Abstract
Genetic and biochemical studies have established that Fur and iron mediate repression of Bordetella alcaligin siderophore system (alc) genes under iron-replete nutritional growth conditions. In this study, transcriptional analyses using Bordetella chromosomal alc-lacZ operon fusions determined that maximal alc gene transcriptional activity under iron starvation stress conditions is dependent on the presence of alcaligin siderophore. Mutational analysis and genetic complementation confirmed that alcaligin-responsive transcriptional activation of Bordetella alcaligin system genes is dependent on AlcR, a Fur-regulated AraC-like positive transcriptional regulator encoded within the alcaligin gene cluster. AlcR-mediated transcriptional activation is remarkably sensitive to inducer, occurring at extremely low alcaligin concentrations. This positive autogenous control circuit involving alcaligin siderophore as the inducer for AlcR-mediated transcriptional activation of alcaligin siderophore biosynthesis and transport genes coordinates environmental and intracellular signals for maximal expression of these genes under conditions in which the presence of alcaligin in the environment is perceived.
In the majority of bacterial species characterized to date, the iron starvation stress response is controlled at the transcriptional level by the ferrous iron-activated Fur repressor protein (16, 22). Additional transcriptional regulators have been identified that can act as positive regulators of siderophore biosynthesis and transport gene expression, all of which are Fur controlled and responsive to the presence of the cognate iron chelate (14, 18, 23, 24, 28). The concerted actions of negatively and positively acting regulators in bacterial species capable of utilizing diverse iron sources may ensure that genes encoding specific nutritional iron transport functions are expressed maximally only under appropriate conditions in which the particular iron source is perceived in the environment, thus conserving energy and precursors. This general type of priority regulation is an established function of positive regulators controlling assimilation of available nutrients (35).
Known positive regulators of iron acquisition systems are of three general mechanistic classes: alternative sigma factors, exemplified by the Escherichia coli FecI regulator of the ferric citrate utilization system (28), classical two-component sensory transduction systems such as the PfeR-PfeS enterobactin utilization system of Pseudomonas aeruginosa (14), and AraC-like transcriptional regulators. AraC-like regulators may be capable of acting positively or negatively depending on the presence or absence of inducers and the position of the regulator binding site on the DNA (20, 35). In P. aeruginosa, the AraC-like protein PchR regulates expression of pyochelin siderophore biosynthesis and transport genes; transcriptional activity responds to pyochelin, and induction requires a functional pyochelin receptor (23, 24). Under iron starvation conditions, the Yersinia pestis AraC-like regulator YbtA is required for full expression of genes encoding the Psn yersiniabactin siderophore receptor and yersiniabactin siderophore biosynthesis activities (18). The Bordetella alcR gene also encodes an AraC-like regulatory protein and is required for maximal expression of alcaligin siderophore biosynthesis (6, 34) and transport activities (6, 10) during iron starvation stress. The mechanism of transcriptional activation by these AraC-like regulators of siderophore genes is thought to involve the cognate siderophore functioning as the inducer.
Bordetella pertussis and Bordetella bronchiseptica are gram-negative bacterial pathogens that inhabit the respiratory mucosae of humans and nonhuman mammals. When nutritional iron is limiting in availability, these organisms produce and utilize the macrocyclic dihydroxamate siderophore alcaligin (12, 31). B. pertussis and B. bronchiseptica are also capable of utilizing iron complexed with the heterologous siderophores enterobactin (4), ferrichrome, and desferrioxamine B (5). Several other iron-regulated genes encoding putative siderophore receptors with undefined specificities have been identified in B. pertussis (3) and B. bronchiseptica (3, 5), suggesting that the iron-scavenging potential of these organisms may include the ability to utilize additional heterologous siderophores as iron sources. In addition to ferric siderophores, the mammalian host-derived molecules lactoferrin (29, 36), transferrin (29, 36), hemin (1), and hemoglobin (33) have been reported as nutritional iron sources for these bacteria.
The Bordetella alcaligin biosynthesis genes alcABCDE comprise part of a Fur-regulated operon and encode proteins with amino acid sequence similarities to other known siderophore synthesis enzymes (21, 26, 34). The alcR gene encoding the AlcR positive regulator of alcaligin biosynthesis (6, 34) and transport activities is located immediately downstream of alcABCDE and is operonic with alcABCDE (6, 25). The majority of alcR transcription initiates at the Fur-controlled promoter immediately upstream from alcA (T. J. Brickman and S. K. Armstrong, Abstr. 97th Ann. Meet. Am. Soc. Microbiol. 1997, abstr. B-241, p. 70, 1997), but alcR is also transcribed from a weaker secondary Fur-regulated promoter located immediately upstream from its own coding region (6, 25).
Although the Bordetella alcaligin system genes are repressible by Fur (7; Brickman and Armstrong, Abstr. 97th Gen. Meet. Am. Soc. Microbiol.), as are other microbial siderophore systems (16), AlcR imposes an additional level of control that is required for full expression of the alcaligin siderophore system genes. In the present study, we establish that maximal transcription of the alc operon under iron starvation growth conditions is dependent on the AlcR regulator and requires the presence of the cognate siderophore alcaligin acting as the inducer. Furthermore, AlcR-mediated alc transcriptional activation is shown to be exquisitely sensitive, responding to extremely low concentrations of inducer.
MATERIALS AND METHODS
Bacterial strains and plasmids.
B. bronchiseptica strains and alcR plasmids used in this study are described in Table 1. The isolation of alcaligin siderophore-deficient mutants BRM1, BRM6, and BRM9, generated by mini-Tn5 lacZ1 (15) transposon mutagenesis of B. bronchiseptica B013N, has been described previously (2). B. bronchiseptica ΔalcR1 mutants BRM13, BRM14, and BRM15, derived from BRM1, BRM6, and BRM9, respectively, were each produced by allelic exchange essentially as described previously for construction of B. bronchiseptica B013N ΔalcR1 mutant BRM11 (6). Presumptive ΔalcR1 mutants were identified by in situ DNA hybridization analysis using the deleted DNA fragment as the probe; Southern hybridization analysis of genomic DNA samples using appropriate DNA probes confirmed that the wild-type alcR allele had been correctly replaced by the ΔalcR1 mutant allele. E. coli DH5α [F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17(rK−mK+) deoR thi-1 supE44 λ− gyrA96 relA1] (Gibco BRL, Gaithersburg, Md.) was used as the host strain for routine plasmid construction and propagation, and as the donor strain in conjugal transfer of plasmids to Bordetella recipient strains. DH5α (pRK2013) provided plasmid-encoded mobilization functions (19) in triparental matings to transfer plasmid vector pRK415 derivatives to Bordetella strains.
TABLE 1.
B. bronchiseptica strains and plasmids used in this study
| Strain or plasmid | Relevant genotype, phenotype, or description | Reference or source |
|---|---|---|
| Strains | ||
| B013N | Nalidixic acid-resistant derivative of strain B013, derived from swine isolate B | 2 |
| BRM1 | B013N alcA::mini-Tn5 lacZ1; Kanr; alcaligin siderophore deficient | 2 |
| BRM6 | B013N alcC::mini-Tn5 lacZ1; Kanr; alcaligin siderophore deficient | 2 |
| BRM9 | B013N alcA::mini-Tn5 lacZ1, with mini-Tn5 fusion element in antisense orientation with respect to alcA transcription; Kanr; alcaligin siderophore deficient | 2 |
| BRM13 | BRM1 ΔalcR1 | This study |
| BRM14 | BRM6 ΔalcR1 | This study |
| BRM15 | BRM9 ΔalcR1 | This study |
| Plasmids | ||
| pRK415 | Mobilizable broad-host-range plasmid cloning vector; Tetr; RK2 origin | 27 |
| pRK21 | pRK415 carrying 1.6-kb KpnI-PstI DNA insert fragment of B. pertussis UT25; alcR+; Tetr; formerly designated pP9KP | 6 |
| pRK15 | pRK415 carrying 2.3-kb EcoRI-PstI DNA insert fragment of B. bronchiseptica B013N; alcR+; Tetr | This study |
| pRK16 | pRK15, except 2.1-kb ΔalcR1 EcoRI-PstI DNA insert fragment of B. bronchiseptica B013N | This study |
Bacterial culture conditions.
B. bronchiseptica strains were maintained on blood agar plates or standard Luria-Bertani agar plates. Modified Stainer-Scholte (SS) medium (38) was used for broth cultures of B. bronchiseptica. Iron-replete and iron-depleted SS culture conditions were achieved by the methods of Armstrong and Clements (2); SS medium was deferrated by batch treatment using Chelex 100 resin (Bio-Rad Laboratories, Richmond, Calif.). Tetracycline was used at 15 μg/ml to select for pRK415 plasmid derivatives, and kanamycin was used at 50 μg/ml for maintenance of pRK2013 and for selection of kanamycin resistance markers of mini-Tn5 lacZ1 mutants. Ampicillin was used at 100 μg/ml for maintenance of other plasmid cloning intermediates in E. coli. In analyses of induction of alc gene transcription by alcaligin, SS culture medium was supplemented as required with purified alcaligin siderophore. All glassware was acid cleaned and rinsed repeatedly in distilled deionized water prior to use. Optical densities of SS cultures were monitored with a Klett-Summerson colorimeter fitted with a no. 54 filter (Klett Mfg. Co., Long Island City, N.Y.).
Bordetella alcaligin siderophore purification and detection.
Alcaligin was purified from B. bronchiseptica culture supernatants by a modification of the benzyl alcohol-ether extraction method (32) as previously described by Brickman and coworkers (12) and was recrystallized at least eight times from ethanolic solution. The chrome azurol S (CAS) universal siderophore detection assay (39) was used to monitor siderophore production by B. bronchiseptica grown in liquid culture as reported previously (2).
Conjugation.
Conjugal transfer of pRK415 plasmid derivatives to Bordetella strains was accomplished by triparental matings using E. coli DH5α as the plasmid donor strain and DH5α(pRK2013) as the source of mobilization functions as described previously (9). Transconjugants were selected on agar plates containing the appropriate selective antibiotics and crude colicin B (8).
Routine DNA procedures.
DNA cloning and hybridization analyses were performed using standard methods (37). DNA probes used in nucleic acid hybridizations were radiolabeled by the random-priming method (17) using the Random Primers DNA Labeling System (Gibco BRL) and [α-32P]dCTP (ICN Radiochemicals, Irvine, Calif.). Transformation of E. coli was carried out by the CaCl2 method of Cohen and coworkers (13).
β-Galactosidase assays.
B. bronchiseptica alc::mini-Tn5 lacZ1 fusion strains were assayed for β-galactosidase activity by the method of Miller (30) as modified by Brickman and coworkers (11) after culture in iron-replete or iron-depleted SS medium. In experiments examining the responsiveness of alc gene transcription to alcaligin siderophore, SS cultures were supplemented with purified alcaligin at the specified final concentrations, ranging from 0 to 50 μg/ml. β-Galactosidase activities presented are means from triplicate assays.
RESULTS
alc operon transcriptional activity is not increased by iron starvation in alcaligin siderophore-deficient mutants.
B. bronchiseptica mini-Tn5 lacZ1 insertion mutants BRM1, BRM6, and BRM9 have been identified previously on the basis of alcaligin siderophore production defects resulting from alc biosynthesis gene disruption (2). BRM1 carries a chromosomal mini-Tn5 lacZ1-encoded lacZ transcriptional fusion to the alcA cistron of the alc operon, and BRM6 carries a similar fusion to the downstream alcC cistron (Fig. 1 and Table 1). The positions and orientations of the fusion elements in both of these mutants place the promoterless lacZ reporter genes under the control of the alc operon control region located immediately upstream of alcA. The mini-Tn5 lacZ1 fusion element of BRM9 is inserted into alcA at a position approximately 200 bp downstream from the insertion site in BRM1, but the element is oriented antisense to alc operon transcription (Fig. 1 and Table 1). Although the BRM1, BRM6, and BRM9 mini-Tn5 lacZ1 insertions served to define genes required for alcaligin siderophore production (2) and led to the discovery of the alcaligin siderophore gene clusters of B. bronchiseptica and B. pertussis (26), iron-regulated lacZ reporter gene expression associated with the mini-Tn5 lacZ1 operon fusion elements was not observed. These data were inconsistent with findings that alc transcription monitored by RNA hybridization methods (25, 26) and alcaligin siderophore production (2) were strongly iron repressible in the alcaligin-producing parent strain B013N. Subsequent genetic and biochemical studies determined that the AlcR regulator protein was required for full expression of alcaligin biosynthesis and transport activities (6) and that the alc genes were cotranscribed from a Fur- and iron-regulated promoter-operator region located immediately upstream from alcA (25, 26; Brickman and Armstrong, Abstr. 97th Gen. Meet. Am. Soc. Microbiol.). Although the alcR regulatory gene is transcribed at a low level from another Fur-controlled promoter immediately upstream from the alcR coding sequences, the majority of alcR expression results from transcription emanating from the alc operon promoter (6, 25; Brickman and Armstrong, Abstr. 97th Gen. Meet. Am. Soc. Microbiol.). Therefore, we hypothesized that the failure to observe elevated alc operon transcriptional activity under iron starvation conditions using the chromosomal alc::mini-Tn5 lacZ1 transcriptional fusions as reporters was likely due to polar effects of the transposon insertions in alcA or alcC on expression of the downstream alcR regulatory gene.
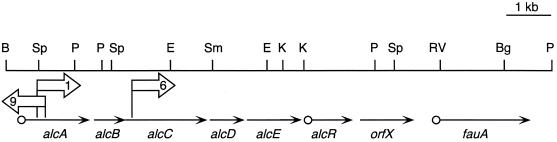
FIG. 1.
Spatial organization of the Bordetella alcABCDER alcaligin siderophore operon. The linear genetic map depicts an approximately 8-kb BamHI-PstI chromosomal DNA region of B. bronchiseptica B013N. Arrows indicate the transcriptional orientations and genetic limits of genes of the alcABCDER operon, and open circles represent the locations of known Fur-regulated promoter-operator regions upstream from alcA, alcR, and fauA. Numbered open arrows indicate the positions and transcriptional orientations of the promoterless lacZ reporter genes associated with the mini-Tn5 lacZ1 transposon insertions of alcaligin-deficient mutants BRM1, BRM6, and BRM9. Abbreviations: B, BamHI; Sp, SphI; P, PstI; E, EcoRI; Sm, SmaI; K, KpnI; RV, EcoRV; Bg, BglII.
The alcR+ plasmid pRK21 complements the alcaligin biosynthesis and transport defects of B. bronchiseptica and B. pertussis alcR mutants (6). We introduced alcR as plasmid pRK21 to B. bronchiseptica fusion strains BRM1, BRM6, and BRM9 to ascertain whether alcR provided in trans, thus circumventing the hypothesized polar influence of the mini-Tn5 lacZ1 insertions on the chromosomal copy of alcR, could result in elevated expression of the alc-lacZ operon fusions under iron-depleted growth conditions. Transcriptional activity of the alc operon in the BRM1 and BRM6 mini-Tn5 lacZ1 fusion strains was not significantly altered by the presence of plasmid pRK21 compared with that in fusion strains bearing the control plasmid vector pRK415 (Fig. 2). Elevated β-galactosidase reporter gene activities were not observed when bacteria were cultured under iron-depleted conditions compared with iron-replete conditions. Thus, relief of polar effects of insertions on alcR by the alcR+ plasmid pRK21 was insufficient to effect iron-regulated alc transcription in fusion strains BRM1 and BRM6 carrying mini-Tn5 lacZ1 fusion elements in the alc sense orientation.
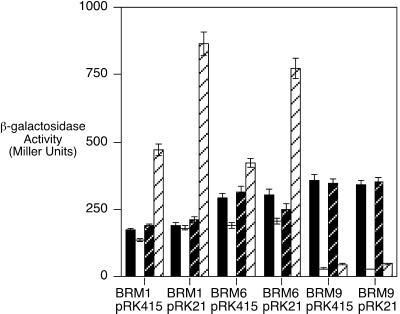
FIG. 2.
Transcriptional activity of the alc operon promoter. Alcaligin siderophore-deficient alc::mini-Tn5 lacZ1 mutants BRM1, BRM6, and BRM9, each carrying the alcR+ plasmid pRK21 or the control plasmid vector pRK415, were cultured in iron-replete or iron-depleted SS medium with or without supplementation of the SS medium with 20 μg of purified alcaligin/ml. Transcriptional activities of the fusion genes were monitored using β-galactosidase assays, with results expressed in Miller units (means ± 1 standard deviation, n = 3). Solid bars, iron-replete cultures; hatched solid bars, iron-replete cultures supplemented with alcaligin; open bars, iron-depleted cultures; hatched open bars, iron-depleted cultures supplemented with alcaligin.
alc operon transcription in siderophore-deficient mutants responds to alcaligin as an inducer under iron starvation conditions.
At the time that alcR was identified as a key regulator of alcaligin siderophore biosynthesis and transport activities, nucleotide sequencing revealed it to be a member of the AraC family of transcriptional regulators (6). With the observation that relief of the polarity of alc::mini-Tn5 lacZ1 insertions by the alcR plasmid pRK21 was insufficient to result in elevated alc-lacZ fusion gene activity in mutants BRM1 and BRM6 under iron starvation growth conditions, it was hypothesized that as an AraC-like regulator, AlcR might require a small-molecule inducer in order to function as a transcriptional activator of alc gene expression. Because other known positive regulators of microbial iron acquisition systems appear to respond to their cognate chelator as the inducer, it was further hypothesized that AlcR might respond to alcaligin siderophore. Since the alc::mini-Tn5 lacZ1 fusion reporter strains are alcaligin siderophore deficient, replicate sets of iron-replete and iron-depleted β-galactosidase assay cultures were supplemented with purified alcaligin at a final concentration of 20 μg/ml to assess the responsiveness of alc transcription to alcaligin. Supplementation of the culture medium with alcaligin resulted in robust elevation of alc::mini-Tn5 lacZ1 transcription in BRM1 and BRM6 under iron-depleted growth conditions, but, as predicted, β-galactosidase activity in BRM9, which carries the lacZ reporter gene fusion in the alc antisense orientation (Fig. 2), was not increased but instead was significantly decreased, likely due to antisense transcription of lacZ. Moreover, supplying alcR in trans as plasmid pRK21 augmented alcaligin-responsive alc::mini-Tn5 lacZ1 transcriptional activity in BRM1 and BRM6, possibly by relief of the polarity of alc::mini-Tn5 lacZ1 insertions on alcR as well as by increased alcR expression resulting from multicopy gene dosage (Fig. 2). Although the alcR+ plasmid pRK21 enhanced alc operon transcription in BRM1 and BRM6 in response to alcaligin, pRK21 did not relieve the absolute requirement for alcaligin as an inducer of alc operon transcription, even though overexpression of some AraC-like regulators due to multicopy gene dosage may suppress the regulator's inducer requirements for activation (35). CAS siderophore detection assays of supernatants from cultures used for β-galactosidase assays confirmed that pRK21 did not complement the alcaligin siderophore biosynthesis defects of alc::mini-Tn5 lacZ1 mutants BRM1, BRM6, and BRM9; thus, induction of alc transcription in β-galactosidase assays was dependent on the exogenously supplied alcaligin. In control CAS siderophore detection assays, alcR overexpression due to multicopy gene dosage did not result in deregulated alcaligin production in the wild-type alcaligin-producing parent strain B013N; B013N(pRK21) produced alcaligin at normal levels and only under iron-depleted culture conditions. These results indicate that alc operon transcription under iron-depleted growth conditions is dependent on the presence of alcaligin and that alc operon transcriptional activity is AlcR responsive. Alcaligin supplementation did not increase alc transcriptional activity under iron-replete culture conditions (Fig. 2), indicating that derepression of Fur- and iron-repressible alc transcription in response to an iron starvation signal is a prerequisite for alcaligin inducer responsiveness and AlcR-mediated activation of alc transcription.
Induction of alc operon transcription by alcaligin is AlcR dependent.
To extend the observations that alc transcriptional activity was alcaligin dependent and responsive to AlcR, it was necessary to establish whether induction of transcription by alcaligin was absolutely dependent on AlcR function. The B. bronchiseptica ΔalcR1 mutant allele was crossed into alc::mini-Tn5 lacZ1 mutants BRM1, BRM6, and BRM9 to create isogenic ΔalcR1 mutant derivatives BRM13, BRM14, and BRM15, respectively, for transcriptional analysis (Table 1). The ΔalcR1 mutation is a nonpolar in-frame deletion mutation created by deletion of a 264-bp NgoAIV fragment internal to the B. bronchiseptica alcR gene (6). The same mutation was previously introduced into B. bronchiseptica strain B013N and B. pertussis UT25 to construct ΔalcR1 mutant strains BRM11 and PM10, respectively (6). The regulatory defect of ΔalcR1 mutant strains can be complemented using plasmid pRK15, which carries a 2.3-kb EcoRI-PstI B. bronchiseptica alcR+ insert DNA fragment, but not by the related plasmid derivative pRK16, which carries the corresponding 2.1-kb EcoRI-PstI ΔalcR1 mutated insert DNA fragment (Table 1). In β-galactosidase assays monitoring alc transcription in response to iron starvation, the presence of the ΔalcR1 mutation in the alcA::mini-Tn5 lacZ1 strain BRM13 abrogated the transcriptional responsiveness of the fusion gene to alcaligin inducer that was observed with the parental alcR+ strain BRM1 (Fig. 3). Furthermore, responsiveness to alcaligin inducer was restored to BRM13 by the alcR+ plasmid pRK15, but not by the ΔalcR1 plasmid derivative pRK16 encoding a defective AlcR regulator. Alcaligin-responsive expression of the alcC::mini Tn5 lacZ1 fusion of BRM14 was likewise found to be alcR dependent, and expression of the antisense-oriented reporter gene of alcA::mini Tn5 lacZ1 control strain BRM15 was negligible, as predicted (data not shown). These results indicate that iron-regulated alc transcription is alcaligin and AlcR dependent; thus, alcaligin participates in a positive autogenous control circuit regulating its own production and utilization through the action of the AlcR regulator.
FIG. 3.
alcR-dependent induction of alc operon transcription. β-Galactosidase reporter activities associated with expression of the alcA::mini-Tn5 lacZ1 fusion element of BRM1(pRK415) were compared with those of the isogenic ΔalcR1 derivative BRM13 carrying the control plasmid vector pRK415, the alcR+ plasmid pRK15, or the ΔalcR1 mutated plasmid pRK16. β-Galactosidase activities were measured for cells cultured in iron-replete SS medium or in iron-depleted SS medium supplemented with 20 μg of purified alcaligin/ml. β-Galactosidase activities are expressed in Miller units (means ± 1 standard deviation; n = 3). Solid bars, iron-replete cultures; hatched bars, iron-depleted cultures supplemented with alcaligin.
Relationship between alcaligin inducer concentration and alc operon transcriptional activity.
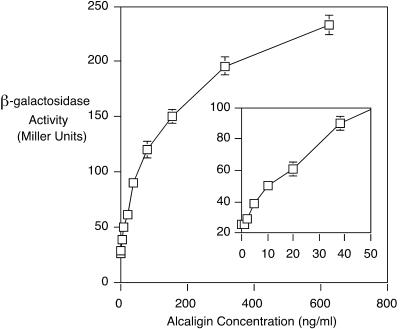
Alcaligin siderophore concentrations measured in iron-depleted SS culture supernatants of wild-type B. bronchiseptica normally range from approximately 25 to 50 μg/ml (12). The relationship between alcaligin inducer concentration and alc operon transcriptional activity was examined using BRM13(pRK21), by monitoring alcA::mini-Tn5 lacZ1 fusion gene expression under iron-depleted conditions in the presence of various concentrations of alcaligin ranging from 0 to 50 μg/ml. Transcriptional activity of the alcA-lacZ fusion of BRM13(pRK21) increased as a function of alcaligin inducer concentration, approaching a maximum reporter gene activity at approximately 20 μg of alcaligin/ml (data not shown). Induction of alc operon transcription by much lower alcaligin concentrations, ranging between 0 and 625 ng/ml, is shown in Fig. 4. A 10-ng/ml threshold concentration of alcaligin inducer nearly doubled the transcriptional activity of the alcA-lacZ fusion gene compared with the basal level of expression under iron-depleted conditions without alcaligin supplement. These findings reveal that alc transcriptional responsiveness to alcaligin inducer is extremely sensitive and that measurable induction of alc transcription occurs at an alcaligin concentration that approximates the minimal concentration required for detectable transport of ferric alcaligin in quantitative [55Fe]ferric alcaligin uptake assays (12). Remarkably, the threshold concentration of alcaligin for induction of alc operon transcription is more than 3 orders of magnitude lower than the concentration of alcaligin required to effect measurable growth stimulation in siderophore bioassays (10, 12).
FIG. 4.
Transcriptional activity of the alc operon varies with alcaligin inducer concentration. BRM13(pRK21) cells were cultured in iron-depleted SS medium supplemented with different concentrations (0, 1, 2, 5, 10, 20, 39, 79, 156, 313, and 625 ng/ml) of purified alcaligin, and transcriptional activity of the alcA::mini-Tn5 lacZ1 fusion gene was monitored by β-galactosidase assay. (Inset) Enlarged scale in the concentration range from 0 to 50 ng of alcaligin/ml. β-Galactosidase activities, expressed in Miller units, are shown as means ± 1 standard deviation (n = 3).
DISCUSSION
The experimental results obtained in this study establish the roles of the regulatory protein AlcR and alcaligin siderophore in the control of alcaligin siderophore system gene expression in Bordetella species. Strong iron-regulated expression of alc transcriptional fusions was achieved by circumventing the polar effects of mini-Tn5 lacZ1 insertions on the alcR gene and by exogenously supplying the alcaligin siderophore inducer that was lacking in the B. bronchiseptica alc::mini-Tn5 lacZ1 fusion strains. AlcR is an AraC-like transcriptional regulator that is necessary for maximal expression of alcaligin siderophore biosynthesis and transport activities under iron starvation stress conditions, and AlcR function requires the presence of alcaligin siderophore acting as the inducer.
The precise mechanism for siderophore induction of transcription involving any of the known iron-related AraC-like regulators YbtA, PchR, and AlcR remains unknown at this time. Since AlcR is an AraC-like protein with a predicted DNA-binding helix-turn-helix structural motif (6), it is presumed that its function involves a sequence-specific DNA-binding activity. It is further hypothesized that the role of alcaligin as an inducer is to modify AlcR activity or DNA-binding site selection to effect transcriptional activation of alcaligin system genes under the appropriate conditions, although it is formally unknown whether alcaligin functions as a coactivator by direct interactions with AlcR. Examination of the nucleotide sequence near the alc operon promoter region revealed the presence of two copies of an 11-nucleotide direct repeat sequence, 5′-TTCTTCGCACA-3′, occupying nucleotide positions −41 to −31 and +71 to +81 with respect to the alc operon major transcription initiation site (Fig. 5). The position of the upstream copy of the repeat with respect to the alc operon promoter is consistent with a potential role (20) in AlcR binding and transcriptional activation of the alc operon; the downstream copy is centered in the alcA initial transcribed region, separated from the upstream copy by 10 integral B-DNA turns. Although it is unknown whether either of these sequences represents actual AlcR-binding sites, the phasing of these two sequences on the DNA helix could potentially allow an interaction between proteins bound at both of these DNA positions. Alternatively, the upstream copy of the putative AlcR-binding site may be directly involved in alc transcriptional activation, and the downstream copy could function as an enhancer-like sequence serving to recruit AlcR to the vicinity of the alc operon promoter. At this time, no evidence exists for AlcR binding to these sequences, and no other candidate AlcR-binding sequences have been identified by nucleotide sequence analysis or mutation. Experimental evidence suggests that expression of the AraC-like regulators YbtA and PchR is negatively autoregulated (18, 24). Examination of potential AlcR autoregulation using an alcR::mini-Tn5 lacZ1 fusion plasmid that placed lacZ reporter gene expression under the control of the secondary promoter-operator region immediately upstream of alcR (Fig. 1) revealed that alcR-lacZ fusion gene expression was not significantly altered in a B. bronchiseptica ΔalcR1 mutant host strain compared with that in an alcR+ strain, regardless of nutritional iron status or the presence of alcaligin inducer in the culture (data not shown). Lack of evidence for AlcR autoregulation acting at the alcR upstream promoter is consistent with the absence of putative AlcR-binding sequences or other identifiable nucleotide sequence similarities shared by the alcA and alcR upstream regions, with the exception of the Fur-binding sites (Brickman and Armstrong, Abstr. 97th Gen. Meet. Am. Soc. Microbiol.). Thus, although alcR expression is negatively regulated by Fur acting at the alcABCDER operon control region as well as at the secondary promoter-operator in the alcR upstream region, no evidence for negative autoregulation of alcR was observed in this study. AlcR positively regulates its own expression by activating transcription of the alcABCDER operon. For the YbtA-regulated psn promoter of Y. pestis, two 18-bp inverted repeat sequences located 48 and 68 bp upstream from the transcription initiation site have been implicated as YbtA-binding sequences (18) by mutational analysis of a psn-lacZ fusion construct. In the psn system, it appears that the promoter-proximal copy of the repeat that overlaps the −35 promoter region alone is sufficient for significant YbtA-mediated activation of transcription but that both repeats are required for maximal expression of psn. Other potential YbtA-binding sequences were identified upstream of irp2, a gene involved in yersiniabactin biosynthesis, as well as in the ybtA initial transcribed region. Positional effects of the two putative YbtA-binding sites in the ybtA initial transcribed region are thought to be responsible for the observed negative autoregulation of ybtA. Experiments in progress are aimed at examination of the putative DNA-binding activity of AlcR, and the influence of alcaligin on AlcR DNA binding and transcriptional activation.
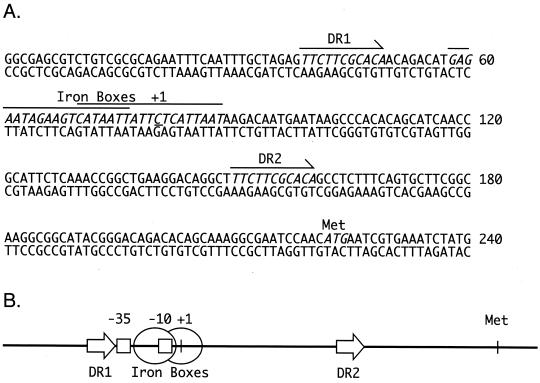
FIG. 5.
The alc operon control region of B. bronchiseptica B013N. (A) Nucleotide sequence of a 240-bp DNA segment representing nucleotide positions −79 to +161 with respect to the alc operon major transcription initiation site (underlined C residue designated +1) (26). Key nucleotide sequence features are italicized. Overlapping nucleotide sequences with similarity to Fur-binding sites (Iron Boxes) are overlined. Two copies of an 11-nucleotide direct repeat sequence (5′-TTCTTCGCACA-3′), designated DR1 and DR2, occupy nucleotide positions −41 to −31 and +71 to +81. The predicted translation initiation methionine (Met) codon for alcA is indicated. (B) Simplified schematic representation of the 240-bp region shown in panel A, showing the spatial relationships of known and predicted regulatory elements. Direct repeat sequences DR1 and DR2 (arrows) and Fur-binding sites (Iron Boxes, designated by ovals) are shown in relation to the major alc operon −35 and −10 promoter determinants (squares) and the transcription initiation site (+1). The relative position of the proposed alcA translation initiation codon (Met) is also indicated.
It was established in this study that activation of alc operon transcription by AlcR can occur at extremely low concentrations of alcaligin inducer. This suggests that Bordetella species, and perhaps other bacterial species having similar regulatory mechanisms controlling iron uptake systems, have evolved a remarkable capacity to sense and respond to the presence of siderophores in their environment. Since a siderophore produced by one bacterium might be sensed by another bacterium of the same or different species expressing the cognate positive regulator, siderophores as small diffusible molecules can mediate a type of intercellular and interspecies communication. Transcriptional activation of the chelate-specific iron transport system in the sensing cell occurs in response to the perceived presence of the siderophore in a manner analogous to the responsiveness of transcriptional regulators involved in perception and response to classical intercellular signaling molecules.
Transcription of the alc operon has previously been shown to be Fur and iron repressible, (7, 25, 26; Brickman and Armstrong, Abstr. 97th Gen. Meet. Am. Soc. Microbiol.) and is now known to be alcaligin and AlcR dependent. Thus alcaligin, as the end product of the siderophore biosynthesis pathway, is a key participant along with AlcR in a positive autogenous control circuit regulating its own production and transport. Since AlcR production is Fur controlled, this positive regulatory mechanism can be viewed simply as subroutine of the global Fur- and iron-regulated negative-control circuit in which the essential nutrient iron, as corepressor with Fur, participates directly in the genetic control of its own assimilation. A major role of positive control of transcription initiation is to establish priorities between pathways that serve the same final purpose (35). Priority regulation of iron acquisition system gene expression could be particularly important when bacteria that are capable of utilizing a variety of potential iron sources are confronted with a mixture of those iron sources, some of which may be more effectively utilized than others in that particular microenvironment. The predominant role of chelate-specific positive regulators may be to allow bacteria to sample their environment, perceive which iron source is available, and selectively activate expression of genes involved in assimilation of the effective iron source. Such regulatory mechanisms may be common to many bacterial species capable of utilizing multiple alternative sources of nutritional iron.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant AI-31088 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Agiato L-A, Dyer D W. Siderophore production and membrane alterations by Bordetella pertussis in response to iron starvation. Infect Immun. 1992;60:117–123. doi: 10.1128/iai.60.1.117-123.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong S K, Clements M O. Isolation and characterization of Bordetella bronchiseptica mutants deficient in siderophore activity. J Bacteriol. 1993;175:1144–1152. doi: 10.1128/jb.175.4.1144-1152.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beall B. Two iron-regulated putative ferric siderophore receptor genes in Bordetella bronchiseptica and Bordetella pertussis. Res Microbiol. 1998;149:189–201. doi: 10.1016/s0923-2508(98)80079-x. [DOI] [PubMed] [Google Scholar]
- 4.Beall B, Sanden G N. A Bordetella pertussis fepA homologue required for utilization of exogenous ferric enterobactin. Microbiology. 1995;141:3193–3205. doi: 10.1099/13500872-141-12-3193. [DOI] [PubMed] [Google Scholar]
- 5.Beall B, Hoenes T. An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors. Microbiology. 1997;143:135–145. doi: 10.1099/00221287-143-1-135. [DOI] [PubMed] [Google Scholar]
- 6.Beaumont F C, Kang H Y, Brickman T J, Armstrong S K. Identification and characterization of alcR, a gene encoding an AraC-like regulator of alcaligin siderophore biosynthesis and transport genes in Bordetella pertussis and Bordetella bronchiseptica. J Bacteriol. 1998;180:862–870. doi: 10.1128/jb.180.4.862-870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brickman T J, Armstrong S K. Bordetella pertussis fur gene restores iron repressibility of siderophore and protein expression to deregulated Bordetella bronchiseptica mutants. J Bacteriol. 1995;177:268–270. doi: 10.1128/jb.177.1.268-270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brickman T J, Armstrong S K. Colicins B and Ia as novel counterselective agents in interspecies conjugal DNA transfers from colicin-sensitive Escherichia coli donors to other gram-negative recipient species. Gene. 1996;178:39–42. doi: 10.1016/0378-1119(96)00331-9. [DOI] [PubMed] [Google Scholar]
- 9.Brickman T J, Armstrong S K. The ornithine decarboxylase gene odc is required for alcaligin siderophore biosynthesis in Bordetella spp.: putrescine is a precursor of alcaligin. J Bacteriol. 1996;178:54–60. doi: 10.1128/jb.178.1.54-60.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brickman T J, Armstrong S K. Essential role of the iron-regulated outer membrane receptor FauA in alcaligin siderophore-mediated iron uptake in Bordetella species. J Bacteriol. 1999;181:5958–5966. doi: 10.1128/jb.181.19.5958-5966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brickman T J, Ozenberger B A, McIntosh M A. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J Mol Biol. 1990;212:669–682. doi: 10.1016/0022-2836(90)90229-F. [DOI] [PubMed] [Google Scholar]
- 12.Brickman T J, Hansel J-G, Miller M J, Armstrong S K. Purification, spectroscopic analysis, and biological activity of the macrocyclic dihydroxamate siderophore alcaligin produced by Bordetella pertussis and Bordetella bronchiseptica. BioMetals. 1996;9:191–203. doi: 10.1007/BF00144625. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S N, Chang A C Y, Hou L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli R-factor DNA. Proc Natl Acad Sci USA. 1972;69:2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean C R, Poole K. Expression of the ferric enterobactin receptor PfeA of Pseudomonas aeruginosa: involvement of a two-component regulatory system. Mol Microbiol. 1993;8:1095–1103. doi: 10.1111/j.1365-2958.1993.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 15.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escolar L, Perez-Martin J, de Lorenzo V. Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 18.Fetherston J D, Bearden S W, Perry R D. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol Microbiol. 1996;22:315–325. doi: 10.1046/j.1365-2958.1996.00118.x. [DOI] [PubMed] [Google Scholar]
- 19.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallegos M-T, Michan C, Ramos J L. The XylS/AraC family of regulators. Nucleic Acids Res. 1993;4:807–810. doi: 10.1093/nar/21.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giardina P C, Foster L-A, Toth S I, Roe B A, Dyer D W. Identification of alcA, a Bordetella bronchiseptica gene necessary for alcaligin production. Gene. 1995;167:133–136. doi: 10.1016/0378-1119(95)00659-1. [DOI] [PubMed] [Google Scholar]
- 22.Hantke K. Regulation of ferric iron transport in Escherichia coli K-12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 23.Heinrichs D E, Poole K. Cloning and sequence analysis of a gene (pchR) encoding an AraC family activator of pyochelin and ferripyochelin receptor synthesis in Pseudomonas aeruginosa. J Bacteriol. 1993;175:5882–5889. doi: 10.1128/jb.175.18.5882-5889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinrichs D E, Poole K. PchR, a regulator of ferripyochelin receptor gene (fptA) expression in Pseudomonas aeruginosa, functions both as an activator and as a repressor. J Bacteriol. 1996;178:2586–2592. doi: 10.1128/jb.178.9.2586-2592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang H Y, Armstrong S K. Transcriptional analysis of the Bordetella alcaligin siderophore biosynthesis operon. J Bacteriol. 1998;180:855–861. doi: 10.1128/jb.180.4.855-861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang H Y, Beaumont F C, Brickman T J, Armstrong S K. Identification and characterization of iron-regulated Bordetella pertussis alcaligin siderophore biosynthesis genes. J Bacteriol. 1996;178:4877–4883. doi: 10.1128/jb.178.16.4877-4884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 28.Kim I, Stiefel A, Plantör S, Angerer A, Braun V. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol Microbiol. 1997;23:333–344. doi: 10.1046/j.1365-2958.1997.2401593.x. [DOI] [PubMed] [Google Scholar]
- 29.Menozzi F D, Gantiez C, Locht C. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect Immun. 1991;59:3982–3988. doi: 10.1128/iai.59.11.3982-3988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 352–355. [Google Scholar]
- 31.Moore C H, Foster L A, Gerbig J G, Dyer D W, Gibson B W. Identification of alcaligin as the siderophore produced by Bordetella pertussis and Bordetella bronchiseptica. J Bacteriol. 1995;177:1116–1118. doi: 10.1128/jb.177.4.1116-1118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neilands J B. A crystalline organo-iron pigment from a rust fungus (Ustilago sphaerogena) J Am Chem Soc. 1952;74:4846–4847. [Google Scholar]
- 33.Nicholson M L, Beall B. Disruption of tonB in Bordetella bronchiseptica and Bordetella pertussis prevents utilization of ferric siderophores, haemin and haemoglobin as iron sources. Microbiology. 1999;145:2453–2461. doi: 10.1099/00221287-145-9-2453. [DOI] [PubMed] [Google Scholar]
- 34.Pradel E, Guiso N, Locht C. Identification of AlcR, an AraC-type regulator of alcaligin siderophore synthesis in Bordetella bronchiseptica and Bordetella pertussis. J Bacteriol. 1998;180:871–880. doi: 10.1128/jb.180.4.871-880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raibaud O, Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- 36.Redhead K, Hill T, Chart H. Interaction of lactoferrin and transferrins with the outer membrane of Bordetella pertussis. J Gen Microbiol. 1987;133:891–898. doi: 10.1099/00221287-133-4-891. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Schneider D R, Parker C D. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect Immun. 1982;38:548–553. doi: 10.1128/iai.38.2.548-553.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]