Summary
Epithelial folding is a fundamental process where initially flat monolayers transform into functional 3D structures. This protocol details fabrication steps for a polycarbonate microfluidic platform which enables triggering epithelial folds that recapitulate stereotypical cell shape changes and folding-associated mechanical stresses. We describe the steps for cell seeding to form a monolayer on the chip, and subsequent approach to trigger calcium waves in the epithelial monolayer through local epithelial deformation. Lastly, we outline quantitative analysis steps of the epithelial response.
For complete details on the use and execution of this protocol, please refer to Blonski et al. (2021).
Subject areas: Cell biology, Cell culture, Cell isolation, Single cell, Developmental biology, Biotechnology and bioengineering
Graphical abstract

Highlights
-
•
Microfluidic system that recapitulates epithelial folding in vitro
-
•
Epithelial monolayer seeded on-chip on an elastic membrane is locally deformed
-
•
Locally triggered epithelial folds induce spreading of intercellular calcium waves
-
•
Design of the system ensures compatibility with the live and high-resolution imaging
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Epithelial folding is a fundamental process where initially flat monolayers transform into functional 3D structures. This protocol details fabrication steps for a polycarbonate microfluidic platform which enables triggering epithelial folds that recapitulate stereotypical cell shape changes and folding-associated mechanical stresses. We describe the steps for cell seeding to form a monolayer on the chip, and subsequent approach to trigger calcium waves in the epithelial monolayer through local epithelial deformation. Lastly, we outline quantitative analysis steps of the epithelial response.
Before you begin
The protocol below describes the specific steps for the fabrication of the microfluidic system enabling epithelial monolayer folding. The basic techniques and methods described here are commonly available in the laboratory equipment. Solely, the pre-fabrication of the polycarbonate microfluidic chip (a sheet with an engraved channel) requires the use of specialized equipment such as milling machine. We are aware that such equipment is not available widely in all biology oriented laboratories. To overcome this obstacle of the protocol, whenever a specialized equipment or tools are necessary to proceed, we propose commercially available solutions. Additionally, our protocol described here was designed and optimized to induce folding-associated calcium waves within the MDCK monolayers. We are convinced that the system can be used with other cells lines known to form a monolayer, however, certain conditions/parameters of the protocol might need to be adjusted.
Preparation for fabricating the polycarbonate microfluidic systems
Timing: 1–3 days
-
1.Prepare a polycarbonate microfluidic chip (Figure 1) using a milling machine before starting the procedure. We designed the device in AutoCAD (Autodesk Inc.). The size of the microfluidic chip (in plane) is designed to fit a steel holder that provides a reservoir for cell culture on the chip (selfmade product). We used a polycarbonate sheet (thickness of 375 μm) to ensure compatibility with high-resolution microscopy techniques.Alternatives: similar commercial holders are available i.e., Pecon Cultivation System (www.pecon.biz; model- Open Cultivation Chamber) or Ske Cell Culture chambers (www.ske.it; model - CC020) chip area and shape need to be adjusted to specific setting of the commercial holders.
-
a.To mimic cell in-plane and out-of-plane deformations of the physiological epithelial folding (Blonski et al., 2021) design a single channel system (width 400 μm and depth 200 μm) with an inlet (our design is available in Mendeley Data, V2, under name “Brun-Cosme-Bruny et al., Star Protocol, Microfluidic system”).
-
b.The system is fabricated by mechanical milling of the channel directly in the polycarbonate. We used a vertical CNC milling machine (MFG4025P, Ergwind, Poland) and a 2-flute fish-tail end-mill cutter (diam. 400 μm or 200 μm; InGraph, Poland).Alternatives: If no milling equipment is available we suggest to contact external private companies providing service of micro-milling in numerous materials including polymers/plastics.
 CRITICAL: It is essential for subsequent bonding with the membrane to clean out the remaining swarfs from the engraved channels. To do so you can use a pressure washer (K7 Premium, Karcher, Germany) followed by soap and isopropanol washes.
CRITICAL: It is essential for subsequent bonding with the membrane to clean out the remaining swarfs from the engraved channels. To do so you can use a pressure washer (K7 Premium, Karcher, Germany) followed by soap and isopropanol washes.
-
a.
-
2.Fabricate tubing inlets (connectors) that will be bonded with the system during fabrication. In our configuration we used PDMS (polydimethylsiloxane) cubes with punched holes as inlets for tubes and pressure control (Figure 1).
-
a.Prepare 20–30 g of 10:1 Sylgard 184 by vigorously mixing (for 1 min) elastomer with the curing agent. Degas the solution.
-
b.Pour the degassed Sylgard 184 mix into a Petri dish (radius 10 cm) to obtain a 0.5 cm thick-layer and let it polymerize under cover in an oven at 60°C for 2 h.
-
c.Once polymerized, using scalpel cut cubes of ∼1 cm × 1 cm (with the ∼0.5 cm height) that will serve to connect to the microfluidic system the pressure tubing.
-
d.Using a biopsy punch (inner radius 0.8 mm), create a hole-through. Ensure that the puncher is positioned perfectly vertically, that PDMS did not break around the hole during the procedure, and that the created hole is smooth (see Figure 1 – step 4).
-
a.
CRITICAL: During pre-cutting with the scalpel wear protection gloves and glasses. For microfabrication the use of clean-area is not necessary but if available it is recommended.
Figure 1.
Fabrication of the microfluidic system
In the first step the polycarbonate microfluidic system is activated with the oxygen plasma and subsequently put-in-contact with 1% solution of APTES (step 2) which enables strong bonding of polycarbonate with PDMS. The elastic membrane is exposed to oxygen plasma and the activated surface is put in contact with the APTES treated polycarbonate to close the channel (step 3). To enable connection of the tubes with the system, a PDMS cube with a punched channel is bonded above the inlet to the PDMS membrane of the system using the oxygen plasma (step 4). Two photographs present the final system after fabrication (left) and once the system is installed in the steel holder (with the tubing connected [right]).
Cell culture maintenance for later use in the device
Timing: 2–3 days
-
3.
Prepare complete media for MDCK cells – MEM (Thermo Fisher Scientific, 31095052), 5% FBS and 1% antibiotic (Penicillin/Streptomycin).
-
4.
Two to three days before the experiment, seed MDCK cells at the concentration of 4 × 105 cells in complete media in a T75 flask and incubate at 37°C in 5% CO2 incubator. The culture should reach at least 3 × 106 cells at the time of the seeding.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Fluo-4 Direct Calcium Assay Kit | Thermo Fisher Scientific | ref #F10471 https://www.thermofisher.com/order/catalog/product/F10471?SID=srch-hj-F10471 |
| Deionized water | N/A | N/A |
| MEM (Minimal Essential Medium) | Thermo Fisher Scientific | ref #31095052 https://www.thermofisher.com/order/catalog/product/31095052 |
| MEM, suspension, no calcium, no glutamine (S-MEM) | Thermo Fisher Scientific | ref #11380037 https://www.thermofisher.com/order/catalog/product/11380037?SID=srch-hj-11380037 |
| Calcium Chloride solution (CaCl2); 1 M | Honeywell | ref #21114; CAS: 10043-52-4 https://lab.honeywell.com/shop/calcium-chloride-solution-21114 |
| Trypsin - EDTA 10× - 100 mL | Dutscher | ref #X0930-100 https://www.dutscher.com/frontoffice/article/X0930-100 |
| Dulbecco’s Phosphate Buffered Saline (PBS without Ca2+, Mg2+) | Merck | ref #D8537 https://www.sigmaaldrich.com/FR/fr/product/sigma/d8537?gclid=CjwKCAjwrqqSBhBbEiwAlQeqGvLyhOJR9S-2gniYubfDfVLmk5zl6V-fIUDZ3sm-B0FYvp5RR7jkERoCsxIQAvD_BwE |
| Penicillin/streptomycin 10 000 U/10 mg/mL | Dutscher | ref #P06-07100 https://www.dutscher.com/frontoffice/article/P06-07100# |
| RIPA Lysis Buffer, 10× | Merck | ref #20-188; CAS: 127087-87-0, 83-44-3 https://www.sigmaaldrich.com/FR/fr/product/mm/20188 |
| Sylgard 184 (Elastomer + curing agent) | Neyco | ref #DC184-1.1 https://www.neyco.fr/en/our-products/silicones-1/microfluidic-pdms/sylgard-184-silicone/silicones-2/microfluidique-pdms/silicone-sylgard-184/sylgard-184 |
| 3-aminopropyltriethoxysilane (APTES) | Merck | ref #440140; CAS: 919-30-2 https://www.sigmaaldrich.com/FR/en/product/aldrich/440140 |
| FluoSpheres™ Carboxylate-Modified Microspheres (2.0 μm, blue fluorescent 365/415 nm) | Thermo Fisher Scientific | ref #F8824 https://www.thermofisher.com/order/catalog/product/F8824 |
| Deposited data | ||
| Microsystem designs | N/A | Mendeley Data, V1, https://doi.org/10.17632/vdhwrpxw2s.1 |
| Experimental models: Cell lines | ||
| MDCK–II Cell line canine | Merck | ref #00062107 https://www.sigmaaldrich.com/FR/en/product/sigma/00062107 |
| Software and algorithms | ||
| ImageJ | National Institutes of Health | https://imagej.nih.gov/ij/download.html |
| Microsoft Excel | Microsoft | https://www.microsoft.com/en-gb/microsoft-365/excel |
| AutoCAD Software | Autodesk | https://www.autodesk.com/products/autocad/overview?term=1-YEAR&tab=subscription |
| Other | ||
| Polycarbonate - Film (Thickness: 0.375 mm) | Goodfellow | ref #CT30-FM-000140 https://www.goodfellow.com/fr/en-us/displayitemdetails/p/ct30-fm-000140/polycarbonate-film |
| Elastosil Film 2030 250/20 (Thickness: 20 μm) | WACKER | https://www.wacker.com/h/en-us/c/elastosil-film-2030/p/000038005 |
| Low pressure plasma system (Plasma Cleaner) Zepto Model 3 13.56 MHz base unit type B incl. rotary switch control |
Diener | https://www.plasma.com/en/low-pressureplasmasystem-zepto/ |
| Microfluidic pump AF1 VAN & AF1 VAC | ELVEFLOW | https://www.elveflow.com/microfluidic-products/microfluidics-flow-control-systems/high-accuracy-vacuum-pressure-pumps-af1/ |
| Biopsy punch 1 mm | World Precision Instruments | ref #504646 https://www.wpiinc.com/var-949-reusable-biopsy-punch-various-sizes |
| PTFE pipe ø int. x ø ext. = 0.5 × 1 mm length 25 m | Dutscher | ref #091927 https://www.dutscher.com/frontoffice/article/091927 |
| Ultrasound tank, 1.4 L | Dutscher | ref #053275 https://www.dutscher.com/frontoffice/article/053275 |
Materials and equipment
| Reagent | Final concentration | Amount |
|---|---|---|
| Sylgard 184 Elastomer | 10:1 Elastomer: Curing Agent
|
20 g |
| (3-Aminopropyl)triethoxysilane APTES |
1% (v/v) in ddH2O
|
1 mL |
| Fibronectin solution | 40 μg/mL in PBS
|
1.5 mL |
| FluoSpheres® 660/680, 0.2 μm | 1:200 (v/v) in isopropanol
|
100 μL |
| Fluo-4 Direct Calcium Assay Kit | Follow the protocol available from the supplier: https://www.thermofisher.com/order/catalog/product/F10471
|
10 mL |
| Calcium chloride, CaCl2 | 1 mM in ddH2O
|
20 mL |
| RIPA lysis buffer X10 | Dilute 1:10 in ddH2O
|
50 mL |
-
•Plasma cleaner is essential for the fabrication process. Each of the plasma cleaner machines have different parameters such as chamber volume, generator type, and power settings. We used Diener Zepto 13.56 MHz plasma cleaner. For polycarbonate surface functionalization with 3-Aminopropyltriethoxysilane (APTES) or further bonding with the PDMS we used the following parameters:
-
○We activated plasma with the pressure in the chamber being stabilized between 0.3 to 0.4 mbar and with the continuous oxygen flow of 0.2 nL/h.
-
○We used 10% power of the plasma during 1 min.
-
○
CRITICAL: The efficiency of plasma activation depends strictly on the numerous parameters such as generator type, size of the chamber, power and plasma type. It is necessary to optimize the parameters of use before starting the protocol. The good starting conditions are the parameters used to bond two PDMS surfaces together. Proper activation renders surfaces highly hydrophilic.
CRITICAL: Using a low pressure plasma cleaner is not harmful in terms of ozone release since the O2 pressure required inside the chamber is weak. However, a standard atmospheric plasma cleaner MUST evacuate the released ozone under a chemical hood to avoid intoxication.
Step-by-step method details
Microfluidic system preparation
Timing: 2–3 h
Once micro-channels are milled in the polycarbonate sheets (as described in the before you begin SECTION), the system is bonded with the elastic PDMS membrane, and subsequently the pressure tubing connector is installed.
-
1.Activation of the Polycarbonate disk with the engraved microchannel and inlet (Figure 1).
-
a.Put the polycarbonate disk in the plasma chamber, the crafted side facing up. Close the chamber and turn on the vacuum pump to decrease the pressure inside the chamber to 0.1 mbar.Note: Depending on the plasma system and the vacuum pump, it can take up to several minutes to stabilize the pressure inside the chamber.
-
b.Meanwhile, prepare the station for polycarbonate APTES functionalization.
-
i.On a bench fix a parafilm that is slightly bigger than the polycarbonate disk.
-
ii.Place a drop of 400 μL of freshly prepared APTES 1% v/v solution (see materials and equipment section) onto the parafilm.
-
i.
-
c.When the pressure is 0.1 mbar in the chamber, purge the chamber with pure O2:
-
i.Open the O2 bottle and the valves connected to the chamber.
-
ii.Turn on the O2 flow-meter to 2.0 nL/h and wait for the pressure to increase to ∼1 mbar.
-
i.
-
d.Decrease the O2 flow-meter to 0.2–0.3 nL/h in order to reach a pressure of 0.3–0.4 mbar in the chamber and subsequently activate the plasma for a total of 60 s at the power of 10%. Troubleshooting 1.Note: Depending on the plasma cleaner system the parameters might vary, see materials and equipment section.
-
e.Terminate the plasma cleaner cycle by turning off the pump and filling the chamber with the air.
-
a.
-
2.Using forceps, take the polycarbonate disk and put it on the 400 μL APTES drop with activated side (side with engraved channel) facing down. Incubate the polycarbonate for 20 min at 20°C–30°C.
 CRITICAL: You must proceed fast and put in contact the activated polycarbonate with APTES solution rapidly, since the plasma treatment is reversible. Usually it takes around 1 min to finalize this step.
CRITICAL: You must proceed fast and put in contact the activated polycarbonate with APTES solution rapidly, since the plasma treatment is reversible. Usually it takes around 1 min to finalize this step. CRITICAL: Very often at first attempt of putting the system in contact with APTES an air bubble is entrapped at the channel void. Reposition the system several times to remove the air and secure complete contact between the system and the APTES solution.
CRITICAL: Very often at first attempt of putting the system in contact with APTES an air bubble is entrapped at the channel void. Reposition the system several times to remove the air and secure complete contact between the system and the APTES solution.-
a.Meanwhile prepare a container with 50–100 mL of ddH2O, and a laboratory wash bottle with the pure isopropanol; both solution will be used for washing the polycarbonate after the APTES treatment.
-
b.5 min before the end of system incubation with the APTES proceed to the activation of the PDMS membrane; step 3.
-
a.
-
3.Activation of the PDMS membrane and bonding with the polycarbonate disk.
-
a.Cut a piece of 20 μm-thick PDMS membrane (available commercially) slightly bigger than the polycarbonate disk.
-
b.Put the PDMS film into the plasma chamber, with the PDMS layer facing up.Note: PDMS membrane is supplied on a removable plastic (thin-film) support on one side and protected by the non-sticking paper on the other side. During manipulation, it is important to keep the protective film as long as possible to limit the deposition of dust and debris on the PDMS membrane. We suggest removing the protection paper once the plastic film with the PDMS is already positioned inside the chamber.
-
c.Activate the PDMS film with the plasma cleaner following the steps 1 c–e.
 CRITICAL: If ventilation of the plasma chamber is too rapid the PDMS membrane on the plastic film can be displaced or even turned upside-down. To avoid it, secure the PDMS membrane at the corners with the Magic scotch TAPE.
CRITICAL: If ventilation of the plasma chamber is too rapid the PDMS membrane on the plastic film can be displaced or even turned upside-down. To avoid it, secure the PDMS membrane at the corners with the Magic scotch TAPE. -
d.Meanwhile, when plasma chamber equilibrates to atmospheric pressure, using forceps, place the polycarbonate disk into a ddH2O water container and gently rinse the system with water. Subsequently, while still holding the polycarbonate disk with the forceps, wash the APTES treated side thoroughly (30 s continuous flow) with the isopropanol from a manual dispenser (wash bottle).
-
e.Dry the polycarbonate disk with the compressed air.
-
f.Place the polycarbonate disk with the channel facing down on the activated PDMS membrane (obtained from step 3c) and press firmly.
 CRITICAL: Start the bond from the one side of the polycarbonate disk in order to avoid entrapment of air bubbles. Some bubbles can be removed by pressing them away locally with the fingers.
CRITICAL: Start the bond from the one side of the polycarbonate disk in order to avoid entrapment of air bubbles. Some bubbles can be removed by pressing them away locally with the fingers. -
g.Let it react for 30 min at 20°C–30°C.
-
h.Inspect the fabricated system under the microscope to identify potential problems such as entrapped dust or not perfectly bound membrane. Discard any system that presents such problems. Troubleshooting 2.
-
a.
-
4.Installation of the pressure tubing connector (PDMS cube) to the system.
-
a.Remove the protection film from the PDMS by peeling it off gently from the edge of the system. At this stage the channel and the inlet are closed by the membrane.
-
b.Using forceps and scalpel remove the PDMS membrane from above the inlet area to enable control over pressure in the channel.
 CRITICAL: If you are not working in the clean-room environment, keep fabricated systems in a closed Petri-dish or multi well plates to avoid any dust deposition. Wear laboratory gloves. To ensure successful bonding with the connector, avoid touching any PDMS surface to be bound and activated.
CRITICAL: If you are not working in the clean-room environment, keep fabricated systems in a closed Petri-dish or multi well plates to avoid any dust deposition. Wear laboratory gloves. To ensure successful bonding with the connector, avoid touching any PDMS surface to be bound and activated. -
c.Place the tubing connector and the polycarbonate system with the PDMS membrane already bonded (result of steps 2 and 3) facing up in the plasma chamber.
-
d.Activate both PDMS surfaces (connector and system) by following the steps 1c–e.
-
e.Align the connector and the inlet from the polycarbonate/PDMS system and put the two activated surfaces in contact for bonding. Subsequently, press gently to ensure full contact. Troubleshooting 1.
 CRITICAL: After step 3d, the surface of the PDMS membrane is activated with the plasma cleaner and therefore remains highly hydrophilic up to 3 h. Before proceeding to cell culture, it is necessary to wait at least 3 h for the PDMS membrane to become hydrophobic again.
CRITICAL: After step 3d, the surface of the PDMS membrane is activated with the plasma cleaner and therefore remains highly hydrophilic up to 3 h. Before proceeding to cell culture, it is necessary to wait at least 3 h for the PDMS membrane to become hydrophobic again.
-
a.
-
5.Quality verification of the system fabrication.
-
a.Insert the tubing into the fabricated connector on the system and further connect the system with the pressure controller (pressure pump). Subsequently insert the system to the Petri dish (diameter 10 cm) with water so that the connector can be fully submerged.
-
b.Place the system submerged in the water-bath in the Petri dish under the inverted microscope and observe visually if the PDMS membrane detaches from the polycarbonate upon increased pressure (we usually use +100 mbar for the testing). Subsequently, verify by eye if no air bubbles are produced in the water-bath at the level of tubing connector or channel. Troubleshooting 3.
-
a.
-
6.Quantification of the membrane deformation.
-
a.Sonicate the beads in the sonicator on the high power to destroy clusters of beads formed in the tube.
-
b.Dilute the nano-beads 1/200 in pure isopropanol and deposit a 20 μL drop on a microscope slide. Using a pipette tip, spread the solution to create a larger area over which the beads will be deposited. Let it dry at RT (or to accelerate in the oven at 40°C).
-
c.Once dry, put in direct contact the microscope slide with the deposited beads and the microsystem. Ensure that the region containing the channel is in contact with the beads. Repeat this step several times to deposit maximum number of beads on the PDMS membrane.
-
d.To observe the membrane deformation with the standard inverted confocal microscope, install the system upside down – channel facing the objective - in the coverslip holder (i.e., Ske company or Pecon Cultivation System). Connect the tubing and the pressure controller.
-
e.Use a confocal microscope with tile or multi-position option to create Z-stacks of the deformed membrane over the channel. Typically, we used a 20× objective (air, N.A 0.5) on a Spinning Disk Andromeda RILL-FEI (EMCCD iXon 897 Camera). Standard Z-range limits of the confocal microscope are sufficient for the imaging.
-
f.Take a Z-stack over the non-deformed system, and scan with different pressures the deformation of the membrane. From the obtained Z-stack it is possible to calculate the curvature at the channel border, and from the relative position of the nanobeads it is possible to calculate the in-plane strain of the membrane (Figure 2).Note: Similarly, the in-plane strain can be calculated by directly observing the changes in the area of the fluorescently stained cells.
-
g.Wash-out the nanobeads from the PDMS system by using soap and scrubbing the surface with hands covered with laboratory gloves (i.e., nitrile or latex). Subsequently, rinse thoroughly with water.
-
a.
Figure 2.
Observation of the membrane deformation
On the left, confocal orthogonal (XZ) images of nano beads deposited on the membrane for control (0 mbar) and under pressure (ΔP = -450 mbar). Scale bar 100 μm. On the right, superposition of fluorescent images of nano beads before (cyan) and after (magenta) the application of the pressure. Scale bar 10 μm. The relative displacement in respect to image border or between the beads can be used to calculate the strain.
Seeding cells to form a monolayer on the system
Timing: 3 h preparation and 24 h incubation of cell culture
Once the system is fabricated and functional, the next step accomplishes formation of the epithelial monolayer on the system.
CRITICAL: Once the system is prepared, before proceeding to coating it is necessary to wait at least 3 h for the PDMS membrane to recover from the plasma membrane treatment.
-
7.
Under the laminar hood, place the system in a sterile container, such as a Petri-dish or a 6-well plate. Wash the system three times by fully submerging the system in the ethanol 70%. Once sterile, using forceps, transfer the system into a new sterile container and wash the system three times with the sterile PBS.
-
8.
Coat the system with a sufficient amount of 40 μg/mL Fibronectin solution in PBS to cover the surface of the PDMS but not the connector. Typically for a single well of a 6-well plate we used 1.5 mL of fibronectin solution. Leave the system one hour at 37°C then rinse it carefully with PBS without introducing PBS inside the PDMS cube. Remove the PBS.
Note: Systems will have tendency to float on top of the fibronectin solution. Use sterile forceps or pipette tip to push the system toward the well bottom to cover the system with the coating solution.
Alternatives: Other than fibronectin coating (i.e., collagen, matrigel, laminin etc.) can be performed on the microfluidic system during step 8 to provide cell-specific adhesion to the system.
-
9.Prepare MDCK cells to be seeded on the system:
-
a.Remove the medium of your 80% confluent T75 flask MDCK culture and rinse twice the cells with 5 mL of PBS.
-
b.Add 2 mL of trypsin on the cells and wait at 37°C until the cells are no longer attached to the surface (this step can take up to 20 min).
-
c.Centrifuge cells (260 × g for 4 min) and re-suspend the pellet in 2–3 mL of the fresh culture medium.
-
d.Count MDCK cells and adjust the concentration to deposit 3 × 105 cells per 1 cm2 on the fibronectin-coated system in S-MEM completed with 5% FBS, 1% antibiotic and 5 μM CaCl2. Low calcium helps to form an instant monolayer as described in literature (Benham-Pyle et al., 2015). After two hours, gently wash the system once with the normal MEM complete medium to remove non-adhered cells and let the monolayer to be formed in the MEM complete medium. Typically, we used 2–3 mL of the media per well of a 6-well plate. Troubleshooting 4.
-
e.Incubate the system in the cell incubator (5% CO2; at 37°C) for 24 h. Troubleshooting 5.
-
a.
CRITICAL: Longer incubations will lead to higher cell density and can affect the formation of calcium waves. In that case it might be necessary to optimize the in-channel pressure change and therefore the resulting membrane deformation to trigger calcium waves in denser monolayers.
Optional: Other type of cells can be used to form a monolayer on the system.
Triggering calcium waves in the epithelial monolayer
Timing: 3 h
When the monolayer is successfully formed, cells are incubated with a calcium fluorescent probe (Fluo-4 Direct Calcium) which allows following changes in calcium concentration upon triggered epithelial deformation.
Note: Before you begin, turn on the CO2 and temperature control on the microscope that will be used for the observation of calcium waves. This will help stabilize the focus during the experiment.
-
10.Incubate cells with the calcium fluorescent probe:
-
a.Filter dead or non-attached cells from the culture medium in which cells on the system were maintained during the last 24 h. Aspirate the medium into a syringe, fix the 0.20 μm filter and gently put 500 μL of the medium back to the well with the system.Note: Use of conditioned medium and not fresh at this stage improves the reproducibility in calcium wave generation between the experiments.
-
b.Using forceps install the system in the microscopy holder (see before you begin and materials and equipment SECTION, and Figure 1). Make sure that the system is correctly installed and no medium leakage occurs from the bottom.
-
c.Following the supplier protocol.
-
d.(https://www.thermofisher.com/order/catalog/product/F10471) double concentrated Fluo-4 Direct Calcium probe solution is added to an equal volume of medium (i.e., total volume of 1 mL composed of 500 μL Fluo-4 Direct Calcium probe for 500 μL of the conditioned medium as described in the step 10a), mixed gently, and the system is incubated for 1 h (or as stated by the supplier) in the cell incubator.Note: The Fluo-4 Direct reference that we used does not need to be washed away before imaging, however, some other references can require this step.
-
a.
-
11.Install the system on the inverted microscope stage and connect the system via tubing (as on Figure 1) to the pressure controller. As showed in Figure 1, when installed in the holder, the setup resembles a normal Petri dish chamber in which cells grow on the top of the chamber covered with the culture medium.
-
a.Use 20× objective for visualization (observation setup as in step 6e). Adjust the focus and use minimal excitation source power that ensures the good quality of the fluorescence signal. Longer excitation times are less phototoxic when used with a lower power excitation source. Low heterogeneity of fluorescence between cells is typically observed with this probe (troubleshooting 6).
-
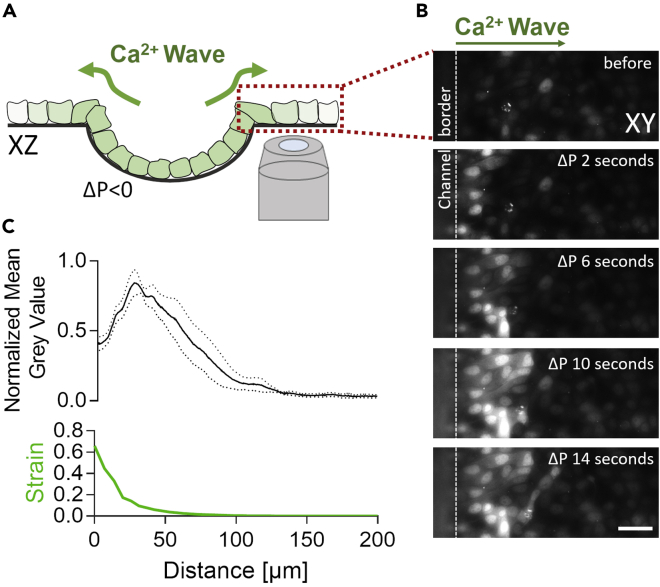
b.Position the field of view so that the border of the channel is visible on the image. The calcium wave spreads away from the channel (Figure 3).
-
c.Adjust time-lapse observation settings. We typically captured an image every two seconds for one minute.
 CRITICAL: Verify if observation settings are not phototoxic by taking a control time-lapse observation very far from the channel. If excitation power is too strong, it alone can cause an increase in calcium signal.
CRITICAL: Verify if observation settings are not phototoxic by taking a control time-lapse observation very far from the channel. If excitation power is too strong, it alone can cause an increase in calcium signal. -
d.Start a time-lapse and acquire the first 5 frames before you turn on the pressure (ΔP = -450 mbar). This serves to quantify the starting level of calcium signal. Additionally, acquiring these initial frames helps to observe if no fluorescence fluctuations occur before any stretching is triggered.Note: Once the pressure is on, you should be able to see a cellular deformation followed by local increase in the calcium signal fluorescence. The wave spreads during ∼30 s and in our system reaches ∼150 μm distance.
-
e.Turn off the pressure controller to bring the pressure in the channel to the atmospheric level, and subsequently detach the tubing.Note: At this stage you can fix cells on the system with the standard procedures and proceed to immunofluorescence. At the end of the staining protocol, mount with the mounting medium and a coverslip. Using a scalpel cut off the connector. For the observation you can use the same system holder as for the live experiments.
-
a.
-
12.Wash the system to reuse it for another experiment.
-
a.Scrub the system gently with gloves under water and with soap. This will remove the majority of the cell layer and cell culture reagents.
 CRITICAL: Do not wash the system with alcohol as it can fix the proteins on the surface of the system.
CRITICAL: Do not wash the system with alcohol as it can fix the proteins on the surface of the system. -
b.Secure the inlet with a tubing closed up at the other end with a needle and a luer lock.
-
c.Insert the system in a small plastic bag with a zip and fill with the RIPA buffer.
-
d.Place the bag in a sonicator bath and make sure that the system is fully submerged in the lysis buffer. Subsequently run two cycles of 4 min at the highest power.Note: Systems can be reused up to 6–8 times.
-
e.To reuse the system for a new experiment, proceed directly starting from step 6. Troubleshooting 7.
-
a.
Figure 3.
Calcium waves spreading upon induced epithelial folding
(A) Schematic representation of the observation setup and positioning of the field of view.
(B) Calcium waves are initiated above and by the border of the channel and spread laterally. Scale bar 50 μm.
(C) With the imposed negative pressure, the membrane deflates and stretches (quantification of the strain in C; described in step 6) causing the activation of calcium signals. Changes in calcium level were quantified by following the fluorescence of Fluo-4 Direct Calcium in the function of distance from the channel border. The graph presents the mean value +/-SEM.
Analysis of calcium waves
Once movies are acquired it is possible to analyze the spatial and temporal properties of calcium waves. Using the steps described below you will be able to obtain the graph as presented in Figure 3C.
-
13.
Install and Open ImageJ.
-
14.
Upload the acquired video into the ImageJ.
Note: ImageJ supports majority of file formats from the image acquisition software. If it is not a TIFF format transform it by saving your movie as a 16-bit TIFF file.
-
15.Adjust the scale and time resolution of your experiment.
-
a.Go to Image>Image Properties.
-
i.Set the scale of the image by filling pixel width and pixel height (set units to μm).
-
ii.Set the frame interval (in this protocol we recommended acquiring a time-point every 2 s).
-
iii.Select “global” if you will analyze more images of the same properties from the experiments. This way the scale and time-point information will be conserved.
-
i.
-
a.
-
16.Rotate the image so that the channel is positioned parallel to the left border of the image.
-
a.Go to Image>Transform>Rotate…
-
i.Set the angle of rotation and check with Preview if the transformation was correct. Then validate with OK.
-
i.
-
a.
-
17.Subtract the Fluo-4 Direct base signal to analyze only changes in fluorescence triggered by epithelial deformation.
-
a.Duplicate last image in sequence before pressure was turned on. To do so, go to Image>Duplicate. Choose to not duplicate the whole stack by unclicking stack.
-
b.Go to Process>Image calculator and choose your time-lapse image as Image 1 and Duplicated image as Image 2. Choose Subtract in the Operation panel. Validate with OK (Figure 4).
-
a.
-
18.Analyze distance calcium wave have spread.
-
a.Select the time-point of interest.
-
b.Using Rectangle tool from the main menu, draw a rectangle that starts at the channel border and covers the whole area of the system except the channel.
-
c.Use Ctrl+K to plot a profile of Intensity over the distance. A new window with a graph will open. To obtain raw data click on Data>Save Data (Figure 4).
-
a.
Note: The average gray value (as showed in Figures 3 and 4) is calculated automatically by the software. The error bar is a result of the analysis of experimental replicates.
Figure 4.
Analysis of calcium waves in ImageJ software
Top images show the operation to remove the basal level of the fluorescence (step 17). From the image at the maximal wave distance (in here at 14 s) subtract the image at 2 s before the cell deformation is triggered (in here 2 s) to obtain an image with the corrected fluorescence level being the signal related only to calcium wave. The image at the bottom shows how the image should be transformed by rotation to obtain calcium wave spreading in horizontal direction (from left to right) in order to obtain the mean gray value profile over the distance (step 18). Scale bar 50 μm.
Expected outcomes
The proposed protocol will allow fabricating a microfluidic system that enables triggering local epithelial deformation. The advantage of this protocol as compared to traditional soft-lithography techniques is the thickness of the system (400 μm) that is compatible with high resolution microscopy. Therefore, among expected results is the precise observation of triggered epithelial deformations and quantification of resulting calcium signals (Figure 3). Because the designed system recapitulates naturally occurring gradients of cell deformation, it is well suited to study i) dynamics of triggered calcium waves, ii) the molecular regulators of calcium induced signaling, and iii) to uncover how mechanical stress regulates this signal.
Limitations
The protocol presented here has been optimized for MDCK epithelial monolayers. We observed variations in Calcium response to triggered deformation that were dependent on cell density. The denser the monolayer was the higher was the deformation needed (and thus triggering pressure). To use this system with other monolayers, it is therefore necessary to initially screen for the deformation that activates calcium signaling. Furthermore, the APTES bonding of polycarbonate with PDMS was optimized to a range of pressure up to +900 mbar and -700 mbar. Use of pressures out of this range can result in the membrane detachment.
Troubleshooting
Problem 1
Inefficient oxygen plasma surface activation.
Potential solution
The proper activation of the polycarbonate or PDMS with the oxygen plasma system is crucial for the successful bonding. As described in the materials and equipment section there is a variability in activation efficiency depending on the plasma type. Ask the supplier of your oxygen plasma system to provide you existing protocols for bonding PDMS with PDMS or glass. Parameters used to perform these typical bonding procedures are a good starting point for optimization. Otherwise, perform series of 60 s activation with varied power; start from 100% power and decrease over 10% for each new pair of PDMS slabs to be bonded.
Problem 2
Entrapment of dust and debris upon bonding.
Potential solution
It is possible to perform all the microfabrication steps without a cleanroom environment. However, the risk that dust and other small particles will be deposited on the surfaces that will be bonded is high. Entrapped dust can cause leakage at the connector site or perturb homogenous membrane deformation in the proximity of the channel. Therefore, inspect surfaces before plasma activation. If dust is detected on the PDMS membrane or PDMS connector you can remove it by using a Magic Scotch Tape (3M). Place the tape over the polycarbonate or PDMS connector. The sticky tape will gently remove dust or remaining particles. Repeat this step until visually all particles are removed.
Problem 3
Microfabricated system is leaking.
Potential solution
If air bubbles are detected during the system verification, inspect the place of the leakage.
-
•If leakage is at the tubing/connector side.
-
○Make sure that the tubing is of correct outer diameter in respect to the punched inlets in the connector. We provided the exact references of biopsy punch and tubing used in this study in the key resources table. Pay attention that you use a perfectly sharp biopsy punch, as otherwise cracks of PDMS can appear.
-
○When preparing the connector, make sure that the biopsy punch is positioned perfectly vertically in respect to the surface, and that making the hole in the PDMS did not cause any breaks. If successful, you should be able to remove from the biopsy punch a single-piece of a PDMS cut-out from the connector cube.
-
○
-
•
If the leakage is at the connector/system side, verify if dust or other small particles were not entrapped (problem 2). Otherwise, the bonding efficiency might not be strong enough (problem 1), pay attention to put in contact two activated surfaces as quickly as possible after oxygen plasma treatment. Make sure also, that during oxygen plasma activation, you activated surfaces that will be in contact for bonding (i.e., surface with the channel, etc.).
-
•
If the leakage is at the channel/membrane side, it is possible that the polycarbonate system was not perfectly cleaned post milling procedure, and sharp and rough edges of the channel remain that can punch the membrane. Increase the step of cleaning the polycarbonate system before starting with the step 1.
Problem 4
Cells do not attach to the system.
Potential solution
If after following steps 7–9 cells are not attached to the system, verify that:
-
•
you have properly washed the system after sterilization with ethanol. Failure at this stage can result in the decreased efficiency of coating and therefore cell attachment.
-
•
during the coating step, the system is not floating on top of the fibronectin coating solution. Since PDMS is highly hydrophobic, make sure to use a sufficient amount of fibronectin solution and push the system towards the bottom of the well with the sterile tip to ensure full coating of the future cell-culture area on the system.
-
•
you used fibronectin at the right concentration and that after incubation time, you washed the system thoroughly with PBS.
-
•
your cells after passaging are in good shape. If the source cells do not adhere in the standard cell culture flask, it suggests other than microfabrication problems.
Problem 5
Epithelial monolayer is contaminated.
Potential solution
Insufficient sterilization can trigger cell culture contamination on the system. Follow the step 7 of system sterilization and pay attention to fully submerge the system in the ethanol. If contamination persists check the stock solutions (fibronectin solution, cell culture media and PBS) as other potential sources of contamination. Pay attention if your standard culture is not contaminated.
Problem 6
Poor fluorescent signal of Fluo-4 Direct Calcium marker.
Potential solution
-
•
Adjust the settings of the exposure time and power of the excitation source.
-
•
Use objectives dedicated to fluorescence imaging.
-
•
Wipe the bottom of the system with ethanol once it is installed in the steel holder.
-
•
Make sure that you followed the concentrations to be used of Fluo-4 Direct Calcium reagents according to suppliers’ technical notes.
Problem 7
Cells do not attach to the system anymore.
Potential solution
If you reuse the system after performing step 12, check how many times you have used it since fabrication. By experience we observed that RIPA washing with sonication is efficient for cleaning the system and reuse for ∼4–5 times. Afterward, a decreased cell adhesion and monolayer formation was observed. If this is the case, fabricate new systems.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Monika Dolega (monika.dolega@univ-grenoble-alpes.fr).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We thank Mylène Pezet, Alexei Grichine, and Jacques Mazzega for their technical assistance in imaging. D.Z. and S.B. were supported by First Team grant (POIR.04.04.00-00-3FEF/17-00) of the Foundation for Polish Science co-financed by the EU under the Smart Growth Operational Programme. M.E.D. is supported by French National Agency of Research (grant ANR-17-CE13-0006).
Author contributions
M.E.D. designed the study and created the protocol. S.B. and D.Z. fabricated milled polycarbonate substrates. M.B.-C.-B. contributed to microsystem fabrication optimization. L.P. and S.F. contributed to optimization of the cell culture for calcium waves observation. M.E.D. and M.B.-C.-B. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Data and code availability
The published article includes all datasets and code generated or analyzed for this study.
References
- Benham-Pyle B.W., Pruitt B.L., Nelson W.J. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonski S., Aureille J., Badawi S., Zaremba D., Pernet L., Grichine A., Fraboulet S., Korczyk P.M., Recho P., Guilluy C., Dolega M.E. Direction of epithelial folding defines impact of mechanical forces on epithelial state. Dev. Cell. 2021;56:3222–3234. doi: 10.1016/j.devcel.2021.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The published article includes all datasets and code generated or analyzed for this study.