Abstract
Cisplatin spearheads the anticancer chemotherapeutics in present-day use although acute toxicity is its primary impediment factor. Among a plethora of experimental medications, a drug as effective or surpassing the benefits of cisplatin has not been discovered yet. Although Oxaliplatin is considered more superior to cisplatin, the former has been better for colorectal cancer while cisplatin is widely used for treating gynaecological cancers. Carcinoma imposes a heavy toll on mortality rates worldwide despite the novel treatment strategies and detection methods that have been introduced; nanomedicine combined with precision medicine, immunotherapy, volume-regulated anion channels, and fluorodeoxyglucose-positron emission tomography. Millions of deaths occur annually from metastatic cancers which escape early detection and the concomitant diseases caused by highly toxic chemotherapy that causes organ damage. It continues due to insufficient knowledge of the debilitative mechanisms induced by cancer biology. To overcome chemoresistance and to attenuate the adverse effects of cisplatin therapy, both in vitro and in vivo models of cisplatin-treated cancers and a few multi-centred, multi-phasic, randomized clinical trials in pursuant with recent novel strategies have been tested. They include plant-based phytochemical compounds, de novo drug delivery systems, biochemical/immune pathways, 2D and 3D cell culture models using small molecule inhibitors and genetic/epigenetic mechanisms, that have contributed to further the understanding of cisplatin's role in modulating the tumour microenvironment. Cisplatin was beneficial in cancer therapy for modulating the putative cellular mechanisms; apoptosis, autophagy, cell cycle arrest and gene therapy of micro RNAs. Specific importance of drug influx, efflux, systemic circulatory toxicity, half-maximal inhibition, and the augmentation of host immunometabolism have been identified. This review offers a discourse on the recent anti-neoplastic treatment strategies to enhance cisplatin efficacy and to overcome chemoresistance, given its superiority among other tolerable chemotherapies.
Keywords: Cisplatin, Cancer therapy, Chemotherapy, Chemoresistance, Cancer biology, Cancer, Immunology, Cancer epidemiology, New advances in cisplatin therapy
Graphical abstract
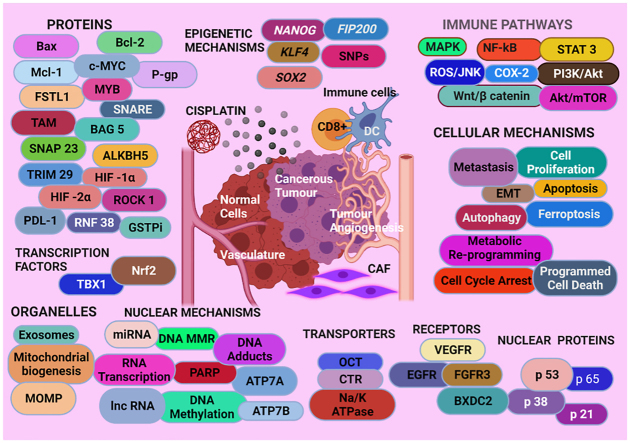
The Graphical Abstract gives an overview of proteins, nuclear proteins, epigenetic, cellular, and nuclear mechanisms, immune pathways, organelles, transporters, receptors, and transcription factors discussed in this review.
Cisplatin; Cancer therapy; Chemotherapy; Chemoresistance; Cancer biology; Cancer; Immunology; Cancer epidemiology; New advances in cisplatin therapy.
1. Introduction
1.1. Cisplatin
Cisplatin is a front-line chemotherapeutic medication prescribed for multiple cancers for many decades [1]. The cisplatin-treated cancers are attenuated with the suppression of tumorigenesis and metastases [2]. However, the biological applications of cisplatin are limited to eliminating the cancers because the average morbidity and mortality statistics are high [3], which may be due to prolonged usage that influence tumour adaptability [4] drug efflux [5] or acclimatisation in various biochemical [6] and immune networks [7] that modulate cancer -induced pathophysiology with widespread toxicity in the organs [8].
1.2. Discovery
Cisplatin was first synthesized by the Italian chemist, Michele Peyrone in 1844 and was named the Peyrone's chloride [9]. The synthesis of cisplatin was incidental to his experimentation with the magnus green salt, a platinum compound given the chemical formula PtCl2(NH3)2 or C12H6N2Pt [10, 11, 12]. Cisplatin was extracted by adding excess ammonia to an acidified PtCl2 solution that yielded yellow and green compounds based on the insolubility of the magnus green salt in hydrochloric acid [13]. Cisplatin was the yellow precipitate having the formula (PtCl2(NH3)2) that is isomeric to the magnus green salt with a completely different set of physicochemical properties [12].
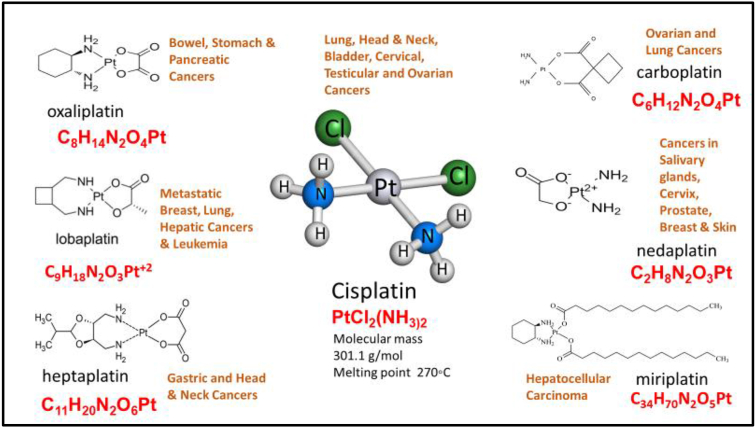
In 1893, Alfred Werner, (a Nobel Laureate-Swiss chemist), designed the chemical structure of Cisplatin (Figure 1) [14], and in 1965, the bioactivity of cisplatin was identified and characterised by Barnett Rosenberg [15]. The first anticancer activity of cisplatin was demonstrated by Rosenberg in a sarcoma mouse model in which the mice given a repeated low dose of cisplatin had no traces of disease recurrence [16]. In 1971 the first clinical trial was performed, after cisplatin was effective in a cancerous mouse model in 1968 [17]. The Food and Drug Agency (FDA) of the United States (US) approved its use in some cancers in 1978 followed by the United Kingdom and several European countries in 1979 [17]. The molecular structure of cisplatin determines its anticancer efficacy in a dose-dependent manner by inhibiting the synthesis of nuclear and mitochondrial deoxyribonucleic acid (DNA) that represses transcription in cancer cells [18].
Figure 1.
Molecular structure of cisplatin, several chaperone platinum-based anti-cancer compounds, and the cancer types for which they are used [17].
1.3. Statistics
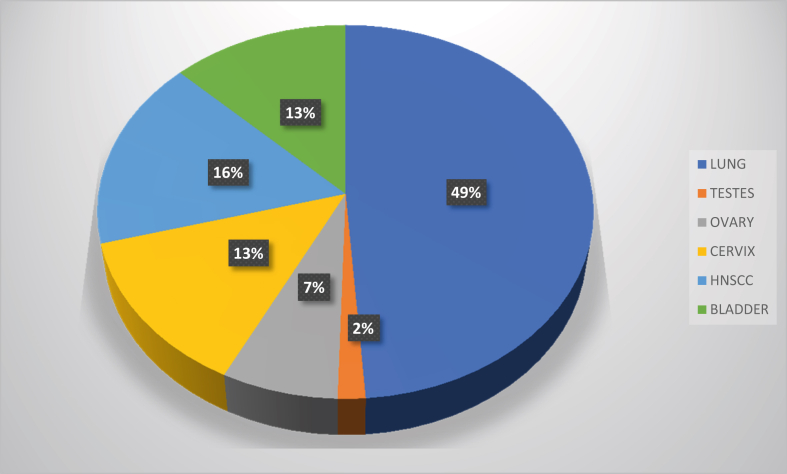
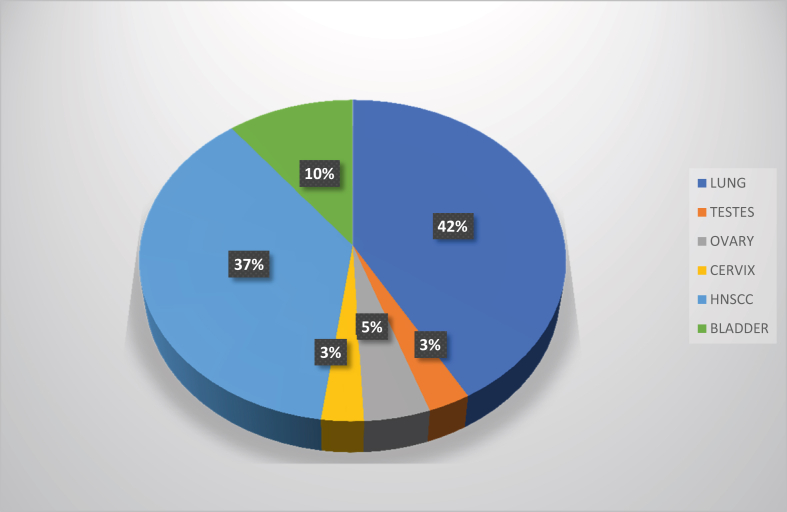
A total of 19,292,789 cancer incidence and 9,958,133 cancer-deaths were reported by the GLOBOCAN cancer surveyor in which, the incidence of cisplatin-related cancers mentioned in this review was 4,519,909 in the year 2020 with 3,253,524 deaths. In Australia and New Zealand, the incidence of those cancers was 37,372 with 15,164 mortalities (Figures 2, 3, 4, and 5) [11], [19]. Platinum-based chemotherapeutics have increased the standardized mortality ratios evidenced in; (i) a decade - long testicular cancer study after chemotherapy and radiation therapy that yet displayed 1-to-6-fold increase in mortality rates from the primary cancer itself and up to 15% increase in mortality from second cancers [10], (ii) 100 postoperative patients of non-small cell lung cancer (NSCLC), given adjuvant cisplatin reported the 5-year survival rate after recurrence at 29%, and the median survival time after recurrence as 37 months [20], (iii) a metanalysis of a clinical trial of NSCLC patients reported a 30% increase in non-lung cancer-related deaths with adjuvant cisplatin therapy [21]. In a 2014 report by the US National Institute of Cancer, cisplatin and other platinum-based anti-cancer drugs were prescribed up to 10–20% of patients of many cancer types [22]. Cisplatin was first utilized against testicular and ovarian cancers but was extended to include bladder, gastric, colorectal, cervical, head and neck cancers, melanoma of the skin and triple negative breast cancer [1]. Notwithstanding its high toxicity in multiple systems; excretory [23], nervous [24], cardiovascular [25], and enteric [26], modern day use of cisplatin produces a 90% cure rate in the cancers for which it is prescribed [27]. Combined therapy with cisplatin has been the standard treatment for many cancer types such as gynaecological, bladder, lung, head and neck cancer and even for metastatic disease [28]. Second cancers, old age, acute toxicity, genetic predisposition (mutations in BRCA1 and BRCA2), cardiovascular and lung disease and metabolic syndrome are listed as high risk factors for mortality in cancer patients treated with cisplatin [28, 29, 30, 31, 32]. Three to four cycles of cisplatin, in combination with bleomycin and etoposide showed a significant progression-free 5-year survival rate of more than 90% in disease-specific metastatic testicular cancer [28]. Another published case report on combined cisplatin chemotherapy in metastatic urothelial cancer reported an overall response rate of 60–70% with 14–15 months of overall survival [31]. Cisplatin is also classified as a carcinogenic compound that forms crosslinks with DNA which is its prime anticancer mechanism and causes mortality by itself. The continuous exposure to platinum increased gastrointestinal tract polyposis in childhood cancer survivors and caused mortality by cardiovascular and respiratory disease, particularly in testicular cancer patients [29]. In a retrospective multi-institutional clinical trial conducted on locally advanced head and neck squamous cell carcinoma (HNSCC) patients between 2004 and 2015, were treated with a 3- weekly dose of 100 mg/m2 and 30 mg/m2 dose weekly of cisplatin in a combined regimen of chemoradiotherapy [30]. Those patients showed dose-responsive survival rates of 58% and 73% respectively while the authors noticed more toxicity in the 3-weekly dosing regimen than in the weekly dosing regimen because acute toxicity is a high-risk factor for cancer mortality. The survival rates for one-year and two-year periods of this study was 89 % and 80% respectively which had a median follow-up of 39 months [30]. In a similar study of cisplatin-treated HNSCC, patients who had received cisplatin doses of 3-weekly 100 mg/m2 and a weekly 40 mg/m2, between 2005-2017 with a median follow-up of 74 months, showed 5-year and 10-year survival rates of 49% and 31% and progression-free survival rates of 35.5% and 22% respectively [33]. Cisplatin has been an effective single-agent drug in treating stages III B to IV in NSCLC which was more potent when combined with paclitaxel. With no significant difference between high and low dose paclitaxel, the patients showed a median survival of 9.9 months and a one-year survival rate of 38.9% when given cisplatin 75 mg/m2 combined with paclitaxel in 3 weekly cycles [34]. Cisplatin in combined therapy (with paclitaxel) has been the cornerstone of ovarian cancer chemotherapy regimens with 70–80% of killing or shrinking of tumours after cytoreduction surgery [32]. Yet a 10–15% of the patients receiving cisplatin will relapse and invariably develop chemoresistance to platinum-based drugs and survive no longer than a year [32]. The second cancers in patients receiving chemotherapy with cisplatin regimens is a risk factor of mortality, although the incidence of cisplatin-associated second cancers was low in statistics reported in 2017. In 28 registered clinical trials including an approximate 7000 patients, second primary cancers appeared in 143 patients in total, in which only 75 had received cisplatin treatment [35]. The authors suggested that these statistics could be used in providing patient counselling and shared decision-making, which reiterates the importance of using cisplatin for cancer therapy. Given these statistics, the number of cancer patients in remission is high in the cisplatin cohort which provides an impetus for its use as a frontline chemotherapy in all cancers [35].
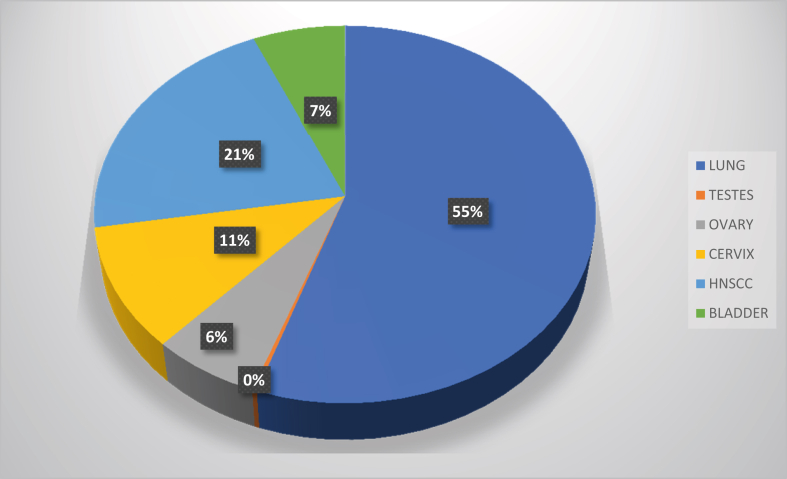
Figure 2.
The incidence of major cisplatin-treated cancers worldwide in 2020 [11].
Figure 3.
The incidence of major cisplatin-treated cancers in Australia & New Zealand in 2020 [11].
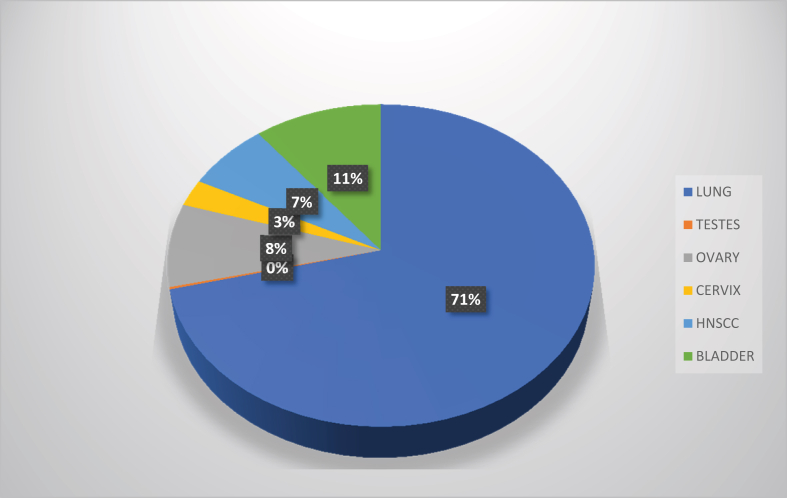
Figure 4.
The overall percentage mortality from major cisplatin-treated cancers worldwide in 2020 [11].
Figure 5.
The overall percentage mortality from major cisplatin-treated cancers in Australia & New Zealand in 2020 [11].
1.4. Biochemical structure
Cisplatin, cis-diamminedichloroplatinum (II), is a coordination compound with square planar geometry, that is a white or deep yellow to yellow-orange crystalline powder at room temperature [12]. Under normal temperature and pressure, it is stable at a water solubility of 2.53 g/L at 25 degrees Celsius [12].
A coordination complex has a central molecule or atom, mostly a metallic ion that is bound to anions or neutral molecules called ligands [2]. The central atom forms coordinate covalent bonds with the ligands that are Lewis bases which have a pair of electrons to donate to the central metal ion which behaves as a Lewis acid (such as a H+) that accepts pairs of electrons from the ligands. The coordination complex is formed as a result of a Lewis acid-base reaction [36]. The chemical properties of the molecules or atoms are different to the coordinating complex, once formed. There are three different ligand types in the platinum anticancer complex of cisplatin [17]. One is the non-leaving ligand type, which are nitrogen donors that form thermodynamically stable bonds with the central platinum atom and contribute to the final DNA-platinum adduct formation, that is responsible for the anticancer efficacy of cisplatin. Modification of those ligands determine the cellular repair mechanisms in circumventing the DNA adducts [17]. The second ligand type is the leaving group ligands that can alter the aquation kinetics and the overall stoichiometry of the platinum anticancer reaction complex [17]. The third ligand type, the axial ligands, bind with platinum complexes with higher valency; platinum (III) and platinum (IV). They can dissociate after the biological reduction of the platinum complex and offer structural moieties for DNA adduction and nanoparticle attachment for targeted drug delivery [13, 37].
Modification of those three ligand types alters the lipophilicity, water solubility, reaction kinetics, toxicity profile and drug resistance of the platinum anticancer complex and the reactivity of cisplatin as an anti-tumorigenesis agent [27]. Quick - reacting ligands increase the toxicity of the compound by binding with off - target biological nucleophiles [27].
1.5. Mechanism of action
Cisplatin interferes with DNA synthesis and repair mechanisms in cancer cells. It forms 1–2 and 1–3 intra-strand adducts and inter-strand cross links between the purine bases, through covalent bond formation at N7 of adenine and guanine [38]. DNA adducts arrest the cell cycle at G2 phase and induce apoptosis by blocking DNA replication [39]. The mechanism of action begins with the aquation of the cisplatin complex and the loss of one or both chloride ligands. The resulting platinum (II) aqua complex is strongly electrophilic and readily binds with several biological ligands by counter-exchanging the already bound water molecules [40]. The platinum-DNA adduct formation causes the distortion of the DNA strands leading to transcription inhibition and subsequent cell death [40].
1.6. Biological activity
After the elucidation of the chemical structure of cisplatin, the biological activity was discovered with the suppression of cell division in E. coli bacteria [41]. When platinum electrodes were activated in a beaker containing bacteria, cell division was abrogated although the prokaryotes continued to grow in length. The platinum in the electrodes had caused DNA damage that induced cell cycle arrest. It was particularly effective against solid malignant tumours [42] which suppressed recurrence despite dose-dependent systemic toxicity [43], for which, the drug is now used in adjuvant [44] or combination therapy [45].
Many different platinum-based compounds have been tested [46] for effectiveness in the targeted killing of solid tumours [47], but only two other cisplatin-related platinum compounds have been authorized for the use against the cancers. Carboplatin [48] is preferred over cisplatin owing to its low toxicity profile [49] and its permissible high dosage. The other is Oxaliplatin [50], which contains a different amine group to that of the amines in cisplatin. Both these therapeutics display different stereoisomerisms and spectra of activity that do not cross-react with cisplatin (Figure 1) [51]. However, cisplatin remains in the forefront of chemotherapy as a commonly prescribed, potent anticancer agent [52].
1.7. Adverse effects
The adverse effects of cisplatin treatment include; severe nephrotoxicity [53] that leads to end-stage dialysis, neurotoxicity which arises as ototoxicity [54], vestibulopathy [55] and peripheral neuropathies (that occur less in younger patients) [24]. Cardiovascular co-morbidities arise in cardiomyocytes [56], as hypertension [57], vascular damage [58], and the metabolic syndrome [59]. The drawback of cisplatin use is that the circulating plasma levels of the drug continues for several decades even after the cessation of therapy [60] and particularly induce myocardial damage as a consequence of mitochondrial dysfunction [61], necrosis, apoptosis, and oxidative stress stimulated by reactive oxygen species (ROS) in the cardiomyocytes [62]. These deficiencies impede the overall survival rate from cisplatin-based therapies in cancer for which counteracting-treatment does not exist [63].
Drug resistance in malignant tumours arises due to a host of factors and the resistance of cancers to cisplatin therapy is determined based on those characteristic elements. They occur primarily by altered drug sites and receptors, drug metabolism, stress-induced genetic and epigenetic mechanisms, DNA repair mechanisms, drug efflux mechanisms, pharmacokinetics, and the tumour microenvironment [64, 65]. Cancer cells can be initially resistant to cisplatin or can acquire resistance [65]. The high-affinity copper transporter (CTR1) is involved in the intracellular uptake of cisplatin [66]. Knockdown of CTR1 reduces the intracellular accumulation of cisplatin and induces cisplatin resistance [67]. Moreover, the epithelial mesenchymal transition (EMT) induces cisplatin resistance. Despite the intense study of cisplatin resistance, it has not been overcome [68]. The deterioration of function in organs increases the risk of mortality in cancer patients [69]. Cisplatin is excreted from the kidneys and accumulates within the proximal renal tubular cells [70]. Nephrotoxicity develops after about 10 days of treatment with cisplatin [70]. The accumulation of cisplatin in the renal tubular cells leads to other clinical features. Those include increased tumour necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), mitochondrial toxicity, hypomagnesemia, glucosuria, phosphaturia, aminoaciduria, electrolyte imbalance, renal salt - wasting, and chronic renal failure [71].
Nephrotoxicity is the major adverse side effect of cisplatin treatment and affects nearly 30–50% of the cancer patients given a dose equal to or above 80 mg/m2 [72], [73]. Nephrotoxicity thus limits the dosage and mortality may be caused by end-stage dialysis rather than from the cancers, such as localized tumours. The nephrotoxicity-causing mechanisms are poorly understood without an effective prognostic biomarker to identify the patients at high risk of developing nephrotoxicity. Acute Kidney Injury (AKI) is the most common disease that manifests in nephrotoxic kidneys and depends upon the age, cumulative dosage, previous exposure to cisplatin, baseline estimated glomerular filtration rate (eGFR), co-morbidities such as diabetes, hypertension, CVD, concomitant medication, pre-hydration, echocardiogram performance status, hypoalbuminemia, and magnesium supplementation [74, 75, 76, 77].
Several mechanisms have been proposed for nephrotoxicity in cisplatin-treated cancer patients including; (i) the increased production of ROS released from the damaged mitochondrial DNA [78], (ii) the activation of apoptosis in damaged renal tubules [79], (iii) the accumulation of cisplatin inside renal tubules due to its high affinity towards the copper transporters and organic cation transporter-2 which aid cisplatin influx [80] and (iv) the inhibition of the membranous Na+/K+-ATPase pumps [81].
1.8. Cisplatin resistance in tumours
The tumour environment represents a different entity compared to the normal cells and presents many metabolic checkpoints that are related to cisplatin resistance in the cancer cells [65]. Cisplatin resistance is a direct outcome of decreased drug-uptake and increased drug efflux which are related to DNA salvage and repair mechanisms of the resistant cancer cells [65]. The exact mechanism of cisplatin influx is unknown but involves the transmembrane carriers such as the copper transporter proteins (Ctrl -1, Ctrl-2, ATP7A and ATP7B) [82]. Cisplatin enters the cell as an inert compound which requires hydration of the chloride molecules for it to be activated [83]. The cisplatin concentration in blood and extracellular tissue fluid is low but the aquation within the cells make it expeditiously bind with the DNA to produce highly reactive mono or bi-hydrated cisplatin forms. Cisplatin induces autophagic vacuoles within the cells which markedly increase under hypoxic conditions [84].
Cisplatin exerts its effects on tumour cells by inhibiting DNA replication, RNA transcription, cell cycle arrest and programmed cell death [82]. These mechanisms involve mitochondrial and nuclear DNA remodelling that induce apoptosis and autophagy [85]. The intracellular accumulation of cisplatin is as low as about 1%, yet the toxicity evoked is high [83]. Intracellular cisplatin interacts with a range of molecules such as mitochondrial DNA, proteins, phospholipids, phosphatidylserines and the cytoplasmic nucleophile, glutathione from which ROS is produced [83]. A direct influence of cisplatin-generated ROS is an increase of mitochondrial outer membrane permeability (MOMP) and DNA damage [65].
The phenomenon of cancer cell bioenergetics involve the remodelling of the DNA and the oxidative processes of glucose, amino acids, and lipid metabolism [86]. Rapid cell proliferation and differentiation are mostly energy-dependent processes which determine tumour development and metastasis. ROS metabolites provide the tumours with a selective advantage, such as inducing the metabolic pathways involving the amino acids and lipids which play key roles in modulating anabolic substrate oxidation and enhancing enzyme catalytic activities [87]. Lipid metabolism is directly involved in membrane functions such as MOMP leading to deregulated drug influx and efflux mechanisms by influencing membrane-operative ion pumps and drug uptake mechanisms. Protein metabolites such as glutathione activate autophagy resulting in redox reactions of which are necessary to activate immune responses [87]. The connection between cisplatin resistance and cancer progression is the ability for remodelling DNA repair mechanisms for which methylation and acetylation of the DNA is considered important [88].
1.9. New advances
Cisplatin prodrug synthesis is an option that targets drug release, enhance efficacy, reduce cytotoxicity and multi-drug resistance [89, 90, 91]. The most preferred cisplatin prodrugs belong to the platinum-S-thiol group and those in use consist of diethyldithiocarbamate, thiourea, thiosulfate, glutathione, cysteine, biotin and amifostine [92]. Glutathione-responsive prodrugs such as glutathione tripeptide are widely used for having enhanced cytoplasmic drug release in tumours and a double or triple-fold increase in the intracellular accumulation compared to the tumour extracellular environment [93, 94]. Metronomic therapy consists of delivering fractionated dosages of a highly toxic drug for an uninterrupted duration to maintain a low cytotoxicity profile while exposing the patients to a continuous regimen of chemotherapy [95, 96]. It has inhibited tumour neovascularisation, angiogenesis, and endothelial cell proliferation also with immunogenic modulation of tumour suppression by presenting neoantigens and increasing programmed tumour cell death [97]. A recent study on ovarian cancer that assessed chemosensitivity of tumours to cisplatin had used in vitro 3D models for faster diagnosis [98]. They had introduced simple cell spheroids, synthetic hydrogels/Peptigels, and polymeric scaffolds with the cell lines A2780 derived from the primary site which was the ovary and SK-OV-3 derived from ascites fluid that represented the metastatic site [98]. Several recent studies on cisplatin in combination with other anticancer drugs are given below (Table 1).
Table 1.
The efficacy of cisplatin combined with several anticancer drugs, the cisplatin dose, metabolic mechanisms, and the outcome reported in recent studies.
| Model | Drugs and Dose | Cancer Type | Mechanisms | Outcome | Ref |
|---|---|---|---|---|---|
| In vitro (A549) and In vivo mouse model | Cisplatin prodrug (20 mg) and Paclitaxel as co-loaded nanoparticles | Lung Cancer (NSCLC) | Low pH and glutathione-responsive cisplatin prodrug produced sustained slow drug release, reduction of drug concentration in blood, low ALT, SCr, and WBC | Remarkable anti-tumour effects in vivo with synergistic tumour cell inhibition with low systemic cytotoxicity | [99] |
| SiHa (BALB/c) Xenograft mouse | Cisplatin prodrug 4 mg/kg and paclitaxel combined therapy as co-loaded nano – particles | Cervical Cancer | Active and passive targeting of TMTP1 (a tumour homing peptide) and enhanced permeability and retention effect | Prolonged blood circulation, reduced toxicity, less side effects, increased accumulation in the tumours with antitumour effects | [100] |
| Human ovarian cancer cell lines A2780, SKOV3, CAOV3, and OVCAR5 | Combined therapy by AMLSD with Stock concentration of cisplatin was 5mM in ddH2O | Ovarian Cancer | Inhibited STAT3 phosphorylation (tyrosine 705) and the expression of its downstream targets c-MYC, CyclinD1, Survivin, and cleaved caspase-3 | LLL12B small molecule inhibitor produced decreased cell viability, proliferation, and migration | [101] |
| Human ovarian cancer cell lines, A 2780, and Ovcar-3 | Cisplatin and Olaparib in combined therapy in concentrations set to 0.0625x, 0.125x, 0.25x, 0.5x, 1.0x and 2.0x IC50 | Ovarian Cancer | Inhibited cell proliferation and induced apoptosis | Synergistic anticancer effect was observed | [102] |
| A549 and MRC5 lung cancer cell lines | Cisplatin 0.05 % w/w and Gemcitabine in noisome - entrapped aerosol formulation | Lung Cancer | Physical characteristics of the formulation, drug uptake, release, stability of carrier, entrapment efficiency was evaluated | Reduced cytotoxicity and cell growth inhibition was observed | [103] |
2. Recent advances in cisplatin-associated testicular cancer
2.1. Testicular cancer
Testicular cancer (TC) was the first to effectively respond to cisplatin treatment despite the high risk of premature mortality from TC treated with platinum-based cancer therapeutics in the early years of its usage [104]. The present-day statistics reveal over 90% survival in cisplatin-treated TC that affects the younger male population more by endowing a near-normal life expectancy [105]. A recent population-based analysis of TC patients in Norway, between the years of 1989–2009 revealed a greater risk of long-term, non-TC-related mortality arising from second cancers when given greater than 4 cycles of annual platinum-based cancer treatment, after a decade from cessation of therapy [10]. The worldwide incidence of TC is around 74,000 new cases in the last year with around 9000 annual mortalities. In Australia and New Zealand, approximately 1000 new cases were diagnosed last year [11].
Cardiovascular morbidity and mortality are long-term risk factors associated with cisplatin-based chemotherapy in metastatic TC [106]. The TC survivors who had received a median cumulative cisplatin dose of 780 mg also required anti-hypertension and lipid-lowering medication, added to worsened diastolic function, longer mitral valve deceleration time and maximal tricuspid regurgitation velocity [106]. Significantly, these long -term cardiovascular complications were reported after 3 decades from discontinuing cisplatin therapy [106]. A genome-wide-association study (GWAS) was performed on single nucleotide polymorphisms (SNP) related to cardiovascular disease (CVD) in TC survivors treated with platinum-based medications, being less than 55 years old at diagnosis and having greater than 3 years of refraction-free survival. They identified 179 SNPs related to CVD; having prominent gene activity associated with the Rac/Rho family small GTPase-2/3 (RAC2/RAC3) network, adiposity, metabolic and immune deficiencies, and apoptosis [107]. RAC2/RAC3 pathways involve leukocyte adhesion to endothelial cells, endothelial activation, neovascularisation, and prevention of calcified plaque formation in arthrosclerosis [107]. A higher risk of venous thromboembolism and bleeding in cisplatin-treated metastatic TC patients exist, particularly in patients having enlarged retroperitoneal lymph nodes [108]. A retrospective study of the testicular germ cell tumours had high cure rates from cisplatin therapy in men (aged between 18-39). It revealed predisposition to thromboembolic events in higher pathological stages (such as the stage III carcinoma), and had been exposed to a greater number of treatment cycles for which prophylactic treatment with anti-coagulants was required [109].
Germ cell tumours demonstrate up to 80% cure rate with the conventional platinum-based therapies although the metastatic versions caused high mortality and presented poor prognosis (such as mediastinal germ cell cancers) even in younger patients [110]. The germ cell tumours display increased DNA methylation and drug resistance which can be reversed with re-sensitization of the tumours to hypomethylating agents in platinum-refractory germ cell cancers [110]. A phase 1 study of the testicular germ cell cancers treated with the hypomethylation-sensitizing drug, guadecitabine (30 mg/m2) followed by 100 mg/m2 cisplatin reported a clinical benefit of up to 46% [110]. Preclinical studies have also revealed that pre-treatment with hypomethylating agents could reverse cisplatin-resistance in germ cell tumours [111].
A study on post-cisplatin-treated fertility in testicular germ cell cancer survivors demonstrated a higher sperm preservation rate and higher paternity rate compared to those not using cryopreserved sperms, treated with 4 cycles of cisplatin therapy [112]. The researchers suggested the clinicians should consider not only the prevention of recurrence, but also the post-treatment fertility status, particularly in younger male patients [112]. Contrarily, a rat study of chemically induced TC reported a decrease in fertility following cisplatin treatment, which was recovered by the anticancer drug, quercetin [113]. Quercetin treatment in combination with cisplatin had improved TC pathology and synergistically activated the anticancer and antioxidant pathways in the testes [113].
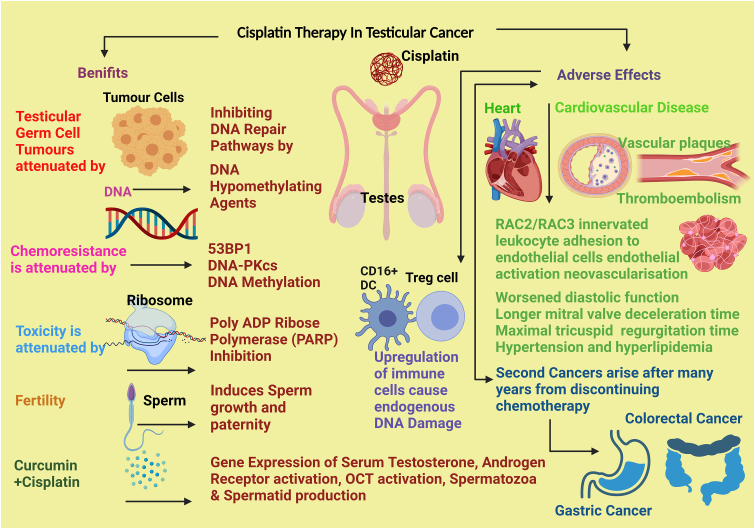
The long-life expectancy achieved by cisplatin-based treatment in TC survivors can be compromised by long-term adverse effects of platinum compound therapy that persists in blood plasma and urine which could give rise to second cancers [114]. TC survivors are thus at high risk of developing gastrointestinal (GI) cancers, and specifically colorectal carcinoma as a secondary complication of cisplatin treatment, although the originating mechanisms are not understood [114]. A dose-response relationship in cisplatin therapy and the emergence of second GI tract malignancies have been reported extending up to 35 years after recovery from TC [114]. A multicentre study of TC survivors treated with cisplatin-based regimens proposed that colorectal cancer surveillance with faecal immunochemical testing after 8 years from recovery would safeguard against the development of GI tract-malignancies in TC patients that are in remission [114]. The major adverse effect of cisplatin therapy is high systemic toxicity that debilitates organ physiology in cancer patients from haematological toxicity, nephrotoxicity, and neurotoxicity which increases mortality [115, 116]. The standard treatment has been 100 mg/m2 of cisplatin given three weekly up to 300 mg/m2 combined with radiotherapy from which toxicity purportedly arise [117]. Cisplatin is effective in ameliorating TC and induce testicular toxicity, evidenced by a rat study, in which cisplatin had reduced serum testosterone levels, androgen receptor and the organic cation transporter gene expression in the testis [118]. These deleterious effects of cisplatin -induced testicular toxicity could be reversed by treatment with curcumin, a naturally - occurring, phytochemical biological agent having proven efficacy in attenuating cisplatin-induced kidney injury (CIAKI) and increasing spermatid and spermatozoa development via the modulation of the testosterone and androgen receptors [118]. The benefits and the adverse effects of cisplatin-treated TC that were reported recently are summarised in Figure 6.
Figure 6.
The latest findings on cisplatin-treated testicular cancer.
2.2. Chemoresistance to cisplatin in testicular cancer
Despite the success rate achieved by cisplatin therapy in germ cell cancers, a subset of patients have a poor prognosis and develop cisplatin-resistance and succumb to death [111]. A study of cisplatin resistance in TC germ cell tumours reported that interference with the mechanisms of salvage and repair pathways of DNA, holds the key to determining drug resistance [119, 120]. Resistance to cisplatin increased in cells which (i) repaired the DNA double strand breaks via the homologous recombination repair pathway, (ii) had reduced expression of the tumour protein 53 binding protein 1 (53BP1), and (iii) inhibited DNA-dependent protein kinase protein (DNA-PKcs) activity that decreased cisplatin cytotoxicity [119]. Thus, by modulating 53BP1 and, by inhibiting poly (ADP-ribose) polymerase (PARP) activity (which is often used in anticancer salvage therapy), the cisplatin-resistance could be counteracted, and cisplatin given in combination with a PARP inhibitor would be potentially beneficial to cisplatin-refractory patients [121]. An evaluation of the crosstalk between the immune cell subsets and endogenous DNA damage was linked to chemoresistance in testicular germ cell tumours to cisplatin therapy [122].
Legend: TC- testicular cancer, 53BP1- p-53 binding protein 1 (DNA double stranded break signalling and repair), DNA-PKcs-deoxyribo nucleic acid -dependent protein kinase-catalytic subunit (overall survival and proliferation of cells), OCT- organic cation transporter (drug delivery protein), NK cell – natural killer cell (tumour regulatory effector lymphocyte promoting immunity), T cell – T lymphocyte (adaptive immunity), DC- dendritic cell (antigen-presentation), RAC2/RAC3 – Rho GTPases member – Rac family small GTPases-2/3 (tumour growth and metastasis).
An increase in the natural killer cells, CD16-positive dendritic cells and regulatory T cells was associated with the level of endogenous DNA damage which modulated the tumour microenvironment and influenced sensitivity to cisplatin therapy [122].
3. Recent advances in cisplatin-associated lung cancer
3.1. Lung cancer
Lung cancer is the second-most diagnosed cancer worldwide which comprises 11% (which is 2,206, 771) of the total cancer incidence and is the most common cause of global cancer deaths (totalling 1.8 million) [11]. The total of new lung cancer cases in Australia and New Zealand was around 15,000 with the total deaths being around 10,000 in the year 2020 [11]. Among the novel approaches in lung cancer therapeutics is an aerosolized drug delivery system. It had low cytotoxicity and was introduced as a novel treatment strategy for lung cancer which had over 95% success in drug penetration in a combination of both cisplatin and gemcitabine (that are highly toxic at high dosage when administered separately) [103]. The tumour extracellular environment is more acidic from having low pH values in its extracellular fluids and as a result, the lysosomes and endosomes contain high acidity [99]. A cisplatin prodrug was synthesized to be redox-responsive and pH-sensitive. It was co-loaded with paclitaxel into nanoparticle drug carriers for targeted drug delivery and drug-responsiveness in NSCLC, resulting in enhanced drug release, low systemic toxicity, and in vivo anticancer efficiency [99]. Another hydrophobic cisplatin prodrug formulation was co-loaded with etoposide (which is highly toxic when administered as monotherapy) in a nanoparticular drug carrier that was found to be more effective than small molecule chemoradiotherapy for the treatment of both small and NSCLC [123]. The metronomic dosing of oral vinorelbine combined with tri-weekly cisplatin displayed low cytotoxicity in patients with locally advanced unresectable stage III NSCLC compared to standard doses of monotherapy [124].
3.2. Chemoresistance to cisplatin in lung cancer
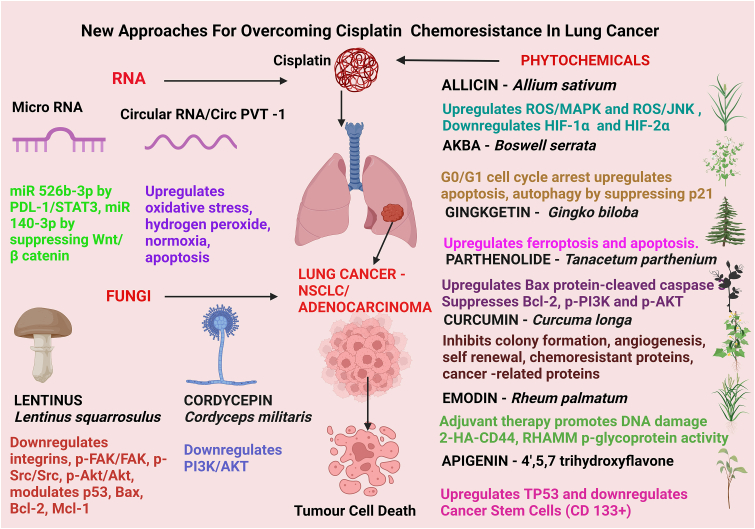
Cisplatin is the first-line chemotherapy utilized in treating advanced and non-targetable NSCLC- which is the most common of the lung cancers comprising 80% of the total lung cancers that are diagnosed. As one of its anti-cancer strategies, cisplatin induces ferroptosis to increase the chemosensitivity of tumour cells to the drug. As such, ferroptosis enhances cisplatin's therapeutic potential as an efficacious anticancer treatment (Figure 7) [125]. Ferroptosis is an iron-mediated mechanism of activating cell death by lipid peroxidation that enhances the action of classical chemotherapeutics. In lung cancer, cisplatin triggered ferroptosis by initiating glutathione-dependent antioxidant mechanism dysfunction, resulting in uncontrolled lipid peroxidation leading to cell death [126]. Cisplatin mediates ferroptosis by depleting intracellular GSH which inhibits glutathione peroxidase activity [126]. Cisplatin - induced ferroptosis was further enhanced by a natural bioflavonoid, Ginkgetin (extracted from Gingko biloba leaves), that induced anticancer effects through activating autophagy in NSCLC [127]. Ferroptosis is a mechanism that is distinct from apoptosis, necrosis, and autophagy. Therefore, induction of ferroptosis is a novel molecular strategy that can be used to overcome chemoresistance in tumour cells to efficacious chemotherapeutics [128]. It is confirmed that both ferroptosis and apoptosis mechanisms together could enhance tumour cell chemosensitivity to cisplatin treatment [127].
Figure 7.
Recent strategies for attenuating cisplatin chemoresistance in lung cancer.
Cisplatin resistance arises from two key processes; an increase in apoptosis resistance and the resetting of redox homeostasis, which can be reversed by naturally - occurring phytochemicals [69, 127]. An evaluation of a traditional Chinese medicinal drug, cordycepin, has shown synergistic effects in NSCLC combined with cisplatin to overcome cisplatin resistance by inhibiting PI3K/AKT pathway [129]. Chemoresistance of lung cancer cells to cisplatin is also influenced by the programmed death ligand-1 (PDL-1) expression which showed negative regulation of the micro - RNA, miR 526b-3p [130]. The overexpression of miR 526b-3p served to upregulate drug sensitivity by suppressing metastasis and T cell activity (CD8+) via the PDL-1/STAT3 axis [130]. Micro-RNA 140- 3p (miR 140-3p) overexpression was found to attenuate cisplatin resistance in lung adenocarcinoma, a solid tumour which manifests commonly among the heterogenous NSCLC [131]. A decrease in miR140-3p was observed in these tumours which stimulated cancer progression by the Wnt/beta catenin signalling, as beta catenin overexpression on the other hand led to the reversal of the anticancer effects displayed by miR 140-3p in lung adenocarcinoma lesions [131].
Parthenolide (a plant-derived anticancer formulation under clinical development) when combined with cisplatin exhibited synergistic action in enhancing chemosensitivity of NSCLC by upregulating the proteins; Bax, and cleaved caspase-3 whilst suppressing Bcl-2, phosphorylated PI3K, AKT and caspase-3 [132]. Cisplatin in combination with acetyl-11-keto-β-boswellic acid (AKBA) in vitro exhibited enhanced chemosensitivity in NSCLC by activating the G0/G1 phase arrest, apoptosis induction, and inhibition of p-21-signalling pathway – dependent autophagy [133]. Emodin is an anthraquinone chemical compound (derived from rhubarb, buckthorn, and Japanese knotweed) that exerts a remarkable effect as an adjuvant to cisplatin therapy [134]. Emodin in combination with cisplatin enhanced chemosensitivity in lung cancer cells in vitro, by inducing DNA damage and cellular apoptosis through inhibiting P-glycoprotein activity [134]. Additionally, emodin level was found to be high in cisplatin – resistant NSCLC cells which was reversed by activating the hyaluronan (HA) synthase 2-HA-CD44/receptor for hyaluronic acid-mediated motility (RHAMM) - mediated signal transduction mechanism [135].
Apigenin (API) is a natural flavone derived from plants that displayed efficacy as a tumour suppressor in an in vitro study of the cisplatin-resistant NSCLC cell lines (such as A549R) [136]. API repressed the expansion of cancer stem cells (bearing the marker CD133 in lung tumours) and enhanced the anticancer effect of cisplatin by inducing the tumour suppressor activity of TP53 (which was reversed by adding the TP53 inhibitor, Pifithrin-α, and siRNA targeting the p53 gene in A549R cells) [136]. The expression of the chemokine receptor, CXCR2 is elevated in carcinomatous lung tumours and its inhibition has promoted apoptosis, senescence, EMT and suppressed cell proliferation in vitro [137]. These effects were found reversed in the presence of a CXCR2 inhibitor in vivo by downregulating; neutrophil infiltration and stimulating anti-tumour activity by inducing CD8+ T cell mediated cytotoxic mechanisms [137].
CircPVT-1 is a circular RNA that is overexpressed in cisplatin-resistant cancers, which when decreased improved the chemosensitivity, with a significant difference between their expression and the advancement of tumour progression in NSCLC patients [138]. The combined treatment with cisplatin and gemcitabine attenuated chemoresistance by the downregulation of circPVT-1 expression in NSCLC [138]. Nanocarriers loaded with cisplatin and glucose oxidase delivered to chemo -resistant NSCLC cell line, A549R induced oxidative stress significantly augmented chemosensitivity to cisplatin therapy without cytotoxicity [139]. The diffusion of oxygen and glucose molecules to A549R cells at a low pH of 6.8 induced in situ superfluous hydrogen peroxide which increased cisplatin-sensitivity by activating pro-apoptotic pathways [139]. The ring finger protein 38 (RNF38) is associated with poor prognosis in NSCLC [140]. It is overexpressed in cisplatin-resistant NSCLC and its downregulation in A549R cell line promoted chemosensitivity to cisplatin by suppressing cell apoptosis [140]. Curcumin possessed therapeutic properties in NSCLC by promoting chemosensitivity in cancer stem -like cells. Curcumin thus inhibited; colony formation, self-renewal, angiogenesis, chemoresistance proteins and cancer-related protein levels with subsequent downregulation of the gene expression of SOX2, NANOG AND KLF4 [141]. A cisplatin prodrug (named cisplatin - poly (D, L-lactide-co-glycolide -CDDP – PLGA) combined with curcumin co-encapsulation with layer-by-layer nanoparticles produced enhanced anti-cancer effects. It increased cisplatin-sensitivity in the lung adenocarcinoma cell line A549, compared to the drugs administered as monotherapy and single drug loaded nano-capsulations [142].
Legend: miR-micro Ribo nucleic acid (RNA)- single stranded non-coding RNA which regulates RNA silencing and post -transcriptional regulation of gene expression), PDL-1 – programmed death ligand -1 (transmembrane protein on immune cells mediating cancer immunity escape), STAT3-signal transducer and activator of transcription -3 (transcription factor controlling cell growth, proliferation, migration and apoptosis), ROS – reactive oxygen species (by product of cellular oxidative metabolism in modulation of cell survival/death, signalling), MAPK -mitogen - activated protein kinase (cellular signalling to stimuli, cell growth, survival & proliferation), JNK – c-JUN-N- terminal kinase (phosphorylation of many mitochondrial/nuclear proteins), HIF- hypoxia inducible factor 1/2 protein (increases oxygen delivery, angiogenesis), p-21 – (major target of TP53 activity-protein linking DNA damage to cell cycle arrest), p-53 – tumour protein 53 –(tumour suppressor mechanisms), Bax-protein of the bcl-2 family protein 4 – (apoptosis regulator), Bcl-2 - B cell lymphoma 2 (protein regulating cell death), Mcl-1 – myeloid cell leukemia– 1 (protein regulating mitochondrial homeostasis), p-FAK/FAK – focal adhesion kinase (high expression in advanced cancers), p-SCr/SCr- Proto-oncogene tyrosine-protein kinase, p-akt/akt – protein kinase B, 2-HA-CD44 - hyaluronan (HA) synthase 2-HA-CD44, RHAMM – receptor for hyaluronic acid-mediated motility, PI3K/Akt - phosphoinositide 3-kinase)/Akt (Protein kinase B) signalling pathway (activation of anti-apoptosis proteins, inhibition of p53 and disruption of apoptosis cell death& augment cisplatin potency).
Chemosensitivity was enhanced by activating apoptosis and in vivo anticancer benefits was demonstrated in A549-xenografted mice [142]. Sensitisation to cisplatin was upregulated in vitro, when cisplatin-treated human lung cancer cells were pre-treated with peptide from Lentinus squarrosulus, a fungus in the family Polyporaceae that induced apoptosis. Apoptosis was achieved through the decrease of integrins (β1, β3, β5, α5, αV) and down-stream signals (p-FAK/FAK, p-Src/Src, p-Akt/Akt) leading to the modulation of p53, Bax, Bcl-2 and Mcl-1 -regulated pathways [143].
Cisplatin resistance is also mediated by a hypoxic tumour microenvironment [144]. Allicin, an organosulfur compound extractable from garlic (Allium sativum) has attenuated cell viability, proliferation, and migration by repressing hypoxia in the tumour microenvironment in NSCLC [144]. It enhanced chemosensitivity to cisplatin therapy through activating apoptosis and autophagy by ROS/MAPK and/or ROS/JNK pathways and suppressing HIF-1α and HIF-2α (master transcription factors that regulate cellular responses to hypoxia) in hypoxic A549 cells to overcome hypoxia and restore cisplatin sensitivity [144].
4. Recent advances in cisplatin-associated ovarian cancer
4.1. Ovarian cancer
Ovarian cancer (OC) is a malignancy among the repertoire of familial cancers affecting the female populations worldwide that also recurs in up to 80% of the patients leading to high mortality [145]. It is the most fatal among the gynaecological cancers and the highest in gynaecological cancer-related deaths [146]. Platinum -based therapies are its most effective cure with most patients, up to 70% responding well to cisplatin therapy, yet relapse upon developing drug resistance [145]. The annual incidence of OC worldwide was around 300,000 new cases with a mortality of 200,000 patients as per the GLOBOCAN statistics of the year 2020. The incidence of OC in Australia and New Zealand was approximately 1700 cases last year with around 1200 mortalities [11].
4.2. Chemoresistance to cisplatin in ovarian cancer
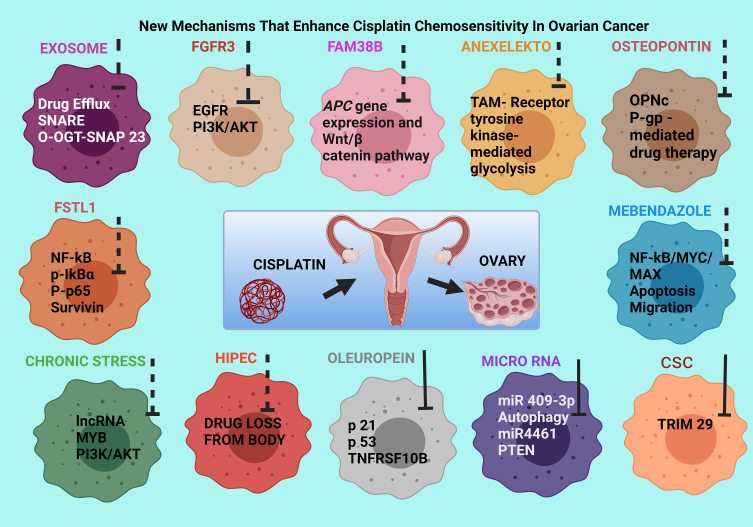
Ovarian cancer cells induce chemoresistance by inhibiting cellular apoptosis through modulating the cell cycle and DNA damage repair pathways (Figure 8) [147]. The family with sequence similarity 83 member B (FAM83B), is an oncogene (that promotes pancreatic ductal adenocarcinoma, endometrial cancer cell proliferation and the progression of lung squamous cell carcinoma) known to play a role in OC drug resistance towards cisplatin therapy [148]. A recent study found that the ovarian cancer cells with high FAM38B levels had low cisplatin resistance, mediated via the activation of the adenomatous polyposis coli gene expression and the Wnt immune pathway [148]. Anexelekto (AXL), which is a member of the TYRO3-AXL-MER (TAM) family of the receptor tyrosine kinases, is overexpressed in several different tumour types and influenced the cisplatin chemoresistance in OC cells [149]. AXL modulated the glycolysis pathway in cancer cells and was comparatively abundant in cisplatin-resistant OC cells [149]. Cisplatin therapy enriched with an inhibitor of AXL served to overcome the platinum compound-resistance displayed by OC [149]. Cancer stem cells (CSC) induced chemo - resistance to cisplatin therapy in OC and aberrations in the tripartite motif protein, TRIM29, was increased in CSC that led to loss of drug sensitivity and after-therapy recurrence [150]. TRIM29 promotes carcinomatous proliferation in pancreatic, cervical, glioma, and colorectal cancers via the Wnt-beta catenin pathway. It may prevail within the OC tumour microenvironment to downregulate sensitivity to cisplatin by inducing tumour recurrence in OC [150].
Figure 8.
Recently reported mechanisms of enhancing cisplatin sensitivity in ovarian cancer.
Micro RNA 409 (miR 409-3p) is a non-coding RNA that is downregulated in cisplatin-resistant OC tumours compared to the normal cisplatin-sensitive ovarian cells and suppresses autophagy. In chemo-sensitive cells, it binds with the Family interacting protein of 200 kD (FIP 200) and activates autophagy resulting in tumour destruction [151]. Cellular miR 409-3p overexpression augments sensitivity to cisplatin by conjugation of miR-409-3p and 3′-UTR in FIP 200 mRNA and hence, enhance FIP 200-mediated autophagy to alleviate chemoresistance in OC [151]. Furthermore, miR 4461 induces chemoresistance to cisplatin therapy in OC [152]. It augments in vitro OC cell proliferation and metastases where the knockdown of it in OC produced sensitivity to the drug via the stimulation of lipid and protein phosphatase and tensin homolog (PTEN), thereby suppressing cancer progression [152].
Cisplatin-loaded nanoparticular lipid carriers have successfully augmented the chemosensitivity in cisplatin-resistant OC cells via downregulating the cisplatin-resistance markers. They included glutathione-s-transferase pi (human) (GSTPi, a family of enzymes that play an important role in detoxification by catalysing the conjugation of many hydrophobic and electrophilic compounds with reduced glutathione) and adenosine triphosphatase copper transporting β gene (ATP7B), (known to decrease cisplatin efflux). ATP7B allowed accumulation of the drug in cytoplasm and modulating reactive oxygen species (ROS) generation that induced apoptotic pathways [153]. Osteopontin (OPN) is a protein that acts on various receptors associated with different signalling pathways in cancer progression and is heavily upregulated in several tumour types [154]. An in vitro study on an ACRP OC cell line that is resistant to cisplatin found OPN-c isoform and the P-glycoprotein multidrug transporter as the most upregulated proteins compared to its chemo-sensitive parental cell line in which the knocking down of the OPN isoform led to increased chemosensitivity to cisplatin therapy [154].
Mitochondrial biogenesis and the mitochondrial-dependent cellular energy metabolism were found to be increased in OC cells resistant to cisplatin treatment [155]. Chemo-resistant OC is dependent upon mitochondrial oxidative phosphorylation for its energy supply and intracellular Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha (PGC1α) -mediated mitochondrial biogenesis. They are increased by PGC1α which is a molecule that plays a central role in regulating cellular energy metabolism, integrating and coordinating nuclear DNA and mitochondrial DNA transcriptional machinery in OC tissues [155]. The inhibition of PGC1α in an in vitro cell line of OC displayed a decrease in cellular oxygen levels with increased lactic acid production, which indicated that chemo-resistant mechanisms in ovarian cancers are regulated by evoking mitochondrial resistance combined with the bioactivity of PGC1α [155].
Follistatin-like protein 1 (FSTL1) is a member of the extracellular matrix protein family which is significantly downregulated in the chemo-resistant OC epithelium (from which up to 90% of ovarian cancers arise), and that chemo-sensitivity of those cells are restored by overexpressing this protein. FSTL1 showed concurrent decrease in the protein levels in the NF-kB pathway (p-IκBα, p-p65 and survivin) and the upregulation of cellular apoptosis-associated proteins, Bax/Bcl-2 and caspase 3 [156]. Thus, FSTL-1 induces cisplatin sensitivity in OC with reduced colony formation and selective apoptosis achieved by the suppression of the NF-kB pathway [156].
The fibroblast growth factor 3 (FGFR3) protein increases cisplatin-resistance in OC [157]. The overexpression of it promotes the function of the epidermal growth factor receptor (EGFR), of which phosphorylation activates the PI3K/AKT pathway of cell proliferation and development of tumours [157]. The silencing of FGFR3 in vitro, and in nude mice, enhanced chemosensitivity of the ovarian tumours to cisplatin treatment [157].
Chronic stress aggravates OC in females and the overexpression of long non-coding RNA (lncRNA) induces cisplatin-resistance in stress-related OC, which is a dominant tumour suppressor molecule [158]. The inhibition of the lncRNA (named LOC102724169) suppressed my elob lastosis transcription factor family (MYB) transcription and the downstream targets of PI3K/AKT pathway, thereby increasing the curative effect of cisplatin therapy in stress - elevated-OC. Exosomes increase the efflux of drugs which cause cellular damage to lower the chemosensitivity of cancer cells [158]. Exosome secretion occurs under the control of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) family of proteins which anchors the exosomes on cytoplasmic membrane. The interactions between these proteins induce the exosome secretory process that is mediated by O-GlcNAcylation transferase (OGT) and reduces O-GlcNAcylation of SNAP-23 (a SNARE family protein) and enhances exosome release causing chemoresistance to cisplatin in ovarian tumours [159].
The first-line treatment for ovarian cancer consists debulking surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin [160]. A study estimated the utility rate and the most suitable cumulative dose of cisplatin to be administered in HIPEC to counteract the proportion of the drug that is lost from the body at the end of the perfusate. It revealed a total dose of cisplatin (70 mg/m2) at 43 °C for 90 min with no impact on the prognosis of disease [160]. The HIPEC model that was adapted could forecast the bioactive utilisation of cisplatin by regression analysis [160]. Cisplatin combined with the taxanes is another chemotherapeutic regimen in attenuating OC, but its efficacy is reduced by chemoresistance of the OC cells [101]. LLL12B is a small molecule inhibitor of chemoresistance that showed improved sensitivity when administered in combination with both cisplatin and paclitaxel together compared to either cisplatin or paclitaxel alone [101]. It suppressed cell viability and proliferation by downregulating the signal transducer and activator of transcription-3 (STAT3) pathway [101].
Legend: The dotted lines show downregulation or inhibition of the mechanisms given within the cancer cells and the solid black arrows show upregulation of the mechanisms given by the agents mentioned above the cells. SNARE-soluble N-ethylmale-imide-sensitive factor-attachment protein receptors 1, 4–5 (regulation of cancer cell invasion, chemo-resistance, autophagy, apoptosis, and the phosphorylation of kinases essential for cancer cell biogenesis), O-OGT-SNAP 23 - O-GlcNAcylated transferase of SNAP-23 (SNAP 23 is a member of the SNARE family of proteins is related to exosome production and cisplatin resistance), EGFR -epidermal growth factor receptor (cell signalling pathway that controls cell division and survival), PI3K/AKT - phosphoinositide 3-kinase)/Akt (Protein kinase B) signalling pathway (activation of anti-apoptosis proteins, inhibition of p53 and disruption of apoptosis cell death & augment cisplatin potency), APC gene – adenomatous polyposis coli gene (tumour suppressor), TAM- TYRO3, AXL, MERTK receptors (overexpressed by cancer cells, to promote oncogenic characteristics and tumour cell aggressiveness (proliferation, migration, cell survival, drug resistance), OPNc -osteopontin isoform -c (related to cisplatin chemoresistance), P-gp - permeability glycoprotein/multidrug resistance protein (membrane transporter with capability to efflux drug molecules out of the cancer cell leading to reduced efficiency of chemotherapy), NF-kB-MYC- MAX - Nuclear factor kappa beta – myelocytomatosis - viral oncogene homolog (carcinogenesis), TRIM 29 - tripartite motif protein 29 (associated with cisplatin resistance), miR -micro RNA (single stranded non-coding RNA which regulates RNA silencing and post -transcriptional regulation of gene expression), Bcl-2 - B cell lymphoma 2 (protein regulating cell death), Mcl-1 – myeloid cell leukemia– 1 (protein regulating mitochondrial homeostasis), PTEN - Phosphatase and tensin homolog (a tumour suppressor gene having lipid and protein phosphatase activities), p-IκBα – phosphorylated I kappa B (tumour promotion and metastasis), NF-kB – nuclear factor kappa beta - (transcription of genes involved in cell proliferation, survival, angiogenesis, inflammation and tumour promotion and metastasis), P- p65 – phosphorylated protein 65 (Ser 536 - promotes apoptosis), lncRNA – long non coding RNA (regulate cell proliferation, apoptosis, migration, invasion and maintenance of stemness during cancer development), MYB - my elob lastosis transcription factor (increases cisplatin chemosensitivity), p-21 – protein 21 (cell cycle inhibitor), p-53 – protein 53 (tumour suppressor protein), TNFRSF 10B – tumour necrosis factor receptor superfamily 10 B (associated with malignant progression of lung cancer), FAM83B - family with sequence similarity 83 member B (biomarker for progression of lung cancer), FSTL1 - Follistatin-like protein 1 (member of the extracellular matrix protein family which is significantly downregulated in the chemo-resistant OC epithelium), FGFR3 - fibroblast growth factor 3 (protein which increases cisplatin-resistance in OC), HIPEC - hyperthermic intraperitoneal chemotherapy (minimises drug loss from the body), CSC- cancer stem cells (possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer).
Mebendazole (MBZ) is a multi-purpose anti-parasitic drug that reduces cisplatin resistance in OC and inhibit tumour proliferation, wound healing, cell migration and apoptosis by activating multiple signalling pathways. They consist of a member of the Ets (E twenty-six) oncogene family of transcription factors/dimer of serum response factor (ELK/SRF), Nuclear factor kappa beta – myelocytomatosis - viral oncogene homolog (NF-kB/MYC/MAX), and E2F/DP1) associated with human ovarian cancer [161]. Oleuropein, a phenolic ingredient, upregulated the expression of p21, p53, TNF Receptor Superfamily Member 10b (TNFRSF10B), miR-34a, miR-125b and miR-16 with the suppression of Bcl-2 and myeloid cell leukemia protein-1 (Mcl-1). The outcome was cell proliferation inhibition, induction of apoptosis and enhancing chemosensitivity to cisplatin in OC [147].
The standard chemotherapy utilized in OC comprises cytoreductive surgery augmented by platinum-based chemotherapeutics such as cisplatin. However, they lead to recurrence due to the development of chemoresistance because of poor prognosis and deficiencies in early detection since patients by then had reached mid stage of the OC [162]. A member of the Bcl-2 athanogene family of proteins, BAG-5, was downregulated in cisplatin-administered OC resulting in metabolic programming towards maintaining the cancer stem cell -like features and inducing chemoresistance [163]. BAG5 is a multifunctional protein which induces stress signals, cell cycle and tumour development, mediated by Bcl6/BAG5/Rictor-mTORC2 signalling pathway in cisplatin-resistant ovarian cancer cells and hence, provides a therapeutic target in clinical therapy of OC [163]. The levels of glutamine, glutamate and glutathione were elevated in cisplatin-resistant OC in vitro, whereas the level of glutathione transferase, the enzyme responsible for the glutamine conversion to glutamate was decreased [164]. Restoration of the enzyme activity by DNA methylating agents was observed and thus the intracellular glutamate availability could be modulated to enhance the chemosensitivity of OC to cisplatin treatment [164].
5. Recent advances in cisplatin-associated bladder cancer
5.1. Bladder cancer
Bladder cancer (BC) occurs mostly as muscle-invasive BC, in the older adults (in the age bracket of 70–90) with bloodied urine as its preliminary diagnostic factor. BC incidence is 3–4 times more in males than in the females [165]. BC comprises urothelial BC, squamous cell carcinoma in the bladder epithelium and muscle-invasive BC (MIBC) [166]. The standard treatment is a radical cystectomy followed by conventional chemotherapy [167] such as cisplatin, for which there is increased chemoresistance [168]. Yet it is highly treatable if detected during the early stages and the most common risk factor is smoking, as the tobacco in cigarettes contain carcinogens. For advanced unresectable BC and/or metastatic BC patients, cisplatin-based chemotherapy remains the first choice of treatment [169]. The best cisplatin eligibility criterion is considered as having a creatinine clearance rate above 60 mL/min [170]. The statistics have revealed a 500,000 new cases of BC diagnosed with around 200,000 mortalities worldwide in 2020, as per the estimates of the GLOBOCAN cancer registry [11]. The BC incidence in Australia and New Zealand was reported at 3900 new cases with around 1200 deaths in the last year [11].
5.2. Chemoresistance to cisplatin in bladder cancer
Perioperative treatment by chemotherapeutics have been suggested after the evaluation of several chemotherapeutics: vinblastine, doxorubicin, methotrexate, and cisplatin given as either neoadjuvant or adjuvant therapy in BC, that displayed around 5% effectiveness in overall survival [171]. Cisplatin treatment developed higher responsiveness in metastatic MIBC and therefore, was effective as much in the perioperative setting [171]. The demethylation of m6A, the most common internal RNA modification in eukaryotes is conspicuously elevated in various tumour types [172]. AlkB homolog 5 RNA demethylase (ALKBH5), a m6A demethylase, belonging to the AlkB family of dioxygenases is markedly downregulated in BC tissues and cell lines, both in vitro and in vivo, and promotes chemoresistance to cisplatin therapy [172]. The overexpression of ALKBH5 was associated with drug sensitivity by casein kinase 2 (CK2) α-mediated glycolysis pathway in a m6A-dependent manner and provides new clinical insights to mediate the survivability in BC [172]. The efficacy of cisplatin in BC was reduced with the elevation of androgens, and the androgen receptor activity was suppressed by a molecule identified as BXDC2, a potential downstream target in the androgen receptor stimulation pathway [173]. The modulation of BXDC2 activity via inhibiting the androgen receptor pathway combined with extracellular signal-induced kinase (ERK) signalling was suggested as means to improve cisplatin sensitivity in BC [173].
Gene profiling of the cisplatin resistance-related genes targeted by the tumour-suppressive micro-RNAs, has found Enoyl-CoA, hydratase/3-hydroxyacyl CoA dehydrogenase (EHHADH), involved in the degradation of fatty acids as a direct target of miRNA 486-5p. Its transfection into in vitro BC cell lines (induced tumour suppression) and the knockdown of EHHADH had profoundly increased the sensitivity of those cells to cisplatin [168]. MIBC arises often from the mutations of the CDKN1A gene which encodes for the tumour suppressive protein, p21 without which the cells are unable to regulate the cell cycle or the DNA repair mechanisms [174]. An in vitro study of BC cell lines in which different p21 peptide truncating was evaluated, found that BC cells deficient in p21 were more chemo-sensitive to cisplatin than the distally truncated p21 harbouring cells which were more chemo-resistant to cisplatin. This response indicated disparate peptide biology arising from the CDKN1A aberrations, the outcome of which is mutation context - dependent in BC [174]. The exosomes derived from cancer-associated fibroblasts promote chemoresistance to cisplatin therapy in BC. An exosome derived from cancer-associated fibroblasts (LINCOO355) displayed enhanced resistance mediated by miR34b-5p. The outcome was upregulated adenosine-triphosphate (ATP) binding cassette subfamily member B -1 [ABCB1- a protein which possesses P-glycoprotein (P-gp) activity related to multi-drug resistance in certain cancers] [175]. However, the chemoresistance was abrogated by the overexpression of miR34b-5p and the silencing of ABCB1 mechanism in an in vitro study which utilised BC cell lines (T24 and 5367) [176].
The glycoprotein 130 (GP130) increases chemoresistance to cisplatin, when overexpressed in BC cells [177]. Downregulation of GP 130 increases cisplatin-chemosensitivity, by inducing DNA repair mechanisms via stimulation of Ku 70, an initiator of canonical non-homologous end joining repair (c-NHEJ) and suppressor of apoptosis. Ku 70 and GP 130 exhibited a positive correlation such that the knockdown of GP130 by a small molecule inhibitor combined with cisplatin promoted DNA double stranded breaks, apoptosis, and reduced cell viability in BC, with an inhibitory effect on Ku 70 [177]. Furthermore, in vitro hypoxic conditions were inhibitory towards cisplatin chemo-sensitization of BC cells where hypoxia induced cisplatin resistance by upregulating autophagy in the BC cell line, BIU-87 [178]. The cisplatin-stimulated apoptosis and autophagy was abrogated by the addition of a hypoxia-inducible factor (HIF-1α) - inhibitor, which indicated the hypoxia-autophagy pathway could provide new insights for the clinical management of BC chemoresistance towards cisplatin therapy [178]. The activation of the ubiquitin proteasome systems (UPS) is correlated with chemoresistance of cancers and exist in BC as well [179]. Cisplatin induced the activation of the UPS and hence chemoresistance in an in vitro BC cell line with the activation of endoplasmic reticulum -induced stress through the unfolded protein accumulation, which indicated UPS as a potential target to modulate cisplatin resistance in BC [179].
6. Recent advances in cisplatin-associated head and neck cancer
6.1. Head and neck cancer
Head and neck squamous cell carcinoma (HNSCC) is the sixth common cause of cancer -related mortality worldwide with an annual 600,000 cases diagnosed consisting a 50% mortality rate [11]. HNSCC arise in the upper digestive and airway associated surfaces such as the oral cavity, pharynx, and larynx which in most patients manifest localised within the site up to 60% of the occurrences and the relapsing and metastatic patients comprise about 25% of the diagnosed population [180]. Early detection within the stages I and II is common although some cases are undetected until it had reached stages III and IV and cisplatin remains the frontline chemotherapy combined with radiotherapy as its best treatment strategy [180, 181].
Chemoradiotherapy (CRT) in HNSCC is associated with low skeletal muscle mass that decreases after CRT and is of value as a prognosis biomarker compared with the body-mass index (BMI) of healthy individuals that is higher than 30 kg/m2 [182]. Ultrasound -guided delivery of cisplatin-loaded microbubbles was effective in HNSCC with reduced systemic cytotoxicity and the same microbubbles loaded with both cisplatin and statins (atorvastatin) acted synergistically inhibitory towards HNSCC progression. These inhibitory anticancer effects were achieved by inducing cancer cell apoptosis that reduced tumour viability with a concurrent decrease in cellular glutathione [183]. An evaluation of cisplatin efficacy in the head and neck carcinoma-mimicking cell lines (FaDu and PE/CA-PJ49) combined with resveratrol (shown to reduce cisplatin toxicity and increase cell viability in normal HaCaT cells) revealed the induction of apoptosis in FaDu cells. Cellular apoptosis was induced by upregulating the gene expression of TP53 and cellular myelocytomatosis (c-MYC- which regulates apoptotic cell death) with a decrease in the B cell lymphoma 2 (BCl-2- shows context-dependent induction or inhibition of apoptosis) [184]. In the PE/CS-PJ49, the expression of c-MYC was reduced with cell cycle blockade at G0/G1 phase [184]. Circulating micro RNAs are single stranded RNA molecules consisting of up to 22 non-coding nucleotides that are abundant in blood plasma and aid in post-transcriptional modifications of pro-cancerous genes. They are therefore considered as promising biomarkers of cancer-associated nephrotoxicity [185]. A study of the miRNAs isolated from blood plasma of head & neck cancer patients having lesions in oral cavity, oropharynx, hypopharynx, and larynx, that received one cycle of high dose cisplatin found miR-3168, miR-6125, and miR-4718 to be potential biomarkers of cisplatin-induced nephrotoxicity in head and neck cancers [185].
A randomized phase II trial, on newer therapeutics found the programmed death ligand-1 (PD-1) monoclonal antibody, Pembrolizumab, with concurrent cisplatin radiotherapy was feasible in tumour resected head and neck cancer [186]. Pantoprazole used for treating acid reflux in the gut was shown to reduce cisplatin-induced nephrotoxicity, in head and neck cancer. Thus, it was identified as a candidate for suppressing the increase of biomarkers [serum creatinine and soluble FasL (sFasL), urinary neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1)] that are associated with renal damage [187]. In a randomized phase III trial on locoregionally advanced HNSCC patients treated with cetuximab and radiotherapy compared to that of cisplatin, was considered superior to cetuximab [181].
Head and neck cancer patients run a risk of 35% in developing acute kidney injury as an adverse side effect of cisplatin treatment in which the eGFR is markedly reduced. The eGFR is considered reduced when fallen below 60 ml/min/1.73 m2 within 3 months from the initiation of treatment and did not have the baseline eGFR restored to normal levels even 12 months after cessation of therapy [71]. Another recent study on head and neck cancer reported the importance of a second glomerular filtration rate reading as a prognostic biomarker for detecting the onset of renal dysfunction associated with cisplatin therapy [188].
Ototoxicity is another side effect of cisplatin therapy which comprises an 88% risk in experiencing hearing loss in head and neck carcinoma patients [189]. An effective model of hearing loss arising from cisplatin toxicity in head and neck cancer was confirmed as a model able to predict hearing complications with which pre-treatment counselling and post-treatment evaluations could be performed [189]. Selenium is an important trace element associated with cancer incidence due to its inclusion in glutathione peroxidase and thioredoxin reductase which are oxidative stress-related enzymes, and the level of those seleno-proteins influenced the achievement of the full dose of cisplatin in HNSCC [190]. Selenium attenuated cisplatin-induced nephrotoxicity in HNSCC patients and thus, selenium supplementation before cisplatin chemoradiotherapy is independently related to the effectiveness of high -dose cisplatin in HNSCC [190].
6.2. Chemoresistance to cisplatin in head and neck cancer
The head and neck cancers comprise a group of heterogeneous tumours and cancer-associated fibroblasts (CAF) are a dominant cell type found in the stroma of these tumours and secrete various substances that can modulate the tumour microenvironment. CAF exert a direct effect on chemoresistance to cisplatin therapy [191]. Therefore, treating chemo-resistant tumours with cisplatin produces no curative effect but serve to increase adverse side effects and cancer progression [191]. Further, genotyping had revealed a link between the differential cisplatin resistance of HNSCC with the vascular endothelial growth factor A (VEGFA), prostaglandin E synthase 2 (PGE2S), cyclooxygenase 2 (COX2), epithelial growth factor receptor (EGFR), and Nanog homeobox genes (NANOG – associated with teratocarcinoma, germ cell and embryonal cancer) in cancer cells which are promoted through modulating gene expression and paracrine signalling [191]. Micro RNA (miR) 136-5p has been evaluated with its downstream target Rho-associated coiled-coil containing protein kinase 1 (ROCK 1) in laryngeal and hypopharyngeal squamous cell carcinoma (LSCC and HSCC) as a potential therapeutic strategy for attenuating chemoresistance to cisplatin [192]. The overexpression of miR 136-5p increased sensitivity to cisplatin by reducing cell viability and increasing cellular apoptosis and autophagy [192]. The knockdown of miR 136-5p showed negative regulation of ROCK 1 levels with the suppression of AKT/mTOR pathway that elevated chemoresistance which was reversed with a ROCK 1 molecular inhibitor [192]. Protein 38, an integral component of the mitogen-activated protein kinase (MAPK) pathway has attenuated cisplatin resistance in HNSCC by interfering with the DNA repair mechanisms [193]. It has also activated the Wnt/beta – catenin signalling and the expression of cancer stem cell markers in HNSCC resistant to cisplatin therapy [193].
7. Recent advances in cisplatin-associated cervical cancer
7.1. Cervical cancer
Cancer of the uterine cervix (CUC) is the seventh most common cancer that has reported 600,000 cases worldwide and approximately 50% of this number consisted cervix-related deaths in the year 2020 [11]. In Australia and New Zealand, around 1000 new cases are reported each year with about 400 mortalities occurring per annum [11]. Cervical cancer is the fourth common cancer that affects the females for which cisplatin therapy remains the front-line treatment strategy either alone or as an adjuvant or neoadjuvant combined with radiotherapy [194, 195]. Cisplatin combined with radiation therapy comprises of the standard treatment for cervical carcinoma but its anti-neoplastic success is obliterated by cisplatin resistance induced by multi-drug resistant transporters and the high activity of the cervical cancer stem cell marker, aldehyde dehydrogenase [196].
7.2. Chemoresistance to cisplatin in cervical cancer
Reduced chemosensitivity to cisplatin is encountered in cervical cancer treatment as its major impediment to overcoming cervical neoplasm which may be due to long-term usage combined with factors such as immunity, physiology, and drug metabolism [83]. Lycopene, a naturally-occurring carotenoid, was screened for its efficacy in augmenting sensitivity to cisplatin in HeLa, cancer-resistant cervical carcinoma cell line [197]. Lycopene could suppress the NF-kB pathway-mediated cell viability and the apoptosis-regulator, Bcl-2 while upregulating B-cell-associated X protein (Bax – which penetrate the mitochondrial outer membrane to mediate cell death by apoptosis) and the nuclear factor erythroid 2-related factor (Nrf2) -regulated antioxidant mechanisms [197]. The inhibition of nuclear import via Karyopherin beta 1 (Kpnβ1) displayed anticancer activity and sensitised cervical cancer cells to cisplatin [198]. In vitro pre-treatment with an inhibitor of Kpnβ1, INI-43, improved cisplatin sensitivity when combined, by suppressing NF-kB transcription and augmenting the suppressor effects of TP53 (with increased p21 and reduced MCl-1 activity) [198]. A nanocarrier-mediated cisplatin and JQI (an inhibitor of bromodomain containing protein -4 responsible for acetylating histones, remodelling chromatin, and recruiting factors necessary for transcription), treatment identified as PGP -CDDP/JQ1, showed promising results in overcoming cisplatin resistance in cervical cancer in vivo by exerting synergistic cytotoxicity mediated through the polo-like kinase-1 (Plk1-a regulator of the mitotic cell cycle) – mutant TP53 axis [199].
Cyanadin-3-O-glucoside (an anthocyanin named chrysanthemin) together with cisplatin showed synergistic anticancer potential by inducing G1 cell cycle arrest and apoptosis [200]. In the Hela cell line, it enhanced the effects of cisplatin by decreasing BCl-2 and cyclin D activation with simultaneous upregulation of TP53 mechanisms, Bax -cleaved caspase 3 (an indicator of cell death activity and propagate signals for apoptosis by enzymatic activity on downstream targets), tissue inhibitor matrix metalloproteinase -1 (TIMP-1 – a regulator of cell biology functions by cytokine-like activity) and PI3K/AKT/mTOR pathway [200]. The T-box transcription factor 1 (TBX1), a member of the T-box family, exerted anti-tumour effects was decreased in cervical cancer and showed resistance towards cisplatin treatment with poor prognosis [201]. TBX1 overexpression was linked with increased sensitivity to cisplatin in cervical cancer cells by the downregulation of MAPK and AKT pathways and could bind directly with miR 6727-5p of which it is a target gene and enhanced the tumour suppression activity of cisplatin treatment [201]. Methyltransferase-like 3 (METTL3) is a protein which catalyses m6A methylation on mRNA or non-coding RNA that affect mammalian RNA metabolism, and abnormal m6A levels caused by METTL3 were involved in cancer development [202]. It increased cisplatin sensitivity in cervical cancer cell lines by promoting apoptosis and the suppression of cell viability via downregulating receptor for advanced glycation end-product (RAGE), associated with metastasis and poor prognosis of various types of cancer [202].
Tumour necrosis factor α-induced protein 8 (TNFAIP8)-like protein 1 (TIPE1) belongs to the TIPE family and is an apoptotic factor involved in necroptosis [203]. However, a recent study has reported enhanced chemoresistance of TIPE1 in cervical cancer cells both in vivo and in vitro, and a factor that determines the transition from cisplatin chemosensitivity to chemoresistance in a wild-type TP53 -dependent manner [203]. Cisplatin treatment delivered in combination with photodynamic therapy (using lasers with methylene blue as the photosensitizing agent) to cervical cancer cell lines, A2780 (sensitive to cisplatin) and A2750-CP (resistant to cisplatin) enhanced drug sensitivity by decreasing the half-maximal inhibitory level (IC50) by 2-fold that caused cell membrane disruption through lipid peroxidation [204]. miR143 was found to synergise cisplatin activity in CaSki cell line which are derived from cervical cancers by the upregulation of the apoptosis-related genes Bax, BCl-2 and caspase 9 [205]. miR143 was overexpressed in chemo-sensitive cells which together with cisplatin increased cell cycle arrest at the sub-G1 and G2-M phases, induced autophagy activation, downregulated vimentin - inhibited CaSki cell migration and c-Myc expression in the treatment group [205]. A tumour -suppressor transcription factor ZBTB28 (which is often silenced in cervical cancer cells by CpG methylation of its promoter) was identified as a potential therapeutic target in cervical cancer that increased chemosensitivity to cisplatin therapy [206]. It was found to mediate cellular apoptosis by interacting with the autophagy-related gene FIP200 [206].
8. Conclusions
Cancer is a complex disease which causes high mortality for which cisplatin has been an effective treatment. The therapeutic value of cisplatin is restricted by the dose-limiting toxicity and the failure to overcome the mechanisms of tumour adaptability and evasion of its drug activity, for which efficacious remedies must be found. The miRNA, siRNA, and circular RNA have been widely recognised as having potential to overcome chemoresistance by which epigenetic modulation via enhancing DNA salvage and repair mechanisms as well as multi-drug resistance could offer useful insights. The new phenomenon of immune editing of tumours could promote the interplay of cisplatin action with immune responsivity in manipulating the tumour-destructive mechanisms by metabolic re-programming of carcinomatous lesions. This review has identified two strategies to improve cisplatin therapy; (1) Reduce the toxicity of cisplatin to non-cancer cells to enable higher doses or more extended treatment with cisplatin to be used and (II) Increase the toxic effect of cisplatin specifically for cancer cells so that the dose of cisplatin can be lowered, thereby reducing damage to non-cancer cells. The cumulative anticancer efficacy of cisplatin has not waned yet but must be strengthened as tumour-specific adjuvant or combinatorial therapy.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
First author is a recipient of the Research Training Programme (RTP) Scholarship from the Australian Government for Ph.D. candidature.
References
- 1.Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tchounwou P.B., et al. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Exp. Pharmacol. 2021;13:303. doi: 10.2147/JEP.S267383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Sayyed A.A., et al. MiR-155 inhibitor-laden exosomes reverse resistance to cisplatin in a 3D tumor spheroid and xenograft model of oral cancer. Mol. Pharm. 2021;18(8):3010–3025. doi: 10.1021/acs.molpharmaceut.1c00213. [DOI] [PubMed] [Google Scholar]
- 5.Hong B., et al. Oncolytic HSV therapy modulates vesicular trafficking inducing cisplatin sensitivity and antitumor immunity. Clin. Cancer Res. 2021;27(2):542–553. doi: 10.1158/1078-0432.CCR-20-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morelli A.P., et al. Metformin impairs cisplatin resistance effects in A549 lung cancer cells through mTOR signaling and other metabolic pathways. Int. J. Oncol. 2021;58(6):1–15. doi: 10.3892/ijo.2021.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabosch S., et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019;38(13):2380–2393. doi: 10.1038/s41388-018-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razak S., et al. Molecular docking, pharmacokinetic studies, and in vivo pharmacological study of indole derivative 2-(5-methoxy-2-methyl-1H-indole-3-yl)-N′-[(E)-(3-nitrophenyl) methylidene] acetohydrazide as a promising chemoprotective agent against cisplatin induced organ damage. Sci. Rep. 2021;11(1):1–23. doi: 10.1038/s41598-021-84748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fennell D., et al. Cisplatin in the modern era: the backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat Rev. 2016;44:42–50. doi: 10.1016/j.ctrv.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Hellesnes R., et al. Wolters Kluwer Health; 2021. Testicular Cancer in the Cisplatin Era: Causes of Death and Mortality Rates in a Population-Based Cohort. [DOI] [PubMed] [Google Scholar]
- 11.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 12.Kauffman G.B., Pentimalli R., Hall M.D. Michele Peyrone (1813-1883), discoverer of cisplatin. Platin. Met. Rev. 2010;54(4):250–256. [Google Scholar]
- 13.Johnston, L., Characterizing drug design: a review of platinum-based chemotherapy drugs. Mom. Quest.. 2: p. 13.
- 14.Barry N.P., Sadler P.J. 100 years of metal coordination chemistry: from Alfred Werner to anticancer metallodrugs. Pure Appl. Chem. 2014;86(12):1897–1910. [Google Scholar]
- 15.Rosenberg B. Cisplatin. Elsevier; 1980. Cisplatin: its history and possible mechanisms of action; pp. 9–20. [Google Scholar]
- 16.de Biasi A.R., Villena-Vargas J., Adusumilli P.S. Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin. Cancer Res. 2014;20(21):5384–5391. doi: 10.1158/1078-0432.CCR-14-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg. Chem. 2019;88 doi: 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- 18.Petrović M., Todorović D. Biochemical and molecular mechanisms of action of cisplatin in cancer cells. Facta Univ. Ser. Med. Biol. 2016;18(1) [Google Scholar]
- 19.GLOBOCAN 2020 . 2020. Global Cancer Observatory: Lyon Cedex. [Google Scholar]
- 20.Kenmotsu H., et al. Survival data for postoperative adjuvant chemotherapy comprising cisplatin plus vinorelbine after complete resection of non-small cell lung cancer. Cancer Chemother. Pharmacol. 2017;80(3):609–614. doi: 10.1007/s00280-017-3400-z. [DOI] [PubMed] [Google Scholar]
- 21.Petrelli F., Barni S. Non-cancer-related mortality after cisplatin-based adjuvant chemotherapy for non-small cell lung cancer: a study-level meta-analysis of 16 randomized trials. Med. Oncol. 2013;30(3):641. doi: 10.1007/s12032-013-0641-5. [DOI] [PubMed] [Google Scholar]
- 22.Group, U.C.S.W. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2017. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-Based Report. [Google Scholar]
- 23.Cohen A., et al. Mechanism and reversal of drug-induced nephrotoxicity on a chip. Sci. Transl. Med. 2021;13(582) doi: 10.1126/scitranslmed.abd6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albany C., et al. Cisplatin-associated neuropathy characteristics compared with those associated with other neurotoxic chemotherapy agents (Alliance A151724) Support. Care Cancer. 2021;29:833–840. doi: 10.1007/s00520-020-05543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain Y., et al. Phytotherapy Research; 2021. Curcumin–cisplatin Chemotherapy: A Novel Strategy in Promoting Chemotherapy Efficacy and Reducing Side Effects. [DOI] [PubMed] [Google Scholar]
- 26.Shahid F., Farooqui Z., Khan F. Cisplatin-induced gastrointestinal toxicity: an update on possible mechanisms and on available gastroprotective strategies. Eur. J. Pharmacol. 2018;827:49–57. doi: 10.1016/j.ejphar.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Wilson J.J., Lippard S.J. Synthetic methods for the preparation of platinum anticancer complexes. Chem. Rev. 2014;114(8):4470–4495. doi: 10.1021/cr4004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bucher-Johannessen C., et al. Cisplatin treatment of testicular cancer patients introduces long-term changes in the epigenome. Clin. Epigenet. 2019;11(1):1–13. doi: 10.1186/s13148-019-0764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groot H.J., et al. Platinum exposure and cause-specific mortality among patients with testicular cancer. Cancer. 2020;126(3):628–639. doi: 10.1002/cncr.32538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y.-G., et al. Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: a nationwide retrospective cohort study (KCSG HN13–01) BMC Cancer. 2020;20(1):1–9. doi: 10.1186/s12885-020-07297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdelhakam D., et al. Complete remission with immunotherapy: case report of a patient with metastatic bladder cancer to the humerus. Urol. Case Rep. 2020;30 doi: 10.1016/j.eucr.2020.101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keener A.B. Innovative therapies to tackle platinum-resistant ovarian cancer. Nature. 2021;600(7889):45–47. [Google Scholar]
- 33.Zapata I., et al. Causes of death in patients with locally advanced head and neck cancer treated with radiotherapy and systemic therapy. BMC Cancer. 2019;19(1):1–10. doi: 10.1186/s12885-019-6427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonomi P., et al. Comparison of survival and quality of life in advanced non–small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 2000;18(3):623. doi: 10.1200/JCO.2000.18.3.623. 623. [DOI] [PubMed] [Google Scholar]
- 35.Liang F., et al. Risk of second primary cancers in cancer patients treated with cisplatin: a systematic review and meta-analysis of randomized studies. BMC Cancer. 2017;17(1):1–12. doi: 10.1186/s12885-017-3902-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alderden R.A., Hall M.D., Hambley T.W. The discovery and development of cisplatin. J Chem. Educ. 2006;83(5):728. [Google Scholar]
- 37.Johnstone T.C., Suntharalingam K., Lippard S.J. The next generation of platinum drugs: targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs. Chem. Rev. 2016;116(5):3436–3486. doi: 10.1021/acs.chemrev.5b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamble D.B., et al. Repair of cisplatin− DNA adducts by the mammalian excision nuclease. Biochemistry. 1996;35(31):10004–10013. doi: 10.1021/bi960453+. [DOI] [PubMed] [Google Scholar]
- 39.Sarin N., et al. Cisplatin resistance in non-small cell lung cancer cells is associated with an abrogation of cisplatin-induced G2/M cell cycle arrest. PLoS One. 2017;12(7):e0181081. doi: 10.1371/journal.pone.0181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuertes M., et al. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr. Med. Chem. 2003;10(3):257–266. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- 41.Hoeschele J.D. Dr Barnett rosenberg–a personal perspective. Dalton Trans. 2016;45(33):12966–12969. doi: 10.1039/c6dt02152b. [DOI] [PubMed] [Google Scholar]
- 42.Peters G.J., et al. Seminars in Oncology. 1995. Interaction between cisplatin and gemcitabine in vitro and in vivo. [PubMed] [Google Scholar]
- 43.Tsang R.Y., Al-Fayea T., Au H.-J. Cisplatin overdose. Drug Saf. 2009;32(12):1109–1122. doi: 10.2165/11316640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Pignon J.-P., et al. Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews. Centre for Reviews and Dissemination; UK: 2008. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. [DOI] [PubMed] [Google Scholar]
- 45.Boulikas T., Vougiouka M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs. Oncol. Rep. 2004;11(3):559–595. [PubMed] [Google Scholar]
- 46.Weiss R.B., Christian M.C. New cisplatin analogues in development. Drugs. 1993;46(3):360–377. doi: 10.2165/00003495-199346030-00003. [DOI] [PubMed] [Google Scholar]
- 47.Hato S.V., et al. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin. Cancer Res. 2014;20(11):2831–2837. doi: 10.1158/1078-0432.CCR-13-3141. [DOI] [PubMed] [Google Scholar]
- 48.Lokich J., Anderson N. Carboplatin versus cisplatin in solid tumors: an analysis of the literature. Ann. Oncol. 1998;9(1):13–21. doi: 10.1023/a:1008215213739. [DOI] [PubMed] [Google Scholar]
- 49.Anderson H., et al. Comparative toxicity of cisplatin, carboplatin (CBDCA) and iproplatin (CHIP) in combination with cyclophosphamide in patients with advanced epithelial ovarian cancer. Eur. J. Cancer Clin. Oncol. 1988;24(9):1471–1479. doi: 10.1016/0277-5379(88)90338-0. [DOI] [PubMed] [Google Scholar]
- 50.Stordal B., Pavlakis N., Davey R. Oxaliplatin for the treatment of cisplatin-resistant cancer: a systematic review. Cancer Treat Rev. 2007;33(4):347–357. doi: 10.1016/j.ctrv.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Rottenberg S., Disler C., Perego P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer. 2021;21(1):37–50. doi: 10.1038/s41568-020-00308-y. [DOI] [PubMed] [Google Scholar]
- 52.Brown A., Kumar S., Tchounwou P.B. Cisplatin-based chemotherapy of human cancers. J. Cancer Sci. Ther. 2019;11(4) [PMC free article] [PubMed] [Google Scholar]
- 53.Casanova A.G., et al. Regression modeling of the antioxidant-to-nephroprotective relation shows the pivotal role of oxidative stress in cisplatin nephrotoxicity. Antioxidants. 2021;10(9):1355. doi: 10.3390/antiox10091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kınal M.E., et al. Investigation of astaxanthin effect on cisplatin ototoxicity in rats by using otoacoustic emission, total antioxidant capacity, and histopathological methods. Ear Nose Throat J. 2021;100(4):NP198–NP205. doi: 10.1177/0145561319866826. [DOI] [PubMed] [Google Scholar]
- 55.Kobel M.J., et al. Vestibular thresholds: a review of advances and challenges in clinical applications. Front. Neurol. 2021;12:203. doi: 10.3389/fneur.2021.643634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albini A., et al. bioRxiv; 2021. A Polyphenol-Rich Extract of Olive Mill Wastewater Enhances Cancer Chemotherapy Effects while Mitigating Cardiac Toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang C.-y., et al. Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021:1–19. doi: 10.1038/s41401-021-00620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dieckmann K.-P., Struss W.J., Budde U. Evidence for acute vascular toxicity of cisplatin-based chemotherapy in patients with germ cell tumour. Anticancer Res. 2011;31(12):4501–4505. [PubMed] [Google Scholar]
- 59.Haugnes H.S., et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann. Oncol. 2007;18(2):241–248. doi: 10.1093/annonc/mdl372. [DOI] [PubMed] [Google Scholar]
- 60.Gietema J., et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet. 2000;355(9209):1075–1076. doi: 10.1016/s0140-6736(00)02044-4. [DOI] [PubMed] [Google Scholar]
- 61.Zhao L. Protective effects of trimetazidine and coenzyme Q10 on cisplatin-induced cardiotoxicity by alleviating oxidative stress and mitochondrial dysfunction. Anatol. J. Cardiol. 2019;22(5):232. doi: 10.14744/AnatolJCardiol.2019.83710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian P., et al. Cyanidin ameliorates cisplatin-induced cardiotoxicity via inhibition of ROS-mediated apoptosis. Exp. Ther. Med. 2018;15(2):1959–1965. doi: 10.3892/etm.2017.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trendowski M.R., et al. Genetic and modifiable risk factors contributing to cisplatin-induced toxicities. Clin. Cancer Res. 2019;25(4):1147–1155. doi: 10.1158/1078-0432.CCR-18-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaemmerer E., Loessner D., Avery V.M. Drug Discovery Today; 2020. Addressing the Tumour Microenvironment in Early Drug Discovery: a Strategy to Overcome Drug Resistance and Identify Novel Targets for Cancer Therapy. [DOI] [PubMed] [Google Scholar]
- 65.Galluzzi L., et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 66.Kuo M.T., et al. Role of the human high-affinity copper transporter in copper homeostasis regulation and cisplatin sensitivity in cancer chemotherapy. Cancer Res. 2012;72(18):4616–4621. doi: 10.1158/0008-5472.CAN-12-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blair B.G., et al. Copper transporter 2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Mol. Pharmacol. 2011;79(1):157–166. doi: 10.1124/mol.110.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashrafizadeh M., et al. Association of the epithelial–mesenchymal transition (EMT) with cisplatin resistance. Int. J. Mol. Sci. 2020;21(11):4002. doi: 10.3390/ijms21114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun C.-Y., et al. Phytochemicals: current strategy to sensitize cancer cells to cisplatin. Biomed. Pharmacother. 2019;110:518–527. doi: 10.1016/j.biopha.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 70.McSweeney K.R., et al. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers. 2021;13(7):1572. doi: 10.3390/cancers13071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patimarattananan T., et al. Risk and impact of delayed renal impairment in patients with locally advanced head and neck squamous cell carcinoma receiving chemoradiotherapy with cisplatin. Support. Care Cancer. 2021;29(2):877–887. doi: 10.1007/s00520-020-05566-y. [DOI] [PubMed] [Google Scholar]
- 72.Ghorbani A., Omidvar B., Parsi A. Protective effect of selenium on cisplatin induced nephrotoxicity: a double-blind controlled randomized clinical trial. J. Nephropathol. 2013;2(2):129. doi: 10.12860/JNP.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oguri T., et al. The retrospective analysis of nephrotoxicity for cisplatin dose of CRT compared 100 mg/m2 to 80 mg/m2 for head and neck cancer (HNC) patients. Ann. Oncol. 2018;29:viii395. [Google Scholar]
- 74.Manohar S., Leung N. Cisplatin nephrotoxicity: a review of the literature. J. Nephrol. 2018;31(1):15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 75.Casanova A.G., et al. Systematic review and meta-analysis of the efficacy of clinically tested protectants of cisplatin nephrotoxicity. Eur. J. Clin. Pharmacol. 2020;76(1):23–33. doi: 10.1007/s00228-019-02771-5. [DOI] [PubMed] [Google Scholar]
- 76.Hayati F., et al. Prevention of cisplatin nephrotoxicity. J. Nephropharmacol. 2016;5(1):57. [PMC free article] [PubMed] [Google Scholar]
- 77.Zazuli Z., et al. Genetic variations and cisplatin nephrotoxicity: a systematic review. Front. Pharmacol. 2018;9:1111. doi: 10.3389/fphar.2018.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernández-Rojas B., et al. C-phycocyanin prevents cisplatin-induced mitochondrial dysfunction and oxidative stress. Mol. Cell. Biochem. 2015;406(1):183–197. doi: 10.1007/s11010-015-2436-9. [DOI] [PubMed] [Google Scholar]
- 79.Topcu-Tarladacalisir Y., Sapmaz-Metin M., Karaca T. Curcumin counteracts cisplatin-induced nephrotoxicity by preventing renal tubular cell apoptosis. Ren. Fail. 2016;38(10):1741–1748. doi: 10.1080/0886022X.2016.1229996. [DOI] [PubMed] [Google Scholar]
- 80.Saito Y., et al. Magnesium co-administration decreases cisplatin-induced nephrotoxicity in the multiple cisplatin administration. Life Sci. 2017;189:18–22. doi: 10.1016/j.lfs.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 81.Šeflová J., et al. Identification of cisplatin-binding sites on the large cytoplasmic loop of the Na+/K+-ATPase. J. Enzym. Inhib. Med. Chem. 2018;33(1):701–706. doi: 10.1080/14756366.2018.1445735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen S.-H., Chang J.-Y. New insights into mechanisms of cisplatin resistance: from tumor cell to microenvironment. Int. J. Mol. Sci. 2019;20(17):4136. doi: 10.3390/ijms20174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L., et al. The role of tumour metabolism in cisplatin resistance. Front. Mol. Biosci. 2021;8:603. doi: 10.3389/fmolb.2021.691795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu H.-M., et al. Hypoxia-induced autophagy mediates cisplatin resistance in lung cancer cells. Sci. Rep. 2015;5(1):1–15. doi: 10.1038/srep12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu H., et al. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des. Dev. Ther. 2016;10:1885. doi: 10.2147/DDDT.S106412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siddik Z.H. Clinically Relevant Resistance in Cancer Chemotherapy. 2002. Biochemical and molecular mechanisms of cisplatin resistance; pp. 263–284. [DOI] [PubMed] [Google Scholar]
- 87.Florea A.-M., Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3(1):1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeller C., et al. Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene. 2012;31(42):4567–4576. doi: 10.1038/onc.2011.611. [DOI] [PubMed] [Google Scholar]
- 89.Chen Q., et al. Platinum (iv) prodrugs with long lipid chains for drug delivery and overcoming cisplatin resistance. Chem. Commun. 2018;54(42):5369–5372. doi: 10.1039/c8cc02791a. [DOI] [PubMed] [Google Scholar]
- 90.Min Y., et al. Combating the drug resistance of cisplatin using a platinum prodrug based delivery system. Angew. Chem. 2012;124(27):6846–6851. doi: 10.1002/anie.201201562. [DOI] [PubMed] [Google Scholar]
- 91.Shi Y., et al. Pt (IV) complexes as prodrugs for cisplatin. J. Inorg. Biochem. 2012;107(1):6–14. doi: 10.1016/j.jinorgbio.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 92.Bugarčić Ž.D., et al. Mechanistic studies on the reactions of platinum (II) complexes with nitrogen-and sulfur-donor biomolecules. Dalton Trans. 2012;41(40):12329–12345. doi: 10.1039/c2dt31045g. [DOI] [PubMed] [Google Scholar]
- 93.Ling X., et al. Glutathione-responsive prodrug nanoparticles for effective drug delivery and cancer therapy. ACS Nano. 2018;13(1):357–370. doi: 10.1021/acsnano.8b06400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Q., et al. In situ supramolecular self-assembly of Pt (IV) prodrug to conquer cisplatin resistance. Adv. Funct. Mater. 2021 [Google Scholar]
- 95.Su N.-W., Chen Y.-J. Metronomic therapy in oral squamous cell carcinoma. J. Clin. Med. 2021;10(13):2818. doi: 10.3390/jcm10132818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pramanik R., Sharma A., Kumar A. Oral metronomic therapy in head and neck cancer. Lancet Global Health. 2021;9(1):e19. doi: 10.1016/S2214-109X(20)30463-0. [DOI] [PubMed] [Google Scholar]
- 97.Kim J.Y., Kim Y.-M. Tumor endothelial cells as a potential target of metronomic chemotherapy. Arch Pharm. Res. (Seoul) 2019;42(1):1–13. doi: 10.1007/s12272-018-01102-z. [DOI] [PubMed] [Google Scholar]
- 98.Gupta P., et al. A systematic comparative assessment of the response of ovarian cancer cells to the chemotherapeutic cisplatin in 3D models of various structural and biochemical configurations—does one model type fit all? Cancers. 2022;14(5):1274. doi: 10.3390/cancers14051274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang B., et al. Lung cancer chemotherapy using nanoparticles: enhanced target ability of redox-responsive and pH-sensitive cisplatin prodrug and paclitaxel. Biomed. Pharmacother. 2021;136 doi: 10.1016/j.biopha.2021.111249. [DOI] [PubMed] [Google Scholar]
- 100.Jiang G., et al. TMTP1-Modified, tumor microenvironment responsive nanoparticles Co-deliver cisplatin and paclitaxel prodrugs for effective cervical cancer therapy. Int. J. Nanomed. 2021;16:4087. doi: 10.2147/IJN.S298252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang R., et al. A novel small molecule LLL12B inhibits STAT3 signaling and sensitizes ovarian cancer cell to paclitaxel and cisplatin. PLoS One. 2021;16(4):e0240145. doi: 10.1371/journal.pone.0240145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao J., et al. Combination treatment with cisplatin, paclitaxel and olaparib has synergistic and dose reduction potential in ovarian cancer cells. Exp. Ther. Med. 2021;22(3):1–9. doi: 10.3892/etm.2021.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mohamad Saimi N.I., et al. Aerosolized niosome formulation containing gemcitabine and cisplatin for lung cancer treatment: optimization, characterization and in vitro evaluation. Pharmaceutics. 2021;13(1):59. doi: 10.3390/pharmaceutics13010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hanna N., Einhorn L.H. Testicular cancer: a reflection on 50 years of discovery. J. Clin. Oncol. 2014;32(28):3085–3092. doi: 10.1200/JCO.2014.56.0896. [DOI] [PubMed] [Google Scholar]
- 105.King J., Adra N., Einhorn L.H. Cancer Research; 2021. Testicular Cancer: Biology to Bedside. [DOI] [PubMed] [Google Scholar]
- 106.Bjerring A.W., et al. The cardiac impact of cisplatin-based chemotherapy in survivors of testicular cancer: a 30-year follow-up. Eur. Heart J. Cardiovasc. Imag. 2021;22(4):443–450. doi: 10.1093/ehjci/jeaa289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steggink L.C., et al. Genome-wide association study of cardiovascular disease in testicular cancer patients treated with platinum-based chemotherapy. Pharmacogenomics J. 2021;21(2):152–164. doi: 10.1038/s41397-020-00191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shields L.B., et al. Thromboembolic events in metastatic testicular cancer treated with cisplatin-based chemotherapy. World J. Clin. Oncol. 2021;12(3):183. doi: 10.5306/wjco.v12.i3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haugnes H.S., et al. Thromboembolic events during treatment with cisplatin-based chemotherapy in metastatic testicular germ-cell cancer 2000–2014: a population-based cohort study. Eur. Urol. Open Sci. 2021;32:19–27. doi: 10.1016/j.euros.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Albany C., et al. A phase 1 study of combined guadecitabine and cisplatin in platinum refractory germ cell cancer. Cancer Med. 2021;10(1):156–163. doi: 10.1002/cam4.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kozakova K., et al. Promising novel therapies for relapsed and refractory testicular germ cell tumors. Expet Rev. Anticancer Ther. 2021;21(1):53–69. doi: 10.1080/14737140.2021.1838279. [DOI] [PubMed] [Google Scholar]
- 112.Yamashita S., et al. Wiley Online Library; 2021. Fertility and Reproductive Technology Use in Testicular Cancer Survivors in Japan: A Multi-institutional, Cross-sectional Study. [DOI] [PubMed] [Google Scholar]
- 113.El-Diasty H.H., et al. Efficacy of quercetin-sensitized cisplatin against N-nitroso-N-methylurea induced testicular carcinogenesis in wistar rats. Asian Pac. J. Cancer Prev. APJCP. 2021;22(1):75. doi: 10.31557/APJCP.2021.22.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ykema B.L., et al. Diagnostic yield of colonoscopy surveillance in testicular cancer survivors treated with platinum-based chemotherapy: study protocol of a prospective cross-sectional cohort study. BMC Gastroenterol. 2021;21(1):1–7. doi: 10.1186/s12876-021-01639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pınar N., et al. Protective effect of dexpanthenol on cisplatin induced nephrotoxicity in rats. Biotech. Histochem. 2021:1–5. doi: 10.1080/10520295.2021.1890215. [DOI] [PubMed] [Google Scholar]
- 116.Mirzaei S., et al. Pharmacological Research; 2021. Nrf2 Signaling Pathway in Cisplatin Chemotherapy: Potential Involvement in Organ protection and Chemoresistance. [DOI] [PubMed] [Google Scholar]
- 117.Szturz P., Vermorken J.B. Critical Issues in Head and Neck Oncology. Springer; Cham: 2021. High-dose three-weekly or low-dose weekly cisplatin during radiation, what to prefer? pp. 139–153. [Google Scholar]
- 118.Fard A.A., Samadi M., Biabangard A. Possible protective effects of curcumin via modulating of androgen receptor (AR) and Oct2 gene alterations in cisplatin-induced testicular toxicity in rat. Endocr. Metab. Immune Disord. Drug Targets. 2021;21(3):458–463. doi: 10.2174/1871530320666200511073302. [DOI] [PubMed] [Google Scholar]
- 119.Caggiano C., et al. Testicular germ cell tumors acquire cisplatin resistance by rebalancing the usage of DNA repair pathways. Cancers. 2021;13(4):787. doi: 10.3390/cancers13040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fazal Z., et al. AACR; 2021. Genome Wide Remodeling of DNA Methylation and CpG Hypermethylation Is Tightly Linked to Acquired Resistance in Testicular Cancer. [Google Scholar]
- 121.Lobo J., et al. Promoter methylation of DNA homologous recombination genes is predictive of the responsiveness to PARP inhibitor treatment in testicular germ cell tumors. Mol. Oncol. 2021;15(4):846–865. doi: 10.1002/1878-0261.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kalavska K., et al. Are changes in the percentage of specific leukocyte subpopulations associated with endogenous DNA damage levels in testicular cancer patients? Int. J. Mol. Sci. 2021;22(15):8281. doi: 10.3390/ijms22158281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang M., et al. Co-delivery of etoposide and cisplatin in dual-drug loaded nanoparticles synergistically improves chemoradiotherapy in non-small cell lung cancer models. Acta Biomater. 2021;124:327–335. doi: 10.1016/j.actbio.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Provencio M., et al. Phase II clinical trial with metronomic oral vinorelbine and tri-weekly cisplatin as induction therapy, subsequently concomitant with radiotherapy (RT) in patients with locally advanced, unresectable, non-small cell lung cancer (NSCLC). Analysis of survival and value of ctDNA for patient selection. Lung Cancer. 2021;153:25–34. doi: 10.1016/j.lungcan.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 125.Liang Z., et al. Cisplatin synergizes with PRLX93936 to induce ferroptosis in non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2021;569:79–85. doi: 10.1016/j.bbrc.2021.06.088. [DOI] [PubMed] [Google Scholar]
- 126.Guo J., et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer research and treatment. Off. J. Korean Canc. Assoc. 2018;50(2):445. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lou J.-S., et al. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine. 2021;80 doi: 10.1016/j.phymed.2020.153370. [DOI] [PubMed] [Google Scholar]
- 128.Zhang C., et al. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol. Cancer. 2022;21(1):1–12. doi: 10.1186/s12943-022-01530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liao X.-Z., et al. Cordycepin reverses cisplatin resistance in non-small cell lung cancer by activating AMPK and inhibiting AKT signaling pathway. Front. Cell Dev. Biol. 2021;8:1640. doi: 10.3389/fcell.2020.609285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen K.-b., et al. miR-526b-3p inhibits lung cancer cisplatin-resistance and metastasis by inhibiting STAT3-promoted PD-L1. Cell Death Dis. 2021;12(8):1–10. doi: 10.1038/s41419-021-04033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu S., et al. miR-140-3p enhances cisplatin sensitivity and attenuates stem cell-like properties through repressing Wnt/β-catenin signaling in lung adenocarcinoma cells. Exp. Ther. Med. 2020;20(2):1664–1674. doi: 10.3892/etm.2020.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu L.-M., et al. Parthenolide augments the chemosensitivity of non-small-cell lung cancer to cisplatin via the PI3K/AKT signaling pathway. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.610097. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 133.Lv M., et al. Acetyl-11-keto-β-boswellic acid enhances the cisplatin sensitivity of non-small cell lung cancer cells through cell cycle arrest, apoptosis induction, and autophagy suppression via p21-dependent signaling pathway. Cell Biol. Toxicol. 2021;37(2):209–228. doi: 10.1007/s10565-020-09541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peng S., et al. Emodin enhances cisplatin sensitivity in non-small cell lung cancer through Pgp downregulation. Oncol. Lett. 2021;21(3):1. doi: 10.3892/ol.2021.12491. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li M., et al. Emodin regulates cell cycle of non-small lung cancer (NSCLC) cells through hyaluronan synthase 2 (HA2)-HA-CD44/receptor for hyaluronic acid-mediated motility (RHAMM) interaction-dependent signaling pathway. Cancer Cell Int. 2021;21(1):1–12. doi: 10.1186/s12935-020-01711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Y., et al. Apigenin enhanced antitumor effect of cisplatin in lung cancer via inhibition of cancer stem cells. Nutr. Cancer. 2021;73(8):1489–1497. doi: 10.1080/01635581.2020.1802494. [DOI] [PubMed] [Google Scholar]
- 137.Cheng Y., et al. Targeting CXCR2 inhibits the progression of lung cancer and promotes therapeutic effect of cisplatin. Mol. Cancer. 2021;20(1):1–21. doi: 10.1186/s12943-021-01355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu H., et al. Clinical significance of circPVT1 in patients with non-small cell lung cancer who received cisplatin combined with gemcitabine chemotherapy. Tumori J. 2021;107(3):204–208. doi: 10.1177/0300891620941940. [DOI] [PubMed] [Google Scholar]
- 139.Mukerabigwi J.F., et al. Cisplatin resistance reversal in lung cancer by tumor acidity-activable vesicular nanoreactors via tumor oxidative stress amplification. J. Mater. Chem. B. 2021;9(13):3055–3067. doi: 10.1039/d0tb02876b. [DOI] [PubMed] [Google Scholar]
- 140.Wu C., et al. RING finger protein 38 induces the drug resistance of cisplatin in non-small-cell lung cancer. Cell Biol. Int. 2021;45(2):287–294. doi: 10.1002/cbin.11423. [DOI] [PubMed] [Google Scholar]
- 141.Abdul Satar N., Ismail M.N., Yahaya B.H. Synergistic roles of curcumin in sensitising the cisplatin effect on a cancer stem cell-like population derived from non-small cell lung cancer cell lines. Molecules. 2021;26(4):1056. doi: 10.3390/molecules26041056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hong Y., et al. Combination therapy of lung cancer using layer-by-layer cisplatin prodrug and curcumin co-encapsulated nanomedicine. Drug Des. Dev. Ther. 2020;14:2263. doi: 10.2147/DDDT.S241291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Khine H.E.E., et al. Chemosensitizing activity of peptide from Lentinus squarrosulus (Mont.) on cisplatin-induced apoptosis in human lung cancer cells. Sci. Rep. 2021;11(1):1–17. doi: 10.1038/s41598-021-83606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pandey N., et al. Allicin overcomes hypoxia mediated cisplatin resistance in lung cancer cells through ROS mediated cell death pathway and by suppressing hypoxia inducible factors. Cell. Physiol. Biochem. 2020;54(4):748–766. doi: 10.33594/000000253. [DOI] [PubMed] [Google Scholar]
- 145.Shannon N.B., et al. A machine learning approach to identify predictive molecular markers for cisplatin chemosensitivity following surgical resection in ovarian cancer. Sci. Rep. 2021;11(1):1–10. doi: 10.1038/s41598-021-96072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tendulkar S., Dodamani S. Chemoresistance in ovarian cancer: prospects for new drugs. Anti Cancer Agents Med. Chem. 2021;21(6):668–678. doi: 10.2174/1871520620666200908104835. [DOI] [PubMed] [Google Scholar]
- 147.Sheikhshabani S.H., et al. Oleuropein reduces cisplatin resistance in ovarian cancer by targeting apoptotic pathway regulators. Life Sci. 2021;278 doi: 10.1016/j.lfs.2021.119525. [DOI] [PubMed] [Google Scholar]
- 148.He S., et al. FAM83B inhibits ovarian cancer cisplatin resistance through inhibiting Wnt pathway. Oncogenesis. 2021;10(1):1–10. doi: 10.1038/s41389-020-00301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tian M., et al. Inhibition of AXL enhances chemosensitivity of human ovarian cancer cells to cisplatin via decreasing glycolysis. Acta Pharmacol. Sin. 2021;42(7):1180–1189. doi: 10.1038/s41401-020-00546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hao L., et al. m6A-YTHDF1-mediated TRIM29 upregulation facilitates the stem cell-like phenotype of cisplatin-resistant ovarian cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021;1868(1) doi: 10.1016/j.bbamcr.2020.118878. [DOI] [PubMed] [Google Scholar]
- 151.Cheng Y., et al. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics; 2021. MiRNA-409-3p Enhances Cisplatin-Sensitivity of Ovarian Cancer Cells by Blocking the Autophagy Mediated by Fip200. [DOI] [PubMed] [Google Scholar]
- 152.Dou L., Zhang Y. miR-4461 regulates the proliferation and metastasis of ovarian cancer cells and cisplatin resistance. Front. Oncol. 2021:11. doi: 10.3389/fonc.2021.614035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mariniello M., et al. Synthetic lethality screening identifies FDA-approved drugs that overcome ATP7B-mediated tolerance of tumor cells to cisplatin. Cancers. 2020;12(3):608. doi: 10.3390/cancers12030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brum M.C.M., et al. Osteopontin-c isoform inhibition modulates ovarian cancer cell cisplatin resistance, viability and plasticity. Oncol. Rep. 2021;45(2):652–664. doi: 10.3892/or.2020.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shen L., et al. PGC1α regulates mitochondrial oxidative phosphorylation involved in cisplatin resistance in ovarian cancer cells via nucleo-mitochondrial transcriptional feedback. Exp. Cell Res. 2021;398(1) doi: 10.1016/j.yexcr.2020.112369. [DOI] [PubMed] [Google Scholar]
- 156.Liu Y.-K., et al. FSTL1 increases cisplatin sensitivity in epithelial ovarian cancer cells by inhibition of NF-κB pathway. Cancer Chemother. Pharmacol. 2021;87(3):405–414. doi: 10.1007/s00280-020-04215-9. [DOI] [PubMed] [Google Scholar]
- 157.Zhao J., et al. FGFR3 phosphorylates EGFR to promote cisplatin-resistance in ovarian cancer. Biochem. Pharmacol. 2021;190 doi: 10.1016/j.bcp.2021.114536. [DOI] [PubMed] [Google Scholar]
- 158.Zhou X., et al. lncRNA LOC102724169 plus cisplatin exhibit the synergistic anti-tumor effect in ovarian cancer with chronic stress. Mol. Ther. Nucleic Acids. 2021;24:294–309. doi: 10.1016/j.omtn.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Qian L., et al. Reduced O-GlcNAcylation of SNAP-23 promotes cisplatin resistance by inducing exosome secretion in ovarian cancer. Cell Death Dis. 2021;7(1):1–11. doi: 10.1038/s41420-021-00489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang W.-y., et al. Calculating the dose of cisplatin that is actually utilized in hyperthermic intraperitoneal chemotherapy among ovarian cancer patients. J. Ovarian Res. 2021;14(1):1–8. doi: 10.1186/s13048-021-00764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang L., et al. Antiparasitic mebendazole (MBZ) effectively overcomes cisplatin resistance in human ovarian cancer cells by inhibiting multiple cancer-associated signaling pathways. Aging (Albany NY) 2021;13(13) doi: 10.18632/aging.203232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Choi S.H., et al. Combined use of cisplatin plus natural killer cells overcomes immunoresistance of cisplatin resistant ovarian cancer. Biochem. Biophys. Res. Commun. 2021;563:40–46. doi: 10.1016/j.bbrc.2021.05.066. [DOI] [PubMed] [Google Scholar]
- 163.Wang J.-M., et al. Implication of BAG5 downregulation in metabolic reprogramming of cisplatin-resistant ovarian cancer cells via mTORC2 signaling pathway. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021 doi: 10.1016/j.bbamcr.2021.119076. [DOI] [PubMed] [Google Scholar]
- 164.Guo J., et al. Reprogramming of glutamine metabolism via glutamine synthetase silencing induces cisplatin resistance in A2780 ovarian cancer cells. BMC Cancer. 2021;21(1):1–11. doi: 10.1186/s12885-021-07879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhu C.-Z., et al. A review on the accuracy of bladder cancer detection methods. J. Cancer. 2019;10(17):4038. doi: 10.7150/jca.28989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bertz S., et al. Specific types of bladder cancer. Pathologe. 2016;37(1):40–51. doi: 10.1007/s00292-015-0129-5. [DOI] [PubMed] [Google Scholar]
- 167.Jensen B.T., Lauridsen S.V., Jensen J.B. Optimal delivery of follow-up care after radical cystectomy for bladder cancer. Res. Rep. Urol. 2020;12:471. doi: 10.2147/RRU.S270240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Okamura S., et al. EHHADH contributes to cisplatin resistance through regulation by tumor-suppressive microRNAs in bladder cancer. BMC Cancer. 2021;21(1):1–13. doi: 10.1186/s12885-020-07717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Manyanga J., et al. Electronic cigarette aerosols alter the expression of cisplatin transporters and increase drug resistance in oral cancer cells. Sci. Rep. 2021;11(1):1–14. doi: 10.1038/s41598-021-81148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jiang D.M., et al. Defining cisplatin eligibility in patients with muscle-invasive bladder cancer. Nat. Rev. Urol. 2021;18(2):104–114. doi: 10.1038/s41585-020-00404-6. [DOI] [PubMed] [Google Scholar]
- 171.Pfister C., et al. Randomized phase III trial of dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur. Urol. 2021;79(2):214–221. doi: 10.1016/j.eururo.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 172.Yu H., et al. ALKBH5 inhibited cell proliferation and sensitized bladder cancer cells to cisplatin by m6A-CK2α-mediated glycolysis. Mol. Ther. Nucleic Acids. 2021;23:27–41. doi: 10.1016/j.omtn.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jiang G., et al. Identification of BXDC2 as a key downstream effector of the androgen receptor in modulating cisplatin sensitivity in bladder cancer. Cancers. 2021;13(5):975. doi: 10.3390/cancers13050975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sikder R.K., et al. Differential effects of clinically relevant N-versus C-terminal truncating CDKN1A mutations on cisplatin sensitivity in bladder cancer. Mol. Cancer Res. 2021;19(3):403–413. doi: 10.1158/1541-7786.MCR-19-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Luo G., et al. Exosomal LINC00355 derived from cancer-associated fibroblasts promotes bladder cancer cell resistance to cisplatin by regulating miR-34b-5p/ABCB1 axis. Acta Biochim. Biophys. Sin. 2021;53(5):558–566. doi: 10.1093/abbs/gmab023. [DOI] [PubMed] [Google Scholar]
- 176.Zhang Y., et al. Exosomal LINC00355 derived from cancer-associated fibroblasts promotes bladder cancer cell proliferation and invasion by regulating miR-15a-5p/HMGA2 axis. Acta Biochim. Biophys. Sin. 2021;53(6):673–682. doi: 10.1093/abbs/gmab041. [DOI] [PubMed] [Google Scholar]
- 177.He S., et al. Down-regulation of GP130 signaling sensitizes bladder cancer to cisplatin by impairing Ku70 DNA repair signaling and promoting apoptosis. Cell. Signal. 2021;81 doi: 10.1016/j.cellsig.2021.109931. [DOI] [PubMed] [Google Scholar]
- 178.Mao X., et al. Hypoxia-induced autophagy enhances cisplatin resistance in human bladder cancer cells by targeting hypoxia-inducible factor-1α. J. Immunol. Res. 2021:2021. doi: 10.1155/2021/8887437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Okubo K., et al. Ubiquitin-proteasome system is a promising target for killing cisplatin-resistant bladder cancer cells. Anticancer Res. 2021;41(6):2901–2912. doi: 10.21873/anticanres.15072. [DOI] [PubMed] [Google Scholar]
- 180.Pfister D.G., et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2020;18(7):873–898. doi: 10.6004/jnccn.2020.0031. [DOI] [PubMed] [Google Scholar]
- 181.Gebre-Medhin M., et al. Artscan A randomized phase III study comparing chemoradiotherapy with cisplatin versus cetuximab in patients with locoregionally advanced head and neck squamous cell cancer. J. Clin. Oncol. 2021;39(1):38–47. doi: 10.1200/JCO.20.02072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chargi N., et al. Patterns, predictors, and prognostic value of skeletal muscle mass loss in patients with locally advanced head and neck cancer undergoing cisplatin-based chemoradiotherapy. J. Clin. Med. 2021;10(8):1762. doi: 10.3390/jcm10081762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liao A.H., et al. Synergistic effects of combined treatment with ultrasound-mediated cisplatin-loaded microbubbles and atorvastatin on head and neck cancer. Head Neck. 2021;43(1):15–26. doi: 10.1002/hed.26445. [DOI] [PubMed] [Google Scholar]
- 184.Bostan M., et al. Resveratrol modulation of apoptosis and cell cycle response to cisplatin in head and neck cancer cell lines. Int. J. Mol. Sci. 2021;22(12):6322. doi: 10.3390/ijms22126322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Quintanilha J.C., et al. MiR-3168, miR-6125, and miR-4718 as potential predictors of cisplatin-induced nephrotoxicity in patients with head and neck cancer. BMC Cancer. 2021;21(1):1–10. doi: 10.1186/s12885-021-08317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bauman J., et al. 2019. Phase I and Expansion Cohort Study of Adjuvant Cisplatin, Intensity-Modulated Radiation Therapy (IMRT), and MK-3475 (Pembrolizumab) in High-Risk Head and Neck Squamous Cell Carcinoma (HNSCC) [Google Scholar]
- 187.Ghonaim E., El-Haggar S., Gohar S. Possible protective effect of pantoprazole against cisplatin-induced nephrotoxicity in head and neck cancer patients: a randomized controlled trial. Med. Oncol. 2021;38(9):1–12. doi: 10.1007/s12032-021-01558-y. [DOI] [PubMed] [Google Scholar]
- 188.Akmar L., et al. Concomitant weekly cisplatin-based chemoradiotherapy in head and neck cancer: the value of a second measured glomerular filtration rate during treatment. Br. J. Radiol. 2021;94(1118) doi: 10.1259/bjr.20200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Deutsch B.C., et al. Validation of hearing loss prediction tool for cisplatin chemotherapy and radiation in head and neck cancer treatment. JAMA Otolaryngol. Head Neck Surg. 2021;147(2):182–189. doi: 10.1001/jamaoto.2020.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ohkoshi A., et al. Serum selenium predicts achievement of full-dose cisplatin in concurrent chemoradiotherapy for locally advanced head and neck squamous cell carcinoma: a prospective, observational study. Oral Oncol. 2021;121 doi: 10.1016/j.oraloncology.2021.105475. [DOI] [PubMed] [Google Scholar]
- 191.Peltanova B., et al. Sensitivity to cisplatin in head and neck cancer cells is significantly affected by patient-derived cancer-associated fibroblasts. Int. J. Mol. Sci. 2021;22(4):1912. doi: 10.3390/ijms22041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang B., et al. The miR-136-5p/ROCK1 axis suppresses invasion and migration, and enhances cisplatin sensitivity in head and neck cancer cells. Exp. Ther. Med. 2021;21(4):1. doi: 10.3892/etm.2021.9748. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Roy S., et al. p38 Mitogen-activated protein kinase modulates cisplatin resistance in Head and Neck Squamous Cell Carcinoma cells. Arch. Oral Biol. 2021;122 doi: 10.1016/j.archoralbio.2020.104981. [DOI] [PubMed] [Google Scholar]
- 194.Laios A., et al. Best Practice & Research Clinical Obstetrics & Gynaecology; 2021. Ovarian Transposition and Cervical Cancer. [DOI] [PubMed] [Google Scholar]
- 195.Federico C., et al. Localized delivery of cisplatin to cervical cancer improves its therapeutic efficacy and minimizes its side effect profile. Int. J. Radiat. Oncol. Biol. Phys. 2021;109(5):1483–1494. doi: 10.1016/j.ijrobp.2020.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Masadah R., et al. The role of microRNAs in the cisplatin-and radio-resistance of cervical cancer. Cancers. 2021;13(5):1168. doi: 10.3390/cancers13051168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Aktepe O.H., et al. Lycopene sensitizes the cervical cancer cells to cisplatin via targeting nuclear factorkappa B (NF- Turk. J. Med. Sci. 2021;51(1):368–374. doi: 10.3906/sag-2005-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chi R.-p.A., et al. Inhibition of Kpnβ1 mediated nuclear import enhances cisplatin chemosensitivity in cervical cancer. BMC Cancer. 2021;21(1):1–16. doi: 10.1186/s12885-021-07819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang Y., et al. Synergistic therapy for cervical cancer by codelivery of cisplatin and JQ1 inhibiting plk1-mutant Trp53 Axis. Nano Lett. 2021;21(6):2412–2421. doi: 10.1021/acs.nanolett.0c04402. [DOI] [PubMed] [Google Scholar]
- 200.Li X., et al. Cyanidin-3-O-glucoside and cisplatin inhibit proliferation and downregulate the PI3K/AKT/mTOR pathway in cervical cancer cells. J. Food Sci. 2021 doi: 10.1111/1750-3841.15740. [DOI] [PubMed] [Google Scholar]
- 201.Liu H., et al. T-box transcription factor TBX1, targeted by microRNA-6727-5p, inhibits cell growth and enhances cisplatin chemosensitivity of cervical cancer cells through AKT and MAPK pathways. Bioengineering. 2021;12(1):565–577. doi: 10.1080/21655979.2021.1880732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li R., et al. Molecular Therapy-Oncolytics; 2021. METTL3 Increases Cisplatin Chemosensitivity of Cervical Cancer Cells via Downregulation of the Activity of RAGE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jiang J., et al. TIPE1 promotes cervical cancer cell chemoresistance to cisplatin in a wild-type p53-dependent manner. Front. Oncol. 2021;10:3094. doi: 10.3389/fonc.2020.593615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jouni F.J., et al. Combination of cisplatin treatment and photodynamic therapy attenuates cisplatin-induced cell toxicity in A2780 and A2780-CP cervical cancer cell lines. Laser Med. Sci. 2021:1–6. doi: 10.1007/s10103-021-03369-z. [DOI] [PubMed] [Google Scholar]
- 205.Esfandyari Y.B., et al. MicroRNA-143 sensitizes cervical cancer cells to cisplatin: a promising anticancer combination therapy. Reprod. Sci. 2021;28(7):2036–2049. doi: 10.1007/s43032-021-00479-5. [DOI] [PubMed] [Google Scholar]
- 206.Li L., et al. ZBTB28 induces autophagy by regulation of FIP200 and Bcl-XL facilitating cervical cancer cell apoptosis. J. Exp. Clin. Cancer Res. 2021;40(1):1–20. doi: 10.1186/s13046-021-01948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article