000;37:1159–1171. doi: 10.1046/j.1365-2958.2000.02069.x. [DOI] [PubMed] [Google Scholar]
- 35.van Sinderen D, Luttinger A, Kong L, Dubnau D, Venema G, Hamoen L. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol Microbiol. 1995;15:455–462. doi: 10.1111/j.1365-2958.1995.tb02259.x. [DOI] [PubMed] [Google Scholar]
- 36.van Sinderen D, ten Berge A, Hayema B J, Hamoen L, Venema G. Molecular cloning and sequence of comK, a gene required for genetic competence in Bacillus subtilis. Mol Microbiol. 1994;11:695–703. doi: 10.1111/j.1365-2958.1994.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 37.Van Valen L. A new evolutionary law. Evol Theory. 1973;1:1–30. [Google Scholar]
- 38.Weinrauch Y, Guillen N, Dubnau D A. Sequence and transcription mapping of Bacillus subtilis competence genes comB and comA, one of which is related to a family of bacterial regulatory determinants. J Bacteriol. 1989;171:5362–5375. doi: 10.1128/jb.171.10.5362-5375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinrauch Y, Msadek T, Kunst F, Dubnau D. Sequence and properties of comQ, a new competence regulatory gene of Bacillus subtilis. J Bacteriol. 1991;173:5685–5693. doi: 10.1128/jb.173.18.5685-5693.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinrauch Y, Penchev R, Dubnau E, Smith I, Dubnau D. A Bacillus subtilis regulatory gene product for genetic competence and sporulation resembles sensor protein members of the bacterial two-component signal-transduction systems. Genes Dev. 1990;4:860–872. doi: 10.1101/gad.4.5.860. [DOI] [PubMed] [Google Scholar]
- 41.Whatmore A M, Barcus V A, Dowson C G. Genetic diversity of the streptococcal competence (com) gene locus. J Bacteriol. 1999;181:3144–3154. doi: 10.1128/jb.181.10.3144-3154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng G, Slavik M F. Isolation, partial purification and characterization of a bacteriocin produced by a newly isolated Bacillus subtilis strain. Lett Appl Microbiol. 1999;28:363–367. doi: 10.1046/j.1365-2672.1999.00545.x. [DOI] [PubMed] [Google Scholar]