Abstract
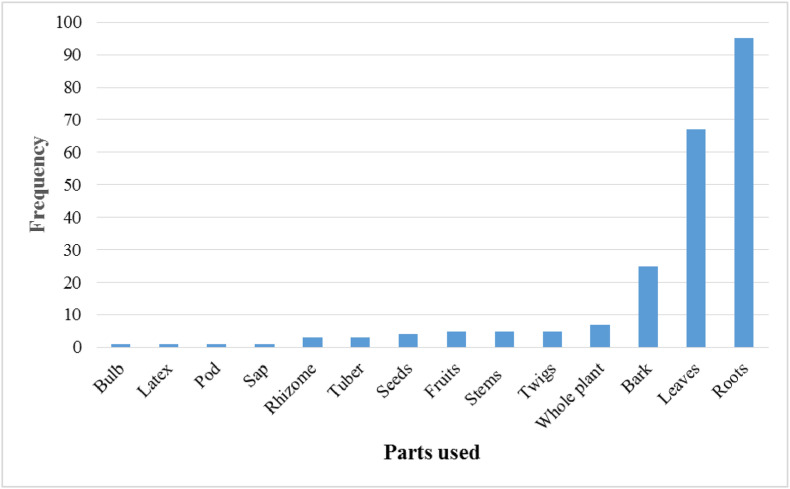
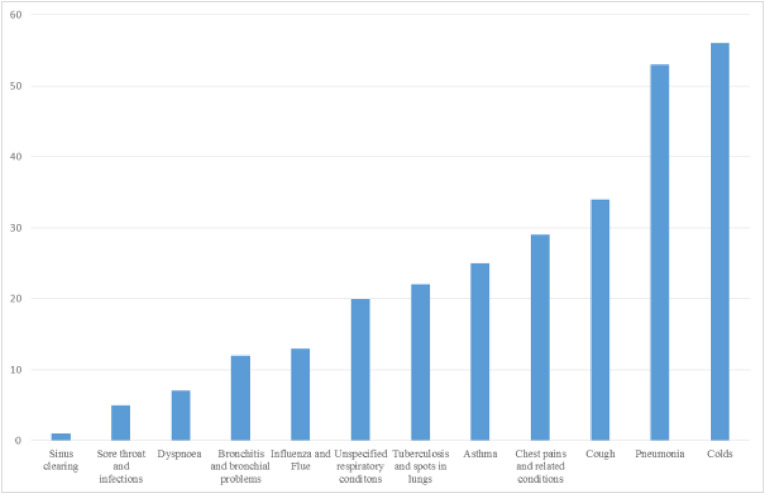
Respiratory diseases have in the recent past become a health concern globally. More than 523 million cases of coronavirus disease (COVID19), a recent respiratory diseases have been reported, leaving more than 6 million deaths worldwide since the start of the pandemic. In Zimbabwe, respiratory infections have largely been managed using traditional (herbal) medicines, due to their low cost and ease of accessibility. This review highlights the plants’ toxicological and pharmacological evaluation studies explored. It seeks to document plants that have been traditionally used in Zimbabwe to treat respiratory ailments within and beyond the past four decades. Extensive literature review based on published papers and abstracts retrieved from the online bibliographic databases, books, book chapters, scientific reports and theses available at Universities in Zimbabwe, were used in this study. From the study, there were at least 58 plant families comprising 160 medicinal plants widely distributed throughout the country. The Fabaceae family had the highest number of medicinal plant species, with a total of 21 species. A total of 12 respiratory ailments were reportedly treatable using the identified plants. From a total of 160 plants, colds were reportedly treatable with 56, pneumonia 53, coughs 34, chest pain and related conditions 29, asthma 25, tuberculosis and spots in lungs 22, unspecified respiratory conditions 20, influenza 13, bronchial problems 12, dyspnoea 7, sore throat and infections 5 and sinus clearing 1 plant. The study identified potential medicinal plants that can be utilised in future to manage respiratory infections.
Keywords: Medicinal plants, Zimbabwe, Respiratory disorders, Coronavirus disease 2019, Pharmacology, Toxicology
Graphical abstract
1. Introduction
Respiratory diseases are among the top ten major causes of mortality and morbidity worldwide (FIRS, 2017; WHO, 2018). The spectrum of these respiratory ailments ranges from acute communicable infections to chronic non-communicable diseases (Xie et al., 2020). Zimbabwe is predominantly affected by acute respiratory infections, chronic obstructive pulmonary disease, asthma, tuberculosis (TB), and lung cancer (Boutayeb, 2006; Rivera-Ortega and Molina-Molina, 2019). Both adults and children alike have over the years been vulnerable to respiratory diseases. A review by Salim et al. (2008) reported the most common recorded causes of respiratory mortality in Zimbabwean children were Pneumocystis carinii pneumonia, acute pyogenic pneumonia and TB with underreporting in asthma and other atopic conditions. Adults on the other hand were reportedly most vulnerable to acute respiratory infections, chronic obstructive pulmonary disease COPD, asthma, lung cancer, and nasopharyngeal and laryngeal cancer associated with exposure to indoor air pollution from burning biomass fuels Zimbabwe. Chronic cough and TB are the most commonly diagnosed conditions among HIV-positive adults with lower respiratory tract infections and asthma more common among HIV-negative patients.
The Coronavirus disease 2019 (COVID-19) is a new infectious respiratory disease caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was discovered between November and December 2019 in Wuhan China (Chen et al., 2020; Di Gennaro et al., 2020; Rothan and Byrareddy, 2020). As at June 14, 2022, this disease had resulted in 541,124,794 confirmed cases globally with at 6,332,729 deaths worldwide (Worldometer, 2022); Zimbabwe has reported 254,155 confirmed cases and 5521 deaths (Worldometer, 2022). This disease has so far been contagious and lethal to the extent that it has become a global emergency. As such, there has been a rising need for the urgent development of an effective treatment to address this pandemic. So far, nations in the global south such as India, China, Malaysia and Africa have taken a unique approach to drug repurposing and antiviral development by leveraging their extensive traditional medicines portfolios and mining them for potential anti-coronaviral drug candidates (Ren et al., 2020).
The study of ethnomedicine has in the past revealed that plant based remedies can ease chest and nasal congestion, soothing irritated airways, suppressing symptoms such as sneezing, coughing and swollen glands (WHO, 2001). Other studies have reported that herbal remedies also exhibit antihistamine and antioxidant properties which are important in alleviating respiratory disorders (Cunningham, 1988; Taur and Patil, 2011). With the background of plant secondary metabolites having been used in the past as sources of lead compounds for the development of effective and valuable conventional drugs such as chloroquine phosphate, originally extracted from the bark of cinchona trees (Redeploying plant defences, 2020), the reason why people turn towards herbal medicine for therapeutic intervention makes logical sense.
Organic herbal remedies are widely used as alternative medicines for primary health care management by 80% of the populations living in low- and medium-income countries (LMICs) (Mahomoodally, 2013; Oyebode et al., 2016; James et al., 2018). As such, most low-income societies in Zimbabwe rely, to a greater extent, on these low cost and easy-to-access alternative medicines (Maroyi, 2013a; Batisai, 2016). In Zimbabwe, indigenous knowledge systems (IKS) provide alternative medicines used to manage a variety of ailments in primary health care (Dimene et al., 2020). A vast repository of these diverse indigenous medicinal plants is consumed as nutraceuticals (Maroyi, 2013a; Bhebhe et al., 2015). While this is so, there still remains a plethora of indigenous knowledge systems to be explored. The highly infectious COVID-19 causes respiratory illness similar to the normal flu with symptoms such as cough, fever and in most severe cases the patients have difficulty in breathing (Cascella et al., 2020). Most infected people usually experience mild to moderate respiratory illness and they are able to recover from the disease without any special treatment (Singhal, 2020).
The respiratory system is a delicate system crucial for gaseous exchange, but it is vulnerable to infectious agents like bacteria, viruses and air pollution (Kim et al., 2018). The emergence of new highly contagious respiratory infections, as well as the high incidence of antimicrobial resistance (AMR) to current drugs against agents causing respiratory infections has led to the increased prevalence of patients with respiratory disorders (Ayukekbong et al., 2017; MacIntyre and Bui, 2017). This, coupled with the high cost of pharmaceuticals (Gronde et al., 2017), has necessitated the need to identify new targets for the development of novel, effective, safe, affordable and accessible alternative medications (Olorunnisola et al., 2011).
Unfortunately, maximum utilization of indigenous knowledge systems especially relating to medicinal plants is seriously hampered by the unavailability of scientific studies to validate the folklore claims as well as their limited scale of domestication and documentation (Maroyi, 2013a). According to (Shoko, 2018) only a handful of studies have been carried out to unravel the full medicinal property spectrum in Zimbabwean plants. This study sought to profile the plant species that are used for the management of existing respiratory ailments in Zimbabwe and modes of formulation. The study also focused on the respiratory conditions that are most commonly managed using the medicinal plant species in Zimbabwe. Moreover, the previous phytochemical profiling studies that were conducted to identify bioactive compounds, pharmacological and toxicological studies were also considered.
Constraints have been cited on the limited active plantation of indigenous medicinal plants which include the lack of tree nurseries, lack of processing facilities and poorly developed marketing pathways, lack of biodiversity studies and inadequate information about the nutritional and therapeutic benefits (Bodeker et al., 1997; Kehlenbeck et al., 2013). In view of a potential need to grow the candidate plants on a large scale, the current study also sought to find out whether agricultural and biotechnological studies have been conducted especially relating to the cultivation and propagation of the candidate traditional medicinal plants. The economic and conservation status of each plant species under consideration was also considered.
In a quest to identify the potential alternative medicines for further exploration in mitigation of the COVID-19 pandemic and future outbreaks, the available ethno-medicinal data of plants used to treat respiratory infections in Zimbabwe was gathered for the identification of under-investigated plant species that have the potential to be explored. This study sought to establish the plant species that are most often used for the management of respiratory disorders in Zimbabwe and their modes of preparation. The study also touched on the respiratory conditions that are most commonly managed using the medicinal plant species in Zimbabwe. The previous phytochemical profiling studies that have already been conducted to identify the bioactive compounds in medicinal plants, pharmacological and toxicological studies that have been recorded were considered. In light of a potential need to grow the candidate plants on a large scale, the current study sought to find out whether agricultural and biotechnological studies have been conducted especially relating to the cultivation and propagation of the candidate traditional medicinal plants. The economic and conservation status of each plant species under consideration was also considered. The plant species with the highest potential for prioritisation for the development of herbal preparations and ultimately drug development for the management of COVID-19 were then identified. This study aimed to identify Zimbabwean plants used traditionally to treat respiratory diseases in humans.
2. Materials and methods
2.1. Research protocol and reporting
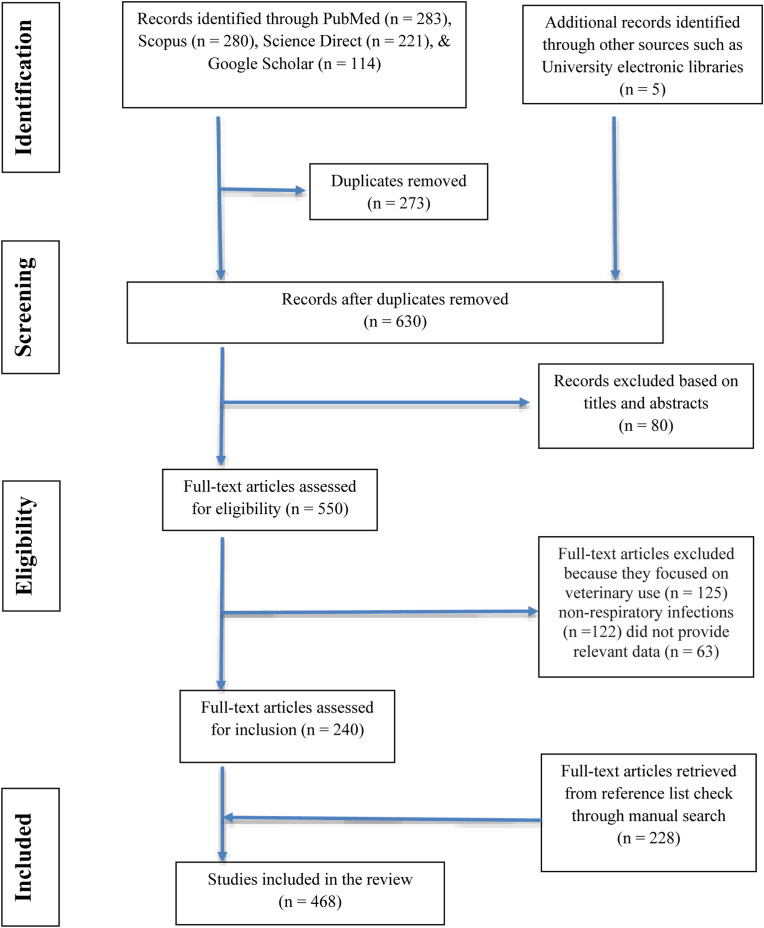
The Preferred Reporting Items for the Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used in the reporting of this study (Fig. 1 ). The protocol used in this systematic review was based on Moher et al., 2009.
Fig. 1.
PRISMA Flow diagram showing the search and retrieval steps of the study.
2.2. Literature search
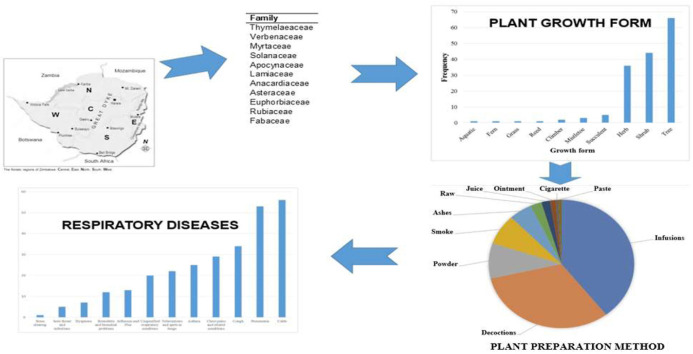
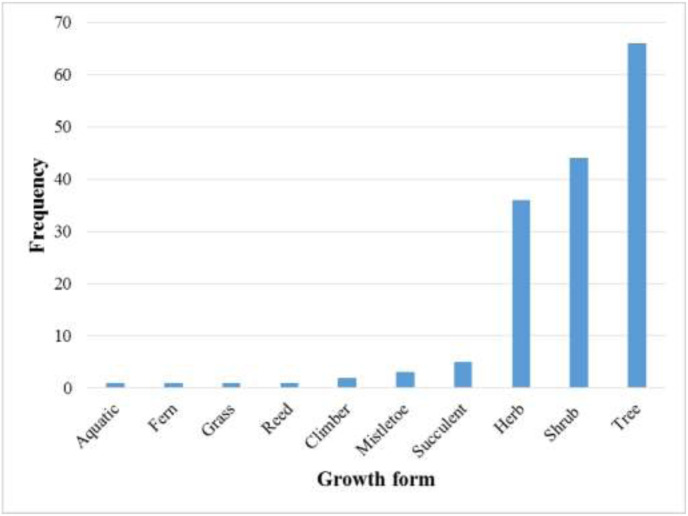
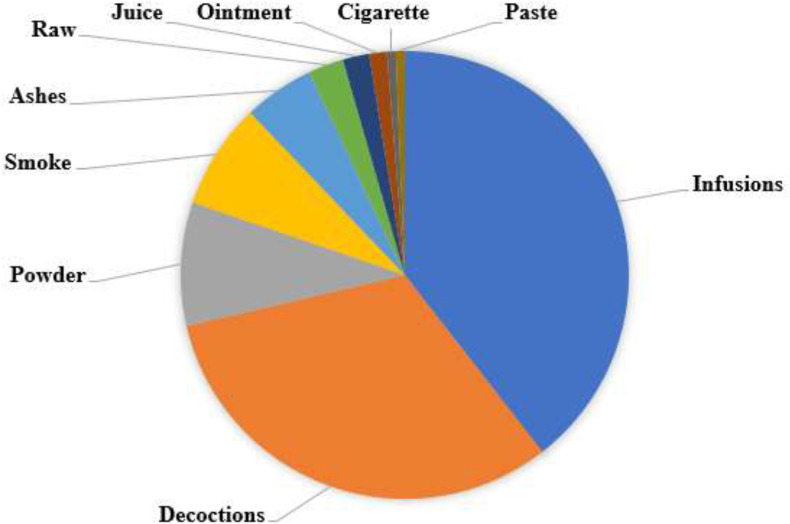
A systematic search was undertaken using a variety of published papers and abstracts up to March 31, 2020 that were retrieved from the online bibliographic databases that included PubMed, Google Scholar, and ScienceDirect. These databases were searched using the following search terms: “traditional use of plants”, “medicinal uses of plants”, “indigenous use of plants”, “herbs to treat respiratory disorders”, “ethnobotanical surveys” and “ethno-pharmacological studies'' and “Zimbabwe”. (For search terms used see: https://docs.google.com/document/d/1ivetFJI0TdAbshvuoagblaqC6nbXlLF3/edit?usp=sharing&ouid=107026948292171124800&rtpof=true&sd=true. Other sources utilised in this study included books (Wild and Gelfand, 1959; Watt and Breyer-Brandwijk, 1962; Williamson, 1975; Chavunduka et al., 1978; Gelfand et al., 1985; Van Wyk et al., 2009; Neffati et al., 2017), book chapters, scientific reports and theses available at universities (Matongo, 2012; Viol, 2013) and National Herbarium and Botanic Gardens (SRGH) libraries. The search was limited to studies published in English or containing at least an abstract written in English. The plant names have been verified with http://www.theplantlist.org and https://www.zimbabweflora.co.zw. Plants with the reported traditional usage against respiratory diseases were identified from the data gathered. A master list was generated enlisting all the medicinal plants used in Zimbabwe for the treatment of respiratory disorders (Table 1 ). The above-mentioned databases were also searched for pharmacological studies providing supporting evidence of medicinal uses for each species. Only reference(s) were provided because of the massive number of studies being consulted and complete information on pharmacological properties can be retrieved from the original studies. All the data has been summarized in five tables (Table 1, Table 2, Table 3, Table 4, Table 5 ) and six figures (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6 ).
Table 1.
Medicinal plants used for the management of respiratory disorders in Zimbabwe.
| Family | Scientific Name | Growth form | Local name Shona- Sh Ndebele- Nd English- Eng |
Parts used | Modes of preparation | Ethnomedicinal uses for respiratory disorders | Ethnomedicinal uses for non- respiratory disorders | General distribution North (N), West (W), Central (C), East (E), South (S) | Conservation status | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Acanthaceae | Barleria spinulosa Klotzsch | Shrub | No information | Whole plant | Applied to incision made on chest | Pneumonia | N, W, C, E, S | Least Concern | Gelfand et al. (1985) | |
| Acanthaceae | Thunbergia oblongifolia Oliv. | Herb | Mufurambudzi, Mukuvamvura, Musvisvinwa (Sh) Early blue thunbergia (Eng) |
Roots | Decoction taken orally | Asthma | Diarrhoea, abdominal pain, depressed fontanelle, nausea, dysmenorrhoea, to prevent abortion, headache and swollen stomach (Dropsy) | N, C, E | Gelfand et al. (1985) | |
| Alliaceae | Tulbaghia leucantha Baker | Herb | Mhondya (Sh) Wild garlic (Eng) |
Whole plant | Decoction taken orally | Asthma | W, C, S | Gelfand et al. (1985) | ||
| Anacardiaceae | *Mangifera indica L. | Tree | Mumango (Sh) Mango (Eng) |
Leaves and twigs | Roasted, powdered decoction drunk and ash licked Decoction and powder |
Asthma, colds, cough and tuberculosis | Diarrhoea, astringent, gonorrhoea, asthma, prolongs ejaculation, anthelmintic | N, W, C, E, S | Least Concern | Chimponda and Mukanganyama (2010); Mangoyi et al. (2014); Maroyi (2018b) |
| Anacardiaceae | Lannea discolor (Sond.) Engl. | Tree | Chizhenje, Mugan'acha, Muhumbukumbu, Mumbumbu, Mupuri, Mushamba (Sh) Tree grape, Live-long (Eng) |
Roots | Cough | Constipation, diarrhoea, dysentery, stomach ailments, convulsions, cough, fever, female infertility, gonorrhea, menstrual problems, bilharzia, bladder problems, and malaria | N, W, C, E, S | Maroyi (2018d) | ||
| Anacardiaceae | Lannea edulis (Sond.) Engl. | Shrub | Mutsambatsi, Tsombori (Sh) Intakubomvu (Nd) Wild grape (Eng) |
Roots | Hot water extract drunk three times a day Decoction |
Cough and Bronchitis | Abdominal pains, amenorrhea, dysmenorrhea, dizziness, hematuria, wounds, rheumatism, bilharziasis and Sexually transmitted infections (gonorrhea, syphilis, and venereal disease) | N, W, C, E, S | Least concern | Maroyi (2011, 2019c) |
| Anacardiaceae | Searsia chirindensis (Baker f.) Moffett | Shrub | Mubikasadza, Mutsodzo (Sh) Red currant rhus (Eng) |
Leaves and Roots | Chest pains and cough | Measles and syphilis | W, C, E, S | Least Concern | Viol (2013) | |
| Anacardiaceae | Searsia lancea (L.f) F.A.Barkley | Tree | Mufokosiana (Sh) Bastard willow (Eng) Uchane, InHlokoshiyane (Nd) |
Roots | Infusion taken orally | Chest pains | Abdominal pain, diarrhoea with blood, measles | N, W, E, S | Gelfand et al. (1985) | |
| Anacardiaceae | Searsia longipes (Engl.) Moffett | Tree | Mudzambuya, Mufokosiana, Mutungahove (Sh) Large-leaved rhus (Eng) Inhlokotshiyane (Nd) |
Leaves | Decoction taken orally | Cough | Abdominal pains, aphrodisiac for women, diarrhoea, syphilis, infertility in women, to dilate the birth canal | N, C, E | Gelfand et al. (1985) | |
| Anacardiaceae | Searsia pyroides (Burch.) Moffett | Tree | Mufokosiana (Sh) Common wild currant, (Eng) |
Roots | Extract drunk as cough medicine. Decoction | Cough | W, C, E, S | Least Concern | Maroyi (2011, 2014) | |
| Annonaceae | Annona stenophylla Engl. & Diels | Shrub | Muroro (Sh) Dwarf custard-apple (Eng) |
Roots | Crushed, mixed with water, extract drunk. Infusion | Chest pains | Boils and Sexually transmitted diseases | N, W, C, E, S | Not Evaluated | Maroyi (2011, 2014) |
| Annonaceae | Hexalobus monopetalus (A. Rich.) Engl. & Diels | Shrub | Muhodzongwa, mukorongwa, mukwingiziri, munyani, mupodzongo, mupodzongwa, musakama (Sh) Baboons' breakfast, Shakama plum (Eng) |
Stems, Roots, bark, leaves, fruit | Colds, bronchitis and pulmonary troubles | Stomach pains, snakebites, headaches, diabetes, diuretic, laxative, antipyretic, insomnia, colic, constipation and venereal diseases, cataracts, expectorant, bloody vomiting, diarrhoea, dysentery, naso-pharyngeal affections |
N, W, C, E, S | Dzoyem et al. (2016) | ||
| Apiaceae | Alepidea amatymbica Eckl. & Zeyh | Herb | Kataza (Sh) Giant alepidea, larger tinsel flower (Eng) Inkatsankatsa (Swazi) |
Leaves and roots | Chewed or decoction drunk. | Asthma, chest pains, influenza, colds and cough | Rheumatism and wounds | E | Critically Endangered |
Gelfand et al. (1985) |
| Apiaceae | Alepidea cordifolia B.-E. van Wyk | Herb | Kataza (Sh) | Rhizome and Roots | Colds and influenza | E | Neffati et al. (2017) | |||
| Apiaceae | Diplolophium zambesianum Heirn | Herb | Ruvhuniti (Sh) | Roots | Infusion taken orally | Pneumonia | Constipation, diarrhoea and sore eyes. | N, W, C, E, S | Gelfand et al. (1985) | |
| Apiaceae | Heteromorpha arborescens (Spreng.) Cham. & Schltdl | Tree | Mhingano (Sh) Livelong, Parsley tree (Eng) Imfenkulu (Nd) |
Bark, leaves, and Roots | Infusion taken orally and roasted the chewed and juice is swallowed | Respiratory problems (asthma, chest pains, coughs, and tuberculosis) | Aphrodisiac, abdominal pains in infants, infertility, cancer, backache, headache and fever. | N, W, C, E | Gelfand et al. (1985); Maroyi (2018g) | |
| Apocynaceae | Carissa edulis (Forssk.) Vahl | Shrub | Mudyabveni, Mudzambara, Muhlababzunzi, Mumbingwa, Muruguru, Mutsamviringa (Sh) Simple-spined num-num (Eng) Umlugulu (Nd) |
Roots | Crushed, mixed with hot water, extract drunk Decoction |
Cough, chest pains, pneumonia and tuberculosis | Diarrhoea | N, W, C, E, S | Least Concern | Maroyi (2011, 2013a); Sharifi-Rad et al. (2020) |
| Apocynaceae | Carissa bispinosa (L.) Desf. ex Brenan | Shrub | Muruguru, Mudyabveni, Mudzambara, Mumbingwa, Murambamhunga, Mutsamviringa (Sh) Y-thorned carissa, Forest num-num (Eng) | Roots | Hot water extract drunk three times a day. Decoction |
Cough | Diarrhoea | W, C, S | Least Concern | Maroyi (2011, 2014) |
| Apocynaceae | Diplorhynchus condylocarpon (Muell. Arg.) Pich. | Tree | Musikanyimo, Mutohwa, Tsowa (Sh) Rhodesian rubber tree, horn-pod tree, wild rubber (Eng) Inkamamasane (Nd) |
Roots | Infusion taken orally and salt added to decoction and drunk. | Cough, pneumonia | Abdominal pain, venereal diseases, infertility, measles anorexia and to rest sterility in men. | N, W, C, E, S | Gelfand et al. (1985) | |
| Apocynaceae | Holarrhena pubescens Wall. ex G. Don | Shrub | Chigafusi, Mugashu, Muhatsu, Mukashumukono, Mukashumurume (Sh) Fever-pod, Jasmine-tree (Eng) |
Roots | Asthma | Aphrodisiac, galactagogue, laxative, constipation, abdominal pains and infertility | N, W, C, E, S | Maroyi (2012a) | ||
| Apocynaceae | *Nerium oleander L | Shrub | Oleander (Eng) | Pneumonia | Cultivated | Gelfand et al. (1985) | ||||
| Apocynaceae |
Tabernaemontana elegans Stapf |
Tree | Muchanga (Sh) Toad tree (Eng) UmKhahlwana, umKhadlu (Zulu) |
Sap, leaves, Roots, rhizome | Lung ailments and tuberculosis | Styptic, aphrodisiac, stomach ache, venereal diseases, cancer | E, S | Dzoyem et al. (2016) | ||
| Asclepiadaceae | Ectadiopsis oblongifolia (Meisn.) Bullock | Shrub | Rukangadza (Sh) Inkamamasane enduna (Nd) |
Roots | Paste applied to painful parts. | Pneumonia | Abdominal pain, diarrhoea, constipation, aphrodisiac, gonorrhoea, sore eyes, cataracts, antiemetic in infants and medicine for premature infants to strengthen them. | N, W, C, E, S | Gelfand et al. (1985) | |
| Asclepiadaceae | Gomphocarpus glaucophyllus Sclechter | Herb | Gwendere (Sh) Blue milkweed (Eng) |
Roots | Infusion taken orally | Asthma | Antiemetic in infants | N, W, C, E | Gelfand et al. (1985) | |
| Asparagaceae | Asparagus africanus Lam. | Climber | Rukato (Sh) Bush asparagus (Eng) |
Roots | Crushed, mixed with hot water, extract drunk Decoction or infusion taken orally |
Pneumonia and tuberculosis. | Diarrhoea and to dilate birth canal | N, W, C, E, S | Least Concern | Gelfand et al. (1985); Maroyi (2011, 2013a); Sharifi-Rad et al. (2020) |
| Asphodelaceae | Aloe ferox Mill. | Succulent | Gavakava (Sh) Bitter aloe, Red aloe (Eng) iNhlaba (Zulu) iKhala (Xhosa) |
Leaves | Tuberculosis | Skin afflictions (burns, wounds, abrasions, irritations), cardiovascular diseases, cancer, neuro-degeneration, and diabetes | C, E, S | Least Concern | Chigora et al. (2007) | |
| Asphodelaceae | Aloe vera (L.) Burm.f. | Succulent | Gavakava (Sh) Aloe (Eng) |
Leaves | Tuberculosis | Minor wounds and inflammatory skin disorders. | C, E, S | Not Evaluated | Chimponda and Mukanganyama (2010); Maroyi (2013a); Mangoyi et al. (2014) | |
| Asphodelaceae | Aloe excelsa Berger | Succulent | Gavakava (Sh) Tree aloe (Eng) Ihlaba (Nd) |
Leaves | Infusion of ashes | Asthma | N, W, C, E, S | Gelfand et al. (1985) | ||
| Asphodelaceae | Aloe spp. | Succulent | Gavakava (Sh) Aloe (Eng) Icena (Nd) |
Leaves | Infusion | Cough | N, W, C, E, S | Gelfand et al. (1985) | ||
| Asteraceae | Artemisia afra Jacq. ex Willd. | Shrub | Wild wormwood, African wormwood (Eng) Umhlonyane (isiXhosa) Mhlonyane (isiZulu) Lengana (Tswana) |
Leaves | Extract drunk Infusion |
Respiratory ailments | Digestive ailments | N, C, E, S | Neffati et al. (2017) | |
| Asteraceae | Aspilia pluriseta Schweinf. subsp. pluriseta | Herb | Mukushamvura, Mumharadzi, Ruhwati (Sh) Dwarf aspilia (Eng) |
Roots | Burnt and smoke inhaled | Dyspnoea (shortness of breath) | Abdominal pains, diarrhoea, delirium, body pains, poor appetite, cessation of senses, prolonged labour, pains during pregnancy and swelling of body | N, C, E | Gelfand et al. (1985) | |
| Asteraceae | Laggera crispata (Vahl) Hepper & J.R.I. Wood | Herb | Rutapatsikidzi (Sh) Bug catcher (Eng) |
Roots | Infusion taken orally and applied to incisions made around the chest. | Pneumonia | Convulsions, headache, bleeding from nose, abdominal pains, fever, painful legs, madness, heart pains and to fatten infants. | N, W, C, E | Gelfand et al. (1985) | |
| Asteraceae | Dicoma anomala Sond. | Herb | Fever bush, stomach bush (Eng) Isihlabamakhondlwane, Umuna (Zulu) |
Roots | Decoction taken orally Infusion taken orally |
Colds, cough and pneumonia | Abdominal pains, antidote for poison, bladder problems in women, sore throat, cataracts, diarrhoea, dysentery, induce labour, pain, painful uterus, malaria, madness, skin sores schistosomiasis, stomach problems and wasting in infants. | N, W, C, E, S | Gelfand et al. (1985); Maroyi (2018f) | |
| Asteraceae | Helichrysum caespititium (DC.) Harv. | Herb | Golden everlasting (Eng) | Leaves, Roots and whole plant | Cough and pulmonary tuberculosis Respiratory infections (chest pains, colds, cough, flu, pneumonia, sinuses and tuberculosis) |
Depressed fontanelle, sexually transmitted infections, nausea, headache, wounds, ulceration, and used as an aphrodisiac | C, E | Watt and Breyer-Brandwijk (1962); Gelfand et al. (1985); Maroyi (2019b) | ||
| Asteraceae | Helichrysum kraussii Schultz Bip. | Shrub | Mupumhanhuka, Mutsvairo, Rusakadzi (Sh) Umawewana (Nd) Curry bush (Eng) |
Whole plant | Burnt ashes mixed with salt and taken orally | Cough | N, W, C, E, S | Gelfand et al. (1985) | ||
| Asteraceae | Inula glomerata Oliv. & Hiern | Herb | Zeveratsuro, Zheveratsuro (Sh) Hare's ears (Eng) |
Roots | Infusion taken orally and rubbed on incisions made on painful parts. | Pneumonia | Constipation, abdominal pain around the umbilicus, ear ache infertility in women, tonic for premature babies, to dilate the birth canal | N, W, C, E, S | Gelfand et al. (1985) | |
| Asteraceae |
Lopholaena coriifolia (Sond.) Phillips & C.A.Sm. Lopholaena dehniae Merxm. |
Shrub | Chigunguru, Mugakatombo, Mukwiradundu, Nyakatondo (Sh) Small-leaved fluff-bush (Eng) |
Roots | Burnt ashes taken orally in porridge | Cough, pneumonia | Abdominal pains, diarrhoea with blood, measles, convulsions and burns | W, C, S | Gelfand et al. (1985) | |
| Asteraceae | Vernonia adoensis Walp. var. adoensis | Shrub | Musikavakadzi (Sh) Bitter leaves, Common bitter leaves (Eng) |
Leaves | Tuberculosis | N, C | Not Evaluated | Chimponda and Mukanganyama (2010); Mangoyi et al. (2014) | ||
| Asteraceae | Vernonia amygdalina Del. | Shrub | Dembezeko, Musikavakadzi, Muzhozho, Nyareru (Sh) Tree vernonia, Bitter-tea vernonia (Eng) Inyathelo (Nd) |
Roots | Infusion taken orally | Cough | Painful uterus, infertility in women, abdominal pain, venereal diseases, cessation of menses, aphrodisiac, weak joints, bilharziasis, fever, diarrhoea and swelling of the body. | N, W, C, E, S | Gelfand et al. (1985) | |
| Bignoniaceae | Kigella africana (Lam.) Benth | Tree | Mubveve, Musonya, Muvhumati (Sh) Sausage tree (Eng) Umvebe (Nd) |
Bark and Roots | Infusion taken orally and applied to incision made on painful part. | Pneumonia | Tropical ulcers, backache, toothache, fits (epilepsy) and antidote for snake bite. | N, W, C, E, S | Gelfand et al. (1985) | |
| Bignoniaceae | Stereospermum kunthianum Cham. | Tree | Kabvevesango, Mutandangozi (Sh) Pink jacaranda (Eng) |
Pod | Chewed with salt | Cough | N, W, C, E | Gelfand et al. (1985) | ||
| Canellaceae | Warburgia salutaris (Bertol. f.) Chiov. | Tree | Muranga (Sh) Pepper-bark tree (Eng) Isibhaha (Zulu) |
Bark | Infusion or decoction | Colds, cough, influenza, sinus clearing, spots in the lungs and chest pains | Panacea(remedy), venereal disease, to increase blood in the body abdominal pains, headache, to cause abortion, aid to divination | E | Critically Endangered |
Maroyi (2008, 2013b); Viol (2013) |
| Cannabaceae |
Trema orientalis (L.) Blume |
Tree | Elm, Pigeonwood (Eng) Umdindwa, Umsekeseke, Umvangazi (Zulu) |
Fruit, Stems, leaves, bark, twigs and seeds | Coughs, sore throats, asthma, bronchitis | Gonorrhea, yellow fever, toothache, antidote, dysentery | N, W, C, E, S | Dzoyem et al. (2016) | ||
| Celastraceae | Elaedendron matabelicum Loes. | Tree | Murunganyama, Murungamunyu (Sh) Condiment saffron (Eng) Umgugudu (Nd) |
Roots | Infusion taken orally | Chest complaints | Aphrodisiac, menorrhagia, diarrhoea with blood, to reduce size of orifice, cavity, venereal diseases, syphilis, abdominal pain, diarrhoea, abscesses, carbuncles, purgative and dysentery remedy | N, W, C, E, S | Least Concern | Gelfand et al. (1985); Viol (2013) |
| Celastraceae | Gymnosporia senegalensis (Lam.) Loes | Tree | Chishuzhu, Chivhunabadza, Musosawafa (Sh) Confetti tree, Red spike-thorn (Eng) Isihlangu (Nd) |
Leaves, twigs and Roots | Coughs, pneumonia and tuberculosis | Chickenpox, measles, varicella, mumps, fever and malaria | N, W, C, E, S | Least Concern | Chimponda and Mukanganyama (2010) | |
| Celastraceae | Sclerocarya birrea subsp. caffra (Sond.) Kokwaro. | Tree | Mupfura, Mutsomo (Sh) Marula (Eng) Umganu (Nd) |
Bark | Cough, pneumonia | Heart pains, diarrhoea, bilharziasis, malaria and antiemetic | N, W, C, E, S | Viol (2013) | ||
| Chenopodiaceae | *Chenopodium ambrosiodes L. | Herb | Munhuhwenhuhwe (Sh) Wormseed (Eng) |
Leaves | Infusion taken orally | Chest pains | N, W, C, E, S (Introduced) | Gelfand et al. (1985) | ||
| Chrysobalanaceae | Parinari curatellifolia Benth. | Tree | Muhacha, Mubuni, Muchakata, Muisha (Sh) Hissing tree, Mobola plum (Eng) Umkhuna (Nd) |
Roots, leaves and bark | Tuberculosis | Fever, toothache, wounds, sores and cuts | N, W, C, E, S | Least Concern | Chimponda and Mukanganyama (2010) | |
| Clusiaceae | Garcinia huillensis Welw | Tree | Mutunduru (Sh) Granite garcinia, Granite mangosteen (Eng) |
Leaves | Treatment of cough, pneumonia and tuberculosis | N, C, E, S | Not Evaluated Lower risk – Near threatened (Zambia) |
Chimponda and Mukanganyama (2010) | ||
| Clusiaceae | Psorospermum febrifugum Spach | Tree | Mumhinu, Munyamharadzi, Muparadzamusha, Musvasva (Sh) Christmas berry (Eng) Umchithamuzi (Nd) |
Roots and Leaves | Ground into powder and added to porridge | Dyspnoea (shortness of breath), pneumonia | Head wound, diarrhoea, earache, syphilis and constipation | N, C, E, S | Gelfand et al. (1985) | |
| Combretaceae | Combretum apiculatum Sond. | Tree | Muruka, Chikukute, Mudziyaishe, Mugodo, Mugoro, Tsingidzi (Sh) Red bushwillow (English) Umbhondo (Nd) |
Leaves | Cough | Snake bites stomach ache | N, W, C, E, S | Least Concern | Maroyi (2013a); Mangoyi et al. (2014); Sharifi-Rad et al. (2020) | |
| Combretaceae |
Combretum platypetalum subsp. oatesii (Rolfe) Exell Combretum oatesii Rolfe |
Shrub | Bepu (Sh) Dwarf red combretum, Red wings (Eng) |
Roots | Porridge prepared with the infusion is applied over painful area | Pneumonia | To dilate birth canal, abdominal pain, diarrhoea, dysmenorrhoea, infertility in women, ear ache, burns, bleeding of the nose, kidney pain, vomiting blood and dilated veins around the umbilicus. | N, W, C, E | Gelfand et al. (1985) | |
| Combretaceae | Combretum zeyheri Sond. | Tree | Muruka, Mupembere-kono, Muchenja (Sh) Large-fruited bushwillow (Eng) Umbhondo, Umbula (Nd) |
Leaves | Cough | Diarrhoea, rectal prolapse, snake bites and stomach ache | N, W, C, E, S | Least Concern | Mangoyi et al. (2014) | |
| Combretaceae |
Terminalia sericea DC. Terminalia velutina sensu Eyles |
Tree | Mangwe, Mukonono, Mususu, Mutabvu (Sh) Silver cluster-Leaves, Silver terminalia (Eng) Umangwe (Nd) |
Roots | Infusion | Sore throat | Wounds, diarrhoea, abdominal pains, worms in anus, antiemetic, infertility in women, to dilate the birth canal, to prevent abortion, tonic, general body weakness, gonorrhoea, bilharziasis, bleeding from nose and depressed fontanelle. | N, W, C, E, S | Gelfand et al. (1985) | |
| Convolvulaceae | Astripomoea malvacea (Klotzsch) Meesu | Herb | Umdandanyane (Nd) | Roots | Decoction | Cough | Abdominal pains in infants, madness, headache and dizziness | N, C, E, S | Gelfand et al. (1985) | |
| Crassulaceae | Kalanchoe spp. | Herb | Kalanchoe (Eng) | Leaves | Ground into powder | Pneumonia | W, C, E, S | Gelfand et al. (1985) | ||
| Cucurbitaeae | *Cucurbita pepo L. | Herb | Nhanga, Muboora (Sh) Pumpkin, Squash (Eng) |
Leaves | Boiled and used as hot compress on painful parts | Pneumonia | Rheumatism | Cultivated | Gelfand et al. (1985) | |
| Cyperaceae | Coleochloa setifera (Ridl.) Gilly. | Herb | Rufuri (Sh) | Roots | Ground into powder and taken orally. | Pneumonia | W, C, E, S | Chigora et al. (2007); Van Wyk (2011); Maroyi (2014) | ||
| Ebenaceae | Diospyros lycioides Desf. | Shrub | Eastern blue-bush, Red star-apple (Eng) Mumbune, Mushawa, Mushumadombo, Musvotamhungu, Mutsvirikiti, Mutsvitsva, Nyatsvipa (Sh) Umbongisa, Umqathuva, Umsungampule (Nd) |
Roots | Infusion | Pneumonia and sore throat | Infertility in women | N, W, C, E, S | Maroyi (2018a) | |
| Ebenaceae |
Euclea crispa (Thunb.) Sond. ex Gürke |
Shrub | Muvhinji (Sh) Blue guarri, Blue-leaved euclea (Eng) |
Roots | Hot water extract drunk three times a day Decoction |
Cough | N, W, C, E, S | Least Concern | Maroyi (2011, 2013a) | |
| Ebenaceae | Euclea natalensis A. DC. | Shrub | Murunze, Nyakabvuri, Mushangura, Chipambati (Sh) Large-leaved guarri, Natal guarri (English) |
Roots and leaves | Asthma, bronchitis, tuberculosis | Anthelminthic, chewing sticks, gonorrhoea, hookworm, malaria, mouthwash, rabies, schistosomiasis, scrofulous swellings, sexually transmitted infections (STIs), toothache, venereal diseases, yellow fever | N, W, C, E, S | Maroyi (2017g) | ||
| Euphorbiaceae | *Ricinus communis L. | Tree | Mupfuta (Sh) Castor oil, Castor bean (Eng) |
Leaves | Pneumonia | Sore eyes and toothache | N, W, C, E, S | Maroyi (2012a) | ||
| Euphorbiaceae | Acalypha petiolaris Hoschst. | Herb | Chitambura (Shona) Heart-leaved Brooms and Brushes (English) Ubukubelo (Ndebele) Umsekezelo (Ndebele) |
Roots | Infusion mixed with salt and taken orally | Asthma | N, W, C, E, S | Gelfand et al. (1985) | ||
| Euphorbiaceae | Antidesma membranaceum Muell. Arg. | Shrub | Mungamunyu (Sh) Pink tassle-berry (Eng) |
Roots and leaves | Infusion taken orally | Cough | E | Gelfand et al. (1985) | ||
| Euphorbiaceae | Bridellia mollis Hutch. | Shrub | Mutuzvidzembwa, Mudenhanyani, Mufukusi, Muhumbakumba, Munzvarawauya, Musosoriondo (Sh) Umkumbakumba, Umwane (Nd) Velvet sweetberry, Velvet-leaved bridelia (Eng) |
Roots | Hot water extract drunk three times a day Decoction |
Cough | N, W, C, E, S | Least Concern | Maroyi (2011, 2019d) | |
| Euphorbiaceae | Croton gratissimus Burch | Shrub | Gunukira, Mufandemenge, Mugugu, Mubangwa, Mufarata (Sh) Lavender croton (Eng) |
Roots and bark | Respiratory disorders | N, W, C, E, S | Least Concern | Chimponda and Mukanganyama (2010) | ||
| Euphorbiaceae | Euphorbia ingens E. Mey. ex Boiss. | Tree | Candelabra tree (Eng) Mugonde, Muhonde, Mukonde (Sh) Umhlonho (Ndebele) |
Latex | Bronchitis and asthma | N, W, C, E, S | Maroyi (2012a) | |||
| Euphorbiaceae | Euphorbia matabelensis Pax. | Tree | Chisimbo, Murimbo (Sh) Musambamacheche (Tonga) Three-forked euphorbia (Eng) Umhlanziso, Hamwamwa (Nd) |
Cough and respiratory infections | Lactation and antidote for poison. | N, W, C, E, S | Gelfand (1956); Gelfand et al. (1985) | |||
| Euphorbiaceae | Flueggea virosa (Roxb. ex Willd.) Voigt | Shrub | Mushagahuwe, Muchagauwe, Mudyambuzi, Mugurumhanda, Musangaoma, Mushikiti, Musosoti, Muzurumbu (Sh) Snowberry tree, White berry bush (Eng) Umhagawuwe, Umklankomo (Nd) Musosoti (Ndau) |
Roots, fruit,bark and leaves | Crushed, mixed with hot water, extract drunk Decoction |
Pneumonia, respiratory tract infections, bronchitis, cough and pneumonia | Snakebite, contraceptive, syphilis, diarrhoea, rheumatism, sterility, rashes and malaria | N, W, C, E, S | Least Concern | Gelfand et al. (1985); Maroyi (2011, 2014); Dzoyem et al. (2016) |
| Euphorbiaceae |
Margaritaria discoidea (Baill.) Webster var. nitida (Pax) Radcliffe-Smith Phyllanthus discoideus (Baill.) Müll. Arg. |
Tree | Bushveld peacock-berry (English) Common pheasant-berry (English) | Roots | Applied on incisions | Pneumonia | N, W, C, E, S | Gelfand et al. (1985) | ||
| Euphorbiaceae |
Monadenium lugardiae N.E.Br. Euphorbia lugardiae (N.E. Br.) Bruyns |
Succulent | Chitsvotsvo (Sh) | Whole plant | Taken orally | Chest pains | N, W, E, S | Gelfand et al. (1985) | ||
| Fabaceae | Albizia amara (Roxb.) Biov. subsp. sericocephala (Benth.) Brenan | Tree | Muchangiza, Mugarahanga, Mugunduzi, Muora (Sh) Bitter albizia (Eng) Umbola (Ndebele) |
Roots | Infusion taken orally | Pneumonia, TB | N, W, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae | Indigofera spp. | Shrub | Rambatuku, Mukatapeta (Sh) | Roots | Ground into powder and mixed with porridge | Chest pains, cough | N, C, S | Gelfand et al. (1985) | ||
| Fabaceae | Vigna unguiculata (L.) Walp. | Herb | Nyemba (Sh) Cow peas (Eng) |
Roots | Infusion taken orally | Chest pains | Cessation of menses, dysmenorrhoea, constipation in infants, epilepsy, and snake bites | N, W, E, S | Gelfand et al. (1985) | |
| Fabaceae |
Pericopsis angolensis (Bak.) van Meeuwen Afrormosia angolensis (Baker) De Wild. Ormosia angolensis Baker |
Tree | Chivanga, Muwanga, Muanga (Sh) Mubanga (Tonga) Afrormosia (Engl) Ubanga (Nd) |
Roots | Infusion taken orally. Burnt and ashes mixed with water. Decoction taken orally. | Cough, Asthma, TB and dyspnoea (shortness of breath). | N, C, E | Near threatened | Gelfand et al. (1985) | |
| Fabaceae | Bauhinia fassoglensis Schweinf. | Tree | Mutukutupasi (Sh) Umdabule (Nd) |
Tuber | Decoction taken orally | Pneumonia | N, W, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae | Bauhinia petersiana Bolle | Tree | Mubondo, Mumwando, Mun'ando, Mupondo (Sh) Large white bauhinia (Eng) Imonddo (Nd) |
Roots, leaves | Cough | Dysmenorrhea female infertility, wounds, diarrhoea | N, W, C, E | Dzoyem et al. (2016) | ||
| Fabaceae | Burkea africana Hook. | Tree | Mukarati (Sh) Umnondo (Nd) False ash, Burke, Wild syringa (Eng) |
Roots | Infusion taken orally | Pneumonia | N, W, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae |
Cassia abbreviata Oliv. |
Tree | Murumanyama, Muremberembe, Muvheneka (Sh) Long-tail cassia (Eng) Isihaqa (Ndebele) |
Roots or bark | Crushed, mixed with cold water, extract drunk Infusion |
Cough and Pneumonia | Abortion, aphrodisiac constipation diarrhoea, back ache, menorrhagia and gonorrhoea |
N, C, E | Least Concern | Kambizi and Afolayan (2001); Maroyi (2011); Ngarivhume et al. (2015) |
| Fabaceae |
Dalbergia melanoxylon Guill. & Perr. |
Shrub | Mugwiti (Sh) Blackwood dalbergia, Zebrawood (Eng) |
Leaves | Dried and smoked as cigarette | Bronchitis, asthma and inflammation in throat | N, W, C, E, S | Near Threatened | Chigora et al. (2007); Maroyi (2013a) | |
| Fabaceae | Dichrostachys cinereal (L.) Wight & Arn. | Shrub | Mupangara, Musekera, Mumhangara (Sh) Ugagu (Nd) Chilitsenge (Tonga) Chinese lantern (Eng) Sickle bush (Eng) |
Leaves and Roots | Pneumonia | Venereal diseases, impotence, syphilis, eye diseases, wounds and injuries. | N, W, C, E, S | Viol (2013) | ||
| Fabaceae | *Leucaena leucocephala (Lam.) De Wit | Tree | Wild tamarind, White lead tree, Lead tree, Horse tamarind, Jumbie bean, White popinac (Eng.) | Bark, leaves, seeds | Colds, flu and tuberculosis | Internal pain, contraceptive, ecbolic, depilatory, fevers, circulatory problems, to calm nerves, reduce back pain and menstrual cramps |
C (Introduced) | Dzoyem et al. (2016) | ||
| Fabaceae | Peltophorum africanum Sond. | Tree | Muzeze, Dzedze, Mudjiza, Mupumhamauva, Zeze, Musambanyoka, Mutandarombo, Nyakambariro, Nyamanyoka (Sh) African wattle (Eng) Umkahla, Umsehla (Nd) |
Leaves | Flu | Diarrhoea, sore eyes, Sexually transmitted infections-syphilis and toothache | N, W, C, E, S | Least Concern | Shoko (2007) | |
| Fabaceae | Piliostigma thonningii (Schumach.) Milne-Redh. | Tree | Musekesa, Mubaba, Muhuku, Musakasa, Mutukutu (Sh) Camel-foot, Monkey bread (English) Ihabahaba (Nd) |
Bark, leaves or Roots | Hot water extract drunk three times a day Decoction |
Cough | Menorrhagia, convulsions, bilharziasis | N, W, C, E, S | Not Evaluated | Gelfand et al. (1985); Maroyi (2011, 2013a, 2014); Sharifi-Rad et al. (2020) |
| Fabaceae | Pterocarpus angolensis DC. | Tree | No information | Roots | Infusion taken orally | Chest pains | N, W, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae | Acacia rehmanniana (Schinz) Kyal. & Boatwr. | Tree | Muunga (Sh) Silky acacia, Silky thorn (Eng) Iphucula, Mona (Nd) | Roots | Rubbed on incision | Pneumonia | N, W, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae | Albizia antunesiana Harms | Tree | Muriranyenze (Sh) Purple-leaved albizia (Eng) Umnonjwana (Nd) |
Roots | Rubbed on incision | Pneumonia | N, W, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae | Albizia tanganyicensis Bak.f. | Tree | Paperbark albizia (Eng) Umphaphama (Nd) |
Bark | Decoction taken orally | Cough | N, W, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae | Aeschynomene mimosifolia Vatke | Shrub | Rutapatsikidzi (Sh) Bug catcher (Eng) |
Roots | Decoction taken orally | Chest pains | N, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae | Crotalaria laburnifolia L. subsp. laburnifolia | Herb | Dodzidunhu (Sh) Oldland rattlepod, Wild sunhemp (Eng) Amahlwayi (Nd) |
Roots | Infusion taken orally | Cough | N, W, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae |
Erythrina abyssinica Lam. DC Erythrina tomentosa R.Br. ex A. Rich. |
Tree | Munhimbiti, Mutete, Mutiti, Mutsiti (Sh) Lucky-bean tree, Red-hot-poker tree (Eng) Umgqogqogqo (Nd) |
Roots and Bark | Infusion taken orally | Cough | N, C, E, S | Gelfand et al. (1985) | ||
| Fabaceae | Xeroderris stuhlmanni (Taub.) Mendonca & E.P Sousa | Tree | Muchemavanhu, Mudzugu, Mumwambizi, Muriravanhu, Murumanyama (Sh) Wing pod (Eng) Umthundulu (Nd) |
Roots | Decoction taken orally | Chest pains | N, W, C, E, S | Gelfand et al. (1985) | ||
| Flacourtiaceae | Flacourtia indica (Burm.f.) Merr. | Tree | Mududwe, Munhunguru, Mutombototo, Mutudza, Mutunguru (Sh) Batoka plum, Governor's plum (Eng) |
Leaves and Roots | Browsed by mouth Eaten raw |
Cough, chest pains and pneumonia | Venereal disease, bilharziasis, diarrhoea | N, W, C, E, S | Least Concern | Viol (2013); Mangoyi et al. (2014) |
| Heteropyxidaceae | Heteropyxis natalensis Harv. | Tree | Lavender tree, natal lavender (Eng) Mudedede (Venda) Inkunzi, Inkhuzwa (Zulu) |
Bark, leaves and Roots | Colds and respiratory disorders | Aphrodisiac, bleeding disorders, bleeding gums, blood purifier, gum infections, toothache, wounds, weaning and menorrhagia | E | Maroyi (2019f) | ||
| Hyacinthaceae | Urginea sanguinea (Schinz) Jessop | Herb | Chitupatupa (Sh) Isigenama (Nd) |
Bulb | Decoction taken orally | Pneumonia | W, C, S | Gelfand et al. (1985) | ||
| Kirkiaceae | Kirkia acuminata Oliv. | Tree | Mubvumira, Mutsakatidze, Mutuhwa, Mutuva (Sh) White seringa (Eng) Modumela (Tswana) Mubvumala (Venda) |
Roots | Extract drunk Infusion |
Cough | Diarrhoea, malaria, cholera, dysentery, constipation and wounds | N, W, C, E, S | Least Concern | Maroyi (2011, 2016, 2017a) |
| Lamiaceae | *Mentha longifolia (L.) Huds | Herb | Horsemint (Eng) | Leaves | Extract Infusion |
Colds and cough | N, W, C, E | Least Concern | Chigora et al. (2007); Maroyi (2011, 2014) | |
| Lamiaceae | *Mentha spicata L. | Herb | Spearmint (Eng) | Leaves | Added to tea or hot infusion taken by mouth Tea and Infusion |
Cough and flu | N, W, C, E | Least Concern | Maroyi (2017c, 2018b) | |
| Lamiaceae | Ocimum obovatum (Benth.) N.E.Br. | Herb | Chikomamatadza (Sh) | Leaves | Burnt and smoke inhaled | Chest pains | Epistaxis, tropical ulcers, abdominal pain in infants and infertility in men. | E | Gelfand et al. (1985) | |
| Lamiaceae | Leucas milanjiana Gürke | Herb | No information | Leaves | Applied on incisions made on painful parts | Pneumonia | Swelling on the body (edema) | N, W, C, E, S | Gelfand et al. (1985) | |
| Lamiaceae | Tetradenia riparia (Hochst.) Codd | Shrub | Chororwe (Sh) Ginger bush, misty plume bush (Eng) Iboza, ibozane (Zulu) |
Leaves | Cough, colds, bronchitis, respiratory ailments | Stomach ache, diarrhoea, dropsy, angina pectoris, fever, malaria and dengue fever, yaws, headache and toothache | N, W, C, E, S | Neffati et al. (2017) | ||
| Lamiaceae | Vitex payos (Lour.) Merr. | Shrub | Chikubai, Chikubvusike, Mudyagava, Muhubva, Muhubvu, Mukubvu, Mutsere, Mutsubvu (Sh) Umtshwankela (Nd) Chocolate berry (Eng) |
Leaves | Burnt and smoke inhaled Smoke |
Cough, colds, respiratory ailments | Lost appetite | N, W, C, E, S | Chigora et al. (2007); Maroyi (2014) | |
| Loganiaceae | Strychnos potatorum L.f. | Tree | Mudanhapfunye, Mudyagudo, Mudyakuwe, Mudyambira (Sh) Umlombelombe (Nd) Black bitterberry, Grape strychnos (Eng) |
Roots and leaves | Decoction taken orally | Cough | N, W, C, E, S | Gelfand et al. (1985) | ||
| Loganiaceae | Strychnos spinosa Lam | Tree | Mutamba-mun'ono (Sh) Spiny monkey-orange (Eng) Umhahli, Umngono (Nd) |
Bark and leaves. | The dose is orally administered by boiling fresh Bark in water and drink. Besides, the unripe fruits are broken, opened, mixed with water and boiled. |
Pneumonia, bronchitis and chest problems. Caffeine in leaves can help breathing in premature babies. | Immune boosting, stomach-ache, diarrhoea, gastro-intestinal problems, venereal diseases like syphilis and gonorrhea, rushes, skin problems, irritation., bleeding and genital warts. | N, W, C, E, S | Mawere and Nhemachena (2016) | |
| Loranthaceae | Loranthus spp on Brachystegia spiciformis Benth. | Mistletoe | Gomarara, Koma (Sh) Inofi (Nd) |
Whole plant | Smoke inhaled | Asthma | N, W, C, E, S | Gelfand et al. (1985) | ||
| Loranthaceae | Loranthus spp. on Berchemia discolor (Klotzsch) Hemsl. | Mistletoe | Gomarara, Koma (Sh) Inofi (Nd) |
Whole plant | Powder taken in porridge | Pneumonia | N, W, C, E, S | Gelfand et al. (1985) | ||
| Loranthaceae | Loranthus spp. on Cordyla africana Lour. | Mistletoe | Gomarara, Koma (Sh) Inofi (Nd) |
Whole plant | Applied in incisions in powder form | Pneumonia | N, W, C | Gelfand et al. (1985) | ||
| Malvaceae | *Abelmoschus esculentus (L.) Moench | Herb | Derere rechipudzi, Derere (Sh) Okra, Lady's finger (Eng) Idelele (Nd) |
Fruit | Bronchitis and tuberculosis | Heart diseases | N, W, E (Introduced) | Least Concern | Chimponda and Mukanganyama (2010) | |
| Malvaceae | Azanza garckeana (F.Hoffm.) Exell & Hillc. | Tree | Mugurura (Sh) Mutohwe (Sh) Snot apple (Eng) Uxakuxaku (Nd) |
Roots | Chest pains and cough | Other- menstruation, retained placenta, mental illness, earache, antiemetic | N, W, C, E, S | Maroyi (2017e) | ||
| Malvaceae | Sida acuta Burm.f. | Shrub | Common wireweed (Eng) Isinama (Nd) |
Roots | Decoction taken orally | Chest pains | N, C, E, S | Gelfand et al. (1985) | ||
| Meliaceae | Ekebergia benguelensis C.DC. | Tree | Mudyamhofu, Mudyavarungu, Munyimonyimo, Mupumhanhuka, Mupuri (Sh) Woodland dogplum (Eng) |
Leaves | Infusion taken orally | Pneumonia | N, C, E, S | Gelfand et al. (1985) | ||
| Meliaceae | Khaya anthotheca (Welw.) C.DC. | Tree | Muwawa, Mubarwa, Mururu (Sh) Red mahogany (Eng) |
Bark and Roots | Infusion taken orally | Pneumonia and colds | Venereal diseases, abdominal pains, antihelmentic antiemetic | N, E, S | Vulnerable (VU) | Gelfand et al. (1985); Viol (2013) |
| Meliaceae | Turraea nilotica Kotschy & Peyr. | Shrub | Chipindura, Chirambagavakava Chitsvimbovarisa, Chitunguru, Mudyakuwe, Mukondanyoka, Muzaramhanga (Sh) Miombo honeysuckle-tree, Bushveld honeysuckle-tree, Small mahogany (Eng) Isidlamvundala (Nd) |
Roots | Applied to incision, infusion taken orally, burnt or smoked. Ground into powder and taken in porridge | Dyspnoea (shortness of breath), pneumonia and respiratory disorders | N, W, C, E, S | Gelfand (1956); Gelfand et al. (1985) | ||
| Moraceae | Ficus ingens (Miq.) Miq. | Tree | Mutsamvi (Sh) Red-leaved rock fig (Eng) Idotsi, Inkiwane (Nd) |
Roots | Crushed, mixed with hot water, extract drunk Decoction |
Cough | Fever | N, W, C, E, S | Least Concern | Maroyi (2011, 2013a) |
| Moraceae | Ficus sycomorus L. | Tree | Muonde, Mukuyu (Sh) Sycomore fig, Mulberry Fig (Eng) Umkhiwa (Nd) | Roots | Crushed, mixed with hot water, extract drunk Decoction |
Tuberculosis, cold and other chest problems | Laxative | N, W, S | Least Concern | Maroyi (2011, 2013a); Maroyi and Cheikhyoussef (2015) |
| Moraceae | Ficus thonningii Blume | Tree | Mutsamvi (Sh) Small fig tree, Strangler Fig (Eng) |
Roots, Stems and leaves | Respiratory infections, bronchitis, treating influenza, sore throat, colds, pneumonia and chest pains. | Prevent abortion and stop nose bleed. | N, W, C, E, S | Least Concern | Dangarembizi et al. (2013) | |
| Myrothamnaceae | Myrothamnus flabellifolius Welw. | Shrub | Rufandichimuka, Mufandichimuka (Sh) Resurrection bush (Eng) Umazifisi (Nd) |
Leaves and twigs | Boiled and drunk as remedy for cold Decoction |
Colds and chest complaints | Nosebleeds and fainting | N, W, C, E, S | Least Concern | Chigora et al. (2007); Semenya and Maroyi (2013) |
| Myrsinaceae | Rapanea melanophloeos (L.) Mez | Tree | Mudonera, Mudongera, Mufuro, Mukwiramakoko, Murwiti, Mutomo (Sh) Umhluti-wentaba, UIvukwabafile (Zulu) Cape beech (Eng) |
Bark, Roots and leaves | Respiratory problems | Stomach, muscular and heart complaints | N, C, E | Dzoyem et al. (2016) | ||
| Myrtaceae | *Eucalyptus camaldulensis Dehnh | Tree | Mugamutiri (Sh) Gum Tree (Eng) |
Leaves | Extract drunk with Citrus limon fruits and Psidium guajava L. leaves as cough, flu and fever medicine Decoction or Infusion |
Sore throat, flu, asthma, cough | Fever | N, W, C, E, S | Near Threatened | Maroyi (2011); Semenya and Maroyi (2013a, 2017c, 2018b) |
| Myrtaceae | *Psidium guajava L. | Shrub | Mugwavha (Sh) Guava (Eng) |
Leaves | Extract drunk with Citrus limon fruits and Eucalyptus camaldulensis leaves as cough, flu and fever medicine Decoction or Infusion |
Cough and flu | Fever and diarrhoea | N, W, C, E, S | Least Concern | Maroyi (2011, 2013a, 2014, 2017c, 2018b) |
| Myrtaceae | Syzigium guineense (Willd.) D.C | Tree | Mukute (Sh) Forest waterberry (Eng) Gihlu (Nd) |
Bark and leaves | Tuberculosis and chest complaints | Stomach ailments and diarrhoea | N, W, C, E, S | Least Concern | Van Wyk (2011); Chimponda and Mukanganyama (2010); Mangoyi et al. (2014) | |
| Myrtaceae |
Syzygium cordatum Hochst. ex C. Krauss |
Tree | Mukute, Muisu (Sh) Waterberry (Eng) Gihlu (Hlengwe) Munonyamansi (Tonga: Zimbabwe) Umdoni, Imiswi (Nd) |
Bark | Extract drunk as tuberculosis medicine Infusion |
Cold and fever | Herpes zoster, herpes simplex, skin rashes | N, W, C, E, S | Least Concern | Chigora et al. (2007); Van Wyk (2011); Maroyi (2013a) |
| Nymphaeaceae | Nymphaea nouchali Burm. f. | Aquatic | Hapa, Hobvwe (Sh) Kwibu (Tonga) Waterlily (Eng) Ikalala, Amalebo-emfula (Nd) |
Roots | Ground into powder and mixed with porridge | Asthma | N, W, C, E, S | Gelfand et al. (1985) | ||
| Phyllanthaceae | Bridelia micrantha (Hochst.) Baill. | Tree | Mitzeerie (Eng) Mudzinza, Mufukusi, Mukodokodo, Mukwandu, Mushungunu, Mutorarwizi, Mutsetsauta, Mutugusi (Sh) | Bark, leaves and Roots | Infusion taken orally | Cough | Abortifacient | N, C, E, S | Gelfand et al. (1985); Maroyi (2017f) | |
| Pittosporaceae | Pittosporum viridiflorum Sims | Tree | Mubanda, Muchemedzambuya, Mugarambinga, Mukwenukwenu, Murambatsvina, Murunganyama (Sh) Cheesewood (Eng) Iyoyi (Nd) |
Roots | Infusion taken orally | Chest pains | N, W, C, E, S | Gelfand et al. (1985) | ||
| Plumbaginaceae | Plumago zeylanica L. | Grass | Wild white plumbago (Eng) | Roots | Infusion taken orally | Dyspnoea (shortness of breath) | Aphrodisiac | N, W, C, E, S | Gelfand et al. (1985) | |
| Poaceae | Phragmites mauritianus Kunth | Reed | Reed grass (Eng) | Stems | Ground into powder and rubbed on incision. | Pneumonia | N, W, C, E, S | Gelfand et al. (1985) | ||
| Polygalaceae | Securidaca longipedunculata Fresen. | Tree | Chipvufanana, Mufufu Munyapunyapu, Munyazvirombo, Mutangeni (Sh) Umfufu (Nd) Violet tree (Eng) |
Roots | Tuberculosis and pneumonia | Epilepsy, venereal diseases, pains, fevers, syphilis and snake repellent. | N, W, C, E, S | Maroyi (2012a); Viol (2013) | ||
| Polygonaceae | Oxygonum sinuatum (Hochst. & Steud. ex Meisn.) Dammer | Herb | Oxygonum (Eng) | Cough | N, W, C, E, S | Gelfand et al. (1985) | ||||
| Proteaceae | Faurea saligna Harv. | Tree | Kapfutsana, Mugarahungwe, Munyaganza, Mushangwa, Muzazati (Sh) African beech, Willow beechwood (Eng) Isidwadwa, Umpembele (Nd) |
Roots | Infusion taken orally | Pneumonia | N, W, C, E, S | Gelfand et al. (1985) | ||
| Pteridaceae | Pellaea spp. | Fern | Mumvuriwedombo, Mudziwebwe (Sh) Purple Cliffbrake, Purple Stems Cliffbrake, Hairy Cliffbrake (Eng) |
Leaves and Roots | Burnt and smoke inhaled as remedy for chest pains Smoke |
Chest pains | E | Least Concern | Chigora et al. (2007), Semenya and Maroyi (2013a, 2014); Neffati et al. (2017) | |
| Ranunculaceae | Clematis villosa DC | Herb | Shock-headed peter (Eng) | Roots | Burnt and smoke inhaled | Cough and colds | N, W, C, E, S | Gelfand et al. (1985) | ||
| Rhamnaceae |
Rhamnus prinoides L'Hérit |
Tree | Musvosvadziva (Sh) Camdeboo, Dogwood, Glossy-Leaves, Shiny Leaves, Stinkwood (Eng) Ulenyenye, Umgilindi, Umhlinye, Umnyenye (Zulu). |
Fruit, leaves, bark, Stems, twigs, seeds, Roots |
Pneumonia, cold and respiratory infections | Blood cleaning, rheumatism, sprains, stomach ache, gargle, skin complaints, sexually transmitted disease, arthritis, back pains, stomach ache, headache | N, C, E | Dzoyem et al. (2016) | ||
| Rhamnaceae | Ziziphus mucronata Willd. | Tree | Muchechen, Chinanga (Sh) Buffalo-thorn, Bog-wood, Cat-thorn (Eng) Umpasamala, Umphafa (Nd) Umlahlankosi, Umlahlabantu, Umkhobobonga, Umphafa (Zulu) |
Leaves | Infusion | Chest complaints, Cough | Skin infections and wounds, body pains, infertility in women, boils, carbuncles, sores and swellings | N, W, C, E, S | Least Concern | Chimponda and Mukanganyama (2010); Dzoyem et al. (2016) |
| Rubiaceae | Agathisanthemum bojeri Klotzsch | Herb | Muwanazvapora (Sh) Velabahleka (Nd) |
Leaves | Burnt and smoke inhaled | Asthma, chest conditions, relieve cough and diffulty in breathing | N, C, E | Watt and Breyer-Brandwijk (1962); Gelfand et al. (1985) | ||
| Rubiaceae | Catunaregam taylorii (S.Moore) Bridson | Shrub | Mutsvairachuru, Murovaduri (Sh) Mountain pomegranate (Eng) |
Leaves, bark and Roots | Respiratory ailments and pulmonary infections | N, W, C, E, S | Least Concern | Chimponda and Mukanganyama (2010) | ||
| Rubiaceae | Fadogia ancylantha Schweinf. | Shrub | Makoni tea bush (Eng) | Leaves | Decoction taken orally | Cough, pneumonia | Abdominal pain, constipation, swelling of the body, hiccoughs, antiemetic, tropical ulcers, to prevent conception and bulging fontanelle. | N, C, E, S | Gelfand et al. (1985) | |
| Rubiaceae | Gardenia resiniflua Hiern subsp. resiniflua | Shrub | Mutara, Mutarara (Sh) Chigalamatongo, Chigonondo (Tonga) Gummy gardenia (Eng) Umjalanatanga, Umvalasangwana (Nd) |
Roots | Burnt and applied on incision on chest | Pneumonia, asthma | Headache, convulsions, earache, madness, fits, infertility in women and dysmenorrhoea. | N, W, C, E, S | Gelfand et al. (1985) | |
| Rubiaceae | Gardenia ternifolia Schuamch. & Thonn. subsp. jovis-tonantis (Welw.) Verdc. var goetzei (Stapf & Hutch.) Verdc | Shrub | Mutara, Mutarara, Mutarura (Shona) Powder-bark gardenia, Wild gardenia (Eng) Umvalasangwana (Nd) |
Roots | Burnt and applied on incision on chest | Pneumonia, asthma | Dysmenorrhoea, convulsions, earache, madness, fits, infertility in women and headache. | N, C, E, S | Gelfand et al. (1985) | |
| Rubiaceae | Gardenia volkensii K. Schum. subsp. spatulifolia (Stapf and Hutch.) Verdc. | Tree | Mutara (Sh) Umvalasanganwa (Nd) |
Roots | Burnt and applied on incision on chest | Pneumonia, asthma | Infertility in women, headache, earache, madness, fits, dysmenorrhoea and convulsions. | E, S | Gelfand et al. (1985) | |
| Rubiaceae | Mussaenda arcuata Poir. | Climber | Muridzameso, Musikawakakadzi (Sh) Forest star (Eng) |
Leaves | Boiled and the strained liquid taken by mouth as required | Influenza | N, C, E, S | Chinemana et al. (1985) | ||
| Rubiaceae | Pavetta schumanniana F. Hoffm. | Shrub | Chifukawi, Chinama, Chipindura chiduku, Chitunguru, Chityorabadza, Mufuramhembwe, Murambagaka, Murunganyama, Musauti, Muwana, Mwenje, Nyapuna, Nyaputa (Sh) Poison bride's-bush, Poison pavetta (Eng) Umbodzani (Nd) |
Leaves | Decoction or infusion, ground into powder or chewed and juice swallowed. Applied to incisions. | Chest pains, cough, pneumonia | Abdominal pains, diarrhoea, nausea, headache, aphrodisiac, venereal disease and infertility in women. | N, W, C, E, S | Gelfand et al. (1985) | |
| Rubiaceae | Spermacoce dibrachiata Oliv. | Herb | Chiparurangoma (Sh) Winged forget-me-not (Eng) |
Roots | Infusion taken orally | Pneumonia, cough | Depressed fontanelle, hoarseness, dizziness, dysmenorrhoea and aphrodisiac. | N, W, C, E | Gelfand et al. (1985) | |
| Rubiaceae | Vangueria infausta Burch. subsp. infausta | Tree | False medlar, Velvet wild medlar (English) Umthofu, Umviyo (Nd) Mudzvirungombe, Munjiro, Munzviro, Munzirwa, Munzvirwa, Mutsviru (Sh) |
Leaves, Roots, seed | Pneumonia | Abdominal pains, diarrhoea and stomach problems, inflammation of umbilical cord, menstrual problems | W, C, E, S | Maroyi (2018e) | ||
| Rubiaceae | Vangueriopsis lanciflora (Hiern) Robyns | Shrub | Mutufu, Mutupfu (Sh) False wild medlar (Eng) Umsomosomo, Umviyo, Amadumbutshenene (Nd) |
Roots | Decoction taken orally | Cough | Abdominal pains, constipation, infertility in women, neck pains, to dilate the birth canal, backache, swelling on the body and madness. | N, W, C | Gelfand et al. (1985) | |
| Rubiaceae | Xeromphis obovata (Hochst.) Keay | Shrub | Chizhuzhu-chitsuku (Sh) Isitalagwa (Nd) |
Roots | Infusion or ground into powder and mixed with porridge | Cough, pneumonia | Antidote for snake bite, nausea, depressed fontanelle, fever, hoarseness, toothache, bile emesis, emetic, heavy menstruation, fits and dizziness | N, W, C, E, S | Gelfand et al. (1985) | |
| Rutaceae | *Citrus limon (L.) Burm. f. | Tree | Muremoni (Sh) Lemon Tree (Eng) |
Fruit | Extract of Citrus lemon Infusion, Tea, Decoction, Juice |
Throat infections, cough and flu | Tonsil | N, W, C, E, S | Maroyi (2011, 2013a, 2014); Semenya and Maroyi (2013) | |
| Rutaceae | Zanthoxylum humile (E.A. Bruce) P.G. Waterman | Shrub | Hairy knobwood (Eng) | Roots | Aqueous extracts are used to treat chest pains and flu. | Chest pains and flu | Erectile dysfunction diarrhoea, hypertension, diabetes, wounds and as antivenins against snake bites | W, S | Pamhidzai and Isaac (2013) | |
| Sapindaceae | Zanha africana (Radlk) Exell. | Tree | Muchenya (Sh) Velvet-Fruitszanha (Eng) |
Bark, leaves and Roots | Applied to painful parts | Respiratory problems (asthma, chest pains, colds, cough, flu, pneumonia and tuberculosis) | Gastro-intestinal problems (abdominal pains, constipation, diarrhoea, dysentery and stomach ache), STD, headache, migraine, body pains, dizziness, female reproductive problems (abortion, dysmenorrhoea, facilitating childbirth, infertility, menorrhagia, pregnancy edema and disorders), fever, typhoid fever, painful legs, nausea, rheumatoid arthritis rheumatism and malaria | N, W, C, E, S | Gelfand et al. (1985); Maroyi (2019f) | |
| Solanaceae | *Capsicum spp. | Herb | Green pepper, Sweet pepper (Eng) | Fruits | Pulverised and a little salt added and in water | Respiratory infections | Cultivated | Gelfand et al. (1985) | ||
| Solanaceae | *Nicotiana tabacum L. | Herb | Chikwarimba, Fodya, Hunga (Sh) Igwayi (Nd) Tobacco (Eng) | Leaves | Asthma, respiratory problems | Cultivated | Maroyi (2012a) | |||
| Solanaceae | *Datura stramonium L | Shrub | Chowa (Sh) Jimson weed, Thorn apple (Eng) |
Leaves | Burnt and smoke inhaled while covered with a blanket | Asthma and cough | Sexual Transmitted Infections | N, W, C, E, S | Not Evaluated | Maroyi (2012a, 2017c, 2018b) |
| Solanaceae | *Solanum incanum L. | Herb | Munhomboro, Munhundurwa (Sh) Poison apple, Snake apple, Bitter apple, Sodom apple, Thorn apple (Eng) Umdulukwa, Intume (Nd) |
Roots | Infusion taken orally and applied to incisions on painful parts. | Pneumonia | Venereal diseases, dysmenorrhoea, sore eyes, diarrhoea, antiemetic, headache, tropical ulcers, general body pains, sore throat, toothache, swelling on the body and snake bite. | N, W, C, E, S | Gelfand et al. (1985) | |
| Thymelaeaceae | Gnidia capitata L.f | Herb | Muwito, Katonje (Sh) | Roots | Smoke inhaled | Asthma | Tonsillitis and venereal diseases | N, W, C, E, S | Gelfand et al. (1985) | |
| hymelaeaceae | Gnidia kraussiana Meisn. | Herb | Chitupatupa (Sh) Isidikili (Nd) Yellow-heads (Eng) |
Tuber | Freshly crushed tuber applied to boil | Cough | Measles, swollen stomach, anorexia, depressed fontanelle, boils, earache, emetic, madness, constipation, stomach ailments and weaknesses in joints. | N, W, C, E, S | Gelfand et al. (1985) | |
| Tiliaceae | Triumfetta welwitschii Mast. | Herb | Ibofane (Nd) | Tuber | Ground into powder | Asthma | Venereal disease, fever, generalised body pain, swelling on the body, painful uterus, to prevent abortion, antidote, tropical ulcers, depressed fontanelle, aphrodisiac, diarrhoea and abdominal pain. | N, W, C, E, S | Gelfand et al. (1985) | |
| Ulmaceae | Chaetachme aristata Planch | Tree | Thorny-Elm, Basterwitpeer (Eng) | Leaves | Tuberculosis | Back wounds and spinal weakness | C, E, S | Dzoyem et al. (2016) | ||
| Verbenaceae | Clerodendrum eriophyllum Gürke | Shrub | Munyakachembere, Ruwudziwudzi (Sh) Tinderwood, White cat's whiskers (Eng) Moswaapeba, Umhlahlampethu, Umnukanja, Umxothanja (Nd) |
Roots, leaves, Bark | Cough and colds | Snakebites, prolapse, wounds and diarrhoea | W, C, E, S | Dzoyem et al. (2016) | ||
| Verbenaceae | Lippia javanica (Burm.f.) Spreng | Shrub | Zumbani, Kachigwere, Mumara, Mushani mukuru, Musumba (Sh) Umsuzwane (Nd) Lemon bush, Fever Tea (Eng) |
Leaves and twigs | Ointment rubbed on chest and abdomen Decoction taken orally or body washed with decoction Ointment and Decoction |
Cold, cough, shortness of breath (dyspnoea), respiratory complaints, bronchial problems, fever, asthma, chronic coughs | Measles, malaria and stomach ache | N, W, C, E, S | Gelfand et al. (1985); Chigora et al. (2007); Chimponda and Mukanganyama (2010); Van Wyk (2011); Semenya and Maroyi (2013a, 2017b); Neffati et al. (2017) | |
| Zingiberaceae | *Zingiber officinale Roscoe | Herb | Tsangamidzi (S) Ginger (Eng) |
Rhizome | Rhizome- extract Raw and Infusion |
Cough, flu and colds | Stomach pains, indigestion, colic, abdominal chills | Cultivated | Matongo (2012) |
Table 2.
Families of medicinal plant species used to treat and manage respiratory diseases in Zimbabwe.
| Family | Number of families | Number of species in each family |
|---|---|---|
| Alliaceae, Asparagaceae, Canellaceae, Cannabaceae, Chenopodiaceae, Chrysobalanaceae, Convolvulaceae, Crassulaceae, Cucurbitaeae, Cyperaceae, Flacourtiaceae, Heteropyxidaceae, Hyacinthaceae, Kirkiaceae, Myrothamnaceae, Myrsinaceae, Nymphaeaceae, Phyllanthaceae, Pittosporaceae, Plumbaginaceae, Poaceae, Polygalaceae, Polygonaceae, Proteaceae, Pteridaceae, Ranunculaceae, Sapindaceae, Tiliaceae, Ulmaceae, Zingiberaceae | 30 | 1 |
| Acanthaceae, Annonaceae, Asclepiadaceae, Bignoniaceae, Clusiaceae, Loganiaceae, Rhamnaceae, Rutaceae, Thymelaeaceae, Verbenaceae | 10 | 2 |
| Celastraceae, Ebenaceae, Loranthaceae, Malvaceae, Meliaceae, Moraceae | 6 | 3 |
| Apiaceae, Asphodelaceae, Combretaceae, Myrtaceae, Solanaceae | 5 | 4 |
| Apocynaceae, Lamiaceae | 2 | 6 |
| Anacardiaceae | 1 | 7 |
| Asteraceae, Euphorbiaceae | 2 | 10 |
| Rubiaceae | 1 | 12 |
| Fabaceae | 1 | 21 |
Table 3.
Pharmacological and toxicological evaluation of medicinal plants used to treat and manage respiratory diseases.
| Family | Scientific Name | Ethnomedicinal uses for respiratory disorders | Pharmacology properties | Toxicological evaluations | References |
|---|---|---|---|---|---|
| Acanthaceae | Barleria spinulosa Klotzsch | Pneumonia | no records found | no records found | |
| Acanthaceae | Thunbergia oblongifolia Oliv. | Asthma | Antioxidant, antimicrobial, anti-diabetic, anti-proliferative, hepatoprotective, anti-inflammatory and detoxifying activities. | Safe LD50 > 8000 mg/kg |
Chan et al. (2011); Wonkchalee et al. (2012); Sultana et al. (2015); Rana et al. (2020) |
| Alliaceae | Tulbaghia leucantha Baker | Asthma | Antioxidant, anti-inflammatory, antiulcer, anti-splasmodic, antiviral, antidiarrheal, and antitumor activities. | no records found | Takaidza (2018) |
| Anacardiaceae | *Mangifera indica L. | Asthma, colds, cough and tuberculosis | Antibacterial, anti-inflammatory, antioxidant, radioprotective, antitumor, immunomodulatory, anti-allergic, antidiabetic, lipolytic, analgesic, antibone resorption, monoamine oxidase inhibiting, antifungal, anti-diabetic, anti HIV, antibone resorption, antiviral, gastroprotective, anti-spasmodic, antihyperlipemic, antipyretic, antidiarrheal and anti-parasitic activities. | Safe LD50 > 2000 mg/kg in rats |
Wauthoz et al. (2007); Maroyi (2013a); Parvez (2016); Ediriweera et al. (2017); Ahomadegbe et al. (2018); Reddeman et al. (2019) |
| Anacardiaceae | Lannea discolor (Sond.) Engl. | Cough | Anthelmintic, antibacterial, antifungal, anti-mycobacterial, antioxidant, anti-plasmodial, nematicidal and cytotoxicity activities. | No records found | Kabongo-Kayoka et al. (2016); Maroyi (2018d); Mwamatope et al. (2020) |
| Anacardiaceae | Lannea edulis (Sond.) Engl. | Cough and Bronchitis | Anthelmintic, antimicrobial, antioxidant, anti-HIV and antimalarial. | Weak or low toxicity LC50 = 971 ± 86 μg/mL |
Maroyi (2019c) |
| Anacardiaceae | Searsia chirindensis (Baker f.) Moffett | Chest pains and cough | Antiviral, antifungal, antioxidant, analgesic, antibacterial, and anti-inflammatory activities. | Safe LC50 value of 1023.26 ± 161.69 μg/ml |
Ojewole (2007); Viol et al. (2016) |
| Anacardiaceae | Searsia lancea (L.f) F.A Barkley | Chest pains | Antibacterial, anti-inflammatory, cytotoxic, healing properties antifungal and antioxidant activities. | Weak or low toxicity LC50 = 600 μg/ml |
McGaw et al. (2007); Gundidza et al. (2008); Mulaudzi et al. (2012); Mangoyi et al. (2014); Madzinga and Kritzinger (2020) |
| Anacardiaceae | Searsia longipes (Engl.) Moffet | Cough | Schistosomacidal, antitussive, cytotoxic and antioxidant activities. | Safe LD50 > 5000 mg/kg body weight |
Šutovská et al. (2009); Olorunnisol et al. (2017); Chacha (2019); Chacha and Mbugi (2019); Olasunkanmi and Adegbola (2019) |
| Anacardiaceae | Searsia pyroides (Burch.) Moffett | Cough | Antioxidant activity. | Safe LD50 > 2000 mg/kg body weight |
Mtunzi et al. (2017); Chacha and Mbugi (2019) |
| Annonaceae | Annona stenophylla Engl. & Diels | Chest pains | Antibacterial, antifungal, anti-inflammatory, antioxidant and hypoglycaemic activities. | Safe LC50 μg/ml - 1190 ± 212 - 2300 ± 276 μg/ml Non-toxic LD50 > 2000 mg/kg in rats |
Maroyi (2019a); Munodawafa et al. (2016) |
| Annonaceae | Hexalobus monopetalus (A. Rich.) Engl. & Diels | Colds, bronchitis and pulmonary troubles | Antimycobacterial, antimicrobial and antimalarial activities. | Highly toxic LC50 values ranging from 0.56 to 66.07 μg/mL |
Malebo et al. (2014); Dzoyem et al. (2016); Chauke et al. (2016); Taderera et al. (2016); Souham et al. (2018) |
| Apiaceae | Alepidea amatymbica Eckl. & Zeyh | Asthma, influenza, colds and cough | Antimicrobial, anti-inflammatory and antiviral activities. | Highly toxic LC50–0.002 μg/ml |
Wintola and Afolayan (2014) |
| Apiaceae | Alepidea cordifolia B.-E. van Wyk | Colds and influenza | no records found | no records found | |
| Apiaceae | Diplolophium zambesianum Heirn | Pneumonia | no records found | no records found | |
| Apiaceae | Heteromorpha arborescens (Spreng.) Cham. and Schltdl. | Respiratory problems (asthma, chest pains, coughs, and tuberculosis) | Anthelmintic, anti-arthritic, antibacterial, antifungal, anti-inflammatory, antiviral, anti-mycobacterial, antinociceptive, contractile effects, antioxidant, anti-peptic ulcer, anti-scabies, antispasmodic, cytotoxicity, genotoxicity, and uterotonic activities. | Highly toxic LC50 value of 81.0 μg/ml |
Katerere and Parry (2000); Maroyi (2018g) |
| Apocynaceae | Carissa edulis (Forssk.) Vahl | Cough, chest pains, pneumonia and tuberculosis | Anti-plasmodial, antioxidant, diuretic, anti-inflammatoty, antimicrobial, anti-herpetic and antiviral activities. | Safe LD50 - 2154.1 mg/kg. Non-toxic LD50 > 2000 mg in rats |
Woode et al. (2007); Ibrahim et al. (2010, 2015); Osseni et al. (2016); Kaunda and Zhang (2017) |
| Apocynaceae | Carissa bispinosa (L.) Desf. ex Brenan | Cough | Analgesic, antioxidant, antimicrobial, anti-inflammatory, antiviral and diuretic activities. | no records found | Maroyi (2013a); Muleya et al. (2014a) |
| Apocynaceae | Diplorhynchus condylocarpon (Muell. Arg.) Pich. | Cough, pneumonia | Sympatholytic activity. | no records found | Moura et al. (2018b) |
| Apocynaceae | Holarrhena pubescens Wall. ex G. Don | Asthma | Analgesic, antibacterial, antiviral, antidiabetic, anti-amoebic, anti-inflammatory, antimalarial and antioxidant activities. | Safe 30% mortality rate was reported in 96 h after administration of doses 500, 1000 and 2000 mg/kg. |
Beuscher et al. (1994); Sinha et al. (2013); Singh (2018); Zahara et al. (2020) |
| Apocynaceae | *Nerium oleander L. | Pneumonia | Antinociceptive, anti-inflammatory, antiviral, antioxidant, anti-asthmatic, anticancer, hepatoprotective, antibacterial, diuretic, anti-diarrhoeal, antimicrobial, antileukemic, immunomodulatory, larvicidal, antiulcer, antibacterial, anti-diabetic and molluscicidal activities. | Highly toxic LC50 - 142 ± 68.2 μg/ml |
Garima and Amla (2010); Avci and Dik (2014); Hase et al. (2016); Munodawafa et al. (2016) |
| Apocynaceae |
Tabernaemontana elegans Stapf |
Lung ailments and tuberculosis | Antibacterial activity | no records found however studies revealed that it is relatively safe | Pallant et al. (2012); Dzoyem et al. (2016) |
| Asclepiadaceae | Ectadiopsis oblongifolia (Meisn.) Bullock | Pneumonia | no records found | no records found | |
| Asclepiadaceae | Gomphocarpus glaucophyllus Sclechter | Asthma | no records found | no records found however the leaves and tubers are sources of toxic cardiac glycosides. | Bester and Condy (2017) |
| Asparagaceae | Asparagus africanus Lam. | Pneumonia and tuberculosis. | Analgesic, anti-inflammatory and antimicrobial activities. | Safe LD50 > 5000 mg/kg in rats |
Hassan et al. (2008); Kebede et al. (2016) |
| Asphodelaceae | Aloe ferox Mill. | Tuberculosis | Anti-inflammatory, antimicrobial, analgesic, calming, antiseptic, antioxidant, germicidal, antiviral, anti-parasitic, anti-tumour and anticancer activities. | Safe LD50 - 3000 mg/kg |
Kambizi et al. (2005); Loots et al. (2007); Mahomoodally (2013); Añibarro-Ortega et al. (2019); Hęś et al. (2019); Sánchez et al. (2020) |
| Asphodelaceae | Aloe vera (L.) Burm.f. | Tuberculosis | Antiviral, antibacterial, laxative, protection against radiation, antioxidant, anticancer, anti-inflammation, antidiabetic, antiallergic, immunostimulation antiviral, antimicrobial, immunomodulatory and anti-tussive activities. | Safe LC50 – 3590 μg/ml. LD50 - 3000 mg/kg |
Hamidi et al. (2014); Sharma et al. (2014b); Al-Snafi (2015); Taukoorah and Mahomoodally (2016); Guo and Mei (2016); Hęś et al. (2019), Añibarro-Ortega et al. (2019); Sánchez et al. (2020) |
| Asphodelaceae | Aloe excelsa Berg. | Asthma | Anti-mycological, antibacterial, antifungal and antiseptic activities. | no records found however reported to be safe. | Coopoosamy and Magwa (2007); Coopoosamy (2010); Cock (2015) |
| Asphodelaceae | Aloe spp. | Cough | Antiviral, antibacterial, anti-inflammatory, immunomodulatory, antioxidant, antifungal, gastro-protective and hypoglycemic activities. | Safe LD50 - 2000 mg/kg |
Steenkamp and Stewart (2007); Mukherjee et al. (2013) |
| Asteraceae | Artemisia afra Jacq. Ex Willd. | Respiratory ailments | Antioxidant, antimicrobial, anti-HIV, anti-TB, anti-inflammatory and antimalarial activities. | Moderately toxic The LC50 - 206.97, 277.16, 406.48, and 669.30 μg/ml. |
Patil et al. (2011); Muleya et al. (2014b); Van de Venter et al. (2014); Adeogun et al. (2018) |
| Asteraceae | Aspilia pluriseta Schweinf. subsp. pluriseta | Dyspnoea (shortness of breath) | Antimicrobial, cytotoxicity, antiviral, antimalarial, healing of dermal excision wounds (mouse model) and skin sensitization activities. | no records found | Cos et al. (2002); Sebisubi et al. (2010); Kuria (2014); Kuria et al. (2015); Njeru and Muema (2020) |
| Asteraceae | Laggera crispata (Vahl) Hepper & J.R.I Wood | Pneumonia | Antibacterial and antifungal activities. | no records found | Kazembe and Nkomo (2012) |
| Asteraceae | Dicoma anomala Sond. | Colds, cough and pneumonia | Anthelmintic, anticancer, antimicrobial, anti-hyperglycemic, anti-inflammatory, antioxidant, anti-plasmodial and hepatoprotective activities | Safe LC50 value of 3040 ± 1060 μg/ml |
Becker et al. (2011); Munodawafa et al. (2016); Maroyi (2018f) |
| Asteraceae | Helichrysum caespititium (DC.) Harv | Cough and pulmonary tuberculosis | Antibacterial, anti-gonorrhoea, antioxidant, anti-mycobacterial, antifungal and cytotoxicity activities. | Safe non-toxic - 3.3% and 40.7% mortality was recorded in aqueous and organic extracts | Mamabolo et al. (2018); Maroyi (2019b) |
| Asteraceae | Helichrysum kraussii Schultz Bip. | Cough | Antibacterial activity. | no records found | Bougastos et al. (2003) |
| Asteraceae | Inula glomerata Oliv. & Hiern | Pneumonia | no records found | no records found | |
| Asteraceae | Lopholaena coriifolia (Sond.) Phillips & C.A.Sm. Lopholaena dehniae Merxm. | Cough, pneumonia | Potent antioxidant and anti-inflammatory activities. | no records found | Wijaya et al. (2012) |
| Asteraceae | Vernonia amygdalina Del. | Cough | Antimicrobial, antimalarial, antithrombotic, antioxidant, antipyretic, analgesic, anti-diabetic, laxative, immunomodulatory, hypoglycemic, anti-inflammatory, antiviral, antifertility, anticancer, antihelmintic, cathartic, antifungal and antibacterial activities | Safe LD50 - 5152.3 mg/kg LD50 - 3721 mg/kg. |
Momoh et al. (2012); Chan et al. (2016); Alara et al. (2017); Tijjani et al. (2017); Danladi et al. (2018); Inusa et al. (2018); Asante et al. (2019) |
| Bignoniaceae | Kigella africana (Lam.) Benth | Pneumonia | Anti-plasmodial, antiviral, anticancer, antiulcer, antidiarrheal, antimicrobial, analgesic, antimicrobial, antioxidant, anti-trypanosomal, wound healing and anti-inflammatory activities. | Moderately toxic LC50 value of less than 300 μg/ml. LD50 -785.65 ± 24 mg/kg |
Akah, (1996); Atawodi and Olowoniyi (2015); Bello et al. (2016); Viol et al. (2016) |
| Bignoniaceae | Stereospermum kunthianum Cham. | Cough | Antibacterial, anti-plasmodial, analgesic, anti-inflammatory, anti-diarrhoeal, anticonvulsant and antioxidant activities. | Safe LD50 up to 8000 mg/kg b. wt. |
Ching et al. (2008, 2009a, 2009b); Oloche et al. (2016) |
| Canellaceae | Warburgia salutaris (Bertol. f.) Chiov. | Colds, cough, influenza, sinus clearing, spots in the lungs and chest pains | Antimicrobial, antioxidant, cytotoxic, anti-inflammatory, phytotoxic, piscicidial and molluscicidal activities. | Moderately toxic LC50 - 351.41 ± 29.58 μg/ml and 359.66 ± 14.33 μg/ml. |
Viol (2013); Lawal et al. (2014); Maroyi (2012a), Viol et al. (2016); Soyingbe et al. (2018) |
| Cannabaceae |
Trema orientalis (L.) Blume |
Coughs, sore throats, asthma, bronchitis | Antimicrobial, anti-plasmodial, antioxidant, anti-inflammatory, diuretic, laxative, thrombolytic, anticancer and antidiabetic activities. | Highly toxic LC50 - 11.67 μg/ml and 48.62 μg/ml. |
Adinortey et al. (2013); Parvez et al. (2019) |
| Celastraceae | Elaedendron matabelicum | Chest complaints | Antimicrobial and antioxidant activities. | Safe LC50 - 1012.31 ± 217.69 μg/ml |
Viol (2013); Viol et al. (2016) |
| Celastraceae | Gymnosporia senegalensis (Lam.) Loes | Coughs, pneumonia and tuberculosis | Antioxidant, antiviral, antibacterial, and antifungal activities. | Safe LC50 value of 2185.61 ± 872 μg/ml LD50 > 1600 mg/kg in mice |
Viol (2013); Malebo et al. (2015); Viol et al. (2016); Makgatho et al. (2018) |
| Celastraceae | Sclerocarya birrea (A. Rich.) Hochst. subsp. caffra (Sond.) Kokwaro | Cough, pneumonia | Anti-diarrhoeal, anti-diabetic, anti-inflammatory, antimicrobial, antiviral, anti-plasmodial, antihypertensive, antioxidant,.anticonvulsant and antinociceptive activities. | Safe LC50 - 1112.37 ± 210.04 μg/ml. |
Ojewole et al. (2010); Viol (2013); Russo et al. (2013); Viol et al. (2016) |
| Chenopodiaceae | *Chenopodium ambrosiodes L. | Chest pains | Antimicrobial, anti-inflammatory, anti-aflatoxigenic, antioxidant, analgesic, antiasthmatic, carminative, stomachic and vermifuge activities. | Safe Acute toxicity, rats were administered 300, 1000 or 3000 mg/kg. |
Kumar et al. (2007); Kokanova-Nedialkova et al. (2009); Sousa et al. (2012); da Silva et al. (2014); Degenhardt et al. (2016) |
| Chrysobalanaceae | Parinari curatellifolia Benth. | Tuberculosis | Antioxidant, antibacterial and anti-diabetic activities. | Safe LC50 > 1000 μg/ml. |
Chirisa and Mukanganyama (2016); Mbunde et al. (2017) |
| Clusiaceae | Garcinia huillensis Welw | Treatment of cough, pneumonia and tuberculosis | Chemotherapeutical, antibacterial, anti-mycobacteria, antifungal, antiviral, and anti-trypanosomal activities. | no records found | Bakana et al. (1987); Magadula and Mbwambo (2014) |
| Clusiaceae | Psorospermum febrifugum Spach | Dyspnoea (shortness of breath), pneumonia | Antibacterial, antiprotozoal, anti-acne, antifungal, antiviral, anticancer, anti-psoriatic, immunomodulatory antioxidant, and neuroprotective activities. | Safe LD50 > 2000 mg/kg body weight. |
Epifano et al. (2013); Elufioye et al. (2016); AbdAbomey-Calavi et al. (2019); Asogwa et al. (2020) |
| Combretaceae | Combretum apiculatum Sond. | Cough | Antibacterial, anticancer, antimicrobial antioxidant, antiviral, anti-inflammatory, anthelmintic, anti-schistosomal, antifungal and anti-inflammatory activities. | no records found | McGaw et al. (2001); Aderogba et al. (2012); de Morais Lima et al. (2012); Mangoyi et al. (2012); de Dieu Tamokou et al. (2013); Epifano et al. (2013) |
| Combretaceae |
Combretum platypetalum subsp. oatesii (Rolfe) Exell Combretum oatesii Rolfe |
Pneumonia | Cytotoxic, anti-tuberculosis, anticancer, antibacterial, antioxidant, antifungal, anti-proliferative and anti-inflammatory activities. | no records found | Magwenzi et al. (2014); Chiramba and Mukanganyama (2016),Chirisa and Mukanganyama (2016); Wende et al. (2021) |
| Combretaceae | Combretum zeyheri Sond. | Cough | Antibacterial, anti-inflammatory, cytotoxicity against human cancer cell line, antioxidant, antifungal and anti-proliferative activities. | Highly toxic LC50 - 16 μg/ml to 159 μg/ml |
de Morais Lima et al. (2012); Mapfunde et al. (2016); Moura et al. (2018a) |
| Combretaceae |
Terminalia sericea DC. Terminalia velutina sensu Eyles |
Sore throat | Antibacterial, antifungal, anti-HIV, anti-fungal, antibacterial, anticancer, lipolytic, wound healing, anti-parasitic, anti-inflammatory, antioxidant and antiviral activities. | Highly toxic LC50 < 300 μg/ml. LC50 ranging from 5.4 (3.5–8.4) to 17.4 (11.4–26.5) μg/ml, |
Moshi and Mbwambo (2005); Mongalo et al. (2016); Viol et al. (2016) |
| Convolvulaceae | Astripomoea malvacea (Klotzsch) Meesu | Cough | no records found | no records found | |
| Crassulaceae | Kalanchoe spp. | Pneumonia | Antimicrobial, anti-inflammatory, anti-allergic, antioxidant, antihistamine, antimalarial and immunomodulatory activities. | Weak or low toxicity LD50 - 1925 mg/kg |
Costa et al. (2008) |
| Cucurbitaeae | *Cucurbita pepo L. | Pneumonia | Anti-hypercholesterolemia, antioxidant, anti-hypertensive, anti-inflammatory, anti- parasitic, anti-tumor, antiviral, anti-diabetic, anti-carcinogenic, antimicrobial, anti-bacterial, intestinal and anti-inflammatory activities.. | Safe LD50 > 5000 mg/kg |
Sener et al. (2007); Badr et al. (2011); Malgwi et al. (2014); Adnan et al. (2017) |
| Cyperaceae | Coleochloa setifera (Ridl.) Gilly | Pneumonia | no records found | no records found however studies show that its relatively safe. | Dzoyem et al. (2016); Maroyi (2013a) |
| Ebenaceae | Diospyros lycioides Desf. | Pneumonia and sore throat | Antibacterial, anti-inflammatory, anti-metastatic, antioxidant, anti-adhesive, antifungal and anti-proliferative activities. | no records found | Cai et al. (2000); Maroyi (2013a, 2018a) |
| Ebenaceae |
Euclea crispa (Thunb.) Sond. ex Gürke |
Cough | Antibacterial, antioxidant, amyloid β-peptide lowering effects, antifungal, and cell membrane disruption activities. | no records found | (Magama et al. (2003); Pretorius et al. (2003); Maroyi (2017d, 2018h) |
| Ebenaceae | Euclea natalensis A.DC. | Asthma, bronchitis, tuberculosis | Antibacterial, anti-diabetic, antifungal, anti-mycobacterial, antiviral, antioxidant, anti-plasmodial, dentin permeability and hepatoprotective activities. | Highly toxic LC50 value of 19.33 μg/mL |
Maroyi (2017d) |
| Euphorbiaceae | *Ricinus communis L. | Pneumonia | Antioxidant, immunomodulatory, lipolytic, antimicrobial, anti-asthmatic, diuretic, antiinfertility, laxative, hepatoprotective, antiviral and anti-inflammatory activities. Major phytochemical with include saponins and flavonoids that have been isolated from the plant have bronchodilatory, mast cell stabilizing and smooth muscle relaxation activities. |
Safe LD50 - 2000 mg/kg |
Burgess et al. (1988); Ilavarasan et al. (2011); Ahmad et al. (2016); Kumar (2017), El-Toumy et al. (2018) |
| Euphorbiaceae | Acalypha petiolaris Hoschst. | Asthma | no records found | no records found | |
| Euphorbiaceae | Antidesma membranaceum Muell. Arg. | Cough | Anti-mycobacterial and antibacterial activity. | Highly toxic LC50 - 36.13 μg/ml |
Magadula et al. (2012); Gitu (2013) |
| Euphorbiaceae | Bridellia mollis Hutch. | Cough | Antioxidant, antimicrobial, antiviral, immunomodulatory and anti-inflammatory activities. | Highly toxic LC50 values of 51.4 μg/mL | Maroyi (2019d) |
| Euphorbiaceae | Croton gratissimus Burch | Respiratory disorders | Good antioxidant, anti-inflammatory, antimicrobial and antiviral activities. | no records found | Grace et al. (2003); Mulholland et al. (2010); Njoya et al. (2018) |
| Euphorbiaceae | Euphorbia ingens E. Mey. ex Boiss. | Bronchitis and asthma | no records found | no records found | Grace et al. (2003) |
| Euphorbiaceae | Euphorbia matabelensis Zahlbr. | Cough and respiratory infections | Antiproliferative (on C33a, HeLa, MCF-7, and MDA-MB-231 cell lines) and GIRK channel blocking activities. | no records found | Hammadi et al. (2019) |
| Euphorbiaceae | Flueggea virosa (Roxb. ex Willd.) Voigt | Pneumonia, respiratory tract infections, bronchitis, cough and pneumonia. | Analgesic, anti-inflammatory, aphrodisiac, sedative, anti-arrhythmic, anti-diabetic, anti-malarial, anti-HIV, anti-hepatitis C, anti-diarrheal, cytotoxic, antimicrobial, antifungal, antioxidant, laxative, cytotoxic, antispasmodic, antibacterial, antidiabetic, CNS behavioural effects, analgesic, anti-ulcerogenic, anticancer, anticonvulsant, sedative, antisickling, antivenin, anti-depressant, anti-trypanosomal, FSH and testosterone augmentative effect on LH activities. | Safe LD50 > 10000 mg/kg in rats. LD50 range of 20000–50000 mg/kg body weight |
Tatematsu et al. (1991); Moshi et al. (2000); Magaji et al. (2007, 2008a, b); Tanko et al. (2008); Sanogo et al. (2009); Yerima et al. (2009); Aiyelero et al. (2012); Garba et al. (2013, 2015); Abere et al. (2014); Sempombe et al. (2014); Zhang et al. (2015); Shehu et al. (2017); Ahmad et al. (2018); Ajaib and Wahla (2018); Renu et al. (2018); Misonge et al. (2019); Salawu et al. (2019) |
| Euphorbiaceae | Margaritaria discoidea (Baill.) Webster var. nitida (Pax) Radcliffe-Smith | Pneumonia | Anti-inflammatory, antibacterial, antifungal, analgesic, cytotoxicity, gastroprotective and antioxidant activities. | Safe LD50 > 3200 mg/kg |
Adedapo et al. (2009); Dickson et al. (2010); Diallo et al. (2015); Sofidiya et al. (2015) |
| Euphorbiaceae | Monadenium lugardiae N.E.Br. Euphorbia lugardiae (N.E. Br.) Bruyns | Chest pains | no records found | no records found | El Badwi and Bakhiet (2012) |
| Fabaceae | Acacia rehmanniana (Schinz) Kyal. & Boatwr. | Pneumonia | no records found | no records found | |
| Fabaceae | Aeschynomene mimosifolia Vatke | Chest pains | no records found | no records found | |
| Fabaceae | Albizia amara (Roxb.) Biov. subsp. sericocephala (Benth.) Brenan | Pneumonia, TB | Antibacterial, antifungal, antioxidant, antiviral, antihyperlipidimic, astringent, antiarthritic, antihyperlipidemic, anti-inflammatory anticancer, analgelsic and hepatoprotective activities. | Safe LD50 - 2000 mg/kg |
Indravathi et al. (2016); Nivetha et al. (2017) |
| Fabaceae | Albizia antunesiana Harms | Pneumonia | Anthelmintic and antioxidant activities. | no records found | Maroyi (2013a); Chipiti et al. (2015) |
| Fabaceae |
Albizia tanganyicensis Bak.f. Albizia lebbeck (L.) Benth. var. australis Burtt Davy Albizia rhodesica Burtt Davy |
Cough | Anti-inflammatory, antioxidant, antimicrobial, nootropic antidiarrheal, anti-helminthic, anti-asthmatic, antipyretic, anti-anaphylactic, ulcer healing, anticonvulsant, anti-allergic and wound healing activities. | Safe LD50 > 5000 mg/kg |
Resmi et al. (2006); Meshram et al. (2016); Ali et al. (2018); Jahangir (2018) |
| Fabaceae | Bauhinia fassoglensis Schweinf. | Pneumonia | Antifungal, antibacterial, antioxidant, anti-plasmodial and cytotoxicity activity | Highly toxic LC50 above 100 μg/ml |
Adongo et al. (2012); Owuor et al. (2012); Ochanga and Chacha (2016a, 2016b); Dzoyem et al. (2016); Ochanga and Kilonzo (2018) |
| Fabaceae | Bauhinia petersiana Bolle | Cough | no records found | no records found however it has been reported to be relatively safe | Dzoyem et al. (2016) |
| Fabaceae | Burkea africana Hook. | Pneumonia | Antioxidant, anti-diarrhoeal, antibacterial, analgesic, anti-inflammatory, antiviral and anticholinesterase activities. | Safe LD50 > 5000 mg/kg non-toxic |
Mair et al. (2018); Moura et al. (2018b); Namadina et al. (2020) |
| Fabaceae |
Cassia abbreviata Oliv. |
Cough and Pneumonia | Antiviral, antioxidant, abortifacient, anti-diabetic, anti-inflammatory, hepatoprotective and antimicrobial activities. | Weak or low toxicity LC50 values of 454.93 ± 18.60 μg/ml LC50 values of 445.72 ± 22.15 μg/m. LC50 values of 1319.37 ± 356.63 μg/ml |
Parry and Matambo (1992); Okeleye et al. (2013); Mongalo and Mafoko (2013); Viol et al. (2016); Sobeh et al. (2018) |
| Fabaceae | Crotalaria laburnifolia L. | Cough | Antimicrobial, analgesic and anthelmintic activities. | no records found | Shankar et al. (2008) |
| Fabaceae |
Dalbergia melanoxylon Guill. & Perr. |
Bronchitis, asthma, cough and inflammation in throat | Antimicrobial, antiviral, anti-diarrhoeal, antioxidant, analgesic, antipyretic and anti-inflammatory activities. | Highly toxic LC50 – 6.8 μg/ml |
Maroyi (2013a); Kiondo et al. (2014); Matata et al. (2018); Najeeb et al. (2018) |
| Fabaceae | Dichrostachys cinereal (L.) Wight & Arn. | Pneumonia | Antioxidant, antiviral, antiashmatic, anti-inflammatory, anticonvulsant, analgesic and antimicrobial activities. Bronchoconstriction and bronchodilation activity in the smooth muscle airway. |
Safe LC50 value of 4304.59 ± 685.69 μg/ml. |
Aworet-Samseny et al. (2011); Viol (2013); Viol et al. (2016); Irie-N'guessan et al. (2017, 2018) |
| Fabaceae | Erythrina abyssinica DC | Cough | Anti-mycobacterial, antidiarrheal, anti-HIV 1, anti-diabetic, anti-inflammatory, hepatoprotective, anti-plasmodial, antibacterial, antioxidant, anti-proliferative, antifungal, anti-obesity and antimicrobial activities. | Safe LC50 - 5440 μg/ml. |
Bunalema et al. (2011); Nkeh-Chungag et al. (2013); Nasimolo et al. (2014); Munodawafa et al. (2016); Chitopo et al. (2019); Macharia et al. (2019) |
| Fabaceae | Indigofera spp. | Chest pains, cough | Antimicrobial, insecticidal, phytotoxic, antiulcergenic, hepatotoxic, teratogenic, cytotoxicity, lipoxygenase and gastrointestinal activities. | no records found however reported to be safe/non-toxic | Rahman et al. (2018) |
| Fabaceae | *Leucaena leucocephala (Lam.) De Wit | Colds, flu and tuberculosis | Antimicrobial, diuretic, antiviral, cytotoxicity, antioxidant and anti-inflammatory activities. | no records found however some studies have reported it to be relatively safe. | Ono et al. (2003); Zarin et al. (2016); Dzoyem et al. (2016); Deivasigamani (2018); Zayed et al. (2018) |
| Fabaceae | Peltophorum africanum Sond. | Flu | Antibacterial, antifungal, antiviral, anti-inflammatory, antioxidant and anthelmintic activities. | Weak or low toxicity LC50 - leaves 913 ± 7.32 μg/ml and 1060 ± 106 μg/ml |
Mongalo (2013); Munodawafa et al. (2016); Adebayo et al. (2017) |
| Fabaceae | Pericopsis angolensis (Bak.) van Meeuwen | Cough, Asthma, TB and dyspnoea (shortness of breath). | Antimicrobial activity. | no records found | Constance et al. (2019) |
| Fabaceae | Piliostigma thonningii (Schumach.) Milne-Redh | Cough | Antioxidant, antiviral, antimalarial, antipyretic, anti-leishmanial, analgesic, antibacterial, anxiolytic, anti-pyrectic, nootropic, anthelminthic, cognitive-enhancing, anti-inflammatory, hypocholesterolemic, hematopoietic, immunomodulatory, anti-lipidaemic, anti-cholesterolaemic and hypoglycemic activities. | Safe LD50 > 5000 mg/kg in rats. |
Koné et al. (2007); Madara et al. (2010); Ighodaro and Omole (2012); Adamu et al. (2013); Afolayan et al. (2018); Alagbe et al. (2019); Moriasi et al. (2020); Olela et al. (2020) |
| Fabaceae | Pterocarpus angolensis Sond. | Chest pains | Antioxidant, antibacterial, antifungal, anti-tumor, antiviral and anti-inflammatory activities. | no records found | Beuscher et al. (1994); Okeleye et al. (2013); Mazimba (2014); Sigidi et al. (2016); Chipinga (2018) |
| Fabaceae | Vigna unguiculata (L.) Walp. | Chest pains | Anthelmintic, antibacterial, antioxidant, anti-nociceptive, antimicrobial, anti-diabetic, antiviral, anti-inflammatory, hypocholesterolemic, antifungal, anti-sickling and thrombolytic activities. | Safe LD50 > 2000 mg/kg body weight |
Nwoke et al. (2008); GV et al. (2017); Sayeed et al. (2017); Hus et al. (2019); Zaheer et al. (2020) |
| Fabaceae | Xeroderris stuhlmanni (Taub.) Mendonca & E.P Sousa. | Chest pains | Anti-proliferative, antimicrobial and anti-mycobacterial activities. | no records found | Mukanganyama et al. (2012); Mangoyi et al. (2014); Selemani et al. (2020) |
| Flacourtiaceae | Flacourtia indica (Burm.f.) Merr. | Cough, chest pains and pneumonia | Antimicrobial, antioxidant, pro-apoptotic, anti-inflammatory, anti-proliferative and antiviral activities. | Moderately toxic LC50 - 467.31 ± 39.01 μg/ml |
Viol (2013); Park et al. (2014); Taderera et al. (2015); Hussain et al. (2016); Viol et al. (2016); Perera et al. (2018) |
| Heteropyxidaceae | Heteropyxis natalensis Harv | Colds and respiratory disorders | Anti-inflammatory, antiviral, pro-inflammatory, antioxidant, antifungal, anti-mycobacterial and antibacterial activities. | Safe LD50 > 5000 mg/kg in rats |
Hurinanthan (2013); Henley-Smith et al. (2018); Maroyi (2019e) |
| Hyacinthaceae | Urginea sanguinea (Schinz) Jessop | Pneumonia | No antibacterial and antioxidant activity. | Safe 1000 mg in 10 ml of solvent showed no mortality in shrimps. |
Naidoo et al. (2004) |
| Kirkiaceae | Kirkia acuminata Oliv. | Cough | Antibacterial, antiviral, anti-inflammatory, anti-mycobacterial, antioxidant and anti-plasmodial activities. | no records found | Recio et al. (1995); Maroyi (2016, 2017a) |
| Lamiaceae | *Mentha longifolia (L.) Huds | Colds and cough | Anti-oxidative, anti-microbial, anti-inflammatory, hepatoprotective, antiviral, antispasmodic and antibacterial activities. | Safe LD50 - 3200 mg/kg in rats |
Mikaili et al. (2013); Dawang (2015); Farzaei et al. (2017); Sevindik (2018) |
| Lamiaceae | *Mentha spicata L. | Cough and flu | Strong antioxidant, anti-inflammatory, antimicrobial, antiviral, carminative, antispasmodic, antiradical, chelating and diuretic activities. | Weak or low toxicity LD50 > 1000 mg/kg |
Kee et al. (2017); Caro et al. (2018); Sevindik (2018) |
| Lamiaceae | Ocimum obovatum (Benth) N.E.Br | Chest pains | Antibacterial, antifungal, antioxidant and radio-protective activities. | no records found | Naidoo et al. (2016) |
| Lamiaceae | Leucas milanjiana Gurke | Pneumonia | no records found | no records found | |
| Lamiaceae | Tetradenia riparia (Hochst.) Codd | Cough, colds, bronchitis, respiratory ailments | Antiviral, antifungal, anti-inflammatory, wound healing, antileishmanial, antioxidant and antibacterial activities. | Weak or low toxicity The general toxicity with dose 1000 mg/kg. |
Cardoso et al. (2015); Njau and Ndakidemi (2017); Ghuman et al. (2019) |
| Lamiaceae | Vitex payos (Lour.) Merr. | Cough, colds, respiratory ailments | no records found | no records found however reported to be safe. | Tufts et al. (2015) |
| Loganiaceae | Strychnos potatorum L.f. | Cough | Hypotensive activity, anticonvulsant, anti-inflammatory, antidiarrheal, anti-HIV, antinociceptive, antipyretic, antioxidant, antiprotozoal, antimicrobial and antimalarial activities. | Safe LD50 - 2000 mg/kg body weight orally in mice. |
Yadav et al. (2014); Palshetkar et al. (2020) |
| Loganiaceae | Strychnos spinosa Lam. | Pneumonia, bronchitis and chest problems. Caffeine in leaves can help breathing in premature babies. | Antimicrobial, anti-inflammatory, antiviral, antioxidant, anti-allergic, hepatoprotective, antithrombotic and anti-carcinogenic activities. | Safe LD50 > 5000 mg/kg. |
Isa et al. (2014); Sadau and Eloff et al. (2014) |
| Loranthaceae | Loranthus spp on Brachystegia spiciformis Benth | Asthma | no records found | no records found | |
| Loranthaceae | Loranthus spp. on Berchemia discolor (Klotzsch) Hemsl. | Pneumonia | no records found | no records found | |
| Loranthaceae | Loranthus spp. on Cordyla africana Lour | Pneumonia | no records found | no records found | |
| Malvaceae | *Abelmoschus esculentus Moench | Bronchitis and tuberculosis | Antioxidant, anti-inflammatory, laxative, anti-hyperlipidemic, antifungal, organ protective, analgesic, antioxidant, anti-diabetic, antimicrobial, immunomodulatory, antibacterial, anticancer and neuropharmacological activities. | Safe LD50 > 2000 mg/kg |
Doreddula et al. (2014); Chen et al. (2016); Petropoulos et al. (2017); Islam (2019) |
| Malvaceae | Azanza garckeana (F. Hoffm.) Exell & Hillc. | Chest pains and cough | Antibacterial, antifungal, antimalarial, anti-hyperglycemic, antimicrobial, analgesic, wound healing, anti-inflammatory, anti-arthritic, antioxidant and ion absorption activities. | Highly toxic LC50 - 3.98 μg/ml acetone extract, 47.66 μg/ml methanol, 100 μg/ml ethyl acetate and 138 μg/ml water. |
Dikko et al. (2016); Mshelia et al. (2016); Maroyi (2017e); Chowdhury et al. (2019); Yusuf et al. (2020) |
| Malvaceae | Sida acuta Burm. f. | Chest pains | Antioxidant, antimicrobial, antibacterial, antimalarial, cardiovascular, antiulcer, antiviral, analgesic and anti-inflammatory, anticancer, antipyretic, hepatoprotective, hypoglycemic and insecticidal activities. | Safe LD50 - 2000 mg/kg body weight. |
Hudson et al. (2000); Ekpo and Etim (2009); Tcheghebe et al. (2017); Singh and Navneet (2018) |
| Meliaceae | Ekebergia benguelensis C.DC. | Pneumonia | Antioxidant, cytotoxic and antimalarial activities. | no records found | Chávez et al. (2001); Kinghorn et al. (2003); Chiribagula et al. (2020) |
| Meliaceae | Khaya anthotheca (Welw.) C.DC. | Pneumonia and colds | Antimicrobial, anticancer, antioxidant and antiviral activities. | Moderately toxic LC50 - 482.19 ± 43.49 μg/ml. |
Viol (2013); Viol et al. (2016) |
| Meliaceae | Turraea nilotica Kotschy & Peyr. | Dyspnoea (shortness of breath), pneumonia and respiratory disorders | Anti-plasmodial activity. | Weak or low toxicity LC50 - 701 ± 25.650 μg/ml. |
Munodawafa et al. (2016) |
| Moraceae | Ficus ingens (Miq.) Miq. | Cough | Immunoprotective and antioxidant. | Safe LD50 > 4000 mg/kg in rats |
Abd El Raheim et al. (2013) |
| Moraceae | Ficus sycomorus L. | Tuberculosis, cold and other chest problems | Antiviral, analgesic, antimicrobial, anti-inflammatory, antioxidant, antitussive and immunomodulatory activities. | Weak or low toxicity LD50 - 1500 mg/kg |
Bello et al. (2015); Ramdé-Tiendrébéogo et al. (2015); El-Beltagi et al. (2019a,b); Hossain (2019) |
| Moraceae | Ficus thonningii Blume | Respiratory infections, bronchitis, treating influenza, sore throat, colds, pneumonia and chest pains. | Anti-inflammatory, antimicrobial and antioxidant activities. | Safe LD50 > 5000 mg/kg |
Coker et al. (2009); Dangarembizi et al. (2013); Badiora et al. (2016); Isyaku et al. (2016); Coker and Oaikhena (2020) |
| Myrothamnaceae | Myrothamnus flabellifolius Welw. | Colds and chest complaints | Anti-inflammatory, antioxidant, analgesic, antimicrobial and antiviral activities. | Highly toxic LC50 at 136 μg/ml, |
Viljoen et al. (2002); Bussmann et al. (2011); Bhebhe et al. (2015); Erhabor et al. (2020) |
| Myrsinaceae | Rapanea melanophloeos (L.) Mez | Respiratory problems | Expectorant, emetic, antioxidant, anti-diabetic, astringent, anti-inflammatory, analgesic, anthelmintic, fungicidal, anti-coagulation, antiviral, antifungal and anti-mycobacterial activities. | Weak or low toxicity LD50 > 1000 mg/kg body weight, |
Ohtani et al. (1993); Amenya et al. (2016); Mehrbod et al. (2018); Lotter et al. (2019) |
| Myrtaceae | *Eucalyptus camaldulensis Dehnh | Sore throat, flu, asthma, cough | Anti-inflammatory, antimicrobial, antiviral, antioxidant, antidiarrheal and analgesic activities. | Safe LD50 ≥ 5000 mg/kg in rats. |
Adeniyi et al. (2015); Abu-Jafar and Huleihel (2017); Upreti et al. (2018) |
| Myrtaceae | *Psidium guajava L. | Cough and flu | Antimicrobial, anti-inflammatory, antiviral, analgesic, antioxidant and antitussive activities. | Safe LD50 ≥ 5000 mg/kg in rats |
Chen and Yen (2007); Daswani et al. (2017); Kafle et al. (2018) |
| Myrtaceae | Syzigium guineense (Willd.) DC. | Tuberculosis and chest complaints | Anti-inflammatory, antiviral, antibacterial and antioxidant activities. | Safe LD50 > 5000 mg/kg in rats. |
Tsakala et al. (1996); Moll et al. (2013); Okwuofu et al. (2017); Oladosu et al. (2017); Ezenyi and Igoli (2018) |
| Myrtaceae |
Syzygium cordatum Hochst. ex C. Krauss |
Cold and fever | Antibacterial, anti-plasmodial, antifungal, antidiarrheal, anti-sexually transmitted infections, anti-diabetic, antioxidant, anti-inflammatory, anti-leishmanial, anti-proteus and anticholinesterase activities. | Safe LD50 > 4000 mg/kg in mice |
Cordier et al. (2013); Maroyi (2014, 2018c) |
| Nymphaeaceae | Nymphaea nouchali Burm. f. | Asthma | Anti-inflammatory, anti-spasmodic, anti-cancer, antimicrobial, anti-analgesic, antioxidant and anti-diuretic activities. | Moderately toxic LD50 - 681 mg/kg in albino mice. |
Raja et al. (2010); Parimala and Shoba (2014); Prasad and Savithramma (2016) |
| Phyllanthaceae | Bridelia micranatha (Hochst.) Baill. | Cough | Anthelmintic, antimicrobial, anticonvulsant, sedative, anti-diabetic, anti-diarrhoeal, anti-nociceptive, antioxidant, insecticidal, anti-plasmodial, anti-schistosomal, anti-inflammatory, hepatoprotective and β-lactamase inhibitory activities. | Highly toxic LC50 of 77 μg/mL |
Nwaehujor Chinaka et al. (2014); Maroyi (2017f) |
| Pittosporaceae | Pittosporum viridiflorum Sims. | Chest pains | Antimicrobial, anti-diarrhoeal, antimalarial, anticancer, anti-inflammatory, antiviral, antioxidant and acaricidal activities. | Safe LC50 values - 700 μg/ml and 2440 μg/ml. |
Mehrbod et al. (2018); Madikizela and McGaw (2019) |
| Plumbaginaceae | Plumago zeylanica L. | Dyspnoea (shortness of breath) | Anti-inflammatory, anti-malarial, antiviral, antifertility, antimicrobial, antioxidant, blood coagulation, wound healing, memory enhancer and anti-cancer activities. | Toxic LD50 – 65 mg/kg |
Parker et al. (2007); Mandavkar and Jalalpure (2011); Ganesan and Gani (2013); Jain et al. (2014); Sharma and Kaushik (2014); Singh et al. (2017); Jain et al. (2020) |
| Poaceae | Phragmites mauritianus Kunth | Pneumonia | no records found | no records found | |
| Polygalaceae | Securidaca longipedunculata Fresen. | Tuberculosis and pneumonia | Antiviral, antifungal, antimicrobial, antioxidant, anti-inflammatory and antibacterial activities. | Moderately toxic LC 50–351.89 ± 35.79 μg/ml, giving readings close to 300 μg/ml. |
Muanda et al. (2010); Viol (2013); Sanusi et al. (2015); Viol et al. (2016); Nguta (2019) |
| Polygonaceae |
Oxygonum sinuatum (Hochst. & Steud. Ex Meisn.) Dammer |
Cough | no records found | no records found | |
| Proteaceae | Faurea saligna Harv. | Pneumonia | Antifungal activity. | no records found | Mangoyi and Mukanganyama (2011) |
| Pteridaceae | Pellaea spp. | Chest pains | Antioxidant and anti-inflammatory activities. | no records found | Baskaran et al. (2018) |
| Ranunculaceae | Clematis villosa DC. | Cough and colds | no records found | no records found | |
| Rhamnaceae |
Rhamnus prinoides L'Hérit |
Pneumonia, cold and respiratory infections | Antimalarial, antioxidant, antiviral, antimicrobial, and anti-inflammatory activities. | Highly toxic Chloroform - LC50 - 133.33 μg/ml and methanol/water extract - LC50 - 214.33 μg/ml. |
Parker et al. (2007); Amabye (2015); Molla et al. (2016); Kamanja et al. (2018); Chen et al. (2020) |
| Rhamnaceae | Ziziphus mucronata Willd. | Chest complaints, Cough | Antimicrobial, antiviral, anti-diabetic, anti-inflammatory, antioxidant, anti-plasmodial, anthelmintic, and anti-anaemic activities. | Safe Leaf extracts LC50 of 4560 ± 1540 μg/ml and roots 1180 ± 144 μg/ml. |
Munodawafa et al. (2016); Adebayo and Masoko (2019); Mongalo et al. (2020) |
| Rubiaceae | Agathisanthemum bojeri Klotzsch | Asthma, chest conditions, relieve cough and diffulty in breathing | no records found | no records found | |
| Rubiaceae | Catunaregam taylorii S.Moore) Bridson | Respiratory ailments and pulmonary infections | Anti-allergic, anti-inflammatory, analgesic, immunomodulatory activity, antibacterial, antioxidant, emetic, antipyretic, human cyclooxygenase (COX)-2 inhibitory effects and a prominent protection of DNA activities. | Safe LD50 up to 2000 mg/kg. |
Patil and Khan et al. (2017); Moura et al. (2018b); Saini et al. (2019) |
| Rubiaceae | Fadogia ancylantha Schweinf. | Cough, pneumonia | Antidiabetic, anti-oxidant and antimicrobial activities. | no records found | Nyirenda et al. (2012) |
| Rubiaceae | Gardenia resiniflua Hiern subsp. resiniflua | Pneumonia, asthma | no records found | no records found | |
| Rubiaceae | Gardenia ternifolia Schuamch. & Thonn. subsp. jovis-tonantis (Welw.) Verdc. var goetzei (Stapf & Hutch) Verdc. | Pneumonia, asthma | Antimalarial, antioxidant, anticancer and antimicrobial activity. | Safe LD50 > 2000 mg/kg |
Agbodjento et al. (2018); Nureye et al. (2018) |
| Rubiaceae | Gardenia volkensii K. Schum. subsp. spatulifolia (Stapf and Hutch.) Verdc. | Pneumonia, asthma | no records found | no records found | |
| Rubiaceae | Mussaenda arcuata Poir. | Influenza | no records found | no records found | |
| Rubiaceae | Pavetta schumanniana F. Hoffm. | Chest pains, cough, pneumonia | Cytotoxicity and anti-mycobacterial activity. | no records found | Aro et al. (2015) |
| Rubiaceae | Spermacoce dibrachiata Oliv. | Pneumonia, cough | no records found | no records found | |
| Rubiaceae | Vangueria infausta Burch. subsp. infausta | Pneumonia | Antibacterial, antioxidant, antileishmanial, antimycobacterial, antifungal, anti-TB, anti-inflammatory, and anti-plasmodial activities. | Moderately toxic LC50 values of 338 ± 23.4 μg/mL (leaf) and 416 ± 28.3 μg/mL (root). |
Munodawafa et al. (2016); Maroyi (2018e) |
| Rubiaceae | Vangueriopsis lanciflora (Hiern) Robyns | Cough | no records found | no records found | |
| Rubiaceae | Xeromphis obovata (Hochst.) Keay | Cough, pneumonia | no records found | no records found | |
| Rutaceae | *Citrus limon (L.) Burm. f. | Throat infections, cough and flu | Antiviral, anti-inflammatory, antioxidant, analgesic, antimicrobial and antitussive activities. | Safe LD50 ≥ 5000 mg/kg in rats. |
Otang and Afolayan (2016); Makni et al. (2018); Klimek-Szczykutowicz et al. (2020) |
| Rutaceae | Zanthoxylum humile (E.A. Bruce) P.G. Waterman | Chest pains and flu | Antibacterial and anti-inflammatory activities. | no records found | Pamhidzai and Isaac (2013) |
| Sapindaceae | Zanha africana (Radlk) Excell. | Respiratory problems (asthma, chest pains, colds, cough, flu, pneumonia and tuberculosis) | Antibacterial, antifungal, antiviral, antidiabetic, anti-inflammatory, insecticidal, anti-trypanosomal and cytotoxicity activities. | Highly toxic LC50 values ranging from 41.1 μg/mL and 240.0 μg/mL |
Maroyi (2019g) |
| Solanaceae | *Capsicum spp. | Respiratory infections in chickens. | Antimicrobial, immuno-modulatory, anti-inflammatory, antiviral, cytotoxicity, antioxidant, insecticidal and anthelmintic activities. | Highly toxic LD50 values of Capsaicin ranging 97.4–118.8 mg/kg in mice, and 148.1 mg/kg and 161.2 mg/kg in rats. |
Al-Snafi (2015); Ordaz-Trinidad et al. (2018) |
| Solanaceae | *Nicotiana tabacum L. | Asthma, respiratory problems | Antimicrobial, antispasmodic, antioxidant, emetic, purgative, sedative, analgesic, insecticidal, anti-inflammatory, antiviral, anti-rheumatic and anthelmintic activities. | Safe LD50 > 2000 mg/kg |
Iqbal et al. (2006); Shang et al. (2016); Batoro and Ekowati (2017); Ameya et al. (2017); Khaleel (2019); Popova et al. (2019); Sulaiman et al. (2020) |
| Solanaceae | *Datura stramonium L | Asthma and cough | Anti-inflammatory, antimicrobial, antiviral, analgesic, antioxidant and anti-asthmatic activities. Patients exhibiting mild airway obstruction are treated with isolate atropine from the plant due to its bronchodilatory activity. |
Safe LC50 - 12860 μg/ml |
Gaire and Subedi (2013); Sayyed and Shah (2014, Sharma et al. (2014a) |
| Solanaceae | *Solanum incanum L. | Pneumonia | Antinociceptive effect, antipyretic, antioxidant, anti-inflammatory, antimicrobial, analgesic and anti-cytotoxic activities. | Safe LD50 > 2000 mg/kg body weight. |
Indhumathi et al. (2014); Mwonjoria et al. (2014); Dakone and Guadie (2016); Anwar (2018) |
| Thymelaeaceae | Gnidia capitata L.f | Asthma | no records found | no records found | |
| Thymelaeaceae | Gnidia kraussiana Meisn. | Cough | Antibacterial, antifungal and molluscicidal activities. | no records found | El Kheir and El Tohami (1979); Ndhlala et al. (2013) |
| Tiliaceae | Triumfetta welwitschii Mast. | Asthma | Antibacterial activities. | no records found | Moyo and Mukanganyama (2015) |
| Ulmaceae | Chaetachme aristata Planch | Tuberculosis | no records found | no records found | |
| Verbenaceae | Clerodendrum eriophyllum Gürke | Cough and colds | Antimicrobial, antioxidant, anti-inflammatory, antihypertensive and immunostimulatory activities. | no records found | Machumi et al. (2010); Fouad et al. (2013); Ogundajo et al. (2019) |
| Verbenaceae | Lippia javanica (Burm.f.) Spreng. | Cold, cough, shortness of breath (dyspnoea), respiratory complaints, bronchial problems, fever, asthma, chronic coughs | Anti-inflammatory, antioxidant, anti-amoebic, antimicrobial, antibacterial, antifungal, anti-mycobacterial, antiviral and anti-plasmodial activities. | Safe LC50 1138 ± 1.33 μg/ml |
Endris et al. (2016); Maroyi (2017b); Osunsanmi et al. (2019) |
| Zingiberaceae | *Zingiber offinale Roscoe | Cough, flu and colds | Antioxidant, anticancer, anti-inflammatory, anti-apoptotic, antiviral, antiemetic, antimicrobial, immunomodulatory, anti-tumorigenic, antilipidemic, and anti-hyperlipidemic activities. Isolated essential oils from the plant was reported to initiate a relaxing property on rat's airway and tracheal contraction. |
Safe LD50 > 5000 mg/kg. |
Mascolo et al. (1989); Rehman et al. (2011); San Chang et al. (2013); Bellik (2014); Rahmani (2014); Teles et al. (2019); Mahboubi (2019) |
Table 4.
Supplementary table of pharmacological activities.
| Pharmacological activities | No of plants | Names of the plant species |
|---|---|---|
| Antioxidant, antimicrobial, antiviral and anti-inflammatory | 57 | Albizia amara (Roxb.) Boivin., Aloe ferox Mill, Aloe spp., *Aloe vera (L.) Burm.f., Artemisia afra Jacq. ex Willd., Bridellia mollis Hutch., Burkea africana Hook., Carissa bispinosa (L.) Desf. ex Brenan., Cassia abbreviata Oliv., Carissa edulis (Forssk.) Vahl, *Citrus limon (L.) Burm. f., Combretum apiculatum Sond., Croton gratissimus Burch., *Cucurbita pepo L., Dalbergia melanoxylon Guill. & Perr., *Datura stramonium L., Dichrostachys cinerea (L.) Wight & Arn., Erythrina abyssinica Lam., *Eucalyptus camaldulensis Dehnh., Kigella africana (Lam.) Benth., Kirkia acuminata Oliv., Ficus sycomorus L., Flacourtia indica (Burm.f.) Merr., Flueggea virosa (Roxb. ex Willd.) Voigt, Heteromorpha arborescens (Spreng.) Cham. & Schltdl., Heteropyxis natalensis Harv., Holarrhena pubescens Wall. ex G.Don., Leucaena leucocephala (Lam.) de Wit., Lippia javanica (Burm.f.) Spreng., *Mangifera indica L., *Mentha longifolia (L.) L., *Mentha spicata L., Myrothamnus flabellifolius Welw., *Nerium oleander L., *Nicotiana tabacum L., Peltophorum africanum Sond., Piliostigma thonningii (Schumach.) Milne-Redh., Pittosporum viridiflorum Sims., Plumago zeylanica L., Pterocarpus angolensis DC., Rapanea melanophloeos (L.) Mez,*Ricinus communis L., Rhamnus prinoides L'Hér., Sclerocarya birrea (A. Rich.) Hochst., Searsia chirindensis (Baker f.) Moffett, Securidaca longipedunculata Fresen., Sida acuta Burm.f., Strychnos potatorum L.f., Strychnos spinosa Lam., Syzygium cordatum Hochst. ex C. Krauss, Syzigium guineense (Willd.) DC., Terminalia sericea Burch. ex DC., Tetradenia riparia (Hochst.) Codd., Vernonia amygdalina Delile, Vigna unguiculata (L.) Walp., *Zingiber offinale Roscoe, Ziziphus mucronata Willd., |
| Antioxidant, antimicrobial and anti-inflammatory | 22 | *Abelmoschus esculentus (L.) Moench., Albizia tanganyicensis Baker f., Annona stenophylla Engl. & Diels, Azanza garckeana (F.Hoffm.) Exell & Hillc., Bridelia micranatha (Hochst.) Baill., Catunaregam taylorii (S. Moore) Bridson, *Chenopodium ambrosiodes L., Clerodendrum eriophyllum Gürke, Combretum platypetalum Welw. ex M.A. Lawson, Combretum zeyheri Sond., Dicoma anomala Sond., Diospyros lycioides Desf., Ficus thonningii Blume, Kalanchoe spp, Margaritaria discoidea (Baill.) G.L.Webster, Searsia lancea (L.f.) F.A.Barkley, *Solanum incanum L., Stereospermum kunthianum Cham., Thunbergia oblongifolia Oliv., Trema orientalis (L.) Blume, Vangueria infausta Burch., Warburgia salutaris (G. Bertol.) Chiov., |
| Antioxidant, antimicrobial and antiviral | 4 | Euclea natalensis A.DC., Khaya anthotheca (Welw.) C. DC., Lannea edulis (Sond.) Engl., Psorospermum febrifugum Spach, |
| Antimicrobial, anti-inflammatory and antiviral | 2 | Alepidea amatymbica Eckl. & Zeyh., Zanha africana (Radlk.) Exell, |
| Anti-inflammatory and antimicrobial | 3 | Asparagus africanus Lam., Chenopodium ambrosiodes L., Zanthoxylum humile (E.A. Bruce) P.G. Waterman, |
| Antioxidant and antimicrobial | 10 | Bauhinia fassoglensis Kotschy ex Schweinf., Elaedendron matabelicum Loes., Euclea crispa (Thunb.) Gürke, Fadogia ancylantha Schweinf., Gardenia ternifolia Schumach., Helichrysum caespititium (DC.) Sond., Lannea discolor Engl., Ocimum obovatum E.Mey. ex Benth., Parinari curatellifolia Planch. ex Benth., Vernonia adoensis Sch. Bip. ex Walp., |
| Antioxidant and anti-inflammatory | 2 | Lopholaena coriifolia (Sond.) E.Phillips & C.A.Sm., Pellaea spp. |
| Antimicrobial and antiviral | 2 | Aspilia pluriseta Schweinf., Garcinia huillensis Welw. ex Oliv. |
| Antibacterial | 4 | Helichrysum kraussii Sch. Bip., Tabernaemontana elegans Stapf., Triumfetta welwitschii Mast., Antidesma membranaceum Müll.Arg., |
| Antimicrobial | 8 | Aloe excelsa A. Berger, Crotalaria laburnifolia L., Gnidia kraussiana Meisn., Hexalobus monopetalus (A.Rich.) Engl. & Diels, Indigofera spp, Laggera crispata (Vahl) Hepper & J.R.I.Wood, Pericopsis angolensis (Baker) Meeuwen, Xeroderris stuhlmanni (Taub.) Mendonça & E.P.Sousa |
| Anti-asthmatic, bronchodilatory, mast cell stabilizing and smooth muscle relaxation | 6 | Albizia tanganyicensis Baker f., Chenopodium ambrosiodes L., *Datura stramonium L., Dichrostachys cinerea (L.) Wight & Arn., *Nerium oleander L., *Ricinus communis L. |
| Antifungal | 1 | Faurea saligna Harv. |
| Anti-tussive | 6 | Aloe spp., aAloe vera (L.) Burm.f., *Citrus limon, (L.) Burm. f., Ficus sycomorus L., *Psidium guajava L., Searsia longipes (Engl.) Moffett |
| Antioxidant | 5 | Albizia antunesiana Harms, Ekebergia benguelensis Welw. ex C.DC., Ficus ingens (Miq.) Miq., Searsia longipes (Engl.) Moffett, Searsia pyroides (Burch.) Moffett |
| Immunomodulatory | 17 | *Abelmoschus esculentus (L.) Moench, Aloe spp., Aloe vera (L.) Burm.f., Bridellia mollis Hutch., Catunaregam taylorii (S. Moore) Bridson, *Capsicum spp., Clerodendrum eriophyllum Gürke, Ficus ingens (Miq.) Miq., Ficus sycomorus L., Kalanchoe spp., *Mangifera indica L., *Nerium oleander L., *Ricinus communis L., Piliostigma thonningii (Schumach.) Milne-Redh., Psorospermum febrifugum Spach, Vernonia amygdalina Delile., *Zingiber offinale Roscoe |
| Anti-mycobacterial | 15 | Aloe excelsa A. Berger, Antidesma membranaceum Müll. Arg., Artemisia afra Jacq. ex Willd., Combretum platypetalum Welw. ex M.A. Lawson, Erythrina abyssinica Lam., Kirkia acuminata Oliv., Helichrysum caespititium (DC.) Sond., Heteromorpha arborescens (Spreng.) Cham. & Schltdl., Heteropyxis natalensis Harv., Hexalobus monopetalus (A.Rich.) Engl. & Diels, Lippia javanica (Burm.f.) Spreng., Pavetta schumanniana F.Hoffm. ex K.Schum., Rapanea melanophloeos (L.) Mez, Vangueria infausta Burch., Xeroderris stuhlmanni (Taub.) Mendonça & E.P.Sousa |
Exotic plants.
Table 5.
Toxicological profile of plants used for respiratory diseases.
| Toxicological profile | No of plants | Names of the plant species |
|---|---|---|
| Safe or nontoxic LC50 ≥ 1000 μg/ml 2000 ≤ LD50 ≥ 5000 mg/kg body weight |
56 | aAbelmoschus esculentus; Albizia amara; Albizia tanganyicensis; Aloe ferox;aAloe vera; Aloe spp.; Annona stenophylla; Asparagus africanus; Burkea africana; Carissa edulis; Catunaregam spinosa;aCitrus limon; Chenopodium ambrosiodes;aCucurbita pepo;aDatura stramonium; Dichrostachys cinerea; Dicoma anomala; Elaedendron matabelicum; Erythrina abyssinica;aEucalyptus camaldulensis; Ficus ingens; Ficus thonningii; Flueggea virosa; Gardenia ternifolia; Gymnosporia senegalensis; Helichrysum caespititium; Heteropyxis natalensis; Holarrhena pubescens; Lippia javanica;aMangifera indica; Margaritaria discoidea; Mentha longifolia;aNicotiana tabacum; Parinari curatellifolia; Piliostigma thonningii; Pittosporum viridiflorum;aPsidium guajava; Psorospermum febrifugum;aRicinus communis; Sclerocarya birrea; Searsia chirindensis; Searsia longipes; Searsia pyroides; Sida acuta; Solanum incanum; Stereospermum kunthianum; Strychnos potatorum; Strychnos spinosa; Syzygium cordatum; Syzigium guineense; Thunbergia oblongifolia; Urginea sanguinea; Vernonia adoensis; Vernonia amygdalina; Vigna unguiculata;aZingiber offinale; Ziziphus mucronata; |
| Weak or low toxicity or mildly toxic 500 ≤ LC50 ≥ 999 μg/ml 1000 ≤ LD50 ≥ 2000 mg/kg body weight |
10 | Cassia abbreviata; Ficus sycomorus; Kalanchoe spp.; Lannea edulis;aMentha spicata; Peltophorum angolensis; Rapanea melanophloeos; Searsia lancea; Tetradenia riparia; Turraea nilotica; |
| Moderately toxic 250 ≤ LC50 ≥ 499 μg/ml 300 ≤ LD50 ≥ 1000 mg/kg body weight |
9 | Artemisia afra; Flacourtia indica; Khaya anthotheca; Kigella africana; Nymphaea nouchali; Securidaca longipedunculata; Vangueria infausta; Warburgia salutaris; |
| Toxic 50 ≤ LD50 ≥ 300 mg/kg body weight |
1 | Plumago zeylanica |
| Highly toxic LC50 ≤ 249 μg/ml 0 ≤ LD50 ≥ 50 mg/kg body weight |
18 | Alepidea amatymbica; Antidesma membranaceum; Azanza garckeana; Bauhinia fassoglensis; Bridelia micranatha; Bridellia mollis;aCapsicum spp.; Combretum zeyheri; Dalbergia melanoxylon; Euclea natalensis; Heteromorpha arborescens; Hexalobus monopetalus; Myrothamnus flabellifolius;aNerium oleander; Rhamnus prinoides; Terminalia sericea; Trema orientalis; Zanha africana; |
| No records found | 66 | Acacia rehmannia; Acalypha petiolaris; Aeschynomene mimosifolia; Agathisanthemum bojeri; Albizia antunesiana; Alepidea cordifolia; Aloe excelsa; Aspilia pluriseta; Astripomoea malvacea; Barleria spinulosa; Bauhinia petersiana; Berchemia discolor; Brachystegia spiciformis; Carissa bispinosa; Chaetachme aristata; Clematis villosa; Clerodendrum eriophyllum; Coleochloa setifera; Combretum apiculatum; Combretum platypetalum; Cordyla africana; Crotalaria laburnifolia; Croton gratissimus; Lannea discolor; Diospyros lycioides; Diplolophium zambesianum; Diplorhynchus condylocarpon; Ectadiopsis oblongifolia; Ekerbergia benguelensis; Euphorbia ingens; Euphorbia matabelensis; Euclea crispa; Fadogia ancylantha; Faurea saligna; Garcinia huillensis; Gardenia resiniflua; Gardenia volkensii; Gnidia capitata; Gnidia kraussiana; Gomphocarpus glaucophyllus; Helichrysum kraussii; Indigofera spp.; Inula glomerata; Kirkia acuminata; Laggera crispata; Lopholaena coriifolia; Leucaena leucocephala; Leucas milanjiana; Loranthus spp.; Monadenium lugardiae; Mussaenda arcuata; Ocimum obovatum; Oxygonum sinuatum; Pavetta schumanniana; Pellaea spp.; Pericopsis angolensis; Phragmites mauritianus; Pterocarpus africanum; Spermacoce dibrachiata; Tabernaemontana elegans; Triumfetta welwitschii; Tulbaghia leucantha; Vangueriopsis lanciflora; Vitex payos; Xeroderris stuhlmanni; Xeromphis obovata; Zanthoxylum humile; |
Exotic plants.
Fig. 2.
General distribution of medicinal plants in different floristic regions of Zimbabwe (Source: Mapaura and Timberlake, 2004 p. 4 p. 4).
Fig. 3.
Growth habit of medicinal plant species used for treatment of respiratory diseases in Zimbabwe.
Fig. 4.
Plant parts used for medicinal preparations used for the management of respiratory diseases in Zimbabwe.
Fig. 5.
Mode of preparation of plants used for the management of respiratory diseases in Zimbabwe.
Fig. 6.
Common respiratory diseases treated traditionally by medicinal plants in Zimbabwe.
2.3. Screening
The titles and abstracts were subsequently examined by two reviewers, independently to identify articles reporting medicinal plants used in Zimbabwe to manage respiratory infections. In the case of any discrepancy in their reports, a third reviewer was brought in to resolve the issue. Relevant papers were equally manually cross-checked to identify further references. Articles that reported on veterinary use of medicinal plants were excluded. The eligible articles were then assessed further for inclusion in the study using the inclusion/exclusion criteria.
2.3.1. Research questions
-
a.
What is the role of medicinal plants in Zimbabwe in the prevention and treatment of respiratory tract infections?
-
b.
Is there any evidence suggesting the efficacy and safety of medicinal plants used in Zimbabwe for management of respiratory disorders?
2.4. Inclusion and exclusion criteria
Full-text articles that at least reported on ethnobotany of Zimbabwean medicinal plants published in peer reviewed journals, reports, books, theses, and dissertations dated until June 17, 2022 were considered. All publishing years were included without any geographical restrictions. Articles that reported data not relevant to the study and not written in English were excluded from the study.
2.5. Data extraction
A data collection tool was designed in Microsoft Excel (Microsoft Corporation, USA) to capture data on different aspects of Zimbabwean medicinal plants. Three reviewers independently extracted relevant data from the included articles regarding the ethnobotany of Zimbabwean medicinal plants used for respiratory disorders. For ethnobotanical data, the diseases or ailments managed, parts used, and mode of preparation and administration were captured. The collected data were checked for completeness and processed independently by three reviewers.
2.6. Data analysis
Respiratory disorders were divided into 15 categories according to the diseases enlisted in published research articles on ethnobotanical surveys conducted in Zimbabwe. Disease categories consisting of similar disorders or pharmacological effects were grouped as a single category. Each plant that is found in more than one province in the country is listed once for each ailment that it works on. The conservation status of plant species was determined following the IUCN Red List categories and criteria version 3.1 (IUCN Red List categories and criteria; Dombo et al., 2002; Golding, 2002). Economic value of the plant species was determined using scientific literature based on the commercial value of medicinal plants in Zimbabwe. Descriptive statistical methods were used to analyse the collected data. Results were expressed as percentages and frequencies and subsequently presented as tables and charts. The analyses were performed using SPSS statistical software (version 20, IBM Inc.).
3. Results and discussion
3.1. Assessment of literature
The PRISMA flow chart (Fig. 1) demonstrates the identification and screening of records for this review.
Several scientific papers were reviewed based on ethno-botanical surveys of different areas of Zimbabwe and are presented in the sections below. Although the numbers could be higher, there is increasing knowledge on the use of plant based medicines and these have been documented (Gelfand et al., 1985; Kambizi and Afolayan, 2001; Dombo et al., 2002; Mapaura and Timberlake, 2004; Chigora et al., 2007; Shoko, 2007; Shumba et al., 2009; Matongo, 2012; Mukamuri and Kozanayi, 2014; Bhebhe et al., 2015; Maroyi and Cheikhyoussef, 2015; Ngarivhume et al., 2015; Maroyi, 2008, 2009, 2011, 2012a, 2012b, 2013a, 2017c, 2018c; Dimene et al., 2020).
3.2. Ethnobotanical surveys and distribution of medicinal plants traditionally used against respiratory disorders in Zimbabwe
The current review indicates that there are at least 58 plant families and 160 plant species used to treat respiratory diseases in Zimbabwe (Table 1). Ethnobotanical surveys are important pre-screening tools for the search of pharmacological interests of plants. While numbers of species in Zimbabwe are lower than those documented in Pakistan and South Africa, where 85 and 86 families with 384 and 306 plant species respectively were noted to treat respiratory disorders (Alamgeer et al., 2018; Semenya and Maroyi, 2018), these numbers suggest that Zimbabwe is a vast repository of medicinal plants for respiratory ailments. From the information in Table 1, it appears that several options of plants can be used to treat a single particular respiratory ailment. It is also true from the same Table 1 that a single particular plant has been used to treat several respiratory problems. For example, asthma can be treated with Thunbergia oblongifolia Oliv., Tulbaghia leucantha Baker, Holarrhena pubescens Wall. ex G.Don and Aloe excelsa A. Berger among several other plants. This is a parallel reflection of how conventional drugs have been used in managing disease.
The proportions of plant families found to treat respiratory diseases in Zimbabwe is shown in Table 2. Zimbabwe appears to be endowed with a diverse array of plant families with an ability to treat respiratory conditions. Within each plant family in Table 2, there is a range between 1 and 21 plant species that were identified. While in most cases there were fewer species within each family, the Apocynacea, Lamiacea, Anacardiaceae, Asteraceae, Euphorbiaceae, Rubiaceae and Fabaceae had the highest species diversity. This suggests that these diverse ranges may be distributed in different geographical locations according to their environmental preferences. Fabaceae family appears to be the most diverse and carries the most prevalent species in the country. Typical examples of Fabaceae are usually trees or shrubs which are found throughout the country. These include Xeroderris stuhlmannii Mendonça & E.P. Sousa, Dichrostachys cinerea (L.) Wight & Arn., Piliostigma thonningii (Schumach.) Milne-Redh. and among several others. According to Table 1, most of the Fabaceae plants are distributed throughout the country, which explains their high species diversity. Of note, it is therefore highly likely to make a mistake in classifying the Fabaceae family, hence, extra caution may need to be taken.
Zimbabwe is divided into 5 agro ecological regions, based on rainfall regime, soil and several other factors. About 57% of plants reported in this review are widely distributed throughout Northern, Eastern, Central, Western, Southern regions of Zimbabwe as represented in Fig. 1. The remaining population of plant species are specific to particular regions. About 19% were distributed in 4 regions, 13% in 3 regions, 3% in 2 regions and 5% in 1 region. A remainder 3% of the medicinal plant species (i.e. Nerium oleander L., Nicotiana tabacum L., Zingiber officinale Roscoe, Capsicum spp. and Cucurbita pepo L.) were reported to be cultivated. (Table 1; Mapaura and Timberlake, 2004).
According to Table 1 it is apparent that plant remedies have been relatively useful in managing human ailments. While these listed plants appear to solve respiratory ailments, some have multi-purpose potencies. Generally, the plants identified seem to be evenly distributed throughout the country hence the different names from each ethnic group distributed in the country.
3.3. Diversity, habit and part used, of medicinal plants traditionally used in the management of respiratory disorders in Zimbabwe
According to Fig. 2, the frequency and type of plants used to treat and or manage respiratory disorders is as follows; trees (n = 66), shrubs (n = 44), herbs (n = 36), succulents (n = 5), mistletoes (n = 3), climbers (n = 2), aquatics (n = 1), grass (n = 1), reed (n = 1) and fern (n = 1). Differences in abundance, socio-cultural beliefs, location, ethnic group, ecological status and variations in the practices of the traditional healers are some of the factors which contribute to the use of a particular plant (Shumba et al., 2009).
The high frequency use of trees in Zimbabwe as a source of herbal therapies is often attributed to their abundance and ease of availability throughout the year. According to Mapanda et al. (2012) Zimbabwe is made up of roughly 40% Miombo woodlands which is predominantly savanna largely characterised by trees and shrubs which grow during the wet summer. Zimbabwe is also a landlocked country, hence the very low frequency use of aquatic plants as medicine.
The plant parts that are frequently used are shown in Fig. 3. It appears the roots, leaves and bark are the main target plant parts used for respiratory treatment in Zimbabwe. While use of the root is the least environmentally sustainable, it is the most preferred source of medicine. However, in some instances a whole plant, fruit, twig, stem or any other plant part are used. This suggest that roots have the most potent antimicrobial and other bioactive properties in general as evidenced by these other authors Kambizi and Afolayan (2001) 53%; Maroyi (2011, 2013a) 61.3%; Ngarivhume et al. (2015) 55.3%.
Abundance, ease of collection, conservation policies or ethnic beliefs of local people are some of the factors that may affect choice of plant parts being acquired and used to treat respiratory illnesses. The second most predominantly used plant part is the leaf. Leaves are relatively abundant and have been largely reported to contain pharmacologically active compounds (Adeyemi et al., 2010). Moreover, leaves are preferred in ethno-preparations because collecting them does not adversely affect the life cycle of the plant (Bhat et al., 2013). The bark was found to be the third most frequently used plant part. The bark from trees is relatively abundant since it comes from trees which are predominant. It should be noted that the collection of bark by completely ring barking the tree leads to death due to lack of connecting cambium tissue (Shumba et al., 2009). Most traditional healers and herbal vendors ring-bark the trees and, in some cases, uproot the plant to get as much bark and root as possible which leads to the death of the plant therefore threatening the species to extinction. Fruits are rarely used as plant parts for treatment of respiratory illnesses. The low use of fruit is probably attributed to the seasonal availability from the plant part and they are largely consumed more as food than medicine.
3.4. Use and mode of preparation of medicinal plants traditionally used in the management of respiratory disorders in Zimbabwe
The different modes of preparation of the plants are shown in Table 1 and Fig. 4. The different formulations used by local people for medicinal plant preparations, starting with the most common mode of preparations of phytomedicines, include infusions (n = 62), decoctions (n = 50), powder (n = 14), smoke (n = 12), ashes (n = 8), raw (n = 4), juice (n = 3), ointment (n = 2), cigarette (n = 1) and paste (n = 1). The increased and continuous use of the various methods has perfected the processing of the preparations over the years and the experience has led to greater efficiency and a decrease in the toxicity of the preparations used (Shumba et al., 2009).
Methods of preparation of plant medicines seem to vary according to area and subculture of people in that region. Plant materials may be used fresh or dry. Decoctions are usually prepared by boiling the plant in water until the volume of water is reduced to half, whereas an infusion is a less concentrated version of a decoction. Studies by Maroyi (2011); Ngarivhume et al. (2015) reported predominant use of decoctions and infusions which may be attributed to these preparations being quicker, low cost and easy to administer. When smoke is used, two ways were employed for administration; either as a cigarette or inhalation of the smoke from a burning plant. The high frequency of infusion and decoction use might be related to their efficiency and the efficacy of indigenous medicinal knowledge acquired over many years of such preparations. A paste seems to be the least common method of preparation for most respiratory diseases, possibly because of its intricacy in preparation. While it was possible to pick out how most of these ethno-medicines were prepared it was notable that some papers do not always intrinsically highlight the mode of preparation of the medicinal plants (Chigora et al., 2007; Viol, 2013; Chimponda and Mukanganyama, 2010; Maroyi, 2012a, 2018d; Dangarembizi et al., 2013; Mangoyi et al., 2014; Dzoyem et al., 2016; Neffati et al., 2017).
There are at least 12 common respiratory conditions in Zimbabwe which can be treated using plant based medicine. Fig. 5 below shows the relative frequency of plant solutions for each condition. Respiratory diseases with the highest ethno-medicinal solutions include colds > pneumonia > coughs > chest pains > asthma > tuberculosis.
It is interesting to note that while Zimbabwe is highly endemic of tuberculosis, a common killer disease, there seems to be a relatively high number of plant medicine solutions. While sinuses and sore throats are relatively common diseases in Zimbabwe, the information in Fig. 5 reveals that there are fewer herbal solutions for them. This profile of Zimbabwean medicinal plants therefore suggests that there are more herbal solutions for tuberculosis than there are for sinuses and sore throats. More investment in herbal medicine research for tuberculosis management and treatment may need to be emphasised.
3.5. Conservation status of medicinal plants traditionally used in the management of respiratory disorders in Zimbabwe
While availability of medicinal plants is pertinent, their conservation and sustainable use must also be kept in perspective. Most medicinal plant species traditionally used to manage respiratory diseases in Zimbabwe are categorised as Least Concern according to the IUCN Red data listings (Table 1; Golding, 2002). Two species, A. amatymbica Eckl. & Zeyh and W. salutaris (G. Bertol.) Chiov. belonging to the two families: Apiaceae and Canellaceae respectively are “Critically Endangered” (Golding, 2002; Maroyi, 2008; Semenya and Maroyi, 2019). Maroyi (2008) reported that A. amatymbica and W. salutaris were non-existent in the wild in Mutema Highlands and Engwe farm in the Eastern Highlands of Zimbabwe. The population loss is due to the destruction of their habitats by extensions of human settlements and agricultural practices that are not sensitive to biodiversity conservation (Maroyi, 2008). These two critically endangered medicinal plants have a limited distribution, only occurring in the Eastern part of Zimbabwe.
According to Mapaura and Timberlake (2004), Dalbergia melanoxylon Guill. & Perr. (Fabaceae) is categorised as “Near Threatened” (Golding, 2002) although it has a wide distribution throughout Zimbabwe. Khaya anthotheca (Welw.) C. DC. (Meliaceae) is “Vulnerable” and it occurs in the Northern, Central and Western areas of Zimbabwe. Special attention needs to be given to such threatened species for traditional medicinal plants to be harvested and exploited sustainably.
While it seems that plants stand as one of the critical sources for human health, several competing factors pose a threat to their existence. These include destructive collection of plant species by traditional healers/herbalists, forest decline, invasion by exotic species that compete, industrialization, increased spread of diseases and excessive use of agrochemicals (Hunter, 2007; Morris, 2010; FAO, 2015).
Benefits that can be derived from medicinal plants and herbs in Zimbabwe are diverse as has been highlighted in the illustrations above. Hence, there is a serious need to strike a balance between conservation and utilization of these medicinal plants (Rajasekharan and Ganeshan, 2002). There has been growing worry over the years some of the very important plants, especially indigenous trees and herbs are almost going extinct due to agricultural expansion and human settlements (Maroyi, 2008). Moreover, the younger generations largely shun traditional knowledge systems due to increasing religious inclinations, which have resulted in many of the locals opting for the conventional medicines, thereby downplaying the wealth of resources in the form of affordable and locally obtainable trees and herbs that are locally available in Zimbabwe (Maroyi, 2012b, 2013a). Of commendable note, traditional leaders have been given authority to put in measures that protect some of the endangered medicinal species. One notable measure has been to arrest anyone found cutting down a tree without authority from the traditional leaders or Forestry Commission. Over the years, plant conservationists have made conscious efforts to protect and conserve medicinal plants and thus prevent their extinction. This has not been a straightforward programme due to many problems that militate against it. The alarming rate at which various plant species are removed from their natural habitats has been documented (Orji et al., 2013). However, in the fight for conservation, probably the single most important ‘role’ for medicinal plants in biological and ecological conservation stems from the foundations that they can provide for the involvement of people in conservation of natural habitats (Schopp-Guth and Fremuth, 2001).
Cultures around the world have developed methods on how to benefit from indigenous plants to maintain health and suppress illnesses for many centuries. These ethnically significant traditional medicinal plants are readily obtainable from an inexpensive and accessible health-care system and are an essential basis of livelihood, mainly for indigenous and rural populations. While these indigenous medicinal plants have established growing commercial and scientific consideration in recent years, there is increasing pressure from which most of these medicinal plants are harvested. There is an ever-increasing risk of overharvesting, bio-prospecting for new sources, and destruction of the habitats of known medicinal plant species. It is estimated that every two years we lose at least one important potential drug. At present, nearly 15,000 medicinal plant species may be threatened with extinction worldwide. Hence, the conservation and study of indigenous medicinal plant species has turned out to be increasingly urgent.
There are various propagation techniques that are used in trees and herbs (Davies et al., 2017). These include: budding, grafting, air and ground layering, use of cuttings, suckers, corms, bulbs to mention but a few. Most propagation techniques have only been focusing on exotic fruit trees like mango, guava, litchi, apples, bananas and others (Davies et al., 2017), yet we have vast indigenous trees and herbs that are well adapted to the local environmental conditions and have been used by generations that have passed on. It is widely agreed that the conservation of medicinal plants and biodiversity in general can be achieved through an integrated approach balancing in situ and ex situ conservation strategies. In Zimbabwe, in situ conservation has been achieved both by setting aside areas as nature reserves and national parks (collectively termed “Protected Areas”) and by ensuring that as many wild species as possible can continue to survive in managed habitats such as plantation forests. This is the best means of conservation to ensure that the populations of species of plants continue to grow and evolve in the wild or in their natural habitats. The conservation of the indigenous medicinal plant genetic resources has long been recognized as an integral part of biodiversity conservation. Traditionally, conservation of medicinal plants was regulated by management practices such as taboos, seasonal and social restrictions on harvesting of medicinal plants, which served to limit medicinal plant harvesting. Studies by Cunningham (1993) as well as Mavi and Shava (1997) done in Zimbabwe shows that other factors that limited pressure on the species from being overexploited included: restricted removal of the bark of a tree, sparing collection of roots for medicinal use and use of taboos to regulate over-harvesting. Literature reviewed showed that the traditional conservation measures of medicinal plants have not been well documented among local communities. This is due to the secretive nature of herbalists when it comes to their knowledge. However, these species are also conserved ex situ. The primary purpose of this is as an insurance policy, but it has the advantage that it is usually easier to supply plant material for propagation, for re-introduction, for agronomic improvement, for research and for education purposes from ex situ collection than from in situ reserves. Approaches to ex situ techniques involves; tissue culture or in vitro regeneration, cryopreservation of plant cells and meristems, low temperature germplasm storage, and seed storage models.
3.6. Economic value of commercially available important medicinal plants traditionally used in the management of respiratory disorders in Zimbabwe
Traditional herbal medicines offer an avenue for potential new drug discovery due to their untapped potential, vastness, diversity and inferred efficacy that has been generated through its use for centuries. The widespread use of medicinal plants, their extracts, formulations and chemicals derived from them, in different traditional and modern systems of medicine, nutraceuticals, cosmeceuticals and functional foods is increasing the demand for medicinal plants internationally. As more people realise the beneficial effects of herbal medicines, the demand for these medicinal plants has been increasing steadily (Bhebhe et al., 2015). The adoption of medicinal plants in everyday usage has also resulted in an increase in their economic value. Many people in the world, especially in developing countries, rely chiefly on herbal medicines, while others alternatively gain income from their wild harvest, trading or processing.
A 1995 analysis estimated that each new plant-derived drug is worth an average of US$94 million to drug companies and US$449 million to society (Mendelsohn and Balick, 1995 cited in Daily, 1997). Other estimates have reported sales ranging from US$1.5 to US$5.7 billion annually for non-prescription medicinal plants in the United States, and US$24.4 billion in sales worldwide. The reported market value of prescription and over-the-counter plant-based drugs in 1985 was US$19.8 billion in the United States, and US$84.3 billion worldwide (Pearce and Moran, 1994; Tuxhill, 1999). The annual value of the global export of the thousands of plants with suspected medicinal characteristics and properties was projected to be 2.2 billion USD in 2012 (Awuchi, 2019). In 2017, the potential global market for the botanical medicines and extracts was projected at several hundred billion US dollars; hence, this is an untapped resource (Awuchi, 2019).
The COVID-19 pandemic has opened up opportunities for herbal medicine producers to inevitably produce herbal medicines that have immunomodulatory properties. Its intended growth is supported by an increase in the focus on herbal products that offer relief in stress, support digestion and enhance immunity health with further increasing attention on personalized medicines and easily affordable and available herbal products. In the year 2020, with the ravaging COVID-19 pandemic, the global market for Herbal Medicines was about US$110.2 billion and is estimated to reach a revised size of US$178.4 billion by 2026, growing at a Compound Annual Growth Rate (CAGR) of 8.1%. Last year alone the Herbal Medicines market in the U.S. was estimated at US$22.8 billion accounting for an 18.4% share in the global market. China is estimated to reach a market size of US$32.9 billion by 2026. Canada and Japan geographic markets, each estimated to grow at 7.1% and 7.4% respectively. In Europe, Germany is estimated to grow at about 6.5% CAGR with the rest of the European market reaching a staggering US$35.8 billion. Continuous growth in these regions is increasing due to cultural and social use of herbal products and improved customer confidence in the efficacy and safety of herbal products (Research and Markets, 2022).
Therefore, if Zimbabwe fully exploits indigenous knowledge system-based medicinal plants, a multi-billion-dollar industry will be created from the sale and distribution of traditional herbal medicine across the globe thereby boosting the country's economy. Furthermore, we recommend that the government should establish plantations which will lead to sustainable harvest, uphold medicinal heritage and conservation of the medicinal plants as well as creation of manufacturing companies resulting in creation of employment, improvement of livelihoods and restoration of health of the general public.
3.7. Pharmacological properties of medicinal plants traditionally used in the management of respiratory disorders in Zimbabwe
Pharmacological properties which have been investigated on each of the 160 medicinal plants used in the management of respiratory conditions in Zimbabwe is shown in Table 3, Table 4 The properties profiled included antioxidant, anti-inflammatory, immuno-modulatory, analgesic, antiviral, anti-tussive, antimicrobial, bronchodilator, mast cell stabilizing, anti-allergic, antihistaminic and smooth muscle relaxant activities in medicinal plants as these have been reported to be key in the treatment of respiratory disorders (Younis et al., 2018). Out of all the plants found, 129 of them (80.6%) have been proven to exhibit pharmacological properties which aid in treating respiratory conditions (Table 4). Antioxidant, anti-inflammatory, antiviral and antimicrobial activities were the most common properties among these medicinal plants which suggests why these medicinal plants’ traditional use in alleviating respiratory illnesses has been effective. Antimicrobial activity was the most evaluated property exhibited by 110 plants (85.3%), followed by antioxidant activity which was shown in 96 plants (74.4%), anti-inflammatory activity exhibited by 85 plants (65.9%), while antiviral activity demonstrated in 65 plants (50.4%). Fewer plants demonstrated immuno-modulatory properties (17 plants or 13.2%) and only 6 plants exhibited anti-asthmatic and anti-tussive properties (4.65%) (Table 4).
Noteworthy in the pharmacological evaluation studies explored were antiviral, anti-inflammatory, antioxidant and antimicrobial properties exhibited by some of the medicinal plants. These properties were identified to be present in 57 medicinal plants with 45 of them being of indigenous origin and 12 of exotic origin (Table 4). The potency of these 57 medicinal plants was wide as several of them were scientifically reported to exhibit antitussive, immunomodulatory, anti-mycobacterial and anti-asthmatic properties. Smooth muscle relaxation, mast cell stabilizing and bronchodilator properties were exclusive to a few, namely Zingiber officinale Roscoe, Dichrostachys cinerea (L.) Wight & Arn., Datura stramonium L. and Ricinus communis L. The traditional use of these 57 medicinal plants in ailments like Influenza, herpes simplex, HIV, pneumonia, tuberculosis and asthma therefore attribute much to their reported pharmacological properties (Table 3, Table 4). The effectiveness of such concoctions may be attributed to synergistic antioxidant, anti-inflammatory, antibacterial and antiviral properties of these medicinal fruits and herbs.
The recent review study by Cock and Van Vuuren (2020) found that of the 257 medicinal plants used in Southern African to treat viral respiratory diseases, only 22 (of these plants have been examined for antiviral activity reported in 9 studies only. However, in addition to the review by the former, this study found that 65 out of 160 plants used in Zimbabwe to manage respiratory diseases exhibited antiviral properties. Only 6 of these 65 plants were also described recently by Cock and Van Vuuren (2020). Thus, this study found 59 more plants with antiviral activity which were not described by the recent review by (Cock and Van Vuuren, 2020). This is evidence that there is still a wealth of knowledge on plant medicine that awaits to be unearthed. So far, there have been a handful of ethno-botanical surveys of this nature carried out specifically for Zimbabwe. There is a growing need to unearth more of the Zimbabwean plant species.
3.8. Toxicological profile of medicinal plants traditionally used for the management of respiratory disorders in Zimbabwe
Of all the medicinal plants listed for respiratory conditions (Table 1), 94 species (58.75%) had toxicological evaluation studies documented, meanwhile, no studies were recorded for the remaining 66 species (41.25%) (Table 5). Among these 94 plants which had toxicological profiles, numerous toxicological activities were evaluated, such as effect of medicinal extracts on liver chang cells, cytotoxic activities on human monocyte cells, genotoxicity and anticancer properties among others. However, of all the toxicological evaluations, Brine Shrimp Lethality Test (BSLT) and rodent acute toxicity test were the most prominent among the studies. These two toxicity tests were common possibly because they give reasonably accurate results, are simple and cost-effective frontline indices on the safety of herbal extracts (Munodawafa et al., 2016). While approximately 59% of plants unveiled had their toxicity evaluated, it is concerning that toxicity of more than 40% of these plants which are already being used ethno-medicinally is still not known. There is a need to carry out toxicological screening on the remaining 41% of plants.
In BSLT, toxicity is classified based on herbal plant extract concentration that causes 50% mortality in brine shrimps (LC50) and 50% mortality in mice/rats (LD50) for rodent acute toxicity studies (Munodawafa et al., 2016; Erhabor et al., 2020) and these provide an early hint on the toxicity of plant extracts. Toxic extracts have been reported to elicit pharmacological activities at low non-toxic doses. For BSLT classification is highly toxic if the LC50 is less than 249 μg/mL, moderately toxic- 250 – 499 μg/mL, while values between 500 and 999 μg/mL are considered weak or low in toxicity and those ≥1000 μg/kg safe (Bussmann et al., 2011; Erhabor et al., 2020). Meanwhile, in rodent acute toxicity tests LD50 of: 0 ≤ 50 mg/kg body weight – was classified as highly toxic; 50 ≤ 300 mg/kg body weight - toxic; 300 ≤ 1000 mg/kg body weight - moderately toxic; 1000 ≤ 2000 mg/kg body weight – mildly toxic; and 2000 ≤ 5000 mg/kg body weight – non-toxic (Malebo et al., 2015). Therefore, of the 94 medicinal plants with toxicological profiles, 56 (59.57%) are regarded as safe/non-toxic; 10 plants (10.64%) are weak or low toxicity or mildly toxic; 9 plants (9.57%) are moderately toxic; 1 plant (1.06%) is toxic and 18 plants (19.15%) are highly toxic (Table 5). Variations in medicinal plants toxicity depend on dosage, type of phytochemical constituents (cardiac glycosides and tropane alkaloids), environmental exposure, mode of extraction, preparation and administration, solvent extraction (organic and non-organic solvents), tested animal species and other factors. To combat toxicity, herbalists use lower dosages, prolonged boiling of herbs, preparing mixtures with other plants, avoiding prescribing any herbal medicines to pregnant women and exercising caution in immunocompromised patients.
3.9. Recommendations for future research
While some mileage has been covered on available medicinal plants, more precise profiles on the specific distribution of medicinal plants may need to be carried out in Zimbabwe. This task may be achieved through use of location data from the plant specimens at the National Herbarium and Botanic Gardens (SRGH) or other herbaria housing Zimbabwean specimens. Ethnobotanical surveys need to be carried out in under-investigated areas as this might yield new information on traditional medicinal plants that have potential to curb and treat the emerging respiratory diseases. Local communities, organisations and institutions are encouraged to participate in the ex situ conservation of medicinal plants by establishing nurseries, herbal gardens and even estates. Natural product development of the 56 prioritised medicinal plants requires further toxicological evaluation tests and clinical research. COVID-19, a highly contagious viral respiratory disease, has symptoms that include dyspnoea, nasal congestion, fever, cough, chest pain, diarrhoea, throat and lung infections; complications of the disease may lead to kidney failure and severe respiratory distress. Preliminary reported activities exhibited by these 56 medicinal plants on viral and bacterial infectious respiratory diseases, symptomatic treatment and prophylaxis against COVID-19 and/or any future infectious respiratory diseases is thus recommended.
4. Conclusions
Zimbabwe is a vast repository of medicinal plants which can be used to manage or treat respiratory ailments. So far there are 58 families of medicinal plants with 160 species used to treat respiratory diseases in Zimbabwe. Fabaceae family is the most predominant plant for managing respiratory conditions. A total of 12 different respiratory illnesses have been reported to be treatable by these medicinal plants in Zimbabwe with coughs > pneumonia > coughs > chest pains > asthma and tuberculosis being the most treatable. There has been a gradual increase in scientific research output on the plants found in Zimbabwe and used to treat respiratory conditions. Both indigenous and exotic plant species found have been documented to be in use in Zimbabwe for the management of human diseases. The conservation status of most medicinal plant species traditionally used to manage respiratory diseases in Zimbabwe are categorised as least concern. However, only two species, A. amatymbica and W. salutaris are classified as Critically Endangered. A total of 44 indigenous and 12 exotic medicinal plant species have been identified from this review as candidate targets for further toxicological evaluations and clinical research in terms of their potential to manage COVID-19 and other respiratory conditions.
Authors’ contributions
Elliot Nyagumbo and Michael Bhebhe collated the paper, coordinated the cohesion of information into a manuscript and created an active voice on the manuscript. William Pote was responsible for the design of the manuscript and conceived the idea of carrying out the study. Elliot Nyagumbo, Cephas Mawere, Ian Mutasa, Emmanuel Kademeteme, Bridgett Shopo, and Ruvimbo J. Mapaya undertook the literature review on ethnobotanical surveys and distribution of medicinal plants and assisted in writing the manuscript. Trust Nyirenda and Elliot Nyagumbo carried out the literature review on pharmacological and toxicological evaluation of the medicinal plants and assisted in writing the manuscript. Ignatius Chagonda and Ruvimbo J. Mapaya, undertook the literature review on conservation status and economic status of the medicinal plants and assisted in writing the manuscript. Alfred Maroyi, Fabian Maunganidze, Tafadzwa Taderera and William N. Mavengere verified the consistency of the information in literature reviewed, assisted in analysing and revised the manuscript providing important perspectives.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We really appreciate Moses Mutetwa, Scelo Mguni and Pimpernel Garanganga for supporting the authors during the writing of the thesis. Most importantly, many thanks go to Professor Laurine Chikoko, Professor Doreen Z. Moyo and Dr Reginald B. Matchava-Hove who have been very supportive throughout the course of the study. This work was supported by the Ministry of Higher and Tertiary Education, Science Innovation and Technology Development, Zimbabwe which was awarded to the Midlands State University for, “Beneficiation and Commercialisation of Indigenous Fruits and Herbs in Zimbabwe”.
References
- Abd El Raheim M.D., Soliman G.A., Zaghloul A.M., Alqasoumi S.I., Awaad A.S., Radwan A.M., Basodan O.A. Chemical constituents and protective effect of Ficus ingens (Miq.) Miq. on carbon tetrachloride-induced acute liver damage in male Wistar albino rats. J. Saudi Chem. Soc. 2013;17:125–133. [Google Scholar]
- Abere T.A., Egharevba C.O., Chukwurah I.O. Pharmacognostic evaluation and antisickling activity of the leaves of Securinega virosa Roxb. ex Willd. (Euphorbiaceae) Afr. J. Biotechnol. 2014;13(40) [Google Scholar]
- Abomey-Calavi R., Cotonou R. Ethyl acetate fraction of Psorospermum febrifugum Spach aqueous extract did not exhibit acute or sub-chronic toxicity. Experimental study on Wistar rats. Int. J. Biol. 2019;11(4) [Google Scholar]
- Abu-Jafar A., Huleihel M. Antiviral activity of Eucalyptus camaldulensis leaves ethanolic extract on herpes viruses infection. Int. J. Comput. Vis. 2017;1:1–9. [Google Scholar]
- Adamu M.A., Yahaya T.A., Abigail B., Adeola S.O. Pharmacological effects of Piliostigma thonningii leaf extract on anxiety-like behaviour and spatial memory in Wistar albino rats. Phytopharmacology. 2013;4(3):561–568. [Google Scholar]
- Adebayo S.A., Masoko P. A review on Ziziphus mucronata Willd (Rhamnaceae) commonly used to treat infections and inflammation-related conditions in southern Africa. AJMP. 2019;7(6):135–145. doi: 10.15413/ajmp.2019.0143. [DOI] [Google Scholar]
- Adebayo S.A., Steel H.C., Shai L.J., Eloff J.N. Investigation of the mechanism of anti-inflammatory action and cytotoxicity of a semipurified fraction and isolated compounds from the leaf of Peltophorum africanum (Fabaceae) J. Evid. Based Complement. Alter. Med. 2017;22(4):840–845. doi: 10.1177/2156587217717417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adedapo A.A., Sofidiya M.O., Afolayan A.J. Anti-inflammatory and analgesic activities of the aqueous extracts of Margaritaria discoidea (Euphorbiaceae) stem bark in experimental animal models. Rev. Biol. Trop. 2009;57(4):1193–1200. doi: 10.15517/rbt.v57i4.5456. [DOI] [PubMed] [Google Scholar]
- Adeniyi B.A., Ayepola O.O., Adu F.D. The antiviral activity of leaves of Eucalyptus camaldulensis (Dehn) and Eucalyptus torelliana (R. Muell) Pakis. J. Pharm. Sci. 2015;28(5):1773–1776. [PubMed] [Google Scholar]
- Adeogun O.O., Maroyi A., Afolayan A.J. Variation in the chemical composition of essential oils from Artemisia afra (Jacq) ex-Wild leaf obtained by different methods and the effect of oil extracts on Artemia salina L. Trop. J. Pharmaceut. Res. 2018;17(3):519–528. [Google Scholar]
- Aderogba M.A., Kgatle D.T., McGaw L.J., Eloff J.N. Isolation of antioxidant constituents from Combretum apiculatum subsp. apiculatum. South Afr. J. Bot. 2012;79:125–131. [Google Scholar]
- Adeyemi O.O., Akindele A.J., Yemitan O.K., Aigbe F.R., Fagbo F.I. Anticonvulsant, anxiolytic and sedative activities of the aqueous root extract of Securidaca longepedunculata Fresen. J. Ethnopharmacol. 2010;130(2):191–195. doi: 10.1016/j.jep.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Adinortey M.B., Galyuon I.K., Asamoah N.O. Trema orientalis Linn. Blume: a potential for prospecting for drugs for various uses. Phcog. Rev. 2013;7(13):67. doi: 10.4103/0973-7847.112852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan M., Gul S., Batool S., Fatima B., Rehman A., Yaqoob S., Shabir H., Yousaf T., Mussarat S., Ali N., Khan S.N. A review on the ethnobotany, phytochemistry, pharmacology and nutritional composition of Cucurbita pepo L. J. Phytopharmacol. 2017;6(2):133–139. [Google Scholar]
- Adongo J.O., Omolo J.O., Njue A.W., Matofari J.W. Antimicrobial activity of the root extracts of tylosema fassoglensis schweinf. Torre & Hillc (Caesalpiniaceae) Sci. J. Microbiol. 2012;2012:1–3. 209. [Google Scholar]
- Afolayan M., Srivedavyasasri R., Asekun O.T., Familoni O.B., Orishadipe A., Zulfiqar F., Ibrahim M.A., Ross S.A. Phytochemical study of Piliostigma thonningii, a medicinal plant grown in Nigeria. Med. Chem. Res. 2018;27:2325–2330. doi: 10.1007/s00044-018-2238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbodjento E., Klotoé J.R., Dramane G., Dougnon T.V., Ategbo J.M. Gardenia ternifolia Schumach. & Thonn.: revue sur les aspects ethnobotanique, ethnopharmacologique, phytochimique et toxicologique. Int. J. Brain Cognit. Sci. 2018;12(6):2922–2932. [Google Scholar]
- Ahmad N., Mishra A., Ahsan F., Mahmood T., Hasan N., Khan Z. Ricinus communis: pharmacological actions and marketed medicinal products. World J. Pharmaceut. Life Sci. 2016;2(6):179–188. [Google Scholar]
- Ahmad A., Abubakar A.H.M.E.D., Garba M.M., Jamiu I., Yusuf P.O. Antivenin studies of leaf extracts of Securinega virosa from Zaria, Nothern Nigeria. Innoriginal: Int. J. Sci. 2018:29–32. [Google Scholar]
- Ahomadegbe M.A., Ladekan E.Y., Togbenou N., Assogba F., Agbonon A., Gbenou J. Phytochemical and toxicity studies of the leaves of Mangifera Indica, Cajanus cajan and of Piliostigma thonningii, acclimated in Benin, used against diarrheal disease. J. Pharmacogn. Phytochem. 2018;7:2971–2978. [Google Scholar]
- Aiyelero O.M., Abdu-Aguye S.N., Yaro A.H., Magaji M.G. Behavioural studies on the methanol leaf extract of Securinega virosa (Euphorbiaceae) in mice. J. Pharmacogn. Phytotherapy. 2012;4(2):12–15. [Google Scholar]
- Ajaib M., Wahla S.Q. Phytochemical screening and anthelmintic activity of Flueggea virosa. J. Chem. Soc. Pakistan. 2018;40:702–706. [Google Scholar]
- Akah P.A. Antidiarrheal activity of Kigelia africana in experimental animals. J. Herbs, Spices, Med. Plants. 1996;4(2):31–38. [Google Scholar]
- Al-Snafi The Pharmacological importance of Aloe vera- a review. IJPR. 2015;6(1):28–33. [Google Scholar]
- Alagbe J.O., Sharma D., Xing L. Effect of aqueous Piliostigma thonningii leaf extracts on the heamatological and serum biochemical indices of broiler chicken. Noble Int. J. Agric. Food Technol. 2019;1(2):62–69. [Google Scholar]
- Alamgeer, Younis W., Asif H., Sharif A., Riaz H., Bukhari I.A., Assiri A.M. Traditional medicinal plants used for respiratory disorders in Pakistan: a review of the ethno-medicinal and pharmacological evidence. Chin. Med. 2018;13:48. doi: 10.1186/s13020-018-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alara O.R., Abdurahman N.H., Mudalip S.K.A., Olalere O.A. Phytochemical and pharmacological properties of Vernonia amygdalina: a review. J. Chem. Eng. Indus. Biotechnol. 2017;2(1):80–96. [Google Scholar]
- Ali M.T., Haque S.T., Kabir M.L., Rana S., Haque M.E. A comparative study of in vitro antimicrobial, antioxidant and cytotoxic activity of Albizia lebbeck and Acacia nilotica stem bark. Bull. Fac. Pharm. Cairo Univ. 2018;56(1):34–38. [Google Scholar]
- Amabye T.G. Natural Products Chemistry & Research; 2015. Evaluation of Phytochemical, Chemical Composition, Antioxidant and Antimicrobial Screening Parameters of Rhamnus Prinoides (Gesho) Available in the Market of Mekelle, Tigray, Ethiopia. [Google Scholar]
- Amenya H.Z., Mbaria J.M., Thaiyah A.G., Thoithi G.N., Gathumbi P.K. A 56-day oral toxicity study of the aqueous extract of rapanea melanophloeos (L.) Mez in rats. Evid. Based Complementary Altern. Med. 2016 doi: 10.1155/2016/7403087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameya G., Manilal A., Merdekios B. In vitro antibacterial activity and phytochemical analysis of Nicotiana tabacum L. Extracted in different organic solvents. Open Microbiol. J. 2017;11:352–359. doi: 10.2174/1874285801711010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Añibarro-Ortega M., Pinela J., Barros L., Ćirić A., Silva S.P., Coelho E., Mocan A., Calhelha R.C., Soković M., Coimbra M.A., Ferreira I.C. Compositional features and bioactive properties of aloe vera leaf (Fillet, mucilage, and rind) and flower. Antioxidants. 2019;8(10):444. doi: 10.3390/antiox8100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar S. Pharmacological investigation of solanum incanum against P. Falciparum, L. infantum, T. cruzi and T. brucei: a role of antioxidant effect and clinical overview. Biomed. Pharmacol. J. 2018;11(2):653–660. 2018. [Google Scholar]
- Aro A.O., Dzoyem J.P., Hlokwe T.M., Madoroba E., Eloff J.N., McGaw L.J. Some South African Rubiaceae tree leaf extracts have antimycobacterial activity against pathogenic and non‐pathogenic Mycobacterium species. Phytother Res. 2015;29(7):1004–1010. doi: 10.1002/ptr.5338. [DOI] [PubMed] [Google Scholar]
- Artemisia afra Jacq. ex Willd http://pza.sanbi.org/artemisia-afra (Accessed on 14 May 2020)
- Asante D.B., Henneh I.T., Acheampong D.O., Kyei F., Adokoh C.K., Ofori E.G., Domey N.K., Adakudugu E., Tangella L.P., Ameyaw E.O. Anti-inflammatory, anti-nociceptive and antipyretic activity of young and old leaves of Vernonia amygdalina. Biomed. Pharmacother. 2019;111:1187–1203. doi: 10.1016/j.biopha.2018.12.147. [DOI] [PubMed] [Google Scholar]
- Asogwa F.C., Ibezim A., Ntie-Kang F., Asogwa C.J., Okoye C.O. Anti-psoriatic and immunomodulatory evaluation of psorospermum febrifugum spach and its phytochemicals. Sci. Afr. 2020;7 [Google Scholar]
- Atawodi S.E.O., Olowoniyi O.D. Pharmacological and therapeutic activities of Kigelia africana (Lam.) Benth. Ann. Res. Rev. Biol. 2015:1–17. [Google Scholar]
- Avci O., Dik B. Determination of in vitro antiviral activity of Nerium oleander distillate against to parainfluenza-3 virus. Anim. Vet. Sci. 2014;2(5):150–153. [Google Scholar]
- Aworet-Samseny R.R., Souza A., Kpahé F., Konaté K., Datté J.Y. Dichrostachys cinerea (L.) Wight et Arn (Mimosaceae) hydro-alcoholic extract action on the contractility of tracheal smooth muscle isolated from Guinea-pig. BMC Compl. Alternative Med. 2011;11(1):1–8. doi: 10.1186/1472-6882-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awuchi C.G. Medicinal plants: the medical, food, and nutritional biochemistry and uses. Int. J. Adv. Acad. Res. 2019;5(11):220–241. [Google Scholar]
- Ayukekbong J.A., Ntemgwa M., Atabe A.N. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob. Resist. Infect. Control. 2017;6:47. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiora A., Agbi A., Ogunleye S., Areola J., Babalola O. Toxicological evaluation of Ficus thonningii blume (Moraceae) stem bark extract on the liver, kidney and testes of adult Wistar rats. Am. J. Biochem. 2016;6:59–71. [Google Scholar]
- Badr S.E., Shaaban M., Elkholy Y.M., Helal M.H., Hamza A.S., Masoud M.S., El Safty M.M. Chemical composition and biological activity of ripe pumpkin fruits (Cucurbita pepo L.) cultivated in Egyptian habitats. Nat. Prod. Res. 2011;25(16):1524–1539. doi: 10.1080/14786410903312991. [DOI] [PubMed] [Google Scholar]
- Bakana P., Claeys M., Totté J., Pieters L.A., Van Hoof L., Van Den Berghe D.A., Vlie-tinck A.J. Structure and chemotherapeutical activity of a polyisoprenylated benzophenone from the stem bark of Garcinia huillensis. J. Ethnopharmacol. 1987;21(1):75–84. doi: 10.1016/0378-8741(87)90096-1. [DOI] [PubMed] [Google Scholar]
- Baskaran X.R., Vigila A.V.G., Zhang S.Z., Feng S.X., Liao W.B. A review of the use of pteridophytes for treating human ailments. J. Zhejiang Univ. - Sci. B. 2018;19:85–119. doi: 10.1631/jzus.B1600344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batisai K. Towards an integrated approach to health and medicine in Africa. Sahara J. (J. Soc. Aspects HIV/AIDS Res. Alliance) 2016;13(1):113–122. doi: 10.1080/17290376.2016.1220323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoro J., Ekowati G. An ethnobotanical tobacco (Nicotiana tabacum L.) in Indonesia. A review. Adv. Life Sci. 2017;7(2):26–29. [Google Scholar]
- Becker J.V., Van der Merwe M.M., van Brummelen A.C., Pillay P., Crampton B.G., Mmutlane E.M., Parkinson C., Van Heerden F.R., Crouch N.R., Smith P.J., Mancama D.T. In vitro anti-plasmodial activity of Dicoma anomala subsp. gerrardii (Asteraceae): identification of its main active constituent, structure-activity relationship studies and gene expression profiling. Malar. J. 2011;10(1):1–11. doi: 10.1186/1475-2875-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellik Y. Total antioxidant activity and antimicrobial potency of the essential oil and oleoresin of Zingiber officinale Roscoe. Asian Paci. J. Trop. Dis. 2014;4(1):40–44. [Google Scholar]
- Bello O., Ojediran J., Dada A.O., Olatunya A., Awakan O. In vivo toxicity studies and phytochemical screening of stem bark of Ficus sycomorus Linn (Moraceae) IOSR-JESTFT. 2015;9:72–74. [Google Scholar]
- Bello I., Shehu M.W., Musa M., Asmawi M.Z., Mahmud R. Kigelia africana (Lam.) Benth.(Sausage tree): phytochemistry and pharmacological review of a quintessential African traditional medicinal plant. J. Ethnopharmacol. 2016;189:253–276. doi: 10.1016/j.jep.2016.05.049. [DOI] [PubMed] [Google Scholar]
- Bester S.P., Condy G.S. Gomphocarpus glaucophyllus schtr. Flower. Plants Afr. 2017;65:120–130. [Google Scholar]
- Beuscher N., Bodinet C., Neumann-Haefelin D., Marston A., Hostettmann K. Antiviral activity of African medicinal plants. J. Ethnopharmacol. 1994;42(2):1–109. doi: 10.1016/0378-8741(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Bhat J.A., Kumar M., Bussmann R.W. Ecological status and traditional knowledge of medicinal plants in Kedarnath wildlife sanctuary of Garhwal Himalaya. Ind. J. Ethnobiol. Ethnomed. 2013;9(1):b5. doi: 10.1186/1746-4269-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhebhe M., Chipurura B., Muchuweti M. Determination and comparison of phenolic compound content and antioxidant activity of selected local Zimbabwean herbal teas with exotic Aspalathus linearis. South Afr. J. Bot. 2015;100:213–218. [Google Scholar]
- Bodeker G., Bhat K.K.S., Burley J., Vantomme P. Food and Agriculture Organization of the United Nations; Oxford, UK: 1997. Medicinal Plants for Forest Conservation and Health Care. [Google Scholar]
- Bougatsos C., Meyer J.J.M., Magiatis P., Vagias C., Chinou I.B. Composition and antimicrobial activity of the essential oils of Helichrysum kraussii Sch. Bip. and H. rugulosum Less. from South Africa. Flavour Fragrance J. 2003;18(1):48–51. [Google Scholar]
- Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans. R. Soc. Trop. Med. Hyg. 2006;100(3):191–199. doi: 10.1016/j.trstmh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Bunalema L., Kirimuhuzya C., Tabuti J.R.S., Waako P., Magadula J.J., Otieno N., Orodho J.A., Okemo P. The efficacy of the crude root bark extracts of Erythrina abyssinica on rifampicin resistant. Mycobacterium tuberculosis. African health sciences. 2011;11(4):587–593. [PMC free article] [PubMed] [Google Scholar]
- Burgess E.P., Koha E.M.W.T., Hutchins R.F., Douglas L. Toxicity of leaves from the castor oil plant, Ricinus communis L.(Euphorbiaceae), to adult grass grub, Costelytra zealandica (White)(Coleoptera: scarabaeidae) N. Z. J. Exp. Agric. 1988;16(1):63–66. [Google Scholar]
- Bussmann R.W., Malca G., Glenn A., Sharon D., Nilsen B., Parris B., Dubose D., Ruiz D., Saleda J., Martinez M., Carillo L. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol. 2011;137(1):121–140. doi: 10.1016/j.jep.2011.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Wei G.X., van der Bijl P., Wu C.D. Namibian chewing stick, Diospyros lycioides, contains antibacterial compounds against oral pathogens. J. Agric. Food Chem. 2000;48(3):909–914. doi: 10.1021/jf9909914. [DOI] [PubMed] [Google Scholar]
- Cardoso B.M., Mello T.F.P.D., Lopes S.N., Demarchi I.G., Lera D.S.L., Pedroso R.B., Cortez D.A., Gazim Z.C., Aristides S.M.A., Silveira T.G.V., Lonardoni M.V.C. Antileishmanial activity of the essential oil from Tetradenia riparia obtained in different seasons. Mem. Inst. Oswaldo Cruz. 2015;110(8):1024–1034. doi: 10.1590/0074-02760150290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro D.C., Rivera D.E., Ocampo Y., Franco L.A., Salas R.D. Pharmacological evaluation of Mentha spicata L. and Plantago major L., medicinal plants used to treat anxiety and insomnia in Colombian Caribbean coast. Evid. Based. Complementary. Altern. Med. eCAM. 2018 doi: 10.1155/2018/5921514. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Mauro I., De Blasio E., Crispo A., Del Gaudio A., Bimonte S., Cuomo A., Ascierto P.A. Rapid and impressive response to a combined treatment with single-dose tocilizumab and NIV in a patient with COVID-19 pneumonia/ARDS. Medicina. 2020;56(8):377. doi: 10.3390/medicina56080377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacha M. Schistosomacidal activity of Lannea schimperi and searsia longipes against Cercariae, schistosomula and adult stage of schistosoma mansoni. Chron. Pharmaceut. Sci. 2019;3:787–799. [Google Scholar]
- Chacha M., Mbugi N.O. Acute toxicity, brine shrimp lethality and phytochemical screening of Lannea schimperi and searsia longipes. J. Chem. Health Risks. 2019;9(2):87–95. [Google Scholar]
- Chan E.W., Eng S.Y., Tan Y.P., Wong Z.C. Phytochemistry and pharmacological properties of Thunbergia laurifolia: a review. Phcog. J. 2011;3(24):1–6. [Google Scholar]
- Chan Y.S., Khoo K.S., Sit N.W.W. Investigation of twenty selected medicinal plants from Malaysia for anti-Chikungunya virus activity. Int. Microbiol. 2016;19(3):175–182. doi: 10.2436/20.1501.01.275. [DOI] [PubMed] [Google Scholar]
- Chauke M.A., Shai L.J., Mogale M.A., Mokgotho M.P. Antibacterial and anti HIV 1 reverse transcriptase activity of selected medicinal plants from Phalaborwa, South Africa. Res. J. Med. Plant. 2016;10:388–395. [Google Scholar]
- Chávez D., Chai H.B., Chagwedera T.E., Gao Q., Farnsworth N.R., Cordell G.A., Pezzuto J.M., Kinghorn A.D. Novel stilbenes isolated from the root bark of Ekebergia benguelensis. Tetrahedron Lett. 2001;42(22):3685–3688. [Google Scholar]
- Chavunduka G.L., Gelfand M., Roberts R.S. 1978. Traditional Healers and the Shona Patient. [Google Scholar]
- Chen H.Y., Yen G.C. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 2007;101(2):686–694. [Google Scholar]
- Chen H., Jiao H., Cheng Y., Xu K., Jia X., Shi Q., Guo S., Wang M., Du L., Wang F. In vitro and in vivo immunomodulatory activity of okra (Abelmoschus esculentus L.) polysaccharides. J. Med. Food. 2016;19(3):253–265. doi: 10.1089/jmf.2015.3513. [DOI] [PubMed] [Google Scholar]
- Chen G.L., Mutie F.M., Xu Y.B., Saleri F.D., Hu G.W., Guo M.Q. Antioxidant, anti- inflammatory activities and polyphenol profile of rhamnus prinoides. Pharmaceuticals. 2020;13:55. doi: 10.3390/ph13040055. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigora P., Masocha R., Mutenheri F. The role of indigenous medicinal knowledge (IMK) in the treatment of ailments in rural Zimbabwe: the case of Mutirikwi communal Lands. JSDA. 2007;9(2):26–43. [Google Scholar]
- Chimponda T., Mukanganyama S. Antimycobacterial activities of selected medicinal plants from Zimbabwe against Mycobacterium aurum and Corynebacterium glutamicum. Trop. Biomed. 2010;27(3):595–610. [PubMed] [Google Scholar]
- Chinemana F., Drummond R.B., Mavi S., De Zoysa I. Indigenous plant remedies in Zimbabwe. J. Ethnopharmacol. 1985;14(2–3):159–172. doi: 10.1016/0378-8741(85)90084-4. [DOI] [PubMed] [Google Scholar]
- Ching F.P., Omogbai E.K.I., Ozolua R.I., Okpo S.O. Antidiarrhoeal activities of aqueous extract of Stereospermum kunthianum (Cham, Sandrine Petit) stem bark in rodents. Afr. J. Biotechnol. 2008;7(9) [Google Scholar]
- Ching F.P., Omogbai E.K.I., Okpo S.O., Ozolua R.I. Antiinflammatory activity of aqueous extract of Stereospermum kunthianum (Cham, Sandrine Petit) stem bark in rats. Indian J. Pharmaceut. Sci. 2009;71(1):106. doi: 10.4103/0250-474X.51943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching F.P., Omogbai E.K., Ozolua R.I., Okpo S.O. Analgesic activity of aqueous extract of Stereospermum kunthianum (Cham, Sandrine Petit) stem bark. Acta Poloniae Pharmaceut. Drug Res. 2009;66(1):83–88. [PubMed] [Google Scholar]
- Chipinga J.V. Efficacy of pterocarpus angolensis crude extracts against Candida krusei, Staphylococcus aureus, Streptococcus agalactiae and Escherichia coli. Malawi Med. J. 2018;30(4):219–224. doi: 10.4314/mmj.v30i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipiti T., Ibrahim M.A., Singh M., Islam M.S. In vitro α-amylase and α-glucosidase inhibitory effects and cytotoxic activity of Albizia antunesiana extracts. Phcog. Mag. 2015;11(Suppl. 2):S231. doi: 10.4103/0973-1296.166018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiramba O., Mukanganyama S. Cytotoxic effects of Combretum platypetalum Welw. Ex MA Lawson subsp. oatesii (Rolfe) Exell (Combretaceae) leaf extracts on Jurkat T-cells and reversal of effects by reduced Glutathione. J. Biol. Active Prod. Nat. 2016;6(3):250–265. [Google Scholar]
- Chiribagula V.B., Bakari A.S., Ndjolo P.O., Kahumba B.J., Simbi J.B.L. Phytochemical screening and antioxidant activity of methanolic extracts of 53 antimalarial plants from Bagira in Eastern DR Congo. GSC Biol. Pharmaceut. Sci. 2020;12(2):99–118. [Google Scholar]
- Chirisa E., Mukanganyama S. Evaluation of in vitro anti-inflammatory and antioxidant activity of selected Zimbabwean plant extracts. J. Herbs, Spices, Med. Plants. 2016;22(2):157–172. [Google Scholar]
- Chitopo W., Muchacha I., Mangoyi R. Evaluation of the antimicrobial activity of Erythrina abyssinica leaf extract. J. Microb. Biochem. Technol. 2019;11(2) [Google Scholar]
- Chowdhury N.S., Jamaly S., Farjana F., Begum N., Zenat E.A. A review on ethnomedicinal, pharmacological, phytochemical and pharmaceutical profile of Lady's finger (Abelmoschus esculentus L.) Plant. Pharmacol. Pharm. 2019;10:94–108. http://www.scirp.org/journal/ 2019. [Google Scholar]
- Cock I.E. The genus aloe: phytochemistry and therapeutic uses including treatments for gastrointestinal conditions and chronic inflammation. Novel Nat. Prod.: Therap. Eff. Pain Arthr. Gastro-intestinal Dis. 2015:179–235. doi: 10.1007/978-3-0348-0927-6_6. [DOI] [PubMed] [Google Scholar]
- Cock I.E., Van Vuuren S.F. The traditional use of southern African medicinal plants in the treatment of viral respiratory diseases: a review of the ethnobotany and scientific evaluations. J. Ethnopharmacol. 2020 doi: 10.1016/j.jep.2020.113194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker M.E., Oaikhena A.O. Antimicrobial activity of the crude extracts and fractions of Ficus thonningii (blume) on isolates from urinary tract infections. J. Med. Active Plants. 2020;9(4):310–322. [Google Scholar]
- Coker M., Emikpe B., Adeniyi B., Budale B. The antiinflammatory potential, hematological and histological changes induced in rats due to the administration of methanolic extracts of Ficus thonningii leaves. Afr. J. Pharm. Pharmacol. 2009;3:273–276. [Google Scholar]
- Constance C., Bagar T., Walter C. Aqueous extracts of pericopsis angolensis and swartzia madagascariensis with high antimicrobial activities against Escherichia coli O157, Shigella spp. and Salmonella enterica subsp. enterica (serovar typhi) Afr. J. Biotechnol. 2019;18(29):831–844. [Google Scholar]
- Coopoosamy R.M. Isolation of volatile compounds of Aloe excelsa (Berger) Afr. J. Biotechnol. 2010;9(43):7289–7294. [Google Scholar]
- Coopoosamy R.M., Magwa M.L. Traditional use, antibacterial activity and antifungal activity of crude extract of Aloe excelsa. Afr. J. Biotechnol. 2007;6(20) [Google Scholar]
- Cordier W., Gulumian M., Cromarty A.D., Steenkamp V. Attenuation of oxidative stress in U937 cells by polyphenolic-rich bark fractions of Burkea africana and Syzygium cordatum. BMC Compl. Alternative Med. 2013;13:116. doi: 10.1186/1472-6882-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos P., Hermans N., De Bruyne T., Apers S., Sindambiwe J.B., Witvrouw M., De Clercq E., Berghe D.V., Pieters L., Vlietinck A.J. Antiviral activity of Rwandan medicinal plants against human immunodeficiency virus type-1 (HIV-1) Phytomedicine. 2002;9(1):62–68. doi: 10.1078/0944-7113-00083. [DOI] [PubMed] [Google Scholar]
- Costa S.S., Muzitano M.F., Camargo L.M., Coutinho M.A. Therapeutic potential of Kalanchoe species: flavonoids and other secondary metabolites. Nat. Prod. Commun. 2008;3(12) 1934578X0800301236. [Google Scholar]
- Cunningham A.B. Institute of Natural Resources. University of Naal; Pietermaritzburg: 1988. An Investigation of Herbal Medicine Trade in Natal/Kwazulu. Investigational Report Number 29. [Google Scholar]
- Cunningham A.B. UNESCO; Paris: 1993. African Medicinal Plants: Setting Priorities at the Interface between Conservation and Primary Health Care. Working paper 1. [Google Scholar]
- da Silva M.G.C., Amorim R.N.L., Câmara C.C., Fontenele Neto J.D., Soto-Blanco B. Acute and sub-chronic toxicity of aqueous extracts of Chenopodium ambrosioides leaves in rats. J. Med. Food. 2014;17(9):979–984. doi: 10.1089/jmf.2013.0134. [DOI] [PubMed] [Google Scholar]
- Dakone D., Guadie A. A review on ethnomedicinal use, nutritional value, phytochemistry and pharmacological characteristics of Solanum incanum L. An important medicinal plant. Int. J. Sci. Technol. Res. 2016;5(6):350–354. [Google Scholar]
- Dangarembizi R., Erlwanger K.H., Moyo D., Chivandi E. Phytochemistry, pharmacology and ethnomedicinal uses of Ficus thonningii (Blume moraceae): a review. Afr. J. Tradit., Complementary Altern. Med. 2013;10(2):203–212. doi: 10.4314/ajtcam.v10i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danladi S., Hassan M.A., Masa'ud I.A., Ibrahim U.I. Vernonia amygdalina Del: a mini review. Res. J. Pharm. Technol. 2018;11(9):4187–4190. [Google Scholar]
- Daswani P.G., Gholkar M.S., Birdi T.J. Psidium guajava: a single plant for multiple health problems of rural Indian population. Phcog. Rev. 2017;11:167. doi: 10.4103/phrev.phrev_17_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies F.T., Geneve R.L., Wilson S.B. Hartmann and Kester's Plant Propagation Principles and Practices. ninth ed. Pearson Education, Inc.; New York: 2017. p. 1004. Hardcover. -13: 978-0-13-448089-3. [Google Scholar]
- Dawang N. Phytochemical constituents and toxicological study of vitex doniana leaf. IOSR-JPBS. 2015;10(5):23–27. doi: 10.9790/3008-10542327. [DOI] [Google Scholar]
- de Dieu Tamokou J., Chouna J.R., Fischer-Fodor E., Chereches G., Barbos O., Damian G., Benedec D., Duma M., Efouet A.P.N., Wabo H.K., Kuiate J.R. Anticancer and antimicrobial activities of some antioxidant-rich Cameroonian medicinal plants. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morais Lima G.R., De Sales I.R.P., Caldas Filho M.R.D., De Jesus N.Z.T., de Sousa Falcão H., Barbosa-Filho J.M., Cabral A.G.S., Souto A.L., Tavares J.F., Batista L.M. Bioactivities of the genus Combretum (Combretaceae): a review. Molecules. 2012;17(8):9142–9206. doi: 10.3390/molecules17089142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt R.T., Farias I.V., Grassi L.T., Franchi G.C., Nowill A.E., Bittencourt C.M.D.S., Wagner T.M., de Souza M.M., Cruz A.B., Malheiros A. Characterization and evaluation of the cytotoxic potential of the essential oil of Chenopodium ambrosioides. Revista Brasileira de Farmacognosia. 2016;26(1):56–61. [Google Scholar]
- Deivasigamani R. Phytochemical analysis of Leucaena leucocephala on various extracts. J. Phytopharmacol. 2018;7(6):480–482. [Google Scholar]
- Di Gennaro F., Pizzol D., Marotta C., Antunes M., Racalbuto V., Veronese N., Smith L. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int. J. Environ. Res. Publ. Health. 2020;17(8):2690. doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo M.S.T., Baldé M.A., Camara A., Traoré M.S., Bah M.L., Diallo A.S., Camara A.K., Laurent S., Roch A., Muller R.N., Maes L. Ethnomedical, phytochemical and biological investigations of Margaritaria discoidea (Ball.) Webster, a plant species widely used in Guinean traditional medicine. J. Plant Sci. 2015;3:40–46. [Google Scholar]
- Dickson R.A., Fleischer T.C., Ekuadzi E., Mensah A.Y., Annan K., Woode E. Antibacterial, antioxidant and anti-inflammatory properties of Margaritaria discoidea, a wound healing remedy from Ghana. Phcog. J. 2010;2(17):32–39. [Google Scholar]
- Dikko Y.J., Khan M.E., Tor-Anyiin T.A., Anyam J.V., Linus U.A. In vitro antimicrobial activity of fruit pulp extracts of Azanza garckeana (F. Hoffm.) Exell & Hillc. And isolation of one of its active principles, Betulinic acid. J. Pharmaceut. Res. Int. 2016:1–10. [Google Scholar]
- Dimene L., Mutseyekwa F., Chifamba J., Nyakatawa G., Mahachi C., Marume A., Bhebhe M., Taderera T. A cross-sectional study to determine the use of alternative medicines during pregnancy in the district hospitals in Manicaland, Zimbabwe. Afr. Health Sci. 2020;20(1):64–72. doi: 10.4314/ahs.v20i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombo A., Da Costa E., Neto G. 2002. Zimbabwe Plant Red Data List 2002.https://www.nationalredlist.org/zimbabwe-plant-red-data-list-2002/ [Google Scholar]
- Doreddula S.K., Bonam S.R., Gaddam D.P., Desu B.S.R., Ramarao N., Pandy V. The Scientific World Journal; 2014. Phytochemical Analysis, Antioxidant, Antistress, and Nootropic Activities of Aqueous and Methanolic Seed Extracts of Ladies Finger (Abelmoschus Esculentus L.) in Mice. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzoyem J.P., Aro A.O., McGaw L.J., Eloff J.N. Antimycobacterial activity against different pathogens and selectivity index of fourteen medicinal plants used in Southern Africa to treat tuberculosis and respiratory ailments S. Afr. J. Bot. 2016;102:70–74. doi: 10.1016/j.sajb.2015.08.002. [DOI] [Google Scholar]
- Ediriweera M.K., Tennekoon K.H., Samarakoon S.R. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (Mango) Evid. base Compl. Alternative Med. 2017;2017:1–24. doi: 10.1155/2017/6949835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpo M.A., Etim P.C. Antimicrobial activity of ethanolic and aqueous extracts of Sida acuta on microorganisms from skin infections. J. Med. Plants Res. 2009;3(9):621–624. [Google Scholar]
- El Badwi S.M.A., Bakhiet A.O. Chemotherapeutic and economic potentials of some laticiferous plants. J. Med. Plants Res. 2012;6(17):3278–3281. [Google Scholar]
- El Kheir Y.M., El Tohami M.S. Investigation of molluscicidal activity of certain Sudanese plants used in folk-medicine. II. Molluscicidal activity of the different morphological organs of Gnidia kraussiana Meisn" abu gotna" family Thymelaeaceae. J. Trop. Med. Hyg. 1979;82(11–12):242–247. [PubMed] [Google Scholar]
- El-Beltagi H.S., Mohamed H.I., Abdelazeem A.S., Youssef R., Safwat G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Not. Bot. Horti. Agrobo. 2019;47:493–505. [Google Scholar]
- El-Beltagi H.S., Mohamed H., Elmelegy A.A., Eldesoky S.E., Safwat G. Phytochemical screening, antimicrobial, antiaxidant, anticancer activities and nutritional values of cactus (Opuntia ficus indicia) pulp and peel. Fresenius Environ. Bull. 2019;28:1534–1551. [Google Scholar]
- El-Toumy S.A., Salib J.Y., El-Kashak W.A., Marty C., Bedoux G., Bourgougnon N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci. Hum. Wellness. 2018;7(1):91–101. [Google Scholar]
- Elufioye T.O., Bamgbose M.O., Alabi S.O. Evaluation of antioxidant and Antiacne activity of Psorospermum febrifugum (spach) and Psorospermum corymbiferum (Hochr.) J. Pharmaceut. Res. Int. 2016:1–10. [Google Scholar]
- Endris A., Asfaw N., Bisrat D. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Lippia javanica leaves from Ethiopia. J. Essent. Oil Res. 2016;28(3):221–226. [Google Scholar]
- Epifano F., Fiorito S., Genovese S. Phytochemistry and pharmacognosy of the genus Psorospermum. Phytochemistry Rev. 2013;12(4):673–684. doi: 10.1016/j.phytochem.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Erhabor J.O., Komakech R., Kang Y., Tang M., Matsabisa M.G. Ethnopharmacological importance and medical applications of Myrothamnus flabellifolius Welw. (Myrothamnaceae)-A review. J. Ethnopharmacol. 2020;252 doi: 10.1016/j.jep.2020.112576. 2020 Apr. [DOI] [PubMed] [Google Scholar]
- Ezenyi I.C., Igoli J.O. Antidiarrhoeal properties of Syzygium guineense leaf extract and identification of chemical constituents in its active column fractions. J. Compl. Integr. Med. 2018;16(2) doi: 10.1515/jcim-2016-0074. :/j/jcim.2019.16.issue-2/jcim-2016-0074/jcim-2016-0074.xml. [DOI] [PubMed] [Google Scholar]
- FAO . 2015. Factors Contributing to the Introduction and Spread of Invasive Species.http://www.fao.org/forestry/aliens/52519/en/ [Google Scholar]
- Farzaei M.H., Bahramsoltani R., Ghobadi A., Farzaei F., Najafi F. Pharmacological activity of Mentha longifolia and its phytoconstituents. J. Tradit. Chin. Med. 2017;37:710–720. [PubMed] [Google Scholar]
- FIRS . The Global Impact of Respiratory Disease. second ed. European Respiratory Society; Sheffield: 2017. https://www.firsnet.org/images/publications/The_Global_Impact_of_Respiratory_Disease.pdf [Google Scholar]
- Fouad M.A., Wanas A.S., Khalil H.E. Phytochemical and biological studies of Clerodendrum Glabraum leaves. Int. J. Pharmacogn. Phytochem. 2013;28(2):1164–11688. [Google Scholar]
- Gaire B.P., Subedi L. A review on the pharmacological and toxicological aspects of Datura stramonium L. Integr. Med. 2013;11(2):73–79. doi: 10.3736/jintegrmed2013016. [DOI] [PubMed] [Google Scholar]
- Ganesan K., Gani S. Ethnomedical and pharmacological potentials of plumbago zeylanica LA. Am. J. Phytomed. Clin. Ther. 2013;1(3):313–337. [Google Scholar]
- Garba M.M., Hamza Y.A., Muhammad M.A., Akpojo A.J., Ibrahim A.A., Marte H.I. Neuropharmacological studies on ethyl acetate fraction of Securinega virosa root bark extract. African J. Pharm. Pharmacol. 2013;7(6):275–279. [Google Scholar]
- Garba M.M., Jamilu Y., Muhammad M.A., Akpojo A.J., Ibrahim A.A., Marte H.I. Securinega virosa (Euphorbiaceae) root bark extract inhibits glioblastoma multiforme cell survival in vitro. African J. Pharm. Pharmacol. 2015;9(27):684–693. [Google Scholar]
- Garima Z., Amla B. A review on chemistry and pharmacological activity of Nerium oleander L. J. Chem. Pharmaceut. Res. 2010;2(6):351–358. [Google Scholar]
- Gelfand M. 1956. Medicine and Magic of the Mashona. Cape Town, Juta) [Google Scholar]
- Gelfand M., Mavi S., Drummond R.B., Ndemera B. Mambo Press; Gweru, Zimbabwe: 1985. The Traditional Medical Practitioner in Zimbabwe. His Principles of Practice and Pharmacopoeia. [Google Scholar]
- Ghuman S., Ncube B., Finnie J.F., McGaw L.J., Njoya E.M., Coopoosamy R.M., Van Staden J. Antioxidant, anti-inflammatory and wound healing properties of medicinal plant extracts used to treat wounds and dermatological disorders. South Afr. J. Bot. 2019;126:232–240. [Google Scholar]
- Gitu L.M. 2013. Biological and Phytochemical Studies of Medicinal Plants, Antidesma Venosum (Euphorbiaceae) and Kotschya Africana (Fabaceae) Used in Traditional Medicine in Kenya. Doctoral dissertation. [Google Scholar]
- Golding J.S. SABONET; Pretoria: 2002. Southern African Plant Red Data Lists. Southern African Botanical Diversity Network Report No. 14. [Google Scholar]
- Grace O.M., Prendergast H.D.V., Jäge A.K., Van Staden J. Bark medicines used in traditional healthcare in KwaZulu-Natal, South Africa: an inventory. South Afr. J. Bot. 2003;69(3):301–363. 2003. [Google Scholar]
- Gronde T.V., Uyl-de Groot C.A., Pieters T. Addressing the challenge of high-priced prescription drugs in the era of precision medicine: a systematic review of drug life cycles, therapeutic drug markets and regulatory frameworks. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0182613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundidza M., Gweru N., Mmbengwa V., Ramalivhana N.J., Magwa Z., Samie A. Phytoconstituents and biological activities of essential Oil from Rhus lancea L. F. Afr. J. Biotechnol. 2008;7(16) [Google Scholar]
- Guo Nan Mei. Aloe vera: a review of toxicity and adverse clinical effects. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016;34(2):77–96. doi: 10.1080/10590501.2016.1166826. 2016 April 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gv N.K., Pullaiah C.P., S D., G.D.R. Acute toxicity studies of aqueous seed extract of vigna unguiculata in albino rats. Innovare J. Ayurvedic Sci. 2017;5(2):1–4. [Google Scholar]
- Hamidi M.R., Jovanova B., Panovska T.K. Toxicоlogical evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Macedonian pharmaceut. Bull. 2014;60(1):9–18. 2014) ISSN 1409 – 8695 UDC: 615.322.099. [Google Scholar]
- Hammadi R., Kúsz N., Mwangi P.W., Kulmány Á., Zupkó I., Orvos P., Tálosi L., Hohmann J., Vasas A. Isolation and pharmacological investigation of compounds from Euphorbia matabelensis. Nat. Prod. Commun. 2019;14(7) 1934578X19863509. [Google Scholar]
- Hase G.J., Deshmukh K.K., Murade V.D., Pokharkar R.D., Phatanagre N.D., Hase D.P., Gosavi A.B. Phytopharmacology of Nerium oleander L. A review. Int. J. Phytopharm. 2016;7(2):975–9328. [Google Scholar]
- Hassan H., Ahmadu A., Hassan A. Analgesic and anti-inflammatory activities of Asparagus africanus root extract. Afr. J. Tradit., Complementary Altern. Med. 2008;5:27–31. doi: 10.4314/ajtcam.v5i1.31252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley-Smith C.J., Botha F.S., Hussein A.A., Nkomo M., Meyer D., Lall N. Biological activities of heteropyxis natalensis against micro-organisms involved in oral infections. Front. Pharmacol. 2018;9:291. doi: 10.3389/fphar.2018.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hęś M., Dziedzic K., Górecka D., Jędrusek-Golińska A., Gujska E. Aloe vera (L.) Webb.: natural sources of Antioxidants–A review. Plant Foods Hum. Nutr. 2019;74(3):255–265. doi: 10.1007/s11130-019-00747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.A. A review on Ficus sycomorus: a potential indigenous medicinal plant in Oman. J. King Saud Univ. Sci. 2019;31:961–965. [Google Scholar]
- Hudson J.B., Anani K., Lee M.K., De Souza C., Arnason J.T., Gbeassor M. Further iinvestigations on the antiviral activities of medicinal plants of Togo. Pharmaceut. Biol. 2000;38(1):46–50. doi: 10.1076/1388-0209(200001)3811-BFT046. [DOI] [PubMed] [Google Scholar]
- Hunter P. The human impact on biological diversity. How species adapt to urban challenges sheds light on evolution and provides clues about conservation. EMBO Rep. 2007;8(4):316–318. doi: 10.1038/sj.embor.7400951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurinanthan V. 2013. Anti-HIV Activity of Selected South African Medicinal Plants (Doctoral Dissertation) [Google Scholar]
- Hus S., Mazbah Udd A.H.M., Azmerin M., Tohidul Am M., Hasan M., Das Aka T., Mazumder T. An in vivo and in vitro evaluation of anti-inflammatory action of seeds of vigna unguiculata available in Bangladeshi region. J. Appl. Sci. 2019;19(2):62–67. [Google Scholar]
- Hussain S.M., Hussain M.S., Arbaaz Ahmed L., Arif N. Characterization of isolated bioactive phytoconstituents from Flacourtia indica as potential phytopharmaceuticals-An in silico perspective. J. Pharmacogn. Phytochem. 2016;5:323–331. [Google Scholar]
- Ibrahim H., Oyi R.A., Ehinmidu J.O., Musa K.Y., Bright N.T. Antimicrobial activity of the water extracts of the leaves and fruits of Carissa edulis Vahl (Apocynaceae) J. Med. Plants Res. 2010;4(11):1028–1032. [Google Scholar]
- Ibrahim H., Williams F.E., Salawu K.M., Usman A.M. Phytochemical screening and acute toxicity studies of crude ethanolic extract and flavonoid fraction of Carissa edulis leaves. Biokemistri. 2015;27(1):39–43. [Google Scholar]
- Ighodaro O.M., Omole J.O. International Scholarly Research Notices; 2012. Effects of Nigerian Piliostigma Thonningii Species Leaf Extract on Lipid Profile in Wistar Rats. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavarasan R., Mallika M., Venkataraman S. Toxicological assessment of Ricinus communis Linn root extracts. Toxicol. Mech. Methods. 2011;21(3):246–250. doi: 10.3109/15376516.2010.538752. [DOI] [PubMed] [Google Scholar]
- Indhumathi T., Mohandass S., Shibi A. Acute toxicity study of ethanolic extract of Solanum incanum L. fruit. Asian J. Pharmaceut. Clin. Res. 2014;7:98–100. [Google Scholar]
- Indravathi G., Reddy R.S., Babu P.S. Albizia amara-A potential medicinal plant: a. Int. J. Sci. Res. 2016;5(3):621–627. [Google Scholar]
- Inusa A., Sanusi S.B., Linatoc A.C., Mainassara M.M., Awawu J.J. Phytochemical analysis and antimicrobial activity of bitter leaf (Vernonia amygdalina) collected from Lapai, Niger State, Nigeria on some selected pathogenic microorganisms. Sci. World J. 2018;13(3):15–18. [Google Scholar]
- Iqbal Z., Lateef M., Jabbar A., Ghayur M.N., Gilani A.H. In vitro and in vivo anthelmintic activity of Nicotiana tabacum L. leaves against gastrointestinal nematodes of sheep. Phytother Res.: Int. J. Dev. Pharmacol. Toxicol. Evaluat. Nat. Prod. Derivat. 2006;20(1):46–48. doi: 10.1002/ptr.1800. [DOI] [PubMed] [Google Scholar]
- Irie-N'guessan A.G., Kouakou S.L., Koua K.B., Effo K.E., Djadji A.T., Diarrassouba N. Anticonvulsant and analgesic assessment of Dichrostachys cinerea root bark, an Ivorian anti-asthmatic herbal, in mice. J. Pharmacol. Clin. Res. 2018;6:1–6. [Google Scholar]
- Irié-N’guessan A.G., Kouakou S.L., Effo K.E., Adepo A.A., Kouakou-Siransy N.G. Anti-inflammatory and antioxidant potential of Dichrostachys cinerea root bark, an Ivorian anti-asthmatic herbal. Int. J. Pharmacol. Res. 2017;7(12):248–254. [Google Scholar]
- Isa A.I., Awouafack M.D., Dzoyem J.P., Aliyu M., Magaji R.A., Ayo J.O., Eloff J.N. Some Strychnos spinosa (Loganiaceae) leaf extracts and fractions have good antimicrobial activities and low cytotoxicities. BMC Compl. Alternative Med. 2014;14(1):1–8. doi: 10.1186/1472-6882-14-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.T. Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): a literature‐based review. Phytother Res. 2019;33(1):72–80. doi: 10.1002/ptr.6212. [DOI] [PubMed] [Google Scholar]
- Isyaku A., Abubakar S., Osuji C.E., Mowobi G.G., Onwuka O.E. In vitro efficacy of three ethnomedicinal plants used in Gbagi land against Salmonella enterica serovar typhi. J. Environ. Life Sci. 2016;1(1):56–65. [Google Scholar]
- Jahangir U. Siris (Albizzia lebbeck (L.) Benth.) in view of Unani and contemporary pharmacology. Int. J. Green Pharm. 2018;12(2) [Google Scholar]
- Jain P., Sharma H.P., Basri F., Baraik B., Kumari S., Pathak C. Pharmacological profiles of ethno-medicinal plant: plumbago zeylanica l.-A review. Int. J. Pharmaceut. Sci. Rev. Res. 2014;24(1):157–163. [Google Scholar]
- Jain P., Sharma H.P., Singh P. 2020. Anti-fungal, Anti-oxidant and Phytochemical Analysis of Plumbago Zeylanica L. Vegetos; pp. 1–11. [Google Scholar]
- James P.B., Wardle J., Steel A., Adams J. Traditional, complementary and alternative medicine use in Sub-Saharan Africa: a systematic review. BMJ. Glob. Health. 2018;3 doi: 10.1136/bmjgh-2018-000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabongo‐Kayoka P.N., Eloff J.N., Obi C.L., McGaw L.J. Antimycobacterial activity and low cytotoxicity of leaf extracts of some African Anacardiaceae tree species. Phytother Res. 2016;30(12):2001–2011. doi: 10.1002/ptr.5706. [DOI] [PubMed] [Google Scholar]
- Kafle A., Mohapatra S.S., Reddy I., Chapagain M. A review on medicinal properties of Psidium guajava. J. Med. Plants. Stud. 2018;6:44–47. [Google Scholar]
- Kamanja I.T., Mbaria J.M., Gathumbi P.K., Mbaabu M., Kabasa J.D., Kiama S.G. Cytotoxicity of selected medicinal plants extracts using the brine shrimp lethality assay from Samburu County, Kenya. J. Media Res. 2018;4(5):249–255. [Google Scholar]
- Kambizi L., Afolayan A.J. An ethnobotanical study of plants used for the treatment of sexually transmitted diseases (njovhera) in. Guruve District , Zimbabwe. 2001;77(1):5–9. doi: 10.1016/s0378-8741(01)00251-3. [DOI] [PubMed] [Google Scholar]
- Kambizi L., Sultana N., Afolayan A.J. Bioactive compounds isolated from Aloe ferox.: a plant traditionally used for the treatment of sexually transmitted infections in the eastern cape, South Africa. Pharmaceut. Biol. 2005;42(8):636–639. [Google Scholar]
- Katerere D., Parry O. Pharmacological actions of Heteromorpha trifoliata (" dombwe") on rat isolated muscle preparations. Cent. Afr. J. Med. 2000;46(1):9–13. [PubMed] [Google Scholar]
- Kaunda J.S., Zhang Y.J. The genus Carissa: an ethnopharmacological, phytochemical and pharmacological review. Nat. Prod. Bio[rospect. 2017;7(2):181–199. doi: 10.1007/s13659-017-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazembe T.C., Nkomo S. Use of Blumea alata, Bidens pilosa and Chenopodium ambrosioides as mosquito repellents and mosquitocides. Bull. Environ. Pharmacol. Life Sci. 2012;1:59–66. [Google Scholar]
- Kebede S., Afework M., Debella A., Ergete W., Makonnen E. Toxicological study of the butanol fractionated root extract of Asparagus africanus Lam., on some blood parameters and histopathology of liver and kidney in mice. BMC Res. Notes. 2016;9:49. doi: 10.1186/s13104-016-1861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee L.A., Shori A.B., Baba A.S. Bioactivity and health effects of Mentha spicata. Integr Food Nutr Metab. 2017:1–2. [Google Scholar]
- Kehlenbeck K., Asaah E., Jamnadass R. In: Diversifying Food and Diets: Using Agricultural Biodiversity to Improve Nutrition and Health. Fanzo J., Hunter D., Borelli T., Mattei F., editors. Earthscan Routledge; New York: 2013. Diversity of indigenous fruit trees and their contribution to nutrition and livelihoods in sub-Saharan Africa: examples from Kenya and Cameroon; pp. 257–269. [Google Scholar]
- Khaleel R.I. Bio-toxicity study of some selected plant by Artemia salina (leach) test. Plant. Arch. 2019;19(2):2847–2850. [Google Scholar]
- Kim D., Chen Z., Zhou L.F., Huang S.X. Air pollutants and early origins of respiratory diseases. Chronic. Dis. Transl. Med. 2018;4(2):75–94. doi: 10.1016/j.cdtm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn A.D.E.A., Farnsworth N., Soejarto D., Cordell G., Swanson S., Pezzuto J., Wani M., Wall M., Oberlies N., Kroll D., Kramer R. Novel strategies for the discovery of plant-derived anticancer agents. Pharmaceut. Biol. 2003;41(Suppl. 1):53–67. [Google Scholar]
- Kiondo F., Feyissa T., Ndakidemi P.A., Seth M. In vitro propagation of Dalbergia melanoxylon Guill. & Perr.: a multipurpose tree. Am. J. Res. Commun. 2014;2:181–194. [Google Scholar]
- Klimek-Szczykutowicz M., Szopa A., Ekiert H. Citrus limon (Lemon) phenomenon—a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and Cosmetics industries, and biotechnological studies. Plants. 2020;9(1):119. doi: 10.3390/plants9010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokanova-Nedialkova Z., Nedialkov P., Nikolov S. The genus Chenopodium: phytochemistry, ethnopharmacology and pharmacology. Phcog. Rev. 2009;3(6):280. [Google Scholar]
- Koné D., Cofie O., Zurbrugg C., Gallizzi K., Moser D., Drescher S., Strauss M. Helminth eggs inactivation efficiency by faecal sludge dewatering and co-composting in tropical climates. Water Res. 2007;41(19):4397–4402. doi: 10.1016/j.watres.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Kumar M. A review on phytochemical constituents and pharmacological activities of Ricinus communis L. Plant. Int. J. Pharmacogn. Phytochem. Res. 2017;9(4):466–472. doi: 10.25258/phyto.v9i2.8116. [DOI] [Google Scholar]
- Kumar R., Mishra A.K., Dubey N.K., Tripathi Y.B. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int. J. Food Microbiol. 2007;115(2):159–164. doi: 10.1016/j.ijfoodmicro.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Kuria J.M. Doctoral dissertation, University of Nairobi; 2014. Efficacy of Aspilia Pluriseta Schweinf in Cutaneous Wound Healing in a Mouse Model. [Google Scholar]
- Kuria J.M., Mbaria J.M., Gathumbi P.K., Kiama S.G. 2015. Influence of Aspilia Pluriseta Schweinf (Asteraceae) on the Healing of Dermal Excision Wounds (Mouse Model) and Skin Sensitization Activity (Guinea Pig Model) [Google Scholar]
- Lawal O.A., Ogunwande I.A., Opoku A.R., Kasali A.A., Oyedeji A.O. Chemical composition and antibacterial activities of essential oil of Warburgia salutaris (Bertol. F.) Chiov. From South Africa. J. of. biologically. active. products. from. Nature. 2014;4:272–277. [Google Scholar]
- Loots D.T., van der Westhuizen F.H., Botes L. Aloe ferox leaf gel phytochemical content, antioxidant capacity, and possible health benefits. J. Agric. Food Chem. 2007;55(17):6891–6896. doi: 10.1021/jf071110t. [DOI] [PubMed] [Google Scholar]
- Lotter N., Chivandi E., Lembede B.W., Ndhlala A.R., Nyakudya T.T., Erlwanger K.H. Anti-oxidant activity, alpha-amylase inhibition and toxicity of leaf extracts of cultivated Rapanea melanophloeos (L.) Mez (cape beech) South Afr. J. Bot. 2019;126:261–264. [Google Scholar]
- Macharia F.K., Mwangi P.W., Yenesew A., Bukachi F., Nyaga N.M., Wafula D.K. BioRxiv; 2019. Hepatoprotective Effects of Erythrina Abyssinica Lam Ex Dc against Non Alcoholic Fatty Liver Disease in Sprague Dawley Rats. [Google Scholar]
- Machumi F., Samoylenko V., Yenesew A., Derese S., Midiwo J.O., Wiggers F.T., Jacob M.R., Tekwani B.L., Khan S.I., Walker L.A., Muhammad I. Antimicrobial and antiparasitic abietane diterpenoids from the roots of Clerodendrum eriophyllum. Nat. Prod. Commun. 2010;5(6) 1934578X1000500605. [PMC free article] [PubMed] [Google Scholar]
- MacIntyre C.R., Bui C.M. Pandemics, public health emergencies and antimicrobial resistance - putting the threat in an epidemiologic and risk analysis context. Arch. Publ. Health. 2017;75:54. doi: 10.1186/s13690-017-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara A.A., Ajayi J.A., Salawu O.A., Tijani A.Y. Anti-malarial activity of ethanolic leaf extract of Piliostigma thonningii Schum. (Caesalpiniacea) in mice infected with Plasmodium berghei berghei. Afr. J. Biotechnol. 2010;9(23):3475–3480. [Google Scholar]
- Madikizela B., McGaw L.J. In vitro cytotoxicity, antioxidant and anti-inflammatory activities of Pittosporum viridiflorum Sims and Hypoxis colchicifolia Baker used traditionally against cancer in Eastern Cape, South Africa. South Afr. J. Bot. 2019;126:250–255. [Google Scholar]
- Madzinga M., Kritzinger Q. Underexplored Medicinal Plants from Sub-saharan Africa. Academic Press; 2020. Searsia lancea; pp. 253–259. [Google Scholar]
- Magadula J.J., Mbwambo Z.H. Open science; 2014. Garcinia Plant Species of African Origin: Ethnobotanical, Pharmacological and Phytochemical Studies; pp. 17–70. [Google Scholar]
- Magadula J.J., Otieno J.N., Nondo R.S., Kirimuhuzya C., Kadukuli E., Orodho J.A., Okemo P. Anti-mycobacterial and toxicity activities of some priority medicinal plants from lake victoria basin, Tanzania. Eur. J. Med. Plants. 2012:125–131. [Google Scholar]
- Magaji M.G., Yaro A.H., Mohammed A., Zezi A.U., Tanko Y., Bala T.Y. Preliminary antidiarrhoeal activity of methanolic extracts of Securinega virosa (Euphorbiaceae) Afr. J. Biotechnol. 2007;6(24) [Google Scholar]
- Magaji M.G., Anuka J.A., Abdu-Aguye I., Yaro A.H., Hussaini I.M. Preliminary studies on anti-inflammatory and analgesic activities of Securinega virosa (Euphorbiaceae) in experimental animal models. J. Med. Plants Res. 2008;2(2):39–44. [Google Scholar]
- Magaji M.G., Anuka J.A., Abdu-Aguye I., Yaro A.H., Hussaini I.M. Behavioural effects of the methanolic root bark extract of Securinega virosa in rodents. Afr. J. Tradit., Complementary Altern. Med. 2008;5(2):147–153. doi: 10.4314/ajtcam.v5i2.31266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magama S., Pretorius J.C., Zietsman P.C., van Wyk B.E. Antimicrobial properties of extracts from Euclea crispa subsp. crispa (Ebenaceae) towards human pathogens. South Afr. J. Bot. 2003;69(2):193–198. [Google Scholar]
- Magwenzi R., Nyakunu C., Mukanganyama S. The Effect of selected Combretum species from Zimbabwe on the growth and drug efflux systems of Mycobacterium aurum and Mycobacterium smegmatis. J. Microb. Biochem. Technol. 2014;3(3) [Google Scholar]
- Mahboubi M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clin. Phytoscience. 2019;5:6. [Google Scholar]
- Mahomoodally F.M. Traditional medicines in Africa: an Appraisal of ten potent African medicinal plants. Evid. Based. Complementary. Altern. Med. 2013 doi: 10.1155/2013/617459. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair C.E., Grienke U., Wilhelm A., Urban E., Zehl M., Schmidtke M., Rollinger J.M. Anti-influenza triterpene saponins from the bark of Burkea africana. J. Nat. Prod. 2018;81(3):515–523. doi: 10.1021/acs.jnatprod.7b00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makgatho M., Nxumalo W., Raphoko L. Anti-mycobacterial,-oxidative,-proliferative and-inflammatory activities of dichloromethane leaf extracts of Gymnosporia senegalensis (Lam.) Loes. S. Afr. J. Bot. 2018;114:217–222. [Google Scholar]
- Makni M., Jemai R., Kriaa W., Chtourou Y., Fetoui H. Citrus limon from Tunisia: phytochemical and physicochemical properties and biological activities. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/6251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malebo H.M., Jonker S.A., Waibel R., Nkunya M.H.H. Diprenylated indole alkaloids from fruits of Hexalobus monopetalus. Nat. Prod. Bioprospect. 2014;4:101–105. doi: 10.1007/s13659-014-0010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malebo H.M., Wiketye V., Katani S.J., Kitufe N.A., Nyigo V.A., Imeda C.P., Ogondiek J.W., Sunguruma R., Mhame P.P., Massaga J.J. In vivo antiplasmodial and toxicological effect of Maytenus senegalensis traditionally used in the treatment of malaria in Tanzania. Malar. J. 2015;14:79. doi: 10.1186/s12936-014-0525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgwi I.S., Olorunshola K.V., Hamman W.O. Effects of aqueous Cucurbita pepo Linn seed extract on some haematological parameters and serum electrolytes of lactating albino rats. Sci. J. Microbiol. 2014;3(1):1–8. [Google Scholar]
- Mamabolo M.P., Muganza F.M., Olivier M.T., Olaokun O.O., Nemutavhanani L.D. Evaluation of antigonorrhea activity and cytotoxicity of Helichrysum caespititium (DC) Harv. Whole plant extracts. Biol. Med. 2018;10(422):2. [Google Scholar]
- Mandavkar Y.D., Jalalpure S.S. A comprehensive review on Plumbago zeylanica Linn. Afr. J. Pharm. Pharmacol. 2011;5(25):2738–2747. [Google Scholar]
- Mangoyi R., Mukanganyama S. In vitro antifungal activities of selected medicinal plants from Zimbabwe against Candida albicans and Candida krusei. Afr. J. Plant Sci. Biotechnol. 2011;5(1):1–7. [Google Scholar]
- Mangoyi R., Mafukidze W., Marobela K., Mukanganyama S. Antifungal activities and preliminary phytochemical investigation of Combretum species from Zimbabwe. Microbiol. Biochem. Technol. 2012;4:37–44. [Google Scholar]
- Mangoyi R., Chitemerere T.A., Chimponda T., Chirisa E. In: Novel Plant Bioresources: Applications in Food, Medicine and Cosmetics. Tiwari B.K., Norton T., Holden N.M., editors. JohnWiley & Sons, Ltd; 2014. Multiple anti-infective properties of selected plant species from Zimbabwe; pp. 179–190. [Google Scholar]
- Mapanda P., Wuta M., Nyamangara J., Rees R.M., Kitzler B. Greenhouse gas emissions from savanna (miombo) woodlands: responses to clearing and cropping. Afr. Crop Sci. J. 2012;20:385–400. [Google Scholar]
- Mapaura A., Timberlake J. SABONET; Pretoria and Harare: 2004. A Checklist of Zimbabwean Vascular Plants. Southern African Botanical Diversity Network Report No. 33. [Google Scholar]
- Mapfunde S., Sithole S., Mukanganyama S. In vitro toxicity determination of antifungal constituents from Combretum zeyheri. BMC Compl. Alternative Med. 2016;16:162. doi: 10.1186/s12906-016-1150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Ethnobotanical study of two threatened medicinal plants in Zimbabwe. Int. J. Biodivers. Sci. Ecosys. Serv. Manage. 2008;4:148–153. [Google Scholar]
- Maroyi A. Traditional homegardens and rural livelihoods in Nhema, Zimbabwe: a sustainable agroforestry system. Int. J. Sustain. Dev. World Ecol. 2009;16:1–8. [Google Scholar]
- Maroyi A. An ethnobotanical survey of medicinal plants used by the people in Nhema communal area, Zimbabwe. J. Ethnopharmacol. 2011;136(2):347–354. doi: 10.1016/j.jep.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Maroyi A. Garden Plants in Zimbabwe: their ethnomedicinal uses and reported toxicity. Ethnobot. Res. Appl. 2012;10:45–57. http://ethnobotanyjournal.org/index.php/era/article/view/549/382 [Google Scholar]
- Maroyi A. Local plant use and traditional conservation practices in Nhema communal area, Zimbabwe. Int. J. Afr. Renaissance Stud.-Multi-Inter- and Transdisciplin. 2012;7:109–128. [Google Scholar]
- Maroyi A. Traditional use of medicinal plants in South central Zimbabwe: review and perspectives. J. Ethnobiol. Ethnomed. 2013;9:31. doi: 10.1186/1746-4269-9-31. http://www.ethnobiomed.com/content/9/1/31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Warburgia salutaris (Bertol . f .) Chiov.: a multi-use ethnomedicinal plant species. J. Med. Plants Res. 2013;7(2):53–60. [Google Scholar]
- Maroyi A. vol. 13. 2014. pp. 1527–1536. (Alternative Medicines for HIV/AIDS in Resource-Poor Settings: Insight from Traditional Medicines Use in Sub-saharan Africa). [Google Scholar]
- Maroyi A. Ethnomedicinal uses, phytochemistry and pharmacological properties of the genus, Kirkia. Trop. J. Pharmaceut. Res. 2016;15(11):2507–2516. [Google Scholar]
- Maroyi A. Kirkia acuminata oliv.: a review of its ethnobotany and pharmacology. Afr. J. Tradit., Complementary Altern. Med. 2017;14(2):217–226. doi: 10.21010/ajtcam.v14i2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Lippia javanica (Burm. f .) spreng. Traditional and commercial uses and phytochemical and pharmacological significance in the African and Indian subcontinent. Evid. Based. Complementary. Altern. Med. 2017;2017 doi: 10.1155/2017/6746071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Exotic plants in indigenous pharmacopoeia of south-Central Zimbabwe: traditional knowledge of herbal medicines. Res. J. Bot. 2017;12:46–52. [Google Scholar]
- Maroyi A. Review of ethnomedicinal uses, phytochemistry and pharmacological properties of Euclea natalensis A. DC. Molecules. 2017;22:2128. doi: 10.3390/molecules22122128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Azanza garckeana fruit tree: phytochemistry, pharmacology, nutritional and primary healthcare applications as herbal medicine: a Review. Res. J. Med. Plant. 2017;11:115–123. [Google Scholar]
- Maroyi A. Ethnopharmacology and therapeutic value of Bridelia micrantha (Hochst.) Baill. In tropical Africa: a comprehensive review. Molecules. 2017;22:1493. doi: 10.3390/molecules22091493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Review of ethnomedicinal uses, phytochemistry and pharmacological properties of Euclea natalensis A. DC. Molecules. 2017;22:2128. doi: 10.3390/molecules22122128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Diospyros lycioides Desf .: review of its botany, medicinal uses, pharmacological activities and phytochemistry. Asian Pac. J. Trop. Biomed. 2018;8(2):130–136. [Google Scholar]
- Maroyi A. Ethnomedicinal uses of exotic plant species in South-central Zimbabwe. Indian J. Trad. Med. 2018;17(1):71–77. [Google Scholar]
- Maroyi A. Syzygium cordatum Hochst. Ex Krauss: an overview of its ethnobotany, phytochemistry and pharmacological properties. Molecules. 2018;23:1084. doi: 10.3390/molecules23051084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Lannea discolor: its botany, ethnomedicinal uses, phytochemistry, and pharmacological properties. Asian J. Pharmaceut. Clin. Res. 2018;12(3):8–15. [Google Scholar]
- Maroyi A. Nutraceutical and ethnopharmacological properties of vangueria infausta subsp. Infausta. Molecules. 2018;23:1089. doi: 10.3390/molecules23051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Dicoma anomala Sond.: a review of its botany, ethnomedicine, phytochemistry and pharmacology. Asian J. Pharmaceut. Clin. Res. 2018;11(6):70–77. [Google Scholar]
- Maroyi A. Heteromorpha arborescens: a review of its Botany, medicinal uses, and pharmacological properties. Asian J. Pharmaceut. Clin. Res. 2018;11(11):75–82. [Google Scholar]
- Maroyi A. Euclea crispa: review of its botany, ethnomedicinal uses, and pharmacological properties. Asian J. Pharmaceut. Clin. Res. 2018;11(10):5–9. [Google Scholar]
- Maroyi A. Annona stenophylla Engl. & Diels: review of its botany, medicinal uses and biological activities. J. Pharmaceut. Sci. Res. 2019;11:3385–3390. [Google Scholar]
- Maroyi A. Helichrysum caespititium (DC.) Harv.: review of its medicinal uses, phytochemistry and biological activities. J. Appl. Pharmaceut. Sci. 2019;9(6):111–118. [Google Scholar]
- Maroyi A. Medicinal uses, biological and chemical properties of wild grape (Lannea edulis): an indigenous fruit plant of tropical Africa. Asian J. Pharmaceut. Clin. Res. 2019;12(9):16–20. [Google Scholar]
- Maroyi A. Utilization of Bridelia mollis as herbal medicine, nutraceutical and functional food in Southern Africa: a review. Trop. J. Pharmaceut. Res. 2019;18:203–209. [Google Scholar]
- Maroyi A. Sansevieria hyacinthoides (l.) Druce: a review of its botany, medicinal uses, phytochemistry, and biological activities. Asian J. Pharmaceut. Clin. Res. 2019;12(9):21–26. [Google Scholar]
- Maroyi A. Phytochemical and ethnopharmacological review of Heteropyxis natalensis. Asian J. Pharmaceut. Clin. Res. 2019;12:8–15. [Google Scholar]
- Maroyi A. Zanha africana (Radlk.) Exell: review of its botany, medicinal uses and biological activities. J. Pharmaceut. Sci. Res. 2019;11(8):2980–2985. [Google Scholar]
- Maroyi A., Cheikhyoussef A. A comparative study of medicinal plants used in rural areas of Namibia. Indian. J. Tradit. Know. 2015;14:401–406. [Google Scholar]
- Mascolo N., Jain R., Jain S.C., Capasso F. Ethnopharmacologic investigation of ginger (Zingiber officinale) J. Ethnopharmacol. 1989;27(1–2):129–140. doi: 10.1016/0378-8741(89)90085-8. [DOI] [PubMed] [Google Scholar]
- Matata D.Z., Ngassapa O.D., Machumi F., Moshi M.J. Screening of plants used as traditional anticancer remedies in Mkuranga and same districts, Tanzania, using brine shrimp toxicity bioassay. Evid. Based. Complementary. Altern. Med. 2018;2018 doi: 10.1155/2018/3034612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matongo K. Doctoral dissertation, University of the Western Cape; 2012. Conservation and Use-Values of Medicinal Plants in Rural Eastern Zimbabwe: a Study of Selected Medicinal Plants. [Google Scholar]
- Mavi S., Shava S. Traditional methods of conserving medicinal plants in Zimbabwe. Bot. Gardens Conserv. News. 1997;2(8):36–37. [Google Scholar]
- Mawere M., Nhemachena A. Langaa Research & Publishing; Cameroon: 2016. Theory, Knowledge, Development and Politics: what Role for the Academy in the Sustainability of Africa? [Google Scholar]
- Mazimba O. Pharmacology and phytochemistry studies in Peltophorum africanum. Bull. Fac. Pharm. Cairo Univ. 2014;52(1):145–153. [Google Scholar]
- Mbunde M.V.N., Innocent E., Mabiki F., Andersson P.G. Ethnobotanical survey and toxicity evaluation of medicinal plants used for fungal remedy in the Southern Highlands of Tanzania. J. Intercult. Ethnopharmacol. 2017;6(1):84–96. doi: 10.5455/jice.20161222103956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaw L.J., Rabe T., Sparg S.G., Jäger A.K., Eloff J.N., Van Staden J. An investigation on the biological activity of Combretum species. J. Ethnopharmacol. 2001;75(1):45–50. doi: 10.1016/s0378-8741(00)00405-0. [DOI] [PubMed] [Google Scholar]
- McGaw L.J., Van der Merwe D., Eloff J. In vitro anthelmintic, antibacterial and cytotoxic effects of extracts from plants used in South African ethnoveterinary medicine. Vet. J. 2007;173(2):366–372. doi: 10.1016/j.tvjl.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Mehrbod P., Abdalla M.A., Njoya E.M., Ahmed A.S., Fotouhi F., Farahmand B., Gado D.A., Tabatabaian M., Fasanmi O.G., Eloff J.N., McGaw L.J. South African medicinal plant extracts active against influenza A virus. BMC Compl. Alternative Med. 2018;18(1):1–10. doi: 10.1186/s12906-018-2184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn R., Balick M.J. The value of undiscovered pharmaceuticals in tropical forests. Econ. Bot. 1995;49:223–228. [Google Scholar]
- Meshram G.G., Kumar A., Rizvi W., Tripathi C.D., Khan R.A. Evaluation of the anti-inflammatory activity of the aqueous and ethanolic extracts of the leaves of Albizzia lebbeck in rats. J. Trad. Complement. Med. 2016;6(2):172–175. doi: 10.1016/j.jtcme.2014.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaili P., Mojaverrostami S., Moloudizargari M., Aghajanshakeri S. Pharmacological and therapeutic effects of Mentha Longifolia L. and its main constituent, menthol. Ancient Sci. Life. 2013;33:131. doi: 10.4103/0257-7941.139059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonge O.J., Kamindu G.N., Sabina W. An ethnobotanical survey of plants used for the treatment and management of cancer in Embu County, Kenya. J. Med. Plants. 2019;7:39–46. [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group* Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Moll A., Heyman H.M., Meyer J.J.M. Plants with activity against the live HI virus and the enzyme, reverse transcriptase. South Afr. J. Bot. 2013;86:148. 2013. [Google Scholar]
- Molla Y., Nedi T., Tadesse G., Alemayehu H., Shibeshi W. Evaluation of the in vitro antibacterial activity of the solvent fractions of the leaves of Rhamnus prinoides L'Herit (Rhamnaceae) against pathogenic bacteria. BMC Compl. Alternative Med. 2016;16(1):1–9. doi: 10.1186/s12906-016-1279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momoh M.A., Muhamed U., Agboke A.A., Akpabio E.I., Osonwa U.E. Immunological effect of aqueous extract of Vernonia amygdalina and a known immune booster called immunace® and their admixtures on HIV/AIDS clients: a comparative study. Asian Pac. J. Trop. Biomed. 2012;2(3):181–184. doi: 10.1016/S2221-1691(12)60038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongalo N.I. Peltophorum africanum Sond [Mosetlha]: a review of its ethnomedicinal uses, toxicology, phytochemistry and pharmacological activities. J. Med. Plants Res. 2013;7(48):3484–3491. [Google Scholar]
- Mongalo N.I., Mafoko B.J. Cassia abbreviata Oliv. A review of its ethnomedicinal uses, toxicology, phytochemistry, possible propagation techniques and Pharmacology. African J. Pharm. Pharmacol. 2013;7(45):2901–2906. [Google Scholar]
- Mongalo N.I., McGaw L.J., Segapelo T.V., Finnie J.F., Van Staden J. Ethnobotany, phytochemistry, toxicology and pharmacological properties of Terminalia sericea Burch. Ex DC.(Combretaceae)–a review. J. Ethnopharmacol. 2016;194:789–802. doi: 10.1016/j.jep.2016.10.072. [DOI] [PubMed] [Google Scholar]
- Mongalo N.I., Mashele S.S., Makhafola T.J. Ziziphus mucronata Willd. (Rhamnaceae): it's botany, toxicity, phytochemistry and pharmacological activities. Heliyon. 2020;6(4) doi: 10.1016/j.heliyon.2020.e03708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriasi G.A., Ireri A.M., Ngugi M.P. In vivo cognitive-enhancing, ex vivo malondialdehyde-lowering activities and phytochemical profiles of aqueous and methanolic stem bark extracts of Piliostigma thonningii (schum.) Int. J. Alzheimer’s Dis. 2020;2020:1–15. doi: 10.1155/2020/1367075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.J. Anthropogenic impacts on tropical forest biodiversity: a network structure and ecosystem functioning perspective. Philos. Trans. R. Soc. London [Biol]. 2010;365(1558):3709–3718. doi: 10.1098/rstb.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshi M.J., Mbwambo Z.H. Some pharmacological properties of extracts of Terminalia sericea roots. J. Ethnopharmacol. 2005;97(1):43–47. doi: 10.1016/j.jep.2004.09.056. [DOI] [PubMed] [Google Scholar]
- Moshi M.J., Kapingu M.C., Uiso F.C., Mbwambo Z.H., Mahunnah R.L. Some pharmacological properties of an aqueous extract of Securinega virosa roots. Pharmaceut. Biol. 2000;38(3):214–221. doi: 10.1076/1388-0209(200007)3831-SFT214. [DOI] [PubMed] [Google Scholar]
- Moura A.F., Lima K.S.B., Sousa T.S., Marinho-Filho J.D.B., Pessoa C., Silveira E.R., Pessoa O.D.L., Costa-Lotufo L.V., Moraes M.O., Araujo A.J. In vitro antitumor effect of a lignan isolated from Combretum fruticosum, trachelogenin, in HCT-116 human colon cancer cells. Toxicol. Vitro. 2018;47:129–136. doi: 10.1016/j.tiv.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Moura I., Duvane J.A., Silva M.J.E., Ribeiro N., Ribeiro-Barros I. Woody species from the Mozambican Miombo woodlands: a review on their ethnomedicinal uses and pharmacological potential. J. Med. Plants Res. 2018;12(2):15–31. [Google Scholar]
- Moyo B., Mukanganyama S. Antibacterial effects of Cissus welwitschii and Triumfetta welwitschii extracts against Escherichia coli and Bacillus cereus. Int. J. Bacteriol. 2015;2015:1–10. doi: 10.1155/2015/16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshelia E.H., Watirahyel E.M., Maigari A.U., Yohanna C., Ismail F. Cytotoxicity and antioxidant activity of stem bark extracts of Azanza garckeana (kola of Tula) Eur. J. Pure Appl. Chem. 2016;3(2):16–24. [Google Scholar]
- Mtunzi F.M., Ejidike I.P., Matamela T., Dikio E., Klink M.J. Phytochemical profiling, antioxidant and antibacterial activities of leaf extracts from Rhus leptodictya. Int. J. Phytochem. Pharmacol. Res. 2017;9:1090–1099. [Google Scholar]
- Muanda F.N., Dicko A., Soulimani R. Assessment of polyphenolic compounds, in vitro antioxidant and anti-inflammation properties of Securidaca longepedunculata root barks. Comptes Rendus Biol. 2010;333(9):663–669. doi: 10.1016/j.crvi.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Mukamuri B.B., Kozanayi W. Commercialization and institutional arrangements involving tree species harvested for bark by smallholder farmers in Zimbabwe. Adv. Econ. Bot. 2014;17:247–254. [Google Scholar]
- Mukanganyama S., Dumbura S.C., Mampuru L. Anti-Proliferative effects of plant extracts from Zimbabwean medicinal plants against human Leukaemic cell lines. Afr. J. Plant Sci. Biotechnol. 2012;6(1):14–20. [Google Scholar]
- Mukherjee P.K., Nema N.K., Maity N., Mukherjee K., Harwansh R.K. Phytochemical and therapeutic profile of Aloe vera. J. Nat. Remedies. 2013;14(1):1–26. [Google Scholar]
- Mulaudzi R.B., Ndhlala A.R., Kulkarni M.G., Van Staden J. Pharmacological properties and protein binding capacity of phenolic extracts of some Venda medicinal plants used against cough and fever. J. Ethnopharmacol. 2012;143(1):185–193. doi: 10.1016/j.jep.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Muleya E., Ahmed A.S., Sipamla A.M., Mtunzi F.M. Free radical scavenging and antibacterial activity of crude extracts from selected plants of medicinal value used in Zululand. Pakistan J. Nutr. 2014;13(1):38. [Google Scholar]
- Muleya E., Ahmed A.S., Sipamla A.M., Mtunzi F.M., Mutatu W. Evaluation of anti-microbial, anti-inflammatory and anti-oxidative properties Artemisia afra, Gunnera perpensa and Eucomis autumnalis. J. Nutr. Food Sci. 2014;4(6):1–6. [Google Scholar]
- Mulholland D.A., Langat M.K., Crouch N.R., Coley H.M., Mutambi E.M., Nuzillard J.M. Cembranolides from the stem bark of the southern African medicinal plant, Croton gratissimus (Euphorbiaceae) Phytochemistry. 2010;71:1381–1386. doi: 10.1016/j.phytochem.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Munodawafa T., Moyo S., Chipurura B., Chagonda L. Brine shrimp lethality bioassay of some selected Zimbabwean traditional medicinal plants. Int. J. Phytopharm. 2016;7(4):229–232. [Google Scholar]
- Mwamatope B., Tembo D., Chikowe I., Kampira E., Nyirenda C. Total phenolic contents and antioxidant activity of Senna singueana, Melia azedarach, Moringa oleifera and Lannea discolor herbal plants. Sci. Afr. 2020;9 [Google Scholar]
- Mwonjoria J., Ngeranwa J., Kariuki H., Githinji C., Sagini M., Wambugu S. Ethno medicinal, phytochemical and pharmacological aspects of solanum incanum (Lin.) Int. J. Pharmacol. Toxicol. 2014:2. [Google Scholar]
- Naidoo V., Katerere D.R., Swan G.E., Eloff J.N. Pretreatment of Urginea sanguinea bulbs used in ethnoveterinary medicine influences chemical composition and biological activity. Pharmaceut. Biol. 2004;42(7):529–533. [Google Scholar]
- Naidoo Y., Sadashiva C.T., Naidoo G., Raghu K. Antibacterial, antioxidant and phytochemical properties of the ethanolic extract of Ocimum obovatum E. Mey. ex Benth. Indian J. Trad. Knowl. 2016;15(1):57–61. [Google Scholar]
- Najeeb T.M., Issa T.O., Mohamed Y.S., Ahmed R.H., Makhawi A.M., Khider T.O. Phytochemical screening, antioxidant and antimicrobial activities of dalberegia melanoxylon tree. World Appl. Sci. J. 2018;36(7):826–833. [Google Scholar]
- Namadina M.M., Aliyu B.S., Haruna H., Sunusi U., Kamal R.M., Balarabe S., Ibrahim S., Nuhu Y., Adamu M.M., Aminu M.A., Hotoro A.S. Pharmacognostic and acute toxicity study of Burkea africana root. J. Appl. Sci. Environ. Manag. 2020;24(4):565–573. [Google Scholar]
- Nasimolo J., Kiama S.G., Gathumbi P.K., Makanya A.N., Kagira J.M. Erythrina abyssinica prevents meningoencephalitis in chronic Trypanosoma brucei brucei mouse model. Metab. Brain Dis. 2014;29(2):509–519. doi: 10.1007/s11011-014-9488-5. [DOI] [PubMed] [Google Scholar]
- Ndhlala A.R., Ncube B., Okem A., Mulaudzi R.B., Van Staden J. Toxicology of some important medicinal plants in southern Africa. Food Chem. Toxicol. 2013;62:609–621. doi: 10.1016/j.fct.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Neffati M., Najjaa H., Máthé A. vol. 3. Springer; Netherlands: 2017. (Medicinal and Aromatic Plants of the World – Africa). [Google Scholar]
- Ngarivhume T., Van't Klooster C.I., de Jong J.T., Van der Westhuizen J.H. Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. J. Ethnopharmacol. 2015;159:224‐237. doi: 10.1016/j.jep.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Nguta J.M. In vivo antimalarial activity, toxicity, and phytochemical composition of total extracts from securidaca longepedunculata Fresen.(polygalaceae) Biomed. Biotechnol. Res. J. 2019;3(3):196–201. [Google Scholar]
- Nivetha S., Padmini S., Tamilselvi S. A review on phytopharmacological properties of Arappu. Int. Res. J. Pharm. 2017;8(11) [Google Scholar]
- Njau E.F.A., Ndakidemi P.A. The genus tetradenia (Lamiaceae): a review of its ethnomedicinal, botanical, chemical and pharmacological activities. Int. J. Biol. 2017;9(4):35–41. [Google Scholar]
- Njeru S.N., Muema J.M. Antimicrobial activity, phytochemical characterization and gas chromatography-mass spectrometry analysis of Aspilia pluriseta Schweinf. extracts. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoya E.M., Eloff J.N., McGaw L.J. Croton gratissimus leaf extracts inhibit cancer cell growth by inducing caspase 3/7 activation with additional anti-inflammatory and antioxidant activities. BMC Compl. Alternative Med. 2018;18(1):305. doi: 10.1186/s12906-018-2372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkeh-Chungag B.N., Tiya S., Mbafor J.T., Ndebia E.J., Rusike S., Iputo J.E. Effects of the methanol extract of Erythrina abyssinica on hot flashes in ovariectomized rats. Afr. J. Biotechnol. 2013;12(6) [Google Scholar]
- Nureye D., Assefa S., Nedi T., Engidawork E. In vivo antimalarial activity of the 80 Methanolic root bark extract and solvent fractions of Gardenia ternifolia Schumach. & Thonn.(Rubiaceae) against Plasmodium berghei. Evid. base Compl. Alternative Med. 2018;2018:1–10. doi: 10.1155/2018/9217835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaehujor Chinaka O., Igile Godwin O., Ode Julius O., Udegbunam Rita I. 2014. Anti-Inflammatory Activities of Methanol Leaf Extract of Bridelia Micrantha (Hochst) Baill.(Euphorbiaceae) in Wistar Rats. [DOI] [PubMed] [Google Scholar]
- Nwoke O.C., Diels J., Abaidoo R., Nziguheba G., Merckx R. Organic acids in the rhizosphere and root characteristics of soybean (Glycine max) and cowpea (Vigna unguiculata) in relation to phosphorus uptake in poor savanna soils. Afr. J. Biotechnol. 2008;7(20) [Google Scholar]
- Nyirenda K.K., Saka J.D.K., Naidoo D., Maharaj V.J., Muller C.J.F. Antidiabetic, anti-oxidant and antimicrobial activities of Fadogia ancylantha extracts from Malawi. J. Ethnopharmacol. 2012;143(1):372–376. doi: 10.1016/j.jep.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Ochanga O., Chacha M. Antifungal and cytotoxicity activity of plants used as herbal teas in Tanzania. Eur. J. Med. Plants. 2016:1–8. [Google Scholar]
- Ochanga O., Chacha M. In vitro antibacterial activity of plants used as herbal tea in Tanzania. J. Complement. Altern. Med. Res. 2016:1–10. [Google Scholar]
- Ochanga O., Kilonzo M. Antioxidant properties of aqueous and ethyl acetate extracts of some plants used as herbal tea in Tanzania. Oxidants Antioxid. Med. Sci. 2018;7(1):1–8. [Google Scholar]
- Ogundajo A.L., Ashafa A.O.T. Medicinal properties of Clerodendrum glaburum E may leaf extracts: phytochemical constituents, antioxidant, cytotoxicity, and carbohydrate-metabolizing enzyme inhibitory potentials. Comp. Clin. Pathol. 2019;28(4):927–936. [Google Scholar]
- Ohtani K., Mavi S., Hostettmann K. Molluscicidal and antifungal triterpenoid saponins from Rapanea melanophloeos leaves. Phytochemistry. 1993;33(1):83–86. [Google Scholar]
- Ojewole J.A. Analgesic, anti-inflammatory and hypoglycaemic effects of Rhus chirindensis (Baker F.) [Anacardiaceae] stem-bark aqueous extract in mice and rats. J. Ethnopharmacol. 2007;113:338–345. doi: 10.1016/j.jep.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Ojewole J.A., Mawoza T., Chiwororo W.D., Owira P.M. Sclerocarya birrea (A. Rich) Hochst. [‘Marula’](Anacardiaceae): a review of its phytochemistry, pharmacology and toxicology and its ethnomedicinal uses. Phytother Res.: Int. J. Dev. Pharmacol. Toxicol. Evaluat. Nat. Prod. Derivat. 2010;24(5):633–639. doi: 10.1002/ptr.3080. [DOI] [PubMed] [Google Scholar]
- Okeleye B.I., Mkwetshana N.T., Ndip R.N. Evaluation of the antibacterial and antifungal potential of Peltophorum africanum: toxicological effect on human Chang liver cell line. Sci. World J. 2013 doi: 10.1155/2013/878735. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwuofu E.O., Ozolua R.I., Yibala O.I., Akhigbemen A.M. Evaluation of acute and sub-acute safety profile of Syzygium guineense (Wild) DC methanol stem bark extract in rodents. Pharma Innov. 2017;6(6):174–179. [Google Scholar]
- Oladosu I., Lawson L., Aiyelaagbe O., Emenyonu N., Afieroho O. Anti-tuberculosis lupane-type isoprenoids from Syzygium guineense Wild DC. (Myrtaceae) stem bark. Future. J. Pharm. Sci. 2017;3:148–152. [Google Scholar]
- Olasunkanmi A.A., Adegbola O.O. 2019. Phytochemical Investigation and Invitro Antioxidant Activity of Aqueous and Ethanol Extracts of Rhus Longipes. [Google Scholar]
- Olela B., Mbaria J., Wachira T., Moriasi G. Evidence-Based Complementary and Alternative Medicine; 2020. Acute Oral Toxicity and Anti-inflammatory and Analgesic Effects of Aqueous and Methanolic Stem Bark Extracts of Piliostigma Thonningii (Schumach.) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oloche J.J., Okwuasaba F., Obochi G.O. Review of phytochemical, pharmacological and toxicological profile of stereospermum kunthianum. J. Adv. Med. Pharmaceut. Sci. 2016:1–10. [Google Scholar]
- Olorunnisola O.S., Bradley G., Afolayan A. Ethnobotanical information on plants used for the management of cardiovascular diseases in Nkonkobe Municipality, South Africa. J. Med. Plants Res. 2011;5(17):4256–4260. [Google Scholar]
- Olorunnisola O.S., Adetutu A., Owoade A.O., Adesina B.T., Adegbola P. Toxicity evaluation and protective effect of Rhus longipes Engl. leaf extract in paracetamol induced oxidative stress in wister rats. J. Phytopharmacol. 2017;6(2):73–77. [Google Scholar]
- Ono L., Wollinger W., Rocco I.M., Coimbra T.L., Gorin P.A., Sierakowski M.R. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (Hawaii strain) Antivir. Res. 2003;60(3):201–208. doi: 10.1016/s0166-3542(03)00175-x. [DOI] [PubMed] [Google Scholar]
- Ordaz-Trinidad N., Dorantes-Álvarez L., Salas-Benito J., Barrón-Romero B.L., Salas-Benito M., Nova-Ocampo M.D. Cytotoxicity and antiviral activity of pepper extracts (Capsicum spp) Polibotánica. 2018;(46):273–285. [Google Scholar]
- Orji E.C., Onwughalu G.I., Nweke I.A. Problems associated with conservation of medicinal plants in Anambra state. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013;2(6):61–63. [Google Scholar]
- Osseni R., Akoha S., Adjagba M., Azonbakin S., Lagnika L., Awede B., Bigot A., Diouf A., Darboux R., Lalèyè A. In vivo toxicological assessment of the aqueous extracts of the leaves of Carissa edulis (Apocynaceae) in Wistar Rats. Eur. J. Med. Plants. 2016:1–10. [Google Scholar]
- Osunsanmi F.O., Zharare G.E., Opoku A.R. Phytochemical constituents and antioxidant potential of crude extracts from Lippia Javanica (Burm. f.) Spreng Leaves. Pharmacogn. J. 2019;11(4):803–807. [Google Scholar]
- Otang W.M., Afolayan A.J. Antimicrobial and antioxidant efficacy of Citrus limon L. peel extracts used for skin diseases by Xhosa tribe of Amathole District, Eastern Cape, South Africa. South Afr. J. Bot. 2016;102:46–49. [Google Scholar]
- Owuor B.O., Ochanda J.O., Kokwaro J.O., Cheruiyot A.C., Yeda R.A., Okudo C.A., Akala H.M. In vitro antiplasmodial activity of selected Luo and Kuria medicinal plants. J. Ethnopharmacol. 2012;144(3):779–781. doi: 10.1016/j.jep.2012.09.045. [DOI] [PubMed] [Google Scholar]
- Oyebode O., Kandala N.B., Chilton P.J., Lilford R.J. Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Pol. Plann. 2016;31(8):984–991. doi: 10.1093/heapol/czw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallant C.A., Cromarty A.D., Steenkamp V. Effect of an alkaloidal fraction of Tabernaemontana elegans (Stapf.) on selected micro-organisms. J. Ethnopharmacol. 2012;140(2):398–404. doi: 10.1016/j.jep.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Palshetkar A., Pathare N., Jadhav N., Pawar M., Wadhwani A., Kulkarni S., Singh K.K. In vitro anti-HIV activity of some Indian medicinal plant extracts. BMC Complement. Med. Therap. 2020;20(1):1–11. doi: 10.1186/s12906-020-2816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamhidzai D., Isaac G. TLC separation, antibacterial and anti-inflammatory activity of extracts derived from Zanthoxylum humile roots. Int. J. Res. Ayurveda Pharm. 2013;4(4) [Google Scholar]
- Parimala M., Shoba F.G. In vitro antimicrobial activity and HPTLC analysis of hydroalcoholic seed extract of Nymphaea nouchali Burm. f. BMC Complement. Alternat. Med. 2014;14(1):1–9. doi: 10.1186/1472-6882-14-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.W., Kundu J., Chae I.G., Bachar S.C., Bae J.W., Chun K.S. Methanol extract of Flacourtia indica aerial parts induces apoptosis via generation of ROS and activation of caspases in human colon cancer HCT116 cells. Asian Pac. J. Cancer Prev. APJCP. 2014;15(17):7291–7296. doi: 10.7314/apjcp.2014.15.17.7291. [DOI] [PubMed] [Google Scholar]
- Parker M.E., Chabot S., Ward B.J., Johns T. Traditional dietary additives of the Maasai are antiviral against the measles virus. J. Ethnopharmacol. 2007;114(2):146–152. doi: 10.1016/j.jep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Parry O., Matambo C. Some pharmacological actions of aloe extracts and Cassia abbreviata on rats and mice. Cent. Afr. J. Med. 1992;38(10):409–414. [PubMed] [Google Scholar]
- Parvez G.M. Pharmacological activities of mango (Mangifera Indica): a review. J. Pharmacogn. Phytochem. 2016;5(3):1. [Google Scholar]
- Parvez A., Azad A.K., Islam M.Z., Munna M.R., Shaheen S.M., Rahman M., Azad A.K. A Phytochemical and Pharmacological review on Trema orientalis: a potential medicinal plant. Pharmacologyonline. 2019;3:103–119. [Google Scholar]
- Patil M.B., Khan P.A. Ethnobotanical, phytochemical and fourier transform infrared spectrophotometer (FTIR) studies of Catunaregam spinosa (thunb.) tirven. J. Chem. Pharmaceut. Sci. 2017;10(2):950–955. Print ISSN: 0974-2115. [Google Scholar]
- Patil G.V., Dass S.K., Chandra R. Artemisia afra and modern diseases. J. Pharmacogenomics Pharmacoproteomics. 2011;2:105. [Google Scholar]
- Pearce D., Moran D. Earthscan; London, UK: 1994. The Economic Value of Biodiversity. [Google Scholar]
- Perera H.D.S.M., Samarasekera J.K.R.R., Handunnetti S.M., Weerasena O.V.D.S.J., Weeratunga H.D., Jabeen A., Choudhary M.I. In vitro pro-inflammatory enzyme inhibition and anti-oxidant potential of selected Sri Lankan medicinal plants. BMC Compl. Alternative Med. 2018;18(1):1–15. doi: 10.1186/s12906-018-2335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos S., Fernandes Â., Barros L., Ciric A., Sokovic M., Ferreira I.C. The chemical composition, nutritional value and antimicrobial properties of Abelmoschus esculentus seeds. Food Funct. 2017;8(12):4733–4743. doi: 10.1039/c7fo01446e. [DOI] [PubMed] [Google Scholar]
- Popova V., Tumbarski Y., Ivanova T., Hadjikinova R., Stoyanova A. Tobacco resinoid (Nicotiana tabacum L.) as an active ingredient of cosmetic gels. J. Appl. Pharmaceut. Sci. 2019;9(9):111–118. [Google Scholar]
- Prasad K.S., Savithramma N. Screening of phytochemical constituents of nymphaea Caerulea savigny. An aquatic plant resource for drug development. Am. J. Adv. Drug Deliv. 2016;4:45–54. [Google Scholar]
- Pretorius J.C., Magama S., Zietsman P.C. Purification and identification of antibacterial compounds from Euclea crispa subsp. crispa (Ebenaceae) leaves. South Afr. J. Bot. 2003;69(4):579–586. [Google Scholar]
- Rahman T.U., Zeb M.A., Liaqat W., Sajid M., Hussain S., Choudhary M.I. Phytochemistry and pharmacology of genus Indigofera: a review. Record Nat. Prod. 2018;12(1):1–13. [Google Scholar]
- Rahmani A.H. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014;6(2):125. [PMC free article] [PubMed] [Google Scholar]
- Raja M.M.M., Sethiya N.K., Mishra S.H. A comprehensive review on Nymphaea stellata: a traditionally used bitter. J. Adv. Pharm. Technol. Res. 2010;1(3):311. doi: 10.4103/0110-5558.72424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekharan P.E., Ganeshan S. Conservation of medicinal plant biodiversity - an Indian perspective. J. Trop. Med. Plants. 2002;3:125–140. [Google Scholar]
- Ramdé-Tiendrébéogo A., Ouédraogo N., Tibiri A., Nacoulma O.G., Guissou I.P. Anti-inflammatory activities of total leaf extracts of Ficus sycomorus L.(Moraceae) used in traditional medicine in the treatment of sickle cell disease. J. Young Pharm. 2015;7(4):359. [Google Scholar]
- Rana M.N., Karim N., Changlek S., Rahman M.A., Tangpong J., Hajjar D., Alelwani W., Makki A.A. Thunbergia laurifolia leaf extract partially recovers lead-induced renotoxicity through modulating the cell signaling pathways. Saudi J. Biol. Sci. 2020;27(12):3700–3710. doi: 10.1016/j.sjbs.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio M.C., Giner R.M., Manez S., Rios J.L., Marston A., Hostettmann K. Screening of tropical medicinal plants for antiinflammatory activity. Phytother Res. 1995;9(8):571–574. [Google Scholar]
- Reddeman R.A., Glávits R., Endres J.R., Clewell A.E., Hirka G., Vértesi A., Béres E., Szakonyiné I.P. A toxicological evaluation of mango leaf extract (Mangifera indica) containing 60% mangiferin. J. Toxicol. 2019;2019:1–14. doi: 10.1155/2019/4763015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeploying plant defences Native Plants. 2020;6:177. doi: 10.1038/s41477-020-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman R., Akram M., Akhtar N., Jabeen Q., Saeed T., Shah S.M.A., Ahmed K., Shaheen G., Asif H.M. Zingiber officinale Roscoe (pharmacological activity) J. Med. Plants Res. 2011;5(3):344–348. [Google Scholar]
- Ren X., Shao X., Li X., Jia X., Song T., Zhou W., Wang P., Li Y., Wang X., Cui Q., Qiu P., Zhao Y., Li X., Zhang F., Li Z., Zhong Y., Wang Z., Fu X. Identifying potential treatments of COVID-19 from Traditional Chinese Medicine (TCM) by using a data-driven approach. J. Ethnopharmacol. 2020;258 doi: 10.1016/j.jep.2020.112932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renu S.N., Archana R., Jaswinder K., Santosh K., Fatima N. Taxonomy, phytochemistry, pharmacology and traditional uses of Flueggea virosa (Roxb. ex Willd.) Royle: a Review. Int. J Life Sci. 2018:579–585. [Google Scholar]
- Research and Markets . 2022. Herbal Medicines - Global Market Trajectory & Analytics.https://www.researchandmarkets.com/reports/5302363/herbal-medicines-global-market-trajectory and#:∼:text=Amid%20the%20COVID%2D19%20crisis,the%20analysis%20period%202020%2D2027 [Google Scholar]
- Resmi C.R., Venukumar M.R., Latha M.S. Antioxidant activity of Albizzia lebbeck (Linn.) Benth. in alloxan diabetic rats. Indian J. Physiol. Pharmacol. 2006;50(3):297. [PubMed] [Google Scholar]
- Rivera-Ortega P., Molina-Molina M. Interstitial lung diseases in developing countries. Ann. Glob. Health. 2019;85(1):4. doi: 10.5334/aogh.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo D., Kenny O., Smyth T.J., Milella L., Hossain M.B., Diop M.S., Rai D.K., Brunton N.P. International Scholarly Research Notices; 2013. Profiling of Phytochemicals in Tissues from Sclerocarya Birrea by HPLC-MS and Their Link with Antioxidant Activity. 2013. [Google Scholar]
- Sadau Y., Eloff J.N. In-vitro lipoxygenase inhibitory activity and total flavonoid of Strychnos spinosa leaf extracts and fractions. Niger. J. Pharm. Sci. 2014;13(1) [Google Scholar]
- Saini H., Dwivedi J., Paliwal H., Kataria U., Sharma M. An ethno-pharmacological evaluation of Catunaregam spinosa (thumb.) tirveng for antioxidant activity. J. Drug Deliv. Therapeut. 2019;9(4-s):280–284. [Google Scholar]
- Salawu M.O., Yekeen A., Nafiu M.O., Oloyede H.O. Anti-ulcerogenic potential of aqueous extract of securinega virosa leaf in indomethacin-induced ulcerated rats. Not. Sci. Biol. 2019;11(2):196–204. [Google Scholar]
- Salim A., Bwakura T., Dzvanga N., Gordon S. The epidemiology of respiratory disease in Zimbabwe. Mer (Tokyo): Afr. J. Respirat. Med. 2008:1–7. [Google Scholar]
- San Chang J., Wang K.C., Yeh C.F., Shieh D.E., Chiang L.C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013;145(1):146–151. doi: 10.1016/j.jep.2012.10.043. [DOI] [PubMed] [Google Scholar]
- Sánchez M., González-Burgos E., Iglesias I., Gómez-Serranillos M.P. Pharmacological update properties of Aloe vera and its major active constituents. Molecules. 2020;25(6):1324. doi: 10.3390/molecules25061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanogo R., Vassallo A., Malafronte N., Imparato S., Russo A., Piaz F.D. New phenolic glycosides from Securinega virosa and their antioxidant activity. Nat. Prod. Commun. 2009;4(12) [PubMed] [Google Scholar]
- Sanusi J., Jibia A.B., Runka J.Y., Liadi S., Abubakar A.A., Zurmi R.U.S. Antimicrobial activity of aqueous and ethanol extracts of violet plant (Securidaca longipedunculata Fres) on tested pathogenic bacteria. Int. J. Pharma Sci. Res. 2015;6:3276–3284. [Google Scholar]
- Sayeed V.K.I., Satish S., Kumar A., Hegde K. Pharmacological activities of Vigna unguiculata (L) Walp: a review. Int. J. Pharmaceut. Chem. Res. 2017;3(1):44–49. [Google Scholar]
- Sayyed A., Shah M. Phytochemistry, pharmacological and traditional uses of Datura stramonium L. review. J. Pharmacogn. Phytochem. 2014;2(5):123–125. [Google Scholar]
- Schopp-Guth A., Fremuth W. Sustainable use of medicinal plants and nature conservation in the Prespa National Park area, Albania. Med. Plant Conserv. 2001;7:5–8. [Google Scholar]
- Sebisubi F.M., Odyek O., Anokbonggo W.W., Ogwal-Okeng J., Carcache-Blanco E.J., Ma C., Orjala J., Tan G.T. 2010. Antimalarial Activity of Aspilia Pruliseta, a Medicinal Plant from Uganda. [DOI] [PubMed] [Google Scholar]
- Selemani M.A., Kazingizi L.F., Manzombe E., Bishi L.Y., Mureya C., Gwata T.T., Rwere F. bioRxiv; 2020. Phytochemical Characterization and in Vitro Antibacterial Activity of Xeroderris Stuhlmannii (Taub.) Mendonca & EP Sousa Bark Extracts. [Google Scholar]
- Semenya S.S., Maroyi A. Medicinal plants used for the treatment of tuberculosis by Bapedi traditional healers in three districts of the Limpopo province, South Africa. Afr. J. Tradit., Complement. Altern. Med. 2013;10(2):316–323. doi: 10.4314/ajtcam.v10i2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenya S.S., Maroyi A. Data on medicinal plants used to treat respiratory infections and related symptoms in South Africa. Data Brief. 2018;21:419–423. doi: 10.1016/j.dib.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenya S.S., Maroyi A. Source, harvesting, conservation status, threats and management of indigenous plant used for respiratory infections and related symptoms in the Limpopo Province, South Africa. Biodiversitas. 2019;20:789–810. [Google Scholar]
- Sempombe J., Mugoyela V., Mihale M.J., Zacharia A., Ipagala P., Kilulya K.F. Preliminary in vivo antitrypanosomal activity and cytotoxicity of Entada abyssinica, Securinega virosa and Ehretia amoena. East Central Afr. J. Pharmaceut. Sci. 2014;17(2):37–43. [Google Scholar]
- Sener B., Orhan I., Ozcelik B., Kartal M., Aslan S., Ozbilen G. Antimicrobial and antiviral activities of two seed oil samples of Cucurbita pepo L. and their fatty acid analysis. Nat. Prod. Commun. 2007;2(4) [Google Scholar]
- Sevindik M. Pharmacological properties of Mentha species. J. Tradit. Med. Clin. Nat. 2018;7(259):2. [Google Scholar]
- Shang S.Z., Zhao W., Tang J.G., Xu X.M., Sun H.D., Pu J.X., Liu Z.H., Miao M.M., Chen Y.K., Yang G.Y. Antiviral sesquiterpenes from leaves of Nicotiana tabacum. Fitoterapia. 2016;108:1–4. doi: 10.1016/j.fitote.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Shankar K.R., Gurjar C., Khatoon B.A. Pharmacological investigations of Crotalaria laburnifolia. Biomed. Pharmacol. J. 2008;1(1):239. [Google Scholar]
- Sharifi-Rad J., Salehi B., Stojanović-Radić Z.Z., Fokou P.V.T., Sharifi-Rad M., Mahady G.B., Sharifi-Rad M., Masjedi M.R., Lawal T.O., Ayatollahi S.A., Masjedi J. Medicinal plants used in the treatment of tuberculosis-Ethnobotanical and ethnopharmacological approaches. Biotechnol. Adv. 2020 doi: 10.1016/j.biotechadv.2020.107629. [DOI] [PubMed] [Google Scholar]
- Sharma N., Kaushik P. Medicinal, biological and pharmacological aspects of Plumbago zeylanica (Linn.) J. Pharmacogn. Phytochem. 2014;3(4):117–120. [Google Scholar]
- Sharma P., Bardwaj R., Yadav A., Sharma R.A. Study of antioxidant activity of Datura stramonium Linn. Res. J. Phytochem. 2014;8(3):112–118. [Google Scholar]
- Sharma P., Kharkwal A.C., Kharkwal H., Abdin M.Z., Varma A. A review on pharmacological properties of aloe vera. Int. J. Pharmaceut. Sci. Rev. Res. 2014;29(2):31–37. [Google Scholar]
- Shehu A., Magaji M.G., Sanni B., Abdu-Aguye S.N. Antidepressant activity of methanol root bark extract of Securinega virosa (Ex Willd.) Baill in albino mice. Bayero J. Pure Appl. Sci. 2017;10(2):277–282. [Google Scholar]
- Shoko T. Ashgate Publishing Limited; Hampshire, England: 2007. Karanga Indigenous Religion in Zimbabwe Health and Well-Being. [Google Scholar]
- Shoko T. Traditional herbal medicine and healing in Zimbabwe. J. Trad. Med. Clin. Naturopath. 2018;7(1):1–3. [Google Scholar]
- Shumba E., Carlson A., Kojwang H., Sibanda M., Masuka M., Moyo N. WWF-World Wide Fund For Nature (Formerly World Wildlife Fund) Harare; Zimbabwe: 2009. Traditional Medicinal Plant Practice in Southern Africa: A Situation Analysis in Zambia and Zimbabwe. [Google Scholar]
- Sigidi M.T., Anokwuru C.P., Zininga T., Tshisikhawe M.P., Shonhai A., Ramaite I.D.I., Traoré A.N., Potgieter N. Comparative in vitro cytotoxic, anti-inflammatory and anti-microbiological activities of two indigenous Venda medicinal plants. Transl. Med. Commun. 2016;1(1):1–7. [Google Scholar]
- Singh R.K. Pre-clinical toxicity studies of Holarrhena antidysenterica stem bark in mice and rats. World J. Pharm. Pharmaceut. Sci. 2018;7:912–922. [Google Scholar]
- Singh A., Navneet . 2018. Pharmacological Applications of Sida Acuta (Burm) Pharmacological Benefits of Natural Products; pp. 144–155. Chapter – 9. [Google Scholar]
- Singh M.K., Pandey A., Sawarkar H., Gupta A., Gidwani B., Dhongade H., Tripathi D.K. Methanolic extract of Plumbago Zeylanica-a remarkable antibacterial agent against many human and agricultural pathogens. J. Pharmacopuncture. 2017;20(1):18. doi: 10.3831/KPI.2017.20.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Sharma A., Reddy P.H., Rathi B., Prasad N.V.S.R.K., Vashishtha A. Evaluation of phytochemical and pharmacological aspects of Holarrhena antidysenterica (Wall.): a comprehensive review. J. Pharm. Res. 2013;6(4):488–492. [Google Scholar]
- Sobeh M., Mahmoud M.F., Abdelfattah M.A., Cheng H., El-Shazly A.M., Wink M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018;213:38–47. doi: 10.1016/j.jep.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Sofidiya M.O., Orisaremi C.O., Sansaliyu I., Adetunde T.O. Gastroprotective and antioxidant potentials of ethanolic stem bark extract of Margaritaria discoidea (Euphorbiaceae) in rats. J. Ethnopharmacol. 2015;171:240–246. doi: 10.1016/j.jep.2015.05.059. [DOI] [PubMed] [Google Scholar]
- Souham A.B., Behanzin J., Melanie A., Sezan A. Evaluation of the toxicity of the ethanolic extracts of the leaves of Hexalobus monopetalus on the kidneys and the liver of Wistar rats. Int. J. Eng. Technol. Manag. Res. 2018;5(11):1–12. doi: 10.5281/zenodo.2114569. [DOI] [Google Scholar]
- Sousa Z.L., de Oliveira F.F., da Conceição A.O., Silva L.A.M., Rossi M.H., Santos J.S., Andrioli J.L. Biological activities of extracts from Chenopodium ambrosioides Lineu and Kielmeyera neglecta Saddi. Ann. Clin. Microbiol. Antimicrob. 2012;11(1):1–7. doi: 10.1186/1476-0711-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyingbe O.S., Mongalo N.I., Makhafola T.J. In vitro antibacterial and cytotoxic activity of leaf extracts of Centella asiatica (L.) Urb, Warburgia salutaris (Bertol. F.) Chiov and Curtisia dentata (Burm. F.) CA Sm-medicinal plants used in South Africa. BMC Compl. Alternative Med. 2018;18:1–10. doi: 10.1186/s12906-018-2378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp V., Stewart M.J. Medicinal applications and toxicological activities of Aloe. Products. Pharmaceut. Biol. 2007;45(5):411–420. [Google Scholar]
- Sulaiman F.A., Nafiu M.O., Yusuf B.O., Muritala H.F., Adeyemi S.B., Omar S.A., Dosumu K.A., Adeoti Z.J., Adegbesan O.A., Busari B.O., Otohinoyi D.A. The GC-MS fingerprints of Nicotiana tabacum L. extract and propensity for renal impairment and modulation of serum triglycerides in Wistar rats. J. Pharm. Pharmacogn. Res. 2020;8(3):191–200. [Google Scholar]
- Sultana K., Chatterjee S., Roy A., Chandra I. An overview on ethnopharmacological and phytochemical properties of Thunbergia sp. Med. Aromatic Plants. 2015;4(5):1–6. [Google Scholar]
- Šutovská M., Fraňová S., Prisežnaková L., Nosáľová G., Togola A., Diallo D., Paulsen B.S., Capek P. Antitussive activity of polysaccharides isolated from the Malian medicinal plants. Int. J. Biol. Macromol. 2009;44(3):236–239. doi: 10.1016/j.ijbiomac.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Taderera T., Chagonda L., Gomo E., Shai L. Inhibitory activity of α-glucosidase and α-amylase by Annona stenophylla root extract as a mechanism for hypoglycaemic control of DM. Int. J. Pharm. 2015;106:436–444. [Google Scholar]
- Taderera T., Gomo E., Chagonda L.S. The Antidiabetic activity of an aqueous root extract of Annona stenophylla Engl. And diels in non-diabetic control and alloxan-induced diabetic rats. J. Biol. Active Prod. Nat. 2016;6(4):315–322. [Google Scholar]
- Takaidza S. Doctoral dissertation, Vaal University of Technology; 2018. Phytochemical Analysis and Biological Activities of Crude Extracts from Selected Tulbaghia Species. [Google Scholar]
- Tanko Y., Okasha M.A., Magaji G.M., Yerima M., Yaro A.H., Saleh M.I.A., Mohammed A. Anti-diabetic properties of Securinega virosa (Euphorbiaceae) leaf extract. Afr. J. Biotechnol. 2008;7(1) [Google Scholar]
- Tatematsu H., Mori M., Yang T.-H., Chang J.-J., Lee T.T.-Y., Lee K.-H. Cytotoxic principles of securinega virosa: virosecurinine and viroallosecurinine and related derivatives. J. Pharmaceut. Sci. 1991;80(4):325–327. doi: 10.1002/jps.2600800408. [DOI] [PubMed] [Google Scholar]
- Taukoorah U., Mahomoodally M.F. Crude Aloe vera gel shows antioxidant propensities and inhibits pancreatic lipase and glucose movement in vitro. Adv. Pharmacol. Sci. 2016;2016:1–9. doi: 10.1155/2016/372085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taur D.J., Patil R.Y. Some medicinal plants with antiasthmatic potential: a current status. Asian Pac. J. Trop. Biomed. 2011;1(5):413‐418. doi: 10.1016/S2221-1691(11)60091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcheghebe O.T., Seukep A.J., Tatong F.N. Ethnomedicinal uses, phytochemical and pharmacological profiles, and toxicity of Sida acuta Burm. f.: a review article. Pharma Innov. 2017;6(6):1. Part A) [Google Scholar]
- Teles A.M., dos Santos B.A., Ferreira C.G., Mouchreck A.N., da Silva Calabrese K., Abreu-Silva A.L., Almeida-Souza F. 2019. Ginger (Zingiber Officinale) Antimicrobial Potential: A Review. Ginger Cultivation and its Antimicrobial and Pharmacological Potentials. [Google Scholar]
- Tijjani M.A., Mohammed G.T., Alkali Y.T., Adamu T.B., Abdurahaman F.I. Phytochemical analysis, analgesic and antipyretic properties of ethanolic leaf extract of Vernonia amygdalina Del. J. Herbmed Pharmacol. 2017;6(3):95–99. [Google Scholar]
- Tsakala T.M., Penge O., John K. Screening of in vitro antibacterial activity from Syzygium Guineense (Willd) hydrosoluble dry extract. Ann. Pharm. Fr. 1996;54(6):276–279. [PubMed] [Google Scholar]
- Tufts H.R., Harris C.S., Bukania Z.N., Johns T. Antioxidant and anti- inflammatory activities of Kenyan leafy green vegetables, wild fruits, and medicinal plants with potential relevance for Kwashiorkor. Evid. base Compl. Alternative Med. 2015:9. doi: 10.1155/2015/807158. 2015, Article ID 807158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxhill J. Worldwatch Institute; Washington, DC: 1999. Nature's Conucopia: Our State in Plant Diversity. [Google Scholar]
- Upreti A., Byanju B., Fuyal M., Chhetri A., Pandey P., Ranjitkar R., Bhatta J.J., Pandey B.P. Evaluation of a-amylase, lipase inhibition and in-vivo pharmacological activities of Eucalyptus camaldulensis Dehnh leaf extract. J. Tradit. Complement. Med. 2018;30:1e7. doi: 10.1016/j.jtcme.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Venter M., Pruissen M., Koekemoer T., Sowemimo A., Govender S. In vitro anti-HIV and-TB activities of Annona muricata and Artemisia afra extracts. Planta Med. 2014;80(16):P1L29. [Google Scholar]
- Van Wyk B.E. The potential of South African plants in the development of new medicinal products. South Afr. J. Bot. 2011;77(4):812–829. [Google Scholar]
- Van Wyk B., Van Oudtshoorn B., Gericke N. Medicinal plants of South Africa. Briza Publications; 2009. [Google Scholar]
- Viljoen A.M., Klepser M.E., Ernst E.J., Keele D., Roling E., Van Vuuren S., Demirci B., Baser K.H.C., Van Wyk B.E., Jäger A.K. The composition and antimicrobial activity of the essential oil of the resurrection plant Myrothamnus flabellifolius. South Afr. J. Bot. 2002;68(1):100–105. [Google Scholar]
- Viol D.I. Masters dissertation, University of Zimbabwe; 2013. Screening of Traditional Medicinal Plants from Zimbabwe for Phytochemistry, Antioxidant, Antimicrobial, Antiviral and Toxicological Activities. [Google Scholar]
- Viol D.I., Chagonda L.S., Moyo S.R., Mericli A.H. Toxicity and antiviral activities of some medicinal plants used by traditional medical practitioners in Zimbabwe. Am. J. Plant Sci. 2016;7:1538–1544. [Google Scholar]
- Watt J.M., Breyer-Brandwijk M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa (Edinburg and London. second ed. E. & S. Livingstone; 1962. [Google Scholar]
- Wauthoz N., Balde A., Balde E.S., Van Damme M., Duez P. Ethnopharmacology of Mangifera indica L. bark and pharmacological studies of its main C-glucosylxanthone, mangiferin. Int. J. Biomed. Pharmaceut. Sci. 2007;1(2):112–119. [Google Scholar]
- Wende M., Sithole S., Fru C.G., Stevens M.Y., Mukanganyama S. BioMed Research International; 2021. The Effects of Combining Cancer Drugs with Compounds Isolated from Combretum Zeyheri Sond. And Combretum Platypetalum Welw. Ex MA Lawson (Combretaceae) on the Viability of Jurkat T Cells and HL-60 Cells. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2001. Cough and Cold Remedies for - World Health Organization.https://apps.who.int/iris/bitstream/handle/10665/66856/WHO_FCH_CAH_01.02.pdf [Google Scholar]
- WHO . 2018. The Top 10 Causes of Death.https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 14 May 2020) [Google Scholar]
- Wijaya S., Jin K.T., Nee T.K., Wiart C. In vitro 5-LOX inhibitory and antioxidant activities of extracts and compounds from the aerial parts of Lopholaena coriifolia (Sond.) E. Phillips & CA Sm. J. Compl. Integr. Med. 2012;9(1) doi: 10.1515/1553-3840.1615. [DOI] [PubMed] [Google Scholar]
- Wild H., Gelfand M. Some native herbal remedies at present in use in Mashonaland. Cent. Afr. J. Med. 1959;V:292–305. [PubMed] [Google Scholar]
- Williamson J. Zomba, Univ. of Malawi; 1975. Useful Plants of Malawi. revised and extended edn) [Google Scholar]
- Wintola O., Afolayan A. Alepidea amatymbica Eckl. & Zeyh.: a review of its traditional uses, phytochemistry, pharmacology, and toxicology. Evid. Based. Complementary. Altern. Med. 2014;2014 doi: 10.1155/2014/284517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonkchalee O., Boonmars T., Aromdee C., Laummaunwai P., Khunkitti W., Vaeteewoottacharn K., Sriraj P., Aukkanimart R., Loilome W., Chamgramol Y., Pairojkul C. Anti-inflammatory, antioxidant and hepatoprotective effects of Thunbergia laurifolia Linn. on experimental opisthorchiasis. Parasitol. Res. 2012;111(1):353–359. doi: 10.1007/s00436-012-2846-5. [DOI] [PubMed] [Google Scholar]
- Woode E., Ansah C., Ainooson G., Abotsi W., Mensah A., Duweijua M. Anti-inflammatory and antioxidant properties of the root extract of Carissa edulis (Forsk.) Vahl (Apocynaceae) J. Sci. Technol. 2007;27:5–15. doi: 10.4314/just.v27i3.33054. [DOI] [Google Scholar]
- Worldometer . 2022. Reported Cases and Deaths by Country or Territory.https://www.worldometers.info/coronavirus 14 June 2022. [Google Scholar]
- Xie M., Liu X., Cao X., Guo M., Li X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020;21:49. doi: 10.1186/s12931-020-1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav K.N., Kadam P.V., Patel J.A., Patil M.J. Strychnos potatorum: phytochemical and pharmacological review. Phcog. Rev. 2014;8(15):61. doi: 10.4103/0973-7847.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerima M., Magaji M.G., Yaro A.H., Tanko Y., Mohammed M.M. Analgesic and antiinflammatory activities of the methanolic leaves extract of Securinega virosa (Euphorbiaceae) Niger. J. Pharm. Sci. 2009;8(1):47–53. [Google Scholar]
- Younis W., Asif H., Sharif A., Riaz H., Bukhari I.A., Assiri A.M. Traditional medicinal plants used for respiratory disorders in Pakistan : a review of the ethno medicinal and pharmacological evidence. Chin. Med. 2018;13:48. doi: 10.1186/s13020-018-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf A.A., Lawal B., Sani S., Garba R., Mohammed B.A., Oshevire D.B., Adesina D.A. Pharmacological activities of Azanza garckeana (Goron tula) grown in Nigeria. Clin. Phytosci. 2020;6:1–8. [Google Scholar]
- Zahara K., Panda S.K., Swain S.S., Luyten W. Metabolic diversity and therapeutic potential of Holarrhena pubescens: an important ethnomedicinal plant. Biomolecules. 2020;10(9):1341. doi: 10.3390/biom10091341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer M., Ahmed S., Hassan M.M. Vigna unguiculata (L.) Walp. (Papilionaceae): a review of medicinal uses, Phytochemistry and pharmacology. J. Pharmacogn. Phytochem. 2020;9(1):1149–1152. [Google Scholar]
- Zarin M.A., Wan H.Y., Isha A., Armania N. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Hum. Wellness. 2016;5(2):65–75. [Google Scholar]
- Zayed M.Z., Sallam S.M.A., Shetta N.D. Review article on Leucaena leucocephala as one of the miracle timber trees. Int. J. Pharm. Pharmaceut. Sci. 2018;10(1):1–7. doi: 10.22159/ijpps.2018v10i1.18250. [DOI] [Google Scholar]
- Zhang H., Zhang C.-R., Han Y.-S., Wainberg M.A., Yue J.-M. New Securinega alkaloids with anti-HIV activity from Flueggea virosa. RSC Adv. 2015;5(129):107045–107053. [Google Scholar]