Abstract
Beta thalassemia is associated with decreased immunity possibly due to iron overload. Al-hijamah (Hijamah) is wet cupping therapy (WCT) of prophetic medicine. Prophet Muhammad Peace be upon him said: “The best among your treatments is Al-hijamah”. Al-hijamah is a promising excretory treatment to clear blood of causative pathological substances. Al-hijamah is a three-step technique (skin suction, scarification and suction) i.e. triple S technique). Recently, we introduced Al-hijamah as a novel iron excretion therapy (through pressure-dependent filtration then excretion via the skin dermal capillaries) that significantly decreased serum iron overload and related oxidative stress using a physiological excretory mechanism (Taibah mechanism). Iron overload was reported to impair both humoral immunity and cell mediated immunity in patients with beta thalassemia. In this study, twenty patients having β-thalassemia major (maintained on iron chelation therapy) underwent a single session of Al-hijamah (30-60 minutes) using 4-5 sucking cups only. Another age and sex-matched control group of thalassemic patients received iron chelation therapy only. Al-hijamah enhanced the immunity of thalassemic patients in the form of increased CD4+ T cell count, from 124.10±36.98 to 326.20±57.94 cells/mm3, and an increased CD8+ T cell count from 100.30±36.98 to 272.40±46.37 cells/mm3. CD4/CD8 ratio significantly increased from 1.29 to 1.7 (P<0.001). There was a significant increase of ten times (P<0.001) in serum TAC/MDA ratio (reflects increased antioxidant capacity vs decreased oxidative load and stress) induced by Al-hijamah. After Al-hijamah, both CD4+ and CD8+ T cell counts significantly increased and positively correlated with TAC/MDA ratio (r = 0.246) and (r = 0.190), respectively. Moreover, CD4/CD8 ratio positively correlated with TAC/MDA after Al-hijamah (r = 0.285). In conclusion, Al-hijamah significantly increased CD4/CD8 ratio in thalassemic patients via increasing TAC/MDA ratio. Our study strongly recommends medical practice of Al-hijamah in hospitals for its immune potentiating effects in agreement with the evidence-based Taibah mechanism. Al-hijamah should be generalized for treating other immune-deficiency conditions. Al-hijamah-induced bloody excretion is so minimal and never aggravates the anaemic status.
Keywords: Thalassemia, Al-hijamah, iron chelation therapy, CD4, CD8, Al-hijamah indices
Introduction
Beta thalassemia major is an inherited haemoglobin disorder resulting in chronic hemolytic anaemia that requires lifelong transfusion therapy. Thalassemia is a chronic hemolytic anaemia characterized by iron overload resulting from repeated blood transfusions and RBCs hemolysis (possibly due to enhanced oxidative stress) [1] and decreased immunity (decreased CD4/CD8 ratio and decreased count of CD4+ T cells) [2]. Life-long blood transfusion therapy for thalassemia has a negative effect on the immune and coagulation systems causing the accumulation of iron in the tissues, immunosuppression and intercurrent infections [3]. Anaemic status in thalassemic patients is closely related to their immunological dysfunctions where thalassemic patients exhibit decreased haemoglobin, MCV and MCH level. Iron overload was reported to affect both humoral immunity and cell mediated immunity in patients with beta thalassemia having decreased Immunoglobulin M (IgM), CD3+ and CD4+ T cells and elevation of CD8+ T cells, IgG, and IgA [4]. Disturbed neutrophil function was found in all thalassemia patients and systemic iron status had inverse correlation with phagocytosis capacity of neutrophils [5]. Thalassemia-induced decrease in cellular immunity is a common predisposing factor to infections in patients with thalassemia. Impaired splenic functions and splenectomy render thalassemic patients suffer from immune deficits and may develop stroke thalassemia [6,7].
In a related context, we reported that Al-hijamah is a promising iron excretion treatment via direct excretion of iron-containing proteins (ferritin) in the bloody excretion removed in the sucking cups during Al-hijamah [8]. Al-hijamah is wet cupping therapy (WCT) of prophetic medicine and is strongly recommended in prophetic medicine for the large magnitude of its therapeutic benefits. Merits of prophetic medicine are evident on Al-hijamah methodology, therapeutic indications and preventive indications and the persistent practice of Al-hijamah till now. Prophet Muhammad peace be upon him said: “Cure is in three: a gulp of honey, shartat mihjam (Al-hijamah) and cauterization. I do not recommend my nation to cauterize”. Prophetic medicine granted Al-hijamah durability and eternity. Al-hijamah is unique in its mechanism of action via purifying blood stream and tissues from disease-causing and disease-related substances in agreement with the evidence-based Taibah mechanism (Taibah theory) [9-13]. Taibah mechanism states that Al-hijamah uses a physiological excretory mechanism (pressure-dependent filtration and excretion) through the fenestrated capillaries of the skin dermis (acting as a filter) that resemble the fenestrated capillaries of the renal glomeruli. In Taibah mechanism, Al-hijamah also acts as a super kidney that can excrete all causative pathological substances (CPS) collectively and simultaneously outside the human body. Consequently, tissues, serum and intercellular fluids are purified from CPS and that may enhance the immunity [10,13].
Historic roots of Al-hijamah are deep and original in prophetic medicine where prophetic teachings conferred eternity to Al-hijamah. In China, a different but closely related method of WCT is utilized. Based on that, Al-hijamah has no roots in Chinese medicine. Chinese WCT is formed basically of two steps: skin scarification followed by skin suction. Chinese WCT is so-called the double S technique while Al-hijamah is called the triple S technique. Till now, Al-hijamah (triple S technique) is not practiced in Chinese medicine and can’t be categorized among traditional Chinese medicines. Traditional Chinese WCT is quite different methodologically from Al-hijamah.
In this study, we aimed at investigating Al-hijamah-induced immunological effects and also aimed at investigating the principles of Taibah mechanism: “Whenever immunity is to be increased and enhanced, Al-hijamah is indicated”. Moreover, in this study, we investigated Al-hijamah as a promising treatment for enhancing immunity in thalassemic patients.
Materials and methods
Goals of the study
Our study aimed at investigating the immune potentiating effects of Al-hijamah in thalassemic patients.
Study design and participants
In this study, we aimed at investigating the immune potentiating effects of Al-hijamah. This study enrolled twenty thalassemic patients (15 males and five females) in the treatment group and another 20 age and sex matched thalassemia subjects in the control group. All were diagnosed with β-thalassemia major.
Ethics approval and consent to participate
Ethical committee approval was gained according to the ethical guidelines for performing clinical studies in humans and according to the declaration of Helsinki. Ethical committee approval was gained from Tanta Faculty of Medicine, Tanta University, Gharbeya governorate, Egypt. Written patients’ agreements and consents were done to start this clinical trial. Trial registration number is: NCT 02761395.
Patients’ education regarding Al-hijamah
Al-hijamah education was done in advance for all patients and their families just before starting the study. Inclusion criteria included patients diagnosed with β-thalassemia major, on regular intake of iron chelation therapy, having hemoglobin ≥9 g/dl and willing to join the study. Exclusion criteria excluded from this study any patient presenting with thalassemia-induced complications e.g. liver cirrhosis, endocrinopathy, hypersplenism, presence of associated medical conditions (thalassemia complicated with stroke or viral hepatitis) or severe anemia. All patients had pallor and jaundice (100%). Most patients had splenomegaly (95%) while some patients (25%) had hepatomegaly. Blood transfusion was given regularly to patients every two weeks.
Al-hijamah was done in a pure sterile medical environment
In this study, Al-hijamah was performed to thalassemic patients at Tanta University Hospital hospital, Tanta University, Egypt. Al-hijamah was done in a complete aseptic atmosphere by four physicians including the corresponding author. Before each transfusion, full blood cross-matching, screening for new antibodies and red cell antigen typing of patients (at least for C, E and Kell) were routinely done. The physicians who performed Al-hijamah to the patients were aware of the red cell antibodies, transfusion reactions and annual transfusion requirements for each patient is a routine.
Procedures and variables assessment
Methods of our study included estimation of CD4+ T cell and CD8+ T cell counts and CD4/CD8 ratio in all the investigated thalassemic patients: control subjects (thalassemia patients receiving iron chelation therapy) and also in the treatment group (receiving both Al-hijamah and iron chelation therapy) before and after Al-hijamah.
Precautions and preparations for Al-hijamah
Blood sampling was done just before Al-hijamah and few days after it. Regimen of iron chelation therapy was deferasirox given at 20-30 mg/kg/day (once daily before meals). For patients with serum ferritin levels >3000 ng/ml, deferasirox was combined with deferoxamine (20-40 mg/kg for 8-12-hour) via subcutaneous infusion using an infusion pump or continuous intravenous infusion (8-10 hours/day for 10 days per month). Patients’ compliance was good in general.
Steps of Al-hijamah
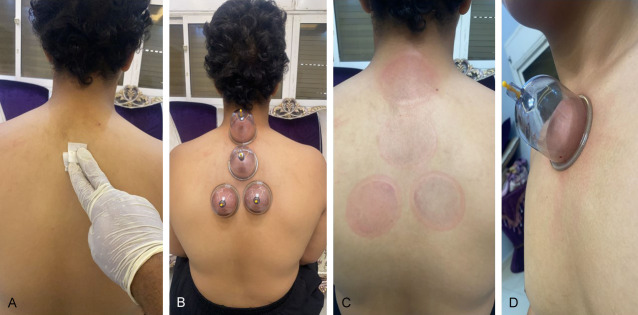
Al-hijamah (triple S therapeutic technique) was done for all patients in the intervention group. Al-hijamah included skin suction, scarification and suction. Methodology of Al-hijamah was discussed in details in all our publications [8-12]. Briefly, skin sterilization was done at the selected anatomical sites: the upper back and upper sternum. First suction was done through applying sucking cups to the indicated anatomical sites (Figure 1A) for 5 minutes using moderate suction induced via a hand-held pump. Skin upliftings were created (beneath which collected and filtered fluids containing CPS were gathered) after lifting the sucking cups (Figure 1A). Sucking cups were also put at the upper sternum few centimeters below the neck. Skin upliftings were created. Immediately after removing the cups, marks for cups applications were evident (Figure 1B).
Figure 1.
Al-hijamah begins with skin sterilization followed by applying the sucking cups at previously decided anatomical sites. A. Skin sterilization using alcohol. B. Upper cup was put at the kahel region, then sub-kahel region and lower two cups were put at the interscapular region. C. Temporary marks at the upper back region (sites of cups application). D. Sucking cup is put at upper sternal region (an anatomical site that is thought by Al-hijamah practitioners to enhance immunity being in close proximity to the thymus gland).
Skin scarifications were done as usual and sucking cups were applied to do the second suction step. Skin scarification was done in all Al-hijamah-induced skin upliftings (Figure 2A). Skin scarification was superficial, vertical (1-3 mm in length), productive and in parallel rows confined to the skin upliftings (Figure 2A, 2B). Second suction step was done immediately following skin scarifications to allow for getting rid of the bloody excretion containing the CPS [8-12].
Figure 2.
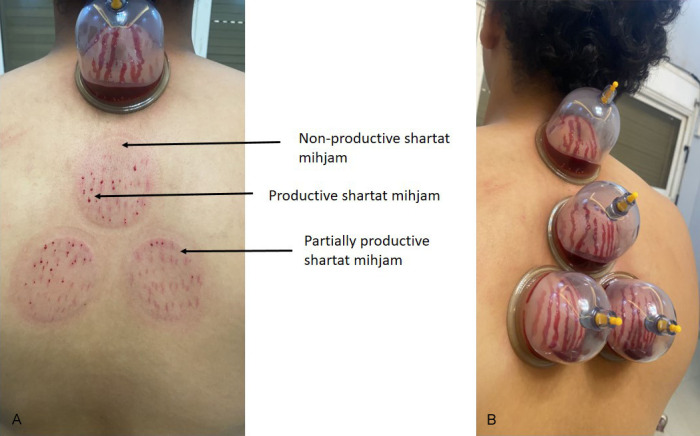
Skin scarifications (shartat mihjam) are the curative skills of Al-hijamah. A. Sharta mihjam should always be productive not partially productive or unproductive. B. Shartat mihjam denotes a skillful practitioner of Al-hijamah (hijamatologist). Scarifications should always be superficial, multiple, vertical (1-3 mm in length), productive, in parallel rows and confined to the skin upliftings.
Data monitoring and adverse events
Al-hijamah was terminated after putting the cups twice or three times. All patients in the intervention group were happy and comfortable. No adverse events were recorded. Finally, strict sterilization was applied. Sucking cups usually leave well-demarcated skin upliftings (Figure 3B). Minimal bloody excretion was collected in all the sucking cups (about 50 ml) in total.
Figure 3.
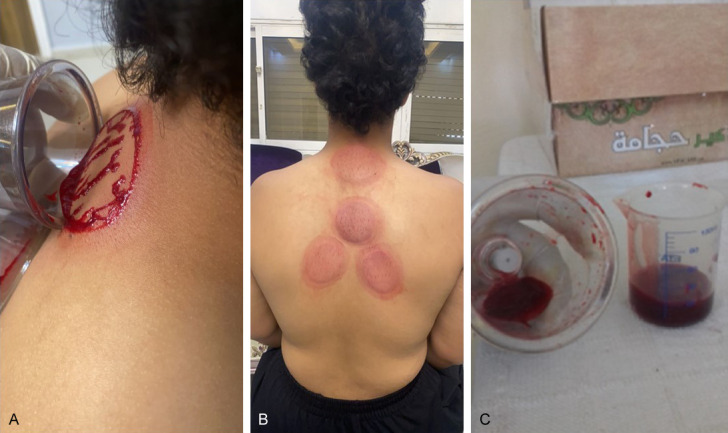
Temporary cupping marks after Al-hijamah. A. The blood excretion that comes out in the sucking cups. B. Cupping marks resolve spontaneously within few days. Skin sterilization is done immediately after Al-hijamah. Cupping marks do not need antibiotics treatment. C. Minimal bloody excretion comes out during Al-hijamah. All collected bloody excretion in the five sucking cups is less than 50 ml.
Endpoints assessment
We assessed Al-hijamah-induced therapeutic effects regarding immunity (CD4, CD8 and CD4/CD8 ratio). That was done within days to less than one month after Al-hijamah.
Evaluation of therapeutic indices of Al-hijamah [8-12]
All the measurements were done within one month duration after the single session of Al-hijamah. Indices of Al-hijamah were estimated as previously describe.
Immunological index measures the degree of immunological potentiation gained after Al-hijamah e.g. lymphocytosis. It can be calculated by measuring the number of CD4 and CD8 cells before and after Al-hijamah.
(II) = [100 × (Immunological response after Al-hijamah)/(Immunological response before Al-hijamah)].
Flow cytometry for CD4 and CD8 cells counting
T-cell subsets (CD4+ & CD8+) were determined using FacsCanto flow cytometer (BD Heidelberg, Germany). Briefly, 100 μl of EDTA blood was stained with 5 μl of the antibodies (anti-CD4-FTIC & anti-CD8-PE & anti-CD45-V500). All antibodies were provided by BD Heidelberg (Germany) in the staining tubes. The tubes were incubated in dark conditions for 20 min then samples were mixed with BD FACS lysing solution (1X) then incubated for 15 min in dark conditions also. Samples were then centrifuged at 1250 rpm for 5 min. The supernatant was discarded to remove the lysed RBCs. Phosphate buffered saline (PBS) was added then samples were centrifuged at 1250 rpm for 5 min where the supernatant was discarded to remove any remaining debris or RBCs then the pellets were re-suspended in 350 µl of PBS. The absolute numbers of cells were calculated using the following formula: The absolute numbers of cells = percentage of cells × total number of white blood cells/100.
Calculating CD4/CD8 ratio
That was done as a simple division calculation.
Calculating total antioxidant capacity/malondialdehyde (TAC/MDA) ratio
Oxidative lipid peroxidation reflecting oxidative stress status was quantified by measurement of serum malondialdehyde (MDA) level using commercially available kits (Biodiagnostic, Giza, Egypt). Serum total antioxidant capacity (TAC) was estimated using commercial kits (elabscience, TX, USA) according to manufacturer’s instructions. Briefly, 100 μL of buffer Solution was added to 1.5 mL eppendorff tubes followed by adding 10 μL of serum. Then, 200 μL of chromogenic Agent working solution and 50 μL of ferric salt stock working solution were added to the sample tubes and control tubes. That was mixed fully to react at 37°C for 30 min. Ferric salt diluent, stop solution and clarificant were sequentially added. Full mixing was done followed by waiting at room temperature. Then, 300 μL of the reaction liquid were used to take the readings at 520 nm OD value using Biotek Synergy multimode microplate reader. TAC/MDA was then calculated.
Statistical analysis
Data was collected, analyzed using SPSS software and presented as mean ± standard error of mean. Paired samples t test was used to compare results before and after Al-hijamah in the treatment group (paired data) i.e. within the same group (intervention group). * indicated P<0.05, ** indicated P<0.01 and *** indicated P<0.001. Independent samples t test was used to compare results between different groups (patients versus controls). # indicated P<0.05, ## indicated P<0.01 and ### indicated P<0.001.
Results
In this study, both Al-hijamah and iron chelation therapy were conducted on twenty patients previously diagnosed as β-thalassmia major. They were 15 males and 5 females. Another twenty patients having thalassemia major with matching age, sex, duration-of-illness served as control patients who were maintained on iron chelation therapy.
Al-hijamah significantly enhanced the natural immunity (Figure 4A, 4B)
Figure 4.
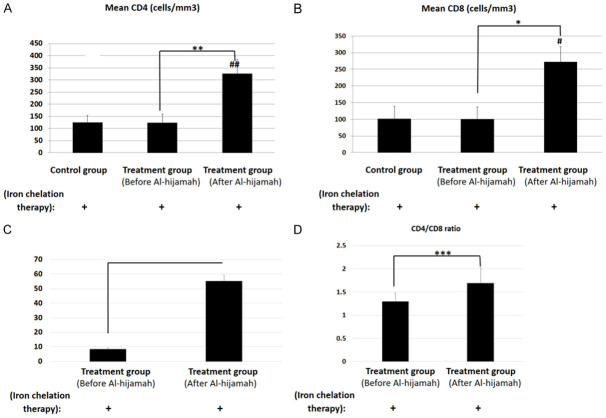
Al-hijamah significantly enhanced immunity in thalassemic patients receiving iron chelation therapy. A. CD4+ T cell count significantly increased when combining iron chelation therapy with Al-hijamah (P<0.01). B. CD8+ T cell count significantly increased when combining iron chelation therapy with Al-hijamah (P<0.05). C. TAC/MDA ratio significantly increased ten times after Al-hijamah. D. CD4/CD8 ratio significantly increased after Al-hijamah. N.B. All studied patients were thalassemic patients receiving iron chelation therapy. Control group did not undergo Al-hijamah treatment. There is no healthy control. * indicated P<0.05 and ** indicated P<0.01 within the same group (intervention group). ## indicated P<0.01 to compare results between different groups (patients versus controls).
Regarding the immunological response in thalassemic patients treated with Al-hijamah, our results revealed that mean CD4+ T-helper cells for the thalassemic patients before the procedure was 124.10±30.27 cells/mm3, which significantly increased to 326.20±57.94 cells/mm3 (P<0.01) after it (Figure 4A). For the control group, CD4+ T-helper cells count was 124.90±28.85 cells/mm3 that did not change significantly from the baseline of the intervention group (before Al-hijamah) (P>0.05). Using independent samples t test, CD4 T-helper cells count (after Al-hijamah) in Al-hijamah group was significantly different from the control (P<0.01) (Figure 4A) (Table 1).
Table 1.
Effects of Al-hijamah on some immunological parameters of thalassemic patients
| Patient’s number | Before Al-hijamah | After Al-hijamah | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CD4+ T cell count (cells/mm3) | CD8+ T cell count (cells/mm3) | CD4/CD8 ratio | TAC/MDA ratio | CD4+ T cell count (cells/mm3) | CD8+ T cell count (cells/mm3) | CD4/CD8 ratio | TAC/MDA ratio | |
| 1 | 150 | 108 | 1.39 | 8 | 183 | 118 | 1.55 | 42.14 |
| 2 | 31 | 28 | 1.11 | 13 | 343 | 258 | 1.33 | 35.08 |
| 3 | 35 | 70 | 0.5 | 13.7 | 185 | 125 | 1.48 | 45.088 |
| 4 | 42 | 88 | 0.48 | 11.5 | 168 | 105 | 1.6 | 51.8 |
| 5 | 44 | 71 | 0.618 | 6.8 | 965 | 729 | 1.32 | 72.86 |
| 6 | 60 | 32 | 1.878 | 10.2 | 150 | 76 | 1.97 | 50.7 |
| 7 | 200 | 184 | 1.09 | 5.4 | 709 | 293 | 2.41 | 30.6 |
| 8 | 53 | 43 | 1.23 | 7.1 | 116 | 550 | 0.21 | 39.33 |
| 9 | 108 | 73 | 1.45 | 13 | 724 | 235 | 3.08 | 47 |
| 10 | 77 | 35 | 2.2 | 9.5 | 532 | 79 | 6.73 | 85.14 |
| 11 | 450 | 552 | 0.82 | 6.2 | 124 | 322 | 0.39 | 80.26 |
| 12 | 15 | 33 | 0.45 | 8.6 | 186 | 113 | 1.65 | 28.14 |
| 13 | 425 | 200 | 2.13 | 0.5 | 350 | 258 | 1.36 | 58.4 |
| 14 | 283 | 73 | 3.88 | 9.6 | 183 | 125 | 1.46 | 42.96 |
| 15 | 31 | 58 | 0.53 | 4.9 | 343 | 105 | 3.27 | 73.45 |
| 16 | 50 | 61 | 0.82 | 8.2 | 185 | 729 | 0.25 | 52.03 |
| 17 | 60 | 32 | 1.88 | 4.7 | 67 | 150 | 0.45 | 52.98 |
| 18 | 300 | 200 | 1.5 | 11.11 | 709 | 293 | 2.42 | 87.34 |
| 19 | 53 | 43 | 1.23 | 10.15 | 116 | 550 | 0.21 | 80 |
| 20 | 15 | 22 | 0.68 | 8.4 | 186 | 235 | 0.79 | 49.29 |
| Mean | 124.1 | 100.3 | 1.29 | 8.5 | 326.2 | 272.4 | 1.70 | 55.23 |
| SD | 135.32 | 120.04 | 0.82 | 3.3 | 259.11 | 207.41 | 1.49 | 18.34 |
| SEM | 30.27 | 26.85 | 0.18 | 0.37 | 57.97 | 46.34 | 0.33 | 4.1 |
Also, the mean CD8+ T-cytotoxic lymphocytes before Al-hijamah was 100.30±36.98 cells/mm3, which increased to 272.40±46.37 cells/mm3 after the procedure with a significant difference (P<0.05). For the control group (receiving iron chelation therapy only), CD8+ T count was 101.30±36.87 cells/mm3 that was not significantly different from the intervention group (before Al-hijamah) (P>0.05) (Figure 4B). Using independent samples t test, CD8+ T-cytotoxic cells count (after Al-hijamah) in Al-hijamah group was significantly different from the control (P<0.01). Interestingly, Al-hijamah increased CD4/CD8 ratio from 1.29 to 1.7 (Table 1). One sample t test revealed a significant difference (P<0.001) between CD4/CD8 ratio before Al-hijamah versus after Al-hijamah.
Efficacy of Al-hijamah as an immune potentiating treatment (therapeutic indices of Al-hijamah)
Immunological index (II) for increasing CD4 cells = [100 × 326.2/124.1] = 262.85%.
i.e. combination of Al-hijamah with iron chelation therapy increases immunity using CD4 in thalassemic patients by about 263% (Table 1).
Immunological index (II) for increasing CD8 cells = [100 × 272.4/100.3] = 272%.
i.e. combination of Al-hijamah with iron chelation therapy increases immunity using CD8 in thalassemic patients by about 272% (Table 1).
CD4/CD8 ratio positively correlated with TAC/MDA ratio
One sample t test revealed a significant difference (P<0.001) between serum TAC/MDA ratio before and after Al-hijamah.
After Al-hijamah, both CD4 and CD8 cells counts positively correlated with TAC/MDA ratio at (r = 0.246) and (r = 0.190), respectively. Moreover, CD4/CD8 ratio positively correlated with TAC/MDA after Al-hijamah (r = 0.285).
Discussion
Immunity is suppressed in thalassemic patients. Potentiation of the natural immunity (physiological leukocytosis and increased number of natural killer cells) was reported when treating rheumatoid arthritis using Al-hijamah [14]. Zhang et al, reported that traditional drug cupping therapy decreased serum levels of the immune inhibitory cytokines e.g. immunoglobin E, interleukin-4 (IL-4) and IL-10. Moreover, WCT increased the production of endogenous immune stimulatory cytokines e.g. interferon-gamma, IL-2, complement-3, complement-4, immunoglobulins (IgA, IgG, and IgM) and activated cells of the immune system e.g. T-helper cells [15]. Recently, we reported that nigella sativa improves anemia, enhances immunity and relieves iron overload-induced oxidative stress as a novel promising treatment in thalassemic patients. Decreasing oxidative stress via the antioxidant ingredients in nigella sativa was associated with increased the immune stimulant cytokine interferon-gamma, CD4 lymphocytes and CD8 lymphocytes [16].
Interestingly, our data proved that Al-hijamah was effective as an immune stimulating treatment that markedly enhanced cell-mediated immunity (Figure 4A, 4B). Immunological therapeutic benefits due to Al-hijamah may be based on different mechanisms. In a previous report, we proved that Al-hijamah significantly decreased iron overload by about 25% following a single session of Al-hijamah using four suction cups [8]. Iron overload is well-known to decrease immunity in thalassemic patients. Iron overload was reported to decrease the proliferative capacity, numbers, and activity of CD4+ T-helper lymphocytes. Moreover, iron overload was reported to impair the generation of CD8+ T-cytotoxic lymphocytes, and the production of immunoglobulins. Iron overload was also reported to be responsible for the poor response to interferon therapy and the reduced functions of the complement system (including both the classic and alternative pathways). Moreover, high plasma ferritin content was reported to induce the development of anti-ferritin antibodies with the production of circulating immune complexes. Iron overload was also reported to tip the immunoregulatory balance unfavourably allowing for increased growth rates of cancer cells and infectious organisms [3].
In this study, our data confirmed that Al-hijamah significantly increased the levels of CD4+ T-helper and T-cytotoxic lymphocytes (Figure 4A, 4B). Moreover, Al-hijamah increased CD4+ and CD8+ T cell percentages and CD4/CD8 ratio (Table 1). This is in agreement with a recent report where Al-hijamah effectively treated Hashimoto’s thyroiditis via significantly decreasing serum autoantibodies e.g. anti-thyroid peroxidase antibody and thyroglobulin antibody. Interestingly, Al-hijamah caused a significant decrease in levels of thyroid stimulating hormone prolactin, and erythrocyte sedimentation rate also [17]. Based on that, we recommend Al-hijamah for immunological potentiation that may be associated with different human diseases.
TAC/MDA represents the antioxidant/oxidative stress ratio, is used as an index of oxidative stress status where increased oxidative stress causes increased tissue damage [18]. Our data revealed a significant difference (P<0.001) between serum TAC/MDA ratio before and after Al-hijamah. There was a significant ten times increase (P<0.001) in serum TAC/MDA ratio (reflects antioxidant excess vs oxidative stress) after Al-hijamah. In addition, both CD4+ and CD8+ T cell counts positively correlated with TAC/MDA ratio after performing Al-hijamah, respectively. Moreover, CD4/CD8 ratio positively correlated with TAC/MDA after Al-hijamah.
Author’s own views are quite convinced with the necessity of practicing Al-hijamah in hospitals for treating different disease conditions particularly when noxious substances are to be excreted outside the human body e.g. percutaneous excretion of excess iron and ferritin in thalassemia and percutaneous excretion of malondialdehyde and harmful oxidants in a wide panel of human diseases. In this study, we do recommend using Al-hijamah for immunological potentiation that may be associated with different human diseases.
Al-hijamah in hospitals for treating different disease conditions is strongly recommended. Al-hijamah proved to be tolerable than many surgical procedures in pediatric practice e.g. abscess evacuation and circumcision. Simplicity of Al-hijamah and its triple S steps (Figures 1, 2 and 3) encourages physicians, especially hematologists to practice it for many therapeutic indications [11-13]. In our study, thalassemia patients treated with Al-hijamah did not exhibit any side effects throughout the procedure. They liked it later and requested the pediatric hematologists to do Al-hijamah again for them.
Finally, we plan to do a larger future study with a larger number of patients to be monitored continuously. Previous reports [8,19] and this report on Al-hijamah confirmed its safety. No practice-related infection was reported in our study. Aseptic medical environment guarantees sterile safe aseptic practice of Al-hijamah. Our data confirmed the second principle of Taibah mechanism: Whenever immunity is to be increased and enhanced, Al-hijamah is also indicated. Al-hijamah proved to be promising when noxious substances are to be excreted outside the human body e.g. percutaneous excretion of excess iron and ferritin in thalassemia and percutaneous excretion of malondialdehyde and harmful oxidants in a wide panel of human diseases.
Conclusion
There was a significant ten times increase in serum TAC/MDA ratio (reflects antioxidant balance vs oxidative stress) after Al-hijamah. After Al-hijamah, both CD4 and CD8 cells counts positively correlated with TAC/MDA ratio at (r = 0.246) and (r = 0.190), respectively. Moreover, CD4/CD8 ratio positively correlated with TAC/MDA after Al-hijamah (r = 0.285). Al-hijamah significantly increased CD4/CD8 ratio in thalassemic patients via increasing TAC/MDA ratio. Our study strongly recommends medical practice of Al-hijamah in hospitals for its immune potentiating effects in agreement with the evidence-based Taibah mechanism. Alhijamah should be generalized for treating other immune-deficiency conditions. Al-hijamah-induced bloody excretion is so minimal and never aggravates the anaemic status. Al-hijamah raises immunity in agreement with the evidence-based Taibah mechanism.
Acknowledgements
We are so grateful to Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia for kindly providing research materials and to Tanta University, Egypt for the efforts to perform this clinical trial. We are very grateful to Mr. Adel Joraibei Al-Obaidi, Mr. Raed Ali Al-Raddadi, Mr Sultan Al-Hussini, Mr. Mohamed Abdelsamad and Mr. Wael Barakat from the administrative department, College of Medicine, Taibah University for their technical help and support to this work. The authors are grateful to the Medical Research Centre and the Deanship of Scientific Research at Taibah University, Saudi Arabia for kindly supporting some kits and research materials for this article in the research project #8016.
Disclosure of conflict of interest
None.
References
- 1.Fibach E, Dana M. Oxidative stress in β-thalassemia. Mol Diagn Ther. 2019;23:245–261. doi: 10.1007/s40291-018-0373-5. [DOI] [PubMed] [Google Scholar]
- 2.Amrita PNA, Bintoro SUY, Sedana MP, Noordiansyah M, Ramadhan PZ, Savitri M, Prayoga AA. Serum ferritin level affects T lymphocyte CD4, CD8, and CD4/CD8 ratio in transfusion-dependent beta-thalassemia. Drug Invention Today. 2020;13:887–892. [Google Scholar]
- 3.Gluba-Brzózka A, Franczyk B, Rysz-Górzyńska M, Rokicki R, Koziarska-Rościszewska M, Rysz J. Pathomechanisms of immunological disturbances in β-thalassemia. Int J Mol Sci. 2021;22:1–13. doi: 10.3390/ijms22189677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagag AA, Elgamsy MA, El-Asy HM, Gamal RM, Elshahaby WN, Abd Elbar ES. Immune status ‘in children with beta thalassemia’ in correlation ‘with iron overload’: single center Egyptian study. Endocr Metab Immune Disord Drug Targets. 2016;16:181–188. doi: 10.2174/1871530317666161107160213. [DOI] [PubMed] [Google Scholar]
- 5.Santhakumar S, Devi V, Barade A, Kulkarni UP, George B, Sindhuvi E. Dysregulated neutrophil iron homeostasis in β-Thalassemia impairs phagocytosis and reactive oxygen species production. Blood. 2021;138:942. [Google Scholar]
- 6.Mettananda S, Gibbons RJ, Higgs DR. α-Globin as a molecular target in the treatment of β-thalassemia. Blood. 2015;125:3694–3701. doi: 10.1182/blood-2015-03-633594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liatsos GD. The immunity features and defects against primary cytomegalovirus infection post-splenectomy indicate an immunocompromised status: a PRISMA-compliant meta-analysis. Medicine. 2019;98:1–7. doi: 10.1097/MD.0000000000017698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Shanshory M, Hablas NM, Shebl Y, Fakhreldin AR, Attia M, Almaramhy HH, Baghdadi H, Ayat M, Albeihany A, El-Dardear A, Ibrahim HA, Mahmoud HS, Nabo MMH, El Sayed SM. Al-hijamah (wet cupping therapy of prophetic medicine) significantly and safely reduces iron overload and oxidative stress in thalassemic children: a novel pilot study. J Blood Med. 2018;9:241–251. doi: 10.2147/JBM.S170523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Sayed SM, Mahmoud HS, Nabo MMH. Methods of wet cupping therapy (Al-Hijamah): in light of modern medicine and prophetic medicine. Altern Integ Med. 2013;2:1–16. [Google Scholar]
- 10.El Sayed SM, Mahmoud HS, Nabo MMH. Medical and scientific bases of wet cupping therapy. Light of modern medicine and prophetic medicine. Altern Integ Med. 2013;2:1–16. [Google Scholar]
- 11.El Sayed SM, Baghdadi H, Abou-Taleb A, Mahmoud HS, Maria RA, Ahmed NS, Nabo MM. Al-hijamah and oral honey for treating thalassemia, conditions of iron overload, and hyperferremia: toward improving the therapeutic outcomes. J Blood Med. 2014;5:219–37. doi: 10.2147/JBM.S65042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Sayed SM, Abou-Taleb A, Mahmoud HS, Baghdadi H, Maria RA, Ahmed NS, Nabo MMH. Percutaneous excretion of iron and ferritin (through Al-hijamah) as a novel treatment for iron overload in beta-thalassemia major, hemochromatosis and sideroblastic anemia. Med Hypotheses. 2014;83:238–246. doi: 10.1016/j.mehy.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 13.El Sayed SM, Al-quliti AS, Mahmoud HS, Baghdadi H, Maria RA, Nabo MMH, Hefny A. Therapeutic benefits of Al-hijamah: in light of modern medicine and prophetic medicine. American Journal of Medical and Biological Research. 2014;2:46–71. [Google Scholar]
- 14.Ahmed SM, Madbouly NH, Maklad SS, Abu-Shady EA. Immunomodulatory effects of blood letting cupping therapy in patients with rheumatoid arthritis. Egypt J Immunol. 2005;12:39–51. [PubMed] [Google Scholar]
- 15.Zhang C, Liang T, Zhang W. Effects of drug cupping therapy on immune function in chronic asthmatic bronchitis patients during protracted period. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26:984–987. [PubMed] [Google Scholar]
- 16.El-Shanshory M, Hablas NM, Aboonq MS, Fakhreldin AR, Attia M, Arafa W, Mariah RA, Baghdadi H, Ayat M, Zolaly M. Nigella sativa improves anemia, enhances immunity and relieves iron overload-induced oxidative stress as a novel promising treatment in children having beta-thalassemia major. J Herb Med. 2019;16:100245. [Google Scholar]
- 17.Obeid AM, Qari FA, Aljaouni SK, Sawsan R, Elsayed AA, Alsayyad MM, Okmi EA. The effect of wet-cupping therapy (hijama) in modulating autoimmune activity of Hashimoto’s thyroiditis. Saudi Med J. 2022;43:45–52. doi: 10.15537/smj.2022.43.1.20210755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Bai H, Deng F, Zhong R, Liu L, Chen L, Zhang H. Chemically protected sodium butyrate improves growth performance and early development and function of small intestine in broilers as one effective substitute for antibiotics. Antibiotics. 2022;11:132. doi: 10.3390/antibiotics11020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Shanshory M, Hablas NM, Shebel Y, Alhadramy O, El-Tahlawi R, Aboonq MS, Soliman TM, Abdel-Gawad AR, El Sayed SM, Abdallah HI, Mahmoud HS, El-Allaf H, El-Sawy S, Yousef RS, Abu-El Naga M, Mariah RA, Nabo MMH, Abdel-Haleem M, Mahmoud AA, Hassan MA, Al Arabi AH, Alnakhli AA, El Sayed SM. Al-hijamah (the triple S treatment of prophetic medicine) exerts cardioprotective, tissue-protective and immune potentiating effects in thalassemic children: a pilot clinical trial. Am J Blood Res. 2020;10:447–458. [PMC free article] [PubMed] [Google Scholar]