Abstract
Rationale & Objective
Previous studies have shown chronic kidney disease (CKD) mortality rates to be lower among females than males. We aimed to examine the extent to which sex differences vary over time, among countries, and with age, using Global Burden of Disease (GBD) Study data.
Study Design
Observational epidemiological study.
Setting & Participants
GBD Study, which used published literature, vital registration systems, kidney replacement therapy registries, and household surveys.
Exposures
Sex.
Outcomes
CKD-associated mortality rate (per 100,000 population).
Analytical Approach
Changes in CKD mortality between 1990 and 2019 were compared between sexes, globally, and separately for the 50 most populous countries. For 2019 only, sex differences in age-standardized and age-specific mortality were compared between countries.
Results
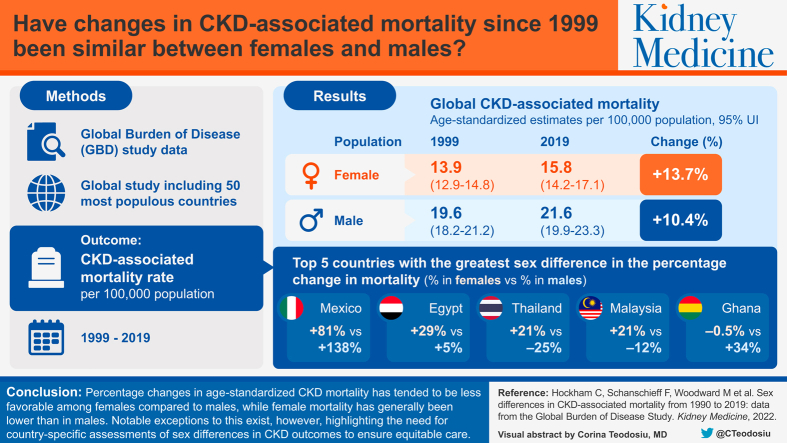
There was no change in global age-standardized mortality for either sex between 1990 and 2019, with female mortality consistently 30% lower than male mortality. Percentage changes were less favorable among females than males in two-thirds of the 50 countries examined, with the greatest change disparities observed in Egypt, Thailand, and Malaysia. Although Mexico exhibited the greatest overall percentage increase, the increase was smaller in females (81% vs 138%). In 2019, female mortality varied between 47% lower and 60% higher than male mortality (in Angola and Egypt, respectively). In most countries, female mortality was lower across all age groups, with no narrowing of sex differences with age.
Limitations
We were not able to assess the sex differences according to CKD stage and we did not explore other disease metrics (eg, disability-adjusted life years).
Conclusions
Percentage changes in age-standardized CKD mortality have tended to be less favorable among females than males, with notable exceptions. Similarly, although female mortality is generally lower than male mortality, there are multiple examples of the opposite. Country-specific assessments of sex differences in CKD-associated outcomes are essential for equitable care.
Index Words: Chronic kidney disease, mortality, sex differences, females, males
Visual Abstract
Plain-Language Summary.
Chronic kidney disease (CKD) is one of the fastest rising causes of death worldwide. Although sex differences in CKD epidemiology and outcomes have been reported, they have not been studied to the same extent as in other noncommunicable diseases. Using the Global Burden of Disease Study data, we show that the global age-standardized CKD mortality rates have not changed for either sex since 1990: female mortality remained around 30% lower than male mortality in 2019. However, our analysis of individual countries showed considerable geographic variation, both in the direction and magnitude of sex-specific changes in CKD mortality between 1990 and 2019 and in the sex differences that existed in 2019. Female mortality is not always lower than male mortality, making country-level assessments important for ensuring equitable care.
Chronic kidney disease (CKD) is one of the fastest rising causes of death worldwide. In 2019, the estimated global prevalence of CKD was 9.4%, with 1.4 million deaths directly attributable to the disease (although this is likely an underestimate). Between 1990 and 2019, CKD rose from being the 19th leading cause of death worldwide to the 11th leading cause.1,2 This rise can be explained by the all-age CKD-associated mortality rate having increased by 64.1% over this period,3,4 as well as by considerable declines in the mortality rates of other major noncommunicable diseases (NCDs) (eg, cardiovascular disease [CVD] and cancer).1,5 Failing to recognize CKD as a global health concern threatens progress toward achieving the United Nations’ Sustainable Development Goal for 2030 of reducing premature mortality from NCDs by a third.1,5, 6, 7
Besides the glomerular filtration rate and albumin-creatinine ratio, the only other variables used in the international Kidney Failure Risk Equation are age and sex, reflecting their central role in disease prognosis.8,9 In 2019, the global age-standardized CKD mortality rate was 15.8 (95% uncertainty interval [UI], 14.2-17.1) deaths per 100,000 females and 21.6 (95% UI, 19.9 to 23.3) deaths per 100,000 males. However, CKD mortality rates are known to vary by country and region.1 Further, age and sex patterns in mortality may similarly vary. The 2019 Global Burden of Disease (GBD) Study estimated the age-standardized female-specific CKD mortality rate to range between 10.7 (95% UI, 9.1-11.7) in high sociodemographic index countries and 21.7 (95% UI, 19.2-24.2) in low sociodemographic index countries; for males, it ranged from 15.2 (95% UI, 13.8-16.1) to 29.3 (95% UI, 26.0-33.2).4
Global recognition of the importance of CKD in reducing life expectancy has lagged behind that of CVD, which shares many of the same risk factors as CKD.5,6 Although the introduction of CKD staging in 2002 has invigorated research,10,11 there remains much that is unknown about sex (and gender) differences in CKD epidemiology and outcomes. For instance, it is unknown whether changes in age-standardized CKD mortality rates over time, or lack thereof, have been the same in females and males. Furthermore, in CVD, it is well established that, in each age category, males die at higher rates than females, but the magnitude of this gap decreases with age.12 Whether this is also true in CKD is less clear.
We used data from the GBD Study to evaluate temporal and geographic trends in sex-specific age-standardized rates of mortality due to CKD between 1990 and 2019. In addition, we used sex- and age-specific rates of CKD-associated mortality to examine whether the magnitude of sex differences varied with age.
Methods
Data Source
Mortality data were obtained from the GBD Study Results tool, which is available through the Global Health Data Exchange.4 The methods used to generate mortality estimates in the GBD Study have been published.1,13 Briefly, data on CKD deaths were compiled from vital registration, verbal autopsy, and surveillance system data available between 1980 and 2017. Deaths directly attributed to CKD (all stages) were defined based on International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision codes, with intermediate or poorly defined International Classification of Diseases codes redistributed where appropriate. Adjustments for differences in case definitions and study methods were also made. For countries where data are limited, information was borrowed from predictive covariates and geographical proximity to countries with data. Mortality rate estimates and 95% UIs were generated using an ensemble modeling process involving mixed-effects and spatiotemporal regression models.1
The freely available GBD Study Results tool allows interactive disaggregation of estimates (unstandardized and standardized by age) by country, year, sex, and age group.
Outcomes
The primary outcome was death directly attributed to CKD, as defined by the GBD Study (Table S1). We also examined changes in mortality from ischemic heart disease (IHD) and stroke.
Statistical Analyses
Age-standardized estimates were predominantly used because they account for differences in population demographic structures over time and among countries, allowing comparisons to be made. In the GBD Study, direct age-standardization is used, whereby the weighted average mortality rate is calculated, using weights that are equal to the proportion of people in each age group in a standard population. Between 1990 and 2010, the World Health Organization World Standard Population was used, whereas in more recent years, the GBD Study has developed their own World Standard Population.13
Global sex-specific CKD mortality rates (all age and age-standardized) were obtained for 1990, 2000, 2010, and 2019 and visualized using a scatter plot. Where the data allow, 95% UIs are also displayed. For the 50 most populous countries for which data were available (Fig S1), the percentage change in age-standardized CKD mortality between 1990 and 2019 was calculated for females and males separately and plotted against each other to assess whether changes were similar between the sexes. Using only 2019 data, we also examined sex differences in age-standardized mortality across the 50 countries.
Using 5-year age groups ranging from 20-24 to 85-89 years, we summarized sex- and age-specific CKD mortality rates globally and for the 50 countries in 2019. Whether the association between age and global CKD mortality is modified by sex was examined using Poisson regression models. In each country, age-specific female-to-male mortality rate ratios (MRRs) were calculated by dividing the female-specific rate by the male-specific rate. Violin plots were used to show the distribution of country-specific MRRs in each age group.
Analyses and data visualizations were performed in R Studio (version 1.4.1717), using the stats, dplyr (version 1.0.6),14 and ggplot2 (version 3.3.3)15 packages. Ethics approval was not required for this study as only publicly available aggregated data were used.
Results
Mortality by Sex, Year, and Country
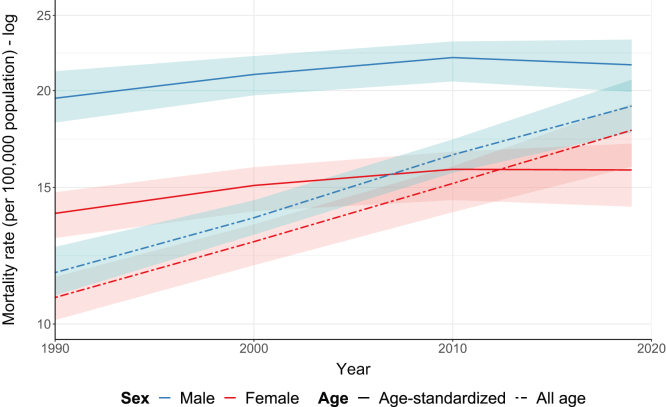
Between 1990 and 2019, the global all-age CKD mortality rate increased from 10.8 (95% UI, 10.1-11.5) to 17.8 (95% UI, 16.0-19.2) per 100,000 females and from 11.7 (95% UI, 10.9-12.6) to 19.1 (95% UI, 17.7-20.7) per 100,000 males, corresponding to a percentage change of 64% in both sexes (Fig 1). In contrast to trends observed for IHD and stroke (Fig S2), there was no significant change in the age-standardized mortality rate for either sex over the same time period (Fig 1). Across all 4 time periods, female mortality was consistently around 30% lower than male mortality.
Figure 1.
Global sex-specific all-age and age-standardized mortality rates (per 100,000 population) for chronic kidney disease in 1990, 2000, 2010, and 2019.
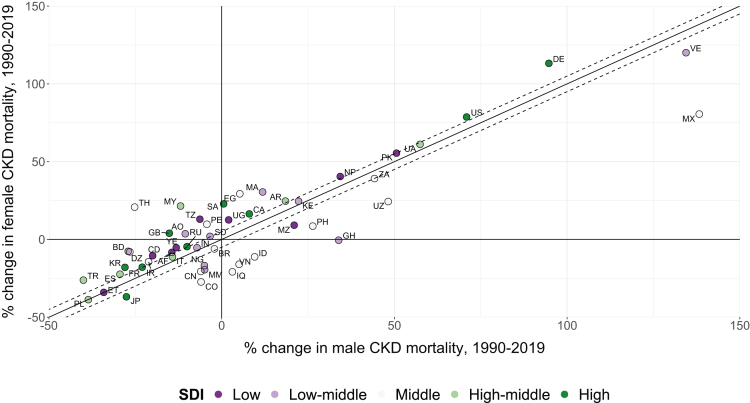
When comparing sex-specific percentage changes in age-standardized mortality in each country, we found that, between 1990 and 2019, 18 (36%) countries showed increasing CKD mortality rates in both sexes. The percentage increase was greater among females than males in 12 countries, and smaller among females in 6 countries (Fig 2; Table S2). Twenty-one countries showed decreasing mortality rates for both sexes. Among these, mortality decreased to a smaller extent among females in 14 countries and to a greater extent in 7 countries. A further 7 countries reported increasing rates in females but decreasing rates in males; the opposite trend was found in 4 countries. Overall, changes in mortality were less favorable among females than males in 33 (66%) of the countries, with no clear trend according to sociodemographic index (Fig 2). In 27 of these, the unequal changes resulted in females having a smaller advantage in 2019 compared with 1990, whereas in 4 countries, the female advantage was reversed, and in 2 countries, the female disadvantage was widened. There were notable examples, however, where changes were worse for males, such as Mexico and Venezuela (Fig 2). The 5 countries with the greatest sex differences in the percentage change in mortality were Mexico (percentage change of +81% among females vs +138% among males), Egypt (+29% vs +5%), Thailand (+21% vs −25%), Malaysia (+21% vs −12%), and Ghana (−0.5% vs +34%).
Figure 2.
Changes in CKD age-standardized mortality rates (per 100,000 population) for females and males between 1990 and 2019 in 50 countries. The x-axis shows the difference in the male mortality rate between 1990 and 2019, and the y-axis shows the equivalent difference in female mortality rate. In countries that fall above the black diagonal line, changes were less favorable among females than males; the opposite is true for countries that fall below the line. Given that countries are unlikely to fall perfectly on the diagonal, guidelines with a y-intercept ±5 percentage points are also shown. Countries are represented by their ISO Alpha-2 codes: Afghanistan (AF), Algeria (DZ), Angola (AO), Argentina (AR), Bangladesh (BD), Brazil (BR), Canada (CA), China (CN), Colombia (CO), Democratic Republic of the Congo (CD), Egypt (EG), Ethiopia (ET), France (FR), Germany (DE), Ghana (GH), India (IN), Indonesia (ID), Iran (IR), Iraq (IQ), Italy (IT), Japan (JP), Kenya (KE), Malaysia (MY), Mexico (MX), Morocco (MA), Mozambique (MZ), Myanmar (MM), Nepal (NP), Nigeria (NG), Pakistan (PK), Peru (PE), Philippines (PH), Poland (PL), the Republic of Korea (KR), Russia (RU), Saudi Arabia (SA), South Africa (ZA), Spain (ES), Sudan (SD), Tanzania (TZ), Thailand (TH), Turkey (TR), Uganda (UG), Ukraine (UA), United Kingdom (GB), United States (US), Uzbekistan (UZ), Venezuela (VE), Vietnam (VN), and Yemen (YE). CKD, chronic kidney disease; SDI, sociodemographic index.
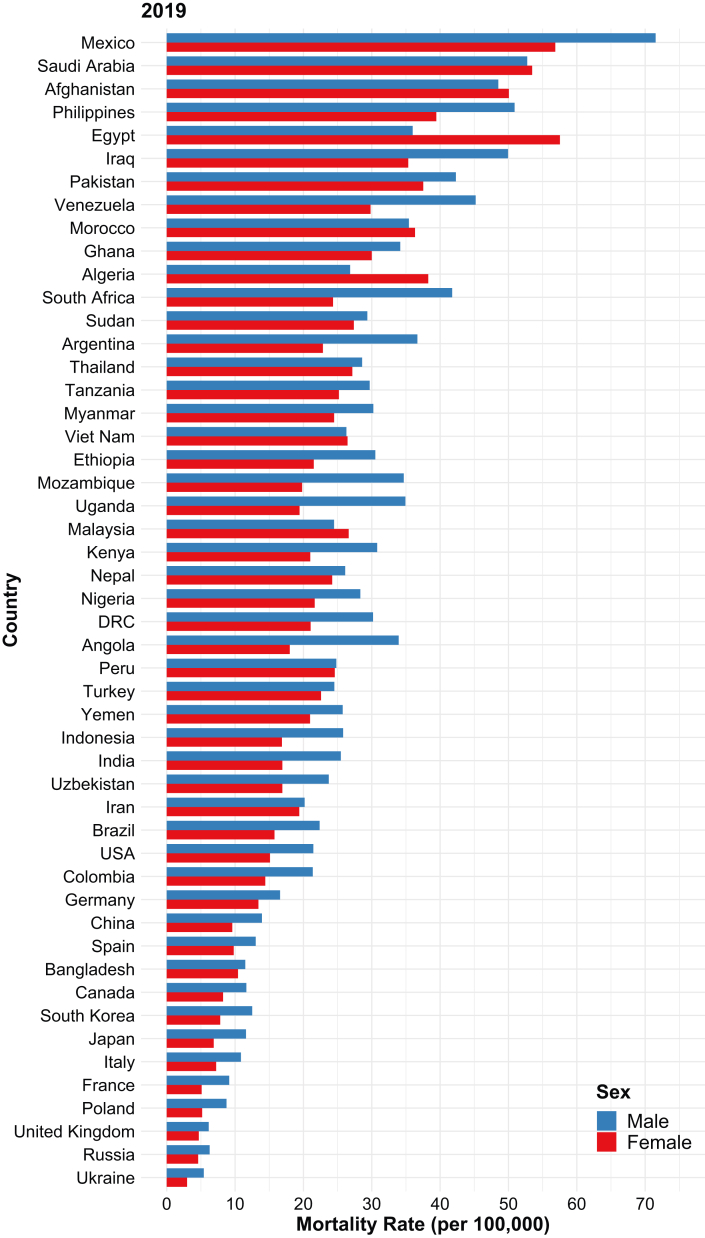
Restricting the analysis to 2019 alone, the countries with the 3 highest female mortality rates were Egypt (57.5 [95% UI, 26.8-81.2] per 100,000 population), Mexico (56.8 [95% UI, 47.5-68.2] per 100,000), and Saudi Arabia (53.5 [95% UI, 40.3-68.0] per 100,000). Those with the highest male mortality rates included Mexico (71.5 [95% UI, 59.1-86.7] per 100,000), Saudi Arabia (52.7 [95% UI, 42.4-64.6] per 100,000), and the Philippines (50.9 [95% UI, 39.4-63.9] per 100,000). The rates were the lowest in Ukraine, the Russian Federation, and the United Kingdom for both sexes (Fig 3). Although countries with high male mortality rates were more likely to have high female mortality rates and vice versa, the sex differences in these rates varied among countries. For example, in the 43 countries where female rates were lower, these were between 1% and 47% lower than those of males (Peru and Angola, respectively). There were 7 exceptions where the rates in females were similar or higher than that in males: Afghanistan, Algeria, Egypt, Malaysia, Morocco, Saudi Arabia, and Vietnam (Fig 3). Among these, female mortality was between 1% and 60% higher than that in males (Vietnam and Egypt, respectively). Of note, Afghanistan, Egypt, and Saudi Arabia were also among the 5 countries with the highest overall CKD-associated mortality rate.
Figure 3.
Sex-specific age-standardized mortality rates (per 100,000 population) in 2019 for chronic kidney disease in each of the 50 selected countries. Countries are ordered in descending order of total mortality rate.
Mortality by Sex and Age
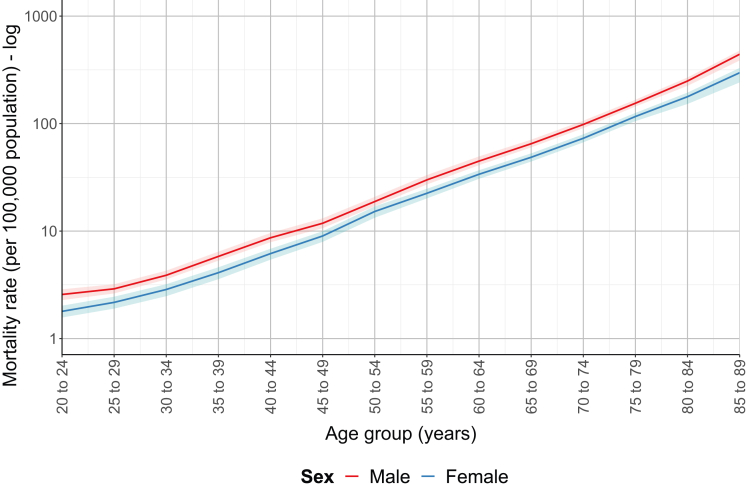
Given the aging global population and strong association of CKD with age, we examined whether sex differences in mortality varied with age, as has been seen for other NCDs. In 2019, the global CKD mortality rate was lower in females than in males across all age groups, with no narrowing of the gap in older ages (Fig 4). This was despite evidence that the association between age and CKD mortality was greater in females than in males in the age groups of 50-54 years and older (Table S3).
Figure 4.
Global sex-specific chronic kidney disease mortality rates (per 100,000 population) in 2019 plotted according to age group.
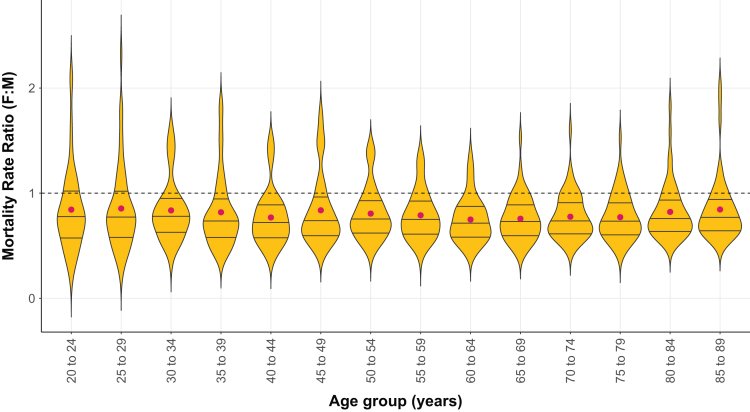
Patterns of sex differences in mortality rates by age were broadly similar among countries. In all age groups, 75% or more country-specific female-to-male MRR values were below unity, indicating a smaller female mortality rate for the specific age group (Fig 5). There was also a little variation in the mean MRRs among the age groups. However, some locations deviated from this trend (Fig S3). For example, mortality rates were similar between the sexes across all ages in Myanmar, Nepal, Nigeria, Pakistan, Peru, Sudan, Tanzania, Vietnam, and Yemen. There were also a handful of countries where either female mortality was higher than male mortality across all ages (Afghanistan and Algeria) or the direction of the sex gap varied with age (Bangladesh, Egypt, and Malaysia).
Figure 5.
Female-to-male MRRs for chronic kidney disease across the 50 selected countries in 2019 according to age. The median and interquartile intervals for the MRR are shown by the solid lines inside the boxes. The mean is depicted by red dots. The dashed line indicates unity, where the MRR is equal to 1. MRR, mortality rate ratio.
Discussion
Using GBD Study data, we found that the global age-standardized CKD mortality rate has not changed in either sex since 1990, which contrasts with well-known improvements of around 30%-40% for IHD and stroke mortality over the same period. However, these global estimates mask important geographic differences, both in terms of the direction and magnitude of sex-specific changes in mortality. Although changes in female mortality were less favorable than changes in male mortality for two-thirds of the 50 selected countries, there were some notable exceptions, for example, Mexico and Venezuela, where the opposite was true. Likewise, although female mortality was lower than male mortality in most countries in 2019, 3 of the 5 countries with the highest CKD-associated mortality rate had higher female rates (Saudi Arabia, Afghanistan, and Egypt). These findings reiterate the need for country-level assessments of sex (and gender) differences in the CKD burden and outcomes to ensure that locally relevant gender-sensitive approaches to CKD prevention and care can be developed.
Geographic disparities in the changes in CKD mortality over time have been previously described.1,16 We extended these findings to examine whether changes in age-standardized CKD mortality rates have affected females and males equally across the globe. What we found was a very heterogeneous picture. Percentage changes between 1990 and 2019 were less favorable among females compared with males in 33 of the 50 countries, either because of females experiencing a greater percentage increase in mortality (12 countries), a smaller decrease (14 countries) or an increase when male mortality decreased (7 countries). In Afghanistan, Malaysia, Morocco, and Saudi Arabia, the female advantage was reversed. In Algeria and Egypt, the pre-existing disadvantage in females was widened.
The sex disparity in the changes in CKD mortality that was observed in each of these countries is likely the result of a complex combination of gendered and social factors, including unequal access to medical care, kidney replacement therapy (KRT), and disease-halting treatment.17 In Egypt, for example, 42% of patients receiving dialysis in 2020 were females, despite the prevalence of CKD (albeit all stages) being 20% higher among females.4,18 However, given that this gender imbalance in KRT recipients is not unique to Egypt or the other countries listed,17 other factors will also be at play and most likely relate to stark gender inequalities that exist. Indeed, with the exception of Malaysia, all of these countries ranked among the lowest 20 countries of the 153 included in the 2020 Global Gender Gap Index.19 Afghanistan, which was not included in 2020, ranked lowest in 2021.20
There were 17 countries where females experienced more favorable changes compared to males. Of note were Mexico and Venezuela, the 2 countries with the greatest overall increase in CKD mortality; here, both sexes experienced considerable increases in CKD-associated mortality, but these were smaller in females than in males (+80.5% vs +138% and +120% vs +134% in Mexico and Venezuela, respectively). In a recently published review of sex and gender differences in access to kidney care around the globe, and with particular attention to Mexico and Central America,17 the authors hypothesized that occupational factors, faster progression to kidney failure, lower compliance with treatment, and lower use of health services among males may explain higher rates of CKD-associated mortality. This is likely compounded by the rising prevalence of diabetes and hypertension, which together account for more than 50% of CKD deaths, along with the presence of hotspots of CKD of unknown etiology, which predominantly affects males of working age.21, 22, 23
The observation that CKD mortality is typically lower among females is consistent with previous reports.24, 25, 26, 27, 28 For example, a 2013 meta-analysis of 46 cohorts, comprising >2 million patients from 5 continents, found that all-cause and cardiovascular-related mortality were lower among females for all levels of estimated glomerular filtration rate and albuminuria, including those in the CKD range.28 More recently, a meta-analysis focusing on cardiovascular-related mortality in patients with CKD found that males had an approximately 13% higher likelihood of mortality than females.25 The geographic consistency with which we observed a lower mortality among females across a range of economically, socially, and culturally diverse countries lends weight to there being a biologic survival advantage in females.29 However, heterogeneity in the magnitude of the sex difference, which, in 2019, ranged between a 47% lower female mortality rate in Angola to a 60% higher female mortality rate in Egypt, suggests that gendered sociocultural factors are also at play.30 The extensive, and costly, health needs of people with CKD to slow their disease progression, manage complications, and prevent death makes this condition particularly susceptible to gender-based differences in health care access. The observation that CKD mortality rates among females were higher than, or similar to, those among males in 7 countries in 2019, including 3 of the 5 countries with the highest overall CKD mortality rates (Saudi Arabia, Afghanistan, and Egypt), is particularly concerning. These are the same countries where changes in CKD mortality were less favorable among females than males—in most cases eliminating the female advantage that existed in 1990. Possible explanations for this disparity have already been discussed, but it is important to emphasize that these findings not only contradict the dogma that mortality from CKD is always lower in females but also highlight the vulnerability of women to losing any biologic advantage that they might have.
When interpreting sex differences in mortality, it is important to consider that a lower female mortality is observed in the general population, as well as for a range of other NCDs.12 The 2019 global all-cause mortality rate among females was around 30% lower than that among males—not dissimilar to what we have observed for CKD. To illustrate why this is important, a study from Australia and New Zealand found that when sex differences in mortality among patients with kidney failure were compared to that in the general population, female patients had greater excess all-cause deaths than their male counterparts.31 To truly understand sex- and gender-based disparities in CKD burden and outcomes, it is essential to contextualize these against wider sex and gender differences in health and disease.
With little progress in CKD-associated mortality and known demographic changes in mean population age across the world, we examined sex differences in CKD mortality according to age—to explore whether the sex gap can be expected to decrease as the population ages. Global female mortality was consistently lower across all age groups, which mirrors the findings of previous studies.32,33 We did not observe any narrowing of sex differences in the older age groups. Although we did observe an interaction between age and sex in those aged >50 years, the effect of age on mortality was only minimally greater in females than in males and was not sufficient to close the sex gap in old age. This is perhaps surprising given that kidney function declines with age and has been shown to be associated with a greater increase in mortality among females than males.28 Alternatively, it might be explained by females with CKD experiencing a more profound competing risk of death because of CVD compared with males or females experiencing a slower decline in kidney function. Nevertheless, our findings demonstrate that sex differences in major chronic diseases like CVD and diabetes cannot be generalized to other, related NCDs.
Once again, the consistency of sex- and age-specific patterns across different country settings suggests a role of biologic factors, with females experiencing a survival advantage across all ages. Where countries deviate from this trend, it might suggest that gender-based inequalities are offsetting this advantage.
There have been several explanations proposed for the lower CKD mortality rates among females. These include biologic reasons, such as protective effects of estrogen or harmful effects of testosterone,34 and sociocultural factors, such as gender differences in health-seeking behavior, quality of care, and treatment adherence.33,35 In general, males with CKD have more comorbid conditions than females (in particular CVD) and are more likely to smoke.28,36 Further, epidemiological data suggest that the prevalence of milder CKD is higher among females than males. The opposite is true for patients with kidney failure, with men accounting for approximately 60% of patients who initiate KRT across a range of settings.33 This has led some to hypothesize that CKD progresses more slowly in females than in males, resulting in a concentration of female patients with CKD in these milder stages and thereby explaining the lower female mortality. However, it is equally plausible that females are underrepresented in KRT recipients because of disparities in access to care or choices around care.37,38
The GBD Study Results tool provides an invaluable resource to obtain cause-specific mortality rates that can be disaggregated in various ways and which are available as all age, age-standardized or age-specific rates. The limitations of the data in relation to CKD have already been described.1 In the present analysis, an important limitation is the fact that the version of the International Classification of Diseases code used in different countries would have changed over time, which could have impacted temporal changes in CKD mortality rates. Nevertheless, such changes in the International Classification of Diseases versions are unlikely to have varied between females and males; thus, our comparisons between the sexes in the same year remain valid. It is important to also consider that CKD diagnosis is itself a gendered variable. A study from Sweden found that, despite having a higher CKD prevalence, females were less likely to have received an International Classification of Diseases, Tenth Revision CKD diagnosis.39 Although it is unclear to what extent this is true in other countries, it has clear implications for our understanding of the true sex differences in CKD outcomes. We have focused on age-standardized mortality to allow comparisons among countries. However, for health planning at the country level, sex differences in all-age CKD mortality should also be explored. Furthermore, we have only been able to examine sex differences in mortality for all-stage CKD. However, there is some evidence that sex differences are diminished, or reversed, in patients with more advanced stages of CKD, particularly those receiving KRT.40 Mortality is also only 1 measure of disease burden; a global assessment of sex differences in terms of disability-adjusted life years is also warranted to understand whether women live more years with the condition. Finally, it would have been preferable to examine the effects of aging on CKD mortality using longitudinal data. However, these are not widely available on a national scale; thus, we elected to use repeat cross-sectional analyses to obtain country estimates as a next best option.
Although global age-standardized CKD mortality rates increased between 1990 and 2019 for both sexes, there was substantial variation in the sex-specific changes that occurred at the country level. Continual monitoring of sex differences in CKD burden, progression, and outcomes will be important to improve understanding of how females and males are differentially impacted by the disease and to develop locally relevant policies that will benefit both sexes equally.
Article Information
Authors’ Full Names and Academic Degrees
Carinna Hockham, DPhil, Florence Schanschieff, MSc, and Mark Woodward, PhD
Authors’ Contributions
Research idea and study design: CH, MW; data acquisition: CH, FS; data analysis: CH, FS; data interpretation: CH, FS, MW; supervision: MW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Prof Woodward is supported by the Australian National Health and Medical Research Council (grants APP1149987 and APP1174120). No funders had any role in study design, data collection, analysis and interpretation, writing of the report, or the decision to submit the report for publication.
Financial Disclosure
Prof Woodward is a consultant to Amgen, Kyowa Kirin, and Freeline. Authors Dr Hockham and Schanschieff declare that they have no relevant financial disclosures.
Peer Review
Received April 21, 2022 as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form July 31, 2022.
Footnotes
Complete author and article information provided before references.
Figure S1: The 50 most populous countries included in the present study. In alphabetical order: Afghanistan, Algeria, Angola, Argentina, Bangladesh, Brazil, Canada, China, Colombia, Democratic Republic of the Congo (DRC), Egypt, Ethiopia, France, Germany, Ghana, India, Indonesia, Iran, Iraq, Italy, Japan, Kenya, Malaysia, Mexico, Morocco, Mozambique, Myanmar, Nepal, Nigeria, Pakistan, Peru, Philippines, Poland, the Republic of Korea, Russia, Saudi Arabia, South Africa, Spain, Sudan, Tanzania, Thailand, Turkey, Uganda, Ukraine, United Kingdom (UK), United States of America (US), Uzbekistan, Venezuela, Vietnam, and Yemen.
Figure S2: Global sex-specific age-standardized mortality rates (per 100,000 population) for chronic kidney disease (CKD), ischemic heart disease (IHD), and stroke in 1990, 2000, 2010 and 2019.
Table S1: The International Classification of Diseases and Injuries (ICD)-9 and ICD-10 codes used in the Global Burden of Disease (GBD) Study to ascertain different categories of chronic kidney disease (CKD) etiology, ischemic heart disease (IHD), and stroke.4
Table S2: Changes in age-standardized chronic kidney disease (CKD) mortality rates (per 100,000 population) for males and females in each of the 50 countries.
Table S3: Poisson regression estimates, with P values, for the association between 5-year age group and sex (coded as 0 for males and 1 for females) and the rate of chronic kidney disease (CKD) mortality, modeling age as a categorical variable.
Descriptive Text for Online Delivery
Supplementary Material
Figure S1–S2, Table S1–S3
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cockwell P., Fisher L.A. The global burden of chronic kidney disease. Lancet. 2020;395(10225):662–664. doi: 10.1016/S0140-6736(19)32977-0. [DOI] [PubMed] [Google Scholar]
- 4.Global Burden of Disease Collaborative Network Global Burden of Disease Study 2019 (GBD 2019) results. http://ghdx.healthdata.org/gbd-results-tool
- 5.Neuen B.L., Chadban S.J., Demaio A.R., Johnson D.W., Perkovic V. Chronic kidney disease and the global NCDs agenda. BMJ Glob Health. 2017;2(2) doi: 10.1136/bmjgh-2017-000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luyckx V.A., Tonelli M., Stanifer J.W. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96(6):414–422D. doi: 10.2471/BLT.17.206441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett J.E., Kontis V., Mathers C.D., et al. NCD Countdown 2030: pathways to achieving Sustainable Development Goal target 3.4. Lancet. 2020;396(10255):918–934. doi: 10.1016/S0140-6736(20)31761-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangri N., Grams M.E., Levey A.S., et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chronic Kidney Disease Prognosis Consortium The kidney failure risk equation 2016. https://kidneyfailurerisk.com/
- 10.Levey A.S., Coresh J., Balk E., et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 11.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 12.Bots S.H., Peters S.A.E., Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2(2) doi: 10.1136/bmjgh-2017-000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickham H., François R., Henry L., et al. dplyr: A Grammar of Data Manipulation. https://dplyr.tidyverse.org
- 15.Wickham H. Springer-Verlag; 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 16.Agudelo-Botero M., Valdez-Ortiz R., Giraldo-Rodríguez L., et al. Overview of the burden of chronic kidney disease in Mexico: secondary data analysis based on the Global Burden of Disease Study 2017. BMJ Open. 2020;10(3) doi: 10.1136/bmjopen-2019-035285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García G.G., Iyengar A., Kaze F., Kierans C., Padilla-Altamira C., Luyckx V.A. Sex and gender differences in chronic kidney disease and access to care around the globe. Semin Nephrol. 2022;42(2):101–113. doi: 10.1016/j.semnephrol.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Farag Y.M.K., El-Sayed E. Global dialysis perspective: Egypt. Kidney360. 2022;3(7):1263–1268. doi: 10.34067/KID.0007482021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global gender gap report 2020 World Economic Forum. https://www3.weforum.org/docs/WEF_GGGR_2020.pdf
- 20.Global gender gap report 2021 World Economic Forum. https://www3.weforum.org/docs/WEF_GGGR_2021.pdf
- 21.Garcia-Garcia G., Briseño-Rentería G., Luquín-Arellan V.H., Gao Z., Gill J., Tonelli M. Survival among Patients with kidney failure in Jalisco, Mexico. J Am Soc Nephrol. 2007;18(6):1922–1927. doi: 10.1681/ASN.2006121388. [DOI] [PubMed] [Google Scholar]
- 22.Mercado-Martinez F.J., da Silva D.G., Correa-Mauricio M.E. A comparative study of renal care in Brazil and Mexico: hemodialysis treatment from the perspective of ESRD sufferers. Nurs Inq. 2017;24(2) doi: 10.1111/nin.12163. [DOI] [PubMed] [Google Scholar]
- 23.Vasquez-Jimenez E., Madero M. Global dialysis perspective: Mexico. Kidney360. 2020;1(6):534–537. doi: 10.34067/KID.0000912020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksen B.O., Ingebretsen O.C. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69(2):375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 25.Shajahan S., Amin J., Phillips J.K., Hildreth C.M. Relationship between sex and cardiovascular mortality in chronic kidney disease: a systematic review and meta-analysis. PloS One. 2021;16(7) doi: 10.1371/journal.pone.0254554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swartling O., Rydell H., Stendahl M., Segelmark M., Trolle Lagerros Y., Evans M. CKD progression and mortality among men and women: a nationwide study in Sweden. Am J Kidney Dis. 2021;78(2):190–199.e1. doi: 10.1053/j.ajkd.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson L.A., Clase C.M. Sex and the incidence and prevalence of kidney disease. Clin J Am Soc Nephrol. 2019;14(11):1557–1559. doi: 10.2215/CJN.11030919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitsch D., Grams M., Sang Y., et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrero J.J., Hecking M., Ulasi I., Sola L., Thomas B. Chronic kidney disease, gender, and access to care: a global perspective. Semin Nephrol. 2017;37(3):296–308. doi: 10.1016/j.semnephrol.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg I., Krause I. The role of gender in chronic kidney disease. EMJ. 2016;1(2):58–64. [Google Scholar]
- 31.De La Mata N.L., Rosales B., MacLeod G., et al. Sex differences in mortality among binational cohort of people with chronic kidney disease: population based data linkage study. BMJ. 2021;375 doi: 10.1136/BMJ-2021-068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarra S., Solini A., Baroni M.G., Frova L., Grande E. A long-term nationwide study on chronic kidney disease-related mortality in Italy: trends and associated comorbidity. J Nephrol. 2022;35(2):505–515. doi: 10.1007/s40620-021-01132-9. [DOI] [PubMed] [Google Scholar]
- 33.Carrero J.J., Hecking M., Chesnaye N.C., Jager K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–164. doi: 10.1038/nrneph.2017.181. [DOI] [PubMed] [Google Scholar]
- 34.Valdivielso J.M., Jacobs-Cachá C., Soler M.J. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28(1):1–9. doi: 10.1097/MNH.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 35.Tong A., Evangelidis N., Kurnikowski A., et al. Nephrologists’ perspectives on gender disparities in CKD and dialysis. Kidney Int Rep. 2022;7(3):424–435. doi: 10.1016/j.ekir.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hecking M., Bieber B.A., Ethier J., et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS) PLoS Med. 2014;11(10) doi: 10.1371/journal.pmed.1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faruque L.I., Hemmelgarn B.R., Wiebe N., et al. Factors associated with initiation of chronic renal replacement therapy for patients with kidney failure. Clin J Am Soc Nephrol. 2013;8(8):1327–1335. doi: 10.2215/CJN.10721012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hödlmoser S., Carrero J.J., Kurnikowski A., et al. Kidney function, kidney replacement therapy, and mortality in men and women. Kidney Int Rep. 2022;7(3):444–454. doi: 10.1016/j.ekir.2021.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gasparini A., Evans M., Coresh J., et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant. 2016;31(12):2086–2094. doi: 10.1093/ndt/gfw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrero J.J., de Mutsert R., Axelsson J., et al. Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant. 2011;26(1):270–276. doi: 10.1093/ndt/gfq386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1–S2, Table S1–S3