Abstract
The discovery of the SARS-CoV-2 Omicron (B.1.1.529) variant has sparked alarm globally because of its rapid rate of infection and trespassing acquired immunity due to vaccination or natural infection. This heavily mutated variant is rapidly spreading around the world. Infected individuals with the Omicron variant may suffer from flu-like symptoms, and infected with the Delta variant frequently report low oxygen levels, high pulse rates, and a loss of smell and taste. Also, the Omicron variant causes asymptomatic or mild disease so far, and not any severe illness as like Delta, and this new variant has a 15% to 80% reduced risk of hospitalization than the Delta variant. Scientists are worried about the possibility of escaping the immunity by the Omicron variants and subvariants among fully vaccinated and recovered COVID-19 patients. Two doses of available vaccines are found to be partially ineffective in protecting this new variant, therefore, the third dose as a booster is recommended to enhance antibody level. Moreover, some antiviral drugs significantly reduce hospitalization or death among mild to severe COVID-19 patients. All authorized antiviral drugs are effective against viral replication for most SARS-CoV-2 variants, and particularly some monoclonal antibodies may not now be effective in treating COVID-19 patients. There is an urgent need to update existing vaccines, develop more effective and newer vaccines as well as additional monoclonal antibodies to counter Omicron. Therefore, along with close monitoring of Omicron characteristics, the present study suggests that health safety guidelines, mass immunization, early diagnosis, and search for effective antiviral drugs should be the approaches to fight against newer SARS-CoV-2 variants.
Keywords: SARS-CoV-2, SARS-COV-2 variants, COVID-19, Omicron, B.1.1.529, Coronavirus, COVID-19 vaccines, antiviral agents
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was initially detected in Wuhan, China, and it is the primary agent of the emergent responsible for coronavirus outbreak-2019 (COVID-19). 1 The SARS-CoV-2 has evolved into many life-threatening variants posing substantial hazards to the global healthcare systems since 2020.2 -7 Subsequently, the discovery of the SARS-CoV-2 Omicron (B.1.1.529) variant in November 2021 sparked alarm globally because of its rapid rate of transmission and antibody escaping capability.8 -10 This heavily mutated variant is rapidly spreading around the world that is of great health concern.11 -13 The World Health Organization (WHO) designated this SARS-CoV-2 variant as the variant of concern (VOC) on November 26, 2021. 14 Since January 2021, the arrival of the subsequent Alpha (α), Beta (β), and Delta (δ) in association with new waves of infections, SARS-CoV-2 VOCs became dominant across the entire world in many countries. 15 As of January 11, 2022, more than 140 nations have already been afflicted by the Omicron variant, 16 sometimes multiple SARS-CoV-2 variants have emerged simultaneously. 17 In an already COVID-19-weary and inflicted society, this new VOC Omicron sparked a new debate about vaccine effectiveness and the current booster campaign.18,19 The distinguishing characteristic of Omicron is the prime immunogenic target of antibodies triggered by immunization or through natural infections, which can hold an astonishingly huge number of genetic alterations (more than 50), of which nearly 30 mutations are on the spike (S) membrane protein; mostly like to aggregate around the site of receptor binding motif; the consensus sequence of this variant has 44 amino acid changes, deletion of 6 amino acids, and insertion of one amino acid when compared to the reference strain of SARS-CoV-2. 20 The presence of a mutation at other sites such as the receptor-binding domain (RBD) and N-terminal domain might induce a neutralizing response on the antibodies. Therefore, Omicron is expected to increase the chances of infecting those who have had antigens based on the primary and original S sequence immunization. 21 We have summarized the characteristics and health hazard of coronavirus variants from Alpha to Omicron in an earlier article. 22 However, Omicron and its subvariants are dominating in the present Covid-19 pandemic. Therefore, the present study only focused on the characteristics of Omicron and its subvariant and their impact on global public health.
Omicron is found to be more contagious than Delta and other SARS-CoV-2 variants and therefore has high transmissibility and infectivity.22,23 Viral escaping of the human immune system is another weapon for Omicron; it can evade natural immunity as well as vaccine-induced immunity that lead to breakthrough infections in vaccinated individuals, and reinfection in recovered patients, which helped it to become the most dominant and common strain on the planet. 24 After detection of the Omicron variant, the reinfection rate in South Africa has dramatically multiplied that rose to nearly 4 times; data suggested that PCR positive tests in people who had previously tested positive have increased in case of reinfection. 25 Also, a rise in the use of quick antigen tests and a failure to capture all negative results might be responsible for the wrong interpretation of positive test rates. Despite this limitation, the rise in reinfection instances is consistent with the immune-escape alterations found in Omicron. To evaluate the variant of Omicron case data, from regions such as South Africa and Botswana, the United Kingdom, and elsewhere, the CDC “Center for Forecasting and Outbreak Analytics” established a synthesis of scenario models undertaken by the US government, academia, and foreign partners, and quicker growth scenarios have been observed as a result of their display. 26 The key takeaway from the research is that daily numbers of new infections could reach new highs, possibly surpassing historical highs and this could cause a nationwide and worldwide uptick in the following weeks. As of June 23, 2022, the ever-high peak of the surge in COVID-19 cases has crossed 3 million cases per day with a cumulative being over 539 million cases and more than 6.3 million deaths reported across the world, and Omicron has spread rapidly to nearly all countries now.13,27 A spike is still likely in scenarios with poorer immune evasion; however, the peak can be pushed back until April 2022. 13 Even if, with minimum severity, the enormous number of estimated cases in a time of short duration projected big surges in hospital demand. 28 The present study aimed to provide brief information about the Omicron variant and its impact on health based on the available literature.
Genetic Mutation of SARS-CoV-2 Omicron Variant
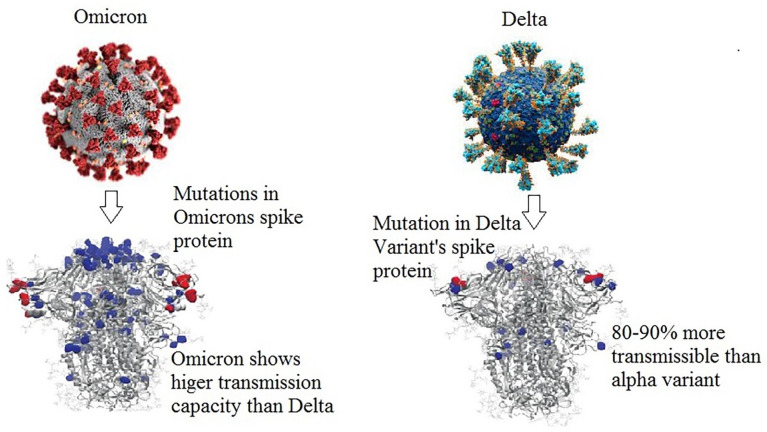
South Africa and Botswana were the first to disclose a new SARS-CoV-2 lineage, “B.1.1.529,” because of the unusually large mutations, it became the variants of concern, including those that have been linked to antibody resistance and enhanced affinity to the receptor of the host, known as angiotensin-converting enzyme-2 (ACE2). 29 The massive quantity of residue mutations observed in the spike protein (herein after spike) (~30) distinguishes this VOC from all previously reported COVID19 strains, the possible changes occurred in many (~15) receptor-binding domain (RBD) of its spike protein. 30 As a result, they may either reduce or escape the immunological responses elicited by vaccination and/or past natural infection in individuals (Figure 1). If the Omicron variant spreads wildly and very rapidly as a dominant variant strain over the world, immune escape or evasion could have serious consequences, such as higher infection rates, reinfection, and/or an increase in the evolution of viral fitness. 16 The Omicron variation replicated at a slower pace than the Delta variant, as seen in the enzyme serine protease 2 (TMPRSS2), which leads to overexpression of the VeroE6 (VeroE6/TMPRSS2) cells found in humans. 21 In TMPRSS2/VeroE6 cells, Delta variant multiplication and fusion are greatly enhanced, whereas the Omicron variant is much less dependent on TMPRSS2. In contrast to the Delta version, the Omicron variant’s replication was not successful in using TMPRSS2 for viral replication, furthermore, the Omicron form might result in lesser lung replication than the Delta type, according to a recent study. 31 Indeed, the preliminary epidemiological evidence indicated that Omicron variation might be conjugated with a milder form of the disease. 32 Some of the mutations detected in Omicron have high transmissibility for the dominating “D614G” and “P681H” mutations.17,33 -35
Figure 1.
Changes in viral spike protein and characteristics of coronavirus variants.
It is promising that Omicron has several other mutations that are so deep in conjunction with the mutations leading to enhanced affinity, which may diminish its affinity for ACE2, and also K417N, G446S, E484A, Q493R, G496S, and Y505H can be included in the list. 36 According to the findings, Omicron’s receptor-binding domain (RBD) has a 2.4-fold higher binding affinity for human ACE2. 36 Although this discovery may provide some reprieve for the time being, subsequent mutations could greatly increase the affinity to binding with the viral receptors at any time in the future, which must be closely monitored. 37
Omicron is currently the dominant variant circulating globally that accounts for more than 98% of viral sequences shared on GISAID after February 2022. It has 5 sub-lineages (BA.1, BA.2, BA.3, BA.4, and BA.5) and several descendent lineages. 38 The latest Omicron sub-lineages BA.4 and BA.5 are considered as VOC lineages under monitoring (VOC-LUM). 38 A recent study has explored that Omicron BA.2.12.1, BA.4, and BA.5 subvariants are capable of escaping antibody responses among the fully vaccinated and coronavirus infected. 39 Therefore, the WHO is recommending reviewing the global epidemiology of VOC-LUM, monitoring and tracking global spread, assisting more laboratory investigations, review viral characteristics of the VOC-LUM. The member states are asked to conduct more investigations to unveil the viral characteristics of these VOC-LUM. 38
COVID-19 Symptoms Due to Omicron Variant
The COVID-19 symptoms due to the Omicron variant are milder ones as similar to previous variants.9,22 However, the Omicron variant and other COVID-19 mutations could raise death rates. 40 People infected with the Omicron variation suffer symptoms comparable to the flu, such as cough, weariness, and head and body pains, whereas those infected with the Delta variant frequently report low oxygen levels, high pulse rates, and a loss of smell and taste. 41 However, shortness of breath is less common in COVID-19 patients due to the Omicron variant. 42 Moreover, tiredness, sore throat, muscle or body aches, loss of taste or smell, and runny nose are being reported frequently. 42 According to the news report, some also reported nausea, shortness of breath, as well as a loss of taste or smell, and diarrhea. At present, we do not have enough epidemiological data about the Omicron variant. However, some preliminary evidence suggested that the illness due to the Omicron variant is mild so far. 42
Disease Severity and Mortality Due to Omicron Variant
One of the current reports from South Africa shows a high transmissible level, with 60% to 80% of the population showing evidence from previous serology to infection or vaccination, implying that Omicron can overcome natural immunity and artificial immunity (vaccine-induced) 43 ; however, early reports do not indicate more severe disease. 44 The symptoms shown by COVID-19 affected patients related to the Omicron variation have been mild so far, according to a physician, from the region of Southern Africa who suspected a different strain of the coronavirus. 37 Nearly 150 countries have already been afflicted by the Omicron variant and the rate of disease severity does not exceed the previous records of other VOC. 31
The findings by research in England, Scotland, and South Africa stated that, that the Omicron variation has a 15% to 80% reduced risk of hospitalization than the Delta variant.23,45 Scientists of Imperial College London analyzed 56,000 and 269,000 Omicron and Delta cases, respectively, in the first 2 weeks of December, estimating overall 15% to 20% infected with Omicron visited the hospital had a 40% to 45% lower probability of hospitalization overnight than those infected with the Delta variant. 46 While due to the high rate of transmissibility of the Omicron virus in recent weeks, researchers from the Imperial College London found that there is a mild decline in the hospitalization rate of COVID-19 patients. 47 However, they assumed that the antibody escaping capability of Omicron variants might compensate for these benefits. Healthcare services across the world may face greater pressure and demand if Omicron infections continue to rise at the current rate.3,48 -52 In the UK, the hospitalization rate is lower among the vaccinated individuals compared to those who have not been vaccinated yet. So, there is a risk of being admitted to the hospital for people who have not been vaccinated yet. Between October and November of 2021 in South Africa, patients diagnosed with Omicron infection showed an 80% lesser likelihood of being admitted to the hospital. 53 Even though case counts were much higher in the latest Omicron-driven wave, the National Institute for Communicable Diseases (NICD) study data shows significantly fewer hospital admissions and deaths than in previous waves. 32 It is ambiguous if the Omicron infection can lead to a more severe form of sickness compared to other variations, such as Delta. According to statistics, it is suggested that prevention is always better than cure, the widely spread variants (Delta and Omicron) of SARS-CoV-2 could turn into serious sickness and eventually result in death to vulnerable patients with compromised immune systems. 54
Effectiveness of COVID-19 Vaccines Against Omicron Variant
Vaccines are now designed to produce antibodies against the spike protein (S), originally based on the SARS-CoV-2 strain. 55 Besides antibodies from previously infected or vaccinated people, the natural immune cell elicited protection from severe disease, from T-cell response. T-cells from previously infected or vaccinated people can recognize viral protein fragments and destroy virus-infected cells as they deploy, thereby restricting the spread of infection. 56 T cells’ ability to recognize and fight infected cells might be impaired by mutations caused by Omicron, however, a recent study suggests that current vaccines are capable of providing considerable protection against Omicron variant. 57 Also, another study revealed that booster vaccination significantly increased T-cell responses to Omicron spike. 58 Therefore, we see some hope according to the recent immunological studies and also, the human immune system can produce several antibodies, which are capable of targeting different portions of the spike protein. If a component of the spike protein alters, a vaccine should still be effective against new variants of coronavirus. However, scientists are assuming about the possible immune escape of the Omicron variant as virtually antibody target sites are different from earlier VOCs. 59
According to early data from the Health Security Agency of the United Kingdom, after receiving 2 doses of vaccine from Oxford–ChAdOx1, known as AstraZeneca’s nCoV-19 (ChAd; n = 22) or, from other companies such as Pfizer–BioNTech’s BNT162b2 (BNT; n = 21) the vaccines’ effectiveness against symptomatic infection has decreased. 60 Due to an increase in the rate of infections among the people who were previously infected or have received double vaccine shots, a new wave of illness may arise. 60 The companies Pfizer and BioNTech reported that their COVID-19 vaccination regimen, consisting of 3 doses was capable of eliminating the new Omicron variant in certain lab tests, and protecting against infection from the recently discovered variant, booster doses may be required. 59 Evidence from Israel, the United Kingdom, and the United States suggest that receiving one shot of booster from vaccines comprising of mRNA base reduces a person’s risk of infection against SARS-CoV-2. 60 A recent Pfizer research over 44 000 people claimed vaccination protection after 6 months dropped from 96.2% to 83.7%. 61 According to these studies, the Pfizer (2 doses) vaccine provides greater than 80% immunity against severe illness and death after 6 months of vaccination, while a booster or additional dose enhances the immune response and restores vaccine effectiveness against the virus.60,61 Indeed, giving vaccines to unvaccinated people in impoverished and developing countries may be preferable because they will receive more than 80% protection against major mortality and morbidity. 61 Another previous study showed that Omicron was more like immune evasion of neutralizing antibodies elicited from ChAdOx-1 (an adenovirus vectored vaccine) than BNT162b2 (Pfizer-BioNTech, which is an mRNA vaccine). 62
Vaccination is still the greatest method to fight with COVID-19 pandemic and protect people from getting infected. 63 Scientists are now looking into Omicron to see how well fully vaccinated people will be protected from hospitalization infection and mortality. CDC recommended vaccination for those who are 5 years and older. However, they recommended a booster dose for persons who are 18 years and older after 6 months of their initial doses.64,65 Notably, the benefits of BNT162b2 vaccination began around 11 days after the first dosage, with 91.7% vaccine efficacy from 11 days after the first dose through the second dose; although, in South Africa, the breakthrough infections have been documented among patients who attained any one of the different types of vaccinations currently in use, from Pfizer-BioNTech, including Johnson & Johnson, and also Oxford-AstraZeneca. 61 More information is needed to completely comprehend the severity of sickness of Omicron in fully vaccinated persons, as well as how reinfections, breakthrough infections, and death in people differ from other variants.
Efficacy of Antiviral Drugs Against Omicron Variant
The interleukin-6 receptor inhibitors and corticosteroids might be effective therapies for individuals who suffer from symptomatic COVID-19, as suggested by WHO. 66 Remdesivir is a nucleotide that inhibits the RNA-dependent polymerase enzyme of SARs, which is present in the epithelial cells of the airways in humans, it shows significant nanomolar action. 67 Remdesivir binds to the viral RNA-dependent RNA polymerase (RDRP), which is a highly conserved enzyme; as a result, it is expected to retain its potency against emerging corona strains. According to in vitro research, all of the different variants of concern are still responsive to remdesivir. Furthermore, recent research found that remdesivir (orally bioavailable prodrug) comprised antiviral activity in respiratory systems (cell lines) and significantly lowered viral infectivity in the lungs of mice infected with SARS-CoV-2 who received this prodrug versus control mice. Over 28 days, the results of this analysis revealed that the remdesivir group had a 72% lower probability of hospitalization compared to a placebo group. 67
In December 2021, the US-FDA approved molnupiravir and paxlovid for emergency use in treating COVID-19 patients. 68 These 2 antivirals are the only orally available authorized drugs for treating mild to moderate COVID-19 patients. These prescription-only oral antiviral drugs may reduce the risk of developing complications and hospitalization. 69 The oral antivirals are effective against various strains of SARS-CoV-2. 70 Molnupiravir targets RdRp and inhibits viral replication. 71 Moreover, molnupiravir shows synergistic effects with other antivirals according to preclinical studies.72,73 However, molnupiravir might be mutagenic and genotoxic. 74 Therefore, physicians should consider molnupiravir based on its comparative risks. 75 Paxlovid contains 2 protease inhibitors, nirmatrelvir, and ritonavir in emergency use in COVID-19 treatment. 67 Nirmatrelvir and ritonavir are protease inhibitors and produce resistance in viral replication for COVID-19 treatment. 76 Moreover, paxlovid reduces hospitalization or death rate by 89% among patients with mild-to-severe COVID-19 symptoms. 77 Scientists assume that paxlovid will be effective against the SARS-CoV-2 Omicron variant at a safety margin. 78 Therefore, along with preventive vaccines, antiviral drugs might be potentially supportive in fighting the new wave of COVID-19 pandemic due to the SARS-CoV-2 Omicron variant.
Vaccination Progress so Far
Recent Omicron variant case statistics consistently show faster spread in the UK, USA, and elsewhere, implying that faster growth scenarios are more plausible.1,8,9 According to the CDC’s analytical methodologies, Omicron’s current quicker relative rate of growth compared to Delta predict that a major outbreak of illnesses might start in early January 2022 in the United States, with the maximum daily number of new infections surpassing previous peaks. 79 The rapid rate of growth in Omicron outbreaks is thought to be due to a combination of enhanced disease transmission and the ability to evade immunity, according to many laboratory trials and clinical investigations. 79 As of June 20, 2022, more than 11.9 billion doses had been administered, with more than 34 million daily doses. 80 As of June 24, 2022, 66.4% population across the world has received any dose of COVID-19 vaccines. However, the vaccination scenario is different for poor and developing countries. 81 Only 17.8% of people have got at least a dose of vaccine in low-income countries. 81 Vaccine hesitancy, COVID-19-related knowledge, risk perceptions, and preventive practices might contribute to the low rate of vaccination in many poor and developing countries.82 -84 People fully vaccinated against COVID-19 in major countries are China (87.24%), the United States (66.90%), the United Kingdom (73.43%), India (65.29%), Brazil (78.77%), Japan (81.17%), and Russia (50.94%). 81 An observational study in the USA reported that higher vaccination rates were associated with the reduced mortality and incidence rates during the waives of Alpha and Delta variants of coronavirus. 85 Another study revealed that vaccination lowered the overall incidence rate to 4.6% from 9.0%. The same study reported that vaccination markedly reduced adverse outcomes and deaths by 63.5% to 69.3% in the same period. 86
Conclusion
Finally, we summarized so far known characteristics of the Omicron variant and presented them in Table 1. There is still insufficient information to understand the transmissibility and mortality of the SARS-CoV-2 Omicron variant. More scientific evidence is required to gain a thorough understanding of this heavily mutated Omicron variant. Till then, scientists should perform research works to reveal the facts that how bad the Omicron is in terms of its transmissibility, diagnosis, disease severity, vaccines and antiviral effectiveness, further mutations, etc. The global healthcare authorities and leadership should take initiatives for mass immunizations of people from poor and developing countries. They should also declare COVID-19 vaccines as global products for emergencies and ensure their equal distribution across the countries. The vaccine manufacturers and the originator countries should show their empathy for humankind regardless of their profit-making tendency and vaccine nationalism. The rich and developed countries are not safe without significant vaccination of people from other parts of the world. Also, healthcare authorities across the world should ensure diagnostics tools and antiviral drugs for their population. Although the Omicron symptoms are mild, however, its high transmission capacity might create an emergency for healthcare services. Therefore, the authorities should ensure more hospital beds, ICUs, and oxygen supplies if such a situation will arise. Finally, to encumber the aggressive spread of any SARS-CoV-2 variant, the authorities should encourage and implement the health safety guidelines such as wearing a face mask, avoiding crowded places, hand washing, and limiting the movement of people to and from highly infected areas.
Table 1.
Summary of the characteristics of Omicron variant.
| COVID-19 variant | Disease severity, symptoms and transmissibility | Immunological response of omicron | anti-viral drugs against omicron |
|---|---|---|---|
| Omicron (B.1.1.529) first identified in Botswana and South Africa in November 24, 2021 and later designated as SARS-CoV-2 VOC on November 26, 202 | • The available epidemiological evidence suggested that the
Omicron variants may cause more mild infections than severe
ones. • Reduced risk of hospitalization and death than the previous waves. • Symptoms are like other COVID-19 variants but milder: headache, fever, cough, weariness, sore throat, muscle or body aches, loss of taste or smell, runny nose. However, shortness of breath is less common. • Omicron have high transmissibility for the dominating “D614G” and “P681H” mutations |
• Fundamentally antibody target sites of Omicron variant are
different from other VOC. • Omicron is more likely possessing immune evasion of neutralizing antibodies elicited from an adenovirus vectored vaccine than in mRNA vaccine. |
• All the variants of concern are still responsive to
remdesivir, molnupiravir, and paxlovid. These antiviral agents
can be used to treat SARS-CoV-2 Omicron variant at a safety
margin. • The protease inhibitors nirmatrelvir and ritonavir can block viral replication in COVID-19 patients. |
Acknowledgments
None.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MRI, WN, and RA conceived the study and wrote the first draft. MS, AR, and KD revised and gave intellectual inputs in the manuscript. MAB and MRI supervised, edited and revised the manuscript. All the authors approved the final version for submission.
Data Availability Statement: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Statement: It was an analysis of online available aggregate data. No Ethical approval was needed.
References
- 1. Islam MR, Hossain MJ. Detection of SARS-CoV-2 Omicron (B.1.1.529) variant has created panic among the people across the world: What should we do right now? J Med Virol. 2022;94:1768-1769. [DOI] [PubMed] [Google Scholar]
- 2. Daria S, Bhuiyan MA, Islam MR. Detection of highly muted coronavirus variant omicron (B.1.1.529) is triggering the alarm for South Asian countries: Associated risk factors and preventive actions. J Med Virol. 2022;94:1267-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahman FI, Ether SA, Islam MR. Upsurge of dengue prevalence during the third wave of COVID-19 pandemic in Bangladesh: pouring gasoline to fire. Clin Pathol. 2022;15:2632010X221076068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahman S, Hossain MJ, Islam MR. The upsurge of diarrhea amid COVID-19 pandemic makes matter worse in Bangladesh: a call to action. Gerontol Geriatr Med. 2022;8:23337214221117419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jannath S, Sohan M, Rahman MA, Islam MR. Suicides among university students during the COVID-19 pandemic: Bangladeshi press reports. Open Health. 2022;3:13-19. [Google Scholar]
- 6. Hossain MJ, Ahmmed F, Sarker MMR, et al. Factors associated with underprivileged E-Learning, session jam phobia, and the subsequent mental distress among students following the extended university closure in Bangladesh. Front Public Health. 2022;9:807474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daria S, Islam MR. Increased suicidal behaviors among students during COVID-19 lockdowns: a concern of student’s mental health in Bangladesh. J Affect Disord Rep. 2022;8:100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daria S, Asaduzzaman M, Shahriar M, Islam MR. The massive attack of COVID-19 in India is a big concern for Bangladesh: the key focus should be given on the interconnection between the countries. Int J Health Plann Manage. 2021;36:1947-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saberiyan M, Karimi E, Khademi Z, Movahhed P, Safi A, Mehri-Ghahfarrokhi A. SARS-CoV-2: phenotype, genotype, and characterization of different variants. Cell Mol Biol Lett. 2022;27:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohapatra RK, Tiwari R, Sarangi AK, Islam MR, Chakraborty C, Dhama K. Omicron (B.1.1.529) variant of SARS-CoV-2: concerns, challenges, and recent updates. J Med Virol. 2022;94:2336-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hossain MS, Islam MR, Islam MT, et al. Knowledge, acceptance and perception about COVID-19 vaccines in Bangladesh: findings from a web-based cross-sectional study. Open Health. 2022;3:73-86. [Google Scholar]
- 12. Hossain MJ, Rahman SMA, Emran TB, Mitra S, Islam MR, Dhama K. Recommendation and roadmap of mass vaccination against Coronavirus disease 2019 pandemic in Bangladesh as a lower-middle-income country. Arch Razi Inst. 2021;76:1823-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daria S, Islam MR. The SARS-CoV-2 omicron wave is indicating the end of the pandemic phase but the COVID-19 will continue. J Med Virol. 2022;94:2343-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. 2021. Accessed December 26, 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- 15. Rahman FI, Ether SA, Islam MR. The “Delta Plus” COVID-19 variant has evolved to become the next potential variant of concern: mutation history and measures of prevention. J Basic ClinPhysiolPharmacol. 2021;33:109-112. [DOI] [PubMed] [Google Scholar]
- 16. European Centre for Disease Prevention and Control. Weekly epidemiological update: Omicron variant of concern (VOC) – week 50. December 19, 2021. Accessed December 26, 2021. https://www.ecdc.europa.eu/en/news-events/weekly-epidemiological-update-omicron-variant-concern-voc-week-50-data-19-december-2021
- 17. Islam MR. The SARS-CoV-2 omicron (B.1.1.529) variant and the re-emergence of COVID-19 in Europe: an alarm for Bangladesh. Health Sci Rep. 2022;5:e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sohan M, Hossain MJ, Islam MR. The SARS-CoV-2 omicron (B.1.1.529) variant and effectiveness of existing vaccines: what we know so far. J Med Virol. 2022;94:1796-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Islam T, Hasan M, Rahman MS, Islam MR. Comparative evaluation of authorized drugs for treating covid-19 patients. Health Sci Rep. 2022;5:e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang L, Cheng G. Sequence analysis of the emerging SARS-CoV-2 variant omicron in South Africa. J Med Virol. 2022;94:1728-1733. [DOI] [PubMed] [Google Scholar]
- 21. Dejnirattisai W, Shaw RH, Supasa P, et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022;399:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Islam S, Islam T, Islam MR. New Coronavirus variants are creating more challenges to global healthcare system: A Brief Report on the current knowledge. Clin Pathol. 2022;15:2632010X221075584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahase E. Covid-19: Hospital admission 50-70% less likely with omicron than delta, but transmission a major concern. BMJ. 2021;375:n3151. [DOI] [PubMed] [Google Scholar]
- 24. Mahase E. Covid-19: Do vaccines work against omicron-and other questions answered. BMJ. 2021;375:n3062. [DOI] [PubMed] [Google Scholar]
- 25. Dyer O. Covid-19: South Africa’s surge in cases deepens alarm over omicron variant. BMJ. 2021;375:n3013. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention. CDC stands up new disease forecasting center. 2021. Accessed January 4, 2022. https://www.cdc.gov/media/releases/2021/p0818-disease-forecasting-center.html
- 27. Taylor L. Covid-19: omicron drives weekly record high in global infections. BMJ. 2022;376:o66. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. WHO Coronavirus (COVID-19) dashboard. Accessed June 25, 2022. https://covid19.who.int/
- 29. Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern Africa. Nature. 2022;603:679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lubin JH, Zardecki C, Dolan EM, et al. Evolution of the SARS-CoV-2 proteome in three dimensions (3D) during the first 6 months of the COVID-19 pandemic. Proteins. 2021;90:1054-1080. doi: 10.1002/prot.26250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao H, Lu L, Peng Z, et al. SARS-CoV-2 omicron variant shows less efficient replication and fusion activity when compared with delta variant in TMPRSS2-expressed cells. Emerg Microbes Infect. 2022;11:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in Tshwane, south Africa. Int J Infect Dis. 2022;116:38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christie B. Covid-19: Early studies give hope omicron is milder than other variants. BMJ. 2021;375:n3144. [DOI] [PubMed] [Google Scholar]
- 34. Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. 2021;25:8012-8018. [DOI] [PubMed] [Google Scholar]
- 35. Surawan DP, Sumohadi D, Budhitresna AA, et al. Titer disparity of anti-Spike receptor binding domain SARS-CoV-2 antibody between vaccinated and naturally infected individuals. Narra J. 2022;2:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Starr TN, Czudnochowski N, Liu Z, et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature. 2021;597:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fang F, Shi PY. Omicron: a drug developer’s perspective. Emerg Microbes Infect. 2022;11:208-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization. Tracking SARS-CoV-2 variants. Accessed June 25, 2022. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 39. Hachmann NP, Miller J, Collier ARY, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387:86-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and delta variant of SARS-CoV-2: a comparative computational study of spike protein. J Med Virol. 2022;94:1641-1649. [DOI] [PubMed] [Google Scholar]
- 41. Iacobucci G. Covid-19: runny nose, headache, and fatigue are commonest symptoms of omicron, early data show. BMJ. 2021;375:n3103. [DOI] [PubMed] [Google Scholar]
- 42. Espenhain L, Funk T, Overvad M, et al. Epidemiological characterisation of the first 785 SARS-CoV-2. Euro Surveill. 2021;26:2101146. doi: 10.2807/1560-7917.ES.2021.26.50.2101146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khandia R, Singhal S, Alqahtani T, et al. Emergence of SARS-CoV-2 omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ Res. 2022;209:112816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kibler KV, Szczerba M, Lake D, et al. Intranasal immunization with a vaccinia virus vaccine vector expressing pre-fusion stabilized SARS-CoV-2 spike fully protected mice against lethal challenge with the heavily mutated mouse-adapted SARS2-N501YMA30 strain of SARS-CoV-2. Vaccines. 2022;10:1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. CNBC. Three new studies suggest omicron has lower hospitalization risk and is milder than other variants. 2021. Accessed January 4, 2022. https://www.cnbc.com/2021/12/23/omicron-variant-has-lower-risk-of-hospitalization-studies-suggest.html
- 46. University of Edinburg THE. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. 2021. Accessed January 4, 2022. https://www.research.ed.ac.uk/en/publications/severity-of-omicron-variant-of-concern-and-vaccine-effectiveness- [DOI] [PMC free article] [PubMed]
- 47. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Daria S, Islam MR. Indiscriminate use of antibiotics for COVID-19 treatment in South Asian countries is a threat for future pandemics due to antibiotic resistance. Clin Pathol. 2022;15:2632010X221099889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rahman FI, Islam MR, Bhuiyan MA. Mucormycosis or black fungus infection is a new scare in South Asian countries during the COVID-19 pandemic: Associated risk factors and preventive measures. J Med Virol. 2021;93:6447-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moona AA, Islam MR. Mucormycosis or black fungus is a new fright in India during covid-19 pandemic: Associated risk factors and actionable items. Public Health Pract. 2021;2:100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Islam MR, Hasan M, Rahman MS, Rahman MA. Monkeypox outbreak – no panic and stigma; only awareness and preventive measures can halt the pandemic turn of this epidemic infection. Int J Health Plann Manage. Published online July 6, 2022. doi: 10.1002/hpm.3539 [DOI] [PubMed] [Google Scholar]
- 52. Islam MR, Asaduzzaman M, Shahriar M, Bhuiyan MA. The spreading of monkeypox in nonendemic countries has created panic across the world: could it be another threat? J Med Virol. Published online June 7, 2022. doi: 10.1002/jmv.27919 [DOI] [PubMed] [Google Scholar]
- 53. Wolter N, Jassat W, Walaza S. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant. MedRxiv, 2021. Accessed January 4, 2022. https://www.medrxiv.org/content/10.1101/2021.12.21.21268116v1 [DOI] [PMC free article] [PubMed]
- 54. Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Preprint. bioRxiv, 2021, 2021.01.15.426911. doi: 10.1101/2021.01.15.426911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. World Health Organization. Update on Omicron. 2021. Accessed January 4, 2022. https://www.who.int/news/item/28-11-2021-update-on-omicron.
- 56. Ledford H. How severe are omicron infections? Nature. 2021;600:577-578. [DOI] [PubMed] [Google Scholar]
- 57. Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 omicron. Nature. 2022;603:493-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Naranbhai V, Nathan A, Kaseke C, et al. T cell reactivity to the SARS-CoV-2 omicron variant is preserved in most but not all individuals. Cell. 2022;185:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Poudel S, Ishak A, Perez-Fernandez J, et al. Highly mutated SARS-CoV-2 omicron variant sparks significant concern among global experts - what is known so far? Travel Med Infect Dis. 2022;45:102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cele S, Jackson L, Khan K, et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. Preprint. medRxiv. 2021;2021.12.08.21267417. [Google Scholar]
- 61. ALJAZEERA. COVID: Study suggests AstraZeneca booster works against Omicron. 2021. Accessed January 4, 2022. https://www.aljazeera.com/news/2021/12/23/astrazeneca-says-booster-jab-works-against-omicron-study-finds
- 62. Thomas SJ, Moreira ED Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meng B, FerreiraI ATM, Abdullahi A, et al. SARS-CoV-2 Omicron spike mediated immune escape, infectivity and cell-cell fusion. bioRxiv. 2021. doi: 10.1101/2021.12.17.473248 [DOI] [Google Scholar]
- 64. Islam MR. Urgent call for mass immunization against coronavirus in Bangladesh. Sci Prog. 2021;104:368504211058562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Centers for Disease Control and Prevention. New SARS-CoV-2 variant of concern identified: Omicron (B.1.1.529) variant. 2021.Accessed January 4, 2022. https://emergency.cdc.gov/han/2021/han00459.asp
- 66. Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases (NCIRD). Accessed January 4, 2022. https://www.cdc.gov/ncird/index.html
- 67. Mohiuddin M, Kasahara K. Investigating the aggressiveness of the COVID-19 omicron variant and suggestions for possible treatment options. Respir Med. 2022;191:106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2022;386:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. FDA. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. FDA News Release, 2021. Accessed December 26, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19
- 70. FDA. Coronavirus (COVID-19) update: FDA authorizes additional oral antiviral for treatment of COVID-19 in certain adults. FDA News Release, 2021. Accessed December 26, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain
- 71. Abdelnabi R, Foo CS, De Jonghe S, Maes P, Weynand B, Neyts J. Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a hamster infection model. J Infect Dis. 2021;224:749-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jayk Bernal A, Gomes da, Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Abdelnabi R, Foo CS, Kaptein SJ, et al. The combined treatment of molnupiravir and favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine. 2021;72:103595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stegmann KM, Dickmanns A, Heinen N, et al. N4-hydroxycytidine and inhibitors of dihydroorotate dehydrogenase synergistically suppress SARS-CoV-2 replication. SSRN Electronic Journal. Published online June 28, 2021. doi: 10.2139/ssrn.4017904 [DOI] [Google Scholar]
- 75. Zhou S, Hill CS, Sarkar S, et al. β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis. 2021;224:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Whitley R. Molnupiravir - a step toward orally bioavailable therapies for covid-19. N Engl J Med. 2022;386:592-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ahmad B, Batool M, Ain QU, Kim MS, Choi S. Exploring the binding mechanism of PF-07321332 SARS-CoV-2 protease inhibitor through molecular dynamics and binding free energy simulations. Int J Mol Sci. 2021;22:9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. [DOI] [PubMed] [Google Scholar]
- 79. Wang Z, Yang L. In the age of omicron variant: Paxlovid raises new hopes of COVID-19 recovery. J Med Virol. 2022;94:1766-1767. [DOI] [PubMed] [Google Scholar]
- 80. Centers for Disease Control and Prevention. Potential Rapid Increase of Omicron Variant Infections in the United States. 2019. Accessed January 4, 2022. https://www.cdc.gov/coronavirus/2019-ncov/science/forecasting/mathematical-modeling-outbreak.html
- 81. Our World in Data. Coronavirus (COVID-19) Vaccinations. 2022. Accessed January 14, 2022. https://ourworldindata.org/covid-vaccinations?country=OWID_WRL
- 82. Ether SA, Emon FA, Roknuzzaman A, Rakibuzzaman M, Rahman FI, Islam MR. A cross-sectional study of COVID-19-related knowledge, risk perceptions, and preventive practices among pharmacy students in Bangladesh. SAGE Open Med. 2022;10:20503121211073014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Islam MR, Hasan M, Nasreen W, Tushar MI, Bhuiyan MA. The COVID-19 vaccination experience in Bangladesh: findings from a cross-sectional study. Int J Immunopathol Pharmacol. 2021;35:20587384211065628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bari MS, Hossain MJ, Ahmmed F, et al. Knowledge, perception, and willingness towards immunization among Bangladeshi population during COVID-19 vaccine rolling period. Vaccines. 2021;9:1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Suthar AB, Wang J, Seffren V, Wiegand RE, Griffing S, Zell E. Public health impact of covid-19 vaccines in the US: observational study. BMJ. 2022;377:e069317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moghadas SM, Vilches TN, Zhang K, et al. The impact of vaccination on Coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clinical Infectious Diseases. 2021;73:2257-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]