Abstract
The pancreatic islet depends on blood supply to efficiently sense plasma glucose levels and deliver insulin and glucagon into the circulation. Long believed to be passive conduits of nutrients and hormones, islet capillaries were recently found to be densely covered with contractile pericytes with the capacity to locally control blood flow. Here, we determined the contribution of pericyte regulation of islet blood flow to plasma insulin and glucagon levels and glycemia. Selective optogenetic activation of pericytes in intraocular islet grafts contracted capillaries and diminished blood flow. In awake mice, acute light-induced stimulation of islet pericytes decreased insulin and increased glucagon plasma levels, producing hyperglycemic effects. Interestingly, pericytes are the targets of sympathetic nerves in the islet, suggesting that sympathetic control of hormone secretion may occur in part by modulating pericyte activity and blood flow. Indeed, in vivo activation of pericytes with the sympathetic agonist phenylephrine decreased blood flow in mouse islet grafts, lowered plasma insulin levels, and increased glycemia. We further show that islet pericytes and blood vessels in living human pancreas slices responded to sympathetic input. Our findings indicate that pericytes mediate vascular responses in the islet that are required for adequate hormone secretion and glucose homeostasis. Vascular and neuronal alterations that are commonly seen in the islets of people with diabetes may impair regulation of islet blood flow and thus precipitate islet dysfunction.
Introduction
Pancreatic islets play a crucial role in maintaining glucose homeostasis. There is general agreement that a functional islet vasculature is needed for adequate nutrient sensing, efficient release of insulin and glucagon into the circulation, and timely responses to changes in glycemia (1,2). There is also compelling evidence that islet blood perfusion and microvascular structure are disrupted in diabetes, but it is not known whether these disturbances are of pathogenic importance (3) because the mechanisms that control islet blood flow and their systemic impact on glucose homeostasis have not been fully elucidated.
Blood flow in the islet was proposed to be controlled by external gates at the level of the arteriole, as well as by internal gates at the level of capillaries (4,5). We recently established that these internal gates are vascular pericytes (6). Indeed, islet capillaries are densely covered with contractile pericytes whose activity is tightly coupled to capillary constriction and dilation and to decreases and increases in blood flow in the islet (6). The islet pericyte could thus be responsible for regulating islet blood flow independently from that of the exocrine pancreas (7–10). Importantly, contractile elements around capillaries, likely pericytes, are the predominant cellular targets of autonomic innervation in the human islet (11). We previously reported that sympathetic agonists activate pericytes to constrict capillaries and reduce blood flow in the mouse islet (6), but we did not investigate the possibility that autonomic nerves control hormone delivery into the circulation by altering capillary blood flow. Of note, islet pericytes change their phenotype under conditions of vascular fibrosis, and pericyte coverage of islet capillaries changes in type 2 diabetes (6,12).
In its vascular context, the islet pericyte could play a pivotal role for islet function that goes beyond providing trophic support for the insulin-secreting β-cell (13–17). The role of pericytes in controlling capillary blood flow, however, is hotly debated not only in islet biology but also in other fields. While some studies clearly showed that pericytes modulate capillary diameter and influence blood flow in tissues such as the brain, retina, and kidney (18–23), other studies reported that most of the control occurs upstream at the level of arterioles (24,25). Studying the functional impact of the pericyte in the islet is particularly important because changes in local blood flow may affect the delivery of glucoregulatory hormones that produce systemic effects. Here, we tested the hypothesis that pericyte regulation of islet blood flow alters circulating levels of insulin and glucagon, thus influencing glucose homeostasis. To achieve selective in vivo manipulation of pericytes in the islet, we transplanted islets bearing pericytes expressing the light-gated ion channel channelrhodopsin (ChR2) into the anterior chamber of the eye of diabetic mice. Once islets engrafted and restored normoglycemia, we manipulated pericytes with light in awake mice. Because pericytes in islets are targets of sympathetic nerves, we also examined whether sympathetic control of islet hormone secretion was mediated by pericytes controlling islet blood flow. To mimic sympathetic activation, we administered the α1-adrenergic receptor agonist phenylephrine topically to islet grafts in the eye. Our results show that activating pericytes decreased islet blood flow, lowered plasma insulin levels, and increased plasma glucagon levels, affecting glycemia and glucose tolerance. Importantly, using living human pancreas slices, we assessed the impact of sympathetic agonists on pericyte activity and blood vessel diameter in the human islet.
Research Design and Methods
Transgenic Mice
Mouse lines used in this study were from The Jackson Laboratory (JAX) (Bar Harbor, ME) and Envigo (Indianapolis, IN). For optogenetic manipulation, we crossed homozygous ChR2-tdTomato–floxed male mice (no. 012567; JAX) with heterozygous female mice that express Cre recombinase under the Cspg4 promoter/enhancer (neuron-glial antigen 2 [NG2]-Cre) (no. 008533; JAX) to produce NG2-ChR2+ and NG2-ChR2− donor mice. Glucose metabolism, islet endocrine cell composition, and vascular architecture was similar in NG2-ChR2+ and NG2-ChR2− donor mice (Supplementary Fig. 3). Islets were isolated from female and male NG2-ChR2 mice (4–6 months) and transplanted into the anterior chamber of the eye of immunocompromised nude mice (Hsd:Athymic Nude-ν, age 8 weeks; Envigo). Male and female nude mice were used as transplant recipients and yielded similar results (Supplementary Fig. 2).
Optogenetics allows a more specific and controlled activation of cellular activity, but it is an artificial manipulation. We therefore also studied the potential effects of sympathetic nervous system activation on pericyte function, islet blood flow, and glucose metabolism using sympathomimetics. To examine the effects of sympathetic agonists on islet pericytes ex vivo, we generated NG2-GCaMP3 mice by crossing male GCaMP3-floxed mice (no. 029043; JAX) with NG2-Cre female mice. For activation of pericytes in vivo with a sympathomimetic, we used C57BL/6 male and female mice (age 12 weeks, no. 000664; JAX) as islet donors and transplant recipients. All animals were housed in virus antibody–free rooms and kept in microisolated cages (five mice per cage) with free access to autoclaved food and water. All experiments were conducted according to protocols approved by the University of Miami Institutional Animal Care and Use Committee.
Human Organ Donors
We obtained living human pancreas slices from deidentified cadaveric donors (Supplementary Table 1) from the Network of Pancreatic Organ Donors with Diabetes (Gainesville, FL). Slices were shipped overnight to Miami and used within 3–30 h after arrival (26,27).
Confocal Imaging of Living Pancreas Slices
Functional imaging of living human pancreas slices was done as previously described (26). Briefly, slices were incubated with Fluo4-AM (6.3 μmol/L) and DyLight 649 lectin from Lycopersicon esculentum (3.3 μg/mL) for 1 h in 3 mmol/L glucose buffer solution containing aprotinin (25,000 IU/mL). To identify pericytes, slices were incubated for 2 h with NG2-Alexa Fluor 647 (1:50). We recorded changes in [Ca2+]i and blood vessel diameter induced by norepinephrine (20 μmol/L), endothelin-1 (10 nmol/L), and tyramine (100 μmol/L) on an upright confocal microscope (Leica TCS SP5 system). Functional data were quantified as described in the Supplementary Methods and Analysis.
Islet Isolation and In Vitro Hormone Secretion
Pancreatic islets were isolated from NG2-ChR2+, NG2-ChR2−, and C57BL/6 mice as previously described (28) and cultured overnight before transplantation. ChR2-expressing pericytes remained in islets after isolation and overnight culture (Supplementary Fig. 9). We assessed responses of C57BL/6 isolated islets to epinephrine (10 μmol/L) and phenylephrine (10 μmol/L) applied in 3 mmol/L glucose solution in perifusion experiments and of NG2-ChR2+ and NG2-ChR2− islets to blue light exposure (for 5 min and for 30 min) in basal (3 mmol/L) and high (16 mmol/L) glucose in static incubation experiments at 37°C. Secreted hormones in supernatant were detected with ELISA kits (Mercodia).
Transplantation Into the Anterior Chamber of the Eye
Recipient mice were rendered diabetic before islet transplantation with streptozotocin (200 mg/kg i.v.). Islets were isolated from five to seven NG2-ChR2+ or NG2-ChR2− donor mice and pooled together before transplantation. Transplantation into the anterior chamber of the eye was performed as previously described (28,29). Only animals with glycemia >450 mg/dL were used. We transplanted 200 mouse islet equivalents from NG2-ChR2+ and NG2-ChR2− mice into diabetic nude mice (optogenetic manipulation) and from C57BL/6 mice into diabetic B6 siblings (for pharmacological manipulation with phenylephrine eye drops). This provided an optimal β-cell mass that allowed reversal of diabetes in 26 of 28 recipient mice (total number of transplanted mice for both manipulations) within 1 month after transplantation. The two animals that never recovered from diabetes received NG2-ChR2+ islets (Supplementary Fig. 2) and were excluded.
In Vivo Imaging of the Mouse Eye
A detailed description of in vivo imaging of intraocular islet grafts is included in the Supplementary Methods and Analysis. To activate and deactivate ChR2, we turned on and off the 488-nm laser during image acquisition (100% laser power). Blood vessels were labeled by tail vein injection of fluorescent dextrans. For monitoring the uptake of glucose analog, 100 μL of 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) (5 mg/mL) was injected i.v. Data analysis was performed using ImageJ software (https://imagej.nih.gov/ij) as described in the Supplementary Methods and Analysis. To estimate changes in blood flow, we used the “Stack Difference” plugin to detect dynamic pixel changes that correspond to the movement of red blood cells (RBCs) (30). We drew regions of interest on different vessels in islet grafts and quantified changes in fluorescence over time (Supplementary Fig. 6). To estimate RBC fluxes, we calculated the area under the curve of fluorescence traces every 5 s after turning on the 488-nm laser, which reflects the total amount of RBCs flowing during that period, and divided it by 5.
Manipulation of Pericyte Activity in Awake Mice and Assessment of Metabolic Effects
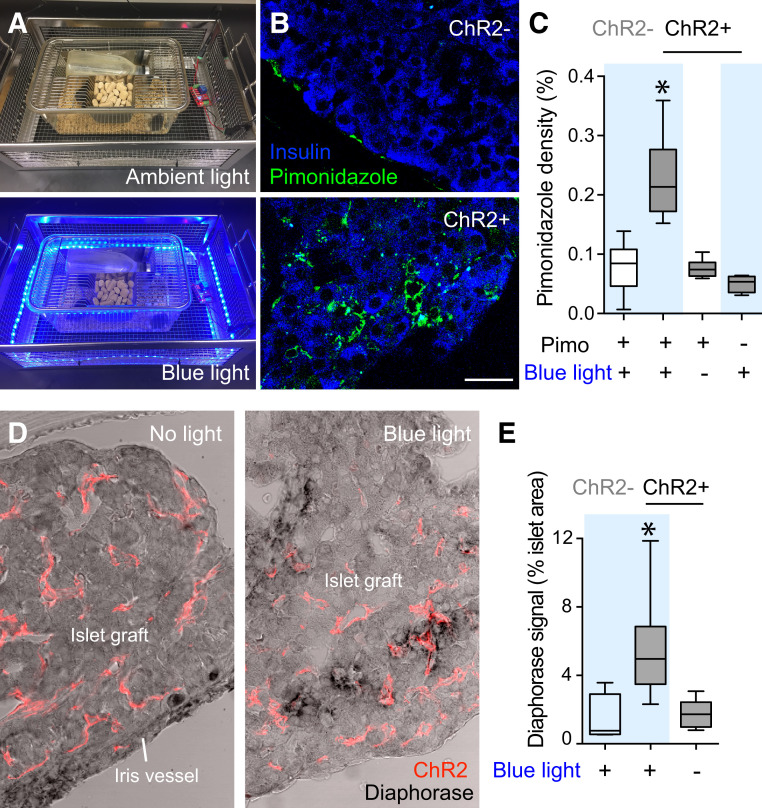
To manipulate pericyte activity in awake, freely moving, transplanted mice, blue light-emitting diodes (LEDs) (emission peak 465 nm) were fixed on a special ion cage as previously described (31) (Fig. 4A). As a control, cages with red LEDs were also built. The output power of each LED was 10 mW, and there were 87 LEDs around the cage’s perimeter (cage dimensions 45.5 × 30.5 cm), yielding an intensity of 0.6 mW/cm2 for the light stimulation. Blue light was delivered chronically for 10 min (Supplementary Fig. 7) or in pulses (1 min on, 4 min off) (Figs. 4 and 5). Stimulation with blue light occurred only acutely; otherwise, transplanted mice were kept in a controlled environment under a normal 12-h light/dark cycle. Mice transplanted with NG2-ChR2+ or NG2-ChR2− islets were housed together and always subjected to the same light stimulation protocol.
Figure 4.
Activation of pericytes with blue light affects vascular perfusion of islet grafts. A: Mice with intraocular islet grafts from NG2-ChR2+ and NG2-ChR2− mice were housed together in cages equipped with a blue LED around their perimeter and subjected to the same light stimulation protocol. B: Stimulation with blue light pulses for 4 h (1 min on, 4 min off) increased levels of the hypoxia marker pimonidazole (Pimo) (60 mg/kg, green) in mice with NG2-ChR2+ islets (bottom) but not in mice with NG2-ChR2− islets (top). C: Quantification of results as in B showing the density of Pimo immunostaining (immunostained area/graft area) in islet grafts (n = 4–6 grafts per six mice). *P < 0.05 between ChR2+ mice Pimo injected and exposed to blue light and all other groups by one-way ANOVA with Tukey multiple comparisons test. D: Optogenetic activation of islet pericytes increased NADPH-diaphorase activity (black staining) in grafts from NG2-ChR2+ mice upon stimulation with blue light (right) but not in the absence of light (left). Note ChR2-tdTomato–expressing pericytes in red and background NADPH-diaphorase activity in iris vessels. E: Quantification of the density of diaphorase activity in ChR2− and ChR2+ grafts (n = 4–6 grafts per six mice). *P < 0.05 between ChR2+ exposed to blue light and the other two groups by one-way ANOVA with Tukey multiple comparisons test. Data are shown in box and whisker plots (min to max) (C and E). Scale bar = 10 μm (B).
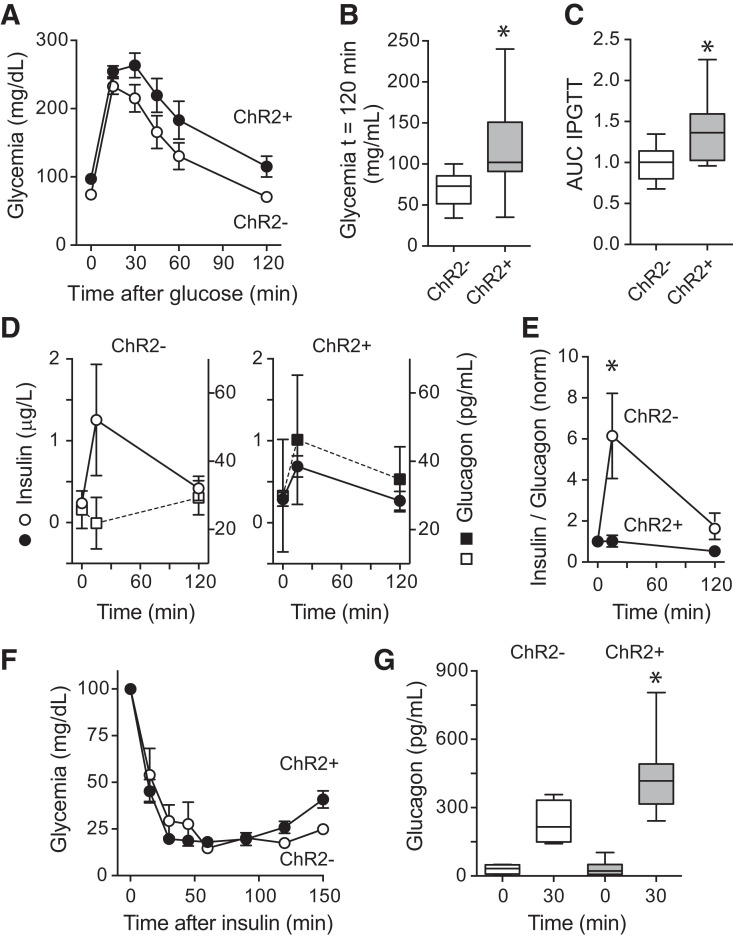
Figure 5.
Optogenetic activation of islet pericytes affects plasma hormone levels and glucose metabolism in awake, freely moving, transplanted mice. A: Glucose excursions of an IPGTT performed in awake, freely moving mice transplanted with islets from NG2-ChR2+ (black) and NG2-ChR2− mice (white) in the presence of blue light pulses (1 min on, 4 min off; light pulses started 1 h before and continued during the IPGTT; n = 7–9 mice/group). B: Glycemia at 120 min after glucose injection. C: Area under the curve (AUC) of IPGTTs performed upon stimulation with blue light (n = 7–9 mice/group). *P < 0.05 by unpaired t test). Values were normalized for AUC values from NG2-ChR2− mice. D: Changes in plasma insulin (circles) and glucagon (squares) induced by glucose in control mice (white symbols, left) and mice with ChR2 in islet pericytes (black symbols, right) during the IPGTT. E: Optogenetic activation of islet pericytes abolished the increase in the insulin-to-glucagon ratio induced by the glucose challenge (n = 5–6 mice). *P < 0.05 by multiple t test. Ratios were normalized to values before glucose administration. F: Insulin tolerance test performed in mice transplanted with islets from NG2-ChR2+ (black) and NG2-ChR2− mice (white) in the presence of blue light pulses (1 min on, 4 min off; light pulses started 4 h before and continued during insulin tolerance test; n = 7–9 mice/group). G: Changes in plasma glucagon levels induced by insulin in transplanted mice. *P < 0.05 between ChR2+ mice 30 min after insulin injection and all other groups by one-way ANOVA with Tukey multiple comparisons test. norm, normalized.
To assess changes in glucose tolerance, transplanted mice were placed in the dark and fasted overnight. Stimulation with pulsed blue light started 1 h before the intraperitoneal glucose tolerance test (IPGTT) and continued throughout the test (glucose 2 g/kg i.p.). As a control, we performed IPGTTs with the same mice in the presence of pulsed red light (Supplementary Fig. 8). To determine changes in glucose-stimulated hormone secretion, transplanted mice were fasted for 6 h. Stimulation with pulsed blue light started 4 h before glucose injection and continued throughout the test. Blood was collected from the tail vein before and 15 and 120 min after glucose injection. To assess changes in glucose counterregulation, animals were fasted for 6 h, and insulin was administered at 0.75 units/kg i.p. (Humulin-R; Eli Lilly). Blood was collected from the tail vein before and 30 min after insulin injection to measure changes in plasma glucagon levels.
To study the effects of sympathetic activation of pericytes in vivo, we used diabetic C57BL/6 mice transplanted with B6 islets. Animals were analyzed 7 days after transplantation (islets had still not engrafted; fed glycemia >300 mg/dL) and 3 months after transplantation (islets were fully revascularized; fed glycemia <200 mg/dL). Transplanted animals were fasted for 6 h and treated with phenylephrine eye drops (2.5%, one drop in transplanted eye). Glycemia and blood were collected before and 30 min after the phenylephrine drop. Control mice were treated with vehicle. To confirm that phenylephrine activates pericytes, we produced living pancreas slices from NG2-GCaMP3 mice as previously described (6) and recorded changes in cytosolic calcium levels induced by phenylephrine (10 and 100 μmol/L) ex vivo.
Assessment of Tissue Hypoxia and Nitric Oxide Production
Mice transplanted with NG2-ChR2+ or NG2-Chr2− islets were placed in the dark and stimulated with pulsed blue light (4 min off, 1 min on) for 4 h. Pimonidazole (Hypoxyprobe Kit, 60 mg/kg i.v.) was injected 90 min before sacrificing the mice. Eyes were collected and processed for immunohistochemistry. To assess local nitric oxide levels, we used the NADPH-diaphorase histochemistry technique (32) (Supplementary Methods and Analysis).
Immunohistochemistry
Pancreata and eyes containing islet grafts from mice expressing ChR2 in pericytes were dissected, fixed overnight in 4% paraformaldehyde, and cryoprotected. Living human pancreata were fixed in 4% paraformaldehyde for 3 h, washed with PBS, and then used for immunohistochemistry. Immunohistochemistry was performed as described in Supplementary Methods and Analysis. We immunostained β-cells (insulin), sympathetic nerves (tyrosine hydroxylase), endothelial cells (CD31), and pericytes with antibodies against different markers: NG2, platelet-derived growth factor receptor-β (PDGFRβ), CD146, and smooth muscle α-actin (αSMA). tdTomato was used as a reporter of ChR2 expression and amplified with an antibody against mCherry. To determine colocalization between pericyte markers NG2, PDGFRβ, and CD146 and ChR2 (tdTomato), we used the ImageJ plugin “intensity correlation analysis” and calculated Mander colocalization coefficients (M1 and M2). To distinguish pericytes from vascular smooth muscle cells that also express NG2 and αSMA, we also analyzed cellular morphology and location (33). Vascular smooth cells have circumferential cytoplasmic processes and form multiple rings around larger blood vessels (diameter ∼10 μm), while pericytes have elongated shapes and cover islet capillaries (vessel diameter ∼5–7 μm) (26) (Supplementary Fig. 1).
Statistical Analysis
Results are expressed as mean ± SE or in box and whisker plots (min to max) from at least three independent biological experiments. P < 0.05 was considered statistically significant. All statistical tests, sample sizes, and P values are provided in the figure legends. All statistics were performed using GraphPad Prism software.
Data and Resources
All data are available in the article or Supplementary Material. Data sets and resources are available from the corresponding author upon reasonable request.
Results
The Islet Vasculature Is Densely Covered With Pericytes
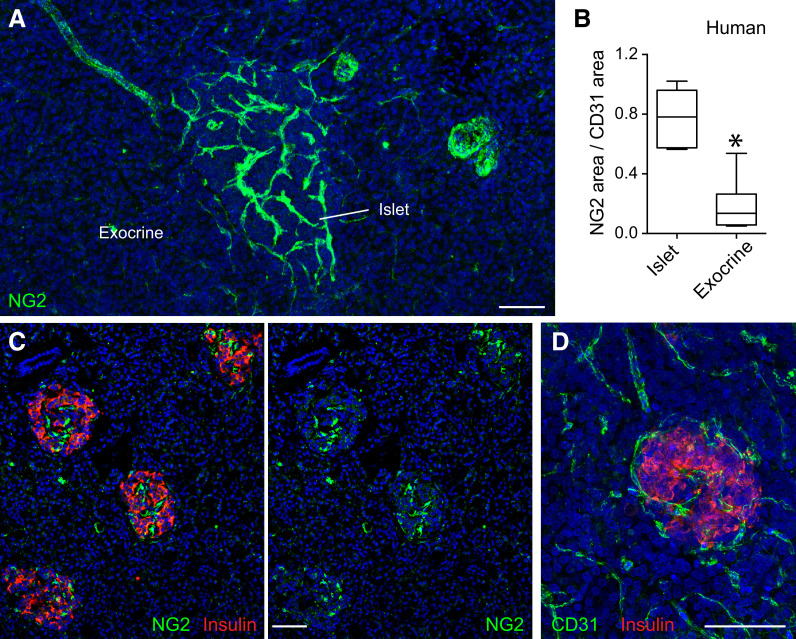
Anatomical and physiological evidence indicates that most islet capillaries are continuous with those in the exocrine pancreas (34–36). This is at odds with studies showing that circulating glucose regulates blood flow in the pancreatic islet without affecting the flow rate in the exocrine pancreas (7–9). We posited that anatomical differences in microvascular elements (endothelial cells and pericytes) could explain differential blood flow regulation. The density of endothelial cells was higher in the endocrine compartment of the human pancreas (CD31 immunostained area was 10.7 ± 1% in islets vs. 5.1 ± 0.5% in exocrine tissue, P < 0.0001 by paired t test, n = 31 islets from four different nondiabetic donors) (Fig. 1D). Nevertheless, even when normalizing for blood vessel density, the abundance of pericytes labeled for NG2 (chondroitin sulfate proteoglycan) was significantly higher in islets than in surrounding exocrine tissue in the human pancreas (Fig. 1A and B). Indeed, islets could be easily distinguished from surrounding tissue by their high abundance of NG2-expressing pericytes (Fig. 1C).
Figure 1.
Blood vessels in human pancreatic islets are densely covered by pericytes. A: Maximal projection of confocal images of a human pancreatic section immunostained for the pericyte marker NG2 (green). The majority of NG2-expressing cells is present in islets in the human pancreas. NG2 is also expressed by smooth muscle cells around arteries and islet-feeding arterioles. B: Quantification of NG2 immunostained area normalized to CD31 immunostained area in endocrine (islets) and exocrine tissue compartments of the human pancreas. Data are shown in a box and whisker plot (min to max) (n = 31 islets from four different donors without diabetes). *P < 0.05 by unpaired t test. C: Confocal images of a human pancreatic section immunostained for insulin (red) and NG2 (green). Regions rich in NG2-expressing cells are islets. D: Maximal projection of confocal images of a human pancreatic section immunostained for the endothelial cell marker CD31 (green) and insulin (red). Scale bars = 50 μm (A, C, and D).
Because there is no single molecular marker that is exclusively expressed by pericytes (33), we immunostained human pancreatic sections for other mural cell markers, such as PDGFRβ (2), CD146 (37), and the contractile protein αSMA. Pericytes in human islets were also immunoreactive for PDGFRβ and CD146, although expression of these markers was not limited to pericytes (Supplementary Fig. 1). Moreover, approximately half of the islet pericyte population expressed αSMA (6,26) (Supplementary Fig. 1). Our data indicate that islets are enriched in pericytes and that pericyte coverage of blood vessels differs greatly between exocrine and endocrine regions of the human pancreas.
Using Optogenetics to Selectively Manipulate Pancreatic Islet Pericytes
We have previously shown that pericytes control capillary diameter and blood flow in the mouse islet (6). To examine the systemic impact of pericyte-mediated control of islet blood flow on plasma hormone levels and glucose metabolism, we used an experimental approach that allowed direct and selective manipulation of islet pericytes (Fig. 2). We transplanted islets into the anterior chamber of the eye of mice rendered diabetic with the β-cell toxin streptozotocin (Fig. 2B). This model has been used extensively to study islet blood vessels because the revascularization of the graft faithfully reconstitutes the structural elements of the vasculature of the islet in the native pancreas (28,38–40). Importantly, pericytes cover the endothelial network in islet grafts as they do in the pancreas (Fig. 2C and D). Of note, intraocular islet grafts restore and maintain normoglycemia a couple of weeks after transplantation (Supplementary Fig. 2), which allowed us to correlate local manipulation of islet cells to systemic effects.
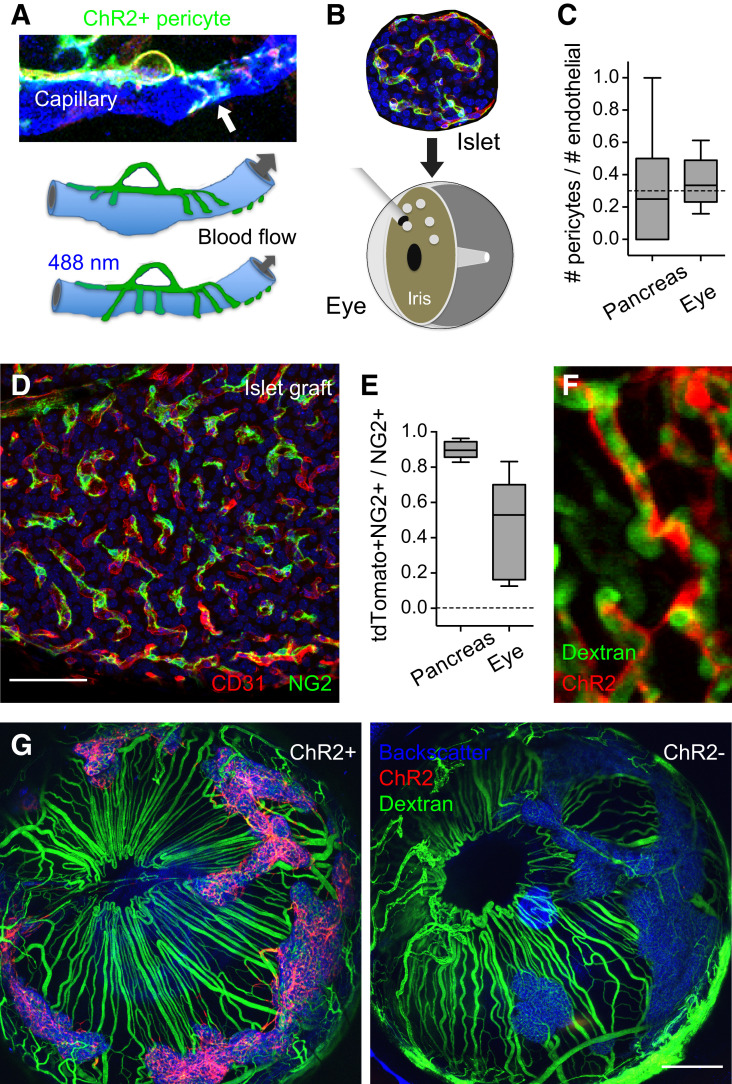
Figure 2.
Experimental strategy to selectively activate islet pericytes. A and B: To visualize and manipulate islet pericytes, we generated mice that expressed ChR2 fused to tdTomato under the control of the NG2 promoter (NG2-ChR2+ mice and control NG2-ChR2− mice) and transplanted 200 islets from these animals into the eyes of nude mice rendered diabetic with streptozotocin. C: Pericyte coverage (pericytes-to-endothelial cells ratio) of capillaries in islets in the pancreas and intraocular grafts is similar. D: Maximal projection of confocal images showing pericytes (NG2, green) covering capillaries (CD31, red) in an intraocular islet graft. E: Mander coefficient reflecting colocalization of ChR2 (tdTomato) and NG2 immunostaining for islet pericytes in the pancreas and in the eye. F: In vivo imaging shows that ChR2-expressing pericytes (red) remain associated with islet capillaries in the islet graft (FITC dextran i.v.). G: Islets (backscatter signal in blue) fully engraft and control glycemia 1 month after transplantation into the eyes of diabetic mice. In mice transplanted with islets from NG2-ChR2+ mice, but not from NG2-ChR2− mice, pericytes can be selectively stimulated with blue light because they express ChR2 (red, left). Because islets were isolated from different NG2-ChR2+ mice and pooled together before transplantation, some variability is observed in the proportion of pericytes that express ChR2 in different grafts on the same transplanted animal (G). Data are shown in box and whisker plots (min to max) (C and E). Scale bars = 50 μm (D) and 500 μm (G).
To manipulate pericytes, we transplanted islets isolated from mouse donors expressing the light-gated ion channel ChR2 under the control of the NG2 promoter/enhancer (NG2-ChR2+ mice) (Fig. 2A and E–G and Supplementary Fig. 3). As a control, we used Cre-negative mice (NG2-ChR2−). In the pancreas, the NG2-Cre mouse line drives expression of genes of interest selectively in smooth muscle cells and pericytes (6,12) (Supplementary Fig. 4). We confirmed that ChR2 is expressed exclusively in pericytes after revascularization of islet grafts in the eye as determined by colocalization with the pericyte markers NG2, PDGFRβ, and CD146 (37) (Supplementary Fig. 4). Approximately 50% of the pericyte population in islet grafts expressed ChR2 (Fig. 2E), indicating that, similarly to what had been described for endothelial cells (41), pericytes from both donor (ChR2+) and host (nude, thus ChR2−) mice participate in the engraftment process. ChR2-expressing pericytes covered the islet endothelial network in grafts as they do in the pancreas (Fig. 2F) and did not colonize the surrounding vasculature (Fig. 2G and Supplementary Fig. 5). Once islets fully engrafted on the iris and took over control of glucose metabolism, we manipulated pericytes optogenetically to determine the effects on capillary diameter, islet blood flow, plasma hormone levels, and glycemia.
Pericytes Control Islet Blood Flow and Glucose Diffusion
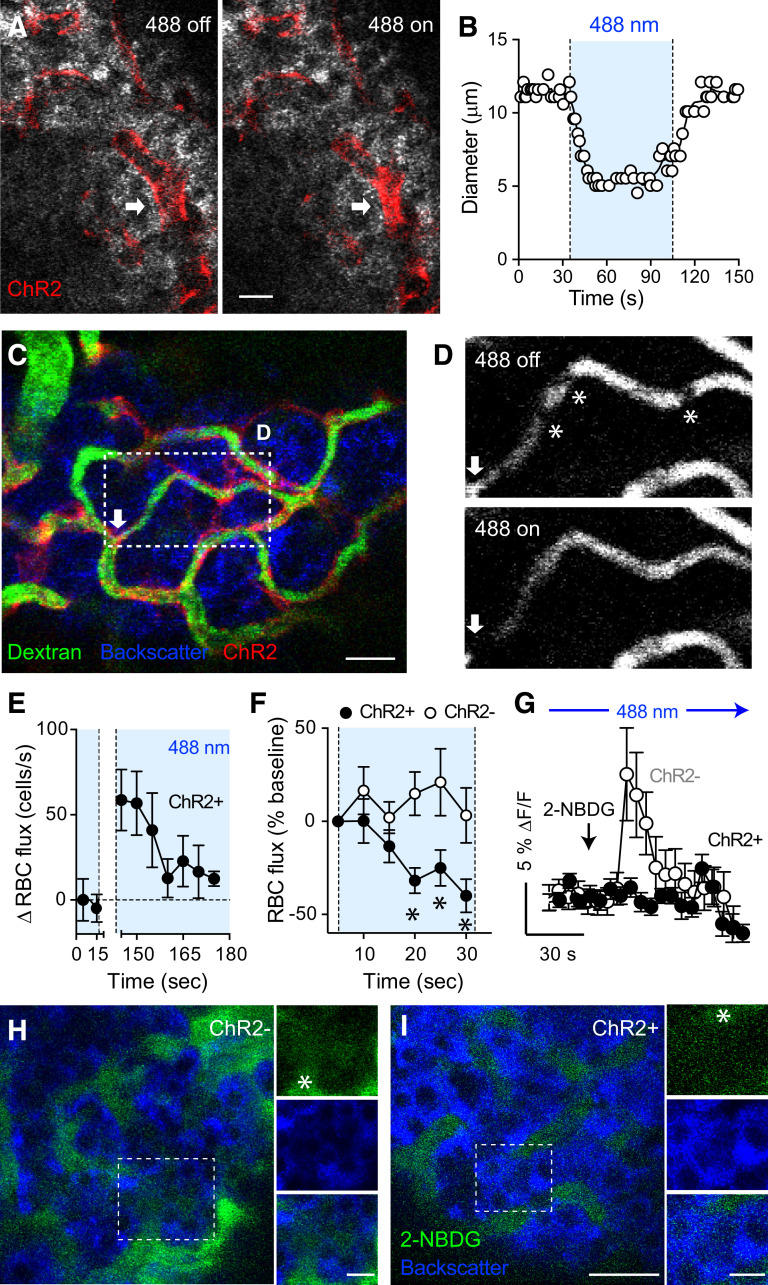
To determine how activation of pericytes affects blood flow in vivo, we placed transplanted mice under a confocal microscope and stimulated intraocular islet grafts with a 488-nm laser. Activating ChR2-expressing pericytes with blue light diminished the diameter of ∼30% of islet capillaries (32.5 ± 10.4%) (Fig. 3A and B and Supplementary Movie 1). The proportion of the microvasculature that was responsive in islet grafts was similar to what we had reported in the native pancreas (6), suggesting that pericytes coming from the iris of the (host) mouse adopted an islet pericyte–like phenotype. Changes in capillary diameter ranged from 20 to 50% reduction of the initial value and were resumed as soon as the 488-nm laser was turned off. We then examined the impact of pericyte activation on islet blood flow. To examine changes in blood flow dynamics, we estimated RBC flux (cell density/time). RBCs were seen moving as nonfluorescent shadows within the fluorescent plasma of islet graft capillaries (Fig. 3D). Pericyte activation decreased RBC flux by ∼50% in islet grafts from ChR2+ mice (Fig. 3C–F, Supplementary Fig. 6, and Supplementary Movies 2–4). We then investigated how light stimulation of pericytes affected plasma glucose diffusion into the islet parenchyma. Activating islet pericytes diminished the diffusion of circulating fluorescent glucose into the islet endocrine parenchyma (Fig. 3G–I). Thus, optogenetic activation of pericytes constricted graft capillaries, decreased blood flow, and limited perfusion of the islet. We could not observe vascular effects when we used red instead of blue light to stimulate or when pericytes did not express ChR2 (NG2-ChR2−) (Fig. 3F and Supplementary Fig. 6).
Figure 3.
Optogenetic activation of islet pericytes constricts capillaries, decreases blood flow, and limits perfusion of the islet. A and B: Confocal images taken during in vivo imaging of an intraocular islet graft show that activating ChR2-expressing pericytes with blue light (488-nm laser) constricted an islet capillary (arrow; changes in diameter are plotted in B). C: In vivo confocal image of an islet graft showing islet vessels (dextran, green) and ChR2-expressing pericytes (red). Arrow indicates a potential “sphincter” pericyte. D: Blood flow through an islet graft capillary (region within rectangle in C) is reduced upon turning on the 488-nm laser. Asterisks indicate different RBCs (shadows). E: Quantification of changes in RBC flux over time in an NG2-ChR2+ islet graft (RBC flux − baseline values are shown). The 488-nm laser had been on for 1 min before starting in vivo recording and was turned off from t = 20–140 s. RBC density was estimated in 5-s intervals (n = 7 vessels). F: Relative changes in RBC flux in capillaries in islet grafts from NG2-ChR2+ (black symbols) and NG2-ChR2− mice (white symbols) induced by the 488-nm laser (n = 14–24 vessels per three mice in each group). *P < 0.05 by multiple t test. G: Quantification of changes in 2-NBDG fluorescence intensity in regions within the islet parenchyma as shown in H and I (fluorescence levels in the islet graft were normalized to those in the aqueous humor; arrow indicates time of injection). H and I: Diffusion of the fluorescent glucose analog 2-NBDG (5 mg/kg i.v.) was reduced by optogenetic stimulation started at 30 s before glucose injection. In vivo images of 2-NBDG fluorescence (green) in islet grafts from NG2-ChR2− (H) and NG2-ChR2+ (I) mice 30 s after injection. Asterisk indicates blood vessels. Data are mean ± SEM (E–G). Scale bars = 10 μm (A) and 20 μm (C, H, and I).
Optogenetic Manipulation of Pericytes Affects Glucose Homeostasis
We then investigated how manipulating pericyte activity and blood flow in the islet affected systemic glucose metabolism by performing studies in awake, freely moving, transplanted mice (Fig. 4A). Animals were placed in cages equipped with blue LEDs for ChR2 activation (intensity 0.6 mW/cm2). To confirm that manipulation with light affected vascular function in the islet, we exposed animals to blue light for 4 h (1 min on, 4 min off) and measured changes in oxygen and nitric oxide in islet grafts. Pimonidazole staining and NADPH-diaphorase activity increased in islet grafts, suggesting that the manipulation increased hypoxic levels and nitric oxide production in islet grafts, likely because of impaired vascular perfusion (Fig. 4B–E). To account for the possible influence of autonomic control of pupillary size on islet graft function, we exposed mice transplanted with islets from NG2-ChR2− donors (control) to acute light stimulation (10 min). In these mice, light increased plasma insulin and glucagon levels. By contrast, in mice transplanted with islets from NG2-ChR2+ donors, light reduced plasma hormone levels (Supplementary Fig. 7). These data indicate that our manipulation of pericyte activity and blood flow overcomes the effects of autonomic input on islet grafts in the context of pupillary light reflex (42). Transplanted animals from both groups were therefore always housed together and subjected to the same light stimulation protocols.
We next addressed the impact of pericyte activation on the islets’ in vivo response to changes in glycemia. We conducted IPGTTs in awake, freely moving, transplanted mice. Activating pericytes in intraocular islet grafts of mice placed in cages equipped with blue light increased the glucose excursion and made mice glucose intolerant (Fig. 5A–C). Red light exposure did not affect glucose tolerance (Supplementary Fig. 8). Mice with control islets from NG2-ChR2− donors showed the typical hormone response to a glucose challenge, that is, an increase in insulin and a decrease in glucagon plasma levels (Fig. 5D). By contrast, mice with activatable pericytes in their islet grafts from NG2-ChR2+ donors showed a tendency toward reduced insulin and increased glucagon plasma levels (Fig. 5D and Supplementary Fig. 8). Importantly, pericyte activation abolished the expected change in the insulin-to-glucagon ratio upon a glucose challenge (Fig. 5E). Under conditions of insulin-induced hypoglycemia, pericyte activation with blue light increased plasma glucagon levels (Fig. 5F and G). In vitro exposure to blue light did not affect either basal or stimulated hormone secretion from islets isolated from NG-ChR2+ mice (Supplementary Fig. 9), indicating that pericyte activation did not directly affect β- and α-cell function. These data indicate that local capillary constriction induced by pericyte activation in vivo affects islet hormone release into the circulation in response to changes in glycemia.
Pericyte Activation With the Sympathetic Agonist Phenylephrine Affects Glucose Homeostasis
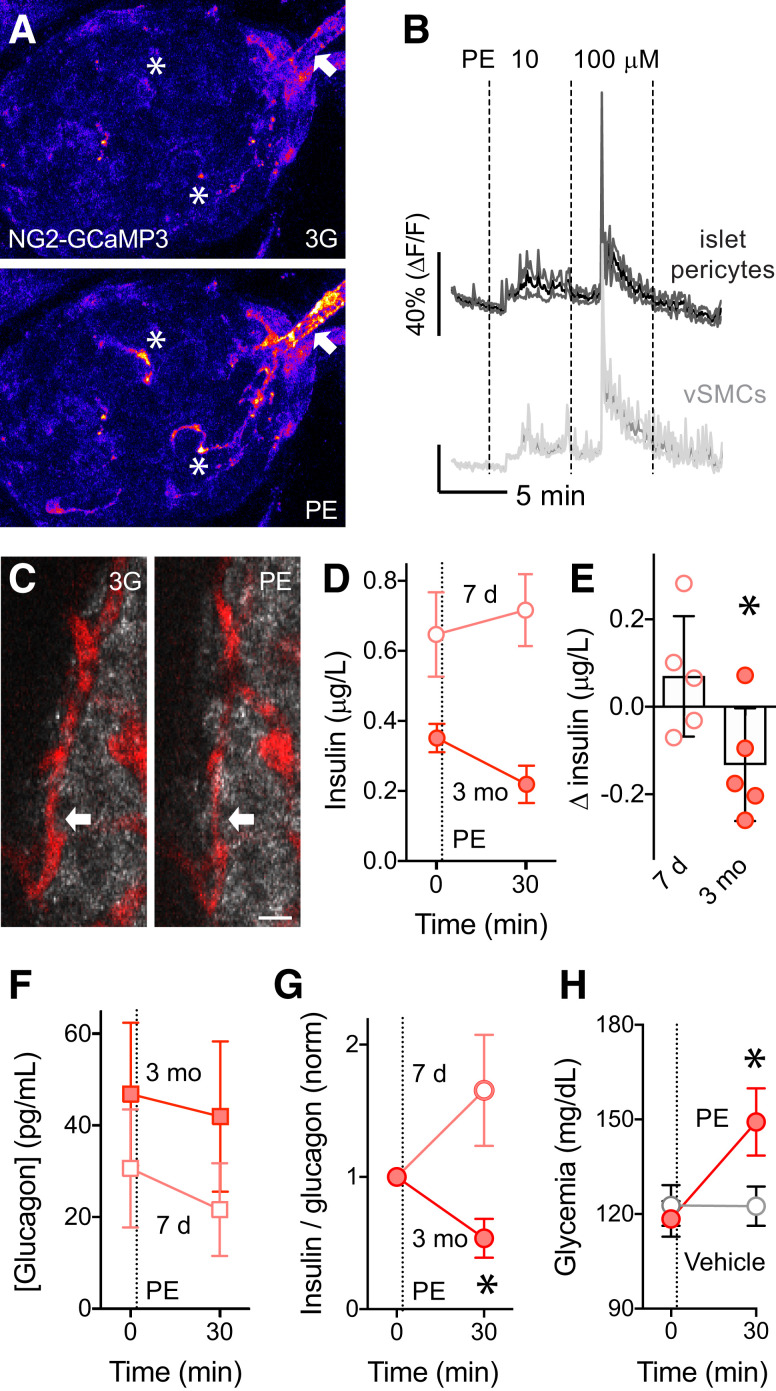
In the pancreas, blood flow is controlled by local sympathetic and parasympathetic nerves (43,44). We had previously shown that sympathetic adrenergic input activates pericytes and decreases blood flow in mouse islets (6), but we had not assessed the impact of sympathetic-mediated control of pericyte function and blood flow on glucose metabolism. To mimic sympathetic nervous input, we targeted adrenergic receptors that are expressed by islet pericytes but not by endocrine cells. Smooth muscle cells and pericytes in various tissues express α1-adrenergic receptors. Endocrine α- or β-cells in rodents express mainly β1- or α2-adrenergic receptors, respectively (45,46) (Supplementary Fig. 10). Accordingly, epinephrine stimulated glucagon secretion and inhibited insulin secretion, but the α1-adrenergic receptor agonist phenylephrine did not affect hormone secretion in vitro (Supplementary Fig. 10). By contrast, phenylephrine increased [Ca2+]i in pericytes and smooth muscle cells in islets in living pancreas slices from NG2-GCaMP3 mice (6) (Fig. 6A and B). We then assessed the in vivo effects of phenylephrine in the syngeneic eye transplantation model (islets from C57BL/6 mice into the eyes of diabetic siblings). Phenylephrine was applied topically as eye drops (2.5%) 7 days after transplantation, when transplanted islets had not been revascularized yet, or 3 months after transplantation, when islets were fully engrafted (29). Importantly, 3 months after transplantation, intraocular islet grafts were also densely innervated, and sympathetic nerves could be seen along ingrowing blood vessels, directly contacting pericytes (42) (Supplementary Fig. 5). In fully revascularized and reinnervated islets, phenylephrine significantly reduced islet capillary diameter (from 9.2 ± 0.6 to 7.8 ± 0.5 μm 10 min after phenylephrine administration, P = 0.0001 by paired t test) (Fig. 6C) and reduced blood flow (6). Importantly, phenylephrine decreased plasma insulin levels in mice with fully engrafted islets (3 months) but not in nontransplanted mice or in the same mice early after transplantation (7 days) (Fig. 6D and E). Although plasma glucagon levels did not change upon phenylephrine administration (Fig. 6F), the insulin-to-glucagon ratio decreased, and glycemia increased (Fig. 6G and H). That the sympathetic agonist acted only on fully vascularized islet grafts demonstrates that the resulting changes in plasma insulin levels were elicited by changes in islet perfusion, not by directly inhibiting insulin secretion from β-cells.
Figure 6.
Activation of pericytes with the sympathetic agonist phenylephrine (PE) decreases plasma insulin levels and increases glycemia. A: In living pancreas slices from NG2-GCaMP3 mice, the α1-adrenergic receptor agonist PE increases [Ca2+]i in islet pericytes (asterisks) and in smooth muscle cells around the islet-feeding arteriole (arrow). B: Traces (mean ± SEM) showing changes in GCaMP3 fluorescence induced by PE (10 and 100 μmol/L) in 3 mmol/L glucose in regions of interest placed around islet pericytes (dark gray) or vascular smooth muscle cells (vSMCs) (light gray) (n = 11 cells per three mice). C: Eyes of C57BL/6 mice transplanted with syngeneic islets were treated with topical application of PE eye drops (2.5%) at 7 days (pink symbols) and 3 months (red symbols) after transplantation. PE eye drops decreased capillary diameter in islet grafts 3 months after transplantation (blood vessels labeled with dextran [red]). D–H: The effects of PE eye drops on plasma insulin (D and E) and glucagon levels (F), their ratios (G), and glycemia (H) were determined after fasting the animals for 6 h. PE-induced changes in plasma insulin (n = 5 mice, *P < 0.05 by paired t test) (E), decreased insulin-to-glucagon ratio (n = 5 mice, *P < 0.05 between 7 days and 3 months 30 min after PE drop by paired t test) (G), and increased glycemia (n = 5 mice, *P < 0.05 between PE treatments at 0 and 30 min by one-way ANOVA Šidák multiple comparisons test) (H) were significantly different at 3 months. Ratios were normalized to values before PE. Data are mean ± SEM. Scale bar = 10 μm (C). 3G, extracellular solution; norm, normalized.
Pericytes Are Major Targets of Sympathetic Nerves, and Sympathomimetics Activate Pericytes and Constrict Blood Vessels in the Human Islet
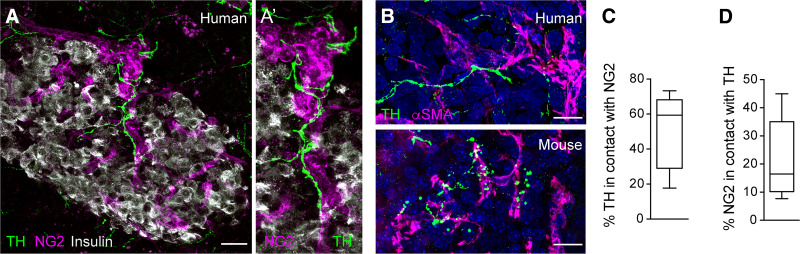
We then examined innervation patterns and responses of pericytes in human islets to sympathetic input. Nerve fibers labeled for the sympathetic marker tyrosine hydroxylase could be seen entering the human islet along arterioles and capillaries, where they contacted NG2-labeled pericytes (Fig. 7A and C). Most of tyrosine hydroxylase–positive fibers were closely associated with pericytes, in particular those labeled for αSMA (Fig. 7B), but not with the endocrine parenchyma (11). Only a subset of islet pericytes was contacted by sympathetic nerve fibers (Fig. 7D).
Figure 7.
Contractile pericytes in human islets are targets of sympathetic nerves. A: Maximal projection of confocal images of a human islet showing that pericytes (NG2, magenta) are contacted by sympathetic nerve fibers (visualized with an antibody against tyrosine hydroxylase [TH], green). A′: Zoomed image (20 μm) of a vessel in the islet in A showing dense sympathetic innervation of pericytes. B: Sympathetic nerve fibers (TH, green) innervate αSMA-expressing pericytes in human (top) and mouse islets (bottom). C: Quantification of the percentage of TH-immunostained area that is in contact with NG2-expressing pericytes. D: Quantification of the percentage of NG2-immunostained area that is in contact with TH-expressing nerve fibers (n = 9 islets from three human donors). Data are shown in box and whisker plots (min to max). Scale bars = 20 μm (A) and 10 μm (B).
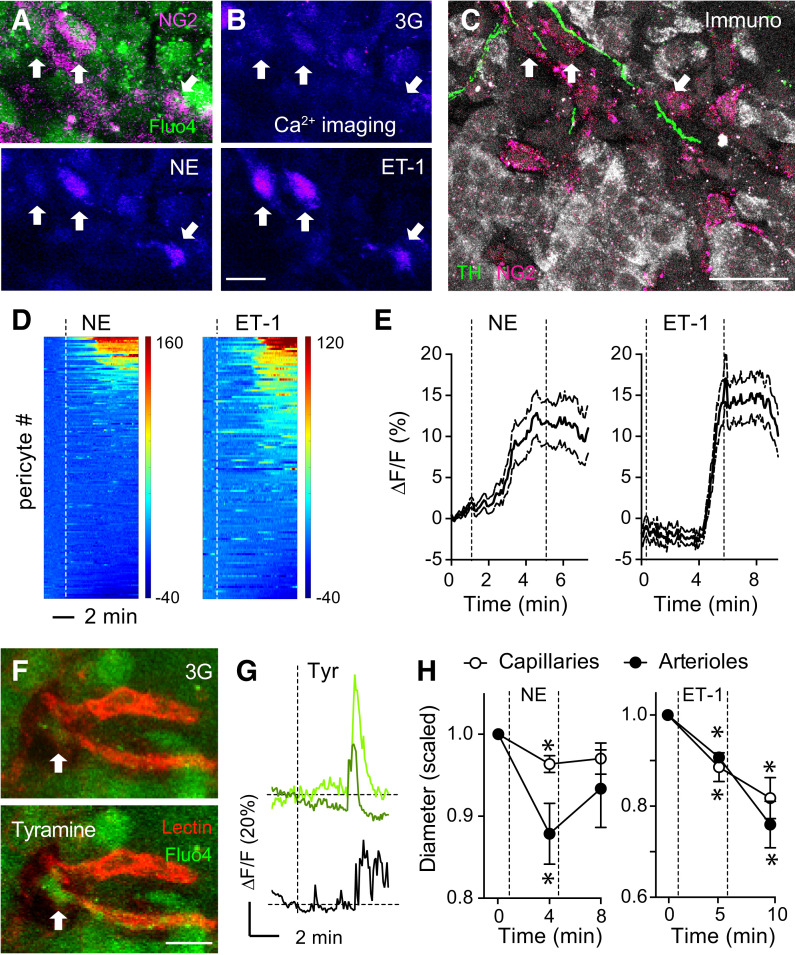
We then recorded islet pericyte activity using [Ca2+]i imaging in living human pancreas slices (26,27). We visualized pericytes and blood vessels with fluorescent antibodies against NG2 (NG2-Alexa Fluor 647) (Fig. 8A) or lectins (from L. esculentum) (Fig. 8F), respectively. Norepinephrine (20 μmol/L) and the potent vasoconstrictor endothelin-1 (10 nmol/L; used as a positive control) increased [Ca2+]i in a subset of islet pericytes (32% and 57% of islet pericyte population, respectively) (Fig. 8B, D, and E). Most pericytes that responded to norepinephrine were contacted by sympathetic nerve fibers, as determined by immunostaining after physiology (Fig. 8C). To assess the effects of endogenously released norepinephrine in human islets, we examined pericyte responses to tyramine, an indirect sympathomimetic agent (47). Tyramine increased [Ca2+]i in a subset of NG2-Alexa Fluor 647–labeled islet pericytes as well as in some endocrine cells (Fig. 8F and G and Supplementary Fig. 11). Norepinephrine administration constricted islet capillaries and feeding arterioles (Fig. 8H) (26). In the human islet, [Ca2+]i increases in pericytes were thus associated with vasoconstriction. Our data further suggest that endogenous norepinephrine, likely from sympathetic nerves, activates islet pericytes. We conclude that the human islet vasculature is generously equipped with contractile pericytes that are major targets of sympathetic innervation. Our study suggests that sympathetic control of hormone secretion in human islets may be mediated by pericytes changing islet blood flow.
Figure 8.
Pericytes in human islets are activated by sympathetic input that leads to vasoconstriction. A and B: Living human pancreas slices were incubated with a fluorescent antibody against NG2 (NG2-Alexa Fluor 647, magenta) and with a membrane-permeant Ca2+ indicator (Fluo4, green) for [Ca2+]i imaging. Pericyte responses to norepinephrine (NE) (20 μmol/L) and endothelin-1 (ET-1) (10 nmol/L) in 3 mmol/L glucose containing extracellular solution (3G) were recorded. C: After physiological experimentation, living slices were processed for immunohistochemistry (Immuno). Antibodies against β-cells (insulin, gray), pericytes (NG2, magenta), and sympathetic nerves (tyrosine hydroxylase [TH], green) were used. Responsive pericytes in B are contacted by sympathetic nerves (arrows point to the same cells in A–C). D: Heat maps showing changes in [Ca2+]i induced by NE (left) and ET-1 (right) in islet pericytes in living pancreas slices from seven donors without diabetes (Supplementary Table 1). Each line corresponds to one pericyte. Dashed lines indicate when stimuli (NE or ET-1) were applied. E: Traces showing changes in [Ca2+]i in islet pericytes induced by NE or ET-1. Data are mean ± SEM (n = 167 [NE] and 133 [ET-1] pericytes). F: Islet pericytes respond to tyramine (Tyr) (100 μmol/L), a sympathomimetic agent that stimulates the release of vesicularly stored NE from sympathetic nerve terminals. Human pancreas slices were incubated with Fluo4 (green) and lectin (red) to label blood vessels. Tyr induces an increase in [Ca2+]i in the pericyte cell body (arrow) and cytoplasmic processes. G: Traces showing changes in [Ca2+]i induced by Tyr in pericytes (green) and in a neighboring endocrine cell (black). H: Quantification of changes in diameter in islet capillaries or arterioles induced by NE or ET-1. Values were normalized to initial diameters (in 3 mmol/L glucose, before stimulus was applied; n = 10–12 vessels per three donors). *P < 0.05 by one-sample t test (values compared with a theoretical mean of 1). Scale bars = 10 μm (A, B, and F) and 20 μm (C).
Discussion
Our study shows that acute activation of islet pericytes, induced either by light or by a sympathetic agonist, decreases islet blood flow, affecting islet hormone secretion and glucose homeostasis. Our results argue against the idea that islet capillaries are only conduits for nutrients, gases, and hormones with little impact on blood flow. On the contrary, we show that the islet vasculature is endowed with pericytes that can actively modulate local blood flow and alter islet function. So far, our understanding of the importance of the islet vasculature for glucose homeostasis was derived from studies based on manipulation of the systemic vasculature and circulation (17,48,49) or on chronic ablation of islet blood vessels (50–52). Although in our study islets were removed from their native location in the pancreas, this report shows that direct and acute manipulation of islet blood flow has systemic effects.
Given their capacity to alter vessel diameter and blood flow, it is surprising that the role of islet pericytes in regulating islet perfusion has been mostly neglected. Studies have mainly focused on how pericytes contribute to the composition of the vascular niche and to β-cell maturation and secretion (12–17). Here, we manipulated islet pericyte activity acutely to demonstrate the importance of regulation of islet blood flow in the maintenance of glucose homeostasis. We did not manipulate pericytes chronically to avoid additional and potentially confounding effects of permanent pericyte loss/dysfunction on endothelial cells and vascular permeability (53,54). Indeed, in a recent study in the brain, pericyte ablation affected blood flow most likely secondary to a substantial breakdown of the blood-brain barrier and edema (55). Their chronic manipulation could also alter the composition of the islet vascular niche and indirectly affect endocrine cell function independently of blood flow. Here, we performed a cause-and-effect study (22) in which pericytes were activated acutely, reversibly, and selectively to elucidate their effects on islet blood flow in real time and in vivo (Fig. 3).
Our anatomical data show that islets can be readily distinguished from the surrounding exocrine tissue by their high density of pericytes (Fig. 1). For blood vessels that are otherwise fully integrated with those in exocrine regions (33), this anatomical feature suggests a structural basis for differential regulation of blood flow in the islet. Blood glucose, as well as autonomic and local vasoactive signals from endothelial and endocrine cells, could target islet pericytes to achieve their reported effects on blood flow (6–9, 56–58). Among these factors, sympathetic input deserves attention. In the human islet, autonomic innervation consists mainly of sympathetic nerves predominantly targeting contractile pericytes (11) (Fig. 7). We now show that norepinephrine activates pericytes and constricts capillaries and feeding arterioles in human islets (Fig. 8). Pericytes that were responsive to adrenergic stimulation were contacted by sympathetic nerves. We further show that the sympathetic agonist phenylephrine activates islet pericytes and decreases blood flow in mouse islets in vivo, reducing insulin plasma levels and increasing glycemia (6) (Fig. 6). We thus propose that sympathetic nerves control hormone release into the circulation by fine-tuning islet blood flow via pericytes. This could help to explain the effects sympathetic nervous input has on plasma insulin levels in humans (47).
Our results show that optogenetic-induced vasoconstriction in the islet limits the diffusion of circulating glucose into the endocrine parenchyma (Fig. 3) and affects plasma levels of islet hormones. The net systemic effect is hyperglycemic, as evidenced by the increased glucose excursion in the glucose tolerance test and the improved glucagon secretion in response to hypoglycemia (Fig. 5). We do not know yet whether these effects are caused by changes in glucose uptake, hormone secretion from endocrine cells, altered delivery into the circulation, or all three. Although pericytes cover ∼30% of the capillaries in the islet, and only half of them may be contractile (6), we estimate that activating pericytes led to a reduction in blood flow in ∼75% of islet vessels (Supplementary Fig. 6). This is possible because the vessel network of mouse islets is highly branched (37), and contractile pericytes are widely distributed, often seen at branching points (6). It is difficult to explain, however, how a reduced blood flow would produce opposite effects on insulin and glucagon levels. Because glucagon-secreting α-cells are preferentially located in the periphery of the mouse islet, it is possible that α-cells are supplied by vessels with a different structure and perfusion pattern. In addition, if α-cells are downstream of β-cells as suggested (59), a reduction of inhibitory factors from β-cells could lead to increases in glucagon secretion (60).
An alternative explanation is that vasoconstriction produces paracrine signals that affect hormone secretion. Although it cannot be considered a definite measure for nitric oxide production (31), our NADPH-diaphorase results suggest that decreased islet blood flow increases nitric oxide levels (Fig. 4), perhaps to compensate for the diminished supply of oxygen and nutrients (61). Nitric oxide is a widely accepted intracellular messenger that has been shown to decrease insulin and increase glucagon secretion (62), but the role of nitric oxide in islet hormone secretion is still controversial (63).
Because pericytes and sympathetic nerves progressively disappear from islets in people with diabetes (6,64), our study has pathophysiological implications. Moreover, other vascular abnormalities, such as immune cell infiltration, amyloid accumulation, and fibrosis, are present in the islets of people with diabetes (65). Together, these alterations may disrupt endocrine and neurovascular coupling and chronically impair local adaptation of islet blood flow to changes in glycemia and metabolic needs. Indeed, islet vascular responses to norepinephrine or high glucose are impaired in a mouse model of islet fibrosis and abnormal pericyte coverage (12). In addition, chronic vascular dysfunction could alter gene expression and affect hormone production or glucose sensing by endocrine cells. In future studies, we will determine whether pericyte function is altered in islets from individuals at different stages of type 1 and type 2 diabetes and assess whether islet vascular dysfunction contributes to diabetes pathogenesis.
Article Information
Acknowledgments. The authors thank Dr. Yuan Liu (Bascom Palmer) for his help building cages for optogenetic manipulation. The authors also thank the Network for Pancreatic Organ Donors With Diabetes, in particular the organ donors and their families, and the whole network slicing team under Dr. Irina Kusmartseva’s supervision. They also thank Dr. Vlad Slepak, Dr. Steve Roper, Dr. Ellen Barrett, and Dr. John Barrett, all from the University of Miami Miller School of Medicine, for carefully reviewing the manuscript.
Funding. This work was funded by National Institutes of Health grants K01DK111757 (J.A.) and R01DK084321, R01DK111538, R01DK130328, and R01DK113093 (A.C.) and by the National Institute of Diabetes and Digestive and Kidney Diseases–supported Human Islet Research Network (Research Resource Identifier: SCR_014393; https://hirnetwork.org; UC4DK104162, New Investigator Pilot Award) (J.A.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.T., L.M.G., E.P., and J.A. performed experiments. R.R.-D performed islet transplantation into the anterior chamber of the eye. M.C. and J.A. analyzed data. A.C. and J.A. designed the study and wrote the manuscript. All authors discussed the results and commented on the manuscript. J.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19706524.
A.T. and L.M.G. contributed equally to this work.
See accompanying article, p. 1611.
References
- 1. Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg 2007;31:705–714 [DOI] [PubMed] [Google Scholar]
- 2. Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev 2010;31:343–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jansson L, Carlsson PO. Pancreatic blood flow with special emphasis on blood perfusion of the islets of Langerhans. Compr Physiol 2019;9:799–837 [DOI] [PubMed] [Google Scholar]
- 4. Brunicardi FC, Stagner J, Bonner-Weir S, et al.; Long Beach Veterans Administration Regional Medical Education Center Symposium . Microcirculation of the islets of Langerhans. Diabetes 1996;45:385–392 [DOI] [PubMed] [Google Scholar]
- 5. McCuskey RS, Chapman TM. Microscopy of the living pancreas in situ. Am J Anat 1969;126:395–407 [DOI] [PubMed] [Google Scholar]
- 6. Almaça J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab 2018;27:630–644.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jansson L, Hellerström C. Stimulation by glucose of the blood flow to the pancreatic islets of the rat. Diabetologia 1983;25:45–50 [DOI] [PubMed] [Google Scholar]
- 8. Moldovan S, Livingston E, Zhang RS, Kleinman R, Guth P, Brunicardi FC. Glucose-induced islet hyperemia is mediated by nitric oxide. Am J Surg 1996;171:16–20 [DOI] [PubMed] [Google Scholar]
- 9. Nyman LR, Ford E, Powers AC, Piston DW. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. Am J Physiol Endocrinol Metab 2010;298:E807–E814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Short KW, Head WS, Piston DW. Connexin 36 mediates blood cell flow in mouse pancreatic islets. Am J Physiol Endocrinol Metab 2014;306:E324–E331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 2011;14:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mateus Gonçalves L, Pereira E, Werneck de Castro JP, Bernal-Mizrachi E, Almaça J. Islet pericytes convert into profibrotic myofibroblasts in a mouse model of islet vascular fibrosis. Diabetologia 2020;63:1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Epshtein A, Rachi E, Sakhneny L, Mizrachi S, Baer D, Landsman L. Neonatal pancreatic pericytes support β-cell proliferation. Mol Metab 2017;6:1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Houtz J, Borden P, Ceasrine A, Minichiello L, Kuruvilla R. Neurotrophin signaling is required for glucose-induced insulin secretion. Dev Cell 2016;39:329–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakhneny L, Epshtein A, Landsman L. Pericytes contribute to the islet basement membranes to promote beta-cell gene expression. Sci Rep 2021;11:2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakhneny L, Rachi E, Epshtein A, et al. Pancreatic pericytes support β-cell function in a Tcf7l2-dependent manner. Diabetes 2018;67:437–447 [DOI] [PubMed] [Google Scholar]
- 17. Sasson A, Rachi E, Sakhneny L, et al. Islet pericytes are required for β-cell maturity. Diabetes 2016;65:3008–3014 [DOI] [PubMed] [Google Scholar]
- 18. Cai C, Fordsmann JC, Jensen SH, et al. Stimulation-induced increases in cerebral blood flow and local capillary vasoconstriction depend on conducted vascular responses. Proc Natl Acad Sci U S A 2018;115:E5796–E5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crawford C, Wildman SS, Kelly MC, Kennedy-Lydon TM, Peppiatt-Wildman CM. Sympathetic nerve-derived ATP regulates renal medullary vasa recta diameter via pericyte cells: a role for regulating medullary blood flow? Front Physiol 2013;4:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzales AL, Klug NR, Moshkforoush A, et al. Contractile pericytes determine the direction of blood flow at capillary junctions. Proc Natl Acad Sci U S A 2020;117:27022–27033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014;508:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hartmann DA, Berthiaume AA, Grant RI, et al. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat Neurosci 2021;24:633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006;443:700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A 2010;107:22290–22295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015;87:95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mateus Gonçalves L, Almaça J. Functional characterization of the human islet microvasculature using living pancreas slices. Front Endocrinol (Lausanne) 2021;11:602519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panzer JK, Hiller H, Cohrs CM, et al. Pancreas tissue slices from organ donors enable in situ analysis of type 1 diabetes pathogenesis. JCI Insight 2020;5:e134525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Almaça J, Molina J, Arrojo E Drigo R, et al. Young capillary vessels rejuvenate aged pancreatic islets. Proc Natl Acad Sci U S A 2014;111:17612–17617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Speier S, Nyqvist D, Cabrera O, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 2008;14:574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santoso F, Sampurna BP, Lai Y, et al. Development of a simple ImageJ-based method for dynamic blood flow tracking in zebrafish embryos and its application in drug toxicity evaluation. Inventions (Basel) 2019;4:65 [Google Scholar]
- 31. Arcuri J, Liu Y, Lee RK, Bhattacharya SK. Lipid profile dataset of optogenetics induced optic nerve regeneration. Data Brief 2020;31:106001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seckler JM, Shen J, Lewis THJ, et al. NADPH diaphorase detects S-nitrosylated proteins in aldehyde-treated biological tissues. Sci Rep 2020;10:21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011;21:193–215 [DOI] [PubMed] [Google Scholar]
- 34. Dybala MP, Kuznetsov A, Motobu M, et al. Integrated pancreatic blood flow: bidirectional microcirculation between endocrine and exocrine pancreas. Diabetes 2020;69:1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henderson JR, Daniel PM. A comparative study of the portal vessels connecting the endocrine and exocrine pancreas, with a discussion of some functional implications. Q J Exp Physiol Cogn Med Sci 1979;64:267–275 [DOI] [PubMed] [Google Scholar]
- 36. Murakami T, Hitomi S, Ohtsuka A, Taguchi T, Fujita T. Pancreatic insulo-acinar portal systems in humans, rats, and some other mammals: scanning electron microscopy of vascular casts. Microsc Res Tech 1997;37:478–488 [DOI] [PubMed] [Google Scholar]
- 37. Smyth LCD, Rustenhoven J, Scotter EL, et al. Markers for human brain pericytes and smooth muscle cells. J Chem Neuroanat 2018;92:48–60 [DOI] [PubMed] [Google Scholar]
- 38. Cohrs CM, Chen C, Jahn SR, et al. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology 2017;158:1373–1385 [DOI] [PubMed] [Google Scholar]
- 39. Diez JA, Arrojo E Drigo R, Zheng X, et al. Pancreatic islet blood flow dynamics in primates. Cell Rep 2017;20:1490–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ilegems E, Dicker A, Speier S, et al. Reporter islets in the eye reveal the plasticity of the endocrine pancreas. Proc Natl Acad Sci U S A 2013;110:20581–20586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nyqvist D, Köhler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 2005;54:2287–2293 [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez-Diaz R, Speier S, Molano RD, et al. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc Natl Acad Sci U S A 2012;109:21456–21461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fontaine AK, Ramirez DG, Littich SF, et al. Optogenetic stimulation of cholinergic fibers for the modulation of insulin and glycemia. Sci Rep 2021;11:3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guyot M, Simon T, Ceppo F, et al. Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nat Biotechnol 2019;37:1446–1451 [DOI] [PubMed] [Google Scholar]
- 45. Baron M, Veres A, Wolock SL, et al. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst 2016;3:346–360.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schuit FC, Pipeleers DG. Differences in adrenergic recognition by pancreatic A and B cells. Science 1986;232:875–877 [DOI] [PubMed] [Google Scholar]
- 47. Gilliam LK, Palmer JP, Taborsky GJ Jr. Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. J Clin Endocrinol Metab 2007;92:4035–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia 1998;41:127–133 [DOI] [PubMed] [Google Scholar]
- 49. Ihoriya C, Satoh M, Kuwabara A, Sasaki T, Kashihara N. Angiotensin II regulates islet microcirculation and insulin secretion in mice. Microcirculation 2014;21:112–123 [DOI] [PubMed] [Google Scholar]
- 50. D’Hoker J, De Leu N, Heremans Y, et al. Conditional hypovascularization and hypoxia in islets do not overtly influence adult β-cell mass or function. Diabetes 2013;62:4165–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iwashita N, Uchida T, Choi JB, et al. Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic beta cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia 2007;50:380–389 [DOI] [PubMed] [Google Scholar]
- 52. Toyofuku Y, Uchida T, Nakayama S, et al. Normal islet vascularization is dispensable for expansion of beta-cell mass in response to high-fat diet induced insulin resistance. Biochem Biophys Res Commun 2009;383:303–307 [DOI] [PubMed] [Google Scholar]
- 53. Hellström M, Gerhardt H, Kalén M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 2001;153:543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Raines SM, Richards OC, Schneider LR, et al. Loss of PDGF-B activity increases hepatic vascular permeability and enhances insulin sensitivity. Am J Physiol Endocrinol Metab 2011;301:E517–E526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nikolakopoulou AM, Montagne A, Kisler K, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci 2019;22:1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jansson L, Hellerström C. Glucose-induced changes in pancreatic islet blood flow mediated by central nervous system. Am J Physiol 1986;251:E644–E647 [DOI] [PubMed] [Google Scholar]
- 57. St Clair JR, Ramirez D, Passman S, Benninger RKP. Contrast-enhanced ultrasound measurement of pancreatic blood flow dynamics predicts type 1 diabetes progression in preclinical models. Nat Commun 2018;9:1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Svensson AM, Ostenson CG, Sandler S, Efendic S, Jansson L. Inhibition of nitric oxide synthase by NG-nitro-L-arginine causes a preferential decrease in pancreatic islet blood flow in normal rats and spontaneously diabetic GK rats. Endocrinology 1994;135:849–853 [DOI] [PubMed] [Google Scholar]
- 59. Stagner JI, Samols E, Bonner-Weir S. beta—alpha—delta pancreatic islet cellular perfusion in dogs. Diabetes 1988;37:1715–1721 [DOI] [PubMed] [Google Scholar]
- 60. Hughes JW, Ustione A, Lavagnino Z, Piston DW. Regulation of islet glucagon secretion: beyond calcium. Diabetes Obes Metab 2018;20(Suppl. 2):127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Casey DP, Joyner MJ. Compensatory vasodilatation during hypoxic exercise: mechanisms responsible for matching oxygen supply to demand. J Physiol 2012;590:6321–6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Akesson B, Mosén H, Panagiotidis G, Lundquist I. Interaction of the islet nitric oxide system with L-arginine-induced secretion of insulin and glucagon in mice. Br J Pharmacol 1996;119:758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bahadoran Z, Mirmiran P, Ghasemi A. Role of nitric oxide in insulin secretion and glucose metabolism. Trends Endocrinol Metab 2020;31:118–130 [DOI] [PubMed] [Google Scholar]
- 64. Mundinger TO, Mei Q, Foulis AK, Fligner CL, Hull RL, Taborsky GJ Jr. Human type 1 diabetes is characterized by an early, marked, sustained, and islet-selective loss of sympathetic nerves. Diabetes 2016;65:2322–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Almaça J, Caicedo A, Landsman L. Beta cell dysfunction in diabetes: the islet microenvironment as an unusual suspect. Diabetologia 2020;63:2076–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]